Abstract
Advancements in breast cancer care have significantly improved survival rates in women of all ages; however, young survivors face unique challenges related to fertility, pregnancy, and maternal-fetal health which may impact on their medical and psychosocial outcomes. Increasingly, young women are diagnosed with breast cancer before completing their reproductive plans and goals and may undergo gonadotoxic therapies and prolonged endocrine therapy with age-related fertility decline. Pretreatment counseling is critical to manage expectations, align reproductive and treatment goals, plan future childbearing opportunities, and refer for fertility preservation interventions when needed. Evidence supports the safety of pregnancy and breastfeeding in posttreatment scenarios, with individual risks and treatment histories carefully evaluated. The growing use of novel agents like CDK4/6 inhibitors, poly-ADP-ribose polymerase inhibitors, and immune checkpoint inhibitors for which there are little to no data regarding impact on fertility highlights the urgent need for further research in this area. Patient-centered, multidisciplinary approaches applied throughout the disease trajectory remain essential to support the reproductive health and overall quality of life of young breast cancer survivors navigating the reproductive complexities of modern breast cancer treatment.
Keywords: young women, breast cancer, gonadotoxicity, fertility, pregnancy
Introduction
The proportion of women diagnosed with breast cancer before completing their reproductive plans is increasing, mostly driven by the rising age at first pregnancy.1–3 Approximately, half of young patients diagnosed with breast cancer under 40 years of age report infertility concerns following anticancer treatments. 1 Premature ovarian insufficiency (POI) and subsequent impaired fertility, known long-term side effects of anticancer treatments, as well as treatment-related delays or postponement of childbearing are particularly concerning among young breast cancer survivors.4,5
Standard (neo)adjuvant chemotherapy regimens for early breast cancer often include gonadotoxic agents such as anthracyclines, alkylating agents, and platinum salts, all of which are associated with POI risk.6,7 Up to one-third of women under 40 years of age will experience treatment-related amenorrhea (TRA) following therapy. 8 Patient-related factors such as age, pre-treatment ovarian reserve, and the presence of a germline BRCA1/2 genetic pathogenic variant (gBRCApv) are associated with an increased risk of treatment-induced gonadotoxicity.9,10 Additionally, most patients receive adjuvant endocrine therapy (i.e., tamoxifen, aromatase inhibitors, and/or gonadotropin-releasing hormone (GnRH) agonists). Although these chronic treatments are not directly gonadotoxic, pregnancy is contraindicated during the standard 5–10-year course due teratogenic risk and ovarian function suppression, further delaying pregnancy and exposing women to age-related fertility decline. Similarly, due to fetal risks, adjuvant HER2-targeted therapies require delaying pregnancy for at least 1 year. Emerging agents such as cyclin-dependent kinase (CDK) 4/6 inhibitors, poly-ADP-ribose polymerase inhibitors (PARPi), and immune checkpoint inhibitors (ICI) have been incorporated into (neo)adjuvant regimens, but their effects on fertility and pregnancy outcomes remain insufficiently studied; thus, pregnancy is also contraindicated during these treatments.7,11
Young breast cancer survivors are less likely to experience a subsequent pregnancy compared to women in the general population.12–14 A recent population-based retrospective study reported birth rates among young breast cancer survivors to be half those expected in the general population (0.49 observed-to-expected ratio; 95% CI 0.47–0.52). 15 Even when compared to other young-onset cancer survivors, those with breast cancer have lower odds of conceiving than survivors of most other cancers. 14 Odds are particularly low among women exposed to adjuvant endocrine therapy, despite the fact that it does not appear to impair ovarian reserve directly.16,17 Fertility concerns can negatively affect quality of life, 3 influence treatment decisions and adherence, and potentially impact long-term disease outcomes. 18 Infertility resulting from anticancer treatment may also be associated with psychological distress, detrimentally affecting the well-being of young survivors.19,20 Fertility counseling, including consideration of fertility preservation and evaluation of postdiagnosis pregnancy feasibility, should be offered before initiating systemic therapy and as needed during follow-up for premenopausal patients of childbearing age.9,10,21,22 Counseling should encompass pretreatment fertility evaluation, family planning goals, treatment implications for fertility and childbearing, breast cancer prognosis, appropriate fertility preservation interventions, and timing of potential future pregnancy.23,24
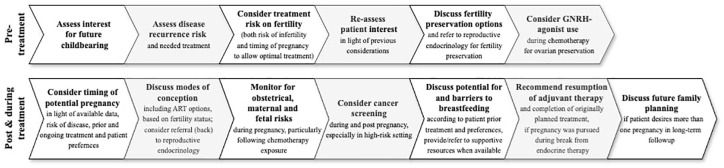
This narrative review synthesizes current evidence and emerging insights on fertility, pregnancy, and reproductive health in young breast cancer survivors. Given the increasing number of young women affected and the evolving landscape of cancer therapies, this review highlights the importance of individualized fertility counseling, thoughtful treatment planning, and ongoing research to optimize survivorship care and quality of life. A summarizing overview of key pregnancy-related considerations before and after breast cancer treatment is illustrated in Figure 1.
Figure 1.
Pregnancy-related considerations before and after breast cancer treatment.
