Abstract
Background
The 2016 National Institute for Health and Care Excellence guidelines recommended the tuberculin skin test (TST), at a 5-mm induration size cut-off, for the diagnosis of latent tuberculosis infection (LTBI) among adult close contacts of active tuberculosis (TB) cases. This study analysed a well-characterised cohort of adult close contacts in London and assessed the cost-effectiveness of LTBI screening strategies with combinations of TST and interferon-γ release assays (IGRAs) in a decision-analytic model.
Methods
Close contacts of pulmonary TB cases who were tested with TST and IGRA between January 2008 and December 2010 were retrospectively reviewed. Using an NHS perspective and lifetime horizon, a decision-analytic Markov model was used to compare costs and quality-adjusted life-years (QALYs) associated with five screening strategies followed by LTBI treatment: 1) TST alone; 2) QuantiFERON-TB Gold In-Tube (QFT) alone; 3) T-SPOT.TB (T-SPOT) alone; 4) TST positive followed by QFT; 5) TST positive followed by T-SPOT.
Results
This study included 381 asymptomatic close contacts aged 18 to 65 years (mean±sd 35.2±11.3). 75.3% had received BCG vaccination. Among the five strategies, for a willingness-to-pay threshold of GBP 25 000 and using incremental net monetary benefit (INMB) with TST as comparator, the IGRA-alone strategies were the most cost-effective, marginally QFT over T-SPOT (QFT: GBP 214; T-SPOT: GBP 199).
Conclusion
Single-step IGRA, particularly QuantiFERON, is preferable for LTBI screening of adult close contacts of pulmonary TB cases.
Shareable abstract
A decision-analytic Markov model was used to compare costs and quality-adjusted life-years associated with five screening strategies for close contacts of pulmonary TB cases in London and found IGRA-alone strategies were the most cost-effective https://bit.ly/4imoUpM
Introduction
Tuberculosis (TB) remains one of the top 10 causes of death worldwide. The estimated number of deaths from TB increased between 2019 and 2021, reversing years of decline between 2005 and 2019 [1]. England is classified as a low incidence country with a rate of 7.3 per 100 000 [2]. However, there is considerable variation in the TB incidence across England with the main burden concentrated in large urban areas. The TB incidence rate is particularly high in London, and the rate in 2012 was the highest among all high-income European countries [3].
Latent TB infection (LTBI) identification and treatment are imperative to achieve a significant reduction in TB deaths and incidence rates globally. However, there is no gold standard for LTBI diagnosis, and the identification of LTBI can only be indicated by indirect approaches which confirm the immunological sensitisation of the individual to TB antigens, such as the tuberculin skin test (TST) and interferon-γ release assay (IGRA) [4]. Defining a close contact as living in the same household or in frequent contact with a source case, UK National Institute for Health and Care Excellence (NICE) guidelines, updated in 2019, recommend that close contacts of pulmonary TB aged 18 to 65 years should be tested by TST, and that a confirmatory IGRA after positive TST can be considered if further evidence is needed to start LTBI treatment [5]. A positive TST is defined as a 5-mm threshold regardless of BCG vaccination status in the updated guidelines, whereas a 6-mm threshold for non-BCG-vaccinated populations and a 15-mm threshold for BCG-vaccinated populations was previously recommended. These updated guidelines could increase the number of unnecessary TSTs and false-positive cases, particularly in urban areas with high TB incidences as the specificity of TST is low in BCG-vaccinated populations [6].
As healthcare resources are scarce even in high-income countries, the cost-effectiveness of LTBI screening has been reported in many countries. Previous health economic analyses of LTBI screening indicated significant differences in the type of analysis, time horizon, outcome measures and modelling methods between the included studies, which resulted in a lack of clarity about the most cost-effective approach for LTBI screening [7]. Though previously assessed in children [8], there is limited evidence about the cost-effectiveness of LTBI screening of adult close contacts in the UK, and it is possible that the screening strategies recommended by the national guidelines might not be optimal in the urban areas with a higher TB incidence, such as London [2, 3].
