Highlights
-
•
The gut virome shapes bacterial populations, metabolism, and immunity, impacting health.
-
•
New sequencing technologies have advanced phage-based therapeutics and diagnostics.
-
•
Phageomics offers personalized therapies against antimicrobial resistance.
-
•
CRISPR-Cas systems and phage diagnostics target antibiotic resistance and pathogens.
Keywords: Gut virome, Bacteriophage, Dysbiosis, Phage therapy, Fecal virome transplantation, CRISPR-Cas system
Abstract
The human gut virome plays a crucial role in the gut and overall health; its diversity and regulatory functions influence bacterial populations, metabolism, and immune responses. Bacteriophages (phages) and eukaryotic viruses within the gut microbiome contribute to these processes, and recent advancements in sequencing technologies and bioinformatics have greatly expanded our understanding of the gut virome. These advances have led to the development of phage-based therapeutics, diagnostics, and artificial intelligence-driven precision medicine. The emerging field of phageomics shows promise for delivering personalized phage therapies that combat antimicrobial resistance by specifically targeting pathogenic bacteria while preserving beneficial microbes. Moreover, CRISPR-Cas systems delivered via phages have shown success in selectively targeting antibiotic resistance genes and enhancing treatment effectiveness. Phage-based diagnostics are highly sensitive in detecting bacterial pathogens, offering significant benefits for human health and zoonotic disease surveillance. This synthesis of the current knowledge highlights the pivotal role of the gut virome in regulating microbial communities and its transformative potential in personalized medicine, emphasizing its importance in advancing therapeutic and diagnostic strategies for improving health outcomes.
Graphical abstract
1. Introduction
The human gut virome, an essential part of the gut microbiome, comprises a diverse range of viruses, including phages, eukaryotic viruses, and endogenous retroviruses. These viruses play crucial roles in shaping microbial communities, supporting immune responses, and influencing metabolic processes [1]. Despite its importance, the gut virome has not been studied as extensively as the bacteria in the microbiome [2]. Recent advances in sequencing technologies and computational tools have provided new insights into the complexity and dynamic nature of the virome, revealing its impact on both gut health and overall physiology [3] (Fig. 1).
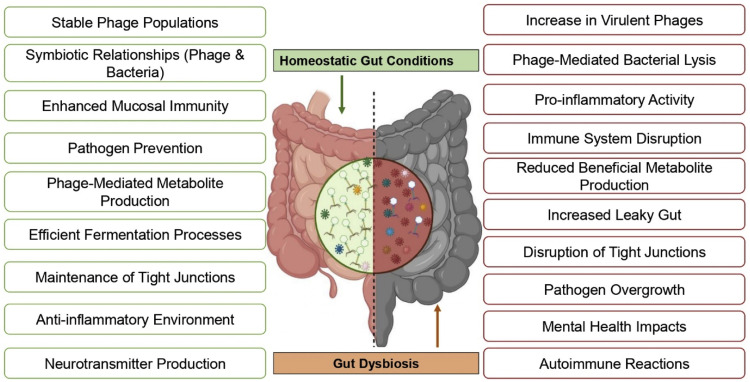
Fig. 1.
Contrasting effects of a healthy gut microbiome on dysbiosis. In the normal gut, diverse microbial populations contribute to optimal metabolic processes, immune regulation, and overall health. Conversely, dysbiosis is characterized by an imbalance in microbial diversity, leading to potential adverse effects on metabolism and immune responses and increased susceptibility to diseases.
Understanding the origin and diversity of the gut virome is critical because it is shaped by evolutionary, environmental, and dietary factors. The virome constantly interacts with other microbiome components, such as bacteria, fungi, and archaea, forming a complex network that influences host health [4]. These interactions are integral to maintaining a stable gut ecosystem and have significant implications for the development of diseases. Changes in the composition of the gut virome have been linked to various human diseases, including inflammatory bowel diseases, autoimmune disorders, metabolic conditions, gastrointestinal disorders, and neurological diseases. The virome also appears to play a role in the body's response to viral infections, such as COVID-19, adding to its relevance in current medical research. Identifying these associations will help us understand the mechanisms through which virome alterations contribute to disease progression [5].
In addition to its role in disease, the gut virome has the potential to be an innovative therapeutic and diagnostic tool. Techniques such as fecal virome transplantation (FVT) offer promising ways to restore a healthy balance in the virome, whereas phage-based therapies have the potential to precisely target harmful bacteria [6]. Advances in the CRISPR-Cas system and artificial intelligence have further enhanced our ability to manipulate and understand the virome, opening new possibilities for personalized medicine and microbiome-based treatments [7].
This review aims to provide a comprehensive understanding of the human gut virome by exploring its origins, diversity, and the environmental and dietary factors that influence its composition. Moreover, we analyzed the interactions between the virome and other components of the microbiome, emphasizing their collective role in maintaining the gut and overall health. Furthermore, this study delved into the association between virome dysbiosis and various human diseases, including inflammatory bowel disease (IBD), autoimmune disorders, metabolic conditions, gastrointestinal and neurological disorders, and coronavirus disease 2019 (COVID-19). Emerging therapeutic and diagnostic approaches, such as FVT, phageomics for personalized medicine, and CRISPR-Cas-based interventions, have been critically examined, along with the potential of artificial intelligence in advancing virome research. By synthesizing current knowledge and identifying gaps, this review aims to highlight the significance of the gut virome in health and disease, paving the way for innovative research and therapeutic strategies.
2. Origin and diversity of the human gut virome
The human gastrointestinal tract contains a wide variety of viruses essential for both health and disease, collectively called the gut virome. These viruses can be classified into two main groups: phages and eukaryotic viruses. Phages that infect bacteria are the most abundant, constituting > 90 % of the gut virome. They significantly influence the composition and function of the gut microbiome by regulating bacterial populations, thereby affecting digestion, immune response, and synthesis of essential nutrients. On the other hand, eukaryotic viruses infect human cells and can range from benign to pathogenic, including viruses like norovirus and rotavirus that cause gastroenteritis [8,9].
The number of viruses in the adult human gut is estimated to be comparable to that of gut bacteria, with over 1012 virus-like particles (VLPs) per individual, adjusted for body size. The gastrointestinal (GI) tract contains the highest concentration of viruses in the human body, with approximately 109–1010 VLPs per gram of feces. Predominant viruses in the gut include Human Parechovirus (HPeV) types 1 and 6, Picobirnaviruses, and Anelloviruses (such as Torque Teno Virus [TTV] and Torque Teno Mini Virus [TTMV]). Other viruses such as rotavirus, astrovirus, Aichi virus, adenoviruses C and F, and bocavirus (HBoV-1) are detected less frequently. The absence of some common enteric viruses in certain studies, such as saliviruses, cosaviruses, coronaviruses, sapoviruses, noroviruses, and cardioviruses, may be due to differences in the detection methods, populations studied, sampling times, and geographical variations. It is essential to consider that viral detection in the gut can vary significantly and may not always capture the full extent of viral diversity present [[10], [11], [12]].
The balance and diversity of the gut virome are essential for maintaining homeostasis. A broad virome is linked to a healthy gut, whereas its disruption or imbalance, referred to as dysbiosis, can contribute to various diseases. These include inflammatory bowel disease, diabetes, obesity, and neurological conditions facilitated by the gut-brain axis. Advances in metagenomic sequencing have allowed a more comprehensive understanding of the gut virome, revealing its dynamic nature and the factors influencing its composition, such as diet, antibiotics, and age [[13], [14], [15]].
The gut virome begins to establish itself at birth and evolves over a person's lifetime, with factors such as breastfeeding, environmental exposure, and genetics playing significant roles in shaping its development. The mode of delivery also affects viral diversity, with vaginal births resulting in greater viral diversity than Cesarean section births [16]. Breast milk contains beneficial viruses such as phages, which regulate the infant's gut microbiome and protect against harmful bacterial and viral infections. Human endogenous retroviruses (HERVs) play regulatory roles in the immune system. Anelloviruses, which are a part of the normal viral flora, are thought to help modulate the immune system [17]. Breast milk provides crucial protection to infants via maternal immune cells and macromolecules. Maternal antibodies in breast milk target a range of viruses, including Rubella, respiratory syncytial virus (RSV), measles, dengue, herpes simplex virus (HSV), varicella zoster virus (VZV), parechovirus, and influenza (Table 1) [18]. Human milk oligosaccharides contribute to the defense against norovirus, rotavirus, and influenza. Additionally, lactoferrin targets rotavirus, norovirus, and SARS-CoV, whereas mucin targets the pox virus. Together, these elements play crucial roles in enhancing the infant immune system and shielding against viral infections during the critical early stages of development [19] (Fig. 2). Initially, the virome was predominantly composed of members of the order Caudovirales, which are double-stranded DNA (dsDNA) phages common in many environments, including the human gut. These phages can either be temperate (integrate into the host genome) or lytic (destroy the host cell to release new phage particles). As infants grow, there is a notable shift in the composition of the gut virome. By the age of 2 years, the Microviridae family of phages is the most prevalent group in the gut virome, a pattern also observed in adult gut viromes. Microviridae are small single-stranded DNA (ssDNA) phages that are typically lytic, meaning that they replicate within and lyse their bacterial hosts to release new viral particles [20,21].
Table 1.
Antiviral components in human breast milk and their mechanisms of action [[25], [26], [27], [28], [29]].
| Molecule | Mechanism of Action | Targeted Viruses |
|---|---|---|
| Maternal Antibodies (IgA, IgG) | Neutralize viruses, prevent attachment to host cells | Respiratory Syncytial Virus (RSV), Herpes Simplex Virus (HSV), VZV, SARS-CoV, Influenza, Enteroviruses, Dengue, Measles, Parechovirus (PeV). |
| Human milk Oligosaccharides (HMOs) | Block pathogen binding, promote beneficial bacteria | Norovirus, Rotavirus, HIV |
| Lactoferrin | Binds iron, inhibits viral replication, direct antiviral activity | RSV, HSV, HIV, Cytomegalovirus (CMV), HCV, HBV, Rotaviruses, Noroviruses, Polioviruses, and Parainfluenza viruses. |
| Mucin | Traps pathogens, prevents adhesion to gut lining | RSV, Influenza, Rotavirus |
| Gangliosides | Inhibit virus binding to host cell receptors | Rotavirus, Influenza |
| Lactadherin | Inactivates virus by binding to virus and host cells | Rotavirus |
| Tenascin C | Binds to viral envelope proteins, neutralizes virus | HIV |
| Glycosaminoglycans | Block virus-host cell binding | HIV |
| Monolaurin | Inactivates viruses by solubilising the lipids and phospholipids in the viral envelop, enhances immune function | HSV, HIV, Influenza Virus |
| Vitamin A | Enhances immune function, reduces viral replication, Cytokine Production. | HIV, Measles Virus, RSV |
| Chondroitin Sulphate | Inhibits virus binding (preventing them from binding to and entering host cells, particularly those that use heparan sulfate proteoglycans for attachment.), anti-inflammatory effects | HIV, HSV, Zika Virus (ZIKV), Dengue Virus |
| Oxysterols | Inhibit viral entry, assembly, and replication | HSV, ZIKA, Ebola, HCV |
| Cytokines | Modulate immune responses, anti-inflammatory effects | Broad antiviral activity |
| Lysozyme | Destroys bacterial cell walls, enhances immune function | Indirectly affects viral infections by reducing bacterial load |
| α-Lactalbumin | Antimicrobial activity, some forms of α-lactalbumin, like HAMLET (Human Alpha-lactalbumin Made Lethal to Tumor cells), can induce apoptosis in virus-infected cells, reducing viral replication. | Broad antimicrobial effects, HSV. HIV, RSV, Papillomavirus |
| MicroRNAs | Regulate immune responses, potential direct antiviral effects | Broad antiviral potential |
RSV – Respiratory Syncytial Virus, HSV – Herpes Simplex Virus, VZV – Varicella-Zoster Virus, SARS-CoV – Severe Acute Respiratory Syndrome Coronavirus, HIV – Human Immunodeficiency Virus, HBV – Hepatitis B Virus, HCV – Hepatitis C Virus, CMV – Cytomegalovirus, ZIKV – Zika Virus, PeV – Parechovirus, HAMLET – Human Alpha-lactalbumin Made Lethal to Tumor cells.
