Abstract
Background
There is a gap between tuberculosis (TB) infection and the onset of clinical TB disease, which makes identifying TB transmission dynamics a prominent challenge. Different reports were made on the concordance of drug-resistance profiles between the household contact and the purported index case. This study investigated the drug-resistance pattern concordance of the index-household contact pair in central Ethiopia.
Method
A laboratory-based cross-sectional study was conducted on Mycobacterium tuberculosis isolates identified from bacteriologically confirmed pulmonary TB patients and their household contacts (HHCs) in central Ethiopia from January to December 2023. Sputum specimens were collected from index cases and presumptive HHCs and examined using the Xpert Ultra assay, Xpert XDR assay, and Mycobacterium tuberculosis culture. Descriptive statistics were used to summarize the data.
Result
Among 902 TB symptoms screened HHCs of 303 index cases, 20.17% (182/902) had Presumptive TB, and 7.14% (13/182) developed active tuberculosis. In index cases, 23.52% (64 /272) showed resistance to at least one of the five first-line anti-TB drugs. The prevalence of mono-resistant to STR, INH, RIF, and PZA was: 2.20% (6 /272), 2.20% (6/272), 6.25% (17/272), and 1.47% (4/272), respectively. Any first-line anti-TB drug resistance was higher among relapse cases than new cases, at 41.67% (10/24) and 21.77% (54/248), respectively. Among the RR/MDR-TB cases tested with the Xpert MTB/XDR assay, 56.81% (25/44) cases showed resistance to INH. Among these 25 INH resistance samples, 5 had no melting point on the wild ahpc gene as well as on the ahpc gene mutant. In HHCs with positive cultures, 23.07% (3/13) displayed resistance to any first-line anti-TB medication. Only 69.23% (9/13) of HHCs had isolates that aligned with the pDST pattern of the index case for all five first-line anti-TB drugs.
Conclusion
Nearly one-third of the household contacts have discordant drug-resistance profiles from the index patients. This study offers compelling proof that it is not advisable to treat close contacts without DST results based on the DST results of the supposed source case. The low drug resistance rate to new oral second-line drugs in this study did not guarantee the absence of resistance to each drug.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-11220-x.
Keywords: Concordance, Drug resistance pattern, Household contact, Mycobacterium Tuberculosis
Introduction
Tuberculosis (TB) remains a major global public health challenge [1]. It is a curable and preventable airborne disease caused by the Mycobacterium tuberculosis Complex (MTBC) with a broad host range [2]. There is a gap between TB infection and the onset of clinical TB disease, which makes identifying TB transmission dynamics a prominent challenge. Around 5% of individuals infected with the TB bacteria will progress to TB disease within the initial two years of infection [1, 3]. Variations in the number of secondary cases per infected person can be a significant indicator of TB transmission [3, 4].
It was usual practice to treat household contacts (HHCs) that tested positive for TB on the assumption that they shared a common strain of Mycobacterium tuberculosis(M. tuberculosis) with the index case, and had concordant drug-resistance profiles [5]. However, members of the same household may have contracted different strains of M. tuberculosis from the community. Studies from high-incidence areas reported that the majority of HHCs have drug-resistance profiles that are different from those of the index patients [6, 7]. A systematic review and meta-analysis study reported a 54.3% pooled drug resistance pattern concordance between Persons with drug-resistant TB (DR-TB) and their HHCs [8].
Among TB cases, DR-TB was the most concerning type. The most significant challenge was resistance to Rifampicin (RIF), the most potent first-line drug [9]. According to the global TB report, in 2023, the prevalence of MDR/RR-TB was 3.3% among new cases and 17% among previously treated cases [1]. In this year, an estimated 400,000 people (95% UI: 360,000–440,000) developed multidrug-resistant or RR-TB (MDR/RR-TB) [1]. A systematic review reported that among patients with MDR-TB, the pooled proportion of resistance to Bedaquiline(BDQ) was 5%, Linezolid (LZD) was 4%, and clofazimine (CFZ) was 4% [10].
Ethiopia was one of the thirty high TB, TB/HIV burden countries [1]. Based on the 2024 global TB report since 2015 there has been a 25% decline in the TB incidence rate in Ethiopia; however, it remains at 146 cases per 100,000 populations. The proportion of new cases with MDR/RR TB was 2.6%, which increased to 15% in previously treated cases [1].
The National Tuberculosis Program (NTP) has established guidelines for the clinical and programmatic management of TB, TB/HIV co-infection, DR-TB, and leprosy in Ethiopia. The NTP has made notable progress in preventing and treating TB, with an emphasis on enhancing service accessibility and involving community participation. The program incorporates TB services into the broader healthcare system, provides these services at no cost, and seeks to tackle the challenges posed by DR-TB and the prevalent co-infection with HIV. Ethiopia’s TB program, which includes DR-TB, employs a decentralized, outpatient model aimed at enhancing access to healthcare services. This strategy seeks to broaden treatment availability by increasing the number of treatment initiation centers (TICs) and treatment follow-up centers (TFCs) [11]. The NTP enhances TB laboratory diagnostics by expanding to over 930 GeneXpert locations and 11 culture and DST facilities [12].
To diagnose DR-TB, the Ethiopian TB diagnostic algorithm suggested TRUENAT, Xpert MTB/RIF Ultra, and MTB/XDR testing at the peripheral level, as well as line probe assay (LPA) (first- and second-line) and phenotypic drug-susceptibility testing (pDST) at the regional laboratory level. Practically, these diagnostic methods were not accessible to all TB diagnostic health facilities, which makes drug susceptibility(DST) test coverage low in the country. The DST methods mentioned above do not detect resistance to the WHO-recently recommended drug regime (Groups A and B), except pDST, which was used as a second-line drug in Ethiopia. In Ethiopia, DR-TB treatment options include shorter, all-oral, Bdq-containing regimens, fully oral longer regimens, and individualized longer regimens [11].
Xpert® MTB/XDR test is capable of detecting resistance using melt curve analysis by targeting associated mutations to isoniazid (INH), ethionamide (ETH), fluoroquinolones (FLQ), and second-line injectable agents (SLID), such as kanamycin (KAN), amikacin (AMK) and capreomycin (CAP) [13].
In Ethiopia, there was scarce data regarding the concordance of genotypic (which assesses drug resistance by pinpointing specific mutations in the bacterial DNA) and phenotypic (which looks at the bacteria’s response to drugs) drug resistance patterns between the household contacts and the index cases. Additionally, limited information was available on the epidemiology of second-line drug-resistant TB (SLDR-TB) and Group A drug-resistant TB (GADR-TB) in Ethiopia in general. This study aimed to assess how well the HHCs and the purported index case from contact investigation match in terms of drug resistance patterns to first- and second-line anti-TB medications.
