Abstract
Our research group identified CTN1122, an imidazo[1,2-a]pyrazine derivative, as a promising antileishmanial agent targeting intramacrophage amastigotes of Leishmania major and Leishmania donovani. CTN1122 selectively inhibits Leishmania casein kinase 1 (L-CK1.2) with a favorable safety profile. Docking studies based on a homology model highlighted key pharmacophoric elements: a 4-pyridyl group at C3, crucial for hydrogen bonding with leucine 90 in the ATP-binding site, and a 4-fluorophenyl moiety at C2, fitting into a hydrophobic pocket. In order to validate these findings, 14 analogs were synthesized with targeted modifications on the imidazo[1,2-a]pyrazine core structure. Three probed the C8 position, three evaluated the impact of C2 substitution, six assessed the C3 4-pyridyl group, and two combined changes at C8 and C3. The study confirmed the critical role of C2 and C3 substituents, as their absence significantly reduced L-CK1.2 inhibition and antileishmanial activity. Additionally, the nitrogen's position within the pyridine ring at C3 proved essential: compound 23, with a meta-pyridyl group, was inactive. Notably, compound 30 exhibited the highest antileishmanial in vitro potency (IC50 = 0.20 μM for L. major; 0.16 μM for L. donovani) alongside enhanced L-CK1.2 inhibition (IC50 = 0.384 μM), with no significant mammalian cytotoxicity.
Docking studies revealed key pharmacophores for L-CK1.2 inhibition. 14 analogs were synthesized; compound 30 showed the best antileishmanial activity and submicromolar L-CK1.2 inhibition without significant cytotoxicity.
Introduction
Leishmaniasis, recognised by the World Health Organisation (WHO) as one of the twenty neglected tropical diseases, is caused by a parasite of the genus Leishmania, transmitted by the bites of infected sand flies. The parasite adopts two forms during its life cycle: an extracellular promastigote form in the sand fly and an intracellular amastigote form in the infected mammal host.1 The disease is endemic in almost 100 countries, covering every continent except Oceania and Antarctica.2 The regions affected are divided between the New World (the Americas) and the Old World (Africa, Asia and Europe). Leishmaniasis has three main clinical forms: cutaneous, mucocutaneous and visceral. The visceral form, caused by L. donovani and L. infantum. Other species, such as L. major and L. braziliensis, mainly cause cutaneous and mucocutaneous forms, respectively.3 An estimated 12 to 15 million people are currently infected, with around 350 million at risk and 70 000 deaths annually.2,4 In 2023, it was estimated that there would be between 700 000 and 1 million new cases of leishmaniasis annually, and, among these, over 90 000 cases of visceral leishmaniasis.5 In Europe, climate changes have recently favoured the spread of the disease, which is described as an emerging disease on the continent.6 There is currently no vaccine available, and treatments are limited to six options: pentavalent antimonials, pentamidine, amphotericin B, paromomycin, miltefosine and azoles, the two latter being the only oral treatment.7 However, these treatments are costly and toxic, and there have been numerous cases of parasite resistance, with failure rates of up to 65% for antimonials in India region.8
The IICiMed team previously discovered the CTN1122 molecule, with an imidazo[1,2-a]pyrazine core, exhibiting significant antileishmanial activity against the intramacrophagic amastigote stage with IC50 values of 0.80 μM against the L. major strain and 2.74 μM against L. donovani. CTN1122 also has a good safety profile and acts effectively as an inhibitor of the target protein, Leishmania casein kinase 1 (L-CK1.2) with an IC50 of 0.72 μM, albeit not selective against the human homolog HsCK1ε.9,10
Leishmania casein kinase 1 isoform 2 (L-CK1.2 or LmCK1.2) is a promising therapeutic target for the treatment of leishmaniasis.11,12 This protein kinase, secreted into macrophages via exosomes, phosphorylates the human receptor IFNAR1, thereby reducing the cellular response for IFNα/β.13,14 In addition, in vitro, L-CK1.2 regulates macrophage apoptosis and RNA metabolism pathways, processes that are often impaired during infection by Leishmania.11,12 Therefore, L-CK1.2 may substitute for human CK1 kinases by phosphorylating host proteins, ultimately manipulating macrophage functions to enhance parasite survival.15 By targeting L-CK1.2, the CTN1122 compound could not only eliminate the intracellular parasite but also restore macrophage defence capabilities by preventing their subversion. Furthermore, as L-CK1.2 plays a crucial role in replacing certain functions of human CK1, it is unlikely that Leishmania could mutate this protein without compromising its own survival, thereby reducing the risk of resistance.16
As the 3D structure of LmCK1.2 has not been obtained, a homology-based structure model was constructed and described in Tisseur et al.17 where the CTN1122 molecule was integrated by docking to better understand its binding position and structure–activity relationships. This model revealed that the 4-pyridyl fragment of CTN1122 is essential for interaction with the target protein. Indeed, the pyridine ring establishes a specific anchor with leucine 90, mimicking the adenine ring of ATP. In addition, the 4-fluorophenyl fragment explores the hydrophobic cavity of the active site, which is not occupied by ATP. This second interaction enables CTN1122 to act as a competitive inhibitor of LmCK1.2 with respect to ATP.
The aim of this study was to validate in vitro the predictions made by molecular docking and to demonstrate that only the fragments located at positions C2 and C3 (4-fluorophenyl and 4-pyridyl) are essential for the inhibitory activity of LmCK1.2. To this end, various pharmacomodulations were carried out around positions C8, C2 and C3 of the reference compound CTN1122, illustrated in Fig. 1.
Fig. 1. Modulation from the hit compound CTN1122.
Firstly, we will examine the effect of successively deleting each of these three groups to confirm their importance. Then, the replacement of these fragments by other chemical groups was studied to assess their ability to preserve inhibitory activity against LmCK1.2 and phenotypic activity. This approach could also make it possible to identify more active and selective analogues than the starting compound.
Results and discussion
Chemistry
Modulation in position C8
Initially, three new compounds were synthesised by modifying the C8 position, as illustrated in Fig. 1. Following the CTN1122 synthesis method described by Marchand et al.,9 omitting one step yields a chlorinated C8 analogue. The synthesis begins similarly than CTN1122 and leads in three steps to compound 4, from which, rather than incorporating dimethylamine by aromatic nucleophilic substitution as in CTN1122, a Suzuki–Miyaura pallado-catalysed coupling with 4-pyridylboronic acid is performed in previously described conditions,9 producing the C8-chlorinated compound (5) in 37% yield (Scheme 1).
Scheme 1. Reagents and conditions: (i) NH4OH, Parr reactor, 100 °C, overnight, 81%; (ii) BrCH2CO(4-FC6H4), CH3CN, 80 °C, 18 h, 55%; (iii) NIS, CH3CN, 80 °C, 40 h, 73%; (iv) 4-pyridylboronic acid, PdCl2(dppf), K3PO4, dioxane/H2O (9 : 1), sealed tube, N2, 100 °C, 24 h, 37%; (v) HCOONa, Pd(PPh3)4, DMSO, 110 °C, 2 h, 95%; (vi) NIS, CH3CN, 80 °C, 40 h, 67%; (vii) 4-pyridylboronic acid, PdCl2(dppf), K3PO4, dioxane/H2O (9 : 1), sealed tube, N2, 100 °C, 20 h, 71%.
Next, from intermediate 3, a compound without dimethylamine was obtained. This step involved the dehalogenation of intermediate 3 by reduction in the presence of sodium formate catalysed by tetrakis(triphenylphosphine)palladium(0)18 to give product 6. Finally, halogenation at the C3 position was then carried out using NIS to give the iodinated analogue 7 and finally, a Suzuki–Miyaura coupling with pyridin-4-ylboronic acid afforded the final product 8 in 71% yield (Scheme 1).
Finally, the third compound modified at the C8 position is compound 12, which features a thioether group. Its synthesis begins with a nucleophilic substitution, where 3-chloropyrazin-2-amine (2) is reacted with sodium methanethiolate to form compound 9 (Scheme 2).19 The process continues with a cyclization step: 3-(methylthio)pyrazin-2-amine (9) is reacted with 2-bromo-4′-fluoroacetophenone to yield compound 10. This intermediate then undergoes iodination in the presence of NIS. Finally, following the same Suzuki–Miyaura coupling conditions, the iodide 11 is arylated with 4-pyridylboronic acid, producing the final compound 12 in a 47% yield (Scheme 2).
Scheme 2. Reagents and conditions: (i) NaSCH3, DMF/EtOH (1 : 1), 85 °C, 2 h, 83%; (ii) BrCH2CO(4-FC6H4), DME/EtOH, reflux, 24 h, 17%; (iii) NIS, CH3CN, 80 °C, 4 h, 56%; (iv) 4-pyridylboronic acid, PdCl2(dppf), K3PO4, dioxane/H2O (9 : 1), sealed tube, N2, 100 °C, 24 h, 47%.
Modulation in position C2
Three new compounds were synthesised for modifications in the C2 position. The first, compound 16, has no 4-fluorophenyl moiety at C2. The second, compound 20, is a positional isomer of 16 with the 4-pyridyl moiety at C3. Finally, compound 21 presents an inversion of the CTN1122 fragments in positions C2 and C3.
For compound 16, the synthesis strategy follows that of CTN1122, except for the cyclisation reaction of the heterocycle. This is carried out using intermediate 2 with 2-bromo-1,1-diethoxyethane in the presence of HBr (48% aq),20 producing the cyclised compound 13 without adding the 4-fluorophenyl at C2, unlike CTN1122. Compound 13 then undergoes iodination with NIS, followed by aromatic nucleophilic substitution with aqueous dimethylamine to integrate this group at C8. Finally, a Suzuki–Miyaura coupling with pyridine gave the final compound in 70% yield (Scheme 3).
Scheme 3. Reagents and conditions: (i) 2-bromo-1,1-diethoxyethane, HBr (48% aq), propan-2-ol, 80 °C, 3 h, 91%; (ii) NIS, DMF, rt to 45 °C, 12 h, 36%; (iii) dimethylamine 40% aq, K2CO3, DMF, 100 °C, 17 h, 60%; (iv) 4-pyridylboronic acid, PdCl2(dppf), K3PO4, dioxane/H2O (9 : 1), sealed tube, N2, 100 °C, 7 h, 68%; (v) dimethylamine 40% aq, 120 °C, 12 h, 92%; (vi) 2-bromoacetic acid, propan-2-ol, 90 °C, 12 h, 100%; (vii) PhNTf2, Et3N, toluene, 110 °C, 20 h, 83%; (viii) 4-pyridylboronic acid, PdCl2(dppf), K3PO4, dioxane/H2O (9 : 1), sealed tube, N2, 100 °C, 24 h, 51%; (ix) 1-fluoro-4-iodobenzene, Pd(OAc)2, PCy3·HBF4, PivOH, K2CO3, DMAc, sealed tube, 100 °C, 12 h, 70%.
Compounds 20 and 21 were obtained using a different synthesis strategy to that of the hit compound. Inspired by the method of Marhadour et al.21 and Tisseur et al.,17 it begins with the incorporation of dimethylamine onto the upstream aminochloropyrazine. After cyclisation and triflation, intermediate 19 is formed. From this intermediate, a Suzuki–Miyaura coupling allows the addition of 4-pyridyl at C2, leading to compound 20 in 51% yield. Finally, 4-fluorophenyl was introduced at C3 via direct arylation in the conditions of Liégault et al.,22 giving compound 21, an isomer of CTN1122, in a satisfactory 70% yield (Scheme 3).
Modulation in position C3
To explore modifications at the C3 position, five novel compounds were synthesized by introducing diverse heterocyclic moieties to evaluate their impact on the biological properties of this compound series. The synthesis began with intermediate 4, where dimethylamine was first introduced at the C8 position via aromatic nucleophilic substitution, yielding iodinated intermediate 22, which retained both C2 and C8 substituents. Subsequent Suzuki–Miyaura cross-coupling reactions on this halogenated intermediate afforded compounds 23 (3-pyridyl), 24 (methylpyrazole), 25 (2-methylpyridine), and 26 (quinoline) (Scheme 4 and Table 1).
Scheme 4. Reagents and conditions: (i) dimethylamine 40% aq, K2CO3, DMF, 100 °C, 5 h, 70%; (ii) (Het)ArB(OH)2, PdCl2(dppf), K3PO4, dioxane/H2O (9 : 1), sealed tube, N2, 100 °C, 20–48 h, 34–85%.
Table 1. Yields of Suzuki–Miyaura coupling reaction (compounds 23–26 and 31–32).

