Abstract
Hepatocellular carcinoma (HCC) is a major cause of cancer-related mortality with limited treatment options. Cannabidiol (CBD), a non-psychoactive compound from Cannabis sativa, has shown anticancer properties. This review analyzes CBD’s therapeutic potential in HCC, focusing on mechanisms, preclinical/clinical findings, and integration into treatment strategies. A systematic search (PubMed, Scopus, Web of Science, Google Scholar) up to March 2025 identified 16 relevant studies (in vitro, in vivo, clinical). CBD exerts antitumor effects via multiple pathways, including apoptosis, autophagy regulation, metastasis suppression, and tumor microenvironment modulation. CBD interacts with the endocannabinoid system (ECS), inhibits oncogenic signaling (PI3K/AKT/mTOR), and enhances chemotherapeutic efficacy (sorafenib, cabozantinib). Studies show CBD induces pyroptosis via caspase-3/GSDME, and modulates autophagy by inhibiting the PI3K/Akt/mTOR pathway. It also sensitizes HCC cells to sorafenib and cabozantinib. Preclinical results are promising, but clinical studies are limited. Challenges like bioavailability and potential hepatotoxicity require investigation. Future research should optimize formulations, determine dosing, and conduct clinical trials to validate CBD’s efficacy/safety in HCC patients. Validated CBD could offer an innovative HCC management option.
Keywords: Cannabidiol (CBD), Liver Cancer, Hepatocellular carcinoma, Combination therapy, Apoptosis, Autophagy, Metastasis, Endocannabinoid system
Background
Liver cancer, particularly hepatocellular carcinoma (HCC), remains a leading cause of cancer-related mortality worldwide, with limited treatment options and a poor prognosis. Despite advances in targeted therapies and immunotherapy, the five-year survival rate for HCC remains low due to late diagnosis, tumor heterogeneity, and resistance to conventional treatments [1]. This has driven significant interest in exploring alternative therapeutic agents, including natural compounds such as cannabidiol (CBD), a non-psychoactive cannabinoid derived from Cannabis sativa.
CBD has gained considerable attention for its anticancer properties, including its ability to inhibit cell proliferation, induce apoptosis, suppress metastasis, and modulate tumor microenvironments in various cancers, including breast, lung, and colorectal cancers [2, 3]. Recent studies suggest that CBD exerts its antitumor effects through multiple molecular pathways, including modulation of the endocannabinoid system (ECS), inhibition of key oncogenic signaling pathways (PI3K/AKT, MAPK/ERK), and activation of apoptotic mechanisms [4, 5].
In liver cancer, CBD has been shown to induce autophagy-mediated apoptosis, reduce angiogenesis, and inhibit epithelial-to-mesenchymal transition (EMT), which is crucial for metastasis [6]. Additionally, CBD interacts with CB1 and CB2 receptors, transient receptor potential vanilloid (TRPV1), and peroxisome proliferator-activated receptors (PPARs), exerting antitumor and immunomodulatory effects through these targets [7, 8]. Preclinical evidence further suggests that CBD can enhance the efficacy of conventional chemotherapeutic agents such as sorafenib, offering a potential combinatorial approach for HCC treatment [9].
Despite promising preclinical findings, clinical studies on CBD’s efficacy in liver cancer are limited, and challenges such as bioavailability, optimal dosing, and potential hepatotoxicity must be addressed before translation into clinical practice [10]. This review aims to provide a comprehensive analysis of current evidence regarding CBD’s therapeutic potential in HCC, discussing its mechanistic pathways, preclinical and clinical findings, and future perspectives for its integration into liver cancer treatment strategies.
Materials and methods
Literature search strategy
This literature review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. A comprehensive search was performed across multiple databases, including PubMed, Scopus, Web of Science, and Google Scholar, up to March 2025. The search terms used included:
“Cannabidiol” OR “CBD”.
“Liver cancer” OR “Hepatocellular carcinoma” OR “HCC”.
“Anticancer effects” OR “Apoptosis” OR “Cell proliferation”.
“Endocannabinoid system” OR “CB1 receptor” OR “CB2 receptor”.
