Abstract
Background and Aims
Antibodies targeting bacterial cytolethal distending toxin subunit B (CdtB) and vinculin are diagnostic of post-infection irritable bowel syndrome (IBS). In this study, we explored the temporal behavior of anti-CdtB and anti-vinculin antibodies and potential relationships to IBS symptoms. The potential impacts of antibody reduction therapies were also assessed.
Methods
A retrospective chart review of 417 IBS patients who had been tested for anti-CdtB and anti-vinculin antibodies was performed. Anti-vinculin and anti-CdtB antibody levels, time to normalization of antibody levels, and IBS symptoms’ burdens and changes were assessed. Use of antibody-depleting therapies (intravenous immunoglobulin [IVIG] or plasmapheresis exchange [PLEX]) vs. usual management was also recorded.
Results
158 subjects (38.5%) were positive for either anti-CdtB or anti-vinculin. In subjects with multiple tests (total N = 38), normalization of anti-vinculin levels over time correlated with improvements in IBS symptoms (p = 0.020). Plasmapheresis (PLEX) or intravenous immunoglobulins (IVIG) treatments were associated with greater antibody normalization than usual management (p = 0.046).
Conclusions
Anti-CdtB and anti-vinculin antibodies are common in post-infection IBS, and anti-vinculin levels may correlate with severity of IBS symptoms.
Keywords: Irritable bowel syndrome, Cytolethal distending toxin B, Vinculin, Antibodies, Symptom severity
Introduction
Irritable Bowel Syndrome (IBS) is a chronic functional gastrointestinal disease characterized by chronic abdominal pain and altered bowel habits, and is subcategorized as constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), or mixed (IBS-M) [1–3]. IBS pathophysiology remains poorly understood; proposed mechanisms include gut-brain axis alterations, small and large bowel dysmotility, and gut microbiome alterations [2–4]. Several studies have assessed links between the microbiome and IBS, as gastrointestinal infections are an important independent risk factor for IBS (termed post-infection IBS) [2–6].
One potential mechanism connecting acute gastroenteritis to post-infection IBS is the development of autoantibodies. Common bacterial causes of gastroenteritis, such as Escherichia, Shigella, Salmonella, and Campylobacter jejuni, release cytolethal distending toxin; when antibodies form against the B subunit (CdtB), autoantibodies targeting vinculin also develop, likely through molecular mimicry. Vinculin, a cytoskeletal protein, is prevalent in interstitial cells of Cajal (ICC) and the myenteric plexus, which are vital components of neuronal cell-driven gut motility [4–6]. Based on this, an enzyme-linked immunosorbent assay (ELISA) was developed to accurately measure circulating levels of anti-CdtB and anti-vinculin in serum samples, and was tested in a large-scale study that included samples from a total of 2681 subjects from 180 centers across the US [7]. The results showed that combining these biomarkers successfully distinguished subjects with IBS-D from those with IBD and celiac disease, as well as healthy controls, and formed the basis for the first diagnostic test for IBS-D [7]. The utility of these antibodies as biomarkers for the diagnosis of IBS-D was subsequently confirmed by several independent groups [8–10], and their utility in also diagnosing IBS-M was later demonstrated [11]. A second-generation test that incorporated epitope stabilization was later developed, for which the post-test probability of having IBS-D rather than IBD was 98.1% when both biomarkers were combined [12]. However, beyond initial IBS diagnosis, little is known about temporal changes in anti-CdtB and anti-vinculin and potential correlations with clinical symptoms.
Here, we assess how these antibodies change over time, including time to normalization of antibody levels, whether changes in anti-CdtB and anti-vinculin antibody levels correlate with changes in IBS symptoms, and whether antibody reduction therapies impact antibody levels.
Methods
We performed a retrospective chart review of subjects from a single GI motility center who underwent second-generation testing for anti-vinculin and anti-CdtB antibodies (Gemelli Biotech, Raleigh, NC) between 10/25/2018 and 7/20/2023. Data included anti-vinculin and anti-CdtB antibody levels, collection times, and qualitative results. Patient demographic, IBS subtype, symptom burden, and intervention data were manually extracted from medical records. This study was approved by the Cedars-Sinai Institutional Review Board.
