Abstract
Background
The most prevalent yet neglected cestode meat-borne parasitic zoonoses are bovine cysticercosis and cystic echinococcosis, while the most common meat-borne protozoan zoonoses are toxoplasmosis and cryptosporidiosis in Ethiopia. In Ethiopia, bovine cysticercosis, cystic echinococcosis, toxoplasmosis, and cryptosporidiosis are the most common but neglected meat-borne parasites. The main transmission route is through contaminated meat products. The aim of this review was to provide an overall prevalence estimation of major food-borne zoonotic parasitic in ruminants in Ethiopia.
Methods
The present meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Articles were searched in PubMed, Google Scholar, Web of Science, and HINARI. A total of 104 eligible articles were included in the final meta-analysis. The pooled prevalence estimates and 95% confidence intervals (CI) were conducted using random effect model, and heterogeneity was assessed using I2 statistics.
Results
Toxoplasmosis had the highest pooled prevalence (38, 95% CI: 30–46%), followed by cystic echinococcosis (25, 95% CI: 18–32%), cryptosporidiosis (14, 95% CI: 9–19%), and bovine cysticercosis (9, 95% CI: 5–13%). In most individual analyses, Egger’s regression test did not reveal significant publication bias, since the p-values were greater than 0.05. Regional subanalysis showed that bovine cysticercosis was most prevalent in the Amhara region (16, 95% CI: 6–13%), whereas cystic echinococcosis was highest in Oromia (33, 95% CI: 22–45%) and Tigray (29, 95% CI: 24–33%). Host-wise analysis indicated that toxoplasmosis was most prevalent in sheep (41%), followed by goats (39%), and cattle (28%). Cryptosporidiosis was most commonly detected in cattle (16%), sheep (11%), and goat (8%). Age-based analysis revealed a higher prevalence in calves and lambs with an estimated pooled prevalence of 15% (I2 = 83%).
Conclusion
The increasing prevalence of meat-borne parasitic zoonoses in Ethiopia highlights the need for urgent intervention. Strengthening disease surveillance, enforcing strict meat inspection protocols, and raising public awareness about zoonotic risks are critical for effective control. A coordinated approach between veterinary professionals, public health authorities, and policymakers is essential to mitigate the burden of these neglected parasitic infections and protect both animal and human health.
Keywords: Ethiopia, foodborne, parasite, pooled prevalence, zoonosis
Introduction
Ethiopia has the largest livestock population and the second largest human population in Africa and has experienced a substantial contribution from its livestock sector to the country’s economy, which also holds promise for further economic development (1). Among the diverse livestock in Ethiopia, cattle and small ruminants hold the utmost importance, with populations of 70 million cattle, 52.5 million sheep, and 42.9 million goats (2).
In developing countries, livestock farming plays a crucial role in ensuring food security; however, it also entails zoonotic risks (3). Zoonosis, a public health concern, has been recognized in numerous developing nations and encompasses a wide range of aetiologies, including parasitic, viral, bacterial, rickettsia, and chlamydial zoonoses. Zoonotic diseases account for a substantial proportion, specifically 60.3%, of infectious diseases (4). Animal-derived foods, such as meat, milk, and eggs, are globally acknowledged as fundamental components of the human diet (5). Nonetheless, the consumption of contaminated animal-based foods poses a significant threat to human health. Mammals, particularly bovine, ovine, and caprine species, serve as the primary sources of zoonotic diseases transmitted through food (6).
Among the health challenges faced, parasitic infections pose a significant burden, resulting in 450 million illnesses and 200,000 deaths annually (7). Among the 1,500 infectious agents identified in humans, 66 are protozoa, and 287 are helminths (8). Currently, food-borne zoonotic parasitic diseases have a global impact on food safety and human health because of the consumption of contaminated food, particularly animal products. These agents significantly affect public health and the economic sector (9).
Parasitic zoonotic diseases encompass a diverse group of infectious diseases with varying host ranges and transmission patterns, and their distribution, prevalence, and transmission patterns are influenced by factors related to humans, animals, and the environment (10). Ethiopia bears a substantial burden of zoonotic diseases, exacerbated by the lack of coordination between the human and animal health sectors and inadequate resources for public health systems. These factors contribute to weak surveillance systems and inefficient responses to public health threats within the country. Specifically, the issue of food-borne parasitic zoonosis in Ethiopia is further complicated by the absence of effective inspection at critical control points in slaughterhouses, limited awareness and knowledge regarding the mode of transmission, and the widespread practice of consuming raw meat in both rural and urban communities (11). Poor hygiene regulations, inadequate food safety laws, and lack of training for food processors contribute significantly to the issue (12). The National Hygiene and Sanitation Strategy Program attribute around 60% of Ethiopia’s disease burden to poor hygiene and sanitation. The absence of strict slaughter regulations and widespread backyard slaughtering worsens the prevalence of meat-borne parasitic zoonoses. Private slaughtering, especially during traditional celebrations, and practices like Kircha, where merchants prioritize profit over hygiene, lead to the consumption of contaminated meat. Several parasitic zoonoses, including bovine cysticercosis, toxoplasmosis, cryptosporidiosis, and echinococcosis, are prevalent in Ethiopia.
Bovine cysticercosis and cystic echinococcosis are major zoonotic diseases with both economic and public health impacts (13, 14). Bovine cysticercosis, caused by the cystic stage of Taenia saginata, affects human health through the consumption of raw or undercooked meat from infected cattle (15). Intestinal taeniosis, linked to this infection, can cause symptoms like abdominal discomfort, intestinal irritation, obstruction, nausea, weight loss, and anal pruritus (16). The disease also leads to financial losses due to carcass condemnation in the dairy industry. Its spread is influenced by cattle rearing practices, meat inspection, and the consumption of raw meat, while poor hygiene and lack of awareness contribute to transmission. Globally, there are about 77 million carriers of Taenia saginata, with 40% in Africa (17). The prevalence of bovine cysticercosis in cattle in Ethiopia varies from 3.1% in the central region to 26.25% in the southern region of the country (18–20). Cystic echinococcosis is a zoonotic parasitic disease that is induced by the cystic form of Echinococcus. granulosus and has considerable public health and economic importance. Echinococcus. granulosus is primarily perpetuated in a domestic cycle involving the dog (Canis familiaris) as the definitive host and domestic ungulates (sheep, cattle) as intermediate hosts (21).
A tapeworm, which is recognized for inhabiting the small intestine of its definitive host, is also found in its larval stage within various organs and tissues of intermediate hosts, including the liver, lungs, brain, muscles, lymph nodes, and other regions (22). This disease is more prevalent in developing countries, especially in rural communities where dogs live in close proximity to humans and domestic herbivores. The highest incidence is reported in regions where sheep and cattle are raised (23). Different regions across the country frequently report human hydatidosis, in which a close relationship exists between dogs and domestic animals in Ethiopia (24). In cattle, studies conducted at various abattoirs across the country have revealed that the prevalence of hydatidosis ranges from 9.4 to 63.7% in Harar and Assella, respectively. This disease plays a significant role in diminishing both the quantity and quality of exported goods. The consequences of this infection in humans and intermediate hosts include the formation of hydatid cysts in the lungs, liver, or other organs (25). While the occurrence of this clinical disease in domestic animals is rare, it poses a greater threat to humans. As reservoirs, domestic animals hold immense importance as hosts for this parasite (22). As the cysts gradually grow in size, they can detrimentally impact the well-being of the host. When located in the lungs, they result in dyspnoea, whereas in the liver, they cause digestive disturbances and ascites (26).
Toxoplasmosis is caused by the intracellular parasite Toxoplasma gondii (T. gondii), which spreads globally through meat transmission (27). Domestic cats serve as the definitive host, while warm-blooded animals and humans act as intermediate hosts. Infection occurs via the consumption of raw or undercooked meat from infected animals like sheep, pigs, and cattle. While generally opportunistic in healthy individuals, T. gondii poses serious risks to those with weakened immune systems, such as HIV/AIDS patients, pregnant women, cancer patients, or organ transplant recipients (28, 29). The prevalence in sheep is about 30%, with active infections estimated at 15% globally. In goats, prevalence can reach up to 77%, varying by region (30). In Ethiopia, various well-documented reports on toxoplasmosis exist. The research conducted an extensive serological survey, revealing the presence of T. gondii infection in sheep and goats, with a prevalence of 43% (31). Additionally, a serological survey performed by Teshale et al. (32) revealed the prevalence of caprine toxoplasmosis in Central and Southern Ethiopia, revealing a remarkable prevalence of 74.8%. In a recent study, a seroprevalence of 70% for toxoplasmosis was reported in goats and sheep in the northwest region of Ethiopia (33).
Toxoplasmosis is considered a disease of significant economic importance because of its ability to cause notable harm through reproductive complications, inflammation of the nervous system, and respiratory infections, particularly in sheep and goats (34). Cryptosporidiosis is a severe parasitic disease caused by protozoan parasites belonging to the Cryptosporidium genus (35). These parasites can cultivate and proliferate within the brush border of gastrointestinal cells in infected animals and humans, leading to severe health issues (36). The genus Cryptosporidium comprises 13 recognized species distinguished on the basis of morphological characteristics, host specificity, and DNA-oriented investigations (37). However, only two species, Cryptosporidium parvum and Cryptosporidium andersoni, are significant in the context of cattle (38). The primary modes of transmission for this zoonotic disease are through contaminated water, contaminated food, and contact with infected animals (39, 40). Calves serve as reservoirs for the zoonotic species Cryptosporidium parvum (C. parvum), which is responsible for causing diarrhea in humans on a global scale. A recent survey conducted in the United Kingdom revealed that 38.5% of cryptosporidiosis cases in humans were attributed to C. parvum, with 25% of these cases occurring through direct contact.
The occurrence of Cryptosporidium infection in cattle can be associated with factors such as age, bedding depth, and environmental cleanliness (41). This parasite follows a homoxenous life cycle and has a significant capacity to reproduce and spread among various animal hosts (18). Reports have indicated that the prevalence of cryptosporidiosis in cattle ranges from 6.25 to 39.65% in different regions of the world. The disease is characterized by symptoms such as loss of appetite, diarrhea, stunted growth, and mortality (42, 43). The increasing human population and socioeconomic shifts have resulted in the migration of people into new ecological niches and changes in animal husbandry practices, thereby significantly impacting the emergence and burden of disease (44). Furthermore, both natural and human-induced environmental changes and disturbances continue to have substantial effects on the prevalence and emergence of zoonotic parasitic diseases. The spread and epidemiology of parasitic zoonoses are significantly influenced by human behavior. Unlike viruses and bacteria, parasites possess unique characteristics that enable them to thrive in the environment and be transmitted by animals and animal products. Some have the potential to cause significant public health problems, whereas others can cause substantial losses in livestock, leading to significant economic and production losses.
This systematic review aims to assist professionals in animal, human, and environmental health in monitoring foodborne parasitic zoonotic diseases. It serves as a resource for developing effective control and prevention strategies and enhancing epidemiological surveillance systems. Additionally, it supports the one health approach and raises awareness about neglected zoonotic diseases in animal-derived food in Ethiopia. The objective was to estimate the prevalence of common foodborne parasitic zoonosis (bovine cysticercosis, toxoplasmosis, cryptosporidiosis, and cystic echinococcosis) in domestic ruminants in Ethiopia, addressing specific research questions.
What is the prevailing frequency of specified parasitic zoonosis contracted through food of ruminant animal origin?
Which specific organ harbors foodborne parasitic zoonosis?
Which region of Ethiopia is more prone to foodborne parasitic zoonosis?
Which foodborne parasite zoonosis is highly distributed in Ethiopia?
Methods
A systematic assessment of published studies reporting the prevalence of toxoplasmosis, cryptosporidiosis, bovine cysticercosis and cystic echinococcosis among different food animal species (Bovine, Ovine and Caprine) was performed based on PRISMA checklist (45) (Supplementary material 1).
Study eligibility criteria
Inclusion criteria
This meta-analysis included primary studies published in English that reported the prevalence of bovine cysticercosis, cystic echinococcosis, toxoplasmosis, and cryptosporidiosis in domestic ruminants (cattle, sheep, and goats) or related food sources. Eligible studies provided a clear estimation of the proportion of these foodborne parasitic zoonoses. Observational studies assessing prevalence were considered, provided the animals were not experimentally infected. Parasite identification was required at least to the genus level, and studies had to be conducted in Ethiopia.
Exclusion criteria
Studies on parasitic zoonoses in pigs, camels, or other non-ruminant species were excluded. Additionally, studies lacking clear prevalence estimates for each parasite–host relationship were omitted. Excluded materials also included review articles, duplicates, and abstracts with insufficient data, qualitative studies, KAP-based studies, book chapters, case reports, editorials, short communications and opinions. Intervention studies without baseline data on animal exposure and disease association were also excluded.
Information sources
The literature search was conducted from October 2022 to January 2024. The database sources PubMed, HINARI, Web of Science, Google Scholar and other manual open internet methods were used. The included studies were reported from Ethiopia. It is based on the reported prevalence of foodborne parasitic zoonosis, mainly in slaughterhouses and other study areas (farms with selected parasites).
Search strategy
We selected the most widespread and public health-relevant foodborne parasitic zoonoses of animal origin in Ethiopia. To ensure a systematic and unbiased approach, two authors (MD and MZ) independently developed a comprehensive search strategy, with any disagreements resolved through discussion with a third author (AB). The search was guided by the CoCoPop (Condition, Context, and Population) framework, where the condition focused on foodborne parasitic zoonoses, the context was Ethiopia, and the population included domestic ruminants such as sheep, goats, and cattle. To enhance the transition between the search strategy and data extraction, we carefully structured the search process using Boolean operators (“AND/OR”) to refine and expand the search results by combining related terms. In PubMed, the search was conducted using the “All Fields” function, employing a query that included keywords related to prevalence, epidemiology, parasitic zoonoses, and specific infections such as toxoplasmosis, cysticercosis, cystic echinococcosis, and cryptosporidiosis. The search was further filtered by animal species, including cattle, sheep, and goats, and restricted to studies conducted in Ethiopia.
In the Web of Science, a broader approach was applied by searching within the “Topic Field,” which includes the title, abstract, and author keywords. The search terms incorporated variations of parasite infections, foodborne transmission, epidemiology, prevalence, and infection rates. Additionally, specific geographic identifiers such as Ethiopia and its regional states (Addis Ababa, Afar, Amhara, Oromia, SNNPR, Gambella, Harari, Somali, and Tigray) were included to ensure comprehensive coverage of relevant studies.
Data extraction
After screening studies based on the eligibility criteria, two investigators (MD and BE) independently extracted relevant data. Any discrepancies were resolved through discussion with a third author. The extracted data encompassed both quantitative and qualitative information and were systematically organized into Word tables and an Excel spreadsheet. The final dataset comprised 104 articles, including details on: Primary author’s name, Publication and study year, Geographical location, Study animal species (host), Sample size and number of positives, diagnostic methods, data collection methodologies, Ethical considerations, prevalence and principal findings of the parasite type.
Study quality assessment
In this review, the quality of the included studies was evaluated using the AMSTAR-2 tool (Supplementary material 2), as the methodological rigor of the studies directly influences the reliability of the meta-analysis results. Two investigators (MD and AB) independently assessed study quality, applying the 15-item AMSTAR-2 checklist, which covers both randomized and nonrandomized trials of health interventions. Some items are applicable to both types of studies, while others are specific to either randomize or nonrandomized trials. Since this meta-analysis focused on nonrandomized (observational) studies, only the relevant items were used. The quality assessment is integral in determining the confidence in the results and ensuring that the conclusions drawn from the meta-analysis are based on studies with sound methodologies. Regarding data extraction, two investigators (MD and BE) independently extracted relevant data from the selected studies. In the case of any discrepancies, the investigators resolved disagreements through discussion and consultation with a third author (AB), ensuring consensus and consistency in the data.
Data synthesis and statistical analysis
The random effects model, which employs the restricted maximum likelihood method (REML) to compute within- and between-study variability, was employed to estimate the aggregated prevalence and 95% confidence intervals. This model was utilized to conduct a comprehensive meta-analysis (overall effect size (ES) or often represented as a percentage or proportion), assess heterogeneity, and determine the weight of each study. Furthermore, graphs and tables were employed to illustrate the prevalence status of the primary parasitic zoonosis on the basis of the geographical distribution of animal species, the type of parasites, and the study years.
To estimate the prevalence, we extracted data from the number of events and the total number of samples. These data were then subjected to proportional meta-analysis via the “metaprop” function of the “meta” package version 4.1.3-0 in Balduzzi et al. (46) R statistical software. Pooled proportions of prevalence were estimated via logit transformation via a logistic-normal random effects regression model. Subgroup analysis was conducted via the mixed effect logistic regression model.
Investigation of heterogeneity
The Cochran’s Q test (indicated by the p value), τ2 (representing between-study variance), and the inverse variance index (I2) were employed to assess the sources of heterogeneity. The I2 index, as elucidated by Higgins and Thompson (47), was calculated to indicate low, moderate, and high levels of heterogeneity, corresponding to I2 values of 25, 50, and 75%, respectively. Heterogeneity was deemed statistically significant if the I2 value exceeded 50%, and the Q test yielded a p value less than 0.10. τ2 (tau-squared) measures the variance between studies. In simple terms, it tells us how much the true effects differ from study to study. I2 (I-squared) indicates the percentage of variability across studies that is due to heterogeneity rather than chance. For example, if I2 = 50%, this means that 50% of the variation between study results is due to true differences rather than random sampling error. By using these indices, we were able to identify the sources and extent of heterogeneity in the meta-analysis.
The extent of study heterogeneity was evaluated through a forest plot diagram, which depicted the weights, effect magnitudes, and 95% confidence intervals for between-study variability. A subgroup analysis of the prevalence of the primary prevalence of toxoplasmosis, cryptosporidiosis, bovine cysticercosis and cystic echinococcosis was performed, considering factors such as publication year, study location or region, species of study animals, and methods of detection.
Publication bias assessment
To assess publication bias, funnel plot diagrams and Egger’s regression test were used. A funnel plot is a scatter plot of the effect sizes from individual studies against their sample sizes. If there is no publication bias, the plot should resemble a symmetrical funnel. Egger’s regression test is a statistical method that helps determine whether the funnel plot is symmetrical. If the plot is asymmetrical (i.e., the studies are skewed in one direction), it suggests potential publication bias, meaning that smaller or less significant studies may be missing from the analysis. A p-value from the test greater than 0.05 typically indicates that the funnel plot is symmetric, suggesting no significant publication bias.
