Abstract
The stimulator of interferon genes (STING) pathway is a promising target in cancer immunotherapy. However, current nanomedicine strategies targeting the STING pathway often suffer from limited tumor specificity and insufficient immune activation. In this study, we developed a novel imaging-guided, self-amplifying photo-immunotherapeutic nanoparticle (SSCOL), comprising a liposome framework that encapsulates the phase-change material perfluoropentane (PFP), the photothermal agent superparamagnetic iron oxide (SPIO), and the STING agonist cGAMP. This nanoparticle exhibits excellent photoacoustic/ultrasound dual-modal imaging capability, enabling precise visualization of tumor tissue. CREKA enables specific binding to fibrin–fibronectin complexes in the tumor stroma, while NIR-induced photothermal effects of SPIO trigger coagulation, amplifying target formation and enhancing nanoparticle accumulation via a positive feedback mechanism. Under photothermal therapy, the phase transition of SSCOL enables the controlled and efficient release of the encapsulated cGAMP, which subsequently activates the STING pathway and triggers a pro-inflammatory cascade, enhances dendritic cell maturation and cytotoxic T lymphocyte activation, and elicits robust immune responses against both primary and metastatic tumors. Collectively, this multifunctional nanoparticle offers a promising strategy that integrates imaging, targeting, and photothermal-enhanced immune activation for STING-mediated cancer immunotherapy.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-025-03536-2.
Keywords: STING pathway, Targeting, Photothermal therapy, Immunotherapy, Dual-modal imaging
Introduction
Tumor metastasis and recurrence present significant challenges in clinical practice [1]. Once metastasis occurs, complete eradication through conventional methods (e.g., surgery and chemotherapy) becomes increasingly difficult [2, 3]. Immunotherapy has demonstrated substantial potential in inhibiting tumor metastasis and preventing recurrence by leveraging anti-tumor immunity [4, 5]. The specific anti-tumor immune response mediated by cytotoxic T lymphocytes (CTLs) constitutes the fundamental mechanism of tumor immunotherapy; however, it necessitates prior activation of the innate immune response [6]. Within the innate immune system, stimulator of interferon genes (STING) serves as a pivotal regulator that subsequently leads to CTL activation, thereby converting “cold tumors” into “hot tumors” [7–9]. Many preclinical studies have demonstrated that exogenous STING agonists, such as cyclic guanosine monophosphate-adenosine monophosphate (cGAMP), can effectively stimulate target cells to release type I interferons (IFNs) and pro-inflammatory cytokines, ultimately facilitating the activation and recruitment of T cells [8, 10]. However, the clinical application of STING agonists is hindered by their poor metabolic and circulatory stability, low cellular uptake efficiency, and insufficient efficacy, which collectively limit their effective intracellular delivery [11, 12]. While intratumoral injection can partially improve targeting specificity, it offers limited therapeutic coverage and fails to induce durable immune responses, particularly against distant metastases [11]. Nanomedicine significantly improves the pharmacokinetics and tumor-targeting efficiency of STING agonists, and it thereby overcomes some in vivo limitations of traditional treatments [13–17]. However, nanomedicine monotherapy still faces challenges such as reversing the immunosuppressive tumor microenvironment, inducing robust systemic immune responses, and eliminating distant metastases [18, 19]. Therefore, the development of multifunctional nanotherapeutic strategies that not only enable precise delivery of STING agonists but also synergistically enhance antitumor immune responses remains a critical focus in cancer immunotherapy.
In this context, photothermal therapy (PTT) has emerged as a promising option for synergistic cancer immunotherapy, owing to its deep tissue penetration, controllability, and noninvasive nature [20–23]. Moreover, the thermal effect of PTT can induce changes in the tumor vasculature and immune microenvironment, promote the release of tumor-associated antigens, and further stimulate a robust adaptive immune response [24, 25]. Therefore, STING-mediated innate immune activation and PTT-induced adaptive immune responses may synergistically enhance antitumor immunity. In our previous study, we employed superparamagnetic iron oxide (SPIO) as a photothermal agent to construct nanoparticles loaded with the immunoadjuvant CpG ODNs, which demonstrated promising outcomes in photothermal-immunotherapy. SPIO exhibits excellent photothermal conversion efficiency under near-infrared (NIR) irradiation, enabling effective ablation of primary tumors while eliciting robust antitumor immune responses to suppress metastatic tumor growth [26]. However, this strategy depends on an external magnetic field to guide nanoparticles to the tumor site, which may limit its applicability to deep tumors and hinder clinical translation. In addition, the combination of thermally responsive phase-change materials with PTT enables controlled drug release and ultrasound imaging. For example, in our previous study, we used SPIO as the photothermal agent and perfluorocarbon as the phase-change material to construct NIR-responsive nanoparticles. Upon NIR irradiation, SPIO effectively triggered the optical droplet vaporization (ODV) of perfluorocarbon, which not only enabled photoacoustic/ultrasound dual-modal imaging but also facilitated the controlled release of antitumor drugs [27]. Therefore, the integration of thermally responsive phase-change materials into PTT nanoplatforms enables precise temporal control of drug release and enhances spatial imaging guidance, thereby amplifying the synergy between photothermal ablation and STING-mediated immune activation.
An optimal targeting strategy plays a pivotal role in image-guided photothermal-immunotherapy, as it critically determines imaging accuracy, photothermal efficacy, and the effective activation of STING-mediated antitumor immune responses. In the majority of solid tumors, the stroma exhibits significant dysregulation, characterized by a high concentration of fibrin-fibronectin complexes that provide essential structural support for the tumor microenvironment [28]. CREKA, a tumor-homing peptide derived from phage display technology, is specifically engineered to bind to the fibrin-fibronectin complexes within the tumor stroma. Its linear pentapeptide structure provides notable advantages over cyclic peptides, including enhanced stability, simplicity, and biocompatibility [29]. Simberg et al.. demonstrated that CREKA-modified nanoparticles selectively accumulate in fibrin-fibronectin complexes in tumor-associated blood clots while remaining absent in normal tissues, underscoring its potential for tumor stroma-targeted delivery [30]. However, the strategy of using CREKA-modified nanoparticles alone to improve the intratumoral accumulation of STING agonists remains limited in efficacy. For instance, CREKA is susceptible to degradation by endogenous vascular proteases, which can compromise its tumor-targeting efficiency [31]. Therefore, new strategies are needed to overcome the limitations of CREKA and enhance the delivery and immunostimulatory efficacy of STING agonists. Notably, tumor vascular damage triggers coagulation, leading to thrombin-mediated cleavage of fibrinogen into fibrin monomers. These fibrin monomers reassemble in the tumor stroma, further associating with fibronectin to form fibrin-fibronectin complexes [28, 29, 32]. Interestingly, PTT has the potential to activate the coagulation cascade through heat-induced damage to tumor tissue and vasculature, thereby enhancing the formation of fibrin–fibronectin complexes [33, 34]. Therefore, we hypothesize that the photothermal effect generated by PTT promotes the formation of additional binding sites for CREKA, thereby attracting more CREKA-modified nanoparticles to accumulate within the tumor. This increased accumulation further reinforces and prolongs the photothermal therapeutic effect. Such a positive feedback mechanism, involving “photothermally induced target amplification” and “self-enhanced nanoparticle accumulation”, offers a promising strategy to enhance intratumoral delivery of STING agonists and maintain sustained photothermal efficacy.
Based on the aforementioned theory, we developed a targeted self-amplifying photo-immunotherapeutic nanoparticle STING-ODVLipo/SPIO-CREKA (SSCOL). This nanoparticle is constructed within a liposome framework, encapsulating perfluoropentane (PFP), SPIO, and the STING agonist cGAMP, and functionalized with the CREKA peptide. We employed the coagulation cascade amplification mechanism as a guiding principle to identify the fibrin-fibronectin complex, which is highly expressed within the tumor stroma, as a specific binding site for CREKA. The outstanding photothermal effect of SPIO disrupts tumor vasculature and initiates coagulation, thereby amplifying the fibrin-fibronectin complex and generating additional targets that further promote accumulation of CREKA-modified nanoparticles. As a result, a positive feedback loop was established between tumor-targeted accumulation of SSCOL and the photothermal effect against tumors, resulting in a reinforced self-amplifying therapeutic cycle. Furthermore, real-time monitoring of SSCOL accumulation within tumors was achieved through photoacoustic/ultrasound imaging techniques. In addition, the photothermal effect induced the phase transition of SSCOL, which enabled the efficient release of cGAMP, thereby activating the cGAS-STING pathway and downstream pro-inflammatory cascades, and ultimately enhancing dendritic cell (DC) maturation and CTL activation. By bridging innate and adaptive immunity, this approach significantly inhibits both primary tumors and distant metastatic lesions.
Materials and methods
Materials
DPPC, DSPE-PEG2000, and SPIO (Fe3O4, 2 mg·mL− 1, 10 nm) were purchased from Xi’an RuiXi Biological Technology Co., Ltd. (Xi’an, China). DSPE-PEG2000-CREKA was purchased from Bioworld Technology Co., Ltd. (Shanghai, China). PFP, Calcein-AM, and propidium iodide (PI) were acquired from Sigma-Aldrich (Shanghai, China). cGAMP was purchased from APExBIO Technology (USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Japan). TGF-β was obtained from Abbkine (Wuhan, China). Anti-CD3+-FITC, anti-CD4+-PerCP, anti-CD8+-APC, anti-CD11c+-FITC, anti-CD80+-APC, and anti-CD86+-PE antibodies were purchased from Thermo Fisher Scientific Inc. (Shanghai, China). ELISA kits for TNF-α, IL-12, and IFN-γ were purchased from Huyu Biological Technology Co., Ltd. (Shanghai, China). ELISA kits for IFN-β and CXCL10 were purchased from Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China). FITC-fibrinogen and Cy5-albumin were obtained from Solarbio Science & Technology Co., Ltd. (Beijing, China). All reagents used in this work were of analytical grade.
Synthesis of SSCOL
The STING-ODVLipo/SPIO-CREKA nanoparticle (SSCOL) was fabricated by using the thin-film hydration method. Briefly, 6 mg of DPPC, 2 mg of DSPE-PEG2000-CREKA, 2 mg of cholesterol, and 300 µL SPIO were dissolved in 10 mL of trichloromethane. Under a water bath at 45 °C, a thin lipid film was formed by vacuum rotary evaporation. PBS buffer was added to hydrate the film, and the flask was shaken to form a homogeneous suspension. Under an ice bath, 200 µL of PFP and 1 mg of cGAMP were added to the suspension, which was then emulsified using an ultrasonic homogenizer (52 W, 3 min). The mixture was subsequently stirred magnetically at room temperature to ensure complete evaporation of the organic solvent. Finally, the suspension was subjected to low-temperature centrifugation to remove free PFP, SPIO, and cGAMP, yielding the SSCOL. By following the same procedure and substituting DSPE-PEG2000 for DSPE-PEG2000-CREKA, SSOL (without CREKA) was prepared.