Our aim was to determine the most cost-effective strategy for LTBI screening of adult close contacts in a London centre. The objectives were to analyse the characteristics and screening test results of adult close contacts in London following the 2016 NICE guidelines and to assess the cost-effectiveness of LTBI screening strategies with various combinations of TST and IGRAs in a decision-analytic model using a cohort of clinical data.
Methods
The study was conducted at the TB clinic in St Mary's Hospital, Imperial College Healthcare NHS Trust, which is a major acute hospital for Northwest London. Ethical approval was not sought as we utilised fully anonymised data that were collected as part of routine delivery of clinical services.
Clinical audit data of all close contacts of active TB cases who visited the TB clinic between January 2008 and December 2010 were retrospectively reviewed. In accordance with the 2016 NICE guidelines, asymptomatic close contacts of pulmonary TB cases aged between 18 and 65 years who were offered both TST and IGRA were included in the data review. A TST induration of at least 5-mm was considered a positive result regardless of BCG vaccination status. One of two commercially available IGRAs (QuantiFERON-TB Gold In-Tube (QFT) or T-SPOT) was performed and results defined as positive, negative or indeterminate depending on the manufacturer's criteria.
A health economic analysis was conducted to compare the cost-effectiveness of LTBI screening strategies with different combinations of TST and IGRAs for adult close contacts of pulmonary TB cases in London from the perspective of the National Health Service (NHS). There was no health economics plan developed before conducting the study. Decision trees and a Markov model were constructed and analysed using Microsoft (MS) Excel and the statistical programming language R (www.R-project.org/). A decision tree is a graphical model that encodes possible patient pathways and outcomes, including costs, health effects and probabilities. A Markov model simulates the progression of patients through different health states over time, accounting for transitions, costs and outcomes associated with each state, to evaluate long-term health interventions. By linking the two types of models we can represent the short-term diagnostic pathway and the subsequent lifetime process. Model assumptions of the analysis are presented in the supplementary material, including the separate decision trees in supplementary figures S1–S3. Posterior probability distributions were obtained using the clinical audit data in a Bayesian model using WinBUGS [9] called from R (see supplementary material Section 2). By applying Bayes’ theorem, the prior knowledge from literature and expert elicitation can be systematically combined with the clinical data to obtain updated estimates. Other model input parameter values used were obtained from the literature (see table 1). The CHEERS (Consolidated Health Economic Evaluation Reporting Standards) checklist [10] is a set of guidelines aimed at enhancing the quality of reporting in health economic evaluations. You can find the checklist in the supplementary material. All model codes and data are publicly available at https://github.com/n8thangreen/LTBIdiagTST.
TABLE 1.
Model parameter values for the decision trees and Markov model
| Name | Label | Base-case value | Range | Reference |
|---|---|---|---|---|
| Probability | ||||
| Probability of accepting LTBI treatment | pAccept_chemo | 0.95 | 0.5–1.0 | [8, 11] |
| Probability of developing hepatitis due to LTBI treatment | pHep | 0.002 | 0.001–0.003 | [11] |
| Probability of completing LTBI treatment | pComp_chemo | 0.8 | 0.5–0.9 | [12] |
| Efficacy of complete LTBI treatment | Eff_comp | 0.65 | 0.5–0.8 | [11] |
| Efficacy of incomplete LTBI treatment | Eff_incomp | 0.21 | 0.1–0.3 | [11] |
| Sensitivity of TST | TST_sens | 0.79 | 0.69–0.89 | [13] |
| Specificity of TST | TST_spec | 0.59 | 0.46–0.73 | [13] |
