Abstract
Background
Individuals with HIV infection often lose weight during the course of their disease. Furthermore, low serum concentrations of testosterone are common in individuals with HIV infection, particularly those with weight loss. Treatment of weight loss with anabolic steroids in HIV‐infected individuals may be beneficial.
Objectives
Our objectives were to assess the efficacy and safety of anabolic steroids for the treatment of weight loss in adults with HIV infection.
Search methods
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, AIDSLINE, AIDSearch, EMBASE, CINAHL, Current Contents, and the National Library of Medicine Gateway Abstracts for controlled trials up to April 2005. We also searched the bibliographies of the identified studies and review the articles. In addition, pharmaceutical manufacturers of anabolic steroids were contacted.
Selection criteria
Randomized controlled trials that compared the use of an anabolic steroid to placebo to treat weight loss in adults with HIV were included. Randomized controlled trials that compared the use of anabolic steroids to placebo for the treatment of weight loss in adults with HIV were selected. Change from baseline in lean body mass or in body weight was reported as on outcome measure.
Data collection and analysis
Two reviewers independently assessed the trials for quality of randomization, blinding, withdrawals, and adequacy of allocation concealment. For continuous data, weighted mean differences (WMD) were calculated. For dichotomous outcomes, risk differences, were calculated. Because of uncertainty as to whether consistent true effects exist in such different populations and treatments, the authors decided a priori to use random effects models for all outcomes.
Main results
Thirteen trials met the inclusion criteria. Two hundred ninety‐four individuals randomized to anabolic steroid therapy and 238 individuals randomized to placebo were included in the analysis of efficacy for change from baseline in lean body mass. Three hundred forty‐three individuals randomized to anabolic steroid and 286 randomized to placebo were included in the analysis of efficacy for change from baseline in body weight. The mean methodologic quality of the included studies was 4.1, of a maximum 5 points. Although significant heterogeneity was present for both outcomes, the average change in lean body mass was 1.3 kg (95% CI: 0.6, 2.0), while the average change in total body weight was 1.1 kg (95% CI: 0.3, 2.0). A total of eight deaths occurred during the treatment period; four in the anabolic steroid treatment groups and four in the placebo‐treatment groups (risk difference 0.00, 95% CI ‐0.03, 0.03). The risk difference for withdrawals or discontinuations of study medication due to adverse events was 0.00 (95% CI: ‐0.02, 0.03).
Authors' conclusions
Although the results of the trials were heterogeneous, on average, the administration of anabolic steroids appeared to result in a small increase in both lean body mass and body weight as compared with placebo. While these results suggest that anabolic steroids may be useful in the treatment of weight loss in HIV infected individuals, due to limitations, treatment recommendations cannot be made. Further information is required regarding the long‐term benefit and adverse effects of anabolic steroid use, the specific populations for which anabolic steroid therapy may be most beneficial, and the optimal regime. In addition, the correlation of improvement in lean body mass with more clinically relevant endpoints, such as physical functioning and survival, needs to be determined.
Keywords: Humans, Anabolic Agents, Anabolic Agents/therapeutic use, HIV Wasting Syndrome, HIV Wasting Syndrome/drug therapy, Randomized Controlled Trials as Topic, Steroids, Steroids/therapeutic use, Weight Loss, Weight Loss/drug effects
Plain language summary
Anabolic steroids for the treatment of weight loss in HIV‐infected individuals
Anabolic steroids may be beneficial in the treatment of weight loss in HIV‐infected individuals. Anabolic steroids include testosterone and its derivatives. One of the functions of testosterone is to help build muscle. Testosterone has been demonstrated to increase muscle mass and lean body mass in testosterone‐deficient but otherwise healthy men. Individuals with HIV infection often lose weight and have low blood levels of testosterone; thus, the use of anabolic steroids in the treatment of weight loss in individuals with HIV infection may be beneficial. The purpose of this review was to evaluate anabolic steroids as a means of treatment of weight loss in individuals with HIV infection. The review includes 13 randomized clinical trials in the primary analysis. The results suggested that anabolic steroids increased both lean body mass and body weight. However, the results were not consistent among individual trials and the average increase was small and may not be clinically relevant. Furthermore, the results need to be interpreted with caution as this meta‐analysis was limited due to small sample sizes; short duration of treatment and of follow‐up; and heterogeneity of the study populations, the anabolic interventions, and concomitant therapies.
Background
HIV‐infected individuals often develop wasting during the course of their disease. In prospective and retrospective cohort studies of HIV‐infected individuals, increasing weight loss was found to be significantly associated with decreased survival, as well as with reduced quality of life (Bhasin 1999, Mulligan 1999). In addition, studies have demonstrated that decreased survival is more clearly associated with changes in lean body mass than with changes in total body weight (Mulligan 1999). Despite the effectiveness of antiretroviral therapy, in particular highly active antiretroviral therapy (HAART), HIV‐associated wasting remains a problem for many individuals (Dworkin 2003, Smit 2002, Wanke 2000).
Hypogonadism, a condition associated with low testosterone levels, may be a factor contributing to wasting in HIV‐infection. Low levels of testosterone occur frequently in men with AIDS, and have also been demonstrated in women with HIV infection and wasting (Grinspoon 1997). Hypogonadism may cause greater loss of lean body mass than fat mass. Corcoran et al reported that, in a study of men with hypogonadism and AIDS wasting (Grinspoon 1996), muscle mass was 75% of the predicted value according to height, while total body weight was 92% of predicted value according to height (Corcoran 1999).
Therefore, it is possible that the therapeutic use of anabolic steroids (androgenic hormones that include or are derivatives of testosterone) will be beneficial in HIV‐infected individuals with weight loss, in terms of increasing lean body mass, body weight, quality of life, and overall survival. However, adverse effects of androgens may include acne, water retention, hepatic toxicity, and endocrine effects including decreased glucose tolerance and alterations in lipid profiles. Additionally adverse effects include thegrowth of facial hair or menstrual irregularities in women, and azoospermia and gynecomastia in men (Snyder 2001).
(For a discussion of the physiologic effects, routes of delivery, and adverse effects of anabolic steroids, see Appendix 1.)
Objectives
To assess the efficacy and safety of anabolic steroids compared to placebo for the treatment of weight loss in HIV‐infected adults.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled clinical trials meeting the inclusion criteria were included in this systematic review. However, the trial published as an abstract (Bucher 1996) was excluded from the primary analysis. It was included in secondary sensitivity analyses only. Trials in which all patients were treated with open label anabolic steroid and subsequently randomized to a discontinuation phase of anabolic steroid or placebo were not included in this review.
Types of participants
Trials involving participants 18 years of age or older with both HIV infection and weight loss of any amount were included in this review. Trials involving either men or women or both were included, as were trials involving either eugonadal or hypogonadal individuals.
Types of interventions
Randomized controlled trials that compared the use of an anabolic steroid to placebo to treat weight loss in adults with HIV were included. Anabolic steroids included any androgen, either synthetic or non‐synthetic, by any route of administration. It was decided by the reviewers, a priori, that the intervention should be given for at least six weeks, as such a time frame was considered necessary to observe clinically significant changes in body weight or body composition (Rabkin 2000b). Other interventions, such as nutritional counselling, exercise, or other drug therapies, were accepted as concomitant therapy, provided they were distributed similarly between anabolic steroid and control treatment groups.
Types of outcome measures
Trials where outcome measures included change from baseline in either lean body mass, fat free mass, or body cell mass and/or change from baseline in body weight were included in this review. However, trials where these outcome measures were reported as medians or without a measure of variability (Phanuphak, Schambelan 2001) were excluded.
Search methods for identification of studies
The following databases were searched: The Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE, AIDSLINE, CINAHL, and Current Contents using the OVID interface; EMBASE; AIDSearch using NISC BiblioLine; and the abstracts in the National Library of Medicine Gateway (NLM). MeSH headings included: HIV infections, acquired immunodeficiency syndrome, body weight, body composition, androgens, androgens synthetic. Keywords used for the search included: HIV, AIDS, weight, wasting, lean body mass, body mass, body composition, cachexia, and the names for each of the anabolic steroids. The Haynes systematic search filter for randomized trials (Haynes 1994) was used in the OVID searches in order to restrict the search to potential randomized studies. A similar filter was used in the EMBASE and AIDSearch searches. A broad search was done of the NLM database. The OVID search strategy used can be found in Appendix 2.
CENTRAL was searched for the time period 1980 to February 2005; MEDLINE for 1980 to April 2005; CINAHL for 1982 to April 2005; Current Contents for 1998 to June 2003; and EMBASE for 1988 to February 2005. AIDSLINE was searched for the time period 1980 to December 1999 as this database was later discontinued; subsequently AIDSearch was searched for 1980 to February 2005. The abstracts contained in the National Library of Medicine were searched to April 2005.
No language restrictions were applied to these searches.
The lists of references from the studies and review articles identified from the searches outlined above were manually searched to include any citations missed by the electronic searches.
Pharmaceutical manufacturers of anabolic steroids were contacted to identify further clinical studies.
Data collection and analysis
Study Selection
Only studies published as full publications were considered in the primary analysis. Trials published as abstracts or as letters to the editor were included in a secondary sensitivity analysis. Duplicate publications or publications involving the same patients but different outcomes were included once. Agreement between the two reviewers, regarding the initial review of the electronic searches, was assessed using the kappa statistic. In the case of disagreement, the two observers discussed the issue and attempted to reach consensus. If necessary, a third adjudicator (RC) was used.
Trials to be included in the review were determined independently by two reviewers (KJ, MB). Each reviewer reviewed the list of references and abstracts obtained from the above search strategy. References considered to possibly meet the inclusion criteria by either reviewer were retrieved and reviewed in full text. All trials meeting the inclusion criteria were included in the review. However, only those published as full peer‐reviewed publications were considered in the primary meta‐analysis, while those published as abstracts or provided by pharmaceutical companies were considered for inclusion in the secondary sensitivity analysis. Duplicate publications, or publications involving the same patients but different outcomes, were included only once. In the case of disagreement, the two reviewers discussed the reference and reached consensus.
Data Collection
The two reviewers extracted data from the trials using a pre‐derived data extraction tool. In the case of disagreement, the two reviewers discussed the issue and attempted to reach a consensus. A third adjudicator was used as necessary. Data were entered into Review Manager Version 4.2 for Windows.
Data Collected
A) Baseline data: Baseline data (means or proportions) were collected for each treatment group for each trial. Data regarding age (years), weight (kilograms), weight loss (kilograms), serum testosterone level (nmol/L), CD4 cell count (cells x 109/ L, and protease inhibitor therapy (%) were extracted.
B) Outcome data: Data (mean change, standard deviation, and number of patients for the continuous variables; and number of participants with an event and total number of participants for the dichotomous variables) were extracted for each treatment group for each change at end of study time point from baseline in lean body mass, change from baseline in body weight, number of deaths, and number of study withdrawals or discontinuations of study medication due to adverse events.
The change from baseline in lean body mass was measured by change in either lean body mass, fat free mass, or body cell mass using any accepted measurement technique. For a further discussion of the definitions and methodology for determining the change in lean body mass, see Appendix 3.
C) Methodologic quality data: The methodologic quality of each trial was assessed using validated assessment tools (Jadad 1996, Schulz 1995,) based on the following parameters: Allocation concealment‐ was allocation concealment adequate, uncertain, or clearly inadequate? Randomization‐ was the trial described as randomized and was the method of randomization described and appropriate? Blinding‐ was the trial described as double‐blind and was the method of double‐blinding described and appropriate? Accounting for withdrawals‐ was there a description of withdrawals and drop‐outs by treatment group? For each study, allocation concealment was determined to be either: adequate, unclear, inadequate, or not used (Schulz 1995); and a numerical score was assigned based on a validated five point scale which included items relating to randomization (2 points), blinding (2 points), and description of withdrawals and dropouts (1 point) (Jadad 1996). Two reviewers reached consensus on the categorizations of allocation concealment and the numerical scores.
Change from baseline in lean body mass and body weight, were generally reported for only those participants that completed the study. Data on deaths and withdrawals or discontinuations of study medication due to adverse events, as well as baseline variables, were generally reported for all randomized participants. Although the authors were contacted in an attempt to obtain data on the efficacy variables for all randomized participants, these data were not available. Therefore, the data available in the published reports were extracted. In addition, although we had planned to extract data on change from baseline in quality of life and on number of participants with adverse drug reactions, these data were generally not available in sufficient detail to allow meta‐analysis.
Data Synthesis
Comparisons between treatment groups for continuous outcomes including change from baseline in lean body mass and change from baseline in body weight were presented as mean differences in the change for each trial. Comparisons between treatment groups for dichotomous outcomes, including deaths and withdrawals from study or discontinuation of study treatment due to adverse events, are presented as risk differences for each trial. Risk ratios were not used because of the significant number of zero cells. One study (Bhasin 2000) had four treatment groups; testosterone verses placebo with or without exercise. Because results were not reported for the overall comparison of anabolic steroid versus placebo, this study was treated as two separate trials of testosterone verses placebo, one with concomitant exercise and one without. In addition, two studies (Hengge 2003a, Miller 1998) had three treatment groups, and compared the effect of two different doses of anabolic steroids verses placebo. For each of these trials, the results for the two anabolic steroid groups were treated as two separate trials, with the results for the placebo group based on half the numbers in that group.
A summary of the weighted treatment effect was calculated across trials using the Cochrane statistical package, RevMan Analyses, Version 1.0 for Windows. The results are expressed as weighted mean differences (WMD and 95% CI) for the continuous outcomes and as pooled risk differences (RD and 95% CI) for the dichotomous outcomes.
A random‐effects model was used due to; the variety of anabolic steroids being tested, the differences in treatment durations, and the differences in study populations. It was suspected that heterogeneity would be significant for most comparisons. Thus, the method of DerSimonian and Laird was used in the calculation of the summary measures of treatment effect.
In order to explore the anticipated heterogeneity, subgroup analyses of trials included in the primary analysis were carried out by the following factors:
·gender (men, women, or men and women);
·gonadal status for trials with men only (hypogonadal men, eugonadal men);
·study treatment duration (6 months, 16 weeks, 12 weeks, 8 weeks);
·study treatment dose (supraphysiologic, physiologic);
·proportion of study participants on protease inhibitors at baseline (less than 60%, 60% or greater);
·measurement technique used to measure lean body mass (DEXA, BIA);
·methodologic quality score (5, 4, 3);
·adequacy of allocation concealment (adequate, uncertain);
·quality score on randomization (2, 1);
·quality score on description of withdrawals (1, 0); and
·total sample size of trial (less than 40, 40 to 49, 50 to 59, 60 or greater).
Subgroup analyses by age could not be carried out as the mean ages of participants in each of the included studies were similar (range 34 to 42 years). Subgroup analysis by the quality score for blinding was not carried out as all studies scored 2. Total sample size was based on total number of randomized subjects in all treatment arms in a trial (i.e., in three arm and factorial design trials, sample size was categorized based upon all subjects in the trial). In the subgroup analyses based on factors with more than one level (i.e. study treatment dosage, study drug duration, methodologic quality score, trial sample size) categories were presented in order to attempt to determine any dosage, duration, quality, or sample size response relationship across trials.
Sensitivity analyses were conducted to evaluate the robustness of the results of the meta‐analysis. These analyses examined the effects of inclusion of abstracts and methodologic quality. Analyses were repeated with abstracts included, as well as excluding studies with quality scores less than 4.
Potential publication bias (or other potential biases associated with smaller studies) as well as potential heterogeneity due to study size was assessed by preparing a funnel plot and examining it for asymmetry.
Results
Description of studies
The electronic literature searches of The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, AIDSLINE, EMBASE, CINAHL, and Current Contents retrieved 184 citations. Of these, 66 were potential studies. Thirteen further potential studies were identified from the search of the Abstracts within the NLM Gateway (Corcoran 2000, Dolan 2004b, Gold 1994, Gold 1999, Hasheeve 1994, Hengge 2002, Hengge 2004, Hernandez‐Lopez 1996, Mulligan 2001, Schambelan 2001, Schrader 2002, Urbina 2004, Wheeler 1998) and seven further citations for potential studies (Berger 1993, Engleson 1996, Jeantils 1993, Jekot 1993, Poles 1997, Rabkin 1995a, Rabkin 1995b) were located by searching the reference lists of the retrieved articles and of review articles. In addition, one unpublished study (Phanuphak) was provided by Organon International. Of these 87 potential studies, 14 (Bhasin 1998, Bhasin 2000, Bucher 1996, Choi 2005, Coodley 1997, Dobs 1999, Dolan 2004a, Grinspoon 1998a, Grinspoon 2000a, Hengge 2003a, Miller 1998, Mulligan 2005, Strawford 1999a, Wagner 2001) were included in the meta‐analysis as controlled clinical trials. However, one (Bucher 1996) of these fourteen studies was published as an abstract only and was therefore included in the secondary sensitivity analyses only.
Of the 73 studies that were excluded: ·three (Berger 1996, Phanuphak, Schambelan 2001) met inclusion criteria but were excluded because data presented were insufficient to allow extrapolation for the meta‐analyses. We were unable to obtain the necessary information from the study authors {for the trial of Berger we could not interpret the outcome data as presented in the publication; the abstract of Schambelan presented outcome data for weight and lean body mass as medians without a measure of variability; the trial report provided by a pharmaceutical company (Phanuphak) presented outcome data for weight and lean body mass as medians with the range (we required the means and standard deviations)}; ·one (Rabkin 2000b) was excluded because the outcomes of interest were not reported for the randomized portion of the study; ·one (Strawford 1999b) was excluded because the treatment duration of the randomized phase was only two weeks; ·one (Batterham 2001) was excluded because comparison was between anabolic steroid vs. dietary counselling, rather than anabolic steroid versus no active therapy; ·one (Schrader 2002) was excluded because the anabolic drug treatment group received Human Chorionic Gonadotropin in addition to anabolic steroids; ·one (Rabkin 2004) was excluded because the entry criteria did not include weight loss and body weight, and composition were either not measured or not reported; ·one (Hengge 1996a) was excluded because although patients were randomly assigned to one of two anabolic steroid therapy treatment groups, these patients were compared to untreated matched controls; ·one (Hernandez‐Lopez 1996) was excluded because it was not a randomized study; ·one (Umar 1998) was excluded as the test treatment was DHEA, which was not considered an anabolic steroid for our meta‐analysis; ·two (Rabkin 1999, Rabkin 2000a) were excluded because they were randomized discontinuation trials; ·eight (Carroll 1997, Jaque 2002, Pharo 1997, Rivera 1999, Romeyn 2000, Sattler 1999a, Sattler 2002, Schroeder 2001 ) were excluded because both treatment groups received an anabolic steroid therapy; ·one (Mwamburi 2004a) was excluded because both treatment groups received an active therapy; ·seventeen were excluded because they were non‐controlled studies (Earthman 2002, Engleson 1996, Fisher 1997, Fisher 1998a, Gold 1994, Gold 1996, Grudzdev 1999, Jeantils 1993, Mooney 1998, Poles 1997, Rabkin 1995a, Rabkin 1995b, Urbina 2004, Vergel 1998, Wagner 1998a, Wagner 1998b, Wagner 1998c); ·one (Fox‐Wheeler 1999) was excluded because it was a non‐controlled study in children ; ·one (Hasheeve 1994) was excluded because it was a non‐controlled study of DHEA, which was not considered an anabolic steroid for our meta‐analysis; ·one (Stute 1994) was excluded because it was a non‐controlled study of a progestin; ·one (Gold 1999a) was excluded because it was a non‐controlled study in patients with lipodystrophy; ·two (Berger 1993, Jekot 1993) were excluded because they were non‐controlled case series; ·thirteen (Batterham 1997, Corcoran 2000, Dolan 2004b, Garsia 1997, Grinspoon 1998c, Hellerstein 1998, Hengge 1996b, Hengge 2002, Hengge 2004, Klibanski 1998, Mulligan 2001, Sattler 1999b, Strawford 1998, Wheeler 1998) were excluded because they were abstracts of studies later published; ·one (Gold 1999b) was excluded as it was the abstract of a study report provided by a drug manufacturer; and ·thirteen (Fairfield 2001a, Fairfield 2001b, Fisher 1998b, Grinspoon 1998b, Grinspoon 1999, Grinspoon 2000b, Hengge 2003b, Mwamburi 2004, Rabkin 1996, Reiter 1996, Schroeder 2003, Van Loan 1999, Wagner 1999) were excluded because they were publications reporting on patients included in an earlier publication.
The 13 trials included in the primary efficacy analyses involved 294 individuals treated with an anabolic steroid and 238 individuals treated with placebo for the analysis of change from baseline in lean body mass. For the analysis of change from baseline in body weight, 343 individuals treated with anabolic therapy and 286 individuals treated with placebo were included. Of these trials, five (Bhasin 1998, Bhasin 2000, Coodley 1997, Dobs 1999, Grinspoon 1998a) included men with hypogonadism, two (Grinspoon 2000a, Strawford 1999a) included men that were eugonadal, one (Hengge 2003a) included both men that were eugonadal and women, one (Wagner 2001) included men with either low normal or low serum testosterone levels, and four (Choi 2005, Dolan 2004a, Miller 1998, Mulligan 2005) included women. One study was published as an abstract (Bucher 1996) and thus included only in the secondary analyses. This study included 56 men (44 treated with anabolic steroid and 12 treated with placebo), with gonadal status not specified.
Risk of bias in included studies
Quality was assessed, as described in the Methods section, by determining the adequacy of allocation concealment (Schulz 1995), and by a validated five‐point scale, which included items relating to randomization (2 points), blinding (2 points), and description of withdrawals and dropouts (1 point) (Jadad 1996). Two reviewers reached consensus on the scores.
For the thirteen studies included in the primary analysis, seven were considered to have described adequate allocation concealment, while six did not provide sufficient description to determine the adequacy of allocation concealment. The mean quality score, on the five‐point scale, of studies included in the primary analysis was 4.3. Six trials scored 5 points, five scored 4 points, and two scored 3 points. Considering the components of the quality score individually, seven studies scored 2 points for randomization while six scored 1 point. All thirteen studies scored 2 points for blinding. Ten studies scored 1 point for description of withdrawals while three scored 0 points.
Effects of interventions
Meta‐analysis Change from baseline in lean body mass: The change from baseline in lean body mass was not measured in all studies included in the meta‐analysis. In the analysis of the eleven studies in which it was measured, the heterogeneity was significant with an I2 of 72%, p < 0.0001, although most of the results were to the right of the no effect line. The weighted mean difference (WMD) calculated using a random‐effects model was 1.3 kg (95% CI: 0.6, 2.0) but, due to the heterogeneity, this needs to be interpreted with caution. In the subgroup analysis of men only, the WMD was 1.5 kg (95% CI: 0.4, 2.6) with significant heterogeneity (I2 = 76%, p = 0.002); and in the subgroup of men with low testosterone levels, the WMD was 0.9 kg (95% CI: ‐0.2, 2.0) with significant heterogeneity (I2 = 61%, p = 0.04). In the subgroup analysis of women only, the WMD was 1.0 kg (95% CI: ‐0.3, 2.2) with significant heterogeneity (I2 = 81%, p = 0.001).
In order to explore the heterogeneity, trial results were subgrouped by treatment duration, treatment dose, proportion of subjects on protease inhibitors, measurement technique used to measure lean body mass, quality score and its individual components, adequacy of allocation concealment and sample size. No trends were noted when trials were ordered by treatment dose or duration. No difference in treatment effect was noted in trials using DEXA versus those using BIA to measure lean body mass, nor in those in which a higher proportion of participants were on protease inhibitors compared with trials in which a lower proportion of participants were on protease inhibitors (although data for this factor were not reported for all trials). When ordered by quality, the study of the lowest quality showed the only negative effect. When the analysis was repeated using only studies with a quality score of 4 or greater, the results were similar to those of the primary analysis. However, when only studies with a quality score of 5 were included, the WMD was 2.2 kg (95% CI: 1.4, 3.1), with an I2 of 22%, p = 0.26. When the individual components of the score were considered, trials with a better score for randomization and trials with a better score for description of withdrawals showed somewhat larger treatment effects than those with low randomization scores or poor descriptions of withdrawals. These subgroup results need to be interpreted with caution given that subgroup analyses are observational by nature, and not based on randomized comparisons (Deeks 2005). Additionally, in this meta‐analysis, multiple subgroup analyses were carried out and many were defined post‐hoc.
No unpublished studies reported results on change in lean body mass.
The funnel plot (see Figure 1) of the standard error of the WMD versus the WMD did not suggest publication bias; nor did it suggest that trials with a smaller standard error of the WMD produced a more accurate estimate of the treatment effect.
1.