Characterization of SSCOL
The microstructure and dispersion of the SSCOL were characterized using an optical microscope and transmission electron microscopy (TEM, Hitachi H-7600, Japan). The particle size distribution, zeta potential, and polymer dispersity index (PDI) were measured using dynamic light scattering (DLS, ZEN3600, Malvern Instruments, UK) with a Malvern particle size analyzer. Continuous monitoring of changes in the distribution, zeta potential, and PDI of SSCOL dispersed in fetal bovine serum (FBS) was performed over a 6-day period. DSPE-PEG2000-CREKA and DSPE-PEG2000 were analyzed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The entrapment efficiency of SPIO was assessed via inductively coupled plasma optical emission spectrometry (ICP-OES, ThermoFisher, USA). The peak areas of cGAMP solutions at various concentrations (10, 20, 30, 40, and 50 µg mL− 1) were measured using high-performance liquid chromatography (HPLC, Shimadzu LC-20AD, Japan). Subsequently, the cGAMP standard curve was constructed, and the entrapment efficiency of cGAMP was evaluated. The loading capacity of the nanoparticles was calculated by the following formulae:
Loading capacity (%) = (mass of SPIO or cGAMP/total mass of nanoparticles) × 100%.
Photothermal conversion of SSCOL
SSCOL solutions at various concentrations were exposed to NIR laser irradiation (2 W·cm− 2, 10 min). Variations in temperature were monitored by infrared thermal imaging during 808 nm NIR laser irradiation at variable power intensities.
Optical droplet vaporization performance of SSCOL
SSCOL solution (50 µg·mL− 1) was dropped onto a glass slide and heated using a heating plate. During the heating process, the liquid-gas phase transition of PFP was monitored in real time under an optical microscope. Subsequently, to investigate the effect of laser irradiation on the optical droplet vaporization (ODV) performance, the SSCOL (2 µg·mL− 1) was added to a 96-well plate and irradiated with an 808 nm laser (2 W·cm⁻²). The number of microbubbles generated at different temperatures was immediately observed under an optical microscope.
Drug-release profile of cGAMP from SSCOL
The 2 mg of SSCOL and 2 mg of SSCL were individually added to sealed dialysis bags and placed in a pre-prepared PBS sustained-release solution (containing 30% v/v anhydrous ethanol, 0.1% v/v Tween-80, and 0.02% w/v sodium azide). The nanoparticles were irradiated with an 808 nm laser (2 W·cm⁻², 5 min). After laser irradiation, the dialysis bags were incubated in a 37 °C shaker (rotation speed 150 rpm). During the drug release process, solution samples were collected from each group at 0, 1, 2, 4, 6, 8, 12, 24, and 48 h, and an equal volume of fresh solution was added to maintain a constant volume. The cGAMP release amounts from the nanoparticles were quantified by HPLC, and the cumulative release percentage of cGAMP was calculated.
In vitro cytotoxicity and biosafety of SSCOL
A standard CCK-8 protocol was used to evaluate the in vitro cytotoxicity of SSCOL. Endothelial cells were seeded in 96-well plates and allowed to adhere until stable. The cells were then co-incubated with varying concentrations of SSCOL (100, 200, 300, 400, 500, and 600 µg·mL− 1). After incubation, the medium was replaced with fresh cell culture medium, and CCK-8 solution was added to each well. Absorbance (OD) was measured at 490 nm using an automatic microplate reader, and the cell survival rate was calculated based on the absorbance values.
For in vivo biocompatibility, healthy female BALB/c mice were intravenously administered SSCOL (2 mg·mL− 1, 200 µL). At different time points (1, 3, 7, 14, 21, 28 d), the mice were euthanized by cervical dislocation. Blood samples were collected for complete blood counts (CBC) and biochemical analysis. Major organs (heart, liver, spleen, lung, kidney) were harvested and fixed in 4% v/v paraformaldehyde. The tissue sections were subjected to hematoxylin − eosin (H&E) staining. Untreated mice were used as blank controls.
Cell culture and animal models
The 4T1 murine breast cancer cell line was originally obtained from JXQ Technology (Chongqing, China) and cultured in RPMI-1640 medium supplemented with 10% v/v FBS and 1% v/v penicillin-streptomycin under standard conditions (5% CO2, 37 °C). Bone marrow-derived dendritic cells (BMDCs) were generated from murine bones according to an established method. In brief, healthy female BALB/c mice (6–8 weeks old) were euthanized via cervical dislocation, and their bilateral femurs and tibias were harvested. The bone marrow cavities were flushed with RPMI-1640 medium, and the flushing solution was collected. Following centrifugation, the supernatant was discarded, and the pellet was resuspended in red blood cell lysis buffer and incubated in the dark to lyse erythrocytes. The lysed cells were washed with culture medium and resuspended to form a cell suspension. GM-CSF and IL-4 were added to the cell suspension at a ratio of 1:10000 to prepare DC-specific culture medium. The BMDCs were seeded into 6-well plates using this medium and cultured at 37 °C with 5% CO2 for 2 days. On day 3, the supernatant was removed and replaced with an equal volume of fresh DC-specific medium. On day 5, half of the supernatant was replaced with an equal volume of fresh DC-specific medium. On day 7, the floating cells were harvested, representing immature BMDCs.
All experimental protocols were approved by the Animal Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (Coren Trial(265), 2021) and conducted at the Laboratory Animal Center of Chongqing Medical University. Female BALB/c mice (aged 6–8 weeks) were obtained from Enswell Biotechnology Ltd. (Chongqing, China). To establish a 4T1 orthotopic murine breast cancer model, 4T1 cells in the logarithmic growth phase were diluted with sterile PBS (2 × 106 cells per mouse). The cell suspension was then slowly injected into the bilateral mammary fat pads of BALB/c mice to simulate primary and metastatic lesions. Tumor size was monitored every 1–2 days using calipers. When the tumor volume reached approximately 60 mm³, the mice were used for therapeutic studies; when the tumor volume reached approximately 100 mm³, the mice were used for imaging studies. In in vivo studies, mice were anesthetized intraperitoneally with 1% pentobarbital sodium, and were euthanized by cervical dislocation.
Photoacoustic/ultrasound dual-modal imaging
To evaluate the ultrasound imaging performance in vitro, the nanoparticle solutions from each group (2 mg·mL− 1, 200 µL) were added to the wells of a 3% w/v agarose gel and subsequently irradiated with an 808 nm laser (2 W/cm², 10 min). Immediately following irradiation, ultrasound images of each group were acquired using the MyLab90 ultrasound diagnostic system (Esaote, Italy), and the intensity values of the ultrasound echo signals in the regions of interest (ROIs) were quantitatively analyzed. Additionally, ultrasound images were collected at various time points post-irradiation (4, 6, 8, and 10 min) for further analysis. To evaluate the photoacoustic (PA) imaging performance in vitro, SSCOL solutions (2 mg·mL− 1, 200 µL) were added to the wells of a 3% w/v agarose gel. Using the Vevo LAZR PAI device (VisualSonics, Toronto, Canada), PA scans were performed within the excitation wavelength range of 680–970 nm to determine the excitation wavelength corresponding to the maximum PA signal intensity of the nanoparticles. This optimal excitation wavelength (700 nm) was then used for subsequent PA imaging experiments. Under this optimized excitation wavelength, in vitro PA images of the different nanoparticle solutions from each group were acquired, and the PA signal intensities of each group were quantitatively measured.
To evaluate the in vivo photoacoustic/ultrasound dual-modal imaging performance of SSCOL, a unilateral orthotopic cancer model was employed. Following intravenous injection via tail vein of SSCOL into tumor-bearing mice (200 µL per mouse), PA images of the tumor were acquired at various time points before and after injection (2, 4, 6, 24, and 48 h). The PA signal intensity within the tumor region was quantitatively measured for each group to determine the optimal time point corresponding to the best PA imaging effect. This optimal time point was then used as the timing for subsequent targeted PTT. The tumor was irradiated with an 808 nm laser (2 W·cm− 2, 10 min), while simultaneously acquiring ultrasound images. The ultrasound echo signal intensity in the tumor region was quantified.
The expression of fibronectin in the tumor
In vitro, 4T1 cells were routinely cultured in 6-well plates. Once the cells had stably adhered, TGF-β was added for co-incubation. In the control group, 4T1 cells were cultured under identical conditions without TGF-β treatment for the same duration. The mesenchymal phenotype transformation of each group of 4T1 cells was observed using an optical microscope, and the expression levels of fibronectin (Fn) in the two groups of 4T1 breast cancer cell samples were quantified by RT-PCR.
In the in vivo experiments, a unilateral orthotopic tumor was employed. Tumor and major organ tissues (liver, kidney, brain) from tumor-bearing mice were collected. These tissues were homogenized in RIPA lysis buffer containing protease inhibitors at 4℃ and then centrifuged. The supernatants of each sample were collected, and rabbit anti-mouse fibronectin polyclonal antibody was added to the lysates. Western blot analysis was performed to detect the expression levels of fibronectin in both tumor tissues and major organ tissues.
In vitro and in vivo photothermal therapeutic effects of SSCOL
In vitro, 4T1 cells were seeded in 96-well plates and co-incubated with nanoparticles from each experimental group. Following this, the cells were exposed to laser irradiation at an intensity of 2 W·cm− 2 for 5 min. Cell viability was subsequently assessed through the CCK-8 assay. 4T1 cells were routinely cultured in confocal microscopy-compatible dishes. After treatment with each group’s nanoparticles, Calcein-AM and PI dyes were added to the respective samples, and cell apoptosis and necrosis were evaluated using confocal laser scanning microscopy (CLSM). Additionally, 4T1 cells were also seeded in 24-well plates. Post-treatment, the cells were trypsinized with 0.25% trypsin, labeled with Annexin V-FITC and PI, and analyzed for apoptosis via flow cytometry.
In the in vivo experiments, a unilateral orthotopic tumor model was utilized. nanoparticle solutions (200 µL, 2 mg·mL− 1) from each group were administered via tail vein injection. Twenty-four hours after injection, tumors were irradiated with an 808 nm laser at a power density of 2 W cm−² for 10 min for PTT. Surface temperature changes of the tumors during irradiation were monitored using an infrared thermal camera (Fotri226, Shanghai, China). Following PTT, tumor tissues from each group were harvested and subjected to H&E staining.