Methods
Study design and setting
A laboratory-based cross-sectional study was conducted using isolates identified from bacteriologically confirmed pulmonary TB patients and their HHCs in central Ethiopia. The study area includes a 200 KM radius from the capital city, Addis Ababa. The study area covers the slums, semi-urban, urban, and rural areas of central Ethiopia. The sites were chosen from Oromia, Addis Ababa city administration, and the Amhara regional state. The study site includes five hospitals (Fitche, Geda, Tullubolo, Debrebirhan, and st Peter hospitals), which served as MDR/RR TB TIC, and 11 public health centers selected from the above regions.
Study population and participants
Once an individual is diagnosed with bacteriologically confirmed pulmonary TB (index case), the health personnel in the TB clinic request the index case to list all HHCs who live with him in the same dwelling. Every consented consecutive bacteriologically confirmed pulmonary TB patient (by Xpert MTB/RIF Ultra assay) who had visited TB clinics at any age, had given a traceable residence location, and had one or more HHCs was eligible for the study. The study included index cases without discriminating by drug resistance profile.
Sampling procedure
The sample size was computed using a single population proportion formula [14] by considering a 14% proportion of INH resistance among smear-positive PTB cases [15], a 95% confidence interval, and a 4% margin of error. Accordingly, the final sample size of index cases with a 5% average contamination rate is 303.
The sample size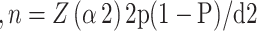
Where n = Sample size
z (α2) 2 = At 95% confidence interval Z value (α = 0.05) = 1.96.
p = Proportion of occurrence of the event to be studied
d = Margin of error at (4%) (0.05)
The average household member in Ethiopia was 4.3 [12]. By subsidizing the index case, on average, one index case will have 3.3 HHC. The total HHCs expected to be screened for this study were 990.
The study was done in fifteen randomly selected public health facilities in central Ethiopia. The total sample size was proportionally distributed to each study site. During the study period, from January 2023 to December 2023, 303 sputum specimens from index cases and 182 sputum specimens from TB presumptive HHCs were collected. From these, 272 pure MTBC isolates were identified from the index cases, and 13 additional isolates were identified from HHCs.
Operational definitions
Any resistance TB means that the TB bacteria are resistant to at least one of the standard first-line TB drugs. Bacteriologically confirmed tuberculosis: TB diagnosed in a biological specimen by a WHO-approved rapid test such as Xpert® MTB/RIF, smear microscopy, or culture. Drug susceptibility testing (DST): In vitro testing using either molecular genotypic techniques to detect resistance-conferring mutations or phenotypic methods to determine susceptibility to a drug. Household contact: A person who shared the same enclosed living space as the index case for one or more nights or for frequent or extended daytime periods during the 3 months before the start of the current treatment. Index case (index patient) of tuberculosis: Initially identified person of any age with new or recurrent TB in a specific household or other comparable setting in which others may have been exposed. An index case is a person on whom a contact investigation is centered, but is not necessarily the source case. Multidrug-resistant tuberculosis means that the bacteria were resistant to only one of the first-line anti-TB drugs. Multidrug-resistant tuberculosis (MDR-TB): TB caused by Mycobacterium tuberculosis strains that are resistant to at least both rifampicin and isoniazid. New case: A newly registered episode of TB in a person who has never been treated for TB or has taken TB medicines for less than 1 month. Rifampicin-susceptible, isoniazid-resistant tuberculosis: TB caused by Mycobacterium tuberculosis strains resistant to isoniazid and susceptible to rifampicin. Rifampicin-resistant tuberculosis (RR-TB): TB caused by Mycobacterium tuberculosis strains resistant to rifampicin. A presumptive TB case was an HHC with cough of two weeks or more or having any two of the following symptoms: fever of two weeks or more, night sweats, and unexplained weight loss of more than 1.5 kg in a month.
Collecting, transporting, and culturing of the specimens
Following the participants’ consent, early morning sputum specimens from bacteriologically confirmed index cases and their presumptive HHCs were collected within one week of the diagnosis of the index case. The specimens were transported to EPHI through a cold chain system (2-8oc). The laboratory activities were conducted at the Ethiopian Public Health Institute (EPHI), the national TB reference laboratory (NTRL). The EPHI-NTRL is an ISO 15,189-accredited laboratory in Xpert MTB/RIF Ultra assay, TB culture, LPA, and phenotypic DST. The specimens were examined using Xpert MTB/RIF Ultra assay, Xpert MTB/RIF XDR assay (for RR cases and INH mono resistance suggestive), and culture.
Specimens were decontaminated using a final NaOH concentration of 1.5%, which was mixed in equal amounts with the sample and concentrated by centrifugation at 3,000 rpm for 15 min. After discarding the supernatant, the sediment was neutralized with PBS. The sediment was inoculated onto MGIT and Löwenstein–Jensen (LJ) egg slant media. The LJ media was checked weekly for growth up to 8 weeks. The MGIT machine automatically reads the growth of the colony every hour after incubation for 42 days. The media with growth was checked for the presence of MTBC using the SD bioline antigen test.
Xpert® MTB/Rif ultra or XDR assay
Molecular analyses were carried out using the input part of the Xpert® MTB/Rif Ultra assay, according to the manufacturer’s instructions, after decontaminating the sample in a diluent solution (2:1 ratio, mixture of NaOH and isopropanol) [16].
The novel Xpert MTB/XDR assay (Cepheid, USA) is a rapid, cartridge-based assay that is intended to be used as a reflex test. The Xpert MTB/XDR assay is an automated in vitro diagnostic test for the detection of XDR MTB complex DNA and resistance-associated mutations [13, 17].
The targets per drug include: the INH gene target, inhA promoter with − 1 to − 32 intergenic nucleotide, katG codon 311–319, nucleotide 939–957, fabG1 codon 199–210, nucleotide 597–630, oxyR-ahpC with nucleotide − 5 to 50 or intergenic − 47 to 92. For ethionamide, the gene target is the inhA promoter with nucleotide − 1 to -32 intergenic. For FLQs, the gene targets are gyrA at codons 87–95, nucleotides 261–285, and gyrB at codons 53–544 or 493–505 with nucleotide 1596–1632 [17].
MGIT first and second line phenotypic drug susceptibility test
Phenotypic DST was done using the MGIT 960 system DST, an AST (antibiotic susceptibility testing) set, that includes one tube for each drug and a growth control tube. First, a known concentration of a drug was added to the MGIT tube, along with the specimen. It was incubated in the machine, where the growth in the drug-containing tube was compared with a drug-free control of the same specimen. The critical concentrations for the drugs were as follows for first-line drugs: Streptomycin (STR) 1.0 µg/mL; INH 0.1 µg/mL; RIF 1.0 µg/mL; EMB 5.0 µg/mL, and PZA 100 µg/mL. For second-line drugs: moxifloxacin (MOX) 0.25 µg/mL and 0.25 µg/mL; LEVO 2.5 µg/mL; BDQ 1.0 µg/mL; delaminid(DLM) 0.06 µg/Ml, pretomanid(p.a.) 2.0 µg/mL, linezolid (LZD) 10 µg/mL; and clofazimine (CFZ) 1.0 µg/Ml [18, 19].
