Abstract
Background
Chinese patent drugs, standardized formulations rooted in traditional Chinese medicine, have gained attention for their potential to induce programmed cell death (PCD) in cancer cells. Emerging evidence suggests that these formulations may affect multiple PCD pathways, including apoptosis, autophagy, ferroptosis, pyroptosis, and necroptosis, thus offering a multifaceted approach to tumor suppression.
Objectives
This study aimed to map the global research landscape on Chinese patent drugs in cancer-related PCD, examining publication trends, principal contributors, and thematic evolutions. The analysis also sought to provide insights that could guide future investigations and clinical applications.
Methods
Bibliometric data were extracted from the Web of Science Core Collection (1998–2023), focusing on articles investigating Chinese patent drugs and PCD in oncological contexts. R-bibliometrix was used for descriptive statistics and trend analyses, while VOSviewer generated network visualizations of co‐occurring keywords, collaboration patterns, and co‐citation clusters.
Results
Overall publication output increased markedly, with China leading in both volume and impact. Collaboration networks revealed extensive international partnerships, underscoring global interest in standardized herbal formulations. Keyword mapping highlighted a shift from early apoptosis-centric studies to more diverse regulated cell‐death pathways, indicating greater mechanistic depth and exploration of synergistic effects with conventional therapies.
Conclusion
Chinese patent drugs are increasingly recognized as promising agents for modulating PCD in cancer cells. Ongoing work focuses on standardized manufacturing, robust clinical validation, and mechanistic elucidation. These trends position Chinese patent drugs at a pivotal juncture for advancing integrative oncology and enhancing patient outcomes.
Keywords: Programmed cell death, Apoptosis, Cancer, Multi-target therapy, Chinese patent drug, Integrative oncology
Introduction
Programmed cell death (PCD) encompasses an array of tightly regulated mechanisms—most notably apoptosis, autophagy, ferroptosis, necroptosis, and pyroptosis—that collectively govern cellular turnover and tissue homeostasis [1–4]. In cancer biology, these pathways have drawn considerable attention because their targeted modulation may halt tumor proliferation, disrupt metastasis, and sensitize cancer cells to existing therapies. Over the past few decades, traditional Chinese medicine (TCM) has shown promise in leveraging bioactive compounds to induce or enhance PCD in malignant cells [5–9]. Of particular interest are Chinese patent drugs (CPDs)—State-approved, standardised formulations of traditional prescriptions that are manufactured under Good Manufacturing Practice (GMP), assigned a unique National Medical Products Administration (NMPA) approval number, and usually supplied as ready-to-use pills, capsules or granules, thereby facilitating dose control and pharmacovigilance within modern regulatory frameworks. Although they combine classical herbal prescriptions with standardized manufacturing, questions remain regarding the underlying mechanistic complexities, dosage standardization, and safety profiles [10–15]. Early investigations of these formulations often focused on apoptosis, recognized as the most canonical form of PCD; however, recent advances highlight the possibility that Chinese patent drugs also engage alternative pathways such as autophagy, ferroptosis, and necroptosis [16–21]. This broadening scope underscores a multifaceted landscape where single formulations can influence numerous molecular targets [22–28]. While those narrative reviews summarise mechanistic details, no dedicated bibliometric synthesis has yet mapped the overall landscape of CPD-induced PCD in oncology. A quantitative bibliometric approach can therefore reveal knowledge hubs, collaboration gaps and emerging themes that traditional reviews may overlook. From an integrative oncology perspective, many clinicians and researchers are increasingly drawn to these compounds as adjuncts or even primary therapies, particularly where conventional regimens face limitations due to resistance or toxicity issues.
Concurrent with this rising interest is the need for rigorous analysis of publication trends, collaboration patterns, and thematic evolutions in the literature. Bibliometric approaches, particularly when supported by robust analytical platforms like VOSviewer and R-bibliometrix, enable a structured mapping of large bibliographic datasets. By extracting metadata from comprehensive databases such as the Web of Science Core Collection, researchers can generate network visualizations of co‐cited articles, keyword co‐occurrences, and geographic collaboration clusters [29–33]. These methods illuminate not only the leading contributors—be they individual scholars, institutions, or countries—but also the central topics that frame this expanding field. A bibliometric perspective thus transcends simple publication counts, providing insight into how research directions are shaped, which core journals and disciplinary fields converge here, and what emergent trends guide present or future inquiry. Since 1998, there has been a noticeable upswing in scientific outputs linking Chinese patent drugs to various PCD pathways in cancer cells. The underlying rationale for investigating these treatments stems from longstanding ethnopharmacological knowledge—handed down through centuries of clinical practice—and the corresponding modernization of TCM. These formulations aim to balance multiple active components, each potentially addressing different molecular hallmarks of cancer. For instance, certain Chinese patent drugs are hypothesized to target NF‐κB signaling, while others affect oxidative stress or specific epigenetic regulators. However, without systematic evaluation, results from individual studies risk being scattered or even contradictory [7, 13, 34, 35]. Moreover, the heterogeneous composition of TCM formulas complicates replication efforts and fosters debates on standardization. To address these issues, contemporary research has increasingly turned to advanced technologies such as genomics and proteomics. These tools reveal that TCM formulations can induce PCD through multiple pathways simultaneously—possibly yielding synergistic effects alongside standard chemotherapy or radiotherapy. Yet verifying these claims calls for high‐throughput screening, consistent manufacturing methods, and clear regulatory guidelines to ensure reproducibility.
An equally critical dimension is the global diffusion of this research. While China leads in publications and historically underpins TCM, other regions—particularly the United States, Europe, Australia, and parts of Asia—have contributed meaningfully through cross-institutional collaborations. Joint projects can bring specialized expertise to bear, whether in pharmacokinetics, immunology, or advanced molecular imaging. This cross‐pollination is evident in multi‐author articles that align ethnopharmacological approaches with Western biomedical paradigms. Over time, the integration of these worldviews has facilitated a shift in scientific discourse: from anecdotal claims toward more empirically rigorous, controlled investigations. VOSviewer‐generated co‐citation and collaboration networks further indicate that an increasing number of researchers are exploring how Chinese patent drugs modulate cell cycle regulation, angiogenesis, and the tumor microenvironment in ways that either complement or enhance conventional therapies. Still, the field must surmount hurdles related to quality assurance, standardization, and acceptance by international regulatory bodies. Critics often question the variability in herbal content, potential drug–drug interactions, and the absence of large‐scale, randomized clinical trials that adhere to global standards. The application of both R‐bibliometrix and VOSviewer in this study broadens analytical capacity. R‐bibliometrix excels in offering customizable statistical analyses of publication patterns, while VOSviewer’s visual maps distill complex relational data into interpretable clusters. Such insights are invaluable for new entrants to the field, established researchers planning collaborative projects, and policy‐makers or funders aiming to channel resources effectively.
