Abstract
Skin cancer is one of the most prevalent types of cancer worldwide, with its global incidence rising despite prevention efforts. Telomere length (TL) has emerged as a potential biomarker for cancer risk; however, its relationship with skin cancer risk remains incompletely understood. To explore the association between TL and the risk of melanoma, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), a systematic review and meta-analysis was conducted. Longer TL was significantly associated with an increased risk in melanoma (pooled odds ratio: 0.51; 95% confidence interval: 0.38–0.69; P<0.0001). A significant association between longer TL and increased melanoma risk was identified in both familial melanoma and the general population. Subgroup analyses revealed consistent associations across sex, population source and adjustments for confounding factors. Geographic stratification indicated stronger associations in studies conducted in the USA compared with those from European populations. A meta-analysis of BCC and SCC studies did not achieve statistical significance, although qualitative synthesis suggested a potential association between shortened TL and increased risk. The significant association of longer TL and increased melanoma risk diverges from the conventional hypothesis that telomere shortening elevates cancer risk, highlighting a cancer-type specific telomeric relationship. The inconclusive findings for BCC and SCC underscore the necessity for further detailed investigation. Large-scale prospective studies with standardized methodologies are imperative to validate these findings and explore the underlying mechanisms. The present findings suggested that TL could potentially serve as a valuable biomarker for melanoma risk stratification in dermatologic oncology.
Keywords: telomere length, telomere biology, biomarker, cancer, skin cancer, melanoma, basal cell carcinoma, squamous cell carcinoma
Introduction
Skin cancer is the most frequently diagnosed cancer among Caucasian individuals, and despite extensive public health campaigns and preventative initiatives, its incidence continues to rise globally (1–3). Skin cancer comprises malignant melanoma and non-melanoma skin cancers (NMSCs), with basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) being the most clinically significant NMSCs due to their potential for local invasion and metastasis (4,5).
BCC, originating from basal epidermal cells, represents 70–80% of skin cancer diagnoses, making it one of the most prevalent types of cancer worldwide (6). BCC is histologically characterised by basophilic nests of basal-like cells arranged in a peripheral palisade pattern and typically exhibits slow growth (7). Although BCC is a malignant tumour, it exhibits almost no metastatic potential (8). SCC constitutes ~20% of NMSC cases and carries a higher risk of metastasis compared with BCC, reported to. Be between 0.1 and 13.7% (9,10). The lifetime risk of SCC in the United States is estimated to be 7–11%, with its incidence increasing over recent decades; risk factors include advanced age, fair skin and cumulative ultraviolet (UV) radiation exposure (11–14). Clinically, SCC may present as enlarging plaques or nodules that may ulcerate, and often arises from the precursor lesion actinic keratosis (AK) (15). Early diagnosis and surgical intervention typically result in excellent outcomes (16).
Melanoma, while less common, is the most aggressive form of skin cancer and poses the greatest mortality risk due to its high metastatic potential and resistance to therapy (17). Cutaneous melanoma originates from melanocytes and constitutes >90% of melanoma cases in Caucasian populations. Pathophysiologically, it is predominantly associated with UV radiation-induced mutations, with UV radiation accounting for >75% of melanoma cases in Caucasian populations; however, non-UV-related subtypes exist (18–20). Risk factors include sun exposure, high nevus count, the presence of atypical moles, genetic predisposition and family history (21,22). The incidence of melanoma has risen sharply since the 1950s, with current estimates reporting ~25 new cases per 100,000 individuals annually in Europe, 30 in the United States, and 60 in Australia and New Zealand (23–25).
Telomeres are specialized nucleoprotein structures capping the ends of eukaryotic chromosomes, consisting of repetitive hexameric sequences (5′-TTAGGG-3′) that span 10–15 kilobases in humans (26–28). Telmoeres serve a critical role in maintaining genomic integrity by safeguarding chromosome ends from degradation and preventing end-to-end fusions (26,29). The shelterin complex ensures telomere protection, regulates their length and affects telomerase activity (27,28). During DNA replication, the inability of DNA polymerase to fully replicate 3′ends results in progressive telomere shortening by 30–200 base pairs per cell division (30). The progressive telomere attrition results in a cellular replicative limit, termed the Hayflick limit (31). In cells with functional cell cycle checkpoints, critical telomere shortening results in replicative senescence, whereas in cells with disrupted checkpoints, telomere crisis ensues, which is resolved either by apoptosis or the activation of telomere maintenance mechanisms (31,32). Telomerase, a ribonucleoprotein enzyme, permits the elongation of telomeres and is tightly regulated, being predominantly active in stem cells, germ cells and certain highly proliferative somatic cell populations, whereas most somatic cells exhibit low or negligible telomerase activity (32–34). The aberrant reactivation of telomerase is found in most malignancies and is considered a critical tumourigenic event (35–37).
The pathophysiology of telomere length (TL) in cancer is complex, with research underscoring its dual role as both a tumour suppressor via induction of apoptosis and senescence, and an enabler of carcinogenesis via genomic instability (38–40). The primary proposed mechanism in carcinogenesis consists of short telomeres inducing chromosomal instability and promoting genomic aberrations, subsequently TL is stabilized above the apoptotic threshold through telomerase activation or alternative lengthening mechanisms, enabling unlimited replicative potential (41,42). The relationship between TL and cancer risk is an area of active investigation, with findings varying by cancer type. Short telomeres have been associated with an increased risk in urological, head and neck, and digestive system cancers (43–46). Studies on breast and colorectal cancers, and NMSCs, have yielded inconclusive results, failing to demonstrate a statistically significant association, whereas studies have indicated a significant association between long TL and increased risk of lung cancer and melanoma (47–51).
Corollary to the rising incidence of skin cancer is the need for novel risk stratification and early detection strategies aimed at prevention and timely therapeutic intervention. Given that TL can be readily assessed through peripheral leukocyte measurements and its centrality to cancer biology, it constitutes a promising cancer biomarker (52–54). The present systematic review and meta-analysis aimed to evaluate and synthesise the current evidence on the association between TL and skin cancer, specifically in melanoma, BCC and SCC, elucidating the potential of TL as a risk biomarker in dermatologic oncology.
Materials and methods
Search strategy
A systematic review and meta-analysis was conducted to investigate the association between TL and skin cancer risk, specifically focusing on melanoma, BCC and SCC. The present study was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (55).
A comprehensive literature search was performed to identify relevant studies published up to October 2024 across the electronic databases PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library (https://www.cochranelibrary.com/) and Scopus (https://www.scopus.com/). The search strategy utilized a combination of Medical Subject Headings terms and key words related to TL and skin cancer risk. The following terms were used: ‘telomere’, ‘telomere length’, ‘skin cancer’, ‘melanoma’, ‘basal cell carcinoma’, ‘squamous cell carcinoma’ and ‘skin neoplasms’. Logical operators ‘AND’ and ‘OR’ were employed to combine the search terms effectively. Additionally, bibliographies of relevant reviews were examined to identify any further eligible studies.
Eligibility criteria
Studies were included based on predefined eligibility criteria, with additional criteria applied for inclusion in the quantitative synthesis. The eligibility criteria were structured based on the PICO framework, as follows:
P (population), eligible studies investigated individuals at risk for developing melanoma, SCC or BCC. Studies focusing on non-malignant or premalignant conditions, such as AK and Bowen's disease (BD), were excluded. Similarly, extracutaneous melanoma, including mucosal and ocular melanoma, and malignancies of the skin other than melanoma, BCC and SCC, such as cutaneous lymphoma, were excluded from both the quantitative and qualitative analyses. All included studies involved human populations, studies involving non-human subjects or cell lines were excluded.
I (intervention/exposure), to be eligible, studies had to explore the relationship between TL and the risk of skin cancer by directly measuring TL in any human biological sample, including tissue or peripheral blood leukocytes (PBLs), while studies reporting indirect measurements, such as genetic risk score, were excluded.
C (comparison), the comparison groups included individuals with and without skin cancer, or patient samples from malignant tissue and normal skin tissue. Studies that lacked a control group or comparator were excluded.
O (outcome), for the qualitative synthesis, a descriptive analysis of the relevant association was required. For inclusion in the quantitative analysis of risk, studies were required to report relative risk estimates, including odds ratios (ORs), risk ratios or hazard ratios (HRs), with corresponding 95% confidence intervals (CIs).
Eligible studies followed an observational design employing a cohort, case-control or nested case-control design. Review articles, meta-analyses, case reports, conference abstracts and dissertations were excluded. Only articles published in English were included, but no restrictions were applied regarding the publication date.
Study selection and data extraction
All retrieved articles were initially screened against the eligibility criteria based on titles and abstracts. Full-text articles were thoroughly examined in studies that appeared to meet the inclusion criteria or when eligibility was uncertain. Two independent reviewers conducted the initial screening and the evaluation of the full-text articles to determine inclusion. Any conflicts were resolved through discussion and consensus.
Data extraction was conducted independently by two reviewers using a standardized data extraction form developed for the present study. Any discrepancies were resolved by consensus. For all eligible studies, the extracted data included: Study name, first author name, year of publication, location, cancer type, the racial or ethnic composition of the study population, information about the source of cases, study design, age and sex distribution among both cases and controls, number of cases and controls, method of TL measurement, tissue source of the DNA, cut-off values used for categorizing TL, relative risk measure and the corresponding 95% CIs. If an article included multiple non-overlapping datasets, they were considered independent individual studies. Between studies with overlapping samples for the same outcome, the study with the largest number of cases was included in the meta-analysis. If sample sizes were similar, the study that adjusted for the greatest number of covariates was selected.
Qualitative synthesis
All studies that met the inclusion criteria, including those incorporated into the quantitative synthesis (meta-analysis), were included in the qualitative synthesis. This synthesis involved systematically reviewing and interpreting the findings of each study.
Quantitative synthesis
Data preparation
In the quantitative synthesis, the relative risk for comparing patients with the longest TLs to the shortest was utilized, with the longest being used as the reference. In studies reporting TL as a continuous variable, the reported effect estimate per unit decrease in TL was utilized. To maintain consistency in the direction of the effect, effect estimates were transformed when necessary, so that long TL was always the reference. In studies with multiple relative risk measures, the most fully adjusted risk measure was included in the meta-analysis.
Meta-analysis
The statistical heterogeneity between studies was assessed using Cochran's Q test and quantified with the I2 statistic. P<0.10 for the Q test was considered indicative of significant heterogeneity. The I2 statistic, describing the percentage of total variation across studies due to heterogeneity rather than chance, was utilised to quantify heterogeneity, with values of 25, 50 and 75% representing low, moderate and high heterogeneity, respectively (56,57). The random-effects model, based on the DerSimonian and Laird method, which accounts for both within-study and between-study variability was used (58). When pooling risk measures, they were transformed by taking the natural logarithm and their standard errors were utilised for the meta-analysis (57).
To explore potential sources of heterogeneity and to assess the robustness of the findings, subgroup analyses were performed based on sex, location, subtype by genetic predisposition (familial vs. sporadic), population source (hospital vs. general population), and adjustment for skin cancer specific confounders. Sensitivity analyses were conducted by sequentially removing each study to evaluate its influence on the overall pooled estimates.