Results
Literature search results
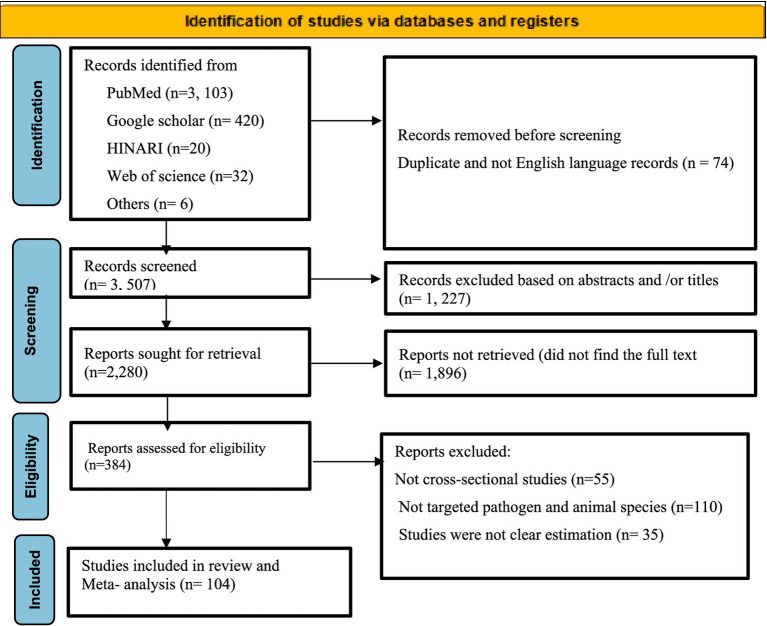
According to the PRISM 2020 flow chart (Figure 1), an initial search identified 3,581 articles across various electronic databases. After excluding 74 articles due to duplicates and non-English records, 3,507 articles remained. Of these, 1,227 were eliminated based on a review of titles and abstracts. A total of 2,280 articles were then sought for retrieval, but 1,896 could not be obtained. Out of the 384 articles assessed for eligibility, 200 were excluded for various reasons. Ultimately, 104 studies were included in the meta-analysis.
Figure 1.
Flow chart of selected studies on foodborne parasitic zoonotic diseases of animal origin.
Overview of the included articles in the meta-analysis
This systematic review included 104 studies focusing on four major zoonotic parasitic diseases affecting domestic ruminants in Ethiopia: bovine cysticercosis, cystic echinococcosis, toxoplasmosis, and cryptosporidiosis. All of the investigations employed an observational study design.
Bovine cysticercosis
The current systematic review includes a total of 34 articles on cysticercosis in cattle, with each article presenting one or multiple examinations of parasites (14). These articles were published between 2008 and 2023. Notably, the individual studies included in this systematic review reported a minimum sample size of 234 in Northeast Ethiopia (48) and a maximum sample size of 4,456 (49) in the Amhara Region in Northwest Ethiopia. The overall number of animals sampled throughout the years of the study was 25,065, of which 3,076 tested positive for bovine cysticercosis. The majority of the data collection methods utilized by observations conducted at abattoirs and surveys conducted through questionnaires. A comprehensive overview of the investigated studies is provided in Table 1.
Table 1.
Characteristics of the studies of Cysticercos bovis (Taenia saginata cysticercos) (n = 34).
| First author | Study year | G. Location | Regions | ST | Methods diagnosis | Data collection | Total examine | Positive | Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Tolosa et al. (80) | 2007–2008 | Southwestern | Oromia | SR | AM and PM | Abattoir survey and questionnaire | 512 | 15 | 0.0293 |
| Regassa et al. (81) | 2007–2008 | Southern | Oromia | SR | AM and PM | Abattoir survey and questionnaire | 415 | 47 | 0.113 |
| Terefe et al. (82) | 2009–2010 | Southern | SNNPR | SYR | AM and PM | Abattoir survey and questionnaire | 898 | 177 | 0.197 |
| Ibrahim and Zerihun (83) | 2010–2011 | Central | AA | SYR | AM and PM | Abattoir survey | 535 | 19 | 0.036 |
| Emiru et al. (84) | 2013–2014 | Central | Oromia | SYR | AM and PM | Abattoir survey | 430 | 24 | 0.056 |
| Hirpha et al. (85) | 2015–2016 | Southern | SNNPR | SR | AM and PM | Mixed (abattoir, questionnaire, and drug sale inventory) | 384 | 33 | 0.086 |
| Edao et al. (86) | 2014–2015 | Southeast | Oromia | SR | AM and PM | Abattoir survey | 430 | 5 | 0.012 |
| Megeresa et al. (87) | 2008–2009 | Southwest | Oromia | SR | PM | Abattoir survey | 500 | 22 | 0.044 |
| Abunna et al. (14) | 2005–2006 | Southern | SNNPR | SR | PM | Abattoir survey, interview, questionnaire | 400 | 105 | 0.263 |
| Bedore and Geinero (88) | 2017–2018 | Central | AA | SYR | PM | Abattoir survey | 317 | 29 | 0.091 |
| Moje et al. (89) | 2012–2013 | Southwest | Oromia | SYR | AM and PM | Abattoir survey | 405 | 203 | 0.5 |
| Sheferaw and Abdu (1) | 2015–2016 | Southwest | Oromia | SYR | PM | Abattoir survey and questionnaire | 1,200 | 80 | 0.067 |
| Tamirat et al. (11) | 2017–2018 | Northwest | Amhara | SYR | AM and PM | Abattoir survey and questionnaire | 480 | 20 | 0.042 |
| Engdaw et al. (90) | 2009–2010 | Northeast | Amhara | SR | PM | Abattoir survey | 421 | 27 | 0.064 |
| Mesele et al. (91) | 2011–2012 | Northwest | Amhara | SR | AM and PM | Abattoir survey | 532 | 92 | 0.173 |
| Tegegne et al. (48) | 2016–2017 | Northeast | Amhara | SR | AM and PM | Abattoir survey and questionnaire | 234 | 21 | 0.09 |
| Yigzaw et al. (92) | 2015–2016 | Northeast | Amhara | SR | AM and PM | Mixed (abattoir, questionnaire, drug sale inventory) | 384 | 26 | 0.068 |
| Bedu et al. (93) | 2010–2011 | Southern | Oromia | SR | PM | Mixed (abattoir, questionnaire, drug sale inventory) | 400 | 96 | 0.24 |
| Kassaw et al. (94) | 2015–2016 | Northeast | Amhara | SR | AM and PM | Abattoir survey | 425 | 20 | 0.047 |
| Tesfaye et al. (95) | 2010–2011 | Southern | SNNPR | SR | AM and PM | Abattoir, questionnaire, and drug sale inventory | 540 | 14 | 0.026 |
| Teklemariam and Debash (96) | 2014–2015 | Central | AA | SR | AM and PM | Mixed (abattoir, questionnaire, drug sale inventory) | 384 | 10 | 0.026 |
| Abay and Kumar (97) | 2007–2008 | Northeast | Tigray | SR | AM and PM | Abattoir survey | 1,023 | 74 | 0.072 |
| Abunna et al. (14) | 2011–2012 | Southern | SNNPR | SR | PM | Abattoir, questionnaire, and drug sale inventory | 400 | 48 | 0.12 |
| Tadesse et al. (98) | 2005–2006 | Southwest | Oromia | SYR | AM and PM | Abattoir, questionnaire, and drug sale inventory | 1,216 | 56 | 0.046 |
| Abera et al. (99) | 2020–2021 | Southwest | Oromia | SYR | AM and PM | Abattoir, questionnaire, and drug sale inventory | 1,108 | 302 | 0.273 |
| Tesfaye et al. (100) | 2013–2014 | Southern | SNNPR | SYR | AM and PM | Abattoir survey | 400 | 17 | 0.043 |
| Bayou and Taddesse (101) | 2016–2017 | Southwest | Oromia | SR | AM and PM | Abattoir survey | 384 | 25 | 0.065 |
| Bekele et al. (102) | 2016–2017 | Southwest | Oromia | SR | AM and PM | Mixedabattoir, questionnaire | 600 | 93 | 0.155 |
| Netsanet et al. (75) | 2018–2019 | Northeast | Amhara | SR | AM and PM | Mixedabattoir, questionnaire | 384 | 37 | 0.096 |
| Kumar et al. (103) | 2005–2006 | Northeast | Tigray | SR | PM | Abattoir survey | 3,711 | 308 | 0.083 |
| Fesseha and Asefa (104) | 2021–2022 | Central | AA | MRS | AM and PM | Abattoir survey, quantitative | 330 | 14 | 0.042 |
| Mussa (105) | 2021–2022 | Southern | SNNPR | SR | PM | Abattoir survey | 422 | 152 | 0.36 |
| Kebede (49) | 2006–2007 | Northwest | Amhara | SYR | PM | Abattoir survey | 4,456 | 824 | 0.1849 |
| Moje et al. (89) | 2011–2012 | Southern | Oromia | SR | AM and PM | Mixed (abattoir, questionnaire, drug sale inventory) | 405 | 41 | 0.101 |
AM, Ante mortem; PM, post-mortem; G. location, Geographical location; SYR, Systematic random; SR, Simple random; MRS, Multistage random sampling; SNNPR, Southern nation and nationalities of peoples region.
Cystic echinococcosis
This systematic review provides a comprehensive analysis of 37 studies on cystic echinococcosis in domestic ruminants, including cattle, sheep, and goats. In this systematic review, the largest sample size examined in a study was 5,194 animals, whereas the smallest study analyzed only 246 animals. A total of 28,024 animals were examined and among them, 7,277 were positive for cystic echinococcosis. Table 2 provides a comprehensive summary of the reviewed studies.
Table 2.
Characteristics of the studies included the prevalence of cystic echinococcosis in Ethiopia (n = 37).
| First author | Publication year | Study year | Location | Region | Host | Study design | Total examine | Positive | Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Tolosa et al. (80) | 2009 | 2007–2008 | Southwestern | Oromia | Cattle | CS | 512 | 161 | 0.314 |
| Regassa et al. (81) | 2009 | 2007–2008 | Southern | SNNPR | Cattle | CS | 415 | 64 | 0.154 |
| Bayu et al. (106) | 2012 | 2011–2012 | Central | AA | Sheep, goat | CS | 1,152 | 31 | 0.027 |
| Belina et al. (107) | 2015 | 2011–2012 | Southwestern | Oromia | Cattle | CS and retrospective | 384 | 36 | 0.094 |
| Asfaw and Afero (108) | 2014 | 2014 | Northeast | Tigray | Cattle | CS | 440 | 141 | 0.32 |
| Guduro and Desta (109) | 2019 | 2016–2017 | Southern | SNNPR | Cattle | CS | 400 | 208 | 0.52 |
| Kebede et al. (110) | 2008 | 2008 | Northeast | Tigray | Cattle | CS | 5,194 | 1,146 | 0.221 |
| Zelalem et al. (111) | 2012 | 2007–2008 | Central | AA | Sheep, cattle | CS | 1,611 | 284 | 0.176 |
| Kumsa (112) | 2019 | 2015–2016 | Central | AA | Cattle | CS | 1,209 | 254 | 0.21 |
| Negash et al. (31) | 2013 | 2010–2011 | Southwestern | Oromia | Cattle | CS | 384 | 190 | 0.495 |
| Abera et al. (113) | 2013 | 2009–2010 | Southern | SNNPR | Cattle | CS | 564 | 205 | 0.363 |
| Temam et al. (114) | 2016 | 2015–2016 | Southwestern | Oromia | Cattle | CS | 400 | 218 | 0.545 |
| Tibebu et al. (115) | 2016 | 2010–2011 | Northwestern | Amhara | Cattle | CS | 415 | 149 | 0.359 |
| Tkubet et al. (70) | 2016 | 2014–2015 | Northeast | Tigray | Cattle | CS | 310 | 80 | 0.258 |
| Abera and Teklebran (116) | 2017 | 2016 | Southern | SNNPR | Cattle | CS | 446 | 50 | 0.112 |
| Hailu et al. (117) | 2016 | 2014–2015 | Southwestern | Oromia | Cattle | CS | 384 | 195 | 0.507 |
| Nasr and Pal (118) | 2016 | 2015 | Northwestern | Amhara | Cattle | CS | 400 | 134 | 0.335 |
| Beyene and Hiko (119) | 2019 | 2017–2018 | Southwestern | Oromia | Cattle | CS | 384 | 128 | 0.333 |
| Atsede and Abebe (120) | 2016 | 2015–2016 | Southwestern | Oromia | Cattle | CS | 384 | 118 | 0.307 |
| Tadesse et al. (121) | 2016 | 2013–2014 | Northwestern | Amhara | Cattle | CS | 384 | 72 | 0.187 |
| Haftu and Kebede (122) | 2016 | 2014–2015 | Southwestern | Oromia | Cattle | CS | 246 | 29 | 0.118 |
| Belisty et al. (123) | 2017 | 2015–2016 | Northwest | Amara | Cattle | CS | 620 | 134 | 0.216 |
| Gemeda et al. (124) | 2020 | 2018–2019 | Southwestern | AA | Sheep, goat | CS | 995 | 216 | 0.217 |
| Yohannes (125) | 2019 | 2017–2018 | Southwestern | Oromia | Cattle | CS | 395 | 159 | 0.402 |
| Moje and Degefa (126) | 2014 | 2011–2012 | Southwestern | Oromia | Cattle | CS | 405 | 41 | 0.101 |
| Berhe (127) | 2009 | 2006–2007 | Northeast | Tigray | Cattle | CS | 4,418 | 1,439 | 0.326 |
| Dana et al. (128) | 2021 | 2019–2020 | Southwestern | Oromia | Cattle | CS | 389 | 206 | 0.53 |
| Akeberegn et al. (129) | 2017 | 2017 | Northern west | Amara | Cattle | CS | 384 | 25 | 0.065 |
| Andualem et al. (130) | 2016 | 2014–2015 | Central | AA | Cattle | CS | 486 | 98 | 0.202 |
| Serda and Jago (131) | 2017 | 2014–2015 | Southeastern | Oromia | Cattle | CS | 450 | 243 | 0.54 |
| Kebede et al. (132) | 2009 | 2005–2006 | Northwest | Amhara | Cattle, sheep | CS | 760 | 176 | 0.2316 |
| Kebede et al. (133) | 2011 | 2007–2008 | Northwest | Amhara | Cattle | CS | 521 | 79 | 0.152 |
| Mathewos et al. (134) | 2022 | 2020–2021 | Southeastern | SNNPR | Cattle | CS | 384 | 69 | 0.18 |
| Kebede et al. (135) | 2009 | 2007–2008 | Northwest | Amhara | Cattle | CS | 413 | 202 | 0.489 |
| Kebede et al. (110) | 2009 | 2007–2008 | Southeastern | SNNPR | Cattle | CS | 400 | 64 | 0.16 |
| Bekele and Butako (136) | 2010 | 2009–2010 | Southeastern | SNNPR | Cattle | CS | 546 | 92 | 0.168 |
CS, cross-sectional study; SNNPR, South Nation and Nationalities of People; AA, Addis Ababa.
Toxoplasmosis
In the present study, 14 articles involving a total of 6,957 animals were examined, with 2,889 of those animals diagnosed as positive for toxoplasmosis. According to Teshale et al. (32), the central region of Ethiopia exhibited the highest prevalence rate of 74.9% at the individual study level. In comparison, Esubalew et al. (33) reported a prevalence rate of 70.5% in the Amhara region, Northwest Ethiopia. With respect to the species of host animals, Esubalew et al. (33) reported a high prevalence of toxoplasmosis (70.5%) in the Amhara region in sheep, followed by goats (74.9%), as indicated by Teshale et al. (32), and cattle (59%), as reported by Maru et al. (50), in the Amhara region. Among the included studies, five did not state ethical considerations. The details of the articles are summarized in Table 3.
Table 3.
Characteristics of the included studies (n = 14) toxoplasmosis.
| First author | Publication year | Study year | G. Location | Region | Host | Ethical consideration | ST | Method diagnosis | Data collection method | Total examine | Positive | Prevalence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tegegne et al. (137) | 2016 | 2014–2015 | Southwest | Oromia | Sheep, goat | Yes | Univariable-logistic regression | LAT | Serological assay | 368 | 212 | 0.576 |
| Tilahun et al. (138) | 2018 | 2011–2013 | Southwest | Oromia | Seep, goat, cattle | Not stated | Multivariable logistic regression | iELISA | Serological and questionnaire survey | 778 | 260 | 0.334 |
| Gebremedhin et al. (139) | 2013 | 2010–2011 | Southwest | Oromia | Sheep | Not stated | Multivariate logistic regression | ELISA | Serological and questionnaire survey | 1,130 | 357 | 0.316 |
| Maru et al. (50) | 2016 | 2014–2015 | Northwest | Amhara | Sheep, goat, cattle | Yes | Chi-square | LAT | Bioassay, serology and microscopy | 335 | 187 | 0.558 |
| Zewdu et al. (140) | 2013 | 2010–2011 | Northwest | Amhara | Goat | Yes | Multivariable logistic regression | ELISA | Serological and questionnaire survey | 927 | 183 | 0.197 |
| Teshale et al. (32) | 2007 | 2005–2006 | Central | AA | Goat | Not stated | Multistage and cluster sampling | DAT | Serological assay | 641 | 480 | 0.749 |
| Negash et al. (141) | 2004 | 1999–2000 | Northeast | Tigray | Sheep, goat | Yes | Simple random | DAT | Serological assay | 174 | 75 | 0.431 |
| Gebremedhin and Gizaw (142) | 2014 | 2013–2014 | Southern | SNNPR | Sheep, goat | Yes | Mantel-haenszell Chi-square | iELISA | Serological assay | 184 | 48 | 0.261 |
| Gebremedhin et al. (143) | 2014 | 2011–2012 | Northeast | Tigray | Sheep, goat | Yes | Multivariable logistic regression | DAT | Serological questionnaire survey | 628 | 111 | 0.177 |
| Jilo et al. (144) | 2021 | 2016–2017 | Southern | Oromia | Sheep, goat | Yes | Simple random | LAT | Serological and questionnaire survey | 400 | 211 | 0.5275 |
| Getachew et al. (61) | 2016 | 2011–2012 | Central | AA | Sheep, goat, cattle | Not stated | Simple random | DAT | Serological and questionnaire survey | 278 | 97 | 0.349 |
| Esubalew et al. (33) | 2022 | 2018–2019 | Northwest | Amhara | Sheep, goat | Yes | Cluster random sampling | LAT | Serological and questionnaire survey | 576 | 406 | 0.705 |
| Gashaw et al. (51) | 2023 | 2019–2021 | Northwest | Amhara | Goat | Yes | Systematic random sampling | LAT | Serological and questionnaire survey | 150 | 65 | 0.433 |
| Zewde et al. (145) | 2022 | 2020–2022 | Southwest | Oromia | Sheep, goat | Not stated | Simple random | ELISA | Serological assay | 388 | 197 | 0.508 |
LAT, latex agglutination test; ST, Sampling technique; IELISA, Indirect enzyme-linked immunoassay; DAT, direct agglutination test; SNNP, South Nation and Nationalities of peoples; G. Location, geographical location.