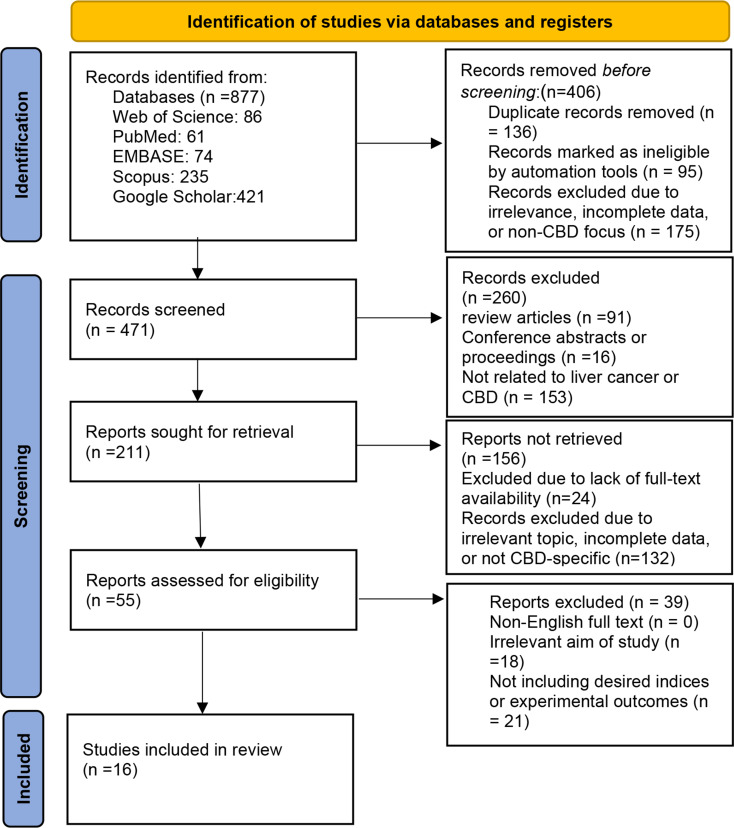
Boolean operators (AND/OR) were applied to refine the search results. The results of the search strategy, including the number of articles retrieved from each database and the subsequent selection process, are summarized in Fig. 1. Additionally, reference lists of included articles were manually screened to identify additional relevant studies.
Fig. 1.
Flowchart Summarizing the Identification and Selection of Studies for Inclusion in the Review
Inclusion and exclusion criteria
Inclusion criteria
Original studies (in vitro, in vivo, or clinical trials) investigating the effects of CBD on HCC or liver cancer models.
Studies published in English in peer-reviewed journals.
Articles discussing mechanistic insights, therapeutic efficacy, or combination therapies involving CBD.
Exclusion criteria
Review articles, meta-analyses, case reports, and commentaries.
Studies focusing on other cannabinoids (e.g., THC) without CBD-specific data.
Articles without full-text availability or those published in non-peer-reviewed sources.
Data extraction and quality assessment
Two independent reviewers screened the titles and abstracts, followed by a full-text review of eligible studies. The extracted data included the following parameters, which are summarized in Table 1:
Table 1.
Summary of studies investigating the effects of Cannabidiol (CBD) on hepatocellular carcinoma (HCC)
| Year | Study Type | Liver Cancer Model | CBD Dose | Mechanistic Insights | Receptor Targets | Combination Therapies | Key Findings | Side Effects | Future Implications | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis induced in HepG2 cells by the synthetic cannabinoid WIN: involvement of the transcription factor PPARγ | 2009 | Experimental (In vitro) | HepG2 cells (Human hepatocellular carcinoma cell line) | WIN 55,212-2 (5–10 µM) | Induction of apoptosis via upregulation of Bax, Bcl-XS, and t-Bid; downregulation of survivin, phospho-AKT, Hsp72, and Bcl-2; activation of JNK/p38 MAPK pathway and mitochondrial depolarization. | CB1, CB2 receptors; PPARγ | GW9662 and T0070907 PPAR antagonists | WIN induces apoptosis in HepG2 cells by activating PPARγ and modulating pro- and anti-apoptotic factors; mitochondrial depolarization is a key event. | Not explicitly reported | Potential therapeutic role for synthetic cannabinoids like WIN in hepatic cancer treatment; further studies needed to confirm clinical relevance. | [11] |
| The Synthetic Cannabinoid WIN 55,212-2 Sensitizes Hepatocellular Carcinoma Cells to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis by Activating p8/CCAAT/Enhancer Binding Protein Homologous Protein (CHOP)/Death Receptor 5 (DR5) Axis | 2010 | Preclinical (In vitro) | HepG2, Hep3B, SK-Hep1 | WIN dose: 2–10 µM | WIN enhances apoptosis through upregulation of CHOP and p8, leading to increased DR5 receptor expression and mitochondrial disruption. | DR5, CHOP, PPARy | WIN combined with TRAIL | The combination of WIN and TRAIL results in synergistic effects, inducing apoptosis in TRAIL-resistant hepatocellular carcinoma cells. | Not discussed | Potential clinical application for cannabinoid-TRAIL combination therapy as a promising anti-cancer strategy. | [12] |
| Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy | 2011 | Preclinical (In vitro/In vivo) | HepG2 and HuH-7 cell lines; xenograft models | Δ9-THC (8 µM), JWH-015 (8 µM) | Induction of autophagy via CB2 receptor activation, involving TRB3 upregulation, inhibition of Akt/mTORC1, and AMPK activation. | CB2 receptor | None reported | Cannabinoids (Δ9-THC and JWH-015) reduced HCC cell viability and tumor growth via autophagy stimulation; autophagy was essential for apoptosis induction. | Not explicitly reported | Potential for cannabinoids as novel therapeutic agents for advanced HCC; further studies needed to confirm clinical applicability. | [13] |