All statistical analyses were conducted using R software (version R 4.4.1; http://www.r-project.org/) with the ‘meta’ (https://cran.r-project.org/package=meta) and ‘metafor’ (https://cran.r-project.org/package=metafor) packages. Two-tailed P<0.05 was considered to indicate a statistically significant difference, except where otherwise specified.
Assessment of publication bias
Publication bias was evaluated using funnel plots for visual inspection, and Egger's regression asymmetry test was conducted to statistically assess the presence of small-study effects (59,60). P<0.05 was considered indicative of potential publication bias.
Quality assessment
Risk of bias was independently assessed by two reviewers using the Newcastle-Ottawa Scale (NOS) for observational studies (61). The NOS evaluates studies based on three domains: Selection of participants, comparability of study groups, and ascertainment of exposure and outcomes. The maximum score a study could receive is nine stars. Studies scoring seven or more stars were considered high quality, those scoring five to six were considered moderate quality, and those scoring below five were considered low quality. Any discrepancies in quality assessments were resolved by consensus.
Results
Study selection
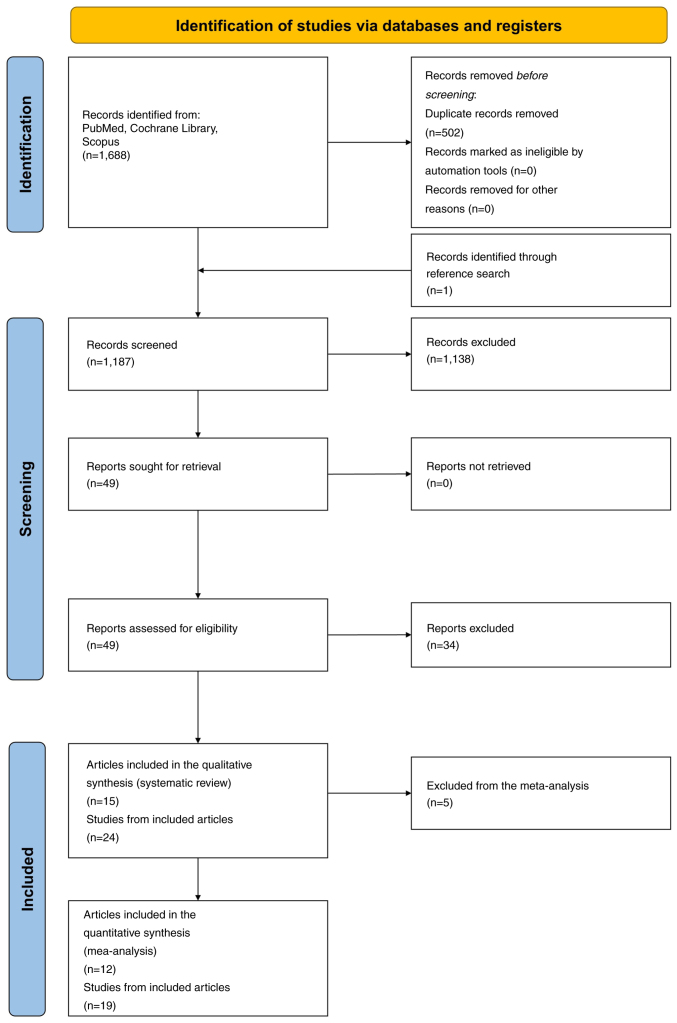
The systematic search yielded a total of 1,688 articles from PubMed, Cochrane Library and Scopus (Fig. 1). One additional study was identified through cross-referencing. After removing duplicates, 1,187 articles were screened based on title and abstract, and 1,138 were excluded as irrelevant to the research question. The full manuscripts for the remaining 49 articles were acquired. Upon thorough examination, 34 articles were excluded based on the eligibility criteria, including lack of a control group, non-human subjects and insufficient information on TL.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the systematic review and meta-analysis.
Subsequently, all studies with overlapping samples for the same outcome were excluded from the present analysis. Specifically, the study by Han et al (2009) (62) on BCC in the Nurses' Health Study (NHS) population, the study by Nan et al (2011) (63) on melanoma in the NHS population, and the study by Weischer et al (2013) (64) on melanoma in the Copenhagen City Heart Study & Copenhagen General Population Study population were not included, thus the included studies were 24 derived from 15 articles.
Study characteristics
The key characteristics of the included studies are detailed in Table ITable IITable III [country, study type and design, population demographics (ethnicity, sex distribution, age of cases and controls), sample sizes, TL measurement methods, DNA source, effect estimates with 95% confidence intervals, TL categorization and covariates adjusted for]. Among the 24 studies included, 12 focused on melanoma, seven on BCC, and five on SCC. The studies eligible for quantitative analysis comprised 19 studies, among which 12 focused on melanoma, four on BCC and three on SCC.
Table I.
General characteristics of studies included in the meta-analysis.
| First author, year | Country | Type | Population | Ethnicity | Male cases | Male controls | Mean age of cases, years | Mean age of controls, years | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Han, 2009 | USA | Melanoma | NHS | European | 0% | 0% | 63.3 | 64.5 | (62) |
| Han, 2009 | USA | SCC | NHS | European | 0% | 0% | 64.7 | 64.5 | (62) |
| Nan, 2011 | USA | Melanoma | WHI-OS | European | 0% | 0% | 68.2 | 63.9 | (63) |
| Nan, 2011 | USA | Melanoma | HPFS | European | 100% | 100% | 65.3 | 59.9 | (63) |
| Liang, 2011 | USA | SCC | HPFS | Caucasian | 100% | 100% | 69 | 63.1 | (75) |
| Liang, 2011 | USA | BCC | NHS | Caucasian | 0% | 0% | 66.1 | 56.3 | (75) |
| Liang, 2011 | USA | BCC | NHS | Caucasian | 0% | 0% | 69.3 | 61 | (75) |
| Bodelon, 2012 | Italy | Melanoma | Hospital-based | Mediterranean Caucasian | Mixed-not specified | Mixed-not specified | Not specified | Not specified | (69) |
| Anic, 2013 | USA | Melanoma | Hospital-based | European | 57% | 35% | 58.6 | 56.3 | (66) |
| Anic, 2013 | USA | SCC | Hospital-based | European | 67% | 35% | 64.8 | 56.3 | (66) |
| Anic, 2013 | USA | BCC | Hospital-based | European | 60% | 35% | 63 | 56.3 | (66) |
| Burke, 2013 | USA | Melanoma | Hospital-based | Caucasian | 50.40% | 41.80% | Not specified | Not specified | (72) |
| Llorca-Cardeñosa, 2014 | Spain | Melanoma | Hospital-based | Caucasian (Spanish origin) | 45.40% | 42.90% | 53 | 48 | (70) |
| Menin, 2016 | Italy | Familial melanoma | Hospital-based | Caucasian | 45.00% | 43.50% | 54.2 | 53.8 | (71) |
| Menin, 2016 | Italy | Sporadic melanoma | Hospital-based | Caucasian | 42.00% | 43.50% | 53.5 | 53.8 | (71) |
| Rode, 2016 | Denmark | Melanoma | CGPS & CCHS/Population | Danish (European) | Mixed-Not specified | Mixed-Not specified | Not specified | Not specified | (50) |
| Rachakonda, 2018 | Germany | Melanoma | Hospital-based | Caucasian | 0.405 | 0.448 | 54 | 44 | (67) |
| Srinivas, 2019 | Hungary/Romania/Slovakia | BCC | Hospital-based | Central/Eastern European | 44.70% | 51.40% | 67 | 61 | (74) |
| Schneider, 2022 | UK | Melanoma | UK Biobank/Population | Predominantly Caucasian | Mixed-not specified | 46% | Not specified | 57 | (68) |
BCC, basal cell carcinoma; SCC, squamous cell carcinoma; NHS, Nurses' Health Study; WHI-OS, Women's Health Initiative Observational Study; HPFS, Health Professionals Follow-up Study; CCHS, Copenhagen City Heart Study; CGPS, Copenhagen General Population Study.
Table II.
Summary of study characteristics and associated risk estimates across studies included in the meta-analysis.
| First author, year | Study design | Cases/controls | Measurement method | DNA source | Relative risk (95% CI) | TL categorization | Factors adjusted | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Han, 2009 Melanoma (NHS) | Nested case-control | 218/870 | RTL (T/S ratio) qPCR | PBLs | 0.62 (0.31–1.23) | RTL Q4 (longest)/Q1 (shortest) | Age, number of moles | (62) |
| Han, 2009 SCC (NHS) | Nested case-control | 285/870 | RTL (T/S ratio) qPCR | PBLs | 0.72 (0.33–1.55) | RTL Q4 (longest)/Q1 (shortest) | Age, number of moles | (62) |
| Nan, 2011 Melanoma (WHI-OS) | Nested case-control | 233/237 | RTL (T/S ratio) qPCR | PBLs | 0.40 (0.18–0.88) | RTL Q4 (longest)/Q1 (shortest) | Age | (63) |
| Nan, 2011 Melanoma (HPFS) | Nested case-control | 120/120 | RTL (T/S ratio) qPCR | PBLs | 0.18 (0.06–0.56) | RTL Q4 (longest)/Q1 (shortest) | Age | (63) |
| Liang, 2011 SCC (HPFS) | Nested case-control | 241/241 | RTL (T/S ratio) qPCR | PBLs | 1.09 (0.62–1.93) | RTL Q4 (longest)/Q1 (shortest) | Age | (75) |
| Liang, 2011 BCC (NHS 1) | Nested case-control | 260/260 | RTL (T/S ratio) qPCR | PBLs | 0.96 (0.49–1.87) | RTL Q4 (longest)/Q1 (shortest) | Age | (75) |
| Liang, 2011 BCC (NHS 2) | Nested case-control | 363/1683 | RTL (T/S ratio) qPCR | PBLs | 0.91 (0.66–1.25) | RTL Q4 (longest)/Q1 (shortest) | Age | (75) |
| Bodelon, 2012 Melanoma | Case-control | 172/196 | RTL (T/S ratio) qPCR | PBLs | 0.87 (0.46–1.64) | RTL as a continuous variable | Age, sex | (69) |
| Anic, 2013 Melanoma | Case-control | 198/372 | RTL (T/S ratio) qPCR | PBLs | 0.22 (0.13–0.38) | RTL Q3 (longest)/Q1 (shortest) | Age, sex | (66) |
| Anic, 2013 SCC | Case-control | 136/372 | RTL (T/S ratio) qPCR | PBLs | 25.00 (11.11–50.00) | RTL Q3 (longest)/Q1 (shortest) | Age, sex | (66) |
| Anic, 2013 BCC | Case-control | 185/372 | RTL (T/S ratio) qPCR | PBLs | 10.00 (5.26–16.67) | RTL Q3 (longest)/Q1 (shortest) | Age, sex | (66) |
| Burke, 2013 Melanoma | Case-control | 119/208 | RTL (T/S ratio) qPCR | PBLs | 0.36 (0.13–0.98) | RTL Q3 (longest)/Q1 (shortest) | Age, sex, DNA source, CDKN2A status, number of moles, solar injury, MC1R genotype | (72) |
| Llorca-Cardeñosa, 2014 Melanoma | Case-control | 406/406 | RTL (T/S ratio) qPCR | PBLs | 0.04 (0.01–0.10) | RTL Q4 (longest)/Q1 (shortest) | Age | (70) |
| Menin, 2016 FM | Case-control | 109/216 | RTL (T/S ratio) qPCR | PBLs | 0.46 (0.23–0.95) | RTL Q4 (longest)/Q1 (shortest) | Age, sex | (71) |
| Menin, 2016 SM | Case-control | 201/216 | RTL (T/S ratio) qPCR | PBLs | 1.74 (1.00–3.04) | RTL Q4 (longest)/Q1 (shortest) | Age, sex | (71) |
| Rode, 2016 Melanoma | Cohort study | 289/64750 | RTL (T/S ratio) qPCR | PBLs | 0.98 (0.95–1.01) | RTL as a continuous variable, per 200 base-pairs decrease | Age, sex, body mass index, smoking status, tobacco consumption, alcohol intake | (50) |
| Rachakonda, 2018 Melanoma | Case-control | 1275/904 | RTL (T/S ratio) qPCR | PBLs | 0.41 (0.33–0.52) | RTL as continuous variable | Age, sex, skin phototype, hair colour, eye colour, sun burns, outdoor occupation, number of nevi | (67) |
| Srinivas, 2019 BCC | Case-control | 506/513 | RTL (T/S ratio) qPCR | PBLs | 4.09 (2.86–5.85) | RTL Q3 (longest)/Q1 (shortest) | Age, sex, country, arsenic exposure, skin complexion, skin response, MC1R genotype, XRCC3 rs861539 | (74) |
| Schneider, 2022 Melanoma | Cohort study | NR/472 432 | RTL (T/S ratio) qPCR | PBLs | 0.80 (0.71–0.89) | RTL as a continuous variable, decreased telomere length (per SD) | Age, sex, body mass index, ethnicity, current smoking, cumulative smoking, alcohol intake, Charlson Comorbidity Index | (68) |
TL, telomere length; RR, relative risk; CI, confidence interval; qPCR, quantitative polymerase chain reaction; RTL, relative telomere length; T/S ratio, telomere repeat copy number/single-copy gene copy number; FM, familial melanoma; SM, sporadic melanoma; BCC, basal cell carcinoma; SCC, squamous cell carcinoma; NHS, Nurses' Health Study; WHI-OS, Women's Health Initiative Observational Study; HPFS, Health Professionals Follow-up Study; PBLs, peripheral blood leukocytes.
