Summary
Cell cycle progression relies on tightly regulated Cyclin synthesis and degradation, with Cyclins A and B activating CDK1 to drive mitosis. Dysregulation of Cyclin levels is linked to tumorigenesis, underscoring the importance of studying cyclin mRNA control for cancer therapy development. Using super-resolution microscopy, we show that cyclin A and cyclin B mRNAs associate with Bruno 1 and Cup in nurse cells, and that depletion of either protein disrupts Cyclin expression and reduces mRNA levels. Both mRNAs also accumulate in Me31B-marked P-bodies; however, Me31B selectively affects cyclin B, causing its stage-specific de-repression and decreased stability, while cyclin A remains unaffected. Loss of Me31B enhances cyclin B mRNA’s association with Cup, suggesting P-body-independent repression mechanisms. These results highlight the nuanced, mRNA-specific roles of P-body condensates in post-transcriptional regulation, challenging the idea of a uniform, binary mechanism of mRNA repression in P-bodies.
Subject areas: Molecular interaction, Cell biology
Graphical abstract

Highlights
-
•
Bru1 and Cup are necessary for CycA and CycB translational repression
-
•
Bru1 and Cup are essential for cycA and cycB mRNAs recruitment into P-bodies
-
•
Bru1 overexpression rescues mitotic re-entry in cup mutant egg chambers
-
•
cycA and cycB mRNAs associate with Cup independently of Me31B
Molecular interaction; Cell biology
Introduction
Under normal physiological conditions, cell cycle progression is tightly regulated by the controlled synthesis and degradation of mitotic cyclins. The cell cycle consists of four distinct phases: G0/G1 (Gap 1), S (DNA synthesis), G2 (Gap 2), and M (Mitosis). Each phase contains critical checkpoints to monitor DNA replication fidelity and chromosome segregation, ensuring proper cell division. Cyclins form complexes with cyclin-dependent kinases (CDKs) which, upon binding, activate the kinase activity of CDKs. These activated kinases subsequently phosphorylate key substrates that propel cell cycle progression. CDKs are highly stable proteins and exhibit consistent levels throughout the cell cycle. Temporal regulation of kinase activity is achieved via oscillation in the levels of their Cyclin binding partners.1
The activation of CDK1, the master regulator of mitosis, is sequentially mediated by Cyclin A (CycA), followed by Cyclin B (CycB), both of which are crucial for initiating and progressing through mitosis. During anaphase, CycB must be degraded by the anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin ligase, to inactivate CDK1 and allow mitotic progression. Failure to degrade CycB results in a shortened G1 phase, abnormal DNA replication, and genomic instability.2 Notably, numerous cancers exhibit elevated levels of CycB, and a meta-analysis of 17 studies found that CycB overexpression correlates with poor survival outcomes in solid tumor patients. This makes CycB a promising therapeutic target, highlighting the need for further investigation into its regulatory mechanisms.3
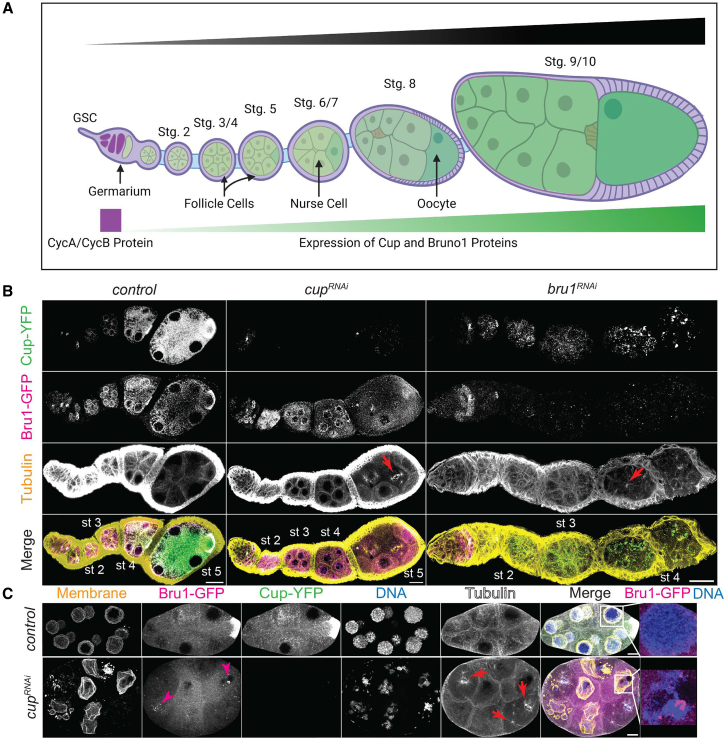
Drosophila melanogaster oogenesis serves as an excellent model system for studying cyclin mRNA regulation, as cycA and cycB mRNAs are post-transcriptionally regulated throughout the majority of oogenesis.4,5,6,7,8 The unique expression pattern of these mRNAs enables the investigation of the specific mechanisms controlling mRNA regulation without the interference of the usual cyclical expression and degradation observed in other tissues. Drosophila oogenesis starts with the asymmetric division of a germline stem cell (GSC) in the germarium, producing a self-renewing stem cell and a differentiating cystoblast. The cystoblast undergoes four rounds of mitotic division with incomplete cytokinesis, forming a 16-cell cyst connected by cytoplasmic bridges known as ring canals. Of these 16 cells, 1 differentiates into the oocyte while the remaining 15 develop into nurse cells. As the germline cyst develops, it becomes encapsulated by somatic follicle cells and exits the germarium as a fully formed egg chamber. This chamber subsequently progresses through 14 distinct developmental stages before being deposited into the oviduct9,10 (Figure 1A).
Figure 1.
Bru1 and Cup knockdowns lead to re-entry of nurse cells into the cell cycle
(A) A schematic model of the Drosophila melanogaster ovariole illustrating the developmental stages from the germarium to a stage 10 egg chamber, highlighting the expression patterns of CycA and CycB proteins, as well as the progressive increase of Cup and Bru1 proteins during oogenesis. Generated via BioRender.
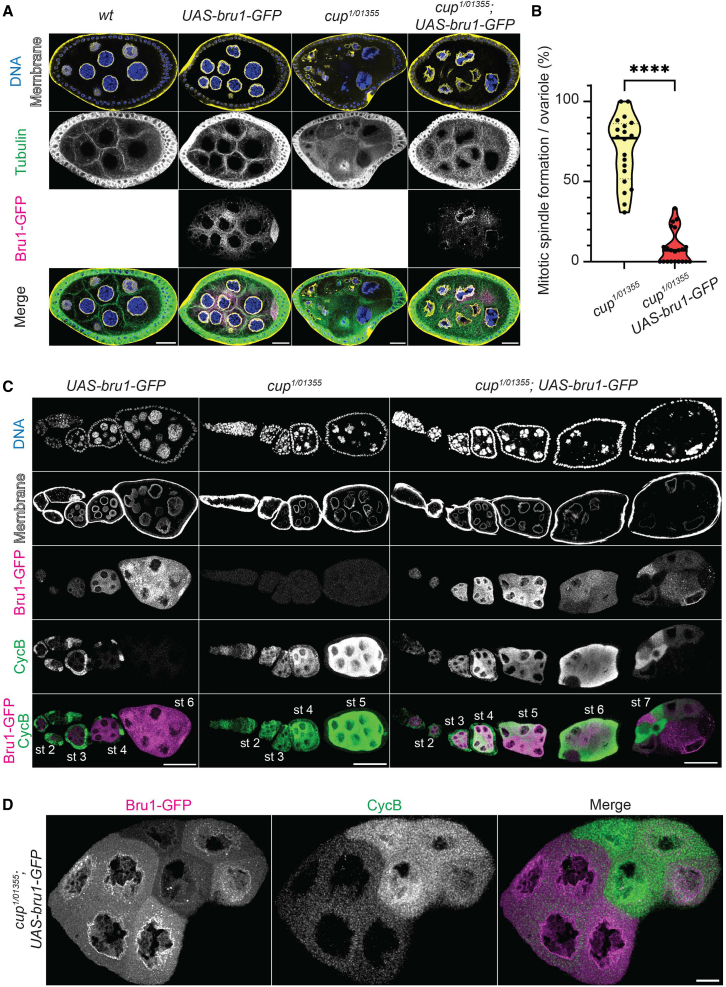
(B) Visualization of ɑ-Tubulin via IF in chains of egg chambers expressing Cup-YFP and Bru1-GFP in the indicated backgrounds. Formation of mitotic spindles in cupRNAi and bruRNAi backgrounds (red arrow). Images are deconvolved, XY max-intensity Z-projections of 5 optical slices (0.3 μm each). Scale bars, 20 μm.
(C) Stage 6 egg chambers expressing Cup-YFP and Bru1-GFP in indicated backgrounds. Membrane (WGA stain). Nuclear aggregation of Bru1-GFP (magenta arrowheads and zoomed-in Merge panels). Formation of mitotic spindles (red arrows). Images are deconvolved, XY max-intensity Z-projections of 5 optical slices (0.3 μm each). Scale bars, 10 μm.
After the egg chamber exits the germarium, only the oocyte nucleus remains in meiosis, arrested in prophase I, while the 15 nurse cells exit meiosis and enter endoreplication. During this process, the nurse cells replicate their DNA without undergoing cell division to supply the oocyte with the necessary factors for its growth. The proper regulation of GSC activity and the four rounds of mitotic division are controlled by the expression and degradation of Cyclins. Although the exact mechanism driving the cyst’s exit from the mitotic cycle remains unclear, the suppression of CycA and CycB protein expression is critical for this transition. CycA and CycB proteins are expressed in the germarium during the four rounds of mitotic divisions, and again starting at stage 12 of oogenesis (Figure 1A). However, their mRNAs are expressed continuously throughout oogenesis and exhibit distinct localization patterns. cycA mRNA is evenly distributed throughout the egg chamber, whereas cycB mRNA preferentially accumulates in the oocyte until stage 7. After stage 7, the levels of both mRNAs decrease until a second round of transcription begins at stage 9, at which point these mRNAs are translated, setting the stage for proper embryonic development.4,7,8,11
The post-transcriptional regulation of cycA mRNA is mediated by Bruno 1 (Bru1), an RNA-binding protein from the CELF1/2 family homolog in Drosophila, which directly binds to the 3′ UTR of cycA mRNA to repress its translation.7 Bru1’s binding partner, Cup, an eIF4E-binding protein with a role in translational repression, also participates in the repression of cycA mRNA. In bru1 and cup mutants, CycA protein is aberrantly expressed, leading to inappropriate mitotic re-entry.7 This finding supports a model in which Bru1 binds to cycA mRNA’s 3′ UTR and recruits Cup, which in turn, binds to eIF4E to inhibit translation, resembling the proposed mechanism for oskar mRNA regulation.12 CycB protein is also ectopically expressed in bru1 and cup mutant egg chambers, but the regulatory mechanism involving Bru1 and Cup in CycB protein expression remains poorly understood. Notably, cycB mRNA lacks a predicted binding site for Bru1, which was found to only weakly bind to the 3′ UTR upon UV crosslinking.7 Although the misregulated translation of cycB mRNA in bru1 and cup mutants suggests both proteins have roles in its regulation, definitive evidence of them interacting with cycB mRNA has yet to be demonstrated.
Bru1 and Cup have been implicated in the regulation of numerous mRNAs and are considered core components of biomolecular condensates known as P-bodies (reviewed by Bayer et al.13). P-bodies are cytoplasmic, membraneless organelles, formed via liquid-liquid phase separation (LLPS), where mRNAs can be stored in a translationally repressed state. Two key elements are essential for P-body formation: RNAs and RNA-binding proteins that contain intrinsically disordered regions. Once a local concentration threshold of these components is reached, LLPS is triggered.14,15,16,17 The functional role of P-bodies remains a topic of ongoing debate. It remains unclear whether P-bodies form as a byproduct of high concentrations of mRNAs and associated proteins, leading to a thermodynamically favorable phase separation or, if they are actively involved in the mRNA life cycle, playing a crucial role in the spatiotemporal regulation of different transcripts.18 This question highlights the need for further research to determine whether P-bodies are passive condensates or active regulators in mRNA processing. Nevertheless, it was found that approximately one-third of mRNAs localize to P-bodies as part of mRNA regulons. These mRNAs are translationally repressed and shielded from 5′ decay. Notably, the proteins encoded by these mRNAs are functionally related.19
The localization of cycA and cycB mRNAs into P-bodies remains to be conclusively unveiled. cycA mRNA, which is bound by Bru1 and regulated by Cup, is a likely target for localization into P-bodies. cycB mRNA has been shown to localize to germ granules, another type of membraneless organelle that forms via LLPS.20 Additionally, cycB mRNA undergoes poly(A) tail shortening via CCR4 and PABP2, followed by re-adenylation via Orb (CPEB protein) beginning at stage 10, prior to translation initiation.21 A shortened poly(A) tail is a hallmark of mRNAs that localize into P-bodies (reviewed by Chen and Shyu22). Moreover, the CCR4-NOT complex, a major deadenylase in the egg chamber, interacts with Cup which facilitates mRNA deadenylation while preventing subsequent decapping and degradation.15,23,24,25,26 Given these regulatory mechanisms, cycB mRNA is also a strong candidate for recruitment into P-bodies.