Fig. 2.
Overview of the human gut virome and factors influencing its composition. The adult gut virome predominantly comprises phages (> 90 %) and eukaryotic RNA and DNA viruses. Virome diversity and composition change across life stages, with high phage diversity in neonates, increasing bacterial diversity in infants, homeostasis in adults, and decreased diversity in aging [56]. Key factors influencing the gut virome are highlighted in red and include diet, infections, age, lifestyle, geographic and environmental exposure, antibiotic use, mode of delivery, and feeding practices. Maternal components, such as mucin, cytokines, and microRNAs, are critical in shaping the neonatal gut virome. Abbreviation: VLPs, virus-like particles.
A study of 53 infants and their mothers revealed that infants had a higher abundance and diversity of gut viruses than their mothers, with phages being the most prevalent. The infant virome evolves over the first three years of life, showing an increase in the abundance of dietary/environmental viruses and a decrease in the abundance of human-host viruses [22]. This diversity likely stems from infants’ immature immune systems and dynamic gut environments, which permit broader viral colonization than adults. Early bacterial colonization combined with dietary and environmental exposure during development contributes to virome evolution. The dominance of phages reflects their pivotal role in shaping the dynamics of the infant gut microbiota. Additionally, crAs-like phages, including prototypical crAssphages (p-crAssphages), are another significant component of the gut virome. These phages are lytic and specifically infect members of the bacterial phylum Bacteroidetes, which are abundant in the human gut. CrAssphages first appear in the gut virome of infants aged 1–3 months. Their prevalence increases as children grow older and becomes widespread among children aged 2–5 years [23].
Despite its importance, the gut virome remains less studied than the bacterial microbiome, partly because of the complexity of viral identification and classification. The lack of a curated database of human gut viral sequences is a major obstacle in metagenomic studies. However, the Global Virome Database (GVD) aims to fill this gap despite limitations in geographic representation and RNA virus detection. The GVD can facilitate ecosystem-wide examination of virome dynamics and has potential applications in personalized medicine, environmental monitoring, and broader viral detection in various ecosystems. Enhanced viral mapping and mechanistic studies are essential to advance our understanding of human and environmental microbiomes [24].
3. Influence of the environment and diet on the gut virome
The human gut virome is a diverse and abundant component of the gut microbiome that plays a significant role in health and disease. Recent research has highlighted how environmental and dietary factors profoundly influence the composition and function of the gut virome. Geographical differences, such as those observed in a cohort of 930 healthy adults across six ethnicities in China, contribute to variations in virome diversity. Urbanization tends to increase dissimilarities in virome composition among individuals, likely due to differences in exposure to environmental factors, such as climate and sanitation [30]. Similarly, research involving a Japanese cohort of 4198 individuals found that geographical and environmental factors, host bacterial distribution, and antiviral defenses significantly affected virome diversity. This study identified numerous factors, including age, sex, and lifestyle, with disease and medication showing the strongest effects on the virome structure [31].
These findings are consistent with observations from studies conducted in the United States, which highlighted that individuality and ethnicity contribute more to taxonomic variation in the gut virome than short-term dietary changes [32,33]. Urban and rural settings also play a role in this phenomenon, with urbanization and industrialization influencing viral diversity and potentially altering the gut virome composition. Additionally, hygiene and sanitation practices, as well as exposure to pets and wildlife, can introduce or reduce specific viral taxa, thereby modulating gut virome diversity and functional potential [34].
Dietary factors also significantly affect gut microbiota. Various diets—omnivorous, vegetarian, and vegan— affect viral composition and interactions differently. For instance, a study on individuals adopting a gluten-free diet revealed notable changes in virome composition, particularly among those with initially lower microbial diversity [35]. Omnivorous diets rich in diverse foods may promote a more varied virome, whereas vegetarian and vegan diets, which often include more fiber and fewer animal products, can influence viral populations in distinct ways [36]. Dietary components such as macronutrients and micronutrients also contribute to this diversity. Probiotics and prebiotics affect the gut virome by introducing beneficial viruses and promoting the growth of beneficial viral communities. Fermented foods, which naturally contain viruses, can also enhance viral diversity. Conversely, artificial additives and preservatives might negatively affect viral populations, whereas alcohol consumption can alter the gut virome composition, potentially disrupting the balance by reducing viral diversity [37,38]. The interplay between diet and the environment further complicates their individual effects on the gut virome. The combined influence of these factors affects viral diversity and function through their effects on the gut microbiota. For instance, dietary patterns that promote beneficial microbial populations may also favor beneficial viral communities, whereas environmental factors, such as urbanization and hygiene practices, can modulate these effects. Understanding the interaction between dietary and environmental factors is crucial for managing health and diseases. The gut virome also influences key physiological processes, including nutrient absorption, immune modulation, and overall digestive health. These observations reveal that variations in virome composition driven by dietary and environmental factors can predispose individuals to or protect them from various diseases, highlighting the importance of personalized nutrition and environmental interventions.
4. Interaction between the gut virome and other microbiome
The interaction between the gut virome and other microbiome components is a complex and dynamic process that affects various aspects of gut health and function. Phages, the predominant elements of the gut virome, play a crucial role in regulating bacterial populations and supporting gut equilibrium [39]. These phages engage in several life cycles. In the lytic cycle, they lyse bacterial cells to release new viral particles, thereby reducing bacterial populations, especially under stress and inflammation. During the lysogenic cycle, phage DNA integrates into the bacterial genome, replicating with the host without immediate destruction, but can switch to the lytic cycle under certain conditions, such as in the presence of antibiotics. The pseudo-lysogenic cycle, which involves phage DNA persisting in a latent state within bacteria until the transition to lytic or lysogenic cycles, is possible. Additionally, the bacterial budding cycle involves the gradual release of new phage particles through budding, which do not immediately lyse the host cell, thereby ensuring a controlled influence on bacterial populations (Fig. 3) [40,41].
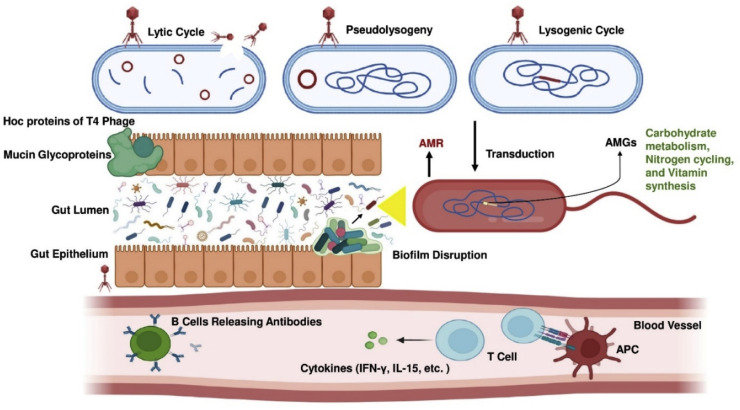
Fig. 3.
Illustration of the multifaceted interactions between phages, the gut microbiome, and the host immune system. Three phage replication strategies— the lytic cycle, pseudolysogeny, and the lysogenic cycle—are depicted, demonstrating the mechanisms through which phages propagate and interact with host bacteria. The highly immunogenic outer capsid proteins (Hoc proteins) of T4 phages facilitate interactions with mucin glycoproteins in the gut epithelium, thereby influencing microbial adhesion and colonization. In the gut lumen, phages play a pivotal role in antimicrobial resistance by transferring resistance genes via mechanisms such as transduction, thereby contributing to the spread of resistant bacterial strains. Phage-encoded auxiliary metabolic genes (AMGs) enhance microbial functions, including carbohydrate metabolism, nitrogen cycling, and vitamin synthesis, which are crucial for maintaining gut microbial balance. Phage-mediated disruption of microbial biofilms further modulates the gut microbial dynamics. On the host immune side, phage-bacteria interactions trigger immune responses. Antigen-presenting cells (APCs) stimulate T cells and B cells, leading to the release of antibodies and cytokines such as interferon-gamma (IFN-γ) and interleukin-15 (IL-15). These immune mediators play key roles in regulating immune responses and maintaining gut homeostasis. This comprehensive depiction underscores the critical role of the gut virome in microbial ecology and immune system modulation.
Phages also contribute to horizontal gene transfer through a process called transduction. During transduction, phages can package bacterial DNA and transfer it to other bacterial cells, spreading beneficial traits such as antibiotic resistance, metabolic capabilities, and virulence factors. Gene transfer can enhance the adaptability and functionality of the gut microbiome [42]. For instance, antibiotic resistance genes are transferred among clinical bacterial pathogens, such as Enterococcus faecalis, Clostridioides difficile, and Staphylococcus aureus, through phage-mediated transduction, exacerbating concerns about antibiotic resistance in medical settings [43].
The gut virome also influences bacterial metabolic function. Some phages carry auxiliary metabolic genes (AMGs) that augment the metabolic capabilities of their host bacteria. These genes can enhance processes such as carbohydrate metabolism, nitrogen cycling, and vitamin synthesis, thereby optimizing the overall metabolic output of the gut microbiome and contributing to the host's nutritional status [44,45]. Moreover, phages can modulate gene expression in bacterial hosts to improve environmental adaptability and virulence, as observed in filamentous phages that alter the biofilm structure and promote toxin production [46].
Phages interact with the host's immune system in a multifaceted manner. Bacterial components of lysed bacteria can stimulate immune responses through phage activity. Certain phages can modulate immune signaling pathways, influencing the balance between pro-inflammatory and anti-inflammatory responses, which is crucial for maintaining immune homeostasis and preventing chronic inflammation associated with diseases such as IBD and autoimmune disorders [47]. Phages can also provide antimicrobial protection against luminal bacterial pathogens and interact with mucosal surfaces, contributing to innate immune defense. Moreover, phages containing immunoglobulin-like domains, such as T4 phages with Hoc proteins, bind to mucin glycoproteins in the intestinal mucosal layer, thereby strengthening the mucosal barrier [48,49].
Phages can also communicate with the immune system through the systemic circulation, where they are recognized by innate immune cells after passing through intestinal epithelial cells. This interaction can improve opsonization and bacterial recognition and control cytokine production, including interferon-gamma (IFN-γ). Phage-derived peptides present in naïve T lymphocytes by antigen-presenting cells (APCs) stimulate cellular and humoral responses. Under certain conditions, phages may induce B cells to produce phage-specific antibodies or increase the production of pro-inflammatory cytokines. For example, E. coli phages can exacerbate colitis in dextran sodium sulfate (DSS)-induced IBD mouse models by stimulating Toll-like receptor 9 (TLR9) and IFN-γ dependent pathways. Conversely, phages possess anti-inflammatory and immunomodulatory properties that may be advantageous in contexts that require immunological tolerance, such as allotransplantation [[50], [51], [52]]. Eukaryotic viruses play a role in maintaining host immunity and gut homeostasis. For instance, intraepithelial lymphocytes (IELs) and various receptors like Toll-like receptors (TLR3, TLR7) and cytosolic receptors such as retinoic acid-inducible gene-1 (RIG-I) recognize enteric viruses and produce IFN-β and interleukin-15 (IL-15), which suppress inflammation and support IEL homeostasis [53,54]. Furthermore, eukaryotic viruses, like murine astrovirus, have been shown to have protective effects by producing IFN-γ in immunocompromised mice [55].
Phages also affect the formation and stability of biofilms. Biofilms are platforms in which bacteria aggregate and adhere to the gut surface, providing a protected environment against stressors, antibiotics, and the host immune system. Phages can disrupt biofilms through lysis or by using depolymerases that target the exopolysaccharides (EPSs) of bacterial host cells. An analysis of 143 phages identified 160 putative depolymerases categorized as lyases (e.g., hyaluronidase and pectin/pectin lyase) and hydrolases (e.g., sialidase and glucanase). These enzymes either act as free agents or as part of phage tail spike proteins, binding to and breaking down EPSs, thereby disturbing the biofilm structure and allowing for deeper phage penetration [56].