Table III.
General characteristics and findings of studies included in the systematic review but not in the meta-analysis.
| First author, year | Country | Type | Cases/controls | Measurement method | DNA source | Findings | (Refs.) |
|---|---|---|---|---|---|---|---|
| Wainwright, 1995 | UK | BCC | 20/19 | Southern blotting | Fresh/frozen skin tissue | Telomeres in 13/20 BCC cases were longer than in the adjacent epidermis (no measure of significance reported) | (76) |
| Perrem, 2007 | Ireland | SCC | 44/10 (RTR) | TEL-FISH | Archival tissue samples | Telomeres in SCC tissue from RTRs were significantly shorter than those in normal skin from RTRs (P=0.0001) | (77) |
| Perrem, 2007 | Ireland | SCC | 44/10 | TEL-FISH | Archival tissue samples | Telomeres in SCC tissue from non-RTRs were significantly shorter than those in normal skin (P=0.0001) | (77) |
| Perrem, 2008 | Ireland | BCC | 35/10 (RTR) | TEL-FISH | Archival tissue samples | Telomeres in BCC tissue from RTRs were significantly shorter than those in normal skin (P=0.0130) | (73) |
| Perrem, 2008 | Ireland | BCC | 35/10 | TEL-FISH | Archival tissue samples | Telomeres in BCC were shorter than those in normal skin, but the observation did not reach statistical significance (P=0.2530) | (73) |
BCC, basal cell carcinoma; SCC, squamous cell carcinoma; TEL-FISH, telomere fluorescence in situ hybridization; RTR, renal transplant recipient.
The majority of the studies included in the present qualitative synthesis were conducted in the United States (11 studies), with additional studies from Ireland (four studies), Italy (three studies), Germany (one study), UK (two studies), Denmark (one study), Spain (one study) and Hungary/Romania/Slovakia (one study). In the meta-analysis, the United States (11 studies) was the most prevalent location, followed by Italy (three studies), Denmark (one study), Spain (one study), Hungary/Romania/Slovakia (one study), Germany (one study) and the UK (one study). Participants in the included studies were predominantly of European and American ancestry. In the meta-analysis, the population was exclusively Western. The age of participants ranged from mid-50s to early 70s. Two studies focused exclusively on male patients and five exclusively on female patients. In studies included in the meta-analysis, a combination of nested case-control designs (seven studies), prospective cohort studies (two studies) and case-control studies (10 studies) was observed. The source of DNA was PBL in 19 studies and malignant tissue samples in five studies. Quantitative polymerase chain reaction (qPCR) was the primary method for TL determination (19 studies), followed by telomere fluorescence in situ hybridization (TEL-FISH) (four studies) and Southern blotting (one study). All studies in the meta-analysis measured TL using qPCR techniques on PBLs, ensuring methodological consistency.
Relative risk measures were reported as ORs with 95% CIs provided, or as HRs in some melanoma studies, where the rare disease principle applies, thus HRs were considered equivalent to ORs (65). In four studies where the short TL was used as the reference, the relative risk was inverted to achieve homogeneity (66,67). Two studies analysed TL as a continuous variable, assessing risk per unit decrease or increase in TL. In studies reporting risk per increase, the relative risk was inverted to maintain homogeneity in the direction of the effect (50,68).
Qualitative synthesis
The association between TL and melanoma risk was investigated in the study by Han et al (2009) (62), where no significant association was found between TL and melanoma risk (OR: 0.62; 95% CI: 0.31–1.23). Similarly, Bodelon et al (2012) (69) reported no significant association (OR: 0.87; 95% CI: 0.46–1.64).
Nan et al (2011) (63) revealed a significant inverse association in the Women's Health Initiative Observational Study cohort (OR: 0.40; 95% CI: 0.18–0.88) and the Health Professionals Follow-up Study cohort (OR: 0.18; 95% CI: 0.06–0.56), indicating that shorter telomeres were associated with reduced melanoma risk. Anic et al (2013) (66) also observed a strong inverse association (OR: 0.22; 95% CI: 0.13–0.38). Llorca-Cardeñosa et al (2014) (70) reported a significant inverse association between shorter telomeres and melanoma risk (OR: 0.04; 95% CI: 0.01–0.10). Rachakonda et al (2018) (67) also demonstrated a significant association (OR: 0.41; 95% CI: 0.33–0.52), reinforcing the hypothesis that shorter telomeres reduce melanoma risk. By contrast, Menin et al (2016) (71) reported that, in sporadic melanoma cases, shorter telomeres were associated with an increased risk (OR: 1.74; 95% CI: 1.00–3.04; P=0.051), although the association did not reach statistical significance, whereas in familial cases, shorter telomeres were associated with a reduced risk (OR: 0.46; 95% CI: 0.23–0.95). Burke et al (2013) (72) also identified an inverse association between short TL and familial melanoma risk (OR: 0.36; 95% CI: 0.13–0.98). Large cohort studies provided mixed results. Rode et al (2016) (50) found no significant association (OR: 0.98; 95% CI: 0.95–1.01) in a Danish population-based cohort. Schneider et al (2022) (68), analysing the UK Biobank data, observed that shorter telomeres were associated with decreased melanoma risk (OR: 0.80; 95% CI: 0.71–0.89).
In studies in BCC, Perrem et al (2008) (73) found significantly shorter telomeres in BCC in renal transplant recipients compared with in normal skin; however, in non-transplant patients the relationship did not reach significance. Anic et al (2013) (66) observed a strong significant association, suggesting that shorter telomeres may increase BCC risk (OR: 10.00; 95% CI: 5.26–16.67). Srinivas et al (2019) (74) concurred, finding a significant association, with shorter telomeres increasing BCC risk (OR: 4.09; 95% CI: 2.86–5.85). By contrast, Liang et al (2011) (75) conducted two nested case-control studies within the NHS and found no significant association (NHS 1: OR: 0.96; 95% CI: 0.49–1.87; NHS 2: OR: 0.91; 95% CI: 0.66–1.25). Wainwright et al (1995) (76) found that in BCC tissue samples, telomeres were longer than in the adjacent epidermis, but no measure of significance was reported.
In SCC, Perrem et al (2007) (77) found significantly shorter telomeres in SCC in both renal transplant recipients and non-transplant patients compared with that in normal skin. Anic et al (2013) (66) observed a strong significant association (OR: 25.00; 95% CI: 11.11–50.00), indicating shorter telomeres increase SCC risk. By contrast, Han et al (2009) (62) found no significant association between TL and SCC risk (OR: 0.72; 95% CI: 0.33–1.55). Liang et al (2011) (75) also reported no significant association (OR: 1.09; 95% CI: 0.62–1.93).
Meta-analysis results
The meta-analysis was conducted using the random-effects model, based on the DerSimonian and Laird method, due to the substantial heterogeneity observed among the studies, as indicated by high I2 statistics and significant Cochran's Q tests. This model accounts for both within-study and between-study variability attributable to heterogeneity across studies (56,58).