| Cannabinoid Receptor Activation Correlates with the Proapoptotic Action of the β2-Adrenergic Agonist (R, R)-4-Methoxy-1-Naphthylfenoterol in HepG2 Hepatocarcinoma Cells | 2012 | Preclinical (In vitro) | HepG2 cell line | Not applicable | MNF reduces cell proliferation and induces apoptosis via cannabinoid receptor (CB1R and CB2R) activation. MNF signaling is sensitive to pertussis toxin, indicating Gi/o protein involvement. MNF acts as a dual β2-AR and CBR ligand, with antiproliferative effects mediated through CBRs. | CB1R, CB2R, β2-AR | WIN55,212-2 (CB1R/CB2R agonist), AM251 (CB1R inverse agonist), AM630 (CB2R inverse agonist) | MNF inhibits proliferation and induces apoptosis in HepG2 cells, unlike Fenoterol, which promotes cell growth. MNF’s effects are mediated by cannabinoid receptors, as shown by the reversal of its antiproliferative effects with CB1R/CB2R inverse agonists. WIN55,212-2 also inhibits HepG2 cell growth. | Not explicitly reported | Highlights the potential of dual β2-AR/CBR ligands like MNF for liver cancer treatment. Further research is needed to explore therapeutic applications. | [14] |
| PPARγ mediates the effects of WIN55,212-2 on HCC cells | 2013 | Preclinical (In vitro) | BEL-7402 cell line | 1–10 µM | Upregulates Bax, Downregulates Bcl-2, induces mitochondrial depolarization, activates caspase pathways | CB1, CB2, PPARγ | Not studied | WIN reduces proliferation of BEL-7402 cells, induces apoptosis via mitochondrial-caspase pathway mediated by PPARγ. Cleavage of caspase-3 and PARP observed. | Not reported | Suggests potential use of WIN in hepatocellular carcinoma (HCC) treatment pending further research. | [15] |
| Involvement of PPARγ in the Antitumoral Action of Cannabinoids on Hepatocellular Carcinoma | 2013 | Preclinical (In vitro/In vivo) | HepG2 and HuH-7 cell lines; xenograft models | THC (8 µM), JWH-015 (8 µM) | Activation of PPARγ pathways, induction of autophagy via TRIB3-mediated ER stress, inhibition of Akt/mTORC1 signaling. | PPARγ, CB2 receptor | THC, JWH-015 (Synthetic CB2 Agonist) | Cannabinoids (THC and JWH-015) reduced HCC cell viability and tumor growth by activating PPARγ-dependent pathways and autophagy. | Potential resistance or altered autophagy-related effects if PPARγ is blocked. | Highlights the therapeutic potential of targeting PPARγ pathways with cannabinoids for HCC treatment; further clinical studies are needed. | [16] |
| Evaluation of Anti-Invasion Effect of Cannabinoids on Human Hepatocarcinoma Cells | 2013 | Preclinical (In vitro) | HepG2 cell line | CB65 and ACEA (0.625, 2.5, 20 nM) | Reduction of MMP-2 and MMP-9 expression, inhibition of cell invasion, and decreased viability at higher doses (20 nM). | CB1 and CB2 receptors | None reported | Cannabinoids ACEA and CB65 reduced cell viability, inhibited invasion, and suppressed MMP-2/MMP-9 expression in HepG2 cells at higher doses. | Potential toxicity with higher doses. | Potential use of CB1 and CB2 receptor agonists as therapeutic agents for HCC treatment; further studies needed to confirm clinical efficacy. | [17] |
| Cannabinoid WIN55, 212-2 induces cell cycle arrest and inhibits the proliferation and migration of human BEL7402 hepatocellular carcinoma cells | 2015 | Experimental (In vitro) | BEL7402 cell line (Human hepatocellular carcinoma) | WIN 55,212-2: 5–10 µM | WIN induces G0/G1 cell cycle arrest via CB2-mediated downregulation of phosphorylated ERK1/2, upregulation of p27, and downregulation of cyclin D1 and Cdk4. Also reduces MMP-9, retinoblastoma protein and E2F1 expression. | CB2 | CB2 antagonist (AM630), CB2 selective agonist (JWH-015) | WIN treatment led to cell cycle arrest at the G0/G1 phase, associated with inactivation of ERK1/2, increased expression of p27, and decreased expression of cyclin D1 and Cdk4. WIN also inhibited cell migration. CB2 inhibition abrogated WIN-induced cell cycle arrest. | Not reported | Cannabinoid receptor agonists, including WIN, may be considered as novel therapeutics for the treatment of HCC. | [18] |
| Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms | 2015 | Experimental (in vivo) | DEN-induced liver cancer in mice | Not applicable (not CBD-specific) | CB1R activation promotes tumor growth via FOXM1 induction, upregulation of tumor-promoting genes (e.g., IDO2), and immune tolerance through T-reg cells. | CB1R | JD5037 (Peripheral antagonist) | CB1R activation drives HCC progression; blocking CB1R reduces tumor growth and alters immune suppression via FOXM1/IDO2 pathway | No major reported side effects | Peripheral CB1R blockade could be a therapeutic target for HCC | [19] |