In this study, we confirm the association of Bru1 and Cup with cycA mRNA in the nurse cells, showing that depletion of either protein results in aberrant CycA protein expression and a reduction in cycA mRNA levels. We show that cycB mRNA also associates with Bru1 and Cup in the nurse cell cytoplasm. Furthermore, downregulation of either Bru1 or Cup leads to rapid and robust CycB protein expression, accompanied by overall decreased mRNA levels in the egg chamber. Both cycA and cycB mRNAs accumulate in P-bodies, marked by the core P-body protein Me31B. We demonstrate that Me31B per se does not play a role in cycA mRNA translational regulation, as no ectopic expression of CycA protein was detected. The downregulation of Me31B does not affect the association of Cup with either mRNA, suggesting that the mRNP complex can form independent of P-body recruitment. On the contrary, stage-specific ectopic CycB protein was detected, suggesting that P-bodies play a supportive, fine-tuning role in regulating cycB mRNA.
Results
Bru1 and Cup knockdowns lead to re-entry into the cell cycle and developmental arrest
In our previous studies on the roles of Bru1 and Cup in the oskar mRNA life cycle, we found that the depletion of both proteins caused developmental arrest.27 We demonstrated that Bru1 and Cup regulated each other’s expression at the mRNA level, as the knockdown of either protein led to a decrease in the levels of the other’s mRNA and protein. Furthermore, in cupRNAi egg chambers, endogenously tagged Bru1-GFP expression levels were decreased, and the cytoplasmic localization was disrupted. Bru1-GFP became diffuse throughout the cytoplasm and accumulated in large nuclear puncta (Figures 1B and 1C – magenta arrowheads).
We wished to more closely assess egg chamber development, using the UAS-Gal4 RNAi system to knock down Bru1 and Cup. We employed the Gal4 driver matalpha4-GAL-VP16 to induce knockdowns specifically during early oogenesis (stages 1–2), allowing Bru1 expression in the germarium. This approach was necessary because loss of Bru1 in the germarium results in a tumorous phenotype and prevents egg chamber formation. We found that Bru1 and Cup knockdowns distinctly affected progress through oogenesis (Figure 1B). Bru1 downregulation led to a “beads on a string” phenotype, where multiple egg chambers in a single ovariole arrested during early stages of oogenesis, progressing just until stage 4. As they reached stage 4, the nurse cells re-entered the cell cycle, indicated by mitotic spindle formation, followed by the rapid degradation of the egg chambers (Figures 1B – red arrows and S1A – red arrows).
Conversely, when Cup levels were reduced using the same driver, the egg chambers developed further, until stage 7 (Figure 1B). In these egg chambers, similarly to the Bru1 knockdown egg chambers, mitotic spindle formation was initiated at around stage 4 in multiple nurse cells as well as within multiple egg chambers in an ovariole (Figures 1C and S1B – red arrows). Unlike in the Bru1 knockdowns, once the nuclei divided, the nuclear envelope reformed, producing nuclei that were smaller in size. Whether this was due to multiple rounds of division, or to the reformation of the nuclei containing only partial chromosomal DNA, remains to be determined (Figures 1C and S1B – red arrowheads).
Altogether, these findings suggest that Bru1 plays a critical role in egg chamber maturation throughout early oogenesis, and not just in the germarium. Since Bru1 has been shown to repress the transcriptional repressor polar granule component (pgc) mRNA, it is possible that this de-repression leads to global transcriptional changes that are detrimental to egg chamber development.28 This activity seems independent of Cup, as cupRNAi egg chambers develop further than those with Bru1 depletion. These results demonstrate that both Bru1 and Cup are essential to suppress the re-entry of nurse cells into the cell cycle, possibly through the regulation of CycA and CycB proteins.
CycA and CycB proteins accumulate at distinct developmental stages in Bru1 and Cup knockdown egg chambers
We aimed to visualize the ectopic accumulation of CycA and CycB proteins, as previous reports in bru1 and cup mutant egg chambers showed the de-repression of cycA and cycB mRNAs starting at the germarium stage.6,7,29 To better understand their roles beyond the germarium, we focused on assessing ectopic CycA and CycB expression and mitotic re-entry in egg chambers where knockdowns were initiated post-germarium stages.
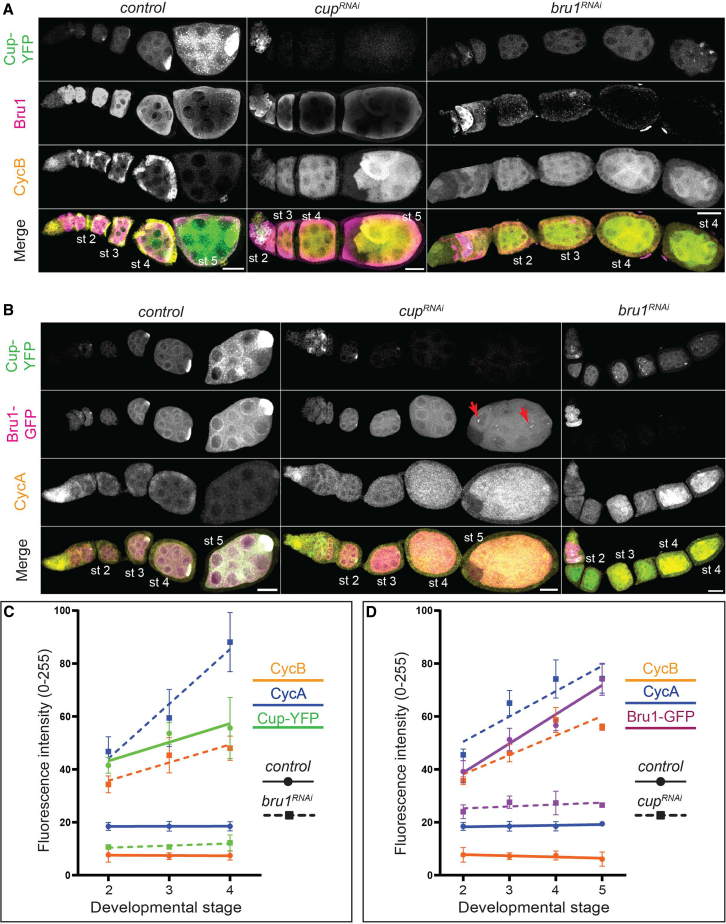
In wild-type egg chambers, CycA and CycB are expressed during the first four germ cell divisions in the germarium (Figure 1A), and then only again after stage 12. Despite this protein-expression gap, cycA and cycB mRNAs are continuously expressed throughout oogenesis.7,30 To further investigate the effect of Bru1 and Cup on their protein expression levels, we used RNAi-mediated knockdowns of Bru1 and Cup in egg chambers co-expressing endogenously tagged Bru1-GFP and Cup-YFP and assessed CycA and CycB distribution. Interestingly, we observed a robust, ectopic expression of CycB starting at stage 2 with either Bru1 or Cup depletion (Figures 2A, 2C, and S2A), highlighting their critical roles in the translational repression of cycB mRNA. In contrast, CycA protein levels were low during early stages but showed the highest increase at stage 4 in both knockdowns (Figures 2B, 2D, and S2B). These patterns mirrored the aberrant mitotic cycle initiation phenotype, with CycB alone being insufficient to trigger mitosis, and CycA being required for the nurse cells to re-enter the cell cycle. This aligns with findings from earlier reports where CycB overexpression did not induce mitosis in nurse cells.31 We confirmed the abnormal expression levels of CycA and CycB in these backgrounds, via immunoblotting. We used 1-day-old mCherryRNAi egg chambers as a control to limit the amount of late-stage egg chambers, where high levels of CycA and CycB are found (Figure S2C).
Figure 2.
Bru1 and Cup knockdowns lead to the ectopic expression of CycA and CycB proteins
(A) Visualization of CycB and Bru1 via IF in chains of egg chambers expressing Cup-YFP in the indicated backgrounds. Images are deconvolved, XY max-intensity Z-projections of 15 (control), 10 (cupRNAi) and 10 (bru1RNAi) optical slices (0.3 μm each). Scale bars, 20 μm.
(B) Visualization of CycA via IF in chains of egg chambers expressing Cup-YFP and Bru1-GFP in the indicated backgrounds. Images are deconvolved, XY max-intensity Z-projections of 15 (control), 11 (cupRNAi) and 10 (bru1RNAi) optical slices (0.3 μm each). Scale bars, 20 μm.
(C) Analysis of fluorescence intensity for CycB, CycA, and Cup-YFP protein expression in the germline cells of egg chambers in the specified developmental stages and backgrounds. Data are represented as mean ± SEM.
(D) Analysis of fluorescence intensity for CycB, CycA, and Bru1-GFP protein expression in the germline cells of egg chambers in the specified developmental stages and backgrounds. Data are represented as mean ± SEM.
To further assess the time course of the ectopic expression of CycA and CycB, we analyzed the fluorescent signal at each developmental stage. We found that the increase in CycB protein expression correlated with the decrease of Cup in bru1RNAi and Bru1 in cupRNAi backgrounds (Figures 2C and 2D). Surprisingly, in bru1RNAi egg chambers, CycA levels increased significantly only when Cup levels were severely reduced at stage 4 (Figure 2C.) Similarly, in cupRNAi egg chambers, robust CycA expression was detected only when Bru1 levels decreased in the cytoplasm, and accumulated in large nuclear puncta at stage 4 (Figures 2B – red arrows and 2D). The low CycA expression in earlier stages suggests that Bru1 or Cup alone can partially repress cycA mRNA translation, but both are required for the strongest suppression. These results demonstrate that both Bru1 and Cup are essential for the translational repression of cycA and cycB mRNAs.
Bru1 and Cup, with cycA and cycB mRNAs, form large complexes in the nurse cell cytoplasm
Previous studies showed that cycA and cycB mRNAs are present throughout the egg chamber, with cycB mRNA accumulating in the oocyte. Additionally, although Bru1 directly binds cycA mRNA in vitro, it remains unclear whether it interacts with cycB mRNA, despite the ectopic accumulation of CycB protein in bru1 mutant egg chambers.4,7 Similarly, the potential association between Cup and cycA or cycB mRNAs has not been explored. To fill these gaps, we aimed to (1) confirm the association between cycA mRNA and Bru1, (2) address whether Bru1 associates with cycB mRNA, and (3) assess the interaction between cycA and cycB mRNAs with Cup in the nurse cell cytoplasm. Since highly disordered proteins, such as Bru1 and Cup, typically form complexes characterized by weak and highly dynamic interactions, co-immunoprecipitation experiments are often unreliable, particularly in Drosophila egg chambers. For example, in a highly sensitive study involving six RNA-binding proteins, numerous novel interactions were identified; however, some previously established interactions were not detected.32 Additionally, the Egl/BicD/Dynein transport complex could not be isolated through conventional immunoprecipitation techniques, although it can be successfully assembled in vitro.33,34 To overcome these limitations, we opted to visualize their association using sensitive single-molecule fluorescence in situ hybridization (smFISH) probes. Interestingly, we found that cycA mRNA, and not only cycB mRNA, localized into the oocyte, and that both mRNAs were expressed throughout oogenesis (Figure S3A).
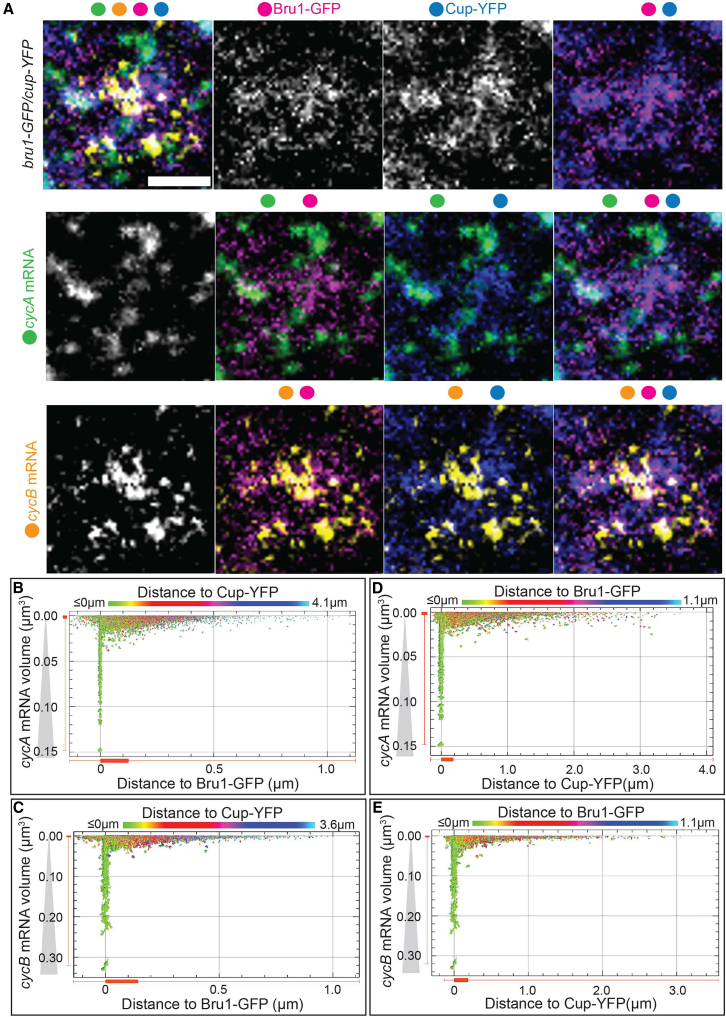
To further assess cycA and cycB mRNAs’ localization and their spatial associations with Bru1 and Cup, we employed STED super-resolution microscopy in the nurse cell cytoplasm. As the large size of Drosophila egg chambers and their surrounding follicle cells presented challenges for STED resolution, we developed a new protocol for sectioning egg chambers that proved to be essential for optimal STED imaging. We represented the zoomed-in images in a Punnett square format to display all possible combinations of colocalization (Figures 3A and S3B). We observed that both cycA and cycB mRNAs colocalized with Bru1-GFP and Cup-YFP predominantly within large cytoplasmic condensates (Figures 3A, S3C, and S3D).
