Abstract
Objective
The advent of biological drugs has revolutionised management of inflammatory bowel disease (IBD). However, the extent to which these novel pharmacological drugs have reduced the need for surgical treatment remains incompletely quantified.
We aimed to investigate the risk of first, major surgery in IBD in a population-based, large epidemiological study.
Methods
We empanelled a cohort comprising all 85 974 patients diagnosed with ulcerative colitis (UC) and 42 760 with Crohn’s disease (CD) in Norway and Sweden in 1987 through 2017. We used log-rank tests to compare the cumulative probability of surgical treatment for UC and CD. Using multivariable Cox proportional hazards models, we estimated hazard ratios (HR) with 95% CIs by year of diagnosis, age, sex and extent of disease.
Results
During a mean follow-up of 9.9 years, surgery was undertaken in 11 187 (13.0%) patients with UC (12.3 per 1000 person-years) and in 11 307 (26.4%) patients with CD (30.0 per 1000 person-years). In UC, the cumulative 5-year probability of surgery decreased from 16.2% in patients diagnosed in 1987–1994 to 5.8% in those diagnosed in 2011–2017 (p<0.001). In CD, the corresponding decline was from 30.1% to 13.9% (p<0.001). In multivariable analyses, the likelihood of surgical treatment decreased during the study period by 61% (HR 0.39, 95% CI 0.36 to 0.42) in UC and by 31% (HR 0.69, 95% CI 0.65 to 0.75) in CD.
Conclusions
Following the introduction of biologic drugs, the need for surgical treatments has been dramatically reduced in patients with UC and moderately reduced in patients with CD.
Keywords: INFLAMMATORY BOWEL DISEASE, ULCERATIVE COLITIS, SURGERY FOR IBD, CROHN'S DISEASE
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies suggest that surgical rates in inflammatory bowel disease (IBD) declined after the introduction of biological treatment. However, many of these studies are small and not strictly population-based.
WHAT THIS STUDY ADDS
With a population-based design, large size, and virtually complete follow-up in nationwide high-quality registers, this study provides reliable evidence regarding trends in the surgical management of IBD over 30 years.
This study finally provides high-quality data of the decreasing trend of surgical treatment in IBD. While the trend is evident and persistent for ulcerative colitis, it seems to have haltered in CD after the millennium.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Trends of treatments provide important information for further research as well as for policy-makers.
INTRODUCTION
Both ulcerative colitis (UC) and Crohn’s disease (CD)—the two main forms of inflammatory bowel disease (IBD)—are associated with onset at young ages and often lifelong chronic inflammation of the bowel. These features, along with increasing incidence, particularly in newly industrialised countries, and decreasing mortality due to more effective medical treatment, cause an increase in the prevalence of IBD globally.1 Hence, optimised management is an important priority not only for individual patients but also for the public health system.2 3
In recent decades, introduction of biologic drugs has revolutionised management of IBD,4 motivating new guidelines that promote intense and proactive ‘top-down’ pharmacologic treatment.5,9 Notwithstanding this progress, many patients both with UC10 and CD11 need surgical treatment because they do not respond sufficiently, although a biological drug that has previously failed can be reconsidered after surgery.12 Surgery also remains a cornerstone in the acute treatment of IBD.13 14
Several studies315,29 but not all30,35 have shown a decrease in rates of surgery in recent decades. Because these studies were predominantly small, covered short time periods, were not population-based and/or did not separately analyse UC and CD, the extent to which novel drugs have reduced the need for surgical treatment remains uncertain. Our primary aim was to provide robust evidence of the trends in surgical treatment in patients with UC and CD. Additionally, we wanted to investigate the types of surgical procedures and the indication for surgery. To this end, we undertook a longitudinal population-based study allowing detailed analyses of temporal trends during three decades in rate and type of surgery separately in patients with UC and CD.
METHODS
Setting
The objective of the study was to investigate the trends of surgical treatment in IBD. This was done through the Scandinavian research collaboration calledIBD-Scandinavian CANcer in IBD study I-SCAN, (I-SCAN), evaluating outcomes of all patients in Norway and Sweden diagnosed with UC or CD since 1987.36,38 These two neighbouring Scandinavian countries are highly homogenous with regards to ethnicity, sociodemographic characteristics, and access to population registers as well as structure of the public healthcare system with virtually no private inpatient care. The individually unique national registration number assigned to all residents in Norway and Sweden enables linkage to these high-quality and virtually complete population registers containing data on morbidity and surgical procedures (see below), mortality (the Norwegian Cause of Death Register and the Swedish Cause of Death Register), and migration (Norway: the national population register; Sweden: the total population register).
Study population
In Norway, we empanelled the analytic cohort based on relevant International Classification of Disease (ICD) codes for IBD collected from all public hospitals from 1987 to 2015, containing information on both inpatient and outpatient care. In Sweden, we used data from the inpatient register (with a nationwide coverage since 1987) from 1987 to 2017 and also information from the outpatient register included from 2001 to 2017.39 Patients aged <10 or >100 at the first registered IBD diagnosis were excluded.
To ensure high specificity of IBD diagnosis, we required at least two inpatient and/or outpatient diagnosis codes of IBD, which yields a positive predictive value of an IBD diagnosis of 90% in our cohort.37 We used the ICD codes from the first two visits to define IBD subtypes at baseline: patients were classified as having UC (ICD-9: 556; ICD-10: K51) or CD (ICD-9: 555; ICD-10: K50) if their first two codes were UC or CD, respectively, and unclassified IBD (IBD-U) if their first codes were indeterminate colitis (ICD10: K523) or any combination of codes (online supplemental table 1). The date of the first IBD diagnosis defined the start of follow-up.