Figure 1. Funnel plot of results for change from baseline in lean body mass
Change from baseline in body weight: All thirteen trials included in the primary analysis for this meta‐analysis reported results for change from baseline in body weight. As expected, the heterogeneity was significant as determined by the I2 of 68% (p < 0.0001). Differences from the individual trials occurred on both sides of the no effect zero line, although the majority occurred to the right of this line. The pooled weighted mean difference (WMD) calculated using a random‐effects model was 1.1 kg (95% CI: 0.3, 2.0). However, although significant, this difference should be interpreted with caution given the heterogeneity present. Subgroup analyses of those trials that included men only, of those trials that included men known to be hypogonadal, and of those that included women only were carried out. In the eight trials that included men only, the heterogeneity remained significant (I2 = 68%, p = 0.002) and the WMD was 0.8 kg (95% CI: ‐0.3, 1.8); in the five trials that included only hypogonadal men, the heterogeneity remained significant (I2 = 66%, p = 0.01) and the WMD was 0.2 kg (95% CI: ‐1.1, 1.4); and in the four trials that included women only, the heterogeneity was large (I2 = 73%, p = 0.005) and the WMD was 1.3 kg (95% CI: ‐0.4, 3.1).
The observed heterogeneity was again explored for potential sources. No trends were noticed when trials were ordered by treatment. When categorized by treatment dose, the results in trials in which the dose was supraphysiologic showed a larger treatment effect than in those in which the dose was considered a physiologic replacement dose. There were no apparent differences in treatment effect between subgroups of studies defined by proportion of participants on protease inhibitors, adequacy of allocation concealment, or sample size. When ordered by quality, the lower quality studies produced negative effects, while the higher quality studies, on average, showed positive effects. In the sensitivity analysis, in which studies with low quality scores were excluded, the heterogeneity remained and the WMD of 1.4 kg (95% CI: 0.6, 2.3) was higher than that of the primary analysis. In the six studies with quality scores of 5, the WMD was 1.6 kg (95% CI: 0.5, 2.7). When the individual components of the quality score were considered, trials with a better score for randomization and trials with a better score for description of withdrawals showed somewhat larger treatment effects. Again, these subgroup results need to be interpreted with caution.
When all published and unpublished trials included in the analysis, the results were very similar to those of the primary analysis (I2 = 66%, p < 0.0001, WMD = 1.2 kg, 95% CI: 0.4, 1.9).
The funnel plots of the standard error of the WMD versus the WMD for change from baseline in body weight for trials in the primary analysis (see Figure 2), and for trials in the sensitivity analysis, in which the unpublished trial was also included (see Figure 3), did not suggest publication bias. Furthermore, the funnel plots did not suggest that trials with smaller WMD standard errors produced more accurate estimate of the treatment effect.
2.

Figure 2. Funnel plot of results for change from baseline in body weight
3.

