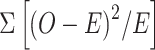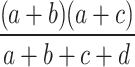Abstract
Background
Gepants have demonstrated notable benefits in migraine therapy, yet their safety profiles are not thoroughly investigated. This study comprehensively analyzed the adverse event (AE) risk signals of the currently approved gepants using the U.S. Food and Drug Administration Adverse Event Reporting System database, aiming to gain better understanding of their post-marketing safety features and potential risks.
Methods
All data of the gepants (rimegepant, atogepant, ubrogepant, and zavegepant) from January 1st 2020 to December 31st 2024 were retrieved from the database. Descriptive analysis was conducted to characterize the features of gepant-associated AEs. Disproportionality analysis and subsequent sensitivity analysis were employed to evaluate the risk signals of the gepants utilizing the algorithms of reporting odds ratio (ROR), proportional reporting ratio (PRR), and information component (IC).
Results
A total of 7766 reports of rimegepant, 3672 reports of atogepant, 1958 reports of ubrogepant, and 463 reports of zavegepant were identified after data processing. Most AEs were occurred within 30 days after gepant administration. The integration of disproportionality analysis and sensitivity analysis indicated that “feeling abnormal” was the most reported AE of rimegepant (n = 185, 6.81%, ROR025 = 6.46, IC025 = 2.59, PRR = 7.24, χ2 = 998.58), while “constipation” was the most common AE of atogepant (n = 288, 16.09%, ROR025 = 19.99, IC025 = 4.10, PRR = 20.72, χ2 = 5418.12). The most prevalent AE of ubrogepant was “fatigue” (n = 60, 7.19%, ROR025 = 1.88, IC025 = 0.84, PRR = 2.38, χ2 = 48.82), whereas “dysgeusia” was the most frequently observed AE of zavegepant (n = 150, 45.18%, ROR025 = 212.07, IC025 = 6.10, PRR = 181.96, χ2 = 26,975.74). Comparative analysis of AEs revealed that two AEs were shared among all gepants and zavegepant had the largest collection of unique AEs (n = 15).
Conclusions
The present pharmacovigilance study systematically revealed the significant risk signals of gepants. The common AEs and unique AEs of the four gepants were also identified and explored. Our results would provide valuable reference for the safe use of gepants, guiding personalized drug selection in clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-025-02091-3.
Keywords: Gepants, Pharmacovigilance, Adverse event, Adverse drug reaction, FAERS
Introduction
Migraine is a prevalent neurological disease affecting more than one billion people worldwide [1]. As a complex neurovascular event, migraine is manifested as recurrent attacks of pulsatile unilateral headache lasting from hours to days, accompanied by symptoms of vomiting, nausea, vertigo, dizziness, phonophobia, and photophobia [2, 3]. There are currently multiple treatment options for migraine, including antiepileptics, analgesics, antidepressants, triptans, and ergots [4]. However, these drugs are still insufficient to fully meet clinical needs [5].
Calcitonin gene-related peptide (CGRP) is a peptide neurotransmitter that is thought to be positively correlated with headache severity [6]. Elevated CGRP level in the trigeminal vascular system is vital for the occurrence and development of migraine [7]. Research on the links between CGRP and migraine has promoted the development of CGRP-based therapies, including anti-CGRP monoclonal antibodies and small molecule CGRP receptor antagonists, unofficially known as gepants [8]. As relatively small CGRP receptor antagonists, gepants have shown great efficacy and advantages in migraine treatment given their short half-lives and ease of frequent administration in acute therapy [9]. Currently, there are four approved gepants, namely rimegepant, atogepant, ubrogepant, and zavegepant. Rimegepant, ubrogepant, and zavegepant are approved by the U.S. Food and Drug Administration (FDA) for acute treatment, while atogepant and rimegepant are approved for migraine prevention [10].
In previous clinical trials, the significant hepatotoxicity of first-generation gepant has led to its discontinuation in development [11]. As a result, the second-generation gepants were developed, including rimegepant, ubrogepant, and atogepant. Zavegepant is the only third-generation gepant approved for acute treatment of migraine, and its nasal spray form further improves patient compliance [12].
At present, the post-marketing safety data of gepants remains limited, with most of the existing data originating from clinical trials and subsequent meta-analysis [13–17]. Previous pharmacovigilance studies have also investigated the adverse events (AEs) of gepants [18–24]. However, these studies were limited to single or partial list of gepants, lacking comprehensive comparisons between gepants. Therefore, it is essential to systematically examine the potential safety profiles of gepants through pharmacovigilance analysis of real-world data. Here, we updated the safety features of all approved gepants by conducting disproportionality analysis based on the U.S. FDA Adverse Event Reporting System (FAERS) database. The FAERS database is a global spontaneous AE reporting system serving as a fundamental tool for drug safety monitoring [25, 26]. By mining and comparing the common and distinctive characteristics of AEs among gepants, our results would provide comprehensive insights into the safe use of gepants, guiding personalized drug selection in clinic.
Materials and methods
Data source and data cleaning
All existing pharmacovigilance data of the four gepants were retrieved from the FAERS database, encompassing the timeframe from January 1 st 2020 to December 31 st 2024. Data extraction was conducted on a single day of February 1 st 2025. The target drugs were restricted to the generic names of gepants, including “rimegepant”, “atogepant”, “ubrogepant”, and “zavegepant”, and reports were included if the gepants were marked as “prime suspect” in the database. We utilized the Medical Dictionary for Regulatory Activities (MedDRA, Version 27.0) to classify and describe AEs using system organ class (SOC) and preferred terminology (PT), respectively.
According to the FAERS database guidelines (https://fis.fda.gov/extensions/FPD-FAQ/FPD-FAQ.html), reports owning the same identifier values (e.g., case ID, date, and drug) were recognized as duplicate reports and were excluded from the analysis. The time-to-onset (TTO) of AE was defined as the interval from the first time of gepant treatment until the onset of AE. In TTO analysis and descriptive analysis, data entries with missing values or incorrectly formatted values were omitted. In addition, since FAERS allows the input of free text entries which results in a large amount of invalid data and abnormal data [27]. Hence, inappropriate PT, such as “drug ineffective”, “off label use”, “no adverse event”, “product quality issue”, and “adverse drug reaction”, were carefully removed to ensure data quality.
