Abstract
Objective
This study is aimed at evaluating the distribution of metastatic bone disease (MBD), with a particular focus on the humerus, and its association with pathological fractures. Factors for contributing to the underestimation of fracture risk were assessed, including their impact on surgical management.
Materials and methods
We retrospectively reviewed patient records of patients undergoing surgical treatment for MBD at our institution between 2005 and 2023. The analysis included factors such as medical history, tumour type, metastatic status, surgical method, lesion location, and imaging. The images of local and staging studies (CT chest/abdomen/pelvis, CT skeleton body, bone scan, PET/CT) were reviewed by two observers. Group comparisons were made based on lesion localisation.
Results
The two most affected bone regions were the proximal femur (39.4%), followed by the proximal humerus (13.5%). Lesions of the proximal humerus were significantly more likely to be associated with pathological fractures compared to those of the proximal femur and other localisations (p < 0.01). Identified potential causes include less frequent depiction of the proximal humerus during staging (29% vs. 79% and 51%; p < 0.01) and overall lower Mirel’s scores despite the number of fractures (8 vs. 10 and 9; p < 0.01).
Conclusion
Metastatic bone disease (MBD) in the proximal humerus is less frequently captured in current staging imaging, particularly CT chest/abdomen/pelvis. Additionally, fracture prediction using Mirel’s scoring often underestimates the actual risk. Staging investigations should include this region more comprehensively, and even when correctly imaged, better tools are needed to evaluate bone metastases effectively.
Keywords: Cancer, Metastases, Metastatic bone disease, Pathological fracture, Staging, Humerus
Introduction
The number of patients living with metastatic bone disease (MBD) is rising, driving up healthcare costs and requiring greater multidisciplinary collaboration and specialised orthopaedic oncology knowledge [1–5]. With improved prognosis and earlier detection, MBD is increasingly treated curatively, often with wide resection, rather than with palliative approaches such as prophylactic stabilisation or fracture fixation [6]. Even in palliative situations, several studies indicate that pathological fractures increase complication rates and healthcare costs while also negatively impacting patient prognosis [7–9].
Improving MBD management faces challenges, especially in enhancing early and accurate detection. Current European Society for Medical Oncology (ESMO) guidelines recommend CT of chest/abdomen and bone scans for staging metastatic breast cancer [10] while staging for renal cell carcinoma includes CT of chest/abdomen/ pelvis but excludes bone scans [11].
After the femur, the humerus is the second most common bone in the extremities to develop bone metastases [12]. However, it receives significantly fewer points on Mirel’s score regarding fracture risk (1 point versus 2 or 3 points, depending on the specific femoral location). Additionally, shoulder pain is common, making it challenging to distinguish between tumour-related pain—an additional parameter of Mirel’s score—and other types of pain [13] (see Fig. 1). Mirel’s score for upper limb lesions has been deemed valid and reproducible by Evans et al. despite a sensitivity of only 14.5% [14]. Recently, modifications to the evaluation criteria, including the addition of new variables and the use of AI tools, have been proposed [15–18].
Fig. 1.
Singular bone metastasis of renal cell carcinoma in a 68-year-old patient, presenting as the primary clinical manifestation of the cancer initially treated as simple shoulder pain. A, B Radiograph images (anteroposterior and lateral views) of the shoulder and humerus demonstrate a pathological fracture through a subcapital osteolytic lesion. C, D Postoperative radiograph images (anteroposterior and lateral views) of the shoulder and humerus following reverse proximal humerus replacement after curative intraarticular resection
The primary objective of this study was to assess the distribution of bone metastases in the body and evaluate how this correlates with pathological fractures, particularly concerning the humerus. Additional secondary outcomes aimed to identify potential reasons for the suspected neglect, such as poorer prognostic markers like metastatic status, staging diagnostic, or the type of surgery performed.