| Opposite roles of cannabinoid receptor 1 and 2 in hepatocarcinogenesis | 2016 | Experimental (in vivo) | DEN-induced hepatocarcinogenesis in mice | Not CBD-specific | CB1 promotes tumor growth via proliferation and fibrosis; CB2 suppresses tumor growth by modulating immune responses and T-cell recruitment. TRPV1 does not influence hepatocarcinogenesis. | CB1, CB2, TRPV1 | None reported | CB1 receptor activation increases hepatocarcinogenesis, while CB2 receptor activation decreases it. FAAH deficiency enhances HCC via increased AEA levels. TRPV1 deletion has no effect. | No major reported side effects | Targeting CB1 for inhibition and CB2 for activation may provide novel therapeutic strategies for HCC treatment. Further research is needed to confirm clinical relevance. | [20] |
| Exogenous hepatitis B virus envelope proteins induce endoplasmic reticulum stress: involvement of cannabinoid axis in liver cancer cells | 2016 | Experimental (in vitro) | Huh-7 and HepG2 liver cancer cell lines | Not CBD-specific | HBV envelope proteins (LHBs, MHBs) induce ER stress via upregulation of BiP, CHOP, PERK phosphorylation, eIF2α phosphorylation, and XBP1 splicing. CB1 receptor expression is essential for ER stress activation. | CB1 | CB1 antagonist (AM251) | HBV envelope proteins trigger ER stress in Huh-7 cells expressing CB1, but not in CB1-deficient HepG2 cells. CB1 inhibition counteracts ER stress markers like BiP and CHOP. | No major reported side effects | Highlights the role of CB1 receptor in HBV-induced ER stress and its potential as a therapeutic target for HCC progression linked to HBV infection. | [21] |
| Endocannabinoid system and the expression of endogenous ceramides in human hepatocellular carcinoma | 2019 | Observational study with human tissue samples | Human HCC tissues and adjacent non-tumor tissues from 67 patients | Not applicable (not CBD-specific) | Downregulation of CB1 and increase in CB2 levels; alterations in ceramide metabolism | CB1, CB2 | None reported | CB1 downregulation may limit anti-tumor apoptosis; CB2 increase could have immune-related implications | No significant side effects reported | CB1 and CB2 serve as potential diagnostic and therapeutic targets; ceramide metabolism requires further exploration | [22] |
| Hepatocyte cannabinoid 1 receptor nullification alleviates toxin-induced liver damage via NF-κB signaling | 2020 | Preclinical Experimental (in vivo) | Con A-induced liver injury in mice | Not applicable | Hepatocyte-specific CB1R nullification reduces liver damage by downregulating NF-κB signaling, decreasing inflammatory markers, and increasing Bcl-2 expression. | CB1 receptor | None reported | Reduced liver necrosis, inflammatory cell infiltration, and apoptosis markers; increased Bcl-2 expression. | Not explicitly reported | Supports the therapeutic potential of targeting CB1 receptors to mitigate toxin-induced liver injury; paves the way for the development of CB1 antagonists in clinical settings | [23] |
| A novel mechanism of cannabidiol in suppressing hepatocellular carcinoma by inducing GSDME dependent pyroptosis | 2021 | Experimental (In vitro and In vivo) | HepG2, HUH7, HCCLM3, MHCC97H cells; xenograft mice | 40 µM (cells); 40 mg/kg (mice) | CBD induces caspase-3/GSDME-mediated pyroptosis; accumulation of ISR and mitochondrial stress contribute to pyroptosis; Represses aerobic glycolysis via ATF4-IGFBP1-Akt axis. | Caspase-3/GSDME, ATF4/CHOP | None mentioned | CBD effectively suppresses HCC cell growth in vitro and in vivo; induces HCC cell pyroptosis in a caspase-3/GSDME-dependent manner; CBD can repress aerobic glycolysis. | Not Reported | Promising potential for CBD in HCC therapy due to dual action on cell death and metabolism | [24] |
| Cannabidiol Enhances Cabozantinib-Induced Apoptotic Cell Death via Phosphorylation of p53 Regulated by ER Stress in Hepatocellular Carcinoma | 2023 | Preclinical (In vitro) | HepG2 and Hep3B cell lines | 0-100 µM | Induction of ER stress leading to p53 phosphorylation, enhancing apoptosis | Not associated with CNR1 or CNR2 pathways | CBD + Cabozantinib | Combination therapy increased apoptosis and cytotoxicity in HCC cells; effects mediated through ER stress and p53 activation. No changes observed in Bax, Bcl-2, or caspase cleavage. | Potential toxicity from high CBD doses | Promising therapeutic strategy for enhancing the efficacy of cabozantinib in HCC treatment; further studies needed for clinical translation | [25] |
Table 2.