Surgery
The outcomes of interest were major abdominal surgeries, defined by procedure codes. In both countries, the inpatient register and hospital data provided information about the surgical procedures by registered procedure codes. These codes were extracted from the Norwegian and Swedish version of the NOMESCO Classification of Surgical Procedures.40 We focused on major abdominal surgeries, including proctectomies, colectomies and intestinal resections. Surgical procedures were divided by type and anatomical site of resection (online supplemental tables 2 and 3). Only surgery after the date of first IBD diagnosis was included.
We used the ICD codes registered at the date of surgery as a proxy for the indication for surgery. We grouped relevant ICD codes into IBD, benign tumours, cancer in situ, obstruction (including ileus), mesenteric thromboembolism, functional intestinal disorders, fissures/fistulas, peritonitis or acute inflammation, bleeding, and stomal dysfunction (online supplemental table 4).
Statistical analysis
We first calculated cumulative probability of major surgery using Kaplan-Meier survival analysis and group differences were assessed using the log-rank test. Follow-up started at the first diagnosis of IBD (UC, CD) and ended at the date of first surgery, date of diagnosis of colorectal cancer (CRC), emigration, death or at the end of follow-up, whichever occurred first.
To assess changes in surgical treatment by year of first diagnosis, the cohort was divided into patients diagnosed in 1987–1994, 1995–2002, 2003–2010, and 2011–2017. We calculated the cumulative probability of surgery at 1, 5 and 10 years after diagnosis in these groups (for patients diagnosed in 2011–2017, cumulative probability at 7 years was calculated). Patients were also analysed separately for UC, CD or IBD-U. Incidence rates of surgery per 1000 patient-years at <1, 1–5, 6–10, and >10 years were calculated for each type of IBD. We used univariate and multivariate Cox proportional hazards model to calculate HRs with 95% CI of major surgery during follow-up, for UC and CD separately. The main exposure was year of diagnosis (groups 1987–1994, 1995–2002, 2003–2010 and 2011–2017). The multivariable regression model was adjusted for age, sex, nationality, cumulative extent of disease (UC), cumulative behaviour of disease (CD) and perianal disease (CD) (online supplemental table 5). We used log-minus-log curves and Schoenfeld residuals to investigate the proportional hazards assumption. Statistical significance of multiplicative interaction was assessed by the likelihood ratio test (p<0.001) comparing models with and without cross-product interaction terms between year of diagnosis and country as the potential effect modifier.
The trends of indication for surgery (ICD codes registered at the date of surgery were used as a proxy for indication) were calculated as a proportion by dividing the number of registered diagnoses, by the total number of major abdominal surgeries for each year.
Analyses were performed using SAS software V.9.4 (Copyright 2016 SAS Institute Cary, North Carolina) and Stata V.18 software (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, Texas: StataCorp LLC). The reporting follows the Strengthening the Reporting of Observational studies in Epidemiology checklist (online supplemental material).
RESULTS
Descriptive characteristics
The analytic cohort included 141 109 patients with at least two diagnostic listings of IBD (online supplemental figures 1 and 2). A total of 85 974 (60.9%) patients had UC, 42 760 (30.3%) CD and 12 375 (8.8%) IBD-U. The proportion of men was slightly higher than women in UC (53.2% vs 46.8%) and lower in CD (47.6% vs 52.4%). Mean age at first diagnosis was 43.9 years (18.5 SD) in UC, 40.1 years (18.8 SD) in CD and 42.9 years (20.0 SD) in IBD-U (table 1).
Table 1. Patient baseline characteristics.
| Number of subjects | Norwaynumber (%) | Swedennumber (%) | Totalnumber (%) |
|---|---|---|---|
| Total IBD cohort | 46 495 | 94 614 | 141 109 |
| Male | 23 690 (51.0) | 48 519 (51.3) | 72 209 (51.2) |
| Female | 22 805 (49.0) | 46 095 (48.7) | 68 900 (48.8) |
| UC | 28 664 | 57 310 | 85 974 |
| Male | 15 233 (53.1) | 30 512 (52.4) | 45 745 (53.2) |
| Female | 13 431 (46.9) | 26 798 (47.6) | 40 229 (46.8) |
| CD | 13 634 | 29 126 | 42 760 |
| Male | 6473 (47.5) | 13 877 (47.6) | 20 350 (47.6) |
| Female | 7161 (52.5) | 15 249 (52.4) | 22 410 (52.4) |
| IBD-U | 4197 | 8178 | 12 375 |
| Male | 1984 (47.3) | 4130 (50.5) | 6114 (49.5) |
| Female | 2213 (52.7) | 4048 (49.5) | 6261 (50.6) |
| Age at diagnosis | Mean (±SD) | Mean (±SD) | Mean (±SD) |
| Number (%) | Number (%) | Number (%) | |
| Full cohort | 42.2 years (18.4) | 42.9 years (19.0) | 42.6 years (18.8) |
| <20 | 4868 (10.5) | 10 738 (11.4) | 15 606 (11.1) |
| ≥20–<40 | 18 598 (40.0) | 35 368 (37.4) | 53 966 (38.3) |
| ≥40–<60 | 13 977 (30.1) | 28 156 (29.8) | 42 133 (29.9) |
| ≥60 | 9052 (19.5) | 20 352 (21.5) | 29 404 (20.8) |
| UC | 44.0 (18.0) | 43.9 (18.7) | 43.9 (18.5) |
| CD | 38.1 (18.1) | 41.0 (19.1) | 40.1 (18.8) |
| IBD-U | 43.2 (19.4) | 42.8 (20.3) | 42.9 (20.0) |
| Numbers and cases by year of diagnosis | Number | ||
| UC | |||
| 1987–1994 (surgeries, %) | 15 913 (4234, 26.6%) | ||
| 1995–2002 (surgeries, %) | 25 246 (3712, 14.7%) | ||
| 2003–2010 (surgeries, %) | 25 574 (2397, 9.4%) | ||
| 2011–2017 (surgeries, %) | 19 241 (844, 4.3%) | ||
| Full cohort (surgeries, %) | 85 974 (11 187, 13.0%) | ||
| CD | |||
| 1987–1994 (surgeries, %) | 8847 (4315, 48.7%) | ||
| 1995–2002 (surgeries, %) | 10 687 (3374, 31.6%) | ||
| 2003–2010 (surgeries, %) | 12 533 (2509, 12.0%) | ||
| 2011–2017 (surgeries, %) | 10 693 (1109, 10.4%) | ||
| Full cohort (surgeries, %) | 42 760 (11 307, 26.4%) |
CD, Crohn’s disease; IBD, inflammatory bowel disease; IBD-U, unclassified inflammatory bowel disease; UC, ulcerative colitis.