The gut virome also influences pathogen colonization. Phages that specifically target pathogenic bacteria can act as barriers to pathogenic colonization, preventing harmful microbes from establishing themselves in the gut [57]. This protective effect is vital for defending against infections and maintaining healthy microbial balance. Conversely, a reduction in the number of protective phages predisposes the host to opportunistic pathogenic infections. The gut virome diversity is directly linked to the bacterial microbiome diversity. A diverse virome, comprising a wide range of phages infecting various bacterial species, supports a stable and diverse bacterial community. This diversity is crucial for resilience against various disturbances, such as antibiotic treatment, dietary changes, and infections. The loss of virome diversity can reduce bacterial diversity and increase susceptibility to dysbiosis and its associated diseases [58,59].
The evolutionary dynamics between phages and their bacterial hosts drive rapid genetic changes in both populations. Bacteria have evolved mechanisms, such as CRISPR-Cas systems, to evade phage infection, whereas phages have developed counter-strategies to overcome bacterial defenses. This coevolution contributes to the genetic and functional diversity of both the virome and microbiome, influencing their roles in health and disease [60]. Phages act as a reservoir or intermediate pool for specific genes, facilitating phenotypic changes through recurrent cycles of phage infection or lysogenic conversion between bacteria and chromosomal exchange, helping bacterial communities in the human gut become more stable, adaptable, and evolve [61].
5. Gut virome-associated human diseases
5.1. Inflammatory bowel disease (IBD)
IBD, which encompasses Crohn's disease (CD) and ulcerative colitis, is increasingly recognized for its association with disturbances in the gut virome (Table 2). Research has provided insights into the correlation between dysbiosis of the gut virome and IBD pathogenesis. Studies have shown a significant reduction in the abundance and diversity of lytic and temperate phages in the ileal mucosa of patients with CD, particularly during disease flare-ups [62]. This dysregulation is characterized by a decrease in the overall phage population and a shift in the composition of phage communities, which contribute to the inflammatory processes observed in IBD. Under normal conditions, phages maintain microbial balance by controlling bacterial populations through various infection cycles, including the lytic, lysogenic, and pseudolysogenic cycles. However, the disruption of this balance is evident in IBD. Certain phages from the order Caudovirales have been implicated in the exacerbation of IBD. These phages can engage TLRs, such as TLR9, leading to the activation of inflammatory pathways and the release of cytokines like IFN-γ, further intensifying mucosal inflammation [63]. The interaction between these phages and the host immune system can trigger a cascade of inflammatory responses that contribute to the chronic nature of IBD.
Table 2.
Association of various diseases with specific viruses and their symptoms.
| Disease Category | Specific Diseases | Associated Viruses | Symptoms |
|---|---|---|---|
| Inflammatory Bowel Disease (IBD) [114,115] | CD, and Ulcerative colitis | Phages (Caudovirales), Lytic and Temperate Phages | Chronic diarrhea, abdominal pain, weight loss, fatigue |
| Autoimmune Disorders [116] | RA, Multiple Sclerosis, SLE, and T1DM | Phages (crAss-like, Podoviridae, Circoviridae-related sequences) | Joint pain, fatigue, skin rashes, neurological symptoms, increased thirst and urination |
| Metabolic Disorders [117] | Obesity, MetS, NAFLD, T2DM, and CVD | Phages (Caudovirales, Microviridae, Siphoviridae) | Weight gain, insulin resistance, hypertension, dyslipidemia, liver fibrosis, cardiovascular issues |
| Gastrointestinal Diseases [118] | Acute Diarrhea, CRC, and NEC | Eukaryotic viruses (Rotavirus, Adenovirus, Calicivirus, Astrovirus), Phages | Diarrhea, vomiting, abdominal pain, bloody stools, rectal bleeding, weight loss |
| Neurological Diseases [119] | MDD, AD, MHE, Stroke, Hypertension, Periodontitis, and GWI | Phages (Siphoviridae, Clostridia-like, Erysipelatoclostridiaceae-like phages), Eukaryotic viruses (Herpesviruses, Parvoviruses, Gillianvirus) | Cognitive impairment, mood disorders, ischemic events, systemic inflammation |
| COVID-19 [120] | COVID-19 | CrAss-like phages, Myoviridae, Siphoviridae, Pepper mild mottle virus, Cytomegalovirus | Respiratory symptoms, gastrointestinal issues, systemic inflammation |
| Liver Diseases [121] | Cirrhosis, Hepatitis | HBV, HCV, and Torque Teno virus | Jaundice, fatigue, liver dysfunction, abdominal pain |
| Respiratory Diseases [122] | COPD, Asthma | Human bocavirus, Torque Teno virus and, Phages | Chronic cough, wheezing, shortness of breath, chest tightness |
| Dermatological Conditions [123] | Atopic Dermatitis, Psoriasis | HPV, Torque Teno virus, Phages | Skin rashes, itching, redness, flaking |
Inflammatory Bowel Disease (IBD), Crohn's Disease (CD), Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), Type 1 Diabetes Mellitus (T1DM), Metabolic Syndrome (MetS), Non-Alcoholic Fatty Liver Disease (NAFLD), Type 2 Diabetes Mellitus (T2DM), Cardiovascular Disease (CVD), Colorectal Cancer (CRC), Necrotizing Enterocolitis (NEC), Major Depressive Disorder (MDD), Alzheimer's Disease (AD), Minimal Hepatic Encephalopathy (MHE), Gulf War Illness (GWI), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Chronic Obstructive Pulmonary Disease (COPD), and Human Papillomavirus (HPV).
In addition, phage-derived factors, including lysins and endolysins, have been identified as potential contributors to mucosal damage. These factors can directly lyse bacterial cells, leading to the release of bacterial antigens and the perpetuation of the inflammatory response. This disruption of the microbial ecosystem results in an altered gut virome composition, exacerbating the inflammatory state [64]. Virome dysbiosis, characterized by changes in phage diversity and abundance, is crucial for understanding the pathophysiology of IBD. The imbalance in phage populations and their interactions with the gut microbiota contributes to the disruption of microbial communities, leading to sustained inflammation and immune system activation. Altered phage populations in patients with IBD may impair the natural regulatory functions of phages, which normally help control bacterial populations and maintain gut homeostasis [65].
The long-term consequences of gut virome dysbiosis in IBD are multifaceted and have profound implications on disease progression and overall gut health. Chronic dysregulation of the gut virome can lead to persistent inflammation, exacerbating tissue damage and increasing the risk of complications such as strictures, fistulas, and even colorectal cancer (CRC) [66]. Over time, the disrupted virome can also impair the ability of the gut to restore microbial equilibrium, further prolonging disease flares and reducing responses to conventional therapies. Additionally, sustained virome imbalances may impact immune system regulation beyond the gut, potentially influencing systemic inflammatory responses and contributing to the extraintestinal manifestations of IBD. Understanding the long-term effects of virome dysbiosis is crucial for developing comprehensive treatment strategies that address immediate symptoms and promote long-term remission and gut homeostasis [67].
Overall, the complex interplay between the gut virome and IBD underscores the need for further investigation into how specific phages influence inflammatory pathways and microbial balance. Understanding these mechanisms could pave the way for novel therapeutic strategies aimed at restoring the virome balance and mitigating IBD symptoms. Targeting the virome, either by modulating phage populations or restoring beneficial phages, represents a promising avenue for developing more effective treatments for IBD.
5.2. Autoimmune disorders
Autoimmune disorders such as systemic lupus erythematosus (SLE), multiple sclerosis (MS), and rheumatoid arthritis (RA) have been increasingly linked to disturbances in the gut virome. Phages have a significant effect on immune system regulation by influencing the development and progression of autoimmune diseases through several mechanisms [68].
One such mechanism involves molecular mimicry, in which peptides encoded by phages bear structural similarities to the host antigens. This can trigger autoimmune reactions by causing the immune system to mistakenly attack the body tissues and perceive them as foreign [69]. In addition to molecular mimicry, phages can also affect immune responses by modulating the balance between pro-inflammatory and anti-inflammatory cytokines. This modulation can disrupt the immune homeostasis, potentially leading to the development or exacerbation of autoimmune diseases [70]. Dysbiosis of the gut virome contributes to the pathogenesis of autoimmune diseases by disturbing the delicate balance of the immune system. Such disturbances can promote the activation of autoreactive T cells and the production of autoantibodies, which are crucial features of autoimmune conditions [71]. For instance, a study that tracked gut virome dynamics in children at risk of type 1 diabetes mellitus (T1DM) found that those who developed T1DM exhibited lower gut virome diversity. In contrast, the controls showed an increased abundance of sequences related to the Circoviridae family of viruses. This shift in the virome composition, particularly the presence of disease-associated viral bacteriophage contigs linked to specific bacterial components, suggests that changes in the gut virome may precede the onset of autoimmunity in patients with T1DM [72].
Further supporting the link between the gut virome and autoimmune disorders, a comprehensive analysis of the gut virome in 476 Japanese individuals, including those with autoimmune diseases such as RA and SLE, revealed a notable decrease in the abundance of crA-like phages. This reduction in phage abundance indicated a potential association between specific phage populations and the development of autoimmune conditions. In addition, we identified a symbiotic relationship between Podoviridae phages and the bacterial genus Faecalibacterium, highlighting the complex interplay between gut viruses and bacteria. This interaction may influence autoimmune disease development by affecting the microbial balance and immune responses within the gut microbiome [73].
Persistent imbalances in the gut virome may lead to the continuous activation of autoreactive immune cells and sustained production of autoantibodies, driving the progression of SLE, MS, RA, and T1DM. In T1DM, long-term virome disturbances can disrupt immune tolerance mechanisms, promoting the activation of autoreactive T cells that target pancreatic β-cells, leading to progressive β-cell destruction and impaired insulin production. This chronic activation accelerates disease onset, complicates long-term glycemic control, and increases the risk of associated complications [74]. Over time, this chronic activation can result in cumulative tissue damage, organ dysfunction, and increased risk of comorbidities. Moreover, long-term disturbances in the virome may compromise the ability of the gut to regulate immune tolerance, exacerbate disease relapse, and reduce the effectiveness of immunosuppressive therapies. Restoring the virome balance through targeted interventions could mitigate these long-term impacts, highlighting the need for a deeper understanding of phage-host interactions in autoimmune disease management [75].
5.3. Metabolic disorders
Metabolic disorders have recently gained attention owing to their association with the gut virome. Although the role of the gut microbiota in metabolic diseases is well established, emerging evidence highlights the significant involvement of the gut virome in conditions such as obesity, metabolic syndrome (MetS), non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM), and cardiovascular disease (CVD) [76].In animal models, particularly in mice, diet-induced changes in the gut virome have been linked to metabolic disorders. For instance, a Western diet promotes the enrichment of temperate phages from the order Caudovirales, which help bacterial hosts adapt to stress conditions associated with obesity [77]. High-fat diets further shift viral communities, favoring phages from the Microviridae family over those from the Siphoviridae family, indicating a possible increase in phage virulence towards host bacteria [78]. FVT experiments have shown the potential to alleviate obesity and T2DM symptoms by reshaping both the bacterial and viral components of the gut microbiome [79].
Studies on humans have corroborated these findings. Obese individuals exhibited enriched phage populations, with overall gut viral richness and diversity lower than those of lean controls. In children with obesity and MetS, although phage richness and diversity were higher, there was a noticeable loss of phages common in normal-weight individuals [80]. Patients with NAFLD show reduced viral diversity and fewer phages, with specific phages correlating with the severity of liver fibrosis [81]. T2DM patients also display significant gut viral dysbiosis, characterized by altered phage populations and disrupted viral-bacterial interactions. This dysbiosis is more pronounced in T2DM patients with obesity, suggesting a compounded effect of both conditions on the gut virome [82]. Research on CVD has revealed a differential abundance of specific phages in patients compared with healthy controls, potentially offering new diagnostic and therapeutic avenues. Viral markers have shown superior discriminatory power over bacterial markers in the early diagnosis of hypertension. These findings suggest a complex and potentially causative role of the gut virome in the development and progression of metabolic disorders [83].