| ||||
|---|---|---|---|---|
| Entry | R8 | R3 | Compound | Yielda (%) |
| 1 |

|

|
23 | 34 |
| 2 |

|

|
24 | 85 |
| 3 |

|

|
25 | 61 |
| 4 |

|

|
26 | 50 |
| 5 |

|

|
31 | 73 |
| 6 |

|

|
32 | 71 |
Isolated yields.
To add a benzamide moiety in the C3 position, a slightly different strategy was employed, requiring an upstream synthesis. Following the conditions of Mologni et al.,23 commercially available 2-amino-4-chloropyridine (27) was treated with benzoyl chloride and N,N-dimethylaminopyridine (DMAP) in acetonitrile. The reaction was carried out under microwave irradiation in a sealed tube at 100 °C and 100 W for 5 minutes to produce intermediate 28 in low yield (Scheme 5). In this reaction, DMAP acts as a catalyst, increasing the electrophilicity of the carbonyl to promote nucleophilic substitution.
Scheme 5. Reagents and conditions: (i) benzoyl chloride, DMAP, CH3CN, MW, 100 °C, 5 min, 30%; (ii) dimethylamine 40% aq, K2CO3, DMF, 100 °C, 5 h, 83%; (iii) 28, Pd(OAc)2, PCy3·HBF4, PivOH, K2CO3, DMAc, sealed tube, 100 °C, 68 h, 8%.
From intermediate 3, dimethylamine is then introduced by nucleophilic aromatic substitution to give compound 29, which unlike the previous intermediates does not contain an iodine atom at C3. To finalize the addition of the benzamide, a direct arylation of 29 was carried out with intermediate 28, forming compound 30 in a low yield of 8%.22 This low yield is attributed to the low reactivity of the chlorine in 28, which is less reactive than the halogens used in the previous arylations, thus explaining the difficulty of this step (Scheme 5).
Finally, the last two compounds, modified both at C3 and without a substituent at C8 compared with the reference compound, were synthesised. The absence of a substituent at C8 is chosen because it generally favours better biological activity (as will be discussed later). Compound 31 has a methylpyrazole group at C3, while compound 32 has a quinoline at C3. These compounds were obtained by Suzuki–Miyaura coupling from intermediate 7, which already contains no substituent in the C8 position (Scheme 6 and Table 1).
Scheme 6. Reagents and conditions: (i) (Het)ArB(OH)2, PdCl2(dppf), K3PO4, dioxane/H2O (9 : 1), sealed tube, N2, 100 °C, 48 h, 73% (31) and 19% (32).
Biological evaluation
Kinase inhibitory activity
Leishmania casein kinase 1 (L-CK1.2) was identified as a key target for the candidate compound CTN1122, with significant inhibitory activity displaying an IC50 of 0.72 μM on the recombinant parasite-specific enzyme.9 The homology model suggested that the 4-pyridyl fragment at position C3 is crucial for this inhibition: the nitrogen of 4-pyridyl establishes a hydrogen bond with leucine 90 in the active site of L-CK1.2, ensuring a stable interaction with the target. In addition, the presence of a phenyl group in the C2 position seems equally essential, as this group fits into a hydrophobic pocket in the active site, thereby enhancing affinity with the kinase. In this study, all the compounds in the series were evaluated for their ability to inhibit L. major CK1 (IC50LmCK1.2, Table 2). In order to measure their selectivity and detect any toxic potential, these compounds were also tested on human CK1 (IC50HsCK1ε), allowing a CK1-specific selectivity index (SICK1) to be calculated.
Table 2. Inhibition of Leishmania major CK1 and human CK1 activities by imidazo[1,2-a]pyrazines CTN1122, 5, 8, 12, 16, 20–26 and 30–32, expressed in enzyme inhibitory concentration 50%, and their selectivity profile.

| ||||||
|---|---|---|---|---|---|---|
| Cpd | R8 | R2 | R3 | IC50a (μM) | IC50a (μM) | SICK1b |
| LmCK1.2 | HsCK1ε | |||||
| CTN1122 |

|

|

|
0.72 | 0.92 | 1.3 |
| 5 |

|

|

|
0.279 | 1.001 | 3.6 |
| 8 |

|

|

|
0.250 | 0.540 | 2.2 |
| 12 |

|

|

|
0.356 | 0.651 | 1.8 |
| 16 |

|

|

|
na | na | — |
| 20 |

|

|

|
na | na | — |
| 21 |

|

|

|
na | na | — |
| 22 |

|

|

|
na | na | — |
| 23 |

|

|

|
na | na | — |
| 24 |

|

|

|
12.42 | na | — |
| 25 |

|
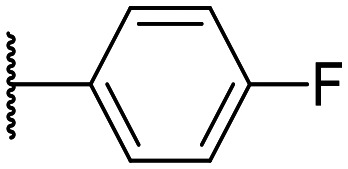
|

|
1.05 | 1.96 | 1.9 |
| 26 |

|

|

|
5.10 | 3.88 | 0.8 |
| 30 |

|

|

|
0.384 | 0.188 | 0.5 |
| 31 |

|

|

|
na | na | — |
| 32 |

|

|

|
2.202 | 1.078 | 0.5 |
Mean from three determinations.
SICK1 = IC50HsCK1ε/IC50LmCK1.2.
Looking first at the modulations at the C8 position, i.e. compounds 5, 8 and 12, we found that replacing dimethylamine with other functions (hydrogen, chlorine or thioether) improved inhibition of the LmCK1.2 target (IC50LmCK1.2 < 0.3 μM) compared to CTN1122, while increasing selectivity towards HsCK1ε (SICK1 > 1.8). Of these, the compound 8, without a substituent (hydrogen) showed the best inhibition, while the chlorine compound (5) displayed the highest selectivity.
Regarding modifications at the C2 position (compounds 16, 20 and 21), we observed a complete loss of inhibitory activity of the target. Compound 16, which retains the 4-pyridyl moiety at C3 but has no aromatic ring at C2, shows that the presence of an aromatic ring at C2 is necessary to interact in the hydrophobic pocket of the ATP active site, as predicted by molecular docking. For compounds 20 and 21, where the 4-pyridyl moiety is in the C2 position, this aromatic structure could potentially be inserted into the hydrophobic pocket. However, the substituent at C3, which is hydrogen for the compound 20 and 4-fluorophenyl for the compound 21, seems to play a crucial role. It is not just about replacing the 4-fluorophenyl group with pyridine at C2, but also the lack of a hydrogen bond donor at C3, as seen with pyridine in CTN1122.
Compounds modified at C3 confirm this hypothesis. In fact, as soon as the substituent at C3 does not correspond to a 4-pyridyl type structure (compounds 22, 23 and 24), we observe a loss of LmCK1.2 inhibitory activity. It therefore seems essential to have a six-membered aromatic ring at C3, with a nitrogen atom in the para position. The isomer of CTN1122 (compound 23) with the nitrogen in the meta position showed no activity. In this series, only compound 30, with a benzamide function, showed greater inhibition than the reference compound. The 3-methyl-4-pyridyl (compound 25) and quinoline fragments (compound 26) also failed to improve inhibition.
Finally, compounds modified at the C8 and C3 positions produced contrasting results. Compound 31, bearing a methylpyrazole, showed no activity on the target, as did its analogue with a dimethylamine at C8. Conversely, compound 32, containing a C3 quinoline without a C8 dimethylamine, showed less inhibition of LmCK1.2 than CTN1122 but more than its analogue with a C8 dimethylamine. This confirms that C8 dimethylamine is probably not the most appropriate substituent for good inhibition of LmCK1.2.
Fig. 2 shows the docking of compound 30, the CTN1122 analogue with a benzamide function in ortho to the 4-pyridyl nitrogen, into the ATP binding site. This model reveals interactions similar to those observed with CTN1122: the pyridine nitrogen in position C3 forms a hydrogen bond with leucine 90 in the hinge region, while the 4-fluorophenyl group in C2 interacts through hydrophobic bonds in the nonpolar pocket. In addition, the benzamide moiety at C3 forms an additional hydrogen bond with leucine 90 aligns opposite the guanidinium chain of arginine 27, an amino acid specific to LmCK1.2. This proximity suggested the formation of a π–cation interaction with this amino acid, potentially conferring selectivity towards human CK1. However, the selectivity index results for compound 30 (SICK1 = 0.5) showed that the expected selectivity was not achieved and the pi–cation interaction is not mentioned in the PoseView 2D diagram. Despite this uncertainty, the presence of this benzamide moiety played a crucial role in improving target affinity compared to compound CTN1122. The binding score values for the compounds CTN1122, 5, 8, 12 and 30 (same binding poses) were also added in the ESI† part (Table ST1). As expected, the bulky compound 30 recorded the highest ChemScore value but no clear correlation between IC50 (LmCK1.2) and the scoring functions is observed. The theoretical complex structure was subjected to 100 ns molecular dynamics in explicit solvent environment. The resulting trajectory showed no major binding mode instability, as demonstrated by protein radius of gyration, solvent accessible surface area, RMSD and binding site residues RMSF (see ESI†), thus validating the binding pose obtained by docking.
Fig. 2. (a) Binding position found by the GOLD docking programme for compound 30 in the ATP pocket of LmCK1.2. Hydrogen bonds are indicated by yellow lines; (b) 2D illustration of the complex generated by PoseView (https://proteins.plus/).
In vitro antileishmanial activity against intracellular amastigotes, cytotoxicity and selectivity indexes
The biological activity of compounds 5, 8, 12, 16, 20–26 and 30–32 (Table 3) was assessed on intramacrophage amastigotes of two Leishmania species: L. major, responsible for cutaneous leishmaniasis, and L. donovani, responsible for the visceral form of the disease. These intramacrophage amastigotes, which represent the infectious form of the parasite and reside in host cells, provide a relevant model for testing the antileishmanial potential of the compounds. At the same time, the toxicity of each compound was assessed on RAW 264.7 macrophages to determine their safety profile. The values obtained were used to calculate the selectivity index (SI) for each compound, based on the ratio between their cytotoxicity (IC50 RAW) and their anti-parasite activity (IC50 on intracellular amastigotes). A selectivity index (SI) greater than 10 is required for a compound to be considered promising. Miltefosine was used as the reference for these tests. To assess relative efficacy, the reference compound CTN1122 was included with an IC50 of 0.80 μM against L. major and 2.74 μM against L. donovani (Table 3). CTN1122 showed low cytotoxicity on RAW 264.7 macrophages (IC50 = 42.03 μM), making it a relevant reference model for comparing the efficacy and safety of novel compounds.
Table 3. In vitro antileishmanial activity against intracellular L. major and L. donovani amastigotes and cytotoxicity evaluation of imidazo[1,2-a]pyrazine derivatives CTN1122, 5, 8, 12, 16, 20–26 and 30–32.