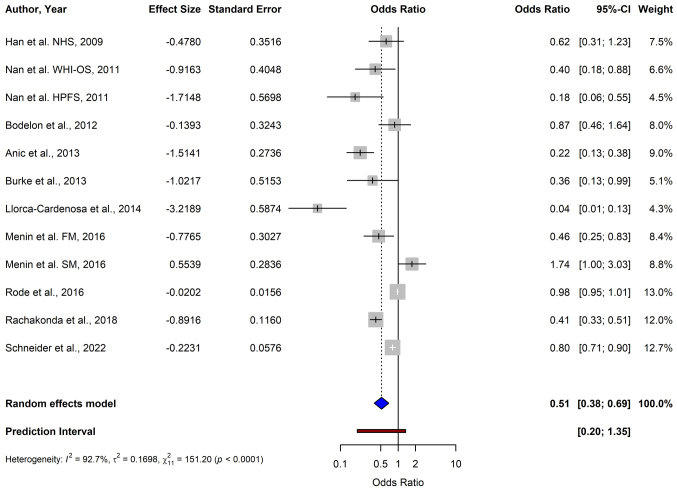
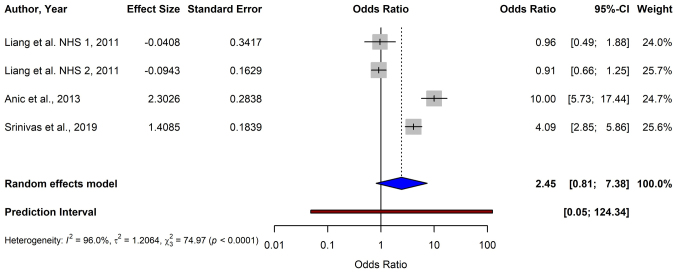
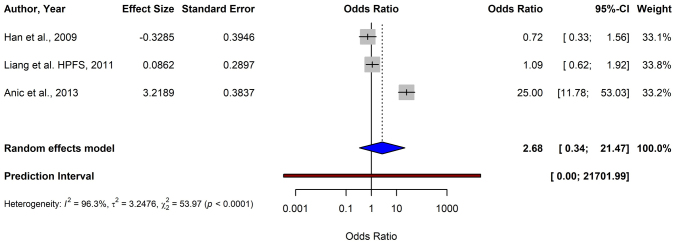
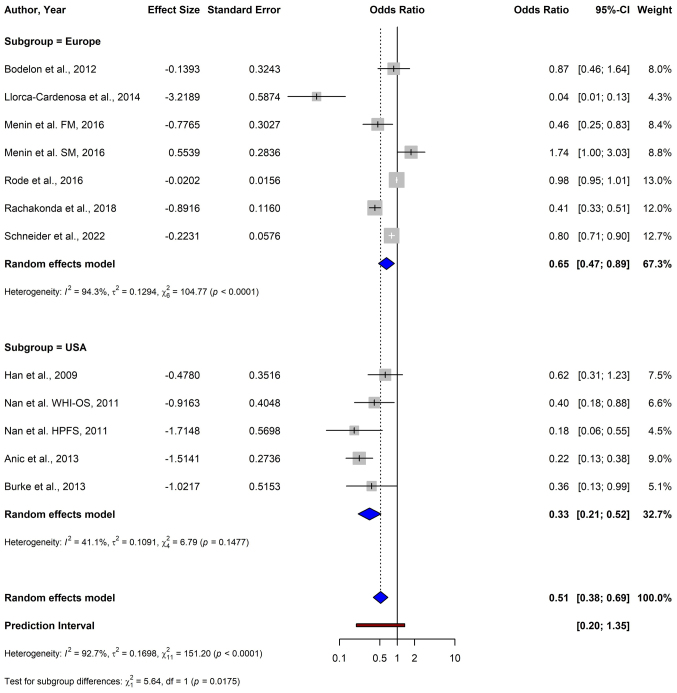
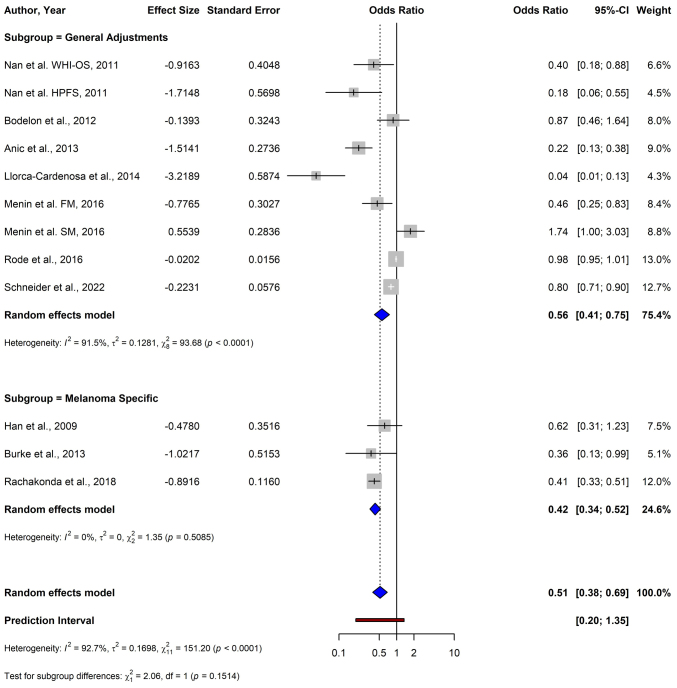
For melanoma, the pooled OR from 12 studies using the random-effects model was 0.51 (95% CI: 0.38–0.69), demonstrating a significant association between shorter TL and a reduced risk of melanoma (P<0.0001) (Fig. 2). For BCC, the data from four studies yielded a pooled OR of 2.45 (95% CI: 0.81–7.38), indicating no significant association between TL and BCC risk (P=0.1124) (Fig. 3). The meta-analysis of SCC included three studies, and resulted in a pooled OR of 2.68 (95% CI: 0.34–21.47), which was not statistically significant (P=0.3519) (Fig. 4).
Figure 2.
Forest plot of a meta-analysis depicting the association between telomere length and melanoma risk using a random-effects model. CI, confidence interval; FM, familial melanoma; SM, sporadic melanoma; NHS, Nurses' Health Study; WHI-OS, Women's Health Initiative Observational Study; HPFS, Health Professionals Follow-up Study.
Figure 3.
Forest plot of a meta-analysis depicting the relationship between telomere length and basal cell carcinoma risk based on a random-effects model. CI, confidence interval; NHS, Nurses' Health Study.
Figure 4.
Forest plot of a meta-analysis depicting the association between telomere length and squamous cell carcinoma risk using a random-effects model. CI, confidence interval; HPFS, Health Professionals Follow-up Study.
Subgroup analysis results
To further elucidate the relationship between TL and melanoma risk, subgroup analyses were conducted based on geographic location, sex, genetic predisposition, population source and level of adjustment. Subgroup analyses for BCC and SCC were not performed due to paucity of data.
Geographic location
In subgroup analyses based on geographic location, a significant inverse association between short TL and melanoma risk was observed across geographic locations. Studies conducted in the USA demonstrated a stronger association, with a pooled OR of 0.33 (95% CI: 0.21–0.52), compared with studies conducted in Europe, which reported a pooled OR of 0.65 (95% CI: 0.47–0.89) (Fig. 5). The test for subgroup differences was statistically significant (P=0.0175), indicating that while the association was significant in both groups, the magnitude of the effect may vary by geographic region.
Figure 5.
Forest plot of a subgroup analysis depicting the association between telomere length and melanoma risk stratified by geographic location (USA vs. Europe) using a random-effects model. CI, confidence interval; FM, familial melanoma; SM, sporadic melanoma; WHI-OS, Women's Health Initiative Observational Study; HPFS, Health Professionals Follow-up Study.
Sex
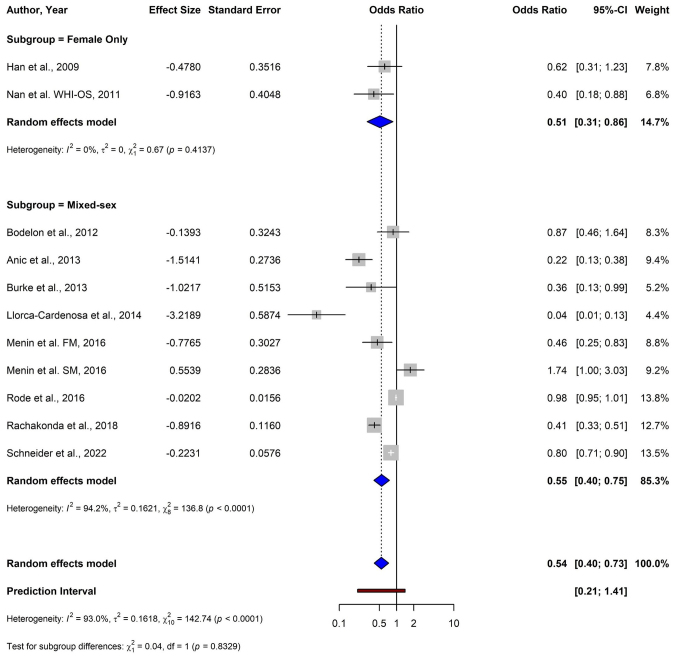
In studies restricted to female patients, a significant inverse association between shorter TL and melanoma risk, with a pooled OR of 0.51 (95% CI: 0.31–0.86) was reported (Fig. 6). Mixed-sex studies demonstrated a similar association, with a pooled OR of 0.55 (95% CI: 0.40–0.75). The test for subgroup differences between female-only studies and mixed-sex studies was not statistically significant (P=0.833), suggesting that the effect of TL on melanoma risk was consistent across the sexes.
Figure 6.
Forest plot of a subgroup analysis depicting the association between telomere length and melanoma risk stratified by sex (female-only vs. mixed-sex studies) using a random-effects model. CI, confidence interval; FM, familial melanoma; SM, sporadic melanoma; WHI-OS, Women's Health Initiative Observational Study.
Genetic predisposition
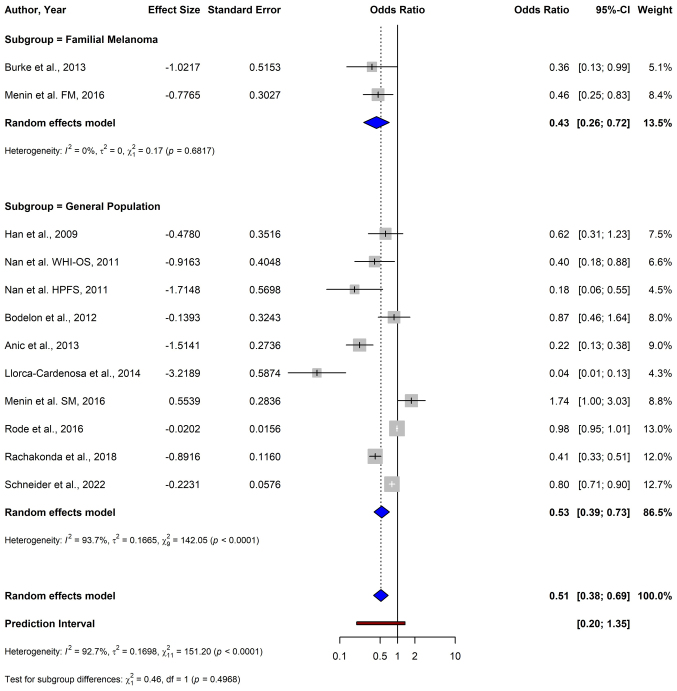
In familial melanoma, a significant inverse association between shorter TL and melanoma risk was shown (pooled OR: 0.43; 95% CI: 0.26–0.72) (Fig. 7). In the general population studies, the pooled OR was 0.53 (95% CI: 0.39–0.73), indicating a similar significant association. The test for subgroup differences was not significant (P=0.497). Additionally, to examine the potential impact of familial melanoma cases that may have been included in the general population studies, an analysis was performed assuming 10% familial melanoma cases in the general population. It was assumed that familial cases in the general population had an effect of OR=0.43, as indicated by the subgroup analysis, and sporadic cases had an OR of 1, indicating no association. As ORs are multiplicative, the calculation was performed on the logarithmic scale: A weighted average of the log(OR) values was computed, with weights reflecting the assumed proportions of familial and sporadic cases. The resulting weighted log(OR) was then exponentiated to obtain the expected overall OR of 0.92.
Figure 7.