Altogether, this introduction underscores the significance of methodical bibliometric assessment in shining light on Chinese patent drugs that induce PCD in cancer cells. The interplay between TCM tradition and cutting-edge biomedical techniques is already reshaping therapeutic paradigms. Yet the path forward hinges on robust scientific validation, transparent collaboration, and consistent regulatory oversight. By synthesizing the existing literature—from the earliest publications in the late 1990 s to the prolific outputs of recent years—this article provides an evidence‐based narrative of how interest in these compounds has evolved, how global actors are collaborating, and how new technologies catalyze deeper mechanistic revelations.
Research objectives
The present study was designed to chart the developmental trajectory of research on Chinese patent drugs that promote programmed cell death in cancer cells, drawing upon a comprehensive bibliometric analysis to reveal publication trends, principal contributors, and thematic shifts. By combining data from the Web of Science Core Collection with analytical tools such as VOSviewer and R-bibliometrix, we aim to generate a nuanced view of global collaboration networks, keyword co‐occurrences, and evolving research themes.
Methods
Data source and overall framework
All bibliometric data were retrieved from the Web of Science Core Collection (WOSCC), chosen for its comprehensive coverage of high-quality scholarly outputs across multiple disciplines. We targeted the timespan from 1998 to 2023 because a preliminary scoping search showed that the first Web-of-Science-indexed article linking a CPD to cancer-related PCD appeared in 1998, whereas 1995–1997 returned zero records; we therefore focused on publications addressing “Chinese patent drug” interventions intended to induce programmed cell death in cancer cells. The underlying rationale for this timeframe was to capture the evolutionary trajectory of research activity, as reflected in Table 1, which documents publication counts per year. Prior to formal data retrieval, we determined the core concepts—namely, (1) Chinese patent drug–related terms, (2) regulated cell death processes, and (3) oncology‐associated terminology—to ensure that the resulting dataset would be both relevant and sufficiently broad. We exported all retrieved records (including authors, titles, abstracts, keywords, and references) in plain‐text format for subsequent processing. This overarching data‐acquisition framework was designed to accommodate detailed screening and to streamline the subsequent bibliometric procedures described in Sect. 2.4. To maintain uniformity and minimize discrepancies due to database updates, the final download and dataset lock occurred on a single day. After confirming data integrity, we compiled a consolidated repository of references suitable for advanced bibliometric analyses. This repository provides the empirical foundation for assessing publication trends, exploring the international scope of research collaborations, and examining the intellectual progression of Chinese patent drug applications targeting cancer cell death pathways.
Table 1.
Annual number of publications on Chinese patent drugs promoting programmed cell death in Cancer cells (1998–2024)
| Year | Volume of publication |
|---|---|
| 1998 | 2 |
| 1999 | 6 |
| 2000 | 5 |
| 2001 | 7 |
| 2002 | 12 |
| 2003 | 9 |
| 2004 | 14 |
| 2005 | 24 |
| 2006 | 24 |
| 2007 | 28 |
| 2008 | 44 |
| 2009 | 54 |
| 2010 | 72 |
| 2011 | 77 |
| 2012 | 87 |
| 2013 | 134 |
| 2014 | 141 |
| 2015 | 163 |
| 2016 | 202 |
| 2017 | 228 |
| 2018 | 260 |
| 2019 | 300 |
| 2020 | 336 |
| 2021 | 404 |
| 2022 | 473 |
| 2023 | 416 |
| 2024 | 551 |
| Add | 4073 |
Retrieval strategy and search formula
A meticulous retrieval strategy was established using the “Topic Search” function in WOSCC to capture all publications pertinent to Chinese patent drugs that promote programmed cell death in cancer cells. We employed a Boolean combination of synonyms for “Chinese patent drug,” regulated cell death mechanisms, and cancer-related terms, ensuring the search was inclusive of diverse terminological variations (e.g., “Chinese patent drug” OR “Chinese proprietary medicine” OR “traditional Chinese medicine,” coupled with apoptosis, autophagy, ferroptosis, and other emerging forms of cell death). The final search expression was:
TS=((“Chinese patent drug” OR “Chinese patent medicine” OR “Chinese proprietary medicine” OR “Chinese proprietary drug” OR “Proprietary Chinese medicine” OR Zhongchengyao OR “traditional Chinese medicine” OR TCM OR “Chinese herbal medicine” OR CHM OR “Chinese herbal formula” OR “Chinese herbal remedy” OR “Chinese herbal compound”) AND (“programmed cell death” OR “regulated cell death” OR “cell death” OR apoptosis OR autophag OR ferroptos OR pyroptos OR necroptos OR anoikis OR cuproptosis) AND (cancer OR tumor OR tumour OR neoplas OR carcinoma OR malignan OR oncolog OR sarcoma OR adenocarcinoma OR leukemia OR lymphoma OR myeloma OR glioma OR metastat)).
All items matching this criterion between 1998 and 2023 were extracted in a single batch. Implementing advanced filtering options (e.g., limiting document types to articles and reviews, refining language to English) minimized the presence of nonrelevant records and enhanced the consistency of the metadata fields. The entire retrieval process was anchored to one execution date to prevent database drift. Only English-language records were retained to ensure consistency in subsequent text-mining, and emerging modalities such as cuproptosis and anoikis were deliberately kept in the string to capture the earliest evidence of these nascent pathways, even though they yielded comparatively few hits. This systematic approach—grounded in a carefully formulated query and a clearly defined temporal range—produced the raw dataset that underpins the subsequent screening and analysis steps.
Literature screening and inclusion/exclusion
Following data retrieval, an extensive literature-screening protocol was adopted to refine the initial corpus. First, duplicates and incomplete records were identified and removed. Two authors each performed an independent preliminary screen by scanning titles and keywords to eliminate obviously irrelevant items (Patents, conference abstracts, or studies unrelated to the intersection of Chinese patent drugs, programmed cell death, and oncology). Next, two additional authors reviewed the abstracts and full texts, applying explicit inclusion criteria: (1) the paper had to involve Chinese patent drug or related TCM formulations, (2) it must discuss some regulated cell death pathway such as apoptosis or autophagy in cancer contexts, and (3) it should report original findings, systematic reviews, or meta‐analyses contributing novel insights into this domain. Studies focusing solely on chemical synthesis without reference to cell death mechanisms or papers on non‐oncological indications were excluded. Two authors who do not overlap with the above four conducted a final review, re‐examining the chosen articles to confirm their thematic relevance and ensuring no essential documents were omitted. Through this tiered selection procedure, we arrived at a well‐defined dataset that reconciles breadth of coverage with topical specificity. The resulting corpus of publications was consolidated into a single file, marking the final set of references used for all subsequent bibliometric assessments.