In disproportionality analysis, we noticed that the top ranked PTs, such as “migraine”, “headache”, “vomiting”, “nausea”, “vertigo”, “dizziness”, “photophobia”, and"phonophobia", actually belong to the medication indication and symptoms associated with migraine. Since different AE signals are interdependent in disproportionality analysis [28], the strong signals of the above PTs might mask the positive signals of other PTs, resulting in potential reporting bias. Therefore, we performed a subsequent sensitivity analysis in which the above PTs were excluded.
Data mining and analysis
To assess the risk signals of the four gepants, three distinct algorithms, namely reporting odds ratio (ROR), proportional reporting ratio (PRR), and Bayesian confidence propagation neural network (BCPNN), were employed in the disproportionality analysis and sensitivity analysis. Information component (IC) is the measure of BCPNN algorithm, and confidence interval (CI) is a range of upper and lower limits which describes the possible mean of a sample [29–31]. The ROR method is computationally simple and highly sensitive, whereas the BCPNN and PRR methods provide more stable and specific results through Bayesian logic and neural network structure [31, 32].
In this study, the three algorithms were integrated to minimize the potential bias of single-algorithm approach. ROR, PRR, and IC were calculated based on the 2 × 2 contingency table (Table 1). According to the formulas and significant criteria listed in Table 2, a significant signal was determined if the lower CI limits of ROR (ROR025) and the lower CI limits of IC (IC025) were greater than 1 and 0, respectively [30]. A significant PRR signal was considered if the value of PRR ≥ 2, and the value of chi-squared (χ2) ≥ 4 [30]. Data analysis was performed using SAS software (Version 9.4), and data visualization was conducted with GraphPad Prism (Version 9.3.1) and online plotting platform (https://www.chiplot.online/, https://bioincloud.tech/).
Table 1.
The 2 × 2 contingency table of disproportionality analysis
| Target adverse events | Other adverse events | Total | |
|---|---|---|---|
| Target drugs | a | b | a + b |
| Other drugs | c | d | c + d |
| Total | a + c | b + d | a + b + c + d |
Table 2.
Algorithms, formulas, and positive signal criteria of disproportionality analysis
| Algorithms | Formulas | Criteria |
|---|---|---|
| ROR |
ROR = 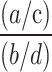 = = 
|
ROR025 > 1, 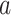 ≥ 3 ≥ 3 |
95%CI = 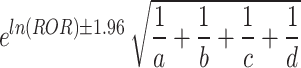
|
||
| BCPNN |
IC = 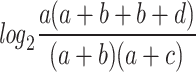
|
IC025 > 0, 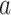 > 0 > 0 |
IC025 = 
|
||
| PRR |
PRR = 
|
χ2 ≥ 4, PRR ≥ 2, 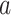 ≥ 3 ≥ 3 |
|
χ2 = O = |
Abbreviations: ROR Reporting odds ratio, ROR 025 the lower limit of the 95% confidence interval of the ROR, 95%CI 95% confidence interval, BCPNN Bayesian confidence propagation neural network, IC Information component, IC025 the lower limit of the 95% confidence interval of the IC, PRR Proportional reporting ratio, χ2 chi-squared
Results
Descriptive analysis
After data processing, a total of 13,859 gepants-related AEs were obtained from the FAERS database. Rimegepant had the highest number of AEs reports (n = 7766), followed by atogepant (n = 3672), ubrogepant (n = 1958), and zavegepant (n = 463). The most common age group was 45–64 for rimegepant (n = 1735, 22.34%), atogepant (n = 521, 14.19%), and ubrogepant (n = 201, 10.27%), whereas the most prevalent age range was 18–44 for zavegepant (n = 71, 15.33%). The majority of reports were originated from North America (n = 7538, 97.06% for rimegepant; n = 3434, 93.52% for atogepant; n = 1906, 97.34% for ubrogepant; n = 459, 99.14% for zavegepant), and were mostly reported by consumers (n = 6498, 83.67% for rimegepant; n = 2997, 81.62% for atogepant; n = 1574, 80.39% for ubrogepant; n = 170, 36.72% for zavegepant). The AEs of rimegepant (n = 2965, 38.18%) and ubrogepant (n = 604, 30.85%) were mostly reported in 2022. The majority of reports were observed in 2023 for atogepant (n = 1517, 41.31%), while most reports were submitted in 2024 for zavegepant (n = 236, 50.97%). Regarding reaction outcome, most of the cases were non-serious (n = 7150, 92.07% for rimegepant; n = 2900, 78.98% for atogepant; n = 1627, 83.09% for ubrogepant; n = 445, 96.11% for zavegepant). The detailed demographic characteristics of the reported cases are depicted in Table 3.
Table 3.