Forest plot of a subgroup analysis depicting the association between telomere length and melanoma risk stratified by genetic predisposition (familial vs. general population studies) using a random-effects model. CI, confidence interval; FM, familial melanoma; SM, sporadic melanoma; WHI-OS, Women's Health Initiative Observational Study; HPFS, Health Professionals Follow-up Study.
Population source
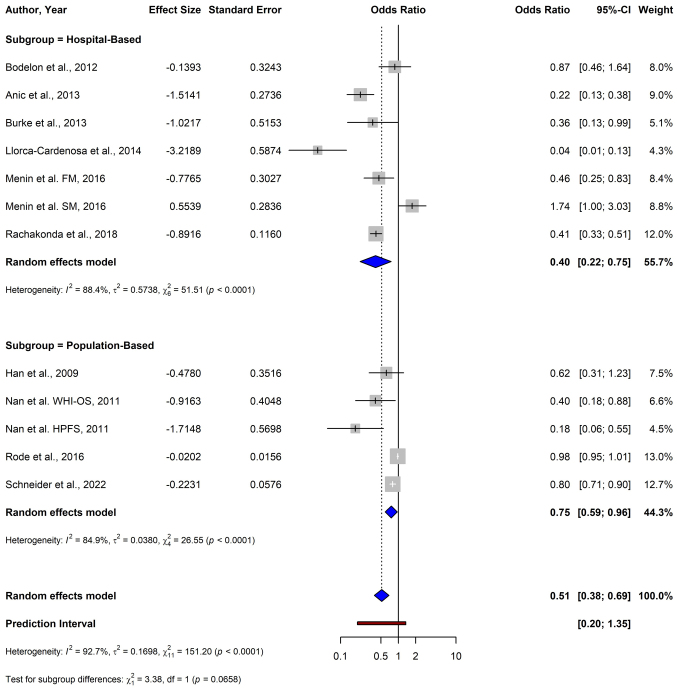
Subgroup analysis based on the source of the sample reported a significant inverse association between shorter TL and melanoma risk, with a pooled OR of 0.40 (95% CI: 0.22–0.75) in hospital-based studies (Fig. 8). Similarly, in population-based studies, a significant protective association, with a pooled OR of 0.75 (95% CI: 0.59–0.96) was shown. The test for subgroup differences between hospital- and population-based studies was not significant (P=0.066).
Figure 8.
Forest plot of a subgroup analysis depicting the association between telomere length and melanoma risk stratified by population source (hospital-based vs. population-based studies) using a random-effects model. CI, confidence interval; FM, familial melanoma; SM, sporadic melanoma; WHI-OS, Women's Health Initiative Observational Study; HPFS, Health Professionals Follow-up Study.
Adjustment level
Subgroup analysis based on stratification by adjustment level demonstrated significant inverse associations between TL and melanoma risk, with a pooled OR of 0.42 (95% CI: 0.34–0.52) in the melanoma-specific subgroup and a pooled OR of 0.56 (95% CI: 0.41–0.75) in the subgroup with general adjustments [non-melanoma-specific adjustments, such as demographic factors (e.g., age, sex) and lifestyle variables (e.g., smoking, alcohol consumption)] (Fig. 9). The test for subgroup differences was not significant (P=0.151).
Figure 9.
Forest plot of a subgroup analysis depicting the association between telomere length and melanoma risk stratified by adjustment level (melanoma-specific vs. general adjustments) using a random-effects model. CI, confidence interval; FM, familial melanoma; SM, sporadic melanoma; WHI-OS, Women's Health Initiative Observational Study; HPFS, Health Professionals Follow-up Study.
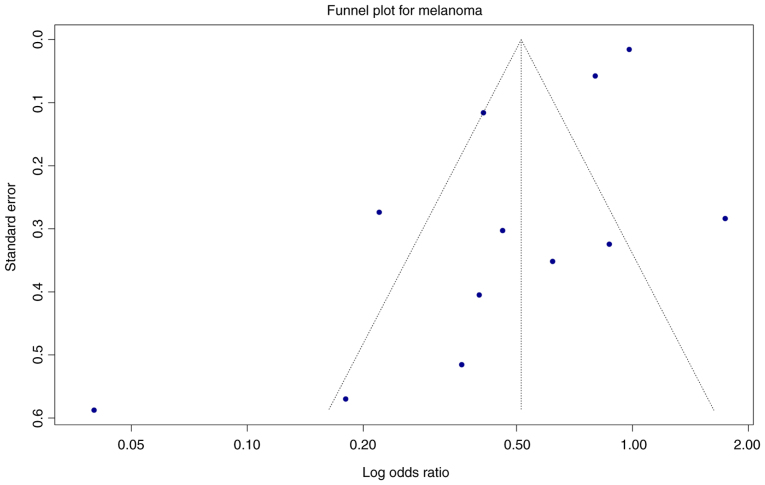
Publication bias
The visual inspection of the funnel plot suggested some asymmetry, especially at the lower end among the smaller studies (Fig. 10). Publication bias was evaluated quantitatively using Egger's test for funnel plot asymmetry. For melanoma studies, Egger's test indicated significant evidence of funnel plot asymmetry, which can be suggestive of publication bias, with a bias estimate of −2.94 (SE=0.89), suggesting potential small-study effects (59,60). For BCC and SCC, publication bias could not be assessed due to the small number of included studies (n=4 studies for BCC, n=3 studies for SCC); this limitation reflects the methodological constraints that require a minimum of 10 studies to produce reliable results (59,60).
Figure 10.
Funnel plot of studies included in the melanoma meta-analysis.
Sensitivity analysis
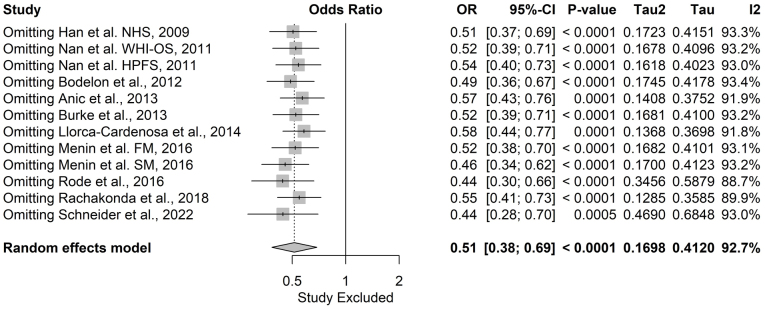
A sensitivity analysis was conducted to evaluate the robustness of the meta-analysis by systematically omitting each study and recalculating the pooled effect size and heterogeneity measures.
In melanoma, the pooled OR ranged from 0.44 (95% CI: 0.28–0.70) to 0.58 (95% CI: 0.44–0.77), indicating that no single study disproportionately influenced the overall findings (Fig. 11). The sensitivity analysis confirmed the stability of the meta-analysis, with the association between shorter TL and reduced melanoma risk remaining statistically significant under all scenarios.
Figure 11.
Leave-one-out sensitivity analysis for the association between telomere length and melanoma risk. OR, odds ratio; CI, confidence interval; FM, familial melanoma; SM, sporadic melanoma; NHS, Nurses' Health Study; WHI-OS, Women's Health Initiative Observational Study; HPFS, Health Professionals Follow-up Study.
For BCC, the sensitivity analysis showed a wide range of pooled ORs, from 1.55 (95% CI: 0.52–4.57) to 3.46 (95% CI: 1.13–10.58) (Fig. S1). In the case of SCC, the pooled OR ranged from 0.94 (95% CI: 0.60–1.49) to 5.17 (95% CI: 0.24–111.31) (Fig. S2). The results for BCC and SCC reflected the substantial variability and suggested that the findings may not be robust.
Quality assessment
Quality assessment using the NOS showcased a predominance of high-quality studies. All studies included in the meta-analysis were high quality, demonstrating robust methodologies. Among the studies included only in the qualitative analysis, most were categorized as of moderate quality (Table SI, Table SII, Table SIII).
Discussion
The present meta-analysis revealed a significant association between longer TL and increased melanoma risk, whereas the associations for BCC and SCC, did not reach statistical significance. Nonetheless, a trend towards increased risk being linked with shorter telomeres was observed in the qualitative synthesis for BCC and SCC.
The association between longer TL and melanoma risk demonstrated in the current meta-analysis may appear counterintuitive, as telomere shortening is generally associated with genomic instability and increased cancer risk (28,33,78,79). Telomere biology exhibits a complex role in oncogenesis; while short telomeres can promote chromosomal instability and tumourigenesis, they may also inhibit tumour progression by enforcing replicative limits (40,80). The present findings align with those of a previous meta-analysis in melanoma by Caini et al (2015) (49), and similar associations have been noted in other types of cancer (49–51). Further exploration of the underlying biological mechanisms is warranted.
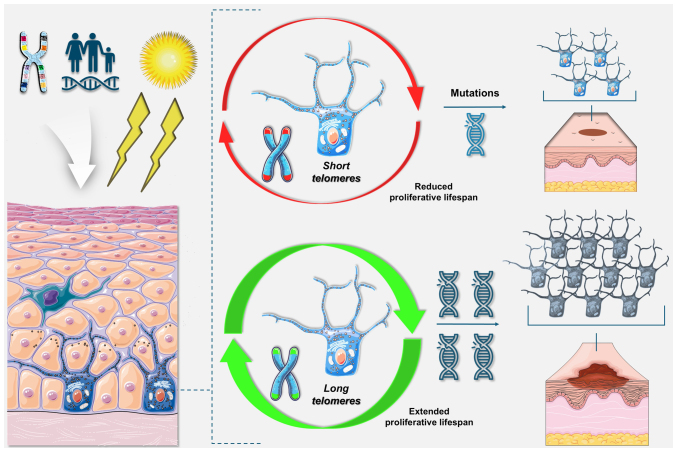
Melanocytes are characterised by low proliferative rates and limited apoptotic capacity, and they may be prone to senescence in response to oncogenic stress rather than apoptosis (62,63,81). Longer telomeres might extend the proliferative lifespan of melanocytes harbouring oncogenic mutations, thereby increasing the likelihood of accumulating additional genetic alterations that could precipitate malignant transformation (Fig. 12) (19,62,63). While an association has been demonstrated between increased TL and total number of nevi, there is evidence that longer TL is associated with increased melanoma risk independently of nevus count, as the protective role of shorter telomeres persists even after adjusting for nevus count and other risk factors (22,62,63,82).
Figure 12.
Telomere dynamics in melanoma tumourigenesis. Schematic diagram illustrating a potential mechanism underlying the protective effect of shorter telomeres in melanoma. Exposure to ultraviolet radiation induces mutations in melanocytes, particularly in individuals with familial melanoma or those harbouring predisposing mutations. Mutated melanocytes with shorter telomeres have a restricted proliferative capacity, limiting their potential for malignancy. Mutated melanocytes with long telomeres exhibit an increased proliferative lifespan, allowing for the accumulation of additional mutations, which may be corollary to melanoma development. Elements of the figure were created using components from Servier Medical Art. Servier Medical Art by Servier is licensed under Creative Commons Attribution 4.0 (https://creativecommons.org/licenses/by/4.0/).