In vivo biodistribution of SSCOL
To assess tumor accumulation and biodistribution of SSCOL before PPT, DiR fluorescent dye-labeled targeted SSCOL and non-targeted SSOL were prepared. A unilateral orthotopic cancer model was used, with tumor-bearing mice anesthetized via isoflurane inhalation. Fluorescence images of tumor sites were captured using an imaging system at different time points post-injection (2, 4, 6, 24, and 48 h) of nanoparticles (2 mg·mL− 1, 200 µL). Fluorescence intensities at tumor sites were quantified for both groups. At 48 h, tumor tissues and major organs (heart, liver, spleen, lung, kidney) were harvested for fluorescence intensity analysis of nanoparticles in excised tissues.
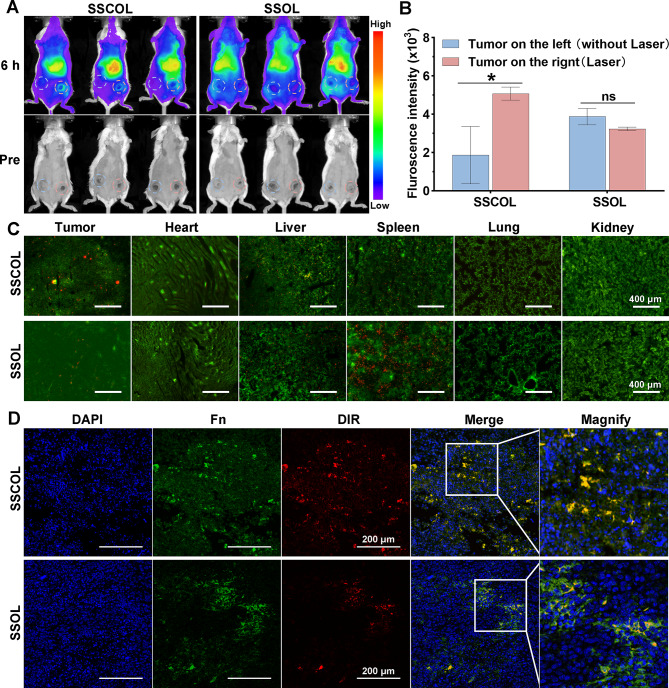
To evaluate tumor accumulation and biodistribution of SSCOL after PTT, a bilateral carcinoma in situ model was used. Unlabeled SSCOL or SSOL (200 µL, 2 mg·mL− 1) were injected via tail vein into tumor-bearing mice. Based on in vivo PA imaging, the optimal time point for imaging was identified, and one tumor site was irradiated with a laser (2 W cm−², 10 min). Following PTT, 200 µL of DiR-labeled SSCOL or SSOL was re-injected via tail vein. Six hours post-injection, fluorescence images of bilateral tumors were captured, and signals were quantified to compare SSCOL and SSOL accumulation. Additionally, FITC-labeled fibrinogen and Cy5-labeled albumin were injected post-PTT. Tumors were harvested, and fluorescence of FITC-fibrinogen and Cy5-albumin was observed ex vivo.
To further evaluate the targeting efficacy of SSCOL towards the fibrin-fibronectin complex within the tumor stroma, tumor-bearing mice were injected with DiR-labeled SSCOL or SSOL (200 µL, 2 mg·mL− 1) via the tail vein. After 24 h, tumors were harvested and subjected to immunofluorescence staining to detect fibronectin. Fibronectin was identified using an alpha-chain antibody and TYR-570 secondary antibody, and the co-localization of nanoparticles with fibronectin in the tumor microenvironment was analyzed using a laser confocal microscope. Furthermore, under the same PTT conditions as previously described, DiI-labeled SSCOL or SSOL were re-injected via the tail vein. Tumors and major organs (heart, liver, spleen, lung, and kidney) were harvested to examine the distribution of DiI-labeled nanoparticles. This analysis was also performed using a laser confocal microscope to evaluate nanoparticle accumulation in tumors and non-target organs.
In vitro and in vivo activation of the STING pathway
In in vitro experiments, immature BMDCs were isolated from mice and seeded into 6-well plates. The SSCOL or SSOL solutions (1 mL, 600 µg·mL− 1) were added to the corresponding wells for co-incubation with DCs. The expression levels of interferon (IFNb1, IFNa1), tumor necrosis factor (TNF), interleukin (IL1b, IL6, IL12b), and chemokine (CXCL9, CXCL10) in the DCs were quantified using RT-PCR. The phosphorylation levels of TBK1 and IRF-3 in the DCs were assessed by Western blot analysis.
In in vivo experiments, a unilateral orthotopic carcinoma model was utilized. The SSCOL and SSOL solutions (200 µL, 2 mg·mL− 1) from each group were administered to tumor-bearing mice via tail vein injection, and the tumor was subjected to laser irradiation (2 W·cm− 2, 10 min). On the second day after PTT, the blood from the eye sockets of the mice was collected. The expression levels of IFN-β and CXCL10 in the serum were determined using an ELISA kit.
In vitro and in vivo DC maturation and related cytokine secretion
A Transwell system was used to co-culture 4T1 cells and BMDCs. Briefly, 4T1 cells were seeded in the upper chamber until adherent and then incubated with SSCOL, SCOL (without cGAMP), SCOL with free cGAMP and free cGAMP solutions (SSCOL = 600 µg·mL− 1, SCOL = 600 µg·mL− 1, cGAMP = 16 µg·mL− 1), respectively. After 8 h of incubation, the medium was replaced with fresh medium, and the 4T1 cells were irradiated with a laser at 2 W·cm−2 for 5 min. Subsequently, the treated 4T1 cells were transferred to the lower chamber of the plates containing DCs for co-culture. After 24 h, DCs were collected and stained with anti-CD11c FITC, anti-CD80 APC, and anti-CD86 PE antibodies. The proportion of CD11c⁺CD80⁺CD86⁺ DCs was analyzed by flow cytometry. Additionally, the levels of cytokines (IFN-γ, TNF-α, and IL-12) in the DC suspension were quantified using an ELISA kit according to the standard protocol.
In vivo anti-tumor immunity
The 4T1 cells were subcutaneously inoculated into left mammary pads of each BALB/c female mouse as the primary tumor (1st tumor). An equivalent volume of 4T1 cells was injected into the right mammary pad as a distant tumor (2nd tumor) on the 7th days after inoculation of the primary tumor. The mice were randomly divided into six groups: control, SCOL (without cGAMP), free cGAMP, SCOL + Laser, SCOL + free cGAMP + Laser, and SSCOL + Laser (SSCOL = 600 µg·mL− 1, SCOL = 600 µg·mL− 1, cGAMP = 16 µg·mL− 1). Once the tumor volume reached approximately 50 mm³, mice in each group received injections of different solutions. Laser treatment groups underwent laser irradiation (2 W·cm− 2, 10 min) on the primary tumor at 24 h post-injection. In each group, the same treatment for the primary tumors was repeated on the 3rd and 6th days following the initial therapy.
The maturation status of DCs and the activation status of CTLs were determined by flow cytometry. On day 7 post-treatment, tumor tissues, inguinal draining lymph nodes, and spleens were harvested from each group and processed into single-cell suspensions. The suspensions were then stained with anti-CD11c FITC, anti-CD80 APC, and anti-CD86 PE antibodies to evaluate the expression levels of co-stimulatory molecules CD11c+, CD80+, and CD86+ on DCs. Additionally, the single-cell suspensions of tumors and spleens were stained with anti-CD3 FITC, anti-CD4 PerCP, and anti-CD8a APC. The serum concentrations of IFN-γ, TNF-α, and IL-12 in tumor-bearing mice in each group were detected by using an ELISA kit. Furthermore, tumor tissues were harvested and subjected to immunofluorescence staining using anti-CD8 FITC and anti-IFN-γ Cy3 antibodies. Co-expression of the fluorescent markers was analyzed under a laser confocal microscope to assess IFN-γ secretion and CD8⁺ T cell infiltration within the tumors.
In vivo anti-tumor efficacy
During the treatment period, mouse body weight was recorded every other day, and the long and short diameters of bilateral tumors were measured. Tumor volume was calculated using the formula: [(width)² × length]/2. On day 19 of treatment, mice were euthanized via cervical dislocation. The primary tumor tissues from each group were harvested for H&E staining and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay to assess tumor cell necrosis. The metastatic tumor tissues were collected for proliferating cell nuclear antigen (PCNA) staining to evaluate tumor cell proliferation.
Statistical analysis
Data are presented as mean ± SD. Comparisons between groups were performed using Student’s t-test, one-way ANOVA, or two-way ANOVA, as appropriate. Statistical significance was defined as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. All experiments were performed independently at least three times.
