Abstract
Introduction/Objective
Schizophrenia with substance use disorder is a complex clinical condition that may increase treatment resistance. Cannabis use disorder is frequently associated with psychosis and the causal link has still to be defined. Partial D2/3 agonists may ensure limbic dopamine release normalization while avoiding reduced frontocortical dopamine release, which would contribute to negative symptoms. We aimed to observe the clinical course of patients with schizophrenia comorbid with cannabis use disorder while being treated with oral or long-acting injectable D2/3 partial agonists.
Methods
We observed 96 young adults with schizophrenia/schizoaffective disorder comorbid with cannabis use disorder during 18 months of treatment with aripiprazole long-acting injectable or oral aripiprazole or brexpiprazole. The assessment comprised Clinical Global Impressions-Severity, Positive And Negative Syndrome Scale, Brief Psychiatric Rating Scale, Barratt Impulsiveness Scale, and Visual Analog Scale for Craving.
Results
Included were 17 women and 79 men (mean age = 26.89 ± 4.74 years). The sample responded favorably to treatment as assessed by all clinical scales, save for the impulsiveness scale which showed no significant change. The four treatment samples responded well without differences, but employing a general linear model, long-acting injectable aripiprazole and brexpiprazole were better and similar on all clinical and craving scales compared to oral aripiprazole and to other antipsychotics. Long-acting injectable aripiprazole fared better than brexpiprazole on general psychopathology, negative symptoms, and craving, while the reverse was true for global severity. However, the sample size imbalance did not allow for drawing strong conclusions. We found no significant treatment resistance in our 96-patient sample.
Conclusion
Partial D2/3 agonists may treat comorbid schizophrenia/schizoaffective disorder and cannabis use disorder, improving the symptoms of both disorders and substance craving.
Keywords: Partial dopamine agonist antipsychotic drugs, aripiprazole, oral, aripiprazole, long-acting injectable, substance use disorders, cannabis use disorder, schizophrenia, schizoaffective disorder, comorbidity
1. INTRODUCTION
Schizophrenia is a chronic illness that requires lifelong treatment. The primary goal in therapy is to treat the acute phase, prevent relapse, and guide the patient toward recovery and reintegration into the community. Approximately 50% of patients with schizophrenia may experience a significant clinical relapse within one year of drug discontinuing [1-3]. Careful selection of antipsychotics is an individualized risk-benefit decision based on both short- and long-term concerns, including the relative clinical efficacy, safety, and tolerability of antipsychotic medications [4]. Antipsychotics often need to be taken for very long periods of time, increasing the likelihood of adverse events that cause patients to discontinue medication and decrease adherence [5]. To overcome low adherence, clinicians may use long-acting injectable (LAI) formulations, which release the active drug during the 15 days to 6 months. There was hope that second-generation antipsychotics (SGAs) would be associated with lower relapse and rehospitalization rates compared to first-generation antipsychotics (FGAs), but this was not found to be the case [6].
Substance use disorders (SUDs) are common in patients with schizophrenia. A meta-analysis [7] found that the prevalence of any SUD in patients with schizophrenia or first-episode psychosis was 42%, with the most common SUDs being those related to illicit drugs (28%), cannabis (26%), alcohol (24%), and stimulants (7%). The treatment of patients with schizophrenia and a co-occurring SUD is particularly challenging. According to current guidelines, the best approach is to combine antipsychotic treatment with psychosocial interventions for addictive behaviors [8-10]. Currently, there is a lack of data on antipsychotics in adolescents with dual diagnosis [11], but SGAs are more acceptable than FGAs [12].
Although there are few advantages of one antipsychotic over another, when treating patients with co-occurring schizophrenia spectrum disorders and SUDs, SGAs may be preferable to FGAs because of their better tolerability and reduced tendency to induce extrapyramidal adverse effects. A comparison between the FGA LAI zuclopenthixol and the SGA LAI risperidone showed that the latter was superior in reducing both substance use and psychotic symptoms [13]. In addition, the potent dopaminergic blockade of FGAs may exacerbate craving [14].
Antipsychotics that are characterized by partial agonism of dopamine D2 receptors show a biphasic effect that includes functional antagonism in the mesolimbic dopamine pathway (where excessive dopamine activity is associated with positive symptoms) and functional agonist activity in the mesocortical pathway (where reduced dopamine activity is associated with negative symptoms and cognitive impairment). In addition, by avoiding complete blockade of the nigrostriatal or tuberoinfundibular pathways, these medications are associated with a low incidence of extrapyramidal symptoms and hyperprolactinemia [15].
Aripiprazole is the prototype of D2/D3 receptor partial agonists used in the treatment of schizophrenia and bipolar disorder [16, 17]. A large body of evidence indicates that is as effective as haloperidol and FGAs in the treatment of positive symptoms, more effective in the treatment of negative symptoms of schizophrenia, and causes fewer adverse effects [18]. In addition, aripiprazole acts as a partial agonist at serotonin 5-HT1A receptors and as a potent antagonist at 5-HT2A and 5-HT2B receptors [19, 20].
Brexpiprazole shares with aripiprazole the partial agonism activity at D2, D3, and serotonin 5-HT1A receptors with a lower intrinsic efficacy at D2 receptors with respect to aripiprazole. Brexpiprazole also acts as an antagonist of 5-HT2A, 5.HT2B, 5-HT7, α1A and α1D adrenergic receptors [19, 20]. 5-HT7 blockade by brexpiprazole is predictive of an effect of the drug in improving cognitive dysfunction [21], whereas α1 adrenergic receptor blockade underlies the FDA-approved clinical application of brexpiprazole in agitation associated with Alzheimer’s disease [22-25].
Aripiprazole, brexpiprazole, and cariprazine share the D2/D3 receptor partial agonist property and for this reason, the term “third-generation antipsychotics” has been advanced [26]. We believe that this sort of proliferation of terms that may well be expressed otherwise does harm to the scientific debate, focusing on form rather than substance. We will maintain the FGA/SGA distinction and pool all newer than clozapine drugs among the latter. The atypical vs. typical (neuroleptic) antipsychotics distinction also did not find a reasonable neurochemical basis. For this reason, in this paper, we will maintain the term D2/D3 receptor partial agonists.
Dopaminergic dysfunction is known to be involved in addiction [27]. Thus, partial agonists of D2 receptors may be beneficial in reducing the rewarding effects of illicit drugs and restrain substance craving and relapse after withdrawal [28].
These properties, together with their efficacy on schizophrenia symptoms, lower risk of extrapyramidal side effects compared to FGAs, and lower risk of metabolic side effects compared to most SGAs [29], make aripiprazole and brexpiprazole attractive alternatives for the treatment of schizophrenia with comorbid SUDs. Despite differences in the neurobiology of each substance use–associated psychosis (our sample consisted of people with at least cannabis use disorder, but also other substance use was possible), partial dopamine D2/3 agonists have been proposed as potentially useful treatments in psychoses comorbid with SUD [30]. Brexpiprazole, thanks to its broader clinical target [31], is one of the substances that can address other comorbidities which often coexist in patients with psychosis and SUD [32].
