Abstract
Multiple sclerosis (MS) is an autoimmune condition characterized by inflammatory demyelination that leads to irreversible neurological damage within the central nervous system (CNS). This review examines the therapeutic potential and clinical efficacy of cladribine tablets (CladT) for treating MS, focusing on the immune reconstitution mechanism and CNS effects. CladT represents a notable advance among disease-modifying therapies for MS due to its selective targeting of lymphocytes, resulting in sustained yet reversible immune modulation. This action leads to a substantial reduction in memory B cells while preserving the innate immune system and maintaining immunoglobulin levels, thereby mitigating the risks of secondary autoimmunity and infection. Cladribine appears to penetrate the blood–brain barrier, as indicated by cerebrospinal fluid (CSF) studies from parenteral cladribine use. In MS, CladT is associated with reductions in CSF immunological markers, such as oligoclonal bands and neurofilament light chain levels; it also mitigates acute and chronic inflammation, as evidenced, respectively, by consistent reductions in unique active lesions, and significant decrease in slowly expanding lesions in patients with predominant grey matter damage. These findings underscore the potential of CladT in reducing disability accumulation and improving long-term clinical outcomes in patients with highly active disease. By synthesizing data from clinical trials and real-world studies, this review underscores the effectiveness of CladT in reducing relapse rates, disability progression and magnetic resonance imaging-detected disease activity and emphasizes the importance of early high-efficacy treatment for optimizing long-term outcomes. Furthermore, emerging biomarkers are discussed as potential tools for predicting individual responses to therapy, thereby enabling more personalized treatment strategies. This review also provides valuable insights into the positive impact of CladT on quality of life measures, long-term outcomes and safety profile, all of which support the use of CladT in the evolving landscape of MS management.
Keywords: B cells, cladribine tablets, disease-modifying therapy, multiple sclerosis, T cells
Plain language summary
Cladribine tablets: a breakthrough in multiple sclerosis treatment
This review examines the effectiveness of cladribine tablets (CladT) as a treatment for multiple sclerosis (MS), a chronic autoimmune disease that damages the central nervous system. The article discusses how CladT works by selectively targeting specific immune cells, reducing harmful memory B cells while preserving the overall immune system’s function. Notably, CladT can cross the blood-brain barrier, help reduce key markers of MS, and mitigate acute and chronic inflammation in the brain. Evidence from clinical trials and real-world studies demonstrates that CladT significantly decreases relapse rates and slows disability progression, suggesting that early treatment with CladT can lead to improved long-term outcomes. Additionally, the review explores potential biomarkers that could personalize MS treatment and highlights how CladT’s benefits and safety contribute to improving the quality of life for people with MS.
Introduction
Multiple sclerosis (MS) is an autoimmune disorder of the central nervous system (CNS), characterized by inflammatory demyelination and axonal/neuronal damage, resulting in irreversible neurological damage in the brain and spinal cord. 1 The formation of focal lesions in MS is attributed to the infiltration of immune cells, including T cells, B cells and myeloid cells, into the parenchyma of the CNS, causing associated tissue damage.2–4 Following acute inflammation, MS lesions may evolve into chronic states characterized by various outcomes, such as remyelination, inflammation resolution without repair or a ‘smoldering’ state where inflammation and myelin degeneration coexist. 5
In 2020, approximately 2.8 million people worldwide were affected by MS, with a prevalence of 35.9 per 100,000 population, showing a 30% increase since 2013. 6 Typically diagnosed in young adults aged 20–30 years, MS significantly impacts physical function, cognition, health-related quality of life (HRQoL) and employment. 7 Females are affected at twice the rate of males, a ratio that has recently increased. 6
Disease symptoms vary and may include unilateral optic neuritis, limb weakness, ataxia, sensory loss and brainstem dysfunction, among others. 8 Progressive neurological decline in individuals with MS primarily manifests as impaired ambulation, loss of bladder control and slowed cognitive processing. 8 The estimated life expectancy for individuals with MS is 75.9 years, compared with 83.4 years in a matched population. 9
MS follows different disease courses, ranging from relapsing-remitting to progressive disease forms, with relapses occurring at variable intervals often accompanied by temporary or permanent deterioration of symptoms. 10 Relapsing-remitting MS (RRMS) may progress to secondary progressive MS (SPMS) in 20% of individuals, 11 typically within 15 years if untreated, 12 while a smaller proportion (15%) of patients experience primary progressive MS (PPMS) from disease onset. 7 Although the aetiology of MS remains unknown, risk factors include Epstein–Barr virus infection, smoking, low vitamin D, adolescent obesity and genetic predisposition. 13 Genes within the human leukocyte antigen complex are the most influential genetic risk factors. 13
The immune response in MS involves complex processes encompassing T cells, B cells, antibodies and innate immune cells, such as blood-borne macrophages that infiltrate active MS lesions, all of which play a significant role in the development of the disease.14,15 In particular, pathogenic lymphocytes are activated peripherally and subsequently infiltrate the CNS, initiating local inflammation and demyelination. 16 While T cells have traditionally been seen to exert the predominant influence over MS, 17 due to their presence within MS lesions, 18 extensive evidence now highlights the multifaceted role of B cells in MS pathophysiology, particularly in antibody production and adaptive and innate immune responses. 19 This evolving understanding of MS immunology has guided the development of disease-modifying therapies (DMTs),20,21 which help manage the disease course, symptoms and relapses, thereby improving patients’ QoL. 22 Despite such advancements, treatments capable of halting or reversing neurodegenerative processes in MS remain an unmet need.
The heterogeneous presentation of MS results in variable treatment responses and risk of progressive disease, which is marked by deteriorating neurological function and escalating disability. Current MS treatments entail various mechanisms of action (MoAs) including immunomodulation, continuous immunosuppression or transient immunosuppression, as seen with immune reconstitution therapies (IRT). Understanding the distinct MoA of MS treatment is crucial for tailoring therapy to individual patients and optimizing treatment outcomes while managing potential adverse effects (AEs). 23 Dosing and administration frequency of DMTs affect patient adherence, 24 and hence treatment efficacy. No reliable biomarkers currently exist to predict individual responses to therapy and inform treatment decisions.
Selective IRTs are administered as short courses and produce durable immune effects.25,26 Currently, selective IRTs include cladribine tablets (CladT) and alemtuzumab. Cladribine is given in two short oral treatment courses during the first 2 years, 27 delivering a cumulative dose of 3.5 mg/kg over a 2-year period, with no further treatment required in years 3 and 4.27,28 Retreatment with CladT beyond year 5 is becoming an increasingly relevant topic, particularly for patients who exhibit ongoing disease activity. Recent expert opinion and consensus recommend a structured approach to retreatment based on an individual basis.29–32 While clinical experiences with retreatment are emerging, they remain limited.33–37
Alemtuzumab is administered intravenously in two courses 12 months apart, with up to two additional courses allowed if needed.38,39 However, the FDA has issued warnings about rare but serious risks of stroke and arterial dissection with alemtuzumab. 40
Compared with platform therapies, selective IRTs have shown greater adherence and efficacy25,26; risks associated with their use are frontloaded and primarily relate to the initial immune depletion phase, 41 with immunosuppression lasting only temporarily.25,26
The IRT mechanism is characterized by a depletion phase, followed by an immune repopulation/reconstitution phase. 42 This short-term immunosuppression allows the immune system to regain normal effector functions during reconstitution, preserving immune competence, supporting normal responses to both inactivated and live vaccinations and permitting safe pregnancy and lactation after drug elimination.25,42 During reconstitution, the immune system acquires a qualitatively and quantitatively different profile that may induce long-term remission, reduce hospital visits and minimize treatment and monitoring needs, with some MS patients experiencing remission for over 10 years.42–45 Early initiation of IRT in MS has been associated with better long-term outcomes, including reduced disability progression, and decreased serum neurofilament light chain (NfL).46–48
Cladribine, which induces immune reconstitution, shows promise in restoring a balanced immune environment, controlling disease activity and potentially promoting neuroprotection, as suggested by reduction of cerebrospinal fluid (CSF) NfL, 49 Magnetic Resonance Imaging (MRI) activity, 50 and grey matter loss, 51 especially when administered early in the disease course.52–58
Cladribine is available orally in tablet form for MS treatment. 27 As a pro-drug analogue of adenosine, cladribine selectively targets peripheral B and T lymphocytes, 59 rapidly and sustainably reducing B-cell levels, 59 particularly memory B cells, 60 with minimal effects on T cells and immunoglobulins (Ig).28,61–64 Reconstitution occurs at variable rates depending on cell subtype.28,61–64 These specific immune depletion and repopulation dynamics, associated with rapid and sustained effects, have been shown to reduce MS relapse rate, slow disability progression and mitigate MRI-detected disease activity in randomized clinical trials52–56 and real-world studies.51,57,58,65–71
Administered as a short course on a 2-year annual dosing schedule, CladT has shown efficacy and a favourable safety profile across different MS patient categories, ranging from individuals at high risk of disease progression to those experiencing their first demyelinating event.55,72,73 CladT treatment is associated with improvements in HRQoL, stabilization in cognitive function 74 and maintenance of overall immune competence,41,63,75,76 as evidenced by robust vaccine responses,60,77,78 without secondary autoimmunity. 42
In this review, we integrate findings from clinical studies, current real-world analyses and the latest available data on CladT. Moreover, we provide an assessment of emerging potential biomarkers that may support treatment efficacy in patients with MS. Our aim is to summarize the evidence of the high efficacy of CladT and to offer valuable insights into its value as a therapeutic agent for MS, thereby further informing treatment decisions.
Review method
The authors conducted a comprehensive non-systematic review of the existing literature on PubMed/MEDLINE using the following keywords and Medical Subject Headings related to MS and treatment: ‘multiple sclerosis’ AND ‘cladribine’ OR ‘disease-modifying therapy’ OR ‘treatment’. Articles in languages other than English were excluded. Additional articles were identified by the authors through their literature expertise and citation review. We included peer-reviewed studies as the primary sources, while congress presentations were used to provide recent insights where peer-reviewed data were unavailable.
Cladribine mechanism of action: immune reconstitution and the impact on the CNS
Cladribine (2-chlorodeoxyadenosine, 2CdA) is a synthetic chlorinated purine nucleoside analogue; it functions as a pro-drug and requires intracellular phosphorylation for activation.61,79 Cladribine is resistant to the adenosine deaminase (ADA) enzyme and is incorporated into target cells.80–82 Through a series of phosphorylation steps, cladribine is converted into its phosphorylated active form, with deoxycytidine kinase (DCK) catalyzing the rate-limiting step. 82 Conversely, cytosolic 5′-nucleotidase (5′-NT) dephosphorylates cladribine into its inactive form. 59 T and B lymphocytes show a relatively high intracellular DCK to 5′-NT ratio compared with other cell types,52,59,82–86 making them more vulnerable to 2CdA-induced apoptosis.59,79,82,83 2CdA is designed to achieve lymphocyte depletion through a selective mechanism that leads to rapid, sustained, yet transient depletion of lymphocytes, which reach their nadir at month 3. 64 However, the impact of 2CdA on the immune system likely extends beyond lymphocyte depletion, potentially influencing the cytokine milieu, reducing chemokine production and inhibiting T cell migration and proliferation.60,87–89
Numerous studies90–92 suggest that specific subsets of CD4+ and CD8+ T cells may have distinct roles in the MS disease process, contributing to myelin loss, oligodendrocyte damage and axonal injury, ultimately leading to neurological dysfunction. 93 B cells, alongside their interplay with T cells, are crucial in the pathobiology of MS,94,95 given that circulating B cells from individuals with MS effectively activate autoreactive T cells.96,97 CladT treatment leads to a rapid 70%–90% decrease in CD19+ B cells within the first 2–4 months,62,98,99 followed by repopulation primarily with newly produced immature and mature naïve B cells (Figure 1).60,99–102 Notably, memory B cells remain consistently low for over 24 months.60,99–102 The correlation between the timing of B-cell subtype depletion and the inhibition of new lesion formation, as seen on MRI, suggests a key role of memory B cells in MS disease control.103,104
Figure 1.