Demographic characteristics of cases with gepant-related adverse events
| Characteristics | Rimegepant | Atogepant | Ubrogepant | Zavegepant |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Number of reports | 7766 | 3672 | 1958 | 463 |
| Gender | ||||
| Female | 4807 (61.90) | 2809 (76.50) | 1294 (66.09) | 344 (74.30) |
| Male | 754 (9.71) | 458 (12.47) | 253 (12.92) | 39 (8.42) |
| Unknown | 2205 (28.39) | 405 (11.03) | 411 (20.99) | 80 (17.28) |
| Age | ||||
| < 18 | 44 (0.57) | 5 (0.14) | 37 (1.89) | 0 (0.00) |
| 18–44 | 1297 (16.70) | 385 (10.48) | 157 (8.02) | 71 (15.33) |
| 45–64 | 1735 (22.34) | 521(14.19) | 201 (10.27) | 56 (12.10) |
| ≥ 65 | 676 (8.70) | 143 (3.89) | 95 (4.85) | 11 (2.38) |
| Unknown | 4014 (51.69) | 2618(71.30) | 1468 (74.97) | 325 (70.19) |
| Median (Q1, Q3) | 51 (40, 61) | 50 (39, 60) | 50 (37, 62) | 44 (35, 54) |
| Geographical distribution | ||||
| North America | 7538 (97.06) | 3434 (93.52) | 1906 (97.34) | 459 (99.14) |
| South America | 16 (0.21) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Europe | 73 (0.94) | 49 (1.33) | 0 (0.00) | 0 (0.00) |
| Asia | 11 (0.14) | 4 (0.11) | 2 (0.10) | 0 (0.00) |
| Unknown | 128 (1.65) | 185 (5.04) | 50 (2.55) | 4 (0.86) |
| Reporter type | ||||
| Consumer | 6498 (83.67) | 2997 (81.62) | 1574 (80.39) | 170 (36.72) |
| Physician | 519 (6.68) | 306 (8.33) | 150 (7.66) | 136 (29.37) |
| Pharmacist | 734 (9.45) | 361 (9.83) | 230 (11.75) | 157 (33.91) |
| Unknown | 15 (0.19) | 8 (0.22) | 4 (0.20) | 0 (0.00) |
| Reporting year | ||||
| 2020 | 586 (7.55) | 0 (0.00) | 289 (14.76) | 0 (0.00) |
| 2021 | 1677 (21.59) | 19 (0.52) | 350 (17.88) | 0 (0.00) |
| 2022 | 2965 (38.18) | 1463 (39.84) | 604 (30.85) | 0 (0.00) |
| 2023 | 1388 (17.87) | 1517 (41.31) | 388 (19.82) | 227(49.03) |
| 2024 | 1150 (14.81) | 673 (18.33) | 327 (16.70) | 236 (50.97) |
| Reaction outcomea | ||||
| Non-serious | 7150 (92.07) | 2900 (78.98) | 1627 (83.09) | 445 (96.11) |
| Life-Threatening | 21 (0.27) | 11 (0.30) | 2 (0.10) | 0 (0.00) |
| Hospitalization | 111 (1.43) | 159 (4.33) | 87 (4.44) | 5 (1.08) |
| Disability | 31 (0.40) | 39 (1.06) | 28 (1.43) | 0 (0.00) |
| Death | 69 (0.89) | 28 (0.76) | 30 (1.53) | 0 (0.00) |
| Congenital Anomaly | 4 (0.05) | 1 (0.03) | 0 (0.00) | 0 (0.00) |
| Required Intervention | 11 (0.14) | 21 (0.57) | 6 (0.31) | 0 (0.00) |
| Other | 452 (5.82) | 625 (17.02) | 245 (12.51) | 13 (2.81) |
Abbreviations: Q1 Lower Quartile, Q3 Upper Quartile
aSome cases may contain more than one outcome
Disproportionality analysis of AE signals
Figure 1 exhibits the top 30 most common PTs of gepants, and the ROR025 values were transformed to logarithms to ensure readability of the graph. We found that “migraine” was the most frequently occurred PT of atogepant (n = 763, 20.78%, ROR025 = 64.50, IC025 = 5.74, PRR = 63.07, χ2 = 46,252.62) and ubrogepant (n = 286, 14.61%, ROR025 = 46.49, IC025 = 5.20, PRR = 48.69, χ2 = 13,333.40). The PT of “nausea” was most prevalent in rimegepant (n = 765, 9.85%, ROR025 = 4.20, IC025 = 2.00, PRR = 4.33, χ2 = 1981.19), while “dysgeusia” was the most common PT of zavegepant (n = 153, 33.05%, ROR025 = 131.45, IC025 = 5.90, PRR = 130.83, χ2 = 19,693.09). Moreover, we found consistent results of pharmacovigilance metrics across the three algorithms (Table S1).
Fig. 1.
The top 30 preferred terminologies and reporting odds ratio (ROR) of the four gepants in disproportionality analysis
Sensitivity analysis of AE signals
In order to eliminate reporting bias mediated by the indication and symptoms of migraine, a subsequent sensitivity analysis was conducted. Figure 2 demonstrates the top 30 most common PTs of gepants. We found that “feeling abnormal” was the most reported PT of rimegepant (n = 185, 6.81%, ROR025 = 6.46, IC025 = 2.59, PRR = 7.24, χ2 = 998.58), while “constipation” was the most common PT of atogepant (n = 288, 16.09%, ROR025 = 19.99, IC025 = 4.10, PRR = 20.72, χ2 = 5418.12). The most prevalent PT of ubrogepant was “fatigue” (n = 60, 7.19%, ROR025 = 1.88, IC025 = 0.84, PRR = 2.38, χ2 = 48.82), whereas “dysgeusia” was the most frequently observed PT of zavegepant (n = 150, 45.18%, ROR025 = 212.07, IC025 = 6.10, PRR = 181.96, χ2 = 26,975.74). Furthermore, we found similar pharmacovigilance data among all three algorithms (Table S2).
Fig. 2.
The top 30 preferred terminologies and reporting odds ratio (ROR) of the four gepants in sensitivity analysis
The AE signals in SOC level indicated that “gastrointestinal disorders” was most common in rimegepant (n = 968, ROR025 = 1.99) and atogepant (n = 633, ROR025 = 1.83). The “nervous system disorders” was the most frequently detected SOC in ubrogepant (n = 314, ROR025 = 1.92) and zavegepant (n = 217, ROR025 = 5.48) (Table 4).
Table 4.