Time between first and second registered ICD code of IBD was less than 1 year for 72.1% of the patients (online supplemental figure 3). The total follow-up time included 1 395 459 person-years with a mean follow-up of 9.9 years, median of 8.5 years and a maximum of 31 years.
The cumulative extent of disease among patients with UC was 15.7% with proctitis, 16.7% with left-sided colitis, 48.1% with extensive disease, and 19.3% were undefined. In CD, the location of the disease was terminal ileum in 22.3%, colon in 20.6%, 42.0% had ileocolonic involvement, and 14.2% were not defined. In CD, 74.3% had no stricturing or penetrating disease, 16.5% had stricturing disease, 5.9% had penetrating disease with fistulation, and 3.3% had both penetrating and stricturing disease.
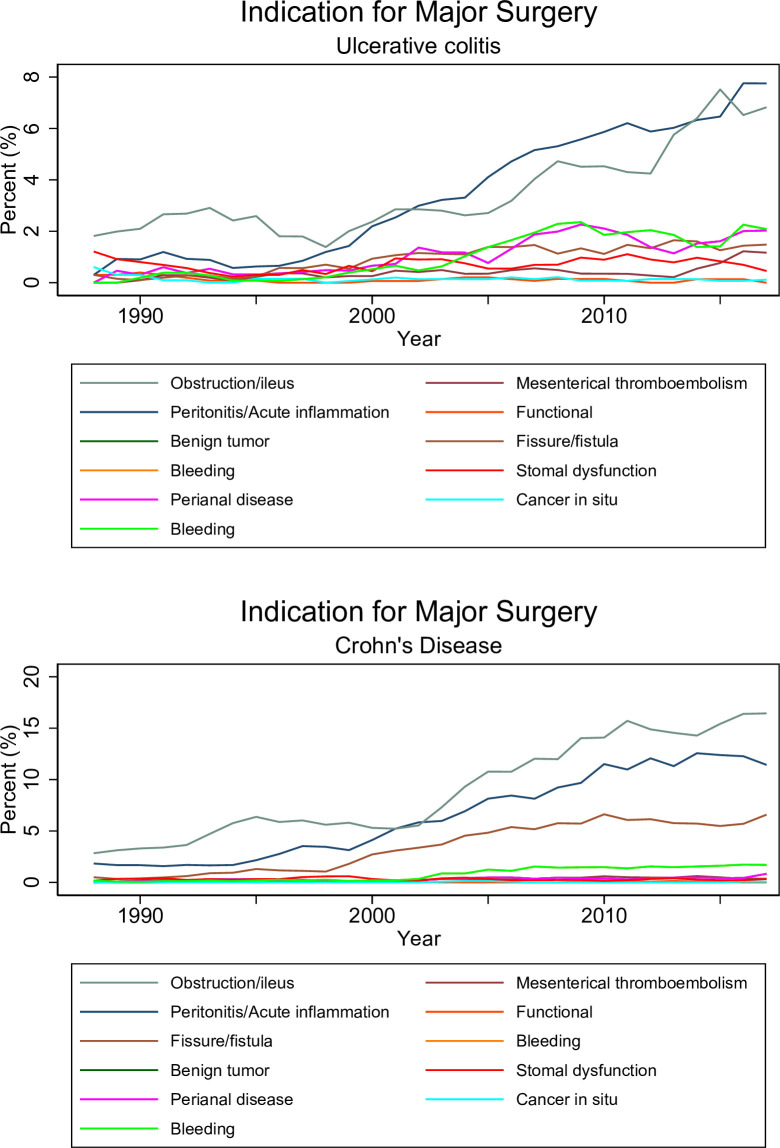
Major surgery was undertaken in 11 187 patients with UC (12.3 per 1000 person-years), 11 307 with CD (30.0 per 1 000 person-years), and 1 761 with unclassified IBD-U (table 1 and online supplemental tables 6–8). A total of 4679 patients had their first surgery at the same date as the date of IBD diagnosis. The dominating indication for surgery (ICD codes registered at the date of surgery were used as a proxy for indication) was the underlying disease accounting for 79% in UC and 87% in CD. In both UC and CD, the second most common indication for surgery was obstruction/ileus (figure 1, IBD-U, see online supplemental figure 4).
Figure 1. Indication for major surgery in patients with ulcerative colitis and Crohn’s disease. ICD codes registered at the date of surgery were used as a proxy for indication. Percentage of procedures with specific diagnoses, by year. IBD diagnosis excluded. Smoothed curve over average of 3 years. For IBD-U, see online supplemental figure 4. IBD, inflammatory bowel disease; IBD-U, unclassified IBD; ICD, International Classification of Disease.
Time trends
In both patient groups, the rate of surgery per 1000 patients decreased over the study period. In patients with UC, 38.5 surgeries per 1000 patients were performed in 1990, compared with 5.6 per 1000 patients in 2017. The most common procedure in UC was total colectomy, followed by proctocolectomy (online supplemental table 9 and figure 5). In CD, there were 82.4 surgeries per 1000 in 1990, compared with 11.2 per 1000 in 2017. In CD, the most common procedure was ileocaecal resection, followed by small bowel resection (online supplemental table 9 and figure 6).