Materials and methods
Study population
We retrospectively reviewed the records of patients who underwent surgical treatment for metastatic bone disease, excluding cases involving spinal metastases, between January 2005 and December 2023 at our university hospital. The review was conducted in July 2024. Haematological disorders, such as lymphoma and multiple myeloma, were included in the analysis because their imaging, fracture risk assessment, and surgical treatment are comparable to those for MBD from visceral cancers. Of the 341 patients eligible for inclusion, 90 were excluded due to incomplete data, high-energy trauma unrelated to MBD, biopsy as the only procedure, or inadequate preoperative imaging. Consequently, 251 patients were included in the final analysis (see Fig. 2). This research was approved by the ethics committee of the authors’ affiliated institution (2023/3080-Daten).
Fig. 2.

Flowchart of study population
Data collection and statistical analysis
Patient characteristics, history, pathology, and imaging results were retrieved from patient records. Mirel’s scoring of x-ray imaging was independently performed by two experienced orthopaedic oncology consultants. The long bones were segmented according to the AO classification into proximal end segment, proximal diaphyseal segment, middle diaphyseal segment, distal diaphyseal segment, and distal end segment. Proximal end segment and proximal diaphyseal segment were labelled ‘proximal’ in the further text. Metastatic disease was defined as either single, oligometastatic (2–5 bone metastasis), or polymetastatic (> 5 bone metastasis) as per staging imaging reports. Metastatic status was not evaluated in patients with lymphoma or multiple myeloma. Previous staging imaging was reassessed by two observers in cases of known malignancy. Treatment was deemed suboptimal if the operated bone lesion was not identified in the report, if Mirels’ scores exceeded 7 without a recommendation for orthopaedic consultation in the multidisciplinary team meeting, or if surgery was recommended but not performed, with no medical justification documented in the patient records.
Statistical analysis was performed with SPSS 29.0 (IBM©, New York, NY, USA). We included all patients with inclusion and without exclusion criteria in our analysis. The interobserver reliability of Mirel’s scores between the two observers was evaluated using the kappa coefficient, which yielded a value of 0.49, indicating moderate reliability. Discrepancies were resolved through consensus with the inclusion of a third observer. Categorial data were compared with Chi2 test. Continuous data showed no normal distribution so non-parametric tests had to be used to determine differences; Mann–Whitney U-test in case of two; Kruskal–Wallis test in case of more than two categories. For significant differences, Cramer’s V was used to calculate the effect size for ordinal data and Cohen’s r for metric data. The respective median with interquartile range is shown in the text. A p < 0.05 was considered statistically significant.
Results
Baseline characteristics
The study population consisted of 124 females and 127 males: age ranging from 35 to 91 years with a median of 67 years (IQR 59–74 years). There was no significant difference regarding age among genders (p = 0.52). In 62 cases, the treated bone lesion represented the first clinical manifestation of the tumour disease (referred to as ‘primary manifestation’), while in 189 cases, the malignant disease was already known. Further baseline characteristics at the time of bone lesion treatment are given in Table 1.
Table 1.