The distribution and risk signals in system organ class of the four gepants
| No | SOC | N | ROR025 | |
|---|---|---|---|---|
| Rimegepant | 1 | Gastrointestinal disorders | 968 | 1.99 |
| 2 | Nervous system disorders | 675 | 1.32 | |
| 3 | General disorders and administration site conditions | 640 | 0.72 | |
| 4 | Skin and subcutaneous tissue disorders | 586 | 2.01 | |
| 5 | Psychiatric disorders | 471 | 1.54 | |
| 6 | Respiratory, thoracic and mediastinal disorders | 237 | 0.78 | |
| 7 | Immune system disorders | 230 | 3.20 | |
| 8 | Musculoskeletal and connective tissue disorders | 215 | 0.69 | |
| 9 | Investigations | 185 | 0.43 | |
| 10 | Eye disorders | 146 | 1.03 | |
| Atogepant | 1 | Gastrointestinal disorders | 633 | 1.83 |
| 2 | Nervous system disorders | 450 | 1.24 | |
| 3 | General disorders and administration site conditions | 409 | 0.65 | |
| 4 | Psychiatric disorders | 392 | 1.88 | |
| 5 | Skin and subcutaneous tissue disorders | 211 | 0.93 | |
| 6 | Investigations | 202 | 0.71 | |
| 7 | Musculoskeletal and connective tissue disorders | 168 | 0.78 | |
| 8 | Metabolism and nutrition disorders | 125 | 1.17 | |
| 9 | Eye disorders | 109 | 1.09 | |
| 10 | Respiratory, thoracic and mediastinal disorders | 105 | 0.46 | |
| Ubrogepant | 1 | Nervous system disorders | 314 | 1.92 |
| 2 | General disorders and administration site conditions | 242 | 0.80 | |
| 3 | Gastrointestinal disorders | 211 | 1.13 | |
| 4 | Psychiatric disorders | 128 | 1.15 | |
| 5 | Skin and subcutaneous tissue disorders | 106 | 0.93 | |
| 6 | Musculoskeletal and connective tissue disorders | 102 | 0.96 | |
| 7 | Respiratory, thoracic and mediastinal disorders | 80 | 0.73 | |
| 8 | Eye disorders | 70 | 1.41 | |
| 9 | Investigations | 69 | 0.45 | |
| 10 | Cardiac disorders | 43 | 0.62 | |
| Zavegepant | 1 | Nervous system disorders | 217 | 5.48 |
| 2 | Respiratory, thoracic and mediastinal disorders | 157 | 6.37 | |
| 3 | Gastrointestinal disorders | 33 | 0.42 | |
| 4 | Eye disorders | 24 | 1.28 | |
| 5 | Skin and subcutaneous tissue disorders | 21 | 0.44 | |
| 6 | General disorders and administration site conditions | 17 | 0.11 | |
| 7 | Immune system disorders | 10 | 0.79 | |
| 8 | Musculoskeletal and connective tissue disorders | 8 | 0.14 | |
| 9 | Psychiatric disorders | 8 | 0.13 | |
| 10 | Ear and labyrinth disorders | 7 | 1.18 |
Abbreviations: SOC System organ class, ROR025 the lower limit of the 95% two-sided reporting odds ratio of the information component
TTO analysis
After data processing, 449 reports of rimegepant, 354 reports of atogepant, 150 reports of ubrogepant, and 43 reports of zavegepant were included in the TTO analysis. We found that the majority of AEs occurred within 30 days after gepant administration (n = 389, 86.64% for rimegepant; n = 219, 61.86% for atogepant; n = 121, 80.67% for ubrogepant; n = 43, 100.00% for zavegepant). The median TTO of the four gepants were as follows: rimegepant (median, [Lower Quartile (Q1), Upper Quartile (Q3)]) = 0 [0, 6]; atogepant (median, [Q1, Q3]) = 13 [0, 84]; ubrogepant (median, [Q1, Q3]) = 0 [0, 15]; zavegepant (median, [Q1, Q3]) = 0 [0, 0] (Fig. 3).
Fig. 3.
The onset time of adverse events of the four gepants
Comparisons of AE signals at the PT level
A comparative analysis of PTs was performed to evaluate the overlapping and distinctive features of AEs among gepants. Only the top 20 positive PTs of each gepant were shown considering readability of the graph. As depicted in Fig. 4, “abdominal discomfort” and “abdominal pain upper” were shared among all four gepants, and zavegepant had the greatest number of unique positive PTs (n = 15), including “lacrimation increased”, “oropharyngeal pain”, “oropharyngeal discomfort”, “nasal congestion”, “eye swelling”, “rhinorrhea”, “upper-airway cough syndrome”, “sinus pain”, “taste disorder”, “nasal discomfort”, “epistaxis”, “rhinalgia”, “eye irritation”, “dysgeusia”, and “burning sensation”.
Fig. 4.
The Venn network of the top 20 positive preferred terminologies of the four gepants
Discussion
To the best of our understanding, this is the first real-world pharmacovigilance study investigating the AEs of all the approved gepants. In descriptive analysis, the higher number of AEs in female patients echoed the epidemiological evidence that migraine predominantly affects women, and the mechanism behind it is likely to be related to the fluctuations in estrogen levels [33–35]. Our data regarding age of patients also supported the view that migraine shows higher prevalence in young and middle-aged women [12]. Of note, we found that the vast majority of reports were from North America and were mainly reported by consumers rather than healthcare professionals, which might affect the generalizability and reliability of the data. Therefore, the inclusion of AE data from other databases (e.g., VigiBase and EudraVigilance) and education of AE reporting among healthcare professionals would be important for future studies.
After integrating disproportionality analysis with sensitivity analysis, we found more diverse AEs compared to the FDA instructions (Table S3). In neurological and psychiatric signals, we found significant PTs of “insomnia”, “somnolence”, and “anxiety” in rimegepant, atogepant, and ubrogepant. The AEs associated with sleep alteration might be linked to the neuromodulatory effects of CGRP on glutamatergic signaling which affecting central sensitization [36]. In addition, CGRP-related anxiety-like behaviors in stress and pain states are also thought to be closely associated with sleep function [37]. The PTs of “feeling abnormal”, “paresthesia”, “burning sensation”, “hypoesthesia”, “memory impairment”, and “brain fog” might be related to the widespread expression of CGRP in brain regions associated with memory and sensory function [38]. Indeed, there is a strong relationship between sleep quality, sensory input, and migraine [39]. Hence, future studies collecting detailed records of relevant medical history would contribute to the deeper understanding of these AEs.