Gonadotoxicity of breast cancer therapies
Pathogenesis and risk factors
POI is characterized by the loss of normal ovarian function before the age of 40, leading to hypoestrogenisn and amenorrhea. Underlying mechanisms are multifactorial, including genetic, autoimmune, and iatrogenic causes, such as cytotoxic agents and radiotherapy.25,26 Age at the time of treatment is a major factor in the evaluation of the risk for treatment induced gonadotoxicity: the younger the patient, the lower the risk of developing POI with the same treatment, due to larger promordial follicle stockpile.27,28 The presence of pathogenic variants in the BRCA1/2 genes may also influence the risk of treatment-induced POI, due to the role of these genes in the repair of DNA double-strand breaks.29–31
The consequences of POI to cancer survivors are significant, encompassing infertility, increased cardiovascular risk, and osteoperosis due to prolonged hypoestrogenism.24,32,33 Moreover, POI can exacerbate menopausal symptoms such as vasomotor instability sexual dysfuntion, and psychological distress, which can further impact the quality of life in these patients.34,35
Cytotoxic agents
Standard chemotherapy for early breast cancer often includes anthracycline- and/or taxane-based regimens. These agents may lead to gonadal failure through the accelerated and premature depletion of germ cells in the ovaries, through toxic insults to primordial follicle oocytes. 36 Additionally, indirect damage may be caused by impairment of the ovarian microenvironment and microvasculature altering and reducing the ovarian blood supply to the germ cells. 36
In a meta-analysis evaluating chemotherapy-induced amenorrhea (CIA) in premenopausal breast cancer patients, cyclophosphamide showed the highest gonadotoxic potential, more than doubling CIA likelihood (OR 2.25; 95% CI 1.26–4.03, p = 0.006). 6 Anthracyclines and taxane-based regiments also increased CIA risk, with pooled ORs of 1.39 (95% CI 1.15–1.70, p = 0.0008) and 1.24 (95% CI 1.03–1.50, p = 0.02), respectively. 6 These significant associations persisted regardless of variable CIA definitions across studies (e.g., >6 months or <3 months without menstruation). 6 Platinum-based regimens, increasingly used in the treatment of early-stage triple-negative breast cancer (TNBC) and HER2-positive breast cancer,26,37 lack robust data on their long-term impact on ovarian reserve and fertility. 7 Of concern, in the Young Women’s Breast Cancer Study (YWS), 1-year CIA rates associated with docetaxel–carboplatin–trastuzumab (TCH) were numerically higher compared to other routinely used chemotherapy regimens. 8
Anti-HER2 agents
Anti-HER2 monoclonal antibodies (i.e., trastuzumab, pertuzumab) are routinely used in early HER2-positive breast cancer, usually in combination with chemotherapy. In an early study by Abusief et al., the addition of trastuzumab to chemotherapy was not found to increase the likelihood of TRA. 38 In a large meta-analysis, Lambertini et al. found similar rates of CIA after chemotherapy combined with either trastuzumab, lapatinib, or the combination. 39 These data as well as the lack of a clear biologically plausible mechanism for gonadotoxicity are reassuring concerning the potential impact of these agents on CIA. Antibody-drug conjugates, increasingly used in the treatment of early HER2-positive breast cancer, have not been well studied to date regarding CIA outcomes despite their evolving role in early-stage breast cancer. 40 In the adjuvant ATEMPT trial, CIA was lower following a year of single-agent Trastuzumab emtansine (T-DM1)compared to the control arm of paclitaxel for 12 weeks and trastuzumab for 1 year (24% vs 50%, respectively; p = 0.045) suggesting the impact of T-DM1 alone on CIA is likely to be limited. 41
Cyclin-dependent kinase 4/6 inhibitors
For women with hormone receptor-positive (HR-positive) breast cancer at high risk for recurrence, the addition of CDK4/6 inhibitors, abemaciclib for 2 years, or ribociclib for 3 years, to adjuvant endocrine therapy improve long-term outcomes.42,43 Data regarding the impact of these agents on healthy ovarian cells are limited. As inhibitors of key cell-cycle regulators, CDK4/6 inhibitors may affect ovarian primordial follicles with potential associated gonadotoxicity. 44 In a subanalysis of the PENELOPE-B study the addition of palbociclib to endocrine therapy did not seem to impact estradiol, Follicle-stimulating hormone (FSH), or anti-Mullerian hormone levels in premenopausal women following chemotherapy. 45 While encouraging, additional data are needed to understand the impact of these agents on young women for whom they are increasingly being recommended.
Poly ADP ribose polymerase inhibitors
For patients with high-risk HER2-negative breast cancer carrying gBRCApv, 1 year of olaparib, a PARPi, has been shown to improve long-term outcomes. 8 In a preclinical murine study, exposure to olaparib significantly depleted primordial oocyte follicles. 46 Clinical reports regarding PARPi gonadotoxicity are sparse. In one study, among 20 gBRCApv positive patients treated with 6 months of neoadjuvant talazoparib, none reported a change in their menstrual cycles during treatment. 47 Most recently, Garber et al reported on-study pregnancies and outcomes among participants in the OLYMPIA trial. Following 1 year of adjuvant olaparib, 4.5% reported a pregnancy compared to 4.4% in the control arm in long-term follow-up, and most were born at full-term. 48
Immune checkpoint inhibitors
ICIs are increasingly used for early-stage TNBC49,50 and may also benefit HR-positive disease.51,52 Information on the impact of ICIs on fertility and pregnancy outcomes is sparse, despite their rapid adoption for multiple oncologic indications. In a cohort study of 3558 reports, exposure to ICI during pregnancy (91 reports) was not associated with specific adverse pregnancy, fetal, or newborn outcomes, compared to other anticancer treatments (3467 reports). 53 Similarly, no signal of disproportionate reporting of spontaneous abortion, fetal growth restriction, and prematurity was detected in a review of 56 safety reports involving ICI exposure during the peri-pregnancy period and filed in the World Health Organization’s spontaneous reporting system. 54 However, a case of severe immune-related gastroenterocolitis following in utero exposure to pembrolizumab, has been described. 55 Preclinically, ICIs enhance immune cell infiltration and tumor necrosis factor-α expression in the ovary. 56 These observations may indicate a negative impact of ICIs both on present and future fertility, as well as on immune-related adverse events in offsprings, highlighting the need for great caution when considering these agents during pregnancy. Further research in women receiving these agents is needed. 23
Endocrine therapy
Adjuvant endocrine therapy is recommended to most patients with HR-positive breast cancer. Although several studies have shown that endocrine therapy does not have a direct gonadotoxic effect, 57 pregnancy is unfeasible or contraindicated during the time it takes to receive it due to risk of teratogenicity. The resulting necessity to postpone pregnancy to complete 5–10 years of standard endocrine therapy exposes women to the inevitable physiologic age-related decline of ovarian reserve.
The POSITIVE trial designed to evaluate the temporary interruption of adjuvant endocrine therapy to attempt pregnancy in breast cancer survivors, 58 provided insight on the risk of POI following adjuvant endocrine therapy. Of 518 women aged ⩽42 years at inclusion, 273 patients (52.7%) reported amenorrhea at enrolment, with most (85%) treated with a GnRH agonist. Within 12 months, 94% resumed menses, and on multivariable analysis, no variable including age, chemotherapy receipt, or type of endocrine therapy was associated with menses recovery in this select clinical trial population. 58 These findings suggest only limited impact of modern chemotherapy on menses in very young premenopausal women in follow-up. Despite menses resumption however, 43% of women enrolled required some form of assisted reproductive technologies (ART) procedures to conceive and given the rapidly changing breast cancer treatment landscape including in the adjuvant setting for young women, our limited knowledge of impact of modern therapies on fertility remains a concern.
