Abstract
Obstructive sleep apnea (OSA) is characterized by repetitive airway closure, which can lead to hypoxemia and sympathetic nervous system activation. This can result in significant cardiovascular and neurologic morbidity. Therefore, early recognition and treatment are extremely important. The gold standard treatment of OSA is positive airway pressure, which has been shown to decrease the risk of these sequelae. Alternatives to positive airway pressure therapy are not commonly discussed but are available to those who are not able to tolerate positive airway pressure. The goal of this review is to discuss the pathophysiology, epidemiology, screening and testing, and treatment considerations for OSA.
Keywords: obstructive sleep apnea, positive airway pressure, Berlin, STOP-BANG, uvulopalatopharyngoplasty, hypoglossal nerve stimulator
“Treating OSA is important in preventing development of related medical conditions and improving sleep quality.”
Introduction
Obstructive sleep apnea (OSA) is a sleep disorder characterized by cyclic cessations or reductions in breath amplitude caused by closure of the upper airway. 1 These closures lead to hypoxemia, hypercapnia, and subsequent sympathetic nervous system activation, which can over time lead to serious cardiovascular and neurologic conditions, such as coronary heart disease, heart failure, uncontrolled hypertension, and stroke. Therefore, recognition and treatment are extremely important. 2 The gold standard treatment of OSA is continuous positive airway pressure (CPAP) device, which has been shown to decrease the risk of these sequelae. 3 The goal of this review is to discuss the pathophysiology of OSA, screening and testing for OSA, and treatment considerations.
OSA is defined by the apnea-hypopnea index (AHI), which is the number of times an individual has a cessation or reduction in breath secondary to upper airway closure. 4 When an individual goes to sleep, there is decreased tonic activity of the upper airway dilator muscles. This leads to increased airway compliance and airway closure. When the airway closes, the mechanoreceptors in the larynx experience negative pressure during inspiration. This negative pressure triggers a protective reflex that causes the airway dilators to re-establish airway patency. When this cycle happens frequently, there is an autonomic compensatory response. This sympathetic nervous system activation is thought to contribute to the cardiovascular effects of untreated OSA. 5
The prevalence of OSA is estimated to be 9% in women and 17% in men aged 50 to 70 years. OSA is more prevalent in men than in women in all age groups, and AHI is generally higher in men as compared to women at any given age. 6 Obesity is the most common risk factor for OSA. It is estimated that 50-60% of people who are obese have OSA. 7 As BMI increases, the prevalence of OSA increases. 8 Male sex is the next most common risk factor for OSA. Neck size can predispose to OSA, with higher risk associated with males who have greater than 17 cm neck circumference and females who have greater than 16 cm neck circumference. Craniofacial abnormalities can also increase risk of OSA. 2
OSA is important to recognize and treat because it has several cardiovascular impacts on health. OSA has a significant impact on systemic hypertension. When patients with untreated OSA were compared to patients with treated OSA, there was a significantly lower risk of hypertension in the treated group. 9 OSA is an independent risk factor for atrial fibrillation. While it is difficult to determine if atrial fibrillation could be caused by OSA, it has been suggested that treatment of OSA can decrease the burden of atrial fibrillation. 2 Additionally, OSA is associated with a twofold increase in cardiovascular deaths, though it is unclear whether treatment with CPAP therapy decreases the risk of an event.2,10 Lastly, OSA has also been identified as a risk factor for stroke, independent of hypertension. While it is also unclear in stroke whether CPAP therapy decreases risk of primary stroke, trials are currently in progress to determine if it prevents recurrent stroke. 2
Initial Evaluation of Daytime Sleepiness
Patients with OSA usually present with a chief complaint of excessive daytime sleepiness. 4 Daytime sleepiness must be distinguished from fatigue when evaluating for OSA. It refers to the likelihood of falling asleep during a particular activity. In order to make this distinction, the Epworth Sleepiness Scale (ESS) could be useful. 11 This scale provides a standardized way of measuring sleep propensity, or the tendency to fall asleep. The ESS consists of eight items from a score of 0 to 24. Higher scores are associated with higher severities of sleep apnea (Table 1). Therefore, this tool can be useful to determine the presence of daytime sleepiness, a common symptom of OSA. 12 However, the performance of the ESS in predicting OSA in general is poor, with a sensitivity of 38% and a specificity of 71%. 13
Table 1.