| Sensitivity of QFT | QFT_sens | 0.886 | 0.812–0.944 | [14] |
| Specificity of QFT | QFT_spec | 0.995 | 0.959–1 | [14] |
| Sensitivity of T-SPOT | TSPOT_sens | 0.872 | 0.643–0.991 | [14] |
| Specificity of T-SPOT | TSPOT_spec | 0.998 | 0.996–1 | [14] |
| Lifetime risk of TB re-activation (for individual aged 35 years) | pTB | 0.12 | 0.08–0.19 | [15] |
| Annual probability of TB re-activation | ||||
| Without treatment | pReact | 0.0072 | [16] | |
| Partial treatment | pReact_incomp | 0.0059 | [11] | |
| Completed treatment | pReact_compl | 0.0026 | [11] | |
| Age-specific annual probability of death for TB active patients % | pDeath_tb | |||
| 15–44 | 0.02 | [17] | ||
| 45–64 | 0.087 | |||
| >64 | 0.31 | |||
| Cost, GBP | ||||
| TST# |

|
25.02 | 12.51–50.05 | [18] |
| QFT (including phlebotomy)# |
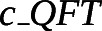
|
29.11 | 15.16–55.67 | Imperial College NHS Trust [19] |
| T-SPOT (including phlebotomy)# |

|
43.17 | 22.21–83.87 | Imperial College NHS Trust [19] |
| TB nurse appointment (Band 6) |

|
53 | 43–63 | [20] |
| Outpatient consultation (first visit; WF02B) |

|
224 | [21] | |
| Outpatient consultation (follow-up visit; WF02A) |

|
166 | [21] | |
| Chest radiograph (service code 812) |

|
46 | 40–52 | [19] |
| Liver function tests# |

|
3.95 | 2.63–5.27 | [19] |
| TB treatment# |

|
6055 | 3028–12 110 | [5] |
| Hepatitis treatment# |

|
984 | 492–1968 | [18] |
| Utility | ||||
| Age-specific utilities for normal health years | ||||
| <25 | 0.94 | [22] | ||
| 25–34 | 0.93 | |||
| 35–44 | 0.91 | |||
| 45–54 | 0.85 | |||
| 55–64 | 0.8 | |||
| 65–74 | 0.78 | |||
| >74 | 0.73 | |||
| Utility loss | ||||
| LTBI treatment (complete) | 0.001 | [8, 23] | ||
| Hepatitis B (per year) | 0.14 | [24] | ||
| Active TB | 0.15 | [8, 23] |
LTBI: latent tuberculosis infection; TST: tuberculin skin test; QFT: QuantiFERON-TB Gold In-Tube; T-SPOT: T-SPOT.TB assay; TB: tuberculosis. #: inflated to 2024 value.
Five screening strategies were considered based on the combination of TST and IGRAs: “TST alone”, “QFT alone”, “T-SPOT alone”, “TST positive followed by QFT (TST/QFT)” and “TST positive followed by T-SPOT (TST/T-SPOT)”. The TST-alone strategy was used as the reference in line with the current UK NICE guidelines [5]. For those who have positive screening results without active TB disease, 3 months of LTBI treatment with daily isoniazid (INH) and rifampicin (RFP) was offered [25]. Decision trees were used to represent the LTBI screening and treatment stage. As there is no gold standard for LTBI diagnosis, the prevalence of LTBI in the model population was estimated based on the clinical data in the TB clinic (supplementary table S2).
After the LTBI screening and treatment stage, a Markov model was used to simulate the whole cohort to death. Six exhaustive and mutually exclusive Markov states were defined (see figure 1): 1) Active TB, 2) LTBI with complete treatment, 3) LTBI with incomplete treatment, 4) LTBI without treatment, 5) No LTBI and 6) Death. The cycle of the Markov model was 1 year. Cycles continue until time of death to capture all quality-adjusted life-years (QALYs) and costs. Lifetimes were constrained to no more than 100 years old. We assumed that there would be no drug-resistant TB case in the model population and all of the individuals with active TB would complete the standard regimen of the intensive phase with INH, RFP, pyrazinamide (PZA) and ethambutol (EB) for 2 months followed by the continuation phase with INH and RFP for 4 months. Transition probabilities in the decision trees and Markov model were calculated using relevant literature and the clinical data.
FIGURE 1.
Schematic of the simplified screening decision tree and the population-level discrete time Markov model. States with bold lines represent starting states. TST: tuberculin skin test; QFT: QuantiFERON-TB Gold In-Tube, T-SPOT: T-SPOT.TB assay; TST/QFT: TST positive followed by QFT; TST/T-SPOT: TST positive followed by T-SPOT; LTBI: latent tuberculosis infection; TB: tuberculosis; Tx: treatment.