Quality assurance
The sample was collected, stored, and transported following NTRL’s standard operating procedure. The sterility of the culture media was checked by incubating the whole media at 37 0C for 48 h, and the performance of the media was checked by known susceptible M. tuberculosis (H37Rv). The sterility of sample processing reagents was checked by inoculating all reagents in a separate BHI medium. Positive and negative controls were included in every run of MTBC culturing.
For molecular operational activities, sterile molecular grade water and reagent control were used as negative control, and H37Rv ATCC 25,177 was used as a positive control. Moreover, to check the quality of the PCR and reverse hybridization process, we see the presence of the amplification control (AC) band indicates that the DNA extraction and PCR procedures were carried out successfully, and the conjugate control (CC) documents two steps in the procedure.
Data management and analysis
Demographic and clinical data were recorded using a paper-based case record form (CRF) system at each study site. Subsequently, the CRF was transported to EPHI with the samples, where data was entered into the database. All laboratory results were recorded in a logbook during the study period. The collected data were analyzed and interpreted accordingly after it was checked for completeness, accuracy, and clarity. The anonymized data were subsequently imported into STATA version 17 software for further statistical analysis. Descriptive statistics were employed to determine the frequency and percentage of the variables. A logistic regression model was conducted to assess the association of any drug resistance with patient clinical and demographic characteristics. The model validation was done using the chi-square goodness-of-fit test. The overall test agreement was determined using Cohen’s kappa value. The odds ratio, along with 95% CI, was determined, and a P-value < 0.05 was considered for the presence of a statistically significant association.
Ethical approval
The Ethiopian Public Health Institute and Addis Ababa University, Aklilu Lema Institute of Pathobiology, granted ethical approval for the study under approval numbers EPHI-IRB-456-2022 and ALIP IRERC/94/2015/23, respectively. The study also received an official letter of support from the Addis Ababa Health Bureau. Following oral and written explanations of the study, voluntary index patients, HHCs, and guardians (when the index case or the HHC was a child aged less than 15 years) signed written informed consent and assent. The confidentiality of individual records was strictly maintained, and aggregate analysis was used to guarantee data anonymization. Treatment of bacteriologically confirmed active TB patients (HHCs) and administration of TPT to children fifteen years of age or under who do not have active TB were conducted according to the national TB guidelines [11].
Result
Demographic and clinical characteristics of index cases
In this study, the most affected people were in the age bracket of reproductive age group (16–44 years). The study participants had an average age of 33 years (95%CI: 32.00-35.09), with ages ranging from 1 year to 83 years. Pulmonary TB was higher in males (55.45%) than in females. The study found that 44.22% of TB patients were malnourished (underweight). Farmers, students, teachers, and factory workers comprised 7.59%, 9.24%, 1.98%, and 3.96% of the study participants, respectively. The finding showed that 10% of the participants had no formal education. In this study, 14.85% of study participants were HIV positive. In this result, 46.86% of the study participants live in single rooms with their HHCs. Relapse TB cases accounted for 8.25% of the total index cases. A significant portion (85.15%) of the index cases had 2–4 HHCs living with them. Among the HHCs, 28.71% share a bed with a household member. To come to the health facility, one-third (30.69%) of the TB patients were delayed for one to three months after they had the TB symptoms (Table 1).
Table 1.
Socio-demographic and clinical characteristics of bacteriologically confirmed pulmonary index case in central ethiopia, January 2023 to December 2023
| Characteristics | Index case; N(%) = 303 | |
|---|---|---|
| Sex | Male | 168(55.45) |
| Female | 135(44.55) | |
| Age | Mean (95%) | 33.54 (32.00-35.09) |
| < 15 | 7(2.31) | |
| 16–24 | 70(23.10) | |
| 25–34 | 114(37.62) | |
| 35–44 | 60(19.80) | |
| 45–65 | 40(13.20) | |
| > 65 | 12(3.96) | |
| BMI | Underweight(< 18.5) | 134(44.22) |
| Normal weight(18.5–24.9) | 165(54.46) | |
| Overweight(> 25.0) | 4(1.32) | |
| Educational status | Illiterate | 36(11.88) |
| Read and write | 15(4.95) | |
| Primary | 70(23.10) | |
| Secondary | 97(32.01) | |
| Certificate and above | 85(28.05) | |
| Occupation | Daily laborer | 25(8.25) |
| Driver | 11(3.63) | |
| Student | 28(9.24) | |
| Teacher | 6(1.98) | |
| Factory worker | 12(3.96) | |
| Farmer | 23(7.59) | |
| Government | 16(5.28) | |
| Private | 68(22.44) | |
| Other | 61(20.13) | |
| No work | 53(17.49) | |
| Marital status | Single | 142(46.86) |
| Married | 144(47.52) | |
| Divorced | 17(5.61) | |
| No of HHCs | 1 | 9(2.97) |
| 2–4 | 258(85.15) | |
| > 4 | 36(11.88) | |
| TB type | New | 278(91.75) |
| Relapse | 25(8.25) | |
| TB symptom duration | < 2 week | 43(14.19) |
| 2 week-1 month | 157(51.81) | |
| 1–3 months | 93(30.69) | |
| > 3 months | 10(3.30) | |
| HIV status | Positive | 45(14.85) |
| Negative | 255(84.16) | |
| Unknown | 3(0.99) | |
| Bacillary load by Xpert MTB/RIF ultra | Low | 75(24.75) |
| Medium | 157(51.82) | |
| High | 71(23.43) | |
| No rooms in the Household | 1 | 142(46.86) |
| 2–3 | 136(44.88) | |
| > 3 | 25(8.25) | |
| Share a bed with household members | Yes | 87(28.71) |
| No | 216(71.29) | |
| Current/previous smoker | Yes | 23(7.59) |
| No | 280(92.41) | |
| Drink alcohol daily | Yes | 44(14.52) |
| No | 259(85.48) | |
BMI: Body mass index; GX: Xpert ultra assay; HIV: Human immunodeficiency virus; No: Number
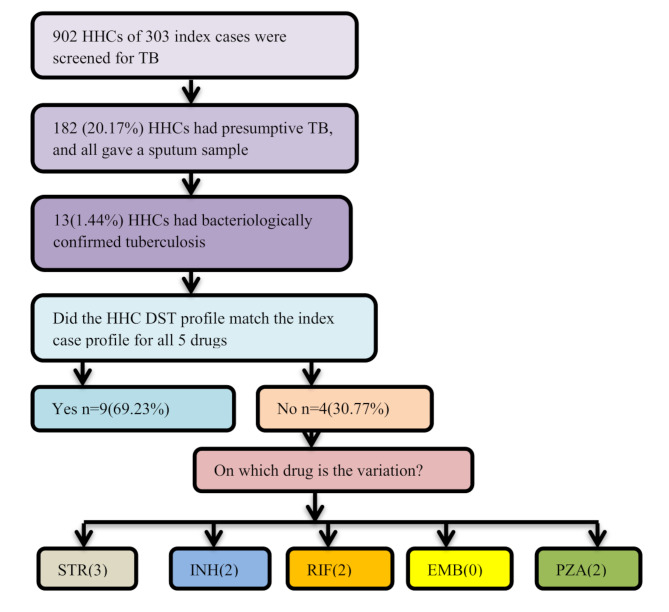
Among 902 TB symptom-screened HHCs of 303 index cases, 20.17% (182/902) had Presumptive TB, and all of them submitted a morning sputum sample. Every sample was examined by Xpert MTB/RIF Ultra assay and M. tuberculosis culture test. Tuberculosis was bacteriologically confirmed in 7.14% (13/182) of the presumptive HHCs using Xpert MTB/ultra and M. tuberculosis culture test. Culture-positive results were found in 94.71% (287/303) of the index cases. The remaining 5.29% (16/303) were positive by Xpert MTB/RIF Ultra assay but did not have growth on culture media. All the 13 HHC’s index cases were culture positive, and we had thirteen index-HHC pairs (Fig. 1 and S Fig. 1).