With some exceptions, the most common surgical procedure remained stable over the years. The most frequent procedure was total colectomy in UC and ileocaecal resection in CD. However, in patients with UC, there was a shift in 2015, when distal colonic resection replaced proctocolectomy as the second most common procedure. In CD, proximal colonic resection was the second most procedure between 1997 and 2015 when small bowel resection was once again the second most common procedure (online supplemental table 9, figures 5 and 6). The mean age at first surgical procedure was 47.7 years (SD 18.3) among patients with UC, and 42.9 years (SD 17.5) in patients with CD. In both patient groups, the mean age at surgery increased throughout the study period (online supplemental table 10).
In patients with UC, the overall cumulative probability of major surgery was 4.4% at 1 year, 9.5% at 5 years, and 12.9% at 10 years after first diagnosis. The cumulative 5-year probability of surgery decreased over time from 16.2% in patients diagnosed in 1987–1994 to 5.8% in those diagnosed in 2011–2017 (table 2). In patients with CD, the overall cumulative probability of major surgery was 10.4%, 20.0% and 27.1% at 1, 5 and 10 years, respectively (table 2). The 5-year cumulative probability of surgery decreased over time from 30.1% in patients diagnosed in 1987–1994 to 13.9% in those diagnosed in 2011–2017 (table 2).
Table 2. Cumulative probability of surgery at 1, 5 and 10 years in ulcerative colitis, Crohn’s disease and unclassified IBD.
| Cumulative probability | 1 year % (95% CI) | 5 years % (95% CI) | 10 years % (95% CI) |
|---|---|---|---|
| Ulcerative colitis | 4.4 (4.3 to 4.5) | 9.5 (9.3 to 9.7) | 12.9 (12.7 to 13.2) |
| 1987–1994 | 8.0 (7.6 to 8.4) | 16.2 (15.7 to 16.8) | 21.5 (20.9 to 22.2) |
| 1995–2002 | 4.8 (4.6 to 5.1) | 9.8 (9.4 to 10.1) | 12.8 (12.3 to 13.2) |
| 2003–2010 | 3.1 (2.9 to 3.3) | 7.3 (6.9 to 7.6) | 9.7 (9.4 to 10.1) |
| 2011–2017 | 2.5 (2.3 to 2.7) | 5.8 (5.4 to 6.2) | 6.7 (6.2 to 7.4) |
| Crohn’s disease | 10.4 (10.2 to 10.7) | 20.0 (19.6 to 20.4) | 27.1 (26.6 to 27.6) |
| 1987–1994 | 16.3 (15.5 to 17.1) | 30.1 (29.9 to 31.8) | 40.3 (39.3 to 41.3) |
| 1995–2002 | 11.9 (11.3 to 12.6) | 21.2 (20.4 to 22.0) | 27.5 (26.7 to 28.4) |
| 2003–2010 | 8.5 (7.6 to 8.5) | 15.3 (14.7 to 16.0) | 20.8 (20.0 to 21.5) |
| 2011–2017 | 6.7 (6.3 to 7.3) | 13.9 (13.0 to 14.7) | 16.6 (15.1 to 18.2) |
| Unclassified IBD | 6.3 (5.9 to 6.8) | 10.7 (10.2 to 11.3) | 14.4 (13.7 to 15.2) |
| 1987–1994 | 9.0 (7.7 to 10.5) | 18.2 (16.4 to 20.1) | 25.7 (23.7 to 27.9) |
| 1995–2002 | 6.7 (5.8 to 7.8) | 12.3 (11.1 to 13.7) | 16.5 (15.1 to 18.1) |
| 2003–2010 | 4.8 (4.1 to 5.6) | 9.3 (8.4 to 10.3) | 13.2 (12.1 to 14.5) |
| 2011–2017 | 3.7 (3.2 to 4.3) | 8.3 (7.3 to 9.4) | 9.7 (8.5 to 11.2) |
IBD, inflammatory bowel disease.
Multivariable analyses
Finally, we fitted multivariable Cox proportional hazards models to eliminate mutual confounding between variables that affected the probability of major surgery (table 3). Because findings from univariate models were similar to results from multivariable-adjusted models, we only discuss the multivariable-adjusted results. In patients with UC, the probability of surgical treatment declined by 61%; the HR decreased monotonically and was 0.39 (95% CI 0.36 to 0.42) in patients diagnosed in 2011–2017 compared with those diagnosed in 1987–1994 (p for trend <0.001). The probability of major surgery was slightly but significantly lower in patients diagnosed at ages 20–60 years (table 3). The need for major surgery was more than two times higher in patients with extensive disease, as compared with those with only left-sided colitis (table 3).
Table 3. Univariate and multivariate HRs and 95% CIs for major surgery in ulcerative colitis and Crohn’s disease, by time of diagnosis.
| Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Ulcerative colitis | ||||
| 1987–1994 | 1.0 | 1.0 | ||
| 1995–2002 | 0.56 | 0.54 to 0.59 | 0.59 | 0.56 to 0.62 |
| 2003–2010 | 0.42 | 0.40 to 0.44 | 0.45 | 0.42 to 0.47 |
| 2011–2017 | 0.33 | 0.31 to 0.36 | 0.39 | 0.36 to 0.42 |
| p<0.001 | p<0.001 | |||
| Age at diagnosis | ||||
| <20 | 1.0 | 1.0 | ||
| 20–40 | 0.81 | 0.76 to 0.86 | 0.90 | 0.84 to 0.96 |
| 40–60 | 0.74 | 0.69 to 0.79 | 0.85 | 0.79 to 0.90 |
| >60 | 0.82 | 0.77 to 0.89 | 1.01 | 0.94 to 1.09 |
| p<0.001 | p<0.001 | |||
| Sex | ||||
| Female | 1.0 | 1.0 | ||
| Male | 0.97 | 0.95 to 1.0 | 0.95 | 0.91 to 0.99 |
| Nationality | ||||
| Sweden | 1.0 | 1.0 | ||
| Norway | 0.95 | 0.92 to 0.99 | 0.90 | 0.85 to 0.95 |
| Extent of disease | ||||
| Proctitis | 1.0 | 1.0 | ||
| Left-sided colitis | 0.46 | 0.42 to 0.51 | 0.50 | 0.45 to 0.56 |
| Extensive | 2.36 | 2.21 to 2.51 | 2.33 | 2.18 to 2.48 |
| Not defined | 1.73 | 1.61 to 1.87 | 1.53 | 1.42 to 1.66 |
| Crohn’s disease | ||||
| 1987–1994 | 1.0 | 1.0 | ||
| 1995–2002 | 0.63 | 0.61 to 0.66 | 0.81 | 0.77 to 0.85 |
| 2003–2010 | 0.46 | 0.43 to 0.48 | 0.69 | 0.65 to 0.72 |
| 2011–2017 | 0.39 | 0.37 to 0.42 | 0.69 | 0.65 to 0.75 |
| p<0.001 | p<0.001 | |||
| Age at diagnosis | ||||
| <20 | 1.0 | 1.0 | ||
| 20–40 | 1.06 | 1.01 to 1.12 | 1.00 | 0.94 to 1.06 |
| 40–60 | 0.95 | 0.90 to 1.01 | 0.92 | 0.86 to 0.97 |
| >60 | 0.82 | 0.76 to 0.88 | 0.95 | 0.89 to 1.02 |
| p<0.001 | p<0.001 | |||
| Sex | ||||
| Female | 1.0 | 1.0 | ||
| Male | 0.99 | 0.96 to 1.02 | 0.96 | 0.93 to 1.00 |
| Nationality | ||||
| Sweden | 1.0 | 1.0 | ||
| Norway | 0.97 | 0.94 to 1.01 | 0.91 | 0.86 to 0.96 |
| Location of disease | ||||
| Terminal ileum | 1.0 | 1.0 | ||
| Colon | 0.69 | 0.64 to 0.73 | 0.74 | 0.69 to 0.79 |
| Ileocolonic | 1.63 | 1.55 to 1.71 | 1.29 | 1.23 to 1.36 |
| Not defined | 0.38 | 0.34 to 0.42 | 0.43 | 0.39 to 0.48 |
| Behaviour of disease | ||||
| Non-stricturing, non-penetrating | 1.0 | 1.0 | ||
| Stricturing | 4.19 | 4.02 to 4.38 | 3.43 | 3.28 to 3.59 |
| Penetrating | 3.39 | 3.19 to 3.61 | 2.81 | 2.62 to 3.01 |
| Both stricturing and penetrating | 6.50 | 6.09 to 6.94 | 4.70 | 4.34 to 5.03 |
| Perianal disease | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.92 | 1.84 to 2.00 | 0.99 | 0.94 to 1.04 |
HRs for major surgery during follow-up in a cohort of 128 834 patients with ulcerative colitis and Crohn’s disease.
Adjusted for year of diagnosis (groups 1987–1994, 1995–2002, 2003–2010 and 2011–2017), age, sex, nationality, extent of disease (UC), behaviour of disease (CD) and perianal disease (CD).
CD, Crohn’s disease; UC, ulcerative colitis.
In patients with CD, the reduction in major surgery over time was lower than for UC, with a 31% decline (HR 0.69, 95% CI 0.65 to 0.75) in patients diagnosed in 2003–2017 compared with those diagnosed in 1987–1994 (p for trend <0.001) (table 3). In contrast to patients with UC, the decline in trends for surgery stopped in 2010 (HR 0.69 for both groups). Age at diagnosis and sex affected probability of surgery only marginally. Patients with ileocolonic disease had significantly 29% higher probability of major surgery than patients with disease affecting only terminal ileum; the probability of surgery was about three to five times higher among patients with stricturing and/or penetrating disease compared with those without (table 3).
DISCUSSION
In this study of 141 109 patients with IBD followed for 1 395 459 person-years, our most salient findings, supporting our à priori hypothesis, were the substantial, temporal decline in surgical treatment of patients with UC. We also observed a much higher need for surgical treatment in patients with CD with a more modest decline, apparently halting at the beginning of this millennium. Our study further provides information on surgical interventions and diagnoses associated with these procedures. The findings in relation to sex, age at diagnosis and extent of disease are also novel. Important findings from the multivariate analysis are the increased probability of surgery for patients with extensive disease in UC and for patients with stricturing or both stricturing and penetrating diseases in CD. The largely similar results in Norway and Sweden with no statistically significant difference in temporal trends of surgical treatment support the robustness and generalisability of our findings.
