Abstract
The corrosion of anode materials caused by dissolved chloride ions (Cl–) is considered one of the most significant issues in the long term durability of seawater electrolysis systems. This study examined the corrosion behaviors of Ni-based electrodes based on a dynamic potential–pH (Pourbaix) diagram with various buffered electrolytes (borate, carbonate, and phosphate). It was found that NiFeO x /Ni plate electrodes degraded faster in media with mild pH than those with alkaline pH, and the corrosion rate depended on the dissolved ionic species in the presence of Cl–, which could be explained in terms of the thermodynamic stability of the formed compounds on the surface. No significant corrosion was observed in phosphate-containing electrolytes, which could be explained by the high formation constant and the low solubility of the Ni-PO4 product with the formed complex. The density functional theory calculation was used to clarify the preferable phosphate adsorption on the Ni site over Cl–, which stabilized Ni.


Water splitting systems powered by electricity generated by renewable energy sources can produce green hydrogen, which can be considered as an alternative to the currently mass-produced gray hydrogen. Electrolysis is composed of two reactions: a sluggish oxygen evolution reaction (OER) with multielectron transfer steps and a facile hydrogen evolution reaction (HER) with lower overpotential than the OER. Thus, many researchers have focused on developing highly active OER catalysts/electrodes in various electrolyte conditions to target the commercially required current density and operation lifetime.
Electrolysis with mildly treated seawater has attracted much attention for the use of abundant water sources such as seawater to minimize the use of freshwater, which is essential for humans and the environment. , However, using seawater electrolysis faces problems in implementation related to the OER, HER, membrane, and low-conductivity solution. − More significant problems are the corrosion from Cl– and low efficiency from the generation of oxygen, which competes with oxidation reactions of Cl– at the anode. Impurities are also a problem in seawater electrolysis, and components of the electrolysis system and even purified water may contain trace amounts of impurities. Therefore, the development of impurity-resistant water electrolysis systems is essential for long-term operation.
Ni-based electrodes are commonly used for alkaline electrolysis because of their high activity and earth-abundant components and have been extensively investigated to elucidate the origin of high activity, especially Ni–Fe mixed electrodes. − However, it is well-known that Ni-based anodes are corroded by Cl– regardless of pH levels, , highlighting that reaction field design is required to maintain high durability while maintaining performance. One approach to suppress the corrosion is to make an overlayer like CeO x and MnO x on active electrocatalysts like NiFeO x . , Other methods use in situ reconstruction of electrocatalysts. For example, Chen et al. reported that W-NiFeS/WC under OER conditions undergoes in situ structural evolution to produce anticorrosive tungstate and sulfate species on the surface of NiFe oxide. Another attractive approach is introducing anions into the electrolyte, which potentially prevents electrode corrosion by the charge repulsion effect of highly charged ions like sulfate, especially in mediums with alkaline pH. , This approach has been revealed to be applicable at non-extreme pH for NiFeO x catalysts in our previous report. However, at the investigated pH level of 9.2, the introduced phosphate worked as not a charge repulsion layer but as a selective stabilizer for the Ni matrix, which suppressed Ni corrosion even under commercially relevant operating conditions. The interaction between the dissolved ions and Ni matrix is a clear indication that it improves the durability of electrodes, regardless of dissolved Cl–.
Qi et al. reported on the corrosion mechanism of carbon steel in saline soil. They described that Cl– facilitates the dissolution of Fe2+ from bulk steel and Fe2+ reacts with OH– and forms continuous Fe hydro (oxide) species such as akaganeite (β-FeOOH), which precipitates on the surface. Zhang et al. summarized the corrosion mechanism of Ni-based anodes under anodic potential in the presence of Cl– and bromide ions (Br–). They estimated that Ni corrosion potentially proceeds with a combination of two possible mechanisms: (1) an adsorption-induced mechanism and (2) an ion migration and penetration model. The adsorption-induced mechanism is described as halide ions sticking to the anode surface and replacing oxygen in the passive layer, leading to the formation of a complex with metal ions that dissolve, diffuse into the solution, and react with hydroxide. The higher the corrosion proceeds, the thinner the passive layer becomes. Finally, the passive film breaks down and the underlying substrate corrodes. In the ion migration and penetration model, halide ions can penetrate the oxide film. Vacancies may form in the passive film, potentially leading to a rapid release of cations or an accumulation of vacancies at the surface. Consequently, the passive film breaks down. Leng et al. reported on the corrosion of steel in a carbonate-containing solution with Cl–. Interestingly, a protective layer of FeCO3 formed on the surface, which led to a lower corrosion rate. The formation of the FeCO3 protection layer was explained by the solubility product of FeCO3. If the amounts of Fe2+ and CO3 2– generated surpass the thermodynamic threshold, FeCO3 would be deposited on the matrix surface.