Data analysis and visualization
This study employed R-bibliometrix and VOSviewer for a multifaceted bibliometric and network analysis workflow. This study employed R-bibliometrix (version 4.3.3) and VOSviewer (version 1.6.20) for a multifaceted bibliometric and network analysis workflow. R‐bibliometrix enabled automated data cleaning, deduplication verification, and descriptive statistical summaries, including the frequency of publications across years, authorship patterns, and keyword distributions. Meanwhile, VOSviewer facilitated a nuanced examination of co‐occurrence networks (keywords, authors, and cited references), co‐citation clusters, and country‐level collaboration diagrams. To ensure internal consistency, the author and affiliation fields were standardized and cross‐checked, mitigating biases arising from variations in naming conventions or data entry errors. We additionally implemented standardized term‐merging protocols for the synonyms of Chinese patent drugs and regulated cell death processes, thus avoiding artificial fragmentation of key concepts. Through these integrative approaches, we illuminated the intellectual structure of this research domain and identified pivotal areas of inquiry that connect Chinese patent drug therapies with multiple pathways of cell death in oncological contexts. Taken together, the combination of R‐bibliometrix for robust data management and VOSviewer for network visualization provided a comprehensive platform to track global trends, spotlight significant collaborators, and highlight emerging themes relevant to cancer therapeutics grounded in traditional Chinese medicinal philosophies.
Result
Analysis of field development trends
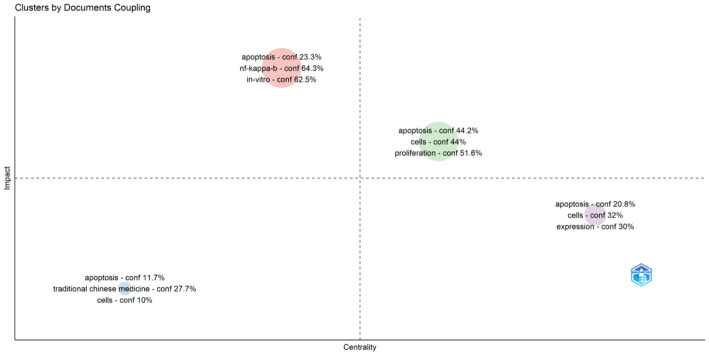
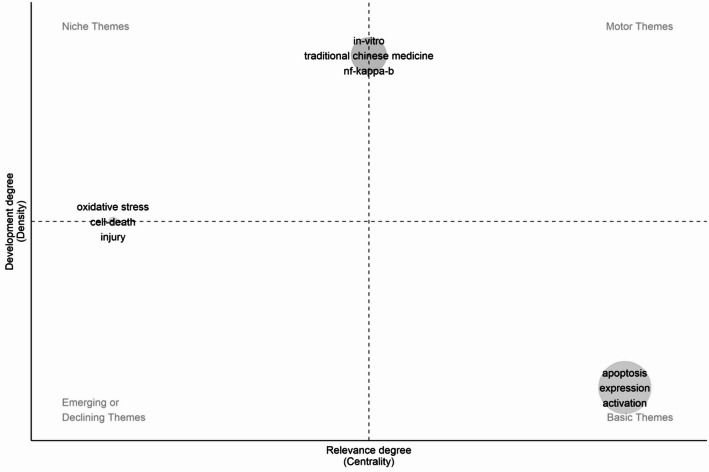
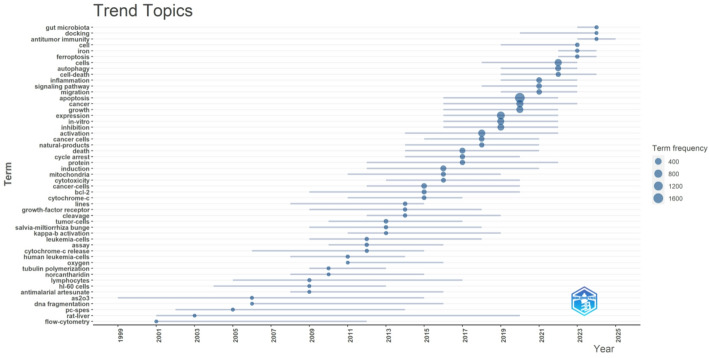
As shown in Fig. 1, the document-coupling analysis identifies multiple thematic clusters revolving around cell-death mechanisms, with ‘apoptosis’ prominently featured across quadrants in conjunction with ‘cells,’ ‘in vitro,’ and ‘nf-kappa-b. The bottom-left grouping, which includes “traditional Chinese medicine,” suggests that certain publications emphasize TCM formulations while maintaining relatively lower centrality and impact, possibly reflecting specialized or preliminary explorations of how TCM‐based compounds induce cell death. By contrast, apoptosis‐related terms associated with higher centrality and impact underscore the essential, widespread investigation of programmed cell death in cancer research. In Fig. 2, themes are plotted based on their development density and relevance: “apoptosis,” “expression,” and “activation” occupy the Basic Themes quadrant, indicating their foundational role in elucidating how Chinese patent drugs trigger signaling cascades within cancer cells. Meanwhile, “oxidative stress,” “cell death,” and “injury” appear in the Emerging or Declining Themes quadrant, implying that these areas either represent nascent directions requiring further investigation or are increasingly absorbed into more specific mechanistic discussions such as ferroptosis.TCM-focused terms (e.g., ‘traditional Chinese medicine’) cluster near the center, highlighting ongoing work that addresses both mechanistic depth (e.g., ‘nf-kappa-b’) and preclinical validation (‘in vitro’). The keyword timeline in Fig. 3 provides further insight into how these concepts have evolved over time: early research predominantly emphasized apoptosis and cytotoxicity, while more recent publications show an increasing frequency of terms such as “ferroptosis,” “autophagy,” and “signaling pathway,” implying a growing appreciation for diverse regulatory networks through which Chinese patent drugs might modulate tumor cell fate. The appearance of broader immunological and metabolic terms (e.g., “inflammation,” “gut microbiota,” “iron”) also suggests that investigators are examining cell death in a broader physiological context, in line with expanding interest in tumor microenvironment and holistic treatment strategies. Taken together, these trends reflect a field that—while grounded in fundamental apoptosis research—is steadily branching out to incorporate a wider spectrum of regulated cell death mechanisms and sophisticated experimental techniques, thereby refining our understanding of how Chinese patent drugs may enhance cancer therapy.
