Abstract
As our understanding of the role of the gut microbiome in human diseases deepens, precision engineering of the gut microbiome using bacteriophages has gained significant attention. Herein, we review the recent advances in bacteriophage-mediated modulation of the gut microbiome, discuss approaches at the ecological and genetic levels, and summarize the challenges and strategies pertinent to each level of intervention. Drawing on the structural attributes of bacteriophages in the context of precision engineering, we examined the latest developments in the field of phage administration. Gaining a nuanced understanding of microbiome manipulation will yield tailored strategies and technologies. This could revolutionize the prevention and treatment of diseases linked to gut pathogens and offer new avenues for the therapeutic use of bacteriophages.1
Keywords: Bacteriophages, Microbiome engineering, Human gut microbiome
Graphical abstract
1. Introduction
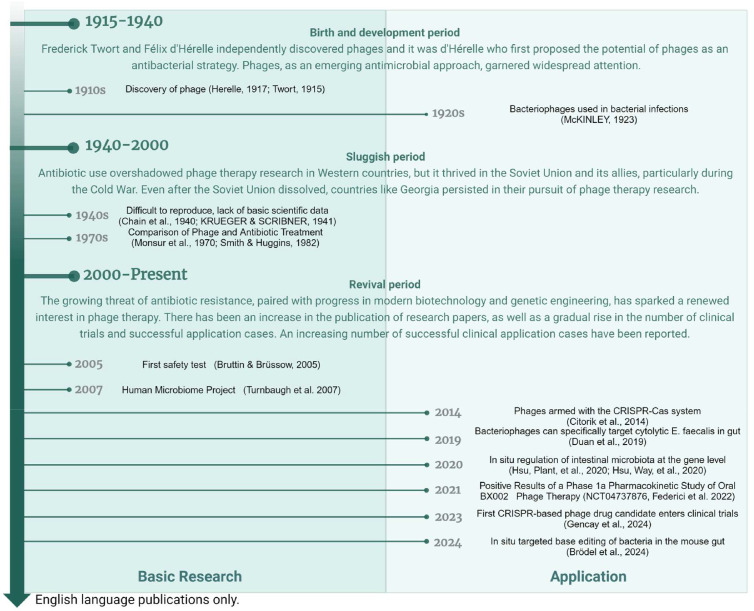
The gut microbiome has become a key focus of scientific research, largely because of the launch of the Human Microbiome Project (Fig. 1) [1]. Extensive research has highlighted its crucial role in human health, influencing a variety of conditions including gastrointestinal disorders, as well as neurodegenerative [2], metabolic [3], and cardiovascular diseases [4]. The literature strongly associates certain pathogenic strains in the gut, including Streptococcus anginosus [5], Desulfovibrio vulgaris [6], and Peptostreptococcus anaerobius with a range of diseases [7]. Many of these pathogenic strains are commensal and can become pathogenic under specific conditions. Pathogenic bacteria have been observed to employ diverse strategies to harness their host. Some produce toxins that can damage the host cells [8], whereas others alter cellular functions by secreting metabolites [7]. Some induce inflammation through surface proteins [9], and others facilitate cellular adhesion through secretion systems [10]. These direct pathogenic mechanisms offer valuable insights that can guide us in addressing the specific virulence genes responsible for these harmful actions.
Fig. 1.
Timeline of phage-based engineering of the gut microbiome [1,[17], [18], [19], [20], [21], [22], [23], [24], [25],[25], [26], [27], [28], [29], [30]].
However, conventional approaches for modulating the gut microbiome, including the use of antibiotics, probiotics [11], fecal microbiota transplants (FMT) [12,13], fecal virus transplantation (FVT) [14,15] and prebiotics [16], offer limited precision and do not permit targeted modulation at the strain or genetic level within the bacterial community. Bacteriophages (phages), the natural enemies of bacteria, offer a high degree of specificity, making them promising candidates for the precise manipulation of the gut microbiome. Their tractability also allows for the direct editing of the gut microbiome and in situ genetic-level regulation. Early phage therapy research focused on the development of antimicrobial substances, as depicted in Fig. 1. The field has since been expanded to address the nuanced regulation within complex microbial ecosystems, notably the gut microbiome [17]. Phages provide a precise method for targeting harmful genes in the gut microbiota without disrupting the entire community, thus offering significant potential for antimicrobial therapy and intestinal health preservation. Rapid progress in molecular biology, bioinformatics, and structural biology, along with advancements in analytical and gene-editing technologies, has significantly enhanced our understanding of phages and their intricate relationships with bacteria. These developments have solidified the foundation for phage therapy research and spurred the investigation of engineered phage technologies.
This review aims to provide a thorough overview of the current landscape of phage-mediated precision engineering of the gut microbiome, also known as intestinal phage therapy. The discussion is structured into three sections. First, we delve into the ecological dimensions of phage-based microbiome engineering, discussing the foundational concepts, appropriate bacterial targets, status of ongoing clinical trials, applications beyond human gut health, and challenges and strategies therein. Second part concentrates on the intricacies of genetic-level engineering with phages, recounting the research advancements to date and contemplating potential hurdles in the field's evolution. The last part addresses the considerations for oral phage administration, along with an overview of current strategies and methodologies.
2. Precision engineering at the ecological level
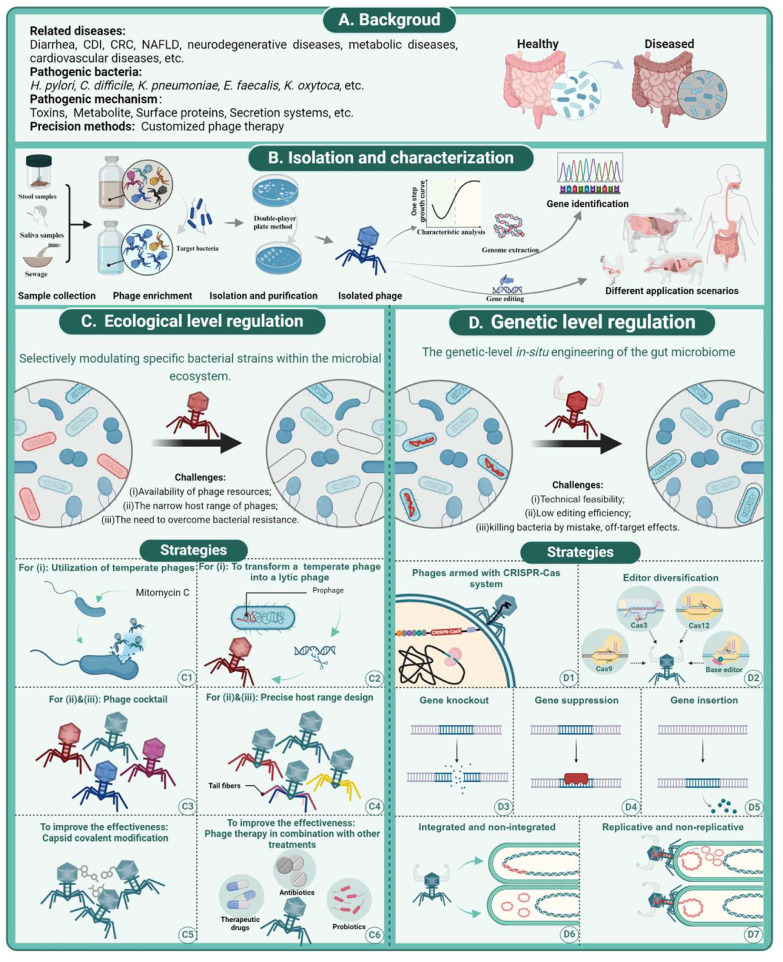
Precision engineering of the gut microbiome focuses on the deliberate elimination and manipulation of certain bacterial strains to treat the diseases they cause (Fig. 2C). Most studies and applications of phage therapy have focused on ecological regulation, as detailed in Table 1. Conditions, such as diarrhea caused by diarrheagenic Escherichia Coli [31], Clostridium difficile infection (CDI) [32], and colorectal cancer (CRC) associated with Fusobacterium nucleatum [33], serve as relevant examples. This strategy includes the isolation and characterization of phages, their subsequent modification to target specific bacterial strains, and their delivery to the gut (Fig. 2B).
Fig. 2.
Phage-based ecological and genetic engineering of the gut microbiome. (A) Background of precision engineering of gut microbes. (B) Workflow of phage isolation and characterization. (C) Precision engineering at the ecological level. (D) Precision engineering at the genetic level.
Table 1.
Recent studies of phage-based microbiome engineering.