Figure 3.
Bru1 and Cup form large complexes with cycA and cycB mRNAs in the nurse cell cytoplasm
(A) Co-visualization via STED of cycA and cycB mRNAs in nurse cells of a sectioned egg chamber at mid-oogenesis expressing Bru1-GFP and Cup-YFP. Images are laid out as a ‘punnett square’ for depicting colocalization combinations of RNAs and proteins. Colored dots indicate the individual channels merged per image. Images are deconvolved, XY max-intensity Z-projections of 3 optical slices (0.1 μm each). Scale bar, 1 μm.
(B–E) 2D-plots representing the distance to (μm - on the x axis) and the volume of (μm3-on the y axis) (B) Bru1-GFP and cycA mRNA, (C) Bru1-GFP and cycB mRNA, (D) Cup-YFP and cycA mRNA, and (E) Cup-YFP and cycB mRNA, respectively. The color spectrum of the heatmap indicates the distance to Cup-YFP (B, C) or to Bru1-GFP (D, E).
We quantified these associations, and generated 2D plots for all confocal image data, where the x axis represents the shortest distance between cycA or cycB mRNA particles to Bru1-GFP particles, and the y axis reflects mRNA particle volume, while the boxplots represent the distribution of the particles (Figures 3B and 3C). Based on the spatial distribution analyses and volumetric properties, two distinct populations of mRNAs were delineated. The first subset consisted of smaller mRNA puncta, which were positioned at a greater distance from Bru1-GFP, likely corresponding to individual mRNA molecules. In contrast, the second population consisted of larger mRNA assemblies, indicative of multiple mRNA molecules, that were closely associated with or entirely localized within Bru1-GFP condensates (Figures 3B and 3C). Quantitative analysis using boxplots revealed that the majority of these particles resided in close proximity to Bru1-GFP, with the median distance of cycA mRNA measured at 0.007 μm3 and cycB mRNA at 0.020 μm3 to Bru1-GFP. Furthermore, heatmap analysis indicated that this population exhibited a higher degree of colocalization with Cup-YFP as well, suggesting a functional relationship. Quantitative analysis of mRNA localization revealed a nearly equivalent distribution of mRNA particles within as well as outside of Bru1-GFP condensates (cycA mRNA – in: 49% vs. out: 51%, cycB mRNA – in: 46% vs. out: 54%) (Table 1). However, the volumetric analysis indicated a significant reduction in the size of mRNA particles that did not colocalize with Bru1-GFP, with a 61% decrease for cycA mRNA and a 65% decrease for cycB mRNA (Table 1). Analysis of cycA and cycB mRNAs in relation to Cup-YFP revealed patterns highly similar to those observed with Bru1-GFP (Figures 3D and 3E). Quantitative analysis using boxplots demonstrated that the majority of mRNA particles were localized in close proximity to Cup-YFP condensates, with median distances of 0.002 μm3 for cycA mRNA and 0.024 μm3 for cycB mRNA. Heatmap data further indicated that Bru1-GFP exhibited enhanced colocalization with mRNAs positioned nearer to Cup-YFP, suggesting a spatial coordination between these factors. Localization analysis showed an approximately equal distribution of mRNA particles within and outside of Cup-YFP condensates (cycA mRNA – in: 50% vs. out: 50%, and cycB mRNA – in: 45% vs. out: 55%). However, volumetric measurements revealed a marked reduction in the size of mRNA particles that did not colocalize with Cup-YFP, with a 61% decrease for cycA mRNA and a 62% decrease for cycB mRNA (Table 1), indicating that larger mRNA assemblies were preferentially enriched within Cup-containing condensates. Although the numbers of particles observed within and outside of Bru1-GFP and Cup-YFP–labeled structures were comparable, the volumes of particles within the condensates were approximately 3-fold greater. This suggests that a substantially larger proportion of mRNA was concentrated within P-bodies, indicating preferential localization or enrichment within these granules.
Table 1.
Particle volume analysis for cycA and cycB mRNAs
| Measurements | Cup-YFP | Bru1-GFP |
|---|---|---|
| cycA mRNA | ||
| Total inside mRNA particle count | 44558 | 43403 |
| Total outside mRNA particle count | 44871 | 46026 |
| Percent value inside (%) | 50% | 49% |
| Percent value outside (%) | 50% | 51% |
| Total volume of mRNA inside condensates (μm3) | 160 | 156 |
| Total volume of mRNA outside condensates (μm3) | 63 | 66 |
| Average volume of mRNA inside condensates (μm3) | 0.0036 | 0.0036 |
| Average volume of mRNA outside condensates (μm3) | 0.0014 | 0.0014 |
| % Decrease of mRNA average volume outside vs. inside condensate | 61% | 61% |
| cycB mRNA | ||
| Total inside mRNA particle count | 55031 | 55163 |
| Total outside mRNA particle count | 66116 | 65988 |
| Percent value inside (%) | 45% | 46% |
| Percent value outside (%) | 55% | 54% |
| Total volume of mRNA inside condensates (μm3) | 217 | 221 |
| Total volume of mRNA outside condensates (μm3) | 97 | 93 |
| Average volume of mRNA inside condensates (μm3) | 0.0039 | 0.004 |
| Average volume of mRNA outside condensates (μm3) | 0.0015 | 0.0014 |
| % Decrease of mRNA average volume outside vs. inside condensate | 62% | 65% |
Our mRNA particle analysis revealed that a subset of cycA and cycB mRNA puncta colocalized with one of the two proteins, as indicated by positions at or below 0 on the x axis. However, in the heatmaps for the alternate protein, some of these same mRNA particles appeared at greater distances — represented by red coloring. This suggests that there exists a population of mRNAs that colocalize with only one of the two proteins, but not both. It remains unclear whether the single mRNA puncta located outside of condensates represent a distinct subpopulation that never interacts with both Bru1-GFP and Cup-YFP, or whether they are transient intermediates en route to P-bodies. Given that smaller mRNA puncta frequently localized to the periphery of P-body foci, we propose that these mRNAs are actively transported toward larger condensates.
Bru1 and Cup are necessary for the formation of cycA and cycB mRNPs
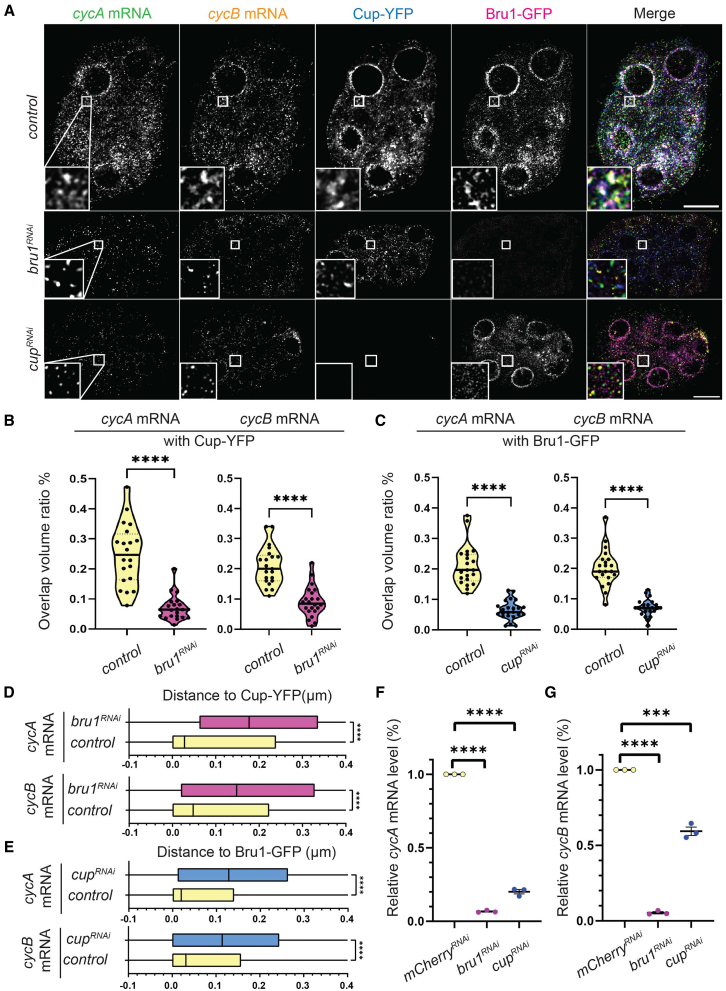
Given that the knockdown of either Bru1 or Cup led to ectopic expression of both CycA and CycB proteins, we next investigated the association of cycA and cycB mRNAs with either Bru1 or Cup in the absence of the other protein (Figure 4A). This allowed us to assess their respective roles in the formation of cytoplasmic mRNP complexes. We performed smFISH experiments, in combination with the RNAi-mediated knockdown of either protein. To ensure consistency, we quantified the colocalization of cycA and cycB mRNAs in egg chambers co-expressing Bru1-GFP and Cup-YFP at similar developmental stages, focusing on stages 2–4. We found that the knockdown of either Bru1 or Cup significantly diminished the association of the other protein with cycA and cycB mRNAs (Figure 4A). We previously demonstrated that the knockdown of either protein results in a reduction of the other, suggesting a mutual dependence in their stability or expression.27 Consequently, we opted to analyze the overlap ratios of mRNAs with these proteins to account for the decreased protein concentration within the egg chambers. This approach allowed for the assessment of mRNA-protein interactions under conditions of diminished protein availability. In the bru1RNAi egg chambers, the mean overlap ratio of the total particle volume of the cycA mRNA with Cup-YFP decreased by 74%, while in that of cycB mRNA it decreased by 58% (Figure 4B). Similarly, in cupRNAi, the mean overlap with Bru1-GFP was reduced by 70% for cycA mRNA and 63% for cycB mRNA (Figure 4C). We also performed analysis of the later developmental stages (stage 5–7) which revealed that Bru1-GFP and mRNA overlap was also severely reduced, with an 81% decrease for cycA mRNA and 74% for cycB mRNA (Figures S4A and S4B).
Figure 4.
Bru1 and Cup are necessary for the formation of cycA and cycB mRNPs
(A) Co-visualization of cycA and cycB mRNAs in stage 4 egg chambers expressing Bru1-GFP and Cup-YFP in the indicated backgrounds. White box indicates the location of the zoomed-in images. Images are deconvolved, XY max-intensity Z-projections of 5 optical slices (0.3 μm each). Scale bars, 10 μm.
(B) Analysis of the ratio of overlap volume for cycA and cycB mRNAs with Cup-YFP performed in control and bru1RNAi egg chambers (stage 2–4). Violin plots represent each individual point, and the median (line) and quartiles (dash lines). Statistics were calculated using Mann-Whitney statistical tests. (∗∗∗∗p < 0.0001; control vs. bru1RNAi: n = 20 and n = 22).
(C) Analysis of the ratio of overlap volume for cycA and cycB mRNAs with Bru1-GFP performed in control and cup1RNAi egg chambers (stage 2–4). Violin plots represent each individual point, and the median (line) and quartiles (dash lines). Statistics were calculated using Mann-Whitney statistical tests. (∗∗∗∗p < 0.0001; control vs. cup1RNAi: n = 21 and n = 23).
(D) Shortest distance analysis of cycA and cycB mRNAs with Cup-YFP performed in control and bru1RNAi egg chambers (stage 2–4). Boxplots provide a zoomed-in representation of the 25th to 75th percentile particle localization, with the median indicated by a vertical line. Statistics were calculated using Mann-Whitney statistical tests. (∗∗∗∗p < 0.0001; control vs. bru1RNAi, cycA mRNA: n = 39105 and n = 53913, cycB mRNA: n = 54036 and n = 14529).
(E) Shortest distance analysis of cycA and cycB mRNAs with Bru1-GFP performed in control and cup1RNAi egg chambers (stage 2–4). Boxplots provide a zoomed-in representation of the 25th to 75th percentile particle localization, with the median indicated by a vertical line. Statistics were calculated using Mann-Whitney statistical tests. (∗∗∗∗p < 0.0001; control vs. cup1RNAi, cycA mRNA: n = 39105 and n = 75524, cycB mRNA: n = 54036 and n = 76198).