Fig. 1.
Cluster analysis of documents by coupling
Fig. 2.
Strategic diagram: thematic development and positioning
Fig. 3.
Trend topics and their temporal evolution
Bibliometric analysis of country
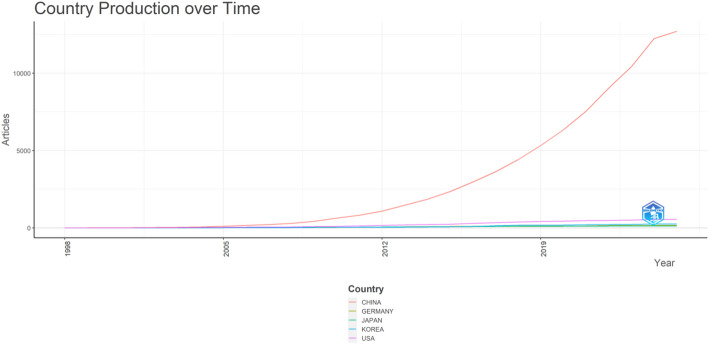
As shown in Fig. 4, China overwhelmingly dominates the citation landscape, amassing a substantially higher number of citations than other nations, with the United States, Germany, Korea, and Japan following at considerable distance. This citation pattern underscores China’s pivotal role in the investigation of Chinese patent drugs aimed at inducing programmed cell death. Cultural and institutional investment in traditional Chinese medicine research may partly explain the magnitude of Chinese outputs in this field, a conclusion further reinforced by the temporal trends shown in Fig. 5, where China’s publication growth accelerates rapidly after 2010 and surpasses other major contributors by an expansive margin. Although the United States and select European countries (e.g., Germany) continue to maintain steady output levels, the gap widens each year, suggesting that Chinese institutions lead in both sheer volume and impact within this specialized research domain. Collaboration patterns, depicted in Fig. 6, illustrate strong international networks linking China to the United States, Taiwan, Japan, and various European countries, reflecting a globalization of research interests and the dispersal of Chinese patent drug investigations beyond domestic settings. Notably, this network showcases hub-like roles for both China and the United States, hinting at extensive scientific exchange and potential co‐financing or cross‐institutional partnerships. Korea, Australia, and Singapore also appear as increasingly visible collaborators, suggesting a broader regional interest in harnessing the therapeutic potential of Chinese herbal formulations for oncological applications. Taken together, this geographic distribution of citations, publications, and collaborations highlights both the rapid ascent of Chinese research institutions in traditional medicine–based cancer therapies and the importance of multinational efforts to validate, standardize, and innovate upon these compounds. The conspicuous leadership of China, however, does not diminish the contributions of other countries; rather, it underscores the field’s expanding global influence, where longstanding knowledge of Chinese patent drugs merges with advanced translational research to shape emerging paradigms in cancer cell‐death modulation.
Fig. 4.
Most cited countries in the field
Fig. 5.
Country output over time
Fig. 6.
Global collaboration network of countries
Bibliometric analysis of keywords
As shown in Fig. 7, the overall frequency of key terms such as “apoptosis,” “activation,” and “traditional Chinese medicine” has soared over the past two decades, underscoring the centrality of regulated cell-death mechanisms in research on Chinese patent drugs for cancer therapy. Early on, “apoptosis” dominated the literature, but more recent years reveal a broadening scope, as “expression,” “inhibition,” and “autophagy” gain traction. Simultaneously, ‘in vitro’ studies remain prevalent, reflecting the importance of laboratory-based validation before transitioning to clinical contexts. The co‐occurrence network in Fig. 8 highlights “apoptosis” as a prominent hub linked with “cells,” “proliferation,” and “nf‐kappa‐b,” suggesting that researchers are probing both mitochondrial and immune signaling pathways to elucidate how Chinese patent drugs initiate tumor cell death. “Traditional Chinese medicine” clusters closely with “expression” and “invasion,” indicating a convergence of pharmacological exploration and molecular studies that map natural compounds to distinct cancer pathways. According to Fig. 9, “apoptosis” stands out with the highest occurrence count, followed by “expression,” “activation,” and “cells,” illustrating a consistent focus on death‐inducing processes, gene‐regulatory events, and experimental verifications. Meanwhile, “cancer,” “in‐vitro,” and “growth” remain integral to the thematic landscape, pointing to sustained efforts in mechanistic assays and proliferation studies. “Proliferation” and “inhibition” likewise occupy considerable positions on this list, reinforcing that cell‐cycle disruption is a shared focal point. In Fig. 10, the mapped keyword clusters reveal at least three distinct groupings: one rich in references to oxidative stress, injury, and cell‐death; another featuring in‐vitro, nf‐kappa‐b, and TCM terms; and a third centered on apoptosis, activation, and expression. This partitioning underscores a thematic interplay wherein TCM formulations may induce multiple regulated cell‐death pathways, from classical mitochondrial apoptosis to more recent autophagy and ferroptosis paradigms. The green cluster around oxidative stress suggests interest in reactive‐oxygen‐species‐mediated cytotoxicity, while the red cluster focused on in‐vitro experimentation underscores the heavy reliance on laboratory models for screening and mechanistic elucidation. These keyword trajectories and co‐occurrence patterns reflect a dynamic research field that, while anchored by apoptosis, increasingly investigates alternative pathways, advanced in‐vitro strategies, and multifaceted TCM interventions to optimize cell‐death induction in cancer cells. Notably, the term ‘Multi-Target Therapy’ bridges several clusters, underscoring a growing research focus on exploiting the broad pharmacodynamic spectrum of Chinese patent drugs to synchronously modulate multiple regulated cell-death pathways.
Fig. 7.
Temporal changes in keyword frequency
Fig. 8.
Keyword co-occurrence network
Fig. 9.
Ranking of the most relevant keywords
Fig. 10.