The dynamics of immune cell populations across different phases during CladT treatment: reduction phase, repopulation phase and reconstitution phase. The adaptive and innate immune cells are depicted, highlighting changes in B cells (blue), T cells (green), neutrophils (lilac) and monocytes (light purple). The left side outlines the transitions in immune cell populations, indicating a decrease in memory B cells and T cell subsets, followed by an increase in regulatory, transitional and naïve B cells. The right-side details cytokine-producing cells, differentiating between pro-inflammatory and anti-inflammatory responses. The diagram shows a decrease in IL-6+ and GM-CSF+ CD4+ and CD8+ T cells, while memory IL-10+ B cells and IL-4+ CD4+ and CD8+ T cells increase, indicating a shift towards an anti-inflammatory phenotype.
CladT, cladribine tablets; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-6, interleukin-6.
The MAGNIFY-MS sub-study,50,60 an open-label, single-arm, multicentre, 2-year phase IV study, evaluated immune cell subtypes dynamics over 24 months following the initial CladT administration in patients with highly active relapsing MS (RMS), defined by one relapse in the past year and at least one T1 gadolinium-enhancing lesion (Gd+) or nine or more T2 lesions, while on therapy with other DMT, or two or more relapses in the past year, regardless of prior treatment, Expanded Disability Status Scale (EDSS) score less than equals to (⩽)5.0. 105 The MRI response aligned with a swift reduction in median CD19+, CD20+ and activated CD69+ B-cell counts, reaching the lowest level by month 3. 106 While total CD19+, CD20+ and activated CD69+ B-cell counts gradually recovered, 60 memory B cells decreased to their nadir, reaching a 96% reduction by month 15, which coincided with the end of the second CladT cycle. 106 Notably, in contrast with memory B cells, the trend of naïve, transitional and regulatory B cells tended to increase compared with baseline (Figure 1).60,106 Similarly, T-cell subtype depletion was slower and less pronounced than that of B-cell depletion, reaching its nadir by month 15, underscoring the importance of completing the second CladT cycle. 106
Given the beneficial role of natural killer (NK) cells in immunomodulation, particularly in inhibiting the proliferation of autoreactive T cells in MS as well as their cytotoxic function during infection, 107 the MAGNIFY-MS sub-study 106 further examined NK cell subtypes. The results revealed an increase in CD16-/CD56bright and Nkp46 cell levels after CladT treatment, suggesting an immune reconstitution shift towards an anti-inflammatory and immunoregulatory state in MS patients treated with CladT (Figure 1). 106 Additionally, throughout the study, no clinically significant changes were noted in serum IgG and IgM levels, 106 which remained within normal ranges for most patients. 60 Importantly, CladT treatment did not result in secondary hypogammaglobulinemia thereby lowering the risk of serious infections.102,106,108
This study confirmed that CladT treatment significantly impacts memory B cells, while leaving the innate immune system, as well as IgG or IgM levels, unaffected over the 24-month course. 106 CladT has not been linked to secondary autoimmunity, probably due to its milder impact on T cells, 42 which prevents the escape of autoreactive immature B cells from T cell-mediated control109,110 and allows B cells to recover with adequate T-cell regulation.64,111 Additional factors contributing to this immune stability include the slow recovery of B cells after CladT treatment,62,63,112 along with the CladT-specific dynamics of certain B-cell subsets,111,112 and the expansion of regulatory B, T and NK immune cell subsets. These factors may indicate an attempt by the reconstituted immune system to restore a balanced immunological environment and suppress autoreactive lymphocytes. 8
CladT induces substantial changes in cytokine secretion patterns, 106 reducing pro-inflammatory cytokine-producing B cells (interleukin-6+ (IL-6+)) and T cells (granulocyte-macrophage colony-stimulating factor+ (GM-CSF+), tumour necrosis factor-α+ (TNF-α+), Interferon-γ+) while increasing anti-inflammatory cytokine-producing (IL-4+) T cells beyond baseline levels, suggesting a positive treatment response (Figure 1). 106 Data from MAGNIFY-MS 106 on this anti-inflammatory immune shift in MS are supported by real-world evidence. In a study by Balasa et al., 113 the authors examined the secretory cytokine profiles from cultured and stimulated peripheral blood mononuclear cells (PBMCs) of treatment-naïve RRMS patients during their remitting phase and treated with CladT. They found that CladT induces a shift towards an anti-inflammatory profile, characterized by increased production of IL-4 and decreased TNF-α production in surviving T cells. 113 Additionally, Holm Hansen et al. 114 observed a transition towards a naïve and anti-inflammatory phenotype in the peripheral B-cell pool of newly diagnosed, naïve to DMTs RRMS patients after 1 year of CladT treatment.
A recent phase IV prospective study (CLADIN) investigated the impact of CladT on innate immunity. 115 This study provided new in vivo evidence of the acute and reversible effect of CladT on monocytes in patients with RRMS, 56.1% of whom were DMT-naïve, particularly CD14(lo), CD16+ ‘non-classical’ monocytes, which recovered within 2 months. 115 However, lymphocyte counts (CD4+, CD8+, CD19+) remained low for up to 12 months. 115 Among the 14 cytokine panels tested, only CCL2 increased 1 week after CladT administration. 115 In vitro experiments further demonstrated that CladT affects the purinergic P2X7 receptor, significantly inhibiting its channel and pore conductance states 115 ; this finding introduces a potential new mechanism of action for CladT which may reduce CNS inflammation in RRMS.
By impacting innate immune cells, CladT may also favourably influence chronic active lesions, where innate immunity and smouldering inflammation are believed to drive disease progression. 115
In vitro data of the effects of cladribine on murine microglia indicated that lower concentrations (0.1–1 µM) significantly reduced granularity, phagocytotic ability and microglia motility, and a higher concentration (10 µM) increased the expression of pro- and anti-inflammatory genes without affecting protein secretion. 116 Overall, cladribine appears to influence gene expression and functional properties of activated microglia, potentially contributing to its therapeutic effects, while not impacting naïve microglia. 116
A more recent study highlighted the significant impact of CladT on the transmigration of monocytes and dendritic cells; in particular, classical monocytes preferentially transmigrated across the stimulated blood–brain barrier (BBB), while non-classical monocytes did not, and dendritic cells showed no active migration in healthy subjects. 117 Further study is needed to assess the impact of CladT on chronic macrophage and microglia activation.
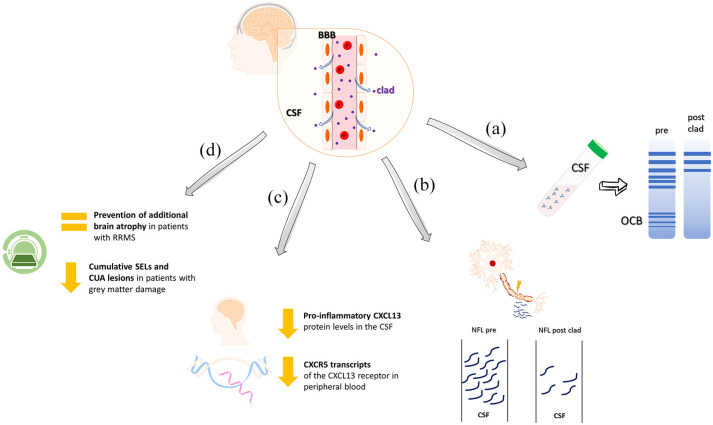
In a recent study, Marastoni et al. 118 presented interim findings from a long-term prospective study assessing CSF inflammatory markers as potential predictors of early disease activity following CladT treatment in patients with RRMS, 56.4% of whom were DMT-naïve and 56% had high disease activity. Notably, they found that CSF Chitinase-3-like 1 may offer valuable prognostic insights into disease activity during the initial treatment years. 118 CSF inflammatory markers mirror persistent CNS processes often unaffected by DMTs, indicating a possible central effect of CladT. Cladribine has been shown to cross the BBB, reaching CSF concentrations of approximately 25% of plasma levels,26,119–121 and rapidly dispersing into tissues shortly after administration. 120 Reduction in Ig oligoclonal bands (OCBs), a key diagnostic marker in MS linked to Ig-producing B cells in the CNS, 122 has been observed in nearly half of a DMT-naïve RRMS patient cohort undergoing subcutaneous cladribine treatment over 10 years. 123 This phenomenon, which is associated with lower disability levels, 123 was recently confirmed by preliminary CSF biomarker data from the MAGNIFY-MS sub-study, 124 where OCBs were reduced or eliminated at least once in 76.5% of participants at post-baseline visits (M12, M24; Figure 2(a)), with NfL levels showing a sustained reduction of over 55% (Figure 2(b)). 124 Moreover, CSF median IgG and IgM levels remained within normal ranges, indicating preservation of immune response capacity. 124
Figure 2.
The central effects of CladT. (a) Reduction of OCB in the CSF of patients with MS treated with CladT (clad). (b) Reduction of NfL levels in CSF samples collected pre- and post-treatment. (c) Reduction of protein levels of CXCL13 in the CSF and the transcripts of the CXCL13 receptor (CXCR5) in peripheral blood. (d) The role of CladT in preventing further brain atrophy is associated with a reduction in cumulative SELs and CUA lesions in patients with MS with grey matter damage.
CladT, cladribine tablets; CSF, cerebrospinal fluid; CUA, combined unique active; MS, multiple sclerosis; NfL, neurofilament light chain; OCB, oligoclonal bands; SEL, slowly expanding lesion.
Recent updates on the MAGNIFY-MS study reported a decrease of proinflammatory CXCL13 chemokine levels in the CSF accompanied by a corresponding reduction in CXCR5 transcripts of the CXCL13 receptors in peripheral blood (Figure 2(c)). 125
Together, these findings suggest that CladT may exert a direct effect within the CNS although the clinical significance of this remains to be investigated. As a small molecule with the ability to penetrate the BBB and tertiary lymphoid organs (TLOs), cladribine potentially targets inflammatory immune cells within the CNS as well as other tissues that monoclonal antibodies are unable to penetrate. Several recent studies have demonstrated the efficacy of CladT in reducing serum NfL (sNfL) levels over a 2-year treatment period in RRMS patients, including those with highly active disease, with DMT-naïve patients ranging from 14% to 56%.47,111,115,126 This effect has also been observed with other DMTs, such as alemtuzumab, 127 natalizumab128,129 and ocrelizumab. 130 These findings support the potential use of sNfL as a predictor of long-term treatment response in clinical practice.47,111,124,126
Preclinical studies are essential for validating clinical benefits, especially when direct evidence of these effects within the CNS is lacking. Indeed, a preclinical study aimed to clarify cladribine’s potential direct effect on the brain by administering intracerebroventricular injections of cladribine in mice with experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. 131 Notably, cladribine showed neuroprotective effects through direct modulation of central neurons, as demonstrated by improvements in both clinical scores and synaptic transmission abnormalities. 131 The observed reduction in synaptic hyperexcitability in EAE mice was attributed to cladribine’s disruption of IL-1β activity at glutamatergic synapses. 131 It is noteworthy that pharmacological agents capable of attenuating IL-1β-mediated synaptic effects in EAE mice are also likely to confer neuroprotective benefits in MS patients.132,133 Importantly, cladribine appears to exert these neuroprotective effects independently of its immunosuppressive effects on peripheral lymphocytes. 131
Furthermore, another in vitro study revealed that, beyond its impact on the peripheral immune system, cladribine preferentially triggers naïve and activated microglial cell apoptosis or the suppression of proliferation, while selectively reducing the secretion of pro-inflammatory cytokines. 134 These results suggest that cladribine may promote neuroprotective effects in MS by modulating the immune response of microglial cells.
