Abstract
Delaying the initiation of cancer treatment increases the risk of mortality, particularly in colorectal cancer (CRC), which is among the most common and deadliest malignancies. This study aims to explore the impact of treatment delays on mortality in CRC. A systematic literature search was conducted in PubMed, Web of Science, and Scopus for studies published between 2000 and 2025. Meta-analyses were performed using random-effects models with inverse variance method to calculate hazard ratios (HRs) for both overall and cancer-specific survival at 4-, 8-, and 12-week treatment delay intervals, with heterogeneity assessed through I2-statistics and publication bias evaluated using funnel plots and Egger’s test. A total of 20 relevant studies were included in the meta-analysis. The analyses of all patients demonstrated a progressively increasing risk of 12–39% with longer treatment delays (4 weeks, HR = 1.12; 95% CI, 1.08–1.16; 8 weeks, HR = 1.24; 95% CI, 1.16–1.34; 12 weeks, HR = 1.39; 95% CI, 1.25–1.55). In particular, incrementally higher hazard ratios were observed for all–cause mortality at 4 weeks (HR = 1.14; 95% CI, 1.09–1.18), 8 weeks (HR = 1.29; 95% CI, 1.20–1.39), and 12 weeks (HR = 1.47; 95% CI, 1.31–1.64). In contrast, cancer-specific survival analysis showed a similar trend but did not reach statistical significance (4 weeks, HR = 1.07; 95% CI, 0.98–1.18; 8 weeks, HR = 1.15; 95% CI, 0.95–1.39; 12 weeks, HR = 1.23; 95% CI, 0.93–1.63). Treatment delays in colorectal cancer patients were associated with progressively worsening overall survival, with each 4-week delay increment leading to a substantially higher mortality risk. This study suggests that timely treatment initiation should be prioritized in clinical practice, as these efforts can lead to substantial improvements in survival rates.
Keywords: Colorectal cancer, Treatment delay, Cancer survival, Oncology care pathways, Healthcare inefficiencies, Geriatric oncology, Frailty, Multimorbidity, Patient navigation, Referral pathways, Telemedicine, Diagnostic delays, Healthcare disparities, Time-to-treatment benchmarks, Cancer treatment access
Introduction
Timely initiation of cancer treatment is essential for optimizing survival outcomes, yet treatment delays remain a persistent challenge in oncology [1–4]. Colorectal cancer (CRC) is a disease of aging [5, 6], with the majority of cases occurring in individuals over 60 [7, 8]. Despite advances in screening and treatment, CRC remains one of the leading causes of cancer-related mortality worldwide [7], and growing evidence suggests that even modest delays in treatment initiation significantly worsen survival outcomes [4, 9]. However, delays in CRC treatment continue to occur due to a complex interplay of patient-, provider-, and healthcare system-related factors, underscoring the urgent need to better understand and mitigate these barriers [10].
Delays in CRC treatment arise at multiple levels [11–15]. Patient-related factors include low symptom awareness, misattribution of early symptoms to benign conditions, reluctance to seek medical attention, and logistical or financial constraints [12, 16–18]. Older patients, in particular, often face additional challenges such as frailty, cognitive impairment, and difficulties in accessing care. Healthcare system inefficiencies, including prolonged wait times for specialist referrals, limited access to diagnostic procedures (e.g., colonoscopies and imaging), and overburdened oncology services, further contribute to treatment delays [19–22]. For instance, in Great Britain, the proportion of cancer patients waiting over 104 days for treatment has increased nearly fourfold, from 6000 in 2016 to 22,000 in 2024 [23]. In 2024, only 62% of National Health Service patients started treatment within 62 days of an urgent suspected cancer referral [23]. Provider-related factors, such as misdiagnosis, delayed decision-making, and concerns over treatment tolerance—particularly in older or medically complex patients—can also extend time to treatment [24, 25]. Additionally, global crises such as the COVID- 19 pandemic have significantly disrupted cancer care delivery, leading to widespread postponements in surgery, chemotherapy, and radiotherapy [26–29].
The consequences of treatment delays in CRC are profound [4, 9], with studies suggesting that each additional week of delay increases the risk of mortality, likely due to continued tumor progression and reduced effectiveness of treatment interventions. While delays in surgery, chemotherapy, and radiation therapy have all been linked to poorer survival outcomes, there remains a lack of standardized benchmarks for defining acceptable treatment timelines. Addressing these delays is particularly crucial in aging populations, where cancer progression may be influenced by age-related declines in immune surveillance, systemic inflammation, and multimorbidity.
This study aims to provide a comprehensive meta-analysis quantifying the impact of treatment delays on both overall and cancer-specific mortality in CRC. By systematically analyzing data across multiple studies, we assess how incremental delays of 4, 8, and 12 weeks affect survival outcomes. Our findings underscore the critical need for timely treatment initiation, inform clinical decision-making, and highlight areas where healthcare policies can be optimized to improve cancer care delivery.
Methods
Data collection
In this meta-analysis, we aimed to investigate the relationship between delays in initiating treatment and mortality in colorectal cancer. We included different therapeutic approaches, including surgical intervention, systemic chemotherapy, and radiation therapy. To gather relevant literature, we conducted a systematic search across three major electronic databases: PubMed, Scopus, and Web of Science, covering publications from 2000 to 2025. Our search strategy combined relevant MeSH terms and keywords for colorectal cancer, treatment delays, and survival outcomes, ensuring comprehensive coverage of available studies. The definition of treatment delay varied across studies, but was most commonly reported as the time from diagnosis to surgery. A minority of studies assessed delays to systemic therapy or radiotherapy.
We applied strict inclusion and exclusion criteria to select studies for analysis. We included prospective and retrospective cohort studies that assessed the impact of treatment delays on mortality in colorectal cancer patients. Only studies reporting hazard ratios, odds ratios, or risk ratios to quantify this relationship and those with a minimum follow-up duration of 4 weeks were considered. Conversely, we excluded experimental studies involving animal models, in vitro investigations, or theoretical simulations. Non-English publications and studies with inadequate methodological rigor or improperly defined patient populations were also omitted. The study was conducted in accordance with key elements of the PRISMA 2020 guidelines. However, we did not submit a completed checklist, which we note as a limitation.
Data extraction
Data extraction was carried out independently by two researchers, who systematically collected information on study characteristics such as author names, publication year, and study outcome. Additionally, we recorded details on outcome measures, and hazard ratios describing the association between treatment delays and survival outcomes. The duration and classification of treatment delays were also noted. In cases where discrepancies arose during data extraction, we resolved them through discussion until consensus was achieved. When agreement could not be reached, a third independent reviewer was consulted to ensure objectivity.
Statistical analyses
We applied two different methods to calculate the hazard rate as an outcome variable. In the absence of a reference period, the reported HR/OR/RR values were standardized using the formula HR per X-month delay = (HR per 4 weeks delay)^(X weeks delay/4 weeks delay) [4]. For studies with a reported reference time, a weighted linear regression was applied to assess the association between treatment delay (weeks) and the log-transformed hazard ratio (HR) for patient outcomes. HR estimates with 95% confidence intervals were computed for delays of 4, 8, and 12 weeks.
To estimate aggregated risk measures, particularly the hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs), we applied a random-effects model. This approach accounts for variations across studies, thereby enhancing the generalizability of the findings. A random-effects model was selected because the included studies differed in terms of population characteristics, definitions of delay, and healthcare settings—introducing clinical and methodological heterogeneity that warranted this modeling approach. To visualize individual study results alongside the overall summary estimate, we generated forest plots, which facilitated data interpretation and helped identify potential heterogeneity between studies. The statistical evaluation was conducted using the online platform MetaAnalysisOnline.com [30].