| ||||||||
|---|---|---|---|---|---|---|---|---|
| Cpd | R8 | R2 | R3 | Macrophages RAW 264.7 IC50a (μM) ± SD | L. major IC50 (μM) ± SD | SIRAWb | L. donovani IC50 (μM) ± SD | SIRAWb |
| Miltefosine c | 43.01 ± 5.16 | 1.44 ± 0.25 | 29.9 | 1.83 ± 0.27 | 23.5 | |||
| CTN1122 |

|

|

|
42.03 ± 4.5 | 0.80 ± 0.20 | 52.5 | 2.74 ± 0.14 | 15.3 |
| 5 |

|

|

|
>100 | 57.22 ± 2.88 | >1.7 | >100 | — |
| 8 |

|

|

|
>100 | 11.87 ± 1.57 | >8.4 | 27.57 ± 2.21 | >6.6 |
| 12 |

|

|
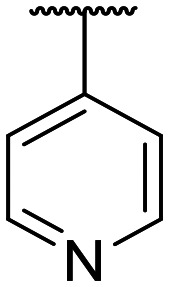
|
>100 | >100 | — | >100 | — |
| 16 |

|

|

|
45.11 ± 2.56 | 48.32 ± 3.87 | 0.9 | 33.01 ± 1.99 | 1.4 |
| 20 |

|

|

|
30.78 ± 1.28 | 5.67 ± 2.2 | 5.4 | 18.32 ± 3.25 | 1.6 |
| 21 |

|

|

|
>100 | 47.22 ± 3.20 | >2.1 | 56.89 ± 4.15 | >1.8 |
| 22 |

|

|

|
44.00 ± 2.01 | >50 | <0.9 | >50 | <0.9 |
| 23 |

|

|

|
43.91 ± 3.94 | >50 | <0.9 | >50 | <0.9 |
| 24 |

|

|

|
15.95 ± 2.55 | 8.99 ± 1.89 | 1.8 | 1.27 ± 0.03 | 12.6 |
| 25 |

|

|

|
>100 | >100 | — | >100 | — |
| 26 |

|

|

|
>100 | 59.84 ± 4.64 | 1.7 | 61.48 ± 2.20 | 1.6 |
| 30 |

|

|

|
10.85 ± 3.43 | 0.20 ± 0.18 | 54.2 | 0.16 ± 0.08 | 67.8 |
| 31 |

|

|

|
>100 | 56.28 ± 4.18 | >1.8 | >100 | — |
| 32 |

|

|

|
>100 | 12.98 ± 0.39 | 7.7 | 25.98 ± 2.30 | 3.8 |
Mean from two determinations.
SIRAW = cytotoxicity on RAW cells (IC50)/amastigotes (IC50).
Miltefosine was used as antileishmanial reference drug. Its cytotoxicity on MRC-5 cells was in accordance with the literature.24
Regarding to modifications at the C8 position, although compounds 5, 8 and 12 showed improved inhibition of LmCK1.2, their activity on intramacrophage amastigotes was clearly inferior to that of CTN1122 for L. major and L. donovani strains. In particular, compound 12, with its thioether substituent, showed no phenotypic activity with an IC50 > 100 μM for the two strains tested. Furthermore, these three compounds showed no toxicity on RAW 264.7 macrophages. This disparity between LmCK1.2 inhibition and phenotypic activity suggests problems with cell penetration or solubility, preventing the compounds from effectively reaching the parasite target protein.
For the modulations at position C2 of the heterocycle, compounds 16 and 20 showed some toxicity on RAW 264.7 macrophages, comparable to CTN1122 for compound 16 and slightly higher for compound 20. In addition, these modifications also led to a decrease in activity on intramacrophage amastigotes. In this case, the loss of inhibition of the LmCK1.2 target for these three compounds was expected, as was the decrease in phenotypic activity. However, although inactive on LmCK1.2, these compounds still showed some activity against parasite strains, although less so than CTN1122. This suggests that they may interact with another unidentified parasite molecular target, explaining their persistent activity against parasite strains despite their inactivity on LmCK1.2.
Finally, modulations at position C3 showed variable results depending on the substituents. On the one hand, compounds 22 and 23, which showed no inhibition of the LmCK1.2 target, also showed no activity on intramacrophage amastigotes, and no apparent cytotoxicity. In contrast, compounds 25 and 26, despite retaining the 6-membered aromatic ring and the nitrogen in the para position required for inhibition of the target, showed much lower inhibition of LmCK1.2 than CTN1122 and also showed a marked drop-in phenotypic activity for the two species studied, with a total absence of activity for compound 25. Two hypotheses could explain this difference in activity. The first could be an inability of the compounds to cross the parasite membranes, due to a problem of solubility or a lack of lipophilicity. The second hypothesis is that the inhibition of LmCK1.2 by these compounds is too weak to cause a significant effect on the parasite at cellular level.
A comparison between compounds 24 and 31, both of which have a methylpyrazole in the C3 position, reveals a notable difference in their activity against the parasite. As reported in the work of Bazin et al.,10 compound 24 showed an IC50 of 1.27 μM on the L. donovani strain, while compound 31, without dimethylamine in position C8, showed no activity. This difference is difficult to explain, because although these two compounds do not inhibit the LmCK1.2 target, it seems that another target is involved. The disparity could be the result of compound 31 having difficulty penetrating the parasite effectively, or it could be linked to the differences in toxicity observed on RAW 264.7 macrophages: compound 24 has a IC50 of 15.95 μM, which could lead to increased cytotoxicity in the tests and thus influence the phenotypic activity values obtained. In contrast, the comparison between compounds 26 and 32 showed a correlation between activity on parasitic strains and target inhibition. Compound 32, although more active on both the target and the parasite strains than compound 26, nevertheless showed lower phenotypic activity than the hit compound CTN1122.
Finally, compound 30, which carries a benzamide moiety, stands out for its strong inhibitory activity on LmCK1.2, and proves to be the most active of this series with IC50 of 0.20 μM on L. major and 0.16 μM on L. donovani. Although some toxicity was observed on RAW 264.7 macrophages, this does not appear to cause a major problem for the future, as the selectivity indices are greater than 50 for both parasitic species. The addition of this type of benzamide substituent therefore considerably improves activity on both the enzymatic target and the parasites. Various simple physicochemical parameters (molecular weight, clog P, clog S, H-bond acceptor/donor count, polar surface area (PSA) and rotatable bond) were also computed for compounds CTN1122, 5, 8, 12 and 30 (see ESI,† Table ST1). Once again, there is no clear correlation between phenotypic data and PhysChem values. Only the lipophilic compound 30 which occupies a different physicochemical space combining all the highest PhysChem values also shows the best phenotypic activities.
Conclusions
In conclusion, this series of 14 new compounds confirmed the interest of the presence of the pharmacophoric structure of the initial compound CTN1122, established by docking modelling on a model built by homology. The study by our group had demonstrated the need to retain two aromatic rings: 4-fluorophenyl in position C2, which is housed in the hydrophobic pocket of the ATP active site, and 4-pyridyl in C3, which provides a hydrogen bond between the para nitrogen and leucine 90 of the active site.17 These two groups appeared to be essential for ensuring optimal interaction with the LmCK1.2 target.
The compounds synthesised in which these substituents were removed or modified (compounds 16, 20 and 21 for the replacement of 4-fluorophenyl in C2; compounds 22, 23, 24 and 31 for the replacement of 4-pyridyl in C3) showed a loss of inhibitory activity on LmCK1.2. This loss also correlated, for a large number of these compounds (16, 21, 22, 23 and 31), with a significant reduction in activity on parasites of the species L. major and L. donovani. These results therefore confirm that the 4-fluorophenyl C2 and 4-pyridyl C3 fragments are essential for interaction with the target and, consequently, for antiparasitic activity.
The structure homology docking study had revealed that the dimethylamine in the C8 position of imidazo[1,2-a]pyrazine was not essential for activity on the LmCK1.2 target, as it was located in a region accessible to the solvent. Indeed, the three compounds in this study in which dimethylamine was substituted with chlorine, hydrogen or thioether (compounds 5, 8 and 12 respectively) not only maintained but improved LmCK1.2 inhibition, with IC50 values of less than 0.36 μM for each, compared with an IC50 of 0.72 μM for the initial compound CTN1122. However, this improvement in target inhibition was not associated with improved phenotypic antiparasite activity. Of these three compounds, compound 5, with a hydrogen at the C8 position, was the most active on the target and also showed the best cellular activity (IC50 = 11.87 μM on L. donovani), although lower than that of CTN1122 (IC50 of 2.74 μM on the same strain). Thus, although C8 dimethylamine is not crucial for kinase inhibition, it appears to play an essential role in the membrane permeability and solubility of the compound, contributing significantly to the phenotypic efficacy on the parasite.
Finally, four of the compounds synthesised in this series, compounds 25, 26, 30 and 32, retained the essential elements for target inhibition: a 4-fluorophenyl group in the C2 position and a 4-pyridyl-like moiety in C3, with the nitrogen in the para position. Although these four compounds should logically have an activity close to that of CTN1122, the results observed turned out to be quite different. Compounds 26 and 32, with a quinoline at C3, and compound 25 which incorporates a 3-methyl-4-pyridyl, showed significantly lower inhibition of LmCK1.2 and activity against intramacrophagic amastigotes than CTN1122. Docking studies were unable to explain this difference, as these compounds appear to occupy the ATP pocket in a similar way to CTN1122. It is possible that the rigidity of the quinoline prevents optimal positioning in the active site. However, no satisfactory explanation could be put forward for methylpyridine. The addition of a bond ortho to the pyridine nitrogen does not appear to be an unfavourable factor, since compound 30, bearing a benzamide group ortho to this nitrogen, shows improved activity against LmCK1.2 (IC50 = 0.384 μM). In addition, compound 30 showed the best phenotypic activity in this series, with IC50 values of 0.20 μM on L. major and 0.16 μM on L. donovani, as well as a selectivity index greater than 54 on RAW 264.7 macrophages, indicating a lack of cytotoxicity for this cell line. These encouraging results justify to evaluate compound 30 on the L. major and L. donovani in vivo models.