Results and discussion
Preparation, characterization, and biosafety of SSCOL
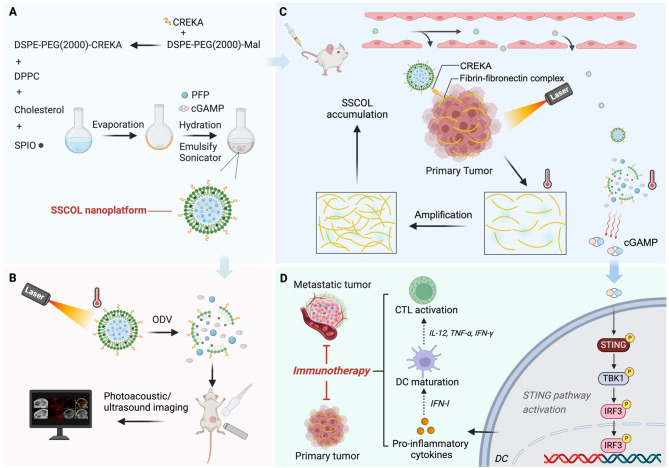
The novel functional nanoparticle STING-ODVLipo/SPIO-CREKA (SSCOL) was successfully synthesized according to the synthetic route (Scheme 1). TEM analysis revealed that SSCOL exhibited a uniform size, regular spherical morphology (Fig. 1A), and an interior filled with SPIO upon magnification (Fig. 1B). Elemental analysis confirmed the presence of iron (Fe) and fluorine (F) within the nanoparticle (Fig. 1C-D), validating the successful encapsulation of SPIO and PFP. The polydispersity indices were recorded at 0.062 and 0.047 for SSOL (without CREKA) and SSCOL respectively, indicating favorable dispersibility characteristics. SSCOL appeared reddish-brown without visible aggregation when resuspended in saline (inset, Fig. 1E). DLS measurements indicated that the particle sizes for SSOL and SSCOL were 274.7 ± 8.77 nm (PDI: 0.062 ± 0.044) and 276.2 ± 18.67 nm (PDI: 0.047 ± 0.032) (Fig. 1E), respectively—dimensions conducive to extravasation from blood vessels into tumor tissues. Zeta potentials were − 17.7 ± 0.75 mV for SSOL and − 8.28 ± 0.98 mV for SSCOL (Fig. S1A). No significant changes were observed in either the size distribution or zeta potential of SSCOL in FBS over the 6-day observation period, confirming the in vitro stability of SSCOL under physiological conditions (Figure S1B, D). Low PDI values further reflected the uniformity and homogeneity of SSCOL (Figure S1C). DSPE-PEG2000-CREKA was synthesized by conjugating DSPE-PEG2000-MAL with CREKA. MALDI-TOF-MS analysis revealed a mass shift from ~ 2500–2700 Da to ~ 3300–3400 Da following conjugation of DSPE-PEG2000 with CREKA, confirming the successful synthesis of the precursor DSPE-PEG2000-CREKA for SSCOL (Fig. 1F). Meanwhile, the drug loading capacities of SPIO and cGAMP within the SSCOL were quantified using ICP-OES and HPLC. The measured drug loading capacities were 382.5 ± 8.76 µg for SPIO and 267.3 ± 13.47 µg for cGAMP, respectively (Fig. 1G and S1E). These results demonstrate that the SSCOL was successfully prepared, effectively encapsulating both lipophilic and hydrophilic agents. In addition, the biosafety of SSCOL was evaluated through a series of in vitro and in vivo experiments. Cytotoxicity on vascular endothelial cells was evaluated using the CCK-8 assay. After 24 h of co-incubation with SSCOL at various concentrations not exceeding 600 µg·mL− 1, the cell survival rate remained above 80% (Fig. S1F), indicating that 600 µg·mL⁻¹ is a safe concentration for in vitro applications. To further validate the in vivo safety of SSCOL, healthy BALB/c mice were administered SSCOL via tail vein injection, followed by laboratory examination and H&E staining. Laboratory results indicated that all parameters were within normal ranges compared to untreated controls (Fig. S2), and H&E staining revealed no significant pathological changes in the cellular morphology of major organs (Fig. S3). In conclusion, SSCOL demonstrated favorable biosafety profiles.
Scheme 1.
(A) Schematic of SSCOL synthesis. (B) Mechanism of photoacoustic/ultrasound dual-modal imaging. (C) Positive feedback between fibronectin-fibrin complex formation and SSCOL targeting. (D) PTT-induced STING pathway activation for enhanced immunotherapy against primary and metastatic tumors
Fig. 1.
Characterization, photothermal conversion and ODV of SSCOL. (A) The TEM micrograph of SSCOL. Scale = 500 nm. (B) Magnified TEM micrographs. Scale = 100 nm. (C, D) Elemental mapping of SSCOL. Scale = 100 nm. (E) Diameters of SSCOL and SSOL (inset: digital photo of SSCOL). (F) MALDI-TOF-MS spectra of DSPE-PEG2000-CREKA and DSPE-PEG2000. (G) HPLC spectra of different concentrations of cGAMP. (H) Photothermal temperature-time curves of SSCOL at various concentrations under 808 nm laser irradiation (2 W·cm⁻²). (I) Photothermal temperature-time curves of SSCOL (2 mg·mL⁻¹) at different power densities of 808 nm laser. (J) The accumulative drug release of cGAMP from SSCOL. (K) Optical microscopy images of SSCOL before and after heating to different temperatures. Scale = 200 μm. (L) Optical microscopy images (upper) of SSCOL at various time points and the corresponding infrared thermal images (lower). Scale = 100 μm
Photothermal conversion and ODV performance of SSCOL
Efficient photothermal conversion is a prerequisite for effective PTT [35]. In vitro experiments showed that the temperature increase in SSCOL was positively correlated with its concentration under 808 nm NIR laser irradiation. At a concentration of 2.5 mg·mL− 1, SSCOL reached a temperature of 61.1 °C after 10 min of irradiation; even at a lower concentration of 1 mg·mL− 1, the laser induced a temperature rise to 41.2 °C (Fig. 1H and S4A-B). Additionally, the temperature changes in SSCOL under varying power irradiations were analyzed, revealing a positive correlation between laser power and SSCOL temperature, indicating favorable thermal controllability (Fig. 1I and S4 C-D). These results confirm that SSCOL has the potential to serve as an excellent photothermal conversion agent for PTT in tumors.
Optical droplet vaporization (ODV) refers to the process where laser energy is absorbed by materials and converted into heat, inducing evaporation or a phase transition in liquid droplets [36]. Optimal ODV performance is essential for efficient drug release and enhanced ultrasound imaging. PFP exists as a liquid at ambient temperature, with a boiling point of 29 °C. When the surrounding temperature exceeds this threshold, liquid PFP undergoes a liquid-to-gas phase transition, resulting in the formation of microbubbles [27, 37, 38]. Thus, we initially investigated the thermo-induced phase transition of PFP-loaded SSCOL by exposing the samples to varying temperatures. At 36 °C, SSCOL retained their original morphology without noticeable changes. At 39 °C, slight volumetric expansion was observed, indicating the enhanced stability of PFP encapsulated within the phospholipid membrane. However, at 47 °C, a large number of SSCOL nanoparticles transformed into microbubbles, demonstrating their thermo-responsive behavior (Fig. 1K). Subsequently, leveraging the favorable photothermal performance of SSCOL, their ODV capability under laser irradiation was further explored. Under a laser intensity of 2 W cm−2 for 5 min, significant microbubbles were observed microscopically, highlighting the remarkable ODV potential of SSCOL (Fig. 1L). This property was hypothesized to enable controlled release of cGAMP. As shown in Fig. 1J, drug release started at the beginning of the experiment. At the second hour, the nanoparticles were irradiated with an 808 nm infrared laser (2 W·cm− 2 for 5 min). No significant changes were observed in the SSCOL alone and SSCL (without PFP) + Laser groups, whereas a dramatic burst release of cGAMP was observed in the SSCOL + Laser group. The significant increase in the cGAMP release rate is primarily attributed to the ODV of a large number of nanoparticles during laser irradiation. This process initiates photo-induced cavitation, leading to the formation of microbubbles and subsequently enhancing cGAMP release. More specifically, the microbubbles generated under laser irradiation originate from dielectric breakdown at the laser focal point, which produces plasma. The rapid expansion of this plasma then generates an acoustic shock wave, which further facilitates the disruption of nanocarriers and promotes cGAMP release [39, 40]. Collectively, these findings demonstrate that SSCOL enables precise, laser-controllable release of cGAMP through ODV-mediated microbubble formation.
Cellular uptake and photothermal cytotoxicity of SSCOL
Given the photothermal properties of SSCOL, we further investigated their cellular uptake and photothermal cytotoxicity. Fluorescently labeled SSCOL or SSOL (without CREKA) were separately co-incubated with 4T1 cells, and their fluorescence signals were observed. The results showed significant nanoparticle fluorescence accumulation in both groups after 6 h, reaching a peak at 8 h (Fig. 2A). Similarly, flow cytometry analysis revealed comparable fluorescence expression across various time points, with the highest values recorded at 8 h: 84.92 ± 0.56% for SSCOL and 83.25 ± 1.96% for SSOL (Fig. 2B-C). Moreover, there was no statistically significant difference in fluorescence between the two groups, which indirectly indicates that the targeting effect of CREKA is mainly not dependent on the specificity of the cell surface. CREKA can specifically recognize the fibrin-fibronectin complex within the tumor stroma. Its targeting efficacy is primarily modulated by the features of the tumor microenvironment, particularly the abnormal extracellular matrix, rather than specific antigens expressed on the surface of tumor cells [29, 30, 41]. On this basis, we further investigated the targeting capability of CREKA toward the fibrin-fibronectin complex in subsequent experiments.
Fig. 2.
Cellular uptake and photothermal cytotoxicity of SSCOL. (A) CLSM images of 4T1 cells after co-incubation with SSCOL and SSOL at various time points. Scale = 100 μm. (B) Flow cytometry analysis of SSCOL and SSOL in 4T1 cells at various time points. (C) The corresponding quantitative data. (D) Cell viability of 4T1 cells after different treatments. (E) CLSM images of 4T1 cells after Calcein-AM/PI staining. Red fluorescence refers to dead cells, and green fluorescence refers to live cells. Scale = 100 μm. (F) Flow cytometry analysis of 4T1 cell apoptosis ratios after different treatments. (G) The corresponding quantitative data. (ns: not significant; **p < 0.01, ****p < 0.0001)
In vitro experiments were performed to evaluate the anti-tumor efficacy of SSCOL, with or without the application of PTT. The results from the CCK-8 assay indicated that neither SSCOL nor laser treatment alone had a significant effect on tumor cell viability (Fig. 2D). However, the SSCOL + Laser group markedly enhanced tumor cell death, leading to a significantly decreased cell viability of 43.95 ± 8.35%. Live-dead cell staining corroborated these findings, revealing a substantial number of dead cells exclusively within the group of SSCOL + Laser (Fig. 2E). Furthermore, flow cytometry confirmed extensive apoptosis and necrosis induced by the combined treatment (Fig. 2F-G). These results collectively demonstrate that SSCOL combined with PTT exerts potent anti-tumor effects in vitro.
The expression of fibronectin in the tumor and in vivo biodistribution of SSCOL
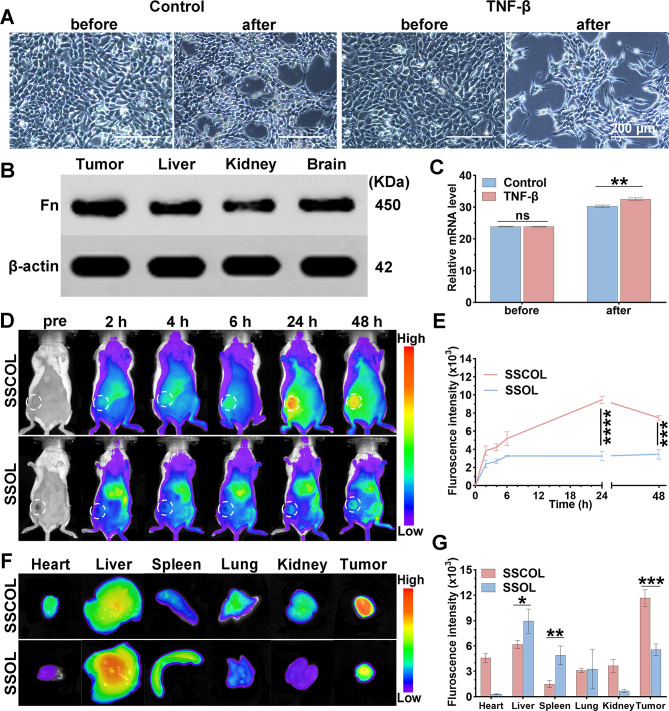
Epithelial-mesenchymal transition (EMT) represents a critical step in the initiation of invasion and metastasis in high-risk breast cancer. Transforming growth factor beta (TGF-β) serves as the primary inducer of EMT, facilitating the transformation of tumor cells to mesenchymal phenotypes while simultaneously upregulating the specific expression of fibronectin [28, 42]. Therefore, this section of the study aims to validate the high specificity of fibronectin expression within tumors. In vitro experiments showed that 4T1 cells treated with TGF-β underwent significant morphological changes compared to untreated cells. These cells became more fibrous and elongated, with reduced cell-cell junctions, closely resembling a mesenchymal-like morphology (Fig. 3A). Such changes signify the induction of EMT, which enhances the migratory capacity of the cells [43]. Western blot analysis was employed to assess fibronectin expression in tumor tissues and normal organ tissues, revealing significantly elevated levels of fibronectin in tumor tissues compared to those in normal organs (Fig. 3B and S5). Additionally, the results of RT-PCR analysis confirmed that fibronectin expression was significantly higher in TGF-β treated cells compared to the control group (Fig. 3C).