Clusters of co-occurring keywords
Bibliometric analysis of journal
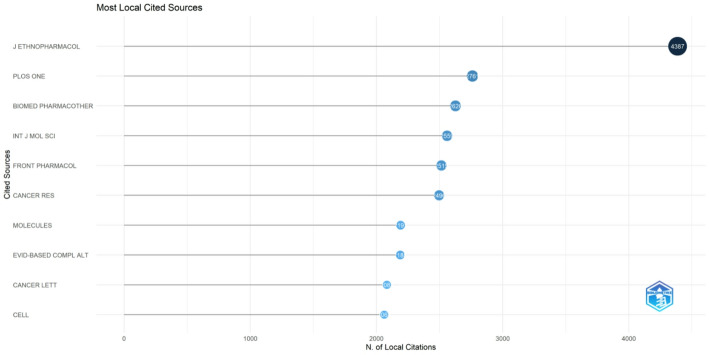
As shown in Fig. 11, the Journal of Ethnopharmacology leads all sources in number of documents, reflecting the enduring importance of ethnopharmacological perspectives in unveiling how Chinese patent drugs may induce programmed cell death in cancer cells. Frontiers in Pharmacology and Evidence-Based Complementary and Alternative Medicine follow closely, suggesting strong editorial focus on integrative or complementary approaches, where traditional Chinese compounds are often investigated alongside modern biochemical and pharmaceutical methodologies. Phytomedicine and Biomedicine & Pharmacotherapy also rank highly, underscoring a growing interest in plant‐derived or combinatorial therapeutics that target cancer at the molecular level. By concentrating publications in these journals, researchers are able to bridge ethnomedical traditions with cutting‐edge experimental science, thereby illuminating diverse mechanisms—such as modulating apoptotic pathways, regulating oxidative stress, or impeding tumor growth. This pattern is further corroborated in Fig. 12, where Journal of Ethnopharmacology emerges as the most frequently cited source, implying broad acknowledgment that traditional pharmacology provides a foundational framework for exploring the cell‐death–promoting effects of Chinese patent drugs. Meanwhile, Biomedicine & Pharmacotherapy, International Journal of Molecular Sciences, and Frontiers in Pharmacology each record hundreds of local citations, highlighting a synergy between fundamental mechanistic studies and translational efforts to refine Chinese herbal formulas for clinical oncology. The noticeable citations in PLOS ONE and Cancer Research reveal ongoing efforts to disseminate these findings in broader, high‐impact venues, possibly in an attempt to standardize TCM approaches and highlight reproducibility. Together, these journal trends point to the field’s multidimensional character: investigations are anchored in ethnopharmacological roots, yet advanced through interdisciplinary platforms that emphasize molecular mechanisms and evidence‐based validation. The dominance of the Journal of Ethnopharmacology in both publication count and citation frequency reinforces the pivotal role of culturally informed pharmacological insight, suggesting that ongoing collaboration between ethnopharmacologists and molecular oncologists may pave the way for future breakthroughs in harnessing Chinese patent drug therapies for programmed cell death in cancer.
Fig. 11.
Most relevant journals by publication count
Fig. 12.
Most frequently cited journals
Analysis of cooperation models
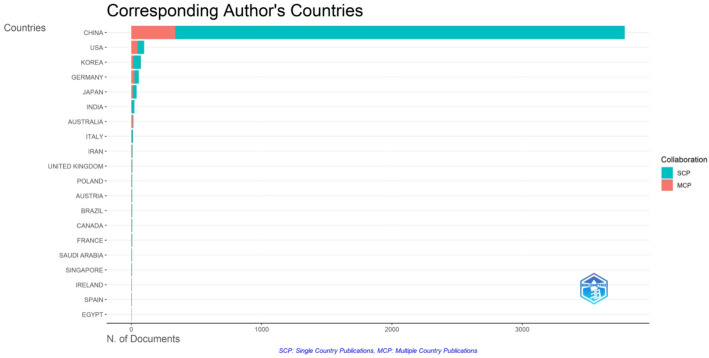
As shown in Fig. 13, China serves as the primary collaboration hub, forging extensive research linkages with North America, Western Europe, and select Asia-Pacific countries in the study of Chinese patent drugs that induce programmed cell death in cancer cells. These cross‐regional connections suggest that both developed and emerging economies are eager to validate, refine, and adapt traditional Chinese formulations for modern oncological applications. Notably, collaborations often involve broad‐spectrum research teams, encompassing pharmacology, molecular biology, and clinical science, indicating that multidisciplinary efforts are essential to translate laboratory findings into viable therapeutic interventions. At the same time, Fig. 14 reveals that most corresponding authors hail from China, reinforcing China’s leading position in both single‐country (SCP) and multi‐country (MCP) publications. While Chinese‐affiliated authors clearly dominate in terms of sheer output, the United States, Korea, Germany, and Japan make meaningful contributions, frequently entering multi‐institutional collaborations that pool expertise and resources. This pattern of authorship confirms a willingness among global research teams to leverage China’s deep ethnopharmacological heritage alongside cutting‐edge Western biomedical approaches, thereby accelerating progress in targeted cancer therapies. The increasing presence of Australia, India, and other nations in the MCP category also signifies a growing diversity of institutional perspectives. Such broad engagement potentially facilitates rigorous evaluation of Chinese patent drugs across varied experimental systems, patient populations, and regulatory environments. From a thematic standpoint, cross‐border partnerships appear to focus on refining mechanistic understanding—elucidating how these compounds modulate apoptotic and autophagic pathways—while simultaneously exploring clinical efficacy and safety. In doing so, they integrate high‐throughput screening, genomic profiling, and immunological assays, echoing a larger shift within oncology research toward precision medicine. Consequently, this network of collaborations fosters not only the standardization and global recognition of these therapeutic agents but also the development of novel combination strategies that may extend beyond classical apoptosis to incorporate complementary treatment modalities.
Fig. 13.
World map of collaborations
Fig. 14.