In the SOC of “gastrointestinal disorders”, the PT of “constipation” was frequently reported in second-generation gepants. CGRP serves as a regulatory factor of gastrointestinal activity and is widely discovered in the gastrointestinal system [36, 40]. Results from phase III clinical trials revealed that 6.9–7.7% of patients had atogepant-related constipation [41]. Another post-marketing investigation showed that 4.7% of patients developed ubrogepant-induced constipation [42]. Importantly, FDA instruction has noted a warning on the risk of atogepant-induced constipation [43]. Consistent with these findings, we found that atogepant (ROR025 = 19.99) displayed the strongest signal of constipation, followed by rimegepant (ROR025 = 3.09) and ubrogepant (ROR025 = 2.45). Although these results could be attributed to the notoriety effect that severe or labeled AEs tend to be overreported [44], gepant-related constipation remains a significant AE that warrants attention.
As for skin and subcutaneous tissue disorders, “pruritus” was the most common PT among second-generation gepants. This PT might be related to the modulating role of CGRP in pruritus that CGRP can affect itch sensation by regulating interleukin-31 and thymic stromal lymphopoietin [45, 46]. Another new PT “alopecia” was detected in rimegepant and atogepant. A large body of evidence revealed the mechanism of microvascular circulation disorders in alopecia, that is, decreased cutaneous CGRP levels can cause vasoconstriction and hair growth dysfunctions [47–49]. Thus, close collaboration between clinicians and dermatologists would be highly beneficial for patients receiving second-generation gepants.
In terms of cardiac disorders, the PT of “palpitations” was reported in all second-generation gepants. CGRP receptors are considered to regulate smooth muscle cells of the cardiovascular system, though no severe cardiovascular AE associated with anti-CGRP drugs have been reported so far [50, 51]. Clinical evidence suggested that cardiovascular AEs caused by anti-CGRP drugs are mainly palpitations, flushing, hypotension, and warm sensation [52]. Considering the complex confounding factors of this category of AE, the background characteristics of patients, including age, basal metabolism, vasomotor, cardiovascular parameters, and menopausal status, should be comprehensively weighed when receiving second-generation gepants [53].
Moreover, given the hepatotoxicity of first-generation gepant, we additionally examined the hepatic safety profiles of the four gepants. Previous study using physiologically based pharmacokinetic modeling has reported no significant hepatotoxicity of the four gepants [54]. Our results aligned with previous studies that hepatotoxicity-related AEs were rarely observed (rimegepant, n = 14; atogepant, n = 27; ubrogepant, n = 12; zavegepant, n = 0). However, although there is no FDA warning for hepatotoxicity of gepants, since hepatobiliary elimination is their primary excretion route [55], clinically based evidence suggested that gepants are not recommended for patients with severe hepatic impairment [15, 56].
Zavegepant is an effective non-oral option that may benefit individuals requiring rapid symptom relief or those unable to tolerate oral medications [57]. Given the recent market entry of zavegepant, its real-world safety profiles are currently lacking. In this study, we collected the largest sample size of zavegepant-related AEs to date. Our real-world data covered broader range of populations compared to clinical trials, aiding in the discovery of unknown AE signals of zavegepant. Clinical trial-based data indicated that zavegepant exhibits good tolerability over 52 weeks of long-term treatment [58], and the common AEs were mild or moderate levels of dysgeusia, nasal discomfort, nausea, nasal congestion, throat irritation, and back pain [59, 60]. In this study, we found that zavegepant did not exhibit the common AEs of second-generation gepants. Notably, “dysgeusia” and “taste disorder” were recognized as the unique PTs of zavegepant, and the underlying mechanism could be that zavegepant affects the taste buds via nasopharyngeal route [61]. Previous clinical trial data showed that 39.1% of patients experienced zavegepant-related dysgeusia [59], which was generally consistent with our results (45.18%). Other clinical trial evidence suggested that the AEs of taste disorders are mostly mild and can be resolved without intervention [59]. Our results supported this evidence that AEs of zavegepant were mostly non-serious (96.11%). We also identified other unique PTs of zavegepant, including “nasal discomfort” “rhinorrhea”, “epistaxis”, “nasal congestion”, and “rhinalgia”, which were considered as local irritation AEs associated with nasal administration [62]. However, we should emphasize that the robustness of signal detection might be constrained due to the limited number of zavegepant-related AE reports collected. Furthermore, we should reinforce the fact that continuous monitoring of zavegepant-related AE is critical given its novel administration routine.
TTO analysis is an important method for healthcare professionals to predict and identify AEs [63]. Due to the large number of missing or invalid values in FAERS database, only 7.19% of the collected reports were included in the TTO analysis of this study. Our results indicated that most AEs occurred within 30 days following gepant administration, suggesting the necessity of close monitoring of the patients, especially during the first month. We also noticed that atogepant had the largest median TTO value among all gepants. Further subgroup analysis revealed greater TTO values of atogepant in certain PTs, including “alopecia” (108.00, [14.00,274.00]), “memory impairment” (89.00, [88.00,345.00]), and “seizure” (50.00, [29.00,61.00]). Such delayed TTO of atogepant appeared to be associated with its relatively longer elimination half-life compared to other gepants [64]. Therefore, education of potential delayed AEs and long-term periodic monitoring should be required in patients receiving atogepant treatment.
This study has several limitations that require acknowledgement. First, since FAERS relies on spontaneous reporting, reports with incomplete or incorrect data are thereby inevitable. This resulted in an insufficient number of cases in descriptive analysis and TTO analysis. Furthermore, due to the intrinsic limitation that FAERS lacks the information of detailed medical records and denominator information, all results only allowed us to determine potential drug-AE correlations rather than confirmed causality. Follow-up studies in combination with other sources, such as medical claims and electronic health records, would provide more comprehensive assessment of AE causality. Lastly, the extensive disproportionality analysis of gepants and PTs in this study might potentially increase the risk of false-positive signals. Subsequent studies applying multiple false discovery rate corrections (e.g., Benjamini–Hochberg and Bonferroni) would be helpful in limiting false-positive signals.