Pretreatment fertility preservation
Controlled ovarian stimulation (COS) for oocyte/embryo cryopreservation and to a much lesser extent, ovarian tissue cryopreservation (OTC), are the principal techniques to preserve fertility prior to the start of cancer treatment. 23 COS is indicated when sufficient time exists before the start of anticancer treatments, given it requires at least 2 weeks for adequate oocyte collection.9,10,59 OTC does not require COS and therefore is an option for prepubertal girls/pre-menarche adolescents or patients with oncological urgency for which treatment must start imediately.9,10
Several COS strategies are available (with tamoxifen/gonadotropin with letrozole/gonadotropin only) resulting in overall similar rates of oocyte retrieval and potential management of hormonal levels. Pregnancy rates using cryopreserved oocytes and embryos from cancer survivors are similar to those of women undergoing elective fertility preservation, 60 with OTC, around 200 live births have been reported. 61 A 37% (95% CI 32–43) pregnancy rate has been reported with OTC and a live birth rate of 28% (95% CI 24–34), including pregnancies naturally achieved as well as through in vitro fertilization (IVF). 62
Cotreatment with a GnRH agonist during cytotoxic chemotherapy can help preserve ovarian function following chemotherapy, reduce the risk of early menopause, and improve prospects of fertility. In a meta-analysis investigating the efficacy and safety of this strategy, including patient-level data from five major clinical trials, POI rate was 14.1% in the GnRH agonists group and 30.9% in the control group (aOR 0.38; 95% CI 0.26–0.57; p < 0.001). Although data on attempts to conceive were unavailable, 10.3% of patients treated with GnRH agonists had at least one posttreatment pregnancy, compared to 5.5% in the control group (p = 0.030). 16 The role of GnRH agonist cotreatment for ovarian function preservation during treatment with newer targeted therapies is unclear.
Breast cancer outcomes following postdiagnosis pregnancies
Many studies over the years have addressed the safety of pregnancy after breast cancer. In a recent large meta-analysis, 14 breast cancer survivors who achieved a posttreatment pregnancy demonstrated superior disease-free survival (DFS; HR 0.66; 95% CI 0.49–0.89) and overall survival (OS; HR 0.56; 95% CI 0.45–0.68), compared to those without subsequent pregnancy. Pregnancy in breast cancer survivors was not associated with detrimental outcomes, regardless of prior tumor characteristics including hormone-receptor status, previous treatment, pregnancy outcome, time to pregnancy from breast cancer diagnosis, or BRCA status. However, concerns have remained given the retrospective nature of most of the studies and the “healthy mother effect” whereby healthier women with a more favorable prognosis may be more inclined to opt to conceive and receive support to do so. 63 Additionally, a “guarantee-time” bias may arise in analyses of time-dependent outcomes like DFS when compared across groups defined by events occurring during follow-up. 64 Lambertini et al. 14 conducted a secondary analysis including only studies with DFS and/or OS data and tried to adjust for these biases and still demonstrated that breast cancer survivors with subsequent pregnancy showed better DFS and OS rates (HR 0.68; 95% CI 0.51–0.91 and HR 0.53; 95% CI 0.42–0.67, respectively).
Historically, the safety of pregnancy following HR-positive breast cancer has been called into question due to concerns about the potential effects of the pregnancy-related hormonal changes on risk of recurrence in the setting of a history of a hormonally driven tumor.65,66 Reassuringly, in a systematic review and meta-analysis of eight studies, including 3805 patients with HR-positive disease, Arecco et al. 67 found no detrimental effect of subsequent pregnancy on DFS (HR 0.96; 95% CI 0.75–1.24, p = 0.781) and OS (HR 0.46; 95% CI 0.27–0.77, p < 0.005). 13
The POSITIVE trial (ClinicalTrials.gov identifier: NCT02308085) is an international, prospective single-arm study designed to evaluate risk of recurrence among women with HR-positive breast cancer following a temporary interruption of endocrine therapy to attempt pregnancy. 68 Women included in the trial (⩽42 years) completed 18–30 months of adjuvant endocrine and temporarily interrupted endocrine therapy for up to 2 years to allow for conception, pregnancy, delivery, and breastfeeding. The initial treatment period was required given data showing that among premenopausal women with HR at least 3 months; even a short 2-year exposure to endocrine therapy provides a long-term survival benefit. 69 After a treatment interruption of up to 2 years, the protocol stipulates that patients resume and complete 5–10 years of endocrine therapy, with duration dependent on usual clinical factors and patient preference/tolerance. At 41 months follow-up from enrolment, participants in POSITIVE had no increased risk of breast cancer recurrence compared to an external control cohort. Longer-term follow-up of the POSITIVE cohort is critical, and the safety of this strategy in high-risk populations was not adequately tested given 93.4% had stages I or II disease. As might have been expected, the risk of recurrence in the patients on POSITIVE was associated with higher risk features (e.g., higher grade, positive lymph node status). While 73% of participants in the POSITIVE trial resumed endocrine therapy following an interruption to become pregnant, rates of real-world rates, outside of a clinical trial, may be lower. In a retrospective study California-based study, of women who become pregnant before completing 5 years of endocrine therapy, only 34% were receiving endocrine therapy by 2 years postdelivery and 48% by 10 years postdelivery. 70 These findings suggest that some women may extend the interruption of endocrine therapy beyond the 2-year period, guided by individual factors such as time to conception or broader family planning considerations. This underscores the importance of close oncologic follow-up to ensure patients understand the critical role of adjuvant endocrine therapy and receive support for its resumption. While the POSITIVE trial provides valuable data on the safety of a 2 year interruption of endocrine treatment, consequences of a longer interruption period remain unknown.
The safety of conception following breast cancer in the subset of patients who are gBRCApv carriers has also been a concern. 11 In a recent international retrospective study involving 4732 gBRCApv carriers, 71 among the 22% of carriers who conceived within 10 years after diagnosis, subsequent pregnancy was not associated with adverse maternal prognosis. However, in the subgroup of BRCA2 carriers (N = 170), an adverse association between pregnancy and DFS was observed (adjusted HR 1.55; 95% CI 1.12–2.16). Reproductive factors may have dissimilar effects on breast cancer risks associated with BRCA1 versus BRCA272–75 mutations, which may also differ from risks among non-gBRCApv carriers. Accordingly, additional research is necessary to offer optimal counseling for gBRCApv carriers, especially those with a BRCA2pv.