The influence of the gut virome on metabolic health is also multifaceted. Phages can modulate host metabolism by interacting with gut microbiota. Dysbiosis within the virome can disrupt the microbial communities, leading to alterations in energy harvesting, insulin sensitivity, and inflammation. In addition, phage-mediated horizontal gene transfer can facilitate the spread of genes associated with metabolic disorders, such as those encoding insulin resistance and adipogenesis. Phages may also produce endotoxins during bacterial lysis, contributing to low-grade inflammation and metabolic dysfunction, characteristic of metabolic disorders [84].
Long-term gut virome dysbiosis in metabolic disorders, such as obesity, NAFLD, T2DM, and CVD, can exacerbate disease progression by sustaining chronic inflammation and disrupting metabolic homeostasis. Persistent imbalances may worsen insulin resistance, promote adipogenesis, and accelerate liver fibrosis and cardiovascular complications. In T2DM, prolonged virome disturbances impair glucose regulation and increase the risk of complications. Restoring the virome balance through interventions such as FVT or precision phage therapy could mitigate these impacts, highlighting the need for further research into virome-host interactions for effective metabolic disorder management [85].
5.4. Gastrointestinal diseases
Gastrointestinal diseases are significantly influenced by gut virome. These viral components play crucial roles in various gastrointestinal (GI) conditions ranging from acute diarrheal disease to CRC. Acute diarrhea is a major global health concern, especially among children, with prominent pathogens including rotavirus, adenovirus, calicivirus, and astrovirus [86]. Metagenomic analyses have expanded our understanding of viral communities in diarrheal diseases, revealing additional agents, such as picobirnavirus and TTV, which underscore the complexity of these viral communities [87]. Studies from Shanghai and Taizhou in China have shown that enteric viruses such as Adenoviridae and Picornaviridae are predominant in diarrheal cases. However, the alpha diversity of the diarrheal virome in these studies did not differ significantly from that in healthy controls [88]. In Cameroon, where many diarrheal episodes remain unexplained, research has suggested the potential zoonotic origins of some enteric viruses, highlighting the diverse and possibly animal-derived nature of these pathogens [89]. Similarly, a study of febrile children in Tanzania indicated that unexplained febrile illnesses could be associated with novel viruses, such as herpesviruses and parvoviruses, reflecting the complex viral landscape of GI conditions [90].
CRC is another GI condition associated with the gut virome. Virome dysbiosis, which is characterized by increased diversity and altered viral communities, is frequently observed in patients with CRC [91]. Specific bacteria-targeting bacteria, such as Bacteroides fragilis and Fusobacterium nucleatum, have been implicated in CRC development through mechanisms such as biofilm formation and horizontal gene transfer [92]. Viral markers for CRC diagnosis and prognosis have been identified, with certain phages and eukaryotic viruses showing differential abundance in patients with CRC, suggesting their potential as diagnostic tools. Notably, virome dysbiosis has been shown to persist even after surgical intervention in patients with CRC, potentially influencing patient outcomes and recurrence rates [93].
Recent advancements in computational biology and bioinformatics have facilitated the identification of consistent viral signatures across multiple cohorts, aiding CRC risk assessment and diagnosis [94]. Phages associated with Fusobacterium, Porphyromonas, and Hungatella, which are enriched in patients with CRC, have emerged as potential viral markers [95]. Additionally, specific viral proteins, such as Hepatitis B virus X protein (HBx), have been linked to CRC, promoting carcinogenesis through mechanisms involving DNA damage and activation of oncogenic pathways [96]. Eukaryotic viruses also play an important role in the development of GI diseases. Pathogenic viruses such as enteroviruses and noroviruses are well recognized for inducing acute gastroenteritis with symptoms including vomiting, diarrhea, and stomach pain. Dysbiosis within the gut virome can disrupt the mucosal barrier integrity, allowing pathogenic viruses to translocate and exacerbate gastrointestinal inflammation. Conversely, certain viruses, such as murine norovirus, have shown protective effects by restoring immune and epithelial cell functions during dysbiosis, illustrating the complex role of the gut virome in gastrointestinal health and disease [97].
Persistent imbalances in the gut virome in gastrointestinal diseases, such as chronic diarrheal conditions and CRC, can exacerbate disease progression and hinder recovery. Prolonged viral disruption may weaken mucosal barriers, increase the risk of infection, and sustain chronic inflammation. In CRC, ongoing virome alterations can drive biofilm formation, horizontal gene transfer, and oncogenic pathways, contributing to tumor growth and recurrence, even after surgery. Targeted therapeutic strategies to restore viral balance could enhance disease management, highlighting the importance of deeper insights into virome-host interactions for effective gastrointestinal interventions [98].
5.5. Neurological diseases
Neurological diseases have increasingly been linked to disturbances in the gut virome through interactions within the microbiota-gut-brain (MGB) axis [99]. Research on this relationship highlights how changes in gut viral communities can influence neurological health and disease progression. Major depressive disorder (MDD) is associated with significant changes in gut virome. In studies using Macaca fascicularis monkeys exhibiting depression-like behavior, metagenomic analyses identified 33 viral and 14 bacterial species that distinguished these monkeys from controls [100]. Notably, changes in lipid metabolism, particularly in 1, 2-diacylglyceride (DG) levels in the prefrontal cortex (PFC), are linked to virome disturbances. This suggests that disruptions in DG pathways in the PFC could contribute to depressive behaviors, emphasizing the impact of the gut virome on brain lipid metabolism and mood regulation.
Alzheimer's disease (AD) is associated with viral dysbiosis of the gut [101]. Metagenomic studies of amyloid-positive patients with AD have revealed reduced alpha diversity and a significant decrease in the abundance of phages from the Siphoviridae family [102]. Specifically, various Lactococcus phages are less abundant in patients than in healthy controls. These viral alterations could serve as potential biomarkers of AD and provide insights into how gut virome changes are associated with AD pathology [103]. Minimal hepatic encephalopathy (MHE), a cognitive dysfunction associated with cirrhosis, is linked to changes in the gut virome. Studies have shown correlations between cognitive impairment and specific phases associated with Lactobacillus, Faecalibacterium, and Streptococcus. These phages may affect neuroactive compounds such as short-chain fatty acids and ammonia, influencing cognitive function [104]. Longitudinal studies support these findings, suggesting that the virome plays a role in both the development and potential reversal of MHE.
Stroke affects the gut virome, and studies have shown altered virome composition following transient focal ischemia in mice [105]. Specific viral taxa, including Clostridia-like and Erysipelatoclostridiaceae-like phages, exhibit changes in abundance after stroke. These alterations indicate that the gut virome may respond to ischemic events and influence stroke outcomes through interactions between gut bacteria and host metabolism. Hypertension (HTN) and periodontitis (PD) have been linked to changes in the oral and gut virome. A study of patients with HTN, PD, or both found distinct viral compositions compared with healthy controls [106]. Notable viruses, such as Gillianvirus and Torbevirus, were associated with the clinical parameters of HTN, suggesting that viral-bacterial interactions might contribute to the exacerbation of these conditions. This underscores the role of the virome in modulating systemic health through local and gut microbiota interactions. Gulf War Illness (GWI) is another example of how gut virome dysbiosis affects neurological health. In a murine model, increased viral richness and alpha diversity correlated with gut bacterial dysbiosis and elevated pro-inflammatory cytokine levels [107]. Viral dysbiosis is associated with weakened intestinal barriers and heightened immune responses, including TLR signaling. Therapeutic interventions targeting the virome have demonstrated improvements in gut barrier integrity and reduction in inflammation, suggesting that modulation of the gut virome could be a viable strategy for managing GWI symptoms.
Chronic gut virome disruption can have lasting effects on neurological health by influencing the MGB axis. In MDD, prolonged virome imbalance may exacerbate mood disorders and increase vulnerability to other mental health conditions. Sustained changes in the virome can accelerate cognitive decline and amyloid accumulation in patients with AD. MHE may lead to persistent cognitive impairment due to ongoing virome alterations, whereas stroke recovery can be impaired by continuous viral shifts. In hypertension and periodontitis, ongoing virome disturbances may increase the risk of cardiovascular and neurological complications. In GWI, prolonged virome imbalances can increase inflammation and worsen neurological symptoms [108].
5.6. COVID-19
Recent research has highlighted the significant impact of COVID-19 on the gut virome, revealing notable alterations in viral communities compared with healthy controls. Studies have indicated an increase in the abundance of crAs-like phages and members of the families Myoviridae and Siphoviridae, along with a decrease in the abundance of various phages including podophages, and eukaryotic DNA viruses in infected individuals [109]. This dysbiosis persists up to 30 days after recovery and correlates with inflammatory markers and disease severity. The influence of antibiotics exacerbates this issue by reducing viral diversity and increasing opportunistic pathogens such as cytomegalovirus [110]. Additionally, significant differences in the gut virome between severe and mild COVID-19 cases have been observed, with patterns mirrored in mouse models [111]. Persistent virome dysbiosis, particularly in older patients, underscores the potential of targeting the gut microbiome as part of comprehensive COVID-19 treatment and prevention strategies [112].
The long-term effects of COVID-19 on the gut virome are still being explored, with evidence suggesting that these changes could lead to persistent immune dysregulation and a higher risk of secondary infections and GI issues. As viral diversity in the gut decreases, the abundance of opportunistic pathogens may increase, exacerbating symptoms such as fatigue, digestive disturbances, and neurological dysfunction. An altered gut composition may also affect metabolism and immune responses, potentially increasing the risk of autoimmune diseases, cardiovascular issues, and metabolic disorders. These long-term consequences are particularly concerning for COVID-19 survivors, highlighting the need to monitor and address the post-infection gut health to mitigate these risks [113].
6. Faecal virome transplantation (FVT)
FVT restores the gut microbial balance through a multifaceted mechanism that involves introducing a diverse range of viruses, primarily phages, into the recipient's gut. Phages play a critical role in shaping the gut microbiome by regulating bacterial populations. During dysbiosis, an imbalance in bacterial and viral communities can lead to the overgrowth of pathogenic bacteria, depletion of beneficial microbes, and disruption of gut homeostasis. FVT addresses this by introducing virome-rich fecal material from healthy donors, which re-establishes viral diversity and helps control the overgrowth of harmful bacteria [124]. This modulation fosters a more stable microbial environment, enhances microbial diversity, and facilitates the proliferation of commensal bacteria essential for gut health.
In addition, the phage-containing fraction of the transplant influences bacterial gene expression and metabolism, driving the selection of bacterial populations that support optimal gut function. Phages also contribute to the horizontal transfer of genetic material, enhancing bacterial adaptability and resilience to environmental stressors [125]. Furthermore, FVT helps modulate the immune response by altering the gut microbial and viral composition and reducing inflammation associated with dysbiosis-related conditions, such as IBD and small intestinal bacterial overgrowth. This immunomodulatory effect stems from the interaction between phages, bacterial components, and the gut epithelium, which collectively strengthen the intestinal barrier and regulate immune signaling pathways [126].
The transplantation process also affects metabolic processes in the gut by promoting a balance of microbial communities involved in nutrient absorption and metabolism. For instance, FVT restores bacterial and viral interactions that enhance short-chain fatty acid production, which is vital for maintaining gut integrity and reducing systemic inflammation. Recent studies have highlighted the role of the gut virome in modulating host metabolism and immune responses, suggesting that FVT can affect metabolic health by restoring the viral and bacterial balance [127]. For example, FVT improves glucose tolerance and reduces obesity-related metabolic disturbances in mouse models [128]. The phage-containing fraction of fecal material in FVT has been noted for its ability to reshape the gut microbiome, enhance microbial diversity, and restore balance during dysbiosis [129].