Although the mentioned articles reported on the corrosion of materials and corrosion mechanisms, they did not discuss the interaction between dissolved ions and anodes as a function of the electrolyte pH in detail. Many studies have tested the OER in the presence of Cl– at around pH 8, which is close to the pH of seawater, or pH 14, which is the pH of conventional alkaline mediums containing 1.0 mol L–1 KOH, but they have not elaborated on the behavior at pH 9–13. ,, Thus, we report on the pH dependence of the corrosion behavior of Ni-based materials and describe the potential descriptors on the corrosion and anticorrosion mechanism induced by dissolved ions. Furthermore, we discuss the dynamic potential–pH diagram of the Ni-based anode. This work could help with the design of electrolytes and electrodes for efficient water splitting.
We employed a NiFeO x -deposited Ni plate as the working electrode. The preparation method is described in the Supporting Information. Figure a–d shows the cyclic voltammetry (CV) profiles of the NiFeO x /Ni plate electrodes in a single potassium borate (K-borate) buffer electrolyte with chloride salts at different pH levels, and Figure e shows photos of the electrodes after CV. We did not perform CV testing below pH 9 because the electrode component of both Ni and Fe would not be thermodynamically stable based on the Pourbaix diagram.
1.
Corrosion behavior of nickel-based oxygen evolution anodes in the presence of chloride ions (Cl–) in mediums with various pH. Redox behaviors were recorded by cyclic voltammetry (CV) using a NiFeO x /Ni plate as working electrodes in 1.0 mol kg–1 K-borate with and without 1.0 mol kg–1 K-phosphate at pH (a) 9.2, (b) 10.5, (c) 12, and (d) 13. All electrolytes contained 0.5 mol L–1 KCl. The scan rate of CV was 5 mV s–1. (e) Photos of NiFeO x /Ni plate electrodes after CV cycling. (f) Illustration of estimated corrosion mechanisms in the presence of Cl– and buffer anions. Az– is the dissolved buffer ions.
The tested electrolytes were 1.0 mol kg–1 K-borate electrolytes at pH 9.2, 10.5, 12, and 13 and all contained 0.5 mol L–1 K–Cl as a chloride source. Pronounced corrosion current over NiFeO x /Ni plate electrodes was observed at pH 9.2, and the sample formed green solids after the reaction, which can be attributed to corrosion of the underlying Ni substrate. This result matched well with previously reported results. ,, Interestingly, the onset potentials of corrosion current shifted to more anodic potentials (i.e., ≈1.2 VRHE at pH 9.2, ≈1.4 VRHE at pH 10.5, and ≈1.45 VRHE at pH 12), and the corrosion current became smaller with increased pH levels. At pH 12, even though the corrosion current seemed negligible, there was a visible degree of corrosion in the photographs, which was likely because of the overlapping of the corrosion current, the catalyst oxidation current, and the OER current. Corrosion happened on the entire electrode at pH 10.5, while it happened at a spot at pH 12, which corresponded with the corrosion current (i.e., reaction rate). At pH 13, significant corrosion was not observed in the photographs, and stable CV results were observed. The Cl– oxidation reaction would proceed in the presence of Cl–, and corrosive ClO– would be produced at 1.72 VRHE. , In our corrosion assessment during the CVs as illustrated in Figures and S1, the maximum anodic potentials during CVs were set below 1.72 VRHE, which theoretically does not trigger the Cl– oxidation reaction. Thus, the contribution of ClO– is negligible in this study. The observations highlight the pH dependence of chloride-induced Ni-based electrode corrosion and that the corrosion is faster at near-neutral pH levels than at alkaline pH.
We also used carbonate buffer electrolytes. Figure S1a–d exhibits the CV behaviors of NiFeO x /Ni plate electrodes in single carbonate electrolytes at pH 9.2–13 with chloride salts. Figure S1e shows photos of spent samples. Although corrosion of the electrodes was also observed at pH 9.2, the onset potential of the corrosion current shifted more positively and the volume of green solid on the surface was smaller than those seen in the single borate electrolytes. When the pH increased to more than 12, stable CV results were recorded, and there was no obvious corrosion-derived degradation on the electrodes in the photograph. This result indicates that the type of dissolved ions has a big impact on the stability of Ni-based electrodes in Cl–-containing electrolytes.
The introduction of phosphate can reduce the corrosion of Ni-based anodes. Stability testing was conducted using CV in K-borate/phosphate and K-carbonate/phosphate mixed buffer electrolytes at the same pH range, as shown in Figures a–d, S1a, and S1b. The electrolytes contained 1.0 mol kg–1 K-borate or K-carbonate with 1.0 mol kg–1 K-phosphate. The corrosion current was drastically suppressed; stable CV results were observed, and the electrode did not show green solids at all tested pH, as shown in Figures e and S1e. Consequently, the best magnitude of stabilization on the Ni-based material in the presence of Cl– was obtained with phosphate, followed by carbonate and borate anions. This highlights that stability improvement can be attributed to the specific interaction of the dissolved ions with the electrode component.