Cryptosporidiosis
A total of 19 studies were discovered for the estimation of the pooled prevalence of cryptosporidiosis. The majority of the studies are located in the southern region of Ethiopia, specifically within the Oromia region. The study subjects included calves, kids, lambs, and adult sheep and cattle, which consisted of a total of 6,972 and 959 cryptosporidiosis infections (event). Notably, most of the literature has focused on calves and lambs. The most frequently isolated species were C. bovis, C. andersoni, C. ubiquitum, and ryane. The southwest region of Ethiopia, specifically the Oromia regional state, recorded a high prevalence rate (24%) of cryptosporidiosis with calves documented by Gashaw et al. (51) (Table 4).
Table 4.
Characteristics (n = 19) of the included studies of cryptosporidiosis.
| First author | Study year | Location | Region | Host | Total examine | Positive | Prevalence | Method detection | Molecular characterization | ST | Data collection | Ethical consideration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hailu et al. (20) | 2017–2018 | Southern | SNNPR | Calves | 330 | 43 | 0.13 | DIFAT | Not done | SR | Mixed | Yes |
| Wegayehu et al. (18) | 2009 | Southwest | Oromia | Calves | 384 | 30 | 0.0781 | MZN | Not done | MRS | Quantitative | Yes |
| Kifleyohannes et al. (52) | 2018–2019 | Northeast | Tigray | Kids, calves, lambs | 726 | 53 | 0.073 | MZN, PCR | C. ubiquitum, C. bovis, C. andersoni, C. ryanae | COS | Mixed | Yes |
| Manyazewal et al. (53) | 2018 | Central | AA | Cattle | 392 | 73 | 0.1862 | MZN, PCR, PCR-RFLP | C. parvum, C. andersoni | SR | Quantitative | Yes |
| Abebe et al. (55) | 2004–2005 | Central | AA | Calves | 580 | 102 | 0.176 | SF, MZN | Not done | CLS | Mixed | Not stated |
| Ayele et al. (146) | 2014–2015 | Northwest | Amhara | Calves | 360 | 67 | 0.186 | SF, MZN | Not done | SR | Quantitative | Yes |
| Birhanu et al. (147) | 2014–2015 | Southwest | Oromia | Calves | 384 | 92 | 0.24 | MZN | Not done | SR | Mixed | Not stated |
| Manyazewal et al. (148) | 2014–2015 | Southwest | Oromia | Calves | 270 | 40 | 0.148 | MZN, PCR | C. bovis, C. ryane, C. andersoni | PS | Quantitative | Yes |
| Wegayehu et al. (149) | 2014 | Southwest | Oromia | Calves | 449 | 91 | 0.203 | MZN, PCR | C. parvum, C. andersoni | SR | Mixed | Yes |
| Gashaw et al. (150) | 2018–2019 | Southwest | Oromia | Cattle | 378 | 41 | 0.108 | MZN | Not done | SR | Mixed | Yes |
| Wegayehu et al. (149) | 2014 | Southwest | Oromia | Lambs | 389 | 8 | 0.021 | MZN, nested PCR | C. ubiquitum | SR | Quantitative | Yes |
| Berhanu et al. (151) | 2021–2022 | Southwest | Oromia | Cattle | 434 | 76 | 0.175 | SF, MZN | Not done | SR | Mixed | Yes |
| Ali et al. (152) | 2017–2018 | Southern | SNNPR | Sheep | 384 | 31 | 0.081 | SF, MZN | Not done | SR | Mixed | Not stated |
| Ebiyo and Haile (153) | 2020–2021 | Southwest | Oromia | Calves | 384 | 53 | 0.138 | MZN | Not done | SYR | Quantitative | Yes |
| Regassa et al. (13) | 2010–2011 | Southwest | Oromia | Calves, lambs, kids | 237 | 56 | 0.236 | SF, MZN | Not done | SYR | Quantitative | Yes |
| Ayana and Alemu (154) | 2014–2015 | Central | AA | Calves, lambs, kids | 364 | 27 | 0.136 | MZN | Not done | SYR | Mixed | Yes |
| Wudu et al. (155) | 2004 | Southwest | Oromia | Calves | 55 | 4 | 0.073 | MZN | Not done | SR | Quantitative | Not stated |
| Tekle (156) | 2020–2021 | Northwest | Amhara | Calves | 193 | 30 | 0.155 | MZN | Not done | SR | Mixed | Not stated |
| Belfa et al. (157) | 2021–2022 | Central | Oromia | Calves | 279 | 42 | 0.151 | MZN | Not done | CLS | Mixed | Not stated |
ST, sampling technique; MZN, modified Zeehl–Nielsen; CLS, cluster sampling; COS, convenient sampling; SR, simple random sampling; SYR, systematic random sampling; MRS, multistage sampling; PS, purposive sampling; mixed, quantitative and qualitative; SF, flotation; DIFAT, direct immuno-flurecent antigen test.
Diagnostic methods used across studies
The diagnostic approaches varied across the four parasitic diseases, with post-mortem meat inspection, serological tests, microscopy, and molecular techniques being the primary methods. Post-mortem meat inspection was the gold standard for diagnosing bovine cysticercosis and cystic echinococcosis, as both diseases were primarily identified through abattoir-based surveys (Tables 1, 2). The presence of cystic lesions in infected organs served as a key diagnostic indicator. Since data were collected from slaughtered animals, ethical approval was generally not required for these studies. For toxoplasmosis, serological tests were the primary diagnostic tool, with ELISA, Latex Agglutination Test (LAT), and Direct Agglutination Test (DAT) commonly used across studies (Table 3). These methods detect specific antibodies in blood samples, making them suitable for screening live animals. In several studies, serological tests were supplemented with questionnaire surveys to assess risk factors, transmission dynamics, and disease prevalence in different livestock populations.
Cryptosporidiosis was predominantly diagnosed using microscopy (modified Zeehl–Nielsen), which detects oocysts in fecal samples. Some studies employed PCR and nested PCR to achieve molecular characterization of Cryptosporidium species, including C. bovis, C. andersoni, C. ubiquitum, and C. ryanae (18, 52, 53) as shown Table 4. However, molecular diagnostics were used in only a limited number of studies. Additionally, questionnaire surveys were commonly employed as a supplementary diagnostic tool across all four parasitic diseases. These surveys provided valuable insights into risk factors, transmission patterns, and disease awareness among livestock owners. By integrating observational, serological, molecular, and questionnaire-based approaches, the studies provided a comprehensive assessment of zoonotic parasitic diseases in Ethiopia.
Summary of findings from the meta-analysis
This meta-analysis of the included studies provides an in-depth assessment of the pooled prevalence, heterogeneity, and potential sources of variability for each parasitic disease. The findings highlight significant variations in prevalence rates across regions, host species, and diagnostic methods, emphasizing the need for subgroup analyses to better understand disease distribution and impact. The substantial heterogeneity were observed in this meta-analysis is likely driven by several factors, including variations in study design, host species, diagnostic techniques, and geographic distribution. The heterogeneity test confirmed significant differences between species, suggesting that biological and ecological factors play a major role in prevalence estimates.
Regional differences in prevalence are likely influenced by climate, altitude, and management practices. Warmer, humid environments may enhance oocyst survival and transmission, while variations in animal husbandry (e.g., free grazing vs. confined feeding) impact exposure risk. Differences in veterinary healthcare infrastructure further affect disease detection and control.
Heterogeneity also arises from methodological variations among studies. Differences in sampling strategies (random, convenience, stratified), population characteristics, and study periods contribute to variability. Sample sizes ranged from small farms to large epidemiological studies, affecting prevalence estimates. Study designs varied, with some focusing on controlled farm settings and others on free-grazing animals, leading to inconsistencies in exposure risk. Additionally, the use of different diagnostic assays influenced reported prevalence rates.
The following sections summarize the key results for each disease, incorporating heterogeneity measures, statistical findings, and publication bias assessments.
Meta-analysis results for toxoplasmosis
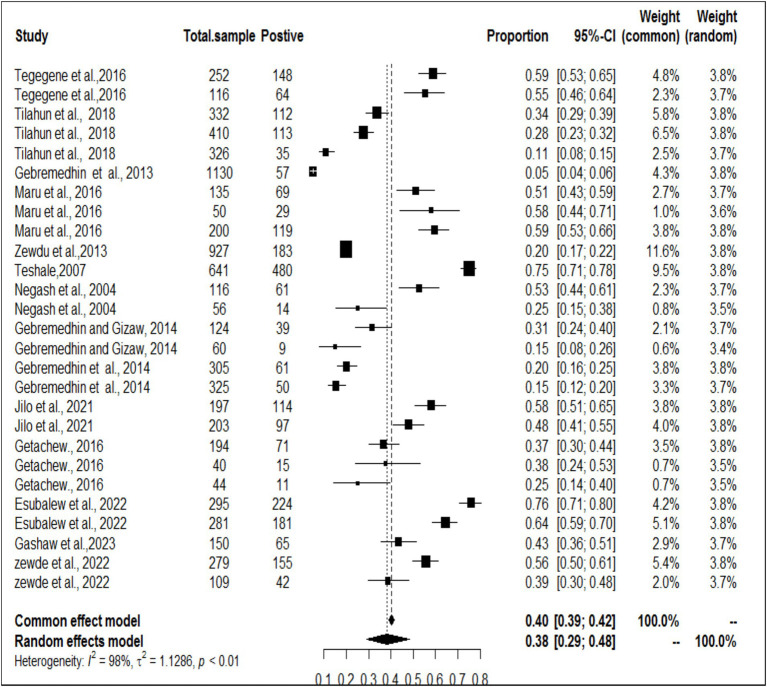
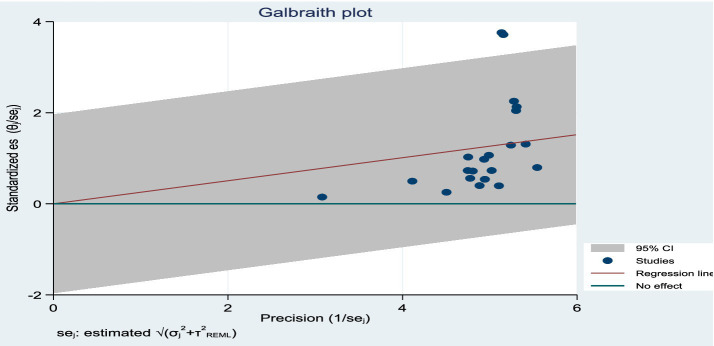
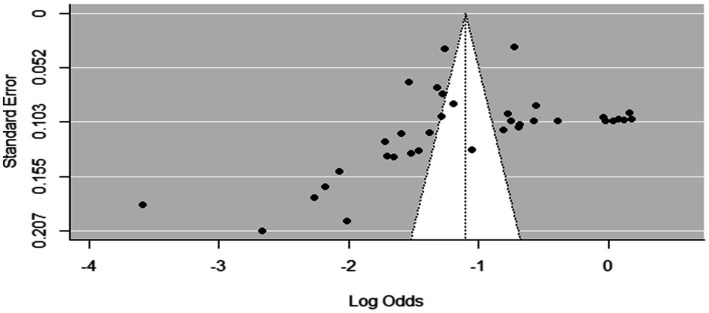
The estimated pooled prevalence was 38% (95% CI: 29–48%, Figure 2), indicating a relatively high burden of toxoplasmosis in domestic ruminants. However, significant heterogeneity was observed (I2 = 98%; τ2 = 1.1286; p < 0.01), suggesting variations in study design, host species, or diagnostic methods across the included studies. The Galbraith plot confirmed high inter-study heterogeneity, as nearly 95% of the studies fell outside the confidence interval (Figure 3). Further subgroup analyses based on host species and geographic location are essential to understand which animal groups or regions experience higher prevalence rates. Despite the heterogeneity, no significant publication bias was detected, as indicated by Egger’s regression asymmetry coefficient (b = 2.38; 95% CI: −2.19, 4.43; p > 0.05). The funnel plots (Figure 4) also demonstrated a symmetrical distribution, reinforcing the robustness of the meta-analysis findings.
Figure 2.
Random forest plots with overall prevalence for toxoplasmosis in ruminants.
Figure 3.
Galbraith plot showing the heterogeneity of the prevalence of toxoplasmosis.
Figure 4.
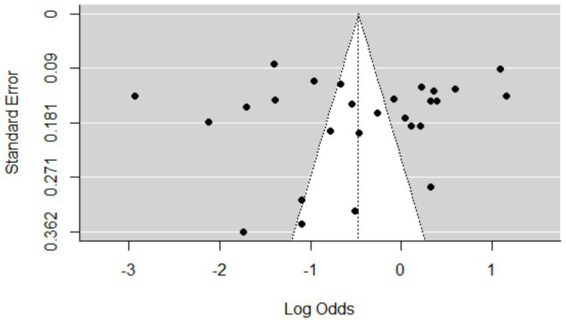
Funnel plots of the standard error by log odds of the prevalence of toxoplasmosis.
Sensitivity
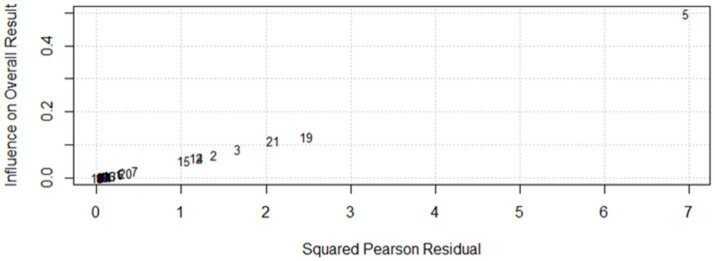
Baujat diagnostic plots (54) was used to detect studies that overly contribute to the heterogeneity in a meta-analysis. The plot shows the contribution of each study to the overall heterogeneity (as measured by Cochran’s Q) on the horizontal axis and its influence on the pooled effect size on the vertical axis. Accordingly, a study by author ID number 5 which denotes Sheferaw and Abdu (1) was found to be an influential study graphically which contributed overly to the overall heterogeneity, though it did not affect the pooled effect size significantly as shown in the leave-one-out meta-analysis result (Figure 5). High heterogeneity (I2 = 98%) suggests further subgroup is needed to explore sources of variability. Finally, a leave-one-out analysis was conducted to evaluate the influence of a single study on the overall effect size estimate. In this analysis, a specific study was excluded, and a meta-analysis was performed on the remaining (n−1) studies. If the confidence interval of the study did not encompass the overall effect size estimate, it was deemed that the study had a substantial impact on it. In this study, the overall effect size estimate was 0.38, and it fell within the confidence intervals of all the studies. As a result, excluding one study did not have a substantial impact on the overall effect size estimate (Figure 2). Similarly, sensitivity analyses have been conducted in a comparable manner for toxoploamosis disease studies to assess the impact of individual studies on the overall pooled prevalence estimates. For example, in the study by Abebe et al. (55), the graphical sensitivity analysis indicated that this study had a notable influence on the reported prevalence of cryptosporidiosis, suggesting that its inclusion may have contributed to variations in prevalence estimates. Likewise, in the study by Higgins and Thompson (47), sensitivity analysis revealed that this study was influential in the context of cystic echinococcosis, demonstrating a similar pattern of influence.
Figure 5.
Diagnosis of potential outliers from the included studies.
However, despite these individual studies showing some degree of influence in graphical representations, their exclusion did not significantly alter the overall pooled prevalence estimates. This suggests that while certain studies may introduce variability at a localized level, the robustness of the meta-analysis remains intact, and the general conclusions drawn from the pooled data are not substantially affected. These findings reinforce the stability of the prevalence estimates and highlight the reliability of our analytical approach in accounting for potential outliers.
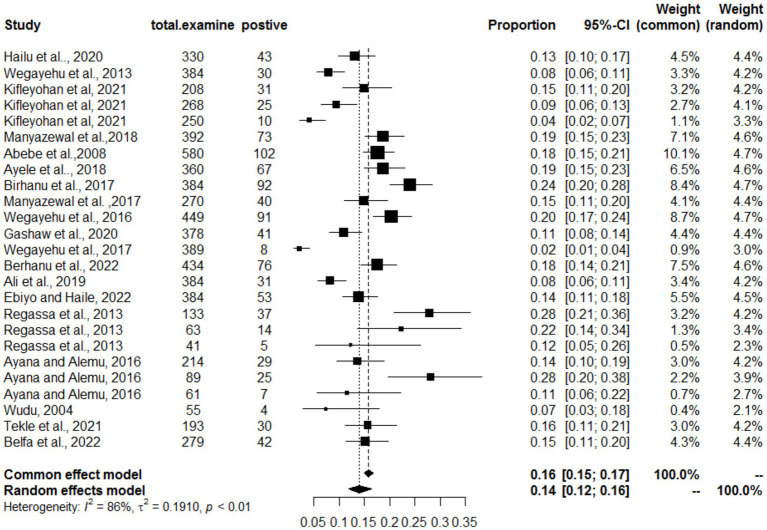
Meta-analysis results for cryptosporidiosis
For cryptosporidiosis, 19 studies were analyzed, revealing a pooled prevalence of 14% (95% CI: 12–16%). The heterogeneity was substantial (I2 = 86%; τ2 = 0.1910; p < 0.01), indicating that study-level differences significantly influenced the estimated prevalence rates. The forest plot (Figure 6) illustrates variations in prevalence across studies, while the funnel plot (Figure 7) suggests that the studies were evenly distributed without asymmetry, indicating no significant publication bias. The differences in diagnostic techniques (microscopy vs. molecular methods) and host species (calves, lambs, and adult ruminants) may contribute to the heterogeneity. Additionally, environmental factors such as hygiene, management practices, and seasonal variations could also influence prevalence rates. The Egger regression test (b = 1.2953; CI: 0.1792, 1.4114; p = 0.2007) further confirmed the absence of statistically significant publication bias in the meta-analysis.