Genetic contributions to telomere dynamics also serve a crucial role in melanoma susceptibility, with genetic loci regulating TL implicated in melanoma pathogenesis (83–85). Evidence has suggested that telomere elongation appears to be the mechanism linking telomerase reverse transcriptase (TERT) promoter and shelterin mutations to cancer, rather than telomere deprotection (86). Carriers of TERT promoter and protection of telomeres 1 (POT1) mutations exhibit longer telomeres compared with unaffected relatives, and families with POT1 and TPP1 mutations demonstrate genetic anticipation for both cancer onset and mortality, suggesting successive telomere elongation across generations (67,86,87). The association between long telomere-associated single nucleotide polymorphisms and increased cancer risk has been observed in multiple types of cancer, including melanoma, glioma and chronic lymphocytic leukaemia (50,86,88,89). Concurrently, short telomeres have been associated with poorer survival outcomes in patients with melanoma (85,90). A potential explanation for this is that longer telomeres confer an increased melanoma risk; however, once the cancer has developed, shorter telomeres may lead to increased genomic instability, thus contributing to worse survival outcomes (85,90).
It has been suggested that the mechanism of interaction between telomere dynamics and melanoma risk may vary between sporadic and familial melanoma (71). Menin et al (2016) (71) demonstrated the association between increased familial melanoma risk and longer telomeres, concurring with evidence in familial melanoma from another study (72); however, Menin et al (71) also reported an inverse relationship in sporadic melanoma, with shorter TL being associated with increased risk. Studies have observed a similar phenomenon in other types of cancer, including breast and ovarian cancers, where short telomeres are linked to increased risk in familial but not in sporadic cancer (91,92).
In the subgroup analysis of exclusively familial melanoma and melanoma in the general population, the test for subgroup differences was not significant. In both subgroups, there was a significant inverse relation between TL and melanoma risk, and the difference in magnitude of the effect did not reach significance. The exact distribution of familial cases in each general population study was not available; however, familial melanoma constitutes 5–10% of melanoma cases (93,94). To further examine the validity of the present findings, an analysis was performed assuming 10% familial melanoma cases in the general population and concluded that familial cases alone cannot account for the observed overall association. The analysis showed that familial cases alone could not account for the overall association as the expected overall OR would have been 0.92 not the observed 0.51 (95% CI: 0.38–0.68). Therefore, the present study indicated that there may be no significant difference in the association of TL and melanoma risk between sporadic and familial melanoma cases. Nevertheless, this analysis is speculative in nature and further large cohort studies that explicitly delineate between familial and sporadic melanoma are imperative to validate the results.
The subgroup analysis based on geographic location revealed significant differences in the magnitude of the association between TL and melanoma risk, with studies conducted in the USA showing a stronger inverse association between TL and melanoma risk (test for subgroup difference P=0.0175). In both groups the subjects were primarily of European or Caucasian descent, so while ethnic differences in TL have been documented, they may not account for the findings (95). Environmental factors may serve a role, particularly UV radiation exposure, the intensity of which varies geographically, and has been documented to impact telomere dynamics and serve a crucial role in melanoma development (21,96–98). Methodological differences among studies, including study design and adjustment for confounders, might also explain part of the variation. Standardization of methodologies is essential to reduce heterogeneity and enable more accurate comparisons across regions, as well as the inclusion of environmental exposure biomarkers.
It has been suggested that telomere dynamics may differ between the sexes, with women having longer telomeres compared with men (48,99); a potential mechanism for this is the potential protective role oestrogen may serve, as it can stimulate telomerase activity through the oestrogen-responsive element present in the human TERT gene (100). In a meta-analysis of the association of colorectal cancer risk and TL, a shift in effect estimates was observed in female patients (48). However, the present subgroup analysis revealed no statistically significant difference in the association between TL and melanoma risk between female-only and mixed-sex studies (test for subgroup differences P=0.833). The similar OR suggested that the reduced risk of melanoma associated with shorter TL is consistent across the sexes, although due to the limited number of male-only studies further research with sex-stratified analyses is warranted to confirm this observation.
Subgroup analysis based on the source of cases revealed significant associations in both hospital-based studies and population-based studies. The test for subgroup differences was not significant (P=0.0658), suggesting that the source of cases may not substantially influence the overall findings. The consistent findings across sample populations indicate the robustness in the association between longer TL and melanoma risk. To explore potential sources of heterogeneity and to assess the potential effect of confounding factors on the robustness of the results, a subgroup analysis was performed on the basis of adjustments for melanoma-specific confounding factors. All studies included in the analysis provided risk estimates adjusted for age, one of the most critical factors affecting TL; however, a minority adjusted for melanoma-specific risk factors (such as number of nevi and UV exposure) (22,31). In the subgroup analysis, the test for the adjustment stratified subgroups found no significant difference (P=0.151), indicating the relationship between melanoma risk and longer telomeres is true and not a confounding artefact. Due to the limited number of melanoma-specific adjusted studies, future research should prioritize collecting comprehensive data on both general and melanoma-specific confounders, enhancing the validity of future findings.
To enable the clinical translation of TL as a skin cancer biomarker, it is recommended that future research implements a multicentre validation framework across geographically and ethnically diverse cohorts. Prospective studies should standardize TL measurement methods and integrate additional biomarkers, such as UV exposure biomarkers, and genomic profiling to refine risk stratification. Measurable clinical endpoints should be prospectively collected to establish the predictive validity of TL. These studies may facilitate the development of comprehensive risk models and the translation of TL measurements into actionable clinical screening and preventative strategies.
In the meta-analysis for associations between TL and BCC risk, and TL and SCC risk, the results did not reach statistical significance and high heterogeneity was observed. Among studies included only in qualitative synthesis, significantly shorter telomeres were detected in SCC tissues compared with in normal skin, whereas the results were mixed in BCC. Given the non-significant quantitative results, caution is warranted in interpreting these findings.
The different associations between TL and skin cancer types may be attributed to the unique proliferative and apoptotic characteristics of melanocytes, basal cells and squamous keratinocytes. Squamous keratinocytes exhibit a high apoptotic threshold and primarily respond to DNA damage by programmed cell death, especially in response to UV-induced genotoxic stress (66,101). Basal cells have a lower senescence rate and reduced apoptotic susceptibility compared with melanocytes (102). These characteristics, combined with the high proliferative demand, may predispose skin cells with shorter telomeres to genomic instability under UV exposure, increasing the risk of malignancy (62,66,102). Conventionally, telomere crisis results in genomic aberrations leading to cancer; however, the specific mechanisms in NMSC remain unclear, the absence of a significant association between TL and telomerase activity suggesting that telomerase-independent mechanisms may serve a role (35,66,101). Moreover, it has been observed that SCC and BCC exhibit significantly shorter TL compared with BD and AK (101,103). Yamada-Hishida et al (2018) (103) suggested that TL may be associated with the malignant potential of NMSCs, with shorter telomeres in SCC and BCC being associated with higher invasive and metastatic risks, positioning TL as a valuable parameter in understanding the biological behaviour of NMSCs (101).
The lack of significant associations for NMSCs in the present meta-analysis may be attributed to the limited number of studies and the substantial heterogeneity observed. Large-scale studies are needed to clarify the relationship between TL and the risk of BCC and SCC, and to ascertain if TL could potentially serve as a biomarker for risk stratification.
Significant heterogeneity was observed in the present meta-analyses, which may stem from numerous factors, including differences in study design, population characteristics and adjustments for confounding variables (104,105). Although subgroup and sensitivity analyses were conducted to explore the sources of heterogeneity, residual heterogeneity remained. A potential contributor to heterogeneity may be the differences in melanoma genomic subtype, unfortunately due to the paucity of data, subgroup analysis on the basis of genomic subtypes was not possible. To address the issue of heterogeneity, future research should aim to standardize methodologies, including genomic subtypes, and to ensure comprehensive adjustment for confounding variables.
Despite the homogeneity in TL measurement methodology and DNA tissue source in the quantitative synthesis, a potential limitation of the present systematic review is the heterogeneity of methods used to measure TL among the qualitative synthesis-only studies. In the quantitative study, qPCR methods were universally employed to measure TL, but studies in the qualitative synthesis used Southern blotting and TEL-FISH analysis, which may vary in precision and sensitivity (106,107). Furthermore, while the quantitative study exclusively used PBLs as the DNA source with independent healthy controls, qualitative synthesis-only studies measured TL in tumour tissue and utilised adjacent normal skin as the control group. These methodological differences could contribute to variability in TL estimates and may partly explain the discrepancy between findings in the quantitative and qualitative synthesis for SCC and BCC.
The present meta-analysis may also be limited by the possibility of publication bias. The results of the Egger's test of melanoma studies suggested funnel plot asymmetry, which can be suggestive of publication bias (59,60). Publication bias may lead to inflation of the estimated effect size in meta-analyses, indicating caution in interpreting the magnitude of the association between TL and melanoma risk (60). However, the reliability of the Egger's test in this context is limited, given the substantial variability in sample sizes, which can inflate the type I error rate. Furthermore, the current analysis included large-scale cohort studies and studies reporting non-significant associations, suggesting that studies with null results were not systematically excluded (60,108). The visual inspection of the funnel plot revealed moderate asymmetry. For BCC and SCC, the small number of included studies (n=4 for BCC, n=3 for SCC) did not allow for formal assessments of publication bias (60). Caution is recommended in interpreting the magnitude of the association as publication bias cannot be entirely ruled out.
Quality assessment using the NOS revealed that almost all studies included in the meta-analysis were of high quality, underscoring the reliability of the present meta-analysis. However, among the qualitative-only studies, most were categorized as of moderate quality. One other potential limitation could be the differences in parametrization of TL, while the majority reported the risk estimates based on quartiles, some studies reported tertiles or continuous measurements. Unfortunately, the studies did not provide sufficient data to recalculate ORs and homogenize TL parametrization, which may contribute to heterogeneity. The present findings may also have limited applicability in diverse ethnic groups, as most studies were conducted in Western countries and had primarily Caucasian patient populations. The lack of diversity highlights the need for further studies with populations from different ethnic backgrounds.
Adjustments for confounding variables also varied among studies. All studies included in the analysis provided risk estimates adjusted for age; however, a minority adjusted for melanoma-specific risk factors. In subgroup analysis, the test for subgroup differences was non-significant (P=0.151), indicating that adjustment for confounders does not influence the observed associations. Nevertheless, due to the limited number of melanoma-specific adjusted studies, future research should prioritize collecting comprehensive data on both general and melanoma-specific confounders, enhancing the validity of future findings.
