Abstract
Diabetic foot ulcers (DFUs) are a devastating complication of diabetes mellitus (DM) that affect millions of people worldwide every year. They have a long-term impact on patients' quality of life and pose a significant challenge for both patients and clinicians, alongside negative economic implications on affected individuals. The current therapeutic approaches are costly and, in many cases, ineffective, highlighting the urgent need to develop novel, affordable, more efficient, and personalized treatments. Recent advances in high-throughput omics technologies, including proteomics, bulk RNA sequencing (bulk RNA-seq), single-cell RNA sequencing (scRNA-seq), and spatial transcriptomics in both preclinical animal and human clinical studies, have enhanced our understanding of the molecular function and mechanisms of DFUs, thereby offering potential for targeted therapies. Additionally, these technologies provide valuable insights behind the mechanism of action of novel wound dressings and treatments. In this review, we outline the latest application of omics technologies in DFU preclinical animal and human clinical research on diabetic wound healing, and spotlight recent findings.
A graphical abstract is available with this article.
Graphical Abstract
Keywords: Bulk RNA sequencing, Diabetic foot ulcers, Diabetic wound healing, Proteomics, Single cell RNA sequencing, Spatial transcriptomics
Key Summary Points
| Diabetic foot ulcers (DFUs) are a devastating complication of diabetes mellitus (DM) that affect millions of people worldwide every year. |
| By leveraging advanced omics technologies, including bulk RNA-seq, scRNA-seq, spatial transcriptomics, and proteomics, the pathophysiology of DFUs is better understood. |
| Omics technologies also help identify new therapeutic targets, and to provide valuable insights into the mechanism of action of novel wound dressings and treatments. |
Digital Features
This article is published with digital features, including an infographic to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.28563710.
Introduction
DFUs affect approximately 18.6 million people worldwide annually, with 1.6 million cases in the United States alone [1]. Due to their unique pathology, healing of DFUs differs from the normal skin-healing process, often leading to delayed or non-healing wounds [2]. They are consequently associated with reduced physical function, decreased quality of life, and increased demand on healthcare resources [3]. Approximately 20% of individuals with a DFU will require a lower extremity amputation, either minor (such as a portion of the foot) or major (above the foot) [4]. The 5-year mortality rate for those with a DFU is approximately 30%, rising to over 70% for those who undergo an above-foot amputation [5]. The estimated annual direct costs for managing DFUs in the United States are between US$9 billion to $13 billion [6].
Surgical debridement and wound dressings, offloading of the DFU, adjunctive antibiotics in the presence of clinical infection, and revascularization when peripheral arterial disease is present are the cornerstones of DFU management [2, 7]. Current advanced treatments that have been approved by the Food and Drug Administration include a recombinant growth factor rhPDGF-BB, becaplermin [8], two bio-engineered skin substitutes, Apligraf [9] and Dermagraft [10], and an acellular, bilayer matrix, Integra [11, 12]. Despite these strategies, which are costly and reserved for non-healing ulcers, treatment failure rates continue to be high, and almost half of DFUs fail to heal [11]. Over the past two decades, progress in materials science, stem cell biology, and tissue engineering has prompted research towards the development of new therapeutic strategies for diabetic wound healing [13, 14]. These include the development of functional biomaterials, delivery of growth factors, stem cell transplantation, and the creation of tissue-engineered constructs with both living cells and synthetic scaffolds [15]. The main impetus is that, when combined with traditional wound management and antimicrobial interventions, these innovative approaches will significantly enhance skin repair and wound healing [2]. Nevertheless, only a small number of biomaterials have been successfully translated into clinical practice, while most still face various challenges, including concerns about biosafety, ethical issues, and low treatment efficacy [16].
Advances in high-throughput omics technologies over the past decade have provided valuable insights into the pathogenesis of DFUs and the understanding of diabetic wound healing, and hold promise for discovering more targeted therapeutics [17]. Multi-omics approaches, including genomics, transcriptomics, proteomics, and metabolomics, are being widely used in both preclinical animal and clinical studies to investigate variations in genomic and expression profiles across entire systems [18–21]. Proteomics give information on protein functions, structures, interactions, compositions, and their roles in cellular activities [22]. Bulk RNA-seq analysis has been used to generate the overall gene expression profile in a population of cells in the wound site [23]. scRNA-seq analysis allows us to evaluate the transcriptional profiles of individual cells and to investigate cell type diversity and the intricate interactions involved in wound healing [24, 25]. Spatial transcriptomics is used to analyze the gene expression patterns across different cell types in the wound micro-environment. By combining spatial transcriptomics with tissue staining, researchers can gain insights into tissue structure, the spatial arrangement of cells, and cellular interactions within the tissue that play important roles in the healing process (Fig. 3) [26, 27]. Recently, scRNA-seq technology has emerged, offering unprecedented opportunities to explore molecular functions within the complex and heterogeneous environment of DFUs [27]. These advancements deepen our understanding of the underlying mechanisms of diabetic wound healing and aid in the development of the next generation of wound dressings and other therapeutic approaches. In this review, we summarize the current applications of omics technologies in DFU research, and highlight recent discoveries.
Fig. 3.
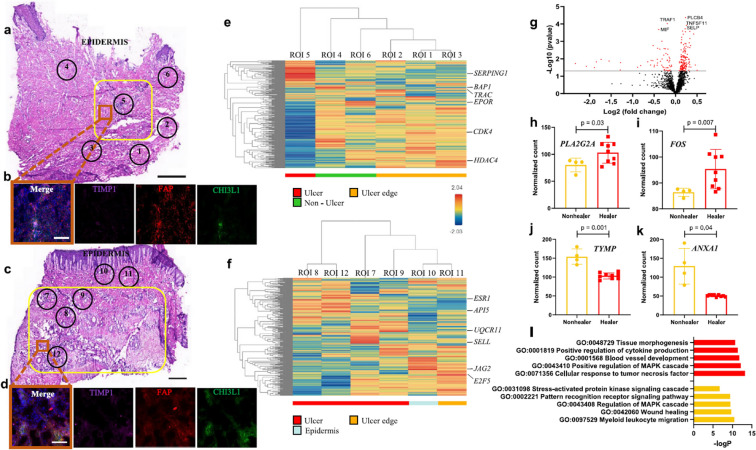
Spatial transcriptome of DFU healers and DFU non-healers. a, c Representative H&E-stained sections from a non-healing and healing DFU, respectively. The yellow boxes indicate the ulcer regions, while the numbered circles indicate the sequenced ROIs. b, d Immunofluorescence staining for HE-Fibro markers TIMP1 (purple), CHI3L1 (green), and pan-fibroblast marker FAP (red). DAPI was used as a nuclear counterstain. The image capture location is indicated by an orange box in (a) and (c). e, f Hierarchical clustering heatmaps display the transcriptomic profiles of the selected ROIs, with the most highly expressed gene in each ROI highlighted. ROIs are categorized based on their location as ulcer (red), non-ulcer (green), ulcer edge (orange), and epidermis (light blue). Expression levels are represented according to the gradient on the middle right (ranging from blue for low to red for high expression). g A volcano plot illustrates the DE analysis results from ROIs within the ulcer in healers (2 patients, 9 ROIs) versus non-healers (2 patients, 4 ROIs). Each dot represents a gene, with red dots indicating genes that exceed the significance threshold. The top five genes are highlighted. h–k Selected significant genes that are upregulated in healers (h, I) and non-healers (j, k) are shown. Data are presented as mean ± SD, with n = 4 ulcer ROIs from 2 non-healers and n = 9 ulcer ROIs from 2 healers. A two-tailed unpaired t test with the Benjamini–Hochberg procedure for adjusted p values was used to calculate the p values. l Gene ontology (GO) analysis shows the biological processes enriched in healers (top, red) and non-healers (bottom, yellow). Staining experiments were repeated three times with two biologically independent patient samples per group. Scale bars are 1 mm in (a, c) and 100 μm in (b, d). This figure was published in [27]
Methods
A comprehensive literature search was conducted using the PubMed database to identify relevant studies on the application of -omics techniques in diabetic wound healing. The search terms included "Single-cell RNA sequencing AND diabetic wound healing," "Single-cell RNA sequencing AND diabetic foot ulcer," "bulk RNA sequencing AND diabetic wound healing," "bulk RNA sequencing AND diabetic foot ulcers," "Spatial transcriptomics AND diabetic wound healing," "Spatial transcriptomics AND diabetic foot ulcers," "Proteomics AND diabetic wound healing," and "Proteomics AND diabetic foot ulcers." Studies published from 2018 onward that discussed the application of -omics technologies on diabetic wound healing were included to highlight recent advancements in the field. Earlier studies were excluded due to the use of outdated techniques that were not considered reliable. Studies unrelated to diabetic wounds or lacking experimental data were excluded.