Conclusions
Our study comprehensively analyzed the significant safety signals of the approved gepants. By mining and comparing the common and distinctive features of AEs among gepants, our results provided valuable reference for the safe use of gepants, offering guidance for clinicians to tailor individualized treatments.
Supplementary Information
Acknowledgements
We sincerely thank FAERS for the free access of data.
Abbreviations
- AE
Adverse event
- BCPNN
Bayesian confidence propagation neural network
- CGRP
Calcitonin gene-related peptide
- CI
Confidence interval
- FAERS
FDA Adverse Event Reporting System
- FDA
Food and Drug Administration
- IC
Information component
- PRR
Proportional reporting ratio
- PT
Preferred terminology
- ROR
Reporting odds ratio
- SOC
System organ class
- TTO
Time-to-onset
Authors’ contributions
YT contributed to the conception and design of the study. QS, SG, and YT collected the data. QS analyzed the data. QS and YT drafted the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82104223 and 82204372), Guangdong Basic and Applied Basic Research Foundation (2020A1515110008), Science and Technology Program of Guangzhou (202102021022, 2024A04J10001, and 2025A03J3308), Medical Scientific Research Foundation of Guangdong (A2024618), and Guangzhou Municipal Key Discipline in Medicine (2025–2027).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Since all data of our study was originated from public databases with non-identifiable datasets, no ethical approval is required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al-Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A (2022) Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol 21(3):284–294. 10.1016/s1474-4422(21)00409-9 [DOI] [PubMed] [Google Scholar]
- 2.Cohen F, Yuan H, Silberstein SD (2022) Calcitonin Gene-Related Peptide (CGRP)-targeted monoclonal antibodies and antagonists in migraine: current evidence and rationale. BioDrugs 36(3):341–358. 10.1007/s40259-022-00530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar-Martinez MD, Goadsby PJ (2022) Pathophysiology and therapy of associated features of migraine. Cells 11(17):2767. 10.3390/cells11172767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstantinos S, Vikelis M, Rapoport A (2020) Acute care and treatment of migraine. J Neuroophthalmol 40(4):472–484 [DOI] [PubMed] [Google Scholar]
- 5.Diener HC (2020) The risks or lack thereof of migraine treatments in vascular disease. Headache 60(3):649–53. 10.1111/head.13749 [DOI] [PubMed] [Google Scholar]
- 6.Nisar A, Ahmed Z, Yuan H (2023) Novel therapeutic targets for migraine. Biomedicines. 11(2). 10.3390/biomedicines11020569 [DOI] [PMC free article] [PubMed]
- 7.Liu J, Wang G, Dan Y, Liu X (2022) CGRP and PACAP-38 play an important role in diagnosing pediatric migraine. J Headache Pain 23(1):68. 10.1186/s10194-022-01435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ailani J, Kaiser EA, Mathew PG, McAllister P, Russo AF, Vélez C et al (2022) Role of calcitonin gene-related peptide on the gastrointestinal symptoms of migraine—clinical considerations. Neurology 99(19):841–853. 10.1212/WNL.0000000000201332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rissardo JP, Caprara ALF (2022) Gepants for acute and preventive migraine treatment: a narrative review. Brain Sci 12(12):1612. 10.3390/brainsci12121612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altamura C, Brunelli N, Marcosano M, Fofi L, Vernieri F (2022) Gepants—a long way to cure: a narrative review. Neurol Sci 43(9):5697–5708. 10.1007/s10072-022-06184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno-Ajona D, Villar-Martínez MD, Goadsby PJ (2022) New generation Gepants: migraine acute and preventive medications. J Clin Med 11(6):1656. 10.3390/jcm11061656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.dos Santos JBR, da Silva MRR (2022) Small molecule CGRP receptor antagonists for the preventive treatment of migraine: a review. Eur J Pharmacol 922:174902. 10.1016/j.ejphar.2022.174902 [DOI] [PubMed] [Google Scholar]
- 13.Haghdoost F, Puledda F, Garcia-Azorin D, Huessler E-M, Messina R, Pozo-Rosich P (2023) Evaluating the efficacy of CGRP mAbs and gepants for the preventive treatment of migraine: A systematic review and network meta-analysis of phase 3 randomised controlled trials. Cephalalgia. 2023(4). 10.1177/03331024231159366 [DOI] [PubMed]
- 14.Lee S, Staatz CE, Han N, Baek IH (2022) Safety evaluation of oral calcitonin-gene–related peptide receptor antagonists in patients with acute migraine: a systematic review and meta-analysis. Eur J Clin Pharmacol 78(9):1365–76. 10.1007/s00228-022-03347-6 [DOI] [PubMed] [Google Scholar]
- 15.Baraldi C, Dagmar B, Paolo M, Pellesi L (2024) The preclinical discovery and development of Atogepant for migraine prophylaxis. Expert Opin Drug Discov 19(7):783–788. 10.1080/17460441.2024.2365379 [DOI] [PubMed] [Google Scholar]
- 16.Pellesi L, Budour J, Fadia B, Samah A-A, Paolo M, Xiao Z (2025) Head-to-head relief: ubrogepant, rimegepant, and zavegepant in migraine treatment. Pain Manag 15(5):279–284. 10.1080/17581869.2025.2494494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells-Gatnik WD, Lanfranco P, Martelletti P (2024) Rimegepant and atogepant: novel drugs providing innovative opportunities in the management of migraine. Expert Rev Neurother 24(11):1107–1117. 10.1080/14737175.2024.2401558 [DOI] [PubMed] [Google Scholar]