List of abbreviations (Alphabetical Order)
| Abbreviation | Full Term |
|---|---|
| AEA | Anandamide |
| Akt | Protein Kinase B |
| AMPK | AMP-activated Protein Kinase |
| ATF4 | Activating Transcription Factor 4 |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell Lymphoma 2 |
| CB1 | Cannabinoid Receptor Type 1 |
| CB2 | Cannabinoid Receptor Type 2 |
| CBD | Cannabidiol |
| CHOP | C/EBP Homologous Protein |
| DR5 | Death Receptor 5 |
| ECS | Endocannabinoid System |
| EMT | Epithelial-to-Mesenchymal Transition |
| ER | Endoplasmic Reticulum |
| ERK | Extracellular Signal-Regulated Kinase |
| E2F1 | E2F Transcription Factor 1 |
| GSDME | Gasdermin E |
| HCC | Hepatocellular Carcinoma |
| Hsp72 | Heat Shock Protein 72 |
| IDO2 | Indoleamine 2,3-Dioxygenase 2 |
| ISR | Integrated Stress Response |
| JNK | c-Jun N-terminal Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| MMP | Matrix Metalloproteinase |
| mTOR | Mechanistic Target of Rapamycin |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B cells |
| PARP | Poly (ADP-ribose) Polymerase |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| p53 | Tumor Protein 53 |
| ROS | Reactive Oxygen Species |
| S6K | Ribosomal Protein S6 Kinase |
| THC | Δ9-Tetrahydrocannabinol |
| TRIB3 | Tribbles Homolog 3 |
| TRPV1 | Transient Receptor Potential Vanilloid Type 1 |
| XBP1 | X-box Binding Protein 1 |
Study type (in vitro, in vivo, clinical).
Liver cancer model used.
CBD concentration, exposure duration, and administration method.
Mechanistic insights (e.g., apoptosis induction, autophagy, inhibition of metastasis).
Combination therapies (e.g., CBD + sorafenib or other anticancer agents).
Key findings and conclusions.
The quality of included studies was assessed using the SYRCLE’s risk of bias tool for animal studies and the Newcastle-Ottawa Scale (NOS) for clinical studies, with the results presented in Table 1. Any discrepancies in study selection or data extraction were resolved through discussion among the reviewers.
Results
Following the systematic search, a total of 16 studies were identified, of which 16 met the inclusion criteria. The selected studies included in vitro (n = 10), in vivo (n = 4), and clinical (n = 2) studies investigating the effects of cannabidiol (CBD) on hepatocellular carcinoma (HCC) and liver cancer models. These studies provided insights into CBD’s mechanisms of action, its therapeutic efficacy, and its potential use in combination with conventional treatments.
The studies reviewed utilized various liver cancer models, including human HCC cell lines (HepG2, Huh7, SMMC-7721, etc.), animal models such as murine xenografts and chemically induced HCC models, as well as patient-derived tissue samples. CBD was administered in different concentrations ranging from 1 to 50 µM for in vitro studies and dosages of 5–50 mg/kg in animal models. These methodologies ensured the generation of comprehensive preclinical evidence.
Discussion
Additional evidence from preclinical and clinical studies
Recent studies suggest that in addition to classical cannabinoid receptors (CB1 and CB2), alternative molecular targets significantly contribute to the anticancer effects of cannabidiol (CBD). Notably, TRPV1, a calcium-permeable ion channel, facilitates CBD-induced calcium influx, triggering apoptotic signaling in cancer cells. Although in vivo studies indicate that TRPV1 may not directly influence hepatocarcinogenesis [20], its activation remains relevant in in vitro models of liver and other cancers. Another key pathway involves PPARγ, a nuclear receptor regulating metabolism and cell differentiation. Evidence shows that CBD and related cannabinoids activate PPARγ, thereby inducing apoptosis and autophagy in HCC models [11, 15, 16]. These findings collectively support the idea that CBD’s anticancer mechanisms extend beyond CB1/CB2 receptor modulation and encompass broader molecular signaling pathways.
Moreover, various preclinical investigations have elucidated how cannabinoids induce cytotoxicity in HCC. For instance, synthetic cannabinoids like WIN 55,212-2 trigger apoptosis via PPARγ-mediated mitochondrial depolarization and caspase activation [11, 15]. This was further reinforced by findings that WIN enhances TRAIL-induced apoptosis through upregulation of CHOP and death receptor 5 (DR5), suggesting potential for combination therapies [12].