| Level | Pathogens (objective) | Phage | Strategy | Target genes | Application (Host) | Reference |
|---|---|---|---|---|---|---|
| Ecological | NOT FOR THESE (to evaluate the impacts of phage) | MS2/P22 | Phage cocktail | / | Type 2 diabetes (Human) | [54] |
| Ecological | non-pathogenic E. coli | P1/ P2/ P3/ P4 | phage cocktail with probiotics | / | Feed additives (Broiler Chickens) | [87] |
| Ecological | NOT FOR THESE (to evaluate the impacts of phage) | vB_KpnP_K1-ULIP33 | / | / | Antimicrobial resistances (Human) |
[91] |
| Ecological | high alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) | HiAlc Kpn-specific phage | / | / | non-alcoholic fatty liver disease (Human) | [92] |
| Ecological | Bacteroides fragilis | F766P4, BF486P1, BF494P1, BF766P1, BF702P1, BF695P2, BF698P1, BF344P1 | Phage cocktail | / | Demonstration of feasibility | [69] |
| Ecological | E. coli GXXW-1103 | A221 | / | / | Post-weaning diarrhea (PWD)(piglets) | [58] |
| Ecological | Shiga toxin-producing E. coli (STEC) | vB_EcoS_Ace (Ace) | / | / | Kill foodborne pathogen (Human) | [93] |
| Ecological | E. coli strain MG1655 | W1 / W3 | Phage cocktail | / | Microbial knockout | [94] |
| Ecological | S. enterica serovar Typhimurium | vB_SenM-2 and vB_Sen-TO17 | Phage cocktail | / | Poultry infections(chickens) | [95] |
| Ecological | Enterohemorrhagic E. coli (EHEC) | λ | Armed with Cas3; cro gene knock-out |
Ege gene | Intestinal infection (Human) | [77] |
| Ecological | Listeria monocytogenes | 15 distinct lytic phages (FOP) | Phage cocktail | / | Foodborne illness (Human) | [96] |
| Ecological | Salmonella Pullorum | Salmonella phage CKT1 | / | / | Body weight loss (chickens) | [97] |
| Ecological | Klebsiella pneumoniae | five-phage combination (5E) | Phage cocktail | / | IBD(Human) | [22] |
| Ecological | Flavobacterium psychrophilum | FpV4 / FPSV-D22 | Phage cocktail | / | Antibiotic therapies (Rainbow trout fry) | [62] |
| Ecological | Salmonella strains | SalmoFree | Phage cocktail | / | Foodborne pathogen (Chickens) | [98] |
| Ecological | E. coli LF82 and E. faecalis OG1RF | isolates | Phage cocktail | / | IBD(Human) | [55] |
| Ecological | Salmonella enterica subsp. enterica serovar Typhimurium | SalmoFresh and FOP | Phage cocktail | / | Foodborne illness (Human) | [59] |
| Ecological | Multidrug-resistant Klebsiella pneumoniae | vB_ KpnM_GF | / | Multidrug-resistant | [99] | |
| Ecological | Clostridioides difficile | ΦCD24–2 | Engineering with a lethal genome-targeting CRISPR array (Cas3 from host); from temperate to obligately lytic: region encoding the cI repressor and integrase gene was deleted | RNase Y | CDI (Human) | [73] |
| Ecological | Fusobacterium nucleatum | Fusobacterium nucleatum phage (P2) | Biorthogonal chemistry strategy | / | CRC (Human) |
[90] |
| Ecological | Campylobacter jejuni | CP20 and CP30A | Phage cocktail | / | Foodborne illness (Chickens) | [100] |
| Ecological | Salmonella Typhimurium | ListShield (six lytic phages), EcoShield PX (three lytic phages), and SalmoFresh (six lytic phages) | phage Cocktail | / | Foodborne illness (Human) | [101] |
| Ecological | Cytolysin-positive (cytolytic) E. faecalis | Efmus1, Efmus2, Efmus3 and Efmus4 | phage Cocktail | / | Alcoholic liver disease (Human) | [21] |
| Ecological | pathogenic E. coli strain O157:H7 | F.O.P. | phage Cocktail | / | Foodborne illness (Human) | [102] |
| Ecological | Chelonia mydas | isolates | phage Cocktail | / | Animal protection (C. mydas) | [63] |
| Ecological | Salmonella Enteritidis/ Salmonella Pullorum | PA13076 /BP96115 | / | / | Antimicrobial resistances (Human) |
[103] |
| Ecological | E. coli induced diarrhea | phages NPC 1000, 1002, 1003, 1006, 1007, 1008, 1009, 1024, 1031/ Microgen phage cocktail | phage Cocktail; given in six split doses | / | Bacterial diarrhea | [104] |
| Ecological | E. coli induced diarrhea | eleven T4-like phages (AB2, 4, 6, 11, 46, 50, 55; JS34, 37, 98, D1.4/Microgen phage cocktail | phage Cocktail | / | Bacterial diarrhea | [105] |
| Ecological | Clostridium difficile | phiCDHM(1–6) phiCDHS1 | phage Cocktail | / | CDI | [106] |
| Ecological | Shigella spp. | ShigActiveTM | phage Cocktail | / | Deadly bacterial pathogens (Human) | [107] |
| Ecological and genetic | Shigella flexneri 2457T/M90T and E. coli O157:H7 | P2/P4 | Tail-Engineered; Armed with cas9 | Ege gene | Antimicrobial resistance | [108] |
| Ecological and genetic | Escherichia coli | M13 | Armed with cas9 | Deletion: GFP | Demonstration of feasibility | [109] |
| Genetic | Shiga toxin (Stx)-producing E. coli | λ | genetic hybrids of λ with other lambdoid phages; inserted genes for 933W·cIind- | Repression: stx2 | Pathogenic infection (Human) | [110] |
| Genetic | E. coli | λ | Engineered with dCas9; | Repression: GFP | Modification of bacterial function | [25] |
| Genetic | E. coli | λ | Engineered a variant of the Ur-λ capsid; Engineered with Cas9; cos site deleted; | Delivery: GFP Deletion: β-lactamase |
in situ modification | [17] |
2.1. Appropriate bacterial targets
Ecological regulation within the gut microbiome is particularly well suited for combating infectious bacteria, which are pathogens capable of spreading and infecting hosts through various transmission routes. Examples include certain serotypes of E. coli [34], Helicobacter pylori [35], and Salmonella [36]. These bacteria are not typically part of a healthy gut microbiota, and they can be eradicated with minimal risk, aligning with the goal of maintaining an ecological balance. To target these bacteria, strictly virulent phages should be employed instead of temperate phages, as this approach ensures efficient and complete elimination of pathogenic bacteria without the risk of lysogeny. In a 2017 personalized phage therapy case report, a 68-year-old patient developed necrotizing pancreatitis complicated by multidrug-resistant Acinetobacter baumannii infection. After attempting multiple treatments for four months without success, phage therapy was started with the Food and Drug Administration (FDA)’s emergency new drug investigation approval, and the patient recovered after a few months. This case highlights a major turning point in the use of phage therapy, attracting widespread attention, and offering new hope for its application in the treatment of pathogenic bacteria [37]. Phages have been successfully used to combat pathogenic bacteria (especially multidrug-resistant bacteria), such as Pseudomonas aeruginosa [38] and Mycobacterium abscessus [39,40], in a variety of diseases other than gastrointestinal infections.
In contrast to pathogenic bacteria that cause infections, there exists a category of disease-contributing commensal bacteria that are naturally part of the human microbiota, such as Helicobacter hepaticus [41], Klebsiella oxytoca [42,43], Klebsiella pneumoniae, and Bacteroides fragilis. These organisms can be advantageous under typical circumstances, but may contribute to noncommunicable diseases (NCDs) under certain conditions [44,45]. One example is the contrast between enterotoxigenic Bacteroides fragilis (ETBF) and non-toxigenic B. fragilis (NTBF). Although B. fragilis is a key beneficial component of the human gut microbiota [46], ETBF, which is capable of producing B. fragilis toxin (BFT), is associated with the progression of CRC [47]. These insights suggest that strains of the same species may play divergent roles within the intestine, influenced by their phenotypes, such as the expression of toxic genes. Interestingly, recent findings suggest that NTBF may pose risks to the host in specific scenarios [48]. This complexity underscores the notion that the role of a single bacterial strain within the gut microbiota is not fixed but can manifest in various ways depending on the environmental context [41]. Studies have also shown that a significant proportion of the healthy Chinese population, ranging from 4% to 5.19%, carries hypervirulent Klebsiella pneumoniae [49], which has been associated with conditions, including primary sclerosing cholangitis (PSC) [50], nonalcoholic fatty liver disease (NAFLD) [51], and inflammatory bowel disease (IBD) [22]. The transition of commensal bacteria from harmless to harmful in the gut microbiota is not yet fully understood [52], implying that the outright removal of these bacteria could entail unforeseen risks. Therefore, the judicious application of ecological-level clearance should be guided by therapeutic outcomes and the extent of restoration of intestinal homeostasis, with the understanding that such interventions may precipitate an alternative state of imbalance. For instance, phage therapy tailored to cytolytic Enterococcus faecalis in alcoholic hepatitis demonstrates the efficacy of precise ecological targeting, in which phages specifically target pathogenic strains while sparing non-cytolytic variants [21].
2.2. Emergence in scientific advances, clinical trials, and beyond
Phage-based intestinal microbiome modulation is burgeoning in academic circles, as evidenced by the data presented in Table 1. With progress in scientific and technological research, strategies for managing infectious bacteria have evolved from the initial use of single or combined natural phages [53] to the current utilization of phage cocktails informed by synthetic biology [23]. Furthermore, the scope of the diseases targeted by these phage therapies is broadening. They are no longer limited to infectious diseases but are increasingly being applied to non-infectious conditions [44] such as type 2 diabetes [54] and IBD [22,55]. There are already numerous studies that have advanced to clinical trials. Clinical trials, such as NCT03269617, have explored the use of a phage cocktail (PreforPro, comprising four E. coli-targeting phages) as a probiotic to adjust intestinal bacteria and improve gut health. Additionally, trials such as NCT03808103 are currently recruiting participants to evaluate the effectiveness of an oral phage (EcoActive) against adherent-invasive E. coli (AIEC) in treating Crohn's disease. In a notable development, BiomX reported positive results from a phase 1a clinical trial (NCT04737876) for BX002, which targets Klebsiella pneumoniae, for the treatment of IBD and PSC. This trial is the first phage therapy conducted in accordance with FDA-recommended investigational new drug (IND) standards [22]. Eligo Bioscience, a frontrunner in this field, is developing phage therapies that leverage CRISPR systems. CTheir candidate EB003 uses the precision of CRISPR-Cas9 to target and eliminate Shiga toxin-producing E. coli (STEC) in the intestine, thereby reducing Shiga toxin levels and preventing hemolytic uremic syndrome.
Moreover, phage therapy is expanding beyond human healthcare, making inroads into animal agriculture. It is becoming increasingly prevalent in livestock such as cattle [56], chickens [57], and swine [58]. In swine farming, phage therapy effectively combats diarrhea caused by E. coli and Salmonella enterica [59,60]. Prophylactic use of phages before pathogen exposure has shown increased efficacy in multiple animal species [61]. Precision engineering of intestinal ecosystems through phage therapy is also advancing in aquaculture [62] and wildlife conservation [63]. However, research on pet healthcare remains in its early stages, with most studies focusing on areas beyond the intestinal tract [64]. As a regulatory milestone, the European Medicines Agency (EMA) issued guidelines on the quality, safety, and effectiveness of phages as veterinary drugs in October 2023 [65]. These guidelines provide a regulatory framework to aid in the approval and marketing of veterinary phage products and ingredients. Overall, the field of precision engineering of intestinal ecology through phage therapy has considerable market potential and is a highly promising area for future research and development.
2.3. Challenges and strategies
Phage-based ecological modulation of the gut microbiome presents a broad spectrum of applications and has shown significant progress. However, there are challenges in the implementation of this technology. Although phages are the most abundant life form on Earth, access to specific phage resources can be difficult. The isolation of specific phages, especially those that target commensal gut bacteria, is not straightforward. The isolation of phages targeting the majority of commensal bacterial species in the gut occurred much later, with researchers in the 1980s isolating phages of B. fragilis from environmental sewage [66] and feces [67]. Recently, there has been increased interest in isolating phages that target commensal gut bacteria because of the potential they offer to study bacterial-phage interactions and regulate the gut microbiome. For example, using the soft agar overlay method, researchers have successfully isolated 27 phages targeting Bacteroides thetaiotaomicron from sewage and pond water [68]. In addition, 209 phages capable of infecting 42 different species of human gut bacteria were isolated from environmental and fecal samples, indicating the potential to further explore commensal phage resources [69]. Current methods for phage isolation have become relatively mature, including co-culturing to enrich phages specific to certain hosts and using tangential flow and protein concentrators to concentrate all phages, followed by phage isolation and purification using double-layer agar plates. The purified phages are then subjected to genome sequencing and annotation. Notably, temperate phages, which are important components of the gut virome [70], generally have a lower lytic efficiency [71] and carry the risk of horizontal gene transfer when integrated into the host genome [40,72,73], making them less desirable for phage therapy. However, it is possible to increase the available phage resources by knocking out the integrating genes [40]. Inducers such as mitomycin C can be used to promote the excision and release of prophages from chromosomes, thereby increasing the chances of isolating temperate phages.
Another challenge associated with phages is their limited host range. This high specificity can hinder their practical application and prevent rapid identification of suitable phages from existing libraries in urgent medical situations. Consequently, designing phages with an appropriate host range is essential to enhance the precision and efficacy of phage therapy. The specificity and efficiency of phage targeting are primarily governed by receptor-binding proteins such as tail fibers, tail spikes, and tail tips, as well as bacterial cell surface receptors, including lipopolysaccharides (LPS), peptidoglycans, and porins [74]. Several studies have successfully broadened the host range of phages by modifying their receptor-binding proteins [17,23]. Additionally, designing phage cocktails to target various strains can provide more comprehensive coverage of bacterial targets. Furthermore, creating phage cocktails that target different receptors on specific bacteria can mitigate the development of phage resistance and minimize potential antagonism among phages.
