Abstract
The prognosis for women with ovarian cancer (OC) is particularly poor if resistance to platinum compounds, the mainstay of standard-of-care therapy, develops. Inhibitors of the Nudix hydrolase MuT Homolog 1 (MTH1) have previously been shown to arrest cancer cells in mitosis, increase 8-oxo-2’-deoxyguanosine (8-oxo-dG) incorporation into DNA, and selectively kill neoplastic cells while sparing normal cells. Here we explored the cytotoxic mechanism of these agents as well as their activity against platinum-resistant OC in vitro and in vivo. Two mitotic MTH1 inhibitors (mMTH1is), TH588 and karonudib, decreased colony formation indistinguishably in platinum-sensitive OC cell lines and their platinum-resistant counterparts in vitro but had limited effects on fallopian tube and immortalized ovarian surface epithelial cells. Treatment with karonudib stalled OC cells in mitosis and caused elevated 8-oxo-dG levels in DNA followed by activation of base excision repair, induction of BAX, and apoptotic cellular demise. This cytotoxicity was blunted by overexpression of the pre-mitotic checkpoint protein CHFR, which inhibits other anti-mitotics, or treatment with the antioxidant N-acetylcysteine, which diminishes nuclear 8-oxo-dG staining, suggesting a role for both mitotic stalling and increased nuclear incorporation of oxidized nucleotides in karonudib efficacy. In three orthotopic OC patient-derived xenograft models, karonudib monotherapy induced growth delay in vivo. Moreover, addition of karonudib to carboplatin doubled median overall survival in two models and prolonged survival for the duration of the study (110 days) in the third. These results demonstrate activity of mMTH1is as monotherapy and in combination with carboplatin in OC that warrants further investigation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-025-00681-0.
Keywords: MTH1 inhibitors, Ovarian cancer, Platinum resistance, PDX models
To the Editor,
Resistance to platinum compounds, the mainstay of standard-of-care ovarian cancer (OC) therapy [1, 2], develops in a majority of cases and is associated with a one-year median survival [3, 4], highlighting the need for new therapies. The Nudix hydrolase MutT Homolog 1 (MTH1) is elevated in various cancers [5] and required for survival of cells transformed by a number of oncogenes [6], but not normal cells, leading to efforts to inhibit this enzyme [7, 8]. Recent studies demonstrated that some MTH1 inhibitors concomitantly arrest neoplastic cells in mitosis, reflecting a previously unappreciated role of MTH1 in cell division, and increase 8-oxo-2’-deoxyguanosine (8-oxo-dG) incorporation into DNA [8, 9]. Here we explored the activity of these mitotic MTH1 inhibitors (mMTH1is) against platinum-resistant OC in vitro and in vivo.
To assess antiproliferative activity, OC cell lines (Supplementary Table S1) were treated with the first-generation mMTH1i TH588 (Supplementary Fig. S1A) or second-generation inhibitor karonudib (Fig. 1A) in colony forming assays (Supplementary Methods). All lines were sensitive, with IC50s of 0.9-4 µM for TH588 and 60-200 nM for karonudib. Mechanistic studies in the A2780 cells indicated these effects on colony formation reflected a process that involved incorporation of 8-oxo-dG into DNA (see below), activation of base excision repair as manifested by XRCC1 foci, activation of DNA damage-activated phosphatidylinositol-3 kinase-related kinases (γH2Ax foci), TP53 accumulation, increased expression of TP53 target genes, increased levels and mitochondrial translocation of the proapoptotic protein BAX, mitochondrial outer membrane depolarization, and development of apoptotic morphological changes in live cell imaging (Figs. S2 and S3). In contrast, nontransformed immortalized ovarian surface epithelial (IOSE) and fallopian tube epithelial cells demonstrated minimal response (Fig. 1B, S1B and S1C).
Fig. 1.
mMTH1 inhibitor sensitivity in platinum-sensitive and -resistant OC cell lines. A, B, cell lines corresponding to the indicated OC histological subtypes (A) or IGROV1 and immortalized normal ovarian surface epithelial cells (IOSE, B) were treated continuously with the mMTH1i karonudib in clonogenic assays. C-F, paired parental IGROV1 and platinum-selected IGROV1/CP cells (C, D) or A2780 and platinum-selected A2780/CP200 cells (E, F) were treated continuously with the indicated concentrations of cisplatin (C, E) or karonudib (D, F). Numbers indicate IC50 values (mean ± sd) from 2 (OVISE) or ≥3 independent replicates (all other lines). IC50 values for IGROV1 and A2780 cells vary slightly between panels because resistant lines were directly compared to parental lines, which were repeated for those experiments. *, ** and *** indicate p < 0.05, 0.01 and 0.001 by 2-sided t tests, respectively. t tests comparing IC50s in panels D and F were not significant. Assays of TH588 sensitivity are presented in Fig. S1
To determine whether mMTH1is also demonstrate efficacy in platinum-resistant OC lines, paired parental and cisplatin-resistant cell lines were compared. Despite a >10-fold difference in cisplatin IC50s (Fig. 1C), responses of parental IGROV and cisplatin-resistant IGROV1/CP cells to mMTHis were similar (Fig. 1D, S1D). Comparable results were observed when parental A2780 and PEO1 cells were compared to their respective platinum-resistant counterparts (Fig. 1E, 1 F, S1E-G).