This article is based on previously conducted studies and does not include any new studies involving human participants or animals performed by the authors.
Bulk RNA-Seq and scRNA-seq in Diabetic Wound Healing: Preclinical Animal and Human Clinical Studies
Acute wound healing is a well-coordinated process that encompasses four distinct overlapping phases: hemostasis, inflammation, proliferation–angiogenesis, and extracellular matrix remodeling. Various cell types with distinct roles are involved in the different phases of wound healing. Any disruption in these phases could compromise the repair process and potentially lead to the formation of non-healing ulcers [7]. In chronic wounds, such as DFUs, the lineal progression from one phase to the next is disrupted. These wounds are characterized by persistent low-grade inflammation.
Several studies have employed bulk RNA-seq and scRNA-seq to investigate the molecular mechanisms of wound healing, particularly in the context of diabetic wounds (Table 1). Both are essential to elucidate the immune components that participate in wound healing and are important tools in revealing the mechanism of action of potential therapeutic approaches in wound healing [28]. bulk RNA sequencing analysis is utilized to study the overall gene expression profile of the wounded tissue while scRNA-seq profiles gene expression at the single-cell level and provides crucial insights into the cellular heterogeneity, rare cell populations and dynamics of wound healing (Fig. 1) [28].
Table 1.
Summary of bulk RNA-seq and scRNA-seq findings (cell types, genes, and pathways) related to diabetic wound healing, grouped by the wound healing phase to which they correspond
| Wound healing phase | Cell types | Genes | Pathways | |
|---|---|---|---|---|
| Inflammation | Fibroblasts |
IFN-γ, VEGF, sVCAM-1 |
Theocharidis et al. [41] | |
|
HE-Fibro M1 inflammatory macrophages in healers anti-inflammatory M2 macrophages in non-healers |
MMP1, MMP3, MMP11, HIF1A, CHI3L1, TNFAIP6 |
Theocharidis et al. [27] | ||
| Fibroblasts |
Il17a, Il17f, Il19, Il21, Il22 |
Pathways associated to muscle contraction and metal ion transport | Theocharidis et al. [45] | |
| CD8 T cells and B cells were less in non-healing ulcers | IL-17 signaling pathway was linked to ulcers | Cheng et al. [47] | ||
| Macrophages |
Cd74, Tnfsf9, Tnfsf12, Tnfrsf12a |
Ma et al. [48] | ||
| M1 macrophages higher in the non-healing group |
NFE2L2, REL, ETV6, MAF, NF1B |
Li et al. [50] | ||
| CGNL1 | Wang et al. [51] | |||
|
CLU, RABGEF1, ENPEP |
Tan et al. [45] | |||
| Inflammatory and proliferative phases |
CDKN1A, CXCL8, IGFBP2, IL1A, MMP10, SER-PINE1, TGFA upregulated in DFUS, TP53 downregulated in DFUS |
Yu et al. [53] | ||
|
Macrophages, Fibroblasts |
SLC31A1 upregulated in macrophages, ADNP upregulated in fibroblasts |
Copper metabolism | Yi et al. [54] | |
| Proliferative phase | Endothelial cells |
CCND1, ENO1, MMP2, HIF1a, SERPINE1 |
Lu et al. [66] | |
| HDMECs |
RAB17, and CD200 related to impaired angiogenesis in DFU-HDMECs, RAB17 expression reduced in DFU-HDMECs |
Du et al. [67] | ||
| Extracellular matrix remodeling |
CYP1A higher in non-healers, SLCO2A1 are present in vascular endothelial cells and associated with faster wound healing when inhibited, MMP2 upregulation in healers, IL13 and IFNG major upstream regulators |
Theocharidis et al. [41] |
Fig. 1.
Identification and characterization of unique healing enriched fibroblasts in DFUs by scRNA-seq. a Schematic of the study design. b Uniform Manifold Approximation and Projection (UMAP) representation of the entire dataset, which includes 174,962 cells. The cells are color-coded according to orthogonally generated clusters and labeled based on manual cell type annotation: BasalKera basal keratinocytes, B-Lympho B-lymphocytes, CD14-Mono CD14 + monocytes, CD16-Mono CD16 + monocytes, DCs dendritic cells, DiffKera differentiated keratinocytes, Erythro erythrocytes, Fibro fibroblasts, HE-Fibro healing enriched fibroblasts, LymphEndo lymphatic endothelial cells, , M1-Macro M1 macrophages, M2-Macro M2 macrophages, Mast mast cells, Melano/Schwann melanocytes and Schwann cells, NK natural killer cells, NKT NK cells and T lymphocytes, Plasma plasma cells, SMCs smooth muscle cells, Sweat/Seba sweat and sebaceous gland cells, T-Lympho T-lymphocytes, VasEndo vascular endothelial cells. Dotted lines indicate groups of cells with similar lineage. c Dot plot depicting the expression of various cell type-specific marker genes used for annotating cell types. The size of the dots shows percentage of cells in each cell cluster expressing the marker gene, the color represents the averaged scaled expression levels, cyan low expression, red high expression. This figure was published in [27]
The methods used to generate high-throughput sequencing libraries for bulk RNA-seq involve several steps to capture the overall transcriptome of a population of cells. RNA was extracted from tissue samples, and techniques for library preparation applied such as poly-A selection, which isolates mRNA by targeting the polyadenylated tails of most eukaryotic transcripts, and rRNA depletion, which removes ribosomal RNA to enrich for mRNA and non-coding RNA species [29]. The RNA was then fragmented and reverse-transcribed into complementary DNA (cDNA), the cDNA was amplified, and adapters were added to prepare it for sequencing [30]. The sequencing libraries that were generated were then subjected to high-throughput sequencing on platforms such as Illumina, allowing for the profiling of gene expression across the entire transcriptome of a cell population [30]. These approaches ensured accurate and comprehensive data for studying gene expression at the population level. The main steps of scRNA-seq involve tissue digestion for single-cell suspension generation, isolating and lysing of single cells, performing reverse transcription that converts RNA into cDNA, amplifying the cDNA, and preparing the library (29, 30). Various methods have used to generate high-throughput sequencing libraries for scRNA-seq, including microfluidic platforms like 10× Genomics and Fluidigm C1, as well as methods like Drop-Seq and Smart-Seq. Each of these techniques utilizes different approaches for capturing and analyzing gene expression at the single-cell level, and enable the sequencing of thousands to millions of individual cells, providing a large volume of data for studying gene expression, cellular heterogeneity, and complex biological processes [31]. More specifically, 10× Genomics offers Chromium, which is a widely used platform that utilizes microfluidics and barcoding to sequence RNA from individual cells, ideal for large-scale studies [32]. Fluidigm C1 combines automated microfluidics with single-cell RNA sequencing, enabling high-throughput analysis by isolating cells in microfluidic chips [33]. Drop-seq employs microdroplets with barcoded beads to encapsulate cells, allowing high-throughput sequencing while preserving cell identity [34]. Smart-seq is suitable for full-length cDNA synthesis for comprehensive transcriptome analysis of individual cells. Seq-Well uses a microchamber system for scalable, cost-effective cell isolation [33]. Although bulk RNA-seq is a cost-effective and high-throughput method, scRNA-seq provides deeper insights into the diversity of gene expression within heterogeneous tissues or cell populations, making it ideal for studying complex biological processes such as diabetic wound healing (33).