Corresponding authors’ country distribution
Analysis of the volume of literature published
As indicated in Table 1, the cumulative number of published articles on Chinese patent drugs that promote programmed cell death in cancer cells has expanded dramatically from just 2 papers in 1998 to a total of 4073 by 2024. During the initial phase (1998–2002), publication outputs were minimal, hovering in single to low double digits (2 to 12 articles per year), suggesting that the research focus in this era was only beginning to recognize the potential synergy between traditional Chinese formulations and targeted oncological therapies. From 2003 to 2008, the field experienced modest yet steady growth, reaching 44 annual publications in 2008; this period likely reflects gradual interest in elucidating the underlying mechanisms—particularly apoptosis—that make these compounds attractive for cancer treatment. A second inflection point emerges between 2009 and 2014, when annual publications rose from 54 to 141, which may be attributed to broader acceptance of complementary and alternative therapies in mainstream scientific discourse, as well as the advent of more sophisticated laboratory techniques capable of dissecting cellular pathways. The transition from 2015 to 2019 marks another significant surge, with articles climbing from 163 to 300 per year, consistent with expanding global collaborations and deeper mechanistic insights into regulated cell death processes (e.g., autophagy, ferroptosis). Notably, the jump between 2019 and 2023—rising from 300 to 416 articles—coincides with the COVID‑19 pandemic, a period during which research into traditional Chinese medicine (TCM) overall saw increased funding and attention, potentially boosting publications on TCM-derived cancer therapeutics as well. By 2024, the number of articles soars to 551, reflecting a culmination of factors including heightened cross‐disciplinary collaborations, technological advances in screening bioactive compounds, and continuing interest in combining TCM with standard anticancer regimens. The overall trajectory suggests that initial skepticism has given way to robust scientific inquiry, with many groups now viewing Chinese patent drugs as a complementary avenue to enhance the precision and efficacy of cancer treatments through targeted induction of programmed cell death.
Discussion
Field development trends and field frontiers
Over the last quarter century, investigations into Chinese patent drugs that promote programmed cell death in cancer cells have undergone a striking evolution, driven by escalating publication volumes, expanding global collaboration networks, and a widening array of mechanistic insights. In the earliest years, research outputs were sparse and largely descriptive, focusing on how traditional formulations might align with fundamental apoptotic pathways. Over time, as in-vitro assays and basic molecular biology techniques became more sophisticated, investigators delved deeper into the precise mechanisms by which these agents influence cancer cell fates, uncovering the roles of signaling cascades such as NF‐κB and the mitochondrial pathway.
With the emergence of autophagy, ferroptosis, pyroptosis, and other regulated cell-death processes, the field began to embrace a more diverse set of targets, moving beyond classical apoptosis to explore how Chinese patent drugs might modulate oxidative stress, immune activation, and tumor metabolism. Notably, a critical turning point arose in the late 2000 s and early 2010 s, when publication counts started climbing more rapidly and articles began appearing in interdisciplinary and high‐impact journals, signaling a broader acceptance of traditional Chinese medicine (TCM) within mainstream cancer research. This shift coincided with growing interest in complementary and alternative therapeutics, the development of high‐throughput screening platforms, and an uptick in multi‐omics approaches that mapped TCM formulations to gene expression profiles and signaling pathways relevant to cell‐death induction. China soon emerged as the leading contributor both in volume of articles and citation impact, a phenomenon partly explained by considerable institutional support for TCM research and the presence of an extensive knowledge base on indigenous herbal remedies [36–41]. Nevertheless, the expanding collaboration map included substantial participation from the United States, Germany, Korea, Japan, Australia, and other regions, reflecting a recognition that Chinese patent drugs hold promise in global oncology. These multinational partnerships frequently encompassed interdisciplinary teams bridging pharmacology, genomics, immunology, and clinical oncology, enabling more robust testing of TCM compounds under diverse laboratory and patient settings. At the same time, a surge of interest in immunomodulation and the tumor microenvironment prompted researchers to examine whether TCM‐derived products could synergize with immune checkpoint inhibitors or complement standard chemotherapeutics. In parallel, keyword analysis demonstrated how terms like “activation,” “expression,” and “inhibition” became increasingly prevalent, highlighting a collective focus on fine‐tuning cellular processes that ultimately lead to tumor cell death. Journals that historically centered on ethnopharmacology were joined by others emphasizing experimental therapeutics and molecular medicine, signifying a dual progression: scientists are no longer content solely with cataloging plant extracts but seek precise mechanisms and potential clinical applicability. COVID‐19 also exerted an indirect influence by elevating general interest in TCM, thus spurring further research into cancer applications of Chinese patent drugs, possibly accelerating the publication rate in the early 2020s. Despite this growth, the field faces challenges regarding standardization of formulations, quality control, and regulatory acceptance across different healthcare systems. Nevertheless, a clear frontier has emerged around combining TCM‐based interventions with advanced molecular diagnostics to tailor treatments for specific cancer subtypes, with particular attention paid to novel or underexplored cell‐death pathways such as ferroptosis and pyroptosis. This frontier demands not only robust in‐vitro validation but also well‐designed preclinical and clinical studies that can capture efficacy, pharmacokinetics, and safety profiles across diverse patient populations.
As research continues to broaden across geographic and disciplinary boundaries, it appears increasingly likely that Chinese patent drugs will be positioned as key components in integrative oncology protocols that harness multiple regulated cell-death mechanisms. By leveraging modern technologies to probe the bioactive compounds within these formulas, and by pairing them with conventional or immunotherapeutic agents, investigators are crafting new paradigms for cancer care that unite time‐honored ethnopharmacological knowledge with cutting‐edge biomedical innovation. The steadily diversifying pool of keywords, the rising rates of international co‐authorship, and the surge in publication counts together suggest that this domain is poised for further growth, and that the frontiers of research now hinge on elucidating the multi‐target, systems‐level interactions by which Chinese patent drugs may reshape cancer cell fate.In parallel with efficacy, pharmacovigilance remains paramount. Recent NMPA bulletins and the 2023 WHO herbal-medicine report both flag batch variability, heavy-metal contamination and herb–drug interactions as recurring concerns for CPDs. Strengthening post-marketing surveillance, lot-release testing and GMP enforcement will be essential to translate experimental efficacy into safe clinical practice.
