Abstract
Psychological stress significantly impacts colorectal cancer (CRC), and chronic stress is known to decrease treatment efficacy and survival rates in patients with CRC. Previous studies have linked psychological stress to changes in the gut microbiota, and the role of the microbiota in CRC progression is well characterized. Despite this, the mechanistic link between chronic stress and CRC remains unclear. In this issue of Cancer Research, Cao and colleagues reveal that chronic stress exacerbates CRC progression by reducing the presence of Lactobacillus johnsonii (L. johnsonii) and its metabolite protocatechuic acid (PCA). The authors demonstrate an increase in β-catenin expression as the major mechanism by which chronic stress potentiates cancer stemness and pathogenesis. Administration of L. johnsonii or PCA to stressed mice decreased β-catenin activity and CRC progression. This study defines a precise mechanism underlying chronic stress and CRC progression, emphasizing the relevance of psychological well-being in CRC outcome. Additionally, the study demonstrates the potential efficacy of L. johnsonii or PCA supplementation as promising therapeutics for CRC treatment.
Chronic stress stands as a significant risk factor for various conditions, like cancer, cardiovascular diseases, and premature mortality. Colorectal cancer (CRC) ranks third in cancer-related deaths, with notable modifiable risk factors, such as smoking, alcohol consumption, and obesity. Among these, depression and chronic stress are known to exacerbate CRC progression, yet the precise mechanisms remain elusive. Understanding how psychological disorders influence CRC progression is crucial, particularly as up to 57% of patients exhibit clinically significant depression (1).
The gut-brain axis signifies a heterocellular interaction between the intestine, microbiota, and the central nervous system. Studies have observed individuals with depression exhibiting dysbiosis, marked by elevated levels of Firmicutes, Bacteroides, and Actinobacteria (2). Furthermore, the microbiome plays a substantial role in overall physical health and systemic inflammation. Extensive research underscores the influence of the microbiome on gastrointestinal cancers, including CRC (3). Notably, distinct microbiota have been observed to play roles across different stages of CRC (4). Fusobacterium nucleatum has been associated with CRC, influencing various pathways that ultimately promote metastasis. Other species, such as Lactobacillus gallinarum and Lactobacillus reuteri, exhibit inhibitory or protective effects against CRC progression, respectively (5, 6).
The significance of the work by Cao and colleagues in this issue of Cancer Research lies in its pioneering effort to delineate the mechanistic connection between stress, the gut microbiota, and tumorigenesis (7). Using inflammation induced and sporadic CRC mouse models in addition to chronic restraint stress, the authors observed elevated levels of circulating cortisol and norepinephrine accompanied by increased depressive behaviors in chronically stressed mice. These findings strongly suggest that chronic stress alters the psychological state of these mice. Remarkably, mice with chronic restraint stress exhibited significantly increased tumor burden, indicating an exacerbation of CRC progression. Analysis revealed stress-induced heightened levels of β-catenin and other stemness markers in tumor tissues and in crypt-derived organoids, implying that chronic stress amplifies cancer cell stemness.
Exploring the underlying mechanisms, the authors noted a decrease in microbial diversity in the gut of chronically stressed mice compared to controls. Treating mice with antibiotics reversed the effects of chronic stress in models of CRC. This suggests that chronic stress drives CRC progression by altering the gut microbiota. Additionally, 16S rRNA analysis indicated significantly reduced Lactobacillus johnsonii (L. johnsonii) levels in mice specifically following chronic stress. Supplementation with L. johnsonii reduced tumor growth induced by chronic stress and reduced the expression of β-catenin and other stemness markers.
The communication between microbes and host cells has been an active area of research. The best- characterized mechanisms involve direct activation of pattern recognition receptors, such as toll-like receptors, which bind microbial antigens. However, recent research has highlighted that microbes can use dietary nutrients to generate a large compendium of novel metabolites that profoundly impact host cells. The metabolomics analysis performed by Cao and colleagues revealed a significant reduction in protocatechuic acid (PCA) levels in colon tissue from mouse models of CRC subjected to chronic stress (7). Subsequent experiments confirmed that L. johnsonii produces PCA and treating tumor organoids with PCA notably inhibited their growth while lowering the expression of β-catenin. Moreover, supplementing with PCA inhibited stress-induced CRC growth in mice, correlating with decreased β-catenin expression. These findings, alongside earlier research, suggest that PCA impedes stress-induced CRC progression by blocking the activation of β-catenin.
Transcriptome and metabolome analyses to define the mechanistic link between chronic stress and β-catenin unveiled decreased cyclic guanosine monophosphate kinase G (cGMP/PKG) signaling. Typically, this pathway promotes β-catenin degradation, suggesting that chronic stress hampers cGMP levels and thereby leads to increased β-catenin expression. However, supplementation with PCA or L. johnsonii restored cGMP levels, indicating a negative regulatory axis between PCA, cGMP, and β-catenin expression (Figure 1).