Screening Questionnaires for OSA
| Epworth Sleepiness Score 12 | STOP-BANG 14 | Berlin Questionnaire 16 |
|---|---|---|
| Scale 0—No chance of dozing 1—Mild chance of dozing 2—Moderate chance of dozing 3—High chance of dozing ⁃ Sitting and reading. ⁃ Watching TV. ⁃ Sitting in public in an inactive place. ⁃ Riding in a car for an hour as passenger. ⁃ Lying down in the afternoon. ⁃ Sitting and talking to someone. ⁃ Sitting quietly after lunch without alcohol. ⁃ Stopped in traffic for a few minutes. |
Answer Yes or No Snoring Tiredness Observed Apneas High Blood Pressure BMI > 35 kg/m2 Age > 50 Neck Circumference > 40 cm Gender male |
Category 1 Has your weight changed?—Yes/No Do you snore?—Yes/No Snore loudness? ⁃ Loud as breathing ⁃ Loud as talking ⁃ Louder than talking ⁃ Very loud How often do you snore? ⁃ Never or nearly never ⁃ 1-2 times a week ⁃ 3-4 times a week How often have your breathing pauses been noticed? ⁃ Never or nearly never ⁃ 1-2 times a month ⁃ 1-2 times a week ⁃ 3-4 times a week ⁃ Category 2 How often are you tired or fatigued after sleeping? ⁃ Never or nearly never ⁃ 1-2 times a month ⁃ 1-2 times a week ⁃ 3-4 times a week During your awaking time, do you feel tired, fatigued or not up to par? ⁃ Never or nearly never ⁃ 1-2 times a month ⁃ 1-2 times a week ⁃ 3-4 times a week ⁃ Nearly every day Have you ever nodded off or fallen asleep while driving a vehicle?—Yes/No Category 3 Do you have high blood pressure?—Yes/No |
| Score > 10 = Daytime sleepiness | High risk of OSA—Yes 5-8 Moderate risk of OSA—Yes 3-4 Low risk of OSA—Yes 0-3 |
Category 1 is positive with 2 or more positive responses to questions 1-6 Category 2 is positive with 2 or more positive responses to questions 7-9 Category 3 is positive with 1 or more positive responses and/or a BMI>30 kg/m2 2 or more positive categories indicates high risk of OSA |
Other common symptoms of OSA that may indicate the need for testing include snoring, gasping for breaths at night, witnessed apneas, and morning headaches. The presence of comorbid conditions that are known to be associated with OSA can also be useful in suspecting OSA, including atrial fibrillation, diabetes, hypertension, heart failure, obesity, and stroke. 2
Screening and Diagnostic Evaluation for OSA
The gold standard diagnostic evaluation for OSA is polysomnography (PSG). However, this test is costly and time-consuming. Therefore, screening tools have been developed to help identify high-risk patients. The American Academy of Sleep Medicine (AASM) recommends against using these types of screening tools in asymptomatic patients. However, they could be useful in patients presenting with symptoms consistent with OSA.
There are several screening tools that are used to identify patients with a high pre-test probability of OSA. A commonly used tool is the STOP-BANG questionnaire. The STOP-BANG questionnaire consists of 2 parts: symptoms and demographics. If the patient scores greater than or equal to 3, then they are considered high-risk for OSA and should be considered for further evaluation (Table 1). This questionnaire has a sensitivity of 82% and a specificity of 42% for detecting mild OSA. 14
Another common screening tool for OSA is the Berlin Questionnaire. This consists of 10 questions on snoring, daytime sleepiness, obesity, and hypertension. In contrast to the STOP-BANG, which is a series of “yes/no” questions, the Berlin Questionnaire assesses the frequency of symptoms (Table 1). 15 When evaluated in a primary care setting, this screening tool has a sensitivity of 77% and a specificity of 89% for predicting mild obstructive sleep apnea. (Table 2) 16
Table 2.
Sensitivity and Specificity of Screening Tools in Diagnosis of OSA. 15
| AHI > 5 | AHI > 15 | AHI > 30 | ||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| ESS | 54% | 65% | 47% | 62% | 58% | 60% |
| STOP-BANG | 88% | 42% | 90% | 36% | 93% | 35% |
| Berlin Questionnaire | 76% | 59% | 77% | 44% | 84% | 38% |
While these tools are useful in identifying patients who need to have further evaluation, they are not diagnostic for OSA. The AASM guidelines strongly recommend that a sleep study be used to diagnose OSA, rather than screening tools and questionnaires. 17 There are several types of sleep studies that can be performed. The gold standard is an in-lab attended PSG. This consists of measurement of air flow through a nasal cannula with a pressure transducer or thermal sensor, respiratory efforts using thoracic and abdominal bands, oxygen saturation monitoring, snoring with a microphone, sleep stage with an electroencephalogram (EEG), cardiac conduction with an electrocardiogram (ECG), and body position and leg movement with dedicated sensors. 18 The in-lab attended test could be performed as a split-night protocol, where the first half of the night is diagnostic, and the second half of the night requires application of a CPAP to determine the optimal settings. The AASM makes a weak recommendation to consider the split-night protocol as a first diagnostic step if choosing to perform an in-lab PSG, as it could save the patient a second night of testing. However, this must be balanced with the risk of having a non-diagnostic result from a shorter test.
Home sleep apnea testing (HSAT) is another option for diagnostic evaluation. This consists of measurement of airflow, respiratory effort, and oxygen saturation. 18 The AASM recommends that patients with high pre-test probability of OSA without significant comorbid conditions should start off with a HSAT as opposed to an in-lab attended PSG. Those with significant cardiopulmonary disease; potential respiratory muscle weakness with or without awake hypoventilation; other sleep disorders, such as restless legs or parasomnias; or on oxygen therapy are excluded from having HSAT as the first test. Also, patients who have any personal or environmental factors that preclude them from self-administering the test at home are also excluded. Home sleep apnea testing is considered less sensitive than in-lab PSG, therefore, a negative test in a patient with a high pre-test probability of OSA requires subsequent in-lab PSG. 17
OSA is diagnosed by measuring the AHI in an in-lab attended PSG and the respiratory event index (REI) in a HSAT. The AHI is determined by the number of apneas and hypopneas over the total sleep time, whereas the REI is determined by the number of apneas and hypopneas over the total recording time. Often, recording time is longer than sleep time, causing REI to be lower and underdiagnosing OSA. 19 Therefore, AASM recommends considering repeat in-lab attended PSG in patients with a non-diagnostic HSAT. 15 The severity of OSA is determined by AHI or REI measured during sleep test (Table 3).