Previous studies have demonstrated two actions of karonudib: (i) stalling of cells in mitosis and (ii) accumulation of oxidized nucleotides, including 8-oxo-dGTP, into DNA [9]. To assess the contributions of these effects to karonudib activity in OC, MTH1i-treated cells were examined for mitotic stalling by flow cytometry (Fig. S4A-S4C) or morphological examination (Fig. S4D, S4E). Both parental and platinum-resistant A2780 cells were transiently stalled in mitosis, although not to the same extent as by paclitaxel (Fig. S4A-S4E). The potential impact of this mitotic stalling was assessed by comparing the action of MTHis in A2780 cells that differ in expression of CHFR, a premitotic checkpoint protein that imparts the ability of cells to arrest prior to mitosis and avoid mitotic catastrophe [10, 11]. A2780 clones expressing CHFR (Fig. S4F) had decreased sensitivity to karonudib as well as paclitaxel (Figs. S4G, S4H), suggesting an important role for mitotic arrest in karonudib-induced killing.
To assess the role of increased nucleotide oxidation in mMTH1i-induced killing, we examined the impact of the antioxidant N-acetylcysteine (NAC) on nuclear 8-oxo-dG levels and clonogenic survival. NAC pretreatment blunted the mMTH1i-induced increase in 8-oxo-dG fluorescence (Fig. S5A-D) and diminished the impact of karonudib on colony formation (Fig. S5E, S5F), supporting a role for 8-oxo-dG incorporation in mMTH1i-induced killing as well.
Based on this role of oxidative damage in mMTH1i-induced killing and previous reports indicating that platinum agents increase cellular reactive oxygen species [12–14], we assessed activity of the karonudib/carboplatin combination in vivo. For these studies, three genomically distinct high grade serous OC patient-derived xenograft (PDX) models (Table S1) with varying platinum sensitivity were implanted orthotopically, allowed to engraft, and monitored by transabdominal ultrasound during and after treatment with diluent, karonudib, carboplatin, or the combination.
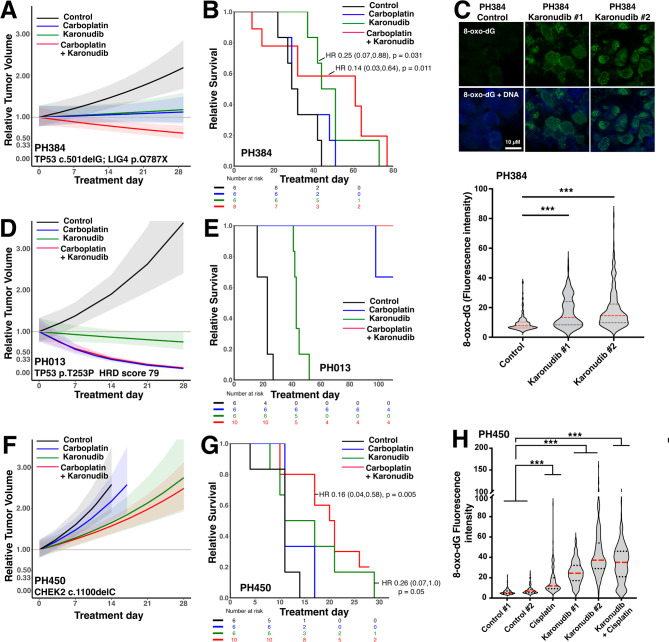
Karonudib enhanced the effects of carboplatin in all three models (Figs. 2 and S6) without enhancing toxicity at the final dose and schedule (Fig. S7). In PH384, a platinum tolerant model harboring TP53 and LIG4 mutations, the karonudib/carboplatin combination induced tumor regressions (Fig. 2A, p < 0.0001 relative to control) and increased overall survival relative to platinum monotherapy [Hazard ratio (HR) for combination versus carboplatin monotherapy 0.22 (0.05–0.91), p = 0.037, Fig. 2B]. Moreover, karonudib also increased nuclear 8-oxo-dG in vivo (Fig. 2C), as anticipated from the cell line studies. In the highly platinum sensitive PH013 model, karonudib induced tumor shrinkage, providing the first evidence for regressions induced by karonudib monotherapy in a carcinoma model (Fig. 2D). In addition, there was a trend toward increased survival in the combination arm relative to carboplatin (Fig. 2E) that did not reach statistical significance due to the small number of events. In PH450, a CHEK2 mutated model that is highly platinum-resistant, addition of karonudib enhanced carboplatin-induced slowing (Fig. 2F, p = 0.002 for combination vs. carboplatin) and increased overall survival (Fig. 2G) [HR for combination vs. carboplatin 0.27 (0.08–0.88), p = 0.03] while also increasing levels of nuclear 8-oxo-dG relative to the increase with carboplatin alone (Fig. 2H).
