Abstract
Purpose
Kimura disease (KD) is a rare, chronic inflammatory disorder that primarily affects the head and neck regions, often mimicking neoplastic conditions. This study aims to provide a comprehensive review of KD, focusing on its clinical presentation, diagnostic challenges, optimal management strategies, and primary histopathologic differential diagnosis.
Methods
A systematic review of literature was conducted using PubMed, Scopus, and Google Scholar databases. We analyzed case reports, retrospective studies, and clinical trials published in English. We extracted data on epidemiology, clinical presentation, laboratory findings, histologic features, current understanding of the pathogenesis, treatment, and prognosis.
Results
KD predominantly affects young Asian males, presenting with painless subcutaneous masses, peripheral eosinophilia, and elevated serum IgE levels. Histopathology reveals lymphoid follicular hyperplasia with eosinophilic infiltration. Biopsy is required for diagnosis. The pathogenesis of KD is poorly understood, but recent studies have elucidated some potentially important mechanisms of the disease. Treatment options include systemic corticosteroids, surgical excision, radiotherapy, and cytotoxic therapies, with recurrence rates varying among modalities.
Conclusion
KD remains a diagnostic challenge due to its overlapping features with a variety of neoplastic and non-neoplastic conditions. While corticosteroids offer temporary relief and can be useful in cases with renal involvement, surgical excision remains the most definitive treatment. Future research should focus on targeted therapies to improve long-term disease control and reduce recurrence.
Keywords: Eosinophilia, Kimura disease, Hematopathology, Head and neck pathology
Introduction
Kimura disease (KD) is a rare, benign, chronic inflammatory condition of uncertain etiology, predominantly affecting subcutaneous tissues, salivary glands, and lymph nodes in the head and neck region [1]. First described in 1937 by Kimm and Szeto [2] and later named by Kimura et al. in 1948 [3], this disease is characterized by painless subcutaneous nodules, peripheral blood eosinophilia, and elevated serum IgE levels [4]. It predominantly affects young to middle-aged men, and is endemic to regions such as Japan, China, and Southeast Asia. It is occasionally reported in other populations [1, 3, 4]. KD has clinical and histomorphologic overlap with both benign and malignant lesions that are commonly localized to the head and neck, making familiarization with the disease process and accurate diagnosis important.
The first account of KD was in 1937 when Kimm and Szeto described a unique inflammatory lesion in the head and neck [2]. In 1948, Kimura et al. expanded on this description in their paper titled “On the Unusual Granulation Combined with Hyperplastic Changes of Lymphatic Tissue,” coining the term eosinophilic hyperplastic granuloma [3]. In 1969, Wells and Whimster described an ill-defined subcutaneous inflammatory and vascularized process associated with peripheral and tissue eosinophilia, introducing the name subcutaneous angiolymphoid hyperplasia with eosinophilia (ALHE) [5].
Wells and Whimster believed that KD and ALHE represented the same disease process with the early stages of the disease presenting as epithelioid vascular change with a lack of lymphoid aggregates that progressed to mature vessels and lymphoid hyperplasia. By the early 1980s, it became increasingly clear that KD and ALHE represented different disease processes due to their distinct clinical and histomorphological features [6–8]. From this point onwards, KD was recognized as a distinct entity, separating it from other vascular proliferations and inflammatory processes. The term ALHE persisted in the literature and in usage by pathologists to denote epithelioid hemangioma (EH).
Despite being recognized as a unique entity, KD has undergone little diagnostic evolution since its inception, and the pathogenesis of the disease remains unclear. This review provides a detailed discussion of the clinical presentation, laboratory findings, histologic features, current understanding of the pathogenesis, treatment, prognosis, and differential diagnosis of KD.