The third challenge is combating bacterial resistance, which manifests in two primary ways: prevention of phage entry and inhibition of phage replication. Resistance to phage entry includes blocking phage adsorption and genome injection [75]. As discussed previously, the main strategies for addressing this issue involve modifying the receptor-binding proteins of phages or creating a phage cocktail. However, the inhibition of phage replication involves complex mechanisms that stem from the co-evolutionary “arms race” between bacteria and phages [76]. To overcome bacterial resistance, targeted modifications have been made to enhance the bactericidal capabilities. One common approach is to equip phages with the CRISPR-Cas system, which targets specific genes, often virulence or resistance genes, to weaken their survival capabilities. As early as 2014, Citorik et al. introduced ΦRGN, a CRISPR-Cas9-equipped phage designed for the precise control of antibiotic resistance and virulence factors in carbapenem-resistant Enterobacteriaceae and enterohemorrhagic E. coli (EHEC) [20]. Bikard et al. successfully developed a phage capable of delivering the RNA-guided nuclease Cas9, which could efficiently eradicate virulent strains of Staphylococcus aureus while sparing non-virulent strains [116]. Initially targeting the resistant bacteria, this method was extended to include intestinal pathogens. Among the CRISPR-Cas systems used, type I systems were predominantly employed. For example, researchers designed phage delivery systems targeting the strain chromosome CRISPR array based on the type I-B CRISPR-Cas system in C. difficile, enabling the endogenous CRISPR-Cas3 in C. difficile to target its own chromosome [73]. Another example involves enhancing the killing ability of phage Eλ by incorporating a CRISPR-Cas3 system to target EHEC, with specificity improved by introducing multiple EHEC-targeting CRISPR spacers. In vitro experiments have demonstrated that Eλ can kill EHEC cells with nearly 100% efficiency [77]. A comprehensive summary of the applications of CRISPR-Cas systems in armed phages is presented in Table 1. Notably, bacteria have evolved a diverse array of defense mechanisms to counter phage attacks [[78], [79], [80]]. Consequently, phages have evolved strategies to overcome these bacterial anti-phage mechanisms [81]. These dynamic interactions often result in a balance that allows for the coexistence of phages and their bacterial hosts in the gut, rather than the outright eradication of bacteria [82], which could potentially compromise therapeutic outcomes. Fortunately, researchers are already working to leverage defense systems against phages in industrial applications. They demonstrated a strategy for developing phage-resistant E. coli through simultaneous genomic integration of a DNA phosphorothioation-based Ssp defense module and mutations in components essential for the phage life cycle, resulting in strong resistance against the diverse phages tested without affecting cell growth [83]. This strategy indirectly confirms the feasibility of enhancing the bactericidal efficacy of phage therapy by modifying the defense system. Moreover, researchers have explored the use of additional functional loads, including extracellular and intracellular payloads, to augment the bactericidal effects of phages [84]. Strategies such as designing phages to disrupt biofilms [85] or infecting hosts with payload-carrying enzymes [86] have inspired the precise regulation of the gut ecosystem by phages. However, the formulation of these strategies requires a deeper understanding of the spatial distribution of the gut microbiome [82]. Although both phage-specific modifications and combinatorial strategies have been employed, complex situations remain inevitable.
Researchers are also exploring combinations of phage therapy with other treatments to maximize their potential. These combination therapies leverage the precision of phage targeting, along with the broader effects of additional interventions, such as the combined application of phages with probiotics [87], antibiotics [88,89], and therapeutic drugs. In an innovative approach, irinotecan-loaded dextran nanoparticles were linked to azide-modified phages, which effectively inhibited the growth of Fusobacterium nucleatum and significantly enhanced the efficacy of chemotherapy for CRC [90]. Although these examples extend beyond the realm of intestinal ecology, they provide valuable insights for future research on the precise manipulation of the gut microbiome using phage therapy.
3. Part B precision engineering at the genetic level in situ
The targeted elimination of specific commensal gut bacteria can trigger a cascade of unintended and far-reaching consequences for the broader microbial community. Research has delineated these effects across diverse settings, including ex vivo fecal models [69], in vivo synthetic communities [111], and natural in vivo environments [112]. Maintaining a nuanced equilibrium in the targeted killing of pathogenic commensal bacteria is imperative for avoiding excessive removal or insufficient specificity. Targeting disease-causing genes allows for the achievement of therapeutic goals while minimizing perturbations to the gut microbiome. Furthermore, classical functional genomics, which focuses on introducing mutations and observing phenotypic changes, often overlooks the impact of indigenous microbes and their local environments. Phage-based gene editing targeting specific members of a microbial community has immense potential for uncovering the diverse biological roles and functional genomics of individual members within the microbial network. Given the intricacies of disease-related commensal bacteria and the burgeoning field of precision medicine, there is an urgent need to develop precision engineering technologies for the gut microbiome based on phage-mediated genetic manipulation at the genetic level.
Phage-based in-situ gene editing can be employed to adjust the genetic makeup of specific gut bacterial strains (Fig. 2D), similar to how viral vectors, such as adeno-associated viruses (AAVs) are designed for the targeted delivery of genetic material to specific human cells for intracellular gene editing [113]. Compared with delivery tools such as engineered bacteria [114] and liposomes [115], the most significant advantage of phage-based in-situ delivery tools is their high host specificity, which allows them to precisely target bacterial strains for gene editing in a complex microbial community. This strategy involves the use of phages to introduce gene-editing systems, enabling gene knockout [109], suppression [25], and insertion.
3.1. Progress
The pioneering approach of utilizing engineered phages for the genetic manipulation of intricate bacterial communities was first documented in 2014, as mentioned above. By applying DNA-level selective pressure, Citorik et al. and Bikard et al. aimed to reduce the prevalence of undesirable genes, minimize off-target effects, and enable the programmable reconfiguration of the microbiota [20,116]. As editing systems continue to evolve, phage-delivered editing systems are being refined and expanded to include a variety of tools, such as Cas3, Cas12a, dCas9, and base editors. Selection of an appropriate editor is crucial and depends on the specific requirements for reprogramming the gut microbiota. For instance, Cas3, a component of the type I CRISPR-Cas system, is prevalent in prokaryotes, and we can leverage the native gene-editing systems of prokaryotes for precise manipulation [73]. Unlike Cas9, Cas3, which combines helicase and nuclease activities, can traverse the genome and degrade adjacent DNA sequentially upon targeting, highlighting its potential for the efficient knockout of large genomic segments [117]. This makes Cas3 an ideal candidate for arming phages to enhance their bactericidal effects. In contrast, type II CRISPR-Cas systems such as Cas9 and Cas12a (or Cpf1) typically function with a single Cas protein [118]. Their ease of use makes them useful for gene editing. The inefficiency of bacterial DNA repair pathways can lead to species- or strain-specific depletion of the target bacterial host population after Cas9-induced double-strand breaks (DSBs) [119]. It has been observed that cells can evade CRISPR-Cas9 targeting through various mechanisms, such as loss of the spacer in the CRISPR array, mutations in the target site, or even deletion of the entire CRISPR-Cas9 system [109]. Base editors, which enable precise single-base substitutions without generating DSBs or requiring donor DNA templates [120], help reduce the loss and lysis of target bacteria and mitigate the impact of post-lysis endotoxin release. Several studies have successfully applied base editing for efficient editing across multiple bacterial species [[121], [122], [123]], and the use of armed phages remains limited.
In addition, phages can be categorized into lytic and temperate types based on their life cycle, which influences how they can be engineered for genetic manipulation. This distinction allows us to classify engineered phages into integrating and non-integrating categories. Integrating phages, such as the λ phage, have been genetically modified to overcome prophage resistance in target bacteria, thereby achieving the transcriptional inhibition of the Shiga toxin. Studies have shown that a single dose of this engineered λ phage can propagate throughout bacterial communities and reduce Shiga toxin production in intestinal mouse infection models without significantly affecting bacterial concentrations [110]. Additionally, a λ phage also equipped with programmable dCas9 has been designed to inhibit specific E. coli genes within the mammalian intestine [25]. Furthermore, researchers have engineered λ phages to express an APOBEC-1-based cytosine base editor (CBE) and integrate the modified phage into the bacterial host genome. This approach uses C to T point mutations to introduce premature stop codons, thereby inactivating both chromosomal- and plasmid-encoded genes without disrupting adjacent genomic regions [71].
Non-integrating phages can be categorized into replicating and non-replicating subtypes. Traditional phage therapy typically involves replicating phages capable of normal replication cycles. However, a key advantage of in situ-directed mutagenesis is its capacity to curb the dissemination of transgenes [17]. Non-replicating phages, which infect and edit target bacteria without establishing persistent infections, are attracting increasing interest. For example, researchers engineered a DNA payload that leverages the replication machinery of phage-inducible chromosomal islands. This ingenious approach ensures that the introduced DNA is non-replicative within the host bacteria, allowing for efficient expression of base editors and promoting successful editing outcomes [17].
3.2. Challenges
Research on the gene-level modulation of the intestinal microbiota is still in its nascent phase. This field grapples with challenges not only in ecological regulation but also in the realm of gene editing (Table 2). The first challenge is technical feasibility; a large number of gastrointestinal bacterial species have not been extensively studied using current editing technologies, rendering strain-specific editing unfeasible [124]. This limitation underscores the urgent need to consider fully synthetic approaches [125]. Although this has been realized for a limited number of species, researchers have already developed fully synthetic processes. This pipeline begins with the generation of overlapping DNA fragments, including segments containing tailored mutations or random mutant libraries through de novo synthesis or PCR amplification. These fragments are then assembled into a complete synthetic phage genome. Assembly can be conducted using yeast-based methods [126] or in vitro techniques such as Gibson [127] or Golden Gate assemblies [128] to ensure that fragments are precisely joined to form an intact genome. The assembled genome is then transformed into natural hosts, such as E. coli [129] or L-form bacteria [130], to reactivate phage particles in a process known as rebooting. The engineered phages are released by lysis with chloroform or similar methods. An interesting case was presented by Cheng et al., who developed a widely applicable framework known as SHAPE. This framework utilizes engineered E. coli strains as stepping-stone hosts to promote genome refactoring of viruses in one pot, validated by successful cross-genus and cross-order rebooting of 90 phages infecting four orders of popular pathogens [129]. Moreover, despite advances in the understanding of classical phage genomes through CiPGr, a CRISPR/Cas9-based strategy for genome reduction [131], our understanding of gene functions in most phage genomes remains limited. This limitation hinders the effective minimization of phage genomes. The requirement to incorporate additional editing machinery into phage-delivered systems and the limited packaging capacity of phage capsids further complicate the construction of engineered phages, making in situ gene manipulation a complex endeavor. One potential solution is to convert phages into cosmids that deliver only essential genes and beneficial elements to the bacteria, thus circumventing packaging constraints. However, this approach is particularly challenging for non-model phages and hosts. It is also essential to develop genetic elements suitable for phage- and strain-specific editing, such as promoters, ribosome-binding sites, and terminators [132]. These components must be deployed within the phages to facilitate precise and effective editing.
Table 2.