Figure 6.
Random forest plots with overall prevalence of cryptosporidiosis in animals.
Figure 7.
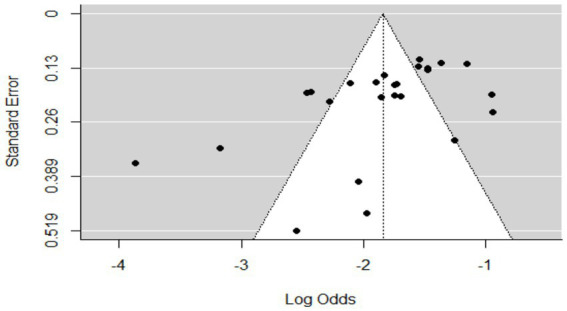
Funnel plots of the standard error by log odds of the prevalence of cryptosporidiosis.
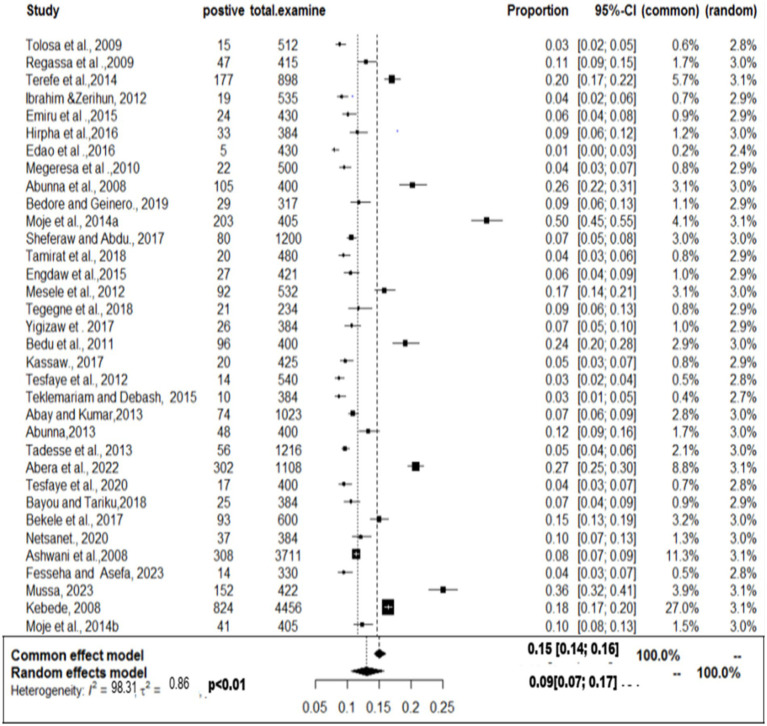
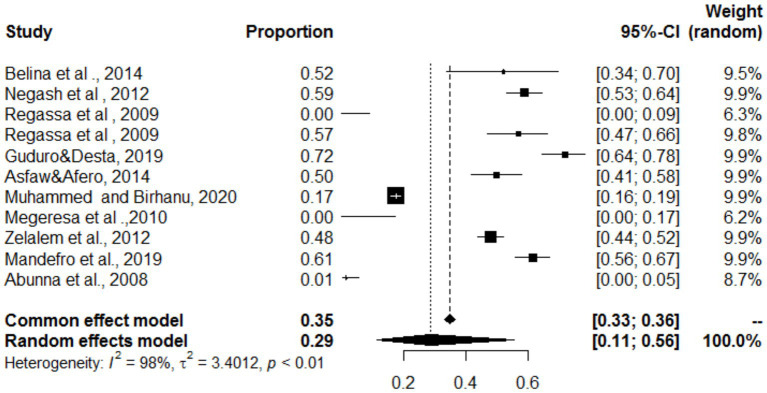
Meta-analysis results for bovine cysticercosis
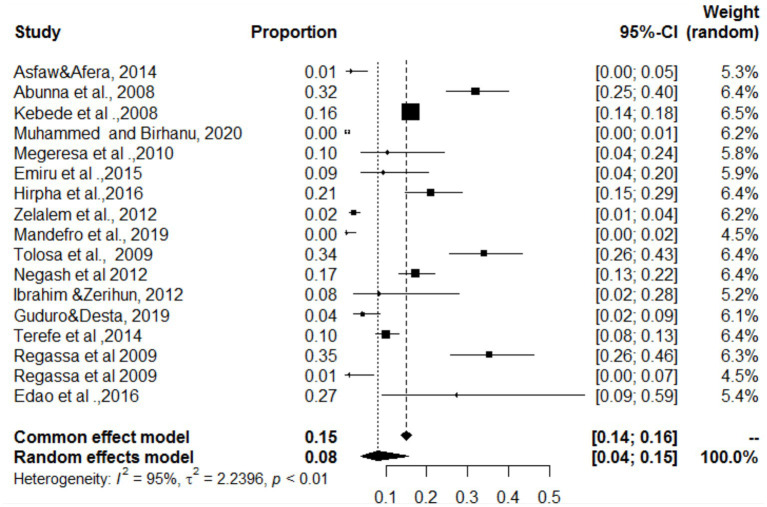
The overall pooled prevalence was 9% (95% CI: 7–17%: Figure 8), highlighting a moderate burden of bovine cysticercosis in Ethiopia. The analysis showed high between-study variability (τ2 = 0.861; H2 = 59; I2 = 98.31%), reinforcing the need for subgroup analyses based on host characteristics and geographic factors. The funnel plot (Figure 9) suggested asymmetry, indicating the possibility of publication bias, which was confirmed by Egger’s regression test (b = 0.3229; 95% CI: 0.1792, 0.4114; p = 0.02812). This suggests that smaller studies may be underreported or that there is selective reporting of significant results, potentially influencing the pooled prevalence estimate.
Figure 8.
Random forest plots showing the overall prevalence of bovine cysticercosis in animals.
Figure 9.
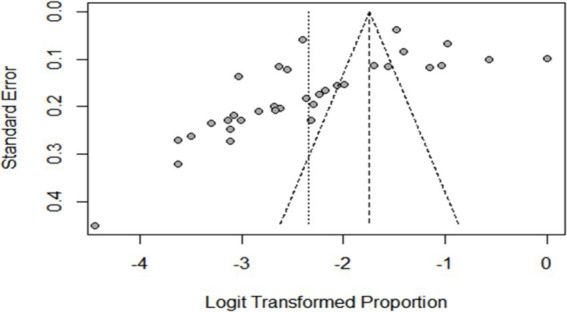
Funnel plot of standard errors by logit of the prevalence of bovine cysticercosis.
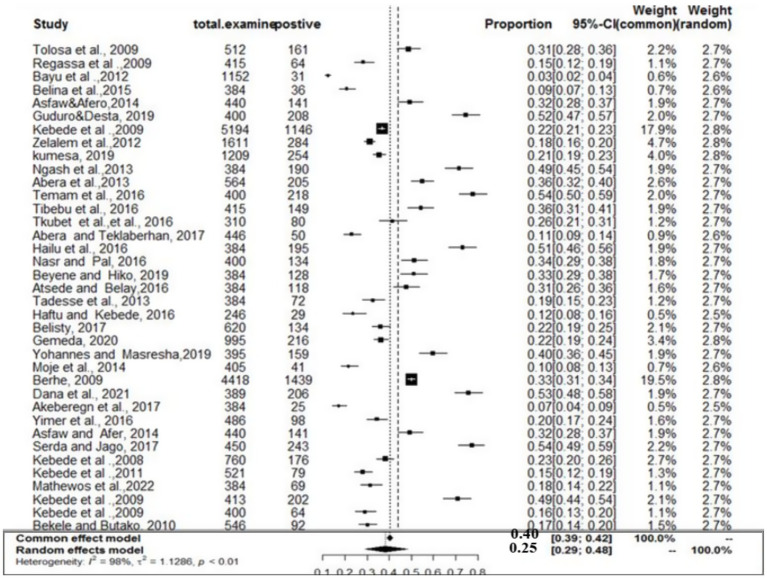
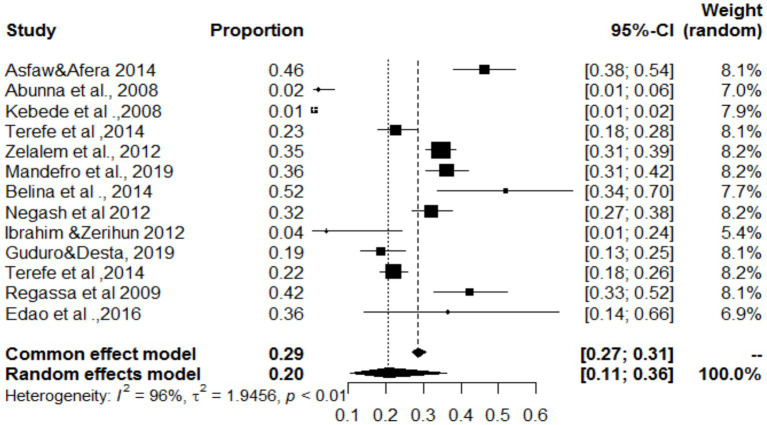
Meta-analysis results for cystic echinococcosis
The pooled prevalence was estimated at 25% (95% CI: 29–48%: Figure 10), indicating a substantial disease burden among domestic ruminants. High heterogeneity (τ2 = 0.7104; I2 = 98.0%) was observed, likely due to differences in study locations, sample sizes, and diagnostic approaches. The funnel plot (Figure 11) showed no asymmetry, suggesting that smaller studies were not systematically underrepresented. Similarly, Egger’s test (b = −2.58; 95% CI: 0.17–0.31; p = 0.08075) confirmed that publication bias was not statistically significant. The high prevalence and widespread distribution of cystic echinococcosis underscore its public health and economic impact, reinforcing the need for improved disease control strategies in Ethiopia.
Figure 10.
Random forest plots showing the overall prevalence of cystic echinocococcosis in domestic food ruminants.
Figure 11.
Funnel plot of standard error by log odds of prevalence estimates of cystic echinococcosis.
Subgroup analysis
A subgroup analysis of each of the four parasitic diseases (toxoplasmosis, cryptosporidiosis, bovine cysticercosis and cystic echinococosis) was conducted on the basis of year, species, and region. All of the analyses of exploratory outcomes showed considerable heterogeneity (I2 > 71). Significant statistical heterogeneity in the subgroup analysis reveals a likely interaction among exploratory variables.
Subanalysis results of toxoplasmosis
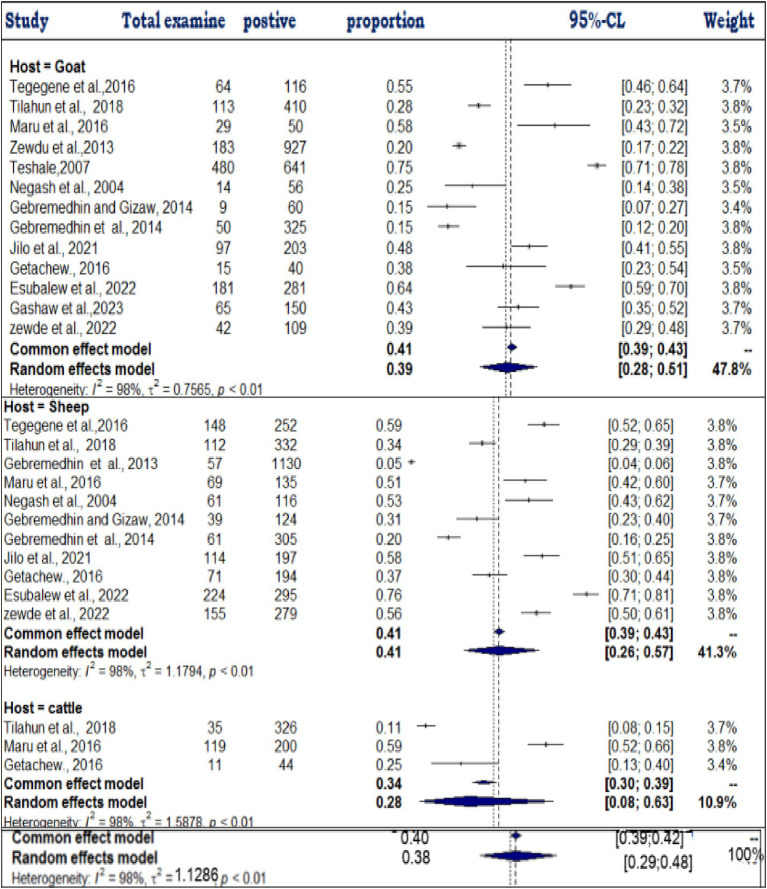
The subanalysis of toxoplasmosis prevalence across different animal species revealed substantial variability, which we now interpret more clearly. The studies were categorized into three groups based on animal species: goats (n = 13), sheep (n = 11), and cattle (n = 3). The pooled prevalence estimates for toxoplasmosis varied across these species, with sheep showing the highest prevalence at 41% (95% CI: 26–57%; I2 = 98%), followed by goats at 39% (95% CI: 28–51%) and cattle at 28% (95% CI: 8–63%) (Figure 12). Despite the high level of within-study variability across all species (I2 = 98%), the heterogeneity test revealed statistically significant differences (Q = 180.28; D.F. = 18; p < 0.001). However, no significant difference in the number of studies between species was observed (Q = 0.8; D.F. = 2; p = 0.7855), indicating that the observed variation in prevalence is not due to the number of studies per group but likely reflects other factors, such as ecological or methodological influences.
Figure 12.
Prevalence of toxoplasmosis among different species of animals.
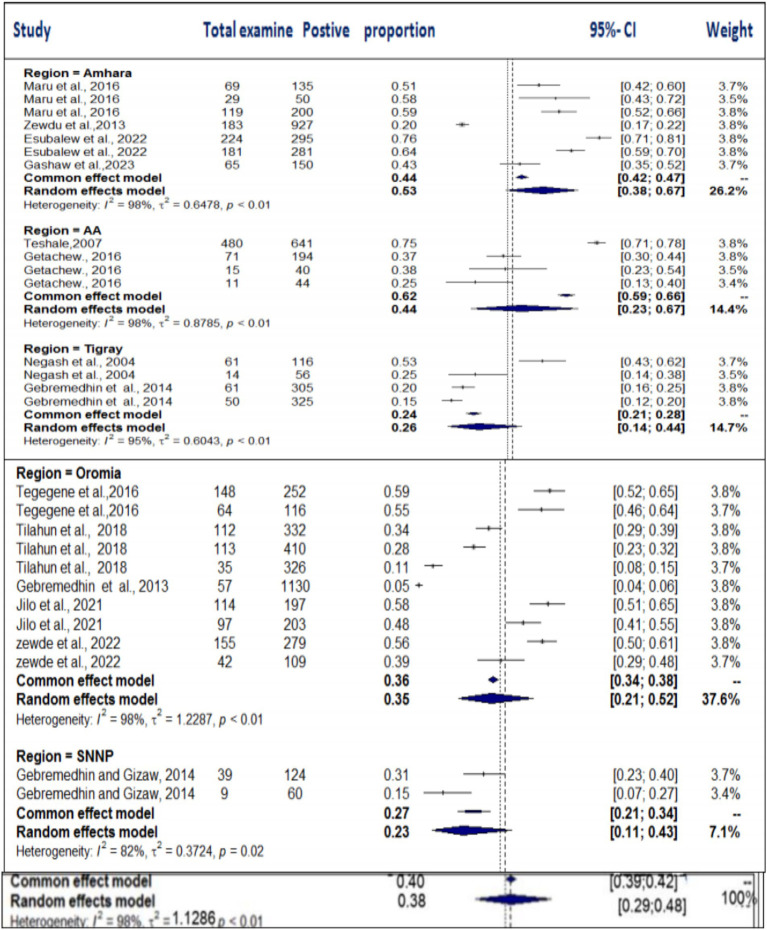
The regional variability analysis also provided important insights. We found similar levels of variability (I2 = 98%) across the Amhara, AA, and Oromia regions. The pooled prevalence for toxoplasmosis was highest in the Amhara region (53%), followed by AA (44%), Tigray (26%), Oromia (35%), and SNNP (23%) (Figure 13). The regional heterogeneity test (Q = 8.21; D.F. = 4; p < 0.00841) indicated statistically significant differences, suggesting that regional factors, such as climate and management practices, may influence the prevalence of toxoplasmosis.
Figure 13.
Subgroup analysis of the distribution of toxoplasmosis based on the study region.