The published literature offers only limited data about population-based long-term trends in the need for surgical treatment separately for UC and CD. Our study extends, however, the evidence in the most informative prior study with a similar design undertaken in Denmark, covering the period 1979–2011.20 Although the Danish study focused on trends in medical treatment, it also documented a more modest decline in surgical treatment of UC and generally higher rates of surgery for CD than we found. This difference may arise because follow-up ended in 2011, before the positive impact of advanced therapies became evident. Advantages of our study include its much larger size, analysis of 6 more years of follow-up and the comparison between two countries. We further required two IBD diagnoses for inclusion, to increase the positive predictive value while the former Danish study only required one diagnosis of IBD for inclusion.20 A French study of therapeutic management, including surgery, from 2009 to 2014, showed numbers of cumulative probability of major surgery that were similar to ours for the corresponding, more recent, time period.21 The study was large, 210 001 patients, but had a shorter follow-up time (median 4.9 years). The majority (if not all) of other studies on the topic did not censor patients at date of CRC diagnosis, which we think is essential to isolate surgery due to IBD and not CRC in these patients.15,1820
At least two factors could explain the more modest decline in surgical treatment among patients with CD compared with UC. First, the use of effective biologic treatments might have penetrated management of CD less effectively than UC, although previous studies show similar usage of biologic treatment in CD and UC.21 28 Further studies might test this hypothesis by individual linkage to the prescription registers now available in both Norway and Sweden. Second, novel drugs may be less effective in reducing the need for surgical treatment in CD than in UC. In the era of biologic treatment, up to 30% of CD patients never achieve good disease control,11 and CD patients with inflammation located to the ileum are thought not to respond as well to TNF-alpha inhibitors compared with CD located elsewhere.41,43 This reflects the larger need for surgical treatment in CD patients with ileal or ileocolonic disease compared with those with colonic involvement that we saw in our study. It has also been proposed that in CD, TNF-alpha inhibitors have anti-inflammatory effects but do not stop the fibrosis initiated by inflammation, leading to excessive repair processes, with fibrosis and strictures.44
Our study shows that total colectomy is by far the most common procedure in UC, potentially a curative treatment for these patients. In the situation of emergency surgery of acute severe UC, subtotal colectomy with reconstruction in a two-step or three-step approach is advised, while in refractory UC, there is currently less consensus regarding the surgical approach.14 In immunosuppressed patients, there is an increased risk for anastomotic failure, which the surgeons need to consider when choosing the surgical method.45 46 In CD, ileocaecal resection is the most common procedure, and studies have shown that this therapy is a good alternative to biologic agents for patients with ileocaecal involvement.47 48
Strengths of our study include its large size, population-based design and virtually complete follow-up in nationwide high-quality registers covering the entire period when new biologic drugs were introduced. The public healthcare systems in Norway and Sweden guarantee all residents equal access to competent inpatient care. We also aimed to overcome or reduce some of the fundamental methodological challenges in studies of IBD.49 In particular, we tried to define the date of disease onset as precisely as possible and to distinguish incident from prevalent cases of IBD. Finally, we required two similar diagnoses to optimise the predictive value when patients were classified as having UC or CD.
Our study also has limitations. First, left censoring in enrolment to our analytic cohort likely entails that some patients identified early had prevalent rather than incident disease. Because the need for surgical treatment is highest in the first years following diagnosis (figure 2, IBD-U see online supplemental figure 7), this might have biased quantification of surgical treatment downward particularly during the first period of enrolment (1987–1994). Therefore, temporal trends might indeed have been slightly underestimated. Second, from 2001, we identified patients in Sweden also from the outpatient register who had not required inpatient care. These patients were likely to have less severe disease with a lower need for surgical treatment, thereby potentially augmenting the downward temporal trend for surgical treatment. The similar results in Norway and Sweden indicate, however, that such a bias, if any, is small. Finally, we were unable to analyse the most recent time period because we did not have any data after 2015 in Norway and 2017 in Sweden. Hence, future research is needed to clarify if the temporal trends, particularly in UC, have continued beyond 2017.
Figure 2. Cumulative probability of first major surgery in patients with ulcerative colitis and Crohn’s disease. Blue line diagnosed 1987–1994, red line 1995–2002, green line 2003–2010, yellow line 2011–2017. P-value for homogeneity performed by log-rank test, p-value compares rates within the first year after diagnosis, from 1 to 5 years and 6–10 years after diagnosis. For IBD-U, see online supplemental figure 7. IBD-U, unclassified inflammatory bowel disease.
In conclusion, the management of IBD has been dramatically transformed during the last decades, particularly in patients with UC, although surgery still plays an important role both in elective care and acute treatment. The decreasing trend for mutilating surgical treatment—probably predominantly attributable to the advent of improved pharmacological therapy—is indeed a success story not only for the individual patients with IBD but also for the less burdened healthcare system. Future research should investigate if optimised treatment with biologic drugs can further reduce the need for surgical intervention in patients with IBD.
Supplementary material
Acknowledgements
The authors are grateful to Zengliang Ruan from Karolinska Institutet for assistance in SAS programming language and to Mikael Andersson Franko from Karolinska Institutet for his expertise and assistance regarding statistical questions.
Footnotes
Funding: This work was supported by The Swedish Cancer Society (2018/703, 2018/735), The Swedish Research Council (2018-02614), Region Stockholm (FoUI-961412), The South-Eastern Norway Regional Health Authority (2018061 and 2015051) and The Norwegian Cancer Society (190345). Additionally, TJ is supported by a Centre of Excellence grant from the Danish National Research Foundation (DNRF148).
Provenance and peer review: Not commissioned; externally peer-reviewed.
Patient consent for publication: Not applicable.
Ethics approval: The study was approved by the Regional Ethics Committee of South-East Norway (2016/359) and the Swedish Ethical Review Authority in Stockholm (2018/1426-31/1 and 2024-03768-02). Participants gave informed consent to participate in the study before taking part.
Data availability free text: Individual-level data cannot be made publicly available due to their containing information that could compromise the privacy of research participants. Summary data can be made available from the corresponding author on reasonable request. To access the codes generated for this study, please contact the corresponding author.
Data availability statement
Data are available upon reasonable request.