Baseline characteristics at time of treatment of bone lesion (median with interquartile ranges presented for metric data and number of cases presented for nominal data)
| Proximal femur | Proximal humerus | Other localisation | p-values | |
|---|---|---|---|---|
|
Gender, male Gender, female |
46 53 |
22 12 |
59 59 |
0.18 |
|
Age, < 61 Age, 61–70 Age > 71 |
33 33 33 |
7 10 17 |
31 40 47 |
0.43 |
|
Primary manifestation Known malignancy |
27 72 |
10 24 |
25 93 |
0.46 |
| Most recent staging imaging within 24 months before treatment of bone lesion | 0.79 | |||
|
- No staging - CT chest/abdomen/pelvis - CT skeleton body - Bone scan - PET/CT |
26 28 5 3 7 |
10 8 3 1 2 |
26 38 8 8 13 |
|
|
- Lesion on staging - Lesion not on staging |
34 9 |
4 10 |
34 33 |
< 0.01 |
|
- Correct treatment - Suboptimal treatment |
23 11 |
1 3 |
27 9 |
0.05 |
| Time after primary diagnosis of cancer (months) | 11 (0–64) | 5 (0–34) | 27 (1–81) | 0.05 |
|
Renal cell carcinoma Multiple myeloma Breast cancer Lung cancer Other cancers |
18 22 26 11 22 |
9 8 1 6 10 |
24 22 21 14 37 |
0.23 |
| Clinical presentation | < 0.01 | |||
|
- Pain - Unstable fracture - Swelling - Other |
41 53 1 4 |
5 28 1 0 |
32 70 6 10 |
|
|
Pathological fracture No pathological fracture |
57 42 |
34 0 |
75 43 |
< 0.01 |
| Mirel’s Score | 10 (9–10) | 8 (8–9) | 9 (8–10) | < 0.01 |
|
Bone quality, mixed Bone quality, lytic |
25 74 |
4 30 |
27 91 |
0.26 |
|
Singular metastatic Oligometastatic Polymetastatic |
7 12 56 |
4 3 17 |
21 12 58 |
0.23 |
|
Visceral metastases No visceral metastases |
41 34 |
13 11 |
42 49 |
0.51 |
|
Wide resection Intralesional operation |
17 82 |
7 27 |
29 89 |
0.41 |
Figure 3 illustrates the reasons for imaging, the distribution of bone lesions within the body, and the most common bones and segments of long tubular bones affected. In 15 cases, the fracture presented clinically as pain but was not unstable, bringing the total number of pathological fractures to 166 (66.1%). The distribution of lesions across different bones and segments shows that 99 (39.4%) of all relevant bone metastases were located in the proximal third of the femur and 34 (13.5%) in the proximal humerus.
Fig. 3.
Clinical presentation (A), anatomic region (B), affected bone (C), and distribution of relevant bone lesion within the long tubular bones (D)
Correlation of localisation of MBD and pathological fracture
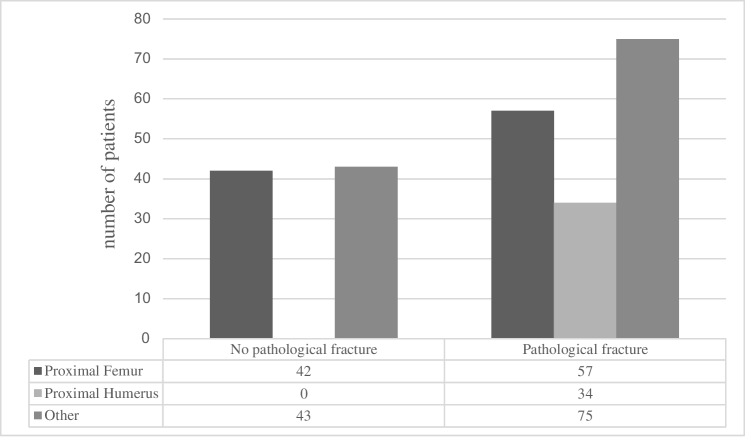
Figure 4 clearly shows that proximal humerus lesions are typically operated on for pathological fractures, whereas other locations tend to be treated earlier (p < 0.01; Cramer’s V = 0.29; see Fig. 4). The same applies to the reasons for orthopaedic consultation, as shown in Table 1, which illustrates a clear difference in the distribution of patients with pain and unstable fractures. In contrast, as expected, Mirels’ scores were significantly higher for lesions in the lower extremities (p < 0.01; see Fig. 5). The respective effect sizes were r = 0.57 (proximal femur – proximal humerus), r = 0.41 (proximal femur – other), and r = 0.18 (proximal humerus – other). There was no difference between the groups in terms of underlying tumour type, metastatic load, and the presence of visceral metastases as prognostic factors or the proportion of curative metastatic resections that were performed. The rate of new diagnoses and the time since the initial diagnosis of known tumour diseases also showed no significant differences between the groups (see Table 1).
Fig. 4.
Distribution of patients presenting with pathological fractures among the most common sites of bone metastases
Fig. 5.