Prospects and predictions for future research directions
Future studies on Chinese patent drugs that induce programmed cell death in cancer cells will likely concentrate on systematically integrating these formulations into mainstream oncology, with a core emphasis on elucidating their multifaceted molecular targets and optimizing clinical applications. While apoptosis remains the earliest and most extensively characterized form of cell death in this domain, ongoing work is increasingly scrutinizing nonapoptotic pathways—such as ferroptosis, pyroptosis, and necroptosis—to determine how specific herbal compounds interact with or modulate these mechanisms at the molecular level. Enhanced collaboration among ethnopharmacologists, molecular oncologists, and clinicians could expedite breakthroughs by refining drug formulations through chemical profiling, ensuring consistent bioactive ingredient concentrations, and mapping each compound’s impact on gene expression signatures relevant to tumor progression. Integrating multi-omics technologies and advanced data analytics, including proteomics, metabolomics, and transcriptomics, may reveal novel synergistic interactions when Chinese patent drugs are combined with chemotherapy, radiotherapy, or immunotherapeutic agents like checkpoint inhibitors. Furthermore, personalized treatment protocols could become a reality if more comprehensive studies examine how patient‐specific factors—ranging from genetic polymorphisms to gut microbiota composition—influence the efficacy and toxicity of TCM interventions. Another promising direction involves tackling tumor heterogeneity by exploring combinatorial regimens that target multiple regulated cell‐death pathways, which may circumvent or delay resistance mechanisms that often undermine single‐agent therapies. However, clinical translation will require careful attention to standardization and regulatory compliance, necessitating rigorous good manufacturing practices and well‐structured clinical trials that assess safety, pharmacokinetics, and long‐term outcomes. As global interest in complementary medicine rises, researchers must also address potential drug–drug interactions and integrative protocols that balance TCM’s holistic benefits with evidence‐based conventional treatment. Interdisciplinary teams can capitalize on international collaborations to conduct large‐scale, multicenter studies that confirm reproducibility and build robust datasets capable of informing meta‐analyses and systematic reviews. Emphasis on translational pipelines—from bench studies that elucidate intricate signaling cascades to clinical trials that validate therapeutic outcomes—will be critical for instilling confidence in broader medical communities and regulatory bodies. A remaining challenge, particularly relevant amid heightened healthcare scrutiny, is demonstrating cost‐effectiveness and scalability, particularly for resource‐limited regions that could benefit from the accessibility of TCM‐derived treatments. To strengthen acceptance and integration of Chinese patent drugs into global clinical practice, researchers may develop novel drug delivery methods, such as nanoparticles or liposomal carriers, designed to enhance targeted tumor accumulation while minimizing systemic toxicity. Ultimately, the convergence of advanced molecular biology tools, international scientific networks, and growing patient demand for holistic cancer care sets the stage for Chinese patent drugs to transition from supplementary options to integral components of precision oncology. By continuously refining formulations, assessing multi‐target mechanisms, and conducting rigorously controlled trials, the field is poised to unlock new therapeutic possibilities that span diverse cancer types and patient populations. Regulatory heterogeneity will further influence these trajectories. In the European Union, CPD analogues would need to comply with EMA traditional-herbal or well-established-use monographs, whereas in the United States they must follow the FDA Botanical Drug Development guidance and IND route. These divergent pathways are likely to shape where multinational trials are conducted and which partners are best placed to navigate overlapping jurisdictions.
Advantages and limitations of this study
This study’s strengths include its comprehensive retrieval strategy from the Web of Science Core Collection, a meticulously curated search formula encompassing multiple cell death pathways, and the use of both R-bibliometrix and VOSviewer to examine collaboration networks, keyword co‐occurrences, and thematic evolutions. By capturing articles published over a 25‐year period, the analysis offers a robust longitudinal view of how research on Chinese patent drugs has progressed and diversified. Nonetheless, the study is not without limitations. Restricting the search to English-language publications inevitably excluded roughly 4 200 Chinese-language papers located in CNKI and VIP, representing about one-third of the total CPD–PCD corpus and potentially containing region-specific evidence. Limiting the data source to WoSCC also omitted an estimated 1 100 additional records present only in Scopus or PubMed, highlighting a measurable database bias that future multi-platform studies should address. Moreover, focusing solely on one database risks missing some relevant studies, and any biases in the metadata (e.g., authors’ institutional affiliations) could influence the results. Finally, the reliance on bibliometric measures cannot fully capture the clinical applicability or comparative efficacy of these interventions, highlighting the need for additional experimental and clinical validation.
Conclusion
This bibliometric study demonstrates a rapid expansion in research on Chinese patent drugs that induce programmed cell death in cancer cells, with publication outputs climbing steadily since the 1998. The application of R-bibliometrix and VOSviewer revealed that China maintains a dominant position in both production and impact, driven by sustained institutional support and long‐standing ethnopharmacological knowledge. International collaborations are broadening, with a notable rise in multi‐country projects that integrate traditional Chinese formulations into diverse oncology frameworks. Co‐occurrence analyses highlight a shifting focus from early apoptosis‐centric investigations to broader regulated cell‐death pathways such as ferroptosis, pyroptosis, and autophagy, suggesting that researchers are exploring increasingly complex molecular targets. The most frequently cited journals underscore a balance between ethnopharmacological roots and emerging evidence‐based requirements, reflecting the transition toward more rigorous, mechanistic validation of these compounds. By tracing keyword trajectories and thematic clusters, this study shows that multiple disciplines—ranging from molecular biology to clinical oncology—are converging on the promise of combining traditional preparations with mainstream therapeutic regimens. The field’s continued growth signals a strong impetus for deeper mechanistic dissection, standardization, and integrative clinical trials. It appears likely that Chinese patent drugs will evolve into a cornerstone of precision oncology, transforming cancer treatment strategies worldwide.
Author contributions
MS, CT conceived and designed the study. MS, CT analyzed data. ST, YG collected data. MS, CT, XZ, ST, YG wrote. ST, YG helped with the final revision of this manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was not funded.
Data availability
The data used to support the findings of this study are included within the article.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Human Ethics and Consent to Participate declarations
Not applicable.
Ethics and Consent to Participate declarations
Not applicable.
Ethics declaration
Not applicable.