This investigation provides insights into treatment strategies for patients with psychotic symptoms associated with SUD. Our intention was to evaluate the efficacy of aripiprazole and brexpiprazole in reducing psychotic symptoms and substance craving. In this study, we focused on the impact of aripiprazole and brexpiprazole on clinical and psychopathological outcomes compared to other antipsychotics.
2. MATERIALS AND METHODS
We conducted a retrospective observational study of inpatients and outpatients with a diagnosis of schizophrenia hospitalized at the Villa Von Siebenthal Neuropsychiatric Hospital in Rome, Italy. Recruitment started in June 2021 and ended in December 2022. Patients aged 18-35 years were eligible if they had a diagnosis of DSM-5/DSM-5-TR schizophrenia or schizoaffective disorder and cannabis use disorder.
This sample of patients is part of a sample used in a previously published study [33]; of the initial sample of 323, we followed 96 patients for 18 months. All were initially inpatients who were discharged after one month and then followed up as outpatients. Included were patients with cannabis use disorder and schizophrenia or schizoaffective disorder. Exclusion criteria were the presence of a comorbid major psychiatric disorder other than schizophrenia or schizoaffective disorder; suicide risk as assessed by the Columbia-Suicide Severity Risk Scale (C-SSRS) [34], i.e., a score of 4 or 5 on the suicidal ideation items or any “yes” response to the suicidal behaviour items; comorbidity with major organic diseases (autoimmune or systemic connective tissue disease, treatment-resistant hypertension, type 1 diabetes, metabolic syndrome, major cardiovascular disease, or major neurological disorders like Alzheimer’s disease and other dementias, cerebrovascular diseases, such as stroke, multiple sclerosis, Parkinson's disease, brain tumours, traumatic head injury, and neurological disorders related to malnutrition); history of epilepsy, head injury, electroencephalographic (EEG) abnormalities, and neurodevelopmental disorders; intelligence quotient (IQ)<75, as assessed by the Wechsler Adult Intelligence Scale (WAIS) [35]; unwillingness to participate and inability to sign the informed consent for themselves or, in the case of inability, unwillingness/refusal of the legal guardian to sign. Of the original 323-patient sample, 272 did not have schizophrenia or schizoaffective disorder (116 bipolar disorder, [36] depressive disorders, 57 borderline personality disorder, 55 attention-deficit/hyperactivity disorder, 5 other personality disorders, 2 mild intellectual disability, 1 autism spectrum disorder), thus leaving 119 patients to analyze. Of these, not all had a follow-up of 18-months available. Only 96 patients had this, the other 23 had still to reach the 18-month interval. This was due to the fact that some cases were included in the original study [33] until December 2022, so their 18-month data were not available at the time of our statistical analyses.
Patients provided written informed consent before participating in any study procedure. Both the informed consent form and the experimental procedures were approved by the Ethics Committee of the Health Authority of Rome 2 (study 331-306-00387; 19-November-2019), in accordance with internationally accepted criteria for ethical research. The study was conducted in accordance with the human rights principles adopted by the World Medical Association at the 18th WMA General Assembly, Helsinki, Finland, June 1964, and subsequently amended at the 64th WMA General Assembly, Fortaleza, Ceará, Brazil, October 2013.
We collected sociodemographic data on an appropriate datasheet. We assessed the psychopathology of the 96 follow-up patients using the Clinical Global Impressions-Severity Scale (CGI-S) [36], the Positive And Negative Syndrome Scale (PANSS) [37, 38], and the 24-item Brief Psychiatric Rating Scale (BPRS) (original version [39], Italian version, [40]) at baseline, 1 month, and 3, 6, 12, and 18 months. The Visual Analog Scale for Craving (VAScrav) [41] was used to assess craving. The latter rates craving from 0 (no craving) to 10 (the most intense craving according to the patient's experience). Impulsivity was assessed using the Barratt Impulsiveness Scale (BIS-11) [42]. We used clinical face-to-face or telephone interviews for all recruited patients. DSM-5 psychiatric diagnoses were made using the Structured Clinical Interview for DSM-5-Clinician Version (SCID-5-CV) [43]. Patients were regularly screened for drug use at enrolment and throughout the study.
Eleven patients were on oral aripiprazole, 13 patients on brexpiprazole, and 38 on LAI aripiprazole. The other 34 patients took FGAs, i.e., haloperidol, promazine, and amisulpride, or other SGAs, i.e., clozapine, lurasidone, olanzapine, paliperidone, quetiapine, and risperidone.
We enrolled SUD patients who were receiving specific pharmacological treatment for their SUD, such as methadone, buprenorphine, and naltrexone, or benzodiazepines and gabapentinoids. All drugs used in this study adhered to clinical prescription guidelines as recommended by each product’s monograph. Aripiprazole was used as oral 10-30 mg/day according to patients’ need, and as LAI at 400 mg/month, while brexpiprazole was rapidly titrated up to 4 mg/day, remaining stable thereafter. Despite our study was real-world, multiple pharmacotherapies were not allowed; patients received one of the aforementioned antipsychotic treatments and were allowed to add only their specific drug treatment for their SUD. All patients, after they were discharged from the hospital, were followed up as outpatients by the same treating physicians who adhered to monotherapy treatment patterns.
Our primary objective was to evaluate the efficacy of antipsychotics characterized by partial agonism of dopamine D2 receptors, specifically aripiprazole (oral formulation and LAI) and oral brexpiprazole (there is no LAI for this drug) in a group of patients diagnosed with schizophrenia/schizoaffective disorder and cannabis use disorder (CUD), using CGI-S, PANSS, BPRS, BIS-11, and VAScrav scores.
2.1. Statistical Analysis
Frequency distributions and descriptive statistics were used to analyze the sample. After testing the sample for normality of distribution with the Shapiro-Wilk test [44], we proceeded with parametric tests.
First, preliminary analyses were performed to verify the internal consistency of the instruments used in the study by calculating Cronbach's alpha. Then, descriptive analyses were performed, which included the calculation of frequencies, means, and standard deviations (SDs) of sociodemographic and clinical variables, including the entire sample and its subdivisions (aripiprazole oral vs. LAI vs. brexpiprazole).
Where possible, differences between groups were tested using multivariate analysis of variance (MANCOVA), and the significance of the main effect was analyzed using Pillai's Trace index. The Bonferroni correction of α for multiple comparisons was applied in case of significance.
To evaluate the effect of partial agonist treatment on CGI-S, BPRS, BIS-11, and PANSS scores, we used a general linear model (GLM) with repeated measures to assess changes over time.