Boxplots of Mirels’ scores of affected bone regions
Potential influence of staging of MBD
Lesions of the proximal humerus were less likely to be detected using standard staging diagnostics (p < 0.01, Cramer’s V = 0.34). There was no difference in the diagnostic modality used across bone regions (p = 0.79). Additionally, there was no statistically significant difference in the quality of assessment according to existing scoring systems between the proximal femur, proximal humerus, and other locations (p < 0.05).
Discussion
In this retrospective study, we confirmed that proximal humerus lesions are typically treated orthopaedically when fractured, while lesions in other locations are often addressed earlier (n = 251). We found that this delay is not solely due to significantly lower Mirels’ scores for upper limb lesions; most proximal humerus lesions were not captured by staging imaging in patients with known cancers (see Fig. 6).
Fig. 6.
Bone lesion undetected during staging in a 71-year-old patient with lung cancer, presenting with a pathological proximal humerus fracture 5 months post-diagnosis. A Coronal CT of the chest, abdomen, and pelvis was performed 1 month prior for staging, showing the cranial extension of the scan without evidence of the lesion. B Anteroposterior radiograph image of the proximal humerus and shoulder demonstrating a pathological fracture caused by an osteolytic bone lesion. C Postoperative anteroposterior radiograph image of the humerus following closed reduction and fixation with a proximal humerus nail
This is the first study to focus on the two most common sites of metastatic bone disease (MBD) in the extremities, specifically examining the reasons for the inadequate identification and stratification of MBD in the proximal humerus. In a multivariate analysis of 245 patients with MBD and 126 pathological fractures, Crenn et al. identified localisation in the upper extremity as a risk factor for pathological fractures, though they did not provide a cause [17]. This effect was also observed in our study group, where all lesions of the proximal humerus undergoing orthopaedic surgery were fractured (n = 34).
It was notable that there was a significant difference in the depiction of bone lesions among patients with known tumour disease across the staging examinations performed. Over 75% of treated MBDs at the proximal femur were detected, compared to approximately 50% of MBDs in other locations, and only 29% of lesions at the proximal humerus. The effect size was moderate, with a Cramer’s V of 0.34. This finding has not previously been described. The retrospective evaluation of treatment based on staging imaging showed no significant difference in quality regarding these locations; however, with only four proximal humeral lesions depicted, the statistical reliability of these figures is very limited.
No differences were observed between the groups in metastatic load, presence of visceral metastases, or surgical methods, suggesting that a substantial proportion of patients in the proximal humerus group also underwent metastasectomy with curative intent. Poorer imaging and the associated fracture risk at the proximal humerus pose significant challenges, as fractures complicate curative resections by opening compartments and potentially spreading tumour cells, often requiring more extensive surgery. This can adversely affect postoperative function and potentially prognosis, even with curative resection, as demonstrated by Saglam et al. and Salunke et al. for osteosarcomas [19, 20]. Given the similar surgical approach for bone metastases, these findings are relevant and, for example, suggest reconsidering the limitation of staging beyond chest CT in renal cell carcinoma patients only when clinically indicated [11, 21]. The number of undetected cases where lesions went unnoticed until a fracture occurred remains unclear. Even in palliative settings, prophylactic stabilisation before a fracture occurs is preferable, as it is more cost-effective and pathological fractures have limited healing potential, negatively impacting the patient’s quality of life [7, 22]. Therefore, early detection of MBD is valuable in these cases as well.
We were also able to confirm a known contributing factor: Mirels’ scores are significantly lower at the proximal humerus than at other sites, despite all patients in our cohort experiencing fractures there. Both Hoban et al. and Tat et al. have attempted to address this issue by adjusting Mirel’s score, either by lowering the cut-off or adding a cortical breach factor [15, 16]. The high effect size shown in our study, particularly in comparison to the proximal femur (r = 0.57), confirms the significantly different assessment of fracture risk for lesions in the proximal humerus. However, the assessment of fracture risk at the proximal femur itself has also recently been questioned, with a modified Mirel’s score proposed and validated using finite element models. This adjusted score identifies the subtrochanteric-diaphyseal region of the proximal femur as significantly more at risk of fracture [23, 24]. Additionally, the initial Mirel’s scoring was validated in a small population, and all bone metastases in this group were treated with radiation therapy. Overall, assessing fracture risk using only Mirel’s score now seems overly simplistic, and further efforts are needed to enhance predictive accuracy by integrating CT imaging and AI methods [18].