The entire study population was used to assess for independence between age and antibody positivity rate via Chi-square testing. Subjects with multiple tests were used to assess changes in antibody levels over time via linear regression. Symptom burdens and changes were determined from medical records as reported by patients’ physicians at encounters where antibody levels were checked. The frequency of symptom improvement was compared between subjects with abnormal antibody levels that later normalized and subjects whose antibody levels become newly abnormal or increased between tests. Subjects were classified as having either improvement or no improvement in symptoms due to small sample size. Fisher’s exact test was used to determine independence.
Lastly, the frequency of antibody normalization in patients with multiple tests who received antibody-depleting therapies (intravenous immunoglobulin [IVIG] or plasmapheresis exchange [PLEX]) between tests was compared to that in patients who initially had positive vinculin or CdtB antibodies and underwent usual management, via Chi-square testing. For the antibody-depleting therapies group, the first antibody level and nearest post-treatment level were used. For the control group, the first antibody level and lowest level thereafter were used.
Results
In total, 434 subjects underwent antibody testing, 417 of whom had valid tests (66% female, age 48 ± 16 years). Of these, 46 (11.2%) were positive for anti-CdtB alone; 91 (22.2%) were positive for anti-vinculin alone; 21 (5.12%) were positive for both; and a total of 158 (38.5%) were positive for either test. Older subjects appeared more likely to have a positive test than younger subjects (p < 0.001).
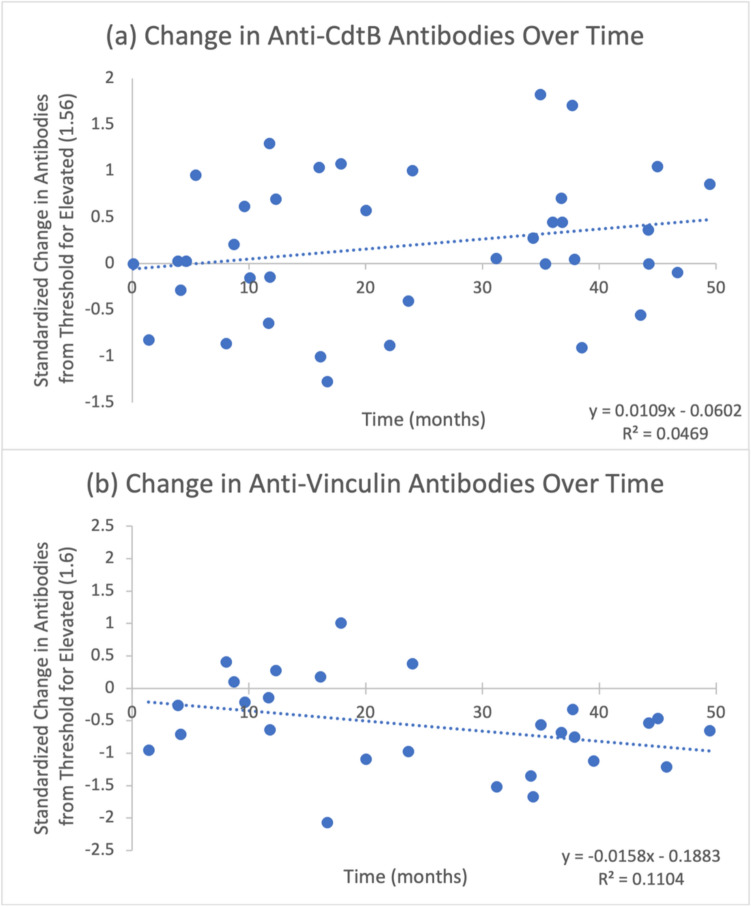
Thirty-eight (9.11%) subjects had multiple tests (range 2–5 tests). Time between first and last tests ranged from 1.4 to 50.2 months. Anti-vinculin levels exhibited a nonsignificant negative relationship with time (95% confidence interval of linear regression coefficient (CI): - 0.033, 0.0041; p = 0.14) (Fig. 1), whereas anti-CdtB levels exhibited a nonsignificant positive relationship with time (95% CI: -0.005, 0.0268; p = 0.192) (Fig. 1).
Fig. 1.
a Change in anti-CdtB antibodies over time. b Change in anti-vinculin antibodies over time. All data points are standardized as levels above upper limits of normal
Anti-vinculin levels changed over time in 20 subjects. Anti-vinculin levels normalized in 12 subjects (mean time to normalization: 23.7 ± 15 months); 9 of these subjects had improved symptoms at follow-up and 3 had no change in symptoms. The remaining 8 subjects developed newly elevated or further elevated anti-vinculin levels between tests. There were 3 subjects with no change in symptoms, four subjects with worsened symptoms, and only 1 subject had improved symptoms at follow-up (Table 1, p = 0.020). Anti-CdtB levels were initially elevated in 4 subjects, and normalized in 2 of these subjects, after 25.6 and 1.4 months, respectively; 1 of these also had improved symptoms at follow-up.