Evaluation of variability and publication bias
To assess inter-study variability, we utilized Cochran’s Q test and the I2 statistic. Cochran’s Q test, based on a chi-squared distribution, was employed to determine whether observed differences in effect sizes exceeded those expected by random variation. Additionally, the I2 statistic quantified the proportion of total variance attributed to actual study differences rather than chance fluctuations.
To examine potential publication bias, we constructed funnel plots to graphically depict the relationship between study effect sizes and their precision. Any asymmetry in these plots could indicate the presence of bias. Furthermore, we performed Egger’s regression analysis to statistically evaluate the correlation between effect sizes and their standard errors, providing a quantitative measure of publication bias.
Subgroup analyses
We conducted subgroup analyses to explore potential variations in effect estimates across different endpoints, including overall and cancer-specific survival. For each subgroup, we calculated pooled effect estimates along with heterogeneity metrics to evaluate the specific impact within each category. Additionally, we extended our analyses to the combined cohort, allowing for a comprehensive assessment of overall effects across all included conditions.
Results
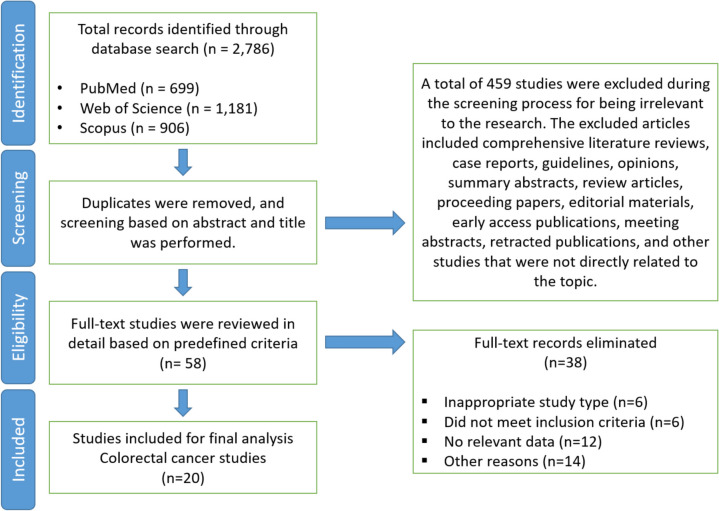
A total of 20 relevant studies were included in this meta-analysis [31–50]. Figure 1 presents the flowchart of the study selection process. The impact of treatment delays of 4, 8, and 12 weeks on survival outcomes in colorectal cancer patients were examined using the same set of studies—22 cohorts for overall survival, nine cohorts for colorectal cancer-specific mortality, and a total of 31 cohorts for the pooled analysis. Studies assessing the impact of delayed treatment on survival outcomes are summarized in Table 1.
Fig. 1.
Flow diagram of study selection process
Table 1.
Summary of studies assessing the impact of delayed treatment on survival outcomes in colorectal cancer patients
| Author | Year | Mortality | 4 weeks delay | 8 weeks delay | 12 weeks delay | HR/OR/RR | Total N | Case N | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | |||||||||
| Ahmed et al | 2010 | All-cause | 1.01 | 0.89 | 1.14 | 1.02 | 0.80 | 1.30 | 1.03 | 0.72 | 1.48 | HR | 663 | 275 |
| Ahmed et al | 2010 | Cancer-specific | 0.97 | 0.87 | 1.09 | 0.94 | 0.75 | 1.19 | 0.91 | 0.65 | 1.30 | HR | 663 | 275 |
| Bagaria et al | 2019 | All-cause | 1.08 | 1.06 | 1.10 | 1.17 | 1.13 | 1.22 | 1.27 | 1.20 | 1.34 | OR | 4685 | 2497 |
| Bayraktar et al | 2010 | All-cause | 1.40 | 1.01 | 1.95 | 1.97 | 1.02 | 3.81 | 2.77 | 1.03 | 7.43 | HR | 186 | 41 |
| Bayraktar et al | 2010 | Cancer-specific | 1.09 | 0.82 | 1.44 | 1.19 | 0.68 | 2.09 | 1.31 | 0.56 | 3.02 | HR | 186 | 32 |
| Becerra et al | 2017 | All-cause | 1.16 | 1.10 | 1.23 | 1.34 | 1.22 | 1.52 | 1.55 | 1.35 | 1.87 | HR | 1133 | 730 |
| Becerra et al | 2017 | Cancer-specific | 1.11 | 1.03 | 1.20 | 1.24 | 1.07 | 1.45 | 1.38 | 1.11 | 1.75 | HR | 1133 | 403 |
| Chau et al | 2005 | All-cause | 1.13 | 1.01 | 1.27 | 1.29 | 1.02 | 1.62 | 1.46 | 1.04 | 2.07 | HR | 801 | 220 |
| Cheung et al | 2009 | All-cause | 1.21 | 1.13 | 1.30 | 1.46 | 1.27 | 1.69 | 1.77 | 1.42 | 2.21 | HR | 6059 | 693 |
| Cheung et al | 2009 | Cancer-specific | 1.21 | 0.99 | 1.48 | 1.46 | 0.97 | 2.18 | 1.76 | 0.96 | 3.22 | OR | 6059 | 245 |
| Edwards et al | 2022 | All-cause | 1.01 | 0.81 | 1.27 | 1.03 | 0.65 | 1.60 | 1.04 | 0.52 | 2.03 | HR | 1031 | 167 |
| Flemming et al | 2017 | All-cause | 1.03 | 0.95 | 1.11 | 1.07 | 0.91 | 1.24 | 1.11 | 0.87 | 1.38 | RR | 4326 | 292 |
| Flemming et al | 2017 | Cancer-specific | 0.91 | 0.81 | 1.01 | 0.82 | 0.66 | 1.03 | 0.74 | 0.54 | 1.05 | RR | 4326 | 352 |
| Hershman et al | 2006 | All-cause | 1.15 | 1.10 | 1.20 | 1.32 | 1.21 | 1.44 | 1.52 | 1.33 | 1.73 | HR | 4382 | 665 |
| Hershman et al | 2006 | Cancer-specific | 1.03 | 0.88 | 1.19 | 1.05 | 0.78 | 1.41 | 1.08 | 0.69 | 1.68 | HR | 4382 | 665 |
| Lima et al | 2011 | All-cause | 1.21 | 1.11 | 1.30 | 1.45 | 1.24 | 1.70 | 1.75 | 1.38 | 2.22 | HR | 1053 | 475 |
| Lima et al | 2011 | Cancer-specific | 1.14 | 1.02 | 1.27 | 1.30 | 1.05 | 1.61 | 1.48 | 1.08 | 2.04 | HR | 1053 | 475 |
| Lo et al | 2021 | All-cause | 1.16 | 1.14 | 1.18 | 1.34 | 1.29 | 1.39 | 1.55 | 1.46 | 1.65 | HR | 187,394 | 6746 |
| Massarweh et al | 2015 | All-cause | 1.16 | 0.98 | 1.38 | 1.35 | 0.96 | 1.90 | 1.57 | 0.94 | 2.61 | HR | 51,331 | 16,570 |
| Murchie et al | 2014 | All-cause | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 0.99 | 0.99 | OR | 958 | 762 |
| Roder et al. (surgery) | 2019 | All-cause | 1.05 | 0.98 | 1.13 | 1.11 | 0.96 | 1.28 | 1.16 | 0.94 | 1.45 | HR | 1675 | 1278 |
| Roder et al. (radiotherapy) | 2019 | All-cause | 1.14 | 1.06 | 1.22 | 1.29 | 1.11 | 1.49 | 1.46 | 1.18 | 1.82 | HR | 616 | 438 |
| Roder et al. (systemic therapy) | 2019 | All-cause | 1.01 | 0.99 | 1.02 | 1.01 | 0.98 | 1.04 | 1.02 | 0.97 | 1.07 | HR | 1556 | 1101 |
| Roder et al. (any treatment) | 2019 | All-cause | 1.11 | 0.95 | 1.29 | 1.23 | 0.91 | 1.66 | 1.36 | 0.86 | 2.14 | HR | 1675 | 1278 |
| Shin et al | 2013 | All-cause | 1.22 | 1.00 | 1.49 | 1.49 | 1.00 | 2.23 | 1.83 | 1.00 | 3.33 | HR | 7529 | 701 |
| Strous et al | 2019 | All-cause | 1.16 | 0.90 | 1.49 | 1.34 | 0.82 | 2.21 | 1.55 | 0.74 | 3.28 | HR | 790 | 179 |
| Trepanier et al | 2019 | All-cause | 1.62 | 1.08 | 2.42 | 2.61 | 1.16 | 5.88 | 4.22 | 1.25 | 14.26 | HR | 408 | 39 |
| Trepanier et al | 2019 | Cancer-specific | 0.93 | 0.90 | 0.96 | 0.86 | 0.81 | 0.92 | 0.80 | 0.73 | 0.88 | HR | 408 | 39 |
| Xu et al | 2014 | All-cause | 1.21 | 1.09 | 1.33 | 1.45 | 1.19 | 1.78 | 1.75 | 1.29 | 2.37 | OR | 4209 | 636 |
| Xu et al | 2014 | Cancer-specific | 1.62 | 1.30 | 2.02 | 2.62 | 1.69 | 4.07 | 4.23 | 2.19 | 8.20 | OR | 4209 | 464 |
| Yun et al | 2012 | All-cause | 1.42 | 1.27 | 1.59 | 2.03 | 1.61 | 2.52 | 2.89 | 2.04 | 4.01 | HR | 147,682 | 20,084 |