Clinical Presentation and Laboratory Findings
KD presents as painless, ill-defined subcutaneous nodules or mass-like lesions (Fig. 1a) that occur in the head and neck region (greater than 75% of cases) or occasionally localized to the extremities [4, 9–12]. Lymphadenopathy is present in most cases, and salivary gland involvement is seen in a smaller subset of cases [1, 4, 10, 11]. Systemic symptoms are uncommon, but pruritus, eczematous rashes, and other skin changes occasionally occur [4, 13]. Other systemic symptoms, such as fever, weight loss, and night sweats, are uncommon but have been reported [13–15]. When these uncommon systemic symptoms are present, neoplastic and infectious etiologies are important clinical considerations.
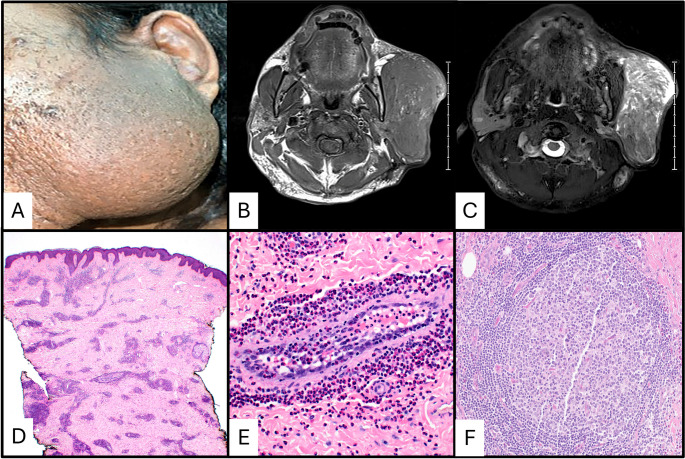
Fig. 1.
41-year-old male (Marshal Islander/Asian/Pacific Islander) presented with a large, recurrent parotid mass eight years after initial surgical excision (a). The patient’s serum IgE level was 5783 IU/ml (normal range 6-495 IU). Magnetic resonance imaging revealed a large T1 isointense mass in the left parotid region (b). The mass is hyperintense on the T2-weighted image (c). An additional lesional focus is present in the postauricular region. The mass on punch biopsy (d) showed a deeply seated lesion composed of a mixed inflammatory infiltrate with prominent eosinophilia and indistinct margins. Eosinophilic vasculitis (e) and subcutaneous secondary follicles with surrounding eosinophils (f) were present
Magnetic resonance imaging (MRI) can be a useful adjunct in the evaluation of KD lesions. They are mostly circumscribed, are characteristically isointense on T1-weighted images (Fig. 1b), and are T2 hyperintense (Fig. 1c). Post contrast enhancement most frequently shows a heterogeneous pattern [16, 17]. Laboratory findings can aid in making the diagnosis of KD with serum IgE levels and peripheral eosinophilia being the most useful. Serum IgE levels are elevated in greater than 90% of cases, commonly many times above the upper limit of normal, and there is frequently moderate to marked peripheral eosinophilia [4, 13]. Markers of inflammation may also be elevated [18]. Interestingly, a small portion of cases are associated with proteinuria and nephrotic syndrome with a wide range of pathologies on kidney biopsies reported to include minimal change disease, focal segmental glomerulosclerosis, IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis [9, 19–22]. Although laboratory findings can help in the diagnosis, a biopsy of the masses associated with KD remains the cornerstone for diagnosis, as it must be differentiated from both lymphomas and other inflammatory and reactive etiologies.