However, as with any emerging medical intervention, FMT raises complex ethical, legal, and social issues, particularly in the contexts of clinical research, therapy, and organ transplantation. Ethical concerns revolve around informed consent, patient vulnerability (especially in conditions such as IBD), and the risks associated with unproven treatment. The process of FMT, although FDA-approved for recurrent CDI, remains experimental for other conditions, such as IBD, leading to the potential exploitation of desperate patients [130]. The challenges include defining a “suitable healthy donor” with limited knowledge of the microbiota, stringent screening protocols, and difficulties in donor recruitment. Moreover, concerns about privacy, confidentiality, and the potential biological effects of donor characteristics (e.g., gender or health background) complicate this process. Although stool banks have been established to mitigate donor issues, logistical problems such as shipping, storage, and the availability of suitable donors remain significant. The risks related to FMT's safety, efficacy, and long-term effects are still poorly understood, further complicating its application as a treatment [131].
7. Phageomics and personalized medicine
Phageomics, the study of bacteriophage genomes, is rapidly advancing, particularly in the context of personalized medicine, and offers potential solutions to the global challenge of antimicrobial resistance [132]. Phage therapy involves highly specific interactions between phages and their host bacteria. The process begins when phages recognize and bind to specific receptors on the surface of bacterial cells using their tail fibers for adsorption. Once attached, the phage injects genetic material into the bacterial cell, hijacking the bacterial machinery to replicate the genome and produce new phage particles. This often leads to bacterial lysis, in which the bacterial cell wall is degraded, releasing progeny phages into the environment to infect other bacteria. However, some phages can enter the lysogenic cycle by integrating their genome into the host DNA and remain dormant. Phage specificity allows for the targeted treatment of pathogenic bacteria while preserving beneficial microbiota, making it a promising therapeutic approach, particularly in personalized medicine. The potential for phage resistance exists with bacteria-developing mechanisms such as receptor site modification or CRISPR-Cas systems to counteract phage attack. However, strategies, such as using phage cocktails or combining phage therapy with other treatments, such as antibiotics or CRISPR-based gene editing, can overcome these resistance mechanisms and enhance treatment efficacy. The ability of phage therapy to target specific bacterial strains, coupled with its adaptability, offers a powerful tool for combating multidrug-resistant infections [133]. Advances in metagenomics and whole-genome sequencing have revealed vast phage diversity and functions, enabling their use in tailored therapies. Phage therapy, which dates back to the early 20th century, has experienced a resurgence, particularly in multidrug-resistant bacterial infections. Personalized phage therapy can be customized for a patient's microbiome, targeting pathogenic bacteria while preserving beneficial microorganisms [134].
CRISPR-Cas systems have emerged as a versatile tool with broad applications in genetic manipulation and therapy [135]. The CRISPR-Cas9 system, when delivered via phage vectors, effectively targets and disrupts resistance genes within bacterial populations, demonstrating the potential of combining genome editing with phage therapy to combat drug resistance [136]. Research has shown that CRISPR-Cas9 can be engineered to recognize specific antibiotic-resistant plasmids, leading to their degradation and subsequent reduction in bacterial resistance [137]. Meanwhile, the CRISPR-Cas13a system, which targets RNA, exhibits strong antiviral activity against many phages. This capability facilitates the engineering of phages with enhanced targeting precision and potential for novel therapeutic strategies [138]. Additionally, the discovery of bacteriophage-encoded CRISPR systems, such as the Casλ RNA-guided nuclease, reveals new possibilities for genetic manipulation across diverse fields, including agriculture, where it can be used to create genetically modified crops with improved traits, and cancer therapy, where it holds promise for precise gene editing in tumor cells [139]. Furthermore, the natural role of CRISPR systems in bacterial immunity, as exemplified by the CRISPR-Cas subtype II-A in Lacticaseibacillus rhamnosus, illustrates their evolutionary significance and adaptability [140]. These systems defend against phage infections and offer a model for engineering phages for therapeutic purposes, emphasizing the broad and evolving applications of CRISPR technologies in both basic research and clinical settings.
The growing recognition of the role of gut viruses in health underscores their potential to transform diagnostic and therapeutic approaches. Viruses within the gut microbiome, particularly phages, regulate bacterial populations and affect immune system functions, positioning them as critical elements in understanding the overall influence of the microbiome on human health. Phage-based therapeutics could potentially be developed to modulate the gut bacteria associated with diseases, including IBD and obesity, offering tailored approaches to restore microbial balance [141]. Traditional culture methods often fail to detect pathogens, such as mycobacteria, which are significant concerns in both animal health and food security. These limitations highlight the need for a more sensitive and direct detection method. Phage-based diagnostics have emerged as a promising solution, offering enhanced sensitivity and specificity for detecting Mycobacterium tuberculosis and other mycobacteria in the agricultural context [142]. These diagnostic tools are crucial for early detection in food production systems to ensure both animal welfare and public health. For instance, phage amplification assays have demonstrated a significant potential for detecting Mycobacterium infections in livestock, providing a valuable tool for disease surveillance and control [143].
Phage therapy, particularly when personalized and complemented by companion diagnostics, is gaining renewed interest as a strategy against antibiotic-resistant infections [144]. By leveraging phage-bacteria specificity, personalized phage therapies can be tailored to individual patient infections, ensuring a targeted treatment that avoids disrupting the surrounding microbiome [145]. This targeted approach not only addresses specific bacterial infections, but also reduces the risk of resistance development in non-target bacterial populations, a common issue with broad-spectrum antibiotics. As phage therapy continues to evolve, its potential as a personalized treatment for drug-resistant bacterial infections has become clear. In this context, researchers developed SNIPR001, a combination of four engineered phages designed to target and kill Escherichia coli, including biofilms, while reducing phage tolerance (Fig. 4) [146]. In preclinical studies using mice and minipigs, SNIPR001 outperformed its individual components in reducing E. coli load. Phage therapy is currently under clinical development to treat potentially fatal E. coli infections in patients with cancer, further showcasing the therapeutic promise of phage-based treatments.
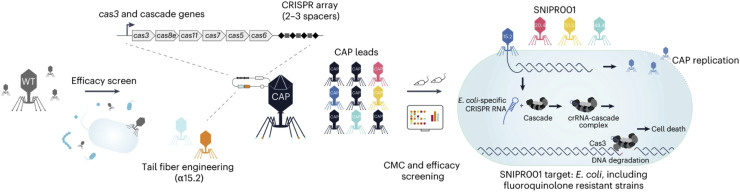
Fig. 4.
Development of SNIPR001, a precision antibiotic designed to selectively target Escherichia coli in patients with hematological cancers. Initially, a library of 162 wild-type phages was screened against a panel of E. coli strains to identify phages with broad coverage and complementary binding to bacterial surface receptors. The selected phages were engineered with modified tail fibers and CRISPR-Cas systems to enhance their specificity and efficacy. The resulting complementary armed phages (CAPs) were evaluated for host range, in vivo efficacy, and compliance with CMC specifications. SNIPR001, composed of four CAPs, aims to reduce E. coli load and prevent bacteremia in patients with neutropenia. Reproduced with permission from Refs. [146].
Advances in phage display technology have also demonstrated promise in identifying cancer-specific biomarkers, such as pancreatic cancer, thus facilitating early detection and potentially improving patient outcomes [147]. Phage display involves engineering phages to present proteins or peptides on their surface, thereby enabling the discovery of molecules that specifically bind to disease-related targets. This technology is currently being explored in cancer research to develop early diagnostic tools and targeted treatments. For example, phage display systems have been used to identify tumor-specific antigens in cancers, such as pancreatic cancer, which are difficult to detect in the early stages, potentially leading to more timely and effective interventions. Other versatile applications of phages include bioengineering, tissue engineering, vaccine development, and immunotherapy [148]. Moreover, engineered phages are currently being explored for targeted cancer therapy, which can be designed to selectively deliver therapeutic agents to cancer cells and minimize damage to healthy tissues. This approach is being investigated in breast and prostate cancer, where modifications to phage particles enhance their ability to recognize and bind to tumor-specific markers, potentially improving the efficacy of both diagnostic and therapeutic applications [149]. Moreover, innovations in luminescence-modulating phages present novel methods for detecting biomarkers in diseases such as prostate cancer [150]. Phage-based biosensors engineered to produce detectable luminescence in the presence of specific cancer biomarkers offer promising noninvasive screening alternatives [151]. These technologies can revolutionize early cancer detection and provide highly sensitive, rapid, and minimally invasive diagnostic tools that improve patient outcomes by enabling earlier and more accurate disease identification [152].
Phage-based therapies and diagnostics have also made significant strides in veterinary medicine and food security. In the agricultural context, phage amplification assays have shown promise for detecting bacterial infections in livestock with high sensitivity. These assays are especially valuable for monitoring zoonotic diseases that can spread from animals to humans and pose significant public health risks [153]. Phage displays have also been used to develop novel antigen-display systems for vaccines. These systems can be engineered to present antigens that stimulate the immune system to recognize and combat diseases, such as cancer or viral infections. In cancer immunotherapy, phages are developed to deliver tumor-specific antigens, triggering an immune response that targets and destroys cancer cells. This application of phage technology highlights its potential not only as a therapeutic tool but also as a preventive measure, offering new avenues for vaccine development [154].
The integration of artificial intelligence (AI) into phageomics further accelerates these advancements by analyzing large datasets of phage-bacterial interactions and predicting which phages are most effective against specific bacterial strains [155]. AI can also predict bacterial resistance to phages, allowing clinicians to adjust treatment strategies in real-time and improve the adaptability and success rates of phage therapies. By incorporating machine learning algorithms, researchers can rapidly identify optimal phage candidates for personalized therapies, ensuring patients receive the most effective treatment for specific infections [156]. Recent advancements in AI have revolutionized phage identification using metagenomic datasets. Tools based on deep learning, such as Seeker, can detect a wide range of phages, including those with low sequence similarity to known families [157]. VIBRANT combines machine learning with protein similarity methods to annotate phage genomes accurately, surpassing conventional techniques [158]. Similarly, VirSorter2 and PhageBoost employ sophisticated classifiers and feature-based methods to improve viral sequence detection and annotation [159,160]. AI also plays a key role in predicting PVPs, which is crucial for understanding phage structure and functionality [161]. Techniques such as artificial neural networks (ANNs) and specialized phage ANN models (PhANNs) enable the identification and functional annotation of structural proteins, even with limited sequence conservation [162]. Tools such as STEP3 and SCORPION leverage evolutionary data and feature selection strategies to enhance PVP prediction, thereby refining phage therapy approaches [163,164]. For host prediction, AI models such as VirHostMatcher-Net use network-based frameworks and CRISPR sequences to improve the accuracy of phage-host identification. HostG employs semi-supervised learning and knowledge graphs to predict hosts for novel viruses, expanding our understanding of phage-host interactions [165]. To predict phage lifestyles, AI tools, such as PHACTS and BACPHLIP, utilize similarity algorithms and conserved protein domains to classify phages as virulent or temperate [166,167]. PhaTYP employs advanced embedding models inspired by natural language processing to enhance the prediction of phage lifestyles using fragmented genomic data [168].
However, challenges remain, including the potential for bacteria to develop phage resistance. This is a natural evolutionary response; however, unlike antibiotics, phages can co-evolve with bacteria, offering a dynamic and adaptable therapeutic approach. Regulatory hurdles and ethical concerns need to be addressed, particularly regarding the use of genetically engineered phages for human treatment. Ensuring the safety and efficacy of engineered viruses requires comprehensive regulatory frameworks and ethical guidelines. The convergence of phageomics and personalized medicine holds immense promise for revolutionizing treatment strategies and offering dynamic patient-specific therapies that can adapt to evolving bacterial threats. The potential applications of phages span various fields, from diagnostic tools to therapeutic interventions, including infectious disease management, cancer treatment, and agricultural biosecurity. Future research and innovation are crucial for overcoming current challenges and fully realizing the potential of phage-based therapies in both human and animal health.