(F) RT-qPCR quantification of endogenous cycA mRNA normalized to rp49 mRNA. Statistical significance was determined by unpaired, two-tailed Student’s t test (mean ± SEM; ∗∗∗∗p < 0.0001; mCherryRNAin = 3, cupRNAin = 3).
(G) RT-qPCR quantification of endogenous cycB mRNA normalized to rp49 mRNA. Statistical significance was determined by unpaired, two-tailed Student’s t test (mean ± SEM; ∗∗∗∗p < 0.0001; mCherryRNAin = 3, cupRNAin = 3).
To comprehensively characterize the alterations in mRNA-protein associations, we assessed colocalization through spatial distance measurements and volumetric analysis. Our findings revealed a significant shift in the minimal distance of cycA and cycB mRNA particles to Bru1-GFP and Cup-YFP in knockdown conditions. Specifically, in the bru1RNAi background, the median distance of cycA mRNA to Cup-YFP increased from 0.028 μm3 to 0.117 μm3, while for cycB mRNA it increased from 0.0477 μm3 to 0.148 μm3 compared to control (Figures 4D, S4C, and S4D). Concomitantly, we observed a substantial increase in the proportion of cycA and cycB mRNA particles localized outside Cup-YFP (control vs. bru1RNAi, for cycA mRNA: 55% vs. 86%, and for cycB mRNA: 59% vs. 79%) (Table 2). Additionally, the fold change increase of the mRNA volume localized inside vs. outside Cup-YFP was closely resembling the wildtype for cycA mRNA, but slight decreased for cycB mRNA (control vs. bru1RNAi, for cycA mRNA: 2.8 vs. 2.6-fold, and for cycB mRNA: 3.0 vs. 2.3-fold). Notably, cycA mRNA displayed a striking volumetric reduction from 0.0039 μm3 in control to 0.0011 μm3 in bru1RNAi inside Cup-YFP particles, and shrinking from 0.0014 μm3 to 0.0004 μm3, respectively, outside Cup-YFP. However, the mRNA volumes of cycB particles remained similar to the wild-type (control vs. bru1RNAi, in: 0.0038 μm3 vs. 0.0033 μm3, and out: 0.0013 μm3 vs. 0.0015 μm3). These findings suggest a potential differential regulation of cycA and cycB mRNAs by Bru1.
Table 2.
Particle volume analysis for cycA and cycB mRNAs in the knockdown backgrounds
| Measurements | Cup-YFP Stages 2-4 |
Bru1-GFP Stages 2-4 |
Bru1-GFP Stages 5-7 |
|||
|---|---|---|---|---|---|---|
| control | bru1RNAi | control | cupRNAi | control | cupRNAi | |
| cycA mRNA | ||||||
| mRNA particle inside condensate (%) | 45% | 14% | 45% | 23% | 51% | 29% |
| mRNA particle outside condensate (%) | 55% | 86% | 55% | 77% | 49% | 71% |
| Average volume of mRNA inside condensates (μm3) | 0.0039 | 0.0011 | 0.0038 | 0.0034 | 0.0035 | 0.0033 |
| Average volume of mRNA outside condensates (μm3) | 0.0014 | 0.0004 | 0.0015 | 0.0018 | 0.0014 | 0.0020 |
| Fold increase of mRNA volume inside vs. outside condensate | 2.8 | 2.6 | 2.5 | 1.9 | 2.5 | 1.7 |
| cycB mRNA | ||||||
| mRNA particle inside condensate (%) | 41% | 21% | 43% | 25% | 48% | 27% |
| mRNA particle outside condensate (%) | 59% | 79% | 57% | 75% | 52% | 73% |
| Average volume of mRNA inside condensates (μm3) | 0.0038 | 0.0033 | 0.0038 | 0.0036 | 0.0041 | 0.0041 |
| Average volume of mRNA outside condensates (μm3) | 0.0013 | 0.0015 | 0.0012 | 0.0018 | 0.0016 | 0.0022 |
| Fold increase of mRNA volume inside vs. outside condensate | 3.0 | 2.3 | 3.1 | 2.0 | 2.5 | 1.9 |
In cupRNAi egg chambers at stages 2–4, the median distance of cycA mRNA particles to Bru1-GFP increased from 0.0845 μm3 to 0.1750 μm3, while for cycB mRNA particles, it increased from 0.0312 μm3 to 0.1140 μm3 relative to control egg chambers (Figures 4E, S4E, and S4F). Similar to bru1RNAi conditions, a greater proportion of cycA and cycB mRNA particles were excluded from Bru1-GFP (control vs. cupRNAi, for cycA mRNA: 55% vs. 77%, and for cycB mRNA: 57% vs. 75%) (Table 2). Interestingly, in cupRNAi egg chambers, the fold change increase of the mRNA volume localized inside vs. outside Bru1-GFP was less pronounced compared to control (control vs. cupRNAi, for cycA mRNA: 2.5 vs. 1.9-fold, and for cycB mRNA: 3.1 vs. 2.0-fold) (Table 2). In cupRNAi egg chambers at stages 5–7, the median distance to Bru1-GFP increased from 0.000 μm3 to 0.092 μm3 for cycA mRNA, and from 0.0105 μm3 to 0.107 μm3 for cycB mRNA compared to control (Figures S4G and S4H). Consistent with the earlier developmental stages, a larger proportion of mRNA particles were localized outside Bru1-GFP (control vs. cupRNAi, for cycA mRNA: 49% vs. 71%, and for cycB mRNA: 52% vs. 73%). Moreover, the fold change increase of the mRNA volumes inside vs. outside Bru1-GFP indicated that the mRNA particles outside of Bru1-GFP were larger relative to control (control vs. cupRNAi for cycA mRNA: 2.5 vs. 1.7-fold, and for cycB mRNA: 2.5 vs. 1.9-fold). Collectively, these findings indicate that both Bru1 and Cup are essential for the association of cycA and cycB mRNAs with P-bodies, highlighting their role in mRNA sequestration and post-transcriptional regulation. Additionally, the overall mRNA levels for both the cycA and cycB transcripts were markedly reduced, as confirmed by RT-qPCR. Limited Cup expression led to a reduction of 80% in cycA mRNA and 40% in cycB mRNA levels (Figures 4F and 4G). The downregulation of Bru1 resulted in even more pronounced reductions, with a 93% decrease in cycA mRNA and a 95% decrease in cycB mRNA levels (Figures 4F and 4G). The pronounced reduction in mRNA levels could be attributed to two primary mechanisms. First, the formation of the silencing complex, particularly in the presence of Cup, is known to inhibit the mRNA degradation machinery. In the absence of Cup, this suppression is relieved, leading to accelerated mRNA degradation.24 Second, following mRNA translation, the canonical mRNA decay pathways are activated, contributing to the normal turnover of the transcript. Whether the observed reduction in mRNA levels was due to altered transcription, mRNA destabilization, or degradation after translation remains to be discerned in future studies. Altogether, these results demonstrate that both Bru1 and Cup are essential for establishing and/or maintaining the association between cycA and cycB mRNAs with the other component of the mRNP complex, as well as for preserving normal mRNA levels.
Re-entry into mitosis of Cup mutant egg chambers is rescued by Bru1 overexpression
In our analysis of knockdown egg chambers, mitosis was only observed at the beginning of stage 4 when both CycA and CycB were ectopically expressed, suggesting that the presence of both proteins is required for mitotic re-entry. cycB mRNA was rapidly derepressed, while cycA mRNA de-repression only occurred when the levels of both Bru1 and Cup were significantly reduced. This raised the question of whether the overexpression of Bru1 or Cup could rescue the mitotic phenotype. To test this hypothesis, we generated fly lines to assess mitosis in a cup mutant background in combination with Bru1 overexpression.
In contrast to the cupRNAi egg chambers, the cup mutants developed further (∼ stage 8), with approximately 70% of ovarioles displaying at least one mitotic event. The overexpression of UAS-Bru1-GFP alone did not cause a phenotype at these stages when using the matalpha4-GAL-VP16 driver. However, in the cup1/01355 mutant background, UAS-Bru1-GFP overexpression significantly reduced the number of ovarioles with mitotic spindle formation to just 9% (Figures 5A and 5B). These egg chambers also progressed further in oogenesis, with most culminating in the "cup" phenotype. This differed from cup mutants alone, where most egg chambers died before reaching later stages, with only a few ovarioles displaying the "cup" morphology (Figure S5A).
Figure 5.
Bru1 overexpression rescues mitotic re-entry of cup mutant egg chambers
(A) Visualization of ɑ-Tubulin via IF in stage 6 egg chambers, where UAS-bru1-GFP transgene is expressed in the indicated backgrounds. Membrane (WGA stain). Images are deconvolved, XY max-intensity Z-projections of 3 (wt), 3 (UAS-bru1-GFP), 3 (cup1/01355) and 4 (cup1/01355; UAS-bru1-GFP) optical slices (0.3 μm each). Scale bars, 20 μm.
(B) Analysis of mitotic spindle formation in cup1/01355 mutant egg chambers and in cup1/01355 mutant egg chambers expressing the UAS-Bru1-GFP transgene. Each data point represents the percentage of ovarioles containing at least one mitotic spindle formation event in cup1/01355 (n = 20) and cup1/01355; UAS-bru1-GFP (n = 21) ovaries. Statistics were calculated using Mann-Whitney statistical tests. (∗∗∗∗p < 0.0001).
(C) Visualization of CycB via IF in chains of egg chambers, where UAS-bru1-GFP transgene is expressed in the indicated backgrounds. Membrane (WGA stain). Images are deconvolved, XY max-intensity Z-projections of 5 (UAS-bru1-GFP), 6 (cup1/01355) and 6 (cup1/01355; UAS-bru1-GFP) optical slices (0.3 μm each). Scale bars, 50 μm.
(D) Visualization of CycB via IF in stage 7 egg chamber, where UAS-bru1-GFP transgene is expressed in cup1/01355 mutant background. Images are deconvolved, XY max-intensity Z-projections 4 optical slices (0.3 μm each). Scale bar, 10 μm.
To determine whether this rescue is related to changes in aberrant Cyclin expression, we assessed the ectopic expression of CycB. Although ectopic CycB protein was still detected with UAS-Bru1-GFP overexpression, its levels were reduced (Figure 5C). It is of note that the UAS-Gal4 system is known for generating mosaic transgene expression. This results in variable levels of Bru1-GFP across nurse cells. Strikingly, we observed an inverse relationship between Bru1-GFP and CycB expression: nurse cells with higher Bru1-GFP levels exhibited lower CycB expression, while those with lower Bru1-GFP levels showed increased CycB expression (Figures 5C and 5D). This suggests that Bru1 plays a role in the translational repression of cycB mRNA, despite the lack of detectable direct binding via UV crosslinking.7
Bru1 and Cup mediate accumulation of cycA and cycB mRNAs with Me31B
We found that cycA and cycB mRNAs associate with Bru1 and Cup within large cytoplasmic condensates, and such interactions are critical for the transcripts’ stability and translational repression. Both Bru1 and Cup are known components of P-bodies, membraneless cytoplasmic organelles, where mRNAs are stored in a translationally repressed state. Me31B, another core P-body component, forms a complex with Cup and is implicated in mRNA translational repression. A global analysis of early embryos revealed that Me31B associates with the majority of maternal mRNAs.35,36,37,38
This led us to further explore the link between cycA and cycB mRNAs and Me31B. Using STED microscopy, we assessed the colocalization of these transcripts in egg chambers co-expressing Me31B-GFP and Cup-YFP, and found that larger mRNA puncta accumulated within Me31B/Cup-bodies, while single mRNA copies were not associated with either protein (Figures 6A and S6A), mimicking the results with Cup-YFP alone (Figure S3D). To confirm their localization in P-bodies, we performed combined smFISH-IF experiments, additionally detecting Bru1 with cycA/cycB mRNPs in sectioned tissue. As anticipated, all three proteins (Me31B, Cup, and Bru1) and both mRNAs colocalized in large cytoplasmic complexes, suggesting that cycA and cycB mRNAs were indeed recruited and stored in P-bodies (Figures 6B and S6B).
Figure 6.
cycA and cycB mRNAs accumulate in Me31B-bodies in a Bru1-and Cup-dependent manner
(A) Co-visualization of cycA and cycB mRNAs with smFISH via STED in the nurse cell of a sectioned egg chamber at mid-oogenesis expressing Cup-YFP and Me31B-GFP. Colored dots indicate individual channels merged per image. Images are deconvolved, XY max-intensity Z-projections of 3 optical slices (0.1 μm each). Scale bar 1μm.
(B) Co-visualization of cycA and cycB mRNAs via smFISH and Bru1 protein via IF in the nurse cell of a sectioned egg chamber at mid-oogenesis, expressing Cup-YFP and Me31B-GFP. Colored dots indicate the channels merged in the images. Images are deconvolved, XY max-intensity Z-projections of 5 optical slices (0.2 μm each). Scale bar 1 μm.