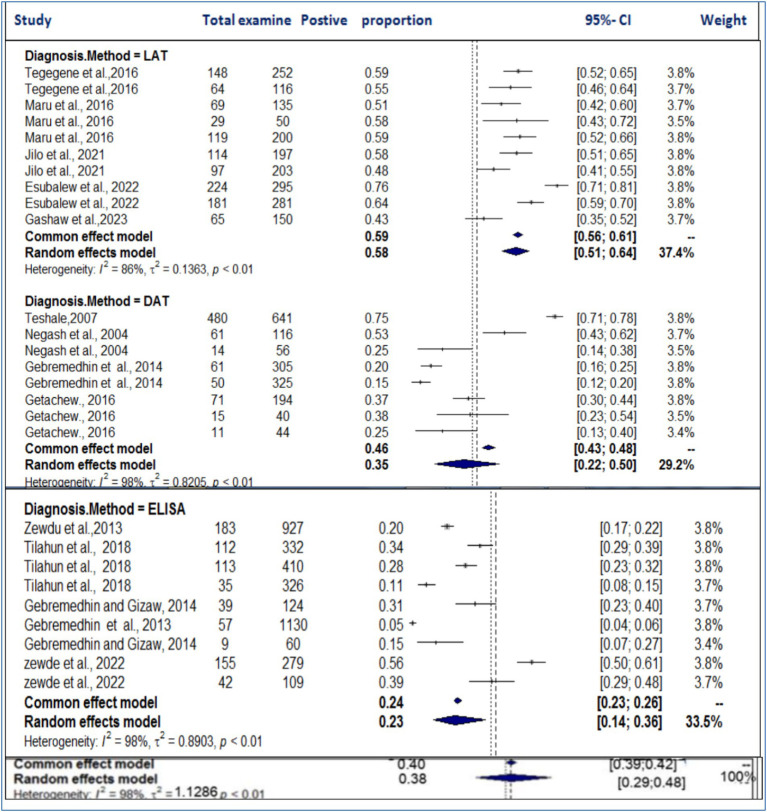
Furthermore, subgroup analysis based on diagnostic techniques (LAT, DAT, and ELISA) revealed considerable heterogeneity (I2 > 86%) across all methods (Figure 14). The highest degree of heterogeneity (I2 = 98%) was observed in studies using the DAT and ELISA diagnostic methods. The pooled prevalence was highest in studies using the LAT method (58%), followed by DAT (35%) and ELISA (23%). The subgroup difference test showed a statistically significant group effect (Q = 23.49; D.F. = 2; p < 0.0001), suggesting that the choice of diagnostic method plays a significant role in the variation in prevalence estimates. We have emphasized the potential clinical and ecological implications of these findings and noted that the variability observed may reflect underlying biological, environmental, or methodological factors that were not fully addressed in the analysis. Regional factors such as climate, management practices, and differences in diagnostic methods could contribute to the observed discrepancies in prevalence estimates. We also acknowledge that further research is needed to explore the biological, ecological, and methodological factors that contribute to these variations. This additional research would help provide a more comprehensive understanding of the true prevalence of toxoplasmosis and its implications for livestock health and management.
Figure 14.
Subanalysis of the pooled prevalence of toxoplasmosis by method of diagnosis.
Subanalysis results of cryptosporidiosis
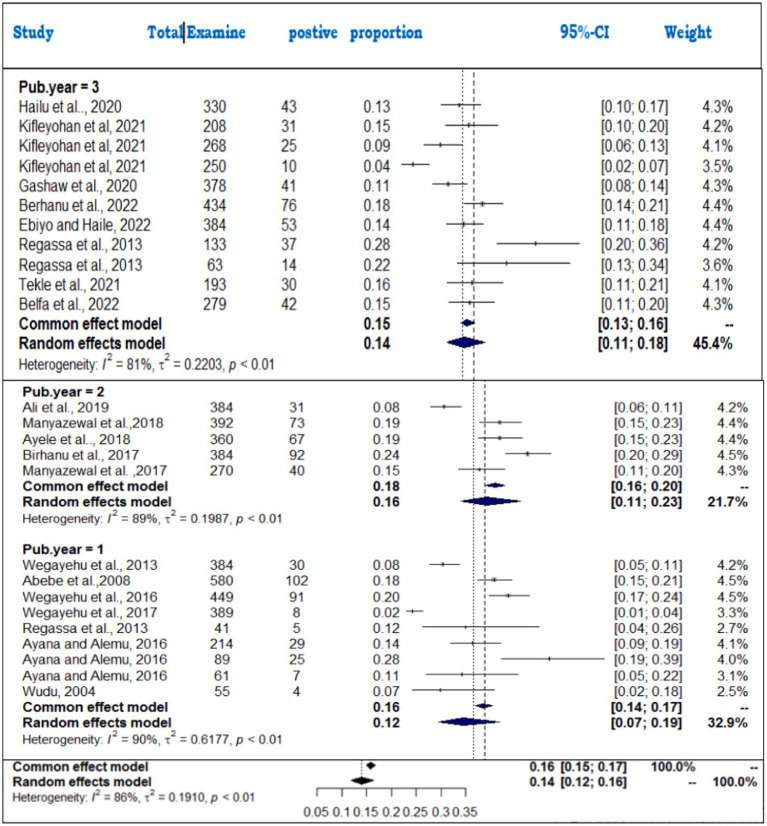
The subanalysis of cryptosporidiosis prevalence across different publication years revealed the highest prevalence between 2017 and 2019 (16%) compared to other periods (14% for 2020–2023 and 12% for 2004–2016) (Figure 15). Statistically significant differences were found between the time periods (Q = 8.69; D.F. = 2; p = 0.001), with high heterogeneity (I2 = 89% for 2017–2019, I2 = 81% for 2020–2023, and I2 = 89% for 2004–2016). The variation in prevalence could reflect improvements in detection methods over time, but further investigation is needed to understand the driving factors.
Figure 15.
Forest plots of subgroup analysis of cryptosporidiosis by publication year. 1 = 2004–2016, 2 = 2017–2019, 3 = 2020–2023.
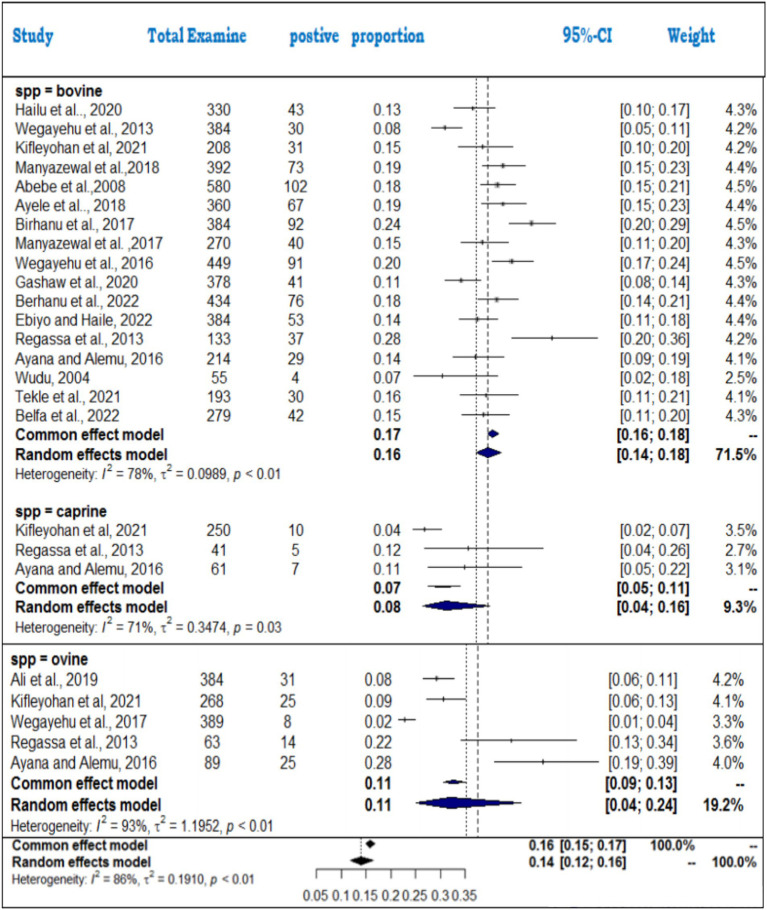
For the animal species analysis, bovines had the highest pooled prevalence (16%), followed by ovines (11%) and caprines (8%) (Figure 16). The heterogeneity was highest in ovine (I2 = 93%), followed by bovines (I2 = 78%) and caprines (I2 = 71%). The significant subgroup effect (Q = 33.3; D.F. = 2; p < 0.001) indicates that species differences play a key role in prevalence rates, and specific factors, such as immune responses and management practices, likely contribute to this variability.
Figure 16.
Subgroup analysis of cryptosporidiosis by animal species.
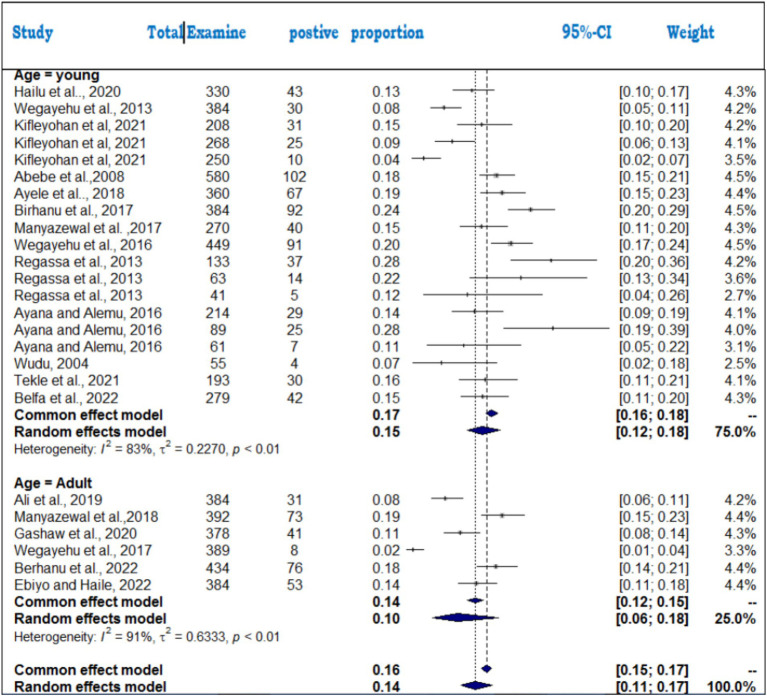
The age category subanalysis showed more variability in adults (I2 = 91%) than in young animals (I2 = 83%). The pooled prevalence for young animals (15%) was slightly higher than for adults (14%) (Figure 17), suggesting that age-related differences might influence susceptibility to cryptosporidiosis. These findings emphasize the need for further exploration of factors such as species, age, and time period to better understand cryptosporidiosis prevalence and improve control strategies.
Figure 17.
Subgroup analysis by age category to examine cryptosporidiosis in foods of animal origin.
Meta-regression
Uni-variable meta-regression was conducted with animal species (goat, sheep and cattle), and region, sample size (continuous variable) and diagnosis method used as categorical variables using the mixed effect model, in case of toxoplasmosis (Table 3). These variables were subjected to assessment to see a linear relationship with the dependent variable that is the effect sizes. Those variables with a p-values < 0.1 were used in the multivariable meta-regression analysis. Only region and diagnostic methods were found to be significant at p-values < 0.1 and therefore were included in the final multivariable analysis. The multivariable meta-regression model as a whole explained 21.96% amount of heterogeneity, while using diagnostic method and regional variability. Even when controlling for diagnostic methods compared to antibody detections (Table 5).
Table 5.
Final multivariable meta-regression model for toxoplasmosis.
| Variables | Coefficient (95% CI) | P-value | Adjusted R2 |
|---|---|---|---|
| Diagnostic methods | 21.96% | ||
| DAT | Ref. | ||
| LAT | 6.8845 (2.0784, 11.6906) | 0.0102 | |
| ELISA | 7.5426 (2.3562, 12.7290) | 0.0094 | |
| Region | 34.7% | ||
| Amhara | Ref. | ||
| AA | −6.5243 (−11.3697, −1.6788) | 0.0129 | |
| Oromia | 0.26 [−0.504, 1.03] | 0.5029 | |
| SNNP | 0.85 [0.138, 1.55] | ||
| Tigray | 0.26 [−0.504, 1.03] | 0.01303 | |
| Sample size | −0.0008 (−0.0034, 0.0019) | 0.5353 | 4.7% |
R2, Coefficient of determination.
In case of cryptosporidiosis meta-regression was performed based on animal species (bovine, ovne and caprine), publication year (2004–2016, 2017–2019, and 2020–2023) and age category of the animals (Adult and Yung). Unfortunately, all the moderators were found to be significant at p-values < 0.1 at univariable meta-regression. Therefore, all of the three variables were included in multivariable meta-regression analysis (Table 6).
Table 6.
Final multivariable meta-regression model (cryptosporidiosis).
| Variables | Component | Coefficient | 95%CI | P-value | Adjusted R2 |
|---|---|---|---|---|---|
| Publication year | Intercept | −1.7494 | 2.2691–1.2297 | <0.0001 | 0.2651 |
| 2004–2016 | Ref | 23% | |||
| 2017–2019 | 0.5992 | −0.2325, 1.4308 | 0.01579 | ||
| 2020–2023 | 1.6383 | 0.5072, 2.7693 | 0.0045 | ||
| Age | Adult | Ref. | 18.4% | ||
| Yung | −0.4068 | −1.5234, 0.7098 | 0.04752 | ||
| Animal species | Bovine | Ref. | 28.5% | ||
| Caprine | −0.6475 | 3.0017, 1.7067 | 0.5898 | ||
| Sheep | 0.0880 | −1.4906, 1.6665 | 0.9130 |
Subanalysis results of cysticercosis and cystic echinococcosis
Subgroup analysis of bovine cysticercosis and cystic echinococcosis based on study region and publication year was performed. The results of the subanalysis, such as the heterogeneity (I2), proportion, 95% CI, and test of between-study heterogeneity (Q), are summarized in Table 7. The subgroup difference test suggested that there was a statistically significant subgroup effect (Q = 11.69; DF = 1; p = 0.0029), implying that the publication year category was significantly associated with the prevalence of bovine cysticercosis in cattle, whereas the subgroup difference test suggested that there was a statistically significant subgroup effect (Q = 11.69; DF = 1; p = 0.0029) in the study region and that the prevalence of cystic echinococcosis (Q = 6.67; df = 4; p = 0.023).
Table 7.
Summary of the results of the subgroup analysis of bovine cysticercosis and cystic echinococcosis according to study region and publication year.
| Moderators | No studies | Proportion | 95%CI | tau2 | I2 (%) | Test for subgroup differences | |
|---|---|---|---|---|---|---|---|
| Bovinecysticercosis | |||||||
| Region wise | SNNPR | 7 | 12% | (0.057; 0.234) | 1.1723 | 97 |
Q = 5.94; df = 4 p = 0.2037 |
| Oromia | 13 | 9% | (0.0510; 0.158) | 1.2863 | 97 | ||
| Tigray | 2 | 8% | (0.0713; 0.090) | 0.0021 | 3 | ||
| AA | 4 | 5% | (0.026; 0.077) | 0.2728 | 84 | ||
| Amhara | 8 | 16% | (0.0579; 0.128) | 0.3570 | 97 | ||
| Year wise | 2015) | 19 | 8.12% | (0.05; 0.121) | 0.770 | 98 |
Q = 11.69; df = 1 p = 0.0029 |
| 2015) | 14 | 9.02% | (0.061; 0.146) | 0.86 | 97 | ||
| Cystic echinococcosis | |||||||
| Region-wise | SNNPR | 7 | 22% | 0.1359; 0.3278 | 0.5679 | 97 |
Q = 6.67; df = 4 p = 0.023 |
| Oromia | 12 | 33% | (0.2245; 0.4531) | 0.8474 | 98 | ||
| Tigray | 5 | 29% | (0.2434; 0.3338) | 0.0565 | 97 | ||
| AA | 5 | 14% | (0.0649; 0.2773) | 0.9401 | 97 | ||
| Amhara | 8 | 23% | (0.1491; 0.3428) | 0.6043 | 98 | ||
| Year-wise | 2008–2013 | 13 | 22% | (0.1480; 0.3185) | 0.8172 | 98 |
Q = 0.66; df = 2 p = 0.7206 |
| 2014–2016 | 14 | 26% | (0.1891; 0.3370) | 0.5374 | 97 | ||
| 2017–2023 | 10 | 28% | (0.1756; 0.4137) | 0.9190 | 98 | ||
Estimated proportions of cystic echinococcosis and Cysticercos bovis found in the same organ
In this study, C. bovis and hydatid cysts were identified as the most important foodborne parasites at post-mortem examination at the slaughterhouse. The main organs affected by these parasites include the lungs, heart, liver, kidneys, and shoulder and masseter muscles. The included studies were categorized as (i) articles examining both parasites simultaneously in the same organ, (ii) studies treating only C. bovis in a single organ, and (iii) articles addressing only the distribution of cystic echinococcosis in a single organ (kidney).
Simultaneous distribution of Cysticercos bovis and cystic echinococcosis in the lungs, heart, and liver
In the present study, the lungs, heart and liver were affected by both C. bovis and cystic echinococcosis (same study). The overall pooled prevalence rates of both C. bovis co-infections with cystic echinococcosis of the lungs, liver and heart were 29, 20 and 8%, respectively (Figures 18–20). In the case of the lung, as shown in Table 6, a subanalysis was performed on the basis of the categorization of studies under C. bovis (eight articles) and cystic echinococcosis (only three articles), and the pooled prevalence was found to be 51 and 1%, respectively. Similarly, subanalyses of the included studies were based on parasite type, and the pooled prevalence rates of C. bovis and hepatic hydatosis were 8 and 36%, respectively (Table 6). In addition, subgroup analysis of studies based on parasite type revealed that the pooled prevalence of C. bovie with cystic echinococcosis in the heart was 14 and 3%, respectively (Table 6).
Figure 18.
Pooled prevalence of C. bovis and cystic echinococcosis in the lungs.
Figure 20.
Pooled prevalence of bovine cysticercosis and cystic echinococcosis in the liver.
Figure 19.
Pooled prevalence of bovine cysticercosis and cystic echinococcosis in the heart.
Overall estimation of the distribution of cystic echinococcosis in the kidney
In our study, the kidney was the only organ affected only by cystic echinococcosis. Among a total of 37 studies, six reported infection of the kidney by cystic echinococcosis. The pooled proportion of cystic echinococcosis in the kidney was 2% (95% CI; 1–6%), with high inter-study heterogeneity (I2 = 96%; p < 0.01) (Supplementary material 3).
Overall distribution of Cysticercos bovis alone in different organs at post-mortem inspection
In this meta-analysis, the highest levels of C. bovis were observed in the shoulder muscle, and the tongue was the second most susceptible organ. Regarding the anatomical distribution of the cysts, 32% of the affected organs were located in the shoulder region, with minimal heterogeneity observed in this proportion (Cochran’s Q = 11.21 and p value of 0.0474, I2 statistic of 55%). The deltoid, 21% (I2 = 83%; Q = 53.32, p = 0.000) of the tongue, 19% (I2 = 66.3%, Q = 26, 72, p = 0.0016) and 6% (I2 = 86.3%, Q = 46.52, p = 0.0001) of the tongue (Supplementary materials 4a–d). Among the organs examined, the diaphragm was least affected by C. bovis, as summarized in Table 8.