Efficacy and safety of CladT: From clinical studies to real-world experience
The efficacy and safety of CladT have been extensively demonstrated in clinical trials50,52,135–137 and real-world studies.57,66,68,138–144 In the 96-week CLARITY trial, 52 a randomized, double-blind, three-arm, placebo-controlled, multi-centre study, involving 1326 patients with highly active RRMS 70% of whom were DMT-naïve, CladT significantly reduced the annualized rate of relapse compared with placebo, increased relapse-free rates and led to notable reductions in MRI lesion counts. 52 Findings from a 2-year extension of the CLARITY study further highlighted the sustained clinical benefits of CladT, with no evidence of disease activity (NEDA-3) persisting up to 6 years. 135 Post hoc analyses of the CLARITY and CLARITY Extension trials showed that CladT continued to reduce the incidence of all relapses, including severe ones, over a 2-year period. 136
The CLASSIC-MS trial, 137 an exploratory, low-interventional, multicentre, ambispective, phase IV study, also demonstrated the long-term efficacy of CladT in patients with RRMS (73.8%) and SPMS (26.2%), 78.4% of whom were DMT-naïve. The study showed sustained treatment benefits over a median follow-up period of 10.9 years, with relative stability in EDSS scores. 137 The MAGNIFY-MS, a 2-year, phase IV, open-label, single-arm study, was conceived to assess the onset of action of CladT at a dose of 3.5 mg/kg by monitoring changes in combined unique active (CUA) lesion counts via frequent MRI at months 1, 2, 3 and 6. 53 From month 1, substantial reductions in mean CUA lesion counts and mean T1 gadolinium-enhancing (Gd+) lesion counts were observed compared with baseline. 53 The maximum reductions in mean CUA, T1Gd+ and annualized new/enlarging T2 lesion counts were reached by month 6 and remained stable over the 48-month follow-up, regardless of the patient’s baseline relapse activity or previous DMT use.50,138 Moreover, the NEDA-3 rate, defined as no qualifying relapses, no 6-month confirmed disability progression (6mCDP) and no new T1Gd+ or active T2 MRI lesions, was 78.6% and 79.2% during years 3 and 4, respectively. 138 These findings underscore the early and sustained efficacy of CladT in MS without the need for continuous immunosuppression. Recently, De Stefano et al. 139 assessed CladT’s effects on composite-based progression independent of relapse activity (c-PIRA) and confirmed disability accumulation (CDA) over 2 years in the MAGNIFY-MS study. The c-PIRA outcome included 6mCDP or a 20% confirmed progression in the timed 25-foot walk or 9-hole peg test, providing a more comprehensive measure of disease progression. 139 The findings show minimal overall disability accumulation among patients with highly active MS treated with CladT, with over 85% of patients free from c-PIRA. 139 Treatment-naïve and younger patients with MS had lower rates of PIRA, relapse-associated worsening and CDA, emphasizing the benefits of early CladT treatment to minimize overall disability accumulation. 139
Safety data from the MAGNIFY-MS and CLARITY Extension trials indicate that lymphopenia, expected due to CladT’s MoA, was the most common AE, with most cases classified as mild or moderate.50,55 Patients treated with CladT had a higher incidence of herpes zoster, though cases were dermatomal and without complications. 140 Importantly, the malignancy rate in the CladT group did not exceed that of other DMTs for MS. 141 A comprehensive safety analysis combining data from several phase III studies and a real-world registry did not reveal any significant new safety concerns for RRMS patients treated with CladT. 141 In a safety analysis by Leist et al., 142 combining data for CladT at a dose of 3.5 mg/kg from three phase III studies (CLARITY, CLARITY Extension and ORACLE-MS), and the PREMIERE registry (operational from November 2009 to October 2018, comprising patients who had participated in at least one of the phase III trials), no new significant safety concerns emerged. 142 As of July 7, 2024, the post-approval safety profile of CladT in patients with RMS has been revised (Table 1). The results are consistent with data obtained from the clinical development programme and prior safety assessments, demonstrating no increased risk of serious infections. 145 The AE of special interest with the highest prevalence was hypersensitivity. Of note: 98% of the 566 cases (674 AEs) in the latest reporting period (July 8, 2023–July 7, 2024) were non-serious.
Table 1.
Cumulative to July 2024, post-approval safety of Cladribine tablets in the treatment of patients with multiple sclerosis.
| AEs of special interest (total reports) | Adjusted reporting rate a per 100 patients-years (95% CI) |
|---|---|
| Hypersensitivity (2858) | 1.13 (1.09; 1.18) |
| Serious infections (1270) | 0.50 (0.48; 0.53) |
| Herpes zoster (830) | 0.33 (0.31; 0.35) |
| Liver injury (623) | 0.25 (0.23; 0.27) |
| Malignancies b (397) | 0.16 (0.14; 0.17) |
| Serious lymphopenia (259) | 0.10 (0.09; 0.12) |
| Seizures (160) | 0.06 (0.05; 0.07) |
| Opportunistic infections (excluding PML c and tuberculosis) (39) | 0.02 (0.01; 0.02) |
| Tuberculosis (35) | 0.01 (0.01; 0.02) |
Post-approval data reflect clinical experience from ~101,132 patients who have received CladT since EMA approval in 2017, with 251,900 patients-years of exposure.
The reporting rate is adjusted for the cumulative duration of patient exposure to CladT.
The spectrum of malignancies resembled the distribution of cancer types seen in the general population, without any clustering of specific tumour types.
As of July 7, 2024, there were no confirmed cases of PML.
AE, adverse event; CI, confidence interval; CladT, cladribine tablets; PML, progressive multifocal leukoencephalopathy.
Real-world studies, in which the vast majority of patients had RRMS, and 10%–36% where DMT-naïve,57,66,68,143,144,146–150 further support the clinical efficacy and safety of CladT seen in trials. These studies report high rates of NEDA-3, reduced disability progression, fewer relapses and lower MRI lesion activity.57,66,68,143,144,146–150 Effectiveness was particularly evident in treatment-naïve individuals and those who switched early from other DMTs.58,65–70,143,144,147
The findings from these real-world studies provide additional support for the sustained efficacy of CladT, showcasing its potential in altering the disease course of MS.
Table 2 summarizes the main efficacy results from various real-world cohorts, categorized by years of follow-up. The overall findings from the clinical practice confirmed a higher effectiveness of CladT when placed early in the therapeutic scenario and the effects of timing of initiation highlight both short and long-term outcomes in patients with MS.33,58,65–68,70,143,146,151–153
Table 2.
Main efficacy outcomes from real-world experience summarized according to the years of follow-up (up to 2 years, between the 3rd and 4th year, and beyond year 4).
| Follow-up period | Reference | Study type | Population | Main efficacy outcomes |
|---|---|---|---|---|
| Up to 2 years | Petracca et al. 68 | Multicentre, observational, retrospective post-marketing study | 243 patients (100% RRMS; 29.3% DMT-naïve) | - 64% NEDA-3: Higher number of prior treatments reduced NEDA-3 retention; clinical relapses were more likely. Median time to NEDA-3 loss: 2.6 years. - 89% relapse-free - 77% MRI activity-free - 87% disability progression-free |
| Sorensen et al. 57 | Prospective registry-based observational cohort study | 268 patients (97.8% RRMS; 12.7% DMT-naïve) | - 84.8% ARR reduction compared to the year before CLAD initiation. - NEDA-3 achieved: 71.7% at 1 year; 49.0% at 2 years. - Risk factors for relapse: Switching from HeDMT or high baseline disease activity. |
|
| Zanetta et al. 66 | Retrospective and prospective monocentric, observational study | 114 patients (100% RRMS; 50% DMT-naïve) | - 74.9% NEDA-3 at 24 months: Higher gadolinium-enhancing lesions at baseline predicted NEDA-3 loss - 90.9% relapse-free - 76.7% MRI activity-free - 96.2% disability progression-free |
|
| Arena et al. 144 (REWIND) | Prospective multicentre study | 217 patients (100% RRMS; 23% DMT-naïve) | - 80% EDSS progression-free - 88% relapse-free at 24 months - 48% MRI activity-free - NEDA-3 outcomes: Higher in the MAT group, with greater freedom from disease progression, clinical relapses and radiological worsening - ARR analysis: Lowest in MAT patients compared to naïve and HAT groups |
|
| Adamec et al. 58 | Retrospective observational multi-centre, multi-national study | 320 patients (100% RRMS; 26.6% DMT-naïve) | - 54.2% NEDA-3 - 72.5% MRI activity-free - 86.5% relapse-free - 90.2% disability progression-free |
|
| Al-Hashel et al. 65 | Observational, longitudinal prospective study | 72 patients (100% RRMS; 44.4% DMT-naïve) | - 75% NEDA-3 - 90% MRI activity-free - 85% relapse-free - 90% disability progression-free |
|
| Conway et al. 184 | Prospective multicentre study | 117 (64.2% RRMS; 9.4% SPMS; 1.9% PPMS; 13.2% PRMS; 11.3% CIS) | - Walking speed improvement over time (p < 0.001) with better scores compared to other DMTs - Stable patient determined disease steps and walking aid use - Stable MRI activity - 11% started a post-CladT DMT |
|
| Years 3–4 | Rauma et al. 69 | Non-interventional cohort study based on the Finnish MS registry | 179 (98.9% RRMS; 29.6% DMT-naïve) | - 86% relapse-free at last follow-up - 92.5% of DMT-naïve patients remained relapse-free until the end of follow-up - Mean ARR 0.1 during follow-up - 66.7% of patients with early relapse have switched from fingolimod |
| Magalashvili et al. 70 | Retrospective exploratory analysis from the Sheba MS Computerized Data Registry |
128 patients (80.5% RRMS; 14.8% SPMS; 4.7%CIS; 15.6% DMT-naïve) | - 59.0% NEDA-2 at Year 3 and 74.3% at Year 4 - 68.9% relapse-free patients at Year 3 and 82.9% at Year 4 - 83.6% EDSS stability or improvement at Year 3 and 85.7% at Year 4 |
|
| Aerts et al. 67 | Retrospective single centre study | 84 patients (100% RMS; 9.5% DMT-naïve) | - 72.5% NEDA-3 during observation period - 37 months median event-free survival - 72.6% remaining disease activity-free at the mean follow-up time (22.6 months) - 16.7% reported relapses - 93.3% MRI activity-free at 22.6 months - 83.3% and 66.7% relapse-free, 96.7% and 88.9% MRI activity-free, 82.8% and 77.8% CDW-free,72.4% and 55.6% disease activity-free at year 2 and 3, respectively - 88.3% SC lesion-free at 22.6 months |
|
| Schiavetti et al. 152 (CladSTOP) | Retrospective, multicentre, observational study | 204 PwMS (15.1% DMT-naïve) | - 91% ARR reduction at Month 36 - 28.0% EDSS improvement by ⩾0.5 points at Month 36 - 18.8% experienced disability progression at Month 36 - 70.4% no MRI activity or relapses |
|
| Manni et al. 47 | Prospective and retrospective single-centre, observational analysis of the Italian MS Registry | 88 patients (100% RMS; 20.5% DMT-naïve) | - 55.7% NEDA-3 at last follow-up - 75.0% NEDA-3 status retention and did not restart DMTs after >2 years post-last CladT dose - 90.9% relapse-free - 80.7% MRI activity-free - 70.4% disability progression-free |
|
| Beyond year 4 | Kowarik et al. 33 | Multicentre retrospective study | 187 patients (100% RMS; 21% DMT-naïve) | - 63% Monitored without therapy at Year 5 - 14% Treatment switch at Years 1–4 - 19% Continued CladT at Year 5 - 4% Therapy switch at Year 5 |
| Wallace et al. 153 | Observational multi-centre study | 116 patients (100% RRMS; 34.5% DMT-naïve) | - 89% ARR reduction at Year 5 - 75% Relapse-free patients at Year 5 - 83.6% No subsequent DMT up to 5 years post-CLAD initiation |
|
| Cañibano et al. 158 | Retrospective single-centre observational study | 46 (100% RMS; 48% DMT-naïve) | - ⩾88% ARR reduction at year 5 - Lower ARR in patients switching to cladribine from HeDMTs compared with platform therapies - 91% were free of relapses at year 5 - 100% MRI activity-free at year 5 - 80% NEDA-3 at year 5. |
ARR, annualized relapse rate; CDW, confirmed disability worsening; CIS, clinically isolated syndrome; CladT, cladribine tablets; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; HeDMT, high efficacy disease-modifying therapy; MRI, magnetic resonance imaging; MS, multiple sclerosis; NEDA-3, no evidence of disease activity-3; PPMS, primary progressive multiple sclerosis; PRMS, progressive relapsing multiple sclerosis; PwMS, patients with multiple sclerosis; RMS, relapsing multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SC, spinal cord; SPMS, secondary progressive multiple sclerosis.