Interestingly, compounds 5, 8 and 12 showed IC50 values against LmCK1.2 comparable to compound 30, whereas they remained almost inactive against Leishmania strains, when compound 30 showed submicromolar antileishmanial properties. These results clearly indicate that its mechanism of action involves an additional molecular target.
Experimental protocols
Chemistry
All commercial reagents were used without further purification. All solvents were reagent or HPLC grade. Analytical TLC was performed on silica gel 60 F254 plates. Open column chromatography was performed on silica gel 60 (70–230 mesh ASTM). The flash column chromatography was performed using a Reveleris® X2 Buchi system, with Buchi cartridge (FlashPure 12 g, 40 μm irregular silica). Yields refer to chromatographically and spectroscopically pure compounds. Melting points were determined on an electrothermal melting point apparatus. 1H NMR and 13C NMR spectra were recorded in CDCl3 or in DMSO-d6 on a 400 MHz spectrometer. Chemical shifts are reported as δ values in parts per million (ppm) relative to tetramethylsilane as internal standard and coupling constants (J) are given in hertz. Multiplicities are reported as follows: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, mt = multiplet, bs = broad singlet, tapp = apparent triplet. Low resolution mass spectra were recorded using an Electrospray Ionization (ESI) Method with Waters ZQ 2000 spectrometer. UPLC column used was an Acquity UPLC® BEH Phenyl (2.1 mm i.d., 50 mm length, 1.7 μm particle size) from Waters. A linear mobile phase gradient was used with a mobile phase A as 100% of acetonitrile in water (at 2%) and mobile phase B as 100% acetonitrile. The gradient table was: 0–0.5 min, 0% B; 0.5–4.0 min 0 → 100% B; 4.0–5.5% 100% B; 5.5–5.7 min 100 → 0% B; 5.7–7.5 min 0% B at flow rate 0.5 mL min−1 and column temperature 35 °C. Formic acid (0.1%) was added in diluent to improve ionization. Pure compounds 5, 8, 12, 16, 20, 21, 25, 26, 30–32 were analyzed at 0.1 mg mL−1 in UPLC grade MeOH (Biosolve), following a 3 μL injection on a UFLC-ESIHRMS (IT-TOFMS) Shimadzu instrument (Prominence Ultra Fast Liquid Chromatography coupled to High Resolution Electrospray Ionization Mass Spectrometry combining Ion trap and Time of Flight analyzers). Analyses were performed by Flow Injection (FIA) in the isocratic flow consisting of H2O + 0.1% formic acid (v/v)/acetonitrile + 0.1% formic acid 10 : 90 (v/v) at 300 μL min−1. The detection using ESI was performed both in positive and negative ionization modes in the mass range m/z 100–1000 with an accumulation time of 10 ms. The m/z values were corrected after calibration with a solution of sodium formate (5 mM in an acetonitrile/water mixture (90/10)) as an external reference, giving an accuracy of approximately 5 ppm.
Compounds 2, 3, 4, and 22 were previously described in Marchand et al.9 Compounds 23 and 24 were previously described in Bazin et al.,10 and compounds 17, 18, and 19 were prepared according the procedures described by Tisseur et al.17
8-Chloro-2-(4-fluorophenyl)-3-(pyridin-4-yl)imidazo[1,2-a]pyrazine 5
To a solution of 4 (100 mg, 0.27 mmol, 1 eq.) in dioxane/water (9 : 1) (3.5 mL) were added 4-pyridylboronic acid (36 mg, 0.29 mmol, 1.1 eq.), potassium phosphate tribasic (142 mg, 0.67 mmol, 2.5 eq.) and [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (95 mg, 0.01 mmol, 0.05 eq.). The vial was purged with argon for 10 min and then sealed. The mixture was heated at 100 °C for 24 h and then it was cooled to room temperature. Water was added and the aqueous layer was extracted with ethyl acetate (2 × 10 mL). The combined organic extracts were washed with brine and water, dried over sodium sulfate, filtered, concentrated in vacuo. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (1 : 1) as eluent to afford 8-chloro-2-(4-fluorophenyl)-3-(pyridin-4-yl)imidazo[1,2-a]pyrazine 5 (32 mg, 37%) as a beige powder. Rf = 0.62 (CH2Cl2/MeOH 9 : 1); Mp: 249–250 °C; 1H NMR (400 MHz, CDCl3) δ: 8.84 (d, 3J = 6.1 Hz, 2H), 7.95 (dd, 3J = 4.5 Hz, 1H), 7.73 (d, 3J = 4.5 Hz, 1H), 7.63 (dd, 3J = 8.8 Hz, 4JC–F = 5.5 Hz, 2H), 7.39 (d, 3J = 6.1 Hz, 2H), 7.05 (t, 3J = 3JC–F = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 163.87 (d, 1JC–F = 246.1 Hz, C), 151.83 (C), 145.85 (2CH), 144.67 (C), 138.21 (C), 136.66 (C), 130.94 (d, 3JC–F = 8.5 Hz, 2CH), 129.14 (CH), 128.57 (d, 4JC–F = 3.1 Hz, C), 124.57 (2CH), 121.73 (C), 116.28 (d, 2JC–F = 21.8 Hz, 2CH), 116.00 (CH); MS (ESI) m/z (%): 325.06 (92) [M + H]+; RT (min): 2.27; UPLC purity: 92%; IR (ATR), cm−1: 3050 (νC–Har), 2922 (νCH3), 1600 (νC N), 1226 (νC–F), 707 (νC–Cl); HRMS (TOF MS ES+): calcd. for C17H10ClFN4 [M + H]+ 325.0651, found: 325.0646.
2-(4-Fluorophenyl)imidazo[1,2-a]pyrazine 6
To a solution of 3 (100 mg, 0.40 mmol, 1 eq.) in DMSO (2 mL) were added sodium formate (548 mg, 0.80 mmol, 2 eq.) and tetrakis(triphenylphosphine)palladium (23 mg, 0.02 mmol, 0.05 eq.). The reaction was heated to 80 °C for 2 h. The reaction mixture was cooled to room temperature, the residue was filtered through a pad of Celite®, washed with distilled water and extracted with dichloromethane (3 × 100 mL). The combined organic layers were dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (30 : 70) as eluent to afford 2-(4-fluorophenyl)imidazo[1,2-a]pyrazine 6 (80 mg, 95%) as a beige powder. Rf = 0.24 (cyclohexane/EtOAc 2 : 8); Mp: 161–162 °C; 1H NMR (400 MHz, DMSO-d6) δ: 9.07 (d, 4J = 0.8 Hz, 1H), 8.60 (s, 1H), 8.59 (dd, 3J = 4.5 Hz, 4J = 0.8 Hz, 1H), 8.09 (dd, 3J = 8.8 Hz, 4J = 5.6 Hz, 2H), 7.90 (d, 3J = 4.5 Hz, 1H), 7.31 (tapp, 3J = 3JC–F = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 163.40 (d, 1JC–F = 247.5 Hz, C), 147.20 (C), 143.81 (CH), 141.12 (C), 132.22 (d, 3JC–F = 9.9 Hz, 2CH), 129.97 (CH), 129.26 (d, 4JC–F = 3.4 Hz, C), 118.75 (CH), 116.15 (d, 2JC–F = 21.7 Hz, 2CH), 109.03 (CH); MS (ESI) m/z (%): 214.1 (100) [M + H]+, 215.1 (8) [M + H + 1]+; RT (min): 1.83; UPLC purity: 99%; IR (ATR), cm−1: 3055 (νC–Har), 1483 (νC C), 1222 (νC–F).
2-(4-Fluorophenyl)-3-iodoimidazo[1,2-a]pyrazine 7
To a solution of 6 (200 mg, 0.94 mmol, 1 eq.) in anhydrous DMF (4.7 mL) was added N-iodosuccinimide (211 mg, 0.94 mmol, 1 eq.). The reaction mixture was stirred at room temperature for 1 h then heated at 45 °C for 3 h. The reaction mixture was diluted with distilled water and extracted with ethyl acetate (3 × 300 mL). The combined organic layers were washed with water, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (30 : 70) as eluent to afford 2-(4-fluorophenyl)-3-iodoimidazo[1,2-a]pyrazine 7 (215 mg, 67%) as a yellow powder. Rf = 0.44 (cyclohexane/EtOAc 3 : 7); Mp: 172–173 °C; 1H NMR (400 MHz, DMSO-d6) δ: 9.03 (d, 4J = 1.4 Hz, 1H), 8.47 (dd, 3J = 4.6 Hz, 4J = 1.4 Hz, 1H), 8.12 (dd, 3J = 8.8 Hz, 4J = 5.5 Hz, 2H), 8.04 (d, 3J = 4.6 Hz, 1H), 7.39 (tapp, 3J = 3JC–F = 8.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ: 161.02 (d, 1JC–F = 243.7 Hz, C), 147.21 (C), 143.64 (CH), 142.56 (C), 130.88 (d, 3JC–F = 11.1 Hz, 2CH), 130.71 (CH), 129.44 (d, 4JC–F = 2.5 Hz, C), 120.17 (CH), 115.46 (d, 2JC–F = 21.8 Hz, 2CH), 67.61 (C); MS (ESI) m/z (%): 340.0 (100) [M + H]+, 341.0 (20) [M + H + 1]+; RT (min): 2.92; UPLC purity: 97%; IR (ATR), cm−1: 3014 (νC–Har), 1602 (νC N), 1454 (νC C), 1211 (νC–F), 665 (νC–I).
2-(4-Fluorophenyl)-3-(pyridin-4-yl)imidazo[1,2-a]pyrazine 8
To a solution of 7 (100 mg, 0.29 mmol, 1 eq.) in dioxane/H2O (9 : 1) (3.5 mL) were added 4-pyridylboronic acid (40 mg, 0.32 mmol, 1.1 eq.), potassium phosphate tribasic (156 mg, 0.73 mmol, 2.5 eq.) and [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (10 mg, 0.01 mmol, 0.05 eq.). The vial was purged with N2 for 10 min and then sealed. The mixture was heated at 100 °C for 7 h and then it was cooled to room temperature. Water was added and the aqueous layer was extracted with ethyl acetate (2 × 10 mL). The combined organic extracts were washed with brine and water, dried over sodium sulfate, filtered and concentrated in vacuo. The crude product was purified by silica gel column chromatography using CH2Cl2/MeOH (9 : 1) as eluent to afford 2-(4-fluorophenyl)-3-(pyridin-4-yl)imidazo[1,2-a]pyrazine 8 (61 mg, 71%) as a brown powder. Rf = 0.62 (CH2Cl2/MeOH 9 : 1); Mp: 164–165 °C; 1H NMR (400 MHz, DMSO-d6) δ: 9.21 (d, 4J = 1.3 Hz, 1H), 8.80 (d, 3J = 6.1 Hz, 2H), 8.32 (dd, 3J = 4.5 Hz, 4J = 1.4 Hz, 1H), 7.90 (d, 3J = 4.6 Hz, 1H), 7.62 (dd, 3J = 8.8 Hz, 4J = 5.5 Hz, 2H), 7.59 (d, 3J = 6.1 Hz, 2H), 7.24 (tapp, 3J = 3JC–F = 8.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ: 161.11 (d, 1JC–F = 243.1 Hz, C), 150.55 (2CH), 143.45 (C), 143.18 (CH), 139.98 (C), 135.87 (C), 130.11 (d, 3JC–F = 8.5 Hz, 2CH), 130.00 (CH), 129.27 (d, 4JC–F = 3.1 Hz, C), 124.66 (2CH), 117.43 (CH), 115.55 (d, 2JC–F = 21.8 Hz, 2CH), 69.67 (C); MS (ESI) m/z (%): 291.2 (100) [M + H]+, 292.2 (20). [M + H + 1]+; RT (min): 1.60; UPLC purity: 90%; IR (ATR), cm−1: 3043 (νC–Har), 1600 (νC N), 1510 (νC C), 1213 (νC–F); HRMS (TOF MS ES+): calcd. for C17H11FN4 [M + H]+ 291.1041, found: 291.1031.
3-(Methylthio)pyrazin-2-amine 9
A mixture of 2 (10.00 g, 77.19 mmol, 1 eq.) and sodium methanethiolate (8.12 g, 115.78 mmol, 1.5 eq.) in DMF (78 mL) and EtOH (78 mL) was stirred at 85 °C for 2 h. The mixture was concentrated, diluted with water and filtered. The filtrate was extracted with EtOAc. The organic layer was washed with water, dried over Na2SO4, filtered and concentrated. The crude product was then purified by column chromatography (100% H2O to H2O/CH3CN 9 : 1) to afford 9 (9.08 g, 83%) as a white powder. Rf = 0.43 (cyclohexane/EtOAc 6 : 4); Mp: 91–92 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.68 (d, 3J = 2.8 Hz, 1H), 7.65 (d, 3J = 2.8 Hz, 1H), 6.20 (s, 2H), 2.48 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 152.32 (C), 140.77 (CH), 136.74 (CH), 131.61 (C), 11.96 (C); MS (ESI) m/z (%): 142.10 (100) [M + H]+; RT (min): 1.16; UPLC purity: 100%; IR (ATR), cm−1: 3419 (νNH2), 1625 (δN–H), 1514 (νC C), 1120 (νC–N), 719 (νS–C).