Table 8.
Summary of parasite zoonotic distributions in different organs at post-mortem inspection.
| Parasite | Organ | PP (%) | N | 95%CI | I 2 | tau2 | Test of heterogeneity |
|---|---|---|---|---|---|---|---|
| Q, d.f., and P value | |||||||
| Hydatosis and C. bovis | Lung | 29 | 11 | (11–56%) | 98 | 3.4 | |
| Hydatosis and C. bovis | Liver | 20 | 13 | (11–36%) | 96 | 1.94 | 282.80, 12, and <0.0001 |
| Hydatosis and C. bovis | Heart | 8 | 17 | (4–15%) | 95 | 2.23 | 340.1, 16, and <0.0001 |
| C. bovis | Shoulder | 32 | 6 | (27–37%) | 55 | 0.039 | 11.21, 5, and 0.0474 |
| C. bovis | Masseter | 19 | 10 | (16–22%) | 66 | 0.045 | 26.72, 9, and 0.0016 |
| C. bovis | Tongue | 21 | 10 | (15–30%) | 83 | 0.43 | 53.32, 9, and 0.000 |
| C. bovis | Diaphragm | 6 | 6 | (3–14%) | 98 | 0.98 | 46.5, 5, and 0.0001 |
| C. bovis | Lung | 1 | 11* (2) | (0–4%) | 0 | 0 | 629.82, 1, and <0.0001 |
| Hydatosis | Lung | 51 | (38–64%) | 99 | 0.54 | ||
| C. bovis | Liver | 8 | 13* (2) | (2–26%) | 97 | 2.58 | 6.62, 1, and 0.0101 |
| Hydatosis | Liver | 36 | (29–44%) | 82 | 0.17 | ||
| C. bovis | Heart | 14 | 17* (2) | (7–26%) | 96 | 1.76 | 6.35, 1, and 0.0117 |
| Hydatosis | Heart | 3 | (1–8%) | 93 | 1.6 | ||
| Hydatosis | Kidney | 2 | 6 | (1–6%) | 96 | 1.46 |
Discussion
The present systematic review aimed to assess the prevalence of common animal-origin foodborne parasitic zoonoses in the meat of domestic ruminants in Ethiopia from 2008 to 2023. Our meta-analysis reveals a concerning picture, with pooled prevalence estimates of 38% for toxoplasmosis, 25% for cystic echinococcosis, 14% for cryptosporidiosis, and 9% for bovine cysticercosis. These figures underscore the significant public health and economic burdens imposed by these infections on Ethiopian livestock production. The highest pooled prevalence was for toxoplasmosis (38%), a finding that is likely linked to the limited availability of specific treatments for Toxoplasma gondii in animals, as well as the frequent cohabitation of small ruminants with cats, the parasite’s definitive host, especially in rural areas where most abattoir meat originates. It’s worth noting that this pooled prevalence falls within the range reported in previous Ethiopian studies, being lower than some (57, 74.9, 70.5, 43.1%) and higher than others (31, 33, 19.7%), which can be attributed to variation in serological tests employed, intermediate/definitive host relationship, sample size used, and geography. As reported by Woldesenbet and Harito (56), Toxoplasma gondii is a significant public health concern in Ethiopia, contributing to the high pooled prevalence observed in this meta-analysis. Almuzaini (57) emphasizes the role of food chain contamination in the widespread prevalence of zoonotic toxoplasmosis. The incidence rate of toxoplasmosis in Ethiopia exceeds those reported in some other African countries like Somalia (15.9%) and Sudan (32%), which points to the effect of sociocultural practices, husbandry methods, and public awareness. Despite the increasing knowledge and awareness of the disease there is still an increase in outbreaks because of poor and improper food handling. The need for further DNA vaccines is supported by Chen et al. (58). Dubey et al. (59) recently updated the worldwide rate of congenital toxoplasmosis in humans.
In terms of species, the pooled seroprevalence was highest in sheep (41%), followed by goats (39%) and cattle (28%). Factors contributing to the observed differences include variations in environmental factors, cat density, study design, management practices, diagnostic methods, host species, animal status, age, breed, serological tests, parasite/host genetics, immune response, cultural practices, and feeding habits. While our finding for goats (39%) aligns with some prior reports from Northwest and Southwest Ethiopia, it differs from others, underscoring the regional variation in Toxoplasma gondii seroprevalence. Hussein et al. (60) identification of Sarcocystis species in sheep and goats shows a clear connection with foodborne parasitic zoonoses. The seroprevalence of T. gondii in sheep (41%) was similar to findings from a study that reported a 37.9% seroprevalence in Central Ethiopia (61). The variation in seroprevalence between countries and continents is possibly due to wild animal contact and environmental conditions which influence the epidemiology of toxoplasmosis. Prevention in accessing pastures to grazing areas reduces the epidemiology of toxoplasmosis.
Cattle exhibited a lower pooled seroprevalence (28%) compared to small ruminants, possibly due to reduced contact with cats, with sheep and goats being often raised at higher altitudes, increasing infection risk. Diagnostic methods also played a role, with ELISA yielding the lowest (23%) and LAT the highest (58%) prevalence rates, due to technical factors and inherent test characteristics. Overall, the prevalence of toxoplasmosis in our study exceeds that reported in a molecular meta-analysis worldwide (14.7% in sheep tissues), which is possibly due to meat consumption methods and the slaughter system used.
Our meta-analysis also found an overall pooled prevalence of Cryptosporidium infection of 14% among commonly consumed domestic red meat animals. This aligns with a previous report from Bishoftu, Ethiopia (14%), but contrasts with findings from Egypt (20%). It’s worth noting that molecular characterization of Cryptosporidium is increasingly refining our understanding of transmission (62–65). Given the importance of this parasite especially in the immunocompromised population, a review of the global burden of Cryptosporidium highlights the ongoing need for improved diagnostics and treatments as recorded by Javed and Alkheraije (66). Giardia and Cryptosporidium spp. are frequently detected together in domestic animals. The difference in animal husbandry and grazing practice may have influenced the exposure and occurrence rates as mentioned by Megersa et al. (67). Stocking rates and husbandry systems are the major contributors to the variations in these countries.
Our analysis revealed higher infection rates in young animals (15%), suggesting that cryptosporidiosis poses a greater risk among calves, lambs, and kids in Ethiopia, this may be associated with colostrum related hygienic and diarrheic conditions. These findings align with numerous prior reports. Lower infection rates were reported in Iran at 4.7% (68), China at 5.09% (69), and Ethiopia at 13% (20). However higher prevalence rates were reported in various countries. Local climatic situations, strategic deworming, sample size, sampling method, study regions, diagnostic methods, feeding habits, environmental/farm hygiene, season, livestock management (production system), colostrum-feeding practices, herd size, composition and breed, confection and levels of close contact contributed to the differences of Cryptosporidium.
For cystic echinococcosis parasites, the overall pooled prevalence was 25%, which was comparable with that reported by Tkubet et al. (70) in the Tigray regional state. A number of factors contribute to this high prevalence of the parasite as reported by Dima and Jemal (71). Epidemiological characteristics and risk factors for cystic echinococcosis have been analyzed in national population-based field surveys (72). Recent studies highlight the ongoing presence of cystic echinococcosis (158) and emphasize the importance of understanding liver cystic echinococcosis. Abdulla (73), notes that the socioeconomic and habit of proper handling of slaughterhouse animals are important factors that influence the variations in prevalence. The variation in prevalence in different regions or localities might be due to differences in agro-ecologic situations. The serology for diagnosing human hepatic cystic echinococcosis and its relation with cyst staging has been reviewed (74).
In the present meta-analysis, the pooled prevalence of bovine cysticercosis was recorded (9%). There are various implementations in the rigorous implementation of meat inspections as noted by Netsanet et al. (75). The need for improved sanitation measures and proper animal hygiene practices should be implemented. The rigor of implementation of meat inspection measures, expansion of public health standards, and various hygienic practices resulted in a higher prevalence than 0.2 and 3% in South Africa and Rwanda, respectively. Mathewos et al. (76) note that the economic significance of the slaughterhouse and handling are important for the economic loss of the bovine.
While Qamar and Alkheraije (77) focused on Haemonchus contortus resistance, and Rehman et al. (78) provide a review of vaccine development against Fasciola it is important to take into considerations that the study also promotes vaccine formulation for other foodborne parasites and the impact of foodborne parasites on public awareness. Kebede et al. (79) noted the importance of risk factors in cattle trematodiasis and how it effects the trematode infection. Netsanet et al. (75) conducted studies on the effects of deltoid location followed by the tongue on masseter in varying degree of location and geographical and environmental conditions and blood kinetics. Organized and strict meat inspection practices in abattoirs and in-depth awareness of society can ensure that meat is free from high levels of metacestode infection. Overall, this investigation revealed that cystic echinococcosis and toxoplasmosis are the most prevalent zoonotic parasites. The control of these parasitic infections is not just an animal health issue; it’s also a public health and economic imperative for Ethiopia. Addressing these challenges requires a multifaceted approach encompassing improved sanitation, better animal husbandry practices, strategic anthelmintic use, and comprehensive public health education.
Limitations
Although this study has certain limitations, it provides significant epidemiological data on the prevalence and spread of endemic foodborne parasitic zoonosis in Ethiopia, which will be useful for disease prevention and control. First, there was an uneven distribution of studies across regions, hosts, study periods, study types, and sample sizes; this shows that the results may not accurately reflect the situation for Ethiopia. The second constraint is that cattle, sheep and goats can only be labeled or consumed by humans according to Ethiopian norms, and the third constraint could be derived from Ethiopian norms, e.g., Dogs, cats, horses, donkeys, mules and others that may not be consumed by Ethiopia do not help to draw representative results. Another important limitation of this review may be that the selected parasites do not represent all zoonotic parasitic diseases of animal origin. In addition, the diagnostic procedures and the sources of the data/samples may also introduce bias and may not represent the accurate distribution of the zoonotic foodborne parasite of animal origin, as most of the samples were collected at the slaughterhouse.
Conclusion
This meta-analysis indicated that the overall pooled prevalence of bovine cysticercosis and cystic echinococcosis among red meat sources from domestic ruminants in Ethiopia has increased in recent years. The subgroup analysis reveals a high overall pooled prevalence of toxoplasmosis in ovine species and cryptosporidiosis in cattle. These findings are expected to enhance on-going efforts to address food security in Ethiopia and support the implementation of food and nutrition security control strategies. The results emphasize the need for critical attention to communities with habits of consuming partially cooked or raw meat. Additionally, this study provides valuable insights that could shape future research and policy decisions related to food safety, particularly concerning the origin of animal-based foods. To address these public health and economic impacts effectively, multisectoral collaboration involving public health scientists, ecologists, veterinarians, economists, and other relevant stakeholders is essential. Furthermore, it is important to plan and implement joint in-service training programs for targeted workers across various sectors.
Funding Statement
The author(s) declare that no financial support was received for the research and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MF: Conceptualization, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. BE: Methodology, Validation, Writing – review & editing, Writing – original draft. AB: Conceptualization, Writing – original draft, Writing – review & editing. HD: Software, Validation, Writing – review & editing. MK: Methodology, Validation, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1501940/full#supplementary-material
References
- 1.Sheferaw D, Abebe R, Fekadu A, Olbamo G, Anjulo A, Yigebahal Z. Major causes of organ and carcass condemnation and associated financial losses in cattle slaughtered at Kombolcha ELFORA abattoir from 2008-2012, Ethiopia. Ethiop Vet J. (2019) 21:54–66. doi: 10.4314/evj.v21i1.5 [DOI] [Google Scholar]
- 2.CSA (2021). Federal Democratic Republic of Ethiopia central statistical agency agricultural sample survey 2020/21 (2013 E.C.) volume II report on livestock and livestock characteristics (private peasant holdings). Statistical bulletin 589, Addis Ababa. [Google Scholar]
- 3.Makita K, Fèvre EM, Waiswa C, Kaboyo W, Eisler MC, Welburn SC. Evidence-based identification of the most important livestock related zoonotic diseases in Kampala, Uganda. J Vet Med Sci. (2011) 73:991–1000. doi: 10.1292/jvms.11-0049, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. (2008) 451:990–3. doi: 10.1038/nature06536, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girma S. Assessment of awareness on food borne zoonoses and its relation with veterinary public health services in and around Addis Ababa, Ethiopia. J Public Health Epidemiol. (2012) 4:48–51. doi: 10.5897/JPHE12.004 [DOI] [Google Scholar]
- 6.Otte J, Pica-Ciamarra U. Emerging infectious zoonotic diseases: the neglected role of food animals. One Health. (2021) 13:100323. doi: 10.1016/j.onehlt.2021.100323, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rega S, Melese Y, Geteneh A, Kasew D, Eshetu T, Biset S. Intestinal parasitic infections among patients who visited Woldia comprehensive specialized Hospital’s emergency department over a six-year period, Woldia, Ethiopia: a retrospective study. Infect Drug Resist. (2022) 15:3239–48. doi: 10.2147/IDR.S369827, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomel BB. Control and prevention of emerging parasitic zoonoses. Int J Parasitol. (2008) 38:1211–7. doi: 10.1016/j.ijpara.2008.05.001, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, Chemaly M, et al. Public health risks associated with food-borne parasites. EFSA J. (2018) 16:e05495. doi: 10.2903/j.efsa.2018.5495, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komba EV. A literature survey of common parasitic Zoonoses encountered at post-mortem examination in slaughter stocks in Tanzania: economic and public health implications. Biomed J Sci Tech Res. (2017) 1:1–6. doi: 10.26717/BJSTR.2017.01.000419 [DOI] [Google Scholar]
- 11.Tamirat Bhabtamu T, Mu-uz G. Prevalence, financial impact and public health significance of Cysticercus bovis at Bahir Dar municipal abattoir, Ethiopia. J Vet Med Anim Health. (2018) 10:14–20. doi: 10.5897/JVMAH2017.0650, PMID: 38147025 [DOI] [Google Scholar]
- 12.WHO-FAO-OIE . Report of the WHO/FAO/OIE joint consultation on in collaboration with the Health Council of the Netherlands. (2004), 1–72. Available online at: http://apps.who.int/iris/bitstream/10665/68899/1/WHO_CDS_CPE_ZFK_2004.9.pdf.