Fig. 3.
The fibronectin expression in tumor and in vivo biodistribution of SSCOL. (A) Optical microscopy images of 4T1 cells before and after co-incubation with TGF-β. Scale = 100 nm. (B) Western blot assay of fibronectin (Fn) expression in tumor, liver, kidney, and brain tissues. (C) RT-PCR assay of fibronectin expression in 4T1 cells before and after co-incubation with TGF-β. (D) NIR fluorescence images of 4T1 tumor-bearing BALB/c mice after administrations of SSCOL and SSOL (without CREKA) at different time points. (E) The corresponding time-fluorescence intensity curves. (F) Ex vivo NIR fluorescence images of SSCOL and SSOL. (G) The corresponding quantitative fluorescence data. (ns: not significant; *p < 0.05; **p < 0.01, ***p < 0.001, ****p < 0.0001)
Enhancing the tumor accumulation of STING agonists and improving their bioavailability are essential for augmenting anti-tumor immune responses. To verify the targeting ability of SSCOL to tumors in the absence of laser irradiation, fluorescence signals were detected using an in vivo fluorescence imaging system after the injection of DiR-labeled nanoparticles in tumor-bearing mice. The results demonstrated that the fluorescence intensity at the tumor site was consistently higher in the SSCOL group compared to the SSOL (without CREKA) group at all time points (Fig. 3D-E). Additionally, 24 h post-injection of SSCOL, the fluorescence intensity at the tumor site reached its peak. Consequently, 24 h was selected as the optimal time point for subsequent in vivo PTT. Ex vivo fluorescence analysis of tumors and major organs at 48 h revealed that fluorescence-labeled SSCOL mainly accumulated at the tumor site, whereas fluorescence-labeled SSOL exhibited a tendency to accumulate in the liver and spleen (Fig. 3F-G). These findings confirm that targeting efficiency of SSCOL is markedly superior to that of SSOL, demonstrating that CREKA modification enhances the targeting capability of SSCOL.
Photoacoustic/ultrasound dual-modal imaging
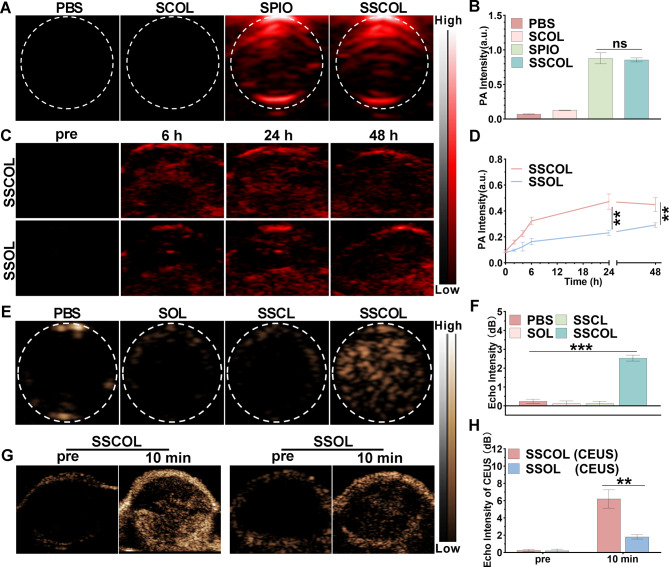
To maximize the effectiveness of PTT and enable precise tumor diagnosis and treatment, multimodal imaging visualization is indispensable. The photoacoustic (PA) imaging performance of SSCOL was investigated, and their targeted aggregation within tumor tissue was assessed to determine the optimal time point for PTT. In vitro results showed that SCOL (without SPIO) exhibited no detectable PA signal, while SSCOL generated PA signals comparable to those of free SPIO, with no significant differences observed between the two groups (Fig. 4A-B and S6). The result is primarily attributed to the broad and high absorption peaks of SPIO particles in the NIR wavelength range [44], which enhance SSCOL’s contrast capabilities for PA imaging. In vivo, the PA signal at the tumor site in the SSCOL group was significantly higher than that in the SSOL (without CREKA) group at all time points. Notably, 24 h post-injection, the PA signal at the tumor site reached its peak before gradually decreasing (Fig. 4C-D and S7). This finding is consistent with in vivo fluorescence imaging results (Fig. 3D-E), further confirming that 24 h post-injection is the optimal time point for PTT. Overall, SSCOL demonstrates excellent tumor PA imaging performance under PTT, which relies on the synergistic contributions of SPIO for imaging contrast and CREKA for targeted delivery.
Fig. 4.
Photoacoustic/ultrasound dual-modal imaging. (A) In vitro PA images of SSCOL, free SPIO, SCOL (without SPIO) and PBS. (B) The corresponding PA signal intensities. (C) In vivo PA images of tumor regions at various time points after intravenous injection with SSCOL and SSOL (without CREKA). (D) The corresponding time-PA signal intensities curves. (E) In vitro ultrasound images of SSCOL, SSCL (without PFP) and SOL following 808 nm laser irradiation. (F) The corresponding echo signal values. (G) In vivo ultrasound images of the tumor regions at 24 h after intravenous injection with SSCOL and SSOL pre-irradiation and post-irradiation for 10 min. (H) The corresponding echo signal values. (ns: not significant; **p < 0.01, ***p < 0.001)
Subsequently, the ultrasound imaging performance of SSCOL was investigated. The in vitro results showed that the ultrasonic echo intensity of SSCOL following laser irradiation is significantly greater than that of SOL (without SPIO) and SSCL (without PFP) (Fig. 4E-F and S8A-B). These findings suggest that laser-induced ultrasound imaging depends on the coexistence of SPIO and PFP, as laser irradiation raises the temperature of SPIO, causing PFP to undergo a phase transition from liquid to gas, thereby enhancing ultrasound imaging performance. In vivo, the tumor was irradiated with a laser 24 h post-injection to evaluate the role of SSCOL in enhancing ultrasound imaging. The results indicated that the ultrasonic echo intensity at the tumor site in the SSCOL group peaked after 10 min of laser irradiation, whereas the SSOL group (without CREKA) exhibited only a modest increase in echo intensity, presumably due to insufficient nanoparticle accumulation (Fig. 4G-H and S8C-D). These results demonstrated that SSCOL, incorporating the combination of SPIO and PFP, achieved optimal ultrasound imaging under laser irradiation. Collectively, the SSCOL exhibits effective tumor-targeting capabilities and photoacoustic/ultrasound dual-modal imaging potential.
Enhanced self-amplifying tumor targeting of SSCOL following PTT
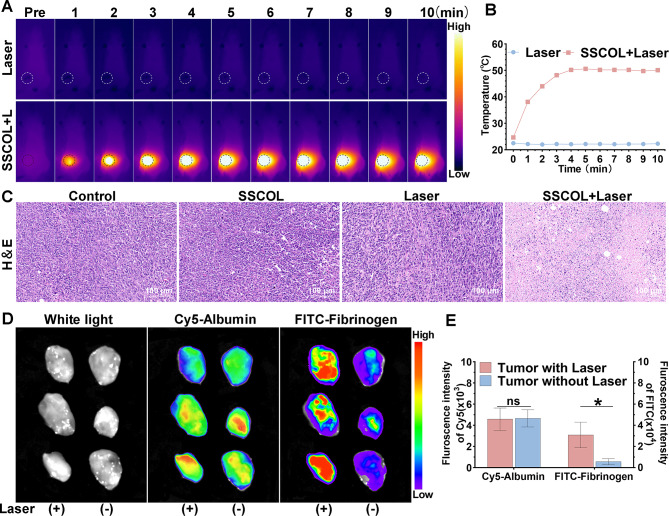
Encouraged by the promising tumor accumulation of SSCOL, its photothermal therapeutic efficacy and the potential for PTT-induced tumor-targeting enhancement were further evaluated in a 4T1 tumor mouse model. The results of infrared thermal imaging showed that the tumor temperature in the laser-alone group remained low, while in the group of SSCOL + Laser, it increased significantly from 24.7 °C to 50.1 °C (Fig. 5A-B). H&E staining revealed nuclear condensation and fragmentation exclusively in tumor cells within the group of SSCOL + Laser, while no significant pathological changes were observed in the other groups (Fig. 5C). These findings demonstrate that the anti-tumor effect of SSCOL is activated by PTT, and that their combination significantly enhances therapeutic efficacy.
Fig. 5.
In vivo photothermal efficacy and the activation of the coagulation cascade induced by PTT. (A) Infrared thermal images of tumor-bearing mice under 808 nm laser irradiation at various time points. (B) The corresponding time-photothermal temperature curves. (C) H&E-stained images of tumor tissues after different treatments. Scale = 100 μm. (D) Ex vivo NIR fluorescence images of Cy5-albumin and FITC-fibrinogen in tumor tissues with laser irradiation versus without laser irradiation. (E) The corresponding quantitative fluorescence data. (ns: not significant; *p < 0.05)
Building upon the concept of “targeted efficacy and positive feedback” in PTT, we further investigated the self-amplifying targeting effect of SSCOL following PTT. Initially, we verified the activation of the coagulation cascade induced by PTT. In brief, PTT was administered to one side of the tumor, while FITC-labeled fibrinogen (a precursor to fibrin involved in coagulation) and Cy5-labeled albumin (a plasma protein unrelated to coagulation) were concurrently injected. Ex vivo fluorescence imaging results demonstrated that the level of FITC-labeled fibrinogen in the laser-irradiated tumor was significantly elevated compared to the non-irradiated side, indicating the accumulation tendency of coagulation-related proteins in damaged tumor tissue. Conversely, this difference in fluorescence intensity was not observed in mice administered with Cy5-labeled albumin (Fig. 5D-E), thereby providing indirect evidence for the effective activation of the coagulation response induced by PTT.