Table 3.
Classification of Severity of OSA. 18
| Severity | AHI |
|---|---|
| None | <5/h |
| Mild | 5-15/h |
| Moderate | 15-30/h |
| Severe | >30/h |
Primary care providers may want to consider referral to a sleep specialist in certain circumstances. Often, patients may have another suspected sleep disorder in addition to OSA, such as restless leg syndrome, insomnia, or narcolepsy. A sleep specialist may be helpful in diagnosing and treating OSA within the context of these disorders. Additional considerations for referral include patients with a high pre-test probability of OSA with negative initial testing, with mixed obstructive and central sleep apnea on initial testing, and with intolerance to CPAP therapy.
Myths Surrounding OSA
Given the prevalence of OSA in the general population, there are many misconceptions surrounding the diagnosis and treatment both in the patient population and the general medical community. (Table 4) Addressing these misconceptions may improve understanding of the condition and possibly increase the chances of PAP therapy adherence.
Table 4.
Common Myths Surrounding OSA
| Myth | Fact |
|---|---|
| Everyone who snores has sleep apnea. | • Even though snoring has the highest sensitivity for OSA, it is not specific for OSA.
69
• Imagine snoring on a continuum with simple or non-apneic snoring on one end, and OSA on the other—patients with OSA are more likely to have loud snoring occurring nightly that significantly bothers their bed partner. 16 |
| Sleep apnea only occurs in overweight patients. | • Obesity is thought to increase the load on the pharynx thus increasing the risk for developing sleep apnea.
70
However, estimates have shown that sleep apnea is due to a BMI >25 kg/m2 in a little more than half of patients
71
and about one-third of OSA patients are non-obese.
72
• Other risk factors include craniofacial anatomy resulting in narrowing of the airways; family history and thus genetic predisposition; and smoking. • In non-obese patients with OSA, in addition to anatomical factors contributing to OSA, the following non-anatomical factors may also play a role: minimal responsiveness of upper airway muscle during sleep, low respiratory arousal threshold, and high loop gain. 73 • The severity of OSA tends to be less in patients who are non-obese compared to patients who are obese. 74 CPAP compliance tends to be lower in this population due to the prevalence of non-anatomic factors and may require different treatment strategies. 75 |
| Sleep apnea is of little concern. | • Increased sleepiness and daytime fatigue as seen in patients with OSA increase the risk for motor vehicle accidents and occupational accidents.
76
• OSA is associated with an increased risk for hypertension, heart failure, stroke, atrial fibrillation, type 2 diabetes, and Alzheimer disease. 77 • While asymptomatic mild OSA has not been shown to increase cardiovascular risk, these patients may not benefit from treatment. 78 • Non-sleepy patients with moderate or severe OSA have not been shown to have a decrease in cardiovascular risk when treated with CPAP therapy. 79 Patients with excessive sleepiness have the most cardiovascular risk. 80 A major limitation of the study was CPAP adherence (mean duration of adherence was 3.3 hours per night). 79 • PAP therapy remains the treatment of choice. Additional studies are needed to investigate if CPAP use of greater than 4 hours a night or longer and consistency would improve cardiovascular outcomes. 81 |
| Sleep apnea only occurs in men. | • While sleep apnea is more common in men than in women at a 2:1 ratio,
82
it has been reported that up to 50% of women aged 20-70 years old can have the disease.
83
• The male-to-female referral ratio is 9:1 indicating that clinicians often overlook this diagnosis in women. 84 |
Positive Pressure Therapy for OSA
The mainstay of treatment for OSA is positive airway pressure (PAP), which encompasses continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), and auto-titrating devices. Simply, these devices use pressure created from a stream of air to splint open the airways. The most used PAP device for the treatment of OSA is CPAP, and studies show that CPAP use decreases not only cardiovascular risk factors associated with OSA but also risk of fatal and non-fatal cardiovascular events.20,21,22 In fact, termination of CPAP therapy has recently been shown to increase all-cause mortality. 23
Furthermore, while patients may believe they only need to use their device occasionally, consistent, nightly use is beneficial as studies show that hours of CPAP use correlates directly with improvement in sleepiness and thus quality of life. 24 Improvement in subjective daytime sleepiness as measured by the Epworth Sleepiness Scale, required a minimal of 4 hours of nightly use, but increased further with longer nightly usage with 92.9% of subjects normalizing their Epworth score with average duration of use > 7 hours. Using the Functional Outcomes of Sleep Questionnaire to assess resolution of functional impairment, optimal mean duration of nightly CPAP use needed was 7.5 hours. 24 In a meta-analysis of subjects with minimally symptomatic OSA, improvement in diastolic BP was confined to those who used CPAP for >4 hours each night. 25 Based on these and similar studies, the Center for Medicare and Medicaid Services and insurance payers define a minimum of PAP therapy for a minimum of 4 hours each night for at least 70% of nights as the standard for adherence to therapy.