Post hoc comparisons were performed using Bonferroni's correction [45]. Contingency tables were used for nonparametric variables, and the significance of the main effect was analyzed by the chi-squared test. We used SPSS v.27 for Mac software to perform all statistical analyses (IBM Corporation, Armonk, New York, 2019). Significance was set at p < 0.05.
3. RESULTS
Demographic and clinical variables of the sample are shown in Table 1. The sample consisted of 17 women and 79 men. The mean age was 26.89 (SD = 4.74) years. The age of onset of cannabis use was 15.75 years (SD = 2.36), earlier than the mean ages of onset of psychotic symptoms and first hospitalization, which were 18.68 (SD = 2.97) and 19.81 years (SD = 4.15), respectively.
Table 1.
Demographic and clinical variables of the sample (17 women [17.7%] and 79 men [82.3%]).
| Variable | Mean | SD |
|---|---|---|
| Age | 26.89 | 4.74 |
| Age at onset of psychosis | 18.68 | 2.97 |
| Age at first hospitalization | 19.81 | 4.15 |
| Age at onset of cannabis use | 15.75 | 2.36 |
| Time since beginning of cannabis use and onset of psychosis | 2.92 | 2.61 |
| Relapses | 0.84 | 1.18 |
Abbreviation: SD, standard deviation.
We also examined the use of psychoactive substances other than cannabinoids (Table 2). Most of our sample (72%) reported polysubstance use. In particular, 58.3% of patients also used cocaine and 33.3% used alcohol.
Table 2.
Substance use in the entire sample*.
| - | N | % |
|---|---|---|
| Polysubstance use | 70 | 72.2% |
| Type of Substance | ||
| Cocaine | 56 | 58.3% |
| Opioids | 8 | 8.3% |
| Amphetamines | 24 | 25% |
| Hallucinogens | 14 | 14.6% |
| Alcohol | 32 | 33.3% |
| Benzodiazepines | 4 | 4.2% |
Note: *The total number of substance use patterns exceeds the total number of patients because multiple substance use patterns may exist for the same patient.
3.1. Efficacy of Antipsychotic Treatment Over Time
To investigate the effect of partial agonist treatment on the clinical variables studied at different time points, we used a general linear model (GLM) with repeated measures, comparing assessments at the time of hospitalization (baseline) with those at 18 months. The analysis showed a significant main effect for all variables, allowing comparisons within and between groups, corrected with the Bonferroni method for multiple comparisons.
Table 3 shows a general decrease in the scores of all psychometric scales in the whole sample from baseline to 18 months. Statistically significant changes between baseline and endpoint are shown for all the scales. Moreover, the effect attributable to the interaction between time and partial agonists is significant only for the BPRS (77.67 at baseline vs. 45.08 at endpoint), and the PANSS Positive subscale (24.73 at baseline vs. 13.42 at endpoint), although it went close to statistical significance for the CGI-S and the PANSS Total Score. BIS-11 and PANSS Negative and General Psychopathology scores did not change significantly from baseline to 18 months.
Table 3.
Repeated-measures General Linear Model (GLM), estimated marginal means, baseline vs. 18-month endpoint in whole sample.
| - | Time | Time x Partial Agonists | ||||
|---|---|---|---|---|---|---|
| - | Baseline* | 18 Months* | F | p | F | p |
| CGI-S | 5.54 | 3.53 | 468.14 | <.001 | 3.24 | 0.075 |
| BIS-11 Total score | 90.56 | 73.61 | 166.33 | <.001 | 0.05 | 0.829 |
| BPRS | 77.67 | 45.08 | 66.18 | <.001 | 5.21 | 0.025 |
| PANSS Total score | 109.85 | 63.10 | 86.92 | <.001 | 3.43 | 0.067 |
| PANSS Negative scale | 28.94 | 16.83 | 165.79 | <.001 | 2.28 | 0.134 |
| PANSS Positive Scale | 24.73 | 13.42 | 67.66 | <.001 | 4.61 | 0.034 |
| PANSS General Psychopathology | 56.35 | 32.75 | 119.82 | <.001 | 1.83 | 0.179 |
Abbreviations: BIS-11, Barratt Impulsiveness Scale; BPRS, 24-item Brief Psychiatric Rating Scale; CGI-S, Clinical Global Impressions scale, Severity; PANSS, Positive And Negative Syndrome Scale. Note: Significance in bold. *Estimated marginal means, representing the average predicted outcomes of the model for each level of the independent variables, taking into account the effects of time and partial agonists in the model.
3.2. Efficacy of Different Antipsychotics vs. Partial Dopamine Receptor Agonists
First, we evaluated the efficacy of aripiprazole and brexpiprazole by comparing them with various FGAs and other SGAs. The assessment was based on the administration of the above psychometric scales at the beginning of hospitalization and after 18 months. We also compared the specific effect of each class (aripiprazole, brexpiprazole, and other antipsychotics) on different psychopathological dimensions using a general linear model (GLM) with repeated measures.
Table 4 shows a greater effect of brexpiprazole on CGI-S scores (Figs. 1 and 2), whereas aripiprazole LAI significantly affected BPRS and VAScrav scores (Fig. 3). Regarding the PANSS dimensions, patients on aripiprazole LAI scored 56.27 on the Negative subscale, which was statistically significant (p = 0.031) vs. other antipsychotics (25.00), aripiprazole (22.19), and brexpiprazole (22.04). Similarly, on the General Psychopathology subscale, patients on aripiprazole LAI scored better (20.42) vs. 47.37 for other antipsychotics, 47.99 for aripiprazole, and 44.79 for brexpiprazole (Fig. 2). Scores on the PANSS positive symptoms subscale and the BIS-11 did not differ between the different drug regimens.
Table 4.
Repeated measures general linear model (GLM).
| - | OAPs | Aripiprazole | Aripiprazole LAI | Brexpiprazole | F | p |
|---|---|---|---|---|---|---|
| CGI-S | 4.68 | 4.75 | 4.38 | 4.36 | 4.87 | 0.003 |
| BIS-11 Total | 82.26 | 83.25 | 81.36 | 82.61 | 0.10 | 0.961 |
| BPRS | 63.77 | 70.71 | 56.27 | 61.71 | 3.10 | 0.031 |
| PANSS Total | 91.45 | 91.00 | 79.70 | 85.50 | 2.60 | 0.057 |
| PANSS Negative | 25.00 | 22.19 | 20.84 | 22.04 | 3.10 | 0.031 |
| PANSS Positive | 19.26 | 21.81 | 18.42 | 18.50 | 0.715 | 0.546 |
| PANSS General Psychopathology | 47.36 | 47.00 | 40.42 | 44.79 | 3.53 | 0.018 |
| VAScrav frequency | 5.83 | 6.44 | 4.58 | 5.29 | 3.89 | 0.012 |
| VAScrav intensity | 6.78 | 7.38 | 5.61 | 5.79 | 4.87 | 0.004 |
Abbreviations: BIS-11, Barratt Impulsiveness Scale; BPRS, 24-item Brief Psychiatric Rating Scale; CGI-S, Clinical Global Impressions scale, Severity; OAPs, other antipsychotics; PANSS, Positive And Negative Syndrome Scale; VAScrav, visual analogue scale for craving. Note: Significance in bold.