The safety profile of CladT in real-world settings mirrors clinical trial data, with AEs generally mild to moderate.58,65–69,143,147 Additionally, patients treated with CladT did not experience increased risk of severe illness or adverse outcomes from COVID-19 compared with the general population,154–157 even after 5 years of follow-up. 158
CladT’s safety and efficacy across different patient needs, including pregnancy considerations, remain important aspects of its overall treatment profile. Prescribing guidelines require exclusion of pregnancy prior to starting CladT, with both female and male patients advised to use effective contraception during treatment and for 6 months after the last dose. 27 However, CladT is regarded as an ideal option for family planning among patients of childbearing age. A recent Delphi Consensus from Oreja-Guevara et al. 159 endorsed CladT as a suitable choice for patients with active disease planning pregnancy in the medium or long term, recommending conception 6 months after completing the second treatment year. 159 Data from clinical and real-world settings show that pregnancy outcomes in MS women to CladT align with those of the general MS population.160,161 Additionally, Signoriello et al. 162 reported that none of the women who completed two CladT cycles before conception showed clinical disease activity postpartum and MRI scans (when available) confirmed radiological stability.
CladT effectiveness readouts in improving cognition and quality of life
MS affects well-being and QoL by causing cognitive impairment, depression, anxiety, fatigue, and pain in addition to motor, sensory, visual, brainstem and sphincter issues assessed by the EDSS. Cognitive impairment is particularly challenging and disabling for MS patients.
CLARITY study data highlight the role of CladT in reducing brain damage and neurodegeneration associated with MS progression.163,164 CladT reduced overall brain atrophy compared with placebo, which correlated with a lower likelihood of disability progression over a 2-year follow-up. 163 Between months 6 and 24, patients treated with CladT experienced smaller mean percentage changes in brain volume than those receiving placebo. 163 Furthermore, Cortese et al. 164 found that, following a short period of pseudoatrophy, grey matter volume decline slowed in CladT-treated RRMS patients from the CLARITY study compared with placebo recipients (Figure 2(d)). 164 A more recent study of Achiron et al. 165 assessed the long-term effects of CladT on brain volume reduction in a real-world setting of patients with RRMS. This study is the first to examine long-term brain atrophy rates in highly active RRMS patients who transitioned from other immunomodulatory drugs (IMDs) to CladT treatment. 165 Prior to switching, these patients were on IMDs known to have only modest effects on brain atrophy. 165 The analysis revealed a consistent breakpoint approximately 2 years after initiating CladT treatment, indicating that the full therapeutic benefits of CladT become evident during this timeframe. 165 This finding underscores CladT’s substantial efficacy in reducing brain atrophy compared to the less effective treatments administered prior to the breakpoint. 165
The complex interplay between brain atrophy, cognitive decline, NfL levels, disease progression, and the emerging role of slowly expanding lesions (SELs) and neuroinflammation characterizes MS. Chronic neuroinflammation, characterized by immune cell infiltration and cytokine release, is a key driver of MS pathology which can lead to structural damage in the CNS, directly impacting neural function and connectivity, and contributing to cognitive impairment. NfL protein, a biomarker for axonal damage, correlates with disease activity and disability progression.166–170 CladT has shown a sustained reduction in serum and CSF NfL levels, 124 highlighting its potential to mitigate neuro-axonal damage.106,111,126,171 Data from real-world studies also support NfL as a valuable biomarker for treatment response and long-term disease outcomes.111,126,168
SELs, which may be missed by conventional MRI scans, contribute to long-term disability progression and cognitive decline by affecting brain regions involved in cognitive function.172–174 The presence of these lesions emphasizes the complexity of MS and the importance of effective treatments such as CladT in addressing both immediate and long-term aspects of the disease process.
The CLARIFY-MS and MAGNIFY-MS studies assessed acute and chronic inflammation in MS through the cumulative number of CUA (cum_CUA) and SELs (cum_SEL). 175 Findings indicate that CladT effectively slows the accumulation of acute inflammation, as demonstrated by consistent rates of cum_CUA over the first 6 months (Figure 2(d)). 175 Additionally, chronic inflammation, measured by cum_SEL, significantly decreased only in patients with grey matter-driven damage during an 18-month period (Figure 2(d)). 175 This suggests that CladT not only mitigates acute inflammatory processes but also targets chronic inflammation specifically in cases with predominant grey matter involvement, underscoring its therapeutic potential across diverse MS phenotypes. 175 These findings align with results from the CLARIFY-MS Extension study 176 where approximately 68% of treated patients experienced minimal or no decline in cognitive function, as measured by the Symbol Digit Modalities Test (SDMT), at month 48 compared with baseline. In this study, 63.9% of participants maintained stable or improved SDMT scores with a 4-point change from baseline, and 77.5% with an 8-point change. 176 Understanding and addressing these interconnected elements is vital for developing comprehensive strategies to manage and mitigate the impact of MS on cognitive function and overall disease progression.
Given the efficacy of CladT in reducing physical and cognitive disability risk, its use in the treatment of MS has demonstrated significant improvements in HRQoL, a key efficacy outcome. 177 The phase III CLARITY trial showed notable gains in Euro Quality of Life 5 Dimensions (EQ-5D) scores, especially in the self-care domain. 178
In 2023, Brochet et al. 74 evaluated HRQoL in highly active RMS patients, 31% of whom were DMT-naïve, treated with CladT in the CLARIFY-MS study, finding significant improvements in both physical and mental health after 2 years of treatment, 74 consistent with outcomes from the CLARITY trial. 52 Patient-reported outcomes (PROs) play a vital role in clinical care, helping both patients and healthcare providers focus on patient-centred care,179,180 offering insights into treatment success from the patient’s perspective, 181 and improving patient-provider communication. 182 In the CLARIFY-MS trial, an open-label, single-arm, phase IV study, PROs showed improvements over 2 years post-treatment initiation. 74
The CLAWIR study, a 2-year observational study in highly active RMS patients beginning CladT, 26.6% of whom were DMT-naïve, also demonstrated benefits in fatigue, physical function, treatment satisfaction and work productivity within the first 12 months of treatment. 183
The CLAWIR study showed that cladribine treatment may contribute to fatigue reduction in RMS patients (97.7% RRMS, 2.3% SPMS; 26.6% treatment-naïve), with more than half of the participants achieving the minimal important difference within 12 months. 183 Afolabi et al. 178 investigated the effects of CladT on quality of life in patients enrolled in the CLARITY trial. Their findings indicated that after 2 years, patients with RMS receiving CladT at doses of 3.5 and 5.25 mg/kg exhibited significantly higher EQ-5D index scores compared to those receiving placebo. 178 These results were further supported by the CLARIFY-MS study, which demonstrated statistically significant improvements in both physical and mental health composite scores of the MSQoL-54 over a 24-month period. 74 The ongoing CLADFIT-MS study aims to assess changes in self-reported physical functioning 52 weeks after switching to CladT in patients with highly active MS. 179 The study’s secondary outcomes will include the Patient-Reported Outcomes Measurement Information System (PROMIS), which evaluates seven health domains, including fatigue. 179 A real-world study by Conway et al. 184 reported improved NeuroQoL fatigue scores in patients treated with CladT at 6, 12, 18 and 24 months post-treatment compared to those receiving other DMTs. However, the difference reached statistical significance only at 6 months. 184 Notably, a systematic review did not observe any difference in the occurrence of fatigue as an AE during CladT treatment compared to other DMTs. 185
New biomarkers in MS patients treated with CladT and future perspectives
Biomarkers capable of predicting disease progression and monitoring treatment efficacy are crucial – particularly with IRTs such as CladT – when treatment courses are short, and assessment of disease activity is essential for promptly determining the need for additional treatment courses.
sNfL protein and glial fibrillary acidic protein are promising biomarkers for neuro-axonal damage and astrocytic activation, 186 respectively, and have shown potential to predict subclinical disease activity after DMT discontinuation in stable MS patients. 187 Manni et al. 47 recently observed sustained reduction in sNfL levels up to 40 months in highly active RMS patients, 20.4 of whom were DMT-naïve, treated with CladT, supporting a role for sNfL in personalized disease management.
Emerging research is identifying additional biomarkers with the potential to predict MS progression.188,189 These candidates, including proteins and other molecular markers, could enhance our understanding of disease trajectory and improve patient care.188,189
Research has also explored molecular changes in PBMCs following CladT treatment to identify potential molecular signatures of treatment efficacy.89,190 Fissolo et al. 190 conducted a study analysing the impact of CladT on transcriptomic and proteomic profiles in blood cells and identifying potential biomarkers for CladT treatment response in 10 RRMS patients, 7 of whom were DMT-naïve. Using multiomics methods, researchers examined the complete transcriptome and proteome profiles of PBMCs obtained from MS patients treated in vitro with CladT. 190 Ex vivo validation was then performed with samples from CladT-treated patients collected at baseline, 3 months and 12 months after treatment. 190 The in vitro study identified a distinct multiomics molecular signature primarily characterized by gene expression downregulation and revealed four candidate biomarkers (NIBAN2, NHLRC2, PPIF and JUN) and 22 microRNAs (miRNAs) modulating their expression, which were dysregulated after CladT treatment. 190
Particularly noteworthy, PPIF and NHLRC2 genes showed significant down- and upregulation, respectively, in ex vivo analysis of PBMCs from CladT-treated patients. 190 A decrease in cyclophilin D, encoded by PPIF – a positive regulator of the mitochondrial permeability transition pore linked to cell death from calcium overload and increased reactive oxygen species 191 – may relate to CladT benefits in MS, as suggested by preclinical studies in MS animal models.192,193
Despite limited knowledge of the function of NHLRC2, altered expression of this gene has been observed in neurodegenerative diseases such as Parkinson’s disease (PD) 194 and Alzheimer’s disease. 195 Regarding miRNAs, miR-30b-5p showed persistent downregulation throughout the first year of CladT treatment, while miR-30e-5p and miR-21-5p were initially downregulated after 3 months but then upregulated at 12 months. 193 Previous studies link miR-21-5p with neurodegenerative diseases 196 and its upregulation in PBMCs of RRMS patients. 197 MiR-30b-5p may help distinguish RRMS from progressive MS, 198 while the role of miR-30e-5p in MS remains unclear. 198 Notably, miR-30e-5p levels were found to be downregulated in the blood of PD patients. 199
Further insights came from a small cohort study examining miRNA profiles in PBMCs from RRMS patients before and after CladT treatment, which revealed 40 dysregulated miRNAs in untreated RRMS patients compared with healthy controls. 200 Many of these miRNAs are involved in inflammatory and immune pathways, suggesting potential roles in MS pathogenesis. 200 CladT treatment was shown to revert some dysregulated miRNAs towards a protective phenotype, indicating drug-specific miRNA profiles that might drive these protective mechanisms. 200 CladT restored levels of several dysregulated miRNAs in RRMS patients before treatment, including miR-199a-3p, miR-199a-5p, miR-29b-3p and miR-23b-3p, suggesting a shift toward an anti-inflammatory and neuroprotective profile. 200 For instance, both miR-23b-3p and miR-199a-3p correlated with urinary symptoms, 89 miR-199a, downregulated in RRMS but upregulated by CladT, plays a role in Treg differentiation, 201 while miR-29b-3p, the only miRNA downregulated after CladT treatment, is linked to the platelet-derived growth factor (PDGF) pathway and associated with enhanced long-term potentiation and brain lesion mitigation in RRMS. 202 MiR-151a-5p was the miRNA most extensively modulated by CladT treatment; however, this miRNA remains uncharacterized in MS pathophysiology and warrants further investigation. 200
Pathway enrichment analysis of CladT-modulated miRNAs identified the involvement of IL-4 and IL-13 signalling pathways, 200 which are known for their potent anti-inflammatory roles in MS. 203 Additionally, collagen and oestrogen receptor signalling pathways were implicated in CladT-modulated miRNA pathways, 200 suggesting roles in repair processes 204 and the production of anti-inflammatory cytokines, as well as the expansion of Treg cells, 205 respectively.