- 18.Liang Q, Liao X, Wu H, Huang Y, Liang T, Li H (2024) Real-world study of adverse events associated with gepant use in migraine treatment based on the VigiAccess and U.S. Food and Drug Administration’s adverse event reporting system databases. Front Pharmacol. 15. 10.3389/fphar.2024.1431562 [DOI] [PMC free article] [PubMed]
- 19.Battini V, Carla C, Emilio C, Sessa M (2023) Ubrogepant and rimegepant: signal detection using spontaneous reports of adverse events from the food and drug administration adverse event reporting system. Expert Opin Drug Saf 22(11):1105–1112. 10.1080/14740338.2023.2223958 [DOI] [PubMed] [Google Scholar]
- 20.Hu J-L, Wu J-Y, Xu S, Qian S-Y, Jiang C, Zheng G-Q (2024) Post-marketing safety concerns with rimegepant based on a pharmacovigilance study. J Headache Pain 25(1):169. 10.1186/s10194-024-01858-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao B, Shanshan G, Zhisen S, Yuna Z, Yiming S, Chen H (2024) Evaluating Ubrogepant-related adverse events using the FDA adverse event reporting system. Expert Opin Drug Saf 23(3):297–303. 10.1080/14740338.2023.2251390 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Shengzhu S, Wang Y (2024) Adverse events associated with Atogepant: a FAERS-based pharmacovigilance analysis. Expert Opinion on Drug Safety. 1–7. 10.1080/14740338.2024.2393268 [DOI] [PubMed]
- 23.Wen H, Yitian D, Chen F (2024) A real-world pharmacovigilance study of FDA adverse event reporting system events for atogepant. Expert Opin Drug Saf. 1–8. 10.1080/14740338.2024.2377347 [DOI] [PubMed]
- 24.Pan H, Lin S (2024) A real-world adverse events study of rimegepant from the FAERS database. Expert Opin Drug Saf. 1–6. 10.1080/14740338.2024.2418319 [DOI] [PubMed]
- 25.Wong CK, Ho SS, Saini B, Hibbs DE, Fois RA (2015) Standardisation of the FAERS database: a systematic approach to manually recoding drug name variants. Pharmacoepidemiol Drug Saf 24(7):731–737. 10.1002/pds.3805 [DOI] [PubMed] [Google Scholar]
- 26.Sakaeda T, Tamon A, Kadoyama K, Okuno Y (2013) Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci 10(7):796–803. 10.7150/ijms.6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarangdhar M, Tabar S, Schmidt C, Kushwaha A, Shah K, Dahlquist JE et al (2016) Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat Biotechnol 34(7):697–700. 10.1038/nbt.3623 [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Zheng X, Zhang M, Huang J, Huang P, Wang J (2024) Drug-induced gynecomastia: data mining and analysis of the FDA adverse event reporting system database. Clin Epidemiol 16:617–630. 10.2147/clep.S470959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC (2002) A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 11(1):3–10. 10.1002/pds.668 [DOI] [PubMed] [Google Scholar]
- 30.Evans SJ, Waller PC, Davis S (2001) Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 10(6):483–486. 10.1002/pds.677 [DOI] [PubMed] [Google Scholar]
- 31.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A et al (1998) A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 54(4):315–321. 10.1007/s002280050466 [DOI] [PubMed] [Google Scholar]
- 32.Dias P, Penedones A, Alves C, Ribeiro CF, Marques FB (2015) The role of disproportionality analysis of pharmacovigilance databases in safety regulatory actions: a systematic review. Curr Drug Saf 10(3):234–250. 10.2174/1574886310666150729112903 [DOI] [PubMed] [Google Scholar]
- 33.Tsai C-K, Tsai C-L, Lin G-Y, Yang F-C, Wang S-J (2022) Sex differences in chronic migraine: focusing on clinical features, pathophysiology, and treatments. Curr Pain Headache Rep 26(5):347–355. 10.1007/s11916-022-01034-w [DOI] [PubMed] [Google Scholar]
- 34.Vetvik KG, MacGregor EA (2017) Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 16(1):76–87. 10.1016/S1474-4422(16)30293-9 [DOI] [PubMed] [Google Scholar]
- 35.Amiri P, Kazeminasab S, Nejadghaderi SA, Mohammadinasab R, Pourfathi H, Araj-Khodaei M et al (2022) Migraine: a review on its history, global epidemiology, risk factors, and comorbidities. Front Neurol. 12:2021. 10.3389/fneur.2021.800605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo AF, Hay DL (2023) CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev 103(2):1565–1644. 10.1152/physrev.00059.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durham PL (2016) Diverse physiological roles of calcitonin gene-related peptide in migraine pathology: modulation of neuronal-glial-immune cells to promote peripheral and central sensitization. Curr Pain Headache Rep 20(8):48. 10.1007/s11916-016-0578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papiri G, Vignini A, Capriotti L, Verdenelli P, Alia S, Di Paolo A et al (2022) Cerebrospinal Fluid α-calcitonin gene-related peptide: a comparison between alzheimer’s disease and multiple sclerosis. Biomolecules 12(2):199. 10.3390/biom12020199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Błaszczyk B, Martynowicz H, Więckiewicz M, Straburzyński M, Antolak M, Budrewicz S et al (2024) Prevalence of headaches and their relationship with obstructive sleep apnea (OSA) - Systematic review and meta-analysis. Sleep Med Rev 73:101889. 10.1016/j.smrv.2023.101889 [DOI] [PubMed] [Google Scholar]
- 40.Holzer P, Holzer-Petsche U (2022) Constipation caused by anti-calcitonin gene-related peptide migraine therapeutics explained by antagonism of calcitonin gene-related peptide’s motor-stimulating and prosecretory function in the intestine. Front Physiol 12:820006. 10.3389/fphys.2021.820006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ailani J, Lipton RB, Goadsby PJ, Guo H, Miceli R, Severt L et al (2021) Atogepant for the preventive treatment of migraine. N Engl J Med 385(8):695–706. 10.1056/NEJMoa2035908 [DOI] [PubMed] [Google Scholar]