Subsequently, the ability of PTT to enhance the formation of fibrin–fibronectin complexes following coagulation activation and to further facilitate the accumulation of SSCOL in tumors was evaluated. Firstly, a bilateral tumor-bearing mouse model was established. Twenty-four hours after tail vein injection of SSCOL, PTT was applied to one side of the tumor to induce a thermal effect. DiR-labeled SSCOL was re-injected via the tail vein, followed by in vivo fluorescence imaging 6 h post-injection. The results showed that in the targeted group (SSCOL), the fluorescence intensity on the tumor side treated with PTT was significantly higher than that on the contralateral side without PTT. Conversely, in the non-targeted group (SSOL, without CREKA), no significant difference in fluorescence intensity was observed between the two tumor sides, despite one side having already received PTT (Fig. 6A-B). Furthermore, confocal imaging of major organs and the laser-irradiated tumor tissue confirmed the previous findings, showing a greater accumulation of SSCOL in the tumor after PTT (Fig. 6C). In addition, immunofluorescence staining was performed to detect the co-localization of SSCOL and fibrinogen in the tumor tissue from the laser-irradiated side. As illustrated in Fig. 6D, fibronectin was highly expressed in the SSCOL group compared to the SSOL group and was co-localized with the nanoparticles. The elevated expression of fibronectin suggests that laser-irradiated SSCOL induces the increased expression of the fibrin-fibronectin complex. Their co-localization with nanoparticles further confirms the targeting capability of nanoparticles toward the increased fibrin-fibronectin complex. These results indicate the existence of a feedback loop under PTT, wherein SSCOL targeting and fibrin-fibronectin complex amplification synergistically reinforce each other.
Fig. 6.
The enhanced tumor-targeting performance of SSCOL following PTT. (A) NIR fluorescence images of bilateral tumor-bearing mice before and 6 h post-injection of SSCOL and SSOL (without CREKA), following PTT on one side of tumor. (B) The corresponding quantitative fluorescence data. (C) CLSM images of SSCOL and SSOL (red) in tumor and major organs. Scale = 400 μm. (D) Immunofluorescence images of fibronectin (Fn, green) and nanoparticles (red). Scale = 200 μm. (ns: not significant; *p < 0.05)
STING pathway activation and enhanced immune responses elicited by SSCOL combined PTT
As an immune activator, cGAMP is capable of stimulating specific antitumor immune responses [8]. By activating the STING pathway, it initiates a series of antitumor immune reactions, such as facilitating the maturation of DCs and amplifying the expression of related pro-inflammatory factors, thereby facilitating CTL activation and proliferation [44, 45]. The activated CTLs, in turn, exert their cytotoxic effects by specifically recognizing and killing tumor cells. As antigen-presenting cells, DCs can directly recognize the DNA of apoptotic or necrotic tumor cells [46]. Importantly, exogenous cGAMP activates the STING pathway in dendritic cells (DCs), thereby eliciting immune responses [47]. This section focuses on DCs to comprehensively evaluate SSCOL-mediated activation of the cGAS-STING pathway and its role in antitumor immunity.
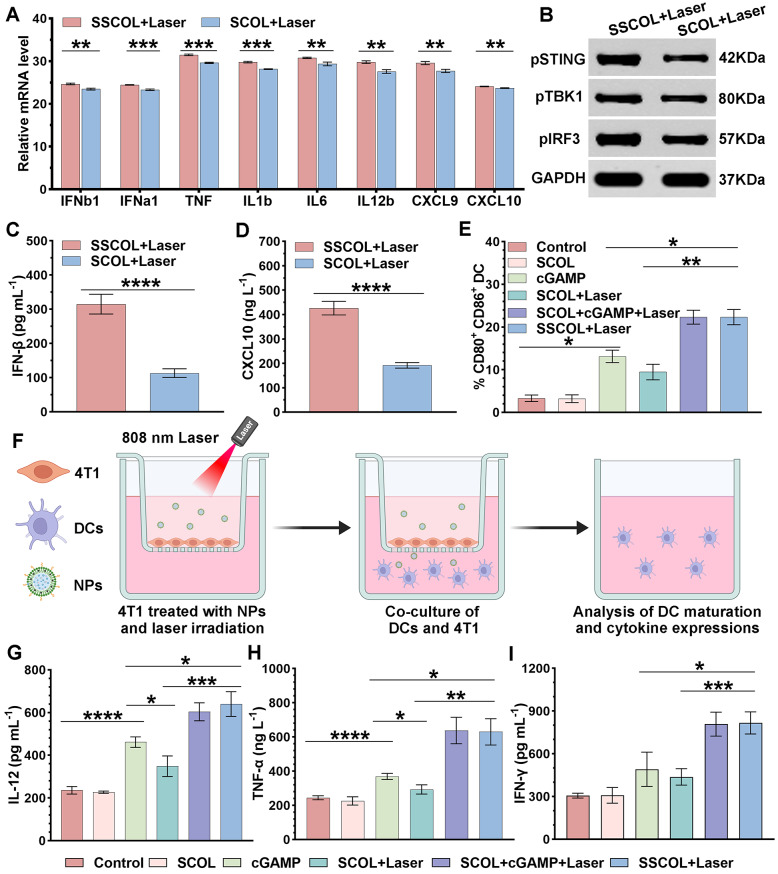
In vitro experiments demonstrated that co-incubation of immature DCs with SSCOL significantly elevated the expressions of IFN-I (IFNb1 and IFNa1), TNF, IL1b, IL6, IL12b, CXCL9, and CXCL10, as revealed by RT-PCR results (Fig. 7A). Western blotting revealed significantly elevated phosphorylation of STING, TBK1, and IRF3 in DCs following SSCOL + Laser treatment, compared to the SCOL (without cGAMP) + Laser group, indicating enhanced activation of the cGAS-STING pathway (Fig. 7B and S9). These results demonstrate that laser-triggered cGAMP release from SSCOL induces a robust pro-inflammatory cytokine response and efficiently activates the cGAS-STING signaling pathway in DCs. As we know, activation of the STING pathway in DCs induces IFN-β (also referred to as IFNb1 in gene nomenclature) secretion and promotes DC maturation [48]. Additionally, it enhances the expression of chemokines (e.g., CXCL10), which prompt T cells to migrate to the tumor site and recruit natural killer (NK) cells to directly kill tumor cells [49, 50]. Therefore, the in vivo serum levels of IFN-βand CXCL10 were evaluated in tumor-bearing mice. The results showed that both IFN-β and CXCL10 levels were significantly higher in the SSCOL + Laser group compared to the SCOL (without cGAMP) + Laser group (Fig. 7C-D). Taken together, these findings suggest that SSCOL effectively initiates STING-dependent innate immune activation both in vitro and in vivo, thereby establishing a favorable immunological context for subsequent antitumor responses.
Fig. 7.
The activation of the STING pathway, and in vitro assessment of DC maturation and related cytokine secretion. (A) RT-PCR assay of the key cytokine levels related to STING pathway in DCs. (B) Western blot assay of the key proteins related to the STING pathway in DCs. (C, D) ELISA assay of cytokine levels of IFN-β and CXCL10 in serum of tumor-bearing mice. (E) Flow cytometry analysis of DC maturation through Transwell systems. (F) Schematic diagram depicted the Transwell systems. (G,H and I) Secretion of IL-12, TNF-α, and IFN-γ in cell supernatants by ELISA assay. (ns: not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
DCs are capable of presenting tumor-associated antigens to naive T cells to stimulate the activation of T cells, and the antigen-presenting ability of DCs depends on the degree of maturation [49, 51]. Hence, based on the effective STING activation by SSCOL, further investigations were conducted to evaluate DC maturation and the activation and proliferation of CTLs. The effect on immune responses was evaluated in vitro by co-culturing immature DCs and 4T1 cells using Transwell chambers (Fig. 7F). Initially, flow cytometry was employed to detect the expression of co-stimulatory molecules CD80 and CD86 on DCs for verifying their maturity (Fig. 7E and S10). The results indicated a modest but statistically significant increase in CD80 and CD86 expression in the SCOL (without cGAMP) + Laser group, suggesting that PTT alone exerts a mild immunostimulatory effect on DCs. Notably, SSCOL + Laser treatment induced significantly higher DC maturation than either free cGAMP or SCOL + Laser, highlighting the synergistic effect of photothermal activation and cGAMP delivery in promoting DC activation. However, no significant difference in DC maturation was observed between the SSCOL + Laser group and the SCOL + cGAMP + Laser group, indicating that photothermally triggered controlled release of cGAMP from SSCOL effectively recapitulates the immune-priming effect of exogenous cGAMP combined with PTT. Upon DC maturation, diverse cytokines are secreted to regulate or activate specific immune cells, thereby further triggering the antigen-specific CTL responses [52]. Hence, to assess the immunostimulatory potential of matured DCs, the levels of IFN-γ, TNF-α, and IL-12—cytokines involved in shaping CTL responses—were measured in the co-culture supernatants using ELISA. The secretion levels of IL-12, TNF-α, and IFN-γ were significantly elevated in the SSCOL + Laser and SCOL + cGAMP + Laser groups compared to all other groups, highlighting the synergistic immunostimulatory effect of cGAMP and photothermal treatment. Similarly, no significant difference was observed between these two groups, further confirming that SSCOL under laser irradiation achieves comparable immunoactivation efficacy to free cGAMP combined with PTT (Fig. 7G-I). Collectively, these results demonstrate that SSCOL not only promotes DC maturation but also enhances the secretion of immunoregulatory cytokines, thereby effectively initiating the antigen-presenting cascade and fostering a cytokine environment conducive to robust CTL priming.