Given the role of ADA in immune regulation, recent research investigated the impact of a single nucleotide polymorphism (SNP) in the ADA gene on disease characteristics and CSF inflammation in MS. 206
The study analysed SNP rs244072 in 561 MS patients and found significant associations with clinical features and central inflammation markers, including increased disability, elevated CSF TNF and RANTES levels (both implicated in MS), heightened IL-5 levels, decreased IL-10 (an anti-inflammatory cytokine) and a trend towards higher lymphocyte counts. 206 Given CladT-mediated ADA inhibition, 207 these findings suggest that ADA modulation may contribute to the benefit of CladT in reducing disability progression and neurodegeneration in MS. The long-term effects of CladT and the management of MS patients beyond 4 years are subjects of significant interest among clinicians. Numerous expert opinion reports29–31,208,209 emphasize the importance of using all available tools and techniques, including clinical, radiological and biological markers, to identify patients who may benefit from additional CladT courses. MRI is highlighted as a key tool in guiding therapeutic decisions and identifying patients suitable for further treatment with CladT.29,30,208,209 The rise of digitalization and the accessibility of user-friendly devices and technology have empowered healthcare professionals to leverage a novel category of ‘biomarkers’ known as digital health technologies. 210 These innovations help predict and interpret health-related outcomes, thereby expanding the scope of biomarker utilization in the context of MS. This approach not only offers a more comprehensive view of disease progression but also opens up new opportunities for personalized and data-driven healthcare interventions in the context of MS.
Integrating these technologies with traditional methods such as clinical and serum markers and MRI offers additional insights into disease progression, treatment response and overall patient well-being, further enhancing the diagnostic landscape and refining patient selection for CladT.
Conclusion
This review provides a detailed examination of the therapeutic role of CladT, emphasizing its effectiveness in altering disease progression, enhancing QoL and preserving immune function in patients grappling with the challenges of MS. CladT has shown significant efficacy in RRMS and highly active RMS,51–58,65–71 with favourable safety profile, in numerous clinical and real-world studies.55,58,65–69,140–143,147 Its unique MoA, characterized by rapid memory B cell reduction with gradual repopulation, alongside minimal impact on T- and other immune cells, likely underpins its ability to maintain innate immune responses and avoid secondary autoimmunity.42,106 The multifaceted role of CladT, encompassing both peripheral and central effects, leads to an overall enhancement of clinical outcomes in patients (Figure 3).
Figure 3.
Peripheral and central combined effects of CladT on clinical outcomes. The distinct yet interconnected peripheral and central effects of CladT on clinical outcomes in patients. The left circle represents peripheral effects, highlighting the involvement of B cells (blue) and T cells (green). The right circle depicts central effects related to neuroinflammation and neuroprotection. The central area showcases key clinical and radiological outcomes, including early onset of action measured by conventional MRI parameters (reduction in T1 Gd+, CUA and active T2 lesions); reduction in relapse-free rates; impact on disability accumulation in terms of EDSS score, HRQoL, SDMT, c-PIRA. These findings underscore the multifaceted role of CladT in improving patient outcomes through both peripheral and central mechanisms.
6mCDP, 6 months confirmed disability progression; 9-HPT, 9-Hole Peg Test; CladT, cladribine tablets; c-PIRA, combined progression independent of relapse activity; CUA, combined unique active lesions; EDSS; Expanded Disability Status Scale; Gd+, gadolinium positive; HRQoL, Health-Related Quality of Life; MRI, magnetic resonance imaging; SDMT, Symbol Digit Modalities Test; T25FW, Timed 25-Foot-Walk.
CladT represents an innovative MS treatment option, effectively permeating the BBB and reducing key markers such as OCBs and NfL levels, which reflect disease activity and neuro-axonal damage. 124 Notably, the reduction of OCBs in approximately 76.5% of patients and sustained NfL decreases underscore the potential of CladT to promote neuroprotection and mitigate disability accumulation. 124 Findings from the MAGNIFY-MS study further show that over 93% of patients receiving CladT remain free from PIRA, underscoring the benefits of early CladT intervention. 139
The convenience of short-course oral administration over 2 years, combined with sustained efficacy, absence of continuous immunosuppression and benefits for pregnancy- and vaccination planning, contribute to high adherence and low discontinuation rates. In an era when patient preference is increasingly important, many individuals opt for more manageable treatment interventions. The sustained efficacy and low treatment and monitoring burden of CladT highlight its value for MS patients in the current treatment landscape.
Evidence suggests CladT is most effective when introduced early in patients with minimal prior DMT exposure or treatment-naïve status.58,65–70,143,144,147 Integrating digital health technologies as ‘biomarkers’ enhances traditional methods for monitoring disease progression and treatment response.
Further research on the direct effects of CladT within the CNS and its long-term benefits will be essential to fully elucidate its role in MS management. Additionally, studies are needed to identify biomarkers that can predict CladT’s effectiveness and help identify patients who may require re-dosing beyond the standard 2-year regimen, thereby maximizing the drug’s potential efficacy. Together, these efforts will enhance our understanding of CladT’s therapeutic impact and optimize treatment strategies for individuals living with MS.
Acknowledgments
Medical writing assistance was provided by Valeria Benedusi of Editamed S.r.l.
Appendix
Abbreviations
AE adverse event
BBB blood–brain barrier
CladT cladribine tablets
CNS central nervous system
CSF cerebrospinal fluid
CUA combined unique active
DMT disease-modifying therapy
EQ-5D euro quality of life 5 dimensions
HRQoL health-related quality of life
IRT immune reconstitution therapy
miRNA microRNA
MoA mechanism of action
MRI magnetic resonance imaging
MS multiple sclerosis
NEDA no evidence of disease activity
NfL neurofilament light chain
OCB oligoclonal bands
PBMC peripheral blood mononuclear cells
PDGF platelet-derived growth factor
PIRA progression independent of relapse activity
PPIF peptidyl-prolyl cis-trans isomerase F (Cyclophilin D)
PRO patient-reported outcome
PROMIS Patient-Reported Outcomes Measurement Information System
RMS relapsing multiple sclerosis
RRMS relapsing-remitting multiple sclerosis
SDMT symbol digit modalities test
SEL slowly expanding lesions
SNP single nucleotide polymorphism
SPMS secondary progressive multiple sclerosis
TLOs tertiary lymphoid organs
Treg regulatory T cells
Footnotes
ORCID iDs: Francesca Romana Rizzo  https://orcid.org/0000-0002-7682-5135
https://orcid.org/0000-0002-7682-5135
Diego Centonze  https://orcid.org/0000-0002-8390-8545
https://orcid.org/0000-0002-8390-8545
Contributor Information
Fabio Buttari, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy; Unit of Neurology, Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy.
Ettore Dolcetti, Unit of Neurology, Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy; Ph.D. Program in Neuroscience, Department of System Medicine, University of Rome Tor Vergata, Rome, Italy.
Francesca Romana Rizzo, Merck Serono S.p.A., an Affiliate of Merck KGaA, Rome, Italy.
Caterina Rizzi, Merck Serono S.p.A., an Affiliate of Merck KGaA, Rome, Italy.
Gianluca Lauritano, Unit of Neurology, Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy.
Angela Borrelli, Unit of Neurology, Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy; Ph.D. Program in Neuroscience, Department of System Medicine, University of Rome Tor Vergata, Rome, Italy.
Federica Azzolini, Unit of Neurology, Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy.
Roberta Fantozzi, Unit of Neurology, Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy.
Francesco Assogna, Merck Serono S.p.A., an Affiliate of Merck KGaA, Rome, Italy.
Antonella Conte, Department of Human Neuroscience, Sapienza University of Rome, Rome, Italy; Department of Neurology and Clinical Neurophysiology, Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy.
Diego Centonze, Department of Systems Medicine, University of Rome Tor Vergata, Via Montpellier 1, Rome 00133, Italy; Unit of Neurology, Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Fabio Buttari: Conceptualization; Writing – original draft; Writing – review & editing.
Ettore Dolcetti: Writing – original draft; Writing – review & editing.
Francesca Romana Rizzo: Conceptualization; Writing – original draft; Writing – review & editing.
Caterina Rizzi: Writing – review & editing.
Gianluca Lauritano: Writing – review & editing.
Angela Borrelli: Writing – review & editing.
Federica Azzolini: Writing – review & editing.
Roberta Fantozzi: Writing – review & editing.
Francesco Assogna: Writing – review & editing.
Antonella Conte: Writing – review & editing.
Diego Centonze: Conceptualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work has been funded by Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA (CrossRef Funder ID: 10.13039/100009945).
Competing interests: F.B. acted as an advisory board member for Teva and Roche and received honoraria for speaking or consultation fees from Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Teva, Biogen Idec, Sanofi and Novartis and non-financial support from Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Teva, Biogen Idec and Sanofi. E.D. was supported with travel expenses for congress attendance from Novartis, Biogen, Roche and Janssen and received honoraria for participation on an advisory board from Lundbeck. G.L., A.B. and F.Azzolini declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article. R.F. has received consulting fees and honoraria for advisory boards from Biogen Idec, Merck-Serono, Novartis, Roche and TEVA. A.C. received speaking honoraria from Roche, Biogen, Novartis, BMS, Almirall, Merck, Sanofi and Lundbeck, and research support from Biogen. A.C. also served as a board member for Roche. D.C. is an advisory board member for Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva. He is also the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva. F.R.R., C.R. and F.Assogna are employees of Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA.
Availability of data and materials: Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1. Correale J, Marrodan M, Ysrraelit MC. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines 2019; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bø L, Vedeler CA, Nyland HI, et al. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 2003; 62: 723–732. [DOI] [PubMed] [Google Scholar]
- 3. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005; 128: 2705–2712. [DOI] [PubMed] [Google Scholar]
- 4. Lucchinetti CF, Popescu BFG, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011; 365: 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009; 132: 1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler 2020; 26: 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA 2021; 325: 765–779. [DOI] [PubMed] [Google Scholar]
- 8. Brownlee WJ, Hardy TA, Fazekas F, et al. Diagnosis of multiple sclerosis: progress and challenges. Lancet 2017; 389: 1336–1346. [DOI] [PubMed] [Google Scholar]
- 9. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology 2015; 85: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalincik T. Multiple sclerosis relapses: epidemiology, outcomes and management. a systematic review. Neuroepidemiology 2015; 44: 199–214. [DOI] [PubMed] [Google Scholar]
- 11. University of California, San Francisco MS-EPIC Team; Cree BAC, Gourraud P-A, Oksenberg JR, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016; 80: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain 2010; 133: 1914–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol 2017; 13: 25–36. [DOI] [PubMed] [Google Scholar]
- 14. Mishra MK, Yong VW. Myeloid cells – targets of medication in multiple sclerosis. Nat Rev Neurol 2016; 12: 539–551. [DOI] [PubMed] [Google Scholar]
- 15. van der Valk P, Amor S. Preactive lesions in multiple sclerosis. Curr Opin Neurol 2009; 22: 207–213. [DOI] [PubMed] [Google Scholar]
- 16. van Langelaar J, Rijvers L, Smolders J, et al. B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers. Front Immunol 2020; 11: 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol 2008; 4: 384–398. [DOI] [PubMed] [Google Scholar]
- 18. Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol 2018; 9: 3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arneth BM. Impact of B cells to the pathophysiology of multiple sclerosis. J Neuroinflammation 2019; 16: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 21. Montalban X, Leist TP, Cohen BA, et al. Cladribine tablets added to IFN-β in active relapsing MS: The ONWARD study. Neurol Neuroimmunol Neuroinflamm 2018; 5: e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kieseier BC, Wiendl H, Hartung H-P. The inflamed peripheral nervous system: update on immune therapies. Curr Opin Neurol 2006; 19: 433–436. [DOI] [PubMed] [Google Scholar]
- 23. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 24. Kołtuniuk A, Chojdak-Łukasiewicz J. Adherence to therapy in patients with multiple sclerosis – review. Int J Environ Res Public Health 2022; 19: 2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol 2018; 31: 233–243. [DOI] [PubMed] [Google Scholar]
- 26. Lünemann JD, Ruck T, Muraro PA, et al. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol 2020; 16: 56–62. [DOI] [PubMed] [Google Scholar]
- 27. Merck Serono Ltd. MAVENCLAD 10 mg tablets summary of product characteristics, https://www.medicines.org.uk/emc/product/8435 (2021, accessed 9 April 2024).