Barriers to PAP Therapy
Based on the above definition, the reported prevalence of non-adherence ranges from 46% to 83%.26,27 Barriers to CPAP use early-on in therapy include claustrophobia, poor mask fit, dry mouth, and nasal congestion. Most importantly, using CPAP nightly requires patient engagement and behavioral changes. One study outlined a higher risk of noncompliance in female patients, those with minimally symptomatic OSA, and those with co-morbidities, compared to patients with severe OSA. 28 Factors such as race, ethnicity, income, educational level are individually inconsistent predictors of adherence 29 but may ultimately contribute to social-psychological factors with stronger correlations to adherence. These include self-efficacy or the patient’s motivation and knowledge to participate in healthy behavior and internal locus of control, that is, the patient’s belief that health outcomes are within their own control. 30 Self-efficacy, in particular, predicts adherence at 1 week and 1 month after starting CPAP use. 31 Although there are conflicting data if patient education and supportive interventions increase adherence, these methods are easily implemented with little risk and possible benefit. 32 Thus, education about the disease and therapy, consistent engagement with positive reinforcement and behavioral interventions are all likely to be especially useful in establishing compliance.
Interventions to Improve PAP Adherence
A pattern of adherence or non-adherence to CPAP is established early after use, usually within the first few days of use and most often, by 1-4 weeks. 33 Indeed, patients who experienced problems during the first night of auto-titration were 3 times less likely to be adherent at 1 month. 34 Conversely, patients whose sleep improved the most on the titration night had the highest levels of compliance, establishing this sleep efficiency change as a powerful predictor of compliance. 35 Therefore, interventions to improve adherence should be initiated prior to the prescription of PAP therapy and within 2 weeks after per the American Academy of Sleep Medicine (AASM) Practice Guideline. 36 A meta-analysis of 30 studies employing educational, supportive and behavioral interventions showed increased nightly CPAP machine use by about .6, .8, and 1.4 hours each night, respectively. 37 Of the behavioral interventions, both cognitive behavioral therapy and motivational enhancement therapy have been demonstrably effective in trials and can be delivered in person and often, via telemedicine. Which therapy is superior remains unclear. 26 A meta-analysis of 11 RCTs showed that telemedicine for up to 6 months enhanced CPAP adherence compared to no intervention. 38 Multi-modality interventions (combined telehealth and automated tele-monitoring, 39 involvement of the patient’s bed partner 40 and including active patient engagement tools, 41 especially when employed early and often in the few two weeks of therapy do improve longer-term CPAP adherence.
Even when appropriately motivated and educated on the benefits of CPAP, common side-effects of CPAP therapy can pose a serious challenge to patients’ adherence and must be addressed in the first week of instituting CPAP therapy. Choosing an optimal interface is essential to prevent discomfort or leaks caused by an ill-fitting mask. Nasal congestion can be treated with decongestants or topical steroids and nasal dryness with heated humidification which is now universal on CPAP devices. Mouth leak contributes to nasal and oral dryness and can be addressed with a chinstrap for mouth breathers, properly re-sizing masks and possibly, switching to a full-face oronasal mask. When nasal masks alone, combined with a chin strap, and oronasal masks are compared, patients reported fewer leaks, mask fit problems and increased satisfaction and sleep quality with the nasal masks. 42 Overall adherence did not differ between the three options at 3 months, however. Notably, oronasal masks were associated with a higher residual AHI in this study, perhaps because of posterior displacement of the tongue. 43 Nasal masks or pillows also reduce the incidence of aerophagy noted in up to 16% of patients, as may avoidance of supine sleep. While it is common to change a patient who cannot tolerate a high fixed pressure to an auto-titrating CPAP device this may not improve compliance with therapy. 44
The non-benzodiazepine sedative eszopiclone, prescribed for the first 14 days of CPAP therapy, has been shown to increase compliance at 6 months (64 vs 45 percent of nights and for 4 instead of 3 hours) compared with placebo. 45 This effect of eszopiclone was confirmed in a meta-analysis of 8 studies but does not extend to zolpidem or zaleplon. 46 While not meant for routine use, this medication is an additional tool for use in patients who have a particularly challenging time with CPAP early in therapy or who have associated insomnia. 47
Technological advances allow physicians to remotely monitor patient’s compliance and optimize pressure settings through cloud-based software. Mobile applications and telemedicine provide easy follow up and give patients a favorable view of PAP therapy. 48 Improving CPAP adherence requires early intervention with a variety of different interventions tailored to the individual patient while recognizing that some patients derive symptomatic benefit with sub-threshold compliance and many, approximately 20%, remain sleepy despite optimal use. 48 When interventions to promote adherence fail, there are other strategies and devices to explore in the treatment of OSA. Medicare coverage for most non-CPAP devices requires that patients have failed a trial of PAP therapy.