Figure f illustrates the estimated corrosion mechanism in buffered electrolytes with Cl–. In a simple Cl–-containing water system, material corrosion can happen with the reaction of M(OH) x with Cl–, which forms a soluble metal-chloride complex. The passivation reaction can proceed through dissolved Mn+ reacting with OH–, which forms M(OH) x on the surface. Uniquely, with buffer ions, the passivation reaction can originate from two reactions: the M(OH) x formation and a metal–buffer complex reaction. Both of these potentially compete with each other as a function of the pH level. Because they have specific thermodynamic stability, the mole fraction of the buffer species varies with the solution pH due to their intrinsic pK a, and the OH– concentration is reflected in the pH levels.
To gain insight into the redox behaviors on the Ni-based electrodes, CV analysis was performed with buffer electrolytes without Cl–. Figure a and Figure S2 show the CV profiles, and Figure S3 shows the reduction potentials in distinct electrolytes with various pH levels by using the NiFeO x /Ni plate electrodes. The Ni oxidation peak overlapped with the OER current, which makes it difficult to determine the exact redox potential. This problem was also observed in the previous literature using the NiFeO x catalyst. They used the reduction peak to quantify changes in the redox characteristics of Ni–Fe films as a function of the composition. Redox events carry kinetic information; it may include the adsorption of ions, capacitor information, and redox of materials, preferably being isolated from catalytic events. A constructed dynamic potential–pH diagram is influenced by factors beyond catalyst properties, including electrolyte ionic strength, the nature of the surfaces, and other system-specific variables. Therefore, a quantitative descriptor is required to capture the “trend” of the shifting nature of the electrode. Herein, we also use the reduction potential to track the redox feature on the surface at this time. The reduction potentials in our electrocatalysts were confirmed to be independent of the scan rate below 10 mV s–1 (Figure S4). However, as we describe below, further effort should be made to determine the redox potential of this kind of catalyst. Figure b shows the measured reduction potential difference between K-borate and phosphate-containing electrolytes. Reduction potentials in phosphate-based electrolytes became more positive than those in single borate electrolytes at lower pH levels. This gap narrowed with increased electrolyte pH levels, suggesting that the unique interaction of Ni and phosphate and Ni hydroxide formation compete at the surface as a function of pH level. Baldovia-Lim et al. tested the pH dependence of precipitation using Ni solution with varying pH levels. Phosphate and Ni2+ in the solution formed a crystalline Ni3(PO4)2·8H2O complex (arupite) at pH 7. In contrast, when the initial Ni2+ solution pH was above 9, there were no significant crystalline arupite peaks, which implies an amorphous phase. They continuously changed the pH from neutral to alkaline. The scanning electron microscopy images suggested the formation of Ni hydroxide species surrounding the arupite, indicating that Ni compound switching happened when varying the solution pH. Ni3(PO4)2 formation was favored at low pH, and Ni(OH)2 was stable at high pH.
2.
Relationship of the reduction potential of Ni-based electrodes and the dissolved Ni amount as a function of electrolyte pH. (a) Reduction behaviors of the NiFeO x /Ni plate. CV profiles were recorded in 1.0 mol kg–1 K-borate (black), 1.0 mol kg–1 K-phosphate (green), and 1.0 mol kg–1 K-borate with 1.0 mol kg–1 phosphate (orange) at pH 9.2 and 13. The scan rate of CV was 5 mV s–1. (b) Reduction potential difference and dissolved Ni amounts as a function of electrolyte pH. The left axis shows the reduction potential of the NiFeO x /Ni plate measured in electrolytes containing phosphate subtracted from that measured in single borate electrolytes. Reduction potentials were calculated based on the peaks of CV profiles. The right axis shows the dissolved Ni2+ concentration calculated from the solubility product of Ni3(PO4)2 and Ni(OH)2.
Figure b displays the expected Ni dissolution amount as a function of pH. The Ni2+ dissolution amount in the solution was calculated using each solubility product of Ni-phosphate and Ni(OH)2. The thermodynamic properties are summarized in Tables S1 and S2. The calculated curves show that Ni2+ is prone to react with phosphate and form Ni3(PO4)2 below pH 11, but it is more likely to react readily with OH– and form Ni(OH)2 above pH 11. This matches well with the linear decrease in the potential difference at different pH values and results from the literature. The potential difference was smaller under alkaline conditions because the species that formed on the surface seemed to be Ni(OH)2, which is likely not affected by the dissolved ions. The difference in reduction potential is considered to directly reflect the stabilization of Ni by introduced ions. This surface stabilization is also supported by the X-ray photoelectron spectroscopy (XPS) results after OER testing. The XPS and OER performance is discussed in the Supporting Information and shown in Figures S5–S7. It is noted that the amount of Ni that can dissolve in the solution used is generally very low. It is estimated to be on the order of nanograms per gram in the tens of mL of solution used in our experiments. Although it is desirable to measure the dissolution behavior of Ni under applied potentials using online quantitative techniques such as inductively coupled plasma mass spectrometry (ICP-MS) or electrochemical quartz crystal microbalancing (EQCM), we reserve these sophisticated investigations for the future. Intriguingly, however, the difference in Ni stabilization measured by CV (in terms of potential difference as a function of pH) matched well with the thermodynamic solubility data (Tables S1 and S2).