Fig. 2.
Effects of karonudib as monotherapy and in combination with carboplatin in orthotopic high grade serous ovarian cancer PDXs. Karonudib was administered at 90 mg/kg as a single agent (all PDXs) or in combination with carboplatin (50 mg/kg/week) at karonudib doses of 90 mg/kg (A-C) or 60 mg/kg (D-H) to mice bearing intraperitoneal high grade serous ovarian cancer PDX models PH384 (A-C), PH013 (D-E), or PH450 (F-H). PDX growth was measured by transabdominal ultra-sound and expressed as cross-sectional area relative to Day 0 (A, D, F). Error bars indicate 95% confidence intervals. Following 28 days of treatment, tumor growth and overall survival (B, E, G) were observed for up to 110 days. Numbers indicate hazard ratios (95% confidence intervals) and corresponding p values for indicated treatments relative to controls. In addition, FFPE samples harvested on day 5 of treatment from PH384 and PH450 were stained for nuclear 8-oxo-dG (e.g., micrographs at top of panel C), with fluorescence intensity (C, H) determined as indicated in the Methods. Fluorescence of 100–250 individual nuclei/sample is indicated. Lines in panels C and H indicate median (red) and interquartile values (black). *** indicates p < 0.001 by Wilcoxon rank sum test after correction for multiple comparisons. Growth curves of PDXs in individual mice are shown in Fig. S6
Collectively, these results demonstrate that mMTH1is exhibit indistinguishable antiproliferative effects in paired platinum sensitive and platinum resistant OC lines in vitro, that both mitotic stalling and incorporation of oxidized nucleotides into DNA are important for these antiproliferative effects, and that antineoplastic effects of carboplatin in three OC PDX models are enhanced by the clinical mMTH1i karonudib in vivo. In view of the safety and monotherapy activity observed in the recently completed karonudib phase I trial (NCT03036228, ref. 15), further study of the karonudib/carboplatin combination might be warranted, providing a potential path toward clinical development of karonudib for OC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Toshi Tanaguchi, Viji Shridhar, Ernst Lengyel and Dominic Scudiero for cell lines; David Toft for anti-HSP90b antibody; Saloni Singh for assistance; and Robert Diasio for encouraging our collaboration.
Abbreviations
- 8-oxo-dG
8-oxo-2’deoxyguanosine
- FBS
Heat-inactivated fetal bovine serum
- HGSOC
High grade serous ovarian cancer
- HR
Hazard ratio
- MTH1
MutT Homolog 1
- MTH1i
MTH1 inhibitor
- mMTH1i
Mitotic MTH1 inhibitor
- NAC
N-acetylcysteine
- OC
Ovarian cancer
- PBS
Calcium- and magnesium-free Dulbecco’s phosphate buffered saline
- PDX
Patient-derived xenograft
- RFT
Resected fallopian tube
- ROS
Reactive oxygen species
Author contributions
Conceptualization: R.M.H., T.H., U.W.B, S.H.K., A.E.W.H; Formal analysis: C.C., M.J.M, E.P.H., M.L., A.L.O.; Investigation: R.M.H., J.M.W., A.K., A.V., A.M.D., P.A.S., K.L.P., E.P.M., X.H., M.A.B, E.M.S.; Resources: U.W.B., S.K., S.J.W., S.H.K.; Writing—original draft preparation: R.M.H., U.W.B., T.H., S.H.K and A.E.W.H.; Writing—review and editing: All authors; Supervision: H.L., U.W.B, T.H., S.H.K. and A.E.W.H.; Funding acquisition: R.M.H., T.H., S.H.K., A.E.W.H. All authors reviewed the manuscript.