| Zeig-Owens et al | 2009 | All-cause | 1.10 | 0.95 | 1.27 | 1.20 | 0.90 | 1.61 | 1.32 | 0.86 | 2.05 | HR | 3006 | 1172 |
The table presents data extracted from individual studies, including author, year of publication, and subgroup classification (all-cause mortality or colorectal cancer-specific mortality). Reported hazard ratios, odds ratios, or risk ratios (HR/OR/RR) are provided for different treatment delay periods (4, 8, and 12 weeks), along with corresponding confidence intervals (lower and upper bounds)
CI confidence interval, HR hazard ratio, OR odds ratio, RR risk ratio
Effect of 4-week delay in treatment
A 4-week treatment delay was associated with a statistically significant reduction in overall survival, with a pooled HR of 1.14 (95% confidence interval [CI], 1.09–1.18) (Fig. 2, upper panel). Statistical assessment demonstrated significant heterogeneity among studies (p < 0.01), with an I2-value of 89%, indicating that most of the heterogeneity between studies was attributable to true heterogeneity rather than random chance variation.
Fig. 2.
Forest plot depicting the association between a 4-week treatment delay and survival outcomes in colorectal cancer patients. The upper section illustrates the impact on all-cause mortality, while the lower section presents colorectal cancer-specific mortality. Hazard ratios (HR) and corresponding confidence intervals (CI) are displayed for each included study, with pooled estimates calculated using a random-effects model. Squares represent individual study estimates, with their size reflecting study weight, while horizontal lines indicate confidence intervals. The diamond represents the pooled effect estimate. Measures of heterogeneity are reported for both subgroups. CI, confidence interval; HR, hazard ratio; IV, inverse variance; SE, standard error
For colorectal cancer-specific mortality, the analysis resulted in a HR of 1.07 (95% CI, 0.98–1.18), indicating no statistically significant impact of delayed treatment on cancer-specific survival (Fig. 2, lower panel). Substantial heterogeneity was evident (p < 0.01), with an I2-value of 86%, demonstrating considerable variation in effect sizes across studies. The pooled analysis demonstrated a statistically significant effect with an HR of 1.12 (95% CI, 1.08–1.16). This analysis also revealed significant heterogeneity (p < 0.01), with an I2-value of 91%, suggesting substantial true differences in effect sizes across the included studies.
Publication bias was thoroughly assessed through multiple methods. The funnel plot analysis for overall survival (Fig. 3A) showed no indication of publication bias, a finding supported by Egger’s test results (intercept, 1.64; 95% CI, − 0.02 and 3.31; t = 1.937; p = 0.067). However, examination of cancer-specific survival revealed a potential publication bias through funnel plot visualization (Fig. 3B), which was corroborated by Egger’s test findings (intercept, 2.82; 95% CI, 0.72–4.91; t = 2.635; p = 0.034).
Fig. 3.
Funnel plots assessing potential publication bias in studies examining the impact of a 4-week treatment delay on colorectal cancer survival. A The relationship between overall survival and hazard ratio (HR); B colorectal cancer-specific survival. Each dot represents an individual study, with standard error plotted against hazard ratio
Effect of 8-week delay in treatment
An increased, statistically significant impact on overall survival was observed for an 8-week treatment delay, with a pooled HR of 1.29 (95% CI, 1.20–1.39), as shown in Fig. 4 (upper panel). Significant heterogeneity was present across studies (p < 0.01), with an I2-value of 89%, indicating substantial variation in treatment effect estimates.
Fig. 4.
Forest plot evaluating the impact of an 8-week treatment delay on survival outcomes in colorectal cancer patients. The top panel illustrates the association with all-cause mortality, and the lower panel presents the effect on colorectal cancer-specific mortality. CI, confidence interval; HR, hazard ratio; IV, inverse variance; SE, standard error
For colorectal cancer-specific survival, the analysis resulted in a non-significant effect with an HR of 1.15 (95% CI, 0.95–1.39). Heterogeneity remained significant (p < 0.01) with an I2-value of 86%, suggesting considerable variation in effect sizes (Fig. 4, bottom panel). The comprehensive evaluation of all 31 cohorts yielded a statistically significant HR of 1.24 (95% CI, 1.16–1.34), with persistent high heterogeneity (p < 0.01; I2 = 91%).
Effect of 12-week delay in treatment
The largest increase in mortality risk was observed for a 12-week treatment delay with an HR of 1.47 (95% CI, 1.31–1.64), as depicted in the upper panel of Fig. 5. Statistical assessment showed significant heterogeneity (p < 0.01) with an I2-value of 90%, indicating considerable variation in effect sizes across studies.
Fig. 5.
Impact of a 12-week treatment delay on survival in colorectal cancer patients, with all-cause mortality displayed in the upper panel and colorectal cancer-specific mortality in the lower panel. A 12-week treatment delay is associated with a significantly increased risk of mortality, with a pooled hazard ratio of 1.39 (95% confidence interval, 1.25–1.55), indicating a 39% higher risk of death compared to timely treatment, with substantial heterogeneity observed (I [2] = 91%). CI, confidence interval; HR, hazard ratio; IV, inverse variance; SE, standard error
For colorectal cancer-specific survival, the wide confidence interval (95% CI, 0.93–1.63) indicates no statistically significant difference in cancer-specific mortality (Fig. 5, lower panel). Heterogeneity remained significant (p < 0.01) with an I2-value of 86%, demonstrating substantial variation in treatment effects. The consolidated analysis of all cohorts revealed a statistically significant effect, with an HR of 1.39 (95% CI, 1.25–1.55). Significant heterogeneity persisted (p < 0.01) with an I2-value of 91%, indicating substantial true differences in effect sizes across studies.