In conclusion, the findings of the present study suggested a significant association between long TL and increased melanoma risk, challenging the conventional view of an association between telomere shortening and increased cancer risk, and highlighting the complex role of telomere dynamics in carcinogenesis. In melanoma, the robustness of the present findings was indicated by their persistence when adjusting for confounding factors and examining different populations. Furthermore, the association was consistent across the sexes and in familial melanoma. By contrast, the associations between TL and the risks of BCC and SCC were not statistically significant in the quantitative analyses. Qualitative synthesis reported non-significant results in BCC and shorter telomeres in SCC tissues compared with in healthy skin. Further research is imperative to clarify the role of telomere dynamics in BCC and SCC, and to explore the mechanisms underlying the association in melanoma. Future large-scale prospective cohort studies with standardized methodology are essential to validate these findings and to ascertain the utility of TL as a clinically significant skin cancer biomarker.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
DAA conceptualized the study. Both DAA and DAS developed the methodology. The investigation was conducted by DAA and DAS. Validation of the data and findings was carried out by both authors. DAA prepared the original draft of the manuscript, while both DAA and DAS contributed to the review and editing process. DAS supervised the overall project. DAA and DAS confirm the authenticity of all the raw data. Both authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
Use of artificial intelligence tools
During the proofreading stage, artificial intelligence-powered tools were used exclusively to improve the readability and language of the manuscript, and subsequently, the authors revised and edited the content produced as necessary, taking full responsibility for the ultimate content of the present manuscript.
References
- 1.Apalla Z, Nashan D, Weller RB, Castellsagué X. Skin cancer: Epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther (Heidelb) 2017;7((Suppl 1)):S5–S19. doi: 10.1007/s13555-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith H, Wernham A, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692. doi: 10.1136/bmj.m692. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski J, Saltzstein SL, Sadler GR, Tahir Z, Blair S. A Comparison of merkel cell carcinoma and melanoma: Results from the California cancer registry. Clin Med Oncol. 2008;2:327–333. doi: 10.4137/cmo.s423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: A review. J Skin Cancer. 2011;2011:210813. doi: 10.1155/2011/210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CSM. Basal cell carcinoma. BMJ. 2003;327:794–798. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, Bichakjian CK, Ward NL, Dlugosz AA, Wong SY. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim RH, Armstrong AW. Nonmelanoma skin cancer. Dermatol Clin. 2012;30:125–139. doi: 10.1016/j.det.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Motley R, Kersey P, Lawrence C, British Association of Dermatologists; British Association of Plastic Surgeons; Royal College of Radiologists, Faculty of Clinical Oncology Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br J Dermatol. 2002;146:18–25. doi: 10.1046/j.0007-0963.2001.04615.x. [DOI] [PubMed] [Google Scholar]
- 10.Preston DS, Stern RS. Nonmelanoma cancers of the Skin. N Engl J Med. 1992;327:1649–1662. doi: 10.1056/NEJM199212033272307. [DOI] [PubMed] [Google Scholar]
- 11.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: Incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/S0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 12.Gloster HM, Brodland DG. The epidemiology of skin cancer. Dermatol Surg. 1996;22:217–326. doi: 10.1016/1076-0512(95)00570-6. [DOI] [PubMed] [Google Scholar]
- 13.Revenga Arranz F, Paricio Rubio J, Mar Vázquez Salvado M, Del Villar Sordo V. Descriptive epidemiology of basal cell carcinoma and cutaneous squamous cell carcinoma in Soria (north-eastern Spain) 1998–2000: A hospital-based survey. J Eur Acad Dermatol Venereol. 2004;18:137–141. doi: 10.1111/j.1468-3083.2004.00829.x. [DOI] [PubMed] [Google Scholar]
- 14.De Vries E, Trakatelli M, Kalabalikis D, Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D, Sotiriadis D, Ioannides D, Aquilina S, Apap C, et al. Known and potential new risk factors for skin cancer in European populations: A multicentre case-control study: Risk factors for skin cancer in European populations. Br J Dermatol. 2012;167((Suppl 2)):S1–S13. doi: 10.1111/j.1365-2133.2012.11081.x. [DOI] [PubMed] [Google Scholar]
- 15.Fargnoli MC, Piccioni A, Neri L, Tambone S, Pellegrini C, Peris K. Long-term efficacy and safety of daylight photodynamic therapy with methyl amninolevulinate for actinic keratosis of the face and scalp. Eur J Dermatol. 2017;27:89–91. doi: 10.1684/ejd.2016.2882. [DOI] [PubMed] [Google Scholar]
- 16.Alam M, Ratner D. Cutaneous Squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 17.Braeuer RR, Watson IR, Wu C, Mobley AK, Kamiya T, Shoshan E, Bar-Eli M. Why is melanoma so metastatic? Pigment Cell Melanoma Res. 2014;27:19–36. doi: 10.1111/pcmr.12172. [DOI] [PubMed] [Google Scholar]
- 18.Cust AE, Armstrong BK, Goumas C, Jenkins MA, Schmid H, Hopper JL, Kefford RF, Giles GG, Aitken JF, Mann GJ. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425–2435. doi: 10.1002/ijc.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold M, De Vries E, Whiteman DC, Jemal A, Bray F, Parkin DM, Soerjomataram I. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int J Cancer. 2018;143:1305–1314. doi: 10.1002/ijc.31527. [DOI] [PubMed] [Google Scholar]
- 20.Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: Detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med. 2020;144:500–522. doi: 10.5858/arpa.2019-0561-RA. [DOI] [PubMed] [Google Scholar]
- 21.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28:1005–1011. [PubMed] [Google Scholar]
- 23.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 24.Chang C, Murzaku EC, Penn L, Abbasi NR, Davis PD, Berwick M, Polsky D. More skin, more sun, more tan, more melanoma. Am J Public Health. 2014;104:e92–e99. doi: 10.2105/AJPH.2014.302185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonardi GC, Falzone L, Salemi R, Zanghï A, Spandidos DA, Mccubrey JA, Candido S, Libra M. Cutaneous melanoma: From pathogenesis to therapy (Review) Int J Oncol. 2018;52:1071–1080. doi: 10.3892/ijo.2018.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 27.Moon IK, Jarstfer MB. The human telomere and its relationship to human disease, therapy, and tissue engineering. Front Biosci J Virtual Libr. 2007;12:4595–4620. doi: 10.2741/2412. [DOI] [PubMed] [Google Scholar]
- 28.O'Sullivan RJ, Karlseder J. Telomeres: Protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–1781. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Lange T. How telomeres solve the End-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monaghan P, Haussmann MF. Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol. 2006;21:47–53. doi: 10.1016/j.tree.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 32.Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21:349–353. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 35.Maciejowski J, de Lange T. Telomeres in cancer: Tumour suppression and genome instability. Nat Rev Mol Cell Biol. 2017;18:175–186. doi: 10.1038/nrm.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson NJ, Schiemann WP. Telomerase in cancer: Function, regulation, and clinical translation. Cancers. 2022;14:808. doi: 10.3390/cancers14030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Goldkorn A. Telomere and telomerase therapeutics in cancer. Genes. 2016;7:22. doi: 10.3390/genes7060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dratwa M, Wysoczańska B, Łacina P, Kubik T, Bogunia-Kubik K. TERT-Regulation and roles in cancer formation. Front Immunol. 2020;11:589929. doi: 10.3389/fimmu.2020.589929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsatsakis A, Oikonomopoulou T, Nikolouzakis TK, Vakonaki E, Tzatzarakis M, Flamourakis M, Renieri E, Fragkiadaki P, Iliaki E, Bachlitzanaki M, et al. Role of telomere length in human carcinogenesis (Review) Int J Oncol. 2023;63:78. doi: 10.3892/ijo.2023.5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Otín C, Pietrocola F, Roiz-Valle D, Galluzzi L, Kroemer G. Meta-hallmarks of aging and cancer. Cell Metab. 2023;35:12–35. doi: 10.1016/j.cmet.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto K, Seimiya H. Revisiting telomere shortening in cancer. Cells. 2019;8:107. doi: 10.3390/cells8020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andreikos D, Kyrodimos E, Kotsinas A, Chrysovergis A, Papacharalampous GX. The Association between telomere length and head and neck cancer risk: A systematic review and Meta-analysis. Int J Mol Sci. 2024;25:9000. doi: 10.3390/ijms25169000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karimi B, Yunesian M, Nabizadeh R, Mehdipour P, Aghaie A. Is leukocyte telomere length related with lung cancer risk?: A Meta-Analysis. Iran Biomed J. 2017;21:142–153. doi: 10.18869/acadpub.ibj.21.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, Svenson U, Roos G, Hosgood HD, III, Shen M, Wei Q. Shortened telomere length is associated with increased risk of cancer: A meta-analysis. PLoS One. 2011;6:e20466. doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X, Han W, Xue W, Zou Y, Xie C, Du J, Jin G. The association between telomere length and cancer risk in population studies. Sci Rep. 2016;6:22243. doi: 10.1038/srep22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benites-Zapata VA, Ulloque-Badaracco JR, Alarcón-Braga EA, Fernández-Alonso AM, López-Baena MT, Pérez-López FR. Telomerase activity and telomere length in women with breast cancer or without malignancy: A systematic review and meta-analysis. Maturitas. 2024;180:107882. doi: 10.1016/j.maturitas.2023.107882. [DOI] [PubMed] [Google Scholar]
- 48.Naing C, Aung K, Lai PK, Mak JW. Association between telomere length and the risk of colorectal cancer: A meta-analysis of observational studies. BMC Cancer. 2017;17:24. doi: 10.1186/s12885-016-2997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caini S, Raimondi S, Johansson H, De Giorgi V, Zanna I, Palli D, Gandini S. Telomere length and the risk of cutaneous melanoma and non-melanoma skin cancer: A review of the literature and meta-analysis. J Dermatol Sci. 2015;80:168–174. doi: 10.1016/j.jdermsci.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Rode L, Nordestgaard BG, Bojesen SE. Long telomeres and cancer risk among 95 568 individuals from the general population. Int J Epidemiol. 2016;45:1634–1643. doi: 10.1093/ije/dyw179. [DOI] [PubMed] [Google Scholar]
- 51.Fabiani R, Chiavarini M, Rosignoli P, Giacchetta I. Leucocyte telomere length and lung cancer risk: A systematic review and Meta-analysis of prospective studies. Cancers (Basel) 2024;16:3218. doi: 10.3390/cancers16183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S, Hu S, Zhou B, Cheng B, Tong H, Su D, Li X, Chen Y, Zhang G. Telomere-related prognostic biomarkers for survival assessments in pancreatic cancer. Sci Rep. 2023;13:10586. doi: 10.1038/s41598-023-37836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holesova Z, Krasnicanova L, Saade R, Pös O, Budis J, Gazdarica J, Repiska V, Szemes T. Telomere length changes in cancer: Insights on carcinogenesis and potential for Non-invasive diagnostic strategies. Genes (Basel) 2023;14:715. doi: 10.3390/genes14030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: A meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Green S, Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. http://training.cochrane.org/handbook. [ March 20; 2025 ]; [Google Scholar]