In the model, only direct costs incurred by the NHS were included [5, 11, 18, 20, 21, 26]. All costs were uplifted to 2024 pound sterling using current annual Consumer Prices Index [27]. Age-specific utilities of normal health states were obtained from the literature [22]. The utility losses by the treatments of LTBI, hepatitis and active TB were fixed during the time horizon and obtained from the literature [8, 23]. Future costs and QALYs were discounted at 3.5% per year as per NICE recommendations (NICE CHTE Methods review discounting). A baseline willingness-to-pay threshold of GBP 25 000 per QALY was used [28]. Net monetary benefit (NMB) calculations were compared to determine the most cost-effective strategy. Probabilistic sensitivity analysis used posterior draws from the Bayesian model. One-way deterministic sensitivity analysis evaluated the impacts of the input parameters on the NMBs varying each parameter within the ranges as shown in table 1. Results are shown in tornado plots where each bar represents the impact of a single input parameter on the INMB. Parameters are ordered from largest to smallest impact. Thus, this highlights which parameters have the most significant impact on the INMB, making it easier to understand model uncertainty [29].
Results
Clinical data review
Overall, 381 asymptomatic close contacts of pulmonary TB cases were analysed. The mean±sd age was 35.2±11.3 years, and 200 participants (52.5%) were male. Among all participants, 179 (47.0%) were categorised as White ethnic, 132 (34.6%) UK born and 287 (75.3%) were BCG-vaccinated (see table 2). Of the 381 participants who were offered both TST and IGRA, 374 (98.2%) accepted TSTs and 366 (97.9%) of those injected attended the TST reading. Further, 378 out of 381 participants (99.2%) accepted the IGRAs. Among 204 participants with positive TST results, 203 (99.5%) accepted the IGRAs. Among the 203 participants, 78 (38.4%) had positive IGRA results. Among 162 participants with negative TST results, 161 (99.4%) accepted the IGRAs. Among the 161 participants, 9 (5.6%) had positive IGRA results. The full set of test results are presented in figure 2.
TABLE 2.
Baseline characteristics of the sample of adult close contacts of pulmonary TB cases
| Description | Value |
|---|---|
| Sample size, n | 381 |
| Age years, mean±sd | 35.2±11.3 |
| Sex, n (%) | |
| Male | 200 (52.5) |
| Female | 181 (47.5) |
| Ethnicity, n (%) | |
| White | 179 (47.0) |
| Black | 108 (28.3) |
| South Asian | 35 (9.2) |
| Other ethnic groups or unknown | 59 (15.5) |
| Country of birth, n (%) | |
| UK | 132 (34.6) |
| Other | 249 (65.4) |
| BCG, n (%) | |
| Vaccinated | 287 (75.3) |
| Unvaccinated | 94 (24.7) |
TB: tuberculosis; BCG: Bacillus Calmette-Guérin.
FIGURE 2.
Screening test results of sample of adult close contacts in London. IGRA: interferon-γ release assay; TST: tuberculin skin test.
Health economic analysis
The model population's age at the start of the simulation was set to 35 years old, based on the clinical cohort's mean age. The estimated prevalence of LTBI was 23% in the base-case analysis (supplementary figure S7). All other transition probabilities in the decision trees and Markov model are presented in supplementary table S1. Table 3 shows total costs, total QALYs and NMBs in the model cohort of 1000 close contacts in the base-case analysis. The QFT-alone strategy was the least costly with GBP 277.05 per person, whereas the TST-alone strategy was the most costly with GBP 451.52. The TST followed by IGRA strategies were the least effective with the lowest QALYS but in fact the QALYs between study were very similar. Among the five strategies, the two IGRA-alone strategies were the most cost-effective with the highest NMB of GBP 213.9 and GBP 198.64 for QFT and T-SPOT, respectively, and at the cost-effectiveness threshold of GBP 25 000 per QALY in the base-case analysis. Figure 3 shows the corresponding cost-effectiveness plane, which shows the difference in costs and QALYs against the TST-alone baseline scenario.