- 28. Giovannoni G. Cladribine to treat relapsing forms of multiple sclerosis. Neurotherapeutics 2017; 14: 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centonze D, Amato MP, Brescia Morra V, et al. Multiple sclerosis patients treated with cladribine tablets: expert opinion on practical management after year 4. Ther Adv Neurol Disord 2023; 16: 17562864231183221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meuth SG, Bayas A, Kallmann B, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets beyond year 4. Expert Opin Pharmacother 2022; 23: 1503–1510. [DOI] [PubMed] [Google Scholar]
- 31. Meca-Lallana V, García Domínguez JM, López Ruiz R, et al. Expert-agreed practical recommendations on the use of cladribine. Neurol Ther 2022; 11: 1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meuth SG, Bayas A, Kallmann B, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets: an expert opinion. Expert Opin Pharmacother 2020; 21: 1965–1969. [DOI] [PubMed] [Google Scholar]
- 33. Kowarik MC, Ernst M, Woitschach L, et al. Real-world therapy management of patients with multiple sclerosis receiving cladribine tablets beyond year 4 – results from a German cladribine cohort. Mult Scler Relat Disord 2024; 88: 105704. [DOI] [PubMed] [Google Scholar]
- 34. Arun T, Shehu A, Pye E, et al. Treatment continuation with cladribine at 5 years after initiation in people with multiple sclerosis: a case series and literature review. Mult Scler Relat Disord 2024; 90: 105837. [DOI] [PubMed] [Google Scholar]
- 35. Korsukewitz C, Richter N, Klehmet J, et al. Reasons for and safety of treatment continuation with cladribine tablets (CladT) in year 5 – first interim analysis of the CLIP-5 study. In: ECTRIMS Conference 2024, ePoster 1761/845, 18–20 September 2024, Copenaghen. [Google Scholar]
- 36. Moral AT, Eichau S, Garcia-Soto JD, et al. Experience with cladribine tablets beyond year 4: the challenge of the long-term management. Abstract Number: 1417/P697. MSMilan 2023, 9th Joint ECTRIMS-ACTRIMS Meeting, October 11–13, 2023, Milan. [Google Scholar]
- 37. Kleinschnitz C, Skuljec J, Kowarik MC, et al. First insights into the safety and effectiveness of additional courses with cladribine tablets under real-world conditions. Mult Scler Relat Disord 2025; 97: 106398. [DOI] [PubMed] [Google Scholar]
- 38. EMA. Summary of Product Caractheristics. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/lemtrada (accessed 4 April 2024).
- 39. EMA. Summary of Product Caractheristics, https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/103948s5184lbl.pdf (accessed 31 March 2025).
- 40. FDA. Summary of Product Caractheristics, https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-warns-about-rare-serious-risks-stroke-and-blood-vessel-wall-tears-multiple-sclerosis-drug (accessed 31 March 2025).
- 41. Boyko AN, Boyko OV. Cladribine tablets’ potential role as a key example of selective immune reconstitution therapy in multiple sclerosis. Degener Neurol Neuromuscul Dis 2018; 8: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giovannoni G, Mathews J. Cladribine tablets for relapsing-remitting multiple sclerosis: a clinician’s review. Neurol Ther 2022; 11: 571–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 2016; 388: 576–585. [DOI] [PubMed] [Google Scholar]
- 44. Muraro PA, Martin R, Mancardi GL, et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol 2017; 13: 391–405. [DOI] [PubMed] [Google Scholar]
- 45. Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 2015; 86: 208–215. [DOI] [PubMed] [Google Scholar]
- 46. Meca-Lallana JE, Álvarez-Cermeño JC, Casanova Estruch B, et al. Early beginning of alemtuzumab: changing the multiple sclerosis treatment paradigm. Interim analysis of the LEMVIDA study. Neurologia (Engl Ed) 2024; 39: 383–391. [DOI] [PubMed] [Google Scholar]
- 47. Manni A, Oggiano F, Palazzo C, et al. Clinical and biological predictors of Cladribine effectiveness in multiple sclerosis: a real-world, single centre study considering a two-year interval from year-2 dosing. J Neurol Sci 2024; 462: 123070. [DOI] [PubMed] [Google Scholar]
- 48. Hassan AM, Hassan AM, Aldhuhoori A, et al. Early use of cladribine tablets impact on disease control in patients with relapsing multiple sclerosis: cohort study from Tawam Hospital, UAE. Mult Scler Relat Disord 2023; 80: 105311. [Google Scholar]
- 49. De Stefano N, Achiron A, Barkhof F, et al. (DMT01) Effect of Cladribine tablets on markers of disease progression, axonal loss, and oligoclonal bands in patients with relapsing multiple sclerosis: results from MAGNIFY-MS. In: Abstracts from the 38th Annual Meeting of the Consortium of Multiple Sclerosis Centers, poster no. DMT01, 29 May–1 June 2024, Nashville, Tennessee. [Google Scholar]
- 50. De Stefano N, Achiron A, Barkhof F, et al. Early onset of action and sustained efficacy of MRI outcomes during Cladribine tablets treatment in highly active relapsing multiple sclerosis: results of the 2-year MAGNIFY-MS study. Mult Scler Relat Disord 2023; 71: 104322. [Google Scholar]
- 51. Cortese R, Testa G, Assogna F, et al. Magnetic resonance imaging evidence supporting the efficacy of Cladribine tablets in the treatment of relapsing-remitting multiple sclerosis. CNS Drugs 2024; 38(4): 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 53. De Stefano N, Barkhof F, Montalban X, et al. Early reduction of MRI activity during 6 months of treatment with Cladribine tablets for highly active relapsing multiple sclerosis: MAGNIFY-MS. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol 2011; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- 55. Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler 2018; 24: 1594–1604. [DOI] [PubMed] [Google Scholar]
- 56. Leist TP, Comi G, Cree BAC, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol 2014; 13: 257–267. [DOI] [PubMed] [Google Scholar]
- 57. Sorensen PS, Pontieri L, Joensen H, et al. Real-world experience of cladribine treatment in relapsing-remitting multiple sclerosis: a Danish nationwide study. Mult Scler Relat Disord 2023; 70: 104491. [DOI] [PubMed] [Google Scholar]
- 58. Adamec I, Brecl Jakob G, Rajda C, et al. Cladribine tablets in people with relapsing multiple sclerosis: a real-world multicentric study from southeast European MS centers. J Neuroimmunol 2023; 382: 578164. [DOI] [PubMed] [Google Scholar]
- 59. Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol 2011; 34: 28–35. [DOI] [PubMed] [Google Scholar]
- 60. Wiendl H, Schmierer K, Hodgkinson S, et al. Specific patterns of immune cell dynamics may explain the early onset and prolonged efficacy of Cladribine tablets: a MAGNIFY-MS substudy. Neurol Neuroimmunol Neuroinflamm 2023; 10: e200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wiendl H. Cladribine – an old newcomer for pulsed immune reconstitution in MS. Nat Rev Neurol 2017; 13: 573–574. [DOI] [PubMed] [Google Scholar]
- 62. Baker D, Herrod SS, Alvarez-Gonzalez C, et al. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol Neuroinflamm 2017; 4: e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord 2019; 29: 168–174. [DOI] [PubMed] [Google Scholar]
- 64. Stuve O, Soelberg Soerensen P, Leist T, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord 2019; 12: 1756286419854986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Al-Hashel J, Ahmed SF, AlMojel M, et al. A prospective observational longitudinal study with a two-year follow-up of multiple sclerosis patients on Cladribine. Clin Neurol Neurosurg 2023; 232: 107885. [DOI] [PubMed] [Google Scholar]
- 66. Zanetta C, Rocca MA, Meani A, et al. Effectiveness and safety profile of cladribine in an Italian real-life cohort of relapsing-remitting multiple sclerosis patients: a monocentric longitudinal observational study. J Neurol 2023; 270: 3553–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aerts S, Khan H, Severijns D, et al. Safety and effectiveness of cladribine tablets for multiple sclerosis: results from a single-center real-world cohort. Mult Scler Relat Disord 2023; 75: 104735. [DOI] [PubMed] [Google Scholar]
- 68. Petracca M, Ruggieri S, Barbuti E, et al. Predictors of Cladribine effectiveness and safety in multiple sclerosis: a real-world, multicenter, 2-year follow-up study. Neurol Ther 2022; 11: 1193–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rauma I, Viitala M, Kuusisto H, et al. Finnish multiple sclerosis patients treated with cladribine tablets: a nationwide registry study. Mult Scler Relat Disord 2022; 61: 103755. [DOI] [PubMed] [Google Scholar]
- 70. Magalashvili D, Mandel M, Dreyer-Alster S, et al. Cladribine treatment for highly active multiple sclerosis: real-world clinical outcomes for years 3 and 4. J Neuroimmunol 2022; 372: 577966. [DOI] [PubMed] [Google Scholar]
- 71. Patti F, Visconti A, Capacchione A, et al. Long-term effectiveness in patients previously treated with cladribine tablets: a real-world analysis of the Italian multiple sclerosis registry (CLARINET-MS). Ther Adv Neurol Disord 2020; 13: 1756286420922685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Freedman MS, Leist TP, Comi G, et al. The efficacy of cladribine tablets in CIS patients retrospectively assigned the diagnosis of MS using modern criteria: results from the ORACLE-MS study. Mult Scler J Exp Transl Clin 2017; 3: 2055217317732802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rammohan K, Giovannoni G, Comi G, et al. Cladribine tablets for relapsing-remitting multiple sclerosis: efficacy across patient subgroups from the phase III CLARITY study. Mult Scler Relat Disord 2012; 1: 49–54. [DOI] [PubMed] [Google Scholar]
- 74. Brochet B, Solari A, Lechner-Scott J, et al. Improvements in quality of life over 2 years with cladribine tablets in people with relapsing multiple sclerosis: the CLARIFY-MS study. Mult Scler 2023; 29: 1808–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Biernacki T, Sandi D, Bencsik K, et al. Medicinal chemistry of multiple sclerosis: focus on cladribine. Mini Rev Med Chem 2020; 20: 269–285. [DOI] [PubMed] [Google Scholar]
- 76. Voo VTF, Butzkueven H, Stankovich J, et al. The development and impact of cladribine on lymphoid and myeloid cells in multiple sclerosis. Mult Scler Relat Disord 2021; 52: 102962. [DOI] [PubMed] [Google Scholar]
- 77. Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021; 14: 17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schmierer K, Wiendl H, Oreja-Guevara C, et al. Varicella zoster virus and influenza vaccine antibody titres in patients from MAGNIFY-MS who were treated with cladribine tablets for highly active relapsing multiple sclerosis. Mult Scler 2022; 28: 2151–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Robertson LE, Chubb S, Meyn RE, et al. Induction of apoptotic cell death in chronic lymphocytic leukemia by 2-chloro-2’-deoxyadenosine and 9-beta-D-arabinosyl-2-fluoroadenine. Blood 1993; 81: 143–150. [PubMed] [Google Scholar]
- 80. Carson DA, Wasson DB, Kaye J, et al. Deoxycytidine kinase-mediated toxicity of deoxyadenosine analogs toward malignant human lymphoblasts in vitro and toward murine L1210 leukemia in vivo. Proc Natl Acad Sci U S A 1980; 77: 6865–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carson DA, Kaye J, Seegmiller JE. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A 1977; 74: 5677–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Beutler E. Cladribine (2-chlorodeoxyadenosine). Lancet 1992; 340: 952–956. [DOI] [PubMed] [Google Scholar]
- 83. Leist TP, Vermersch P. The potential role for cladribine in the treatment of multiple sclerosis: clinical experience and development of an oral tablet formulation. Curr Med Res Opin 2007; 23: 2667–2676. [DOI] [PubMed] [Google Scholar]
- 84. Rice GP, Filippi M, Comi G. Cladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. Cladribine MRI Study Group. Neurology 2000; 54: 1145–1155. [DOI] [PubMed] [Google Scholar]
- 85. Beutler E, Sipe JC, Romine JS, et al. The treatment of chronic progressive multiple sclerosis with cladribine. Proc Natl Acad Sci U S A 1996; 93: 1716–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sigal DS, Miller HJ, Schram ED, et al. Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood 2010; 116: 2884–2896. [DOI] [PubMed] [Google Scholar]
- 87. Jacobs BM, Ammoscato F, Giovannoni G, et al. Cladribine: mechanisms and mysteries in multiple sclerosis. J Neurol Neurosurg Psychiatry 2018; 89: 1266–1271. [DOI] [PubMed] [Google Scholar]
- 88. Kraus SHP, Luessi F, Trinschek B, et al. Cladribine exerts an immunomodulatory effect on human and murine dendritic cells. Int Immunopharmacol 2014; 18: 347–357. [DOI] [PubMed] [Google Scholar]
- 89. Korsen M, Bragado Alonso S, Peix L, et al. Cladribine exposure results in a sustained modulation of the cytokine response in human peripheral blood mononuclear cells. PLoS One 2015; 10: e0129182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sobottka B, Harrer MD, Ziegler U, et al. Collateral bystander damage by myelin-directed CD8+ T cells causes axonal loss. Am J Pathol 2009; 175: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Podbielska M, Banik NL, Kurowska E, et al. Myelin recovery in multiple sclerosis: the challenge of remyelination. Brain Sci 2013; 3: 1282–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Groh J, Abdelwahab T, Kattimani Y, et al. Microglia-mediated demyelination protects against CD8+ T cell-driven axon degeneration in mice carrying PLP defects. Nat Commun 2023; 14: 6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kaskow BJ, Baecher-Allan C. Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med 2018; 8: a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cencioni MT, Mattoscio M, Magliozzi R, et al. B cells in multiple sclerosis – from targeted depletion to immune reconstitution therapies. Nat Rev Neurol 2021; 17: 399–414. [DOI] [PubMed] [Google Scholar]
- 95. Comi G, Bar-Or A, Lassmann H, et al. Role of B cells in multiple sclerosis and related disorders. Ann Neurol 2021; 89: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jelcic I, Al Nimer F, Wang J, et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 2018; 175: 85–100.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fraussen J, Claes N, Van Wijmeersch B, et al. B cells of multiple sclerosis patients induce autoreactive proinflammatory T cell responses. Clin Immunol 2016; 173: 124–132. [DOI] [PubMed] [Google Scholar]
- 98. Rolfes L, Pfeuffer S, Huntemann N, et al. Immunological consequences of cladribine treatment in multiple sclerosis: a real-world study. Mult Scler Relat Disord 2022; 64: 103931. [DOI] [PubMed] [Google Scholar]
- 99. Moser T, Schwenker K, Seiberl M, et al. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann Clin Transl Neurol 2020; 7: 2199–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Baker D, Pryce G, Herrod SS, et al. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult Scler Relat Disord 2019; 30: 176–186. [DOI] [PubMed] [Google Scholar]
- 101. Ceronie B, Jacobs BM, Baker D, et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol 2018; 265: 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Spiezia AL, Cerbone V, Molinari EA, et al. Changes in lymphocytes, neutrophils and immunoglobulins in year-1 cladribine treatment in multiple sclerosis. Mult Scler Relat Disord 2022; 57: 103431. [DOI] [PubMed] [Google Scholar]
- 103. Baker D, Marta M, Pryce G, et al. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017; 16: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. DiSano KD, Gilli F, Pachner AR. Memory B cells in multiple sclerosis: emerging players in disease pathogenesis. Front Immunol 2021; 12: 676686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. https://clinicaltrials.gov/study/NCT03364036?rank=1 (accessed 31 March 2025).