- 13.Regassa A, Gizaw O, Abunna F, Abebe R, Beyene D, Megersa B, et al. Cryptosporidium in calves, lambs and kids at Haramaya, eastern Ethiopia. Ethiop Vet J. (2014) 17:81. doi: 10.4314/evj.v17i1.7 [DOI] [Google Scholar]
- 14.Abunna F, Tilahun G, Megersa B, Regassa A, Kumsa B. Bovine cysticercosis in cattle slaughtered at Awassa municipal abattoir, Ethiopia: prevalence, cyst viability, distribution and its public health implication. Zoonoses Public Health. (2008) 55:82–8. doi: 10.1111/j.1863-2378.2007.01091.x, PMID: [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . WHO/FAO/OIE guidelines for the surveillance, prevention and control of taeniosis/cysticercosis. Paris, France: World Organization for Animal Health; (2005). [Google Scholar]
- 16.Jongwutiwes S, Putaporntip C, Chantachum N, Sampatanukul P. Jejunal perforation caused by morphologically abnormal Taenia saginata saginata infection. J Infect. (2004) 49:324–8. doi: 10.1016/j.jinf.2003.09.003, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Hiko A, Seifu B. Spatiotemporal distribution and economic loss associated with bovine cysticercosis and human taeniasis in Ethiopia. Parasite Epidemiol Control (Internet). (2019) 4:e00078. doi: 10.1016/j.parepi.2018.e00078, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wegayehu T, Adamu H, Petros B. Prevalence of Giardia duodenalis and Cryptosporidium species infections among children and cattle in north Shewa zone, Ethiopia. BMC Infect Dis. (2013) 13:419. doi: 10.1186/1471-2334-13-419, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Work A, Wakjira B, Hailemichael B, Tolossa YH. Epidemiology of Cysticercus bovis/Taenia saginata and community perception about meat borne zoonosis in three selected districts of west Shoa zone of Oromia region, Ethiopia. Int J Curr Res Biol Med. (2021) 6:20–31. doi: 10.22192/ijcrbm.2021.06.02.003 [DOI] [Google Scholar]
- 20.Hailu M, Asmare K, Zewdu E, Sheferaw D, Gizaw D, Di V, et al. Cryptosporidium and Giardia infections in dairy calves in southern Ethiopia. Parasite Epidemiol Control (Internet). (2020) 10:e00155. doi: 10.1016/j.parepi.2020.e00155, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urquhart GM, Armous J, Dunkan JL, Dunn AM JF. Veterinary parasitology (2). Oxford, UK: Black Well Science; (1986), pp. 224–234. [Google Scholar]
- 22.Taylor MA, Coop RL, Wall R. Veterinary parasitology. Oxford: Wiley Blackwell Publishing Ltd; (2007), 23–594. [Google Scholar]
- 23.Teklay M, Berhe M, Tesfay T, Yohannes T. Echinococcosis: the status of cystic Hydatidosis in Ethiopia. Acta Sci Microbiol. (2019) 2:2581–3226. doi: 10.31080/ASMI.2019.02.0279 [DOI] [Google Scholar]
- 24.Fromsa A, Jobre Y. Infection prevalence of hydatidosis (Echinococcus granulosus, Batsch, 1786) in domestic animals in Ethiopia: a synthesis report of previous surveys. Ethiop Vet J. (2011) 15:11–33. doi: 10.4314/evj.v15i2.67691 [DOI] [Google Scholar]
- 25.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. (2003) 16:18–36. doi: 10.1128/CMR.16.1.18-36.2003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. (2004) 17:107–35. doi: 10.1128/CMR.17.1.107-135.2004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenter AM. Toxoplasma gondii in animals used for human consumption. Mem Inst Oswaldo Cruz. (2009) 104:364–9. doi: 10.1590/S0074-02762009000200033, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Leal FE, Cavazzana CL, de Andrade HF, Galisteo AJ, de Mendonça JS, Kallas EG. Toxoplasma gondii pneumonia in immunocompetent subjects: case report and review. Clin Infect Dis. (2007) 44:e62–6. doi: 10.1086/511871, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol. (2009) 39:895–901. doi: 10.1016/j.ijpara.2009.02.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. (2000) 30:1217–58. doi: 10.1016/S0020-7519(00)00124-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negash K, Beyenea D, Kumsa B. Cystic echinococcosis in cattle slaughtered at shashemanne municipal abattoir, south Central Oromia, Ethiopia: prevalence, cyst distribution and fertility. Trans R Soc Trop Med Hyg. (2013) 107:229–34. doi: 10.1093/trstmh/trt003, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Teshale S, Dumètre A, Dardé ML, Merga B, Dorchies P. Serological survey of caprine toxopiasmosis in Ethiopia: prevalence and risk factors. Parasite. (2007) 14:155–9. doi: 10.1051/parasite/2007142155, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Esubalew S, Nigatu SD, Kussa M, Tsegaye AA, Bogale B. Seroepidemiology of toxoplasma gondii in small ruminants in Northwest Ethiopia. Vet Parasitol. (2020) 22:100456. doi: 10.1016/j.vprsr.2020.100456, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Radostitis OM, Done SH. A text book of the disease of cattle, horse, sheep, goats and pigs In. Veterinary medicine. 10th ed. New York: Elsevier; (2007). 1517–5. [Google Scholar]
- 35.Abeywardena H, Jex AR, Gasser RB. A perspective on Cryptosporidium and Giardia, with an emphasis on bovines and recent epidemiological findings. Adv Parasitol. (2015) 88:243–301. doi: 10.1016/bs.apar.2015.02.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafieyan H, Alborzi A, Hamidinejat H, Tabandeh MR, Hajikolaei MR. Prevalence of Cryptosporidium spp. in ruminants of Lorestan province, Iran. J Parasit Dis. (2016) 40:1165–9. doi: 10.1007/s12639-014-0642-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao L, Fayer R, Ryan U, Upton SJ. Cryptosporidium Taxonomy: recent advances and implications for public health. Clin Microbiol Rev. (2004) 17:72–97. doi: 10.1128/CMR.17.1.72-97.2004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindsay DS, Upton SJ, Owens DS, Morgan UM, Mead JR, Blagburn BL. Cryptosporidium andersoni n. sp. (Apicomplexa: Cryptosporiidae) from cattle, Bos taurus. J Eukaryot Microbiol. (2000) 47:91–5. doi: 10.1111/j.1550-7408.2000.tb00016.x, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Slifko TR, Smith HV, Rose JB. Emerging parasite zoonoses associated with water and food. Int J Parasitol. (2000) 30:1379–93. doi: 10.1016/S0020-7519(00)00128-4, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Smith HV, Cacciò SM, Cook N, Nichols RAB, Tait A. Cryptosporidium and Giardia as foodborne zoonoses. Vet Parasitol. (2007) 149:29–40. doi: 10.1016/j.vetpar.2007.07.015, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Brook E, Hart CA, French N, Christley R. Prevalence and risk factors for Cryptosporidium spp. infection in young calves. Vet Parasitol. (2008) 152:46–52. doi: 10.1016/j.vetpar.2007.12.003, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Aboelsoued D, Abdel Megeed KN. Diagnosis and control of cryptosporidiosis in farm animals. J Parasit Dis. (2022) 46:1133–46. doi: 10.1007/s12639-022-01513-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddique F, Abbas RZ, Babar W, Mahmood MS, Iqbal A. Section A: parasitic diseases cryptosporidiosis. Vet Pathobiol Public Health. (2021) 2021:63–75. doi: 10.47278/book.vpph/2021.001 [DOI] [Google Scholar]
- 44.Macpherson CNL. Human behaviour and the epidemiology of parasitic zoonoses. Int J Parasitol. (2005) 35:1319–31. doi: 10.1016/j.ijpara.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma T. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. BMJ Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Tegegne A, Hiko A, Elemo KK. Bovine cysticercosis and human taeniasis: animal-human health and economic approach with treatment trends in Kombolcha town, Wollo, Ethiopia. Int J One Health. (2018) 4:15–21. doi: 10.14202/IJOH.2018.15-21 [DOI] [Google Scholar]
- 49.Kebede N. Cysticercosis of slaughtered cattle in northwestern Ethiopia. Res Vet Sci. (2008) 85:522–6. doi: 10.1016/j.rvsc.2008.01.009, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Maru M, Damtie D, Kenubih A, Maru A, Adugna B, Dagnachew S, et al. Toxoplasma gondii infection in slaughtered domestic ruminants in Northwest Ethiopia: occurrence, bioassay and virulence assessment. J Parasit Dis (Internet). (2022) 46:429–39. doi: 10.1007/s12639-022-01466-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gashaw M, Mitiku BA, Yihun TY. Seroprevalence and associated risk factors of toxoplasma gondii infection in immuno-compromised women and sheep of Bahir Dar City, Ethiopia. Ethiop Vet J. (2023) 27:93–111. doi: 10.4314/evj.v27i1.5 [DOI] [Google Scholar]
- 52.Kifleyohannes T, Nødtvedt A, Debenham JJ, Terefe G, Robertson LJ. Cryptosporidium and Giardia in livestock in Tigray, northern Ethiopia and associated risk factors for infection: a cross-sectional study. Front Vet Sci. (2022) 8:1–11. doi: 10.3389/fvets.2021.825940, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manyazewal A, Francesca S, Pal M, Gezahegn M, Tesfaye M, Lucy M, et al. Prevalence, risk factors and molecular characterization of Cryptosporidium infection in cattle in Addis Ababa and its environs, Ethiopia. Vet Parasitol Reg Stud Rep. (2018) 13:79–84. doi: 10.1016/j.vprsr.2018.03.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baujat B, Mahé C, Pignon JP, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. (2002) 21:2641–52. doi: 10.1002/sim.1221, PMID: [DOI] [PubMed] [Google Scholar]
- 55.Abebe R, Wossene A, Kumsa B. An epidemiological study of Cryptosporidium infection in dairy calves on selected dairy farms of Central Ethiopia. Rev Med Vet (Toulouse). (2008) 159:107–11. doi: 10.47739/2378-931X/1249 [Google Scholar]
- 56.Woldesenbet YB, Harito JB. Epidemiology and public health importance of toxoplasma Gondii in Ethiopia: an overview. J Anim Res Vet Sci. (2023) 7:44. doi: 10.24966/ARVS-3751/10004 [DOI] [Google Scholar]
- 57.Almuzaini AM. Flow of zoonotic toxoplasmosis in food chain. Pak Vet J. (2023) 43:NLINE. doi: 10.29261/pakvetj/2023.010 [DOI] [Google Scholar]
- 58.Chen R, jia Peng J, Mohsin M, Huang X, Lin X, Aguilar-Marcelino L, et al. Construction and Evaluation of the Toxoplasma gondii DNA vaccine targeting DEC-205. Pakistan Veterinary Journal. (2022) 42. [Google Scholar]
- 59.Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Villena I. Congenital toxoplasmosis in humans: an update of worldwide rate of congenital infections. Parasitology. (2021) 148:1406–16. doi: 10.1017/S0031182021001013, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hussein SN, Ibrahim AA, Shukur MS. Molecular identification of Sarcocystis species in sheep (Ovis aries) and goats (Capra hircus) of duhok province, Iraq. Pak Vet J. (2023) 43:248–54. doi: 10.29261/pakvetj/2023.016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Getachew A, Tilahun A, Aylate A, Tesfaye W. Sero-prevalence of toxoplasma gondii infection and associated risk factors in animals presented to Sholla and Akaki-Kality veterinary clinics, Addis Ababa. Glob J Med Res G Vet Sci Vet Med. (2016) 16:10–7. [Google Scholar]
- 62.Chen X, Saeed NM, Ding J, Dong H, Kulyar MFEA, Bhutta ZA, et al. Molecular epidemiological investigation of Cryptosporidium sp., Giardia duodenalis, Enterocytozoon bieneusi and Blastocystis sp. infection in free-ranged yaks and Tibetan pigs on the plateau. Pak Vet J. (2022) 42:533–9. doi: 10.29261/pakvetj/2022.060 [DOI] [Google Scholar]
- 63.Guy RA, Yanta CA, Muchaal PK, Rankin MA, Thivierge K, Lau R, et al. Molecular characterization of Cryptosporidium isolates from humans in Ontario, Canada. Parasit Vectors. (2021) 14:69. doi: 10.1186/s13071-020-04546-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lebbad M, Winiecka-Krusnell J, Stensvold CR, Beser J. High diversity of Cryptosporidium species and subtypes identified in cryptosporidiosis acquired in Sweden and abroad. Pathogens (Basel, Switzerland). (2021) 10:523. doi: 10.3390/pathogens10050523, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryan U, Zahedi A, Feng Y, Xiao L. An update on zoonotic Cryptosporidium species and genotypes in humans. Animals. (2021) 11:3307. doi: 10.3390/ani11113307, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Javed K, Alkheraije KA. Cryptosporidiosis: a foodborne zoonotic disease of farm animals and humans. Pak Vet J. (2023) 43:213–23. doi: 10.29261/pakvetj/2023.038 [DOI] [Google Scholar]
- 67.Megersa B, Shemsu J, Kassahun R, Merera O, Moje N, Edao BM, et al. Trematode infection in ruminants and diversity of snail hosts across three agro-ecological zones in Ethiopia. BMC Vet Res. (2023) 20:197. doi: 10.1186/s12917-024-04049-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong C, Cao XF, Deng L, Li W, Huang XM, Lan JC, et al. Epidemiology of Cryptosporidium infection in cattle in China: a review. Parasite. (2017) 24:1. doi: 10.1051/parasite/2017001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zewdu E, Teshome Y, Makwoya A. Bovine Hydatidosis in ambo municipality Abattior, West Shoa, Ethiopia. Ethiop Vet J. (2010) 14:1–14. doi: 10.5455/JRVS.20240706010641 [DOI] [Google Scholar]
- 70.Tkubet G, Mohammed S, Getaneh G. Bovine hydatidosis in mekelle municipal abattoir. World J Biomed Sci. (2016) 3:1–13. doi: 10.5829/idosi.ejbs.2020.54.63 [Google Scholar]
- 71.Dima FG, Jemal A. Prevalence of Hydatidosis in cattle, sheep, and goats slaughtered in Haramaya municipal abattoir, eastern Ethiopia. Covid Res Treatment. (2023) 2. doi: 10.58489/2836-3604/010 [DOI] [Google Scholar]
- 72.Ma T, Wang Q, Hao M, Xue C, Wang X, Han S, et al. Epidemiological characteristics and risk factors for cystic and alveolar echinococcosis in China: an analysis of a national population-based field survey. Parasit Vectors. (2023) 16:181. doi: 10.1186/s13071-023-05788-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdulla IT. Prevalence of Hydatidosis and its financial losses in cattle slaughtered at Fedis District, eastern Hararghe, Oromia, Ethiopia. Acta Sci Vet Sci. (2023) 6:81–7. [Google Scholar]
- 74.Tamarozzi F, Silva R, Fittipaldo VA, Buonfrate D, Gottstein B, Siles-Lucas M. Serology for the diagnosis of human hepatic cystic echinococcosis and its relation with cyst staging: a systematic review of the literature with meta-analysis. PLoS Negl Trop Dis. (2021) 15:e0009370. doi: 10.1371/journal.pntd.0009370, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Netsanet BG, Yesuf YA, Abrha BH, Lewtnesh BD. Prevalence and public health importance of bovine cysticercosis in and around Dessie, Amhara region, north East Ethiopia. Nigerian J Anim Sci. (2020) 22:173–85. [Google Scholar]
- 76.Mathewos M, Endale H, Kebamo M. Coprological and postmortem assessment and economic significance of bovine Fasciolosis in cattle slaughtered at Tarcha municipal abattoir, southern Ethiopia. Parasite Epidemiol Control. (2023) 22:e00316. doi: 10.1016/j.parepi.2023.e00316, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qamar W, Alkheraije KA. Anthelmintic resistance in Haemonchus contortus of sheep and goats from Asia–a review of in vitro and in vivo studies. Pak Vet J. (2023) 43:376–87. doi: 10.29261/pakvetj/2023.088 [DOI] [Google Scholar]
- 78.Rehman TU, Elsaid FG, Toledo MMG, Gentile A, Gul RA, Rashid M, et al. Fasciolosis: recent update in vaccines development and their efficacy. Pak Vet J. (2023) 43:224–31. doi: 10.29261/pakvetj/2023.034, PMID: 40341896 [DOI] [Google Scholar]
- 79.Kebede IA, Beriso TE, Mengistu TS, Gebremeskel HF. Study on cattle Trematodiasis and related risk factors in Damot Sore District, Wolaita zone, southern Ethiopia. J Parasitol Res. (2023) 2023:6687665. doi: 10.1155/2023/6687665, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tolosa T, Tigre W, Teka G, Dorny P. Prevalence of bovine cysticercosis and hydatidosis in Jimma municipal abattoir, south West Ethiopia. Onderstepoort J Vet Res. (2009) 76:323–6. doi: 10.4102/ojvr.v76i3.37 [DOI] [PubMed] [Google Scholar]
- 81.Regassa A, Abunna F, Mulugeta A, Megersa B. Major metacestodes in cattle slaughtered at Wolaita Soddo municipal abattoir, southern Ethiopia: prevalence, cyst viability, organ distribution and socioeconomic implications. Trop Anim Health Prod. (2009) 41:1495–502. doi: 10.1007/s11250-009-9338-3, PMID: [DOI] [PubMed] [Google Scholar]
- 82.Terefe Y, Redwan F, Zewdu E. Bovine cysticercosis and its food safety implications in Harari People’s National Regional State, eastern Ethiopia. Onderstepoort J Vet Res. (2014) 81:1–6. doi: 10.4102/ojvr.v81i1.676 [DOI] [PubMed] [Google Scholar]
- 83.Ibrahim N, Zerihun F. Prevalence of Tania saginata cysticercosis in cattle slaughtered in Addis Ababa municipal abattoir. Ethiop Glob Vet. (2012) 8:467–71. [Google Scholar]
- 84.Lielt E, Desalew T, Tsegabirhan K, Teshale S, Yohannes H. Prevalence and public health significance of bovine cysticercosis at Elfora abattoir, Bishoftu, Ethiopia. J Public Heal Epidemiol. (2015) 7:34–40. doi: 10.5897/JPHE2014.0681 [DOI] [Google Scholar]
- 85.Hirpha A, Bekele T, Melaku M. Study on bovine Cysticercosis with special attention to its prevalence, economic losses and public health significance in and around Halaba Kulito town, South Ethiopia. World J Agric Sci. (2016) 12:299–307. doi: 10.5829/idosi.wjas.2016.12.4.23762 [DOI] [Google Scholar]
- 86.Edao A, Dima FG, Deressa FB. Prevalence of bovine cysticercosis and status of human taeniasis in and around Asella town, Tiyoworeda, south East Ethiopia. Glob J Med Res. (2016) 16:18–26. [Google Scholar]
- 87.Megersa B, Tesfaye E, Regassa A, Abebe R, Abunna F. Bovine cysticercosis in cattle slaughtered at Jimma municipal abattoir, South Western Ethiopia: prevalence, cyst viability and its socioeconomic importance. Vet World. (2010) 2:257–62. doi: 10.5455/vetworld.2010.257-262, PMID: 36448968 [DOI] [Google Scholar]
- 88.Geinoro T, Bedore B. Prevalence of Cysticercus bovis in cattle slaughtered at Bishoftu municipal abattoir; public health significance and community perception about zoonotic importance of taeniosis in Bishoftu. Int J Adv Res Biol Sci. (2019) 6:52–61. doi: 10.22192/ijarbs.2019.06.04.008 [DOI] [Google Scholar]