ART use following breast cancer
As breast cancer treatment may impair fertility, some patients may require ART to achieve pregnancy. Retrospective data have suggested safety14,76–78 and prospective data on the safety of ART have been reported from the POSITIVE trial. 27 ART modalities in POSITIVE included use of cryopreserved embryo(s) in patients who underwent embryo/oocyte cryopreservation before enrolment, and while on study, COS for IVF, intrauterine insemination, clomiphene use, embryo/egg donation, and ovarian tissue transplantation. Among 497 enrolled patients, 252 (51%) and 215 (43%) underwent some form of pretreatment fertility preservation and posttreatment ART procedures, respectively. Over a median follow-up of 41 months from enrolment, neither intervention detrimentally affected outcomes. Larger populations and longer follow-up to confirm these findings is warranted.
Maternofetal concerns
Most studies support the maternal safety of postdiagnosis pregnancies in breast cancer survivors. In a systematic review and meta-analysis, Lambertini et al. compared pregnancy and neonatal outcomes among 3240 survivors and the general population. 14 No significant differences were observed in terms of pregnancy completion, spontaneous and induced abortion, preeclampsia, or postpartum bleeding. However, breast cancer survivors exhibited a 14% increased risk of cesarean section (OR 1.14; 95% CI 1.04–1.25). Regarding neonatal outcomes, offspring of breast cancer survivors had increased risks of low birth weight (OR 1.50; 95% CI 1.31–1.73), preterm birth (OR 1.45; 96% CI 1.11–1.99), and being small for gestational age (OR 1.16; 95% CI 1.01–1.33) compared to offspring of women in the general population. These risks were primarily observed in women had previously received chemotherapy. No statistically significant differences in congenital abnormalities were found between the groups. Some analyses maybe have been limited by the heterogeneity inherent in retrospective observational studies, including differences in patient age groups, lack of differentiation between spontaneous and induced childbirth, and incomplete data regarding receipt of cancer treatment during pregnancy.
A recent extensive report from The Teenage and Young Adult Cancer Survivor Study (TYACSS)-a retrospective, population-based cohort of 200,945 five-year cancer survivors diagnosed between the ages of 15–39 in England and Wales and linked to the English Hospital Episode Statistics database examined the risk of 27 different obstetric complications among women who survived 1 of 17 different types of cancer. 15 Breast cancer survivors were not significantly more likely to experience preterm labor or birth, but did show an increased risk of unsuccessful induction of labor. However, in a sensitivity analysis, the standardized incidence ratio for preterm birth was consistent with the increased risk previously reported by Lambertini et al. Although rare, breast cancer survivors were found to have an increased risk of intrapartum hemorrhage (standardized incidence ratio of 1.57; 95% CI 1.03–2.41; total observations among breast cancer survivors: 0.5%). Births occurring within the first 9 months after cancer diagnosis were excluded, and risk estimates were stratified by maternal age. The reasons for these differences remain unclear.
Breastfeeding
Breastfeeding is associated with numerous benefits for both the infant and the mother and has been shown to improve the overall quality of life for breast cancer survivors of reproductive age.79,80 Despite these benefits and prior studies demonstrating that breastfeeding is safe for breast cancer survivors,79,81 medical counseling against breastfeeding and lack of support remains common barriers for this population. 82
In a recent survey of survivors who had a live birth following breast cancer treatment, 83 95% of women who had not undergone bilateral mastectomy reported attempting to breastfeed. While most were satisfied with their ability to breastfeed, only half reported receiving specific information about the practice from any source, and fewer than a quarter consulted with a lactation specialist, which might have been beneficial. 84
Breast cancer outcomes following breastfeeding were reported in the POSITIVE trial 85 : of 317 enrolled patients with at least one live birth, 313 did not have bilateral mastectomy and 196 breastfed (62.6%). Breast cancer-free interval at 24 months was 3.6% (95% CI 1.5–8.8) for survivors who breastfed and 3.1% (95% CI 1.0–9.5), for those who did not, supporting the safety of breastfeeding. For gBRCApv carriers, decisions regarding breastfeeding may be more complex due to considerations for risk-reducing mastectomy. In an international retrospective cohort study 86 including 474 young (⩽40 years at diagnosis) gBRCApv carriers with a live birth after breast cancer, 110 (23.2%) women reported breastfeeding, while 225 (47.45%) underwent risk-reducing mastectomy before delivery. With a median follow-up after delivery of 7 years (IQR 3.6–10.5), breastfeeding safety was reassuring, with no difference in locoregional recurrence or second primary breast cancer events between breastfeeding and nonbreastfeeding groups (HR = 1.08, p = 0.82).
Practical considerations
The optimal timing of conception after completion of cancer treatment is crucial for maternal and fetal safety. While the most well-controlled recent data do not demonstrate increased risk of recurrence or worsened survival from pregnancy after breast cancer, 11 some studies have shown an increased risk of disease relapse among women conceiving between 6 and 12 months after diagnosis.87,88 Other evidence suggests that patients conceiving within 2 years from diagnosis may experience improved DFS, compared to those without subsequent pregnancy. 81 In women previously exposed to chemotherapy, an increased risk of low birth weight, preterm birth, and small for gestational age has been observed, compared to the general population as noted previously. 14 This risk appears to be higher when conception occurs within the first year following cytotoxic therapy. 89 Therefore, it is generally recommended to wait at least 12 months after completing chemotherapy before attempting to conceive. Breast cancer treatment has evolved significantly over the past decade, and specific recommendations for time to wait to become pregnant after many cancer therapeutics are lacking. The US Food and Drug Administration offers general guidance recommending a minimum contraception period of 6 months plus the elimination of five half-lives after cessation of potentially teratogenic agents.9,90
For women with HR-positive breast cancer, plans for conception typically follow adjuvant endocrine therapy. Tamoxifen is potentially teratogenic and should be discontinued before attempting pregnancy.91–93 A conservative three-month washout period is generally recommended for all forms of endocrine therapy, though 68 shorter periods may also suffice: Tamoxifen has a 7-day half-life thus complete washout could be considered as soon as 35 days (5 half-lives). The impact of adding CDK4/6 inhibitors to endocrine therapy on subsequent pregnancy outcomes is unknown. These agents are contraindicated while attempting to conceive and during pregnancy. In the MonarcheE trial protocol, women were required to use effective contraception during the study and for 12 weeks following the last dose. 94
Patients with TNBC may be advised to wait 2–3 years after completing adjuvant therapy before trying to conceive primarily to avoid pregnancy during the highest risk of recurrence period. In current practice, many women with TNBC receive adjuvant therapy, including immunotherapy or PARPi. During immunotherapy, contraception is recommended for at least 4 months after termination of pembrolizumab and 5 months for atezolizumab. 95 Olaparib is contraindicated during pregnancy and per the OlympiA study protocol contraception was recommended for at least 1 month after the last dose. 96 Women receiving anti-HER2 therapies should use proper contraception during anti-HER2 treatment and for up to 7 months after completing trastuzumab. 97 The Dana–Farber Breast Oncology Program has developed consensus guidelines with washout recommendations for several of these newer agents. 98 Recommendations are summarized in Table 1.