Alternative and Adjunctive Treatment Options
Lifestyle Modification
There is a strong correlation between obesity and OSA. A BMI >29 kg/m2 increases the risk of OSA 10-fold, and it is estimated that two of every three patients diagnosed with OSA are obese. 49 Weight reduction is associated with improvement in OSA symptoms, as well as improvement in impaired glucose tolerance and type 2 diabetes mellitus. 50 Lifestyle changes resulting in a patient losing 10% of their body weight has been shown to decrease the severity of OSA and losing 15% of body weight has been shown to help achieve remission of the disease. 51 Moreover, a combination of CPAP therapy with lifestyle modifications has better outcomes than with CPAP alone. 52 There is PSG data that supports improvement in daytime sleepiness and reduction in PAP requirements after weight loss surgery. 53 Therefore, the AASM recommends repeat PSG with 10% change in body weight. 54
Weight reduction via lifestyle changes, such as caloric deficit diet and increased physical activity, are recommended at minimum as adjunctive therapy for OSA. Specifically, the Mediterranean diet has been shown to decrease AHI in patients with moderate to severe OSA over a 6-month period. 55 Other lifestyle modifications to improve OSA include avoidance of alcohol, opioids, and sedatives. 56 Additionally, GLP-1 analogs are now increasingly being used for weight loss and some preliminary studies have shown promising results in reduction in AHI. 57
Positional Therapy
Oftentimes, patients with OSA suffer from positional obstructive sleep apnea (POSA) characterized as the collapse of the pharynx and surrounding soft tissue during sleep. In fact, more than half of OSA patients have worse apnea-hypopnea index (AHI) while in the supine position. 56 Positional therapy (PT) encourages patients to avoid supine sleeping position. 56 Positional therapy (PT) encourages patients to avoid supine sleeping position. Approaches to preventing supine positioning include rigid wedges, triangular pillows, battery-powered neck positioning devices, and something as simple as a t-shirt with a tennis ball attached to the backside. Data suggests that encouraging lateral sleep positioning can improve OSA symptoms, especially in patients with mild-moderate OSA. Positional therapy is a cost-effective strategy for reduction in AHI in OSA. 58
Oral Appliance Therapy
Mandibular advancement devices (MAD) represent another option for patients that are unable to tolerate PAP as second-line therapy (even though it has a higher self-reported compliance rate, its efficacy is variable). MADs protrude the lower jaw and increase pharyngeal dimensions, and therefore, reduce airway collapsibility. 59 More research is needed to assess MAD in severe OSA, but in mild-moderate OSA, MADs can provide improvement in clinical symptoms and normalization of AHI. 60 MADs should be tailored to each individual patient. Morbid obesity, older age, and male sex are associated with less improvement using MADs. 61
Hypoglossal Nerve Stimulation
As mentioned above, the pathophysiology of OSA includes a complex neuromuscular and structural collapse of the pharynx during sleep. Studies have shown that hypoglossal nerve stimulation (HNS) can prevent pharyngeal collapse without waking patients from sleep. Upper airway stimulation (UAS) via hypoglossal nerve stimulation causes contraction of the genioglossus muscle which then co-activates other muscles and stabilizes the anterior oropharynx. 62 The UAS device is surgically implanted under general anesthesia; three incisions are required for the placement of the device/generator, the breathing sensor electrode, and a hypoglossal stimulation electrode. (Figure 1) The device has a small remote control to turn the system on before sleep. With each sensed breath, the device stimulates the nerve to contract the muscles to keep the airway open. 63 Upper airway stimulation via HNS has been shown to improve objective and subjective parameters of OSA but unfortunately, this treatment modality is only recommended for moderate to severe OSA patients with a BMI <35 kg/m2 which is limiting for a large portion of OSA patients. Additionally, patient should not have concentric upper airway collapse on drug induced sleep endoscopy. 64
Figure 1.
Depiction of the hypoglossal nerve stimulator. From [Strollo Jr PJ et al. Upper Airway Stimulation for Obstructive Sleep Apnea. 9;370(2):139-49. Copyright © 2014 Massachusetts Medical Society. Reprinted with permission.
Expiratory Positive Airway Pressure Nasal Device
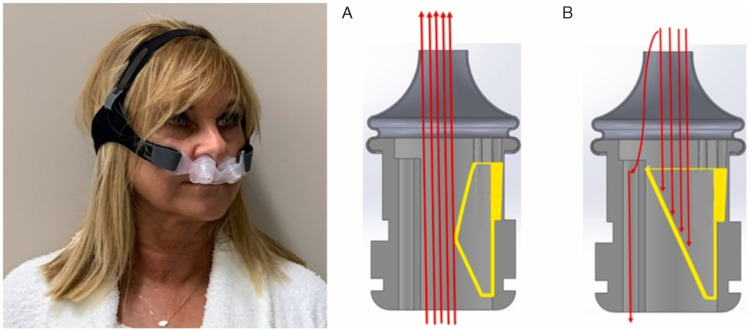
Expiratory positive airway pressure (EPAP) nasal devices, not to be confused with over-the-counter nasal adhesive strips which are not indicated for OSA, were first introduced by Mahadevia et al in 1983. 65 These devices contain a valve which attaches or covers each nostril to increase pressure while exhaling during sleep. (Figure 2) Inspiratory resistance is minimal, and therefore, inspiration is unaffected. There are several EPAP nasal devices approved by the FDA. The expiratory pressures generated by each of them are different. Thus, further clinical trials are needed to determine the necessary amount of expiratory pressure to efficiently reduce apnea and hypopnea events. 66 A prospective, multi-center, parallel group, randomized, placebo-controlled, double-blind trial compared the change in AHI and oxygen desaturation index (ODI) between an EPAP nasal device and a sham. The nasal device was found to significantly reduce AHI and ODI in mild to moderate OSA compared to sham. Significant reduction in AHI was also found in a small subset of subjects with severe OSA. 67 The use of an EPAP nasal device is an alternative treatment in mild to moderate OSA patients intolerant to CPAP therapy. 67
Figure 2.