Fig. 1.
Index–contact pairs, drug resistance profile concordance. The figure showed that there was a drug resistance pattern difference between the index case and an HHC at each first-line drug except for EMB. Three index–HHC pairs have differences in the STR drug resistance pattern. EMB: ethambutol; INH: isoniazid; RIF: rifampicin; STR: streptomycin; PZA: Pyrazinamid
Among the culture-positive samples, 94.77% (272/287) of index cases and 100% (13/13) of the HHC isolates were successfully sub-cultured, and pDST was performed on the BACTEC MGIT™ 960 instrument using the SIRE and PZA kit. Out of the index cases that have pDST results, 16.17% (44/272) were found to have any RR, 2.21% (6/272) have INH mono resistance TB, and 9.55% (26/272) have MDR-TB (S Fig. 1).
Phenotypic DST analysis of index cases
The analysis found that 23.52% (64/272; 95% CI: 18.88–28.92) of the index case isolates tested for susceptibility to first-line anti-TB drugs exhibited resistance to at least one of the five drugs. In this study, the prevalence of mono-resistant to STR, INH, RIF, and PZA was: 2.21% (6/272), 2.21% (6/272), 6.25% (17/272), and 1.47% (4/272), respectively. Any first-line anti-TB drug resistance was higher among relapse cases, 41.67% (10/24; 95%CI: 24.47–61.17), than new TB cases, 21.77% (54/248; 95%CI: 17–27). In the relapse cases, only 12.50% (3/24; 95%CI: 4.34-31.0) showed RIF mono resistance. Among the index cases initially treated as DS-TB by Xpert MTB/RIF Ultra assay, the higher mono resistance was on STR (2.63%; 95%CI: 1.00–5.0), followed by INH and PZA (1.75%; 95%CI: 0.68–4.42) for each drug. The prevalence of any PZA resistance in RR-TB cases was 11.36% (5/44; 95% CI: 4.95–23.97). In the total cases, the highest proportion of poly resistance was observed against STR + INH + PZA (1.10%; 95% CI: 0.37–3.19). Among the tested isolates, 1.47% (4/272) were resistant to EMB. Two isolates were resistant to all first-line anti-TB drugs that were tested (Table 2).
Table 2.
Phenotypic drug resistance pattern of M. tuberculosis complex isolated from index patients to first-line anti-tuberculosis drugs in central ethiopia, January 2023 to December 2023
| Drug resistance pattern | Total case(n = 272) | New case(n = 248) | Relapse case(n = 24) | Non-RR-TB (n = 228) | MDR-TB/RR-TB(n = 44) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | 95% CI | N (%) | 95% CI | N (%) | 95% CI | N (%) | 95% CI | N (%) | 95% CI | ||
| Drug resistance | Any resistance | 64 (23.53) | 18.88-28.92 | 54(21.77) | 17–27 | 10(41.67) | 24.47–61.17 | 20 (8.77) | 5.75–13.16 | 44(100) | - |
| Susceptible (to 5 drugs) | 208(76.47) | 71.08–81.12 | 194(78.23) | 72–82 | 14(58.33) | 38.83–75.53 | 208(91.23 | 86.84–94.25 | - | - | |
| Any resistance | Any STR | 17 (6.25) | 3.94–9.78 | 13(5.24) | 3.09–8.76 | 4(16.67) | 6.68–35.86 | 11(4.82) | 2.71–8.43 | 6(13.64) | 6.41–26.71 |
| Any INH | 37 (13.60) | 10.03–18.18 | 30(12.1) | 8.61–16.75 | 7(29.17) | 14.92–49.17 | 11(4.82) | 2.71–8.43 | 26(59.09) | 44.41–72.31 | |
| Any RIF | 44(16.17) | 12.28–21.02 | 36(14.52) | 10.68–19.45 | 8(33.33) | 17.97–53.29 | - | - | 44(100.0) | ||
| Any EMB | 4(1.47) | 0.57–3.72 | 3(1.21) | 0.41–3.5 | 1(4.17) | 0.74–20.25 | 1(0.44) | 0.08–2.44 | 3(6.82) | 2.35–18.23 | |
| Any PZA | 11(4.04) | 2.27–7.09 | 9(3.63) | 1.92–6.75 | 2(8.33) | 2.31–25.84 | 6(2.63) | 1.21–5.62 | 5(11.36) | 4.95–23.97 | |
| Mono resistance | STR | 6(2.21) | 1.02–4.73 | 6(2.40) | 1.11–5.18 | - | - | 6(2.63) | 1.00–5.0 | - | - |
| INH | 6(2.21) | 1.02–4.73 | 6(2.40) | 1.11–5.18 | - | - | 4(1.75) | 0.68–4.42 | - | - | |
| RIF | 17(6.25) | 3.94–9.78 | 14(5.65) | 3.4–9.26 | 3(12.50) | 4.34-31.0 | - | - | 17(38.64) | 25.72–53.38 | |
| EMB | - | - | - | - | - | - | - | - | - | - | |
| PZA | 4(1.47) | 0.57–3.72 | 4(1.61) | 0.63–4.07 | - | - | 4(1.75) | 0.68–4.42 | - | - | |
| Poly resistance | STR + INH + PZA | 3(1.10) | 0.37–3.19 | 2(0.81) | 0.22–2.9 | 1(4.16) | 0.74–20.25 | 2(0.87) | 0.24–3.15 | 1(2.27) | 0.4–11.8 |
| INH + EMB + PZA | 1(0.37) | 0.07–2.06 | 1(0.40) | 0.07–2.24 | - | - | - | - | 1(2.27) | 0.4–11.8 | |
| STR + INH + PZA + EMB | 1(0.37) | 0.07–2.06 | - | - | 1(4.16) | 0.74–20.25 | 1(0.43) | 0.08–2.44 | - | - | |
| Resistance to all tested drugs | 2(0.74) | 0.2–2.65 | 2(0.81) | 0.22–2.90 | - | - | - | - | 2(4.54) | 1.26–15.14 | |
EMB: Ethambutol; INH: isoniazid; RIF: rifampicin; STR: streptomycin; PZA: Pyrazinamide“-”: not identified; Retreatment cases: relapse case, treatment after failure, and treatment after loss to follow-up; Non RR-TB: drug-susceptible TB that were treated by first-line anti-TB drugs; MDR-TB/RR: Multidrug resistance TB/Rifampicin resistance TB: Any resistance-resistance at least for one drug: Mono resistance-Resistance only for that respective drug only
Second-line pDST testing was carried out on 44 RR/MDR-TB isolates to assess their resistance to the newly WHO-recommended second-line anti-TB drugs. Results showed that 97.73% (95%CI: 88.20–99.60) of the isolates were fully susceptible to the seven tested new second-line anti-TB drugs (groups A and B). Only 2.27% (1/44; 95%CI: 0.4–11.8) of the isolates demonstrated resistance to Lev and Mox, with the remaining isolates being fully susceptible. The prevalence of drug resistance to the new all-oral 6-month regimen, which includes BDQ, PTM, LZD, and Mox, was found to be low (S Table 1).