Cannabinoid receptor agonists such as ACEA and CB65 have been shown to inhibit HCC cell invasion and migration by downregulating MMP-2 and MMP-9 [17]. Additionally, cannabinoids disrupt pro-survival pathways like ERK/MAPK and Akt/mTOR, reducing cancer cell proliferation [13, 14, 16]. Interestingly, MNF, a dual agonist of cannabinoid and β2-adrenergic receptors, exerts antiproliferative effects, indicating a therapeutic role for multi-targeted compounds [14].
In vivo studies emphasize the contrasting roles of cannabinoid receptors: CB1 activation contributes to tumor progression via FOXM1 and IDO2, while CB2 activation inhibits tumor growth, possibly by modulating T-cell recruitment and immune function [19, 20]. Furthermore, CB1 inhibition alleviates inflammation in toxin-induced liver injury models, underscoring its relevance to hepatic pathology [23].
Human studies further support these insights. In HCC patient tissues, altered expression of CB1 and CB2 receptors, alongside dysregulated ceramide metabolism, suggest potential diagnostic and therapeutic utility [22].
Recent evidence has deepened our understanding of CBD-specific pathways in HCC. Shangguan et al. demonstrated that CBD induces pyroptosis via the caspase-3/GSDME axis, while simultaneously repressing aerobic glycolysis through the ATF4-IGFBP1-Akt pathway [24]. Notably, combination therapy of CBD with cabozantinib led to enhanced apoptotic cell death mediated by ER stress and p53 phosphorylation, proposing a chemosensitizing role for CBD [25].
Furthermore, CBD may also modulate drug resistance in cancer by affecting efflux transporter activity. Although direct evidence in hepatocellular carcinoma is limited, preclinical studies in other tumor models, such as breast and colon cancers, have demonstrated that CBD can inhibit the expression and function of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). These effects result in increased intracellular accumulation of chemotherapeutic drugs, suggesting a potential role for CBD in reversing multidrug resistance (MDR) mechanisms in HCC. This hypothesis warrants further experimental validation in liver cancer models [26–29].
Importantly, while caspase-3/GSDME-mediated pyroptosis is supported by strong preclinical data, the potential involvement of canonical inflammasome activation (e.g., NLRP3/caspase-1) remains unclear in HCC. Although evidence from other disease contexts suggests CBD may influence inflammasome activity, further research is required to confirm such effects in liver cancer models.
Collectively, these studies underscore the diverse antitumor mechanisms of cannabinoids, including induction of apoptosis, autophagy, inhibition of invasion and metastasis, immune modulation, and enhancement of standard therapies.
To better illustrate the synergistic mechanisms of CBD with chemotherapeutic agents, we have included a proposed schematic model (Fig. 2), outlining key molecular interactions—such as ER stress, p53 activation, and inhibition of the Akt/mTOR pathway—that contribute to enhanced chemosensitivity.
Fig. 2.
Proposed schematic model showing how cannabidiol (CBD) enhances the anticancer efficacy of sorafenib and cabozantinib in hepatocellular carcinoma (HCC). CBD promotes ER stress and subsequent phosphorylation of p53, which leads to apoptotic cell death. Additionally, CBD inhibits the Akt/mTOR signaling pathway, contributing to increased chemosensitivity. These synergistic effects result in enhanced apoptosis and therapeutic efficacy when combined with standard chemotherapeutic agents
Safety considerations: hepatotoxicity and drug interactions
While cannabidiol (CBD) holds considerable promise as a therapeutic agent in hepatocellular carcinoma (HCC), its safety profile must be carefully evaluated, especially in the context of liver function. Preclinical studies and early-phase clinical trials have reported instances of elevated liver enzymes (ALT, AST) in patients receiving high doses of CBD, raising concerns about hepatotoxicity. These effects are often dose-dependent, with hepatic adverse events more frequently observed at doses exceeding 20 mg/kg/day [10].
Moreover, CBD is primarily metabolized by hepatic cytochrome P450 enzymes, particularly CYP3A4 and CYP2C19, which also mediate the metabolism of many commonly used anticancer drugs, including sorafenib and cabozantinib. As such, drug-drug interactions are a critical consideration. CBD can act as a competitive inhibitor of these enzymes, potentially leading to increased serum concentrations of co-administered chemotherapeutic agents, thereby enhancing toxicity risks [30, 31].
Given these issues, the co-administration of CBD with standard HCC therapies should be approached with caution. Monitoring liver function tests (LFTs) and serum drug levels during treatment is highly recommended. Additionally, novel delivery systems such as lipid-based nanoformulations may help reduce hepatic burden and improve CBD’s pharmacokinetic profile, enhancing both safety and efficacy.
Further clinical studies are needed to define the therapeutic window of CBD, assess long-term safety outcomes, and establish clear guidelines for its use in patients with liver dysfunction or those undergoing polypharmacy.