Table 1.
Recommendations for treatment washout prior to pregnancy and breastfeeding and half-life elimination.
| Drug | Washout period recommended prior to pregnancy and breastfeeding | Half-life elimination time |
|---|---|---|
| Cytotoxic agents (chemotherapy) | 1 year following chemotherapy completion, due to potential teratogenicity | Differs by protocol |
| Tamoxifen | At least 3 months | 5–7 days; its active metabolite N-desmethyl tamoxifen has half-life elimination time of approximately 14 days |
| Letrozole | At least 3 months | 42 h |
| Trastuzumab, Pertuzumab, T-DM1 | At least 7 months | - Trastuzumab concentration decreases to ~3% (~97% washout) by 7 months following discontinuation - Pertuzumab: 18 days T-DM1: 4 days |
| Ribociclib | At least 3 weeks | 33–42 h |
| Abemaciclib | At least 3 weeks | 18.3 h |
| Olaparib | At least 1 months | 15 h |
| Pembrolizumab | At least 4 months; half-life elimination: 22 days | 22 days |
Source: Adapted from Dana–Farber Breast Oncology Program consensus guidelines. 98
Imaging follow-up during pregnancy
The female breast undergoes distinct physiological changes during pregnancy and lactation, which manifest as varied clinical and imaging findings. These changes pose challenges when considering breast surveillance or workup of new signs or symptoms concerning for local recurrence or new primary breast cancer in breast cancer survivors. 99 While mammogram, ultrasound and noncontrast MRI are considered safe during pregnancy, the benefits of screening during a pregnancy are unclear and ideally women considering pregnancy should have their screening studies prior to conception and after pregnancy. In gBRCApv carriers, whole breast ultrasound has been recommended as the screening modality of choice during pregnancy, 100 and this can be considered in high-risk survivors with remaining breast tissue as well.
For diagnostic workup in women who develop concerning signs or symptoms, breast ultrasound is the first-line imaging examination in pregnant or lactating women given its safety and efficacy in a predominantly young patient population where dense breast tissue may decrease the sensitivity of mammography. Ultrasound demonstrates high sensitivity for both malignant and benign abnormalities of the breast101,102 and suspicious finding on ultrasound can be further evaluated with ultrasound-guided tissue biopsy. Mammography, though not a first-line modality in this setting, is associated with a negligible fetal radiation dose and may serve as an adjunct to ultrasound when the cause of a palpable mass remains unclear,103,104 to evaluate disease extent or when suspicious calcifications are present after an abnormal ultrasound finding or diagnosis of local recurrence or new breast cancer. 99 Dynamic contrast-enhanced MRI is contraindicated during pregnancy, but is safe and beneficial for lactating patients with a recent breast cancer diagnosis when evaluating for disease. 105
Conclusion
While advances in breast cancer treatment have significantly improved survival rates, young survivors face unique challenges, particularly regarding fertility preservation, postdiagnosis pregnancies, and maternofetal health. A comprehensive understanding of the gonadotoxic effects of breast cancer therapies and their implications for reproductive outcomes is critical. Current evidence supports the safety of pregnancy and breastfeeding in many posttreatment scenarios, in consideration of patient preference and provided individual risks and treatment histories are carefully evaluated and managed during pregnancy.
The growing use of novel agents such as CDK4/6 inhibitors, PARPi, and ICIs highlights the urgent need to routinely collect fertility and pregnancy outcomes prospectively in seminal clinical trials and the importance of further research to refine fertility preservation strategies and optimize family planning counseling. Tailored, multidisciplinary approaches applied throughout the disease trajectory remain essential to support the reproductive health and overall quality of life of young breast cancer survivors.
Acknowledgments
None.
Footnotes
ORCID iD: Tal Sella  https://orcid.org/0000-0003-2030-9200
https://orcid.org/0000-0003-2030-9200
Contributor Information
Nadia Mordenfeld Kozlovsky, Department of Oncology, Sheba Medical Center, Ramat Gan, Israel.
Ann H. Partridge, Department of Medical Oncology, Program for Young Adults with Breast Cancer, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02115, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Tal Sella, Department of Oncology, Sheba Medical Center, Ramat Gan, Israel.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Nadia Mordenfeld Kozlovsky: Data curation; Investigation; Project administration; Visualization; Writing – original draft.
Ann H. Partridge: Investigation; Methodology; Supervision; Writing – review & editing.
Tal Sella: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
T.S. reports honorarium from Roche, Eli Lilly, Novartis, MSD, Gilead, Astrazeneca and Stemline, advisory from Novartis, Roche and travel support for Roche, Gilead, Oncotest, Pfizer, Stemline, and Astra-Zeneca. A.H.P. reports research support from Patient-Centered Outcomes Research Institute (AFT-25 COMET), Susan G. Komen, the Breast Cancer Research Foundation (BCRF) and the American Cancer Society (ACS); and royalties from Wolters Kluwer. NMK reports no relevant COI.
Availability of data and materials: Not applicable.