Performance of xpert MTB/XDR using pDST as a standard test in RR/MDR-TB index cases isolated
The genes katG, inhA, aphC, and kasA are associated with resistance to INH. Among the total RR-TB cases tested with the Xpert® MTB/XDR assay, 25 cases (56.81%) showed resistance to INH. All cases of INH resistance were linked to katG mutations. Of these 25 INH resistance samples, 5 had no melting point on the wild ahpc gene, but they did not have a melting point on the ahpc gene mutation. Of the 19 INH susceptible samples, 4 have no melting point on the ahpc wild gene. There was one discordant result between Xpert MTB/XDR and pDST(Kappa = 0.94, p < 0.05). It was resistant to pDST and susceptible to the Xpert MTB/XDR assay. The prevalence of MDR-TB by Xpert MTB/XDR assay and pDST was 9.19% (25/272) and 9.55% (26/272), respectively. Only one MTBC isolate (2.27%) showed resistance to FLQ, and it had a melting point on the gyrA3-mutB gene(S. Table 2).
Phenotypic DST analysis of household contact isolates
In the HHCs, the prevalence of any first-line anti–TB drug resistance was 23.08% (3/13; 95% CI: 8.18–50.26). All other contacts were fully susceptible to the five five-line anti-TB drugs. Among the five drugs, STR mono resistance was found in 7.69% (1/13; 95% CI: 1.37–33.31) of HHC isolates. In the contact investigation, 15.38% (2/13; 95%CI: 4.32–42.23) HHC isolates had any INH and PZA resistance. Furthermore, 15.38% (2/13; 95% CI: 4.32–42.23) of the HHCs’ isolates showed resistance to STR, INH, and PZA. Rifampicin-resistant TB was not found among HHCs (S. Table 3).
Concordance of drug resistance profile between the index case and household contacts
The result showed that 69.23% (9/13; 95%CI: 42.37–87.32) of HHCs had isolates matching the index case’s pDST for all 5 drugs. While 30.77% (4/13; 95% CI: 12.68–53.63) HHCs had isolates with different pDST to one or more drugs than their purported index case. Two RR index cases had HHCs with DS-TB cases. Among the 13 index–HHCs pair pDST results, 2 pair isolates had different DST patterns on each STR, INH, RIF, and PZA. No EMB pDST pattern difference was registered in the 13 pairs (Fig. 1 and S Table 4).
Risk of any first-line anti-TB drug resistance among index cases
There was a statistically significant association between TB treatment history and any drug resistance (aOR = 0.36, 95% CI: (0.13–0.96)) (P < 0.05). New TB cases were 0.36 times less likely to develop resistance to any one of the anti-TB drugs compared with those relapse cases. Index cases who have an age greater than 45 are eight times more likely to have any drug resistance than patients under 15 years. Patients who had high bacillary load were 1.47 times more likely to develop any drug resistance than patients who had low bacillary load (Table 3).
Table 3.
Factors associated with any drug-resistant tuberculosis among index cases who had valid DST results
| Variables | Any drug resistance detected(n = 64) | Any drug resistance not detected(n = 208) | aOR(95%CI) | P-value | |
|---|---|---|---|---|---|
| Sex | Female | 28(22.95) | 94(77.05) | 1 | |
| Male | 36(24.0) | 114(76.0) | 1.17(0.60–2.26) | 0.640 | |
| Age group | < 15 | 0 | 7(100.0) | 1 | |
| 16–24 | 15(23.80) | 48(76.20) | 5.16(0.55–48.07) | 0.150 | |
| 25–34 | 18(17.30) | 86(82.70) | 2.52(0.27–22.81) | 0.409 | |
| 35–44 | 17(31.48) | 37(68.52) | 6.772(0.71–64.04) | 0.095 | |
| 45–65 | 13(38.23) | 21(61.77) | 8.11(0.82–79.48) | 0.072 | |
| > 65 | 1(10.0) | 9(90.0) | - | ||
| BMI | < 18.5 | 23(19.32) | 96(80.68) | 1 | |
| 18.5–24.9 | 39(26.19) | 110(73.81) | 1.38(0.71–2.71) | 0.335 | |
| > 24.9 | 2(50) | 2(50) | 2.97(0.36–24.39) | 0.311 | |
| TB type | Relapse | 10(41.67) | 14(58.33) | 1 | |
| New | 54(21.77) | 194(78.23) | 0.36(0.13–0.96) | 0.043 | |
| HIV status | Positive | 9(21.42) | 33(78.58) | 1 | |
| Negative | 54(23.68) | 173(76.32) | 1.70(0.70–4.12) | 0.236 | |
| Unknown | 1(33.33) | 2(66.66) | 6.81(0.35–129.82) | 0.202 | |
| GX bacillary load | low | 17(25.37) | 50(74.63) | 1 | |
| Medium | 25(18.38) | 111(81.62) | 0.67(0.31–1.44) | 0.307 | |
| High | 22(31.88) | 47(68.12) | 1.47(0.62–3.45) | 0.372 | |
| No room in the household | 1–2 | 47(21.96) | 167(68.04) | 1 | |
| > 2 | 17(29.31) | 41(70.69) | 1.40(0.67–2.91) | 0.366 | |
| Current/previous smoker | Yes | 5(25) | 15(75) | 1 | |
| No | 59(23.41) | 193(76.59) | 0.88(0.24–3.22) | 0.854 | |
| Drink alcohol | Yes | 7(19.44) | 29(80.56) | 1 | |
| No | 57(24.15) | 179(75.85) | 2.02(0.69–5.91) | 0.197 | |
| Cough duration | < 1 month | 12(16.43) | 61(73.57) | 1 | |
| > 1 month | 52(26.13) | 147(73.87) | 1.99(0.92–4.33) | 0.08 | |
Discussion
Based on the pDST result, 23.53% of the isolates from index cases exhibited resistance to at least one first-line anti-TB drug (any drug resistance). The prevalence of resistance to any first-line anti-TB drugs was greater in relapse cases compared to new TB cases. No isolates showed resistance to the new BPal drug regimen. The most common mutation in isolates resistant to the INH drug was found in the katG gene using the Xpert ® MTB/XDR assay. The result revealed that one-third of the HHCs had discordant drug-resistance profiles from the index patients.