8. Conclusion
The gut virome plays pivotal roles in maintaining microbial homeostasis, modulating immune responses, and influencing human health across multiple systems. It acts as a gatekeeper by preventing pathogenic colonization, promoting microbial diversity, and driving evolutionary adaptations. Dysbiosis, marked by disruptions in phage abundance, diversity, and function, has been implicated in various conditions, including IBD, metabolic disorders, autoimmune diseases, neurological conditions, and antimicrobial resistance (AMR). The growing threat of AMR has sparked renewed interest in phage therapy as a promising solution. Advances in metagenomics, whole-genome sequencing, and CRISPR-Cas technologies enable the development of precision-targeted phage-based therapies to combat multidrug-resistant pathogens. These therapies offer effective bacterial control, preserve beneficial microbiota, and minimize unintended disruptions. As research on the gut virome advances, its therapeutic potential has become clearer, highlighting its critical role in addressing complex diseases and global health challenges.
Future research should focus on large-scale, longitudinal studies to uncover the precise mechanisms by which the gut virome influences health and disease. Advances in phage engineering, including CRISPR-Cas systems, hold promise for developing tailored therapies to combat AMR while preserving microbial homeostasis. Combining phage therapy with innovative approaches such as chimeric antigen receptor (CAR)-T cell therapy, in which engineered immune cells target bacterial antigens or dysbiotic microbial communities, could revolutionize treatment strategies for chronic infections and systemic diseases. Integrating AI with phageomics will accelerate phage identification and optimize personalized therapies. In addition, expanding virome research beyond the gut to explore its systemic impact will pave the way for holistic diagnostic and therapeutic innovations.
CRediT authorship contribution statement
Rahul Harikumar Lathakumari: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Methodology, Data curation, Conceptualization. Leela Kakithakara Vajravelu: Visualization, Validation, Supervision. Anusha Gopinathan: Visualization, Validation, Supervision. Poornima Baskar Vimala: Formal analysis, Conceptualization. Vishnupriya Panneerselvam: Formal analysis, Conceptualization. Sujith Sri Surya Ravi: Resources, Project administration, Investigation. Jayaprakash Thulukanam: Resources, Project administration, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors would like to thank Dakshina M Nair from Department of Microbiology, SRM Medical College Hospital and Research Centre, Kattankulathur, Tamil Nadu for her invaluable insights and suggestions.
References
- 1.Tiamani K., et al. The role of virome in the gastrointestinal tract and beyond. FEMS Microbiol. Rev. 2022;46(6):fuac027. doi: 10.1093/femsre/fuac027. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shkoporov A.N., et al. The human gut virome is highly diverse, stable, and individual specific. Cell Host. Microbe. 2019;26(4):527–541. doi: 10.1016/j.chom.2019.09.009. e5. [DOI] [PubMed] [Google Scholar]
- 3.Pargin E., et al. The human gut virome: composition, colonization, interactions, and impacts on human health. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.963173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lathakumari R.H., Vajravelu L.K., Satheesan A., Ravi S., Thulukanam J. Antibiotics and the gut microbiome: understanding the impact on human health. Med. Microecol. 2024;20 doi: 10.1016/j.medmic.2024.100106. [DOI] [Google Scholar]
- 5.Friedland R.P., Haribabu B. The role for the metagenome in the pathogenesis of COVID-19. EBioMedicine. 2020;61 doi: 10.1016/j.ebiom.2020.103019. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen T.S., et al. Overcoming donor variability and risks associated with fecal microbiota transplants through bacteriophage-mediated treatments. Microbiome. 2024;12(1):119. doi: 10.1186/s40168-024-01820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali N., Vora C., Mathuria A., Kataria N., Mani I. Chapter four - advances in CRISPR-Cas systems for gut microbiome. Singh V., editor. Chapter four - advances in CRISPR-Cas systems for gut microbiomeProgress in Molecular Biology and Translational Science. 2024;208:59–81. doi: 10.1016/bs.pmbts.2024.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Shkoporov A.N., et al. The human gut virome is highly diverse, stable, and individual specific. Cell Host. Microbe. 2019;26(4):527–541. doi: 10.1016/j.chom.2019.09.009. e5. [DOI] [PubMed] [Google Scholar]
- 9.Camarillo-Guerrero L.F., Almeida A., Rangel-Pineros G., Finn R.D., Lawley T.D. Massive expansion of human gut bacteriophage diversity. Cell. 2021;184(4):1098–1109. doi: 10.1016/j.cell.2021.01.029. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang G., Bushman F.D. The human virome: assembly, composition and host interactions. Nat. Rev. Microbiol. 2021;19(8):514–527. doi: 10.1038/s41579-021-00536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes A., et al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc. Natl. Acad. Sci. 2015;112(38):11941–11946. doi: 10.1073/pnas.1514285112. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecuit M., Eloit M. In: The Microbiota in Gastrointestinal Pathophysiology. Floch M.H., Ringel Y., Walker W.Allan, editors. Academic Press; Boston: 2017. Chapter 21 - the viruses of the gut microbiota; pp. 179–183. [DOI] [Google Scholar]
- 13.Cao Z., et al. The gut ileal mucosal virome is disturbed in patients with Crohn's disease and exacerbates intestinal inflammation in mice. Nat. Commun. 2024;15(1):1638. doi: 10.1038/s41467-024-45794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang K., et al. Alterations in the gut virome in obesity and type 2 diabetes mellitus. Gastroenterology. 2021;161(4):1257–1269. doi: 10.1053/j.gastro.2021.06.056. e13. [DOI] [PubMed] [Google Scholar]
- 15.Bhattarai Y., et al. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson's disease. Gut. Microbes. 2021;13(1) doi: 10.1080/19490976.2020.1866974. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez-Bello M.G., et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaczorowska J., van der Hoek L. Human anelloviruses: diverse, omnipresent and commensal members of the virome. FEMS Microbiol. Rev. 2020;44(3):305–313. doi: 10.1093/femsre/fuaa007. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paquette S.G. Influenza transmission in the mother-infant dyad leads to severe disease, mammary gland infection, and pathogenesis by regulating host responses. PLoS. Pathog. 2015;11(10) doi: 10.1371/journal.ppat.1005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanisch F.-G., Hansman G.S., Morozov V., Kunz C., Schroten H. Avidity of α-fucose on human milk oligosaccharides and blood group–unrelated oligo/polyfucoses is essential for potent norovirus-binding targets. J. Biol. Chem. 2023;293(30):11955–11965. doi: 10.1074/jbc.RA117.001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siranosian B.A., Tamburini F.B., Sherlock G., Bhatt A.S. Acquisition, transmission and strain diversity of human gut-colonizing crAss-like phages. Nat. Commun. 2020;11(1):280. doi: 10.1038/s41467-019-14103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucker F., et al. New microviridae isolated from Sulfitobacter reveals two cosmopolitan subfamilies of single-stranded DNA phages infecting marine and terrestrial Alphaproteobacteria. Virus. Evol. 2022;8(2):veac070. doi: 10.1093/ve/veac070. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walters W.A., et al. Longitudinal comparison of the developing gut virome in infants and their mothers. Cell Host. Microbe. 2023;31(2):187–198. doi: 10.1016/j.chom.2023.01.003. e3Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honap T.P., Jr. Biogeographic study of human gut-associated crAssphage suggests impacts from industrialization and recent expansion. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0226930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregory A.C., Zablocki O., Zayed A.A., Howell A., Bolduc B., Sullivan M.B. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host. Microbe. 2020;28(5):724–740. doi: 10.1016/j.chom.2020.08.003. e8Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francese R., et al. Viruses and human milk: transmission or protection? Adv. Nutr. 2023;14(6):1389–1415. doi: 10.1016/j.advnut.2023.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wedekind S.I.S., Shenker N.S. Antiviral properties of human milk. Microorganisms. 2021;9(4) doi: 10.3390/microorganisms9040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai X., et al. Identified human breast milk compositions effectively inhibit SARS-CoV-2 and variants infection and replication. iScience. 2022;25(4) doi: 10.1016/j.isci.2022.104136. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lembo D., Cagno V., Civra A., Poli G. Oxysterols: an emerging class of broad spectrum antiviral effectors. Mol. Aspects. Med. 2016;49:23–30. doi: 10.1016/j.mam.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Kato D., et al. Antiviral activity of chondroitin sulphate E targeting dengue virus envelope protein. Antiviral Res. 2010;88(2):236–243. doi: 10.1016/j.antiviral.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Zuo T., et al. Human-gut-DNA virome variations across geography, ethnicity, and urbanization. Cell Host. Microbe. 2020;28(5):741–751. doi: 10.1016/j.chom.2020.08.005. e4Nov. [DOI] [PubMed] [Google Scholar]
- 31.Nishijima S., et al. Extensive gut virome variation and its associations with host and environmental factors in a population-level cohort. Nat. Commun. 2022;13(1):5252. doi: 10.1038/s41467-022-32832-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., et al. Individuality and ethnicity eclipse a short-term dietary intervention in shaping microbiomes and viromes. PLoS Biol. 2022;20(8) doi: 10.1371/journal.pbio.3001758. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo T., et al. Human-gut-DNA virome variations across geography, ethnicity, and urbanization. Cell Host. Microbe. 2020;28(5):741–751. doi: 10.1016/j.chom.2020.08.005. e4. [DOI] [PubMed] [Google Scholar]
- 34.M.W. Opere, “Analysing the interplay of environmental virology, public health, and sanitation: a comprehensive review from a Kenyan perspective,” 2023. doi: 10.3389/fcimb.2023.1256822. [DOI] [PMC free article] [PubMed]
- 35.Palmieri O., et al. Adherence to gluten-free diet restores alpha diversity in celiac people but the microbiome composition is different to healthy people. Nutrients. 2022;14(12) doi: 10.3390/nu14122452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomova A., et al. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019;6 doi: 10.3389/fnut.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee E., Lee J.-E. Impact of drinking alcohol on gut microbiota: recent perspectives on ethanol and alcoholic beverage. Curr. Opin. Food Sci. 2021;37:91–97. doi: 10.1016/j.cofs.2020.10.001. [DOI] [Google Scholar]
- 38.Harper A., et al. Viral infections, the microbiome, and probiotics. Front. Cell Infect. Microbiol. 2021;10 doi: 10.3389/fcimb.2020.596166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shkoporov A.N., Hill C. Bacteriophages of the Human gut: the ‘known unknown’ of the microbiome. Cell Host. Microbe. 2019;25(2):195–209. doi: 10.1016/j.chom.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M., Zhang T., Yu M., Chen Y.-L., Jin M. The life cycle transitions of temperate phages: regulating factors and potential ecological implications. Viruses. 2022;14(9) doi: 10.3390/v14091904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howard-Varona C., Hargreaves K.R., Abedon S.T., Sullivan M.B. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISMe J. 2017;11(7):1511–1520. doi: 10.1038/ismej.2017.16. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borodovich T., Shkoporov A.N., Ross R.P., Hill C. Phage-mediated horizontal gene transfer and its implications for the human gut microbiome. Gastroenterol. Rep. (Oxf) 2022;10:goac012. doi: 10.1093/gastro/goac012. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancuso G., Midiri A., Gerace E., Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2021;10(10) doi: 10.3390/pathogens10101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo X.-Q., et al. Viral community-wide auxiliary metabolic genes differ by lifestyles, habitats, and hosts. Microbiome. 2022;10(1):190. doi: 10.1186/s40168-022-01384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown T.L., Charity O.J., Adriaenssens E.M. Ecological and functional roles of bacteriophages in contrasting environments: marine, terrestrial and human gut. Curr. Opin. Microbiol. 2022;70 doi: 10.1016/j.mib.2022.102229. [DOI] [PubMed] [Google Scholar]