2-(4-Fluorophenyl)-8-(methylthio)imidazo[1,2-a]pyrazine 10
To a solution of 9 (1.5 g, 10.63 mmol, 1 eq.) in DME (10 mL) was added 2-bromo-4-fluoroacetophenone (2.78 g, 12.76 mmol, 1.2 eq.). The mixture was stirred at room temperature overnight. The precipitate was collected by filtration and washed with Et2O. The material was used in the next step. To a solution of the precipitate in ethanol (37 mL) was reflux for 4 h then it was cooled to room temperature and the solvent was evaporated in vacuo. NaHCO3 sat was added and the aqueous layer was extracted with chloroform. The combined organic extracts were dried over sodium sulfate, filtered, concentrated in vacuo. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (8 : 2) to afford 2-(4-fluorophenyl)-8-(methylthio)imidazo[1,2-a]pyrazine 10 (472 mg, 17%) as a yellow powder. Rf = 0.41 (cyclohexane/EtOAc 6 : 4); Mp: 129–130 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.53 (s, 1H), 8.34 (d, 3J = 4.6 Hz, 1H), 8.04 (dd, 3J = 8.8 Hz, 3JC–F = 5.5 Hz, 2H), 7.8 (d, 3J = 4.6 Hz, 1H), 7.36 (t, 2H, 3J = 3JC–F = 8.8 Hz, 2H), 2.63 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 162.11 (d, 1J = 245.2 Hz, C), 152.13 (C), 143.75 (C), 137.85 (C), 129.43 (d, 4J = 2.9 Hz, C), 128.46 (CH), 127.98 (d, 3J = 8.3 Hz, 2CH), 116.43 (CH), 115.58 (d, 2J = 21.5 Hz, 2CH), 111.22 (CH), 11.32 (CH3); MS (ESI) m/z (%): 260.06 (100) [M + H]+; RT (min): 2.89; UPLC purity: 99%; IR (ATR), cm−1: 3155 (νC–Har), 2935 (νCH3), 1608 (νC N), 1219 (νC–F), 698 (νS–C).
2-(4-Fluorophenyl)-3-iodo-8-(methylthio)imidazo[1,2-a]pyrazine 11
To a solution of 10 (397 mg, 1.53 mmol, 1 eq.) in acetonitrile (11 mL) was added N-iodosuccinimide (517 mg, 2.29 mmol, 1.5 eq.). The reaction mixture was stirred at 80 °C for 4 h. After cooling, the resulting mixture was diluted with ethyl acetate and washed with water. The organic layer was dried over sodium sulfate, filtered and concentrated in vacuo. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (8 : 2) to afford 2-(4-fluorophenyl)-3-iodo-8-(methylthio)imidazo[1,2-a]pyrazine 11 (330 mg, 56%) as a yellow powder. Rf = 0.34 (cyclohexane/EtOAc 8 : 2); Mp: 172–173 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.18 (d, 3J = 4.6 Hz, 1H), 8.07 (dd, 3J = 8.8 Hz, 4JC–F = 5.5 Hz, 2H), 7.93 (d, 3J = 4.6 Hz, 1H), 7.36 (t, 2H, 3J = 3JC–F = 8.8 Hz, 2H), 2.64 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 162.13 (d, 1JC–F = 245.2 Hz, C), 152.16 (C), 143.77 (C), 137.82 (C), 129.44 (d, 3JC–F = 12.9 Hz, 2CH), 128.46 (CH), 127.99 (d, 4JC–F = 2.9 Hz, C), 116.43 (CH), 115.55 (d, 2JC–F = 21.5 Hz, 2CH) 111.21 (C), 11.36 (CH3); MS (ESI) m/z (%): 385.95 (95) [M + H]+; RT (min): 3.62; UPLC purity: 96%; IR (ATR), cm−1: 3150 (νC–Har), 2926 (νCH3), 1604 (νC N), 1224 (νC–F), 725 (νS–C), 698 (νC–I).
2-(4-Fluorophenyl)-8-(methylthio)-3-(pyridin-4-yl)imidazo[1,2-a]pyrazine 12
To a solution of 11 (300 mg, 0.78 mmol, 1 eq.) in dioxane/water (9 : 1) (10 mL) were added 4-pyridylboronic acid (105 mg, 0.86 mmol, 1.1 eq.), potassium phosphate tribasic (414 mg, 1.95 mmol, 2.5 eq.) and [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (28 mg, 0.04 mmol, 0.05 eq.). The vial was purged with nitrogen for 10 min and then sealed. The mixture was heated at 100 °C overnight and then it was cooled to room temperature. Water was added and the aqueous layer was extracted with ethyl acetate. The combined organic extracts were washed with brine and water, dried over sodium sulfate, filtered, concentrated in vacuo. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (1 : 1 to 2 : 8) to afford 2-(4-fluorophenyl)-8-(methylthio)-3-(pyridin-4-yl)imidazo[1,2-a]pyrazine 12 (123 mg, 47%) as a beige powder. Rf = 0.39 (100% AcOEt); Mp: 233–234 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.78 (d, 3J = 5.5 Hz, 2H), 8.01 (d, 3J = 4.5 Hz, 1H), 7.79 (d, 3J = 4.5 Hz, 1H), 7.58 (d, 3J = 5.5 Hz, 2H), 7.56 (dd, 3J = 8.8 Hz, 4JC–F = 5.5 Hz, 2H), 7.23 (t, 3J = 3JC–F = 8.8 Hz, 2H), 2.64 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 162.13 (d, 1JC–F = 246.1 Hz, C), 152.96 (C), 150.82 (2CH), 141.84 (C), 137.33 (C), 135.84 (C), 130.12 (d, 3JC–F = 8.5 Hz, 2CH), 129.34 (CH), 129.21 (d, 4JC–F = 3.1 Hz, C), 124.66 (2CH), 120.36 (C), 115.55 (d, 2JC–F = 21.8 Hz, 2CH), 113.77 (CH), 11.44 (CH3); MS (ESI) m/z (%): 337.08 (99) [M + H]+; RT (min): 2.66; UPLC purity: 97%; IR (ATR), cm−1: 3035 (νC–Har), 2922 (νCH3), 1598 (νC N), 1510 (νC C); HRMS (TOF MS ES+): calcd. for C18H13FN4S [M + H]+ 337.0918, found: 337.0915.
8-Chloroimidazo[1,2-a]pyrazine 13
To a solution of 2 (4.00 g, 30.97 mmol, 1 eq.) and aqueous hydrobromic acid solution (33% in water, 1 mL) in propan-2-ol (100 mL) was added slowly 2-bromo-1,1-dimethoxyethane (12.20 g, 61.95 mmol, 2 eq.). The reaction was heated to 80 °C for 3 h. The reaction mixture was cooled to room temperature and a saturated solution of sodium bicarbonate was slowly added until the pH = 7 and the aqueous phase was then extracted twice with dichloromethane. The combined organic layers were dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (30 : 70 to 0 : 100) to afford 8-chloroimidazo[1,2-a]pyrazine 13 (4.34 g, 91%) as a white powder. Rf = 0.33 (EtOAc); Mp: 183–184 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.65 (d, 3J = 4.5 Hz, 1H), 8.28 (d, 3J = 0.8 Hz, 1H), 7.88 (d, 3J = 0.8 Hz, 1H), 7.72 (d, 3J = 4.5 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 141.50 (C), 136.91 (C), 135.33 (CH), 127.18 (CH), 120.63 (CH), 117.19 (CH); MS (ESI) m/z (%): 153.9 (100) [M + H]+, 155.9 (35). [M + H + 2]+; RT (min): 0.95; UPLC purity: 97%; IR (ATR), cm−1: 3105 (νC–Har), 1479 (νC C), 736 (νC–Cl).
8-Chloro-3-iodoimidazo[1,2-a]pyrazine 14
To a solution of 13 (1.00 g, 6.51 mmol, 1 eq.) in anhydrous DMF (33 mL) was added N-iodosuccinimide (1.46 g, 9.76 mmol, 1.5 eq.). The reaction mixture was stirred at room temperature for 1 h then heated at 45 °C for 72 h. The reaction mixture was diluted with distilled water and extracted with ethyl acetate (3 × 300 mL). The combined organic layers were dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (30 : 70 to 100% EtOAc) to afford 8-chloro-3-iodoimidazo[1,2-a]pyrazine 14 (0.66 g, 36%) as a yellow powder. Rf = 0.76 (cyclohexane/EtOAc 3 : 7); Mp: 262–263 °C; 1H NMR (400 MHz, CDCl3) δ: 8.05 (d, 3J = 4.5 Hz, 1H), 7.91 (s, 1H), 7.83 (d, 3J = 4.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 143.92 (C), 142.23 (CH), 129.65 (C), 129.02 (CH), 118.89 (CH), 62.36 (C); MS (ESI) m/z (%): 279.9 (100) [M + H]+, 281.9 (35). [M + H + 2]+; RT (min): 1.64; UPLC purity: 97%; IR (ATR), cm−1: 3099 (νC–Har), 1604 (νC N), 783 (νC–Cl).
3-Iodo-N,N-dimethylimidazo[1,2-a]pyrazin-8-amine 15
To a solution of 14 (150 mg, 0.51 mmol, 1 eq.) in DMF (12 mL) was added N,N-dimethylamine (40% in water, 0.2 mL, 1.02 mmol, 2 eq.). The vial was purged with N2 for 10 min and then sealed. The reaction was heated to 100 °C for 3 h. The reaction mixture was cooled to room temperature and the mixture was diluted with distilled water and extracted with ethyl acetate (3 × 300 mL). The combined organic layers were dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (30 : 70) to afford 3-iodo-N,N-dimethylimidazo[1,2-a]pyrazin-8-amine 15 (88 mg, 60%) as a purple solid. Rf = 0.77 (cyclohexane/AcOEt 3 : 7); Mp: 82–83 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.70 (s, 1H), 7.61 (d, 3J = 4.5 Hz, 1H), 7.45 (d, 3J = 4.5 Hz, 1H), 3.49 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ: 149.44 (C), 137.48 (C), 135.96 (CH), 128.97 (CH), 109.77 (CH), 68.12 (C), 39.93 (2CH3); MS (ESI) m/z (%): 289.0 (100) [M + H]+; RT (min): 1.11; UPLC purity: 100%; IR (ATR), cm−1: 3021 (νC–Har), 2808 (νC–CH3), 1602 (νC N), 1535 (νC C), 632 (νC–I).
N,N-Dimethyl-3-(pyridin-4-yl)imidazo[1,2-a]pyrazin-8 amine 16
To a solution of 15 (100 mg, 0.34 mmol, 1 eq.) in dioxane/H2O (9/1) (3.5 mL) were added 4-pyridylboronic acid (46 mg, 0.38 mmol, 1.1 eq.), potassium phosphate tribasic (184 mg, 0.86 mmol, 2.5 eq.) and [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (12 mg, 0.01 mmol, 0.05 eq.). The vial was purged with N2 for 10 min and then sealed. The mixture was heated at 100 °C for 7 h and then it was cooled to room temperature. Water was added and the aqueous layer was extracted with ethyl acetate (2 × 10 mL). The combined organic extracts were washed with brine and water, dried over sodium sulfate, filtered and concentrated in vacuo. The crude product was purified by silica gel column chromatography using (cyclohexane/EtOAc 10 : 90 to 0 : 100) as eluent to afford N,N-dimethyl-3-(pyridin-4-yl)imidazo[1,2-a]pyrazin-8 amine 16 (33 mg, 68%) as a beige powder. Rf = 0.16 (cyclohexane/EtOAc 1 : 9); Mp: 159–160 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.71 (d, 3J = 4.5 Hz, 2H), 7.98 (s, 1H), 7.96 (d, 3J = 4.5 Hz, 1H), 7.65 (d, 3J = 4.5 Hz, 2H), 7.42 (d, 3J = 4.5 Hz, 1H), 3.94 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ: 150.40 (2CH), 150.15 (C), 135.79 (C), 134.77 (C), 132.13 (CH), 129.16 (CH), 124.22 (C), 121.44 (2CH), 108.20 (CH), 39.50 (2CH3); MS (ESI) m/z (%): 240.1 (100) [M + H]+, 241.2 (15), [M + H + 1]+; RT (min): 0.99; UPLC purity: 92%; IR (ATR), cm−1: 3024 (νC–Har), 2924 (νC–CH3), 1608 (νC N), 1544 (νC C); HRMS (TOF MS ES+): calcd. for C13H13N5 [M + H]+ 240.1244, found: 240.1237.
N,N-Dimethyl-2-(pyridin-4-yl)imidazo[1,2-a]pyrazin-8-amine 20