Challenges and future directions
Despite these promising findings, several challenges hinder the clinical translation of CBD for HCC treatment. CBD’s poor bioavailability, rapid hepatic metabolism, and potential hepatotoxicity remain key obstacles. Nanotechnology-based delivery systems and lipid carriers are being explored to overcome these limitations and enhance therapeutic efficacy. Furthermore, additional clinical trials are urgently needed to establish optimal dosing regimens and evaluate long-term safety profiles.
Another critical aspect is CBD’s impact on the tumor microenvironment. Preliminary data suggest that CBD reduces pro-inflammatory cytokines and modulates immune responses, potentially creating a less favorable environment for tumor progression. However, further research is needed to elucidate these interactions and their implications for HCC therapy.ose undergoing polypharmacy.
Conclusion
CBD has emerged as a promising therapeutic agent for HCC through its multifaceted antitumor activities, including the induction of apoptosis and pyroptosis, modulation of autophagy, suppression of metastasis, and disruption of tumor-supportive signaling pathways such as PI3K/Akt/mTOR. Beyond its interaction with classical cannabinoid receptors, CBD also engages alternative molecular targets such as TRPV1 and PPARγ, broadening its therapeutic relevance.
Importantly, CBD enhances the efficacy of chemotherapeutic agents like sorafenib and cabozantinib by promoting endoplasmic reticulum stress and p53 activation, suggesting a potential role as a chemosensitizer. Preliminary evidence also indicates that CBD may counteract multidrug resistance by modulating efflux transporters (P-glycoprotein, BCRP), although this remains to be validated in HCC models.
Despite these promising effects, concerns regarding hepatotoxicity and drug-drug interactions necessitate careful clinical consideration. CBD’s metabolism via hepatic cytochrome P450 enzymes may interfere with standard anticancer therapies, and its hepatic safety profile must be closely monitored.
Overall, while preclinical findings strongly support the therapeutic potential of CBD in HCC, robust clinical trials are urgently needed to confirm its efficacy, safety, optimal dosing strategies, and long-term effects. If validated, CBD could represent an innovative and complementary approach in the management of hepatocellular carcinoma.
Author contributions
M.E and M.D. wrote the protocol. M.E. and M.D. collated the data for the study. The first draft of the manuscript was written by M.E. and M.D. and thoroughly revised by all authors. All authors progressed the concept of this study.
Funding
Nil.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical considerations
The authors have carefully addressed ethical considerations, including monitoring for text plagiarism, duplicate publications, research misconduct, data fabrication, and falsification.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Llovet J, Kelley R, Villanueva A, Singal A, Pikarsky E, Roayaie S et al. Hepatocellular Carcinoma Nat Rev Dis Primers. 7: 6. Article10. 2021;1038. [DOI] [PubMed]
- 2.O’Brien K. Cannabidiol (CBD) in cancer management. Cancers. 2022;14(4):885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seltzer ES, Watters AK, MacKenzie D, Granat LM, Zhang D. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers. 2020;12(11):3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott KA, Dalgleish AG, Liu WM. The combination of Cannabidiol and ∆9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol Cancer Ther. 2014;13(12):2955–67. [DOI] [PubMed] [Google Scholar]
- 5.Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78(16):1665–703. [DOI] [PubMed] [Google Scholar]
- 6.Fu Z, Zhao P-Y, Yang X-P, Li H, Hu S-D, Xu Y-X, et al. Cannabidiol regulates apoptosis and autophagy in inflammation and cancer: A review. Front Pharmacol. 2023;14:1094020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols JM, Kaplan BL. Immune responses regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020;5(1):12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Sharma A, Hoffmann MJ, Skowasch D, Essler M, Weiher H, et al. Discovering single Cannabidiol or synergistic antitumor effects of Cannabidiol and cytokine-induced killer cells on non-small cell lung cancer cells. Front Immunol. 2024;15:1268652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosseinzadeh F, Verdi J, Ai J, Hajighasemlou S, Seyhoun I, Parvizpour F, et al. Combinational immune-cell therapy of natural killer cells and Sorafenib for advanced hepatocellular carcinoma: a review. Cancer Cell Int. 2018;18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eadie L, Lo LA, Boivin M, Deol JK, MacCallum CA. Clinical guidance for cannabidiol-associated hepatotoxicity: A narrative review. J Gastroenterol Hepatol. 2024;39(12):2522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliano M, Pellerito O, Portanova P, Calvaruso G, Santulli A, De Blasio A, et al. Apoptosis induced in HepG2 cells by the synthetic cannabinoid WIN: involvement of the transcription factor PPARγ. Biochimie. 2009;91(4):457–65. [DOI] [PubMed] [Google Scholar]