- 46.Obeng N., Pratama A.A., van Elsas J.D. The significance of mutualistic phages for bacterial ecology and evolution. Trends Microbiol. 2016;24(6):440–449. doi: 10.1016/j.tim.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 47.de Souza E.B., Pinto A.R., Fongaro G. Bacteriophages as potential clinical immune modulators. Microorganisms. 2023;11(9) doi: 10.3390/microorganisms11092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chin W.H., et al. Bacteriophages evolve enhanced persistence to a mucosal surface. Proc. Natl. Acad. Sci. 2022;119(27) doi: 10.1073/pnas.2116197119. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barr J.J., et al. Bacteriophage adhering to mucus provide a non–host-derived immunity. Proc. Natl. Acad. Sci. 2013;110(26):10771–10776. doi: 10.1073/pnas.1305923110. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haq I.Ul, et al. The breadth of bacteriophages contributing to the development of the phage-based vaccines for COVID-19: an ideal platform to design the multiplex vaccine. Int. J. Mol. Sci. 2023;24(2) doi: 10.3390/ijms24021536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gogokhia L., et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host. Microbe. 2019;25(2):285–299. doi: 10.1016/j.chom.2019.01.008. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Górski A., Międzybrodzki R., Jończyk-Matysiak E., Żaczek M., Borysowski J. Phage-specific diverse effects of bacterial viruses on the immune system. Future Microbiol. 2019;14(14):1171–1174. doi: 10.2217/fmb-2019-0222. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao Z., Sugimura N., Burgermeister E., Ebert M.P., Zuo T., Lan P. The gut virome: a new microbiome component in health and disease. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104113. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu P., Hong Y., Yang B., Shrestha P., Sajjad N., Chen J.-L. Induction of the antiviral immune response and its circumvention by coronaviruses. Viruses. 2020;12(9) doi: 10.3390/v12091039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valerie C., Bridgett S., Jiangwei Y., Brandi L., Peter V., Stacey S.-C. Characterizing a murine model for astrovirus using viral isolates from persistently infected immunocompromised mice. J. Virol. 2019;93(13) doi: 10.1128/jvi.00223-19. 10.1128/jvi.00223-19Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S., Lu H., Zhang S., Shi Y., Chen Q. MDPI; 2022. Phages Against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Feb. 01, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lathakumari R.H., Vajravelu L.K., Satheesan A., Ravi S., Thulukanam J. Antibiotics and the gut microbiome: understanding the impact on human health. Med. Microecol. 2024;20 doi: 10.1016/j.medmic.2024.100106. [DOI] [Google Scholar]
- 58.Ritz N.L., et al. The gut virome is associated with stress-induced changes in behaviour and immune responses in mice. Nat. Microbiol. 2024;9(2):359–376. doi: 10.1038/s41564-023-01564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeGruttola A.K., Low D., Mizoguchi A., Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel. Dis. 2016;22(5):1137–1150. doi: 10.1097/MIB.0000000000000750. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duan N., Hand E., Pheko M., Sharma S., Emiola A. Structure-guided discovery of anti-CRISPR and anti-phage defense proteins. Nat. Commun. 2024;15(1):649. doi: 10.1038/s41467-024-45068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alkhalil S.S. The role of bacteriophages in shaping bacterial composition and diversity in the human gut. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1232413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gyriki D., et al. Exploring the gut microbiome's role in inflammatory bowel disease: insights and interventions. J. Pers. Med. 2024;14(5) doi: 10.3390/jpm14050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gogokhia L., et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host. Microbe. Feb. 2019;25(2):285–299. doi: 10.1016/j.chom.2019.01.008. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.ur Rahman M., et al. Endolysin, a promising solution against antimicrobial resistance. Antibiotics. 2021;10(11) doi: 10.3390/antibiotics10111277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Federici S., Kviatcovsky D., Valdés-Mas R., Elinav E. Microbiome-phage interactions in inflammatory bowel disease. Clin. Microbiol. Infect. 2023;29(6):682–688. doi: 10.1016/j.cmi.2022.08.027. [DOI] [PubMed] [Google Scholar]
- 66.Quaglio A.E.V., Grillo T.G., De Oliveira E.C.S., Di Stasi L.C., Sassaki L.Y. Baishideng Publishing Group Inc; 2022. Gut Microbiota, Inflammatory Bowel Disease and Colorectal Cancer. Aug. 14, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tun H.M., et al. BMJ Publishing Group; 2023. Gut Virome in Inflammatory Bowel Disease and Beyond. Nov. 10, [DOI] [Google Scholar]
- 68.Sundaresan B., Shirafkan F., Ripperger K., Rattay K. The role of viral infections in the onset of autoimmune diseases. Viruses. 2023;15(3) doi: 10.3390/v15030782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cusick M.F., Libbey J.E., Fujinami R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012;42(1):102–111. doi: 10.1007/s12016-011-8294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Górski A., Międzybrodzki R., Jończyk-Matysiak E., Kniotek M., Letkiewicz S. Therapeutic phages as modulators of the immune response: practical implications. Clin. Infect. Dis. 2023;77(Supplement_5):S433–S439. doi: 10.1093/cid/ciad483. Nov. [DOI] [PubMed] [Google Scholar]
- 71.Sun L., Su Y., Jiao A., Wang X., Zhang B. T cells in health and disease. Signal. Transduct. Target. Ther. 2023;8(1):235. doi: 10.1038/s41392-023-01471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao G., et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc. Natl. Acad. Sci. 2017;114(30):E6166–E6175. doi: 10.1073/pnas.1706359114. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomofuji Y., et al. Whole gut virome analysis of 476 Japanese revealed a link between phage and autoimmune disease. Ann. Rheum. Dis. 2022;81(2):278–288. doi: 10.1136/annrheumdis-2021-221267. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roep B.O., Thomaidou S., van Tienhoven R., Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system? Nat. Res. 2021 doi: 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen C., et al. Alterations of the gut virome in patients with systemic lupus erythematosus. Front. Immunol. 2023;13 doi: 10.3389/fimmu.2022.1050895. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chuaypen N., et al. Gut microbiota in patients with non-alcoholic fatty liver disease without type 2 diabetes: stratified by body mass index. Int. J. Mol. Sci. 2024;25(3) doi: 10.3390/ijms25031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mukhopadhya I., Segal J.P., Carding S.R., Hart A.L., Hold G.L. The gut virome: the ‘missing link’ between gut bacteria and host immunity? Therap. Adv. Gastroenterol. 2019;12 doi: 10.1177/1756284819836620. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anjelique S., et al. Fecal viral community responses to high-fat diet in mice. mSphere. 2020;5(1) doi: 10.1128/msphere.00833-19. 10.1128/msphere.00833-19Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao X., et al. Transfer of modified gut viromes improves symptoms associated with metabolic syndrome in obese male mice. Nat. Commun. 2024;15(1):4704. doi: 10.1038/s41467-024-49152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujimoto K., Miyaoka D., Uematsu S. Characterization of the human gut virome in metabolic and autoimmune diseases. BioMed Cent. Ltd. 2022 doi: 10.1186/s41232-022-00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lang S., et al. Intestinal virome signature associated with severity of nonalcoholic fatty liver disease. Gastroenterology. 2020;159(5):1839–1852. doi: 10.1053/j.gastro.2020.07.005. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang K., et al. Alterations in the gut virome in obesity and type 2 diabetes mellitus. Gastroenterology. 2021;161(4):1257–1269. doi: 10.1053/j.gastro.2021.06.056. e13Oct. [DOI] [PubMed] [Google Scholar]
- 83.Han M., Yang P., Zhong C., Ning K. The human gut virome in hypertension. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.03150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Vincenzo F., Del Gaudio A., Petito V., Lopetuso L.R., Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern. Emerg. Med. 2024;19(2):275–293. doi: 10.1007/s11739-023-03374-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang G.G., Trevaskis N.L., Murphy A.J., Febbraio M.A. Diet-induced gut dysbiosis and inflammation: key drivers of obesity-driven NASH. iScience. 2023;26 doi: 10.1016/j.isci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Msanga D.R., et al. Adenovirus infection is predicted by prolonged duration of diarrhea among Rotavirus-vaccinated children below five years of age in Mwanza, Tanzania. Int. J. Pediatr. (United Kingdom) 2020;2020 doi: 10.1155/2020/9303216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang S., et al. Viral metagenomics reveals diverse viruses in the fecal samples of children with diarrhea. Virol. Sin. 2022;37(1):82–93. doi: 10.1016/j.virs.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bao S., et al. Viral metagenomics of the gut virome of diarrheal children with Rotavirus A infection. Gut. Microbes. 2023;15(1) doi: 10.1080/19490976.2023.2234653. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwe Y.C., et al. Gut virome analysis of Cameroonians reveals high diversity of enteric viruses, including potential interspecies transmitted viruses. mSphere. 2019;4(1) doi: 10.1128/msphere.00585-18. 10.1128/msphere.00585-18Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cordey S., et al. Blood virosphere in febrile Tanzanian children. Emerg. Microbes. Infect. 2021;10(1):982–993. doi: 10.1080/22221751.2021.1925161. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ho S.X., et al. Alterations in colorectal cancer virome and its persistence after surgery. Sci. Rep. 2024;14(1):2819. doi: 10.1038/s41598-024-53041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.El Haddad L., Mendoza J.F., Jobin C. Bacteriophage-mediated manipulations of microbiota in gastrointestinal diseases. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1055427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siyuan S., Dongxue H., Chenchen M., Shuaiming J., Jiachao Z. Expanding the colorectal cancer biomarkers based on the Human gut phageome. Microbiol. Spectr. 2021;9(3) doi: 10.1128/Spectrum.00090-21. e00090-21Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horaira M.A., Islam Md.A., Kibria Md.K., Alam Md.J., Kabir S.R., Mollah Md.N.H. Bioinformatics screening of colorectal-cancer causing molecular signatures through gene expression profiles to discover therapeutic targets and candidate agents. BMC Med. Genom. 2023;16(1):64. doi: 10.1186/s12920-023-01488-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Z., Dan W., Zhang N., Fang J., Yang Y. Colorectal cancer and gut microbiota studies in China. Gut. Microbes. 2023;15(1) doi: 10.1080/19490976.2023.2236364. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Z., Li N., Cai P., Zhang C., Cao G., Yin J. Mechanism of HBx carcinogenesis interaction with non-coding RNA in hepatocellular carcinoma. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pavia G., Marascio N., Matera G., Quirino A. Does the human gut virome contribute to host health or disease? Viruses. 2023;15(11) doi: 10.3390/v15112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagao-Kitamoto H., Kitamoto S., Kuffa P., Kamada N. Korean Association for the Study of Intestinal Diseases; 2016. Pathogenic Role of the Gut Microbiota in Gastrointestinal Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ullah H., et al. The gut microbiota–brain axis in neurological disorder. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1225875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu J., et al. Changes in gut viral and bacterial species correlate with altered 1,2-diacylglyceride levels and structure in the prefrontal cortex in a depression-like non-human primate model. Transl. Psychiatry. 2022;12(1):74. doi: 10.1038/s41398-022-01836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seo D., Holtzman D.M. Current understanding of the Alzheimer's disease-associated microbiome and therapeutic strategies. Exp. Mol. Med. 2024;56(1):86–94. doi: 10.1038/s12276-023-01146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghorbani M., Ferreira D., Maioli S. A metagenomic study of gut viral markers in amyloid-positive Alzheimer's disease patients. Alzheimers. Res. Ther. 2023;15(1):141. doi: 10.1186/s13195-023-01285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gholamzad A., et al. Exploring the virome: an integral part of human health and disease. Pathol. Res. Pract. 2024;260 doi: 10.1016/j.prp.2024.155466. [DOI] [PubMed] [Google Scholar]