Discussion
This meta-analysis provides compelling evidence that treatment delays in CRC are associated with significantly increased mortality, with progressively worse survival outcomes observed at 4, 8, and 12 weeks of delay. These findings align with prior studies suggesting that delayed initiation of cancer therapy contributes to tumor progression, increased disease burden at treatment onset, and reduced therapeutic efficacy [4, 9].
The impact of treatment delay on CRC outcomes is multifaceted. Delays allow tumors to progress to more advanced stages, increasing the likelihood of lymphovascular invasion [51], perineural invasion [52], and distant metastases. Therefore, minimizing treatment delays is essential to prevent disease progression and improve survival rates in CRC patients. Additionally, delayed treatment can lead to worsened patient performance status, limiting therapeutic options and increasing treatment-related complications.
Delays in CRC treatment arise from a multifactorial interplay of patient-, provider-, and system-related barriers, which often act synergistically to prolong time-to-treatment [10, 53]. Many patients experience delays due to knowledge shortage and low symptom awareness, particularly in early-stage CRC, where signs such as intermittent gastrointestinal discomfort or changes in bowel habits may be misattributed to benign conditions [18, 54, 55]. Psychological factors—including fear of cancer diagnosis, unwillingness to accept colonoscopy [53], concerns about treatment side effects, and reluctance to seek medical attention—further contribute to delays. Socioeconomic challenges such as financial hardship, access to health services, insurance issues, insufficient social support, lack of transportation, visits to alternative medicine practitioners, and inadequate healthcare literacy are also critical factors, disproportionately affecting underserved populations [16, 53, 54].
Delays at the provider level often stem from misdiagnosis or failure to recognize early warning signs, especially in younger patients or those without classic CRC risk factors [56]. In many cases, diagnostic workups are prolonged due to stepwise testing protocols, where patients undergo multiple rounds of noninvasive tests before referral for definitive colonoscopy [56, 57]. Sampling error for biopsy specimens also frequently results in delays [58]. Furthermore, treatment hesitancy—particularly in medically complex patients—may lead to prolonged pre-treatment optimization efforts to ensure they can tolerate aggressive cancer therapies. While these precautions aim to enhance patient safety, they may inadvertently postpone initiation of curative therapy, potentially impacting overall outcomes.
The most substantial delays often arise from structural inefficiencies in healthcare systems [23, 59]. Long wait times for specialist referrals, delays in diagnostic procedures (e.g., colonoscopies, imaging, pathology reports), and bottlenecks in scheduling oncology consultations are persistent issues [20, 60, 61]. These barriers are exacerbated in overburdened healthcare systems with limited resources, particularly in regions with shortages of gastroenterologists, oncologists, and surgical teams. Geographic disparities in healthcare access further contribute to treatment delays, disproportionately affecting patients in rural or underserved areas.
The COVID- 19 pandemic further highlighted the fragility of healthcare systems, with widespread disruptions in elective surgeries, diagnostic procedures, and oncology care, leading to unprecedented delays in cancer treatment worldwide [27, 28, 62, 63]. While some healthcare systems have recovered, residual delays persist, emphasizing the need for resilient, adaptable oncology care pathways that can withstand future crises.
Although CRC affects a broad patient population, older adults are disproportionately impacted by treatment delays due to unique vulnerabilities associated with aging. Age-related frailty [64], multimorbidity [65], and cognitive impairment complicate treatment decision-making, often requiring additional pre-treatment assessments that extend time-to-treatment [66]. Polypharmacy and preexisting conditions may further delay therapy, as oncologists and surgeons navigate potential drug interactions, cardiovascular risk assessments, and anesthesia considerations [67]. Beyond biological factors, older adults also face greater logistical challenges in accessing timely care. Many rely on caregivers for transportation, appointment scheduling, and treatment coordination, which can create additional barriers, particularly for those with limited social support [68]. Moreover, provider biases regarding treatment tolerability in older patients (“age bias”) may contribute to therapeutic nihilism, where aggressive treatments are postponed or withheld due to concerns over toxicity, despite evidence suggesting that appropriately selected older patients benefit from standard therapies [69]. Given the aging global population and the rising burden of CRC in older adults, addressing these vulnerabilities is critical to reducing disparities in cancer treatment outcomes. Strategies such as integrated geriatric-oncology care models, prehabilitation programs, and enhanced multidisciplinary decision-making frameworks can help optimize treatment pathways for older patients while minimizing unnecessary delays [70].
Despite growing evidence that delayed CRC treatment significantly worsens survival, there remains no universally accepted threshold defining an “acceptable” delay [71]. This lack of standardization contributes to substantial variability in clinical practice, with treatment timelines differing across healthcare systems, institutions, and geographic regions. Establishing evidence-based time-to-treatment benchmarks is crucial to ensuring equitable and timely cancer care delivery, particularly for vulnerable patient populations. To address these challenges, policymakers and healthcare institutions must prioritize key reforms aimed at reducing delays and improving patient outcomes. First, streamlining referral pathways by implementing direct-to-specialist triage systems can help expedite diagnostic workups, ensuring that high-risk patients are promptly identified and receive timely intervention [21]. Additionally, expanding access to oncology services, particularly in resource-limited settings, is critical. This can be achieved through telemedicine consultations [72–75], mobile screening programs, and the development of regional cancer networks [76, 77], which would help bridge the gap between urban and rural healthcare infrastructures. Beyond structural improvements, patient-centered strategies must also be implemented to minimize delays. Patient navigation programs—which provide guidance and support to individuals facing logistical, financial, and administrative barriers—have been shown to improve timely access to care, particularly in underserved communities [78, 79]. Finally, integrating comprehensive geriatric assessments into oncology workflows is essential to ensuring that older adults receive timely, personalized, and evidence-based treatment decisions [70]. By systematically evaluating frailty, comorbidities, and functional status, clinicians can make informed treatment choices that balance oncologic urgency with individual patient needs, preventing unnecessary delays while optimizing outcomes. By adopting these multifaceted interventions, healthcare systems can move toward a more standardized, patient-centric approach to CRC treatment, ultimately improving survival rates and reducing disparities in cancer care delivery.
While this meta-analysis provides quantitative evidence that treatment delays significantly increase mortality risk in CRC, further research is needed to refine our understanding of how these delays affect different patient populations and to develop targeted interventions. Future studies should focus on stratifying the risks associated with treatment delays based on key patient characteristics, including age, tumor stage, and comorbidity burden, to create personalized treatment urgency models. Such models could help clinicians prioritize high-risk patients and tailor treatment strategies accordingly. In addition to patient-specific risk assessments, research should examine healthcare system interventions that may mitigate delays. Initiatives such as fast-track diagnostic clinics and multidisciplinary pre-treatment assessments could help streamline care delivery, reduce bottlenecks in oncology services, and ultimately improve patient outcomes [80]. Evaluating the effectiveness of these interventions across different healthcare systems will be crucial for developing evidence-based policies aimed at reducing time-to-treatment disparities. Beyond survival outcomes, future studies should explore the broader consequences of treatment delays, including quality of life, post-treatment functional status, and patient-reported experiences [81, 82]. These factors are particularly relevant for older adults, who may experience greater long-term functional impairment following delayed treatment. Understanding the full spectrum of impacts will help guide patient-centered oncology care and ensure that interventions address both survival and post-treatment well-being. Finally, it is essential to assess real-world data from diverse healthcare settings, particularly in lower-income and rural populations, where barriers to timely treatment are often more pronounced [28]. Expanding research to include these underrepresented groups will provide a more comprehensive, globally applicable understanding of treatment delays and inform policy decisions aimed at reducing disparities in cancer care. By addressing these critical gaps, future research can help refine clinical guidelines, optimize healthcare delivery, and improve outcomes for all patients facing treatment delays in colorectal cancer. While our meta-analysis focused on treatment initiation delays, the impact of chemotherapy interruptions or premature discontinuation—particularly relevant in older adults—also warrants investigation. Emerging data suggest such disruptions may negatively affect survival, and this remains an area for further study.