thics and Consent to Publish declarations
Not applicable.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yuan J, Ofengeim D. A guide to cell death pathways. Nat Rev Mol Cell Biol. 2024;25(5):379–95. [DOI] [PubMed] [Google Scholar]
- 2.Santagostino SF, Assenmacher CA, Tarrant JC, et al. Mechanisms of regulated cell death: current perspectives. Vet Pathol. 2021;58(4):596–623. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Hong M, Li Y, et al. Programmed cell death tunes tumor immunity. Front Immunol. 2022;13:847345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai X, Wang D, Zhang J. Programmed cell death, redox imbalance, and cancer therapeutics. Apoptosis. 2021;26(7):385–414. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Liang Q, Sun G. Traditional Chinese medicine for prevention and treatment of hepatocellular carcinoma: a focus on epithelial-mesenchymal transition. J Integr Med. 2021;19(6):469–77. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Lou Y, Wang J, et al. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol. 2021;11:609705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JF, Wu SW, Shi ZM, et al. Traditional Chinese medicine for colorectal cancer treatment: potential targets and mechanisms of action. Chin Med. 2023;18(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Fu JL, Hao HF, et al. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res. 2021;170:105728. [DOI] [PubMed] [Google Scholar]
- 9.Yao C, Zhang J, Li J, et al. Traditional Chinese medicine (TCM) as a source of new anticancer drugs. Nat Prod Rep. 2021;38(9):1618–33. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Li M, Du K, et al. Traditional Chinese medicine for adjuvant treatment of breast cancer: Taohong Siwu Decoction. Chin Med. 2021;16:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y, Xian Z, Wu F, et al. Traditional Chinese medicine combined with chemotherapy in the treatment of advanced non-small cell lung cancer: key drug screening and mechanism analysis. Naunyn Schmiedebergs Arch Pharmacol. 2025;398(1):843–54. [DOI] [PubMed] [Google Scholar]
- 12.Wei Z, Chen J, Zuo F, et al. Traditional Chinese medicine has great potential as candidate drugs for lung cancer: A review. J Ethnopharmacol. 2023;300:115748. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Zhang Q, Yu L, et al. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J Ethnopharmacol. 2021;264:113249. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Yang P, Jia Y. Molecular mechanisms of astragaloside-IV in cancer therapy. Int J Mol Med. 2021;47(3):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng X, Jiao YN, Hao HF, et al. Taraxacum Mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10/STAT3/PD-L1 signaling pathways. J Ethnopharmacol. 2021;274:113978. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Chen Y, Wu JH, et al. Targeting regulated cell death with plant natural compounds for cancer therapy: A revisited review of apoptosis, autophagy-dependent cell death, and necroptosis. Phytother Res. 2023;37(4):1488–525. [DOI] [PubMed] [Google Scholar]
- 17.Sun S, Yu W, Zhang G, et al. Potential mechanism of traditional Chinese medicine intervention in gastric cancer: targeted regulation of autophagy. Front Pharmacol. 2025;16:1548672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q, Chen Z, Ding Y, et al. Protective effect of traditional Chinese medicine on non-alcoholic fatty liver disease and liver cancer by targeting ferroptosis. Front Nutr. 2022;9:1033129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Liu L, Wang X, et al. Necroptosis inhibits autophagy by regulating the formation of RIP3/p62/Keap1 complex in shikonin-induced ROS dependent cell death of human bladder cancer. Phytomedicine. 2023;118:154943. [DOI] [PubMed] [Google Scholar]
- 20.Xi Z, Dai R, Ze Y, et al. Traditional Chinese medicine in lung cancer treatment. Mol Cancer. 2025;24(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng W, Yang R, Li K, et al. The curative and preventative effects of traditional Chinese medicine on ferroptosis of hepatocellular carcinoma. Nat Prod Commun. 2023;18(12):1934578X231220485. [Google Scholar]
- 22.Wang S, Guo S, Guo J, et al. Cell death pathways: molecular mechanisms and therapeutic targets for cancer. MedComm. 2024;5(9):e693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu B, Lou YM, Wu P, et al. Emerging role of necroptosis, pyroptosis, and ferroptosis in breast cancer: new dawn for overcoming therapy resistance. Neoplasia. 2024;55:101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He, Guangfeng et al. “Single-cell transcriptomics reveals heterogeneity and prognostic markers of myeloid precursor cells in acute myeloid leukemia.” Frontiers in immunology vol. 15 1494106. 16 Dec. 2024, doi:10.3389/fimmu.2024.1494106 [DOI] [PMC free article] [PubMed]
- 25.Tang Y, Zhuang Y, Zhao C, et al. The metabolites from traditional Chinese medicine targeting ferroptosis for cancer therapy. Front Pharmacol. 2024;15:1280779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Lin H, Sun Y, et al. Platycodin D2 mediates incomplete autophagy and ferroptosis in breast Cancer cells by regulating mitochondrial ROS. Phytotherapy Research; 2024. [DOI] [PubMed]
- 27.Li M, Tao J, Qian R, et al. Development of alternative herbals remedy for gastric cancer based on transcriptomic analysis of immune infiltration and ferroptosis. Front Genet. 2023;14:1086368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Q, Li Z, Li Y, et al. Natural products targeting signaling pathways associated with regulated cell death in gastric cancer: recent advances and perspectives. Phytother Res. 2023;37(6):2661–92. [DOI] [PubMed] [Google Scholar]
- 29.McAllister JT, Lennertz L, Atencio Mojica Z. Mapping a discipline: a guide to using VOSviewer for bibliometric and visual analysis. Sci Technol Libr. 2022;41(3):319–48. [Google Scholar]
- 30.Iliescu AN. Conceptual atlas of the Knowmad literature: visual mapping with VOSviewer. Manage Dyn Knowl Econ. 2021;9(3):379–92. [Google Scholar]
- 31.Dereli A. Vosviewer Ile bibliyometrik analiz. Communicata. 2024;28:1–7. [Google Scholar]
- 32.Büyükkıdık S. A bibliometric analysis: a tutorial for the bibliometrix package in R using IRT literature. J Meas Eval Educ Psychol. 2022;13(3):164–93. [Google Scholar]
- 33.Bhat WA, Khan NL, Manzoor A, et al. How to conduct bibliometric analysis using R-studio: a practical guide. Eur Econ Lett (EEL). 2023;13(3):681–700. [Google Scholar]
- 34.Liu L, Xu L, Wang S, et al. Confirmation of inhibitingTLR4/MyD88/NF-κB signalling pathway by Duhuo Jisheng Decoction on osteoarthritis: a network Pharmacology approach-integrated experimental study. Front Pharmacol. 2022;12:784822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu, Yuheng et al. “Revealing the association between East Asian oral microbiome and colorectal cancer through Mendelian randomization and multi-omics analysis.” Frontiers in cellular and infection microbiology vol. 14 1452392. 17 Sep. 2024, doi:10.3389/fcimb.2024.1452392 [DOI] [PMC free article] [PubMed]
- 36.Zhang X, Qiu H, Li C, et al. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15(5):283–98. [DOI] [PubMed] [Google Scholar]
- 37.Xiong, Jingwen et al. “The two-sided battlefield of tumour-associated macrophages in glioblastoma: unravelling their therapeutic potential.” Discover oncology vol. 15,1 590. 25 Oct. 2024, doi:10.1007/s12672-024-01464-5 [DOI] [PMC free article] [PubMed]
- 38.Wei J, Liu Z, He J, et al. Traditional Chinese medicine reverses cancer multidrug resistance and its mechanism. Clin Transl Oncol. 2022;8:1–12. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Li J, Chen R, et al. Active ingredients from Chinese medicine for combination cancer therapy. Int J Biol Sci. 2023;19(11):3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong Z, Vong CT, Chen F, et al. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: helping to fight against cancer via multiple targets. Med Res Rev. 2022;42(3):1246–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu CX. Overview on development of ASEAN traditional and herbal medicines. Chin Herb Med. 2021;13(4):441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.