Even though different studies report higher or lower prevalence of one or more first-line anti-TB drugs in Ethiopia, our result was similar to previous studies conducted in Ethiopia, Zambia, and Kenya, which reported rates of 21.3% [20], 23.5% [21], and 26.2% [22], respectively. The prevalence observed in our study was notably greater than the results reported in studies from Ethiopia, which showed rates of 11.6% [23] and 5.6% [24]. In contrast, other research conducted in Nigeria and Pakistan indicated a significantly higher prevalence of any drug resistance, with rates of 41.1% [25] and 65.9% [26], respectively. These differences might be linked to the co-infection of HIV and TB, the management approaches for TB patients, the variation in applied diagnostic techniques, inadequate treatment adherence by patients, sample size issues (smaller samples can result in an inflation of proportions), and study area differences [27, 28].
The findings indicated that the relapse case group faced a higher risk of developing resistance to any first-line anti-TB medication compared to the new TB case group. The easy availability and misuse of these medications heighten the risk of emerging drug-resistant TB. Extended anti-TB treatment may contribute to the development of drug resistance [29]. Promoting the rational use of anti-TB drugs in clinical practice and tracking patient adherence helps to reduce the emergence of drug-resistant MTB strains.
In this study, INH and STR mono-resistance were low, with a rate of 2.4% each. However, higher INH mono-resistance was reported in other studies in Ethiopia [30, 31]. Another study in Ethiopia also reported lower INH mono resistance [20]. This discrepancy between studies highlights the importance of geographical mapping for TB drug resistance within the country. Although our study showed low mono-resistance rates, further investigation is necessary to gain a clearer understanding of its prevalence, especially considering the continued use of the INH drug in the national TB control program.
The study also showed a RR-TB prevalence of 16.17%. This finding was similar to the report of a study in Ethiopia [20]. However, a much higher prevalence rate (28%) was reported from Southwestern Ethiopia [32], and lower rates were reported by other studies in Benishangul-Gumuz (2.4%) [33] and in central Ethiopia (1.2%) [30]. Unfortunately, some of the health facilities we selected were sites where more MDR-TB patients received treatment compared to DS-TB patients.
The reported no resistance to FLQ, BDQ, CLF, DEL, LZD, and PTM in our study was far from other reports. For BDQ 6.2% in Uzbekistan [34], 2.1% in India (21/1,016) [35], and 2.4% in China (10/425) [36], resistance was reported. For FLQ 15.0% in Uzbekistan [34], 69.2% (703/1,016) in India [35], 73.2% in China (311/425) [36], 37.0% in Russia (59/161) [37], and 31.4% in Ukraine (53/169) [38]. Furthermore, 0.8% in Uzbekistan [34], in India (72/365, 19.7%) [39], and in China (30/425, 7.1%) [35]. These differences may be influenced by contextual factors, sample size, and genetic variations in MTB strains, as well as variances in testing criteria. We recommend future research to explore the drug resistance profile for these new drugs using a large sample size.
The Xpert MTB/XDR assay detected isoniazid (INH) resistance by identifying a mutation in the katG gene at codons 311–319 and nucleotides 939–957. Our results showed that all samples resistant to INH, as identified by the Xpert MTB/XDR assay, exhibited a mutation in the katG gene. This finding aligns with previous studies conducted in Ethiopia, which reported that the katGS315T mutation contributed to around 96.5% [20, 40] of cases of INH resistance. On a global scale, 64% of all identified phenotypic resistance to isoniazid is linked to the katG315 mutation [41].
In this research, five samples from INH-resistant cases and four from INH-susceptible cases showed no melting temperature at the wild-type ahpC gene, nor did they demonstrate a melting temperature for the mutant ahpC gene. This result supports the company’s assertion that the link between mutations in the oxyR-ahpC gene and INH and FLQ resistance has not been definitively proven [17]. The discordance between the Xpert MTB/XDR and pDST suggests that there was a region responsible for INH resistance that the assay did not cover. This aligns with the sensitivity result of the assay, which was claimed by the company [17]. Furthermore, in this study, FLQ resistance was linked to mutations in the gyrA gene, which aligns with findings from another study conducted in Ethiopia [20].
In our analysis, 69.23% of TB-positive HHCs had isolates that matched the index case’s DST across all five drugs. Proportional matching DST profiles have been reported in Russia [42]. However, our results surpass those from Peru and the Philippines, which reported that 36.6% [43] and 18.8% [6] of contacts had isolates matching the index case’s DST for all five drugs, respectively. This variation might be due to the small number of index-HHC pairs in our study, which affected the proportion rate. There are several possible explanations for the differences between index and contact DST results. Firstly, the contact was infected with the strain of the index patient before the index acquired further drug resistance; a previous study in Peru highlighted a similar instance [44]. Second, the person may have contracted the infection from an external source. Third, it’s possible that the index patient had two different strains but only transmitted one strain to the household member. Fourth, the reliability of drug susceptibility testing (DST) varies for certain medications. In Ethiopia, there was high social interaction that contributed to TB transmission from sources other than the household.
In the current study, the previous TB treatment history was associated with resistance against any first-line anti-TB medications. This correlation is consistent with findings from prior studies [45, 46]. Drug-resistant TB could develop when the medications prescribed for TB are not used properly or managed effectively. This is why any resistance to first-line anti-TB drugs was linked to the patient’s previous treatment history. This shows the presence of an issue with patient adherence to the prescribed TB treatment in the study area.
Our study had some limitations to consider. First, the study was primarily designed to assess the concordance of the drug resistance profile using the index case without discrimination by DST result, which may have underestimated the second-line pDST profile in the RR/MDR-TB. Second, because of the contact investigation design, the number of index-HHC pairs was small, and the findings should be interpreted with caution. Third, due to economic reasons, we were unable to do whole-genome sequencing. These limitations suggest that further research with a larger sample size and a focus on second-line drug resistance is warranted to better understand the complexities of TB treatment and resistance patterns.