Although we considered study design, outcome definition clarity, and adjustment for confounders during selection, we did not apply a formal risk of bias assessment tool. We acknowledge this as a limitation and recommend that future meta-analyses incorporate standardized quality assessments, such as the Newcastle––Ottawa Scale. Most studies did not stratify results by cancer stage, limiting our ability to evaluate whether treatment delay has differential effects across early- vs. late-stage CRC. Stage-specific analyses represent an important area for future investigation. While we did not perform formal sensitivity analyses (e.g., excluding outlier studies), informal inspection showed no single study significantly influenced the pooled estimates. We recommend that future work includes predefined sensitivity analyses to further validate findings. Although publication bias was assessed using funnel plots and Egger’s regression, we did not apply Trim-and-Fill or other imputation methods. Incorporating such analyses in future work could enhance detection of small-study effects and potential reporting bias. We did not conduct meta-regression due to limited covariate data across studies. Nevertheless, we recognize that meta-regression could help identify contributors to heterogeneity and should be considered in future reviews. This review was not prospectively registered, which we acknowledge as a limitation. Prospective registration through platforms like PROSPERO would improve transparency and reduce risk of bias and should be pursued in future meta-analyses.
In conclusion, this meta-analysis demonstrates that treatment delays in CRC are associated with significantly worse survival outcomes, reinforcing the importance of timely intervention. Delays arise from a complex interplay of patient, provider, and system-level barriers, with older adults facing additional vulnerabilities that increase their risk of delayed care. Addressing these challenges requires a multidimensional approach, including streamlined healthcare pathways, improved resource allocation, targeted patient support programs, and standardized treatment benchmarks. Future research should further refine age- and risk-stratified treatment guidelines to ensure that all patients, regardless of age or healthcare setting, receive timely and effective CRC treatment.
Author contribution
ZU, MF, AL, AU, and BG contributed to the study conception and design. MF, AL, PV, AB, and AU performed the systematic literature search and data extraction. Statistical analyses were conducted by GM, JTF, AU, and BG. AB, JTF, ZU, GM, MF, BG, AL, and AU drafted the manuscript. JTF and PV provided critical revisions for intellectual content. BG and JTF prepared the figures. All authors reviewed, edited, and approved the final manuscript.
Funding
Open access funding provided by Semmelweis University. This work was supported by grants from the National Institute on Aging (RF1 AG072295, R01 AG055395, R01 AG068295; R01 AG070915), the National Institute of Neurological Disorders and Stroke (R01 NS100782), and the National Cancer Institute (R01 CA255840). This work was also supported by TKP2021-NKTA- 47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by the Hungarian Research Network—HUN-REN (TKCS- 2021/32) through the HUN-REN-DE Public Health Research Group at Department of Public Health and Epidemiolog (Faculty of Medicine, University of Debrecen); by funding through the National Cardiovascular Laboratory Program (RRF- 2.3.1–21 - 2022–00003) and by the National Laboratory for Drug Research and Development (PharmaLab, RRF- 2.3.1–21 - 2022–00015) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; by the Semmelweis Momentum Programme; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K20 funding scheme; and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/EUniWell/EAC-A02 - 2019/EAC-A02 - 2019–1). The computational infrastructure of A5 Genetics Ltd (Kutaso, Hungary) was used for the study. This work was also supported by the EKÖP- 2024–2 and EKÖP- 2024–9 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The 4o version of ChatGPT, developed by OpenAI, and Claude 3.5 Sonnet, developed by Anthropic were used as a language tool to refine our writing and enhance the clarity of our work. The support of ELIXIR Hungary is acknowledged.
Declarations
Ethics approval and consent to participate
NA.
Consent for publication
NA.
Competing interests
Dr. Balázs Győrffy serves as Associate Editor for GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience and has personal relationships with individuals involved in the submission of this paper. The rest of the authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zoltan Ungvari, Mónika Fekete, János Tibor Fekete, and Andrea Lehoczki contributed equally to this work.
References
- 1.Whittaker TM, Abdelrazek MEG, Fitzpatrick AJ, Froud JLJ, Kelly JR, Williamson JS, Williams GL. Delay to elective colorectal cancer surgery and implications for survival: a systematic review and meta-analysis. Colorectal Dis. 2021;23:1699–711. 10.1111/codi.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, Esener S, Fitzgerald RC, Gambhir SS, Kuhn P, Rebbeck TR. Early detection of cancer. Science. 2022 Mar 18;375(6586):eaay9040. 10.1126/science.aay9040 [DOI] [PubMed]
- 3.Holliday HW, Hardcastle JD. Delay in diagnosis and treatment of symptomatic colorectal cancer. Lancet. 1979;1:309–11. 10.1016/s0140-6736(79)90718-9. [DOI] [PubMed] [Google Scholar]
- 4.Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, O’Sullivan DE, Booth CM, Sullivan R, Aggarwal A. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fekete M, Major D, Feher A, Fazekas-Pongor V, Lehoczki A. Geroscience and pathology: a new frontier in understanding age-related diseases. Pathol Oncol Res. 2024;23(30):1611623. 10.3389/pore.2024.1611623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungvari Z, Fekete M, Varga P, Lehoczki A, Fekete JT, Ungvari A, Gyorffy B. Overweight and obesity significantly increase colorectal cancer risk: a meta-analysis of 66 studies revealing a 25–57% elevation in risk. Geroscience. 2024. 10.1007/s11357-024-01375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD. Colorectal Cancer Collaborators: Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2019;2022(7):627–47. 10.1016/S2468-1253(22)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulanja MB, Ntafam C, Beutler BD, Antwi-Amoabeng D, Rahman GA, Ulanja RN, Mabrouk T, Governor SB, Djankpa FT, Alese OB. Race, age, and sex differences on the influence of obesity on colorectal cancer sidedness and mortality: A national cross-sectional study. J Surg Oncol. 2023;127:109–18. 10.1002/jso.27096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–42. 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 10.Langenbach MR, Schmidt J, Neumann J, Zirngibl H. Delay in treatment of colorectal cancer: multifactorial problem. World J Surg. 2003;27:304–8. 10.1007/s00268-002-6678-9. [DOI] [PubMed] [Google Scholar]