Figure 1. Funnel plot for change from baseline in body weight with unpublished trial included
Number of deaths: In the ten trials included in the primary analysis that reported the number of deaths, the pooled risk difference was 0.00 (95% CI: ‐0.02, 0.03). There was no significant heterogeneity for this result.
Number of withdrawals or discontinuations of study medication due to adverse events: Ten studies included in the primary analysis reported the number of withdrawals or discontinuations of study medication due to adverse events. Five adverse events were reported in the anabolic steroid treatment groups while none were reported in the placebo treatment groups. The pooled risk difference was 0.00 (95% CI ‐0.02, 0.03), with no significant heterogeneity.
Descriptive Analysis
We were unable to include all relevant information in the meta‐analysis due to certain limitations; some of this information is summarized below.
Two unpublished studies (Phanuphak , Schambelan 2001) could not be included in the meta‐analysis due to the unavailability of necessary summary statistics (i.e., the mean change in lean body mass and weight and its standard deviation). The study by Phanuphak showed increases in lean body mass and body weight with nandrolone with a dose response as compared to placebo, while the study of Schambelan showed no difference in the change in lean body mass or weight with testosterone compared to placebo, when both were used in conjunction with megestrol. The numerical results are summarized in the table "Characteristics of Excluded Studies."
All studies included in the meta‐analysis documented withdrawals and discontinuations. There was little consistency among the studies in specifying the causes of discontinuations. Additionally, consistent methodology was not used to reporting adverse events. Meaningful analysis was further hampered by the fact that the studies used different anabolic steroid analogues, routes of delivery, doses, duration of therapy, and that events occurring could vary by gender.
Adverse events, when reported, were usually described as mild and reversible. Events were generally related to either application site reactions (pain, skin irritation) or consistent with reported side effects associated with the use of anabolic steroids. Adverse events included such events as increased liver function tests, acne, mild hirsutism, breast tenderness, clitoral enlargement, increased libido, increased aggressiveness/irritability and mood swings among others.
Six studies included statements indicating that any withdrawals or discontinuations were not related to adverse events. Explanations included refusal to continue, personal reasons or subject lost to follow‐up. A further four studies did document the occasional subject in either arm discontinuing due to adverse events such as increased liver function studies, threatened or completed suicide, or anxiety/depression. Only one study clearly indicated that the adverse event causing discontinuation was treatment‐related. This occurred in a subject who developed cannaliculus cholestasis on oral oxymetholone therapy.
In the studies included in the meta‐analysis, there were very few reported deaths that occurred during treatment. In the three studies in which deaths occurred during treatment, a total of eight were documented, including four in the active therapy arms and four in the placebo arms. Seven of the deaths were considered AIDS‐related. Additionally, one subject who committed suicide was considered to have experienced an adverse event.
Discussion
Anabolic steroids are currently being used by HIV‐infected individuals for the treatment of weight loss. The results of this meta‐analysis suggest that these steroids, on average, increase both lean body mass and body weight. However, the magnitude of the increase is not large and may not be considered clinically relevant. Furthermore, these results need to be interpreted with caution because the estimate of the treatment effect was based on a relatively small sample size and significant heterogeneity was present in the meta‐analyses. This heterogeneity was likely introduced by multiple sources of variability across the studies, including differences in study populations (baseline weight, weight loss, testosterone level, and stage of HIV illness), differences in interventions (different dosages, formulations, treatment duration, and concomitant therapies) and differences in the methods of determining the changes in lean body mass. Because of the many sources of variability, it is not possible to determine the relative effects of each from the small number of studies available.
In addition, it is not known whether improvements in the end points analysed in this meta‐analysis correlate with more clinically relevant endpoints such as survival time, increased muscle strength and physical functioning, and overall quality of life. Finally, it should be noted, that in terms of safety and efficacy, the studies reported in this meta‐analysis only studied the short‐term effects of anabolic steroids.
Authors' conclusions
Implications for practice.
While the data available suggest that the use of anabolic steroids in HIV‐infected individuals with associated weight loss will result in small increases in lean body mass and body weight without significant numbers of adverse effects, there are significant limitations in these data and the meta‐analysis. Furthermore, while theoretically an increase in lean body mass should lead to improved physical functioning and quality of life, and ultimately to increased survival, this has not been demonstrated.
Implications for research.
Further research into the use of anabolic steroids to treat HIV wasting disease is required to explore: 1) the effectiveness of anabolic steroids in particular populations (such as those with low testosterone levels versus those with normal levels, those with differing severities of weight loss); 2) the best agent and dosage to be used in particular populations; 3) the optimal duration of treatment including the effects of cycling therapy; 4) the effects of concomitant therapies, particularly exercise and/or appetite stimulants; and 5) potential serious but uncommon adverse effects of anabolic steroids and potential adverse effects resulting from longer periods of administration of anabolic steroids.
In addition, changes in lean body mass or weight need to be correlated with more clinically relevant endpoints such as quality of life, improved physical functioning and survival. It may be possible to obtain some of this information from a systematic review of non‐controlled trials or dose comparison trials in the literature, from observational studies using large clinical databases, and/or from review and pooling of actual individual patient data from the randomized studies conducted to date. However, for definitive answers to many of these questions, it will be necessary to conduct further randomized trials.
APPENDIX 1: The physiological effects, routes of delivery, and adverse effects of anabolic steroids
Anabolic steroids are androgenic hormones that include or are derivatives of testosterone (Kashkin 1989). Androgenic hormones are cholesterol derivatives that act on the androgen receptor and result in masculinizing effects. The main androgen in the plasma of men is testosterone, which is produced by the testis. In women, testosterone is synthesized in small amounts by both the ovary and adrenal gland. Androgens have been shown to have anabolic (nitrogen retaining) effects. These anabolic actions can result in increase strength and muscle mass (Snyder 2001). Studies of otherwise healthy hypogonadal men have shown significant increases in body weight and lean body mass with androgen administration (Bhasin 1997, Brodsky 1996), while studies of eugonadal men have shown increases in lean body mass and strength with administration of supraphysiologic doses of testosterone (Bhasin 1999).
The liver metabolizes testosterone very rapidly. Thus, in order to sustain effective levels, testosterone must be administered either parenterally as a short acting preparation (propionate) or as esterified long acting preparations (enanthate and cypionate) or by a transdermal delivery system. Other chemical modifications of testosterone have led to the production of androgens with greater anabolic than androgenic effects, (for example oxandrolone and nandrolone), and of some which can be taken orally, (for example oxandrolone and oxymetholone) (Snyder 2001).
Adverse effects of androgens in women may include growth of facial hair or menstrual irregularities. In children, disturbances in growth and osseous development can occur. In men, azoospermia may occur due to inhibition of gonadotropin secretion while conversion of androgens to estrogens may cause gynecomastia. Other adverse effects include acne, water retention, cholestatic hepatitis, alterations in various tests of hepatic function, hepatic adenocarcinoma, and endocrine effects including decreased glucose tolerance and alterations in lipid profiles (Snyder 2001).
APPENDIX 2: Search strategy used in OVID Searches
1 exp HIV infections/ 2 exp AIDS/ 3 AIDS.tw. 4 HIV.tw. 5 acquired immun$ syndrome.tw. 6 human immunodeficiency virus.tw. 7 or/1‐6 8 exp body weight/ 9 exp body composition/ 10 lean body mass.tw. 11 weight.tw. 12 body composition.tw. 13 wasting.tw. 14 cachexia.tw. 15 sarcopenia.tw. 16 fat free mass.tw. 17 body cell mass.tw. 18 or/8‐17 19 7 and 18 20 exp HIV wasting syndrome/ 21 slim disease.tw. 22 or/19‐21 23 exp androgens/ 24 exp androgens, synthetic/ 25 androgen$.tw. 26 anabolic steroid$.tw. 27 etiocholanolone.mp. 28 androst$.sh,tw,mp. 29 prasterone.tw,sh,mp. 30 stanolone.tw,sh,mp. 31 testosterone.tw,sh,mp. 32 methyltestosterone.tw,sh,mp. 33 metribolone.tw,sh,mp. 34 ethylestrenol.sh,tw,mp. 35 fluoxymesterone.tw,sh,mp. 36 mesterolone.tw,sh,mp. 37 methandriol.sh,tw,mp. 38 methandrostenolone.tw,sh,mp. 39 methenolone.sh,tw,mp. 40 nandrolone.sh,tw,mp. 41 norethandrolone.sh,tw,mp. 42 oxandrolone.sh,tw,mp. 43 oxymetholone.sh,tw,mp. 44 stanozolol.sh,tw,mp. 45 trenbolone.sh,tw,mp. 46 amafolone.tw,sh,mp. 47 atromid.sh,tw,mp. 48 benorterone.tw,sh,mp. 49 boldenone.sh,tw,mp. 50 calusterone.tw,sh,mp. 51 danazol.tw,sh,mp. 52 drostanolone.tw,sh,mp. 53 etiocholanone.sh,tw,mp. 54 mestanolone.tw,sh,mp. 55 mibolerone.sh,tw,mp. 56 testololactone.sh,tw,mp. 57 hydroxyandrost$.sh,tw,mp. 58 epiandrosterone.sh,tw,mp. 59 oxotestosterone.sh,tw,mp. 60 oxoandrostenedione.tw,sh,mp. 61 or/23‐60 62 22 and 61 63 random$.tw. 64 random allocation/ 65 comparative study/ 66 placebo$.tw. 67 (controlled adj trial).tw. 68 (double adj blind$).tw. 69 clinical trial.pt. 70 randomized controlled trial.pt. 71 or/63‐70 72 or/63‐70 73 62 and 72
APPENDIX 3: Definitions and methodology for determining outcome variables
The change in fat free body mass measured by any accepted technique was extracted. In general, either fat free mass, lean body mass, or body cell mass were measured. Fat free mass (FFM) refers to all mass that is not fat and includes both intracellular and extracellular water, lean body mass (LBM) is fat free mass minus bone mineral mass, and body cell mass (BCM) refers to the mass of metabolically active cells and thus includes intracellular water but excludes extracellular water (Wang 1992). Although each of the terms FFM, LBM, and BCM is different, it is assumed in this review that the change in any of these three measures of body composition reflects a change in the body cell mass or metabolically active tissue (i.e., that the change in bone mineral mass or extracellular fluid is minimal before and after treatment in comparison with the change in body cell mass). In addition, body composition can be measured by various techniques. The most commonly used techniques in the studies included in this review were bioelectrical impedance analysis (BIA) (single or multiple frequency) and dual energy X‐ray absorptiometry (DEXA). Bioelectrical impedance analysis measures the electrical conductivity through the body and uses equations to predict the various components of the body including total body water, fat free mass and body cell mass. Multiple frequency BIA is better able to distinguish extracellular from intracellular water fluids and thus results in better predictions of body composition, particularly in unwell populations. Dual energy X‐ray absorptiometry uses X‐rays with two different energy intensities to measure fat mass, bone mineral mass, and lean body mass, and does not distinguish between extracellular and intracellular water.
What's new
| Date | Event | Description |
|---|---|---|
| 29 October 2008 | Amended | Converted to new review format. |
History
Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 16 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to acknowledge the Cochrane HIV/AIDS Review Group Coordinator and Assistant Coordinator (Gail Kennedy, Tara Horvath), Trials Search Coordinator (Karishma Busgeeth) and referees for assistance and helpful feedback with our review; Bev Shea for the teaching and assistance provided regarding the Cochrane Collaboration and use of its software; George Wells of the University of Ottawa for his teaching of the methods of meta‐analysis; and Jessie McGowan, Donna Dolan, and Connie Barrowclough for the assistance provided with the electronic search strategy and in retrieving references.
Data and analyses
Comparison 1. Anabolic steroid compared to placebo ‐ primary analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 3 Deaths | 13 | 512 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 4 Withdrawals or discontinuations due to adverse events | 13 | 597 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
1.1. Analysis.

Comparison 1 Anabolic steroid compared to placebo ‐ primary analysis, Outcome 1 Change from baseline in lean body mass.
1.2. Analysis.

Comparison 1 Anabolic steroid compared to placebo ‐ primary analysis, Outcome 2 Change from baseline in body weight.
1.3. Analysis.

Comparison 1 Anabolic steroid compared to placebo ‐ primary analysis, Outcome 3 Deaths.
1.4. Analysis.

Comparison 1 Anabolic steroid compared to placebo ‐ primary analysis, Outcome 4 Withdrawals or discontinuations due to adverse events.
Comparison 2. Anabolic steroid compared to placebo ‐ subgroups by gender.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 Men | 8 | 323 | Mean Difference (IV, Random, 95% CI) | 1.47 [0.38, 2.55] |
| 1.2 Women | 4 | 135 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐0.31, 2.24] |
| 1.3 Men and women | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 1.91 [0.06, 3.75] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 2.1 Men | 9 | 359 | Mean Difference (IV, Random, 95% CI) | 0.78 [‐0.28, 1.84] |
| 2.2 Women | 5 | 196 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐0.40, 3.06] |
| 2.3 Men and women | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 2.23 [0.62, 3.84] |
| 3 Deaths | 13 | 512 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 3.1 Men | 7 | 275 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 3.2 Women | 4 | 148 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.07] |
| 3.3 Men and women | 2 | 89 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 4 Withdrawals or discontinuations of study medication due to adverse events | 13 | 597 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
| 4.1 Men | 8 | 398 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
| 4.2 Women | 3 | 110 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 4.3 Men and women | 2 | 89 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.04, 0.13] |
2.1. Analysis.

Comparison 2 Anabolic steroid compared to placebo ‐ subgroups by gender, Outcome 1 Change from baseline in lean body mass.
2.2. Analysis.

Comparison 2 Anabolic steroid compared to placebo ‐ subgroups by gender, Outcome 2 Change from baseline in body weight.
2.3. Analysis.

Comparison 2 Anabolic steroid compared to placebo ‐ subgroups by gender, Outcome 3 Deaths.
2.4. Analysis.

Comparison 2 Anabolic steroid compared to placebo ‐ subgroups by gender, Outcome 4 Withdrawals or discontinuations of study medication due to adverse events.
Comparison 3. Anabolic steroid compared to placebo ‐ men only ‐ subgroups by gonadal status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change for baseline in lean body mass | 8 | 323 | Mean Difference (IV, Random, 95% CI) | 1.47 [0.38, 2.55] |
| 1.1 Hypogonadal men | 5 | 219 | Mean Difference (IV, Random, 95% CI) | 0.86 [‐0.22, 1.95] |
| 1.2 Borderline gonadal status | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.74, 2.14] |
| 1.3 Eugonadal men | 2 | 65 | Mean Difference (IV, Random, 95% CI) | 3.29 [2.11, 4.48] |
| 2 Change from baseline in body weight | 9 | 359 | Mean Difference (IV, Random, 95% CI) | 0.78 [‐0.28, 1.84] |
| 2.1 Hypogonadal men | 6 | 255 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐1.07, 1.43] |
| 2.2 Borderline gonadal status | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 1.1 [‐0.91, 3.11] |
| 2.3 Eugonadal men | 2 | 65 | Mean Difference (IV, Random, 95% CI) | 2.32 [1.04, 3.60] |
3.1. Analysis.
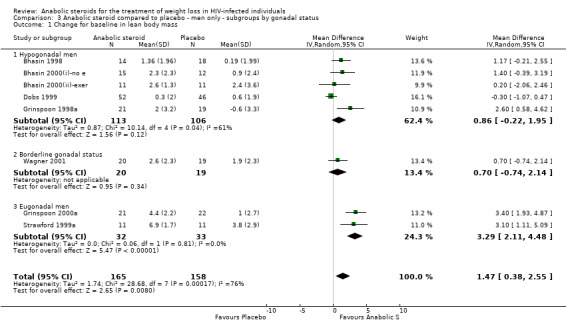
Comparison 3 Anabolic steroid compared to placebo ‐ men only ‐ subgroups by gonadal status, Outcome 1 Change for baseline in lean body mass.
3.2. Analysis.

Comparison 3 Anabolic steroid compared to placebo ‐ men only ‐ subgroups by gonadal status, Outcome 2 Change from baseline in body weight.
Comparison 4. Anabolic steroid compared to placebo ‐ subgroups by treatment duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 6 months | 2 | 97 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐0.80, 3.46] |
| 1.2 16 weeks | 4 | 123 | Mean Difference (IV, Random, 95% CI) | 1.29 [0.18, 2.41] |
| 1.3 12 weeks | 7 | 290 | Mean Difference (IV, Random, 95% CI) | 1.16 [0.11, 2.22] |
| 1.4 8 weeks | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 3.10 [1.11, 5.09] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 2.1 6 months | 3 | 155 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.69, 1.69] |
| 2.2 16 weeks | 4 | 123 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐0.64, 3.64] |
| 2.3 12 weeks | 8 | 329 | Mean Difference (IV, Random, 95% CI) | 0.96 [‐0.29, 2.21] |
| 2.4 8 weeks | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 2.5 [0.47, 4.53] |
4.1. Analysis.

Comparison 4 Anabolic steroid compared to placebo ‐ subgroups by treatment duration, Outcome 1 Change from baseline in lean body mass.
4.2. Analysis.