Strength and limitations
A strength of this study is the relatively large patient cohort for a single-centre study, which ensures uniform treatment by the same team following consistent principles. However, the study is limited by the variability in tumour types and corresponding treatments, the extended observation period, and its retrospective design. Additionally, there is potential bias in Mirel’s scoring, as the analysis included only cases that underwent surgical intervention.
Conclusion
The two most common regions for orthopaedically relevant bone metastases in the extremities are the proximal femur and the proximal humerus. Lesions of the proximal humerus are less frequently captured in standard staging diagnostics and receive lower scores in Mirel’s scoring. As a result, these lesions often go unnoticed until fractures occur. Therefore, staging exams should more effectively cover this region, and fracture risk assessment methods for bone metastases require revision.
Abbreviations
- AI
Artificial intelligence
- CT
Computed tomography
- ESMO
European Society for Medical Oncology
- IQR
Interquartile range
- MBD
Metastatic bone disease
- PET/CT
Positron emission tomography/computed tomography
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data presented in this study are not publicly available but available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biermann JS, Holt GE, Lewis VO, Schwartz HS, Yaszemski MJ. Metastatic bone disease: diagnosis, evaluation, and treatment. J Bone Joint Surg Am. 2009;91:1518–30. [PubMed] [Google Scholar]
- 2.Eastley N, Newey M, Ashford RU. Skeletal metastases - the role of the orthopaedic and spinal surgeon. Surg Oncol. 2012;21:216–22. 10.1016/j.suronc.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Tsukamoto S, Errani C, Kido A, Mavrogenis AF. What’s new in the management of metastatic bone disease. Eur J Orthop Surg Traumatol. 2021;31:1547–55. 10.1007/s00590-021-03136-4. [DOI] [PubMed] [Google Scholar]
- 4.Trompeter A. Management of metastatic bone disease (MBD). Injury. 2022;53:3869–71. 10.1016/j.injury.2022.09.054. [DOI] [PubMed] [Google Scholar]
- 5.Malviya A, Gerrand C. Evidence for orthopaedic surgery in the treatment of metastatic bone disease of the extremities: a review article. Palliat Med. 2012;26:788–96. 10.1177/0269216311419882. [DOI] [PubMed] [Google Scholar]
- 6.Errani C, Mavrogenis AF, Cevolani L, Spinelli S, Piccioli A, Maccauro G, Baldini N, Donati D. Treatment for long bone metastases based on a systematic literature review. Eur J Orthop Surg Traumatol. 2017;27:205–11. 10.1007/s00590-016-1857-9. [DOI] [PubMed] [Google Scholar]
- 7.Blank AT, Lerman DM, Patel NM, Rapp TB. Is prophylactic intervention more cost-effective than the treatment of pathologic fractures in metastatic bone disease? Clin Orthop Relat Res. 2016;474:1563–70. 10.1007/s11999-016-4739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raschka T, Weiss S, Reiter A, Barg A, Schlickewei C, Frosch KH, Priemel M. Outcomes and prognostic factors after surgery for bone metastases in the extremities and pelvis: a retrospective analysis of 140 patients. J Bone Oncol. 2022;34:100427. 10.1016/j.jbo.2022.100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavrogenis AF, Pala E, Romagnoli C, Romantini M, Calabro T, Ruggieri P. Survival analysis of patients with femoral metastases. J Surg Oncol. 2012;105:135–41. 10.1002/jso.22061. [DOI] [PubMed] [Google Scholar]