References
- 1. Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol 2014; 32(11): 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambertini M, Fontana V, Massarotti C, et al. Prospective study to optimize care and improve knowledge on ovarian function and/or fertility preservation in young breast cancer patients: results of the pilot phase of the PREgnancy and FERtility (PREFER) study. Breast 2018; 41: 51–56. [DOI] [PubMed] [Google Scholar]
- 3. Ruggeri M, Pagan E, Bagnardi V, et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European Centers. Breast 2019; 47: 85–92. [DOI] [PubMed] [Google Scholar]
- 4. Lambertini M, Goldrat O, Clatot F, et al. Controversies about fertility and pregnancy issues in young breast cancer patients: current state of the art. Curr Opin Oncol 2017; 29(4): 243–252. [DOI] [PubMed] [Google Scholar]
- 5. Poorvu PD, Frazier AL, Feraco AM, et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr 2019; 3(1): pkz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat 2014; 145(1): 113–128. [DOI] [PubMed] [Google Scholar]
- 7. Martelli V, Latocca MM, Ruelle T, et al. Comparing the gonadotoxicity of multiple breast cancer regimens: important understanding for managing breast cancer in pre-menopausal women. Breast Cancer Targets Ther 2021; 13: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poorvu PD, Hu J, Zheng Y, et al. Treatment-related amenorrhea in a modern, prospective cohort study of young women with breast cancer. NPJ Breast Cancer 2021; 7(1): 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lambertini M, Peccatori FA, Demeestere I, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2020; 31(12): 1664–1678. [DOI] [PubMed] [Google Scholar]
- 10. Anderson RA, Amant F, Braat D, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open 2020; 2020(4): hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perachino M, Poggio F, Arecco L, et al. Update on pregnancy following breast cancer diagnosis and treatment. Cancer J 2022; 28(3): 176–182. [DOI] [PubMed] [Google Scholar]
- 12. Stensheim H, Cvancarova M, Møller B, et al. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer 2011; 129(5): 1225–1236. [DOI] [PubMed] [Google Scholar]
- 13. Anderson RA, Brewster DH, Wood R, et al. The impact of cancer on subsequent chance of pregnancy: a population-based analysis. Hum Reprod 2018; 33(7): 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: a systematic review and meta-analysis. J Clin Oncol 2021; 39(29): 3293–3305. [DOI] [PubMed] [Google Scholar]
- 15. Sunguc C, Winter DL, Heymer EJ, et al. Risks of adverse obstetric outcomes among female survivors of adolescent and young adult cancer in England (TYACSS): a population-based, retrospective cohort study. Lancet Oncol 2024; 25: 1080–1091. [DOI] [PubMed] [Google Scholar]
- 16. Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient–level data. J Clin Oncol 2018; 36(19): 1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril 2010; 94(2): 638–644. [DOI] [PubMed] [Google Scholar]
- 18. Sella T, Poorvu PD, Ruddy KJ, et al. Impact of fertility concerns on endocrine therapy decisions in young breast cancer survivors. Cancer 2021; 127(16): 2888–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosen A, Rodriguez-Wallberg KA, Rosenzweig L. Psychosocial distress in young cancer survivors. Semin Oncol Nurs 2009; 25(4): 268–277. [DOI] [PubMed] [Google Scholar]
- 20. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology Recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24(18): 2917–2931. [DOI] [PubMed] [Google Scholar]
- 21. Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018; 36(19): 1994–2001. [DOI] [PubMed] [Google Scholar]
- 22. Paluch-Shimon S, Cardoso F, Partridge AH, et al. ESO–ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann Oncol 2022; 33(11): 1097–1118. [DOI] [PubMed] [Google Scholar]
- 23. Arecco L, Borea R, Magaton IM, et al. Current practices in oncofertility counseling: updated evidence on fertility preservation and post-treatment pregnancies in young women affected by early breast cancer. Expert Rev Anticancer Ther 2024; 24(9): 803–817. [DOI] [PubMed] [Google Scholar]
- 24. Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up 5 behalf of the ESMO Guidelines Committee. Ann Oncol 2024; 35: 159–182. [DOI] [PubMed] [Google Scholar]
- 25. Touraine P, Chabbert-Buffet N, Plu-Bureau G, et al. Premature ovarian insufficiency. Nat Rev Dis Primers 2024; 10(1): 63. [DOI] [PubMed] [Google Scholar]
- 26. Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (POI). Front Endocrinol (Lausanne) 2021; 12: 626924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Su HI, Haunschild C, Chung K, et al. Prechemotherapy antimullerian hormone, age, and body size predict timing of return of ovarian function in young breast cancer patients. Cancer 2014; 120(23): 3691–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silva C, Caramelo O, Almeida-Santos T, et al. Factors associated with ovarian function recovery after chemotherapy for breast cancer: a systematic review and meta-analysis. Hum Reprod 2016; 31(12): 2737–2749. [DOI] [PubMed] [Google Scholar]
- 29. Lambertini M, Goldrat O, Toss A, et al. Fertility and pregnancy issues in BRCA -mutated breast cancer patients. Cancer Treat Rev 2017; 59: 61–70. [DOI] [PubMed] [Google Scholar]
- 30. Vuković P, Peccatori FA, Massarotti C, et al. Preimplantation genetic testing for carriers of BRCA1/2 pathogenic variants. Crit Rev Oncol Hematol 2021; 157: 103201. [DOI] [PubMed] [Google Scholar]
- 31. Turan V, Oktay K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum Reprod Update 2020; 26(1): 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lambrinoudaki I, Paschou SA, Lumsden MA, et al. Premature ovarian insufficiency: a toolkit for the primary care physician. Maturitas 2021; 147: 53–63. [DOI] [PubMed] [Google Scholar]
- 33. Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc 2016; 91(11): 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Podfigurna-Stopa A, Czyzyk A, Grymowicz M, et al. Premature ovarian insufficiency: the context of long-term effects. J Endocrinol Invest 2016; 39(9): 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henze M, Stuckey BGA. Endocrine consequences of breast cancer therapy and survivorship. Climacteric 2024; 27(4): 333–339. [DOI] [PubMed] [Google Scholar]
- 36. Codacci-Pisanelli G, Del Pup L, Del Grande M, et al. Mechanisms of chemotherapy-induced ovarian damage in breast cancer patients. Crit Rev Oncol Hematol 2017; 113: 90–96. [DOI] [PubMed] [Google Scholar]
- 37. van der Voort A, van Ramshorst MS, van Werkhoven ED, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual ERBB2 blockade in patients with ERBB2-positive breast cancer. JAMA Oncol 2021; 7(7): 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abusief ME, Missmer SA, Ginsburg ES, et al. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer 2010; 116(4): 791–798. [DOI] [PubMed] [Google Scholar]