References
- 1.Park J, Cheon JH. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med J. 2021;62:99–108. doi: 10.3349/ymj.2021.62.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet . 2017;390:2769–78. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhao M, Gönczi L, Lakatos PL, et al. The Burden of Inflammatory Bowel Disease in Europe in 2020. J Crohns Colitis. 2021;15:1573–87. doi: 10.1093/ecco-jcc/jjab029. [DOI] [PubMed] [Google Scholar]
- 4.Olivera P, Spinelli A, Gower-Rousseau C, et al. Surgical rates in the era of biological therapy: up, down or unchanged? Curr Opin Gastroenterol. 2017;33:246–53. doi: 10.1097/MOG.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 5.Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. The Lancet. 2015;386:1825–34. doi: 10.1016/S0140-6736(15)00068-9. [DOI] [PubMed] [Google Scholar]
- 6.Tsui JJ, Huynh HQ. Is top-down therapy a more effective alternative to conventional step-up therapy for Crohn’s disease? Ann Gastroenterol. 2018;31:413–24. doi: 10.20524/aog.2018.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raine T, Bonovas S, Burisch J, et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis . 2022;16:2–17. doi: 10.1093/ecco-jcc/jjab178. [DOI] [PubMed] [Google Scholar]
- 8.Torres J, Bonovas S, Doherty G, et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J Crohns Colitis. 2020;14:4–22. doi: 10.1093/ecco-jcc/jjaa043. [DOI] [PubMed] [Google Scholar]
- 9.Noor NM, Lee JC, Bond S, et al. A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn’s disease (PROFILE): a multicentre, open-label randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9:415–27. doi: 10.1016/S2468-1253(24)00034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotze PG, Heuthorst L, Lightner AL, et al. New insights on the surgical management of ulcerative colitis in the 21st century. Lancet Gastroenterol Hepatol. 2022;7:679–88. doi: 10.1016/S2468-1253(22)00001-2. [DOI] [PubMed] [Google Scholar]
- 11.Wintjens D, Bergey F, Saccenti E, et al. Disease Activity Patterns of Crohn’s Disease in the First Ten Years After Diagnosis in the Population-based IBD South Limburg Cohort. J Crohns Colitis. 2021;15:391–400. doi: 10.1093/ecco-jcc/jjaa173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raine T, Verstockt B, Kopylov U, et al. ECCO Topical Review: Refractory Inflammatory Bowel Disease. J Crohns Colitis. 2021;15:1605–20. doi: 10.1093/ecco-jcc/jjab112. [DOI] [PubMed] [Google Scholar]
- 13.Adamina M, Bonovas S, Raine T, et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J Crohns Colitis. 2020;14:155–68. doi: 10.1093/ecco-jcc/jjz187. [DOI] [PubMed] [Google Scholar]
- 14.Spinelli A, Bonovas S, Burisch J, et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J Crohns Colitis. 2022;16:179–89. doi: 10.1093/ecco-jcc/jjab177. [DOI] [PubMed] [Google Scholar]
- 15.Hong SW, Ye BD, Cheon JH, et al. Clinical Features and Long-term Prognosis of Crohn’s Disease in Korea: Results from the Prospective CONNECT Study. Gut Liver. 2022;16:907–20. doi: 10.5009/gnl210299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Lee HJ, Lee SW, et al. Recent trends in the epidemiology and clinical outcomes of inflammatory bowel disease in South Korea, 2010-2018. World J Gastroenterol. 2024;30:1154–63. doi: 10.3748/wjg.v30.i9.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye BD, Hong SN, Seo SI, et al. Changes in the Long-term Prognosis of Crohn’s Disease between 1986 and 2015: The Population-Based Songpa-Kangdong Inflammatory Bowel Disease Cohort Study. Gut Liver. 2022;16:216–27. doi: 10.5009/gnl210044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacio FGM, de Souza LMP, Moreira JP de L, et al. Hospitalization and surgery rates in patients with inflammatory bowel disease in Brazil: a time-trend analysis. BMC Gastroenterol. 2021;21:192. doi: 10.1186/s12876-021-01781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan GG, Seow CH, Ghosh S, et al. Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am J Gastroenterol. 2012;107:1879–87. doi: 10.1038/ajg.2012.333. [DOI] [PubMed] [Google Scholar]
- 20.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut. 2014;63:1607–16. doi: 10.1136/gutjnl-2013-305607. [DOI] [PubMed] [Google Scholar]
- 21.Kirchgesner J, Lemaitre M, Rudnichi A, et al. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: analysis of the French administrative health databases 2009-2014. Aliment Pharmacol Ther. 2017;45:37–49. doi: 10.1111/apt.13835. [DOI] [PubMed] [Google Scholar]
- 22.Jeuring SFG, van den Heuvel TRA, Liu LYL, et al. Improvements in the Long-Term Outcome of Crohn’s Disease Over the Past Two Decades and the Relation to Changes in Medical Management: Results from the Population-Based IBDSL Cohort. Am J Gastroenterol. 2017;112:325–36. doi: 10.1038/ajg.2016.524. [DOI] [PubMed] [Google Scholar]
- 23.Rönnblom A, Holmström T, Karlbom U, et al. Clinical course of Crohn’s disease during the first 5 years. Results from a population-based cohort in Sweden (ICURE) diagnosed 2005-2009. Scand J Gastroenterol. 2017;52:81–6. doi: 10.1080/00365521.2016.1230777. [DOI] [PubMed] [Google Scholar]
- 24.Zhulina Y, Udumyan R, Tysk C, et al. The changing face of Crohn’s disease: a population-based study of the natural history of Crohn’s disease in Örebro, Sweden 1963-2005. Scand J Gastroenterol. 2016;51:304–13. doi: 10.3109/00365521.2015.1093167. [DOI] [PubMed] [Google Scholar]
- 25.Jeuring SFG, Bours PHA, Zeegers MP, et al. Disease Outcome of Ulcerative Colitis in an Era of Changing Treatment Strategies: Results from the Dutch Population-Based IBDSL Cohort. J Crohns Colitis. 2015;9:837–45. doi: 10.1093/ecco-jcc/jjv129. [DOI] [PubMed] [Google Scholar]
- 26.Rönnblom A, Holmström T, Tanghöj H, et al. Low colectomy rate five years after diagnosis of ulcerative colitis. Results from a prospective population-based cohort in Sweden (ICURE) diagnosed during 2005-2009. Scand J Gastroenterol. 2016;51:1339–44. doi: 10.1080/00365521.2016.1200141. [DOI] [PubMed] [Google Scholar]