Challenges and potential solutions in phage-based intestinal precision engineering.
| Applications | Challenges | Potential solutions |
|---|---|---|
| Natural phages | Specificity: natural phages often exhibit narrow host ranges, limiting their utility against diverse bacterial strains in the human gut; | Using phage cocktails to target multiple bacterial strains simultaneously can reduce the likelihood of resistance; |
| Combining phages with adjuvants like antibiotics can enhance effectiveness; | ||
| Resistance development: bacteria can develop resistance through a variety of mechanisms; | Using synthetic biology to expand phage host range, enhance their bactericidal capabilities, and reduce the likelihood of resistance; | |
| Unpredictable ecological impact: natural phage-bacteria dynamics may lead to unintended shifts in gut microbiota; | Combining engineered phages for precise gene-level regulation with machine learning-based ecological impact predictions can enhance therapeutic specificity while minimizing unintended disruptions to gut microbiota; | |
| Engineered phages | The lack of genome editing platforms for diverse intestinal phages; | Developing phage genome editing platforms independent of phage bacterial host; |
| The limited payload capacity of phages; | Developing more compact genetic tools, streamlining the phage genome, or enhancing the payload capacity of phage particles through methods such as using phage packaging helper plasmids can effectively address the limited payload capacity of phages; | |
| The low editing efficiency in human gut; | Optimizing DNA delivery systems, improving genome editing tool design, and testing different types of genome editors are key strategies to address low editing efficiency; | |
| Engineered phages face stricter regulatory scrutiny due to their modified genetic components, raising concerns about horizontal gene transfer or unintended effects; | Conducting thorough preclinical evaluations, including assessing horizontal gene transfer risks and implementing biosafety measures to comply with regulatory standards are essential steps in ensuring the safety and efficacy of engineered phages for clinical applications; |
Low editing efficiency poses a significant challenge. In particular, the delivery efficiency and specificity of the genome editing cargo are pivotal for the success of in situ editing. The reasons for this low efficiency are multifaceted and include the efficiency of DNA delivery, specificity and activity of editing tools, homology-directed repair (HDR) capacity of the cells, non-specific editing, genotype and physiological state of the bacteria, and complexity of the target sequence. Continuous optimization of the editing system, which involves the design of single-guide RNA, donor DNA templates, and method of delivery of the editing components, is essential. The accidental killing of bacteria is another concern that needs to be addressed. The ultimate goal is to successfully edit the target bacterial strain while ensuring its normal functioning within its environment. However, it is important to recognize that the characteristics of the editing tools or the inherent repair mechanisms within bacteria may lead to an unintended loss of the bacterial strain. Furthermore, following technological advancements, it is crucial to consider the potential off-target and long-term effects of editing or modulation of the bacterial genome.
4. Part C targeted delivery of bacteriophages
Oral phage administration, noted for its convenience and potential to enhance patient compliance [133], is a crucial approach for the precise modulation of the intestinal microbiota. However, a clinical trial indicated that while phage therapy was safe for patients, it did not successfully amplify in the intestine or improve diarrheal outcomes [105]. This could be attributed to the lack of protection for the administered phages, which were dissolved in an oral rehydration solution, leading to a significant reduction in phage activity due to the harsh conditions of the digestive tract. The varying physiological characteristics and transit times of the digestive tract across different segments [134,135] further complicate phage delivery. Given these challenges, an effective phage delivery system should aim to: 1) preserve the quantity and activity of the phage preparation, 2) target a specific segment of the intestine, and 3) prolong the interactions between free phages and their bacterial targets (Fig. 3A). Additionally, phages, which are primarily composed of protein capsids and nucleic acids, are susceptible to denaturation and degradation within the digestive tract, hindering their ability to reach and function in the intended intestinal segment. Other factors such as dietary influences and interindividual variability may also affect the efficacy of phage administration [136]. For phage preparations to be commercially viable, it is essential to consider their stability during transportation and storage and manage drug loading and encapsulation efficiency to control production costs. Consequently, investigating the release mechanisms and delivery materials of phage preparations and enhancing their utilization rate will be instrumental in enhancing the effectiveness, safety, and compliance with phage therapies.
Fig. 3.
Delivery of phages to the gastrointestinal environment. (A) Background on phage administration [133]. (B) Modification of phages. (C) Selection of encapsulation materials. (D) Determination of delivery carriers. (E) Temporal modification of the environment.
4.1. Strategies
With the rapid progress in pharmacy, materials science, and biomedicine, drug delivery systems (DDS) have undergone revolutionary changes, providing abundant innovation inspiration for the field of phage administration. Drug delivery strategies involve three key points: 1) modification of the drug itself, 2) optimization based on the surrounding environment in which the drug is located, and 3) construction of a delivery system that controls the interaction between the drug and its microenvironment [137]. Oral phage preparations can align with these aspects.
Modification of phages. Modification of phages can enhance their delivery efficiency and stability, extend their residence time in the host, and alter their interactions with host cells and the immune system, thus improving their therapeutic efficacy. Notably, a serial passage technique was used to identify phage mutants with prolonged circulation in the circulatory system. A mutation in the head protein E resulted in improved bactericidal effects and enhanced the evasion of host immunity [138]. Additionally, a cell-free phage production method allows for rapid surface modification by incorporating a SpyTag into the capsid. This approach was used to attach two alternative fusion proteins to the phage surface, thereby enhancing the endocytic uptake by bladder epithelial cells and increasing phage bioavailability within the host [139]. These cases illustrate the utility of protein capsid engineering for optimizing phage administration and therapeutics (Fig. 3B).
Selection of encapsulation materials. Encapsulation materials must be safe, biocompatible, biodegradable, and non-irritating to humans and animals [140]. Given the varying pH levels and enzymatic profiles along the digestive tract as well as the rapid transit of materials, the choice of encapsulation materials is critical. They must be stable under harsh digestion conditions yet responsive enough to release phages at the right moment. Materials that are pH-sensitive (e.g., sodium alginate, Eudragit S100®) or enzyme-responsive (e.g., chitosan, pectin) can be selected based on the digestive tract's environmental conditions (Fig. 3A) to facilitate effective phage release (Fig. 3C) [141]. Furthermore, encapsulation materials can be used to alter the surface charge of phages, thereby potentially inhibiting the growth of intracellular pathogenic bacteria [142]. For commercial phage preparation, encapsulation materials should also offer good storage stability.
Determination of delivery carriers. Delivery carriers are systems or structures designed to transport pharmaceuticals to specific sites in the body. A range of phage encapsulation carriers has been developed from a variety of structural materials, including fibers, emulsions, liposomes, hydrogels, and particles, each with distinct properties and applications. For instance, an electrospun nanofiber mesh was developed for the controlled release of iron-doped apatite nanoparticles (IDANPs) and phages to effectively treat topical bacterial infections [143]. Research is underway to develop a microencapsulation system using membrane emulsification technology to shield phages from gastric acid and ensure their release into the intestine [144,145]. In scenarios where phage therapy targets intracellular pathogens, a recent study investigated the use of three common phospholipids as liposome-phage nanocomplexes for aerosolizing phage K for intracellular therapy. The findings indicate that egg phosphatidylcholine (EPC) liposomes enhance the efficacy of phage K in controlling tissue infection in a 3D spheroid model compared to other methods [146]. Fibers and hydrogels provide physical protection and minimize phage inactivation during storage and transport. Emulsions and liposomes provide an internal environment for phage survival. Particle-based carriers facilitate storage and transportation, and can be formulated into tablets for ease of administration (Fig. 3D).
Temporal modification of the environment (Fig. 3E). Researchers have employed methods to adjust the gastric pH of the host before phage administration to address the challenges of acidic pH and enzymatic activity in the gastrointestinal tract. This approach ensures the survival of orally administered phages and has been widely applied in both murine studies [25] and human clinical trials [22]. Common pH adjusters include sodium bicarbonate and medications, such as ranitidine and omeprazole. Międzybrodzki et al. showed that antacids and buffering agents can effectively neutralize gastric acidity, thus protecting therapeutic phages from degradation. Their research demonstrated that a temporary alteration of the stomach environment can shield the Twort-like lytic anti-staphylococcal phage A5/80 from gastric inactivation, allowing a significant portion of phages to reach the intestine after oral administration [147].
In clinical practice, it may be advantageous to leverage the strengths of multiple strategies, in conjunction with the intrinsic properties of phages, to optimize delivery. For example, to mitigate phage degradation in the gastrointestinal tract, Hsu et al. delivered phages by diluting them tenfold in sodium bicarbonate and encapsulating them within alginate microspheres. Then, they applied layer-by-layer assembly technology to coat them with polyethylene imines and pectin. The study showed that both buffered and encapsulated phages provided similar levels of red fluorescent protein repression for the duration of the study [25].
5. Conclusion and prospects
In this review, we explored a multi-dimensional approach to the precision engineering of the gut microbiome through phage-based interventions, encompassing ecological, genetic, and delivery strategies. Although the application of phages as tools for the precise manipulation of the gut microbiome to enhance human health is still emerging, there has been a notable acceleration in the number of clinical trials involving phage therapies. The field is witnessing significant advancements in precision engineering technologies at the strain and gene levels, which not only enhance our capacity to modulate the gut microbiome and its genome in situ but also demonstrate substantial potential to address the rising challenges of antibiotic resistance and NCDs effectively.
To advance further in this domain, it is essential to deepen our understanding of the following key areas: 1) diversity of phages, 2) phage-bacteria interaction networks, 3) phage interactions with eukaryotic cells and the immune system, and 4) interplay of phages with multiple metabolic pathways within the gastrointestinal tract.
Additionally, continuous innovation in associated technological advancements is vital, including: 1) the development of phage culture omics, 2) the characterization, design, standardization, and generalization of synthetic biology genetic elements, and 3) the creation of mathematical models for digestive tract infections and the manipulation of intestinal bacteria. By concentrating on these areas and fostering technological advancements, we can catalyze significant progress in the field and realize full therapeutic potentials.
CRediT authorship contribution statement
Xiaoxian Kuang: Writing – review & editing, Writing – original draft, Visualization. Juntao Shen: Writing – original draft, Funding acquisition, Conceptualization. Linggang Zheng: Writing – review & editing, Writing – original draft. Yi Duan: Writing – review & editing. Yingfei Ma: Writing – review & editing. Elaine Lai-Han Leung: Writing – review & editing. Lei Dai: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the Guangdong Basic and Applied Basic Research Foundation (2024A1515011872) and the National Key R&D Program of China (2019YFA0906700). All illustrations in this work were created using the BioRender platform (©BioRender: biorender.com), which we extend our appreciation for its user-friendly design tools.