- 39. Lambertini M, Campbell C, Bines J, et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst 2019; 111(1): 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380(7): 617–628. [DOI] [PubMed] [Google Scholar]
- 41. Ruddy KJ, Zheng Y, Tayob N, et al. Chemotherapy-related amenorrhea (CRA) after adjuvant ado-trastuzumab emtansine (T-DM1) compared to paclitaxel in combination with trastuzumab (TH) (TBCRC033: ATEMPT Trial). Breast Cancer Res Treat 2021; 189(1): 103–110. [DOI] [PubMed] [Google Scholar]
- 42. Rastogi P, O’Shaughnessy J, Martin M, et al. Adjuvant abemaciclib plus endocrine therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative, high-risk early breast cancer: results from a Preplanned monarchE Overall Survival Interim Analysis, including 5-year efficacy outcomes. J Clin Oncol 2024; 42(9): 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slamon D, Lipatov O, Nowecki Z, et al. Ribociclib plus endocrine therapy in early breast cancer. N Engl J Med 2024; 390(12): 1080–1091. [DOI] [PubMed] [Google Scholar]
- 44. Scavone G, Ottonello S, Blondeaux E, et al. The role of cyclin-dependent kinases (CDK) 4/6 in the ovarian tissue and the possible effects of their exogenous inhibition. Cancers (Basel) 2023; 15(20): 4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Furlanetto J, Marmé F, Thode C, et al. 60MO Ovarian function in young patients (pts) treated with postneoadjuvant palbociclib (PAL) and endocrine therapy (ET) for hormone receptor (HR)-positive, HER2-negative early breast cancer (BC): explorative analysis in Penelope-B. Ann Oncol 2022; 33: S149–S150. [Google Scholar]
- 46. Winship AL, Griffiths M, Lliberos Requesens C, et al. The PARP inhibitor, olaparib, depletes the ovarian reserve in mice: implications for fertility preservation. Hum Reprod 2020; 35(8): 1864–1874. [DOI] [PubMed] [Google Scholar]
- 47. Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J Clin Oncol 2020; 38(5): 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garber J. GS1-09: OlympiA: a phase 3, multicenter, randomized, placebo-controlled trial of adjuvant olaparib after (neo)adjuvant chemotherapy in patients w/ germline BRCA1 & BRCA2 pathogenic variants & high-risk HER2-negative primary breast cancer: longer-term follow. Presented at SABCS 2024, 11 December 2024. [Google Scholar]
- 49. Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med 2022; 386(6): 556–567. [DOI] [PubMed] [Google Scholar]
- 50. Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020; 396(10257): 1090–1100. [DOI] [PubMed] [Google Scholar]
- 51. Cardoso F, McArthur HL, Schmid P, et al. LBA21 KEYNOTE-756: phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2– breast cancer. Ann Oncol 2023; 34: S1260–S1261. [Google Scholar]
- 52. Loi S, McArthur HL, Harbeck N, et al. A phase III trial of nivolumab with neoadjuvant chemotherapy and adjuvant endocrine therapy in ER+/HER2– primary breast cancer: CheckMate 7FL. J Clin Oncol 2020;38(15 Suppl): TPS604. [Google Scholar]
- 53. Gougis P, Hamy AS, Jochum F, et al. Immune checkpoint inhibitor use during pregnancy and outcomes in pregnant individuals and newborns. JAMA Netw Open 2024; 7(4): e245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Noseda R, Müller L, Bedussi F, et al. Immune checkpoint inhibitors and pregnancy: analysis of the VigiBase® Spontaneous Reporting System. Cancers (Basel) 2022; 15(1): 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baarslag MA, Heimovaara JH, Borgers JSW, et al. Severe immune-related enteritis after in utero exposure to pembrolizumab. N Engl J Med 2023; 389(19): 1790–1796. [DOI] [PubMed] [Google Scholar]
- 56. Winship AL, Alesi LR, Sant S, et al. Checkpoint inhibitor immunotherapy diminishes oocyte number and quality in mice. Nat Cancer 2022; 3(8): 1–13. [DOI] [PubMed] [Google Scholar]
- 57. Lambertini M, Olympios N, Lequesne J, et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-müllerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol 2019; 9: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Azim HA, Niman SM, Partridge AH, et al. Fertility preservation and assisted reproduction in patients with breast cancer interrupting adjuvant endocrine therapy to attempt pregnancy. J Clin Oncol 2024; 42: 2822–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boots CE, Meister M, Cooper AR, et al. Ovarian stimulation in the luteal phase: systematic review and meta-analysis. J Assist Reprod Genet 2016; 33(8): 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cobo A, García-Velasco J, Domingo J, et al. Elective and onco-fertility preservation: factors related to IVF outcomes. Hum Reprod 2018; 33(12): 2222–2231. [DOI] [PubMed] [Google Scholar]
- 61. Dueholm Hjorth IM, Kristensen SG, Dueholm M, et al. Reproductive outcomes after in vitro fertilization treatment in a cohort of Danish women transplanted with cryopreserved ovarian tissue. Fertil Steril 2020; 114(2): 379–387. [DOI] [PubMed] [Google Scholar]
- 62. Khattak H, Malhas R, Craciunas L, et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: a systematic review and individual patient data meta-analysis. Hum Reprod Update 2022; 28(3): 400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Valachis A, Tsali L, Pesce LL, et al. Safety of pregnancy after primary breast carcinoma in young women: a meta-analysis to overcome bias of healthy mother effect studies. Obstet Gynecol Surv 2010; 65(12): 786–793. [DOI] [PubMed] [Google Scholar]
- 64. Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013; 31(23): 2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lambertini M, Di Maio M, Pagani O, et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast 2018; 42: 41–49. [DOI] [PubMed] [Google Scholar]
- 66. Razeti MG, Spinaci S, Spagnolo F, et al. How I perform fertility preservation in breast cancer patients. ESMO Open 2021; 6(3): 100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arecco L, Blondeaux E, Bruzzone M, et al. Safety of pregnancy after breast cancer in young women with hormone receptor-positive disease: a systematic review and meta-analysis. ESMO Open 2023; 8(6): 1022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Partridge AH, Niman SM, Ruggeri M, et al. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med 2023; 388(18): 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ekholm M, Bendahl PO, Fernö M, et al. Two years of adjuvant tamoxifen provides a survival benefit compared with no systemic treatment in premenopausal patients with primary breast cancer: long-term follow-up (> 25 years) of the Phase III SBII:2pre Trial. J Clin Oncol 2016; 34(19): 2232–2238. [DOI] [PubMed] [Google Scholar]