Conclusion
Nearly one-third of the household contacts have discordant drug-resistance profiles from the index patients. This study offers compelling proof that it’s not advisable to treat a close contact without DST results based on the DST results of the supposed source case. Therefore, it is crucial to obtain DST results for every contact that develops tuberculosis disease to ensure successful treatment. The country should enhance the tuberculosis drug resistance surveillance by using additional diagnostic strategies like genome sequencing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
AcknowledgmentsWe sincerely appreciate Addis Ababa University, the Addis Ababa Health Bureau, and the various site Health Offices for their ethical approval and support letters that made this study possible. Our profound thanks extend to the Ethiopian Public Health Institute for its guidance, testing facilities, and the ethical endorsement required for this research. Lastly, we want to express our deep gratitude to the study participants, healthcare professionals, and health extension workers across all study sites.
Abbreviations
- AOR
Adjusted odds ratio
- BDQ
Bedaquiline
- CI
Confidence interval
- CLF
Clofazimine
- DEL
Delamanide
- EMB
Ethambutol
- FLQ
Floroquinolone
- HHC
Household contacts
- INH
Isonazid
- LZD
Linozolide
- M. tuberculosis
Mycobacterium tuberculosis
- PTB
Pulmonary Tuberculosis
- PTM
Protinamide
- PZA
Pyrazinamide
- RIF
Rifampicin
- STR
Streptomycin
- TB
Tuberculosis
- WHO
World Health Organization
Author contributions
Author contributionsGS developed the study protocol, participated in data collection, collected and transported samples, conducted data analysis, and wrote the first draft of the manuscript. GD, AA, and BZ performed the laboratory analysis of sputum specimens and reviewed the manuscript. SH and BG reviewed the study protocol and supervised the conduct of the study. GT, GS, AA, GD, BZ and BG critically reviewed the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
We did not get any specific funding to conduct this study.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO). Global Tuberculosis Report 2023; WHO: Geneva, Switzerland. 2024. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024
- 2.Delogu G, Sali M, Fadda G. The biology of mycobacterium tuberculosis infection. Mediterranean J Hematol Infect Dis. 2013;5(1):l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melsew YA, Gambhir M, Cheng AC, McBryde ES, Denholm JT, Tay EL, et al. The role of super-spreading events in Mycobacterium tuberculosis transmission: evidence from contact tracing. BMC Infect Dis. 2019;19(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks-Pollock E, Danon L, Korthals Altes H, Davidson JA, Pollock AMT, van Soolingen D, et al. A model of tuberculosis clustering in low-incidence countries reveals more transmission in the United Kingdom than the Netherlands between 2010 and 2015. PLoS Comput Biol. 2020;16:e1007687. [DOI] [PMC free article] [PubMed]
- 5.Rodriguez CA, Sasse S, Yuengling KA, Azzawi S, Becerra MC, Yuen CM. A systematic review of National policies for the management of persons exposed to tuberculosis. Int J Tuberc Lung Dis. 2017;21:935–40. [DOI] [PubMed] [Google Scholar]
- 6.Sia IG, Buckwalter SP, Doerr KA, Lugos S, Kramer R, Orillaza-Chi R, et al. Genotypic characteristics of Mycobacterium tuberculosis isolated from household contacts of tuberculosis patients in the Philippines. BMC Infect Dis. 2013;13:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Swindells S, Kim S, Hughes MD, Naini L, Wu X, et al. Feasibility of identifying household contacts of rifampin- and multidrug-resistant tuberculosis cases at high risk of progression to tuberculosis disease. Clin Infect Dis. 2019;70:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang SS, Brooks MB, Jenkins HE, Rubenstein D, Seddon JA, van de Water BJ, et al. Concordance of Drug-resistance profiles between persons with Drug-resistant tuberculosis and their household contacts: A systematic review and Meta-analysis. Clin Infect Dis. 2021;73(2):250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soedarsono S, Mertaniasih NM, Kusmiati T, Permatasari A, Ilahi WK, Anggraeni AT. Characteristics of previous tuberculosis treatment history in patients with treatment failure and the impact on acquired Drug-Resistant tuberculosis. Antibiot (Basel). 2023;12(3):598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diriba G, Alemu A, Yenew B, Tola HH, Gamtesa DF, Mollalign H, et al. Epidemiology of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: A systematic review and meta-analysis. Int J Infect Dis. 2023;132:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health. Guidelines for Clinical and Programmatic Management of TB, TB/HIV, DR-TB and Leprosy in Ethiopia. Addis Ababa, Ethiopia 2021 7th edition.
- 12.Ministry of Health. National Tuberculosis leprosy and other lung disease bi-annual review report 2025. Addis Ababa,Ethiopia.
- 13.Cao Y, Parmar H, Guar R, Lieu D, Raghunath S, Via N, et al. Xpert MTB/XDR: a 10-color reflex assay suitable for point-of-care settings to detect isoniazid, fluoroquinolone, and second-line-injectable-drug resistance directly from mycobacterium tuberculosis-positive sputum. J Clin Microbiol. 2021;59:e02314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CHAPTLE, Introductory Biostatics. 2003 by John Wiley & Sons, Inc. ISBN 0-471-41816-1.
- 15.Seyoum B, Demissie M, Worku A, Bekele S, Aseffa A. Prevalence and drug resistance patterns of Mycobacterium tuberculosis among new smear positive pulmonary Tuberculosis patients in Eastern Ethiopia. Tuberculosis Res Treat. 2014:2014;753492. [DOI] [PMC free article] [PubMed]
- 16.Xpert MTBRIF. Ultra assay ENGLISH package insert. 2020;302–3514 Rev A. n.d.
- 17.Xpert MTB-XDRENGLISH. Package Insert 302–3514 Rev A. URL: https://cepheid.widen.net/s/cwc24p8lcl Accessed on 06th october 2024.