- 58.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 59.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sterne JAC, Gavaghan D, Egger M. Publication and related bias in meta-analysis. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 61.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [ March 20; 2025 ]; [Google Scholar]
- 62.Han J, Qureshi AA, Prescott J, Guo Q, Ye L, Hunter DJ, De Vivo I. A Prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. 2009;129:415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nan H, Du M, De Vivo I, Manson JE, Liu S, McTiernan A, Curb JD, Lessin LS, Bonner MR, Guo Q, et al. Shorter telomeres associate with a reduced risk of melanoma development. Cancer Res. 2011;71:6758–6763. doi: 10.1158/0008-5472.CAN-11-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weischer M. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105:459–468. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- 65.Greenland S, Thomas DC, Morgenstern H. The rare-disease assumption revisited. Am J Epidemiol. 1986;124:869–876. doi: 10.1093/oxfordjournals.aje.a114476. [DOI] [PubMed] [Google Scholar]
- 66.Anic GM, Sondak VK, Messina JL, Fenske NA, Zager JS, Cherpelis BS, Lee JH, Fulp WJ, Epling-Burnette PK, Park JY, Rollison DE. Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer Epidemiol. 2013;37:434–439. doi: 10.1016/j.canep.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rachakonda S, Kong H, Srinivas N, Garcia-Casado Z, Requena C, Fallah M, Heidenreich B, Planelles D, Traves V, Schadendorf D, et al. Telomere length, telomerase reverse transcriptase promoter mutations, and melanoma risk. Genes Chromosomes Cancer. 2018;57:564–572. doi: 10.1002/gcc.22669. [DOI] [PubMed] [Google Scholar]
- 68.Schneider CV, Schneider KM, Teumer A, Rudolph KL, Hartmann D, Rader DJ, Strnad P. Association of telomere length with risk of disease and mortality. JAMA Intern Med. 2022;182:291–300. doi: 10.1001/jamainternmed.2021.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bodelon C, Pfeiffer RM, Bollati V, Debbache J, Calista D, Ghiorzo P, Fargnoli MC, Bianchi-Scarra G, Peris K, Hoxha M, et al. On the interplay of telomeres, nevi and the risk of melanoma. PLoS One. 2012;7:e52466. doi: 10.1371/journal.pone.0052466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llorca-Cardeñosa MJ, Peña-Chilet M, Mayor M, Gomez-Fernandez C, Casado B, Martin-Gonzalez M, Carretero G, Lluch A, Martinez-Cadenas C, Ibarrola-Villava M, Ribas G. Long telomere length and a TERT-CLPTM1 locus polymorphism association with melanoma risk. Eur J Cancer. 2014;50:3168–3177. doi: 10.1016/j.ejca.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 71.Menin C, Bojnik E, Del Bianco P, Elefanti L, Gianesin K, Keppel S, Stagni C, Mocellin S, Vecchiato A, De Rossi A. Differences in telomere length between sporadic and familial cutaneous melanoma. Br J Dermatol. 2016;175:937–943. doi: 10.1111/bjd.14652. [DOI] [PubMed] [Google Scholar]
- 72.Burke LS, Hyland PL, Pfeiffer RM, Prescott J, Wheeler W, Mirabello L, Savage SA, Burdette L, Yeager M, Chanock S, et al. Telomere length and the risk of cutaneous malignant melanoma in Melanoma-Prone families with and without CDKN2A mutations. PLoS One. 2013;8:e71121. doi: 10.1371/journal.pone.0071121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perrem K, Lynch A, Al Nooh F, Leader M, Elaine Kay. The different telomere lengths in basal and squamous cell carcinomas also differ between the nontransplant and renal transplant population. Hum Pathol. 2008;39:1034–1041. doi: 10.1016/j.humpath.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Srinivas N, Rachakonda S, Hielscher T, Calderazzo S, Rudnai P, Gurzau E, Koppova K, Fletcher T, Kumar R. Telomere length, arsenic exposure and risk of basal cell carcinoma of skin. Carcinogenesis. 2019;40:715–723. doi: 10.1093/carcin/bgz059. [DOI] [PubMed] [Google Scholar]
- 75.Liang G, Qureshi AA, Guo Q, De Vivo I, Han J. No association between telomere length in peripheral blood leukocytes and the risk of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1043–1045. doi: 10.1158/1055-9965.EPI-11-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wainwright LJ, Rees JL, Middleton PG. Changes in mean telomere length in basal cell carcinomas of the skin. Genes Chromosomes Cancer. 1995;12:45–49. doi: 10.1002/gcc.2870120108. [DOI] [PubMed] [Google Scholar]
- 77.Perrem K, Lynch A, Conneely M, Wahlberg H, Murphy G, Leader M, Kay E. The higher incidence of squamous cell carcinoma in renal transplant recipients is associated with increased telomere lengths. Hum Pathol. 2007;38:351–358. doi: 10.1016/j.humpath.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 78.De Vitis M, Berardinelli F, Sgura A. Telomere length maintenance in cancer: At the crossroad between telomerase and alternative lengthening of telomeres (ALT) Int J Mol Sci. 2018;19:606. doi: 10.3390/ijms19020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andreikos D, Spandidos D, Georgakopoulou V. Telomeres and telomerase in mesothelioma: Pathophysiology, biomarkers and emerging therapeutic strategies (Review) Int J Oncol. 2025;66:23. doi: 10.3892/ijo.2025.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mooi WJ, Peeper DS. Oncogene-induced cell Senescence-halting on the road to cancer. N Engl J Med. 2006;355:1037–1046. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- 81.Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, Marais R, Wynford-Thomas D, Bennett DC. Cellular senescence in naevi and immortalisation in melanoma: A role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bataille V, Kato BS, Falchi M, Gardner J, Kimura M, Lens M, Perks U, Valdes AM, Bennett DC, Aviv A, Spector TD. Nevus size and number are associated with telomere length and represent potential markers of a decreased senescence in vivo. Cancer Epidemiol Biomarkers Prev. 2007;16:1499–1502. doi: 10.1158/1055-9965.EPI-07-0152. [DOI] [PubMed] [Google Scholar]
- 83.Robles-Espinoza CD, Velasco-Herrera M del C, Hayward NK, Adams DJ. Telomere-regulating genes and the telomere interactome in familial cancers. Mol Cancer Res. 2015;13:211–222. doi: 10.1158/1541-7786.MCR-14-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toussi A, Mans N, Welborn J, Kiuru M. Germline mutations predisposing to melanoma. J Cutan Pathol. 2020;47:606–616. doi: 10.1111/cup.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Son N, Cui Y, Xi W. Association between telomere length and skin cancer and aging: A mendelian randomization analysis. Front Genet. 2022;13:931785. doi: 10.3389/fgene.2022.931785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McNally EJ, Luncsford PJ, Armanios M. Long telomeres and cancer risk: The price of cellular immortality. J Clin Invest. 2019;129:3474–3481. doi: 10.1172/JCI120851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stanley SE, Armanios M. The short and long telomere syndromes: Paired paradigms for molecular medicine. Curr Opin Genet Dev. 2015;33:1–9. doi: 10.1016/j.gde.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS. Telomere length and dynamics predict mortality in a wild longitudinal study. Mol Ecol. 2013;22:249–259. doi: 10.1111/mec.12110. [DOI] [PubMed] [Google Scholar]
- 89.Speedy HE, Di Bernardo MC, Sava GP, Dyer MJS, Holroyd A, Wang Y, Sunter NJ, Mansouri L, Juliusson G, Smedby KE, et al. A genome-wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2014;46:56–60. doi: 10.1038/ng.2843. [DOI] [PubMed] [Google Scholar]
- 90.Rachakonda S, Srinivas N, Mahmoudpour SH, Garcia-Casado Z, Requena C, Traves V, Soriano V, Cardelli M, Pjanova D, Molven A, et al. Telomere length and survival in primary cutaneous melanoma patients. Sci Rep. 2018;8:10947. doi: 10.1038/s41598-018-36657-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez-Delgado B, Yanowsky K, Inglada-Perez L, De La Hoya M, Caldes T, Vega A, Blanco A, Martin T, Gonzalez-Sarmiento R, Blasco M, et al. Shorter telomere length is associated with increased ovarian cancer risk in both familial and sporadic cases. J Med Genet. 2012;49:341–344. doi: 10.1136/jmedgenet-2012-100807. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Delgado B, Yanowsky K, Inglada-Perez L, Domingo S, Urioste M, Osorio A, Benitez J. Genetic anticipation is associated with telomere shortening in hereditary breast cancer. PLoS Genet. 2011;7:e1002182. doi: 10.1371/journal.pgen.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rossi M, Pellegrini C, Cardelli L, Ciciarelli V, Di Nardo L, Fargnoli MC. Familial melanoma: Diagnostic and management implications. Dermatol Pract Concept. 2019;9:10–16. doi: 10.5826/dpc.0901a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zocchi L, Lontano A, Merli M, Dika E, Nagore E, Quaglino P, Puig S, Ribero S. Familial Melanoma and susceptibility genes: A review of the most common clinical and dermoscopic phenotypic aspect, associated malignancies and practical tips for management. J Clin Med. 2021;10:3760. doi: 10.3390/jcm10163760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hansen MEB, Hunt SC, Stone RC, Horvath K, Herbig U, Ranciaro A, Hirbo J, Beggs W, Reiner AP, Wilson JG, et al. Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Hum Mol Genet. 2016;25:2324–2330. doi: 10.1093/hmg/ddw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chang YM, Barrett JH, Bishop DT, Armstrong BK, Bataille V, Bergman W, Berwick M, Bracci PM, Elwood JM, Ernstoff MS, et al. Sun exposure and melanoma risk at different latitudes: A pooled analysis of 5700 cases and 7216 controls. Int J Epidemiol. 2009;38:814–830. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/S1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 98.Lundsgaard NU, Cramp RL, Franklin CE. Early exposure to UV radiation causes telomere shortening and poorer condition later in life. J Exp Biol. 2022;225:jeb243924. doi: 10.1242/jeb.243924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Martin-Ruiz C, Shiels P, Sayer AA, Barbieri M, et al. Gender and telomere length: Systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. Estrogen activates telomerase. Cancer Res. 1999;59:5917–5921. [PubMed] [Google Scholar]
- 101.Ventura A, Pellegrini C, Cardelli L, Rocco T, Ciciarelli V, Peris K, Fargnoli MC. Telomeres and telomerase in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:1333. doi: 10.3390/ijms20061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 103.Yamada-Hishida H, Nobeyama Y, Nakagawa H. Correlation of telomere length to malignancy potential in non-melanoma skin cancers. Oncol Lett. 2018;15:393–399. doi: 10.3892/ol.2017.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 105.Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Stat Med. 2002;21:1503–1511. doi: 10.1002/sim.1183. [DOI] [PubMed] [Google Scholar]
- 106.Montpetit AJ, Alhareeri AA, Montpetit M, Starkweather AR, Elmore LW, Filler K, Mohanraj L, Burton CW, Menzies VS, Lyon DE, Jackson-Cook CK. Telomere length: A review of methods for measurement. Nurs Res. 2014;63:289–299. doi: 10.1097/NNR.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gutierrez-Rodrigues F, Santana-Lemos BA, Scheucher PS, Alves-Paiva RM, Calado RT. Direct comparison of Flow-FISH and qPCR as diagnostic tests for telomere length measurement in humans. PLoS One. 2014;9:e113747. doi: 10.1371/journal.pone.0113747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS One. 2018;13:e0204056. doi: 10.1371/journal.pone.0204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study are included in the figures and/or tables of this article.