- 70. Ransohoff JD, Lewinsohn RM, Dickerson J, et al. Endocrine therapy interruption, resumption, and outcomes associated with pregnancy after breast cancer. JAMA Oncol 2025; 11(4): 423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lambertini M, Blondeaux E, Agostinetto E, et al. Pregnancy after breast cancer in young brca carriers: an International Hospital-Based Cohort Study. JAMA 2024; 331(1): 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tryggvadottir L, Olafsdottir EJ, Gudlaugsdottir S, et al. BRCA2mutation carriers, reproductive factors and breast cancer risk. Breast Cancer Res 2003; 5(5): R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cullinane CA, Lubinski J, Neuhausen SL, et al. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer 2005; 117(6): 988–991. [DOI] [PubMed] [Google Scholar]
- 74. Pan H, He Z, Ling L, et al. Reproductive factors and breast cancer risk among BRCA1 or BRCA2 mutation carriers: results from ten studies. Cancer Epidemiol 2014; 38(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 75. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106(6): dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Arecco L, Blondeaux E, Bruzzone M, et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis. Hum Reprod 2022; 37(5): 954–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Anderson RA, Lambertini M, Hall PS, et al. Survival after breast cancer in women with a subsequent live birth: influence of age at diagnosis and interval to subsequent pregnancy. Eur J Cancer 2022; 173: 113–122. [DOI] [PubMed] [Google Scholar]
- 78. Azim HA, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol 2013; 31(1): 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Azim HA, Bellettini G, Liptrott SJ, et al. Breastfeeding in breast cancer survivors: pattern, behaviour and effect on breast cancer outcome. Breast 2010; 19(6): 527–531. [DOI] [PubMed] [Google Scholar]
- 80. de Bree E, Makrigiannakis A, Askoxylakis J, et al. Pregnancy after breast cancer. A comprehensive review. J Surg Oncol 2010; 101(6): 534–542. [DOI] [PubMed] [Google Scholar]
- 81. Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst 2018; 110(4): 426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bhurosy T, Niu Z, Heckman CJ. Breastfeeding is possible: a systematic review on the feasibility and challenges of breastfeeding among breast cancer survivors of reproductive age. Ann Surg Oncol 2021; 28(7): 3723–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sella T, Sorouri K, Rosenberg SM, et al. Breastfeeding experiences among young breast cancer survivors: a survey study. Cancer2025; 131(1): e35585. [DOI] [PubMed] [Google Scholar]
- 84. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact 2016; 32(3): 530–541. [DOI] [PubMed] [Google Scholar]
- 85. Azim HA, Niman S, Partridge AH, et al. 1814O Breastfeeding in women with hormone receptor-positive breast cancer who conceived after temporary interruption of endocrine therapy: results from the POSITIVE trial. Ann Oncol 2024; 35: S1076. [Google Scholar]
- 86. Blondeaux E, Delucchi V, Mariamidze E, et al. 1815O Breastfeeding after breast cancer in young BRCA carriers: results from an international cohort study. Ann Oncol 2024; 35: S1076–S1077. [Google Scholar]
- 87. Kranick JA, Schaefer C, Rowell S, et al. Is pregnancy after breast cancer safe? Breast J 2010; 16(4): 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ives A, Saunders C, Bulsara M, Semmens J. Pregnancy after breast cancer: population based study. BMJ 2007; 334(7586): 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hartnett KP, Mertens AC, Kramer MR, et al. Pregnancy after cancer: does timing of conception affect infant health? Cancer 2018; 124(22): 4401–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. U.S. Food and Drug Administration. Oncology Pharmaceuticals: Reproductive toxicity testing and labeling recommendations † (Guidance for Industry; FDA‑2019‑D‑1234), https://www.fda.gov/media/124829/download (2019, accessed 8 June 2025).
- 91. U.S. Food and Drug Administration. SOLTAMOX® (tamoxifen citrate) oral solution: Highlights of prescribing information (FDA Label No. 021807s005), https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf (2018, accessed 8 June 2025).
- 92. Pagani O, Ruggeri M, Manunta S, et al. Pregnancy after breast cancer: are young patients willing to participate in clinical studies? Breast 2015; 24(3): 201–207. [DOI] [PubMed] [Google Scholar]
- 93. Fabian C, Sternson L, El-serafi M, et al. Clinical pharmacology of tamoxifen in patients with breast cancer: correlation with clinical data. Cancer 1981; 48(4): 876–882. [DOI] [PubMed] [Google Scholar]
- 94. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020; 38(34): 3987–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Garutti M, Lambertini M, Puglisi F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: a systematic review. ESMO Open 2021; 6(5): 100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1—or BRCA2-mutated breast cancer. N Engl J Med 2021; 384(25): 2394–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lambertini M, Martel S, Campbell C, et al. Pregnancies during and after trastuzumab and/or lapatinib in patients with human epidermal growth factor receptor 2–positive early breast cancer: analysis from the NeoALTTO (BIG 1-06) and ALTTO (BIG 2-06) trials. Cancer 2019; 125(2): 307–316. [DOI] [PubMed] [Google Scholar]
- 98. Dana-Farber Breast Oncology Center. Consensus recommendations for patients with a history of breast cancer who desire pregnancy, http://physicianresources.dana-farber.org/flexpaper/patients-with-a-history-of-breast-cancer-who-desire-pregnancy/symposiumId/756D3840-C0D2-11EC-83DAD2C4A0BDF6B5 (2024, accessed 8 June 2025).
- 99. Kieturakis AJ, Wahab RA, Vijapura C, et al. Current recommendations for breast imaging of the pregnant and lactating patient. Am J Roentgenol 2021; 216(6): 1462–1475. [DOI] [PubMed] [Google Scholar]
- 100. Sorin V, Bufman H, Bernstein-Molho R, et al. Breast cancer screening in BRCA1/2 pathogenic sequence variant carriers during pregnancy and lactation. Clin Imaging 2024; 111: 110189. [DOI] [PubMed] [Google Scholar]
- 101. Vashi R, Hooley R, Butler R, et al. Breast imaging of the pregnant and lactating patient: physiologic changes and common benign entities. Am J Roentgenol 2013; 200(2): 329–336. [DOI] [PubMed] [Google Scholar]
- 102. Ahn BY, Kim HH, Moon WK, et al. Pregnancy- and lactation-associated breast cancer. J Ultrasound Med 2003; 22(5): 491–497. [DOI] [PubMed] [Google Scholar]
- 103. Sabate JM, Clotet M, Torrubia S, et al. Radiologic evaluation of breast disorders related to pregnancy and lactation. RadioGraphics 2007; 27(Suppl 1): S101–S124. [DOI] [PubMed] [Google Scholar]
- 104. diFlorio-Alexander RM, Slanetz PJ, Moy L, et al. ACR Appropriateness Criteria® breast imaging of pregnant and lactating women. J Am Coll Radiol 2018; 15(11): S263–S275. [DOI] [PubMed] [Google Scholar]
- 105. Vashi R, Hooley R, Butler R, et al. Breast imaging of the pregnant and lactating patient: imaging modalities and pregnancy-associated breast cancer. Am J Roentgenol 2013; 200(2): 321–328. [DOI] [PubMed] [Google Scholar]