Comparison 4 Anabolic steroid compared to placebo ‐ subgroups by treatment duration, Outcome 2 Change from baseline in body weight.
Comparison 5. Anabolic steroid compared to placebo ‐ subgroups by treatment dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 Supraphysiologic dose | 8 | 292 | Mean Difference (IV, Random, 95% CI) | 1.80 [0.79, 2.81] |
| 1.2 Physiologic replacement dose | 6 | 240 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.20, 1.43] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 2.1 Supraphysiologic dose | 7 | 236 | Mean Difference (IV, Random, 95% CI) | 2.25 [1.31, 3.19] |
| 2.3 Physiologic replacement dose | 9 | 393 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.60, 1.12] |
5.1. Analysis.

Comparison 5 Anabolic steroid compared to placebo ‐ subgroups by treatment dose, Outcome 1 Change from baseline in lean body mass.
5.2. Analysis.

Comparison 5 Anabolic steroid compared to placebo ‐ subgroups by treatment dose, Outcome 2 Change from baseline in body weight.
Comparison 6. Anabolic steroid compared to placebo ‐ subgroups by proportion of study participants on protease inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 8 | 261 | Mean Difference (IV, Random, 95% CI) | 2.43 [1.67, 3.20] |
| 1.1 Proportion on proteases inhibitors less than 60% | 5 | 144 | Mean Difference (IV, Random, 95% CI) | 2.24 [1.18, 3.31] |
| 1.2 Proportion on proteases inhibitors 60% or greater | 3 | 117 | Mean Difference (IV, Random, 95% CI) | 2.82 [1.67, 3.97] |
| 2 Change from baseline in body weight | 9 | 319 | Mean Difference (IV, Random, 95% CI) | 1.92 [0.75, 3.08] |
| 2.1 Proportion on protease inhibitors less than 60% | 5 | 145 | Mean Difference (IV, Random, 95% CI) | 2.07 [‐0.02, 4.15] |
| 2.2 Proportion on protease inhibitors 60% or greater | 4 | 174 | Mean Difference (IV, Random, 95% CI) | 1.59 [0.57, 2.60] |
6.1. Analysis.

Comparison 6 Anabolic steroid compared to placebo ‐ subgroups by proportion of study participants on protease inhibitors, Outcome 1 Change from baseline in lean body mass.
6.2. Analysis.

Comparison 6 Anabolic steroid compared to placebo ‐ subgroups by proportion of study participants on protease inhibitors, Outcome 2 Change from baseline in body weight.
Comparison 7. Anabolic steroid compared to placebo ‐ subgroups by measurement technique.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 Measurement by DEXA | 9 | 288 | Mean Difference (IV, Random, 95% CI) | 1.31 [0.47, 2.14] |
| 1.2 Measurement by BIA | 5 | 244 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐0.21, 2.96] |
7.1. Analysis.

Comparison 7 Anabolic steroid compared to placebo ‐ subgroups by measurement technique, Outcome 1 Change from baseline in lean body mass.
Comparison 8. Anabolic steroid compared to placebo ‐ subgroups by methodologic quality score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 Quality score 5 | 7 | 228 | Mean Difference (IV, Random, 95% CI) | 2.23 [1.36, 3.09] |
| 1.2 Quality score 4 | 6 | 206 | Mean Difference (IV, Random, 95% CI) | 0.94 [0.07, 1.82] |
| 1.3 Quality score 3 | 1 | 98 | Mean Difference (IV, Random, 95% CI) | ‐0.3 [‐1.07, 0.47] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 2.1 Quality score 5 | 8 | 286 | Mean Difference (IV, Random, 95% CI) | 1.57 [0.49, 2.66] |
| 2.2 Quality score 4 | 6 | 210 | Mean Difference (IV, Random, 95% CI) | 1.24 [‐0.26, 2.73] |
| 2.3 Quality score 3 | 2 | 133 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.37, 0.40] |
8.1. Analysis.

Comparison 8 Anabolic steroid compared to placebo ‐ subgroups by methodologic quality score, Outcome 1 Change from baseline in lean body mass.
8.2. Analysis.

Comparison 8 Anabolic steroid compared to placebo ‐ subgroups by methodologic quality score, Outcome 2 Change from baseline in body weight.
Comparison 9. Anabolic steroid compared to placebo ‐ subgroups by adequacy of allocation concealment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 Allocation concealment adequate | 8 | 256 | Mean Difference (IV, Random, 95% CI) | 1.67 [0.67, 2.67] |
| 1.2 Allocation concealment uncertain | 6 | 276 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐0.19, 2.01] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 2.1 Allocation concealment adequate | 9 | 318 | Mean Difference (IV, Random, 95% CI) | 1.31 [0.63, 2.00] |
| 2.2 Allocation concealment uncertain | 7 | 311 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.78, 2.40] |
9.1. Analysis.
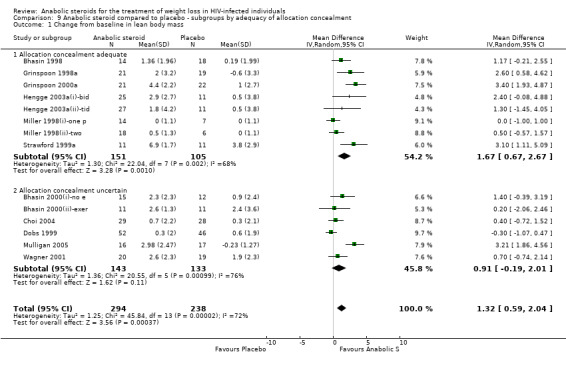
Comparison 9 Anabolic steroid compared to placebo ‐ subgroups by adequacy of allocation concealment, Outcome 1 Change from baseline in lean body mass.
9.2. Analysis.

Comparison 9 Anabolic steroid compared to placebo ‐ subgroups by adequacy of allocation concealment, Outcome 2 Change from baseline in body weight.
Comparison 10. Anabolic steroid compared to placebo ‐ subgroups by score on randomization.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 Randomization score 2 | 8 | 285 | Mean Difference (IV, Random, 95% CI) | 1.84 [0.86, 2.81] |
| 1.2 Randomization score 1 | 6 | 247 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.15, 1.75] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 2.1 Randomization score 2 | 9 | 343 | Mean Difference (IV, Random, 95% CI) | 1.42 [0.41, 2.44] |
| 2.2 Randomization score 1 | 7 | 286 | Mean Difference (IV, Random, 95% CI) | 0.78 [‐0.60, 2.15] |
10.1. Analysis.

Comparison 10 Anabolic steroid compared to placebo ‐ subgroups by score on randomization, Outcome 1 Change from baseline in lean body mass.
10.2. Analysis.

Comparison 10 Anabolic steroid compared to placebo ‐ subgroups by score on randomization, Outcome 2 Change from baseline in body weight.
Comparison 11. Anabolic steroid compared to placebo ‐ subgroups by score on description of withdrawals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 Description of withdrawals score 1 | 12 | 377 | Mean Difference (IV, Random, 95% CI) | 1.60 [0.82, 2.38] |
| 1.2 Description of withdrawals score 0 | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.71, 0.57] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 2.1 Description of withdrawals score 1 | 13 | 439 | Mean Difference (IV, Random, 95% CI) | 1.54 [0.64, 2.43] |
| 2.2 Description of withdrawals score 0 | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.24, 0.42] |
11.1. Analysis.

Comparison 11 Anabolic steroid compared to placebo ‐ subgroups by score on description of withdrawals, Outcome 1 Change from baseline in lean body mass.
11.2. Analysis.

Comparison 11 Anabolic steroid compared to placebo ‐ subgroups by score on description of withdrawals, Outcome 2 Change from baseline in body weight.
Comparison 12. Anabolic steroid compared to placebo ‐ subgroups by total sample size of trial.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 14 | 532 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.59, 2.04] |
| 1.1 Sample size less than 40 | 2 | 55 | Mean Difference (IV, Random, 95% CI) | 3.18 [2.06, 4.29] |
| 1.2 Sample size 40 to 49 | 2 | 71 | Mean Difference (IV, Random, 95% CI) | 0.95 [‐0.05, 1.94] |
| 1.3 Sample size 50 to 59 | 5 | 185 | Mean Difference (IV, Random, 95% CI) | 1.24 [0.03, 2.44] |
| 1.4 Sample size 60 or greater | 5 | 221 | Mean Difference (IV, Random, 95% CI) | 0.68 [‐0.39, 1.74] |
| 2 Change from baseline in body weight | 16 | 629 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.29, 1.96] |
| 2.1 Sample size less than 40 | 3 | 90 | Mean Difference (IV, Random, 95% CI) | 1.89 [‐1.25, 5.02] |
| 2.2 Sample size 40 to 49 | 2 | 71 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.84, 1.76] |
| 2.3 Sample size 50 to 59 | 6 | 247 | Mean Difference (IV, Random, 95% CI) | 1.01 [0.17, 1.86] |
| 2.4 Sample size 60 or greater | 5 | 221 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.65, 2.76] |
12.1. Analysis.

Comparison 12 Anabolic steroid compared to placebo ‐ subgroups by total sample size of trial, Outcome 1 Change from baseline in lean body mass.
12.2. Analysis.

Comparison 12 Anabolic steroid compared to placebo ‐ subgroups by total sample size of trial, Outcome 2 Change from baseline in body weight.
Comparison 13. Anabolic steroid compared to placebo ‐ sensitivity analysis with unpublished trials included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Change from baseline in body weight (subgroups by gender) | 17 | 685 | Mean Difference (IV, Random, 95% CI) | 1.16 [0.39, 1.93] |
| 2.1 Men | 10 | 415 | Mean Difference (IV, Random, 95% CI) | 0.87 [‐0.08, 1.83] |
| 2.2 Women | 5 | 196 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐0.40, 3.06] |
| 2.3 Men and women | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 2.23 [0.62, 3.84] |
13.2. Analysis.

Comparison 13 Anabolic steroid compared to placebo ‐ sensitivity analysis with unpublished trials included, Outcome 2 Change from baseline in body weight (subgroups by gender).
Comparison 14. Anabolic steroid compared to placebo ‐ sensitivity analysis with trials with quality score < 4 excluded.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in lean body mass | 13 | 434 | Mean Difference (IV, Random, 95% CI) | 1.48 [0.76, 2.20] |
| 2 Change from baseline in body weight | 14 | 496 | Mean Difference (IV, Random, 95% CI) | 1.44 [0.59, 2.30] |
14.1. Analysis.

Comparison 14 Anabolic steroid compared to placebo ‐ sensitivity analysis with trials with quality score < 4 excluded, Outcome 1 Change from baseline in lean body mass.
14.2. Analysis.