- 10.Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, Dent R, Fenlon D, Gligorov J, Hurvitz SA, Im SA, Krug D, Kunz WG, Loi S, Penault-Llorca F, Ricke J, Robson M, Rugo HS, Saura C, Schmid P, Singer CF, Spanic T, Tolaney SM, Turner NC, Curigliano G, Loibl S, Paluch-Shimon S, Harbeck N, clinicalguidelines@esmo.org EGCEa. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–95. 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S, Horwich A, clinicalguidelines@esmo.org EGCEa. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:706–20. 10.1093/annonc/mdz056. [DOI] [PubMed] [Google Scholar]
- 12.Kendal JK, Abbott A, Kooner S, Johal H, Puloski SKT, Monument MJ. A scoping review on the surgical management of metastatic bone disease of the extremities. BMC Musculoskelet Disord. 2018;19:279. 10.1186/s12891-018-2210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas J, van Doorn P, Hegedus E, Lewis J, van der Windt D. A systematic review of the global prevalence and incidence of shoulder pain. BMC Musculoskelet Disord. 2022;23:1073. 10.1186/s12891-022-05973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans AR, Bottros J, Grant W, Chen BY, Damron TA. Mirels’ rating for humerus lesions is both reproducible and valid. Clin Orthop Relat Res. 2008;466:1279–84. 10.1007/s11999-008-0200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tat J, Bodansky D, Sheth U, Ung Y, Whyne C, Nam D. Predicting pathological fractures at metastatic humeral lesions. JB JS Open Access. 2023;8. 10.2106/JBJS.OA.22.00070 [DOI] [PMC free article] [PubMed]
- 16.Hoban KA, Downie S, Adamson DJA, MacLean JG, Cool P, Jariwala AC. Mirels’ score for upper limb metastatic lesions: do we need a different cutoff for recommending prophylactic fixation? JSES Int. 2022;6:675–81. 10.1016/j.jseint.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crenn V, Carlier C, Gouin F, Sailhan F, Bonnevialle P, So.F.C.O.T. mot. High rate of fracture in long-bone metastasis: proposal for an improved Mirels predictive score. Orthop Traumatol Surg Res. 2020;106:1005–11. 10.1016/j.otsr.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Confavreux CB, Follet H, Mitton D, Pialat JB, Clézardin P. Fracture risk evaluation of bone metastases: a burning issue. Cancers (Basel). 2021;13. 10.3390/cancers13225711 [DOI] [PMC free article] [PubMed]
- 19.Saglam F, Baysal Ö, Sofulu Ö, Baykan SE, Erol B. The impact of pathological fractures on surgery, morbidity, functional and oncological outcomes in patients with primary bone sarcomas. Injury. 2021;52:1740–7. 10.1016/j.injury.2021.04.062. [DOI] [PubMed] [Google Scholar]
- 20.Salunke AA, Chen Y, Tan JH, Chen X, Khin LW, Puhaindran ME. Does a pathological fracture affect the prognosis in patients with osteosarcoma of the extremities? : a systematic review and meta-analysis. Bone Joint J. 2014;96-B:1396–403. 10.1302/0301-620X.96B10.34370. [DOI] [PubMed] [Google Scholar]
- 21.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF): S3-Leitlinie Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms, Langversion 5.0, 2024, AWMF-Registernummer: 043–017OL https://www.leitlinienprogramm-onkologie.de/leitlinien/nierenzellkarzinom/
- 22.Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983;178:297–302. [PubMed] [Google Scholar]
- 23.Amendola RL, Miller MA, Kaupp SM, Cleary RJ, Damron TA, Mann KA. Modification to Mirels scoring system location component improves fracture prediction for metastatic disease of the proximal femur. BMC Musculoskelet Disord. 2023;24:65. 10.1186/s12891-023-06182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai VS, Amendola RL, Mann KA, Damron TA. Internal validation of modified Mirels’ scoring system for pathologic femur fractures. BMC Musculoskelet Disord. 2024;25:719. 10.1186/s12891-024-07836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are not publicly available but available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.