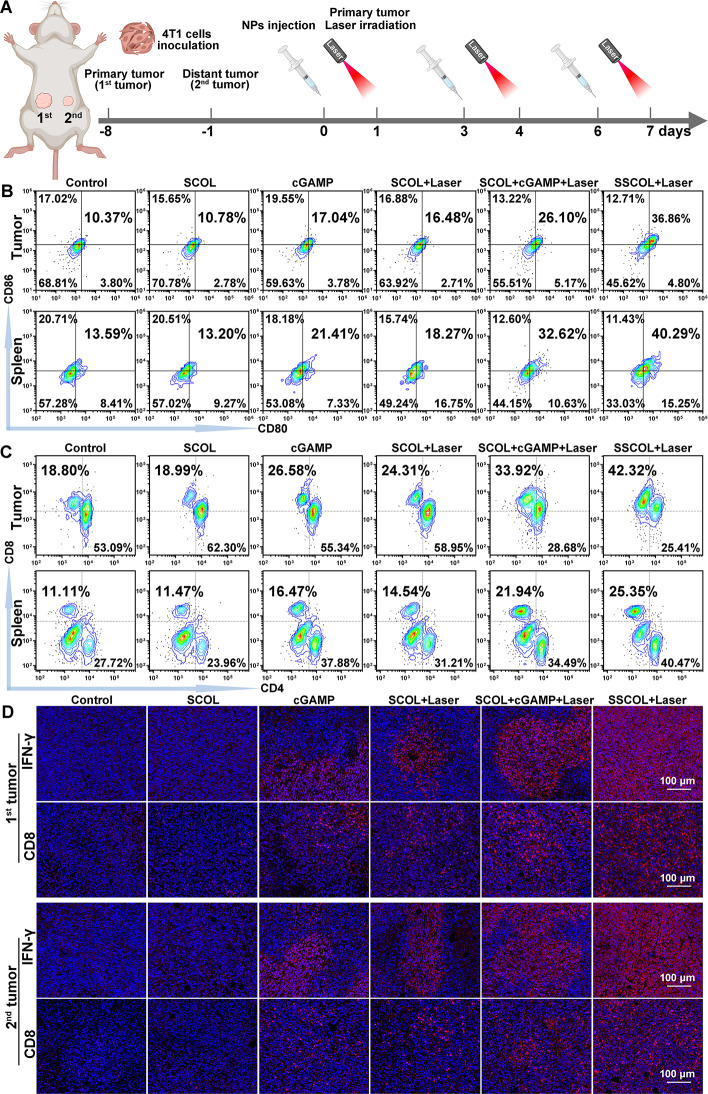
To further verify the antitumor efficacy of SSCOL combined with PTT on both primary and distant metastatic tumors, a bilateral 4T1 orthotopic breast cancer model was established, in which the first tumor served as the site for PTT and the second tumor mimicked distant metastasis (Fig. 8A). Flow cytometry analysis was performed on tumor, spleen and draining lymph node tissues to evaluate immune activation. As shown in Fig. 8B and Fig. S11, SSCOL + Laser treatment significantly enhanced the proportion of mature DCs (CD11c⁺CD80⁺CD86⁺) in both primary tumor and spleen tissues, with a markedly higher percentage than any other group, including the SCOL + cGAMP + Laser group. This indicates efficient STING activation and potent immune priming mediated by SSCOL upon photothermal stimulation. Furthermore, analysis of T cell subpopulations (Fig. 8C and Fig. S12) revealed a pronounced increase in intratumoral CD8⁺ CTLs in the SSCOL + Laser group (42.32% in tumors), along with elevated CD8⁺ CTL levels in spleens, suggesting strong systemic immune activation. The same group also exhibited increased infiltration of CD4⁺ helper T cells in both compartments, supporting the induction of systemic immunity. Immunofluorescence staining corroborated these findings. Strong CD8 and IFN-γ signals were observed in both primary and metastatic tumors following SSCOL + Laser treatment, indicating that the local PTT-triggered immune activation successfully elicited systemic CTL responses capable of targeting distant tumor lesions (Fig. 8D). Meanwhile, among all groups, SSCOL + Laser treatment elicited the highest serum levels of IL-12, TNF-α, and IFN-γ, indicating its superior capacity to stimulate systemic immune responses (Fig. S13). Collectively, these results encompassing DC maturation, T cell activation, immune cell infiltration in both local and distant tumor sites, and systemic cytokine secretion comprehensively demonstrate that SSCOL combined with PTT elicits potent local and systemic antitumor immune responses.
Fig. 8.
In vivo anti-tumor immunity. (A) Schematic illustration of the in vivo experimental schedule. (B) Flow cytometry analysis of DC maturation (CD11c+ CD80+ CD86+) in tumor and spleen to show the populations of matured DCs. (C) Flow cytometry analysis of CD4+T cells and CD8+T cells in tumor and spleen. (D) Immunofluorescence images of IFN-γ and effector CD8+T cell in primary (1st) tumor and metastatic (2nd) tumor after different treatments. Scale = 100 μm
The synergistic anti-tumor efficacy of SSCOL and PTT on primary and metastatic tumors
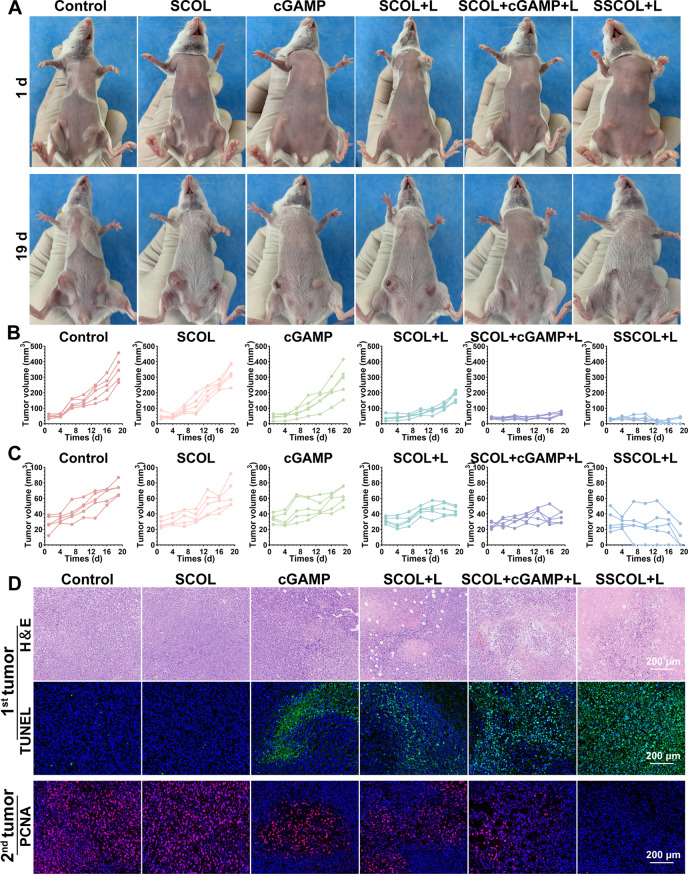
To evaluate the efficacy of photothermal-immune combined therapy for tumor treatment, a bilateral 4T1 breast cancer model was established, and tumor progression was documented throughout the treatment period. After treatment, mice were euthanized, dissected, and tumor tissues were photographed. As shown in Fig. 9A-C, S14 and S15, PTT alone significantly inhibited primary tumor growth but exhibited limited efficacy against metastatic tumors, indicating suboptimal systemic immune activation. In contrast, immunotherapy alone partially suppressed metastatic tumor growth while having weaker effects on primary lesions. Notably, the combined photothermal-immune treatment (SSCOL + Laser) showed potent inhibition of both primary and distant tumors. Additionally, therapeutic outcomes were markedly superior in the SSCOL + Laser group compared to the SCOL + cGAMP + Laser group, suggesting that nanoparticle encapsulation facilitates efficient immune activation and targeted drug release. H&E and TUNEL staining of primary tumors revealed extensive necrosis and apoptosis in the SSCOL + Laser group, accompanied by nuclear condensation and fragmentation, while PCNA staining of metastatic tumors showed minimal proliferative activity, confirming its dual-site therapeutic effect (Fig. 9D). Furthermore, no abnormal conditions, including sudden weight loss, were observed during treatment in any group (Fig. S16), confirming the favorable biosafety of the photothermal-immune therapeutic nanoparticle. Overall, these findings validate the potent therapeutic efficacy and favorable biosafety of SSCOL-mediated photothermal-immunotherapy in eradicating both primary and metastatic tumors.
Fig. 9.
The synergistic anti-tumor efficacy. (A) Digital photos of tumor-bearing mice at day 1 and 19 after different treatments. (B) Growth curves of the primary tumor (1st) and (C) the metastatic tumor (2nd) on mice. (D) H&E and TUNEL staining of primary tumors and PCNA staining of metastatic tumors. Scale = 200 μm
Conclusion
In summary, this study presents a novel imaging-guided, self-amplifying photo-immunotherapeutic nanoparticle (SSCOL) that effectively addresses two critical limitations of current STING-based nanomedicine: limited tumor specificity and insufficient immune activation. To overcome poor tumor targeting, SSCOL is functionalized with CREKA peptides that specifically bind to fibrin–fibronectin complexes in the tumor stroma. The photothermal effect further amplifies these targets via coagulation cascade activation, forming a positive feedback loop that significantly enhances intratumoral accumulation and spatial precision. To improve immune activation, SSCOL encapsulates cGAMP and achieves laser-triggered phase transition, enabling controlled and efficient release of the STING agonist. This process robustly activates the cGAS–STING pathway, induces pro-inflammatory cytokine secretion, promotes dendritic cell maturation and cytotoxic T lymphocyte activation, and elicits potent systemic anti-tumor immunity. Notably, the photothermal effect not only facilitates cGAMP release but also modulates the tumor immune microenvironment, enhancing antigen presentation and amplifying STING-mediated immune responses. Moreover, the integrated photoacoustic/ultrasound dual-modal imaging allows real-time monitoring of nanoparticle distribution and guides precise photothermal treatment.
Together, this study integrates dual-modal imaging with multimodal therapeutic strategies, offering an innovative and effective approach for precise tumor targeting, robust immune activation, and simultaneous suppression of both primary and metastatic tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Scheme 1 was created using BioRender.com with a licensed agreement.
Author contributions
The conception and supervision of the study were conducted by ZQ and GY. The original draft writing, formal analysis, and conceptualization were completed by CQ and YH. Validation, methodology, and investigation were carried out by LL, ZH, TM, ZM, and CL. Methodology and data curation were performed by LW, HY, and CY. RH was responsible for validation and project administration. ZQ and GY were in charge of manuscript review and editing, supervision, and funding acquisition. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82370500, 82102053), the Natural Science Foundation of Chongqing Municipality (CSTB2024NSCQ-MSX0226), and the Science and Technology Research Project of the Chongqing Municipal Education Commission (KJZD-K202400412).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The animal experiments in this study were approved by the Animal Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (Coren Trial(265), 2021) and conducted at the Laboratory Animal Center of Chongqing Medical University.
Consent for publication
All the authors have approved the manuscript to publish.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiaoqi Chen and Huilin Yu contributed equally to this work.
Contributor Information
Qiu Zeng, Email: zengqiu816@hospital.cqmu.edu.cn.