- 89.Moje N, Zewde D, Bacha B, Regassa A. Metacestodes in cattle slaughtered at Shashemene municipal abattoir, southern Ethiopia: prevalence, cyst viability, organ distribution and financial losses. Glob Vet. (2014) 12:129–39. doi: 10.5829/idosi.gv.2014.12.01.8119 [DOI] [Google Scholar]
- 90.Engdaw TA, Alemneh AT, Ambaw ST. Study on the prevalence of Cysticercus bovis in Kombolcha Elfora, northeastern Ethiopia. Eur J Appl Sci. (2015) 7:152–7. doi: 10.5829/idosi.ejas.2015.7.4.96109 [DOI] [Google Scholar]
- 91.Mesele G, Guadu T, Bogale B, Chanie M. Pathological conditions causing organ and carcass condemnation and their financial losses in cattle slaughtered in Gondar, Northwest Ethiopia. Afr J Basic Appl Sci. (2012) 4:200–8. doi: 10.5829/idosi.ajbas.2012.4.6.66146 [DOI] [Google Scholar]
- 92.Yigizaw G, Tefera Y, Tintagu T. Prevalence of Cysticercus bovis at Dessie municipal abattoir, north East Ethiopia. Abyssinia J Sci Technol. (2017) 2:25–9. [Google Scholar]
- 93.Bedu H, Tafess K, Shelima B, Woldeyohannes D, Amare B. Bovine cysticercosis in cattle slaughtered at zeway municipal abattoir: prevalence and its public health importance. J Vet Sci Technol. (2011) 2:108. doi: 10.4172/2157-7579.1000108 [DOI] [Google Scholar]
- 94.Kassaw M, Belay W, Tesfaye W. Prevalence of Cysticercus Bovis in cattle Slaughterd at Kombolcha ELFORA meat processing factory, Northern Ethiopia. Int J Curr Res Biol Med. (2017) 2:1–6. doi: 10.22192/ijcrbm.2017.02.02.001 [DOI] [Google Scholar]
- 95.Tesfaye D, Sadado T, Demissie T. Public health and economic significance of bovine cysticercosis in Wolaita Soddo, southern Ethiopia. Glob Vet. (2012) 9:557–63. doi: 10.5829/idosi.gv.2012.9.5.6547 [DOI] [Google Scholar]
- 96.Teklemariam AD, Debash W. Prevalence of Taenia saginata/cysticercosis and community knowledge about zoonotic cestodes in and Around Batu, Ethiopia. Ethiop J Vet Sci Technol. (2015) 6:27. doi: 10.4172/2157-7579.1000273 [DOI] [Google Scholar]
- 97.Abay G, Kumar A. Cysticercosis in cattle and its public health implications in Mekelle City and surrounding areas, Ethiopia. Ethiop Vet J. (2013) 17:31–40. doi: 10.4314/evj.v17i1.3 [DOI] [Google Scholar]
- 98.Tadesse A, Tolossa YH, Ayana D, Terefe G. Bovine cysticercosis and human taeniosis in south–west Shoa zone of Oromia region, Ethiopia. Ethiop Vet J. (2013) 17:121–33. doi: 10.4314/evj.v17i2.9 [DOI] [Google Scholar]
- 99.Abera A, Sibhat B, Assefa A. Epidemiological status of bovine cysticercosis and human taeniasis in eastern Ethiopia. Parasite Epidemiol Control. (2022) 17:e00248. doi: 10.1016/j.parepi.2022.e00248, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tesfaye F, Bekele J, Abera M, Hawas NM. Public health implications of bovine Cysticercosis from cattle slaughtered at Dilla municipal abattoir, southern Ethiopia. East Afr J Biophys Comput Sci. (2020) 1:54–61. [Google Scholar]
- 101.Bayou K, Taddesse T. Prevalence of bovine Cysticercosis of slaughtered cattle in dale Wabera District municipal abattoir. Western Ethiop Int J Anim Sci. (2018) 1:1–4. [Google Scholar]
- 102.Bekele D, Berhanu B, Pal M. Studies on the prevalence, cyst viability, organ distribution and public health significance of bovine cysticercosis in ambo municipality abattoir, western Shoa, Ethiopia. J Parasitol Vector Biol. (2017) 9:73–80. doi: 10.5897/JPVB2016.0277 [Google Scholar]
- 103.Kumar A, Berhe G. Occurrence of cysticercosis in cattle of parts of Tigray region of Ethiopia. Heart. (2008) 47:88–90. [Google Scholar]
- 104.Fesseha H, Asefa I. Prevalence and associated risk factors of Cysticercosis bovis in Bishoftu municipal abattoir, Central Ethiopia. Environ Health Insights. (2023) 17:17. doi: 10.1177/11786302231164298, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mussa SM. Prevalence of bovine Cysticercosis, human Taeniasis and Asociated risk factors in selected towns of Silte zone, southern Ethiopia. bioRxiv. doi: 10.1101/2023.01.25.525623 [DOI] [Google Scholar]
- 106.Bayu Y, Asmelash A, Zerom K, Ayalew T. Prevalence and economic importance of liver parasites: hydatid cyst, Fasciola species and Cysticercus tenuicolis in sheep and goats slaughtered at Addis Ababa abattoir enterprise in Ethiopia. J Vet Med Anim Health. (2013) 5:1–7. doi: 10.5897/JVMAH11.029 [DOI] [Google Scholar]
- 107.Belina D, Getnet F, Eyasu Z, Sena B. Bovine hydatidosis: prevalence, public health and its economic significance in and around Harar, Ethiopia. J Vet Med Anim Health. (2015) 7:18–26. doi: 10.5897/JVMAH2014.0337 [DOI] [Google Scholar]
- 108.Asfaw B, Afera A. Prevalence of hydatid cyst in cattle at municipal Abbatoir of Shire. J Vet Sci Technol. (2014) 5:5. doi: 10.4172/2157-7579.1000186 [DOI] [Google Scholar]
- 109.Guduro GG, Desta AH. Cyst viability and economic significance of Hydatidosis in southern Ethiopia. J Parasitol Res. (2019) 2019:2038628. doi: 10.1155/2019/2038628, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kebede N, Mekonnen H, Wossene A, Tilahun G. Hydatidosis of slaughtered cattle in Wolaita Sodo abattoir, southern Ethiopia. Trop Anim Health Prod. (2009) 41:629–33. doi: 10.1007/s11250-008-9234-2 [DOI] [PubMed] [Google Scholar]
- 111.Fikire Z, Tolosa T, Nigussie Z, Macias C, Kebede N. Prevalence and characterization of hydatidosis in animals slaughtered at Addis Ababa abattoir, Ethiopia. J Parasitol Vector Biol. (2012) 4:1–6. [Google Scholar]
- 112.Kumsa B. Cystic echinococcosis in slaughtered cattle at Addis Ababa abattoir enterprise. Ethiop Vet Anim Sci. (2019) 7:100050. doi: 10.1016/j.vas.2019.100050, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abera M, Teame S, Sheferaw D. Cystic echinococcosis of cattle in Jimma municipal abattoir, south West Ethiopia. Glob Vet. (2013) 11:771–5. doi: 10.5829/idosi.gv.2013.11.6.81112 [DOI] [Google Scholar]
- 114.Temam A, Deresa B, Abdurahaman M. Study on prevalence and monetary loss attributed to Hydatidosis in cattle slaughtered at Jimma municipal abattoir, Southwestern Ethiopia. Glob J Med Res G Vet Sci Vet Med. (2016) 16:17–24. [Google Scholar]
- 115.Tibebu S, Kinfe M, Tegegne B, Medicine V, Box PO. The prevalence, cyst viability, organ distribution and economic importance of bovine Hydatidosis in cattle slaughtered at Debre Berhan municipal abattoir, north Shewa zone, Amhara regional state, Ethiopia. Adv Biol Res. (2016) 10:315–22. doi: 10.5829/idosi.abr.2016.315.322 [DOI] [Google Scholar]
- 116.Abera A, Teklebran T. Study on prevalence and cyst characterization of hydatidosis in cattle slaughtered at Wolayta Soddo municipal abattoir. Int J Res Granthaalayah. (2017) 5:60–78. doi: 10.29121/granthaalayah.v5.i7.2017.2108 [DOI] [Google Scholar]
- 117.Hailu Tolossa Y, Ayana D, Amin A, Jibat T. Hydatidosis/echinococcosis: its prevalence, economic and public health significance in Dodola district, western Arsi zone of Oromia region, Ethiopia. Sci Parasitol (Internet). (2016) 17:7–15. [Google Scholar]
- 118.Nasr W, Pal M. Research article prevalence, cyst viability, fertility and economic significance of bovine hydatidosis in an abattoir at Kombolcha, Ethiopia. Haryana Vet. (2016) 55:17–22. [Google Scholar]
- 119.Beyene T, Hiko A. Zoonotic metacestodes and associated fi nancial loss from cattle slaughtered at Yabello municipal abattoir. Parasite Epidemiol Control. (2019) 5:e00096. doi: 10.1016/j.parepi.2019.e00096, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Atsede B, Abebe B. Epidemiological investigation of Hepato-Pulmonary bovine Hydatidosis and its economic and zoonotic importance at Jimma municipal abattoir, Ethiopia. J Biol Agricult Healthcare. (2015) 5:11. [Google Scholar]
- 121.Tadesse M, Tesfaye S, Admasu P. Prevalence of bovine hydatidosis and its economic importance in cattle slaughtered at Bahir Dar municipal abattoir, Northern Ethiopia. Int J Livest Res. (2016) 6:1–0. doi: 10.5455/ijlr.20160717032536 [DOI] [Google Scholar]
- 122.Haftu B, Kebede T. Study on prevalence and economic significance of bovine hydatidosis in Bako Muncipal Abattoir, West Shoa Zone, Oromiya Regional State. J Vet Sci Technol. (2014) 5:197. doi: 10.4172/2157-7579.1000197 [DOI] [Google Scholar]
- 123.Belisty S, Hailehizeb C, Bewuketu AAT. Retrospective study on bovine Hydatidosis at Gondar Elfora abattoir. Biomed Nurs. (2017) 3:40–9. doi: 10.7537/marsbnj030417.05 [DOI] [Google Scholar]
- 124.Gemeda BG. A study on the prevalence, distribution and economic significance of echinococcosis/Hydatidosis in livestock (cattle, sheep, goats and pigs) slaughtered at Addis Ababa abattoir, Ethiopia. Int J Adv Res Biol Sci. (2020) 7:135–45. doi: 10.22192/ijarbs.2020.07.11.018 [DOI] [Google Scholar]
- 125.Yohannes G. Study on prevalence and associated risk factors of bovine hydatidosis in Hawassa Municipal Abattoir, Ethiopia. Middle-East J Sci Res. (2019) 1:42–6. doi: 10.5829/idosi.mejsr.2016.3520.3526 [DOI] [Google Scholar]
- 126.Moje N, Degefa A. Prevalence, cyst viability, organ distributions and financial losses due to hydatidosis in cattle slaughtered at Nekemte municipal abattoir, Western Ethiopia. J Vet Med Anim Health. (2014) 6:280–8. doi: 10.5897/JVMAH2014.0316 [DOI] [Google Scholar]
- 127.Berhe G. Abattoir survey on cattle hydatidosis in Tigray region of Ethiopia. Trop Anim Health Prod. (2009) 41:1347–52. doi: 10.1007/s11250-009-9320-0, PMID: [DOI] [PubMed] [Google Scholar]
- 128.Dana D, Suleman S, Workneh N, Massa D, Levecke B, Kifleyohannes T. Original paper cystic echinococcosis, a food-borne zoonotic neglected tropical disease in slaughtered cattle at Jimma town municipal abattoir, Southwest Ethiopia. Ann Parasitol. (2021) 67:627–35. doi: 10.17420/ap6704.379 [DOI] [PubMed] [Google Scholar]
- 129.Akeberegn D, Alemneh T, Kassa T. The prevalence of bovine Hydatidosis among slaughtered cattle at Debre Berhan municipal abattoir, north Shewa zone, Ethiopia. J Vet Sci Med. (2017) 5:1–5. doi: 10.13188/2325-4645.1000030 [DOI] [Google Scholar]
- 130.Andualem Y, Mesfin A, Ali Mohammed NS. Prevalence, cyst characterization and economic importance of bovine Hydatidosis in Addis Ababa abattoirs Enterprise, Ethiopia. J Anim Res. (2016) 6:375. doi: 10.5958/2277-940X.2016.00033.4 [DOI] [Google Scholar]
- 131.Serda B, Jago D. Prevalence and financial loss of bovine hydatidosis from cattle slaughtered at Adama municipal abattoir, south eastern Ethiopia. J Parasitol Vector Biol (Internet). (2017) 9:8–12. doi: 10.5897/JPVB2014.0179 [Google Scholar]
- 132.Kebede NK, Getachew Tilahun GT, Asrat Hailu AH. Current status of bovine cysticercosis of slaughtered cattle in Addis Ababa abattoir, Ethiopia. Trop Anim Health Prod. (2009) 2009:291–4. doi: 10.1007/s11250-008-9188-4 [DOI] [PubMed] [Google Scholar]
- 133.Kebede N, Tilahun G, Wossene A. Prevalence and financial effects of Hydatidosis in cattle slaughtered in Birre-Sheleko and Dangila abattoirs, northwestern Ethiopia. Zoonoses Public Health. (2004) 58:41–6. doi: 10.1111/j.1863-2378.2009.01250.x [DOI] [PubMed] [Google Scholar]
- 134.Mathewos M, Dawa D, Yirgalem M, Denano T, Fesseha H. Cystic echinococcosis in cattle slaughtered at a slaughterhouse in Gessa, southern Ethiopia. Parasite Epidemiol Control. (2022) 18:e00262. doi: 10.1016/j.parepi.2022.e00262, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kebede N, Mitiku A, Tilahun G. Hydatidosis of slaughtered animals in Bahir Dar abattoir, northwestern Ethiopia. Trop Anim Health Prod. (2009) 41:43–50. doi: 10.1007/s11250-008-9152-3, PMID: [DOI] [PubMed] [Google Scholar]
- 136.Bekele J, Butako B. Occurrence and financial loss assessment of cystic echinococcosis (hydatidosis) in cattle slaughtered at Wolayita Sodo municipal abattoir, southern Ethiopia. Trop Anim Health Prod. (2011) 43:221–8. doi: 10.1007/s11250-010-9680-5, PMID: [DOI] [PubMed] [Google Scholar]
- 137.Tegegne D, Abdurahaman M, Yohannes M. Seroepidemiology and associated risk factors for toxoplasma gondii in sheep and goats in southwestern Ethiopia. BMC Vet Res. (2016) 12:4–9. doi: 10.1186/s12917-016-0906-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tilahun B, Tolossa YH, Tilahun G, Ashenafi H, Shimelis S. Seroprevalence and risk factors of toxoplasma gondii infection among domestic ruminants in east Hararghe zone of Oromia region, Ethiopia. Vet Med Int. (2018) 2018:4263470. doi: 10.1155/2018/4263470, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gebremedhin EZ, Agonafir A, Tessema TS, Tilahun G, Medhin G, Vitale M, et al. Seroepidemiological study of ovine toxoplasmosis in east and west Shewa zones of Oromia regional state, Central Ethiopia. BMC Vet Res. (2013) 9:117–8. doi: 10.1186/1746-6148-9-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zewdu E, Agonafir A, Sisay T, Tilahun G, Medhin G, Vitale M, et al. Seroepidemiological study of caprine toxoplasmosis in East and West Shewa Zones, Oromia Regional State, Central Ethiopia. Res Vet Sci. (2013) 94:43–8. doi: 10.1016/j.rvsc.2012.07.020, PMID: [DOI] [PubMed] [Google Scholar]
- 141.Negash T, Tilahun G, Patton S, Prévot F, Dorchies P. Serological survey on toxoplasmosis in sheep and goats in Nazareth, Ethiopia. Rev Med Vet. (2004) 155:486–7. doi: 10.5555/20043202138 [Google Scholar]
- 142.Gebremedhin E, Gizaw D. Seroprevalence of toxoplasma gondii infection in sheep and goats in three districts of southern nations, nationalities and peoples’ region of Ethiopia. World Appl Sci J. (2017) 31:1891–6. doi: 10.5829/idosi.wasj.2014.31.11.83312 [DOI] [Google Scholar]
- 143.Gebremedhin EZ, Abdurahaman M, Hadush T, Tessema TS. Seroprevalence and risk factors of toxoplasma gondii infection in sheep and goats slaughtered for human consumption in Central Ethiopia. BMC Res Notes. (2014) 7:696. doi: 10.1186/1756-0500-7-696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jilo K, Tegegne D, Kasim S, Dabasa G, Zewdei W. Seroprevalence and public health significance of toxoplasmosis in small ruminants of pastoral Community in Yabello District, Borana zone, southern Ethiopia. Vet Med Int. (2021) 2021:1–11. doi: 10.1155/2021/6683797, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zewde D, Deme HM, Ashagrie T, Ismael A, Abie G, Negesu D, et al. Sero prevalence and assessment of risk factors for toxoplasma gondii in small ruminants in and around Sebeta town, Ethiopia. Glob Vet. (2022) 24:79–86. doi: 10.5829/idosi.gv.2022.79.86 [DOI] [Google Scholar]
- 146.Ayele A, Seyoum Z, Leta S. Cryptosporidium infection in bovine calves: prevalence and potential risk factors in Northwest Ethiopia. BMC Res Notes. (2018) 11:1–6. doi: 10.1186/s13104-018-3219-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Birhanu F, Lemma D, Eticha E, Abera B, Adem A. Prevalence and risk factors of cryptosporidiosis in dairy calves in Asella town, south eastern, Ethiopia. Acta Parasitol Glob. (2017) 8:50–7. doi: 10.5829/idosi.apg.2017.50.57 [DOI] [Google Scholar]
- 148.Anberber M, Stomeo Francesca MG, Getachew T. Cryptosporidium infection in dairy cattle calves and its public health significance in Central Ethiopia. J Adv Vet Res. (2017) 7:59–65. [Google Scholar]
- 149.Wegayehu T, Karim R, Anberber M, Adamu H. Prevalence and genetic characterization of Cryptosporidium species and Giardia duodenalis in lambs in Oromia special zone, Central Ethiopia. BMC Vet Res. (2016) 13:1–11. doi: 10.1186/s12917-016-0916-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gashaw M, NegesseWelde DA, Waktole H. Study on eimeria and cryptosporidium infection in dairy cattle farms of Holeta, west shoa zone, Oromia, Ethiopia. J Am Sci. (2020) 16:44–60. doi: 10.7537/marsjas160820.06 [DOI] [Google Scholar]
- 151.Berhanu K, Ayana D, Megersa B, Ashenafi H, Waktole H. Cryptosporidium in human-animal-environment interphase at Adama and Asella areas of Oromia regional state, Ethiopia. BMC Vet Res. (2022) 18:1–14. doi: 10.1186/s12917-022-03497-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ali M, Assefa T, Yimer A. Epidemiological Study of Small Ruminant Cryptosporidium Infection in Ziway Dugda District of East Arsi Zone, Ethiopia. J Vet Sci Technol. (2019) 11:592. [Google Scholar]
- 153.Ebiyo A, Haile G. Prevalence and factors associated with Cryptosporidium infection in calves in and around Nekemte town, east Wollega zone of Ethiopia. Vet Med Int. (2022) 2022:1–7. doi: 10.1155/2022/1468242, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ayana D, Alemu B. Cryptosporidiosis in calves, lambs and goat kids in Bishoftu, Oromia regional state, Ethiopia. Afr J Basic Appl Sci. (2015) 7:233–9. doi: 10.5829/idosi.ajbas.2015.7.5.95160 [DOI] [Google Scholar]
- 155.Wudu T, Kelay B, Mekonnen HM, Tesfu K. Calf morbidity and mortality in smallholder dairy farms in Ada’a Liben district of Oromia, Ethiopia. Trop Anim Health Prod. (2008) 40:369–76. doi: 10.1007/s11250-007-9104-3 [DOI] [PubMed] [Google Scholar]
- 156.Tekle Y. Prevalence and risk factors of cryptosporidiosis in calves and hospitalized human in Libo Kemkem District, northwestern. Ethiopia: Bahirdar University; (2021). [Google Scholar]
- 157.Belfa C, Ashagrie T, Zewde D, Degneh E. Prevalence and assessment of risk factors of cryptosporidiosis in calves in selected districts of Oromia special zone, Central Ethiopia. Glob Vet. (2022) 24:109–27. doi: 10.5829/idosi.gv.2022.109.127 [DOI] [Google Scholar]
- 158.Khan SN, Ali R, Khan S, Norin S, Rooman M, Akbar NU, et al. (2021). Cystic echinococcosis: an emerging zoonosis in southern regions of Khyber Pakhtunkhwa, Pakistan. BMC veterinary research, 17:139. doi: 10.1186/s12917-021-02830-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.