To a solution of 19 (280 mg, 0.90 mmol, 1 eq.) in dioxane/water (9 : 1) (10 mL) were added 4-pyridylboronic acid (122 mg, 0.99 mmol, 1.1 eq.), potassium phosphate tribasic (479 mg, 2.25 mmol, 2.5 eq.) and [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (32 mg, 0.04 mmol, 0.05 eq.). The vial was purged with nitrogen for 10 min and then sealed. The mixture was heated at 100 °C overnight and then it was cooled to room temperature. Water was added and the aqueous layer was extracted with ethyl acetate. The combined organic extracts were washed with brine and water, dried over sodium sulfate, filtered, concentrated in vacuo. The crude product was purified by silica gel column chromatography cyclohexane/EtOAc (1 : 1) to afford N,N-dimethyl-2-(pyridin-4-yl)imidazo[1,2-a]pyrazin-8-amine 20 (110 mg, 51%) as a green solid. Rf = 0.39 (100% AcOEt); Mp: 77–78 °C; 1H NMR (400 MHz, DMSO) δ: 8.62 (d, 3J = 5.9 Hz, 2H), 8.56 (s, 1H), 7.88 (d, 3J = 6.0 Hz, 2H), 7.81 (d, 3J = 4.4 Hz, 1H), 7.33 (d, 3J = 4.4 Hz, 1H), 3.52 (s, 4H); 13C NMR (100 MHz, DMSO) δ: 150.19 (2CH), 149.48 (C), 140.49 (C), 139.54 (C), 133.93 (C), 128.40 (CH), 119.73 (2CH), 113.07 (CH), 110.51 (CH), 39.25 (2CH3); MS (ESI) m/z (%): 240.12 (100) [M + H]+; RT (min): 1.28; UPLC purity: 96%; IR (ATR), cm−1: 3047 (νC–Har), 2914 (νC–CH3), 1610, 1546 (νC C and νC N); HRMS (TOF MS ES+): calcd. for C13H13N5 [M + H]+ 240.1244, found: 240.1238.
3-(4-Fluorophenyl)-N,N-dimethyl-2-(pyridin-4-yl)imidazo[1,2-a]pyrazin-8-amine 21
To a solution of 20 (100 mg, 0.42 mmol, 1 eq.) in N,N-dimethylacetamide (2 mL) were added 1-fluoro-4-iodobenzene (185 mg, 0.83 mmol, 2 eq.), palladium acetate (35 mg, 0.02 mmol, 0.04 eq.), tricyclohexylphosphine tetrafluoroborate (12 mg, 0.03 mmol, 0.08 eq.), pivalic acid (25 mg, 0.25 mmol, 0.6 eq.) and potassium carbonate (173 mg, 1.25 mmol, 3 eq.). The vial was purged with nitrogen for 10 min and then sealed. The mixture was heated at 100 °C for 12 h and then it was cooled to room temperature. Water was added and the aqueous layer was extracted with ethyl acetate (2 × 10 mL). The combined organic extracts were washed with brine and water, dried over sodium sulfate, filtered, concentrated in vacuo. The crude product was then purified by column chromatography (100% H2O to H2O/CH3CN 9 : 1) to afford 3-(4-fluorophenyl)-N,N-dimethyl-2-(pyridin-4-yl)imidazo[1,2-a]pyrazin-8-amine 21 (97 mg, 70%) as a yellow powder. Rf = 0.11 (cyclohexane/EtOAc 7 : 3); Mp: 265–266 °C; 1H NMR (400 MHz, CDCl3) δ: 8.76 (d, 3J = 5.5 Hz, 2H), 7.54 (d, 3J = 5.5 Hz, 2H), 7.42 (dd, 3J = 8.8 Hz, 3JC–F = 5.5 Hz, 2H), 7.33 (dd, 3J = 4.5 Hz, 1H), 7.28 (t, 3J = 3JC–F = 8.8 Hz, 2H), 7.10 (d, 3J = 4.6 Hz, 1H), 3.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 163.55 (1JC–F = 248.0 Hz, C), 150.59 (C), 150.02 (2CH), 141.52 (C), 137.51 (C), 134.07 (C), 132.66 (d, 3JC–F = 8.5 Hz, 2CH), 129.18 (CH), 125.00 (d, 4JC–F = 3.8 Hz, C), 123.35 (C), 121.63 (2CH), 117.14 (d, 2JC–F = 21.9 Hz, 2CH), 107.00 (CH), 39.98 (2CH3); MS (ESI) m/z (%): 334.14 (95) [M + H]+; RT (min): 2.05; UPLC purity: 95%; IR (ATR), cm−1: 1600 (νC N), 1539 (νC C); HRMS (TOF MS ES+): calcd. for C19H16FN5 [M + H]+ 334.1463, found: 334.1464.
2-(4-Fluorophenyl)-N,N-dimethyl-3-(2-methylpyridin-4-yl)imidazo[1,2-a]pyrazin-8-amine 25
To a solution of 22 (100 mg, 0.26 mmol, 1 eq.) in dioxane/H2O (9 : 1) (3 mL) were added 2-methylpyridine-4-boronic acid (39 mg, 0.29 mmol, 1.1 eq.), [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (10 mg, 0.01 mmol, 0.05 eq.) and potassium phosphate tribasic (138 mg, 0.65 mmol, 2.5 eq.). The vial was purged with N2 for 20 min and then sealed. The mixture was heated at 100 °C for 48 h and then cooled to room temperature. Water was added and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were washed with brine and water, dried over sodium sulfate, filtered and concentrated in vacuo. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (50 : 50) as eluent to afford 2-(4-fluorophenyl)-N,N-dimethyl-3-(2-methylpyridin-4-yl)imidazo[1,2-a]pyrazin-8-amine 25 (55 mg, 61%) as a beige powder. Rf = 0.53 (CH2Cl2/MeOH 95 : 5); Mp: 105–106 °C; 1H NMR (400 MHz, CDCl3) δ: 8.67 (d, 3J = 4.5 Hz, 1H), 7.59 (dd, 3J = 8.8 Hz, 4J = 5.5 Hz, 2H), 7.36 (d, 3J = 4.5 Hz, 1H), [7.28–7.24] (m, 2H), 7.17 (d, 3J = 4.8 Hz, 1H), 7.02 (tapp, 3J = 3JC–F = 8.8 Hz, 2H), 3.64 (s, 6H), 2.64 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 162.7 (1JC–F = 247.7 Hz, C), 160.03 (C), 150.61 (C), 150.40 (CH), 140.57 (C), 138.27 (C), 134.25 (C), 129.82 (3JC–F = 8.1 Hz, 2CH), 129.70 (4JC–F = 3.4 Hz, C), 129.23 (CH), 124.19 (CH), 121.97 (CH), 119.82 (C), 115.53 (2JC–F = 21.5 Hz, 2CH), 106.97 (CH), 33.99 (2CH3), 24.61 (CH3); MS (ESI) m/z (%): 348.6 (100) [M + H]+; RT (min): 1.76; UPLC purity: 97%; IR (ATR), cm−1: 3015 (νC–Har), 1605 (νC N), 1221 (νC–F); HRMS (TOF MS ES+): calcd. for C20H18FN5 [M + H]+ 348.1619, found: 348.1615.
2-(4-Fluorophenyl)-N,N-dimethyl-3-(quinolin-4-yl)imidazo[1,2-a]pyrazin-8-amine 26
To a solution of 22 (158 mg, 0.41 mmol, 1 eq.) in dioxane/H2O (9 : 1) (4 mL) were added quinoline-4-boronic acid (78 mg, 0.45 mmol, 1.1 eq.), [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (15 mg, 0.02 mmol, 0.05 eq.) and potassium phosphate tribasic (217 mg, 1.02 mmol, 2.5 eq.). The vial was purged with N2 for 20 min and then sealed. The mixture was heated at 100 °C for 24 h and then cooled to room temperature. Water was added and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were washed with brine and water, dried over sodium sulfate, filtered and concentrated in vacuo. The crude product was purified by reverse phase chromatography using H2O/CH3CN (80 : 20) as eluent to afford 2-(4-fluorophenyl)-N,N-dimethyl-3-(quinolin-4-yl)imidazo[1,2-a]pyrazin-8-amine 26 (78 mg, 50%) as a yellow powder. Rf = 0.29 (cyclohexane/EtOAc 1 : 1); Mp: 180–181 °C; 1H NMR (400 MHz, CDCl3) δ: 9.08 (d, 3J = 4.3 Hz, 1H), 8.28 (d, 3J = 8.6 Hz, 1H), 7.81 (tapp, 3J = 8.5 Hz, 1H), 7.52 (dd, 3J = 8.8 Hz, 4J = 5.5 Hz, 2H), 7.48 (d, 3J = 4.4 Hz, 1H), [7.47–7.42] (m, 2H), 7.28 (d, 3J = 4.4 Hz, 1H), 6.89 (tapp, 3J = 3JC–F = 8.8 Hz, 2H), 6.76 (d, 3J = 4.3 Hz, 1H), 3.70 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 162.65 (d, 1JC–F = 247.3 Hz, C), 161.71 (C), 150.74 (CH), 150.14 (C), 149.26 (C), 141.16 (C), 136.45 (CH), 134.58 (C), 130.55 (d, 4JC–F = 3.6 Hz, C), 129.25 (d, 3JC–F = 8.0 Hz, 2CH), 129.06 (CH), 128.08 (CH), 126.94 (C), 125.28 (CH), 124.03 (CH), 122.58 (C), 117.78 (CH), 115.58 (d, 2JC–F = 21.3 Hz, 2CH), 107.65 (CH), 40.07 (2CH3); MS (ESI) m/z (%): 384.7 (100) [M + H]+; RT (min): 2.20; UPLC purity: 100%; IR (ATR), cm−1: 3001 (νC–Har), 2878 (νCH3), 1606 (νC N), 1221 (νC–F); HRMS (TOF MS ES+): calcd. for C23H18FN5 [M + H]+ 384.1619, found: 384.1612.
N-(4-Chloropyridin-2-yl)benzamide 28
To a solution of 27 (300 mg, 2.33 mmol, 1 eq.) in CH3CN (6 mL) were added benzoyl chloride (0.40 mL, 3.47 mmol, 2 eq.) and 4-dimethylaminopyridine (211 mg, 1.73 mmol, 1 eq.). The solution was irradiated under microwave at 100 °C (100 W) for 5 min. After cooling, the resulting mixture was placed in a cold room for 6 h minimum. The precipitate was filtered to collect N-(4-chloropyridin-2-yl)benzamide 28 as a white powder (163 mg, 30%). Rf = 0.85 (cyclohexane/EtOAc 1 : 1); Mp: 190–191 °C; 1H NMR (400 MHz, DMSO-d6) δ: 11.23 (s, 1H), 8.40 (d, 3J = 5.6 Hz, 1H), 8.32 (d, 4J = 2.1 Hz, 1H), 8.03 (d, 3J = 8.2 Hz, 2H), 7.62 (t, 3J = 5.6 Hz, 1H), 7.52 (tapp, 3J = 3J = 8.5 Hz, 2H), 7.35 (dd, 3J = 5.6 Hz, 4J = 2.1 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 166.21 (C), 152.36 (C), 148.81 (CH), 145.51 (C), 133.01 (C), 132.63 (CH), 129.47 (2CH), 128.32 (2CH), 120.65 (CH), 115.87 (CH); MS (ESI) m/z (%): 233.1 (100) [M + H]+, 235.1 (35) [M + H + 2]+; RT (min): 2.71; UPLC purity: 99%; IR (ATR), cm−1: 3062 (νC–Har), 1680 (νC O), 1600 (νC N), 1571 (νC C), 1280 (νC–N).
2-(4-Fluorophenyl)-N,N-dimethylimidazo[1,2-a]pyrazin-8-amine 29
To a solution of 3 (2.00 g, 8.07 mmol, 1 eq.) in DMF (90 mL) were added sodium carbonate (1.12 g, 8.07 mmol, 1 eq.) and N,N-dimethylamine (40% in water, 8.18 mL, 162 mmol, 20 eq.). The mixture was stirred at 90 °C for 5 h. After the completion, the resulting mixture was diluted in water and ethyl acetate. The aqueous phase was extracted twice with ethyl acetate. The organic layer was washed with water (5×), dried over sodium sulfate, filtered and evaporated to dryness to give the pure 2-(4-fluorophenyl)-N,N-dimethylimidazo[1,2-a]pyrazin-8-amine 29 as a brown powder (1.72 g, 83%). Rf = 0.64 (cyclohexane/EtOAc 1 : 1); Mp: 109–111 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.34 (s, 1H), 7.98 (mt, 2H), 7.79 (d, 3J = 4.4 Hz, 1H), [7.31–7.28] (m, 3H), 3.51 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ: 163.32 (d, 1JC–F = 245.8 Hz, C), 149.98 (C), 141.94 (CH) 133.44 (C), 130.52 (d, 4JC–F = 3.4 Hz, C), 128.74 (C), 127.45 (d, 3JC–F = 8.2 Hz, 2CH), 115.55 (d, 2JC–F = 22.3 Hz, 2CH), 111.60 (CH), 110.37 (CH). 2CH3 are under the solvent peak; MS (ESI) m/z (%): 257.2 (100) [M + H]+; RT (min): 1.76; UPLC purity: 100%; IR (ATR), cm−1: 2926 (νCH3), 1604 (νC N), 1537 (νC C), 1093 (νC–F).
N-(4-(8-(Dimethylamino)-2-(4-fluorophenyl)imidazo[1,2-a]pyrazin-3-yl)pyridin-2-yl)benzamide 30
To a solution of 29 (200 mg, 0.78 mmol, 1 eq.) in DMAc (4 mL) were added N-(4-chloropyridin-2-yl)benzamide (325 mg, 1.56 mmol, 2 eq.), palladium acetate (70 mg, 0.31 mmol, 0.4 eq.), tricyclohexylphosphonium tetrafluoroborate (23 mg, 0.06 mmol, 0.08 eq.), pivalic acid (48 mg, 0.47 mmol, 0.6 eq.), and potassium carbonate (323 mg, 2.34 mmol, 3 eq.). The vial was purged with N2 for 15 minutes and then sealed. The mixture was heated at 110 °C for 68 h. The resulting mixture was filtered on Celite® and washed with MeOH. The organic layer was washed with water and brine, dried over sodium sulfate, filtered, and concentrated in vacuo. The crude product was purified by reverse phase chromatography using (100% H2O to H2O/CH3CN 50 : 50) as eluent to afford N-(4-(8-(dimethylamino)-2-(4-fluorophenyl)imidazo[1,2-a]pyrazin-3-yl)pyridin-2-yl)benzamide 30 (20 mg, 8%) as a white powder. Rf = 0.61 (CH2Cl2/MeOH 95 : 5); Mp: decomposition from 87 °C; 1H NMR (400 MHz, DMSO-d6) δ: 11.10 (s, 1H), 8.56 (d, 3J = 5.0 Hz, 1H), 8.33 (s, 1H), 8.02 (d, 3J = 7.6 Hz, 2H), [7.66–7.60] (m, 3H), [7.53–7.50] (m, 3H), 7.39 (d, 3J = 5.0 Hz, 1H), [7.26–7.20] (m, 3H), 3.56 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ: 166.27 (C), 161.82 (d, 1JC–F = 244.9 Hz, C), 153.25 (C), 149.66 (C), 149.31 (CH), 139.36 (C), 138.50 (C), 133.83 (C), 133.20 (CH), 132.03 (C), 129.67 (d, 4JC–F = 2.1 Hz, C), 129.59 (d, 3JC–F = 8.4 Hz, 2CH), 129.01 (C), 128.33 (2CH), 128.02 (2CH), 120.76 (CH), 119.86 (CH), 115.49 (d, 2JC–F = 21.8 Hz, 2CH), 115.25 (CH), 107.35 (CH). 2CH3 are under the solvent peak; MS (ESI) m/z (%): 453.2 (100) [M + H]+, 454.2 (20) [M + H + 1]+; RT (min): 2.48; UPLC purity: 98%; IR (ATR), cm−1: 3281 (νNH), 3080 (νC–Har), 1672 (νC O), 1604 (νC N), 1529 (νC C); HRMS (TOF MS ES+): calcd. for C26H21FN6O [M + H]+ 453.1834, found: 453.1835.