- 11.Korsgaard M, Pedersen L, Laurberg S. Delay of diagnosis and treatment of colorectal cancer–a population-based Danish study. Cancer Detect Prev. 2008;32:45–51. 10.1016/j.cdp.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Zarcos-Pedrinaci I, Fernández-López A, Téllez T, Rivas-Ruiz F, Rueda A, Suarez-Varela MM, Briones E, Baré M, Escobar A, Sarasqueta C, de Larrea NF. Factors that influence treatment delay in patients with colorectal cancer. Oncotarget. 2016 Nov 24;8(22):36728. 10.18632/oncotarget.13574 [DOI] [PMC free article] [PubMed]
- 13.Macia F, Pumarega J, Gallen M, Porta M. Time from (clinical or certainty) diagnosis to treatment onset in cancer patients: the choice of diagnostic date strongly influences differences in therapeutic delay by tumor site and stage. J Clin Epidemiol. 2013;66:928–39. 10.1016/j.jclinepi.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Zhang FG, Sheni R, Zhang C, Viswanathan S, Fiori K, Mehta V. Association Between Social Determinants of Health and Cancer Treatment Delay in an Urban Population. JCO Oncol Pract. 2024;20:1733–43. 10.1200/OP.24.00118. [DOI] [PubMed] [Google Scholar]
- 15.Padilla-Ruiz M, Morales-Suárez-Varela M, Rivas-Ruiz F, Alcaide J, Varela-Moreno E, Zarcos-Pedrinaci I, Téllez T, Fernández-de Larrea-Baz N, Baré M, Bilbao A, Sarasqueta C. Influence of Diagnostic Delay on Survival Rates for Patients with Colorectal Cancer. Int J Environ Res Publ Health. 2022;19(6):3626. 10.3390/ijerph19063626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langenbach MR, Sauerland S, Krobel KW, Zirngibl H. Why so late?!–delay in treatment of colorectal cancer is socially determined. Langenbecks Arch Surg. 2010;395:1017–24. 10.1007/s00423-010-0664-8. [DOI] [PubMed] [Google Scholar]
- 17.Montiel Ishino FA, Odame EA, Villalobos K, Whiteside M, Mamudu H, Williams F. Applying Latent Class Analysis on Cancer Registry Data to Identify and Compare Health Disparity Profiles in Colorectal Cancer Surgical Treatment Delay. J Public Health Manag Pract. 2022;28:E487–96. 10.1097/PHH.0000000000001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarcos-Pedrinaci I, Tellez T, Rivas-Ruiz F, Padilla-Ruiz MDC, Alcaide J, Rueda A, Bare ML, Suarez-Varela MMM, Briones E, Sarasqueta C, Fernandez-Larrea N, Escobar A, Quintana JM, Redondo M. Factors Associated with Prolonged Patient-Attributable Delay in the Diagnosis of Colorectal Cancer. Cancer Res Treat. 2018;50:1270–80. 10.4143/crt.2017.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiber EE, Camacho F, Zeeshan MF, Kern TT, Fleming ST. Disparities in Colorectal Cancer Treatment Delay Within Appalachia-The Role of For-Profit Hospitals. J Rural Health. 2015;31:382–91. 10.1111/jrh.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-de Castro JD, Baiocchi Ureta F, Fernandez Gonzalez R, Pin Vieito N, Cubiella FJ. The effect of diagnostic delay attributable to the healthcare system on the prognosis of colorectal cancer. Gastroenterol Hepatol. 2019;42:527–33. 10.1016/j.gastrohep.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer. 2008;98:60–70. 10.1038/sj.bjc.6604096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah JP, Valdes M, Rockey DC. Transferred and delayed care of patients with colorectal cancer in a safety-net hospital system–manifestations of a distressed healthcare system. J Gen Intern Med. 2012;27:1142–9. 10.1007/s11606-012-2040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bawden A. Sharp rise in cancer patients in England waiting months for treatment. The Guardian. 13 Feb 2025. https://www.theguardian.com/society/2025/feb/13/sharp-rise-in-cancer-patients-in-england-waiting-months-for-treatment acccessed on 02/20/2025
- 24.Van Hout AM, de Wit NJ, Rutten FH, Peeters PH. Determinants of patient’s and doctor’s delay in diagnosis and treatment of colorectal cancer. Eur J Gastroenterol Hepatol. 2011;23:1056–63. 10.1097/MEG.0b013e32834c4839. [DOI] [PubMed] [Google Scholar]
- 25.Tudor Car L, Papachristou N, Bull A, Majeed A, Gallagher J, El-Khatib M, Aylin P, Rudan I, Atun R, Car J, Vincent C. Clinician-identified problems and solutions for delayed diagnosis in primary care: a PRIORITIZE study. BMC Fam Pract. 2016;17:131. 10.1186/s12875-016-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roderburg C, Loosen SH, Leyh C, Joerdens MS, Mohr R, Luedde T, Alymova S, Klein I, Kostev K. Prevalence of and factors associated with a treatment delay due to the COVID-19 pandemic in patients with gastrointestinal cancer in Europe. J Cancer Res Clin Oncol. 2023;149:11849–56. 10.1007/s00432-023-05062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner H, Weber L, Cardoso R, Heisser T, Hoffmeister M, Holleczek B. Indications of sustained delay of colorectal cancer diagnoses in Germany during the first 2 years of the COVID-19 pandemic. Int J Cancer. 2024;155:595–7. 10.1002/ijc.34943. [DOI] [PubMed] [Google Scholar]
- 28.Mentrasti G, Cantini L, Zichi C, D’Ostilio N, Gelsomino F, Martinelli E, Chiari R, La Verde N, Bisonni R, Cognigni V, Pinterpe G, Pecci F, Migliore A, Aimar G, De Vita F, Traisci D, Spallanzani A, Martini G, Nicolardi L, Cona MS, Baleani MG, Rocchi MLB, Berardi R. Alarming Drop in Early Stage Colorectal Cancer Diagnoses After COVID-19 Outbreak: A Real-World Analysis from the Italian COVID-DELAY Study. Oncologist. 2022;27:e723–30. 10.1093/oncolo/oyac129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greene G, Griffiths R, Han J, Akbari A, Jones M, Lyons J, Lyons RA, Rolles M, Torabi F, Warlow J, Morris ERA, Lawler M, Huws DW. Impact of the SARS-CoV-2 pandemic on female breast, colorectal and non-small cell lung cancer incidence, stage and healthcare pathway to diagnosis during 2020 in Wales, UK, using a national cancer clinical record system. Br J Cancer. 2022;127:558–68. 10.1038/s41416-022-01830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fekete JT, Győrffy B. MetaAnalysisOnline. com: Web-Based Tool for the Rapid Meta-Analysis of Clinical and Epidemiological Studies. Journal of Medical Internet Research. 2025 Mar 6;27:e64016. 10.2196/64016 [DOI] [PMC free article] [PubMed]
- 31.Lima IS, Yasui Y, Scarfe A, Winget M. Association between receipt and timing of adjuvant chemotherapy and survival for patients with stage III colon cancer in Alberta. Canada Cancer. 2011;117:3833–40. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed S, Ahmad I, Zhu T, Arnold FP, Anan GF, Sami A, Yadav SK, Alvi R, Haider K. Early discontinuation but not the timing of adjuvant therapy affects survival of patients with high-risk colorectal cancer: a population-based study. Dis Colon Rectum. 2010;53:1432–8. [DOI] [PubMed] [Google Scholar]
- 33.Hershman D, Hall MJ, Wang X, Jacobson JS, McBride R, Grann VR, Neugut AI. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107:2581–8. 10.1002/cncr.22316. [DOI] [PubMed] [Google Scholar]
- 34.Cheung WY, Neville BA, Earle CC. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Dis Colon Rect. 2009;52(6):1054–64. 10.1007/DCR.0b013e3181a51173. [DOI] [PubMed] [Google Scholar]