Bulk RNA-Seq and scRNA-seq Studies in Inflammatory Phase of Diabetic Wound Healing
The inflammatory phase involves complex interactions between immune cells like neutrophils and macrophages to remove debris, prevent infection, and prepare for the proliferative phase of wound healing, which entails tissue formation and remodeling, as well as mast cell degranulation responsible for releasing pro-inflammatory mediators and cytokines to promote inflammation and leukocytic infiltration [35–37]. The production of hydrogen peroxide (H2O2) is also considered a crucial component of the wound-healing response serving as an early damage signal that regulates multiple key aspects of the healing process [38]. It starts within seconds of injury, during 24 h of the injury, and lasts up to 2 weeks. However, chronic wounds such as DFUs are characterized by a prolonged inflammatory phase and lack of the linear progression to the next phase, the proliferation [39]. Macrophages play a major role in wound healing. The transition between pro-inflammatory (M1) and immuno-modulatory (M2) phenotypes is critical to regulate the different phases of repair [39]. While M1 macrophages are essential for acute inflammation, their persistence in chronic wounds, particularly in diabetes, contributes to impaired healing. M2 macrophages support angiogenesis and collagen synthesis during the proliferation phase [40].
Theocharidis et al. [41] collected discarded foot skin specimens from healthy subjects, patients with diabetes mellitus (DM) but without DFUs, and patients with DFUs. They investigated local factors that are associated with DFU healing. scRNA-seq analysis revealed various fibroblast cell clusters and elevated inflammation in the dorsal skin of patients with DM and DFUs. Additionally, both proteomics and transcriptomics analysis of both serum and forearm skin revealed that higher expression of factors like interferon-γ (IFN-γ), vascular endothelial growth factor (VEGF), and soluble vascular cell adhesion molecule-1 (sVCAM-1) were linked to DFU healers. In a subsequent study, Theocharidis et al. [27] conducted a large-scale unbiased scRNA-Seq study of DFUs and forearm skin biopsies, as well as peripheral blood mononuclear cells from DFU healing and non-healing patients, patients with DM, and healthy controls (Fig. 1a). The authors created a diabetic wound-healing cell atlas and studied the molecular changes in patients with healing and non-healing DFUs. They discovered a distinct fibroblast subpopulation, termed healing fibroblasts, which exhibited upregulated genes related to matrix degradation, hypoxia response, and inflammation, such as MMP1, MMP3, MMP11, HIF1A, CHI3L1, and TNFAIP6 in DFU healing patients (Figs. 1b, 2a–c). They also found a greater presence of M1 inflammatory macrophages in DFU healing patients. In contrast, non-healing patients had higher levels of anti-inflammatory M2 macrophages. Pang et al. performed and unbiased scRNA-seq analysis of wound monocytes/macrophages (Mo/MF) to investigate the impact of diabetes on Mo/MF proliferation in skin wounds and identify potential regulators of this process [42]. They discovered a distinct cluster, Ly6C+F4/80lo/− Mo/MΦ with increased proliferative activity, by cells from diabetic wounds that have enriched expression of cell cycle-related genes. This finding demonstrated that diabetes alters Mo/MF dynamics by promoting excessive proliferation in the wound environment. Further analysis identified CCL2 as a key regulator, with elevated levels in diabetic wounds correlating with enhanced Mo/MF proliferation. These results highlight a novel connection between CCL2/CCR2 signaling and dysregulated Mo/MF proliferation, which may contribute to chronic inflammation and impaired healing in diabetic wounds.
Fig. 2.
Single-cell RNA sequencing highlighting the expression of key genes in the healing enriched fibroblasts in diabetic foot ulcers (DFUs). a Stacked bar plots displaying the distribution of various cell types across the four different clinical groups. Green represents healthy subjects, orange DFU healers, red DFU non-healers, and purple diabetic patients. Cell types that show significant differences between the clinical groups are indicated with an asterisk. b Heatmap highlighting the most highly expressed genes (red) within each cell cluster. c Feature plots illustrating the expression of key genes: (I) MMP1, (II) MMP3, (III) CHI3L1, and (IV) TNFAIP6, which were notably overexpressed in the fibroblasts associated with DFU healing. This figure was published in [27]
In their study, Li et al. [43] used the GSE165816 DFU patient scRNA-seq dataset from the GEO database and the GSE199939 RNA-SEq dataset to elucidate the DFU micro-environment and functional condition. Their analysis revealed a larger population of macrophages, white blood cells, and monocytes in DFU patients, while patients with DM had higher proportions of pluripotent stem cells and stromal cells. Additionally, multipotent stem cells and hematopoietic stem cells were found to be significantly lower in both healing and non-healing patients with DFU compared to those with DM. Finally, genes like ANPEP, BID, CYBA, CYBB, FCER1G, ITGA1, and PLAUR were overexpressed in the DFU micro-environment and could be considered as potential therapeutic targets. In a different study, Keogh et al. [44] investigated Group B Streptococcus (GBS) infection and the various strategies the pathogen adapts in diabetic wounds in mice. Dual RNA sequencing analysis revealed transcriptional changes on both the host and pathogen. The dual RNA-seq approach revealed that GBS exacerbates inflammation in diabetic wounds, characterized by increased cytokine production and neutrophil degranulation. They also described the mechanism by which GBS adapts to the diabetic wound environment, identified key upregulated virulence factors, and provided insights into the GBS pathogenesis and diabetic wound's immune response, which affects healing’s inflammatory stage.
In another study, Theocharidis et al. [45] used bulk RNA-seq to evaluate the efficacy and elucidate the mechanism of action of a novel strain-programmed patch on diabetic mouse wounds. Transcriptomic analysis of diabetic mouse wounds with the strain-programmed patch revealed pathways associated with muscle contraction and metal ion transport and genes like Il17a, Il17f, Il19, Il21, and Il22 that are involved in the inflammatory landscape required to accelerate wound healing. They also showed that the new strain-programmed patch favors pro-regenerative fibroblasts that improve re-epithelialization and angiogenesis, and promote wound closure. Januszyk et al. [46] evaluated the scRNA-seq application on clinically debrided wound tissues that were exposed to extended periods of cold ischemia, without using FACS shorting. They identified cell clusters with unique transcriptional profiles related to fibroblasts subpopulations with varying fibrotic potentials, keratinocytes, neutrophils, monocytes, and endothelial cells, and they observed different distributions between diabetic and non-diabetic cells, validating the effective application of scRNA-seq on clinically obtained wound tissues kept on ice for elongated periods.