- 12.Pellerito O, Calvaruso G, Portanova P, De Blasio A, Santulli A, Vento R, et al. The synthetic cannabinoid WIN 55,212-2 sensitizes hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating p8/CCAAT/enhancer binding protein homologous protein (CHOP)/death receptor 5 (DR5) axis. Mol Pharmacol. 2010;77(5):854–63. [DOI] [PubMed] [Google Scholar]
- 13.Vara D, Salazar M, Olea-Herrero N, Guzmán M, Velasco G, Díaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18(7):1099–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul RK, Ramamoorthy A, Scheers J, Wersto RP, Toll L, Jimenez L, et al. Cannabinoid receptor activation correlates with the proapoptotic action of the β2-adrenergic agonist (R, R′)-4-methoxy-1-naphthylfenoterol in HepG2 hepatocarcinoma cells. J Pharmacol Exp Ther. 2012;343(1):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong Y, Zhou Y, Wang Y, Xiao S, Liao DJ, Zhao Q. PPARγ mediates the effects of WIN55, 212-2, an synthetic cannabinoid, on the proliferation and apoptosis of the BEL-7402 hepatocarcinoma cells. Mol Biol Rep. 2013;40:6287–93. [DOI] [PubMed] [Google Scholar]
- 16.Vara D, Morell C, Rodríguez-Henche N, Diaz-Laviada I. Involvement of PPARγ in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis. 2013;4(5):e618–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pourkhalili N, Ghahremani MH, Farsandaj N, Tavajohi S, Majdzadeh M, Parsa M, et al. Evaluation of anti-invasion effect of cannabinoids on human hepatocarcinoma cells. Toxicol Mech Methods. 2013;23(2):120–6. [DOI] [PubMed] [Google Scholar]
- 18.Xu D, Wang J, Zhou Z, He Z, Zhao Q, Cannabinoid. 212-2 induces cell cycle arrest and inhibits the proliferation and migration of human BEL7402 hepatocellular carcinoma cells. Mol Med Rep. 2015;WIN55(6):7963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukhopadhyay B, Schuebel K, Mukhopadhyay P, Cinar R, Godlewski G, Xiong K, et al. Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms. Hepatology. 2015;61(5):1615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suk K-T, Mederacke I, Gwak G-Y, Cho SW, Adeyemi A, Friedman R, et al. Opposite roles of cannabinoid receptors 1 and 2 in hepatocarcinogenesis. Gut. 2016;65(10):1721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montalbano R, Honrath B, Wissniowski TT, Elxnat M, Roth S, Ocker M, et al. Exogenous hepatitis B virus envelope proteins induce Endoplasmic reticulum stress: involvement of cannabinoid axis in liver cancer cells. Oncotarget. 2016;7(15):20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Tian Y, Zheng R, Li L, Qiu F. Endocannabinoid system and the expression of endogenous ceramides in human hepatocellular carcinoma. Oncol Lett. 2019;18(2):1530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Gautam S, Aseer KR, Kim J, Chandrasekaran P, Mazucanti CH, et al. Hepatocyte cannabinoid 1 receptor nullification alleviates toxin-induced liver damage via NF-κB signaling. Cell Death Dis. 2020;11(12):1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shangguan F, Zhou H, Ma N, Wu S, Huang H, Jin G, et al. A novel mechanism of Cannabidiol in suppressing hepatocellular carcinoma by inducing GSDME dependent pyroptosis. Front Cell Dev Biology. 2021;9:697832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon Y, Kim T, Kwon H, Kim J-K, Park Y-T, Ham J, et al. Cannabidiol enhances cabozantinib-induced apoptotic cell death via phosphorylation of p53 regulated by ER stress in hepatocellular carcinoma. Cancers. 2023;15(15):3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland M, Panetta J, Hoskins J, Bebawy M, Roufogalis B, Allen J, et al. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol. 2006;71(8):1146–54. [DOI] [PubMed] [Google Scholar]
- 27.Anderson LL, Etchart MG, Bahceci D, Golembiewski TA, Arnold JC. Cannabis constituents interact at the drug efflux pump BCRP to markedly increase plasma Cannabidiolic acid concentrations. Sci Rep. 2021;11(1):14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H-J, Wang J-S, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, et al. Characterization of P-glycoprotein Inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther. 2006;317(2):850–7. [DOI] [PubMed] [Google Scholar]
- 29.Holland M, Lau D, Allen J, Arnold J. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br J Pharmacol. 2007;152(5):815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doohan PT, Oldfield LD, Arnold JC, Anderson LL. Cannabinoid interactions with cytochrome P450 drug metabolism: a full-spectrum characterization. AAPS J. 2021;23(4):91. [DOI] [PubMed] [Google Scholar]
- 31.Smith SA, Le GH, Teopiz KM, Kwan AT, Rhee TG, Ho RC, et al. Effects of Cannabidiol and ∆9-tetrahydrocannabinol on cytochrome P450 enzymes: a systematic review. Drug Metab Rev. 2024;56(2):164–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.