Example of an EPAP nasal device. Inhalation (A) with the contracted flexible shell (yellow), and exhalation (B) with the expanded flexible shell (yellow). Airflow direction is indicated with red arrows. Adapted with permission from authors [Sleeper et al. Sleep Med 2022;96:87-92]
Surgery
Various surgical approaches have been used in OSA treatment and are classified into intra- and extrapharyngeal procedures. Intrapharyngeal procedures target the soft palate and tongue base. The most studied intrapharyngeal procedure is uvulopalatopharyngoplasty, which involves resection of the uvula and portions of the soft palate and has been found to significantly reduce AHI. Tonsillectomy, tongue base reduction, and lateral wall pharyngoplasty are other procedures used for OSA therapy. The most well-studied extrapharyngeal surgery is maxillomandibular advancement (MMA). MMA targets the bony structure of the face and aims to enlarge the airway with bilateral mandibular osteotomies and forward fixation of the facial skeleton. 18 MMA is a highly invasive procedure but comes with a reduction in AHI in up to 98% of patients, with the most impact seen in OSA patients with a preoperative AHI of <60 events/hour. 68 Lastly, as weight loss is a recommended therapy for OSA, bariatric surgery should be considered in the overall management of patients with OSA and morbid obesity. 68
Surgical interventions have not been shown to be as effective as PAP in lowering AHI, but there may be a role for surgical intervention in patients who are unable to tolerate PAP. Though positive airway pressure has been shown to be the most effective therapy for OSA, there are several adjunct strategies that can be used when PAP is not tolerated. Importantly, patient engagement is critical to successful therapy, and tailoring of treatment to patients’ preferences will improve outcomes.
Conclusions
Sleep-disordered breathing is a commonly reported problem in the outpatient setting, mostly in the obese population, but it can also be present in the non-obese population. Screening using validated questionnaires will help with prompt recognition, referral for diagnostic testing, and treatment. Treating OSA is important in preventing development of related medical conditions and improving sleep quality. CPAP is the first line treatment for OSA. However, tolerance to this therapy may be difficult due to claustrophobia, poor mask fit, dry mouth, or nasal congestion. There are remedies available for these reported issues.
Despite making changes to improve comfort and tolerability, some patients abandon CPAP use and refuse to restart therapy. If a patient fails to engage or provide behavioral changes to tolerate CPAP, providers should discuss other available modalities of treatment rather than leaving OSA untreated. Referral to a sleep specialist would be helpful in troubleshooting barriers to treatment and providing information on other modalities of treatment. Depending on the severity of OSA, patients may be candidates for positional therapy, mandibular advancement device, uvulopalatoplasty, hypoglossal nerve stimulator, and so forth. Of course, weight loss and lifestyle changes should be encouraged for all patients with OSA. Treatment can be challenging. Future research on new treatment options would be welcome in treating this important disease.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Patrick Koo https://orcid.org/0000-0002-8482-1353
References
- 1.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70-81. [DOI] [PubMed] [Google Scholar]
- 2.Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;144(3):e56-e67. [DOI] [PubMed] [Google Scholar]
- 3.Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5(3):263-276. [PMC free article] [PubMed] [Google Scholar]
- 4.Abbasi A, Gupta SS, Sabharwal N, et al. A comprehensive review of obstructive sleep apnea. Sleep science (Sao Paulo, Brazil). 2021;14(2):142-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fietze I, Laharnar N, Obst A, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences - results of SHIP-Trend. Journal of sleep research. 2019;28(5):e12770. [DOI] [PubMed] [Google Scholar]
- 7.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17(1):32-42. [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14(2):131-136. [DOI] [PubMed] [Google Scholar]
- 11.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. Chest. 1993;103(1):30-36. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. [DOI] [PubMed] [Google Scholar]
- 13.Godoy PH, Nucera APCDS, Colcher AP, de Andrade JE, Alves DDSB. Screening for obstructive sleep apnea in elderly: performance of the Berlin and STOP-Bang questionnaires and the Epworth sleepiness scale using polysomnography as gold standard. Sleep science (Sao Paulo, Brazil). 2022;15(2):136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boynton G, Vahabzadeh A, Hammoud S, Ruzicka DL, Chervin RD. Validation of the STOPBANG questionnaire among patients referred for suspected obstructive sleep apnea. Journal of Sleep Disorders: Treatment & Care. 2013;02(4):1000121. doi: 10.4172/2325-9639.1000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu HY, Chen P-Y, Chuang L-P, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70. [DOI] [PubMed] [Google Scholar]
- 16.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485. [DOI] [PubMed] [Google Scholar]