Funding
This work was supported in part by NIH Grant K12 CA09062 (to AEWH), the Minnesota Ovarian Cancer Alliance (to RMH and AEWH), the Swedish Cancer Society (to TH), a Mayo-Karolinska Institutet Collaborative Grant (to AEWH and TH), the Mayo Clinic Ovarian Cancer Specialized Program of Research Excellence (P50 CA1363939), the Mayo Clinic Cancer Center (P30 CA015083), the Fred C. and Katherine B. Andersen Foundation, and funding to RMH from T32 GM065841 and the Mayo Foundation for Research and Education.
Data availability
RNAseq data (Figure S2) are deposited in the Gene Expression Omnibus database (GSE293549). Other datasets generated during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
PDXs were generated under protocols approved by the Mayo Clinic Institutional Review Board (approval # 09-008768) and Mayo Clinic Animal Care and Use Committee (approval # A37615). Treatments with carboplatin and karonudib were conducted under the aegis of Mayo Clinic Animal Care and Use Committee protocol A00005570-20.
Consent for publication
Not applicable.
Competing interests
U.W.B., T.H. and K.S. are shareholders in Oxcia AB. In addition, U.W.B. is CEO and board member of Oxcia AB; T.H. is board member of Oxcia AB; and A.W.H. serves on the advisory board of Oxcia AB. The other authors report no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Scott H. Kaufmann and Andrea E. Wahner Hendrickson contributed equally to this work as senior authors.
Contributor Information
Scott H. Kaufmann, Email: Kaufmann.Scott@Mayo.edu
Andrea E. Wahner Hendrickson, Email: wahnerhendrickson.andrea@mayo.edu.
References
- 1.Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–53. [DOI] [PubMed] [Google Scholar]
- 3.McMullen M, Karakasis K, Madariaga A, Oza AM. Overcoming platinum and PARP-Inhibitor resistance in ovarian cancer. Cancers (Basel). 2020;12(6). [DOI] [PMC free article] [PubMed]
- 4.Elyashiv O, Aleohin N, Migdan Z, Leytes S, Peled O, Tal O et al. The poor prognosis of acquired secondary platinum resistance in ovarian cancer patients. Cancers (Basel). 2024;16(3). [DOI] [PMC free article] [PubMed]
- 5.Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helleday T. Cancer phenotypic lethality, exemplified by the non-essential MTH1 enzyme being required for cancer survival. Ann Oncol. 2014;25(7):1253–5. [DOI] [PubMed] [Google Scholar]
- 7.Gad H, Koolmeister T, Jemth AS, Eshtad S, Jacques SA, Strom CE, et al. MTH1 Inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature. 2014;508(7495):215–21. [DOI] [PubMed] [Google Scholar]
- 8.Helleday T. Mitotic MTH1 inhibitors in treatment of cancer. Cancer Treat Res. 2023;186:223–37. [DOI] [PubMed] [Google Scholar]
- 9.Rudd SG, Gad H, Sanjiv K, Amaral N, Hagenkort A, Groth P, et al. MTH1 inhibitor TH588 disturbs mitotic progression and induces mitosis-dependent accumulation of genomic 8-oxodG. Cancer Res. 2020;80(17):3530–41. [DOI] [PubMed] [Google Scholar]
- 10.Scolnick DM, Halazonetis TD. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature. 2000;406(6794):430–5. [DOI] [PubMed] [Google Scholar]
- 11.Wahner Hendrickson AE, Visscher DW, Hou X, Goergen KM, Atkinson HJ, Beito TG, et al. CHFR and paclitaxel sensitivity of ovarian cancer. Cancers (Basel). 2021;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh T, Terazawa R, Kojima K, Nakane K, Deguchi T, Ando M, et al. Cisplatin induces production of reactive oxygen species via NADPH oxidase activation in human prostate cancer cells. Free Radic Res. 2011;45(9):1033–9. [DOI] [PubMed] [Google Scholar]
- 13.Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE. 2013;8(11):e81162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Simpson CM, Berner J, Chong HB, Fang J, Ordulu Z, et al. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell. 2023;186(11):2361–e7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yachnin J, Ny L, Sandvall T, Sanjiv K, Scobie M, Platzack B, et al. Abstract CT182: OXC-101 shows favorable safety profile in first in human phase 1 trial in patients with advanced solid cancer. Cancer Res. 2023;83(8Supplement):CT182–CT. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNAseq data (Figure S2) are deposited in the Gene Expression Omnibus database (GSE293549). Other datasets generated during the current study are available from the corresponding authors on reasonable request.