2-(4-Fluorophenyl)-3-(1-methyl-1H-pyrazol-4-yl)imidazo[1,2-a]pyrazine 31
To a solution of 7 (95 mg, 0.28 mmol, 1 eq.) in dioxane/H2O (9 : 1) (3 mL) were added (1-methyl-1H-pyrazol-4-yl)boronic acid (38 mg, 0.30 mmol, 1.1 eq.), [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (10 mg, 0.01 mmol, 0.05 eq.) and potassium phosphate tribasic (149 mg, 0.70 mmol, 2.5 eq.). The vial was purged with N2 for 20 min and then sealed. The mixture was heated at 100 °C for 24 h and then cooled to room temperature. Water was added and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were washed with brine and water, dried over sodium sulfate, filtered and concentrated in vacuo. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (100 : 0 to 20 : 80) as eluent to afford 2-(4-fluorophenyl)-3-(1-methyl-1H-pyrazol-4-yl)imidazo[1,2-a]pyrazine 31 (60 mg, 73%) as a yellow powder. Rf = 0.31 (CH2Cl2/MeOH 95 : 5); Mp: 175–176 °C; 1H NMR (400 MHz, CDCl3) δ: 9.12 (d, 4J = 1.1 Hz, 1H), 7.9 (dd, 3J = 4.7 Hz, 4J = 1.1 Hz, 1H), 7.88 (d, 3J = 4.7 Hz, 1H), 7.78 (dd, 3J = 8.8 Hz, 4J = 5.3 Hz, 2H), 7.68 (s, 1H), 7.55 (s, 1H), 7.07 (tapp, 3J = 3JC–F = 8.6 Hz, 2H), 4.05 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 163.04 (d, 1JC–F = 248.1 Hz, C), 144.71 (C), 143.63 (CH), 140.42 (C), 139.92 (CH), 130.57 (CH), 130.06 (CH), 129.94 (d, 3JC–F = 7.7 Hz, 2CH), 129.60 (d, 4JC–F = 3.2 Hz, C), 116.51 (CH), 115.72 (d, 2JC–F = 21.5 Hz, 2CH), 114.35 (C), 108.48 (C), 39.61 (CH3); MS (ESI) m/z (%): 294.2 (100) [M + H]+, 295.2 (20) [M + H + 1]+; RT (min): 2.96; UPLC purity: 99%; IR (ATR), cm−1: 1527 (νC C), 1215 (νC–F), 1159 (νC–N); HRMS (TOF MS ES+): calcd. for C16H12FN5 [M + H]+ 294.1150, found: 294.1144.
4-(2-(4-Fluorophenyl)imidazo[1,2-a]pyrazin-3-yl)quinoline 32
To a solution of 7 (206 mg, 0.61 mmol, 1 eq.) in dioxane/H2O (9 : 1) (5 mL) were added quinoline-4-boronic acid (116 mg, 0.67 mmol, 1.1 eq.), [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (22 mg, 0.03 mmol, 0.05 eq.) and potassium phosphate tribasic (323 mg, 1.52 mmol, 2.5 eq.). The vial was purged with N2 for 20 min and then sealed. The mixture was heated at 100 °C for 24 h and then cooled to room temperature. Water was added and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were washed with brine and water, dried over sodium sulfate, filtered and concentrated in vacuo. The crude product was purified by silica gel column chromatography using cyclohexane/EtOAc (50 : 50 to 20 : 80) as eluent to afford 4-(2-(4-fluorophenyl)imidazo[1,2-a]pyrazin-3-yl)quinoline 32 (30 mg, 19%) as a beige powder. Rf = 0.13 (EtOAc); Mp: 222–223 °C; 1H NMR (400 MHz, CDCl3) δ: 9.25 (d, 4J = 1.2 Hz, 1H), 9.12 (d, 3J = 4.2 Hz, 1H), 8.31 (d, 3J = 8.5 Hz, 1H), 7.85 (d, 3J = 4.7 Hz, 1H), 7.82 (dd, 3J = 4.7 Hz, 4J = 1.2 Hz, 1H), 7.57 (dd, 3J = 8.8 Hz, 4J = 4.3 Hz, 2H), [7.51–7.47] (m, 3H), 7.38 (d, 3J = 8.1 Hz, 1H), 6.94 (tapp, 3J = 3JC–F = 8.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ: 163.15 (d, 1JC–F = 247.3 Hz, C), 150.75 (CH), 149.32 (C), 145.63 (C), 144.02 (CH), 140.97 (C), 135.02 (C), 130.83 (d, 3JC–F = 10.8 Hz, 2CH), 130.31 (CH), 129.96 (CH), 129.88 (CH), 128.82 (d, 4JC–F = 3.0 Hz, C), 128.44 (CH), 126.43 (C), 124.75 (CH), 123.84 (CH), 117.75 (C), 116.70 (CH), 115.88 (d, 2JC–F = 21.3 Hz, 2CH); MS (ESI) m/z (%): 341.2 (100) [M + H]+, 342.2 (40) [M + H + 1]+; RT (min): 2.73; UPLC purity: 98%; IR (ATR), cm−1: 3041 (νC–Har), 1487 (νC C), 1213 (νC–F); HRMS (TOF MS ES+): calcd. for C21H13FN4 [M + H]+ 341.1197, found: 341.1198.
Biological evaluation
Antileishmanial activity
Cell lines and cultures
The mouse monocyte/macrophage cell line RAW 264.7 (TIB-71™ from ATCC) was maintained in culture in DMEM (Invitrogen Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen Life Technologies).
The Leishmania strains were obtained from the CNR Leish (Centre National de Référence des Leishmania, Montpellier, France). Strains used for in vitro experiments were Leishmania donovani (MHOM/ET/67/HU3) and L. major (MHOM/PT/92/CRE26).
These strains were used for in vitro experiments. Promastigotes forms were grown in RPMI 1640 medium (Gibco, Fisher Scientific) supplemented with GlutaMAX™ and 25 mM HEPES, 0.5 mg L−1 haemin, 10% foetal bovine serum (FBS) (Invitrogen, Life Technologies) at 25 °C in a dark environment under an atmosphere of 5% CO2.
In vitro antileishmanial evaluation on intramacrophage amastigotes
The mouse monocyte/macrophage cell line RAW 264.7 was maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum. RAW 264.7 cells were seeded into a 96-well microtiter plate at a density of 100 000 cells per well in 100 μL of DMEM. After incubation in a 5% CO2 incubator at 37 °C for 24 h, the culture medium was replaced with 100 μL of fresh DMEM containing a suspension of plateau-phase culture of promastigotes to reach a ratio parasite/macrophage of 16 : 1. In each plate, 8 wells contained axenic amastigotes (control of the parasite growth), 8 wells contained only macrophages (control of the macrophage growth) and finally 8 wells contained infected macrophages (control of the growth of intramacrophage parasites). After incubation in a 5% CO2 incubator at 37 °C for 24 h (the time needed by the parasite to infect the macrophage), cells were visualized using an inverted microscope to check the presence of inside parasites. Then, the culture medium was replaced with fresh DMEM to realize the serial dilution. Final concentrations of tested compounds were obtained by serial dilution ranging between 391 nM and 100 μM for pure compounds. After 72 hours of incubation, cells were visualized using an inverted microscope to check the cell lysis, their morphology and the presence of outside parasites. The viability of the amastigotes into macrophages was then assessed using the SYBR1 Green I (Invitrogen, France) incorporation method. Thus, the medium was removed and the cells were lysed in 100 μL lysis buffer. The plates were then subjected to 3 freeze–thaw cycles to achieve complete lysis. The parasite lysis suspension was diluted 1 : 1 in lysis buffer with SYBR Green I as previously described.25 The IC50 value was calculated by nonlinear regression using Icestimator website 1.2 version: https://www.antimalarial-icestimator.net/MethodIntro.htm. Fluorescence obtained was compared to those from the range obtained with parasite, infected cell and non-infected cell densities. The activity of the compounds was expressed as IC50 (concentration of drug inhibiting the parasite growth by 50%, comparatively to the controls treated with the excipient only). Miltefosine (Sigma, France) was used as the reference drug.
Cytotoxicity determination
Evaluation of compounds cytotoxicity using SYBR Green method
Cytotoxicity was evaluated on mouse monocyte/macrophage-like cell line RAW 264.7. The RAW 264.7 cells were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM) (Invitrogen Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum, at 37 °C in a humidified atmosphere containing 5% CO2. Cells were seeded into a 96-well microtitration plate at a density of 100 000 cells per well in 100 μL. After incubation in a 5% CO2 incubator at 37 °C for 24 h, the culture medium was replaced with 100 μL of fresh DMEM containing two-fold serial dilutions of the tested compounds. Final concentrations of tested compounds were obtained by serial dilution ranging between 49 nM and 100 μM for pure compounds. Miltefosine was used as the reference drug and cells without drug treatment as growth control. After incubation, cells were visualized using an inverted microscope to check their morphology and to control the drug solubility. After a 48 h incubation time at 37 °C with 5% CO2, growth was determined by using SYBR Green 1.25 The cytotoxicity of the compounds was expressed as IC50 (half-maximal inhibitory concentration: concentration inhibiting the macrophages growth by 50%). The IC50 was calculated by nonlinear regression using Icestimator website 1.2 version: https://www.antimalarial-icestimator.net/MethodIntro.htm.
Kinase inhibition assays
Kinase enzymatic activities were assayed in 384-well plates using the ADP-Glo™ assay kit (Promega, Madison, WI) according to manufacturer's recommendations (see ref. 26 for details on this method). Briefly, reactions were carried out in a final volume of 6 μL for 30 min at 30 °C in appropriate kinase buffer, with either protein or peptide as substrate and in the presence of 10 μM ATP. The experimental conditions used are described in Ibrahim et al.27 In order to determine the half-maximal inhibitory concentration (IC50), the assays were performed in duplicate in the absence or presence of increasing doses of the tested compounds. Kinase activities are expressed in % of maximal activity, i.e. measured in the absence of inhibitor. IC50 values were determined from the dose response curves using Prism-GraphPad (GraphPad Software, San Diego, CA, USA). The protein kinases and conditions used during this study were: (i) LmCK1.2 (LmjF35.1010, from Leishmania major, recombinant, expressed in bacteria)11 was assayed in buffer “A” (10 mM MgCl2, 1 mM EGTA, 1 mM DTT, 25 mM Tris-HCl pH 7.5, 50 μg mL−1 heparin) with 170 μM of the following peptide: RRKHAAIGSpAYSITA (“Sp” stands for phosphorylated serine) as CK1-specific substrate; (ii) HsCK1ε (human, recombinant, expressed by baculovirus in Sf9 insect cells) was assayed in buffer “A” with 170 μM of the following peptide: RRKHAAIGSpAYSITA. To validate both CK1ε and LmCK1.2 kinase assays, staurosporine from Streptomyces sp. (#S5921, purity 95%, Sigma-Aldrich) was used under the same conditions.