- 27.Tsai L, Ma C, Dulai PS, et al. Contemporary Risk of Surgery in Patients With Ulcerative Colitis and Crohn’s Disease: A Meta-Analysis of Population-Based Cohorts. Clin Gastroenterol Hepatol. 2021;19:2031–45. doi: 10.1016/j.cgh.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao M, Sall Jensen M, Knudsen T, et al. Trends in the use of biologicals and their treatment outcomes among patients with inflammatory bowel diseases - a Danish nationwide cohort study. Aliment Pharmacol Ther. 2022;55:541–57. doi: 10.1111/apt.16723. [DOI] [PubMed] [Google Scholar]
- 29.Anon Changes in surgery rates among hospitalized patients with inflammatory bowel disease in Japan from 2015 to 2019: a nationwide administrative database analysis - yoshida - 2024 - journal of gastroenterology and hepatology - wiley online library. [10-Jan-2025]. https://onlinelibrary-wiley-com.proxy.kib.ki.se/doi/10.1111/jgh.16387 Available. Accessed. [DOI] [PubMed]
- 30.Wewer MD, Arp L, Sarikaya M, et al. The Use and Efficacy of Biological Therapies for Inflammatory Bowel Disease in a Danish Tertiary Centre 2010-2020. Crohns Colitis 360. 2022;4:otac041. doi: 10.1093/crocol/otac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019;68:423–33. doi: 10.1136/gutjnl-2017-315568. [DOI] [PubMed] [Google Scholar]
- 32.Lo B, Vester-Andersen MK, Vind I, et al. Changes in Disease Behaviour and Location in Patients With Crohn’s Disease After Seven Years of Follow-Up: A Danish Population-based Inception Cohort. J Crohns Colitis. 2018;12:265–72. doi: 10.1093/ecco-jcc/jjx138. [DOI] [PubMed] [Google Scholar]
- 33.Manetti N, Bagnoli S, Rogai F, et al. Disease Course and Colectomy Rate of Ulcerative Colitis: A Follow-up Cohort Study of a Referral Center in Tuscany. Inflamm Bowel Dis. 2016;22:1945–53. doi: 10.1097/MIB.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 34.Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: a population-based interrupted time series study. Gut. 2020;69:274–82. doi: 10.1136/gutjnl-2019-318440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anon . Oxford Academic; [18-Dec-2024]. P35 treatment outcomes of inflammatory bowel disease in the biological era—a nationwide retrospective cohort study in three nordic countries: results from the trinordic study | journal of crohn’s and colitis.https://academic.oup.com/ecco-jcc/article/14/Supplement_1/S036/5705315 Available. Accessed. [Google Scholar]
- 36.Yu J, Refsum E, Helsingen LM, et al. Risk of hepato‐pancreato‐biliary cancer is increased by primary sclerosing cholangitis in patients with inflammatory bowel disease: A population‐based cohort study. UEG Journal . 2022;10:212–24. doi: 10.1002/ueg2.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Refsum E, Perrin V, et al. Inflammatory bowel disease and risk of adenocarcinoma and neuroendocrine tumors in the small bowel. Ann Oncol. 2022;33:649–56. doi: 10.1016/j.annonc.2022.02.226. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Refsum E, Wieszczy P, et al. Risk of malignant lymphomas in patients with inflammatory bowel disease: a population-based cohort study. BMJ Open Gastroenterol. 2023;10:e001037. doi: 10.1136/bmjgast-2022-001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anon . Socialstyrelsen; 2024. [03-Jan-2025]. National patient register.https://www.socialstyrelsen.se/en/statistics-and-data/registers/national-patient-register/ Available. Accessed. [Google Scholar]
- 40.Anon NOMESCO classification of surgical procedures. [16-Dec-2024]. https://norden.diva-portal.org/smash/get/diva2:970547/FULLTEXT01.pdf Available. Accessed.
- 41.Bouwman W, Verhaegh W, van de Stolpe A. Improved diagnosis of inflammatory bowel disease and prediction and monitoring of response to anti-TNF alpha treatment based on measurement of signal transduction pathway activity. Front Pharmacol. 2022;13:1008976. doi: 10.3389/fphar.2022.1008976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45:1291–302. doi: 10.1111/apt.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atreya R, Siegmund B. Location is important: differentiation between ileal and colonic Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2021;18:544–58. doi: 10.1038/s41575-021-00424-6. [DOI] [PubMed] [Google Scholar]
- 44.Friedrich M, Pohin M, Powrie F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity. 2019;50:992–1006. doi: 10.1016/j.immuni.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Selvaggi F, Pellino G, Canonico S, et al. Effect of preoperative biologic drugs on complications and function after restorative proctocolectomy with primary ileal pouch formation: systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:79–92. doi: 10.1097/MIB.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 46.Zittan E, Wong-Chong N, Ma GW, et al. Modified Two-stage Ileal Pouch-Anal Anastomosis Results in Lower Rate of Anastomotic Leak Compared with Traditional Two-stage Surgery for Ulcerative Colitis. J Crohns Colitis. 2016;10:766–72. doi: 10.1093/ecco-jcc/jjw069. [DOI] [PubMed] [Google Scholar]
- 47.Ponsioen CY, de Groof EJ, Eshuis EJ, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol. 2017;2:785–92. doi: 10.1016/S2468-1253(17)30248-0. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal M, Ebert AC, Poulsen G, et al. Early Ileocecal Resection for Crohn’s Disease Is Associated With Improved Long-term Outcomes Compared With Anti-Tumor Necrosis Factor Therapy: A Population-Based Cohort Study. Gastroenterology. 2023;165:976–85. doi: 10.1053/j.gastro.2023.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adami H-O, Bretthauer M, Emilsson L, et al. The continuing uncertainty about cancer risk in inflammatory bowel disease. Gut. 2016;65:889–93. doi: 10.1136/gutjnl-2015-311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.