Figure S8 shows the Fe dissolution in response to pH. Fe2+ is readily oxidized to more thermodynamically stable Fe(OH)3 species in water and the tested potential range. It is hypothesized that FePO4 and FeCO3 may form, but it might not happen significantly because of the high stability of the Fe(OH)3 phase. Thus, the interaction between Fe and dissolved buffer ions likely happens less frequently than with Ni-based complexes. Figure S9 also shows the dissolution of Ni with Cl–. The black and purple lines show the dissolution boundary of Ni2+ and NiCl2(aq) from Ni(OH)2 calculated from thermodynamic equilibriums. Corrosion and passivation were observed before Ni2+/3+ redox in the CV results. Thus, Ni(OH)2 was considered a major complex in the calculation. The closer boundary indicates that Ni(OH)2 easily reacts with Cl– at mild pH, which leads to further dissolution of Ni in addition to thermodynamic dissolution (Ni(OH)2 → Ni2+). Once Ni is stabilized by the phosphate, it is 3 orders of magnitude less likely to dissolve than Ni(OH)2. This Ni stabilization is thought to have led to its stability in the presence of Cl– at mild pH. The alkaline pH is very far from the corrosion boundary of Cl–, so the Ni matrix is considered to be stable. Although some Ni passivation can be expected with the addition of carbonate, the degree of Ni passivation is smaller than that with phosphate. This means that the degree of nickel stabilization is uniquely determined by the electrode component and electrolyte. The thermodynamic pH boundary between Fe(OH)3 and FeCl3 was located at a more acidic pH than that of Ni(OH)2 and NiCl2 when we estimated the dissolution of the metal complex as 10–6. This explains the faster corrosion of the Ni matrix at a mild pH.
In this study, the formation of a Ni-phosphate complex plays a key role. We tried to identify the Ni-phosphate complex on the surface. We performed operando Raman experiments. Figure S10a shows the potential-dependent spectra of the Ni electrode in the borate electrolyte. At 1.7 VRHE, a clear oxidation peak was observed at 450–500 cm–1, which can be attributed to NiOOH originating from the oxidation of Ni(II). However, the problem is that the buffer electrolyte itself exhibits Raman peaks, which make it difficult to track the surface-formed complex. This was also the case for borate/phosphate electrolytes as shown in Figure S10b. Based on the previous literature, Ni3(PO4)2 has peaks at 600–1200 cm–1, which are derived from PO4 3–. In our case, the overlap with strong electrolyte peaks at approximately 880 and 1000 cm–1 rendered the Ni3(PO4)2 peak and adsorbed ion species. Alternatively, there was also an oxidation peak at 450–500 cm–1 for the NiOOH conversion, indicating that Ni oxidation took place even in the presence of phosphate ions. We also employed an ex situ X-ray diffraction (XRD) measurement to track the crystalline structure after repeated CV in borate and borate/phosphate electrolytes (see Figure S11). As-made catalyst did not show a pronounced oxidized Ni peak on the Ni plate likely due to the strong background of the Ni plate and the amorphous structure of the formed oxides. Also, there were no significant peaks after CV in both electrolytes. In addition, based on the above-mentioned literature, Ni3(PO4)2 is amorphous at pH above 9. Furthermore, in our recent study, phosphate passivation dynamically proceeded, which makes it more difficult to detect the surface-formed complex with ex situ characterization. Even though XPS measurements were performed under ex situ conditions, the obtained information was close to the surface where the phosphate-induced change is more pronounced. Although the above conducted analysis could not provide conclusive evidence, the Bode diagram analysis in Figure S12 shows that, when phosphate was introduced in the K-borate electrolyte, the phase angle does not change significantly when potential was applied compared to the case without phosphate, indicating that phosphate induced the passivation and kept the capacitive control.
To explore the stability of Ni(OH) x in various buffer systems, density functional theory (DFT) calculations were employed to analyze their interactions. A neutral cluster model of Ni7O24H34 was taken as a starting reference compound to compute computational Pourbaix diagrams and investigate structural transformations under electrochemical conditions (Figures S13–S16). The detailed computational methodologies are provided in the Supporting Information. For PO4 3–-doped systems, the Ni7(μ3OH)6(μ2OH)6(μ1OH)7(H2O)5(PO4) structure emerged as the most stable configuration up to pH ∼ 10. Under highly alkaline conditions, the [Ni7(μ3O)2(μ3OH)4(μ2OH)6(μ1OH)9(H2O)3(PO4)]2– structure remained stable at low potentials, while [Ni7(μ3O)2(μ3OH)4(μ2OH)6(μ1OH)9(H2O)3(PO4)]3– and Ni7(μ3O)1(μ3OH)5(μ2OH)6(μ1OH)8(H2O)4(PO4)]2– structures were stabilized in more alkaline conditions.