Table 1.
Rates of anti-vinculin level normalization and symptom improvement among 20 subjects with initially elevated anti-vinculin levels
| Symptoms improved | Symptoms not improved | Total | |
|---|---|---|---|
| Normalized | 9 | 3 | 12 |
| Not normalized | 1 | 7 | 8 |
| Total | 10 | 10 | P = 0.020 |
Of the subjects who had multiple tests, 8 subjects (6 female, age range 32–75 years) were initially positive for anti-vinculin or anti-CdtB and received antibody-reducing therapies; 2 were treated with PLEX and 6 with IVIG. A further 18 subjects (13 female, age range 21–85 years) who were initially positive for anti-vinculin or anti-CdtB underwent usual management. On subsequent testing, 5 subjects who received antibody reduction therapy (62.5%) exhibited normalized antibody levels, as compared to 4 subjects who underwent usual management (22.2%) (Table 2, p = 0.046). The time to normalization ranged from 2 months to 5.8 years, reflecting wide variation in time between pre-treatment testing, treatment date, and post-treatment testing.
Table 2.
Rates of anti-vinculin level normalization in patients who received antibody-depleting therapies (intravenous immunoglobulin [IVIG] or plasmapheresis exchange [PLEX]) vs. those undergoing usual care
| Antibodies normalized | Antibodies abnormal | Total | |
|---|---|---|---|
| IVIG/PLEX | 5 | 3 | 8 |
| Usual care | 4 | 14 | 18 |
| Total | 9 | 17 | P = 0.046 |
Discussion
Our study investigates temporal changes in anti-vinculin and anti-CdtB levels in post-infection IBS subjects over time. Importantly, we provide evidence that normalization of anti-vinculin antibody levels correlates with improvements in gastrointestinal symptoms. We were unable to demonstrate a clear reduction of antibodies over time among all-comers but did find that antibody-depleting therapies successfully reduced anti-vinculin levels. These findings have important implications for patients with IBS, which has been defined by symptom-based criteria for over 100 years but could instead be defined by serologic testing.
Post-infection IBS is associated with chronic alterations in bowel habits, independent of bacterial infection [4, 13, 14], and evidence suggests that anti-CdtB and anti-vinculin may contribute to mechanisms underlying IBS, such as dysmotility [13]. Vinculin is expressed in interstitial cells of Cajal (ICC) and the myenteric plexus, which control gut motility [4]. Elevated anti-vinculin is linked to decreased ICC density [15], and anti-vinculin is also associated with slowed gastric transit in systemic sclerosis [16, 17]. Our finding of improved GI symptoms following anti-vinculin reduction supports this, but further research is needed. Although it was beyond the scope of our study, examining whether symptom improvement occurred in all study subjects regardless of antibody trend could be valuable in guiding clinical interpretation of these trends.
Monitoring anti-vinculin levels in IBS subjects via affordable serum testing may also assist in IBS management, by identifying subjects for potential antibiotic reduction therapy. Again, further research is needed.
Our study has some limitations. The nature of a retrospective chart review limits our ability to control variation among subjects, e.g., whether subjects experienced gastroenteritis episodes between tests, and variable testing intervals. Small sample sizes limited statistical power to analyze trends over time and correlate symptom changes with antibody levels, which could be improved in future prospective studies. Further, using validated IBS symptom questionnaires may better facilitate correlating symptom severity with anti-vinculin and anti-CdtB levels.
In conclusion, anti-vinculin and anti-CdtB antibodies are commonly found and linked to symptoms in IBS-D and IBS-M. Anti-vinculin in particular may be associated with symptom severity, and antibody-reducing therapies may be helpful in future treatments.
Author Contributions
Study conception and design: MP, AR, RM, and ES. Data collection and analysis: MP, AR, RM, ES, JAB, KM, AH, and MR. Manuscript drafting: ES and JAB. Protocol and manuscript review and important intellectual contribution, including final approval of draft: MP, AR, and RM.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. None.