Maschalidi et al. [19] performed an inter-species approach where they incubated primary mouse bone marrow dendritic cells (DCs) with labeled apoptotic human Jurkat cells and carried out RNA-seq with isolated phagocytic DCs to determine gene profiles induced in DCs during efferocytosis in diabetic wound healing. Transcriptomic analysis revealed increased expression of multiple SLC7 genes, in particular the upregulation of Slc7a11 in innate immune cells of inflamed skin. In addition, transcriptomic analysis of SLC7A11-deficient DCs revealed that GDF15, released by efferocytic DCs, aids wound closure downstream of SLC7A11. These findings highlighted SLC7A11 as an inhibitor of DC phagocytosis and suggested that inhibiting SLC7A11 could enhance wound healing and be considered as a potentially benefiting treatment for diabetic wounds. Cheng et al. [47] performed scRNA-seq to determine the distribution of various cell populations in DFUs and found that CD8 T cells and B cells were fewer in non-healing ulcers compared to healthy skin and healing ulcers. They also found that the IL-17 signaling pathway was linked to ulcers. In their study, Ma et al. [48] performed an unbiased scRNA-seq analysis of CD45 + immune cells isolated from wounds of streptozotocin-induced diabetic mice and skin from wild-type mice across four different time points. By using transcriptomic analysis, the authors studied the heterogeneity of macrophages and created their genetic profile in wounds of diabetic and healthy control mice. They also found expression of the Cd74, Tnfsf9, Tnfsf12, and Tnfrsf12a genes in a cluster of macrophages, indicating their possible role in promoting inflammation. They discovered a new population of macrophages that possess osteoclast gene expression and could be linked to skin renewal and repair responses.
In other research, Audu et al. [49] performed scRNA-seq on wounds from patients with DM and bulk RNA-seq in macrophages isolated from wounds from diabetic and control mice to study the mechanisms underlying the dysregulated macrophage inflammation observed in diabetic wounds. Transcriptomic analysis revealed that JMJD3 regulates inflammation in wound macrophages by lowering H3K27me3 through the JAK1/JAK3/STAT3 pathway. They also showed that targeting JMJD3 in macrophages could reduce STING-mediated inflammation and NFκB cytokines, thereby improving diabetic wound healing and making it a promising target for treating non-healing diabetic wounds. Li et al. [50] used scRNA-seq and bulk RNA-seq sequencing data from the GEO database from DFUs, forearm-skin of DFU patients, and control subjects to study the immune-associated genes (IRGs) regulation mechanisms, and transcriptional features within DFUs. Their findings revealed higher M1 macrophages in the non-healing group compared to healing DFUs or healthy controls and NFE2L2, REL, ETV6, MAF, and NF1B as key transcription factors. In their research, Wang et al. [51] integrated large-scale unbiased scRNA-seq and bulk RNA-seq datasets from GEO database to study the transcriptional characteristics of fibroblast subtypes and the ferroptosis role in DFUs. They used data from control, diabetic, DFU healer, and DFU non-healer groups to explore the complexity and heterogeneity of fibroblasts within DFUs, revealing six distinct fibroblast subtypes with unique functional roles and transcriptional profiles. Their findings also demonstrated a strong association between the ferroptosis-related gene CGNL1 and pro-inflammatory fibroblasts, suggesting CGNL1 as a promising diagnostic biomarker and therapeutic target for DFUs.
In another study, Tan et al. [52] downloaded and analyzed bulk RNA-seq and scRNA-seq [27] data from patients with DFU available in the GEO database to provide insights into the pathogenesis, diagnosis, and treatment of DFUs. Their findings detected dysregulated expressed genes (DEGs) related to DFUs that are linked to lysosomal functions and immune/inflammatory responses. They suggested that CLU, RABGEF1, and ENPEP have potential as new molecular markers for identifying DFUs. By targeting these markers, the authors indicated that latamoxef, parthenolide, meclofenoxate, and lomustine could be used as promising treatments for DFUs. In their research, Yu et al. [53] analyzed publicly available bulk RNA-seq data of biopsies from wound edges of DFUs and uninvolved diabetic foot skin to elucidate the role of senescence-associated genes in DFUs. Their findings showed upregulation in DFUs of genes that are involved in inflammatory and extracellular matrix remodeling phases of wound healing. More specifically, CDKN1A, CXCL8, IGFBP2, IL1A, MMP10, SER-PINE1, and TGFA cellular senescence markers were upregulated, while TP53 was downregulated in DFUs, ultimately suggesting that cellular senescence genes play a critical role in DFU pathogenesis. Yi et al. [54] used bulk RNA-seq and scRNA-seq data from the GEO database to study the DFU mechanism and how it is linked to copper metabolism. Their findings revealed that genes associated with copper metabolism, such as SLC31A1 and ADNP, were differentially expressed in DFUs. More specifically, SLC31A1 was upregulated in macrophages, whereas ADNP was upregulated in fibroblasts and chondrocytes.
In conclusion, different studies have shown that both immune cells, such as macrophages, CD8T cells, B cells, as well as structural cells like fibroblasts play important roles in the inflammatory phase of diabetic wound healing.
Bulk RNA-Seq and scRNA-Seq Studies in the Phase of Proliferation and Angiogenesis of Diabetic Wound Healing
The proliferative phase follows and overlaps the inflammatory phase in acute wounds [55]. This phase typically begins about 3–5 days after the injury and can last for several weeks, depending on the severity of the wound. Keratinocytes, fibroblasts, macrophages, and endothelial cells are involved in this phase to orchestrate wound closure, tissue formation, matrix deposition, and angiogenesis [35].
Studies have shown that macrophages become dysfunctional in a diabetic high-glucose environment, resulting in impaired wound healing [56]. A reduced number and activation of macrophages contribute to disrupted healing, with macrophage depletion during the early inflammatory phase leading to impaired tissue formation and delayed epithelialization [57]. Chronic diabetic wounds often display inadequate M1 macrophage activation in the early stages and excessive M2 activation during the proliferative phase [58]. Introducing activated macrophages into wounds has been shown to enhance healing by promoting vascularization and tissue repair, suggesting that stimulating macrophage recruitment and correcting their abnormal activation could restore effective inflammation and improve wound healing [56, 59]. Theocharidis et al. [39] used bulk RNA-seq to explore the mechanism of action of topically delivering primary macrophages or their secretome through calcium-crosslinked alginate dressings to diabetic mouse wounds. RNA-seq revealed that macrophage treatment and their conditioned media influenced various healing processes, such as keratinization and junction formation, while vascularization and inflammation pathways depended on the type of macrophages used. In general, alginate dressings were effective for topical applications, and polarized macrophages, especially their secretome, improved wound healing. In another study, Singh et al. [60] studied the effect of diabetes on angiogenesis in diabetic wound healing in mice and humans. They co-cultured human umbilical vein endothelial cells with two different mouse dermal fibroblasts, from wild-type mice and db/db mice. Transcriptomic analysis revealed that diabetes inhibits angiogenesis and leads to notable changes in the gene expression of endothelial cells, with a marked expression of pathways related to cytokine signaling, apoptosis, and collagen degradation. Finally, a particular subset of stromal precursor cells (ABCB5+) was identified, and it significantly accelerated healing when injected into the wound.
In their research, Wang et al. [61] performed RNA sequencing of human umbilical cord mesenchymal stem cells (hucMSCs) treated with or without CHIR99021, a Wnt signaling pathway agonist, to underlying the mechanism and assess the therapeutic potency of exosomes from hucMSCs in diabetic wound healing. They showed that SNAP25, a key component of the SNARE complex that is crucial for exocytosis, was among the 24 genes that were upregulated in the CHIR99021-treated group and mediated the increased exosome secretion in this group. In addition, they demonstrated differentially expressed mRNAs that cluster in the TGFß and PI3K-Akt pathways, crucial for regulating wound healing. Escuin-Ordinas et al. [62] performed RNA sequencing on mouse wounds that were treated with the BRAF inhibitor, vemurafenib, to evaluate this therapeutic’s efficacy on diabetic wound healing. RNA sequencing data revealed higher expression of genes related to hair follicle development. More specifically, they highlighted Tcf7 as a key transcription factor for inducing regenerative healing to wounds treated with vemurafenib. Finally, authors indicated upregulation of mRNA genes related to keratinocyte differentiation leading to enhanced re-epithelialization of vemurafenib-treated wounds. Li et al. [63] used scRNA-seq to study cellular events and heterogeneity in the wound micro-environment of diabetic wounds treated with a carbon monoxide (CO)-releasing hyaluronan hydrogel (CO@HAG) that they developed. A novel Cxcl14+ cluster of fibroblast progenitor was found in the wounds that were treated with CO@HAG. Macrophage pro-inflammatory activity decreased, while their anti-inflammatory activity increased.