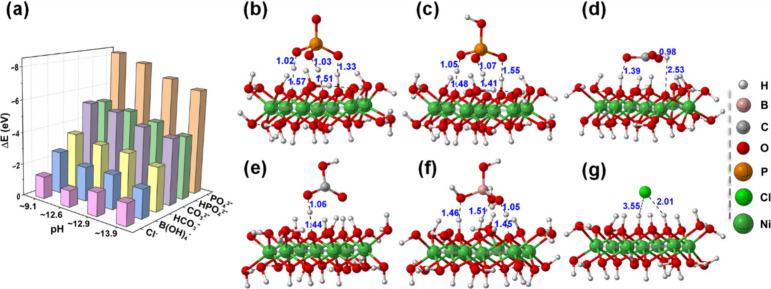
Binding energy analyses were performed for these four structures to evaluate the comparative binding strength of different buffers under alkaline conditions. At pH ∼ 9.1, the binding affinities to [Ni7(μ3OH)6(μ2OH)6(μ1OH)7(H2O)5]3+ had the following sequence: PO4 3– > HPO4 2– ≈ CO3 2– > HCO3 – > B(OH)4 – > Cl–. This is consistent with the experimental observations shown in Figure . While the computed binding energies for the buffer on the surface are relatively high at the current level of theory, these values are smaller than the formation enthalpy of the salts. More importantly, it qualitatively aligns well with the experimental observations, consistent with adsorption driven stabilization. This trend persisted under more alkaline conditions (pH ∼ 12.6 and ∼ 13.9) and highlights phosphate buffers as the most effective stabilizers for the Ni(OH) x surface. Figure S13 also shows the binding modes of PO4 3– on Ni(OH) x at varying pH values and demonstrates strong hydrogen bonding with adjacent surface hydroxyl groups. Similar hydrogen-bonding interactions were inferred for other buffer ions, as shown in Figure . Notably, the binding trends among buffers remained consistent across pH variations, although binding energies systematically decreased with increasing alkalinity due to structural changes in the clusters driven by pH changes. Furthermore, from the optimized geometries, the distance between the H atoms of the Ni(OH) x surface and different buffer ions was detected to be in a range that is quite higher than that of normal O–H bonds. This finding indicates the absence of chemical bond formation between H and O atoms and eliminates the possibility of chemisorption. Instated, the interactions are more likely linked by electrostatic interactions, particularly hydrogen bonding interactions.
3.
(a) Computed binding strength of the different buffers at different pH levels over the Ni(OH) x model structure. (b–g) Optimized structures of the PO4 3–, HPO4 2–, CO3 2–, HCO3 –, B(OH)4 –, and Cl– buffer, respectively, on top of the most stable Ni7(μ3OH)6(μ2OH)6(μ1OH)7(H2O)5 structure at pH ∼ 9.1.
To design reactions for a wide pH range, it is important to understand the stable phase in the potential/pH diagram under the electrochemical conditions of the electrode. The conventional potential–pH relationship for elements is shown by the Pourbaix diagram, which considers thermodynamics under ideal conditions. However, in a real reaction environment, there is a kinetic contribution that forms certain phases, and if a stable phase is formed on the substrate, then the stability of the underlying substrate should be considered, especially with the presence of Cl–. Thus, the potential–pH relationship obtained from the electrochemical outcomes helps to understand the corrosion trend of Ni-based electrodes under applied potentials. This potential–pH relationship, which considers kinetic contribution, is referred to as the “dynamic potential–pH (Pourbaix) diagram” also advocated by Bard and co-workers.
Figure exhibits the potential–pH relationship with Cl– from the result of Figure and Figure S17. We first plotted the potential at which the current begins to flow in the borate-containing (red curve) and phosphate-containing (green curve) electrolyte versus pH on the standard hydrogen electrode (SHE) scale. We plotted the first rise in current at 0.1 mA cm–2 from CV as a representative point because it is difficult to distinguish Ni oxidation, OER, and corrosion currents, which compete as the pH moves toward the alkaline side. In the borate electrolytes, currents contributing to corrosion were observed at a lower pH and lower potential. On the other hand, at a similar pH, the introduction of phosphate shifted the rising potential of the current to a higher potential, indicating the formation of a stable phase. This potential gap is the corrosion area in single borate electrolytes, and the gap is overcome by phosphate additives. In both electrolytes, the potentials at which the current begins to flow were found to be closer toward the alkaline side. This supports that the Cl–-induced corrosion hardly proceeds when considering the emergence of the stable redox feature of Ni electrodes. Figure S18 shows the dynamic potential–pH diagram constructed with various current densities at 1 and 5 mA cm–2 as corrosion thresholds. Even though different current densities were used to define the unstable region (red area), the corrosion trend looks similar. However, using the corrosion threshold at a higher current density pushes up the unstable region more anodically, underestimating the stable region. Thus, to express the stable region in the presence of phosphate with Cl–, using the small current density at 0.1 mA cm–2 was reasonable. The important point of this diagram is that it reflects not only the surface stability but also the stability of the substrate, which can be very useful when using porous materials with large surface areas such as Ni foam.