- 106. Wiendl H, De Stefano N, Barkhof F, et al. Blood biomarker dynamics in highly active relapsing multiple sclerosis patients treated with Cladribine tablets: results of the 2-year MAGNIFY-MS Study (S16.006). Neurology 2023; 100: 3016. [Google Scholar]
- 107. Rodriguez-Mogeda C, van Ansenwoude CMJ, van der Molen L, et al. The role of CD56bright NK cells in neurodegenerative disorders. J Neuroinflamm 2024; 21: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lycett MJ, Lea RA, Maltby VE, et al. The effect of cladribine on immunoglobulin levels compared to B cell targeting therapies in multiple sclerosis. Mult Scler J Exp Transl Clin 2023; 9: 20552173221149688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kinnunen T, Chamberlain N, Morbach H, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood 2013; 121: 1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol 2008; 20: 632–638. [DOI] [PubMed] [Google Scholar]
- 111. Paolicelli D, Ruggieri M, Manni A, et al. Real-life experience of the effects of Cladribine tablets on lymphocyte subsets and serum neurofilament light chain levels in relapsing multiple sclerosis patients. Brain Sci 2022; 12: 1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Baker D, Herrod SS, Alvarez-Gonzalez C, et al. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol 2017; 74: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Balasa R, Maier S, Hutanu A, et al. Cytokine secretion dynamics of isolated PBMC after cladribine exposure in RRMS patients. Int J Mol Sci 2022; 23: 10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Holm Hansen R, von Essen MR, Mahler MR, et al. Cladribine effects on T and B cells and T cell reactivity in multiple sclerosis. Ann Neurol 2023; 94: 518–530. [DOI] [PubMed] [Google Scholar]
- 115. Monif M, Sequeira RP, Muscat A, et al. CLADIN – CLADribine and INnate immune response in multiple sclerosis – a phase IV prospective study. Clin Immunol 2024; 265: 110304. [DOI] [PubMed] [Google Scholar]
- 116. Jørgensen LØ, Hyrlov KH, Elkjaer ML, et al. Cladribine modifies functional properties of microglia. Clin Exp Immunol 2020; 201: 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lin LY, Juillard P, Hawke S, et al. Oral Cladribine impairs intermediate, but not conventional, monocyte transmigration in multiple sclerosis patients across a model blood-brain barrier. Int J Mol Sci 2023; 24: 6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Marastoni D, Foschi M, Eccher C, et al. CSF levels of Chitinase3like1 correlate with early response to cladribine in multiple sclerosis. Front Immunol 2024; 15: 1343892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liliemark J. The clinical pharmacokinetics of cladribine. Clin Pharmacokinet 1997; 32: 120–131. [DOI] [PubMed] [Google Scholar]
- 120. Hermann R, Karlsson MO, Novakovic AM, et al. The clinical pharmacology of cladribine tablets for the treatment of relapsing multiple sclerosis. Clin Pharmacokinet 2019; 58: 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kearns CM, Blakley RL, Santana VM, et al. Pharmacokinetics of cladribine (2-chlorodeoxyadenosine) in children with acute leukemia. Cancer Res 1994; 54: 1235–1239. [PubMed] [Google Scholar]
- 122. Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol 2006; 59: 880–892. [DOI] [PubMed] [Google Scholar]
- 123. Rejdak K, Stelmasiak Z, Grieb P. Cladribine induces long lasting oligoclonal bands disappearance in relapsing multiple sclerosis patients: 10-year observational study. Mult Scler Relat Disord 2019; 27: 117–120. [DOI] [PubMed] [Google Scholar]
- 124. De Stefano N, Achiron A, Barkhof F. Effect of cladribine tablets on markers of disease progression, axonal loss, and oligoclonal bands in patients with relapsing multiple sclerosis: results from MAGNIFY-MS. In: Abstracts from the 38th Annual Meeting of the Consortium of Multiple Sclerosis Centers, Poster no. DMT01, 29 May–1 June 2024, Nashville, Tennessee. [Google Scholar]
- 125. Wiendl H, Derfuss T, Schmierer K, et al. O037/579. Evaluation of the treatment response to cladribine tablets using a transcriptomics and proteomics approach in the cerebrospinal fluid and peripheral blood of people with multiple sclerosis. Mult Scler J 2024; 30(Suppl. 3). https://journals.sagepub.com/doi/full/10.1177/13524585241269218 (accessed 27 November 2024).
- 126. Seiberl M, Feige J, Hilpold P, et al. Serum neurofilament light chain as biomarker for cladribine-treated multiple sclerosis patients in a real-world setting. Int J Mol Sci 2023; 24: 4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Delcoigne B, Manouchehrinia A, Barro C, et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology 2020; 94: e1201–e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sainz-Amo R, Rodero-Romero A, Monreal E, et al. Effect of natalizumab on sNfL and sGFAP levels in multiple sclerosis patients. Int J Mol Sci 2024; 25: 13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Højsgaard Chow H, Petersen ER, Olsson A, et al. Age-corrected neurofilament light chain ratio decreases but does not predict relapse in highly active multiple sclerosis patients initiating natalizumab treatment. Mult Scler Relat Disord 2024; 88: 105701. [DOI] [PubMed] [Google Scholar]
- 130. Bar-Or A, Thanei G-A, Harp C, et al. Blood neurofilament light levels predict non-relapsing progression following anti-CD20 therapy in relapsing and primary progressive multiple sclerosis: findings from the ocrelizumab randomised, double-blind phase 3 clinical trials. eBioMedicine 2023; 93: 104662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Musella A, Mandolesi G, Gentile A, et al. Cladribine interferes with IL-1β synaptic effects in experimental multiple sclerosis. J Neuroimmunol 2013; 264: 8–13. [DOI] [PubMed] [Google Scholar]
- 132. Rossi S, Furlan R, De Chiara V, et al. Interleukin-1β causes synaptic hyperexcitability in multiple sclerosis. Ann Neurol 2012; 71: 76–83. [DOI] [PubMed] [Google Scholar]
- 133. Rossi S, Studer V, Motta C, et al. Inflammation inhibits GABA transmission in multiple sclerosis. Mult Scler 2012; 18: 1633–1635. [DOI] [PubMed] [Google Scholar]
- 134. Aybar F, Julia Perez M, Silvina Marcora M, et al. 2-Chlorodeoxyadenosine (Cladribine) preferentially inhibits the biological activity of microglial cells. Int Immunopharmacol 2022; 105: 108571. [DOI] [PubMed] [Google Scholar]
- 135. Giovannoni G, Singer BA, Issard D, et al. Durability of no evidence of disease activity-3 (NEDA-3) in patients receiving cladribine tablets: The CLARITY extension study. Mult Scler 2022; 28: 1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. De Stefano N, Sormani MP, Giovannoni G, et al. Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: the CLARITY and CLARITY extension studies. Mult Scler 2022; 28: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Giovannoni G, Boyko A, Correale J, et al. Long-term follow-up of patients with relapsing multiple sclerosis from the CLARITY/CLARITY extension cohort of CLASSIC-MS: an ambispective study. Mult Scler 2023; 29: 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. De Stefano N, Vermersch P, Wiendl H, et al. Long-term effectiveness of cladribine tablets over 4 years in relapsing multiple sclerosis: results from the MAGNIFY-MS extension study. In: ECTRIMS 2024, Poster no. P349/560, 18–24 September 2024, Copenaghen, Denmark. [Google Scholar]
- 139. De Stefano N, Wiendl H, Barkhof F, et al. Low rate of progression independent of relapse in patients with relapsing multiple sclerosis treated with cladribine tablets. In: ECTRIMS 2024, Poster no. P326/628, 18–24 September 2024, Copenaghen, Denmark. [Google Scholar]
- 140. Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord 2019; 29: 157–167. [DOI] [PubMed] [Google Scholar]
- 141. Pakpoor J, Disanto G, Altmann DR, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflamm 2015; 2: e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Leist T, Cook S, Comi G, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord 2020; 46: 102572. [DOI] [PubMed] [Google Scholar]
- 143. Pfeuffer S, Rolfes L, Hackert J, et al. Effectiveness and safety of cladribine in MS: real-world experience from two tertiary centres. Mult Scler 2022; 28: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Arena S, Chisari CG, Toscano S, et al. REal-World effectIveNess of claDribine for patients with multiple sclerosis: a Sicilian multicentric experience (REWIND study). Curr Neuropharmacol 2023; 22: 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Leist T, et al. Safety of cladribine tablets in the treatment of people with multiple sclerosis: 2024 post-approval update with a focus on infection risk. In: ACTRIMS 2025, P400, 27 February–1 March 2025, West Palm Beach, FL, USA. [Google Scholar]
- 146. Santos M, Sequeira J, Abreu P, et al. Safety and effectiveness of cladribine in multiple sclerosis: real-world clinical experience from 5 tertiary hospitals in Portugal. Clin Neuropharmacol 2023; 46: 105–111. [DOI] [PubMed] [Google Scholar]
- 147. Lizak N, Hodgkinson S, Butler E, et al. Real-world effectiveness of cladribine for Australian patients with multiple sclerosis: an MSBase registry substudy. Mult Scler 2021; 27: 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Barros A, Santos M, Sequeira J, et al. Effectiveness of cladribine in multiple sclerosis – clinical experience of two tertiary centers. Mult Scler J 2020; 26(Suppl. 3): 278–278. [Google Scholar]
- 149. Moccia M, Lanzillo R, Petruzzo M, et al. Single-center 8-years clinical follow-up of cladribine-treated patients from phase 2 and 3 trials. Front Neurol 2020; 11: 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Santos M, Barros A, Sequeira J, et al. Safety and tolerability of cladribine in multiple sclerosis – clinical experience of two tertiary centers. Mult Scler J 2020; 305–306. [Google Scholar]
- 151. Rojas JI, Alonso R, Luetic G, et al. Real-world effectiveness and safety of cladribine in multiple sclerosis: longitudinal data from the nationwide registry in Argentina. Clin Neuropharmacol 2024; 47: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Schiavetti I, Signori A, Albanese A, et al. Therapeutic choices and disease activity after 2 years of treatment with cladribine: an Italian multicenter study (CladStop). Eur J Neurol 2024; 31: e16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wallace B, David R, Niall M, et al. Cladribine tablets in highly-active relapsing multiple sclerosis real-world effectiveness in UK clinical practice (CAMELOT-MS). J Neurol Neurosurg Psychiatry 2024; 95: A43. [Google Scholar]
- 154. Jack D, Damian D, Nolting A, et al. COVID-19 in patients with multiple sclerosis treated with cladribine tablets: an update. Mult Scler Relat Disord 2021; 51: 102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. De Angelis M, Petracca M, Lanzillo R, et al. Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult Scler Relat Disord 2020; 45: 102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Seferoğlu M, Ethemoğlu Ö, Turan ÖF, et al. MS and COVID-19 challenge: asymptomatic COVID-19 infection during treatment with cladribine. Neurol Sci 2021; 42: 3533–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Drulovic J, Ivanovic J, Martinovic V, et al. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult Scler Relat Disord 2021; 54: 103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cañibano BG, Okar L, Baniamer YZ, et al. Real world experience with cladribine tablets in the management of relapsing multiple sclerosis in Qatar. Clin Neurol Neurosurg 2024; 247: 108615. [DOI] [PubMed] [Google Scholar]