Data Availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
M.P. is a consultant for Bausch Health, Ferring Pharmaceuticals Inc., Salvo Health, Dieta Health, and Cylinder Health Inc. M.P. has received grant support from Bausch Health and Synthetic Biologics. R.M. has received grant support from Valiant Pharmaceuticals. A.R. is a consultant/speaker for, and has received grant support from, Bausch Health. Cedars-Sinai has a licensing agreement with Gemelli Biotech. A.R., M.P., and R.M. have equity in Gemelli Biotech. All other authors report no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. 10.2147/CLEP.S40245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enck P, Aziz Q, Barbara G et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. 10.1038/nrdp.2016.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396:1675–1688. 10.1016/S0140-6736(20)31548-8 [DOI] [PubMed] [Google Scholar]
- 4.Pimentel M, Morales W, Pokkunuri V et al. Autoimmunity links vinculin to the pathophysiology of chronic functional bowel changes following campylobacter jejuni infection in a rat model. Dig Dis Sci. 2015;60:1195–1205. 10.1007/s10620-014-3435-5 [DOI] [PubMed] [Google Scholar]
- 5.Pimentel M, Lembo A. Microbiome and its role in irritable bowel syndrome. Dig. Dis. Sci. 2020;65:829–839. 10.1007/s10620-020-06109-5 [DOI] [PubMed] [Google Scholar]
- 6.Rezaie A, Pimentel M, Cohen E. Autoimmunity as a potential cause of post-infectious gut dysmotility: a longitudinal observation. Am J Gastroenterol. 2017;112:656–657. 10.1038/ajg.2017.8 [DOI] [PubMed] [Google Scholar]
- 7.Pimentel M, Morales W, Rezaie A et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438. 10.1371/journal.pone.0126438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chira A, Dumitrascu DL. Serum biomarkers for irritable bowel syndrome. Clujul Medical. 2015;88:258–264. 10.15386/cjmed-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pike BL, Paden KA, Alcala AN et al. Immunological biomarkers in postinfectious irritable bowel syndrome. J Travel Med. 2015;22:242–250. 10.1111/jtm.12218 [DOI] [PubMed] [Google Scholar]
- 10.Schmulson M, Balbuena R, Corona de Law C. Clinical experience with the use of anti-CdtB and anti-vinculin antibodies in patients with diarrhea in Mexico. Rev Gastroenterol Mex. 2016;81:236–239. 10.1016/j.rgmx.2016.07.001 [DOI] [PubMed]
- 11.Rezaie A, Park SC, Morales W et al. Assessment of anti-vinculin and anti-cytolethal distending toxin b antibodies in subtypes of irritable bowel syndrome. Dig Dis Sci. 2017;62:1480–1485. 10.1007/s10620-017-4585-z [DOI] [PubMed] [Google Scholar]
- 12.Morales W, Rezaie A, Barlow G, Pimentel M. Second-generation biomarker testing for irritable bowel syndrome using plasma anti-CdtB and anti-vinculin levels. Dig Dis Sci. 2019;64:3115–3121. 10.1007/s10620-019-05684-6 [DOI] [PubMed] [Google Scholar]
- 13.Morales W, Triantafyllou K, Parodi G et al. Immunization with cytolethal distending toxin B produces autoantibodies to vinculin and small bowel bacterial changes in a rat model of postinfectious irritable bowel syndrome. Neurogastroenterol Motil. 2020;32:e13875. 10.1111/nmo.13875 [DOI] [PubMed]
- 14.Takakura W, Kudaravalli P, Chatterjee C, Pimentel M, Riddle MS. Campylobacter infection and the link with Irritable Bowel Syndrome: on the pathway towards a causal association. Pathog Dis. 2022;80:ftac003. 10.1093/femspd/ftac003 [DOI] [PubMed]
- 15.Kim JH, Nam SJ, Park SC et al. Association between interstitial cells of Cajal and anti-vinculin antibody in human stomach. Korean J Physiol Pharmacol. 2020;24:185–191. 10.4196/kjpp.2020.24.2.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suliman Y, Kafaja S, Oh SJ et al. Anti-vinculin antibodies in scleroderma (SSc): a potential link between autoimmunity and gastrointestinal system involvement in two SSc cohorts. Clin Rheumatol. 2021;40:2277–2284. 10.1007/s10067-020-05479-5 [DOI] [PubMed]
- 17.Herrán M, Adler BL, Perin J, Morales W, Pimentel M, McMahan ZH. Antivinculin antibodies in systemic sclerosis: associations with slow gastric transit and extraintestinal clinical phenotype. Arthritis Care & Research. 2023;75:2166–2173. 10.1002/acr.25118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.