Fig. (1).
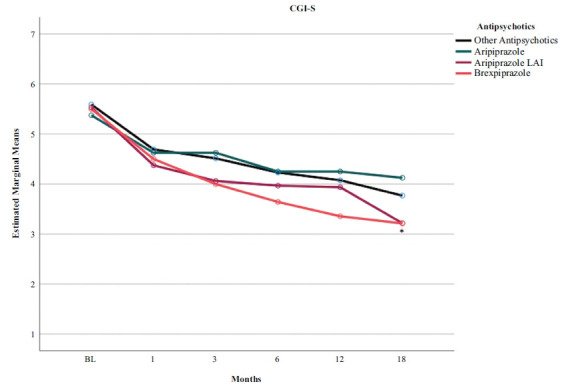
Time course of CGI-S scores according to the type of pharmacotherapy in a sample with comorbid schizophrenia and cannabis use disorder. BL, baseline. *p < 0.05 brexpiprazole vs. all others.
Fig. (2).
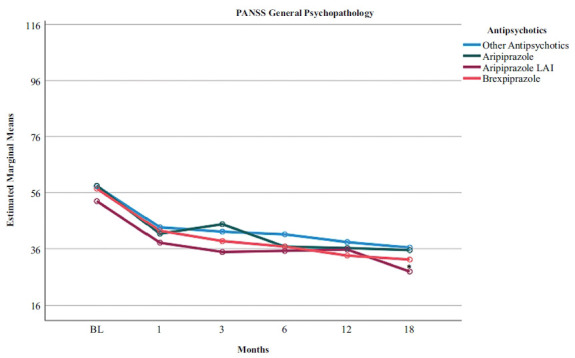
Time course of PANSS-General Psychopathology scores according to the type of pharmacotherapy in a sample with comorbid schizophrenia and cannabis use disorder. BL, baseline. *p < 0.05 LAI aripiprazole vs. all others.
Fig. (3).

Time course of VAScrav scores according to the type of pharmacotherapy in a sample with comorbid schizophrenia and cannabis use disorder. BL, baseline. *p < 0.05 LAI aripiprazole vs. all others.
4. DISCUSSION
In this study, we evaluated 96 patients with schizophrenia/schizoaffective disorder and comorbid SUD. These patients were all treated with partial dopamine receptor agonists, including brexpiprazole, oral aripiprazole, aripiprazole LAI, and other antipsychotics. LAI aripiprazole improved BPRS scores and cravings better than all other treatments, while brexpiprazole improved CGI-S scores better than all others. We found no baseline-to-endpoint differences between brexpiprazole, oral aripiprazole, LAI aripiprazole, and other antipsychotics in terms of impulsiveness symptoms, as assessed by the BIS-11. We could conclude from our results that people with schizophrenia/schizoaffective disorder who have comorbid SUD do not respond less to brexpiprazole or aripiprazole treatment than patients treated with other antipsychotics, but may instead respond better. However, the limited statistical power of our sample and the relative differences between the four groups in sample size, despite sample homogeneity, do not allow us to draw firm conclusions.
Another issue worth to be considered is the efficacy of treatment with aripiprazole and brexpiprazole in reducing psychotic symptoms, and substance craving, and their ability to improve the global clinical status.
In this study, brexpiprazole appeared to be significantly more efficacious than other treatments on global symptom status measured by the CGI-S. Brexpiprazole may be better suited for the long-term treatment of adult schizophrenia because it has a favorable safety and tolerability profile, in addition to reducing both positive and negative symptoms, thereby achieving the goals of increasing patient socialization and community reintegration [46]. Previous studies of brexpiprazole support our findings. Correll et al. [47] carried out a multicentre, randomized, double-blind, placebo-controlled trial to assess the efficacy, safety, and tolerability of oral brexpiprazole 2 and 4 mg/day; they showed superior efficacy and good tolerability compared with placebo in patients with acute exacerbations of schizophrenia. In a recent study, Lombardozzi et al. [48] evaluated treatment response to brexpiprazole in patients with schizophrenia comorbid or not with SUD and substance craving in the SUD subsample. Brexpiprazole was associated with similar significant improvements from baseline in both groups at the 6-month endpoint. In addition, the comorbid and non-comorbid subsamples did not differ in treatment response, suggesting that SUD comorbidity did not inhibit or reduce antipsychotic response to the drug.
In this study, we found the superiority of LAI aripiprazole over other treatments on the 24-item BPRS, PANSS Negative and PANSS General Psychopathology scales, and VAScrav frequency and intensity. In our clinical experience, LAIs have several advantages over oral antipsychotics, including ensuring clinician awareness of nonadherence, reducing pill burden, and reducing the consequences of planned or unplanned treatment gaps [49]. Psychotic relapse is extremely distressing for patients and their families and is associated with multiple downstream effects on disease progression and brain structure, such as progressive reductions in grey and white matter structure and volume [50]. Relapses can also lead to reduced response to previously effective antipsychotics, potentially contributing to treatment resistance [51]. Co-occurring SUDs in people with schizophrenia add to the complexity of psychiatric comorbidity, which is associated with increased symptom severity, poorer course of illness, high rates of treatment nonadherence, and increased rates of relapse. In people with addiction, reduced dopamine function results in decreased sensitivity to nondrug stimuli and reduced inhibitory function of the frontal cortex [52]. These findings suggest that aripiprazole-induced dopamine release may be associated with increased activation of the anterior cingulate, which may control craving for alcohol and other substances [17]. Regarding the serotonergic system, the 5-HT1A partial agonist effect of aripiprazole may modulate the prefrontal cortex to improve impulse control through projections from the raphe nucleus to the ventral tegmental area and nucleus accumbens [53]. Craving, despite the differences in the substance involved, that may extend from cocaine [54-56] and dopaminomimetics [57-59] to cannabinoids [60, 61], to nicotine [62], alcohol [63], and food [64, 65], may share common mechanisms involving dopamine receptors, probably countered by partial dopamine D2/D3 agonists [66].
In this investigation, LAI aripiprazole shows significant efficacy in reducing craving, and our data are clearly supported by various studies on the treatment of schizophrenia comorbid with SUD. The results of this study are consistent with previous literature. Aripiprazole produced significant improvements in psychotic symptoms and substance-related clinical outcomes, demonstrating efficacy in reducing craving and preventing relapse in patients with SUD [67]. LAI aripiprazole improved adherence and functioning in two patients with psychotic disorders and SUD [68], suggesting that it may be an effective and safe treatment option for dual disorders.