(C) Co-visualization of cycA and cycB mRNAs via smFISH in the nurse cell of stage 5 egg chamber expressing Me31B-GFP in indicated RNAi backgrounds. Images are deconvolved, XY max-intensity Z-projections of 4 (control), 5 (bru1RNAi) and 5 (cupRNAi) optical slices (0.3 μm each). Scale bars, 1 μm.
(D) Analysis of average shortest distance for cycA and cycB mRNAs with Me31B-GFP performed in control, bru1RNAi and cupRNAi egg chambers (stage 2–4). Violin plots represent each individual point, and the median (line) and quartiles (dash lines). Statistics were calculated using Mann-Whitney statistical tests. (cycA: ∗∗∗∗p < 0.0001, cycB: ∗∗∗p = 0.0001 (bru1RNAi), ∗∗∗p = 0.0006 (cupRNAi); control (n = 21), bru1RNAi (n = 20) and cupRNAi (n = 21)).
This finding prompted us to investigate why cycA and cycB mRNAs, despite their association with Me31B, underwent premature protein expression and mRNA degradation in the absence of Bru1 or Cup. We presumed that Me31B alone would be insufficient to maintain translational repression. To address this, we knocked down Bru1 or Cup in egg chambers expressing Me31B-GFP and evaluated the colocalization of cycA and cycB mRNAs with Me31B-GFP (Figures 6C, S6C, and S6D).
To further characterize colocalization dynamics, we conducted spatial distance measurements. Our results demonstrated a significant increase in the distance between cycA or cycB mRNAs and Me31B-GFP in both bru1RNAi and cupRNAi backgrounds, suggesting a disruption in their association with P-bodies. Specifically, the average distance of cycA mRNA particles to Me31B-GFP increased by 59% in bru1RNAi and by 50% in cupRNAi egg chambers, accompanied by a notable shift in the overall distribution of detected particles (Figures 6D and S7A–S7C). Similarly, for cycB mRNA, the distance to Me31B-GFP increased by 39% in bru1RNAi and by 32% in cupRNAi egg chambers (Figures 6D and S7D–S7F).
To gain a more comprehensive understanding of how mRNA-P-body interactions are affected, we also analyzed volume overlap ratios. Our data reveal a substantial reduction in the colocalization of cycA mRNA with Me31B, decreasing by 69% in bru1RNAi and by 48% in cupRNAi egg chambers. Similarly, cycB mRNA overlap with Me31B decreased by 48% in bru1RNAi and by 38% in cupRNAi egg chambers (Figure 6D). These findings indicate that while cycA and cycB mRNAs are recruited to Me31B-labeled P-bodies, their stable localization within these structures is critically dependent on both Bru1 and Cup, underscoring their essential roles in mRNA sequestration and post-transcriptional regulation.
Differential translational regulation of cycA and cycB mRNAs by Me31B
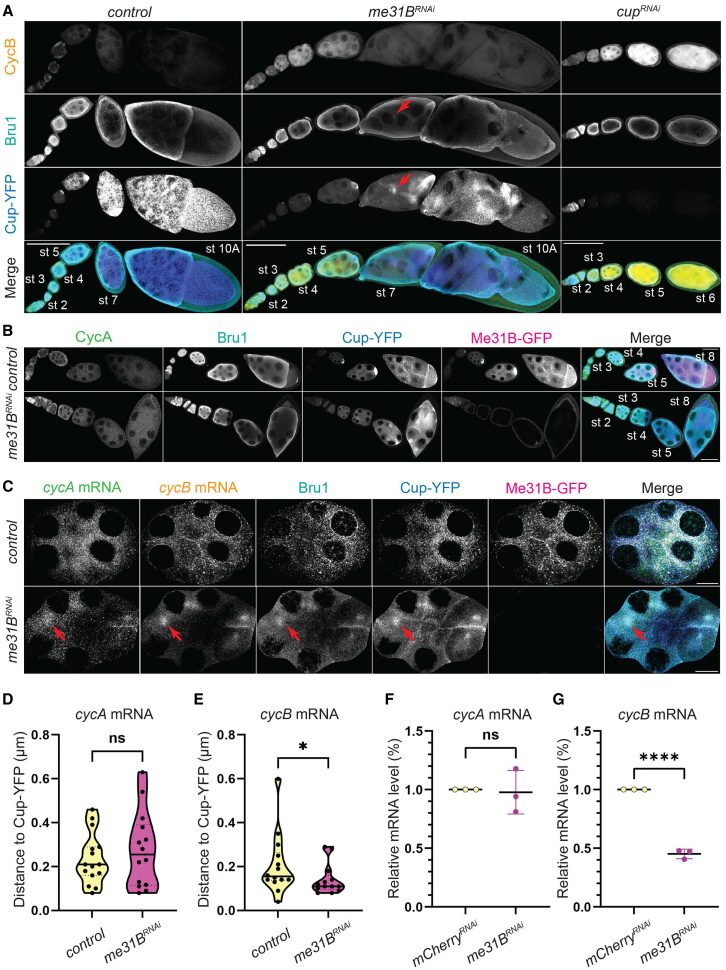
Given the severe reduction in cycA and cycB mRNA association with Me31B-GFP in Bru1- and Cup-depleted egg chambers, we questioned whether this diminished association drives the ectopic expression of CycA and CycB proteins. To address this, we knocked down Me31B in egg chambers expressing Me31B-GFP and Cup-YFP and assessed CycA and CycB expression. Using the same Gal4 driver as in the Bru1 and Cup knockdowns, we observed that these egg chambers progressed to stage 10 without mitotic spindle formation, but exhibited a disrupted actin cytoskeleton and contained multiple nurse cell nuclei within a shared cytoplasm (Figures 7A and S8A – red arrow, red arrowheads). Me31B knockdown caused cycB mRNA derepression during early oogenesis, lasting until stage 6, though this derepression was less pronounced than in cupRNAi egg chambers (Figure 7A). Cup-YFP and Bru1 levels were slightly reduced, and their cytoplasmic localization was altered, accumulating in distinct subcellular regions without forming the characteristic P-body condensates (Figures S8B – red arrows and S8C – 3D Surface plots). Due to limited antibody penetration, Bru1 could not be fully visualized at stage 10 without sectioning the egg chambers, but some redistribution was detectable at stage 7 (Figure 7A – red arrow). Remarkably, no ectopic CycA protein expression was observed following Me31B knockdown (Figure 7B). These findings suggest that Me31B is not essential for maintaining the translational repression of cycA mRNA, but plays a role in cycB mRNA repression, potentially through the regulation of Cup and Bru1 expression levels and cytoplasmic localization.
Figure 7.
Me31B differentially regulates the translation of cycA and cycB mRNAs, but their association with Cup-YFP is independent of Me31B
(A) Visualization of CycB and Bru1 via IF in chains of egg chambers expressing Cup-YFP in the indicated backgrounds. Accumulation of Bru1 and Cup (red arrows). Images are deconvolved, XY max-intensity Z-projections of 15 (control), 13 (me31BRNAi) and 16 (cupRNAi) optical slices (1 μm each). Scale bars, 100 μm.
(B) Visualization of CycA and Bru1 via IF in chains of egg chambers expressing Cup-YFP and Me31B-GFP in the indicated backgrounds. Images are deconvolved, XY max-intensity Z-projections of 6 (control) and 6 (me31BRNAi) optical slices (1 μm each). Scale bars, 50 μm.
(C) Co-visualization of cycA and cycB mRNAs and Bru1 protein via smFISH and IF in a sectioned mid-oogenesis egg chamber expressing Cup-YFP and Me31B-GFP in control and me31BRNAi backgrounds. Accumulation of cycA mRNA, cycB mRNA, Bru1 and Cup (red arrows). Images are deconvolved, XY max-intensity Z-projections of 5 (control) and 9 (me31BRNAi) optical slices (0.2 μm each). Scale bars, 10 μm.
(D) Shortest distance analysis of cycA mRNA to Cup-YFP was performed in control and me31BRNAi backgrounds in stages 6–8 egg chambers. Violin plots represent each individual point, and the median (line) and quartiles (dash lines). Statistics were calculated using Mann-Whitney statistical tests. (NS; control n = 15, me31BRNAin = 14).
(E) Shortest distance analysis of cycB mRNA to Cup-YFP was performed in control and me31BRNAi backgrounds in stages 6–8 egg chambers. Violin plots represent each individual point, and the median (line) and quartiles (dash lines). Statistics were calculated using Mann-Whitney statistical tests. (∗p = 0.049; control n = 14, me31BRNAin = 13).
(F) RT-qPCR quantification of endogenous cycA mRNA normalized to rp49 mRNA. Statistical significance was determined by unpaired, two-tailed Student’s t test (mean ± SEM; NS; mCherryRNAin = 3, me31BRNAin = 3).
(G) RT-qPCR quantification of endogenous cycB mRNA normalized to rp49 mRNA. Statistical significance was determined by unpaired, two-tailed Student’s t test (mean ± SEM; ∗∗∗∗p < 0.0001; mCherryRNAin = 3, me31BRNAin = 3).
cycA and cycB mRNAs association with Cup-YFP is independent of Me31B
Given the absence of detectable CycA and only limited ectopic CycB protein in me31BRNAi egg chambers, we hypothesized that the mRNP complex retained its integrity, thereby maintaining translational repression of both transcripts. To test this, we analyzed the colocalization of cycA and cycB mRNAs with Cup-YFP and Bru1 in me31BRNAi egg chambers. We conducted smFISH-IF experiments on sectioned egg chambers to overcome the limitations posed by antibody penetration. Our results revealed that Bru1, Cup-YFP, and cycA and cycB mRNAs exhibited an altered cytoplasmic distribution, no longer forming large cytoplasmic condensates, yet they accumulated together in subregions within the cytoplasm (Figures 7C-red arrows and S8B – 3D Surface plots). In some cases, these accumulations were observed to occur near the ring canals, likely due to impaired mRNA transport between nurse cells that results from the breakdown of the actin cytoskeleton (Figure S8B – red arrows). Further analysis of the egg chambers beyond stage 7, showed that the association between cycA mRNA and Cup-YFP was not significantly altered (Figure 7D). Interestingly, the observed distance between cycB mRNA particles and Cup particles decreased, indicating that their association was not only preserved, but became more tightly coupled (Figure 7E). To determine whether Me31B plays a role in maintaining wild-type transcript levels of cycA and cycB mRNAs, we performed RT-qPCR. While cycA mRNA levels remained unchanged (Figure 7F), cycB mRNA levels showed a significant reduction (55%) (Figure 7G). These findings suggest that although cycA mRNA accumulated with Me31B, this interaction was not essential for its post-transcriptional regulation. In contrast, cycB mRNA stability appeared to depend on P-bodies for protection from degradation, though P-bodies likely serve to fine-tune its translational repression, which is primarily mediated by Bru1 and Cup.
Discussion
The precise regulation of the cell cycle relies on the proper expression and degradation of Cyclins, including CycA and CycB, two proteins essential for the initiation and progression of mitosis.2 Aberrant expression of either protein can result in developmental arrest and cancer, as tumor-associated mutations often target CDK/Cyclin complexes leading to uncontrolled cell division. Thus, it is critical to deepen our understanding of how these mRNAs are regulated.
We found that the knockdown of either Bru1 or Cup in the Drosophila germline led to the ectopic expression of both CycA and CycB proteins during oogenesis. However, while the CycB protein was detected immediately following the knockdown of either Bru1 or Cup, robust CycA expression was only observed beginning at stage 4, which coincides with the re-entry into mitosis in the knockdown egg chambers. This suggests that both proteins must reach a threshold to initiate mitotic division, or alternatively, that CycA is necessary for mitosis while CycB alone is insufficient. The latter model is more plausible, as previous studies have shown that CycA-deficient embryos that accumulate CycB still fail to enter mitosis.39 Another possible explanation for the onset of mitosis at stage 4 is that nurse cells, at this stage, exit the endocycle and their chromosomes undergo partial mitotic chromatin condensation, possibly rendering the nurse cells more susceptible to mitotic re-entry.5 These explanations are not mutually exclusive and may likely contribute together to the observed phenomenon. Our findings reveal that while both CycA and CycB proteins were ectopically expressed in the absence of Bru1 and Cup, their distinct expression patterns suggest differential regulatory mechanisms governing their mRNAs.
For cycB mRNA, the alternative splicing of its 3′ UTR is a likely regulatory mechanism, resulting in the expression of a short and long isoform. The short transcript is expressed during early oogenesis (<stages 6/7), when Bru1 levels are high and cycB mRNA is translationally repressed, while the long transcript is expressed during late oogenesis (>stages 9), when Bru1 is no longer detectable.4,7 Our analysis, conducted prior to the expression of the long transcript, confirmed that Bru1 colocalizes with the short isoform of cycB mRNA. Interestingly, despite the absence of canonical BREs in the cycB transcript we still observed a clear colocalization between Bru1 and cycB mRNA. This suggests that Bru1 may recognize and bind cycB mRNA through non-canonical sites or an indirect mechanism, highlighting a potentially broader role for Bru1 in cycB mRNA regulation.