4.

Dynamic potential–pH diagram of the NiFeO x /Ni plate in buffered electrolyte with Cl– at various pH. The red and green curves show the measured potential at 0.1 mA cm–2 in K-borate and K-borate/phosphate electrolyte in the presence of Cl–, respectively.
For further improvement of this dynamic potential–pH diagram, the following items should be considered. The first is dissolution and desorption of the catalyst due to external factors. A problem with trying to measure the stability of electrodes above the potential scale of the OER is that the stability of the catalyst is inevitably dominated by external factors. For example, insufficient buffer capacity of the electrolyte triggers local pH shifting, which triggers pH corrosion, and a tremendous amount of bubbles are generated at higher current density, which causes the catalyst to be stripped off. The second issue is redox characterization. The surface state of the catalyst varies depending on the synthesis method and dissolved ion species. For example, at non-extreme pH, the interaction of buffers and electrodes must be considered. Also, for some catalysts such as NiFeO x , the OER and the oxidation current of the catalyst overlap. It would be better to use online gas quantification and spectroscopic approaches to carefully track the redox of the catalyst and the switch between catalyst oxidation and the OER. Figure shows that the redox properties vary significantly with pH, depending on the dissolved ion species. The dynamic stability of passivation induced with dissolved ions at the potential region of the OER also needs to be measured in the future.
This article has discussed the pH-dependent corrosion behaviors over Ni-based material of the NiFeO x /Ni plate electrode in borate, carbonate, and phosphate-containing electrolyte in the presence of Cl–. The Ni-based electrodes were highly invaded and lost their stability due to Cl– at non-extreme pH. However, they were relatively stable at alkaline pH, which is likely due to the pH-dependent thermodynamic stability of the Ni complex. The addition of phosphate into the borate and carbonate electrolytes alleviated the corrosion even at mild pH because phosphate binds more strongly and forms low-solubility products than other Ni-based complexes. This allows Ni to be more stabilized, even in the presence of Cl–. The Ni reduction potential became more positive in phosphate-containing electrolytes than in those with borate, resulting in a unique surface at mild pH. In contrast, this potential difference became smaller with higher pH. These results highlight the competitive passivation of Ni-phosphate versus Ni(OH)2 on the surface.
DFT calculations revealed the stability and structural transformations of Ni(OH) x clusters in various buffer systems and highlighted phosphate as the most effective coordination anion. Binding energy trends and computational Pourbaix diagrams correlated well with experimental observations and demonstrated consistent buffer stabilization across pH variations.
The constructed dynamic potential–pH diagram could help in the design of electrode materials and dissolved ion systems. This article could provide a guideline for overcoming the low durability of Ni-based anodes in the presence of Cl–, which is essential for long-term water electrolysis systems.
Supplementary Material
Acknowledgments
The authors are grateful to the following for their support: GteX Program Japan (Grant Number JPMJGX23H2) and the MEXT Program: Data Creation and Utilization-Type Material Research and Development Project (Grant Number JPMXP1122712807); Mohammed bin Salman Center for Future Science and Technology for Saudi-Japan Vision 2030 at The University of Tokyo (MbSC2030); The Science and Technology Research Partnership for Sustainable Development (SATREPS) in collaboration with Japan Science and Technology Agency (JST, JPMJSA2104); Japan International Cooperation Agency (JICA) and JSPS KAKENHI (24KJ0721). K.B. acknowledges the ANRF-PMECRG grant (ANRF/ECRG/2024/000912/CS), IITG-SRG, and computing facilities from IIT Guwahati.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.5c00704.
H.K. performed all electrochemical experiments and analyses. S.L. and K.B. were in charge of the theoretical calculation section. All the authors wrote the manuscript, and K.T. obtained the funding and supervised the project.
The authors declare no competing financial interest.