- 159. Oreja-Guevara C, Tintoré M, Meca V, et al. Family planning in fertile-age patients with multiple sclerosis (MS) (ConPlanEM Study): Delphi consensus statements. Cureus 2023; 15: e44056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Giovannoni G, Galazka A, Schick R, et al. Pregnancy outcomes during the clinical development program of cladribine in multiple sclerosis: an integrated analysis of safety. Drug Saf 2020; 43: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dost-Kovalsky K, Thiel S, Ciplea AI, et al. Cladribine and pregnancy in women with multiple sclerosis: the first cohort study. Mult Scler 2023; 29: 461–465. [DOI] [PubMed] [Google Scholar]
- 162. Signoriello E, Foschi M, Lanzillo R, et al. Pregnancy effect on disease activity in women with multiple sclerosis treated with cladribine. J Neurol 2024; 271: 4039–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. De Stefano N, Giorgio A, Battaglini M, et al. Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler 2018; 24: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Cortese R, Battaglini M, Sormani MP, et al. Reduction in grey matter atrophy in patients with relapsing multiple sclerosis following treatment with cladribine tablets. Eur J Neurol 2023; 30: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Achiron A, Warszawer Y, Nissan Y, et al. Positive impact of cladribine tablets on reducing brain atrophy in patients with relapsing-remitting multiple sclerosis: a longitudinal study. Mult Scler 2025; 31(6): 689–695. [DOI] [PubMed] [Google Scholar]
- 166. Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141: 2382–2391. [DOI] [PubMed] [Google Scholar]
- 168. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92: e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bittner S, Oh J, Havrdová EK, et al. The potential of serum neurofilament as biomarker for multiple sclerosis. Brain 2021; 144: 2954–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bar-Or A, Montalban X, Hu X, et al. Serum neurofilament light trajectories and their relation to subclinical radiological disease activity in relapsing multiple sclerosis patients in the APLIOS trial. Neurol Ther 2023; 12: 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Moser T, Seiberl M. Serum NfL in MS patients treated with cladribine: course and predictive value (P1-3.006). Neurology 2023; 100: 2694. [Google Scholar]
- 172. Preziosa P, Pagani E, Meani A, et al. Slowly expanding lesions predict 9-year multiple sclerosis disease progression. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Calvi A, Tur C, Chard D, et al. Slowly expanding lesions relate to persisting black-holes and clinical outcomes in relapse-onset multiple sclerosis. Neuroimage Clin 2022; 35: 103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wood H. Slowly expanding lesions are linked to multiple sclerosis progression. Nat Rev Neurol 2022; 18: 252–252. [DOI] [PubMed] [Google Scholar]
- 175. De Stefano N, Gentile G, Cacciante F, et al. Cladribine tablets reduce slowly expanding lesions in gray matter-driven MRI phenotype and active lesions in all sustain-based MRI phenotypes of MS patients. In: ECTRIMS 2024, Poster no. P207/1740, 18–24 September 2024, Copenaghen, Denmark. [Google Scholar]
- 176. Selmaj K, Langdon D, Brochet B, et al. Long-term effects of cladribine tablets treatment on cognition, relapses, MRI, and safety outcomes in patients with relapsing multiple sclerosis: 4-year results from the CLARIFY-MS Extension study. In: ECTRIMS 2024, Poster no. P846/1650, 18–24 September 2024, Copenaghen, Denmark. [Google Scholar]
- 177. Glanz BI, Zurawski J, Gonzalez CT, et al. Comparison of health-related quality of life across treatment groups in individuals with multiple sclerosis. Mult Scler Relat Disord 2020; 40: 101944. [DOI] [PubMed] [Google Scholar]
- 178. Afolabi D, Albor C, Zalewski L, et al. Positive impact of cladribine on quality of life in people with relapsing multiple sclerosis. Mult Scler 2018; 24: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Borriello G, Chisari CG, Maimone D, et al. Cladribine effects on patient-reported outcomes and their clinical and biometric correlates in highly active relapsing multiple sclerosis at first switch: the observational, multicenter, prospective, phase IV CLADFIT-MS study. Front Neurol 2024; 15: 1422078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Aff (Millwood) 2016; 35: 575–582. [DOI] [PubMed] [Google Scholar]
- 181. van Munster CEP, Uitdehaag BMJ. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs 2017; 31: 217–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Jensen RE, Rothrock NE, DeWitt EM, et al. The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care 2015; 53: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rau D, Müller B, Übler S. Evaluation of patient-reported outcomes in patients with relapsing multiple sclerosis treated with cladribine tablets in the CLAWIR study: 12-month interim analysis. Adv Ther 2023; 40: 5547–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Conway DS, Nicholas JA, Thompson NR, et al. Real world experience with cladribine tablets for multiple sclerosis at four academic multiple sclerosis centers. Mult Scler Relat Disord 2025; 94: 106272. [DOI] [PubMed] [Google Scholar]
- 185. Śladowska K, Kawalec P, Holko P, et al. Comparative safety of high-efficacy disease-modifying therapies in relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Neurol Sci 2022; 43: 5479–5500. [DOI] [PubMed] [Google Scholar]
- 186. Rosenstein I, Nordin A, Sabir H, et al. Association of serum glial fibrillary acidic protein with progression independent of relapse activity in multiple sclerosis. J Neurol 2024; 271: 4412–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bose G, Healy BC, Saxena S, et al. Increasing neurofilament and glial fibrillary acidic protein after treatment discontinuation predicts multiple sclerosis disease activity. Neurol Neuroimmunol Neuroinflamm 2023; 10: e200167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Fissolo N, Benkert P, Sastre-Garriga J, et al. Serum biomarker levels predict disability progression in patients with primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2024; 95: 410–418. [DOI] [PubMed] [Google Scholar]
- 189. Yang J, Hamade M, Wu Q, et al. Current and future biomarkers in multiple sclerosis. Int J Mol Sci 2022; 23: 5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Fissolo N, Calvo-Barreiro L, Eixarch H, et al. Molecular signature associated with cladribine treatment in patients with multiple sclerosis. Front Immunol 2023; 14: 1233546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zorov DB, Juhaszova M, Yaniv Y, et al. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res 2009; 83: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Warne J, Pryce G, Hill JM, et al. Selective inhibition of the mitochondrial permeability transition pore protects against neurodegeneration in experimental multiple sclerosis. J Biol Chem 2016; 291: 4356–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Forte M, Gold BG, Marracci G, et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci U S A 2007; 104: 7558–7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. van Dijk KD, Berendse HW, Drukarch B, et al. The proteome of the locus ceruleus in Parkinson’s disease: relevance to pathogenesis. Brain Pathol 2012; 22: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Long J, Pan G, Ifeachor E, et al. Discovery of novel biomarkers for Alzheimer’s disease from blood. Dis Markers 2016; 2016: 4250480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Juźwik CA, Drake SS, Zhang Y, et al. microRNA dysregulation in neurodegenerative diseases: a systematic review. Prog Neurobiol 2019; 182: 101664. [DOI] [PubMed] [Google Scholar]
- 197. Baulina N, Kulakova O, Kiselev I, et al. Immune-related miRNA expression patterns in peripheral blood mononuclear cells differ in multiple sclerosis relapse and remission. J Neuroimmunol 2018; 317: 67–76. [DOI] [PubMed] [Google Scholar]
- 198. Ebrahimkhani S, Vafaee F, Young PE, et al. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep 2017; 7: 14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Yin X, Wang M, Wang W, et al. Identification of potential miRNA-mRNA regulatory network contributing to Parkinson’s disease. Parkinsons Dis 2022; 2022: 2877728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Arisi I, Malimpensa L, Manzini V, et al. Cladribine and ocrelizumab induce differential miRNA profiles in peripheral blood mononucleated cells from relapsing-remitting multiple sclerosis patients. Front Immunol 2023; 14: 1234869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ghadiri N, Emamnia N, Ganjalikhani-Hakemi M, et al. Analysis of the expression of mir-34a, mir-199a, mir-30c and mir-19a in peripheral blood CD4+T lymphocytes of relapsing-remitting multiple sclerosis patients. Gene 2018; 659: 109–117. [DOI] [PubMed] [Google Scholar]
- 202. Mori F, Rossi S, Piccinin S, et al. Synaptic plasticity and PDGF signaling defects underlie clinical progression in multiple sclerosis. J Neurosci 2013; 33: 19112–19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Morawiec N, Techmański T, Tracz K, et al. The comparative analysis of selected interleukins and proinflammatory factors in CSF among de novo diagnosed patients with RRMS. Clin Neurol Neurosurg 2023; 225: 107522. [DOI] [PubMed] [Google Scholar]
- 204. Ghorbani S, Yong VW. The extracellular matrix as modifier of neuroinflammation and remyelination in multiple sclerosis. Brain 2021; 144: 1958–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Maglione A, Rolla S, Mercanti SFD, et al. The adaptive immune system in multiple sclerosis: an estrogen-mediated point of view. Cells 2019; 8: 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Stampanoni Bassi M, Buttari F, Simonelli I, et al. A single nucleotide ADA genetic variant is associated to central inflammation and clinical presentation in MS: implications for cladribine treatment. Genes (Basel) 2020; 11: 1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Spurgeon S, Yu M, Phillips JD, et al. Cladribine: not just another purine analogue? Expert Opin Investig Drugs 2009; 18: 1169–1181. [DOI] [PubMed] [Google Scholar]
- 208. Oreja-Guevara C, Brownlee W, Celius EG, et al. Expert opinion on the long-term use of cladribine tablets for multiple sclerosis: systematic literature review of real-world evidence. Mult Scler Relat Disord 2023; 69: 104459. [DOI] [PubMed] [Google Scholar]
- 209. Habek M, Drulovic J, Brecl Jakob G, et al. Treatment with cladribine tablets beyond year 4: a position statement by southeast European multiple sclerosis centers. Neurol Ther 2023; 12: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Dillenseger A, Weidemann ML, Trentzsch K, et al. Digital biomarkers in multiple sclerosis. Brain Sci 2021; 11: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]