Molecular modelling
Molecular modelling studies were performed using SYBYL-X 2.1.1 software28 running on a Dell precision 7680 workstation. The three-dimensional structure of compound 30 was built from a standard fragments library and optimized using the Tripos force field29 including the electrostatic term calculated from Gasteiger and Hückel atomic charges. Powell's method available in Maximin2 procedure was used for energy minimization until the gradient value was smaller than 0.001 kcal mol−1 Å−1. The crystal structure of HsCK1δ complexed with an inhibitor at 1.98 Å resolution (PDB code: 4KBC, 68% homology)30 was used as the template. The LmCK1.2 3D model was constructed with the SwissModel server.31 Flexible docking of compound 30 into ATP-binding site was performed using GOLD program.32 The most stable docking solutions were selected according to the best scored conformation predicted by the ChemScore_kinase scoring function implemented in GOLD. Biovia Discovery Studio Visualizer was used for graphical display.33
The theoretical complex structure was subjected to a 100 ns molecular dynamics in explicit solvent environment (see ESI†). The resulting trajectory showed no major binding mode instability, thus validating the binding pose obtained by docking and providing extra data in favour of the π–cation interaction.
Author contributions
PM, NR and SC conceived the project. PM, MAB, CL, LT, JT, KG, LK, CC designed and synthesized the compounds. NR, SC, OL, CP, CT, FP, PLP and PML supervised and performed the biological studies. SB, BB and TR conducted the CK1 assays. CL and GB realized the molecular modelling study. JB and JT provided the analytical data. LT, PM and MAB took the lead in writing the manuscript. All authors have provided critical feedback and approved the final manuscript.
Conflicts of interest
The authors declare that there is no conflict of interest.
Supplementary Material
Acknowledgments
This work benefited from the support of the project TEXLEISH ANR-21-CE18-0026 of the French National Research Agency (ANR). The authors gratefully acknowledge the Consortium against Parasites and Fungi (CaPF) for the scientific exchanges. Authors acknowledge the Corsaire-ThalassOMICS Metabolomics Facility (Biogenouest, University of Nantes, France) for HRMS analyses. The authors also thank the Cancéropôle Grand Ouest (“Marines molecules, metabolism and cancer” network), IBiSA (French Infrastructures en sciences du vivant: biologie, santé et agronomie), Biogenouest (Western France life science and environment core facility network supported by the Conseil Régional de Bretagne) for supporting the KISSf screening facility (FR2424, CNRS and Sorbonne Université), Roscoff, France.
Electronic supplementary information (ESI) available: Molecular dynamics experiments. See DOI: https://doi.org/10.1039/d5md00257e
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. All datasets used and analyzed during the current study have been deposited in the ESI† file and are publicly available.
References
- Clos J. Grünebast J. Holm M. Pathogens. 2022;11:1052. doi: 10.3390/pathogens11091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Guerrero E. Quintanilla-Cedillo M. R. Ruiz-Esmenjaud J. Arenas R. F1000Research. 2017;6:750. doi: 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison B. A. Bradley B. T. Lab. Med. 2023;54:363–371. doi: 10.1093/labmed/lmac134. [DOI] [PubMed] [Google Scholar]
- Reithinger R. Dujardin J.-C. Louzir H. Pirmez C. Alexander B. Brooker S. Lancet Infect. Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Leishmaniasis, (n.d.), https://www.who.int/news-room/fact-sheets/detail/leishmaniasis, (accessed March 19, 2025)
- Koch L. K. Kochmann J. Klimpel S. Cunze S. Sci. Rep. 2017;7:13325. doi: 10.1038/s41598-017-13822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. Frasca K. Scherrer S. Henao-Martínez A. F. Newman S. Ramanan P. Suarez J. A. Curr. Trop. Med. Rep. 2021;8:121–132. doi: 10.1007/s40475-021-00232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S. More D. K. Singh M. K. Singh V. P. Sharma S. Makharia A. Kumar P. C. Murray H. W. Clin. Infect. Dis. 2000;31:1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- Marchand P. Bazin M.-A. Pagniez F. Rivière G. Bodero L. Marhadour S. Nourrisson M.-R. Picot C. Ruchaud S. Bach S. Baratte B. Sauvain M. Pareja D. C. Vaisberg A. J. Le Pape P. Eur. J. Med. Chem. 2015;103:381–395. doi: 10.1016/j.ejmech.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Bazin M.-A. Cojean S. Pagniez F. Bernadat G. Cavé C. Ourliac-Garnier I. Nourrisson M.-R. Morgado C. Picot C. Leclercq O. Baratte B. Robert T. Späth G. F. Rachidi N. Bach S. Loiseau P. M. Le Pape P. Marchand P. Eur. J. Med. Chem. 2021;210:112956. doi: 10.1016/j.ejmech.2020.112956. [DOI] [PubMed] [Google Scholar]
- Rachidi N. Taly J.-F. Durieu E. Leclercq O. Aulner N. Prina E. Pescher P. Notredame C. Meijer L. Späth G. F. Antimicrob. Agents Chemother. 2014;58:1501–1515. doi: 10.1128/AAC.02022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieu E. Prina E. Leclercq O. Oumata N. Gaboriaud-Kolar N. Vougogiannopoulou K. Aulner N. Defontaine A. No J. H. Ruchaud S. Skaltsounis A.-L. Galons H. Späth G. F. Meijer L. Rachidi N. Antimicrob. Agents Chemother. 2016;60:2822–2833. doi: 10.1128/AAC.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm T. Meng Z. Haas P. Henne-Bruns D. Rachidi N. Knippschild U. Bischof J. Biosci., Biotechnol., Biochem. 2019;83:1663–1675. doi: 10.1080/09168451.2019.1617105. [DOI] [PubMed] [Google Scholar]
- Smirlis D. Dingli F. Sabatet V. Roth A. Knippschild U. Loew D. Späth G. F. Rachidi N. Front. Cell Dev. Biol. 2022;9:800098. doi: 10.3389/fcell.2021.800098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre A. Shintre S. S. Rachidi N. Front. Cell. Infect. Microbiol. 2022;12:825458. doi: 10.3389/fcimb.2022.825458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachidi N. Knippschild U. Späth G. F. Front. Cell. Infect. Microbiol. 2021;11:655700. doi: 10.3389/fcimb.2021.655700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisseur L. Cojean S. Gassama K. Logé C. Pagniez F. Cavé C. Bernadat G. Loiseau P. M. Bach S. Thiéfaine J. Picot C. Tomasoni C. Leclercq O. Baratte B. Robert T. Le Pape P. Rachidi N. Bazin M.-A. Marchand P. ChemMedChem. 2025:e202400862. doi: 10.1002/cmdc.202400862. [DOI] [PubMed] [Google Scholar]
- Chan L. Jin H. Stefanac T. Wang W. Lavallée J.-F. Bédard J. May S. Bioorg. Med. Chem. Lett. 1999;9:2583–2586. doi: 10.1016/S0960-894X(99)00435-7. [DOI] [PubMed] [Google Scholar]
- Woll M. G. Qi H. Turpoff A. Zhang N. Zhang X. Chen G. Li C. Huang S. Yang T. Moon Y.-C. Lee C.-S. Choi S. Almstead N. G. Naryshkin N. A. Dakka A. Narasimhan J. Gabbeta V. Welch E. Zhao X. Risher N. Sheedy J. Weetall M. Karp G. M. J. Med. Chem. 2016;59:6070–6085. doi: 10.1021/acs.jmedchem.6b00460. [DOI] [PubMed] [Google Scholar]
- Mccomas C. C., Liverton N. J., Habermann J., Koch U., Narjes F., Li P., Peng X., Soll R., Wu H., Palani A., Dai X., Liu H., He S. and Dang Q., WO2013034048, 2013
- Marhadour S. Bazin M.-A. Marchand P. Tetrahedron Lett. 2012;53:297–300. doi: 10.1016/j.tetlet.2011.11.015. [DOI] [Google Scholar]
- Liégault B. Lapointe D. Caron L. Vlassova A. Fagnou K. J. Org. Chem. 2009;74:1826–1834. doi: 10.1021/jo8026565. [DOI] [PubMed] [Google Scholar]
- Mologni L. Dalla Via M. Chilin A. Palumbo M. Marzaro G. ChemMedChem. 2017;12:1390–1398. doi: 10.1002/cmdc.201700243. [DOI] [PubMed] [Google Scholar]
- Van den Kerkhof M. Mabille D. Chatelain E. Mowbray C. E. Braillard S. Hendrickx S. Maes L. Caljon G. Int. J. Parasitol.: Drugs Drug Resist. 2018;8:81–86. doi: 10.1016/j.ijpddr.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomel S. Cojean S. Pons V. Cintrat J.-C. Nguyen L. Vacus J. Pruvost A. Barbier J. Gillet D. Loiseau P. M. J. Antimicrob. Chemother. 2021;76:2640–2650. doi: 10.1093/jac/dkab226. [DOI] [PubMed] [Google Scholar]
- Zegzouti H. Zdanovskaia M. Hsiao K. Goueli S. A. Assay Drug Dev. Technol. 2009;7:560–572. doi: 10.1089/adt.2009.0222. [DOI] [PubMed] [Google Scholar]
- Ibrahim N. Bonnet P. Brion J.-D. Peyrat J.-F. Bignon J. Levaique H. Josselin B. Robert T. Colas P. Bach S. Messaoudi S. Alami M. Hamze A. Eur. J. Med. Chem. 2020;199:112355. doi: 10.1016/j.ejmech.2020.112355. [DOI] [PubMed] [Google Scholar]
- SYBYL-X 2.1.1, Tripos Associates, Inc., 1699 South Hanley Road, St. Louis, MO 63144, U.S.A [Google Scholar]
- Clarck M. Cramer III R. D. Van Opdenbosch N. J. Comput. Chem. 1989;10:982–1012. doi: 10.1002/jcc.540100804. [DOI] [Google Scholar]
- Mente S. Arnold E. Butler T. Chakrapani S. Chandrasekaran R. Cherry K. DiRico K. Doran A. Fisher K. Galatsis P. Green M. Hayward M. Humphrey J. Knafels J. Li J. Liu S. Marconi M. McDonald S. Ohren J. Paradis V. Sneed B. Walton K. Wager T. J. Med. Chem. 2013;56:6819–6828. doi: 10.1021/jm4006324. [DOI] [PubMed] [Google Scholar]
- Biasini M. Bienert S. Waterhouse A. Arnold K. Studer G. Schmidt T. Kiefer K. Gallo Cassarino T. Bertoni M. Bordoli L. Schwede T. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. Willett P. Glen R. C. Leach A. R. Taylor R. J. Mol. Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- Dassault Systèmes Biovia Corp, Discovery Studio Visualizer, v24.1.0.23298, Dassault Systèmes, San Diego, 2023 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. All datasets used and analyzed during the current study have been deposited in the ESI† file and are publicly available.