In a different study, Li et al. [64] used RNA sequencing to evaluate the therapeutic efficacy of a novel engineered miRNA-loaded exosome delivery system on diabetic mouse wounds, and demonstrated effective inhibition of the NF-κB signaling pathway in vitro through IRAK1 targeting that resulted in decreased inflammation with miR146a-loaded exosomes. They also showed anti-inflammatory and regenerative properties, including collagen deposition and neovascularization, which resulted in accelerated wound healing when this treatment was used in vitro with diabetic mice. Deng et al. [65] explored the therapeutic properties of d-SMG, a natural biological adhesive they developed from snail mucus gel, in wounds of streptozotocin-induced diabetic rats. Transcriptomic analysis revealed downregulation of Tnf, Ccl5, Nos2, Il1b, Il18, and Tlr5 genes, upregulation of Vegfb, Tgfb1, Fgf3, and Col7a1 genes, and pathways associated with wound healing and organ regeneration, demonstrating that the treatment diminished inflammation and promoted epithelial renewal, angiogenesis, and healing in diabetic wounds. In their research, Lu et al. [66] performed scRNA-seq at DFU healers, DFU non-healers, and healthy non-diabetic controls data from the GEO dataset (GSE165816) [27] to investigate the molecular and functional properties of endothelial cells in healing and non-healing DFUs. This study revealed downregulation of genes like CCND1, ENO1, MMP2, HIF1a, and SERPINE1, that are crucial for angiogenesis and wound healing, in endothelial cells in non-healing DFUs compared to healing DFUs. Despite similar endothelial cell numbers between the two groups, impaired healing was linked to reduced immune-mediated functions and weaker cellular interactions rather than cell quantity or vascular density. These findings highlighted the critical role of endothelial cell function and interaction over quantity or vascular density in diabetic wound healing.
Du et al. [67] employed a combined approach of bulk RNA-seq and scRNA-seq on human dermal microvascular endothelial cells (HDMECs) isolated from patients with DFU and healthy controls to study functional changes such as proliferation, migration, and tube formation, and to identify potential regulators of angiogenesis in DFUs. Transcriptomic analysis, along with WGCNA and LASSO regression, identified the RAB17 and CD200 genes as being related to impaired angiogenesis in DFU-HDMECs. RAB17 expression was notably reduced in DFU-HDMECs compared to healthy controls. The authors observed that RAB17 overexpression improved angiogenesis, increased HIF-1α and VEGF-A levels, and enhanced diabetic wound healing, partly through the MAPK/ERK signaling pathway, suggesting RAB17 as a potential target for impaired angiogenesis in DFUs.
Overall, various studies have shown that different type of cells, such as endothelial cells, HDNECS, macrophages, and fibroblasts, participate in the proliferation–angiogenesis phase of diabetic wound healing.
Bulk RNA-Seq and scRNA-Seq Studies in the Extracellular Matrix Remodeling Phase of Diabetic Wound Healing
The extracellular matrix (ECM) remodeling phase is a complex process involving multiple cell types, such as fibroblasts, myofibroblasts, macrophages, and endothelial cells, that coordinate collagen deposition, connective tissue and capillary reduction, the epithelium's normal thickness restoration/maturation, ECM reorganization, and wound contraction [35, 68, 69]. This phase can last several weeks to months and even years.
In their research, Theocharidis et al. [41] prospectively followed a large group of patients with DFU to study wound healing in DFUs, collecting forearm skin biopsies from healthy control subjects, patients with DM and without DFUs, and two distinct DFU patient groups classified as healers and non-healers. They performed bulk RNA-seq to explore the systemic effect of DFU healing outcome at a nonulcerative skin site. The bulk RNA-seq revealed that non-healers had higher levels of CYP1A, related to sebaceous glands and skin barrier function. They also found that SLCO2A1 genes are present in vascular endothelial cells and are associated with faster wound healing when inhibited. Meanwhile, healers showed upregulation of ECM-related genes like MMP2, which is linked to better ECM remodeling in chronic wounds. IL13 and IFNG were also noted as major upstream regulators.
Spatial Transcriptomics in Diabetic Wound Healing: Human Clinical Studies
Spatial transcriptomics is an -omic assay in situ that preserves the tissue structure while providing valuable information on tissue architecture, cell localization, and co-localization [28, 70, 71]. Theocharidis et al. [27] conducted a study analyzing skin specimens from healing and non-healing DFUs, through the Nanostring GeoMx DSP platform to spatially profile the transcriptome of wound healing (Fig. 3). An enrichment of a unique population of healing-associated fibroblasts overexpressing MMP1, MMP3, MMP11, HIF1A, CHI3L1, and TNFAIP6, and increased M1 macrophage polarization in the wound bed of DFU patients with healing wounds were identified (Fig. 3h, i). In addition, Spatial transcriptomics revealed a higher abundance of M1 macrophages in healers and M2 macrophages in non-healers (Fig. 3j, k). This study provided insights into the wound-healing micro-environment and identified cell types that could play a key role in promoting DFU healing, offering information about novel therapeutic approaches. In conclusion, spatial transcriptomics is a useful tool to elucidate the complex processes of tissue repair and regeneration during impaired wound healing, and it provides pertinent information on potential therapeutic targets [28].
Proteomics in Diabetic Wound Healing: Preclinical Animal and Human Clinical Studies
Proteomics, the comprehensive study of proteins and their functions, illuminates the underlying mechanisms of DFU pathogenesis and aids in the development of effective therapeutic strategies [72]. Proteomics hold much potential in assessing early disease diagnosis, prognosis, and monitoring the progression of the disease, alongside its contributions to the development of novel therapeutics for DFUs [73, 74]. Proteomics platforms, such as Luminex, mass spectrometry (MS), Olink, and Somagenics, provide powerful tools for protein analysis. Luminex uses bead-based multiplex assays to measure multiple proteins simultaneously with high sensitivity and reliability, effective for biomarker discovery in disease prognosis, diagnosis, and therapeutic applications in patients [75]. MS offers high-resolution protein identification and quantification, including the analysis of post-translational modifications [75]. Olink employs the proximity extension assay for high-throughput, precise protein detection and quantification, commonly used in clinical research [75, 76]. Somagenics focuses on protein biomarker analysis, leveraging technologies like protein micro-arrays for disease biomarker discovery.
Proteomic Studies for the Development of Biomarkers for Diabetic Wound Healing
Proteomic research has revealed various biomarkers that can predict DFU healing outcomes. These biomarkers could be used as early non-invasive clinical diagnostic tools, especially when clinical assessment is difficult, or as therapeutic targets for the wound healing of DFUs [72, 77]. In their study, Theocharidis et al. [11] used serum specimens from a large cohort of patients with DM and DFU who participated in a prospective study for 12 weeks, to identify proteins that could be used as prognostics for DFU healing using a Luminex Magpix apparatus and Millipore multiplex panels. The patients were seen bi-weekly and divided into healer and non-healer groups. Healers being those who healed their DFUs within the duration of the study, and non-healers as those who did not heal their DFUs within the study timeline. The authors identified a panel of proteins that could predict DFU closure, and IL-4, IL-5, IL-6, IL-13, and IFNγ were identified in relation to healing DFUs, while Fractalkine, IL-8, and TNFa were correlated with non-healing DFUs. They also highlighted IL-10 that could be used as a one-time measured, single biomarker for DFU prediction of successful healing given its consistent levels in healing patients. In another study, Zhao et al. [78] conducted a dynamic proteomic analysis of serum and skin samples, to identify biomarkers that promote the healing of DFUs. Four age-matched groups were screened for differential protein expression: normal subjects, diabetic patients, pretreatment DFU patients, and healed DFU patients. Serum and skin proteomics screening revealed that APOA1 plays a significant role in promoting DFU healing through its involvement in coagulation, inflammation, angiogenesis, and wound repair. These findings were also supported by the clinical case follow-up studies, which demonstrated decreased APOA1 levels in patients with more severe DFU.