Once-monthly aripiprazole LAI improved craving and QoL at 1-year follow-up compared with paliperidone palmitate in initially hospitalized patients with psychosis comorbid with SUD [66]. Szerman et al. [69] carried out a multicentre, observational, retrospective study to evaluate the efficacy and impact of aripiprazole LAI in patients with schizophrenia with comorbid SUD. Severity was assessed using the Clinical Global Impressions Severity Scale for schizophrenia, and daily functioning and disability were assessed using the World Health Organization Disability Assessment Scale. At the one-year follow-up, aripiprazole improved both clinical status and quality of life, and reduced drug use, craving, and dependence severity.
In our study, the improvement in psychiatric symptoms is clinically meaningful and suggests that treatment with aripiprazole and brexpiprazole produces clinically relevant improvements in psychopathology. These findings suggest that both drugs may be effective in alleviating withdrawal symptoms associated with dopamine deficiency and, because of their broad receptor activity, may represent a new strategy for achieving balanced dopamine levels in the treatment of drug dependence. The co-occurrence of SUDs and psychosis is increasingly common and has been shown to worsen the longitudinal course of illness, reduce medication adherence and increase relapse rates. The treatment of schizophrenia is challenging, with the primary goal being to treat the acute phase, prevent relapse, and guide the patient to recovery. There are so many antipsychotics available today that it has become a challenge for psychiatrists to choose among them for each patient.
Long-term treatment may improve psychopathology, increase relapse prevention, reduce rehospitalizations, and improve outcomes in individuals with psychotic symptoms and comorbid substance use. Future studies will determine whether there are differences between the marketed partial D2/D3 agonists aripiprazole and brexpiprazole in the treatment of comorbid major psychiatric disorders and SUDs. For the moment, we may produce the following recommendations for clinicians and provide prompts for future research to clinical investigators. When dealing with people with comorbid SUDs and psychotic disorders like schizophrenia or schizoaffective disorder, try to persuade the patient to take an LAI like aripiprazole once monthly, which performed best in our study according to a general linear model; then try oral brexpiprazole that performed slightly better than oral aripiprazole according to the same model. The least recommendable treatments seem to be first-generation antipsychotics and other than D2/3 partial antagonist second-generation antipsychotics. Future research perspectives may consist in extending the observation period to two years. The other D2/3 partial antagonist we did not use here, cariprazine, could be a suitable comparator in future studies. Dealing with individual substances other than cannabinoids could provide clues as to neurobiological differences between various psychotic subtypes.
Another issue to deal with in future studies is how thought disorder responds to specific pharmacotherapy, in particular to those drugs we used in this study. Residual thought disorder is a neglected issue in current literature [70], and the scales used here only partially address it. The development of scales is welcome which could focus on the cognitive aspects of thought disorder and not only based on its positive and negative dimensions.
5. LIMITATIONS
Our study has several limitations. Our sample size was small and needs to be increased in order to draw stronger conclusions. Furthermore, the three dopamine partial agonist groups were not well-balanced, with the LAI group more than doubling the oral groups. In addition to the retrospective and uncontrolled design, another limitation is the allowed concomitant use of different psychotropic medications. In this study, we do not report on safety, which was assessed and will be the subject of a future study. Further, we did not measure plasma levels of aripiprazole or brexpiprazole in our patients nor did we perform genetic investigations regarding the metabolizer status of each patient to each drug received. The latter is not easy to obtain and may create problems with privacy and consent obtainment. While best therapeutic and safety windows have been identified for aripiprazole and its major active metabolite [71, 72], no such windows have been identified for brexpiprazole. It would be interesting to correlate in future studies the serum/plasma levels of brexpiprazole with clinical response. Despite its important limitations, our study suggests that drugs with partial agonist activity at dopamine D2/3 and serotonin 5-HT1A receptors and LAI formulations maintain their antipsychotic efficacy in patients with schizophrenia/schizoaffective disorder and coexisting SUD. Our data should await replication in larger samples.
CONCLUSION
The coexistence of cannabis use disorder and schizophrenia/schizoaffective disorder does not reduce the clinical response to antipsychotics endowed with partial D2/3 agonist properties. The treatment with such agents is associated with a strong reduction of craving symptoms. All antipsychotic treatment regimens were associated with significant improvements on all tested scales. Differences among D2/3 partial agonists oral and LAI and other SGA and FGA formulations were found only with the repeated-measures general linear model analysis and regarded oral brexpiprazole vs. others on global impressions, aripiprazole LAI vs. others on psychiatric symptom rating, general psychopathology, and negative symptomatology.
ACKNOWLEDGEMENTS
We thank Ms. Sara Beomonte for the statistical analyses.
LIST OF ABBREVIATIONS
- EEG
Electroencephalographic
- GLM
General Linear Model
- LAI
Long-acting Injectable
- SDs
Standard Deviations
- SGAs
Second-generation Antipsychotics
- SUDs
Substance Use Disorders
AUTHORS’ CONTRIBUTIONS
G.T. and S.D.F. conceived the study, G.T., G.L., E.A., and V.G. recruited patients and HCs and assessed them, G.T. and G.D.K. performed literature searches, F.P. performed the statistical analysis, G.T. and I.P. implemented the database, G.T., and G.D.K. provided the first draft, G.D.K. and S.D.F. supervised the writing of the manuscript, A.F., G.D.K. and S.D.F. supervised the writing of the manuscript, G.T., G.D.K., A.F. and S.D.F. provided the final draft, which was endorsed by all authors. They all viewed and accepted the manuscript in its final form.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The informed consent form and experimental procedures were approved by the Ethics Committee of the Rome2 Health Authority (Study 331-306-00387, 19-November-2019), in accordance with internationally accepted criteria for ethical research. The studies were conducted in accordance with local laws and institutional requirements.
HUMAN AND ANIMAL RIGHTS
Humans were involved in this study in full respect of their rights, as sanctioned by the World Medical Association at the 18th WMA General Assembly, Helsinki, Finland, June 1964, and subsequently amended at the 64th WMA General Assembly, Fortaleza, Ceará, Brazil, October 2013.