- 35.Zeig-Owens R, Gershman ST, Knowlton R, Jacobson JS. Survival and time interval from surgery to start of chemotherapy among colon cancer patients. J Registry Manag. 2009;36(2):30–41. [PubMed] [Google Scholar]
- 36.Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, Hill M, Hickish T, Lofts F, Jodrell D, Webb A, Oates JR. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005;16:549–57. 10.1093/annonc/mdi116. [DOI] [PubMed] [Google Scholar]
- 37.Massarweh NN, Haynes AB, Chiang YJ, Chang GJ, You YN, Feig BW, Cormier JN. Adequacy of the National Quality Forum’s Colon Cancer Adjuvant Chemotherapy Quality Metric: Is 4 Months Soon Enough? Ann Surg. 2015;262:312–20. 10.1097/sla.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu F, Rimm AA, Fu P, Krishnamurthi SS, Cooper GS. The impact of delayed chemotherapy on its completion and survival outcomes in stage II colon cancer patients. PLoS ONE. 2014;9:e107993. 10.1371/journal.pone.0107993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becerra AZ, Aquina CT, Mohile SG, Tejani MA, Schymura MJ, Boscoe FP, Xu Z, Justiniano CF, Boodry CI, Swanger AA, Noyes K, Monson JR, Fleming FJ. Variation in Delayed Time to Adjuvant Chemotherapy and Disease-Specific Survival in Stage III Colon Cancer Patients. Ann Surg Oncol. 2017;24:1610–7. 10.1245/s10434-016-5622-4. [DOI] [PubMed] [Google Scholar]
- 40.Bayraktar UD, Chen E, Bayraktar S, Sands LR, Marchetti F, Montero AJ, Rocha-Lima CM. Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma? Cancer. 2011;117:2364–70. 10.1002/cncr.25720. [DOI] [PubMed] [Google Scholar]
- 41.Flemming JA, Nanji S, Wei X, Webber C, Groome P, Booth CM. Association between the time to surgery and survival among patients with colon cancer: A population-based study. Eur J Surg Oncol. 2017;43:1447–55. 10.1016/j.ejso.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Bagaria SP, Heckman MG, Diehl NN, Parker A, Wasif N. Delay to Colectomy and Survival for Patients Diagnosed with Colon Cancer. J Invest Surg. 2019;32:350–7. 10.1080/08941939.2017.1421732. [DOI] [PubMed] [Google Scholar]
- 43.Shin DW, Cho J, Kim SY, Guallar E, Hwang SS, Cho B, Oh JH, Jung KW, Seo HG, Park JH. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20:2468–76. 10.1245/s10434-013-2957-y. [DOI] [PubMed] [Google Scholar]
- 44.Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, Choi IJ, Kim YW, Park SJ, Kim JH, Lee DH, Yoon SJ, Jeong SY, Noh DY, Heo DS. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012;23:2731–7. 10.1093/annonc/mds101. [DOI] [PubMed] [Google Scholar]
- 45.Murchie P, Raja EA, Brewster DH, Campbell NC, Ritchie LD, Robertson R, Samuel L, Gray N, Lee AJ. Time from first presentation in primary care to treatment of symptomatic colorectal cancer: effect on disease stage and survival. Br J Cancer. 2014;111:461–9. 10.1038/bjc.2014.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strous MTA, Janssen-Heijnen MLG, Vogelaar FJ. Impact of therapeutic delay in colorectal cancer on overall survival and cancer recurrence - is there a safe timeframe for prehabilitation? Eur J Surg Oncol. 2019;45:2295–301. 10.1016/j.ejso.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Lo BD, Caturegli G, Stem M, Biju K, Safar B, Efron JE, Rajput A, Atallah C. The Impact of Surgical Delays on Short- and Long-Term Survival Among Colon Cancer Patients. Am Surg. 2021;87:1783–92. 10.1177/00031348211047511. [DOI] [PubMed] [Google Scholar]
- 48.Edwards GC, Gamboa AC, Feng MP, Muldoon RL, Hopkins MB, Abdel-Misih S, Balch GC, Holder-Murray J, Mohammed M, Regenbogen SE, Silviera ML, Hawkins AT. What’s the magic number? Impact of time to initiation of treatment for rectal cancer. Surgery. 2022;171:1185–92. 10.1016/j.surg.2021.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trepanier M, Paradis T, Kouyoumdjian A, Dumitra T, Charlebois P, Stein BS, Liberman AS, Schwartzman K, Carli F, Fried GM, Feldman LS, Lee L. The Impact of Delays to Definitive Surgical Care on Survival in Colorectal Cancer Patients. J Gastrointest Surg. 2020;24:115–22. 10.1007/s11605-019-04328-4. [DOI] [PubMed] [Google Scholar]
- 50.Roder D, Karapetis CS, Olver I, Keefe D, Padbury R, Moore J, Joshi R, Wattchow D, Worthley DL, Miller CL, Holden C, Buckley E, Powell K, Buranyi-Trevarton D, Fusco K, Price T. Time from diagnosis to treatment of colorectal cancer in a South Australian clinical registry cohort: how it varies and relates to survival. BMJ Open. 2019;9:e031421. 10.1136/bmjopen-2019-031421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Z, Zheng Y, Yang J, Jin W, Wang J, Wang W, Li S. Prognostic Analysis of Lymphovascular Invasion in Stages I-III Colorectal Cancer: A Retrospective Study Based on Propensity Score Match. Am J Clin Oncol. 2023;46:366–73. 10.1097/COC.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Huo R, He K, Cheng L, Zhang S, Yu M, Zhao W, Li H, Xue J. Perineural invasion in colorectal cancer: mechanisms of action and clinical relevance. Cell Oncol (Dordr). 2024;47:1–17. 10.1007/s13402-023-00857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin Y, Zheng MC, Yang X, Chen TL, Zhang JE. Patient delay to diagnosis and its predictors among colorectal cancer patients: A cross-sectional study based on the Theory of Planned Behavior. Eur J Oncol Nurs. 2022;60:102174. 10.1016/j.ejon.2022.102174. [DOI] [PubMed] [Google Scholar]
- 54.Chow Z, Osterhaus P, Huang B, Chen Q, Schoenberg N, Dignan M, Evers BM, Bhakta A. Factors Contributing to Delay in Specialist Care After Colorectal Cancer Diagnosis in Kentucky. J Surg Res. 2021;259:420–30. 10.1016/j.jss.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rydbeck D, Asplund D, Bock D, Haglind E, Park J, Rosenberg J, Walming S, Angenete E. Younger age at onset of colorectal cancer is associated with increased patient’s delay. Eur J Cancer. 2021;154:269–76. 10.1016/j.ejca.2021.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Yee A, Linton T, Lee MS, Raimes S. The factors that lead to a delay between general practitioner referral of symptomatic patients and specialist diagnosis of colorectal cancer: an audit in the Bay of Plenty District Health Board. N Z Med J. 2019;132:27–37. [PubMed] [Google Scholar]
- 57.Mutneja HR, Bhurwal A, Arora S, Vohra I, Attar BM. A delay in colonoscopy after positive fecal tests leads to higher incidence of colorectal cancer: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1479–86. 10.1111/jgh.15381. [DOI] [PubMed] [Google Scholar]