Proteomic Studies for Investigating the Pathogenesis and Finding Therapeutic Targets in DFUs
Proteomics is pivotal in exploring the pathogenesis of DFUs by identifying and characterizing proteins related to inflammation, wound healing, and tissue regeneration. Detailed protein analysis enables the discovery of potential therapeutic targets, and new approaches to treat DFUs. Wang et al. [79] utilized tandem mass tag (TMT) labeled quantitative proteomics and network pharmacology analysis to evaluate the protein expression profiles on DFU and healthy tissues. More specifically, Kyoto Encyclopedia of Genes and Genomes enrichment analysis was carried out on differentially expressed proteins (DEPs) to identify the potential DFU-related pathways and mechanism of DFU wound healing. This study showed that Staphylococcus aureus infection and leukocyte transendothelial migration is mediated by 3 proteins: matrix metalloproteinase-9 (MMP-9), fatty acid-binding protein-5, and integrin subunit alpha M in DFU wound healing. Consequently, these proteins could be considered as potential therapeutic targets for DFU healing. In an alternative study, Nguyen et al. [80] used affinity resin coupled with proteomics analysis to validate the presence of MMP-9 and its potential use as an important target for inhibition for the treatment of DFUs. Debridement tissues from patients with DM and chronic wounds, dermal tissues from non-diabetic patients, and wound debridement samples from diabetic mice were collected. This study showed that human and mouse MMP-8 and MMP-9 homologs share 99% homology, suggesting similar mechanisms of pathology and repair of diabetic wounds between the two species. Finally, findings of this study indicated that (R)-ND-336 that was synthesized and examined as an inhibitor of the MMP-9 on db/db mice wounds displayed superior efficacy compared to becaplermin, an existing FDA-approved drug, and could be a potential treatment for DFUs. Yu et al. [81] conducted a study where they collected blood serum specimens from Patients with DFU and healthy controls. They utilized TMT method with liquid chromatography–mass spectrometry (LC–MS/MS) for quantitative proteomics to profile serum proteins in DFU patients, ultimately identifying several DEPs. The findings of this study were further validated using parallel reaction monitoring and enzyme-linked immunosorbent assay. The results revealed that the DEPs in the DFU group were primarily associated with potential mechanisms involved in DFU pathogenesis, such as ECM–receptor interaction, and complement and coagulation cascades. More specifically, they found upregulation of proteins such as LRG1, CD5L, CRP, IGHA1, and LBP in DFU patients, which are associated with ECM–receptor interactions and immune response. The novel data from this study have provided insights into DFU pathogenesis and identified potential therapeutic DFU targets among the differentially expressed serum proteins.
Proteomic Studies for Elucidating the Mechanism of Action of Treatments in Diabetic Wound Healing
Proteomics plays a considerable role in elucidating the mechanisms of action of novel treatments for DFUs. In their study, Mrozikiewicz-Rakowska et al. [82] performed a nonrandomized and nonblinded study with 47 patients with neuropathic DFUs; half of them received fibrin gels with adipose-derived stem cells (ADSCs) from a healthy donor and the other half received fibrin gel only. A control group of healthy individuals was assessed to validate the results. Scrapings from wound samples were collected and analyzed for the presence of ADSCs on the wounds at subsequent time points, followed by LC–MS/MS online UPLC and a QExative Orbitrap mass spectrometer proteomics analysis to detect molecular markers of healing in ADSC-modulated wounds at days 0 and 7. The authors identified 34 significant DEPs on ADSC groups after 7 days of the application. Overexpression of GAPDH, CAT, ACTN1, KRT1, KRT9, SCL4A1, and TPI proteins were correlated with the wound-healing rate of the patients. Among these proteins, immunomodulatory proteins (GAPDH and CAT) and molecular re-epithelization markers (regenerative processes related proteins specific to keratinocytes, KRT1 and KRT9, and directional migration of keratinocyte-associated protein ACTN1) were included. Subsequently, researchers performed pathway analysis with the Reactome platform and revealed that these processes could be linked to healing or play a supportive role in this process, such as antimicrobial peptides that modulate chronic inflammation by controlling the wound microbiome. These findings underscore the positive impact of ADSCs on DFU wound healing and provide insights into the mechanism of action of ADSCs as cell-based therapy for DFUs. Loretelli et al. [83] performed proteomic analysis using LC–MS/MS to explore the effects of the topical application of acellular embryonic stem cell extracts (EXTs) on wound healing in diabetic db/db mice. Previous studies showed that EXTs retain the typical immunoregulatory and anti-inflammatory properties of intact stem cells. The proteomic analysis combined with immunological screening revealed that APEX1 is an overrepresented protein in EXTs and plays a key role in their immunological effect. This research highlighted the role of EXTs in promoting re-epithelialization, contraction, and neo-angiogenesis, and wound healing in preclinical db/db mouse. In another study, Liu et al. [74] conducted a multi-omics analysis, including proteomics, transcriptomics, and molecular docking, to assess the effect of β-sitosterol on diabetic wound healing and to elucidate its molecular mechanism of action in rats. Transcriptomics and proteomics analyses revealed that β-sitosterol treatment activated the MAPK, mTOR, and VEGF pathways, leading to improved wound healing in a rat model. They also showed that β-sitosterol enhances alternatively activated macrophages (M2), macrophage proliferation, angiogenesis, and collagen synthesis in diabetic wounds. Ultimately, they revealed that β-sitosterol could be a promising therapeutic option for DFUs and may reduce amputation rates.
Macro-Proteomics and Drug Resistance in DFUs
Proteomics is a particularly useful tool in addressing bacterial drug resistance in DFUs. Sun et al. [84] employed macro-proteomics and MALDI TOF MS to analyze bacterial strains and their resistance profiles in DFU samples. Their findings could be used as an early-stage diagnosis tool of DFUs in clinical settings and aid the selection of appropriate antibiotics. Foremost, 16 necrotic specimens were collected and used for bacterial cultures. In subsequent steps, they utilized the MALDI TOF analysis to reveal 5 strains of Gram-positive bacteria that consisted of Staphylococcus aureus and 11 strains of Gram-negative bacteria consisting of bacillus. Using macro-proteomics analysis, the authors detected biomarkers related to antibiotic resistance and created the bacterial resistance fingerprint for 4 different antibiotics: ceftazidime, piperacillin, levofloxacin, and tetracycline.
Overall, proteomic research provides insights into the molecular mechanisms involved in DFU pathogenesis and healing. The development and validation of biomarkers and therapeutic targets, along with insights into the mechanisms of novel treatments, could improve DFU management and lead to the development of effective therapeutic strategies in clinic.