Two models have been proposed to explain cycB mRNA translational de-repression in the bru1 mutant. The first posits that CycA expression triggers the CycA/CDK1 complex, which subsequently relieves the inhibition of CycB, making CycB protein expression a secondary effect of CycA expression rather than being directly regulated by Bru1. However, we find this model unlikely, as the CycB protein consistently appears in earlier stages of oogenesis than CycA. We favor the second model, which suggests that Bru1 regulates cycB mRNA in a BRE-independent manner, similar to its regulation of germ cell-less (gcl) mRNA.40 Whether this interaction is direct or mediated by another protein, potentially Cup, remains unclear.
Further investigation is required to determine whether the alternative splicing of the 3′ UTR affects Bru1/Cup binding by altering mRNA folding or if the longer UTR recruits a protein that inhibits Bru1/Cup-mediated repression or eliminates their binding. Additionally, since Bru1 has been implicated in splicing regulation in muscle tissue, it would be intriguing to investigate whether Bru1 also regulates cycB mRNA splicing—specifically, whether it promotes the expression of the shorter 3′ UTR isoform during stages of oogenesis when Bru1 protein is present and CycB is not. Moreover, it would be valuable to explore whether the splicing of other Bru1 target mRNAs is altered in cupRNAi egg chambers, where Bru1 accumulates in large condensates inside the nurse cell nuclei.41,42
A key piece of evidence supporting Bru1’s role in cycB mRNA translational regulation is the suppression of CycB protein expression in cup mutants when Bru1 is overexpressed. While the underlying mechanism remains unclear, it is possible that in cup mutant egg chambers, where Cup levels are significantly reduced but not entirely absent, Bru1 overexpression increases the formation of silencing particles, thereby rescuing egg chamber development. Alternatively, in the complete absence of Cup, elevated Bru1 levels may promote Bru1 dimerization, resulting in the formation of silencing complexes, similar to those observed in oskar mRNA translation repression.43 This notion is further substantiated by recent findings that Bru1—through its prion-like domains—promotes phase separation, potentially serving as a key mechanism in the formation and organization of silencing particles.44
How Bru1 is recruited to cycB mRNA remains an unresolved question, and we propose several potential mechanisms for this interaction. First, Cup, which is known to directly bind mRNAs, could act as a mediator, facilitating the recruitment of Bru1 to cycB mRNA.45 Second, Bru1 may bind cycB mRNA in a BRE-independent manner, similar to its interaction with gcl mRNA.40 Third, Bru1 could be recruited to cycB mRNA through interaction with another protein, and in cup mutants —where Bru1 levels are reduced— only the overexpression of Bru1 may provide sufficient levels to restore cycB mRNA repression. While these models suggest plausible mechanisms for cycB mRNA regulation, the precise pathway remains to be clarified through future studies.
In the embryo, cycB mRNA is translationally repressed through the binding of Pumilio and Nanos to the 3′ UTR, with Nanos being the essential factor. Nanos recruits the CCR4-NOT complex specifically in germ cells, where cycB mRNA is deadenylated, but not degraded. Additionally, Nanos directly interacts with Cup, colocalizing in both the germarium and the primordial germ cells of the embryo.46,47 Nanos levels are high in the germarium, but rapidly decrease as the egg chamber exits this region.48 Interestingly, both Nanos and Cup exert their translational repression by recruiting the CCR4-NOT complex and are involved in the sequential regulation of another mRNA, pgc mRNA, during oogenesis. Before differentiation in the germarium, Pumilio and Nanos repress pgc mRNA, while after differentiation this role is taken over by Bru1 and Cup.28 Whether cycB mRNA undergoes similar regulation remains to be determined, yet we believe this mechanism is highly probable. Supporting this hypothesis, in the embryo, cycB mRNA regulation requires not only Nanos, but also an Oskar-dependent factor, which we predict could be Cup.46
The formation of the translationally repressive cycB mRNP is essential not only for Drosophila development, but also for Xenopus, zebrafish, and mouse oocyte development, underscoring its evolutionary conservation.49,50 The canonical model for this regulation involves a 3′ UTR-binding protein recruiting an eIF4E-binding protein, which in turn inhibits the formation of the translational initiation complex. This mechanism has been demonstrated for numerous mRNAs, particularly in germ cell mRNPs.51,52 Additionally, cycB1 mRNA translational control is regulated through the assembly and disassembly of mRNA granules in zebrafish and mouse oocytes.49 These mRNPs can subsequently form higher-order complexes via LLPS, contributing to the formation of P-bodies.15,53,54
P-bodies, where mRNPs accumulate, are cytoplasmic sites for mRNA storage and translational repression.15,19,53 Both Bru1 and Cup are known to localize within P-bodies, but whether their accumulation into P-bodies is essential for maintaining mRNAs in a translationally repressed state remains controversial.12,27,54 Recent research has shown that cyclic mRNAs dynamically accumulate in P-bodies during the cell cycle, with mRNAs in P-bodies being uncoupled from their cytoplasmic expression, suggesting cell cycle-dependent targeting of mRNAs to P-bodies.55
Through our study, we show that cycA and cycB mRNAs are both regulated by Bru1 and Cup and both accumulate in P-bodies, but exhibit differential regulation. For cycA mRNA, maintaining the integrity of the primary mRNP complex is essential for its post-transcriptional regulation, independent of P-body association. In contrast, cycB mRNA regulation is more complex: translational repression is not fully sustained, and transcript levels are significantly reduced in Me31B knockdowns. These findings suggest that cycB mRNA recruitment to P-bodies is important for its regulation. However, since the changes in expression were not as pronounced as in the Bru1 or Cup knockdown egg chambers, P-body recruitment likely serves to fine-tune cycB mRNA translational regulation, while maintaining the integrity of the primary mRNP complex is key. The exact mechanism behind this differential regulation remains unknown, but identifying the variations in mRNP composition will likely provide crucial insights. Finally, given our recent discovery that ER exit sites contribute to P-body organization in the case of Me31B, but not for Cup, it would be intriguing to explore the role of ER exit sites in mediating the association of cyclin mRNAs with Me31B or Cup.56
We highlight that the formation of the mRNP complex is critical for maintaining the repression and stability of both cycA and cycB mRNAs. While P-body accumulation is not required for cycA mRNA regulation, it is important for proper cycB mRNA regulation. This finding underscores the complexity of P-body involvement in mRNA regulation and suggests there is much more to learn about their roles. Future research should aim to uncover the mechanisms behind this differential regulation, which could provide broader insights into P-body functions or P-body subclasses. Our results show that P-bodies modulate mRNA regulation in a transcript-specific manner rather than through a uniform mechanism.
Limitations of the study
Imaging analysis is a powerful tool for detecting larger macromolecular complexes; however, the resolution of image acquisition imposes inherent limitations on the detection of smaller assemblies. Our findings suggest that a subset of cycA and/or cycB mRNAs colocalize independently with either Bru1 or Cup. However, accurately quantifying the proportion of these colocalization events remains challenging at this stage. Additionally, due to early oogenesis arrest of bru1RNAi egg chambers, we are unable to analyze beyond stage 4 of oogenesis and, therefore, cannot expand our model of mRNA regulation occurring throughout later stages.
Resource availability
Lead contact
Requests for further information and resources should be directed to and will be fulfilled by the lead contact, Diana P. Bratu (bratu@genectr.hunter.cuny.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This study does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We thank Dr. A. Nakamura (Riken Center for Developmental Biology) and Dr. M. Lilly (NIH) for the kind gifts of antibodies, as well as Dr. P.M. Macdonald (The University of Texas at Austin) for the kind gift of an antibody and the D. melanogaster line. We thank the BDSC Indiana, VDCR Vienna, and DGRC Kyoto for providing D. melanogaster lines, as well as the TRiP at Harvard Medical School (NIH/NIGMS RO1-GM084947) for the transgenic RNAi stocks. We thank undergraduate student Daniela Villafuerte for her assistance with fly husbandry and the maintenance of stocks. We thank the Bioimaging Facility at Hunter College for access to the Leica TCS SP8 and Imaris Image Analysis Software. We thank Dr. P. Feinstein (Hunter College) for allowing us to use the Roche LightCycler instrument. This work was supported by the National Institutes of Health (1SC1GM135132) and the National Science Foundation (1919829) awards to D.P.B.
Author contributions
Conceptualization, L.V.B. and D.P.B.; methodology, L.V.B. and D.P.B.; validation, L.V.B.; formal analysis, L.V.B.; investigation, L.V.B., S.N.M., H.K., and ZK; data curation, L.V.B. and D.P.B.; writing – original draft, L.V.B.; writing – review and editing, L.V.B., S.N.M., and D.P.B.; supervision, D.P.B.; project administration, D.P.B.; and funding acquisition, D.P.B.
Declaration of interests
The authors declare no competing interests.
Declaration of generative AI and AI-assisted technologies
During the preparation of this manuscript, the authors used ChatGPT 4o in order to improve the language and its readability. After using this tool, the authors reviewed and edited the content as needed, and are taking full responsibility for the content of the publication.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse α-CycA | Deposited to the DSHB (Developmental Studies Hybridoma Bank) by Lehner, C.F. | Cat#A12 |
| Mouse α-CycB | Deposited to the DSHB (Developmental Studies Hybridoma Bank) by O’Farrell, P.H. | Cat#F2F4 |
| Mouse α-Cup | Gift from Dr. A. Nakamura (Institute of Molecular Embryology and Genetics, Kumamoto University) | Nakamura, A. et al.57 |
| Mouse α-Me31B | Gift from Dr. A. Nakamura (Institute of Molecular Embryology and Genetics, Kumamoto University) | Nakamura, A. et al.58 |
| Rabbit α-Bruno 1 | Gift from Dr. M. Lilly (NIH- National Institutes of Child Health and Development) | Sugimura,I. et al.7 |
| Rabbit α-Bruno 1 | Gift from Dr. P.M. Macdonald (The University of Texas at Austin) | Webster,P.J. et al.59 |
| Mouse α-Tubulin | Deposited to the DSHB by Walsh, C. | Cat#AA4.3-s |
| Mouse α-Tubulin | Deposited to the DSHB by Frankel, J./Nelsen, E.M | Cat#12G10 |
| Rabbit α-Tri-methyl-Histone | Cell Signaling Technology | Cat#C42D8 |
| Chemicals, peptides, and recombinant proteins | ||
| Phalloidin Alexa Fluor 647 | Life Technologies | Cat#A22287 |
| Wheat germ agglutinin CFⓇ405S | Biotium | Cat# 29027-1 |
| Wheat germ agglutinin CFⓇ555 | Biotium | Cat#29076-1 |
| Dylight 550 goat anti-Mouse | ThermoFisher | Cat#84540 |
| Dylight 650 goat anti-Mouse | ThermoFisher | Cat#84545 |
| Dylight 650 goat anti-Rabbit | ThermoFisher | Cat#84546 |
| ProLongTM Diamond Antifade Mountant | ThermoFisher | Cat#P36970 |
| RapiClear 1.47 | SUNJin Lab | Cat#RC147001 |
| Aqua-Poly/Mount | Polysciences | Cat#18606-20 |
| O.C.T. Compound | Tissue-Tek | Cat#25608-930 |
| Laemmli Sample Buffer | Bio-Rad | Cat#FB2399 |
| TruBlot ULTRA Anti-Mouse Ig HRP | Rockland | Cat#18-8817-31 |
| TruBlot ULTRA Anti-Rabbit Ig HRP | Rockland | Cat#18-8816-31 |
| SuperSignal West Femto Maximum Sensitivity Substrate | ThermoFisher | Cat#34095 |
| TRIzol | ThermoFisher | Cat#15596018 |
| SuperScript IV | Life Technologies | Cat#11766050 |
| PowerTrack SYBR Green Master Mix | Roche Molecular Systems, Inc. | Cat#A46110 |
| Experimental models: Organisms/strains | ||
| D. melanogaster: RNAi of mCherry y[1] sc[∗] v[1] sev[21]; P{y[+t7.7] v[+t1.8]=VALIUM20-mCherry.RNAi}attP2 | Bloomington Drosophila Stock Center | BL #35785 |
| D. melanogaster: me31B-GFP y[1] w[∗]; P{w[+mC]=PTT-GB}me31B[CB05282] | Bloomington Drosophila Stock Center | BL #51530 |
| D. melanogaster: bru1-GFP y[1] w[67c23]; Mi{PT-GFSTF.1}bru1[MI00135-GFSTF.1] | Bloomington Drosophila Stock Center | BL #60144 |
| D. melanogaster: tub-a(V37)-Gal4 w[∗]; P{w[+mC]=matalpha4-GAL-VP16}V37 | Bloomington Drosophila Stock Center | BL #7063 |
| D. melanogaster: RNAi of Cup y[1] sc[∗] v[1] sev[21]; P{y[+t7.7] v[+t1.8]=TRiP.GL00327}attP2/TM3, Sb[1] | Bloomington Drosophila Stock Center | BL #35406 |
| D. melanogaster: RNAi of Bru1 y[1] sc[∗] v[1] sev[21]; P{y[+t7.7] v[+t1.8]=TRiP.GL00314}attP2 | Bloomington Drosophila Stock Center | BL #35394 |
| D. melanogaster: RNAi of Bru1 y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.HMJ21531}attP40 | Bloomington Drosophila Stock Center | BL #54812 |
| D. melanogaster: RNAi of Me31B y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.GL00695}attP40/CyO | Bloomington Drosophila Stock Center | BL #38923 |
| D. melanogaster: RNAi of Me31B y[1] sc[∗] v[1] sev[21]; P{y[+t7.7] v[+t1.8]=TRiP.HMS00539}attP2 | Bloomington Drosophila Stock Center | BL#33675 |
|
D. melanogaster: cup01355 P{ry[+t7.2]=PZ}cup[01355] cn[1]/CyO; ry[506] |
Bloomington Drosophila Stock Center | BL #12218 |
| D. melanogaster: cup1 cup[1] cn[1] bw[1] speck[1]/CyO, l(2)DTS513[1] | Bloomington Drosophila Stock Center | BL #4978 |
| D. melanogaster: cup-YFP w[1118]; PBac{681.P.FSVS-1}cup[CPTI001409] | Kyoto Drosophila Stock Center | DGRC 115-161 |
| D. melanogaster: UAS-bru1-GFP | Gift from Dr. P.M. Macdonald (The University of Texas at Austin) | Snee, M., et al.60 |
| Oligonucleotides | ||
| Primer: CycA Forward ′GTCACACCCACAAAACGGCA′ | This paper | N/A |
| Primer: CycA Reversed ′GCTGCTGGTGCTCATCCTCT′ | This paper | N/A |
| Primer: CycB Forward ′CGAGGACGAGCACCATACGA′ | This paper | N/A |
| Primer: CycB Reversed ′AGTGCAGCGACAGGAACAGT′ | This paper | N/A |
| cycA mRNA smFISH probe | This paper | Table S1 |
| cycB mRNA smFISH probe | This paper | Table S1 |
| Software and algorithms | ||
| Imaris image analysis software | Oxford Instruments | https://imaris.oxinst.com/ |
| Fiji | Rueden et al.61 | https://imagej.nih.gov/ij/ |
| Schindelin et al.62 | ||
| LAS-X | Leica | NA |
| Prism8 | Graphpad | http://www.graphpad.com/ |
| BioRender | NA | https://www.biorender.com/ |
Experimental model and study participant details
Drosophila stocks
Drosophila melanogaster stocks were maintained on standard cornmeal agar food at 25°C. Prior to dissection, female flies were fed yeast paste in grape vials for 2-5 days. Fly stocks were obtained from Bloomington Drosophila Stock Center. Gal4-inducible TRiP (RNAi) lines: mCherry (BL #35785), cup (BL #35406), bru1 (BL #35394 and BL #54812), me31B (BL #38923 and BL#33675). Mutant lines: cup01355 (BL #12218) and cup1 (BL #4978). Maternal Gal4 driver line matalpha4-GAL-VP16 (V37) (BL#7063) was used throughout the study. Fluorescently tagged lines: bru1-GFP (BL #60144) and me31B-GFP (BL #51530). We acquired cup-YFP (DGRC 115-161) from the Kyoto Drosophila Stock Center. UAS-bru1-GFP fly line - generous gift from Dr. P.M. Macdonald (The University of Texas at Austin).