TABLE 3.
Cost-effectiveness results of LTBI screening strategies per adult close contacts in London
| Strategy | QALY (95% CI) |
Cost, GBP (95% CI) |
ΔQALY | ΔCost GBP | ICER (GBP/QALY) |
INMB, GBP |
|---|---|---|---|---|---|---|
| TST alone (reference) | 18.726 (18.722–18.728) |
451.52 (425.37–486.1) |
- | - | - | - |
| QFT alone | 18.727 (18.724–18.73) |
277.05 (251.82–307.74) |
0.001577 | −174.473 | −110 641 | 213.9 |
| T-SPOT alone | 18.727 (18.724–18.73) |
289.93 (263.55–317.49) |
0.001482 | −161.595 | −109 053 | 198.64 |
| TST/QFT | 18.725 (18.722–18.727) |
332.55 (299.87–361.56) |
−0.00094 | −118.971 | 126 611.2 | 95.48 |
| TST/T-SPOT | 18.724 (18.722–18.727) |
342.71 (312.04–367.23) |
−0.00118 | −108.81 | 92 421.06 | 79.38 |
NMBs were calculated at the cost-effectiveness threshold of GBP 25 000 per QALY. LTBI: latent tuberculosis infection; QALY: quality-adjusted life-year; ICER: incremental cost-effectiveness ratio; NMB: net monetary benefit; TST: tuberculin skin test; QFT: QuantiFERON-TB Gold In-Tube; T-SPOT: T-SPOT.TB assay; TST/QFT: TST positive followed by QFT; TST/T-SPOT: TST positive followed by T-SPOT.
FIGURE 3.

Cost-effectiveness plane of LTBI screening strategies per adult close contacts in London. TST: tuberculin skin test; QFT: QuantiFERON-TB Gold In-Tube; T-SPOT: T-SPOT.TB assay; TST/QFT: TST positive followed by QFT; TST/T-SPOT: TST positive followed by T-SPOT.
The deterministic sensitivity analysis results are presented in tornado plots in supplementary figures S5 and S6. They showed that re-activation probability, test costs and test performance are factors affecting cost-effectiveness but no input parameter was sensitive to the superiority of the IGRA-alone strategies.
Discussion
In the main analysis, we estimated the total costs and QALYs of five strategies, with different combinations of TST and IGRAs, in the lifetime periods of close contacts aged 35 years old at the time of entering the model. Our results suggest that T-SPOT or QFT alone was the best strategy for LTBI screening of adult close contacts in terms of the cost-effectiveness, although the 2016 NICE guidelines recommend TST-based strategies [5].
Two-stage testing may be justified to avoid unnecessary LTBI treatment particularly for individuals with risk of complications due to the treatment. It has been reported that the risk of progression to active TB was significantly higher in individuals with positive IGRA results than in those with positive TST results [30]. This could justify the confirmatory IGRA after positive TST. A cost–utility analysis of LTBI screening in the USA, in which only individuals with positive TST results were included, reported that confirmatory QFT was cost-effective [31]. In Germany, it was shown that TST positive at a 5-mm threshold followed by QFT was the most cost-effective, in close contacts aged 20 years [32]. However, the estimated prevalence of LTBI in this study was 11%, which was significantly lower than our study estimate. In the UK and within a similar population, Pooran et al. [18] reported that TST positive followed by IGRA strategy was more cost-effective for LTBI screening of close contacts than both TST-alone and IGRA-alone strategies. However, the outcome measure was the number of active TB cases prevented rather than QALY and the time horizon was only 2 years.
Reasons for our results are that the TST-alone pathway demonstrates relatively poor performance, yielding a high number of false positives and incurring additional costs due to the requirement of two clinical visits [5]. Given the relatively low prevalence, even in London where this study is based [3], overdiagnosis emerges as a significant concern, particularly in light of the comparatively low rates of progression to active TB [16].