Conclusion
By leveraging advanced omics technologies, including bulk RNA-seq, scRNA-seq, spatial transcriptomics, and proteomics, the pathophysiology of DFUs has been better understood. Various studies have shown that particular phenotypes of macrophages play a critical role in the progression of diabetic wound healing. M1 classically activated macrophages, which promote inflammation, and M2 alternatively activated macrophages, which have anti-inflammatory properties, are key players in this process. Other cells that were found to be crucial for the outcome of wound healing are fibroblasts. More specifically, a unique subpopulation of fibroblasts, called healing fibroblasts, was identified in DFU healing patients, which showed increased expression of genes associated with matrix degradation, hypoxia response, and inflammation. Additionally, several studies have demonstrated differential gene expression of various matrix metalloproteinases, including MMP2, between DFU healing and non-healing patients. The omics technologies also provide insights into the mechanisms by which new therapeutics and biomaterials function, enabling their optimization to effectively promote diabetic wound healing. At the same time, omics technics helps to identify new therapeutic targets, allowing for the design of novel drugs and their delivery via cutting-edge biomaterials, thus enabling more efficient and personalized treatments.
Acknowledgements
The authors would like to acknowledge the Rongxiang Xu Center for Regenerative therapeutics at Beth Israel Medical Center.
Authors Contribution
Eleftheria Angeliki Valsami, and Aristidis Veves conceived and designed the review. Eleftheria Angeliki Valsami conducted the literature search, analyzed relevant studies, and drafted the manuscript. Eleftheria Angeliki Valsami, Guangyu Chu, Ming Guan, Jessica Gilman, Georgios Theocharidis, and Aristidis Veves contributed to the interpretation of the literature and provided critical revisions. All authors reviewed and approved the final manuscript.
Funding
This work was supported by the by National Institutes of Health (NIH) grant R01DK136699 (Aristidis Veves). Aristidis Veves received funding from the National Rongxiang Xu Foundation.
Declarations
Conflict of Interest
Guangyu Chu, Ming Guan, Jessica Gilman, and Georgios Theocharidis have nothing to disclose. Eleftheria Angeliki Valsami's current affiliation is with Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, MA 02114, USA. Aristidis Veves is a Section Editor of Advances in Therapy. Aristidis Veves was not involved in the selection of peer reviewers for the manuscript nor any of the editorial subsequent decisions.
Ethical Approval
This article is based on previously conducted studies and does not include any new studies involving human participants or animals performed by the authors.
References
- 1.Zhang Y, et al. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care. 2020;43(5):964–74. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, et al. Diabetic Foot Ulcers: A Review. JAMA. 2023;330(1):62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376(24):2367–75. [DOI] [PubMed] [Google Scholar]
- 4.McDermott K, et al. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care. 2023;46(1):209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong DG, et al. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice JB, et al. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651–8. [DOI] [PubMed] [Google Scholar]
- 7.Sumpio BJ, et al. Future Directions in Research in Transcriptomics in the Healing of Diabetic Foot Ulcers. Adv Ther. 2023;40(1):67–75. [DOI] [PubMed] [Google Scholar]
- 8.Wieman, T.J., J.M. Smiell, and Y. Su, Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care, 1998. 21(5): p. 822–7. [DOI] [PubMed]
- 9.Veves A, et al. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24(2):290–5. [DOI] [PubMed] [Google Scholar]
- 10.Marston WA, et al. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26(6):1701–5. [DOI] [PubMed] [Google Scholar]
- 11.Theocharidis, G., et al., Use of Serum Protein Measurements as Biomarkers that Can Predict the Outcome of Diabetic Foot Ulceration. Adv Wound Care (New Rochelle), 2024. [DOI] [PubMed]
- 12.Driver VR, et al. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 2015;23(6):891–900. [DOI] [PubMed] [Google Scholar]
- 13.Las Heras K, et al. Modulating the immune system towards a functional chronic wound healing: A biomaterials and Nanomedicine perspective. Adv Drug Deliv Rev. 2024;210: 115342. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, et al. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev. 2019;146:267–88. [DOI] [PubMed] [Google Scholar]
- 15.Berthiaume F, Hsia HC. Regenerative Approaches for Chronic Wounds. Annu Rev Biomed Eng. 2022;24:61–83. [DOI] [PubMed] [Google Scholar]
- 16.Jiang P, et al. Current status and progress in research on dressing management for diabetic foot ulcer. Front Endocrinol (Lausanne). 2023;14:1221705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastar, I., et al., Molecular Pathophysiology of Chronic Wounds: Current State and Future Directions. Cold Spring Harb Perspect Biol, 2023. 15(4). [DOI] [PMC free article] [PubMed]
- 18.Wang S, et al. Accelerating diabetic wound healing by ROS-scavenging lipid nanoparticle-mRNA formulation. Proc Natl Acad Sci U S A. 2024;121(22): e2322935121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maschalidi S, et al. Targeting SLC7A11 improves efferocytosis by dendritic cells and wound healing in diabetes. Nature. 2022;606(7915):776–84. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, et al. Proteomics and transcriptomics explore the effect of mixture of herbal extract on diabetic wound healing process. Phytomedicine. 2023;116: 154892. [DOI] [PubMed] [Google Scholar]
- 21.Deng P, et al. Combined metabolomics and network pharmacology to elucidate the mechanisms of Dracorhodin Perchlorate in treating diabetic foot ulcer rats. Front Pharmacol. 2022;13:1038656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Amrani S, et al. Proteomics: Concepts and applications in human medicine. World J Biol Chem. 2021;12(5):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Wang CY. From bulk, single-cell to spatial RNA sequencing. Int J Oral Sci. 2021;13(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theocharidis G, et al. Single-cell transcriptomics in human skin research: available technologies, technical considerations and disease applications. Exp Dermatol. 2022;31(5):655–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitley SK, Horne WT, Kolls JK. Research Techniques Made Simple: Methodology and Clinical Applications of RNA Sequencing. J Invest Dermatol. 2016;136(8):e77–82. [DOI] [PubMed] [Google Scholar]
- 26.Houser, A.E., et al., The Use of Single-Cell RNA-Sequencing and Spatial Transcriptomics in Understanding the Pathogenesis and Treatment of Skin Diseases. JID Innovations, 2023. 3(4). [DOI] [PMC free article] [PubMed]
- 27.Theocharidis G, et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nat Commun. 2022;13(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pita-Juarez YH, et al. High Content Single Cell and Spatial Tissue Profiling Modalities for Deciphering the Pathogenesis and Treatment of Wound Healing. In: Veves A, Giurini JM, Schermerhorn ML, editors., et al., The Diabetic Foot: Medical and Surgical Management. Cham: Springer; 2024. p. 199–218. [Google Scholar]
- 29.Hrdlickova, R., M. Toloue, and B. Tian, RNA-Seq methods for transcriptome analysis. Wiley Interdiscip Rev RNA, 2017. 8(1). [DOI] [PMC free article] [PubMed]
- 30.Costa-Silva J, Domingues D, Lopes FM. RNA-Seq differential expression analysis: An extended review and a software tool. PLoS ONE. 2017;12(12): e0190152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jovic D, et al. Single-cell RNA sequencing technologies and applications: A brief overview. Clin Transl Med. 2022;12(3): e694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams CG, et al. An introduction to spatial transcriptomics for biomedical research. Genome Medicine. 2022;14(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.See P, et al. A Single-Cell Sequencing Guide for Immunologists. Front Immunol. 2018;9:2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macosko EZ, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161(5):1202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9): 200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egozi EI, et al. Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regen. 2003;11(1):46–54. [DOI] [PubMed] [Google Scholar]