Yuan Guo, Email: 305856@hospital.cqmu.edu.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Yang J, Xu B, Zhang X. Tumor metastasis: Mechanistic insights and therapeutic interventions. MedComm (2020) 2021;2:587–617. [DOI] [PMC free article] [PubMed]
- 4.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yalamandala BN, Moorthy T, Liu ZH, Huynh TMH, Iao HM, Pan WC, Wang KL, Chiang CS, Chiang WH, Liao LD, et al. A Self-Cascading catalytic therapy and antigen capture Scaffold-Mediated T cells augments for postoperative brain immunotherapy. Small. 2025;21:e2406178. [DOI] [PubMed] [Google Scholar]
- 6.Sanmamed MF, Chen L. A paradigm shift in Cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Meng F, Xu Y, Li T, Chen X, Wang H. Chemically programmed STING-activating nano-liposomal vesicles improve anticancer immunity. Nat Commun. 2023;14:4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, Ascano M, Kelley M, Johnson DB, Balko JM, Wilson JT. Endosomolytic polymersomes increase the activity of Cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. [DOI] [PubMed] [Google Scholar]
- 10.Ohkuri T, Kosaka A, Ishibashi K, Kumai T, Hirata Y, Ohara K, Nagato T, Oikawa K, Aoki N, Harabuchi Y, et al. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol Immunother. 2017;66:705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motedayen Aval L, Pease JE, Sharma R, Pinato DJ. Challenges and opportunities in the clinical development of STING agonists for Cancer immunotherapy. J Clin Med. 2020;9. [DOI] [PMC free article] [PubMed]
- 12.Harrington KJ, Brody J, Ingham M, Strauss J, Cemerski S, Wang M, Tse A, Khilnani A, Marabelle A, Golan T. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol. 2018;29:viii712. [Google Scholar]
- 13.Luo T, Nash GT, Jiang X, Feng X, Mao J, Liu J, Juloori A, Pearson AT, Lin W. A 2D nanoradiosensitizer enhances radiotherapy and delivers STING agonists to potentiate Cancer immunotherapy. Adv Mater. 2022;34:e2110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang H, Xu C, Guo D, Peng F, Chen N, Song H, Ji X. Dismantlable coronated nanoparticles for coupling the induction and perception of Immunogenic cell death. Adv Mater. 2024;36:e2313097. [DOI] [PubMed] [Google Scholar]
- 15.Nie C, Ma T, Ye J, He M, Zhang T, Wei K, Jiang J, Chu X. A STING agonist-loaded bispecific nanobioconjugate modulates macrophage immune responses to enhance antitumor immunotherapy. Chem Eng J. 2024;485:149901. [Google Scholar]
- 16.Chiang MR, Hsu CW, Pan WC, Tran NT, Lee YS, Chiang WH, Liu YC, Chen YW, Chiou SH, Hu SH. Reprogramming dysfunctional dendritic cells by a versatile catalytic dual oxide Antigen-Captured nanosponge for remotely enhancing lung metastasis immunotherapy. ACS Nano. 2025;19:2117–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yalamandala BN, Chen YJ, Lin YH, Huynh TMH, Chiang WH, Chou TC, Liu HW, Huang CC, Lu YJ, Chiang CS, et al. A Self-Cascade penetrating brain tumor immunotherapy mediated by Near-Infrared II cell Membrane-Disrupting nanoflakes via detained dendritic cells. ACS Nano. 2024;18:18712–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying X, Chen Q, Yang Y, Wu Z, Zeng W, Miao C, Huang Q, Ai K. Nanomedicines Harnessing cGAS-STING pathway: sparking immune revitalization to transform ‘cold’ tumors into ‘hot’ tumors. Mol Cancer. 2024;23:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan W, Yung B, Huang P, Chen X. Nanotechnology for multimodal synergistic Cancer therapy. Chem Rev. 2017;117:13566–638. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Qu H, Pan Y, Cheng W, Xue X. Manganese-coordinated nanoparticle with high drug-loading capacity and synergistic photo-/immuno-therapy for cancer treatments. Biomaterials. 2025;312:122745. [DOI] [PubMed] [Google Scholar]
- 21.Ban Q, Bai T, Duan X, Kong J. Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers. Biomater Sci. 2017;5:190–210. [DOI] [PubMed] [Google Scholar]
- 22.Sun M, Zhang Z, Yin C, Wei Z-J, Yan Z, Long K, Tian Y, Wang Y, Wang W, Yuan Z. Photosensitizer-Free Nanophosphor-Based theranostic probe for controllable photodynamic and photothermal visualization therapy. Chem Eng J. 2024;490:151581. [Google Scholar]
- 23.Huynh TMH, Luc V-S, Chiang M-R, Weng W-H, Chang C-W, Chiang W-H, Liu Y-C, Chuang C-Y, Chang C-C, Hu S-H. Programmed lung metastasis immunotherapy via Cascade-Responsive cell Membrane-Mimetic Copolymer-Wrapped Nanoraspberry-Mediated Elesclomol-Copper delivery. Adv Funct Mater. 2024;34:2401806. [Google Scholar]
- 24.Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperth. 2005;21:761–7. [DOI] [PubMed] [Google Scholar]
- 25.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Ran Y, Wang Z, Cheng J, Cao Y, Yang C, Liu F, Ran H. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials. 2019;219:119370. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Wang X-Y, Chen Y-L, Liu F-Q, Tan M-X, Ao M, Yu J-H, Ran H-t. Wang Z-X: A light-controllable specific drug delivery nanoplatform for targeted bimodal imaging-guided photothermal/chemo synergistic cancer therapy. Acta Biomater. 2018;80:308–26. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Qutaish M, Han Z, Schur RM, Liu Y, Wilson DL, Lu ZR. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat Commun. 2015;6:7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilch J, Brown DM, Komatsu M, Jarvinen TA, Yang M, Peters D, Hoffman RM, Ruoslahti E. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc Natl Acad Sci U S A. 2006;103:2800–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, Derfus AM, Yang M, Hoffman RM, Bhatia S, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci U S A. 2007;104:932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okur AC, Erkoc P, Kizilel S. Targeting cancer cells via tumor-homing peptide CREKA functional PEG nanoparticles. Colloids Surf B Biointerfaces. 2016;147:191–200. [DOI] [PubMed] [Google Scholar]
- 32.Kruse AM, Meenach SA, Anderson KW, Hilt JZ. Synthesis and characterization of CREKA-conjugated iron oxide nanoparticles for hyperthermia applications. Acta Biomater. 2014;10:2622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overchuk M, Weersink RA, Wilson BC, Zheng G. Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. ACS Nano. 2023;17:7979–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Zhou S, Wu M, Qu R, Wang X, Chen W, Jiang Y, Jiang X, Zhen X. Light-Driven Self-Recruitment of biomimetic semiconducting polymer nanoparticles for precise tumor vascular disruption. Adv Mater. 2023;35:e2210920. [DOI] [PubMed] [Google Scholar]
- 35.Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J Control Release. 2012;164:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang N, Song J, Liu Y, Liu M, Zhang L, Sheng D, Deng L, Yi H, Wu M, Zheng Y, et al. Photothermal therapy mediated by phase-transformation nanoparticles facilitates delivery of anti-PD1 antibody and synergizes with antitumor immunotherapy for melanoma. J Control Release. 2019;306:15–28. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Wang Y, Niu C, Strohm EM, Zheng Y, Ran H, Huang R, Zhou D, Gong Y, Wang Z et al. Laser-Activatible PLGA microparticles for Image‐Guided Cancer therapy in vivo. Adv Funct Mater. 2014;24.
- 38.Wang R, Zhou Y, Zhang P, Chen Y, Gao W, Xu J, Chen H, Cai X, Zhang K, Li P, et al. Phase-transitional Fe(3)O(4)/perfluorohexane microspheres for magnetic droplet vaporization. Theranostics. 2017;7:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viger ML, Sheng W, Dore K, Alhasan AH, Carling CJ, Lux J, de Gracia Lux C, Grossman M, Malinow R, Almutairi A. Near-infrared-induced heating of confined water in polymeric particles for efficient payload release. ACS Nano. 2014;8:4815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abraham E, Minoshima K, Matsumoto H. Femtosecond laser-induced breakdown in water: time-resolved shadow imaging and two-color interferometric imaging. Opt Commun. 2000;176:441–52. [Google Scholar]
- 41.Zhang N, Ru B, Hu J, Xu L, Wan Q, Liu W, Cai W, Zhu T, Ji Z, Guo R, et al. Recent advances of CREKA peptide-based nanoplatforms in biomedical applications. J Nanobiotechnol. 2023;21:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiota M, Zardan A, Takeuchi A, Kumano M, Beraldi E, Naito S, Zoubeidi A, Gleave ME. Clusterin mediates TGF-beta-induced epithelial-mesenchymal transition and metastasis via Twist1 in prostate cancer cells. Cancer Res. 2012;72:5261–72. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayour EJ, Mitchell DA. Manipulation of innate and adaptive immunity through Cancer vaccines. J Immunol Res. 2017;2017:3145742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li A, Yi M, Qin S, Chu Q, Luo S, Wu K. Prospects for combining immune checkpoint Blockade with PARP Inhibition. J Hematol Oncol. 2019;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardner A, Ruffell B. Dendritic cells and Cancer immunity. Trends Immunol. 2016;37:855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XY, Yan Y, Guo XR, Lu A, Jiang LX, Zhu YJ, Shi YJ, Liu XY, Wang JC. Enhanced tumor immunotherapy by triple amplification effects of nanomedicine on the STING signaling pathway in dendritic cells. Adv Healthc Mater. 2024:e2403143. [DOI] [PubMed]
- 48.Prabagar MG, McQueney M, Bommireddy V, Siegel R, Schieven GL, Lu K, Husanov R, Deepak R, Diller D, Huang CY et al. The Sting agonist Vb-85247 induces durable antitumor immune responses by intravesical administration in a Non-Muscle invasive bladder Cancer. Cancer Res. 2024. [DOI] [PMC free article] [PubMed]
- 49.Li A, Yi M, Qin S, Song Y, Chu Q, Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. 2019;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toffoli EC, van Vliet AA, Forbes C, Arns AJ, Verheul HWM, Tuynman J, van der Vliet HJ, Spanholtz J, de Gruijl TD. Allogeneic NK cells induce the in vitro activation of monocyte-derived and conventional type-2 dendritic cells and trigger an inflammatory response under cancer-associated conditions. Clin Exp Immunol. 2024;216:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu H, Liu Z, Guo H, Hu X, Wang Y, Cheng X, Zhang LW, Wang Y. Mechanoimmune-Driven backpack sustains dendritic cell maturation for synergistic tumor radiotherapy. ACS Nano. 2024;18:23741–56. [DOI] [PubMed] [Google Scholar]
- 52.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.