Footnotes
Abbreviations: AAVs: adeno-associated viruses; AIEC: adherent-invasive Escherichia coli; BFT: Bacteroides fragilis toxin; CBE: cytosine base editor; CDI: Clostridium difficile infection; CRC: colorectal cancer; DDS: drug delivery system; DSBs: double-strand breaks; EHEC: enterohemorrhagic Escherichia coli; EMA: European Medicines Agency; EPC: egg phosphatidylcholine; ETBF: enterotoxigenic Bacteroides fragilis; FDA: Food and Drug Administration; FMT: fecal microbiota transplant; FVT: fecal virus transplantation; HDR: homology-directed repair; IBD: inflammatory bowel disease; IDANPs: iron-doped apatite nanoparticles; IND: investigational new drug; LPS: lipopolysaccharides; MOI: Multiplicity of infection; NAFLD: nonalcoholic fatty liver disease; NCDs: noncommunicable diseases; NTBF: non-toxigenic Bacteroides fragilis; PSC: primary sclerosing cholangitis; STEC: Shiga toxin-producing Escherichia coli
References
- 1.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The Human Microbiome Project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu X., Li B., Lou P., Dai T., Chen Y., Zhuge A., Yuan Y., Li L. The Relationship Between the Gut Microbiome and Neurodegenerative Diseases. Neurosci. Bull. 2021;37:1510–1522. doi: 10.1007/s12264-021-00730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J., Wang K., Wang X., Pang Y., Jiang C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. 2021;12:360–373. doi: 10.1007/s13238-020-00814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazemian N., Mahmoudi M., Halperin F., Wu J.C., Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8:36. doi: 10.1186/s40168-020-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu K., Cheung A.H.K., Wong C.C., Liu W., Zhou Y., Wang F., Huang P., Yuan K., Coker O.O., Pan Y., Chen D., Lam N.M., Gao M., Zhang X., Huang H., To K.F., Sung J.J.Y., Yu J. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell. 2024;187:882–896.e17. doi: 10.1016/j.cell.2024.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Xie R., Gu Y., Li M., Li L., Yang Y., Sun Y., Zhou B., Liu T., Wang S., Liu W., Yang R., Su X., Zhong W., Wang B., Cao H. Desulfovibrio vulgaris interacts with novel gut epithelial immune receptor LRRC19 and exacerbates colitis. Microbiome. 2024;12:4. doi: 10.1186/s40168-023-01722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui W., Guo M., Liu D., Xiao P., Yang C., Huang H., Liang C., Yang Y., Fu X., Zhang Y., Liu J., Shi S., Cong J., Han Z., Xu Y., Du L., Yin C., Zhang Y., Sun J., Gu W., Chai R., Zhu S., Chu B. Gut microbial metabolite facilitates colorectal cancer development via ferroptosis inhibition. Nat. Cell Biol. 2024 doi: 10.1038/s41556-023-01314-6. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y., Oh J., Xue M., Huh W.J., Wang J., Gonzalez-Hernandez J.A., Rice T.A., Martin A.L., Song D., Crawford J.M., Herzon S.B., Palm N.W. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science. 2022;378:eabm3233. doi: 10.1126/science.abm3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An Y., Zhai Z., Wang X., Ding Y., He L., Li L., Mo Q., Mu C., Xie R., Liu T., Zhong W., Wang B., Cao H. Targeting Desulfovibrio vulgaris flagellin-induced NAIP/NLRC4 inflammasome activation in macrophages attenuates ulcerative colitis. J. Adv. Res. 2023;52:219–232. doi: 10.1016/j.jare.2023.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor J.C., Gao X., Xu J., Holder M., Petrosino J., Kumar R., Liu W., Höök M., Mackenzie C., Hillhouse A., Brashear W., Nunez M.P., Xu Y. A type VII secretion system of Streptococcus gallolyticus subsp. gallolyticus contributes to gut colonization and the development of colon tumors. PLOS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dronkers T.M.G., Ouwehand A.C., Rijkers G.T. Global analysis of clinical trials with probiotics. Heliyon. 2020;6:e04467. doi: 10.1016/j.heliyon.2020.e04467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panchal P., Budree S., Scheeler A., Medina G., Seng M., Wong W.F., Eliott R., Mitchell T., Kassam Z., Allegretti J.R., Osman M. Scaling Safe Access to Fecal Microbiota Transplantation: Past, Present, and Future. Curr. Gastroenterol. Rep. 2018;20:14. doi: 10.1007/s11894-018-0619-8. [DOI] [PubMed] [Google Scholar]
- 13.Suez J., Zmora N., Zilberman-Schapira G., Mor U., Dori-Bachash M., Bashiardes S., Zur M., Regev-Lehavi D., Ben-Zeev Brik R., Federici S., Horn M., Cohen Y., Moor A.E., Zeevi D., Korem T., Kotler E., Harmelin A., Itzkovitz S., Maharshak N., Shibolet O., Pevsner-Fischer M., Shapiro H., Sharon I., Halpern Z., Segal E., Elinav E. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell. 2018;174:1406–1423.e16. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y., Wang W., Zhang F. The Next Generation Fecal Microbiota Transplantation: To Transplant Bacteria or Virome. Adv. Sci. 2023;10 doi: 10.1002/advs.202301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo T., Wong S.H., Lam K., Lui R., Cheung K., Tang W., Ching J.Y.L., Chan P.K.S., Chan M.C.W., Wu J.C.Y., Chan F.K.L., Yu J., Sung J.J.Y., Ng S.C. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut. 2017 doi: 10.1136/gutjnl-2017-313952. gutjnl-2017-313952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Megur A., Daliri E.B.M., Baltriukienė D., Burokas A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022;23:6097. doi: 10.3390/ijms23116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brödel A.K., Charpenay L.H., Galtier M., Fuche F.J., Terrasse R., Poquet C., Havránek J., Pignotti S., Krawczyk A., Arraou M., Prevot G., Spadoni D., Yarnall M.T.N., Hessel E.M., Fernandez-Rodriguez J., Duportet X., Bikard D. situ targeted base editing of bacteria in the mouse gut. Nature. 2024 doi: 10.1038/s41586-024-07681-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruttin A., Brüssow H. Human Volunteers Receiving Escherichia coli Phage T4 Orally: a Safety Test of Phage Therapy. Antimicrob. Agents Chemother. 2005;49:2874–2878. doi: 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chain E., Florey H.W., Gardner A.D., Heatley N.G., Jennings M.A., Orr-Ewing J., Sanders A.G. Penicillin as a chemotherapeutic agent. The Lancet. 1940;236:226–228. [Google Scholar]
- 20.Citorik R.J., Mimee M., Lu T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014;32:1141–1145. doi: 10.1038/nbt.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan Y., Llorente C., Lang S., Brandl K., Chu H., Jiang L., White R.C., Clarke T.H., Nguyen K., Torralba M., Shao Y., Liu J., Hernandez-Morales A., Lessor L., Rahman I.R., Miyamoto Y., Ly M., Gao B., Sun W., Kiesel R., Hutmacher F., Lee S., Ventura-Cots M., Bosques-Padilla F., Verna E.C., Abraldes J.G., Brown R.S., Vargas V., Altamirano J., Caballería J., Shawcross D.L., Ho S.B., Louvet A., Lucey M.R., Mathurin P., Garcia-Tsao G., Bataller R., Tu X.M., Eckmann L., Van Der Donk W.A., Young R., Lawley T.D., Stärkel P., Pride D., Fouts D.E., Schnabl B. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federici S., Kredo-Russo S., Valdés-Mas R., Kviatcovsky D., Weinstock E., Matiuhin Y., Silberberg Y., Atarashi K., Furuichi M., Oka A., Liu B., Fibelman M., Weiner I.N., Khabra E., Cullin N., Ben-Yishai N., Inbar D., Ben-David H., Nicenboim J., Kowalsman N., Lieb W., Kario E., Cohen T., Geffen Y.F., Zelcbuch L., Cohen A., Rappo U., Gahali-Sass I., Golembo M., Lev V., Dori-Bachash M., Shapiro H., Moresi C., Cuevas-Sierra A., Mohapatra G., Kern L., Zheng D., Nobs S.P., Suez J., Stettner N., Harmelin A., Zak N., Puttagunta S., Bassan M., Honda K., Sokol H., Bang C., Franke A., Schramm C., Maharshak N., Sartor R.B., Sorek R., Elinav E. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185:2879–2898.e24. doi: 10.1016/j.cell.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Gencay Y.E., Jasinskytė D., Robert C., Semsey S., Martínez V., Petersen A.Ø., Brunner K., De Santiago Torio A., Salazar A., Turcu I.C., Eriksen M.K., Koval L., Takos A., Pascal R., Schou T.S., Bayer L., Bryde T., Johansen K.C., Bak E.G., Smrekar F., Doyle T.B., Satlin M.J., Gram A., Carvalho J., Jessen L., Hallström B., Hink J., Damholt B., Troy A., Grove M., Clube J., Grøndahl C., Haaber J.K., Van Der Helm E., Zdravkovic M., Sommer M.O.A. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024;42:265–274. doi: 10.1038/s41587-023-01759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Herelle F. Sur un microbe invisible antagoniste des bacilles dysentériques. C. r. Académie Sci. 1917;165:373–375. [Google Scholar]
- 25.Hsu B.B., Plant I.N., Lyon L., Anastassacos F.M., Way J.C., Silver P.A. In situ reprogramming of gut bacteria by oral delivery. Nat. Commun. 2020;11:5030. doi: 10.1038/s41467-020-18614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.KRUEGER A.P., SCRIBNER E.J. THE BACTERIOPHAGE: ITS NATURE AND ITS THERAPEUTIC USE. J. Am. Med. Assoc. 1941;116:2269–2277. doi: 10.1001/jama.1941.62820200013011. [DOI] [Google Scholar]
- 27.McKINLEY E.B. THE BACTERIOPHAGE IN THE TREATMENT OF INFECTIONS. Arch. Intern. Med. 1923;32:899–910. doi: 10.1001/archinte.1923.00110240092005. [DOI] [Google Scholar]
- 28.Monsur K.A., Rahman M.A., Huq F., Islam M.N., Northrup R.S., Hirschhorn N. Effect of massive doses of bacteriophage on excretion of vibrios, duration of diarrhoea and output of stools in acute cases of cholera. Bull. World Health Organ. 1970;42:723–732. [PMC free article] [PubMed] [Google Scholar]
- 29.Smith H.W., Huggins M.B. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. Microbiology. 1982;128:307–318. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- 30.Twort F.W. AN INVESTIGATION ON THE NATURE OF ULTRA-MICROSCOPIC VIRUSES. Orig. Publ. 1915;2(4814):1241–1243. doi: 10.1016/S0140-6736(01)20383-3. Issue186. [DOI] [Google Scholar]