- 58.Johnson G, Hershorn O, Singh H, Park J, Helewa RM. Sampling error in the diagnosis of colorectal cancer is associated with delay to surgery: a retrospective cohort study. Surg Endosc. 2022;36:4893–902. 10.1007/s00464-021-08841-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suryani ND, Wiranata JA, Puspitaningtyas H, Hutajulu SH, Prabandari YS, Handaya AY, Hardianti MS, Taroeno-Hariadi KW, Kurnianda J, Purwanto I. Determining factors of presentation and diagnosis delays in patients with colorectal cancer and the impact on stage: a cross sectional study in Yogyakarta Indonesia. Ecancermedicalscience. 2024;18:1761. 10.3332/ecancer.2024.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Geest LG, Elferink MA, Steup WH, Witte AM, Nortier JW, Tollenaar RA, Struikmans H. Guidelines-based diagnostic process does increase hospital delay in a cohort of colorectal cancer patients: a population-based study. Eur J Cancer Prev. 2014;23:344–52. 10.1097/CEJ.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 61.Abdulaal A, Arhi C, Ziprin P. Effect of Health Care Provider Delays on Short-Term Outcomes in Patients With Colorectal Cancer: Multicenter Population-Based Observational Study. Interact J Med Res. 2020;9:e15911. 10.2196/15911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishibashi F, Shida D, Suzuki S, Nagai M, Mochida K, Morishita T. A delay in the diagnosis of colorectal cancer screened by fecal immunochemical tests during the COVID-19 pandemic: a longitudinal cohort study. Int J Colorectal Dis. 2022;37:2543–6. 10.1007/s00384-022-04270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ricciardiello L, Ferrari C, Cameletti M, Gaianill F, Buttitta F, Bazzoli F, De’Angelis GL, Malesci A, Laghi L. Impact of SARS-CoV-2 pandemic on colorectal cancer screening delay: effect on stage shift and increased mortality. Clinical Gastroenterology and Hepatology. 2021 Jul 1;19(7):1410-7. 10.1016/j.cgh.2020.09.008 [DOI] [PMC free article] [PubMed]
- 64.Maeda H, Takahashi M, Seo S, Hanazaki K. Frailty and Colorectal Surgery: Review and Concept of Cancer Frailty. J Clinic Med. 2023;12(15):5041. 10.3390/jcm12155041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mounce LTA, Price S, Valderas JM, Hamilton W. Comorbid conditions delay diagnosis of colorectal cancer: a cohort study using electronic primary care records. Br J Cancer. 2017;116:1536–43. 10.1038/bjc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones D, Di Martino E, Bradley SH, Essang B, Hemphill S, Wright JM, Renzi C, Surr C, Clegg A, De Wit N, Neal R. Factors affecting the decision to investigate older adults with potential cancer symptoms: a systematic review. Br J Gen Pract. 2022;72:e1–10. 10.3399/BJGP.2021.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maggiore RJ, Dale W, Gross CP, Feng T, Tew WP, Mohile SG, Owusu C, Klepin HD, Lichtman SM, Gajra A, Ramani R. Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: effect on chemotherapy-related toxicity and hospitalization during treatment. J Am Geriat Soc. 2014;62(8):1505–12. 10.1111/jgs.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clauer J, Ameen K, Incudine A, Newcomer K, Mulvey A, Steinberg K, Geiger A, Campbell S, Calabrese A, Tejani M, Shah M. Treatment experiences and decision-making among patients with metastatic colorectal cancer: Results of an online US patient survey. J Clin Oncol 2025;43, 2025 (suppl 4; abstr 80).
- 69.Dotan E, Lynch SM, Ryan JC, Mitchell EP. Disparities in care of older adults of color with cancer: A narrative review. Cancer Med. 2024;13:e6790. 10.1002/cam4.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Outlaw D, Abdallah M, Gil-Jr LA, Giri S, Hsu T, Krok-Schoen JL, Liposits G, Madureira T, Marinho J, Subbiah IM, Tuch G, Williams GR. The Evolution of Geriatric Oncology and Geriatric Assessment over the Past Decade. Semin Radiat Oncol. 2022;32:98–108. 10.1016/j.semradonc.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee YH, Kung PT, Wang YH, Kuo WY, Kao SL, Tsai WC. Effect of length of time from diagnosis to treatment on colorectal cancer survival: A population-based study. PLoS ONE. 2019;14:e0210465. 10.1371/journal.pone.0210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drastal M, Shaw K, Hyman N, Green M, Baumgart CQ, Diaz D. Colorectal Cancer Screening Decisions From Student-Led Telemedicine Initiative During COVID-19. PRiMER. 2023;7:253936. 10.22454/PRiMER.2023.253936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ekowo OE, Elgabry A, Gouveia N, Khan A. Telemedicine Clinics in General and Colorectal Surgery During the COVID-19 Pandemic: Patient-Reported Outcomes. Cureus. 2024;16:e52441. 10.7759/cureus.52441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gallo G, Picciariello A, Di Tanna GL, Santoro GA, Perinotti R, Grossi U. E-consensus on telemedicine in colorectal surgery: a RAND/UCLA-modified study. Updates Surg. 2022;1:1–8. 10.1007/s13304-021-01139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wise AK, Bhutiani N, Werthmann N, Kavalukas SL, Galandiuk S, Farmer RW. Early experience with focused telemedicine implementation in an academic colorectal surgery practice. Surgery. 2022;172:83–8. 10.1016/j.surg.2022.01.033. [DOI] [PubMed] [Google Scholar]
- 76.Rodrigues R, Geyl S, Albouys J, De Carvalho C, Crespi M, Tabouret T, Taibi A, Durand-Fontanier S, Legros R, Dahan M, Carrier P, Sautereau D, Loustaud-Ratti V, Kerever S, Jacques J. Effect of implementing a regional referral network on surgical referral rate of benign polyps found during a colorectal cancer screening program: A population-based study. Clin Res Hepatol Gastroenterol. 2021;45:101488. 10.1016/j.clinre.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Sharma V, Junejo MA, Mitchell PJ. Current Management of Malignant Colorectal Polyps Across a Regional United Kingdom Cancer Network. Dis Colon Rectum. 2020;63:39–45. 10.1097/DCR.0000000000001509. [DOI] [PubMed] [Google Scholar]
- 78.Ike B, Keppel GA, Osterhage KP, Ko LK, Cole A. Adapting a Remotely Delivered Patient Navigation Program for Colorectal Cancer Screening in Primary Care: Important Considerations for Rural Contexts. J Prim Care Community Health. 2024;15:21501319241288024. 10.1177/21501319241288025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keppel GA, Ike B, Leroux BG, Ko LK, Osterhage KP, Jacobs JD, Cole AM. Colonoscopy Outreach for Rural Communities (CORC): A study protocol of a pragmatic randomized controlled trial of a patient navigation program to improve colonoscopy completion for colorectal cancer screening. Contemp Clin Trials. 2024;141:107539. 10.1016/j.cct.2024.107539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gorin SS. Multilevel Approaches to Reducing Diagnostic and Treatment Delay in Colorectal Cancer. Ann Fam Med. 2019;17:386–9. 10.1370/afm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miles A, McClements PL, Steele RJ, Redeker C, Sevdalis N, Wardle J. Perceived diagnostic delay and cancer-related distress: a cross-sectional study of patients with colorectal cancer. Psychooncology. 2017;26:29–36. 10.1002/pon.4093. [DOI] [PubMed] [Google Scholar]
- 82.Pita-Fernandez S, Pertega-Diaz S, Lopez-Calvino B, Seoane-Pillado T, Gago-Garcia E, Seijo-Bestilleiro R, Gonzalez-Santamaria P, Pazos-Sierra A. Diagnostic and treatment delay, quality of life and satisfaction with care in colorectal cancer patients: a study protocol. Health Qual Life Outcomes. 2013;11:117. 10.1186/1477-7525-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]