Histologic Features and Immunohistochemistry
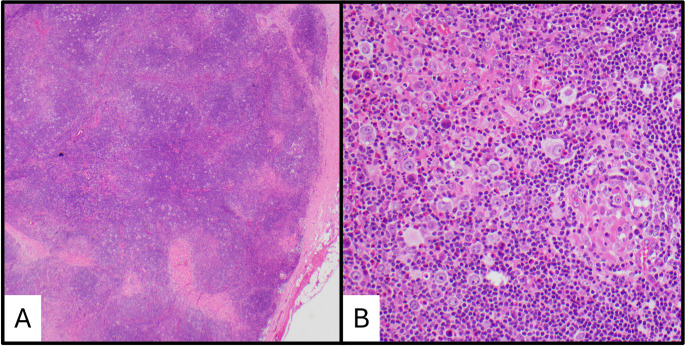
Skin manifestations and lymph node involvement by KD show consistent features (Figs. 1d and f and 2). Florid follicular hyperplasia with preserved nodal architecture, diffuse interfollicular eosinophilic infiltrates, eosinophilic microabscesses, and prominent postcapillary venule proliferation with perivenular sclerosis are the most consistent features [4]. Other frequent findings include stromal sclerosis, vascularization of germinal centers, eosinophilic folliculitis, germinal center proteinaceous deposits, and polykaryocytes within germinal centers and/or interfollicular areas [4, 23, 24]. Germinal center necrosis is uncommon.
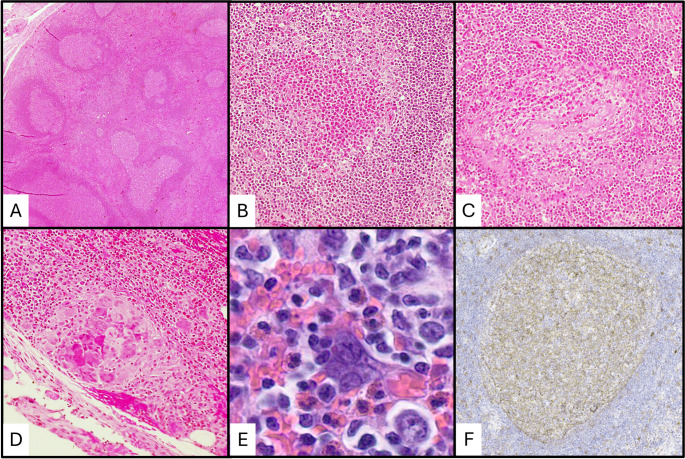
Fig. 2.
Histopathologic features of Kimura disease in a lymph node. (a) Low power showing follicle lysis. (b) High power showing an eosinophilic abscess. (c) High power showing eosinophilic vasculitis. (d) High power showing an eosinophilic granuloma. (e) High power showing a Warthin-Finkeldy polykaryocyte. (f) IgE reticular staining in a germinal center
Immunohistochemistry (IHC) demonstrates germinal centers with typical IHC profiles seen in reactive follicular hyperplasia including immunoreactivity for CD10, CD20, CD79a, BCL-6, and PAX5 and absent immunoreactivity for BCL2 [4, 25]. The mantle zones are well formed in KD and demonstrate a mature B-cell immunoprofile [18]. Kappa and lambda light chain staining will show a polytypic pattern. IgE reticular staining within the germinal centers is a classic IHC feature in nearly all cases (Fig. 2f); although, this finding is not specific and is present in other reactive lymphadenopathies [4, 25, 26].
Pathogenesis
The exact etiology of KD is not known. However, it is likely that T helper (Th) 2 cells and Th2 associated cytokines play an important role in the pathogenesis of KD. Th2 cells release interleukin (IL)-4, IL-5, and IL-13 [27]. IL-4 drives the immunoglobulin (Ig) class switching to IgG1 and IgE [27]. Increased levels of IL-5 stimulate eosinophil development in bone marrow and survival in the blood [28]. IL-4 and IL-13 also act to increase the production of C-C Motif Chemokine Ligand (CCL) 5, CCL11, CCL24, and CCL26, which enhance chemotaxis of eosinophils to tissues [29–32].
The percentage of Th2 cells and the Th2/Th1 ratio in the peripheral blood is significantly increased compared to healthy controls, and the number for Th2 cells in KD correlates directly with the serum IgE titer [33]. Similarly, stimulated peripheral blood T-cells from individuals with KD show a higher expression of Th2 cytokines, including IL-3, IL-4, IL-5, IL-13, and granulocyte-macrophage colony stimulating factor, compared to healthy controls [34]. Moreover, multicolor immunofluorescence staining of formalin-fixed, paraffin-embedded sections has shown that KD lesions are rich in Th2 cells as well as IgE and IL-4 positive mast cells [35].