- 18.Springer B, Lucke K, Calligaris-Maibach R, Ritter C, Böttger EC. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and epicenter instrumentation. J Clin Microbiol. 2009;47(6):1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqi S, Ahmed A, Asif S. Direct drug susceptibility testing of Mycobacterium tuberculosis for rapid detection of multidrug resistance using the Bactec MGIT 960 system: a multicenter study. J Clin Microbiol. 2012;50(2):435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yenew B, Kebede A, Alemu A, Diriba G, Mehammed Z, Amare M, et al. Genotypic and phenotypic drug resistance patterns of Mycobacterium tuberculosis isolated from presumptive pulmonary tuberculosis patients in ethiopia: A multicenter study. PLoS ONE. 2024;19(5):e0303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monde N, Munyeme M, Chongwe G, Wensman JJ, Zulu M, Siziya S, et al. First and Second-Line Anti-Tuberculosis Drug-Resistance patterns in pulmonary tuberculosis patients in Zambia. Antibiot (Basel). 2023;12(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonge SA, Otieno MF, Sharma RR, Nteka SS. Drug susceptibility patterns of Mycobacterium tuberculosis isolates from Tuberculosis patients in coastal Kenya. J Tuberc Res. 2017;5:201–19. [Google Scholar]
- 23.Dagne B, Desta K, Fekade R, Amare M, Tadesse M, Diriba G, et al. The epidemiology of first and second-line drug-resistance Mycobacterium tuberculosis complex common species: evidence from selected TB treatment initiating centers in Ethiopia. PLoS ONE. 2021;16(1):e0245687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diriba G, Kebede A, Tola HH, Alemu A, Tadesse M, Tesfaye E, et al. Surveillance of drug resistance tuberculosis based on reference laboratory data in Ethiopia. Infect Dis Poverty. 2019;8(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzoewulu NG, Ibeh IN, Lawson L, Goyal M, Umenyonu N, Ofiaeli RO, Okonkwo R. Drug resistant Mycobacterium tuberculosis in tertiary hospital South east, Nigeria. J Med Microb Diagn. 2014;3:1. [Google Scholar]
- 26.Saifullah A, Mallhi TH, Khan YH, Iqbal MS, Alotaibi NH, Alzarea AI, Rasheed M. Evaluation of risk factors associated with the development of MDR- and XDR-TB in a tertiary care hospital: a retrospective cohort study. PeerJ. 2021;9:e10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mvelase NR, Balakrishna Y, Lutchminarain K, Mlisana K. Evolving rifampicin and Isoniazid mono-resistance in a high multidrug-resistant and extensively drug-resistant tuberculosis region: a retrospective data analysis. BMJ Open. 2019;9(11):e031663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulis G, Pai M. Isoniazid-resistant tuberculosis: A problem we can no longer ignore. PLoS Med. 2020;17:e1003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang C-Y, Centis R, Migliori GB. Drug-resistant tuberculosis: past, present, future. Respirology. 2010;15:413–32. [DOI] [PubMed] [Google Scholar]
- 30.Tilahun M, Ameni G, Desta K, Zewude A, Yamuah L, Abebe M, et al. Molecular epidemiology and drug sensitivity pattern of Mycobacterium tuberculosis strains isolated from pulmonary tuberculosis patients in and around Ambo town, central Ethiopia. PLoS ONE. 2018;13(2):e0193083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alelign A, Zewude A, Mohammed T, Tolosa S, Ameni G, Petros B. Molecular detection of Mycobacterium tuberculosis sensitivity to rifampicin and Isoniazid in South Gondar zone, Northwest Ethiopia. BMC Infect Dis. 2019;19(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tadesse M, Aragaw D, Dimah B, Efa F, Abdella K, Kebede W, et al. Drug resistance-conferring mutations in Mycobacterium tuberculosis from pulmonary tuberculosis patients in Southwest Ethiopia. Int J Mycobacteriology. 2016;5(2):185–91. [DOI] [PubMed] [Google Scholar]
- 33.Lobie TA, Woldeamanuel Y, Asrat D, Beyene D, Bjørås M, Aseffa A. Genetic diversity and drug resistance pattern of Mycobacterium tuberculosis strains isolated from pulmonary tuberculosis patients in the Benishangul Gumuz region and its surroundings, Northwest Ethiopia. PLoS ONE. 2020;15(4):e0231320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moe S, Azamat I, Allamuratova S, Oluya M, Khristusev A, Rekart ML, et al. Second-line drug-resistant TB and associated risk factors in karakalpakstan, Uzbekistan. IJTLD Open. 2024;1(9):391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreyer V, Mandal A, Dev P, Merker M, Barilar I, Utpatel C, et al. High fluoroquinolone resistance proportions among multidrug-resistant tuberculosis driven by dominant L2 Mycobacterium tuberculosis clones in the Mumbai metropolitan region. Genome Med. 2022;14(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao C, Guo H, Li Q, Zhang X, Shang Y, Li T, Wang Y, Xue Z, Wang L, Li L, Pang Y. Prevalence of extensively drug-resistant tuberculosis in a Chinese multidrug-resistant TB cohort after redefinition. Antimicrob Resist Infect Control. 2021;10(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostyukova I, Pasechnik O, Mokrousov I. Epidemiology and drug resistance patterns of Mycobacterium tuberculosis in High-Burden area in Western siberia, Russia. Microorganisms. 2023;11(2):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butov D, Lange C, Heyckendorf J, Kalmykova I, Butova T, Borovok N, et al. Multidrug-resistant tuberculosis in the Kharkiv region, Ukraine. Int J Tuberc Lung Dis. 2020;24(5):485–91. [DOI] [PubMed] [Google Scholar]
- 39.Vengurlekar D, Walker C, Mahajan R, Dalal A, Chavan V, Galindo MA, et al. Linezolid resistance in patients with drug-resistant TB. Int J Tuberc Lung Dis. 2023;27(7):567–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agonafir M, Belay G, Feleke A, Maningi N, Girmachew F, Reta M, Fourie PB. Profile and frequency of mutations conferring Drug-Resistant tuberculosis in the central, southeastern and Eastern Ethiopia. Infect Drug Resist. 2023;16:2953–61. d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seifert M, Catanzaro D, Catanzaro A, Rodwell TC. Genetic mutations associated with Isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS ONE. 2015;10(3):e0119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raznatovska OM, Moskaliuk AS, Grekova TA, Chernyshova LI, Pushnova OO, Shelestina T. I. The relevance of household contacts tracing among child contacts of patients with multidrug-resistant tuberculosis. Infusion Chemother, 2020;(1):14–23.
- 43.Parr JB, Mitnick CD, Atwood SS, Chalco K, Bayona J, Becerra MC. Concordance of resistance profiles in households of patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2014;58(3):392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furin JJ, Becerra MC, Shin SS, Kim JY, Bayona J, Farmer PE. Effect of administering short-course, standardized regimens in individuals infected with drug-resistant Mycobacterium tuberculosis strains. Eur J Clin Microbiol Infect Dis. 2000;19:132–6. [DOI] [PubMed] [Google Scholar]
- 45.Farazi A, Sofian M, Zarrinfar N, Katebi F, Hoseini SD, Keshavarz R. Drug resistance pattern and associated risk factors of tuberculosis patients in the central province of Iran. Caspian J Intern Med. 2013 Fall;4(4):785-9. [PMC free article] [PubMed]
- 46.Tao NN, Li YF, Song WM, Liu JY, Zhang QY, Xu TT, et al. Risk factors for drug-resistant tuberculosis, the association between comorbidity status and drug-resistant patterns: a retrospective study of previously treated pulmonary tuberculosis in shandong, china, during 2004–2019. BMJ Open. 2021;11(6):e044349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.