- 31.Zhou S.X., Wang L.P., Liu M.Y., Zhang H.Y., Lu Q.B., Shi L.S., Ren X., Wang Y.F., Lin S.H., Zhang C.H., Geng M.J., Zhang X.A., Zhu Y.L., Li Z.J., Fang L.Q., Liu W., Yang W.Z. Characteristics of diarrheagenic Escherichia coli among patients with acute diarrhea in China, 2009‒2018. J. Infect. 2021;83:424–432. doi: 10.1016/j.jinf.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Czepiel J., Dróżdż M., Pituch H., Kuijper E.J., Perucki W., Mielimonka A., Goldman S., Wultańska D., Garlicki A., Biesiada G. Clostridium difficile infection: review. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang N., Fang J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023;31:159–172. doi: 10.1016/j.tim.2022.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Kaper J.B., Nataro J.P., Mobley H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 35.Kusters J.G., Van Vliet A.H.M., Kuipers E.J. Pathogenesis of Helicobacter pylori Infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popa G.L., Popa M.I. Salmonella spp. infection – a continuous threat worldwide. GERMS. 2021;11:88–96. doi: 10.18683/germs.2021.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schooley R.T., Biswas B., Gill J.J., Hernandez-Morales A., Lancaster J., Lessor L., Barr J.J., Reed S.L., Rohwer F., Benler S., Segall A.M., Taplitz R., Smith D.M., Kerr K., Kumaraswamy M., Nizet V., Lin L., McCauley M.D., Strathdee S.A., Benson C.A., Pope R.K., Leroux B.M., Picel A.C., Mateczun A.J., Cilwa K.E., Regeimbal J.M., Estrella L.A., Wolfe D.M., Henry M.S., Quinones J., Salka S., Bishop-Lilly K.A., Young R., Hamilton T. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017;61:e00954. doi: 10.1128/AAC.00954-17. -17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jennes S., Merabishvili M., Soentjens P., Pang K.W., Rose T., Keersebilck E., Soete O., François P.M., Teodorescu S., Verween G., Verbeken G., De Vos D., Pirnay J.P. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—a case report. Crit. Care. 2017;21:129. doi: 10.1186/s13054-017-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.J.A. Nick, R.M. Dedrick, A.L. Gray, E.K. Vladar, B.E. Smith, K.G. Freeman, K.C. Malcolm, L.E. Epperson, N.A. Hasan, J. Hendrix, K. Callahan, K. Walton, B. Vestal, E. Wheeler, N.M. Rysavy, K. Poch, S. Caceres, V.K. Lovell, K.B. Hisert, V.C. De Moura, D. Chatterjee, P. De, N. Weakly, S.L. Martiniano, D.A. Lynch, C.L. Daley, M. Strong, F. Jia, G.F. Hatfull, R.M. Davidson, Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection, Cell 185 (2022) 1860–1874.e12. 10.1016/j.cell.2022.04.024. [DOI] [PMC free article] [PubMed]
- 40.Dedrick R.M., Guerrero-Bustamante C.A., Garlena R.A., Russell D.A., Ford K., Harris K., Gilmour K.C., Soothill J., Jacobs-Sera D., Schooley R.T., Hatfull G.F., Spencer H. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019;25:730–733. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow J., Mazmanian S.K. A Pathobiont of the Microbiota Balances Host Colonization and Intestinal Inflammation. Cell Host Microbe. 2010;7:265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osbelt L., Wende M., Almási É., Derksen E., Muthukumarasamy U., Lesker T.R., Galvez E.J.C., Pils M.C., Schalk E., Chhatwal P., Färber J., Neumann-Schaal M., Fischer T., Schlüter D., Strowig T. Klebsiella oxytoca causes colonization resistance against multidrug-resistant K. pneumoniae in the gut via cooperative carbohydrate competition. Cell Host Microbe. 2021;29:1663–1679.e7. doi: 10.1016/j.chom.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Pötgens S.A., Brossel H., Sboarina M., Catry E., Cani P.D., Neyrinck A.M., Delzenne N.M., Bindels L.B. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci. Rep. 2018;8:12321. doi: 10.1038/s41598-018-30569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finlay B.B. CIFAR Humans, the Microbiome, Are noncommunicable diseases communicable? Science. 2020;367:250–251. doi: 10.1126/science.aaz3834. [DOI] [PubMed] [Google Scholar]
- 45.Kviatcovsky D., Valdés-Mas R., Federici S., Elinav E. Phage therapy in noncommunicable diseases. Science. 2023;382:266–267. doi: 10.1126/science.adh2718. [DOI] [PubMed] [Google Scholar]
- 46.Ramakrishna C., Kujawski M., Chu H., Li L., Mazmanian S.K., Cantin E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019;10:2153. doi: 10.1038/s41467-019-09884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valguarnera E., Wardenburg J.B. Good Gone Bad: One Toxin Away From Disease for Bacteroides fragilis. J. Mol. Biol. 2020;432:765–785. doi: 10.1016/j.jmb.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Sun L., Zhang Y., Cai J., Rimal B., Rocha E.R., Coleman J.P., Zhang C., Nichols R.G., Luo Y., Kim B., Chen Y., Krausz K.W., Harris C.C., Patterson A.D., Zhang Z., Takahashi S., Gonzalez F.J. Bile salt hydrolase in non-enterotoxigenic Bacteroides potentiates colorectal cancer. Nat. Commun. 2023;14:755. doi: 10.1038/s41467-023-36089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J., Li Y., Tang N., Li J., Zhou J., Lu S., Zhang G., Song Y., Wang C., Zhong J., Xu J., Feng J. The human gut serves as a reservoir of hypervirulent Klebsiella pneumoniae. Gut Microbes. 2022;14 doi: 10.1080/19490976.2022.2114739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamoto N., Sasaki N., Aoki R., Miyamoto K., Suda W., Teratani T., Suzuki T., Koda Y., Chu P.S., Taniki N., Yamaguchi A., Kanamori M., Kamada N., Hattori M., Ashida H., Sakamoto M., Atarashi K., Narushima S., Yoshimura A., Honda K., Sato T., Kanai T. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol. 2019;4:492–503. doi: 10.1038/s41564-018-0333-1. [DOI] [PubMed] [Google Scholar]
- 51.Yuan J., Chen C., Cui J., Lu J., Yan C., Wei X., Zhao X., Li N., Li S., Xue G., Cheng W., Li B., Li H., Lin W., Tian C., Zhao J., Han J., An D., Zhang Q., Wei H., Zheng M., Ma X., Li W., Chen X., Zhang Z., Zeng H., Ying S., Wu J., Yang R., Liu D. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019;30:675–688.e7. doi: 10.1016/j.cmet.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Jochum L., Stecher B. Label or Concept – What Is a Pathobiont? Trends Microbiol. 2020;28:789–792. doi: 10.1016/j.tim.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Chan B.K., Abedon S.T., Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013;8:769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- 54.Ye J., Meng Q., Jin K., Luo Y., Yue T. Phage cocktail alleviated type 2 diabetes by reshaping gut microbiota and decreasing proinflammatory cytokines. Appl. Microbiol. Biotechnol. 2024;108:9. doi: 10.1007/s00253-023-12912-7. [DOI] [PubMed] [Google Scholar]
- 55.Buttimer C., Sutton T., Colom J., Murray E., Bettio P.H., Smith L., Bolocan A.S., Shkoporov A., Oka A., Liu B., Herzog J.W., Sartor R.B., Draper L.A., Ross R.P., Hill C. Impact of a phage cocktail targeting Escherichia coli and Enterococcus faecalis as members of a gut bacterial consortium in vitro and in vivo. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.936083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Callaway T.R., Edrington T.S., Brabban A.D., Anderson R.C., Rossman M.L., Engler M.J., Carr M.A., Genovese K.J., Keen J.E., Looper M.L., Kutter E.M., Nisbet D.J. Bacteriophage Isolated from Feedlot Cattle Can Reduce Escherichia coli O157:H7 Populations in Ruminant Gastrointestinal Tracts. Foodborne Pathog. Dis. 2008;5:183–191. doi: 10.1089/fpd.2007.0057. [DOI] [PubMed] [Google Scholar]
- 57.Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mao X., Wu Y., Ma R., Li L., Wang L., Tan Y., Li Z., Liu H., Han K., Cao Y., Li Y., Peng H., Li X., Hu C., Wang X. Oral phage therapy with microencapsulated phage A221 against Escherichia coli infections in weaned piglets. BMC Vet. Res. 2023;19:165. doi: 10.1186/s12917-023-03724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamy-Besnier Q., Chaffringeon L., Lourenço M., Payne R.B., Trinh J.T., Schwartz J.A., Sulakvelidze A., Debarbieux L. Prophylactic Administration of a Bacteriophage Cocktail Is Safe and Effective in Reducing Salmonella enterica Serovar Typhimurium Burden in Vivo. Microbiol. Spectr. 2021;9:e00497. doi: 10.1128/Spectrum.00497-21. -21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thanki A.M., Mignard G., Atterbury R.J., Barrow P., Millard A.D., Clokie M.R.J. Prophylactic Delivery of a Bacteriophage Cocktail in Feed Significantly Reduces Salmonella Colonization in Pigs. Microbiol. Spectr. 2022;10:e00422. doi: 10.1128/spectrum.00422-22. -22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson R.P., Gyles C.L., Huff W.E., Ojha S., Huff G.R., Rath N.C., Donoghue A.M. Bacteriophages for prophylaxis and therapy in cattle, poultry and pigs. Anim. Health Res. Rev. 2008;9:201–215. doi: 10.1017/S1466252308001576. [DOI] [PubMed] [Google Scholar]
- 62.Donati V.L., Madsen L., Middelboe M., Strube M.L., Dalsgaard I. The Gut Microbiota of Healthy and Flavobacterium psychrophilum-Infected Rainbow Trout Fry Is Shaped by Antibiotics and Phage Therapies. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.771296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahasan Md.S., Kinobe R., Elliott L., Owens L., Scott J., Picard J., Huerlimann R., Ariel E. Bacteriophage versus antibiotic therapy on gut bacterial communities of juvenile green turtle, Chelonia mydas. Environ. Microbiol. 2019;21:2871–2885. doi: 10.1111/1462-2920.14644. [DOI] [PubMed] [Google Scholar]
- 64.Choi Y., Lee W., Kwon J.G., Kang A., Kwak M.J., Eor J.Y., Kim Y. The current state of phage therapy in livestock and companion animals. J. Anim. Sci. Technol. 2024;66:57–78. doi: 10.5187/jast.2024.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.European Medicines Agency, Guideline on quality, safety and efficacy of veterinary medicinal products specifically designed for phage therapy, (2023). https://www.ema.europa.eu/en/quality-safety-and-efficacy-bacteriophages-veterinary-medicines-scientific-guideline.