Comparison 14 Anabolic steroid compared to placebo ‐ sensitivity analysis with trials with quality score < 4 excluded, Outcome 2 Change from baseline in body weight.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhasin 1998.
| Methods | Randomized, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Sample size: AS 20, P 21 Withdrawals: AS 6, P 3 Efficacy sample size: AS 14, P 18 | |
| Participants | HIV+ men with low serum testosterone levels with mean pretreatment weight loss 3.9 kg (but weight loss not an inclusion criteria) Mean age: ˜ 40 Mean weight at baseline: AS 74, P 72 Mean weight loss: AS 4.7, P 3.1 Mean testosterone: AS 8.9, P 7.3 Mean CD4 ct: 249 % on PIs: NR |
|
| Interventions | Testosterone transdermal patches, 2 every 24 h (approx testosterone delivery 5 mg per day) applied to abdomen, back, arms, or thighs nightly vs. placebo patches | |
| Outcomes | Change in body weight Change in LBM measured by DEXA Deaths Withdrawals due to AEs | |
| Notes | Quality score=4 (R1, B2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bhasin 2000(i)‐no e.
| Methods | Randomized, double‐blind, placebo‐controlled trial Treatment duration: 16 weeks Sample size: AS 17, P 14 Withdrawals: AS 2, P 2 Efficacy sample size: AS 15, P 12 | |
| Participants | HIV+ men with weight loss >5% and low serum testosterone levels (<12.1 nmol/L (349 ng/dL)) Mean age: AS 41, P 42 Mean weight: AS 77, P 77 Mean weight loss: AS 7.4, P 6.4 Mean serum testosterone: AS 7.1, P 6.1 Mean CD4 ct: AS 357, P 279 % on PIs: AS 60, P 40 |
|
| Interventions | Testosterone enanthate 100 mg IM q 1 week vs. placebo Concomitatnt therapy: no resistance exercise, standardized energy and protein intake | |
| Outcomes | Change in body weight Change in FFM measured by DEXA and D2O Deaths Witdrawals due to AEs | |
| Notes | Quality score =5
(R2, B2, W1) This trial had four treatement groups (placebo + no exercise, testosterone + no exercise, placebo + exercise, and testosterone + exercise); results were not presented for the two testosterone treatment groups combined nor for the two placebo groups combined, therefore we treated the results as coming from two separate trials, each comparing testosterone to placebo (one with concomitant exercise and one without) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bhasin 2000(ii)‐exer.
| Methods | Part of Bhasin 2000 study with resistance exercise included Sample size: AS 15, P 15 Withdrawals: AS 4, P 4 Efficacy sample size: AS 11, P 11 | |
| Participants | Mean age: AS 40, P 44 Mean weight: AS 69, P 72 Mean weight loss: AS 6.7, P 7.1 Mean serum testosterone: AS 7.0, P 7.0 Mean CD4 ct: AS 340, P 229 % on PIs: AS 55, P 45 | |
| Interventions | Testosterone enanthate 100 mg IM q 1 week vs. placebo Concomitant therapy: resistance exercise, standardized energy and protein intake | |
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bucher 1996.
| Methods | Randomized, double‐blind, placebo‐controlled trial Treatment duration: 12 weeks Sample size: AS 44, P 12 Withdrawals: 3 (treatment group not specified) Efficacy sample size: NR | |
| Participants | HIV‐positive homosexual males, gonadal status not specified Mean age: AS 35, P 33 Mean weight AS 76, P 70 Mean weight loss: NR Mean serum testosterone: NR Mean CD4 ct: AS 395, P 249 % on PIs: NR |
|
| Interventions | Nandrolone decanoate 100 mg IM per week vs. placebo | |
| Outcomes | Change in body weight Change in body composition: results not yet available Deaths: NR Withdrawals due to AEs: overall numbers reported but not by treatment group | |
| Notes | Published abstract from conference proceedings only Efficacy sample size unclear‐ we used original sample size for our meta‐analysis Unclear whether variability measure for efficacy outcomes is SD or SE (we assumed it to be the SD) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Choi 2004.
| Methods | Randomized, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Sample size: AS 26, P 26 Withdrawals: AS 6, P 8 Efficacy sample size: AS 26, P 26 | |
| Participants | Premenopausal HIV‐infected women who lost 5‐15% of ususal body weight in prior 6 months and with relative androgen deficiency (morning testosterone < 33ng/dl, the median of refernce range) Mean age: ˜38 Mean weight: AS 64.3, P 68.8 Mean weight loss: Mean serum testosterone: AS 28.9, P 20.4 Mean CD4 ct: AS 481, P 392 % on PIs: NR |
|
| Interventions | Two testosterone patches applied to abdominal skin twice weekly, estimated delivery rate of testosterone 300 ug/d vs. two placebo patches | |
| Outcomes | Change in body weight Change in fat‐free mass measured by DEXA Deaths: NR Withdrawals due to AEs: NR | |
| Notes | Quality score=4
(R2, B2, W0) Efficacy analysis by ITT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Coodley 1997.
| Methods | Randomized, double‐blind, placebo‐controlled cross‐over trial (cross‐over portion not included for the meta‐analysis) Trial duration: 12 weeks Sample size: 39 (treatment groups not specified) Withdrawals: 4 (treatment groups not specified) Efficacy sample size: AS 17, P 18 | |
| Participants | HIV+ men with weight loss >5% usual body weight, CD4 ct<200, almost all hypogonadal Mean age: NR Mean weight: AS 70, P 71 Mean weight loss: NR Mean serum testosterone: NR (did report free testosterone) Mean CD4 ct: AS 133, P 90 % on PIs: NR |
|
| Interventions | Testosterone cypionate 200 mg IM q 2 weeks vs. placebo Concomitant therapy with megestrol, prednisone, or droanibinol allowed (% in which it was used NR) | |
| Outcomes | Change in body weight Change in body composition: not measured Deaths: NR Withdrawals due to AEs: NR | |
| Notes | Quality score=3
(R1, B2, W0) Measure of variability on change in weight not presented (nor we able to obtain it by attempting to contact the author) , only p‐value from Wilcoxan's rank sum test‐ we have assumed the p‐value from t‐test and a common SD for both treatment groups to calculate a SD Many concomitant therapies allowed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dobs 1999.
| Methods | Randomized, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Sample size: AS 67, P 66 Withdrawals: AS 15, P 20 Efficacy sample size: AS 52, P 46 | |
| Participants | Men with AIDS with weight loss 5‐20% of baseline weight, low testosterone levels Mean age: AS 39, P 41 Mean weight: AS 68, P 69 Mean weight loss: AS 5.4, P 4.8 Mean serum testsoterone: AS 15.4, P 14.9 Mean CD4 ct: AS 156, P 177 % on PIs: NR |
|
| Interventions | Testosterone patch 15mg (delivers ˜6 mg /day) applied to the scrotum each morning and worn for 22‐24 hours vs. placebo patch Concomitant therapy: nutritional evaluation at wks 0,4,8, and 12 with nutritional counselling as necessary to both treatment groups | |
| Outcomes | Change in body weight Change in BCM measured by single frequency BIA Deaths: NR Withdrawals due to AEs: NR | |
| Notes | Quality score=3
(R1, B2, W0) Unclear if all patients that did not complete the study were excluded from the efficacy analysis‐ we assumed that they were all excluded Very high drop‐out rate |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dolan 2004a.
| Methods | Randomized, double‐blind, placebo‐controlled trial Treatment duration: 6 months Sample size: AS 29, P 28 Withdrawals: AS 2, P 3 Efficacy sample size: AS 29, P 28 | |
| Participants | HIV‐infected women with weight <90% of ideal or weight loss>10% and with free testosterone < median of reference range Mean age: AS 38, P 38 Mean weight: AS 55, P 54 Mean weight loss: AS 17%, P 21% Mean serum testosterone: AS 22, P 22 Mean CD4 ct: AS 218,P 356 % on PIs: AS 68, P 81 |
|
| Interventions | Testosterone transdermal patch, 4.1 mg/patch, estimated delivery rate of testosterone 150 ug/d, twice weekly vs. placebo patch | |
| Outcomes | Change in body weight Change in FFM: not reported Change in fat mass measured by DEXA Change in muscle mass measured by urinary creatinine excretion on a meat‐free diet Change in fat free mass: not reported Deaths Withdrawals due to AEs | |
| Notes | Quality score=5
(R2, B2, W1) Efficacy analysis by ITT Results for change in FFM not available from author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Grinspoon 1998a.
| Methods | Randomized, double‐blind, placebo‐controlled trial Trial duration: 6 months Sample size: AS 26, P 25 Withdrawals: AS 4, P 6 Efficacy sample size: AS 22, P 19 | |
| Participants | HIV+ men with wasting (weight <90% of ideal or weight loss >10% of baseline) and decreased free testosterone levels Mean age: AS 40, P 44 Mean weight: AS 66, P 72 Mean weight loss: AS 10.5, P 14.3 Mean serum testosterone: AS 11.3, P 10.1 Mean CD4 ct: AS 188, P 161 % on PIs: AS 19, P 16 |
|
| Interventions | Testosterone enanthate 300 mg IM q 3 weeks for 6 months | |
| Outcomes | Change in body weight Change in FFM measured by DEXA Deaths Withdrawals due to AEs | |
| Notes | Quality score=5 (R2, B2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Grinspoon 2000a.
| Methods | Randomized, double‐blind, placebo‐controlled factorial trial Treatment duration: 12 wks Sample size: AS 27, P 27 (AS + RT 13, AS 14; P + RT 14, P 13) Withdrawals: AS 6, P 5 Efficacy sample size: AS 21, P 22 | |
| Participants | HIV‐infected men with AIDS‐related wasting (weight <90% of ideal or self‐reported weight loss >10% and normal serum level of free tesosterone (>42 pmol/L) Mean age: 38 Mean weight: AS 70, P 67 Mean weight loss: NR Mean serum tesotsterone: AS 22.5, P 23.0 Mean CD4 ct: AS 430, P 313 % on PIs: 72 |
|
| Interventions | Testosterone enanthate 200 mg IM q 1 week vs. placebo for 12 weeks; Prgressive resistance training 3 times weekly vs. no training for 12 weeks | |
| Outcomes | Change in body weight Change in LBM measured by DEXA, Deaths | |
| Notes | Quality score=5 (R2, B2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hengge 2003a(i)‐bid.
| Methods | Randomized, double‐blind, placebo‐controlled trial with two AS doses (BID and TID) Treatment duration:16 weeks a) BID dose Sample size: AS 30, P 28 Withdrawals: AS 5, P 6 Efficacy sample size: AS 25, P 22 | |
| Participants | HIV+ men and women with with at least 5% weight loss over 6 months or wieght loss with weight 10% below ideal body weight
All were eugonadal at baseline Mean age: AS 41, P 38 Mean weight: AS 65, P 61 Mean weight loss: NR Mean serum testosterone: AS 20.5, P 24.3 Mean CD4 ct: AS 417, P 529 % on PIs: 100 |
|
| Interventions | Oxymetholone 50 mg po bid vs. placebo po bid | |
| Outcomes | Change in body weight Change in LBM measured by tetrapolar BIA Withdrawals due to AEs Deaths | |
| Notes | Quality score=5
(R2, B2, W1) Study publication indicates that measures of variability are SDs; in fact they are SEMs (confirmed by contacting study author)) Trial included two treatment groups compared to single placebo group‐ we divided placebo group in half for each of the two treatment comparisons |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hengge 2003a(ii)‐tid.
| Methods | Part of Hengge 2003 study ‐second test dose (ii) TID dose Sample size: AS 31, P 28 Withdrawals: AS 4, P 3 Efficacy sample size: AS 27, P 22 | |
| Participants | Mean age: AS 37, P 38 Mean weight: AS 66, P 61 Mean weight loss: NR Mean serum testosterone: AS 24.6, P 24.3 Mean CD4 ct: AS 484, P 529 % on PIs: 100 | |
| Interventions | Oxymetholone 50 mg po tid vs. placebo tid | |
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Miller 1998(i)‐one p.
| Methods | Randomized, double‐blind, placebo‐controlled trial with two different AS doses Treatment duration: 12 weeks a) One AS patch Sample size: AS 18, P 17 Withdrawals: AS 4, P 4 Efficacy sample size: AS 14, P 13 | |
| Participants | Women with HIV infection with weight loss >10% pre‐illness maximun or <90% ideal, serum free testosterone < mean of reference range (3 pg/ml) Mean age: AS 38, P 37 Mean weight: AS 52, P 54 Mean weight loss: AS 9.9, P 10.2 Mean serum testosterone: AS 0.68, P 1.13 Mean CD4 ct: AS 264, P 255 % on PIs: NR |
|
| Interventions | One transdermal testosterone patch containing 4.1 mg of testosterone and expected to deliver 150 ug per day and one placebo patch applied to abdomen twice weekly at 8 am, vs. two placebo patches applied twice weekly | |
| Outcomes | Change in body weight Change in LBM measured by DEXA Deaths Withdrawals due to AEs | |
| Notes | Quality score=4
(R1, B2, W1) Trial included two treatment groups compared to single placebo group‐ we divided placebo group in half for each of the two treatment comparisons |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Miller 1998(ii)‐two.
| Methods | Part of Miller 1998 study (ii) Two AS patches Sample size: AS 18, P 17 Withdrawals: AS 0, P 4 Efficacy sample size: AS 18, P 13 | |
| Participants | Mean age: AS 36, P 37 Mean weight: AS 55, P 54 Mean weight loss: AS 8.8, P 10.2 Mean serum testosterone: AS 0.86, P 1.13 Mean CD4 ct: AS 480, P 255 % on PIs: NR | |
| Interventions | Two transdermal testosterone patches, each containing 4.1 mg of testosterone and expected to deliver 150 ug per day applied to abdomen twice weekly at 8 am vs. two placebo patches applied twice weekly | |
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mulligan 2005.
| Methods | Randomized, double‐blind, placebo‐controlled trial Trial duration: 12 weeks (placebo‐controlled phase) Sample size: AS 19, P 19 Withdrawals (from placebo‐controlled phase): AS 3, P 2 Efficacy sample size: AS 16, P 17 | |
| Participants | HIV‐infected women with weight loss of 5% or greater or with BMI <20 kg/m2 Median age: AS 36, P 36 Median weight: AS 49.4, P 49.0 Median weight loss: NR Median serum testosterone: NR Median CD4 ct: AS 389, P 257 % on PIs: AS 47, P 47 |
|
| Interventions | Nandrolone decanoate 100 mg IM q 2 weeks vs equivalent volume placebo IM q 2 weeks | |
| Outcomes | Change in body weight Change in LBM measured by single frequency BIA Deaths Withdrawals due to ADRs | |
| Notes | Qualtiy score=4
(R1, B2, W1) The outcome results for change in body weight and LBM were provided as medians with interquartile ranges in the study publication; we contacted the authors and obtained these results as means with standard deviations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Strawford 1999a.
| Methods | Randomized, double‐blind, placebo‐controlled trial Treatment duration: 8 weeks Sample size: AS 12, P 12 Withdrawals: AS 1, P 1 Efficacy sample size: AS 11, P 11 | |
| Participants | HIV seropositive men with >5% weight loss during preceding 2 years but stable past 3 months,
eugonadal Mean age: AS 42, P 40 Mean weight: AS 69, P 73 Mean weight loss: AS 6.0, P 7.2 Mean serum testosterone: AS 20.9, P 22.7 Mean CD4 ct: AS 234, P 337 % on PIs: 54 |
|
| Interventions | Oxandrolone 20 mg/day vs. placebo Concomitant treatment: Supervised progressive resistance exercise (PRE) and physiologic testosterone replacement (testosterone enathate 100 mg/wk IM) |
|
| Outcomes | Change in body weight Change in LBM measured by DEXA Deaths Withdrawals due to AEs | |
| Notes | Quality score=5
(R2, B2, W1) All patients received physiological replacement of testosterone‐ we treated this as concomitant therapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wagner 2001.