Even though healthcare systems vary from country to country, many other studies in high-income countries reported the superiority of single-step IGRA strategies. Over a 20-year time horizon, T-SPOT alone was the most cost-effective strategy for 20-year-old and 40-year-old close contacts in Switzerland [33]. In Japan, Kowada et al. concluded that QFT was the most-cost-effective strategy in a model cohort with the age of 20 years over a lifetime horizon [12]. In Canada, QFT was an optimal strategy for close contacts aged 35 years [34].
In the clinical data review, two main findings were made. Firstly, the demographic patterns were highly heterogeneous in the cohort of adult close contacts in London. Almost half of the participants were categorised in the non-White ethnic groups, and only one third had been born in the UK. Considering high TB incidence rates in the non-UK-born populations and the non-White ethnic groups, the demographic results of the present study could suggest that there are more LTBI cases among adult close contacts in London than the rest of England [2].
Secondly, the majority of the participants were BCG-vaccinated in spite of the high proportion of non-UK-born participants, and only 38.4% of the participants with positive TST results had positive IGRA results. International studies have demonstrated a discordance between TST and IGRA results in BCG-vaccinated populations. A study in Saudi Arabia using a cohort with a high BCG vaccination rate indicated that among the participants who had positive TST results, at a 10-mm threshold, 54.8% had negative QFT results [35]. The 2016 NICE guidelines recommend that TST should be performed for LTBI screening of adult close contacts, and that individuals with the induration size of 5 mm or larger are considered positive regardless of BCG vaccination status [5]. The high level of discordance between the TST and IGRA results in our cohort, as well as the high BCG coverage, suggests that TST-alone based strategies may not be appropriate for LTBI screening of adult close contacts in London.
Our analysis was limited by the retrospective nature and the assumption of HIV negativity and fully drug-sensitive TB. The clinical data review was a retrospective analysis at a single institution; therefore, the selection of those who were offered the screening tests might have been biased [36]. In the health economic analysis, we did not consider HIV-infected individuals in the model cohort because the HIV status was not available in the clinical data review and a low and reducing background rate was assumed [37]. Also, we did not consider drug-resistant TB cases in the present study because the number and proportion of multidrug-resistant TB (MDR-TB) cases have been decreasing and the transmission of MDR-TB in the UK is still rare [2, 38]. We have had to make several assumptions and considered several model inputs that had been estimated in different populations and countries. However, the sensitivity analysis showed the robustness of the superiority of the IGRA-alone strategy among adult close contacts in London.
Future work should aim to confirm our findings and obtain sufficient data to enable subgroup analyses among patients who are not BCG vaccinated and with TST cut-off of 10 or 15 mm.
LTBI screening of adult close contacts is essential for TB control in London, which has the highest incidence in England. Considering the high BCG coverage and the high level of discordance between TST and IGRA results in our cohort, TST at a 5-mm threshold might not be appropriate for adult contact screening in London, contrary to the latest recommendation by NICE. Single-step IGRA is preferred with respect to cost-effectiveness according to the results of the present study. Further economic studies in other areas and populations in England are needed to evaluate whether this approach is also applicable to other regions and to allow usage of resources of the NHS most effectively.
Acknowledgements
The authors acknowledge staff members of Imperial College Healthcare NHS Trust, London for their efforts in data collection. We thank members of the immunology department Alison Cox and Panos Pantelides.
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: Study design and set up by N. Green, M. Hayama and M. O'Donoghue with input by O.M. Kon, S. Seneviratne and N. Drey. M. O'Donoghue collected and analysed the clinical data at St Mary's Hospital. N. Green and M. Hayama analysed the data and designed the economic model with input from K. Manalan and O.M. Kon. N. Green, K. Manalan and M. Hayama drafted the manuscript with input from all other authors. All authors approved the final version.
Conflict of interest: All authors have no conflict of interest.
Support statement: This study was undertaken at St Mary's Hospital, Imperial College Healthcare NHS Trust, and supported by the NIHR Biomedical Research Centre.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material
00818-2024.SUPPLEMENT
CHEERS 2022 checklist
00818-2024.SUPPLEMENT2
References
- 1.World Health Organization . Global tuberculosis report 2020. Date last accessed: 4 March. Date last updated: 15 October 2020. 2024. www.who.int/publications/i/item/9789240013131 [Google Scholar]
- 2.UK Health Security Agency . Tuberculosis in England: 2021 Report. London, UK Health Security Agency, 2021.