Figure 1. Chronic stress induced dysbiosis increases colorectal cancer.
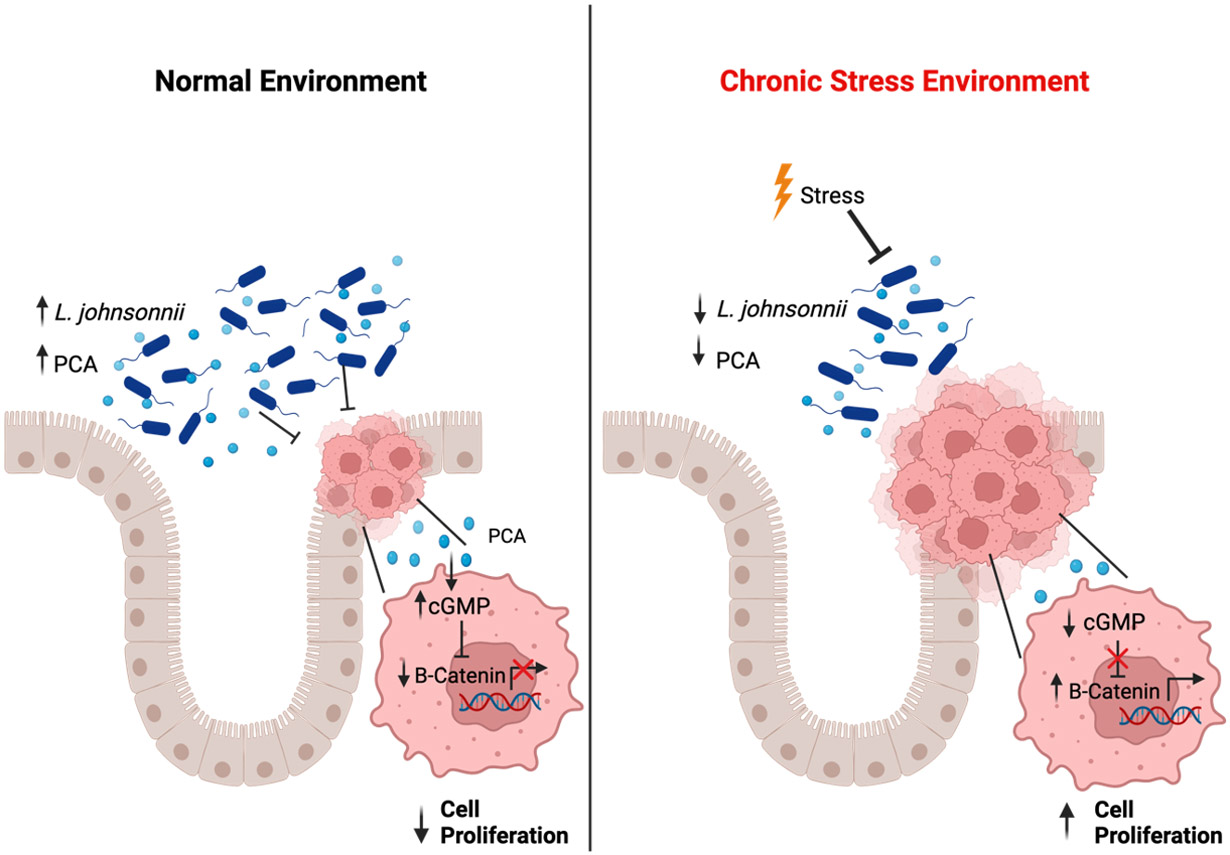
Chronic stress decreases levels of Lactobacillus johnsonii (L. johnsonii) and its beneficial metabolite protocatechuic acid (PCA). Under normal conditions, PCA activates cyclic guanosine monophosphate kinase G (cGMP/PKG) signaling, leading to degradation of β-catenin and decreased cellular proliferation. Under chronic stress, PCA is depleted, leading to less cGMP signaling and increased β-catenin expression that promotes cellular proliferation and tumor growth.
Several questions arise from these findings. How does chronic stress induce dysbiosis and specifically reduce L. johnsonii levels? While intestinal levels of cortisol and norepinephrine are not significantly increased in chronically stressed mice, circulating serum levels were significantly higher, and even transient exposure to stress hormones may alter gut microbiota composition. Stress in rodents has been shown to quickly change the microbiota composition, and in vitro work showed a significant increase in bacterial levels after catecholamine exposure (8). Cortisol and mast cells have been shown to increase “leaky gut,” leading to further inflammation as bacteria enter circulation (9). Additionally, examining the impact of various stressors, such as overcrowding, food insecurity, and impoverished environments, which simulate chronic stress in mouse models, may enhance the generalizability of these findings and provide further insights into the downstream mechanisms of stress and dysbiosis.