| Methods | Randomized , double‐blind, placebo‐controlled three‐treatmenf group trial of anabolic steroid and protein supplements (data from the comparison of AS vs P with concomitant protein supplements used for the meta‐analysis) Treatment duration: 12 weeks Sample size: AS 22, P 22 Withdrawals: AS 2, P 2 Efficacy sample size: AS 20, P 20 | |
| Participants | HIV+ men with loss of at least 10% of normal body weight and serum testosterone <19.1 (550 ng/dl) Mean age:41 Mean weight: NR Mean weight loss: NR Mean serum testosterone: 13.8 Mean CD4 ct: 229 % on PIs: NR |
|
| Interventions | Tesosterone cypionate IM q 2 weeks, 200 mg initially, followed by 400 mg vs. placebo Concomitant therapy with high protein supplements | |
| Outcomes | Change in body weight Change in body composition (FFM and BCM) measured by BIA Deaths Withdrawals due to AEs: NR by treatment group | |
| Notes | Quality score=4
(R1, B2, W1) Data from the third treatment group, which received placebo drug and placebo protein supplement, were not included in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Under participants: mean weight and mean weight loss refers to baseline values measured in kilograms Age is reported in years, weight in kilograms, serum testosterone in ng/dl, CD4 counts in number of cells X 10 to the power 9 per litre
AS=anabolic steroid treatment group P=placebo treatment group
ADR= adverse drug event BCM=body cell mass BIA= bioelectrical impedance analysis DEXA= dual energy X‐ray absorptiometry FFM=fat free mass LBM=lean body mass NR=not reported PIs=protease inhibitors RT=resistance training
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Batterham 1997 | Conference abstract of published study (Batterham 2001) Reason for exclusion: duplicate publication |
| Batterham 2001 | Randomized study of nandrolone decanoate (100 mg IM q 2 weeks) versus megestrol acetate (400 mg/day) versus dietary counselling for 12 weeks in 15 males with HIV‐associated weight loss. Those randomized to dietary counselling alone were further randomized to nandrolone or megestrol after completing the dietary counselling Reason for exclusion: results presented for subjects receiving nandrolone in first phase and following dietary counselling combined |
| Berger 1993 | Case series of three men with wasting and muscle weakness related to HIV infection treated successfully with stanozol 2 to 4 mg TID Reason for exclusion: non‐controlled case series |
| Berger 1996 | Randomized double‐blind study of oxandrolone (5 mg per day or 15 mg per day versus placebo for 16 weeks in 63 HIV‐seropositive men with >10% loss of body weight Reason for exclusion: unable to interpret data for main outcome (change in body weight) ‐ data presented in graph inconsistent with those reported in the text; we were unable to obtain clarification by attempting to contact the author |
| Carroll 1997 | Conference abstract of randomized study of oxandrolone 10 mg/day compared to oxandrolone 20 mg/day for 16 weeks in conjunction with resistance training and nutritional support in 20 HIV positive women with wasting syndrome Reason for exclusion: both treatment groups received anabolic steroid therapy |
| Corcoran 2000 | Conference abstract of published study (Grinspoon 2000a) Reason for exclusion: duplicate publication |
| Dolan 2004b | Conference abstract of published study (Dolan 2004a) Reason for exclusion: duplicate publication |
| Earthman 2002 | Non‐controlled study of oxandrolone therapy for an average of 18.6 weeks on the changes in body cell mass and quality of life in 25 HIV‐infected patients Reason for exclusion: non‐controlled study |
| Engelson 1996 | Letter to the editor describing an open label study of testosterone cypionate 400 mg biweekly for 12 weeks in 29 HIV positive men with sexual dysfunction Reason for exclusion: non‐controlled study |
| Fairfield 2001a | Analysis of patients from the Grinspoon 2000a study to determine effects of testosterone and progressive resistance training on bone mineral density and bone turnover in eugonadal men with AIDS wasting Reason for exclusion: duplicate patients |
| Fairfield 2001b | Analysis of patients from the Grinspoon 2000a study to determine effects of testosterone and progressive resistance training on muscle composition in eugonadal men with AIDS wasting Reason for exclusion: duplicate patients |
| Fisher 1997 | Conference abstract of study of oxandrolone 20 mg daily with glutamine 20 g daily in 16 male subjects with HIV infection and weight loss Reason for exclusion: non‐controlled study |
| Fisher 1998a | Conference abstract reporting on a open‐label, community‐based study of oxandrolone 20 mg daily for up to 12 months in patients with HIV infection associated weight loss‐ preliminary report on 49 patients Reason for exclusion: non‐controlled study |
| Fisher 1998b | Conference abstract describing same study as Fisher 1998a Reason for exclusion: duplicate publication |
| Fox‐Wheeler 1999 | Open label study of oxandrolone 0.1 mg/kg/day for 3 months in 9 children with HIV infection and either malnourishment or risk of malnourishment Reason for exclusion: non‐controlled study, study population was children |
| Garsia 1997 | Conference abstract of published study (Batterham 2001) Reason for exclusion: duplicate publication |
| Gold 1994 | Conference abstract of pilot study combining nandrolone decanoate 100 mg IM q 2 weeks with resistive exercise for 16 weeks in HIV patients with weight loss and no improvement with nutrional counselling Reason for exclusion: non‐controlled study |
| Gold 1996 | Open label study of nandrolone decanoate 100 mg/ml IM q 2 weeks for 16 weeks in HIV‐positive men with 5 to 15% of usual body weight loss who did not respond to nutritional assessment and education Reason for exclusion: non‐controlled study |
| Gold 1999a | Letter to the editor describing a study of nandrolone decanoate 100 mg IM per week for 8 weeks in patients with HIV‐associated lipodystrophy Reason for exclusion: non‐controlled study; population not patients with weight loss |
| Gold 1999b | Conference abstract of unpublished study (Phanuphak) provided by Organon International Reason for exclusion: duplicate report |
| Grinspoon 1998b | Analysis of patients from Grinspoon 1998a study with respect to the GH‐IGH‐I axis in men with AIDS wasting syndrome and the effect of testosterone administration on it Reason for exclusion: duplicate patients |
| Grinspoon 1998c | Conference abstract for published study (Grinspoon 1999a) Reason for exclusion: duplicate publication |
| Grinspoon 1999 | Longer term follow‐up of patients from Grinspoon 1998a study; open label continuation of testosterone enanthate 300 mg IM every 3 weeks for a further 6 months after the initial randomized treatment for 6 months Reason for exclusion: duplicate patients; longer term follow‐up phase was not controlled |
| Grinspoon 2000b | Analysis of patients from Grinspoon 1998a study with respect to depression indices in hypogonadal and eugonadal men with AIDS wasting and the response of these indices to testosterone therapy Reason for exclusion: duplicate patients |
| Grudzdev 1999 | Russian study of nandrolone decanoate 50 mg IM per week for 12 weeks in 13 patients with AIDS and weight loss Reason for exclusion: non‐controlled study |
| Hasheeve 1994 | Conference abstract of study evaluating use of DHEA (75 mg orally QD) as an adjunct therapy for HIV disease in 12 patients with AIDS Reason for exclusion: no control group, DHEA not considered an anabolic steroid for this meta‐analysis, weight and body composition not assessed |
| Hellerstein 1998 | Conference abstract of published study (Strawford 1998) Reason for exclusion: duplicate publication |
| Hengge 1996a | A randomized controlled study in men and women with HIV infection and weight loss of oxymetholone 50 mg po TID plus ketotifen versus oxymetholone versus a control group obtained from patients who did not desire therapy and matched to treated patients by disease characteristics Reason for exclusion: comparison between oxymetholone and no treatment based on non‐randomized treatment groups |
| Hengge 1996b | Conference abstract of published study (Hengge 1996a) Reason for exclusion: duplicate publication |
| Hengge 2002 | Conference abstract of preliminary results of published study (Hengge 2003a) Reason for exclusion: duplicate publication |
| Hengge 2003b | Published report of a published study included in the meta‐analysis (Hengge 2003a) Reason for exclusion: duplicate publication |
| Hengge 2004 | Conference abstract of published study (Hengge 2003a) Reason for exclusion: duplicate publication |
| Hernandez‐Lopez 1996 | Study nandrolone decanoate and testosterone and nutritional support in 39 men with wasting compared to 100 control patients receiving nutrional support without anabolic therapy Reason for exclusion: non‐randomized study |
| Jaque 2001 | Randomized trial of nandrolone (600 mg IM weekly) versus nandrolone plus resistance training for 12 weeks in 30 HIV‐positive men Reason for exclusion: anabolic steroid therapy in both treatment groups |
| Jeantils 1993 | Uncontrolled study of supplementation with oral testosterone undecanoate 40 mg TID for 2 months (as reported by Coodley) Reason for exclusion: non‐controlled study |
| Jekot 1993 | Clinical observations and monitoring of eight patients with HIV/AIDS self administered or administered anabolic steroids (exact drug not specified) in cycles (8 to 12 weeks on drug, 8 to 12 weeks off drug) for periods of 1 to 7 years as part of their HIV/AIDS treatment Reason for exclusion: retrospective case series with no control group |
| Klibanski 1998 | Conference abstract of published study (Grinspoon 1998a and 1999a) Reason for exclusion: duplicate publication |
| Mooney 1998 | Conference abstract of a pilot open label study to evaluate a comprehensive approach to wasting syndrome which included nandrolone decanoate 200 mg/wk and testosterone enanthate 100 mg/wk for 12 weeks in 30 HIV/AIDS patients Reason for exclusion: non‐controlled study |
| Mulligan 2001 | Conference abstract of published study (Mulligan 2005) Reason for exclusion: duplicate publication |
| Mwamburi 2004a | Randomized open‐label study comparing oxandrolone to megestrol acetate for 2 months in HIV positive patients with weight loss receiving HAART Reason for exclusion: active therapy in both treatment groups |
| Mwamburi 2004b | Study in which subjects were randomized to either oxandrolone to megestrol acetate for 2 months followed by both agents and dietary advice for 5 months for all subjects in HIV positive patients with weight loss receiving HAART; continuation of Mwamburi 2004a study Reason for exclusion: duplicate patients |
| Phanuphak | Study report provided by Organon Ltd of a 24 week, randomized, double‐blind placebo‐controlled dose ranging study of nandrolone decanoate given IM every 2 weeks in 91 HIV‐seropositive men with weight loss and CD4 counts less than 500; study carried out in Thailand and Brazil Reason for exclusion: results for change in lean body mass and weight reported as median with range which are insufficient to allow inclusion in the meta‐analysis Results at 12 weeks: change in lean body mass ‐0.4, 1.5, 1.9 kg in nandrolone 50, 100, and 150 mg treatment groups, respectively, 0.3 kg in placebo group; change in weight 0.0, 1.2, and 1.6 kg in nandrolone 50, 100, and 150 mg treatment groups, respectively, ‐0.7 kg in placebo group |
| Pharo 1997 | Abstract report for conference proceedings of a randomized clinical trial of two different doses of oxandrolone (10 mg and 20 mg per day for 16 weeks) in conjunction with resistance training and nutritional support in 20 women with HIV infection and wasting Reason for exclusion: both treatment groups received anabolic steroid therapy |
| Poles 1996 | An open label study of oxandrolone 10 mg po BID for up to 120 days in 20 men and one woman AIDS‐related weight loss Reason for exclusion: non‐controlled study |
| Rabkin 1995a | Non‐controlled study of the effects of testosterone replacement in 81 HIV‐positive men with low levels of testosterone Reason for exclusion: non‐controlled study |
| Rabkin 1995b | Interim results of a 12 week open study of testosterone cypionate 200‐400 mg IM q 2 weeks (as reported by Coodley) Reason for exclusion: non‐controlled study |
| Rabkin 1996 | Preliminary report of results from Rabkin 1999 study Reason for exclusion: duplicate patients |
| Rabkin 1999 | Trial involving 124 men with HIV infection, low serum testosterone (<500 ng/dl), and clinical symptoms of hypogonadism of open label treatment with testosterone cypionate 400 mg IM biweekly for 8 weeks, followed by a further 4 weeks for responders, followed by a 6 week randomized, placebo‐controlled discontinuation phase in 84 testosterone responders Reason for exclusion: randomized portion of the study limited to the discontinuation phase |
| Rabkin 2000a | Study in HIV positive men and women with depressed mood and fatigue of open label DHEA 200 to 500 mg/day for 12 weeks followed by a randomized, double‐blind placebo‐controlled 4 week discontinuation phase Reason for exclusion: randomized portion of the study limited to the discontinuation phase DHEA not included as an anabolic steroid for this meta‐analysis |
| Rabkin 2000b | A 6 week double‐blind, placebo‐controlled study of testosterone cypionate 400 mg IM biweekly versus placebo followed by a 12 week open label phase in which both groups received testosterone. Participants included HIV‐positive men with clinical symptoms of hypogonadism (sexual dysfunction and at least one of depressed mood, low energy of loss of muscle mass) and low or low normal serum testosterone levels. Of the 70 patients who completed the placebo‐controlled portion of the study, 18 had loss of muscle mass. Body composition was reported to be assessed monthly, but the results were presented only using a 12 week time frame thus were not presented for the controlled portion of the study. Reason for exclusion: results for change in body weight and body cell mass not reported for the controlled portion of the study |
| Rabkin 2004 | Double‐blind placebo‐controlled three‐arm trial of fluoxetine, testosterone cypionate 400 mg biweekly for 8 weeks, or double placebo for 8 weeks in 123 HIV‐positive men with depression Reason for exclusion: entry criteria did not include weight loss, change in weight or body composition either not measured or not reported |
| Reiter 1996 | Conference abstract of same study reported in a second abstract (Bucher 1996) Reason for exclusion: duplicate publication |
| Rivera 1999 | Randomized cross‐over trial of IM testosterone (200 mg q 2 weeks) versus trans‐scrotal testosterone (6 mg/day) for 8 weeks with cross‐over the following 8 weeks in 21 HIV patients with weight loss of at least 5% Reason for exclusion: both treatment groups received anabolic steroid therapy |
| Romeyn 2000 | Letter to the editor describing a pilot study on oxandrolone 10 mg twice daily with randomization to either progressive resistance exercise or no exercise in HIV‐positive subjects with weight loss Reason for exclusion: both treatment groups received anabolic steroid therapy |
| Sattler 1999a | Randomized, non‐placebo controlled study of nandrolone decanoate IM weekly (200 mg week 1, 400 mg week 2, 600 mg weeks 3 to 12, 400 mg week 13, 200 mg week 14, 100 mg week 15, and 50 mg week 16) plus resistance training versus nandrolone alone in men with HIV infection with stable weight Reason for exclusion: both treatment groups received anabolic steroid therapy; study population required to have stable weight |