- 3.Kirby T. Tuberculosis rates unacceptably high in UK cities. Lancet Infect Dis 2013; 13: 836–837. doi: 10.1016/S1473-3099(13)70276-7 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) . Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep 2000; 49: 1–51. [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence (NICE) . NICE guideline NG33: Tuberculosis Prevention, diagnosis, management and service organisation. 2016. Date last updated: 16 February 2024. https://www.nice.org.uk/guidance/ng33/ [PubMed]
- 6.Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149: 177–184. doi: 10.7326/0003-4819-149-3-200808050-00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pai M, Riley LW, Colford JM. Interferon-γ assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004; 4: 761–776. doi: 10.1016/S1473-3099(04)01206-X [DOI] [PubMed] [Google Scholar]
- 8.Auguste P, Tsertsvadze A, Pink J, et al. Accurate diagnosis of latent tuberculosis in children, people who are immunocompromised or at risk from immunosuppression and recent arrivals from countries with a high incidence of tuberculosis: systematic review and economic evaluation. Health Technol Assess (Rockv) 2016; 20: 1–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunn DJ, Thomas A, Best N, et al. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000; 10: 325–337. doi: 10.1023/A:1008929526011 [DOI] [Google Scholar]
- 10.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 2022; 25: 3–9. doi: 10.1016/j.jval.2021.11.1351 [DOI] [PubMed] [Google Scholar]
- 11.Pareek M, Bond M, Shorey J, et al. Community-based evaluation of immigrant tuberculosis screening using interferon release assays and tuberculin skin testing: observational study and economic analysis. Thorax 2013; 68: 230–239. doi: 10.1136/thoraxjnl-2011-201542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowada A. Cost-effectiveness of interferon-gamma release assay for entry tuberculosis screening in prisons. Epidemiol Infect 2013; 141: 2224–2234. doi: 10.1017/S0950268812002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahwati LC, Feltner C, Halpern M, et al. Primary care screening and treatment for latent tuberculosis infection in adults. JAMA 2016; 316: 970–983. doi: 10.1001/jama.2016.10357 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhou G, Shi W, et al. Comparing the diagnostic performance of QuantiFERON-TB Gold Plus with QFT-GIT, T-SPOT.TB and TST: a systematic review and meta-analysis. BMC Infect Dis 2023; 23: 40. doi: 10.1186/s12879-023-08008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horsburgh CR, Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 2004; 350: 2060–2067. doi: 10.1056/NEJMsa031667 [DOI] [PubMed] [Google Scholar]
- 16.Ekramnia M, Li Y, Haddad MB, et al. Estimated rates of progression to tuberculosis disease for persons infected with Mycobacterium tuberculosis in the United States. Epidemiology 2024; 35: 164–173. doi: 10.1097/EDE.0000000000001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crofts JP, Pebody R, Grant A, et al. Estimating tuberculosis case mortality in England and Wales, 2001–2002. Int J Tuberc Lung Dis 2008; 12: 308–313. [PubMed] [Google Scholar]
- 18.Pooran A, Booth H, Miller RF, et al. Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC Pulm Med 2010; 10: 7. doi: 10.1186/1471-2466-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NHS England . 2021/22 National Cost Collection Data Publication. 2023. Date last accessed: 4 March 2024. Date last updated: 9 July 2024. www.england.nhs.uk/publication/2021-22-national-cost-collection-data-publication/
- 20.Jones K, Weatherly H, Castelli A, et al. Unit costs of health and social care 2022 manual. Date last accessed: 4 March 2024. https://kar.kent.ac.uk/100519/
- 21.NHS England . 2022/23 National Tariff Payment System [Internet]. Date last accessed: 4 March 2024. Date last updated: 31 March 2022. www.england.nhs.uk/wp-content/uploads/2020/11/22-23-National-tariff-payment-system.pdf