- 66.Tartera C., Lucena F., Jofre J. Human origin of Bacteroides fragilis bacteriophages present in the environment. Appl. Environ. Microbiol. 1989;55:2696–2701. doi: 10.1128/aem.55.10.2696-2701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kai M., Watanabe S., Furuse K., Ozawa A. Bacteroides Bacteriophages Isolated from Human Feces. Microbiol. Immunol. 1985;29:895–899. doi: 10.1111/j.1348-0421.1985.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 68.Hryckowian A.J., Merrill B.D., Porter N.T., Van Treuren W., Nelson E.J., Garlena R.A., Russell D.A., Martens E.C., Sonnenburg J.L. Bacteroides thetaiotaomicron-Infecting Bacteriophage Isolates Inform Sequence-Based Host Range Predictions. Cell Host Microbe. 2020;28:371–379.e5. doi: 10.1016/j.chom.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen J., Zhang J., Mo L., Li Y., Li Y., Li C., Kuang X., Tao Z., Qu Z., Wu L., Chen J., Liu S., Zeng L., He Z., Chen Z., Deng Y., Zhang T., Li B., Dai L., Ma Y. Large-scale phage cultivation for commensal human gut bacteria. Cell Host Microbe. 2023;31:665–677.e7. doi: 10.1016/j.chom.2023.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Gregory A.C., Zablocki O., Zayed A.A., Howell A., Bolduc B., Sullivan M.B. The Gut Virome Database Reveals Age-Dependent Patterns of Virome Diversity in the Human Gut. Cell Host Microbe. 2020;28:724–740.e8. doi: 10.1016/j.chom.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nethery M.A., Hidalgo-Cantabrana C., Roberts A., Barrangou R. CRISPR-based engineering of phages for in situ bacterial base editing. Proc. Natl. Acad. Sci. 2022;119 doi: 10.1073/pnas.2206744119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pfeifer E., Bonnin R.A., Rocha E.P.C. Phage-Plasmids Spread Antibiotic Resistance Genes through Infection and Lysogenic Conversion. mBio. 2022;13:e01851. doi: 10.1128/mbio.01851-22. -22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selle K., Fletcher J.R., Tuson H., Schmitt D.S., McMillan L., Vridhambal G.S., Rivera A.J., Montgomery S.A., Fortier L.C., Barrangou R., Theriot C.M., Ousterout D.G. Vivo Targeting of Clostridioides difficile Using Phage-Delivered CRISPR-Cas3 Antimicrobials. mBio. 2020;11 doi: 10.1128/mBio.00019-20. e00019-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nobrega F.L., Vlot M., De Jonge P.A., Dreesens L.L., Beaumont H.J.E., Lavigne R., Dutilh B.E., Brouns S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018;16:760–773. doi: 10.1038/s41579-018-0070-8. [DOI] [PubMed] [Google Scholar]
- 75.Rostøl J.T., Marraffini L. (Ph)ighting Phages: How Bacteria Resist Their Parasites. Cell Host Microbe. 2019;25:184–194. doi: 10.1016/j.chom.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Madi N., Cato E.T., Sayeed Md.Abu, Creasy-Marrazzo A., Cuénod A., Islam K., Khabir Md.I.U., Bhuiyan Md.T.R., Begum Y.A., Freeman E., Vustepalli A., Brinkley L., Kamat M., Bailey L.S., Basso K.B., Qadri F., Khan A.I., Shapiro B.J., Nelson E.J. Phage predation, disease severity, and pathogen genetic diversity in cholera patients. Science. 2024;384:eadj3166. doi: 10.1126/science.adj3166. [DOI] [PubMed] [Google Scholar]
- 77.Jin M., Chen J., Zhao X., Hu G., Wang H., Liu Z., Chen W.H. An Engineered λ Phage Enables Enhanced and Strain-Specific Killing of Enterohemorrhagic Escherichia coli. Microbiol. Spectr. 2022;10:e01271. doi: 10.1128/spectrum.01271-22. -22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doron S., Melamed S., Ofir G., Leavitt A., Lopatina A., Keren M., Amitai G., Sorek R. Systematic discovery of antiphage defense systems in the microbial pangenome. Science. 2018;359:eaar4120. doi: 10.1126/science.aar4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao L., Altae-Tran H., Böhning F., Makarova K.S., Segel M., Schmid-Burgk J.L., Koob J., Wolf Y.I., Koonin E.V., Zhang F. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science. 2020;369:1077–1084. doi: 10.1126/science.aba0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Millman A., Melamed S., Leavitt A., Doron S., Bernheim A., Hör J., Garb J., Bechon N., Brandis A., Lopatina A., Ofir G., Hochhauser D., Stokar-Avihail A., Tal N., Sharir S., Voichek M., Erez Z., Ferrer J.L.M., Dar D., Kacen A., Amitai G., Sorek R. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe. 2022;30:1556–1569.e5. doi: 10.1016/j.chom.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 81.Landsberger M., Gandon S., Meaden S., Rollie C., Chevallereau A., Chabas H., Buckling A., Westra E.R., Van Houte S. Anti-CRISPR Phages Cooperate to Overcome CRISPR-Cas Immunity. Cell. 2018;174:908–916.e12. doi: 10.1016/j.cell.2018.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lourenço M., Chaffringeon L., Lamy-Besnier Q., Pédron T., Campagne P., Eberl C., Bérard M., Stecher B., Debarbieux L., De Sordi L. The Spatial Heterogeneity of the Gut Limits Predation and Fosters Coexistence of Bacteria and Bacteriophages. Cell Host Microbe. 2020;28:390–401.e5. doi: 10.1016/j.chom.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Zou X., Xiao X., Mo Z., Ge Y., Jiang X., Huang R., Li M., Deng Z., Chen S., Wang L., Lee S.Y. Systematic strategies for developing phage resistant Escherichia coli strains. Nat. Commun. 2022;13:4491. doi: 10.1038/s41467-022-31934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meile S., Du J., Dunne M., Kilcher S., Loessner M.J. Engineering therapeutic phages for enhanced antibacterial efficacy. Curr. Opin. Virol. 2022;52:182–191. doi: 10.1016/j.coviro.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Lu T.K., Collins J.J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. 2007;104:11197–11202. doi: 10.1073/pnas.0704624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du J., Meile S., Baggenstos J., Jäggi T., Piffaretti P., Hunold L., Matter C.I., Leitner L., Kessler T.M., Loessner M.J., Kilcher S., Dunne M. Enhancing bacteriophage therapeutics through in situ production and release of heterologous antimicrobial effectors. Nat. Commun. 2023;14:4337. doi: 10.1038/s41467-023-39612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shaufi M.A.M., Sieo C.C., Chong C.W., Geok Hun T., Omar A.R., Han Ming G., Ho Y.Wan. Effects of Phage Cocktail, Probiotics, and Their Combination on Growth Performance and Gut Microbiota of Broiler Chickens. Animals. 2023;13:1328. doi: 10.3390/ani13081328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Comeau A.M., Tétart F., Trojet S.N., Prère M.F., Krisch H.M. Phage-Antibiotic Synergy (PAS): β-Lactam and Quinolone Antibiotics Stimulate Virulent Phage Growth. PLoS ONE. 2007;2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gordillo Altamirano F., Forsyth J.H., Patwa R., Kostoulias X., Trim M., Subedi D., Archer S.K., Morris F.C., Oliveira C., Kielty L., Korneev D., O'Bryan M.K., Lithgow T.J., Peleg A.Y., Barr J.J. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat. Microbiol. 2021;6:157–161. doi: 10.1038/s41564-020-00830-7. [DOI] [PubMed] [Google Scholar]
- 90.Zheng D.W., Dong X., Pan P., Chen K.W., Fan J.X., Cheng S.X., Zhang X.Z. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 2019;3:717–728. doi: 10.1038/s41551-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 91.Laforêt F., Antoine C., Lebrun S., Gonza I., Goya-Jorge E., Douny C., Duprez J.N., Scippo M.L., Taminiau B., Daube G., Fall A., Thiry D., Delcenserie V. Impact Assessment of vB_KpnP_K1-ULIP33 Bacteriophage on the Human Gut Microbiota Using a Dynamic In Vitro Model. Viruses. 2023;15:719. doi: 10.3390/v15030719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gan L., Feng Y., Du B., Fu H., Tian Z., Xue G., Yan C., Cui X., Zhang R., Cui J., Zhao H., Feng J., Xu Z., Fan Z., Fu T., Du S., Liu S., Zhang Q., Yu Z., Sun Y., Yuan J. Bacteriophage targeting microbiota alleviates non-alcoholic fatty liver disease induced by high alcohol-producing Klebsiella pneumoniae. Nat. Commun. 2023;14:3215. doi: 10.1038/s41467-023-39028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pinto G., Shetty S.A., Zoetendal E.G., Gonçalves R.F.S., Pinheiro A.C., Almeida C., Azeredo J., Smidt H. An in vitro fermentation model to study the impact of bacteriophages targeting Shiga toxin-encoding Escherichia coli on the colonic microbiota. Npj Biofilms Microbiomes. 2022;8:74. doi: 10.1038/s41522-022-00334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ping L., Zhuoya L., Pei J., Jingchao C., Yi L., Guosheng L., Hailei W. Editing of a Specific Strain of Escherichia coli in the Mouse Gut Using Native Phages. Microbiol. Spectr. 2022;10:e01804–e01822. doi: 10.1128/spectrum.01804-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kosznik-Kwaśnicka K., Podlacha M., Grabowski Ł., Stasiłojć M., Nowak-Zaleska A., Ciemińska K., Cyske Z., Dydecka A., Gaffke L., Mantej J., Myślińska D., Necel A., Pierzynowska K., Piotrowska E., Radzanowska-Alenowicz E., Rintz E., Sitko K., Topka-Bielecka G., Węgrzyn G., Węgrzyn A. Biological aspects of phage therapy versus antibiotics against Salmonella enterica serovar Typhimurium infection of chickens. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.941867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jakobsen R.R., Trinh J.T., Bomholtz L., Brok-Lauridsen S.K., Sulakvelidze A., Nielsen D.S. A Bacteriophage Cocktail Significantly Reduces Listeria monocytogenes without Deleterious Impact on the Commensal Gut Microbiota under Simulated Gastrointestinal Conditions. Viruses. 2022;14:190. doi: 10.3390/v14020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang J., Liang L., Cui K., Li P., Hao G., Sun S. Salmonella phage CKT1 significantly relieves the body weight loss of chicks by normalizing the abnormal intestinal microbiome caused by hypervirulent Salmonella Pullorum. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clavijo V., Morales T., Vives-Flores M.J., Muñoz A.Reyes. The gut microbiota of chickens in a commercial farm treated with a Salmonella phage cocktail. Sci. Rep. 2022;12:991. doi: 10.1038/s41598-021-04679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corbellino M., Kieffer N., Kutateladze M., Balarjishvili N., Leshkasheli L., Askilashvili L., Tsertsvadze G., Rimoldi S.G., Nizharadze D., Hoyle N., Nadareishvili L., Antinori S., Pagani C., Scorza D.G., Romanò A.L.L., Ardizzone S., Danelli P., Gismondo M.R., Galli M., Nordmann P., Poirel L. Eradication of a Multidrug-Resistant, Carbapenemase-Producing Klebsiella pneumoniae Isolate Following Oral and Intra-rectal Therapy With a Custom Made, Lytic Bacteriophage Preparation. Clin. Infect. Dis. 2020;70:1998–2001. doi: 10.1093/cid/ciz782. [DOI] [PubMed] [Google Scholar]
- 100.Richards P.J., Connerton P.L., Connerton I.F. Phage Biocontrol of Campylobacter jejuni in Chickens Does Not Produce Collateral Effects on the Gut Microbiota. Front. Microbiol. 2019;10:476. doi: 10.3389/fmicb.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moye Z.D., Woolston J., Abbeele P.V.D., Duysburgh C., Verstrepen L., Das C.R., Marzorati M., Sulakvelidze A. A Bacteriophage Cocktail Eliminates Salmonella Typhimurium from the Human Colonic Microbiome while Preserving Cytokine Signaling and Preventing Attachment to and Invasion of Human Cells by Salmonella In Vitro. J. Food Prot. 2019;82:1336–1349. doi: 10.4315/0362-028X.JFP-18-587. [DOI] [PubMed] [Google Scholar]
- 102.Dissanayake U., Ukhanova M., Moye Z.D., Sulakvelidze A., Mai V. Bacteriophages Reduce Pathogenic Escherichia coli Counts in Mice Without Distorting Gut Microbiota. Front. Microbiol. 2019;10:1984. doi: 10.3389/fmicb.2019.01984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bao H., Pang M., Olaniran A., Zhang X., Zhang H., Zhou Y., Sun L., Schmidt S., Wang R. Alterations in the diversity and composition of mice gut microbiota by lytic or temperate gut phage treatment. Appl. Microbiol. Biotechnol. 2018;102:10219–10230. doi: 10.1007/s00253-018-9378-6. [DOI] [PubMed] [Google Scholar]