Method details
Sectioning of Drosophila egg chambers
Ovaries were dissected into 2% PFA in PBS and fixed for 10 min. They were washed 3 x 10 min in PBST and 1 x 5 min in 0.1 M glycine pH 3, and stored in 30% sucrose overnight at 4°C. The following day, they were flash frozen in O.C.T Compound (Tissue-Tek) before 25 μm sections were prepared on a cryotome.
Immunofluorescence
Ovaries were dissected and fixed in 2% PFA in PBS for 10 min. Egg chambers were washed 3 x 5 min in PBST (0.3% Triton X-100), permeabilized and blocked for 2 hours in PBS with 1% Triton X-100 and 1% BSA, and incubated with primary antibodies overnight at room temperature with rocking. Followed by 3 x 15 min washes in PBST and incubation with fluorescently labeled secondary antibodies (1:1000; DyLight 550 and DyLight 650; ThermoFisher Scientific). Followed by 3 x 15 min washes in PBST and mounting in ProLong Diamond Antifade Mountant (Life Technologies) or in a mixture of 75:25 RapiClear 1.47 (SUNJin Lab):Aqua-Poly/Mount (Polysciences) for clearing the egg chambers. Antibodies used: mouse anti-CycA A12 -deposited to the DSHB by Lehner, C.F. (DSHB Hybridoma Product A12) (1:100); mouse anti-CycB F2F4 -deposited to the DSHB by O’Farrell, P.H. (DSHB Hybridoma Product F2F4) (1:100); mouse anti-Cup (1:1000) and mouse anti-Me31B (1:1000) - gifts from Dr. A. Nakamura (Institute of Molecular Embryology and Genetics, Kumamoto University); rabbit-anti-Bruno 1 (1:5,000) - gift from Dr. M. Lilly (National Institutes of Child Health and Development, NIH); rabbit-anti-Bruno 1 (1:4,000) - gift from Dr. P.M. Macdonald (The University of Texas at Austin); F-actin stain: Phalloidin AlexaFluor 647 (1:500; Life Technologies); nuclear membrane stain: wheat germ agglutinin CFⓇ405S and CFⓇ555 (1:400; Biotium). Actin and nuclear membrane stains were added together with the secondary antibody incubation.
Tubulin staining
Ovaries were dissected into BRB80 (0.5 M K-PIPES pH 6.8, 2 M MgCl2, and 0.5 M K-EGTA) and permeabilized in 1% Triton X-100 in BRB80 without rocking. They were rinsed in BRB80 and fixed with 2% PFA for 10 min. Egg chambers were washed 3 x 10 min in PBST followed by 1 x 10 min in 2X SSC 10% formamide, and incubated overnight in mouse anti-ɑ-Tubulin AA4.3-s deposited to the DSHB by Walsh, C. and anti-ɑ-Tubulin 12G10 deposited to the DSHB by Frankel, J. / Nelsen, E.M (1:100; Developmental Studies Hybridoma Bank). Egg chambers were washed 3 x 10 min followed by 2 hours incubation in a fluorescently labeled secondary antibody (1:1000; DyLight 550 or DyLight 650; ThermoFisher Scientific). Egg chambers were washed 3 x 20 min in PBST and were fixed again in 2% PFA, washed 3 x 5 min in PBST and mounted, as described above.
Single-molecule RNA FISH (smFISH) in ovaries
smFISH were performed as previously described,63 with the following modifications. Ovaries were dissected and fixed in 2% PFA in PBS for 10 min. Egg chambers were washed 3 x 5 min in PBST (0.3% Triton X-100). After fixation, egg chambers were pre-hybridized in wash buffer (2X SSC, 10% formamide) and incubated with the cycA-570 and cycB-610 probes (1:100) (Supplemental_Methods_S1) overnight at 37°C, followed by washing 3 x 10 min in wash buffer and mounting with ProLongTM Diamond Antifade Mountant (Life Technologies) on a glass slide using a #1.5 coverslip glass.
Single smFISH and combined smFISH-IF in sectioned egg chambers
Sectioned egg chambers were washed and re-hybridized in PBS 2 x 10 min and in 2X SSC for 10 min followed by pre-hybridization in wash buffer (2X SSC, 10% formamide) for 10 min. Sections were incubated with the cycA-570 and cycB-610 probes (Supplemental_Methods_S1) for 8 hours or overnight at 37°C, followed by washing with wash buffer and mounting with ProLongTM Diamond Antifade Mountant (Life Technologies) on a glass slide using a #1.5 cover glass. Combined smFISH-IF was carried out following 8 hours of incubation with the smFISH probes. Sections were washed in the wash buffer for 10 min and then 0.05% Triton X-100 in 2X SSC for 10 min. Followed by incubation with primary antibodies overnight at room temperature in 0.05% Triton X-100, 0.2% BSA, 2X SSC. After 3 x 10 min washes with 0.05% Triton X-100 in 2X SSC, the egg chambers were incubated with fluorescently labeled secondary antibodies (1:1000; DyLight 550 or DyLight 650; ThermoFisher Scientific) for 2 hours at room temperature in 0.05% Triton X-100, 0.2% BSA, 2X SSC, and were washed 3 x 10 min with 0.05% Triton X-100 in 2X SSC and mounted with ProLongTM Diamond Antifade Mountant (Life Technologies) on a glass slide using a #1.5 coverslip glass.
Microscopy
All imaging was performed on a TCS SP8 Laser Scanning Microscope (Leica Microsystems) equipped with a white light laser (470-670 nm), a solid-state laser (405 nm), and a STED 660 nm CW high intensity laser. For all laser scanning confocal imaging, the HyD detectors were used in photon counting mode, with 40X/1.4 and 63X/1.4 oil objectives. For all STED imaging, the 100X/1.4 oil objective was used, optical Z slices were 0.1 μm, and zoom was set at 6X. STED samples were prepared from 25 μm ovary sections. Optical sections were acquired using an automated XYZ-piezo stage. Acquisition software: Leica LAS-X. Images were merged using the smooth option with Leica’s ‘Image merger’.
Western blot analysis
Ten ovaries for each genotype were dissected directly into 95 μL of 2X Laemmli Sample Buffer (Bio-Rad) supplemented with 5 μl of BME and immediately mechanically lysed. They were heated at 95°C for 10 min and centrifuged for 10 min at 10,000 RCF at 25°C. Lysates were loaded onto a 10% acrylamide gel. Primary antibodies used: mouse anti-CycA (1:100), mouse anti-CycB (1: 100), and rabbit anti-Tri-methyl-Histone (C42D8) (1:150,000; Cell Signaling Technology). Bands visualized using TrueBlot ULTRA secondary antibodies: anti-mouse and anti-rabbit Ig HRP (1:50,000; Rockland) with SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific).
RNA isolation and RT-qPCR
Whole ovaries were dissected into 4°C PBS. Ovaries were mechanically lysed in TRIzol (ThermoFisher Scientific) to extract total RNA. RNA was washed with ethanol and eluted in RNAse-free water. Reverse Transcriptase reactions using 2.5 μg total RNA were performed using Superscript IV kit (Life Technologies). Primers were designed using DRSC FlyPrimerBank and made by Integrated DNA Technologies (STAR Methods). RT-qPCR was performed with a Roche Lightcycler 480 (Roche Molecular Systems, Inc.). Each reaction contained 1 μl of cDNA, 4 μl of 10 μM primers, and 5 μl of PowerTrack™ SYBR Green Master Mix (ThermoFisher Scientific).
Quantification and statistical analysis
Statistical analyses results can be found in figure legends and results section.
Imaging analysis
Identical image acquisition using laser confocal microscopy was used for the control, as well as each experimental slide. For all analysis, images were taken from 3 slides each prepared from an independent fly cross. All image files were saved as 16-bit data files after deconvolution with Leica’s ‘Lightning’ module. Figure images were processed using Fiji/ImageJ (NIH).61,62 Quantification was performed using Imaris Microscopy Image Analysis software (Oxford Instruments). Imaris’ ‘Surface Detection’ module was used to identify and segment the thresholded signal and create the objects. Once the objects are identified, Imaris can analyze the surface-to-surface overlap to determine the ratio of the total overlap volume, and calculate the distance between the objects and assess the shortest distance (we considered the objects as ‘colocalized’ for values of ≤ 0). Thresholding and sensitivity were carefully determined and the mean value of each image was used for the statistical calculations. Statistics were calculated using Mann-Whitney statistical tests with Prism v8 (GraphPad Software) and results can be found in each figure legend.
RT-qPCR analysis
Data are represented as mean +/- SEM. Statistical significance was determined by unpaired, two-tailed Student’s t-test and results can be found in each figure legend. Data analyses were performed with Prism v8 (GraphPad Software).
Published: May 21, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.112727.