- 17.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical Practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical Practice guideline. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine. 2017;13(3):479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea. JAMA. 2020;323(14):1389-1400. [DOI] [PubMed] [Google Scholar]
- 19.Wittine LM, Olson EJ, Morgenthaler TI. Effect of recording duration on the diagnostic accuracy of out-of-center sleep testing for obstructive sleep apnea. Sleep. 2014;37(5):969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iftikhar IH, Valentine CW, Bittencourt LRA, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea. J Hypertens. 2014;32(12):2341-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233-1241. [DOI] [PubMed] [Google Scholar]
- 22.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046-1053. [DOI] [PubMed] [Google Scholar]
- 23.Pépin JL, Bailly S, Rinder P, et al. Relationship between CPAP termination and all-cause mortality: a French nationwide database analysis. Chest. 2022;161(6):1657-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratton DJ, Stradling JR, Barbé F, Kohler M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax. 2014;69(12):1128-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver TE. Novel aspects of CPAP treatment and interventions to improve CPAP adherence. J Clin Med. 2019;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cistulli PA, Armitstead J, Pepin J-L, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagnadoux F, Le Vaillant M, Paris A, et al. Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest. 2016;149(1):288-290. [DOI] [PubMed] [Google Scholar]
- 29.Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung. 2019;197(2):115-121. [DOI] [PubMed] [Google Scholar]
- 30.Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J. 2004;24(3):461-465. [DOI] [PubMed] [Google Scholar]
- 31.Stepnowsky CJ, Jr., Marler MR, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med. 2002;3(3):239-247. [DOI] [PubMed] [Google Scholar]
- 32.Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP. Chest. 2019;155(6):1272-1287. [DOI] [PubMed] [Google Scholar]
- 33.Villa Alvarez J, Dales R, Kendzerska T. Demographics, sleep apnea and positive airway pressure (PAP) treatment-related characteristics associated with PAP adherence: a large retrospective community-based longitudinal observational study. Sleep Med. 2022;98:139-143. [DOI] [PubMed] [Google Scholar]
- 34.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27(1):134-138. [DOI] [PubMed] [Google Scholar]
- 35.Drake CL, Day R, Hudgel D, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep. 2003;26(3):308-311. [DOI] [PubMed] [Google Scholar]
- 36.Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of sleep medicine clinical Practice guideline. J Clin Sleep Med : JCSM: Official Publication of the American Academy of Sleep Medicine. 2019;15(2):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2014;1:CD007736. [DOI] [PubMed] [Google Scholar]
- 38.Hu Y, Su Y, Hu S, et al. Effects of telemedicine interventions in improving continuous positive airway pressure adherence in patients with obstructive sleep apnoea: a meta-analysis of randomised controlled trials. Sleep Breath. 2021;25(4):1761-1771. [DOI] [PubMed] [Google Scholar]
- 39.Hwang D, Chang JW, Benjafield AV, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The tele-OSA randomized trial. Am J Respir Crit Care Med. 2018;197(1):117-126. [DOI] [PubMed] [Google Scholar]
- 40.Gentina T, Bailly S, Jounieaux F, et al. Marital quality, partner's engagement and continuous positive airway pressure adherence in obstructive sleep apnea. Sleep Med. 2019;55:56-61. [DOI] [PubMed] [Google Scholar]
- 41.Malhotra A, Crocker ME, Willes L, et al. Patient engagement using new technology to improve adherence to positive airway pressure therapy. Chest. 2018;153(4):843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowland S, Aiyappan V, Hennessy C, et al. Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine. 2018;14(1):101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrade RG, Madeiro F, Piccin VS, et al. Impact of acute changes in CPAP flow route in sleep apnea treatment. Chest. 2016;150(6):1194-1201. [DOI] [PubMed] [Google Scholar]
- 44.Bakker JP, Marshall NS. Flexible pressure delivery modification of continuous positive airway pressure for obstructive sleep apnea does not improve compliance with therapy. Chest. 2011;139(6):1322-1330. [DOI] [PubMed] [Google Scholar]
- 45.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. Effects of a short course of eszopiclone on continuous positive airway pressure adherence. Ann Intern Med. 2009;151(10):696-702. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Tang Y, Chen Y, et al. The effect of non-benzodiazepine sedative hypnotics on CPAP adherence in patients with OSA: a systematic review and meta-analysis. Sleep. 2021;44(8):zsab077. [DOI] [PubMed] [Google Scholar]
- 47.Wallace DM, Sawyer AM, Shafazand S. Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath. 2018;22(1):5-15. [DOI] [PubMed] [Google Scholar]
- 48.Suarez-Giron M, Garmendia O, Lugo V, et al. Mobile health application to support CPAP therapy in obstructive sleep apnoea: design, feasibility and perspectives. ERJ open research. 2020;6(1):00220-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pillar G, Shehadeh N. Abdominal fat and sleep apnea. Diabetes Care. 2008;31(Supplement_2):S303-S309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudgel DW, Patel SR, Ahasic AM, et al. The role of weight management in the treatment of adult obstructive sleep apnea. An official American thoracic society clinical Practice guideline. Am J Respir Crit Care Med. 2018;198(6):e70-e87. [DOI] [PubMed] [Google Scholar]