References
- Vorosmarty C. J., McIntyre P. B., Gessner M. O., Dudgeon D., Prusevich A., Green P., Glidden S., Bunn S. E., Sullivan C. A., Liermann C. R.. Global Threats to Human Water Security and River Biodiversity. Nature. 2010;467(7315):555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- Vorosmarty C. J., Green P., Salisbury J., Lammers R. B.. Global Water Resources: Vulnerability from Climate Change and Population Growth. Science. 2000;289(5477):284–288. doi: 10.1126/science.289.5477.284. [DOI] [PubMed] [Google Scholar]
- Kirk D. W., Ledas A. E.. Precipitate Formation during Sea Water Electrolysis. Int. J. Hydrog. Energy. 1982;7(12):925–932. doi: 10.1016/0360-3199(82)90160-4. [DOI] [Google Scholar]
- Liu G., Xu Y., Yang T., Jiang L.. Recent Advances in Electrocatalysts for Seawater Splitting. Nano Mater. Sci. 2023;5(1):101–116. doi: 10.1016/j.nanoms.2020.12.003. [DOI] [Google Scholar]
- Dresp S., Dionigi F., Loos S., Ferreira de Araujo J., Spöri C., Gliech M., Dau H., Strasser P.. Direct Electrolytic Splitting of Seawater: Activity, Selectivity, Degradation, and Recovery Studied from the Molecular Catalyst Structure to the Electrolyzer Cell Level. Adv. Energy Mater. 2018;8(22):1800338. doi: 10.1002/aenm.201800338. [DOI] [Google Scholar]
- Komiya H., Obata K., Wada M., Nishimoto T., Takanabe K.. Electrolyte Engineering Applying Concentrated Chloride Ions with Mixed Buffer Solutions for a Versatile High-Productivity Water-Splitting System. ACS Sustain. Chem. Eng. 2023;11(34):12614–12622. doi: 10.1021/acssuschemeng.3c02322. [DOI] [Google Scholar]
- Dionigi F., Reier T., Pawolek Z., Gliech M., Strasser P.. Design Criteria, Operating Conditions, and Nickel–Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis. ChemSusChem. 2016;9(9):962–972. doi: 10.1002/cssc.201501581. [DOI] [PubMed] [Google Scholar]
- Becker H., Murawski J., Shinde D. V., Stephens I. E. L., Hinds G., Smith G.. Impact of Impurities on Water Electrolysis: A Review. Sustain. Energy Fuels. 2023;7(7):1565–1603. doi: 10.1039/D2SE01517J. [DOI] [Google Scholar]
- McCrory C. C. L., Jung S., Ferrer I. M., Chatman S. M., Peters J. C., Jaramillo T. F.. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015;137(13):4347–4357. doi: 10.1021/ja510442p. [DOI] [PubMed] [Google Scholar]
- Görlin M., Chernev P., Ferreira de Araújo J., Reier T., Dresp S., Paul B., Krähnert R., Dau H., Strasser P.. Oxygen Evolution Reaction Dynamics, Faradaic Charge Efficiency, and the Active Metal Redox States of Ni–Fe Oxide Water Splitting Electrocatalysts. J. Am. Chem. Soc. 2016;138(17):5603–5614. doi: 10.1021/jacs.6b00332. [DOI] [PubMed] [Google Scholar]
- Trotochaud L., Young S. L., Ranney J. K., Boettcher S. W.. Nickel–Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014;136(18):6744–6753. doi: 10.1021/ja502379c. [DOI] [PubMed] [Google Scholar]
- Li Y.-F., Selloni A.. Mechanism and Activity of Water Oxidation on Selected Surfaces of Pure and Fe-Doped NiO x. ACS Catal. 2014;4(4):1148–1153. doi: 10.1021/cs401245q. [DOI] [Google Scholar]
- Friebel D., Louie M. W., Bajdich M., Sanwald K. E., Cai Y., Wise A. M., Cheng M.-J., Sokaras D., Weng T.-C., Alonso-Mori R., Davis R. C., Bargar J. R., Nørskov J. K., Nilsson A., Bell A. T.. Identification of Highly Active Fe Sites in (Ni,Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015;137(3):1305–1313. doi: 10.1021/ja511559d. [DOI] [PubMed] [Google Scholar]
- Chen J. Y. C., Dang L., Liang H., Bi W., Gerken J. B., Jin S., Alp E. E., Stahl S. S.. Operando Analysis of NiFe and Fe Oxyhydroxide Electrocatalysts for Water Oxidation: Detection of Fe4+ by Mössbauer Spectroscopy. J. Am. Chem. Soc. 2015;137(48):15090–15093. doi: 10.1021/jacs.5b10699. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wang Y., Li S., Wang Z., Chen H., Yi L., Chen X., Yang Q., Xu W., Wang A.. Concerning the Stability of Seawater Electrolysis: A Corrosion Mechanism Study of Halide on Ni-Based Anode. Nat. Commun. 2023;14(1):4822. doi: 10.1038/s41467-023-40563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya H., Shinagawa T., Takanabe K.. Electrolyte Engineering for Oxygen Evolution Reaction Over Non-Noble Metal Electrodes Achieving High Current Density in the Presence of Chloride Ion. ChemSusChem. 2022;15(19):e202201088. doi: 10.1002/cssc.202201088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K., Takanabe K.. A Permselective CeOx Coating To Improve the Stability of Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2018;57(6):1616–1620. doi: 10.1002/anie.201712121. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wang C., Ye L., Liu X., Xin L., Yang Y., Wang L., Hou W., Wen Y., Zhan T.. MnOx Film-Coated NiFe-LDH Nanosheets on Ni Foam as Selective Oxygen Evolution Electrocatalysts for Alkaline Seawater Oxidation. Inorg. Chem. 2022;61(38):15256–15265. doi: 10.1021/acs.inorgchem.2c02579. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhou X., Ye C., Ye M., Shen J.. In-Situ Constructing Oxide-Anion Dual-Layer on Ce-B-Containing Electrode Electrolyte Interface towards Highly Corrosive Seawater Splitting. Appl. Catal. B Environ. 2024;343:123560. doi: 10.1016/j.apcatb.2023.123560. [DOI] [Google Scholar]
- Chen Z., Wei W., Xu X., Gu X., Huang C., Wei W., Shao Z., Ni B.-J., Chen H.. Reconstructed Anti-Corrosive and Active Surface on Hierarchically Porous Carbonized Wood for Efficient Overall Seawater Electrolysis. Sci. Bull. 2024;69(15):2337–2341. doi: 10.1016/j.scib.2024.05.044. [DOI] [PubMed] [Google Scholar]
- Ma T., Xu W., Li B., Chen X., Zhao J., Wan S., Jiang K., Zhang S., Wang Z., Tian Z., Lu Z., Chen L.. The Critical Role of Additive Sulfate for Stable Alkaline Seawater Oxidation on Nickel-Based Electrodes. Angew. Chem., Int. Ed. 2021;60(42):22740–22744. doi: 10.1002/anie.202110355. [DOI] [PubMed] [Google Scholar]
- Yu M., Li J., Liu F., Liu J., Xu W., Hu H., Chen X., Wang W., Cheng F.. Anionic Formulation of Electrolyte Additive towards Stable Electrocatalytic Oxygen Evolution in Seawater Splitting. J. Energy Chem. 2022;72:361–369. doi: 10.1016/j.jechem.2022.04.004. [DOI] [Google Scholar]
- Komiya H., Obata K., Honma T., Takanabe K.. Dynamic Stabilization of Nickel-Based Oxygen Evolution Electrocatalysts in the Presence of Chloride Ions Using a Phosphate Additive. J. Mater. Chem. A. 2024;12(6):3513–3522. doi: 10.1039/D3TA05566C. [DOI] [Google Scholar]
- Qi G., Qin X., Xie J., Han P., He B.. Electrochemical Corrosion Behaviour of Four Low-Carbon Steels in Saline Soil. RSC Adv. 2022;12(32):20929–20945. doi: 10.1039/D2RA03200G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng J., Tian Z., Liao K., He T., Liu X., He G., Tang X., Xia G.. Localised Corrosion Failure of an L245N Pipeline in a CO 2 – O 2 – Cl – Environment. Corros. Eng. Sci. Technol. 2023;58(4):372–383. doi: 10.1080/1478422X.2023.2188637. [DOI] [Google Scholar]
- Tong W., Forster M., Dionigi F., Dresp S., Sadeghi Erami R., Strasser P., Cowan A. J., Farràs P.. Electrolysis of Low-Grade and Saline Surface Water. Nat. Energy. 2020;5(5):367–377. doi: 10.1038/s41560-020-0550-8. [DOI] [Google Scholar]
- Mohammed-Ibrahim J., Moussab H.. Recent Advances on Hydrogen Production through Seawater Electrolysis. Mater. Sci. Energy Technol. 2020;3:780–807. doi: 10.1016/j.mset.2020.09.005. [DOI] [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd English.; National Association of Corrosion Engineers: Houston, Texas, 1974. [Google Scholar]
- Grgur B. N., Trišović T. L., Rafailović L.. Corrosion of Stainless Steel 316Ti Tank for the Transport 12–15% of Hypochlorite Solution. Eng. Fail. Anal. 2020;116:104768. doi: 10.1016/j.engfailanal.2020.104768. [DOI] [Google Scholar]
- Louie M. W., Bell A. T.. An Investigation of Thin-Film Ni–Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2013;135(33):12329–12337. doi: 10.1021/ja405351s. [DOI] [PubMed] [Google Scholar]
- Baldovia-Lim J. M., de Luna M. D. G., Abarca R. R. M., Lacson C. F. Z., Grisdanurak N., Lu M.-C.. Integrating Bi-pH Operation to Enhance Ni2+ Removal and Recovery in Fluidized-Bed Non-Seeded Granulation Process. Chem. Eng. J. 2023;472:145102. doi: 10.1016/j.cej.2023.145102. [DOI] [Google Scholar]
- Mirghni A. A., Madito M. J., Oyedotun K. O., Masikhwa T. M., Ndiaye N. M., Ray S. J., Manyala N.. A High Energy Density Asymmetric Supercapacitor Utilizing a Nickel Phosphate/Graphene Foam Composite as the Cathode and Carbonized Iron Cations Adsorbed onto Polyaniline as the Anode. RSC Adv. 2018;8(21):11608–11621. doi: 10.1039/C7RA12028A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguzzi A., Fan F.-R. F., Vertova A., Rondinini S., Bard A. J.. Dynamic Potential–pH Diagrams Application to Electrocatalysts for Water Oxidation. Chem. Sci. 2012;3(1):217–229. doi: 10.1039/C1SC00516B. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.