| Sattler 1999b | Conference abstract of published study (Sattler 2002) Reason for exclusion: duplicate publication |
| Sattler 2002 | Randomized trial of nandrolone plus resistance training versus nandrolone for 12 weeks in 30 HIV‐infected men. Reason for exclusion: both treatment groups received anabolic steroid therapy |
| Schambelan 2001 | Conference abstract of a randomized, placebo‐controlled trial of testosterone enanthate (200 mg q 2 weeks in men, 100 mg q 2 weeks in women) for 12 weeks in conjunction with megestrol in all subjects in 81 HIV‐positive patients (79 male) with weight loss Reason for exclusion: results for change in lean body mass and weight were reported as medians with no measure of variability which are insufficient to allow inclusion in the meta‐analysis Results: change in LBM 3.3 kg in testosterone group, 3.3 kg in placebo group; change in weight 5.3 kg in testosterone group, 7.3 kg for placebo group |
| Schrader 2002 | Randomized trial of a "stacking" regimen of testosterone cypionate, nandrolone decanoate, and Human Chorionic Gonadotropin for 16 weeks in conjunction with resistance exercise, nutrional counselling and dietary supplementation in all patients in 30 HIV‐positive males with weight loss Reason for exclusion: test treatment included anabolic steroids together with HCG |
| Schroeder 2001 | Randomized trial of nandrolone alone versus nandrolone in combination with resistance training for 12 weeks in 23 HIV‐positive men Reason for exclusion: both treatment groups received anabolic steroid therapy |
| Schroeder 2003 | Reanalysis of data from two previous studies of anabolic steroids, one in HIV‐positive men (Sattler #1) and one in older healthy men; in the first study subjects were randomized to nandrolone 600 mg/wk +/‐ resistance training Reason for exclusion: duplicate patients |
| Strawford 1998 | Conference abstract of published study (Strawford 1999a) Reason for exclusion: duplicate publication |
| Strawford 1999b | A study including a randomized, double‐blind, placebo‐controlled phase of nandrolone decanoate 195 mg/wk (240 mg followed by 56 mg q 2 days) versus nandrolone 65 mg/wk (80 mg followed by 19 mg q 2 days) versus placebo for 2 weeks followed by an open label non‐controlled phase of nandrolone 200 mg q 2 weeks for 12 weeks. Study participants were HIV seropositive men with involuntary weight loss >5% and low to low normal serum testosterone concentrations. Reason for exclusion: controlled portion of study only 14 days |
| Stute 1994 | Abstract from conference proceedings of non‐controlled retrospective analysis of 31 cachectic HIV‐infected patients treated with medroxy progesterone acetate (MPA) 1000 mg daily for a mean duration of 11 weeks Reason for exclusion: non‐controlled retrospective study; MPA not an anabolic steroid |
| Umar 1998 | Randomized, placebo‐controlled study of DHEA (50 mg daily) for 6 months in 29 female patients with AIDS Reason for exclusion: DHEA not included as an anabolic steroid in the meta‐analysis |
| Urbina 2004 | Conference abstract report of an open‐label study of switching anabolic steroid therapy to oxymetholone 50 mg QD for 24 weeks in 16 HIV positive subjects with wasting and prior treatment with either nandrolone decanoate or oxandrolone for at least 12 weeks Reason for exclusion: non‐controlled study |
| Van Loan 1999 | Further analysis of results of Strawford 1999b study Reason for exclusion: duplicate subjects |
| Vergel 1998 | Conference abstract of an open trial in 30 HIV positive patients with wasting syndrome of nutritional counselling, enhanced protein nutrition, resistance weight training, daily vitamin/anti‐oxidant supplementation, and a 12 week course of nandrolone decanoate 200 mg/week and testosterone enathate 100 mg/week to assess change in weight, body cell mass and quality of life Reason for exclusion: non‐controlled study |
| Wagner 1998a | Twelve week open trial of testosterone in men with HIV infection, clinical symptoms of hypogonadism, and serum testosterone < 500 ng/dl. Study assessed correlates of fatigue and efficacy of testosterone as a treatment of fatigue. Reason for exclusion: non‐controlled study |
| Wagner 1998b | Open study of testosterone cypionate 200 mg IM followed by 400 mg biweekly for 12 weeks in 23 eugonadal men (serum testosterone > 500 ng/ml) with AIDS with symptoms characteristic of hypogonadism (diminished libido, low mood, low energy, low appetite/weight loss) Reason for exclusion: non‐controlled study |
| Wagner 1998c | A 12 week open trial of testosterone IM biweekly in 54 men with HIV infection, low serum testosterone and clinical symptoms of hypogonadism analyzed by those who engaged in exercise vs. those who did not Reason for exclusion: non‐controlled study |
| Wagner 1999 | Secondary analysis of data from Rabkin 1995a study of open label testosterone 400 mg IM biweekly for 12 weeks in HIV positive men with clinical symptoms of hypogonadism, low serum testosterone levels (<500 ng/dl), and CD4 counts < 400. Analysis limited to only those men with significant depletion of body cell mass to assess effects of testosterone on body composition and weight and predictors of response in that group. Reason for exclusion: duplicate patients Reason for exclusion: non‐controlled study |
| Wheeler 1998 | Conference abstract of preliminary results of published study (Fox‐Wheeler 1999) Reason for exclusion: duplicate publication |
Sources of support
Internal sources
Health Canada, Canada.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Bhasin 1998 {published data only}
- Bhasin S, Storer TW, Asbel‐Sethi N, Kilbourne A, Hays R, Sinha‐Hikim I, et al. Effects of testosterone replacement with a nongenital transdermal system, Androderm, in Human Immunodeficiency Virus‐infected men with low testosterone levels. J Clin Endocrinol Metab 1998;83:3155‐62. [DOI] [PubMed] [Google Scholar]
Bhasin 2000(i)‐no e {published data only}
- Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, et al. Testosterone replacement and resistance exercise in HIV‐infected men with weight loss and low testosterone levels. JAMA 2000;283:763‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhasin 2000(ii)‐exer {published data only}
Bucher 1996 {published data only}
- Bucher G, Berger DS, Fields‐Gardner C, Jones R, Reiter WM. A prospective study on the safety and effect of nandrolone decanoate in HIV‐positive patients. Int Conf AIDS. 1996; Vol. 11(1):26 (abstract Mo.B. 423).
Choi 2004 {published data only}
- Choi H, Gray P, Storer T, Calof O, Woodhouse L, Singh A, et al. Effects of testosterone replacement in Human Immunodeficiency Virus‐infected women with weight loss. J Clin Endocrinol Metab 2005;90(3):1531‐41. [DOI] [PubMed] [Google Scholar]
Coodley 1997 {published data only}
- Coodley GO, Coodley MK. A trial of testosterone therapy for HIV‐associated weight loss. AIDS 1997;11:1347‐52. [DOI] [PubMed] [Google Scholar]
Dobs 1999 {published data only}
- Dobs AS, Cofrancesco J, Nolten WE, Danoff A, Anderson R, Hamilton CD, et al. The use of transscrotal testosterone delivery system in the treatment of patients with weight loss related to human immunodeficiency virus infection. Am J Med 1999;107:126‐32. [DOI] [PubMed] [Google Scholar]
Dolan 2004a {published data only}
- Dolan S, Wilkie S, Aliabadi N, Sullivan MP, Basgoz N, Davis B, Grinspoon S. Effects of testosterone administration in Human Immunodeficiency Virus‐infected women with low weight. Arch Intern Med 2004;164:897‐904. [DOI] [PubMed] [Google Scholar]
Grinspoon 1998a {published data only}
- Grinspoon S, Cocoran C, Askari H, Schoenfeld D, Wolf L, Burrows B, et al. Effects of androgen adminstration in men with the AIDS wasting syndrome. Ann Intern Med 1998;129:18‐26. [DOI] [PubMed] [Google Scholar]
Grinspoon 2000a {published data only}
- Grinspoon S, Corcoran C, Parlman K, Costello M, Rosenthal D, Anderson E, et al. Effects of testosterone and progressive resistance training in eugonadal men with AIDS wasting. Ann Intern Med 2000;133:348‐55. [DOI] [PubMed] [Google Scholar]
Hengge 2003a(i)‐bid {published data only}
- Hengge UR, Stocks K, Wiehler H, Faulkner S, Esser S, Lorenz C, et al. [Double‐blind, randomized, placebo‐controlled phase III trial of oxymetholone for the treatment of HIV wasting]. AIDS 2003;17:699‐710. [DOI] [PubMed] [Google Scholar]
Hengge 2003a(ii)‐tid {published data only}
- BID dose.
Miller 1998(i)‐one p {published data only}
- Miller K, Corcoran C, Armstrong C, Caramelli K, Anderson E, Cotton D, et al. Transdermal testosterone adminstration in women with acquired immunodeficiency syndrome wasting: a pilot study. J Clin Endocrinol Metab 1998;83(8):2717‐25. [DOI] [PubMed] [Google Scholar]
Miller 1998(ii)‐two {published data only}
Mulligan 2005 {published data only}
- Mulligan K, Zackin R, Clark RA, Alston‐Smith B, Liu T, Sattler FR, et al. Effect of nandrolone decanoate therapy on weight and lean body mass in HIV‐infected women with weight loss: a randomized, double‐blind, placebo‐controlled, multicenter trial. Archives Internal Medicine 2005;165(5):578‐85. [DOI] [PubMed] [Google Scholar]
Strawford 1999a {published data only}
- Strawford A, Barbieri T, Loan M, Parks E, Catlin D, Barton N, et al. Resistance exercise and supraphysiologic androgen therapy in eugonadal men with HIV‐related weight loss: a randomized controlled trial. JAMA 1999;281(14):1282‐90. [DOI] [PubMed] [Google Scholar]
Wagner 2001 {published data only}
- Wagner GJ, Rabkin JG, Rabkin R. A randomized comparative trial of testosterone and protein supplements for weight loss in HIV plus men. Nutrition Research 2001;21:159‐69. [Google Scholar]
References to studies excluded from this review
Batterham 1997 {published data only}
- Batterham M, Garcia R. Randomised prospective medium‐term comparison of megestrol acetate, nandrolone decanoate and dietary therapy alone for HIV associated wight loss. Annu Conf Australas Soc HIV Med. 1997; Vol. 9:91 (Abstract no. IS 87).
Batterham 2001 {published data only}
Berger 1993 {published data only}
- Berger JR, Pall L, Winfield D. Effect of anabolic steroids on HIV‐related wasting myopathy. South Med J 1993;86:865‐6. [DOI] [PubMed] [Google Scholar]
Berger 1996 {published data only}
- Berger JR, Pall L, Hall CD, Simpson DM, Berry PS, Dudley R. Oxandrolone in AIDS‐wasting myopathy. AIDS 1996;10(14):1657‐62. [DOI] [PubMed] [Google Scholar]
Carroll 1997 {published data only}
- Carroll E, Sauer L, Pharo AN, Vergel N. Oxandrolone: anabolic steroid use in HIV positive women. Natl Conf Women HIV. 1997:107 (Abstract no. 103.4).
Corcoran 2000 {published data only}
Dolan 2004b {published data only}
Earthman 2002 {published data only}
- Earthman CP, Reid PM, Harper IT, Ravussin E, Howell WH. Body cell mass repletion and improved quality of life in HIV‐infected individuals receiving oxandrolone. JPEN 2002;26(6):357‐64. [DOI] [PubMed] [Google Scholar]
Engelson 1996 {published data only}
- Engelson ES, Rabkin JG, Rabkin R, Kotler DP. Effects of testosterone upon body composition. J Acquir Immune Defic Syndr 1996;11:510‐14. [DOI] [PubMed] [Google Scholar]
Fairfield 2001a {published data only}
- Fairfield WP, Finkelstein JS, Klibanski A, Grinspoon SK. Osteopenia in eugonadal men with acquired immune deficiency syndrome wasting syndrome. J Clin Endocrinol Metab 2001;86:2020‐6. [DOI] [PubMed] [Google Scholar]
Fairfield 2001b {published data only}
- Fairfield WP, Treat M, Rosenthal DI, Frontera W, Stanley T, Corcoran C, et al. Effects of testosterone and exercise on muscle leanness in eugonadal men with AIDS wasting. J Appl Physiol 2001;90:2166‐71. [DOI] [PubMed] [Google Scholar]
Fisher 1997 {published data only}
- Fisher A, Abbaticola M. Effects of oxandrolone and 1‐glutamine on body weight, body cell mass, and body fat in patients with HIV infection‐preliminary analysis. Conf Retroviruses Opportunistic Infect, 4th. 1997:192 (Abstract no. 692).
Fisher 1998a {unpublished data only}
- Fisher AE, Abbaticola MM. Effects of oxandrolone on body weight and composition in patients with HIV‐associated weight loss. 5th Conf Retrovir Oppor Infect. 1998:169 (Abstract no. 477).
Fisher 1998b {unpublished data only}
- Fisher A, Abbatilola M. The effects of oxandrolone on body weight and composition in patients with HIV‐associated weight loss. Int Conf AIDS. 1998; Vol. 12:844 (Abstract no. 42351).
Fox‐Wheeler 1999 {published data only}
- Fox‐Wheeler S, Heller L, Salata CM, Kaufman F, Loro ML, Gilsanz V, et al. Evaluation of the effects of oxandrolone on malnourished HIV‐positive pediatric patients. Pediatrics 1999;104(6):e73. [DOI] [PubMed] [Google Scholar]
Garsia 1997 {published data only}
- Garsia R, Batterham M. Randomized prospective medium‐term comparison of megestrolacetate, nandrolone decanoate and dietary therapy alone for HIV associated weight loss. Annu Conf Australas Soc HIV Med, 9. 1997:91 (Abstract no. IS 87).
Gold 1994 {published data only}
- Gold J, High H, Michelmore H, Oliver C. The effect of the anabolic steroid, nandrolone decanoate, on lean body mass and qulaity of life in HIV+ men ‐ a preliminary analysis. Annu Conf Australas Soc HIV Med 1994;264:unnumbered poster. [Google Scholar]
Gold 1996 {published data only}
- Gold J, High HA, Li Y, Michelmore H, Bodswoth NJ, Finlayson R, et al. Safety and efficacy of nandrolone decanoate for treatment of wasting in patients with HIV infection. AIDS 1996;10(7):745‐52. [DOI] [PubMed] [Google Scholar]
Gold 1999a {published data only}
- Gold J, Batterham M. Nandrolone decanoate; use in HIV‐associated lipodystrophy syndrome: a pilot study. Int J STD AIDS 1999;10(8):558. [PubMed] [Google Scholar]
Gold 1999b {published data only}
- Gold J, Phanuphak P, Castelo A. The use of nandrolone decanoate in patients with HIV wasting: a double blind, placebo controlled trial inThailand and Brazil. Annu Conf Australas Soc HIV Med. 1999; Vol. 11:153:Abstract no P55.
Grinspoon 1998b {published data only}
- Grinspoon S, Corcoran C, Stanley T, Katznelson L, Klibanski A. Effects of androgen administration on the growth hormone‐insulin‐like growth factor I axis in men with acquired immunodeficiency syndrome wasting. J Clin Edocrinol Metab 1998;83:4251‐6. [DOI] [PubMed] [Google Scholar]
Grinspoon 1998c {published data only}
- Grinspoon SK, Corcoran C, Anderson E, Schoenfeld D, Basgoz N, Klibanski A. Long‐term effects of androgen administration in men with AIDS wasting. Int Conf AIDS. 1998; Vol. 12:555 (abstract no. 32176).
Grinspoon 1999 {published data only}
- Grinspoon S, Corcoran C, Anderson E, Hubbard J, Stanley T, Basgoz N, et al. Sustained anabolic effects of long‐term androgen administration in men with AIDS wasting. Clin Infect Dis 1999;28:634‐6. [DOI] [PubMed] [Google Scholar]
Grinspoon 2000b {published data only}
- Grinspoon S, Corcoran C, Stanley T, Baaj A, Basgoz N, Klibanski A. Effects of hypogonadism and testoterone administration on depression indices in HIV‐infected men. J Clin Edocrinol Metab 2000;85:60‐65. [DOI] [PubMed] [Google Scholar]
Grudzdev 1999 {published data only}
- Gruzdev BM, Ivannikov EV, Gorbacheva ES. Anabolic therapy in AIDS patients. Terapevticheskii Arkhiv 1999;71(11):35‐37. [PubMed] [Google Scholar]