- 38.van der Vliet A, Janssen-Heininger YM. Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger? J Cell Biochem. 2014;115(3):427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theocharidis G, et al. Murine macrophages or their secretome delivered in alginate dressings enhance impaired wound healing in diabetic mice. Biomaterials. 2022;288: 121692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sindrilaru A, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theocharidis G, et al. Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes. 2020;69(10):2157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pang J, Maienschein-Cline M, Koh TJ. Enhanced Proliferation of Ly6C(+) Monocytes/Macrophages Contributes to Chronic Inflammation in Skin Wounds of Diabetic Mice. J Immunol. 2021;206(3):621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, et al. Characterization of the micro-environment of diabetic foot ulcers and potential drug identification based on scRNA-seq. Front Endocrinol (Lausanne). 2022;13: 997880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keogh, R.A., et al., Group B Streptococcus adaptation promotes survival in a hyperinflammatory diabetic wound environment. Sci Adv, 2022. 8(45): p. eadd3221. [DOI] [PMC free article] [PubMed]
- 45.Theocharidis G, et al. A strain-programmed patch for the healing of diabetic wounds. Nat Biomed Eng. 2022;6(10):1118–33. [DOI] [PubMed] [Google Scholar]
- 46.Januszyk, M., et al., Characterization of Diabetic and Non-Diabetic Foot Ulcers Using Single-Cell RNA-Sequencing. Micromachines (Basel), 2020. 11(9). [DOI] [PMC free article] [PubMed]
- 47.Cheng Y, et al. Identification of potential immunologic resilience in the healing process of diabetic foot ulcers. Int Wound J. 2024;21(3): e14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, et al. Single-cell RNA-Seq analysis of diabetic wound macrophages in STZ-induced mice. J Cell Commun Signal. 2023;17(1):103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Audu CO, et al. Macrophage-specific inhibition of the histone demethylase JMJD3 decreases STING and pathologic inflammation in diabetic wound repair. Cell Mol Immunol. 2022;19(11):1251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, et al. Role of M1 macrophages in diabetic foot ulcers and related immune regulatory mechanisms. Front Pharmacol. 2022;13:1098041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, et al. Identification of CGNL1 as a diagnostic marker in fibroblasts of diabetic foot ulcers: Insights from single cell RNA sequencing and bulk sequencing data. Int J Immunopathol Pharmacol. 2024;38:3946320241265945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan L, Qu J, Wang J. Development of novel lysosome-related signatures and their potential target drugs based on bulk RNA-seq and scRNA-seq for diabetic foot ulcers. Hum Genomics. 2024;18(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu GT, et al. Mapping cellular senescence networks in human diabetic foot ulcers. Geroscience. 2024;46(1):1071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi WJ, et al. Analyzing Immune Cell Infiltration and Copper Metabolism in Diabetic Foot Ulcers. J Inflamm Res. 2024;17:3143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu XY, et al. Effect of activated autologous monocytes/macrophages on wound healing in a rodent model of experimental diabetes. Diabetes Res Clin Pract. 2013;102(1):53–9. [DOI] [PubMed] [Google Scholar]
- 57.Maruyama K, et al. Decreased Macrophage Number and Activation Lead to Reduced Lymphatic Vessel Formation and Contribute to Impaired Diabetic Wound Healing. Am J Pathol. 2007;170(4):1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miao M, et al. Diabetes-impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen. 2012;20(2):203–13. [DOI] [PubMed] [Google Scholar]
- 59.Danon D, et al. Treatment of human ulcers by application of macrophages prepared from a blood unit. Exp Gerontol. 1997;32(6):633–41. [DOI] [PubMed] [Google Scholar]
- 60.Singh, K., et al., Angiogenin Released from ABCB5(+) Stromal Precursors Improves Healing of Diabetic Wounds by Promoting Angiogenesis. J Invest Dermatol, 2022. 142(6): p. 1725–1736 e10. [DOI] [PMC free article] [PubMed]
- 61.Wang L, et al. Activation of the Wnt/β-catenin signalling pathway enhances exosome production by hucMSCs and improves their capability to promote diabetic wound healing. J Nanobiotechnology. 2024;22(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Escuin-Ordinas H, et al. Wound healing with topical BRAF inhibitor therapy in a diabetic model suggests tissue regenerative effects. PLoS ONE. 2021;16(6): e0252597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, et al. Single-Cell Analysis Reveals Cxcl14(+) Fibroblast Accumulation in Regenerating Diabetic Wounds Treated by Hydrogel-Delivering Carbon Monoxide. ACS Cent Sci. 2024;10(1):184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q, et al. MiR146a-loaded engineered exosomes released from silk fibroin patch promote diabetic wound healing by targeting IRAK1. Signal Transduct Target Ther. 2023;8(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng T, et al. A natural biological adhesive from snail mucus for wound repair. Nat Commun. 2023;14(1):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu Y, et al. Single-cell profiling reveals transcriptomic signatures of vascular endothelial cells in non-healing diabetic foot ulcers. Front Endocrinol (Lausanne). 2023;14:1275612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du, H., et al., Single-cell RNA-seq and bulk-seq identify RAB17 as a potential regulator of angiogenesis by human dermal microvascular endothelial cells in diabetic foot ulcers. Burns Trauma, 2023. 11: p. tkad020. [DOI] [PMC free article] [PubMed]
- 68.Park S, et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat Cell Biol. 2017;19(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cundell, J., Chapter Two - Diabetic Foot Ulcers: Assessment, Treatment, and Management, in Smart Bandage Technologies, J. Davis, et al., Editors. 2016, Academic Press. p. 37–61.
- 70.He S, et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat Biotechnol. 2022;40(12):1794–806. [DOI] [PubMed] [Google Scholar]
- 71.Janesick A, et al. High resolution mapping of the tumor micro-environment using integrated single-cell, spatial and in situ analysis. Nat Commun. 2023;14(1):8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stolzenburg-Veeser L, Golubnitschaja O. Mini-encyclopaedia of the wound healing - Opportunities for integrating multi-omic approaches into medical practice. J Proteomics. 2018;188:71–84. [DOI] [PubMed] [Google Scholar]
- 73.Domon B, Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312(5771):212–7. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, et al. Discovery of β-sitosterol’s effects on molecular changes in rat diabetic wounds and its impact on angiogenesis and macrophages. Int Immunopharmacol. 2024;126: 111283. [DOI] [PubMed] [Google Scholar]
- 75.Cui M, Cheng C, Zhang L. High-throughput proteomics: a methodological mini-review. Lab Invest. 2022;102(11):1170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, et al. Methods and clinical biomarker discovery for targeted proteomics using Olink technology. Proteomics Clin Appl. 2024;18(5): e2300233. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, et al. An update on potential biomarkers for diagnosing diabetic foot ulcer at early stage. Biomed Pharmacother. 2021;133: 110991. [DOI] [PubMed] [Google Scholar]
- 78.Zhao F, et al. Combined with dynamic serum proteomics and clinical follow-up to screen the serum proteins to promote the healing of diabetic foot ulcer. Endocrine. 2024;84(2):365–79. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, et al. Proteomic analysis of foot ulcer tissue reveals novel potential therapeutic targets of wound healing in diabetic foot ulcers. Comput Biol Med. 2023;159: 106858. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen TT, et al. Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing. J Med Chem. 2018;61(19):8825–37. [DOI] [PubMed] [Google Scholar]
- 81.Yu XT, et al. Tandem mass tag-based serum proteomic profiling revealed diabetic foot ulcer pathogenesis and potential therapeutic targets. Bioengineered. 2022;13(2):3171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mrozikiewicz-Rakowska, B., et al., Allogenic Adipose-Derived Stem Cells in Diabetic Foot Ulcer Treatment: Clinical Effectiveness, Safety, Survival in the Wound Site, and Proteomic Impact. Int J Mol Sci, 2023. 24(2). [DOI] [PMC free article] [PubMed]
- 83.Loretelli C, et al. Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetol. 2020;57(7):883–90. [DOI] [PubMed] [Google Scholar]
- 84.Sun H, et al. MALDI-TOF MS Based Bacterial Antibiotics Resistance Finger Print for Diabetic Pedopathy. Front Chem. 2021;9: 785848. [DOI] [PMC free article] [PubMed] [Google Scholar]