- 51.Tuomilehto HPI, Seppä JM, Partinen MM, et al. Lifestyle intervention with weight reduction. Am J Respir Crit Care Med. 2009;179(4):320-327. [DOI] [PubMed] [Google Scholar]
- 52.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes. 2005;29(9):1048-1054. [DOI] [PubMed] [Google Scholar]
- 54.Caples SM, Anderson WM, Calero K, Howell M, Hashmi SD. Use of polysomnography and home sleep apnea tests for the longitudinal management of obstructive sleep apnea in adults: an American Academy of Sleep Medicine clinical guidance statement. J Clin Sleep Med. 2021;17(6):1287-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papandreou C, Schiza SE, Bouloukaki I, et al. Effect of Mediterranean dietversusprudent diet combined with physical activity on OSAS: a randomised trial. Eur Respir J. 2012;39(6):1398-1404. [DOI] [PubMed] [Google Scholar]
- 56.Omobomi O, Quan SF. Positional therapy in the management of positional obstructive sleep apnea-a review of the current literature. Sleep Breath. 2017;22(2):297-304. [DOI] [PubMed] [Google Scholar]
- 57.Blackman A, au fnm, Foster GD, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes. 2016;40(8):1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oksenberg A, Gadoth N, Töyräs J, Leppänen T. Prevalence and characteristics of positional obstructive sleep apnea (POSA) in patients with severe OSA. Sleep Breath. 2019;24(2):551-559. [DOI] [PubMed] [Google Scholar]
- 59.Bamagoos AA, Cistulli PA, Sutherland K, et al. Dose-dependent effects of mandibular advancement on upper airway collapsibility and muscle function in obstructive sleep apnea. Sleep. 2019;42(6). [DOI] [PubMed] [Google Scholar]
- 60.Mohammadieh A, Sutherland K, Cistulli PA. Sleep disordered breathing: management update. Intern Med J. 2017;47(11):1241-1247. [DOI] [PubMed] [Google Scholar]
- 61.Marklund M, Braem MJA, Verbraecken J. Update on oral appliance therapy. Eur Respir Rev. 2019;28(153):190083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bender B. Hypoglossusstimulation bei OSAS. Laryngo-Rhino-Otol. 2016;95(11):795-807. [DOI] [PubMed] [Google Scholar]
- 63.Manchanda S, Neupane P, Sigua NL. Upper airway stimulation/hypoglossal nerve stimulator. Am J Respir Crit Care Med. 2020;202(8):P23-P24. [DOI] [PubMed] [Google Scholar]
- 64.Yu MS, Ibrahim B, Riley RW, Liu SY-C. Maxillomandibular advancement and upper airway stimulation: extrapharyngeal surgery for obstructive sleep apnea. Clinical and experimental otorhinolaryngology. 2020;13(3):225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahadevia AK, Onal E, Lopata M. Effects of expiratory positive airway pressure on sleep-induced respiratory abnormalities in patients with hypersomnia-sleep apnea syndrome. The American review of respiratory disease. 1983;128(4):708-711. [DOI] [PubMed] [Google Scholar]
- 66.Sleeper G, Rashidi M, Strohl KP, et al. Comparison of expiratory pressures generated by four different EPAP devices in a laboratory bench setting. Sleep Med. 2022;96:87-92. [DOI] [PubMed] [Google Scholar]
- 67.Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011;34(4):479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaghi S, Holty J-EC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea. JAMA Otolaryngology-Head & Neck Surgery. 2016;142(1):58. [DOI] [PubMed] [Google Scholar]
- 69.Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea? JAMA. 2013;310(7):731. [DOI] [PubMed] [Google Scholar]
- 70.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148(2):462-466. [DOI] [PubMed] [Google Scholar]
- 71.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592-1599. [DOI] [PubMed] [Google Scholar]
- 72.Lecube A, Sampol G, Lloberes P, et al. Asymptomatic sleep-disordered breathing in premenopausal women awaiting bariatric surgery. Obes Surg. 2009;20(4):454-461. [DOI] [PubMed] [Google Scholar]
- 73.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar P, Rai DK, Kanwar MS. Comparison of clinical and polysomnographic parameters between obese and nonobese obstructive sleep apnea. J Fam Med Prim Care. 2020;9(8):4170-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray EL, McKenzie DK, Eckert DJ. Obstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotype. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine. 2017;13(1):81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terán-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. N Engl J Med. 1999;340(11):847-851. [DOI] [PubMed] [Google Scholar]
- 77.Patel SR. Obstructive sleep apnea. Ann Intern Med. 2019;171(11):ITC81. [DOI] [PubMed] [Google Scholar]
- 78.Chowdhuri S, Quan SF, Almeida F, et al. An official American thoracic society research statement: impact of mild obstructive sleep apnea in adults. Am J Respir Crit Care Med. 2016;193(9):e37-e54. [DOI] [PubMed] [Google Scholar]
- 79.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613-620. [DOI] [PubMed] [Google Scholar]
- 80.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. [DOI] [PubMed] [Google Scholar]
- 81.Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kryger MH, Roth T, Dement WC, Principles and Practice of Sleep Medicine. 6th ed. 2017, Philadelphia, PA: Elsevier. xlv:1730. [Google Scholar]
- 83.Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J. 2012;41(3):610-615. [DOI] [PubMed] [Google Scholar]
- 84.Young T. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1996;156(21):2445-2451. [PubMed] [Google Scholar]