CONSENT FOR PUBLICATION
Written informed consent for participation in this study was obtained from all patients and the legal guardians/next of kin of the participants.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The raw data supporting the conclusions of this article will be provided by the corresponding author upon reasonable request without reservation.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kane J.M., Rifkin A., Quitkin F., Nayak D., Ramos-Lorenzi J. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch. Gen. Psychiatry. 1982;39(1):70–73. doi: 10.1001/archpsyc.1982.04290010048009. [DOI] [PubMed] [Google Scholar]
- 2.Crow T.J., MacMillan J.F., Johnson A.L., Johnstone E.C. A randomised controlled trial of prophylactic neuroleptic treatment. Br. J. Psychiatry. 1986;148(2):120–127. doi: 10.1192/bjp.148.2.120. [DOI] [PubMed] [Google Scholar]
- 3.Weiden P., Glazer W. Assessment and treatment selection for “revolving door” inpatients with schizophrenia. Psychiatr. Q. 1997;68(4):377–392. doi: 10.1023/A:1025499131905. [DOI] [PubMed] [Google Scholar]
- 4.Kasper S. First-episode schizophrenia: The importance of early intervention and subjective tolerability. J. Clin. Psychiatry. 1999;60(Suppl. 23):5–9. [PubMed] [Google Scholar]
- 5.McIntyre R.S. Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: The UNITE global survey. J. Clin. Psychiatry. 2009;70(Suppl. 3):05–11. doi: 10.4088/JCP.7075su1c.02. [DOI] [PubMed] [Google Scholar]
- 6.Stone J.M., Roux S., Taylor D., Morrison P.D. First-generation versus second-generation long-acting injectable antipsychotic drugs and time to relapse. Ther. Adv. Psychopharmacol. 2018;8(12):333–336. doi: 10.1177/2045125318795130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt G.E., Large M.M., Cleary M., Lai H.M.X., Saunders J.B. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol Depend. 2018;191:234–258. doi: 10.1016/j.drugalcdep.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Hasan A., Falkai P., Wobrock T., Lieberman J., Glenthøj B., Gattaz W.F., Thibaut F., Möller H.J. World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of Schizophrenia. Part 3: Update 2015 Management of special circumstances: Depression, Suicidality, substance use disorders and pregnancy and lactation. World J. Biol. Psychiatry. 2015;16(3):142–170. doi: 10.3109/15622975.2015.1009163. [DOI] [PubMed] [Google Scholar]
- 9.Crockford D., Addington D. Canadian Schizophrenia guidelines: Schizophrenia and other psychotic disorders with coexisting substance use disorders. Can. J. Psychiatry. 2017;62(9):624–634. doi: 10.1177/0706743717720196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitali M., Mistretta M., Alessandrini G., Coriale G., Romeo M., Attilia F., Rotondo C., Sorbo F., Pisciotta F., Attilia M.L., Ceccanti M. Pharmacological treatment for dual diagnosis: A literature update and a proposal of intervention. Riv. Psichiatr. 2018;53(3):160–169. doi: 10.1708/2925.29419. [DOI] [PubMed] [Google Scholar]
- 11.Price S.A., Brahm N.C. Antipsychotic treatment of adolescent dual diagnosis patients. J. Pediatr. Pharmacol. Ther. 2011;16(4):226–236. doi: 10.5863/1551-6776-16.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A., Datta S.S., Wright S.D., Furtado V.A., Russell P.S. Atypical antipsychotics for psychosis in adolescents. Cochrane Libr. 2013;2013(10):CD009582. doi: 10.1002/14651858.CD009582.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubio G., Martínez I., Ponce G., Jiménez-Arriero M.A., López-Muñoz F., Álamo C. Long-acting injectable risperidone compared with zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity. Can. J. Psychiatry. 2006;51(8):531–539. doi: 10.1177/070674370605100808. [DOI] [PubMed] [Google Scholar]
- 14.McEvoy J.P., Freudenreich O., Levin E.D., Rose J.E. Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology (Berl.) 1995;119(1):124–126. doi: 10.1007/BF02246063. [DOI] [PubMed] [Google Scholar]
- 15.Stahl S.M. Stahl’s Essential Psychopharmacology. 5th ed. Cambridge, UK: Cambridge University Press; 2021. [DOI] [Google Scholar]
- 16.Burris K.D., Molski T.F., Xu C., Ryan E., Tottori K., Kikuchi T., Yocca F.D., Molinoff P.B. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J. Pharmacol. Exp. Ther. 2002;302(1):381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 17.Riva M.A., Di Sciascio G. Aripiprazole: From pharmacological profile to clinical use. Neuropsychiatr. Dis. Treat. 2015;11:2635–2647. doi: 10.2147/NDT.S88117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swainston Harrison T., Perry C.M. Aripiprazole. Drugs. 2004;64(15):1715–1736. doi: 10.2165/00003495-200464150-00010. [DOI] [PubMed] [Google Scholar]
- 19.Maeda K., Sugino H., Akazawa H., Amada N., Shimada J., Futamura T., Yamashita H., Ito N., McQuade R.D., Mørk A., Pehrson A.L., Hentzer M., Nielsen V., Bundgaard C., Arnt J., Stensbøl T.B., Kikuchi T., Brexpiprazole I. In vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 2014;350(3):589–604. doi: 10.1124/jpet.114.213793. [DOI] [PubMed] [Google Scholar]
- 20.Kishi T., Ikuta T., Matsuda Y., Sakuma K., Iwata N. Aripiprazole vs. brexpiprazole for acute schizophrenia: A systematic review and network meta-analysis. Psychopharmacology (Berl.) 2020;237(5):1459–1470. doi: 10.1007/s00213-020-05472-5. [DOI] [PubMed] [Google Scholar]
- 21.Maeda K., Lerdrup L., Sugino H., Akazawa H., Amada N., McQuade R.D., Stensbøl T.B., Bundgaard C., Arnt J., Kikuchi T., Brexpiprazole II:. Antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 2014;350(3):605–614. doi: 10.1124/jpet.114.213819. [DOI] [PubMed] [Google Scholar]
- 22.Caraci F., Santagati M., Caruso G., Cannavò D., Leggio G.M., Salomone S., Drago F. New antipsychotic drugs for the treatment of agitation and psychosis in Alzheimer’s disease: Focus on brexpiprazole and pimavanserin. F1000 Res. 2020;9:F1000. doi: 10.12688/f1000research.22662.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stummer L., Markovic M., Maroney M. Brexpiprazole in the treatment of schizophrenia and agitation in Alzheimer’s disease. Neurodegener. Dis. Manag. 2020;10(4):205–217. doi: 10.2217/nmt-2020-0013. [DOI] [PubMed] [Google Scholar]