- 104.Sarker S.A., Berger B., Deng Y., Kieser S., Foata F., Moine D., Descombes P., Sultana S., Huq S., Bardhan P.K., Vuillet V., Praplan F., Brüssow H. Oral application of E scherichia coli bacteriophage: safety tests in healthy and diarrheal children from B angladesh. Environ. Microbiol. 2017;19:237–250. doi: 10.1111/1462-2920.13574. [DOI] [PubMed] [Google Scholar]
- 105.Sarker S.A., Sultana S., Reuteler G., Moine D., Descombes P., Charton F., Bourdin G., McCallin S., Ngom-Bru C., Neville T., Akter M., Huq S., Qadri F., Talukdar K., Kassam M., Delley M., Loiseau C., Deng Y., El Aidy S., Berger B., Brüssow H. Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. EBioMedicine. 2016;4:124–137. doi: 10.1016/j.ebiom.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nale J.Y., Spencer J., Hargreaves K.R., Buckley A.M., Trzepiński P., Douce G.R., Clokie M.R.J. Bacteriophage Combinations Significantly Reduce Clostridium difficile Growth In Vitro and Proliferation In Vivo. Antimicrob. Agents Chemother. 2016;60:968–981. doi: 10.1128/AAC.01774-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mai V., Ukhanova M., Reinhard M.K., Li M., Sulakvelidze A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella -challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage. 2015;5 doi: 10.1080/21597081.2015.1088124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fa-arun J., Huan Y.W., Darmon E., Wang B. Tail-Engineered Phage P2 Enables Delivery of Antimicrobials into Multiple Gut Pathogens. ACS Synth. Biol. 2023;12:596–607. doi: 10.1021/acssynbio.2c00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lam K.N., Spanogiannopoulos P., Soto-Perez P., Alexander M., Nalley M.J., Bisanz J.E., Nayak R.R., Weakley A.M., Yu F.B., Turnbaugh P.J. Phage-delivered CRISPR-Cas9 for strain-specific depletion and genomic deletions in the gut microbiome. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu B.B., Way J.C., Silver P.A. Stable Neutralization of a Virulence Factor in Bacteria Using Temperate Phage in the Mammalian Gut. mSystems. 2020;5:e00013–e00020. doi: 10.1128/mSystems.00013-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reyes A., Wu M., McNulty N.P., Rohwer F.L., Gordon J.I. Gnotobiotic mouse model of phage–bacterial host dynamics in the human gut. Proc. Natl. Acad. Sci. 2013;110:20236–20241. doi: 10.1073/pnas.1319470110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsu B.B., Gibson T.E., Yeliseyev V., Liu Q., Lyon L., Bry L., Silver P.A., Gerber G.K. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe. 2019;25:803–814.e5. doi: 10.1016/j.chom.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ronda C., Chen S.P., Cabral V., Yaung S.J., Wang H.H. Metagenomic engineering of the mammalian gut microbiome in situ. Nat. Methods. 2019;16:167–170. doi: 10.1038/s41592-018-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghosh R., De M. Liposome-Based Antibacterial Delivery: An Emergent Approach to Combat Bacterial Infections. ACS Omega. 2023;8:35442–35451. doi: 10.1021/acsomega.3c04893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bikard D., Euler C.W., Jiang W., Nussenzweig P.M., Goldberg G.W., Duportet X., Fischetti V.A., Marraffini L.A. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 2014;32:1146–1150. doi: 10.1038/nbt.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Csörgő B., León L.M., Chau-Ly I.J., Vasquez-Rifo A., Berry J.D., Mahendra C., Crawford E.D., Lewis J.D., Bondy-Denomy J. A compact Cascade–Cas3 system for targeted genome engineering. Nat. Methods. 2020;17:1183–1190. doi: 10.1038/s41592-020-00980-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao R., Liu D., Jia X., Zheng Y., Liu W., Xiao Y. CRISPR-Cas9/Cas12a biotechnology and application in bacteria. Synth. Syst. Biotechnol. 2018;3:135–149. doi: 10.1016/j.synbio.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pawelczak K.S., Gavande N.S., VanderVere-Carozza P.S., Turchi J.J. Modulating DNA Repair Pathways to Improve Precision Genome Engineering. ACS Chem. Biol. 2018;13:389–396. doi: 10.1021/acschembio.7b00777. [DOI] [PubMed] [Google Scholar]
- 120.Liang Y., Chen F., Wang K., Lai L. Base editors: development and applications in biomedicine. Front. Med. 2023;17:359–387. doi: 10.1007/s11684-023-1013-y. [DOI] [PubMed] [Google Scholar]
- 121.Rodrigues S.D., Karimi M., Impens L., Van Lerberge E., Coussens G., Aesaert S., Rombaut D., Holtappels D., Ibrahim H.M.M., Van Montagu M., Wagemans J., Jacobs T.B., De Coninck B., Pauwels L. Efficient CRISPR-mediated base editing in Agrobacterium spp. Proc. Natl. Acad. Sci. 2021;118 doi: 10.1073/pnas.2013338118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Volke D.C., Martino R.A., Kozaeva E., Smania A.M., Nikel P.I. Modular (de)construction of complex bacterial phenotypes by CRISPR/nCas9-assisted, multiplex cytidine base-editing. Nat. Commun. 2022;13:3026. doi: 10.1038/s41467-022-30780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wei Y., Feng L.J., Yuan X.Z., Wang S.G., Xia P.F. Developing a Base Editing System for Marine Roseobacter Clade Bacteria. ACS Synth. Biol. 2023;12:2178–2186. doi: 10.1021/acssynbio.3c00259. [DOI] [PubMed] [Google Scholar]
- 124.Zheng L., Shen J., Chen R., Hu Y., Zhao W., Leung E.L.H., Dai L. Genome engineering of the human gut microbiome. J. Genet. Genomics. 2024 doi: 10.1016/j.jgg.2024.01.002. S1673852724000031. [DOI] [PubMed] [Google Scholar]
- 125.Mahler M., Costa A.R., Van Beljouw S.P.B., Fineran P.C., Brouns S.J.J. Approaches for bacteriophage genome engineering. Trends Biotechnol. 2023;41:669–685. doi: 10.1016/j.tibtech.2022.08.008. [DOI] [PubMed] [Google Scholar]
- 126.Pires D.P., Monteiro R., Mil-Homens D., Fialho A., Lu T.K. J. Azeredo, Designing P. aeruginosa synthetic phages with reduced genomes. Sci. Rep. 2021;11:2164. doi: 10.1038/s41598-021-81580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pulkkinen E.M., Hinkley T.C., Nugen S.R. Utilizing in vitro DNA assembly to engineer a synthetic T7 Nanoluc reporter phage for Escherichia coli detection. Integr. Biol. 2019;11:63–68. doi: 10.1093/intbio/zyz005. [DOI] [PubMed] [Google Scholar]
- 128.Faber M.S., Van Leuven J.T., Ederer M.M., Sapozhnikov Y., Wilson Z.L., Wichman H.A., Whitehead T.A., Miller C.R. Saturation Mutagenesis Genome Engineering of Infective ΦX174 Bacteriophage via Unamplified Oligo Pools and Golden Gate Assembly. ACS Synth. Biol. 2020;9:125–131. doi: 10.1021/acssynbio.9b00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng L., Deng Z., Tao H., Song W., Xing B., Liu W., Kong L., Yuan S., Ma Y., Wu Y., Huang X., Peng Y., Wong N.K., Liu Y., Wang Y., Shen Y., Li J., Xiao M. Harnessing stepping-stone hosts to engineer, select, and reboot synthetic bacteriophages in one pot. Cell Rep. Methods. 2022;2 doi: 10.1016/j.crmeth.2022.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kilcher S., Studer P., Muessner C., Klumpp J., Loessner M.J. Cross-genus rebooting of custom-made, synthetic bacteriophage genomes in L-form bacteria. Proc. Natl. Acad. Sci. 2018;115:567–572. doi: 10.1073/pnas.1714658115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan S., Shi J., Jiang J., Ma Y. Genome-scale top-down strategy to generate viable genome-reduced phages. Nucleic Acids Res. 2022;50:13183–13197. doi: 10.1093/nar/gkac1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Voorhees P.J., Cruz-Teran C., Edelstein J., Lai S.K. Challenges & opportunities for phage-based in situ microbiome engineering in the gut. J. Controlled Release. 2020;326:106–119. doi: 10.1016/j.jconrel.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 133.Chu J.N., Traverso G. Foundations of gastrointestinal-based drug delivery and future developments. Nat. Rev. Gastroenterol. Hepatol. 2022;19:219–238. doi: 10.1038/s41575-021-00539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.N. Procházková, G. Falony, L.O. Dragsted, T.R. Licht, J. Raes, H.M. Roager, Advancing human gut microbiota research by considering gut transit time, Gut 72 (2023) 180–191. 10.1136/gutjnl-2022-328166. [DOI] [PMC free article] [PubMed]
- 135.Shalon D., Culver R.N., Grembi J.A., Folz J., Treit P.V., Shi H., Rosenberger F.A., Dethlefsen L., Meng X., Yaffe E., Aranda-Díaz A., Geyer P.E., Mueller-Reif J.B., Spencer S., Patterson A.D., Triadafilopoulos G., Holmes S.P., Mann M., Fiehn O., Relman D.A., Huang K.C. Profiling the human intestinal environment under physiological conditions. Nature. 2023;617:581–591. doi: 10.1038/s41586-023-05989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koziolek M., Grimm M., Schneider F., Jedamzik P., Sager M., Kühn J.P., Siegmund W., Weitschies W. Navigating the human gastrointestinal tract for oral drug delivery: Uncharted waters and new frontiers. Adv. Drug Deliv. Rev. 2016;101:75–88. doi: 10.1016/j.addr.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 137.Vargason A.M., Anselmo A.C., Mitragotri S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021;5:951–967. doi: 10.1038/s41551-021-00698-w. [DOI] [PubMed] [Google Scholar]
- 138.C.R. Merril, B. Biswas, R. Carlton, N.C. Jensen, G.J. Creed, S. Zullo, S. Adhya, Long-circulating bacteriophage as antibacterial agents., Proc. Natl. Acad. Sci. 93 (1996) 3188–3192. 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed]
- 139.S.B.W. Liyanagedera, J. Williams, J.P. Wheatley, A.Y. Biketova, M. Hasan, A.P. Sagona, K.J. Purdy, R.J. Puxty, T. Feher, V. Kulkarni, SpyPhage: A Cell-Free TXTL Platform for Rapid Engineering of Targeted Phage Therapies, ACS Synth. Biol. 11 (2022) 3330–3342. 10.1021/acssynbio.2c00244. [DOI] [PubMed]
- 140.Durán-Lobato M., Niu Z., Alonso M.J. Oral Delivery of Biologics for Precision Medicine. Adv. Mater. 2020;32 doi: 10.1002/adma.201901935. [DOI] [PubMed] [Google Scholar]
- 141.Yang Y., Du H., Zou G., Song Z., Zhou Y., Li H., Tan C., Chen H., Fischetti V.A., Li J. Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: A review. J. Controlled Release. 2023;353:634–649. doi: 10.1016/j.jconrel.2022.11.048. [DOI] [PubMed] [Google Scholar]
- 142.Meng L., Yang F., Pang Y., Cao Z., Wu F., Yan D., Liu J. Nanocapping-enabled charge reversal generates cell-enterable endosomal-escapable bacteriophages for intracellular pathogen inhibition. Sci. Adv. 2022;8:eabq2005. doi: 10.1126/sciadv.abq2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Andriolo J.M., Sutton N.J., Murphy J.P., Huston L.G., Kooistra-Manning E.A., West R.F., Pedulla M.L., Hailer M.K., Skinner J.L. Electrospun Fibers for Controlled Release of Nanoparticle-Assisted Phage Therapy Treatment of Topical Wounds. MRS Adv. 2018;3:3019–3025. doi: 10.1557/adv.2018.483. [DOI] [Google Scholar]
- 144.Richards K., Malik D.J. Microencapsulation of Bacteriophages Using Membrane Emulsification in Different pH-Triggered Controlled Release Formulations for Oral Administration. Pharmaceuticals. 2021;14:424. doi: 10.3390/ph14050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vinner G.K., Richards K., Leppanen M., Sagona A.P., Malik D.J. Microencapsulation of Enteric Bacteriophages in a pH-Responsive Solid Oral Dosage Formulation Using a Scalable Membrane Emulsification Process. Pharmaceutics. 2019;11:475. doi: 10.3390/pharmaceutics11090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li J., Zheng H., Leung S.S.Y. Investigating the effectiveness of liposome-bacteriophage nanocomplex in killing Staphylococcus aureus using epithelial cell coculture models. Int. J. Pharm. 2024;657 doi: 10.1016/j.ijpharm.2024.124146. [DOI] [PubMed] [Google Scholar]
- 147.Międzybrodzki R., Kłak M., Jończyk-Matysiak E., Bubak B., Wójcik A., Kaszowska M., Weber-Dąbrowska B., Łobocka M., Górski A. Means to Facilitate the Overcoming of Gastric Juice Barrier by a Therapeutic Staphylococcal Bacteriophage A5/80. Front. Microbiol. 2017:08. doi: 10.3389/fmicb.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]