Supplemental information
References
- 1.Pluta A.J., Studniarek C., Murphy S., Norbury C.J. Cyclin-dependent kinases: Masters of the eukaryotic universe. Wiley Interdiscip. Rev. RNA. 2023;15 doi: 10.1002/wrna.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malumbres M., Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 3.Ye C., Wang J., Wu P., Li X., Chai Y. Prognostic role of cyclin B1 in solid tumors: a meta-analysis. Oncotarget. 2017;8:2224–2232. doi: 10.18632/oncotarget.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalby B., Glover D.M. 3’ non-translated sequences in Drosophilacyclin B transcripts direct posterior pole accumulation late in oogenesis and peri-nuclear association in syncytial embryos. Development. 1992;115:989–997. doi: 10.1242/dev.115.4.989. [DOI] [PubMed] [Google Scholar]
- 5.Reed B.H., Orr-Weaver T.L. The Drosophila gene morula inhibits mitotic functions in the endo cell cycle and the mitotic cell cycle. Development. 1997;124:3543–3553. doi: 10.1242/dev.124.18.3543. [DOI] [PubMed] [Google Scholar]
- 6.Lilly M.A., de Cuevas M., Spradling A.C. Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev. Biol. 2000;218:53–63. doi: 10.1006/dbio.1999.9570. [DOI] [PubMed] [Google Scholar]
- 7.Sugimura I., Lilly M.A. Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev. Cell. 2006;10:127–135. doi: 10.1016/j.devcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Chiappetta A., Liao J., Tian S., Trcek T. Structural and functional organization of germ plasm condensates. Biochem. J. 2022;479:2477–2495. doi: 10.1042/bcj20210815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin J.M., Bratu D.P. Drosophila melanogaster Oogenesis: An Overview. Methods Mol. Biol. 2015;1328:1–20. doi: 10.1007/978-1-4939-2851-4_1. [DOI] [PubMed] [Google Scholar]
- 10.Hinnant T.D., Merkle J.A., Ables E.T. Coordinating Proliferation, Polarity, and Cell Fate in the Drosophila Female Germline. Front. Cell Dev. Biol. 2020;8:19. doi: 10.3389/fcell.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little S.C., Sinsimer K.S., Lee J.J., Wieschaus E.F., Gavis E.R. Independent and coordinate trafficking of single Drosophila germ plasm mRNAs. Nat. Cell Biol. 2015;17:558–568. doi: 10.1038/ncb3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura A., Sato K., Hanyu-Nakamura K. Drosophila Cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell. 2004;6:69–78. doi: 10.1016/s1534-5807(03)00400-3. [DOI] [PubMed] [Google Scholar]
- 13.Bayer L.V., Milano S.N., Bratu D.P. The mRNA dynamics underpinning translational control mechanisms of Drosophila melanogaster oogenesis. Biochem. Soc. Trans. 2024;52:2087–2099. doi: 10.1042/bst20231293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banani S.F., Lee H.O., Hyman A.A., Rosen M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y., Na Z., Slavoff S.A. P-Bodies: Composition, Properties, and Functions. Biochemistry. 2018;57:2424–2431. doi: 10.1021/acs.biochem.7b01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittag T., Parker R. Multiple Modes of Protein-Protein Interactions Promote RNP Granule Assembly. J. Mol. Biol. 2018;430:4636–4649. doi: 10.1016/j.jmb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borcherds W., Bremer A., Borgia M.B., Mittag T. How do intrinsically disordered protein regions encode a driving force for liquid-liquid phase separation? Curr. Opin. Struct. Biol. 2021;67:41–50. doi: 10.1016/j.sbi.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putnam A., Thomas L., Seydoux G. RNA granules: functional compartments or incidental condensates? Genes Dev. 2023;37:354–376. doi: 10.1101/gad.350518.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubstenberger A., Courel M., Bénard M., Souquere S., Ernoult-Lange M., Chouaib R., Yi Z., Morlot J.B., Munier A., Fradet M., et al. P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol. Cell. 2017;68:144–157.e5. doi: 10.1016/j.molcel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Trcek T., Lehmann R. Germ granules in Drosophila. Traffic. 2019;20:650–660. doi: 10.1111/tra.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benoit B., Mitou G., Chartier A., Temme C., Zaessinger S., Wahle E., Busseau I., Simonelig M. An essential cytoplasmic function for the nuclear poly(A) binding protein, PABP2, in poly(A) tail length control and early development in Drosophila. Dev. Cell. 2005;9:511–522. doi: 10.1016/j.devcel.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Chen C.Y.A., Shyu A.B. Deadenylation and P-bodies. Adv. Exp. Med. Biol. 2013;768:183–195. doi: 10.1007/978-1-4614-5107-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris J.Z., Hong A., Lilly M.A., Lehmann R. twin, a CCR4 homolog, regulates cyclin poly(A) tail length to permit Drosophila oogenesis. Development. 2005;132:1165–1174. doi: 10.1242/dev.01672. [DOI] [PubMed] [Google Scholar]
- 24.Igreja C., Izaurralde E. CUP promotes deadenylation and inhibits decapping of mRNA targets. Genes Dev. 2011;25:1955–1967. doi: 10.1101/gad.17136311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temme C., Simonelig M., Wahle E. Deadenylation of mRNA by the CCR4-NOT complex in Drosophila: molecular and developmental aspects. Front. Genet. 2014;5:143. doi: 10.3389/fgene.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalabi Hagkarim N., Grand R.J. The Regulatory Properties of the Ccr4-Not Complex. Cells. 2020;9:2379. doi: 10.3390/cells9112379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayer L.V., Milano S., Formel S.K., Kaur H., Ravichandran R., Cambeiro J.A., Slinko L., Catrina I.E., Bratu D.P. Cup is essential for oskar mRNA translational repression during early Drosophila oogenesis. RNA Biol. 2023;20:573–587. doi: 10.1080/15476286.2023.2242650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flora P., Wong-Deyrup S.W., Martin E.T., Palumbo R.J., Nasrallah M., Oligney A., Blatt P., Patel D., Fuchs G., Rangan P. Sequential Regulation of Maternal mRNAs through a Conserved cis-Acting Element in Their 3' UTRs. Cell Rep. 2018;25:3828–3843.e9. doi: 10.1016/j.celrep.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D., Wang Q., Huang H., Xia L., Jiang X., Kan L., Sun Q., Chen D. Effete-mediated degradation of Cyclin A is essential for the maintenance of germline stem cells in Drosophila. Development. 2009;136:4133–4142. doi: 10.1242/dev.039032. [DOI] [PubMed] [Google Scholar]
- 30.Vardy L., Pesin J.A., Orr-Weaver T.L. Regulation of Cyclin A protein in meiosis and early embryogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:1838–1843. doi: 10.1073/pnas.0813237106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashevsky H., Wallace J.A., Reed B.H., Lai C., Hayashi-Hagihara A., Orr-Weaver T.L. The anaphase promoting complex/cyclosome is required during development for modified cell cycles. Proc. Natl. Acad. Sci. USA. 2002;99:11217–11222. doi: 10.1073/pnas.172391099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bansal P., Madlung J., Schaaf K., Macek B., Bono F. An Interaction Network of RNA-Binding Proteins Involved in Drosophila Oogenesis. Mol. Cell. Proteomics. 2020;19:1485–1502. doi: 10.1074/mcp.RA119.001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sladewski T.E., Billington N., Ali M.Y., Bookwalter C.S., Lu H., Krementsova E.B., Schroer T.A., Trybus K.M. Recruitment of two dyneins to an mRNA-dependent Bicaudal D transport complex. eLife. 2018;7 doi: 10.7554/eLife.36306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClintock M.A., Dix C.I., Johnson C.M., McLaughlin S.H., Maizels R.J., Hoang H.T., Bullock S.L. RNA-directed activation of cytoplasmic dynein-1 in reconstituted transport RNPs. eLife. 2018;7 doi: 10.7554/eLife.36312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao M. Me31B: a key repressor in germline regulation and beyond. Biosci. Rep. 2024;44 doi: 10.1042/bsr20231769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kara E., McCambridge A., Proffer M., Dilts C., Pumnea B., Eshak J., Smith K.A., Fielder I., Doyle D.A., Ortega B.M., et al. Mutational analysis of the functional motifs of the DEAD-box RNA helicase Me31B/DDX6 in Drosophila germline development. FEBS Lett. 2023;597:1848–1867. doi: 10.1002/1873-3468.14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M., Ly M., Lugowski A., Laver J.D., Lipshitz H.D., Smibert C.A., Rissland O.S. ME31B globally represses maternal mRNAs by two distinct mechanisms during the Drosophila maternal-to-zygotic transition. eLife. 2017;6 doi: 10.7554/eLife.27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura A., Amikura R., Hanyu K., Kobayashi S. Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development. 2001;128:3233–3242. doi: 10.1242/dev.128.17.3233. [DOI] [PubMed] [Google Scholar]
- 39.Lehner C.F., O'Farrell P.H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore J., Han H., Lasko P. Bruno negatively regulates germ cell-less expression in a BRE-independent manner. Mech. Dev. 2009;126:503–516. doi: 10.1016/j.mod.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Spletter M.L., Barz C., Yeroslaviz A., Schönbauer C., Ferreira I.R.S., Sarov M., Gerlach D., Stark A., Habermann B.H., Schnorrer F. The RNA-binding protein Arrest (Bruno) regulates alternative splicing to enable myofibril maturation in Drosophila flight muscle. EMBO Rep. 2015;16:178–191. doi: 10.15252/embr.201439791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikonova E., DeCata J., Canela M., Barz C., Esser A., Bouterwek J., Roy A., Gensler H., Heß M., Straub T., et al. Bruno 1/CELF regulates splicing and cytoskeleton dynamics to ensure correct sarcomere assembly in Drosophila flight muscles. PLoS Biol. 2024;22 doi: 10.1371/journal.pbio.3002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chekulaeva M., Hentze M.W., Ephrussi A. Bruno acts as a dual repressor of oskar translation, promoting mRNA oligomerization and formation of silencing particles. Cell. 2006;124:521–533. doi: 10.1016/j.cell.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Bose M., Lampe M., Mahamid J., Ephrussi A. Liquid-to-solid phase transition of oskar ribonucleoprotein granules is essential for their function in Drosophila embryonic development. Cell. 2022;185:1308–1324.e23. doi: 10.1016/j.cell.2022.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pekovic F., Rammelt C., Kubíková J., Metz J., Jeske M., Wahle E. RNA binding proteins Smaug and Cup induce CCR4-NOT-dependent deadenylation of the nanos mRNA in a reconstituted system. Nucleic Acids Res. 2023;51:3950–3970. doi: 10.1093/nar/gkad159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadyrova L.Y., Habara Y., Lee T.H., Wharton R.P. Translational control of maternal cyclin B mRNA by Nanos in the Drosophila germline. Development. 2007;134:1519–1527. doi: 10.1242/dev.002212. [DOI] [PubMed] [Google Scholar]
- 47.Bhandari D., Raisch T., Weichenrieder O., Jonas S., Izaurralde E. Structural basis for the Nanos-mediated recruitment of the CCR4-NOT complex and translational repression. Genes Dev. 2014;28:888–901. doi: 10.1101/gad.237289.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forbes A., Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development (Camb.) 1998;125:679–690. doi: 10.1101/gad.237289.113. [DOI] [PubMed] [Google Scholar]
- 49.Kotani T., Yasuda K., Ota R., Yamashita M. Cyclin B1 mRNA translation is temporally controlled through formation and disassembly of RNA granules. J. Cell Biol. 2013;202:1041–1055. doi: 10.1083/jcb.201302139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stebbins-Boaz B., Cao Q., de Moor C.H., Mendez R., Richter J.D. Maskin is a CPEB-associated factor that transiently interacts with elF-4E. Mol. Cell. 1999;4:1017–1027. doi: 10.1016/s1097-2765(00)80230-0. [DOI] [PubMed] [Google Scholar]
- 51.Huggins H.P., Keiper B.D. Regulation of Germ Cell mRNPs by eIF4E:4EIP Complexes: Multiple Mechanisms, One Goal. Front. Cell Dev. Biol. 2020;8:562. doi: 10.3389/fcell.2020.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter J.D., Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 53.Standart N., Weil D. Cytosolic Droplets for Coordinated mRNA Storage. Trends Genet. 2018;34:612–626. doi: 10.1016/j.tig.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Wilby E.L., Weil T.T. Relating the Biogenesis and Function of P Bodies in Drosophila to Human Disease. Genes. 2023;14:1675. doi: 10.3390/genes14091675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safieddine A., Benassy M.N., Bonte T., Slimani F., Pourcelot O., Kress M., Ernoult-Lange M., Courel M., Coleno E., Imbert A., et al. Cell-cycle-dependent mRNA localization in P-bodies. Mol. Cell. 2024;84:4191–4208.e7. doi: 10.1016/j.molcel.2024.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Milano S.N., Bayer L.V., Ko J.J., Casella C.E., Bratu D.P. The role of ER exit sites in maintaining P-body organization and integrity during Drosophila melanogaster oogenesis. EMBO Rep. 2025;26:494–520. doi: 10.1038/s44319-024-00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura A., Sato K., Hanyu-Nakamura K. Drosophila Cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell. 2004;6:69–78. doi: 10.1016/s1534-5807(03)00400-3. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura A., Amikura R., Hanyu K., Kobayashi S. Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development. 2001;128:3233–3242. doi: 10.1242/dev.128.17.3233. [DOI] [PubMed] [Google Scholar]
- 59.Webster P.J., Liang L., Berg C.A., Lasko P., Macdonald P.M. Translational repressor Bruno plays multiple roles in development and is widely conserved. Genes Dev. 1997;11:2510–2521. doi: 10.1101/gad.11.19.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snee M., Benz D.,, Jen J., Macdonald P.M. Two distinct domains of Bruno bind specifically to the oskar mRNA. RNA Biol. 2008;5:1–9. doi: 10.4161/rna.5.1.5735. [DOI] [PubMed] [Google Scholar]
- 61.Rueden C.T., Schindelin J., Hiner M.C., DeZonia B.E., Walter A.E., Arena E.T., Eliceiri K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bayer L.V., Batish M., Formel S.K., Bratu D.P. Single-Molecule RNA In Situ Hybridization (smFISH) and Immunofluorescence (IF) in the Drosophila egg chamber. Methods Mol. Biol. 2015;1328:125–136. doi: 10.1007/978-1-4939-2851-4_9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This study does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.