- 24.Varadharajan A., Davis A.D., Ghosh A., Jagtap T., Xavier A., Menon A.J., Roy D., Gandhi S., Gregor T. Guidelines for pharmacotherapy in Alzheimer’s disease - A primer on FDA-approved drugs. J. Neurosci. Rural Pract. 2023;14(4):566–573. doi: 10.25259/JNRP_356_2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marinheiro G., Dantas J.M., Mutarelli A., Menegaz de Almeida A., Monteiro G.A., Zerlotto D.S., Telles J.P.M. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer’s disease: A meta-analysis of randomized controlled trials. Neurol. Sci. 2024;45(10):4679–4686. doi: 10.1007/s10072-024-07576-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Ricci V., Sarni A., Martinotti G., Maina G. Comparative analysis of third-generation antipsychotics in first-episode schizophrenia: Efficacy, safety, and cognitive impacts. A narrative review. Int. Clin. Psychopharmacol. 2024;2024:14. doi: 10.1097/YIC.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 27.Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergne D., Anton R. Aripiprazole: A drug with a novel mechanism of action and possible efficacy for alcohol dependence. CNS Neurol. Disord. Drug Targets. 2010;9(1):50–54. doi: 10.2174/187152710790966731. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro E.L.A., de Mendonça Lima T., Vieira M.E.B., Storpirtis S., Aguiar P.M. Efficacy and safety of aripiprazole for the treatment of schizophrenia: An overview of systematic reviews. Eur. J. Clin. Pharmacol. 2018;74(10):1215–1233. doi: 10.1007/s00228-018-2498-1. [DOI] [PubMed] [Google Scholar]
- 30.Ricci V., De Berardis D., Maina G. Third-generation antipsychotics and lurasidone in the treatment of substance-induced psychoses: A narrative review. Healthcare (Basel) 2024;12(3):339. doi: 10.3390/healthcare12030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fornaro M., Fusco A., Anastasia A., Cattaneo C.I., De Berardis D. Brexpiprazole for treatment-resistant major depressive disorder. Expert Opin. Pharmacother. 2019;20(16):1925–1933. doi: 10.1080/14656566.2019.1654457. [DOI] [PubMed] [Google Scholar]
- 32.Koskinen J., Hakko H., Riipinen P., Sihvola N., Halt A.H. A letter to the editor: The impact of comorbid depression and substance use disorder on drivers with a psychotic disorder killed in motor vehicle accidents. Schizophr. Res. . 2024;270:246–248. doi: 10.1016/j.schres.2024.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Trovini G., Amici E., Bauco P., Matrone M., Lombardozzi G., Giovanetti V., Kotzalidis G.D., De Filippis S. A comprehensive evaluation of adverse childhood experiences, social–emotional impairments, and neurodevelopmental disorders in cannabis-use disorder: Implications for clinical practice. Eur. Psychiatry. 2023;66(1):e77. doi: 10.1192/j.eurpsy.2023.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Posner K., Brown G.K., Stanley B., Brent D.A., Yershova K.V., Oquendo M.A., Currier G.W., Melvin G.A., Greenhill L., Shen S., Mann J.J. The Columbia-suicide severity rating scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wechsler D.A. Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX: Psychological Corporation; 2008. p. 328. [DOI] [Google Scholar]
- 36.Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised; US Department of Health, Education, and Welfare Publication. Rockville, MD: ADM; 1976. pp. 76–338. [Google Scholar]
- 37.Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 38.Pancheri P., Brugnoli R., Carilli L., Delle Chiaie R., Marconi P.L., Petrucci R.M. Dimensional assessment of schizophrenic symptomatology. Validation of the Italian version of the Positive And Negative Symptoms Scale (PANSS). Giorn. Ital. Psychopathol. 1995;1:60–75. [Google Scholar]
- 39.Ventura J., Lukoff D., Nuechterlein K.H., Liberman R.P., Green M., Shaner A. Training and quality assurance with the Brief psychiatric rating scale: “The drift busters” appendix 1: Brief Psychiatric Rating Scale (BPRS) expanded version (40) scales, anchor points and administration manual. Int. J. Methods Psychiatr. Res. 1993;3:221–244. [Google Scholar]
- 40.Roncone R., Ventura J., Impallomeni M., Falloon I.R., Morosini P.L., Chiaravalle E., Casacchia M. Reliability of an Italian standardized and expanded brief psychiatric rating scale (BPRS 4.0) in raters with high vs. low clinical experience. Acta Psychiatr. Scand. 1999;100(3):229–236. doi: 10.1111/j.1600-0447.1999.tb10850.x. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson A.N. Visual analogue scales and drug effects. Br. J. Clin. Pharmacol. 1978;6:3–4. doi: 10.1111/j.1365-2125.1978.tb01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 43.First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. Structured Clinical Interview for DSM-5 Disorders-Clinician Version SCID-5-CV. Washington, DC: American Psychiatric Association Publishing, Inc.; 2016. [Google Scholar]
- 44.Shapiro S.S., Wilk M.B. An analysis of variance test for normality (complete samples). Biometrika. 1965;52(3-4):591–611. doi: 10.1093/biomet/52.3-4.591. [DOI] [Google Scholar]
- 45.Bonferroni C.E. Statistical class theory and probability calculus;Publications of the Royal Institute of Economic and Commercial Sciences of Florence: Florence, Italy. 1936 [Google Scholar]
- 46.Watanabe Y., Yamada S., Otsubo T., Kikuchi T. Brexpiprazole for the treatment of schizophrenia in adults: An overview of its clinical efficacy and safety and a psychiatrist’s perspective. Drug Des. Devel. Ther. 2020;14:5559–5574. doi: 10.2147/DDDT.S240859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correll C.U., Skuban A., Ouyang J., Hobart M., Pfister S., McQuade R.D., Nyilas M., Carson W.H., Sanchez R., Eriksson H. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: A 6-week randomized, double-blind, placebo-controlled trial. Am. J. Psychiatry. 2015;172(9):870–880. doi: 10.1176/appi.ajp.2015.14101275. [DOI] [PubMed] [Google Scholar]
- 48.Lombardozzi G., Trovini G., Amici E., Kotzalidis G.D., Perrini F., Giovanetti V., Di Giovanni A., De Filippis S. Brexpiprazole in patients with schizophrenia with or without substance use disorder: An observational study. Front. Psychiatry. 2023;14:1321233. doi: 10.3389/fpsyt.2023.1321233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Correll C.U., Kim E., Sliwa J.K., Hamm W., Gopal S., Mathews M., Venkatasubramanian R., Saklad S.R. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: An overview. CNS Drugs. 2021;35(1):39–59. doi: 10.1007/s40263-020-00779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karlsgodt K.H., Sun D., Cannon T.D. Structural and functional brain abnormalities in schizophrenia. Curr. Dir. Psychol. Sci. 2010;19(4):226–231. doi: 10.1177/0963721410377601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vita A., De Peri L., Deste G., Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: A meta-analysis and meta-regression of longitudinal MRI studies. Transl. Psychiatry. 2012;2(11):e190. doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volkow N.D., Fowler J.S., Wang G-J., Swanson J.M. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol. Psychiatry. 2004;9(6):557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 53.Walsh S.L., Cunningham K.A. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl.) 1997;130(1):41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- 54.Saunders B.T., Yager L.M., Robinson T.E. Cue-evoked cocaine “craving”: Role of dopamine in the accumbens core. J. Neurosci. 2013;33(35):13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alonso I.P., O’Connor B.M., Bryant K.G., Mandalaywala R.K., España R.A. Incubation of cocaine craving coincides with changes in dopamine terminal neurotransmission. Addict. Neurosci. 2022;3:100029. doi: 10.1016/j.addicn.2022.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber S.J., Kawa A.B., Beutler M.M., Kuhn H.M., Moutier A.L., Westlake J.G., Koyshman L.M., Moreno C.D., Wunsch A.M., Wolf M.E. Dopamine transmission at D1 and D2 receptors in the nucleus accumbens contributes to the expression of incubation of cocaine craving. Neuropsychopharmacology. 2024, 2024. doi: 10.1038/s41386-024-01992-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morales A.M., Kohno M., Robertson C.L., Dean A.C., Mandelkern M.A., London E.D. Midbrain dopamine D2/D3 receptor availability and drug craving are associated with mesocorticolimbic gray matter volume in methamphetamine users. Mol. Psychiatry. 2015;20(6):658. doi: 10.1038/mp.2015.59. [DOI] [PubMed] [Google Scholar]
- 58.Mojtabai R., Susukida R., Farokhnia M., Nguyen T.Q., Dunn K.E., Amin-Esmaeili M. Trajectories of craving in the course of pharmacotherapy trials for methamphetamine use disorder. Addiction. 2024;119(10):1803–1812. doi: 10.1111/add.16610. [DOI] [PubMed] [Google Scholar]
- 59.Blum K., Badgaiyan R., Braverman E., Dushaj K., Li M., Thanos P., Demetrovics Z., Febo M. Hypothesizing that, a pro-dopamine regulator (KB220Z) should optimize, but not hyper-activate the activity of trace amine-associated receptor 1 (TAAR-1) and induce anti-craving of psychostimulants in the long-term. J. Reward Defic. Syndr. Addict. Sci. 2016;2(1):14–21. doi: 10.17756/jrdsas.2016-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Machielsen M., Beduin A.S., Dekker N., Kahn R.S., Linszen D.H., van Os J., Wiersma D., Bruggeman R., Cahn W., de Haan L., Krabbendam L., Myin-Germeys I. Differences in craving for cannabis between schizophrenia patients using risperidone, olanzapine or clozapine. J. Psychopharmacol. 2012;26(1):189–195. doi: 10.1177/0269881111408957. [DOI] [PubMed] [Google Scholar]
- 61.Filbey F.M., DeWitt S.J. Cannabis cue-elicited craving and the reward neurocircuitry. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;38(1):30–35. doi: 10.1016/j.pnpbp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chukwueke C.C., Kowalczyk W.J., Di Ciano P., Gendy M., Taylor R., Heishman S.J., Le Foll B. Exploring the role of the Ser9Gly (rs6280) Dopamine D3 receptor polymorphism in nicotine reinforcement and cue-elicited craving. Sci. Rep. 2020;10(1):4085. doi: 10.1038/s41598-020-60940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodd Z.A., Anstrom K.K., Knapp D.J., Racz I., Zimmer A., Serra S., Bell R.L., Woodward D.J., Breese G.R., Colombo G. Factors mediating alcohol craving and relapse: Stress, compulsivity, and genetics. Alcohol. Clin. Exp. Res. 2005;29(7):1325–1333. doi: 10.1097/01.ALC.0000171487.62079.A3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blum K., Liu Y., Shriner R., Gold M.S. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr. Pharm. Des. 2011;17(12):1158–1167. doi: 10.2174/138161211795656819. [DOI] [PubMed] [Google Scholar]
- 65.Haddad-Tóvolli R., Ramírez S., Muñoz-Moreno E., Milà-Guasch M., Miquel-Rio L., Pozo M., Chivite I., Altirriba J., Obri A., Gómez-Valadés A.G., Toledo M., Eyre E., Bortolozzi A., Valjent E., Soria G., Claret M. Food craving-like episodes during pregnancy are mediated by accumbal dopaminergic circuits. Nat. Metab. 2022;4(4):424–434. doi: 10.1038/s42255-022-00557-1. [DOI] [PubMed] [Google Scholar]
- 66.Cuomo I., Kotzalidis G.D., de Persis S., Piacentino D., Perrini F., Amici E., De Filippis S. Head-to-head comparison of 1-year aripiprazole long-acting injectable (LAI) versus paliperidone LAI in comorbid psychosis and substance use disorder: Impact on clinical status, substance craving, and quality of life. Neuropsychiatr. Dis. Treat. 2018;14:1645–1656. doi: 10.2147/NDT.S171002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinotti G., Orsolini L., Fornaro M., Vecchiotti R., De Berardis D., Iasevoli F., Torrens M., Di Giannantonio M. Aripiprazole for relapse prevention and craving in alcohol use disorder: Current evidence and future perspectives. Expert Opin. Investig. Drugs. 2016;25(6):719–728. doi: 10.1080/13543784.2016.1175431. [DOI] [PubMed] [Google Scholar]
- 68.Erdogan A., Kulaksizoglu B., Bingol M.S., Cinemre B., Kuloglu M.M. Successful treatment with long-acting injectable aripiprazole monohydrate for two patients with dual diagnosis - Substance use disorder and psychotic disorder. Dusunen Adam. 2021;34:102–106. doi: 10.14744/DAJPNS.2021.00126. [DOI] [Google Scholar]
- 69.Szerman N., Basurte-Villamor I., Vega P., Martinez-Raga J., Parro-Torres C., Cambra Almerge J., Grau-López L., De Matteis M., Arias F. Once-monthly long-acting injectable aripiprazole for the treatment of patients with schizophrenia and co-occurring substance use disorders: A multicentre, observational study. Drugs Real World Outcomes. 2020;7(1):75–83. doi: 10.1007/s40801-020-00178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Özbek S.U., Alptekin K. Thought disorder as a neglected dimension in schizophrenia. Alpha Psychiatry. 2021;23(1):5–11. doi: 10.5152/alphapsychiatry.2021.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirschbaum K.M., Müller M.J., Malevani J., Mobascher A., Burchardt C., Piel M., Hiemke C. Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J. Biol. Psychiatry. 2008;9(3):212–218. doi: 10.1080/15622970701361255. [DOI] [PubMed] [Google Scholar]
- 72.Hart X.M., Hiemke C., Eichentopf L., Lense X.M., Clement H.W., Conca A., Faltraco F., Florio V., Grüner J., Havemann-Reinecke U., Molden E., Paulzen M., Schoretsanitis G., Riemer T.G., Gründer G. Therapeutic reference range for aripiprazole in schizophrenia revised: A systematic review and metaanalysis. Psychopharmacology (Berl.) 2022;239(11):3377–3391. doi: 10.1007/s00213-022-06233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be provided by the corresponding author upon reasonable request without reservation.