β-catenin is typically highly active in CRC due to mutations either in the tumor suppressor APC or through direct activating mutations in β-catenin. The authors have demonstrated that chronic stress further activated β-catenin but that treatments involving PCA or L. johnsonii could suppress β-catenin (7). This suggests that chronic stress amplifies β-catenin activity beyond the mutations observed in CRC. However, it remains unclear whether chronic stress-induced activation of β-catenin predisposes individuals to cancer. Additionally, dysregulation of the β-catenin pathway is observed in various cancers. Chronic stress is known to have a broad impact and other future directions could involve assessing different cancer models to determine whether chronic stress-linked microbial changes extend to peripheral cancers distant from gut microbiota. This exploration could shed light on the broader implications of gut dysbiosis and chronic stress in cancer development.
The primary tumor model assessed by Cao and colleagues involved administering the carcinogen azoxymethane followed by inflammatory injury using dextran sulfate sodium. This model replicates tumors commonly observed in patients with inflammatory bowel diseases. Although the phenotype was confirmed in a sporadic model of CRC driven by APC mutations, both models mainly lead to adenomas. More advanced models of CRC could provide a broader perspective on the impact of chronic stress on tumorigenesis. Moreover, inflammatory bowel disease represents a chronic, relapsing condition affecting the intestine with two main subtypes: Crohn’s disease and ulcerative colitis. While the exact cause of this disease remains not entirely understood, microbes and dysbiosis play central roles in its progression. Furthermore, chronic stress induces flares and exacerbates disease severity in inflammatory bowel disease (10). The observed body weight loss in mice indicates heightened intestinal injury following chronic stress. Assessing changes in L. johnsonii and investigating the effects of L. johnsonii on patients with inflammatory bowel disease could offer deeper insights into other potential diseases where L. johnsonii and PCA could be efficacious.
Lastly, daily administration of L. johnsonii or PCA was necessary due to the selective loss of this microbe in mice with CRC. Identifying microbes present at high levels in the tumor and capable of producing PCA could enhance efficacy of the treatment and reduce the required dosing. There are likely numerous other probiotic or commensal bacteria with the ability to produce anti-tumor metabolites like PCA. Employing metatranscriptomics could help identify additional candidates responsible for generating PCA. Additionally, defining the metabolic pathways through which PCA is generated might pave the way for dietary interventions that could sustain high levels of PCA.
While considerable knowledge exists regarding the ability of the microbiome to influence disease, the underlying mechanisms remain elusive. Cao et al. have identified a specific bacterial species (L. johnsonii) and its metabolite (PCA) that are significantly depleted in the gut during chronic stress. Administering either L. johnsonii or PCA decreased tumor growth in mouse models of chronic stress-induced CRC. This study holds significant translational implications, as both L. johnsonii and PCA supplementation could be explored as potential therapeutic targets for CRC.
Acknowledgements
This work was funded by NIH grants: R01CA148828, R01CA245546, and R01DK095201 (Y. M. S.). S.E.M. was supported by a NIH Cellular and Molecular Biology Training Grant T32-GM145470.
References
- 1.Peng Y-N, Huang M-L, Kao C-H. Prevalence of Depression and Anxiety in Colorectal Cancer Patients: A Literature Review. Int J Environ Res Public Health 2019;16:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016;21:786–96. [DOI] [PubMed] [Google Scholar]
- 3.Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol 2019;16:690–704. [DOI] [PubMed] [Google Scholar]
- 4.Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med 2019;25:968–76. [DOI] [PubMed] [Google Scholar]
- 5.Sugimura N, Li Q, Chu ESH, Lau HCH, Fong W, Liu W, et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 2022; 71:2011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell HN, Rebernick RJ, Goyert J, Singhal R, Kuljanin M, Kerk SA, et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 2022;40:185–200.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Q, Zhao M, Su Y, Liu S, Lin Y, Da H, et al. Chronic stress dampens Lactobacillus johnsonii-mediated tumor suppression to enhance colorectal cancer progression. Cancer Res [DOI] [PubMed] [Google Scholar]
- 8.Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock 2002;18:465–70. [DOI] [PubMed] [Google Scholar]
- 9.Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014;63:1293–9. [DOI] [PubMed] [Google Scholar]
- 10.Ge L, Liu S, Li S, Yang J, Hu G, Xu C, et al. Psychological stress in inflammatory bowel disease: Psychoneuroimmunological insights into bidirectional gut-brain communications. Front Immunol 2022;13:1016578. [DOI] [PMC free article] [PubMed] [Google Scholar]