- 22.Kind P, Hardman G, Macran S. UK population norms for EQ-5D. Centre for Health Economics Discussion Paper Series. 1999. www.york.ac.uk/che/pdf/DP172.pdf
- 23.Kowada A. Cost effectiveness of the interferon-gamma release assay for tuberculosis screening of hemodialysis patients. Nephrol Dial Transplant 2013; 28: 682–688. doi: 10.1093/ndt/gfs479 [DOI] [PubMed] [Google Scholar]
- 24.Woo G, Tomlinson G, Yim C, et al. Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol 2012; 26: 445–451. doi: 10.1155/2012/736452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koufopoulou M, Sutton AJ, Breheny K, et al. Methods used in economic evaluations of tuberculin skin tests and interferon gamma release assays for the screening of latent tuberculosis infection: a systematic review. Value Health 2016; 19: 267–276. doi: 10.1016/j.jval.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 26.Joint Formulary Committee . British National Formulary (BNF) (online). London, BMJ and Pharmaceutical Press. Date last accessed: 11 March 2024. www.medicinescomplete.com [Google Scholar]
- 27.Office for National Statistics . Inflation and price indices. Date last accessed: 24 June 2024. https://www.ons.gov.uk/economy/inflationandpriceindices
- 28.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008; 26: 733–744. doi: 10.2165/00019053-200826090-00004 [DOI] [PubMed] [Google Scholar]
- 29.Briggs AH, Sculpher M, Claxton C. Decision Modelling for Health Economic Evaluation (Handbooks for Health Economic Evaluation). Oxford, UK, Oxford University Press (OUP), 2006; p. 237. [Google Scholar]
- 30.Diel R, Loddenkemper R. Predictive value of interferon-γ release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest 2012; 142: 63–75. doi: 10.1378/chest.11-3157 [DOI] [PubMed] [Google Scholar]
- 31.Shah M, Miele K, Choi H, et al. QuantiFERON-TB gold in-tube implementation for latent tuberculosis diagnosis in a public health clinic: a cost-effectiveness analysis. BMC Infect Dis 2012; 12: 360. doi: 10.1186/1471-2334-12-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diel R, Nienhaus A, Loddenkemper R. Cost-effectiveness of interferon-gamma release assay screening for latent tuberculosis infection treatment in Germany. Chest 2007; 131: 1424–1434. Doi: 10.1378/chest.06-2728 [DOI] [PubMed] [Google Scholar]
- 33.Diel R, Wrighton-Smith P, Zellweger JP. Cost-effectiveness of interferon- release assay testing for the treatment of latent tuberculosis. Eur Respir J 2007; 30: 321–332. doi: 10.1183/09031936.00145906 [DOI] [PubMed] [Google Scholar]
- 34.Oxlade O, Schwartzman K, Benedetti A, et al. Developing a tuberculosis transmission model that accounts for changes in population health. Med Decis Making 2011; 31: 53–68. doi: 10.1177/0272989X10369001 [DOI] [PubMed] [Google Scholar]
- 35.Al Hajoj S, Varghese B, Datijan A, et al. Interferon gamma release assay versus tuberculin skin testing among healthcare workers of highly diverse origin in a moderate tuberculosis burden country. PLoS One 2016; 11: e0154803. doi: 10.1371/journal.pone.0154803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedgwick P. Retrospective cohort studies: advantages and disadvantages. BMJ 2014; 348: g1072. doi: 10.1136/bmj.g1072 [DOI] [PubMed] [Google Scholar]
- 37.Winter JR, Stagg HR, Smith CJ, et al. Trends in, and factors associated with, HIV infection amongst tuberculosis patients in the era of anti-retroviral therapy: a retrospective study in England, Wales and Northern Ireland. BMC Med 2018; 16: 85. doi: 10.1186/s12916-018-1070-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson LF, Tamne S, Brown T, et al. Transmission of multidrug-resistant tuberculosis in the UK: a cross-sectional molecular and epidemiological study of clustering and contact tracing. Lancet Infect Dis 2014; 14: 406–415. doi: 10.1016/S1473-3099(14)70022-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material
00818-2024.SUPPLEMENT
CHEERS 2022 checklist
00818-2024.SUPPLEMENT2