- 42.Chiang CC, Arca KN, Dunn RB, Girardo ME, Quillen JK, Dodick DW et al (2021) Real-world efficacy, tolerability, and safety of ubrogepant. Headache 61(4):620–627. 10.1111/head.14062 [DOI] [PubMed] [Google Scholar]
- 43.Goadsby PJ, Dodick DW, Ailani J, Trugman JM, Finnegan M, Lu K et al (2020) Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol 19(9):727–737. 10.1016/s1474-4422(20)30234-9 [DOI] [PubMed] [Google Scholar]
- 44.Neha R, Subeesh V, Beulah E, Gouri N, Maheswari E (2021) Existence of Notoriety Bias in FDA adverse event reporting system database and its impact on signal strength. Hosp Pharm 56(3):152–158. 10.1177/0018578719882323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahremany S, Hofmann L, Harari M, Gruzman A, Cohen G (2021) Pruritus in psoriasis and atopic dermatitis: current treatments and new perspectives. Pharmacol Rep 73(2):443–453. 10.1007/s43440-020-00206-y [DOI] [PubMed] [Google Scholar]
- 46.Kahremany S, Hofmann L, Gruzman A, Cohen G (2020) Advances in understanding the initial steps of pruritoceptive itch: how the itch hits the switch. Int J Mol Sci. 21(14). 10.3390/ijms21144883 [DOI] [PMC free article] [PubMed]
- 47.Ruiz M, Cocores A, Tosti A, Goadsby PJ, Monteith TS (2023) Alopecia as an emerging adverse event to CGRP monoclonal antibodies: cases series, evaluation of FAERS, and literature review. Cephalalgia. 2023(2). 10.1177/03331024221143538 [DOI] [PubMed]
- 48.Woods RH (2022) Alopecia signals associated with calcitonin gene-related peptide inhibitors in the treatment or prophylaxis of migraine: A pharmacovigilance study. Pharmacotherapy 42(10):758–67. 10.1002/phar.2725 [DOI] [PubMed] [Google Scholar]
- 49.Rossi R, Del Bianco E, Isolani D, Baccari MC, Cappugi P (1997) Possible involvement of neuropeptidergic sensory nerves in alopecia areata. NeuroReport 8(5):1135–1138. 10.1097/00001756-199703240-00015 [DOI] [PubMed] [Google Scholar]
- 50.Falkenberg K, Bjerg HR, Olesen J (2020) Two-Hour CGRP Infusion Causes Gastrointestinal Hyperactivity: Possible Relevance for CGRP Antibody Treatment. Headache 60(5):929–37. 10.1111/head.13795 [DOI] [PubMed] [Google Scholar]
- 51.Favoni V, Giani L, Al-Hassany L, Asioli GM, Butera C, de Boer I et al (2019) CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain 20(1):27. 10.1186/s10194-019-0979-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo S, Vollesen ALH, Olesen J, Ashina M (2016) Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain 157(12):2773–2781. 10.1097/j.pain.0000000000000702 [DOI] [PubMed] [Google Scholar]
- 53.Enomoto H, Terauchi M, Odai T, Kato K, Iizuka M, Akiyoshi M et al (2021) Independent association of palpitation with vasomotor symptoms and anxiety in middle-aged women. Menopause 28(7):741–747. 10.1097/gme.0000000000001776 [DOI] [PubMed] [Google Scholar]
- 54.Woodhead JL, Siler SQ, Howell BA, Watkins PB, Conway C (2022) Comparing the Liver Safety Profiles of 4 Next-Generation CGRP Receptor Antagonists to the Hepatotoxic CGRP Inhibitor Telcagepant Using Quantitative Systems Toxicology Modeling. Toxicol Sci 188(1):108–116. 10.1093/toxsci/kfac051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Migliorati JM, Liu S, Liu A, Gogate A, Nair S, Bahal R et al (2022) Absorption, distribution, metabolism, and excretion of US food and drug administration-approved antisense oligonucleotide drugs. Drug Metab Dispos 50(6):888–897. 10.1124/dmd.121.000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhardwaj R, Donohue MK, Madonia J, Morris B, Marbury TC, Matschke KT et al (2024) Reduced hepatic impairment study to evaluate pharmacokinetics and safety of zavegepant and to inform dosing recommendation for hepatic impairment. Clin Transl Sci 17(7):e13813. 10.1111/cts.13813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greco G, Monteith T (2024) Intranasal zavegepant for the acute treatment of migraine. Expert Rev Neurother 24(12):1131–1140. 10.1080/14737175.2024.2405741 [DOI] [PubMed] [Google Scholar]
- 58.Lipton RB, Croop R, Stock DA, Madonia J, Forshaw M, Lovegren M et al (2023) Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: a phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol 22(3):209–217. 10.1016/s1474-4422(22)00517-8 [DOI] [PubMed] [Google Scholar]
- 59.Croop R, Madonia J, Stock DA, Thiry A, Forshaw M, Murphy A et al (2022) Zavegepant nasal spray for the acute treatment of migraine: A Phase 2/3 double-blind, randomized, placebo-controlled, dose-ranging trial. Headache 62(9):1153–1163. 10.1111/head.14389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhillon S (2023) Zavegepant: first approval. Drugs 83(9):825–831. 10.1007/s40265-023-01885-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doty RL, Shah M, Bromley SM (2008) Drug-induced taste disorders Drug safety 31:199–215. 10.2165/00002018-200831030-00002 [DOI] [PubMed] [Google Scholar]
- 62.Mullin K, Croop R, Mosher L, Fullerton T, Madonia J, Lipton RB (2024) Long-term safety of zavegepant nasal spray for the acute treatment of migraine: A phase 2/3 open-label study. Cephalalgia 44(8):03331024241259456. 10.1177/03331024241259456 [DOI] [PubMed] [Google Scholar]
- 63.Ando G, Taguchi K, Enoki Y, Yokoyama Y, Kizu J, Matsumoto K (2019) Evaluation of the expression time of ganciclovir-induced adverse events using JADER and FAERS. Biol Pharm Bull 42(11):1799–1804. 10.1248/bpb.b19-00156 [DOI] [PubMed] [Google Scholar]
- 64.Boinpally R, Shebley M, Trugman JM (2024) Atogepant: Mechanism of action, clinical and translational science. Clin Transl Sci 17(1):e13707. 10.1111/cts.13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.