Hasheeve 1994 {published data only}
- Hasheeve D, Salvato P, Thompson C. DHEA: a potential treatment for HIV disease. Int Conf AIDS 1994;223:Abstract no PB0322. [Google Scholar]
Hellerstein 1998 {published data only}
- Hellerstein MK, Strawford A, Barton N, King J, Barbieri T, Loan M, Parks EJ. The effects of oxandrolone (OX) plus resistance exercise (RE) on nitrogen balance (N BAL), body composition and strength in men with AIDS wasting syndrome (AWS). Int Conf AIDS, 12. 1998; Vol. Abstract no 42335:841.
Hengge 1996a {published data only}
- Hengge UR, Baumann M, Maleba R, Brockmeyer NH, Goos M. Oxymetholone promotes weight gain in patients with advanced human immunodeficiency virus (HIV‐1) infection. Brit J Nutr 1996;75(1):129‐38. [DOI] [PubMed] [Google Scholar]
Hengge 1996b {published data only}
Hengge 2002 {published data only}
- Hengge UR, Stocks K, Unger S, Faulkenr S, Goos M, Dudley R. Randomized phase III trial of oxmetholone for the treatment of HIV wasting and lipodystrophy. Conf Retroviruses Opportunistic Infect. 2002:Abstract No 705‐T.
Hengge 2003b {published data only}
- Hengge UR, Stocks K, Faulkner S, Wiehler H, Lorenz C, Jentzen W, Hengge D, Ringham G. Oxymetholone for the treatment of HIV‐wasting: a double‐blind, randomized, placebo‐controlled phase III trial in eugonadal men and women. HIV Clin Trials 2003;4:150‐63. [DOI] [PubMed] [Google Scholar]
Hengge 2004 {published data only}
- Hengge UR, Stocks K, Faulkner S, Wiehler H, Lorenz C, Jentzen W, et al. Oxymetholone for the treatment of HIV wasting: a double‐blind, randomized, placebo‐controlled phase 3 trial in eugonadal men and women. 11th Conf Retrovir Opportunistic Infect 2004;Abstract no 725. [Google Scholar]
Hernandez‐Lopez 1996 {published data only}
- Hernandez‐Lopez G, Feregrino‐Goyos M, Eid‐Lidt G, Alvarado‐Diez R, et al. Low levels of testosterone in AIDS wasting syndrome. The effect of anabolic steroids on muscular body mass and better nutritional support. Int Conf AIDS 1996;11(2):454(abstract no. Pub.B.1098). [Google Scholar]
Jaque 2001 {published data only}
- Jaque SV, Schroeder ET, Azen SP, Dube MP, et al. Magnitude and timing of regional body composition changes during anabolic therapies in HIV‐positive men. Clinical Exercise Physiology 2002;4:50‐9. [Google Scholar]
Jeantils 1993 {published data only}
- Jeantils V, Nguyen G, Bacle F, Thomas M, Krivitzky A. weight gain under oral testosterone undecanoate in AIDS. Therapie 1993;48:71‐2. [PubMed] [Google Scholar]
Jekot 1993 {published data only}
- Jekot WF, Purdy DW. Treating HIV/AIDS patients with anabolic steroids. AIDS Patient Care 1993;7(2):68‐74. [Google Scholar]
Klibanski 1998 {published data only}
- Klibanski A, Basgoz N, Schoenfeld D, Grinspoon SK, Corcoran C, Anderson E. Long‐term effects of androgen adminstration in men with AIDS wasting. Int Conf AIDS, 12. 1998; Vol. Abstract no 32176:555.
Mooney 1998 {published data only}
- Mooney M, Salvato P, Vergel NR. Anabolic steroids, resistance exercise and protein supplementation effect on lean body mass in HIV+ patients. Int Conf AIDS, 12. 1998; Vol. Abstract no 32185:557.
Mulligan 2001 {published data only}
- Mulligan K, Zackin R, Clark RA, Sattler FR, et al. Nandrolone decanoate increases weight and lean body mass in HIV‐infected women with weight loss: a randomized, double‐blind, placebo‐controlled, multicenter trial. Conf Retroviruses Opportunistic Infect 2001;8:236(abstract no. 64). [DOI] [PubMed] [Google Scholar]
Mwamburi 2004a {published data only}
- Mwamburi DM, Gerrior J, Wilson IB, Chang H, Scully E, Saboori S, et al. Comparing megestrol acetate therapy with oxandrolone therapy for HIV‐related weight loss: similar results in 2 months. Clin Infect Dis 2004;38(6):895‐902. [DOI] [PubMed] [Google Scholar]
Mwamburi 2004b {published data only}
- Mwamburi DM, Gerrior J, Wilson IB, Chang H, Scully E, Saboori S, et al. Combination megestrol acetate, oxandrolone, and dietary advice restores weight in human immunodeficiency virus. Nutr Clin Pract 2004;19(4):395‐402. [DOI] [PubMed] [Google Scholar]
Phanuphak {unpublished data only}
- Phanuphak P, Castello A, Marliere E, Chuenyam M, Valk‐Cortenraad M, Li Y, Gold J. The use of nandrolone decanoate in patients with HIV wasting: a double‐blind, placebo controlled trial in Thailand and Brazil. Provided by Organon International Unpublished. [Google Scholar]
Pharo 1997 {published data only}
- Pharo AN, Vergel N, Carroll E, Sauer L. Oxandrolone: anabolic steroid use in HIV positive women. Natl Conf Women HIV. 1997:107 (Abstract no. 103.4).
Poles 1996 {published data only}
- Poles MA, Meller JA, Lin A, Weiss WR, Gocke M, Dietrich DT. Oxandrolone as a treatment for AIDS‐related weight loss and wasting. 4th Conf Retro and Opportun Infect. 1997:193 (Abstract no. 695).
Rabkin 1995a {published data only}
- Rabkin JG, Wagner G, Rabkin R. Testosterone replacement therapy in HIV illness. Gen Hosp Psychiatry 1995;17:37‐42. [DOI] [PubMed] [Google Scholar]
Rabkin 1995b {published data only}
- Rabkin JG, Rabkin R. Testosterone replacement therapy for HIV infected men. AIDS Reader 1995;5:136‐44. [Google Scholar]
Rabkin 1996 {published data only}
- Rabkin JG, Wagner G, Rabkin R. Treatment of depression in HIV+ men: literature review and report of an ongoing study of testosterone replacement therapy. Ann Behavioral Med 1996;18(1):24‐29. [DOI] [PubMed] [Google Scholar]
Rabkin 1999 {published data only}
- Rabkin JG, Wagner GJ, Rabkin R. Testosterone therapy for human immunodeficiency virus‐positive men with and without hypogonadism. J Clin Psychopharmacology 1999;19(1):19‐27. [DOI] [PubMed] [Google Scholar]
Rabkin 2000a {published data only}
- Rabkin JG, Ferrando SJ, Wagner GJ, Rabkin R. DHEA treatment for HIV+ patients: effects on mood, androgenic and anabolic parameters. Psychoneuroendocrinology 2000;25(1):53‐68. [DOI] [PubMed] [Google Scholar]
Rabkin 2000b {published data only}
- Rabkin JG, Wagner GJ, Rabkin R. A double‐blind, placebo‐controlled trial of testosterone therapy for HIV‐positive men with hypogonadal symptoms. Arch Gen Psychiatry 2000;57(2):141‐7. [DOI] [PubMed] [Google Scholar]
Rabkin 2004 {published data only}
- Rabkin JG, Wagner GJ, Rabkin R, Lin SH. Testosterone versus fluoxetine for depression and fatigue in HIV/AIDS: a placebo‐controlled trial. J Clin Psychpharmcol 2004;24(4):379‐85. [DOI] [PubMed] [Google Scholar]
Reiter 1996 {published data only}
- Reiter WM, Jones R, Fields‐Gardner C, Berger DS, Bucher G. A prospective study on the safety and effect of nandrolone decanoate in HIV‐positive patients. Int Conf AIDS. 1996; Vol. 11, issue 1:26 (Abstract no. Mo.B.423.
Rivera 1999 {published data only}
- Rivers S, Horton R, Sattler F, Qian D, Olson C, Briggs W, Bhasin S. Block in the generation of dihydrotestosterone from testosterone in patients with weight loss. Conf Retroviruses Opportunistic Infect 6th. 1999:200 (Abstract no. 701).
Romeyn 2000 {published data only}
- Romeyn M, Gunn N. Resistance exercise and oxandrolone for men with HIV‐related weight loss [letter]. JAMA 2000;284(2):176. [PubMed] [Google Scholar]
Sattler 1999a {published data only}
- Sattler FR, Jaque SV, Schroeder ET, Olson C, Dube MP, Martinez C, et al. Effects of pharmacologic doses of nandrolone decanoate and progressive resistance training in immunodeficient patients infected with human immunodeficiency virus. J Clin Endocrinol Metab 1999;84(4):1268‐76. [DOI] [PubMed] [Google Scholar]
Sattler 1999b {published data only}
Sattler 2002 {published data only}
- Sattler FR, Schroeder ET, Dube MP, Jaque SV, Martinez C, Blanche PJ, et al. Metabolic effects of nandrolone decanoate and resistance training in men with HIV. Am J Physiol Endocrinol Metab 2002;283:E1214‐22. [DOI] [PubMed] [Google Scholar]
Schambelan 2001 {published data only}
- Schambelan M, Zackin R, Mulligan K, Sattler FR, et al. Effect of testosterone (T) on the response to megesterol acetate (MA) in patients with HIV‐associated wasting: a randomized, double‐blind placebo‐controlled trial (ACTG 313). Conf Retroviruses Opportunistic Infect 2001;8:236 (Abstract no. 640). [Google Scholar]
Schrader 2002 {published data only}
- Schrader S, Vergel N. A randomized, open label, controlled study on the use of ananbolic steroids, nutritional supplementation and exercise for HIV‐positive males. Int Conf AIDS. 2002:Abstract no. B10287. [Abstract no. B10287]
Schroeder 2001 {published data only}
- Schroeder ET, Jaque SV, Hawkins SA, Olson C, et al. Regional DXA in assessment of muscle adaptation to anabolic stimuli. 2001; Vol. 3:199‐206.
Schroeder 2003 {published data only}
- Schroeder ET, Terk M, Sattler FR. Androgen therapy improves muscle mass and strength but not muscle quality: results from two studies. Am J Physiol Endocrinol Metab 2003;285(1):E16‐24. [DOI] [PubMed] [Google Scholar]
Strawford 1998 {published data only}
- Strawford A, Barbieri T, Loan M, Parks EJ, King J, Barton N, et al. The effects of oxandrolone (OX) plus resistance exercise (RE) on nitrogen balance (N BAL), body composition and strength in men with AIDS wasting syndrome (AWS). Int Conf AIDS. 1998; Vol. 12:841 (Abstract no. 42335).
Strawford 1999b {published data only}
- Strawford A, Barbieri T, Neese R, Loan M, Christiansen M, Hoh R, et al. Effects of nandrolone decanoate therapy in borderline hypogonadal men with HIV‐associated weight loss. J Acquir Immune Defic Syndr Hum Retrovirol 1999;20:137‐46. [DOI] [PubMed] [Google Scholar]
Stute 1994 {published data only}
- Stute A, Poppinger J, Spitz S, Jaegel Guedes E, Jaegel H. Treatment with medroxy‐progesterone‐acetate reverses weight loss but not body cell mass depletion: results of body‐impedance analysis. Int Conf AIDS. 1994; Vol. 10(2):223 (abstract no. PB0907).
Umar 1998 {published data only}
- Umar S, Feleke G, Roginsky MS, Schaeffer P. Effect of dehydroepiandosterone (DHEA) on clinical and laboratory parameters in female patients with AIDS (FPWA). Int Conf AIDS. 1998; Vol. 12:848 (Abstract no. 42373).
Urbina 2004 {published data only}
- Urbina A, Miller M, Hance I. Oxymetholone as therapy to maintain body composition in HIV positive men. Int Conf AIDS. 2004:Abstract no. B12432.
Van Loan 1999 {published data only}
- Loan MD, Strawford A, Jacob M, Hellerstein M. Monitoring changes in fat‐free mass in HIV‐positive men with hypotestosteronemia and AIDS wasting syndrome treated with gonadal hormone replacement therapy. AIDS 1999;13:241‐8. [DOI] [PubMed] [Google Scholar]
Vergel 1998 {published data only}
- Vergel NR, Salvato P, Mooney M. Anabolic steroids, resistance exercise and protein supplementation effect on lean body mass in HIV+ patients. Int Conf AIDS. 1998; Vol. 12:557 (Abstract no. 32185).
Wagner 1998a {published data only}
- Wagner GJ, Rabkin JG, Rabkin R. Testosterone as treatment for fatigue in HIV+ men. Gen Hosp Psych 1998;20(4):209‐13. [DOI] [PubMed] [Google Scholar]
Wagner 1998b {published data only}
- Wagner GJ, Rabkin JG. Testosterone therapy for clinical symptoms of hypogonadism in eugonadal men with AIDS. Int J STD AIDS 1998;9(1):41‐44. [DOI] [PubMed] [Google Scholar]
Wagner 1998c {published data only}
- Wagner G, Rabkin J, Rabkin R. Exercise as a mediator of psychological and nutritional effects of testosterone therapy in HIV+ men. Medicine & Science in Sports and Exercise 1998;30(6):811‐17. [DOI] [PubMed] [Google Scholar]
Wagner 1999 {published data only}
- Wagner GJ, Rabkin JG, Rabkin R. Effects of testosterone on weight and body composition in men with human immunodeficiency virus‐related weight loss. Nutr Res 1999;19(2):227‐33. [Google Scholar]
Wheeler 1998 {published data only}
- Wheeler S, Haight M, Heller LS, Loro ML, Kaufman F, Salata C, Church JA. Effects of oxandrolone on malnutition in HIV‐infected children. Int Conf AIDS 1998;12:35:Abstract no. 12120. [Google Scholar]
Additional references
Bhasin 1997
- Bhasin S, Storer T, Berman N, Yarasheski KE, Clevenger B, Philips J, et al. Testosterone replacement increases fat‐free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 1997;82:407‐13. [DOI] [PubMed] [Google Scholar]
Bhasin 1999
- Bhasin S, Javanbakht M. Can androgen therapy replete lean body mass and improve muscle function in wasting associated with human immunodeficiency virus infection. Jpen 1999;23:S195‐201. [DOI] [PubMed] [Google Scholar]
Brodsky 1996
- Brodsky I, Balagopal P, Nair K. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men ‐ a clinical research center study. J Clin Endocrinol Metab 1996;81:3469‐75. [DOI] [PubMed] [Google Scholar]
Corcoran 1999
- Corcoran C, Grinspoon S. Drug therapy: treatments for wasting in patients with the acquired immunodeficiency syndrome. New Engl J Med 1999;340:1740‐50. [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Higgins JPT, Altman DG, eds. Analysing and presenting results. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]; Section 8.8.1. In: The Cochrane Library, Issue 3. Chichester UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
Dworkin 2003
- Dworkin MS, Williamson JM, and the Adult/Adolescent Spectrum of HIV Disease Project. AIDS wasting syndrome: trends, influence on opportunistic infections, and survival. J Acquir Immune Defic Syndr 2003;33:267‐73. [DOI] [PubMed] [Google Scholar]
Grinspoon 1996
- Grinspoon S, Corcoran C, Lee K, Burrows B, Hubbard J, Katznelson L, et al. Loss of lean body mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab 1996;81:4051‐8. [DOI] [PubMed] [Google Scholar]
Grinspoon 1997
- Grinspoon S, Corcoran C, Miller K, Biller BM, Askari H, Wang E, et al. Body composition and endocrine function in women with acquired immunodeficieny syndrome wasting. J Clin Endocrinol Metab 1997;82:1332‐7. [DOI] [PubMed] [Google Scholar]
Haynes 1994
- Haynes RB, Wilczynski N, McKibbon KA, Walker CJ, Sinclair JC. Developing optimal search strategies for detecting clinically sound studies in MEDLINE. J Am Med Inform Assoc 1994;1:447‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clin Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kashkin 1989
- Kashkin KB, Kleber HD. Hooked on hormones? An anabolic steroid addiction hypothesis. JAMA 1989;262:3166‐70. [DOI] [PubMed] [Google Scholar]
Mulligan 1999
- Mulligan K, Schambelan M. Wasting. In: Cohen PT, Sande MA, Volberding PA editor(s). The AIDS Knowledge Base. 3rd Edition. Philadelphia: Lippincott Williams & Wilkins, 1999:403‐13. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: Dimensions of methodological qualtiy associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Smit 2002
- Smit E, Skolasky RL, Dobs A, Calhoun BC, Visscher BR, Palella FJ, Jacobson LP. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987‐1999. Am J Epidemiol 2002;156:211‐8. [DOI] [PubMed] [Google Scholar]
Snyder 2001
- Snyder PJ. Androgens. In: Hardman JG, Limbird LE, Gillman AG editor(s). Goodman and Gillman's the pharmacological basis of therapeutics. 10th Edition. New York: McGraw‐Hill, 2001:1635‐1648. [Google Scholar]
Wang 1992
- Wang ZM, Pierson RN, Heymsfield SB. The five‐level model: a new approach to organizing body‐composition research. Am J Clin Nutr 1992;56:19‐28. [DOI] [PubMed] [Google Scholar]
Wanke 2000
- Wanke CA, Silva M, Knox TA, Forrester J, Speigelman D, Gorbach SL. Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clin Infect Dis 2000;31:803‐5. [DOI] [PubMed] [Google Scholar]