- 104.Jinato T., et al. Alterations in gut virome are associated with cognitive function and minimal hepatic encephalopathy cross-sectionally and longitudinally in cirrhosis. Gut. Microbes. 2023;15(2) doi: 10.1080/19490976.2023.2288168. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chelluboina B., Kieft K., Breister A., Anantharaman K., Vemuganti R. Gut virome dysbiosis following focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 2022;42(9):1597–1602. doi: 10.1177/0271678.221107702. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hui-Lin Y., et al. Alterations of oral and gut viromes in hypertension and/or periodontitis. mSystems. 2023;9(1) doi: 10.1128/msystems.01169-23. e01169-23, Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seth R.K., et al. Gut DNA virome diversity and its association with host bacteria regulate inflammatory phenotype and neuronal immunotoxicity in experimental Gulf War illness. Viruses. 2019;11(10) doi: 10.3390/v11100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suganya K., Koo B.S. MDPI AG; 2020. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Oct. 02, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu Z.-H., et al. Alterations in the composition of intestinal DNA virome in patients with COVID-19. Front. Cell Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.790422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piazzesi A., et al. The pediatric gut bacteriome and virome in response to SARS-CoV-2 infection. Front. Cell Infect. Microbiol. 2024;14 doi: 10.3389/fcimb.2024.1335450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cao J., et al. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut. Microbes. 2021;13(1) doi: 10.1080/19490976.2021.1887722. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martín Giménez V.M., Modrego J., Gómez-Garre D., Manucha W., de las Heras N. Gut microbiota dysbiosis in COVID-19: modulation and approaches for prevention and therapy. Int. J. Mol. Sci. 2023;24(15) doi: 10.3390/ijms241512249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cao J., et al. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut. Microbes. 2021;13(1) doi: 10.1080/19490976.2021.1887722. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ding Y., Wan M., Li Z., Ma X., Zhang W., Xu M. Comparison of the gut virus communities between patients with Crohn's disease and healthy individuals. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1190172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tun H.M., et al. BMJ Publishing Group; 2023. Gut Virome in Inflammatory Bowel Disease and Beyond. Nov. 10, [DOI] [Google Scholar]
- 116.Campbell A.W. Hindawi Publishing Corporation; 2014. Autoimmunity and the Gut. [DOI] [Google Scholar]
- 117.Fujimoto K., Miyaoka D., Uematsu S. Characterization of the human gut virome in metabolic and autoimmune diseases. Inflamm. Regen. 2022;42(1):32. doi: 10.1186/s41232-022-00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spencer L., Olawuni B., Singh P. Gut virome: role and distribution in health and gastrointestinal diseases. Front. Cell Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.836706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu X., et al. Correlation between the gut microbiome and neurodegenerative diseases: a review of metagenomics evidence. Neural Regen. Res. 2024;19(4) doi: 10.4103/1673-5374.382223. https://journals.lww.com/nrronline/fulltext/2024/04000/correlation_between_the_gut_microbiome_and.28.aspx [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao J., et al. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut. Microbes. 2021;13(1):1–21. doi: 10.1080/19490976.2021.1887722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen L., Hou X., Chu H. The novel role of phage particles in chronic liver diseases. Microorganisms. 2023;11(5) doi: 10.3390/microorganisms11051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chunxi L., Haiyue L., Yanxia L., Jianbing P., Jin S. The gut microbiota and Respiratory diseases: new evidence. J. Immunol. Res. 2020;2020(1) doi: 10.1155/2020/2340670. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Pessemier B., Grine L., Debaere M., Maes A., Paetzold B., Callewaert C. Gut–Skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9(2) doi: 10.3390/microorganisms9020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Draper L.A., et al. Autochthonous faecal viral transfer (FVT) impacts the murine microbiome after antibiotic perturbation. BMC Biol. 2020;18(1):173. doi: 10.1186/s12915-020-00906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang D., et al. Adaptive strategies and ecological roles of phages in habitats under physicochemical stress. Trends Microbiol. 2024;32(9):902–916. doi: 10.1016/j.tim.2024.02.002. [DOI] [PubMed] [Google Scholar]
- 126.Lin D., Hu B., Li P., Zhao Y., Xu Y., Wu D. Roles of the intestinal microbiota and microbial metabolites in acute GVHD. Exp. Hematol. Oncol. 2021;10(1):49. doi: 10.1186/s40164-021-00240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ritz N.L., et al. The gut virome is associated with stress-induced changes in behaviour and immune responses in mice. Nat. Microbiol. 2024;9(2):359–376. doi: 10.1038/s41564-023-01564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mao X., et al. Transfer of modified gut viromes improves symptoms associated with metabolic syndrome in obese male mice. Nat. Commun. 2024;15(1):4704. doi: 10.1038/s41467-024-49152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Draper L.A., et al. Autochthonous faecal viral transfer (FVT) impacts the murine microbiome after antibiotic perturbation. BMC Biol. 2020;18(1):173. doi: 10.1186/s12915-020-00906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abbas Y., et al. Fecal microbiota transplantation: current challenges and future landscapes. Clin. Microbiol. Rev. 2024;37(2) doi: 10.1128/cmr.00060-22. e00060-22May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ma Y., Liu J., Rhodes C., Nie Y., Zhang F. Ethical issues in fecal microbiota transplantation in practice. Am. J. Bioethics. 2017;17(5):34–45. doi: 10.1080/15265161.2017.1299240. May. [DOI] [PubMed] [Google Scholar]
- 132.Jo S.J., Kwon J., Kim S.G., Lee S.-J. The biotechnological application of bacteriophages: what to do and where to go in the middle of the post-antibiotic era. Microorganisms. 2023;11(9) doi: 10.3390/microorganisms11092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hibstu Z., Belew H., Akelew Y., Mengist H.M. Dove Medical Press Ltd; 2022. Phage Therapy: A Different Approach to Fight Bacterial Infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brives C., Pourraz J. Phage therapy as a potential solution in the fight against AMR: obstacles and possible futures. Palgrave Commun. 2020;6(1):100. doi: 10.1057/s41599-020-0478-4. [DOI] [Google Scholar]
- 135.Nair D.M., Vajravelu L.K., Thulukanam J., Paneerselvam V., Vimala P.B., Lathakumari R.H. Tackling hepatitis B Virus with CRISPR/Cas9: advances, challenges, and delivery strategies. Virus Genes. 2024;60(6):592–602. doi: 10.1007/s11262-024-02105-3. [DOI] [PubMed] [Google Scholar]
- 136.Nath A., et al. Phage delivered CRISPR-Cas system to combat multidrug-resistant pathogens in gut microbiome. Biomed. Pharmacotherapy. 2022;151 doi: 10.1016/j.biopha.2022.113122. [DOI] [PubMed] [Google Scholar]
- 137.Javed M.U., Hayat M.T., Mukhtar H., Imre K. CRISPR-Cas9 system: a prospective pathway toward combatting antibiotic resistance. Antibiotics. 2023;12(6) doi: 10.3390/antibiotics12061075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adler B.A., et al. Broad-spectrum CRISPR-Cas13a enables efficient phage genome editing. Nat. Microbiol. 2022;7(12):1967–1979. doi: 10.1038/s41564-022-01258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Al-Shayeb B., et al. Diverse virus-encoded CRISPR-Cas systems include streamlined genome editors. Cell. 2022;185(24):4574–4586. doi: 10.1016/j.cell.2022.10.020. e16Nov. [DOI] [PubMed] [Google Scholar]
- 140.Panahi B., Dehganzad B., Nami Y. CRISPR-Cas systems feature and targeting phages diversity in lacticaseibacillus rhamnosus strains. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1281307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fujiki J., Schnabl B. Phage therapy: targeting intestinal bacterial microbiota for the treatment of liver diseases. JHEP Rep. 2023;5(12) doi: 10.1016/j.jhepr.2023.100909. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beinhauerova M., Slana I. Phage amplification assay for detection of mycobacterial infection: a review. Microorganisms. 2021;9(2) doi: 10.3390/microorganisms9020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ouyang X., et al. Mycobacteriophages in diagnosis and alternative treatment of mycobacterial infections. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mattila S., Ruotsalainen P., Jalasvuori M. On-demand isolation of bacteriophages against drug-resistant bacteria for personalized phage therapy. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fauconnier A. Regulating phage therapy. EMBo Rep. 2017;18(2) doi: 10.15252/embr.201643250. 198-200–200Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gencay Y.E., et al. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024;42(2):265–274. doi: 10.1038/s41587-023-01759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Y., Yang K., Duan H., Du Y., Ye J. Phage-based peptides for pancreatic cancer diagnosis and treatment: alternative approach. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1231503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ragothaman M., Yoo S.Y. Engineered phage-based cancer vaccines: current advances and future directions. Vaccines. (Basel) 2023;11(5) doi: 10.3390/vaccines11050919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang C., et al. Engineered M13 phage as a novel therapeutic bionanomaterial for clinical applications: from tissue regeneration to cancer therapy. Mater. Today Bio. 2023;20 doi: 10.1016/j.mtbio.2023.100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kulpakko J., Juusti V., Rannikko A., Hänninen P.E. Detecting disease associated biomarkers by luminescence modulating phages. Sci. Rep. 2022;12(1):2433. doi: 10.1038/s41598-022-06433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Juusti V., Rannikko A., Laurila A., Sundvall M., Hänninen P., Kulpakko J. Phage biosensor for the classification of metastatic urological cancers from urine. Life. 2024;14(5) doi: 10.3390/life14050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lathakumari R.H., Vajravelu L.K., Thulukanam J., Narasimhan A.K. Next-gen nano biosensor technologies to monitor carbapenem resistance for personalized medicine. Indian J. Microbiol. 2024 doi: 10.1007/s12088-024-01337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jones H.J., Shield C.G., Swift B.M.C. The application of bacteriophage diagnostics for bacterial pathogens in the agricultural supply chain: from farm-to-fork. PHAGE. 2020;1(4):176–188. doi: 10.1089/phage.2020.0042. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Islam Md.S., Fan J., Pan F. The power of phages: revolutionizing cancer treatment. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1290296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu G.-Y., et al. Antimicrobial resistance crisis: could artificial intelligence be the solution? Mil. Med. Res. 2024;11(1):7. doi: 10.1186/s40779-024-00510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bajiya N., Dhall A., Aggarwal S., Raghava G.P.S. Advances in the field of phage-based therapy with special emphasis on computational resources. Brief. Bioinform. 2023;24(1):bbac574. doi: 10.1093/bib/bbac574. Jan. [DOI] [PubMed] [Google Scholar]
- 157.Auslander N., Gussow A.B., Benler S., Wolf Y.I., Koonin E.V. Seeker: alignment-free identification of bacteriophage genomes by deep learning. Nucl. Acids. Res. 2020;48(21) doi: 10.1093/nar/gkaa856. e121–e121, Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kieft K., Zhou Z., Anantharaman K. VIBRANT: automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome. 2020;8(1):90. doi: 10.1186/s40168-020-00867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guo J., et al. VirSorter2: a multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome. 2021;9(1):37. doi: 10.1186/s40168-020-00990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sirén K., Millard A., Petersen B., Gilbert M.T.P., Clokie M.R.J., Sicheritz-Pontén T. Rapid discovery of novel prophages using biological feature engineering and machine learning. NAR. Genom. Bioinform. 2021;3(1):lqaa109. doi: 10.1093/nargab/lqaa109. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bajiya N., Dhall A., Aggarwal S., Raghava G.P.S. Advances in the field of phage-based therapy with special emphasis on computational resources. Brief. Bioinform. 2023;24(1):bbac574. doi: 10.1093/bib/bbac574. Jan. [DOI] [PubMed] [Google Scholar]
- 162.Cantu V.A., et al. PhANNs, a fast and accurate tool and web server to classify phage structural proteins. PLoS Comput. Biol. 2020;16(11) doi: 10.1371/journal.pcbi.1007845. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.T T.Y, et al. Component parts of bacteriophage virions accurately defined by a machine-learning approach built on evolutionary features. mSystems. 2021;6(3) doi: 10.1128/msystems.00242-21. 10.1128/msystems.00242-21May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ahmad S., et al. SCORPION is a stacking-based ensemble learning framework for accurate prediction of phage virion proteins. Sci. Rep. 2022;12(1):4106. doi: 10.1038/s41598-022-08173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang W., et al. A network-based integrated framework for predicting virus–prokaryote interactions. NAR Genom. Bioinform. 2020;2(2):lqaa044. doi: 10.1093/nargab/lqaa044. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McNair K., Bailey B.A., Edwards R.A. PHACTS, a computational approach to classifying the lifestyle of phages. Bioinformatics. 2012;28(5):614–618. doi: 10.1093/bioinformatics/bts014. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hockenberry A.J., Wilke C.O. BACPHLIP: predicting bacteriophage lifestyle from conserved protein domains. PeerJ. 2021;9 doi: 10.7717/peerj.11396. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shang J., Tang X., Sun Y. PhaTYP: predicting the lifestyle for bacteriophages using BERT. Brief. Bioinform. 2023;24(1):bbac487. doi: 10.1093/bib/bbac487. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]