An increase in Th2 cells and related cytokines may explain the tissue and peripheral eosinophilia and increased serum IgE that is seen in KD. IgE mediated mast cell recruitment, activation, and degradation may explain common symptoms seen in KD, including skin rashes and pruritus. The exact mechanism that leads to an increase in the Th2-mediated immune response in KD is not known. It is possibly the result of injury or inflammation that leads to exposure of self-antigens. Dysregulation of the Th2-mediated immune response plays a role in various autoimmune conditions [27, 36–40].
Prognosis and Treatment
KD is considered a benign condition with no risk of metastasis [41]. The lesions of KD can be locally aggressive and may have local recurrence. Surgery is the primary treatment method, but radiation, systemic immunosuppression, and chemotoxic therapies have been employed with variable success [11, 41, 42]. In a meta-analysis by Lee et al., the recurrence rate for surgical excision was 30.5%, for systemic immunosuppression was 45%, and for radiotherapy was 60% [11]. Combinations of these treatment modalities have been employed to attempt to reduce recurrence and limit the need for prolonged systemic immunosuppression.
Adjuvant radiotherapy following surgery may reduce the risk of local recurrence compared to surgery alone. Surgical excision and adjuvant radiotherapy has been shown to reduce recurrence compared to surgery alone in two studies by Ye et al. [42, 43]. In a different study, no significant difference was found between the recurrence rates of radiotherapy and non-radiotherapy groups (p = 0.23) [44]. Further studies are required to determine the efficacy of radiotherapy in reducing the rates of local recurrence.
Proteinuria has been reported in 12–16% of patients with KD, with 60–78% of these patients developing nephrotic syndrome [45]. Steroids have treated the renal disease associated with KD with success in multiple reported cases [19–21, 46, 47]. Although recurrence risk may be higher with the use of steroids alone for treatment of KD, there may be a benefit for the use of steroids in cases with associated renal disease.
Targeted therapies have the potential to be more effective than traditional therapies with fewer systemic and off-target effects. Multiple case reports of successful treatment of Kimura disease with dupilumab have been published [48–52]. Dupilumab is a monoclonal antibody that inhibits the signaling of IL-4 and IL-13, two key cytokines involved in the Th2 immune response. It works by binding to the shared alpha subunit (IL-4Rα) of the IL-4 and IL-13 receptor complexes, preventing the release of proinflammatory cytokines, chemokines, nitric oxide, and IgE [53].
Dupilumab may be a good treatment option for KD because the disease is characterized by a Th2-dominated immune response with elevated levels of IL-4, IL-5, IL-10, and IL-13, which play key roles in its pathogenesis [33–35]. By blocking the signaling of IL-4 and IL-13 through the IL-4Rα receptor, dupilumab can directly inhibit Th2-mediated inflammation, reducing eosinophil recruitment and tissue damage. This targeted mechanism addresses the underlying immune dysregulation, offering a rational and potentially effective treatment approach.
Bulk-RNA sequencing has demonstrated that the extracellular-regulated kinase/mitogen-activated protein kinase (Erk/MAPK) signaling pathway is over-activated in the eosinophils of patients with KD compared to healthy controls [54]. The Erk/MAPK signaling pathway is essential for eosinophil activation, degranulation, and migration, supporting that overactivation of the pathway may play an integral role in the pathogenesis of Kimura disease [55, 56]. The exact mechanism leading to Erk/MAPK signaling pathway over-activation in KD is unknown, but this provides an option for targeted therapy, such as MAPK inhibitors. Further understanding of the pathogenesis of KD may provide more options for targeted therapy in the future.
Differential Diagnosis
The differential diagnosis of KD encompasses a variety of conditions that share clinical and histopathologic features with KD. These can be broadly categorized into non-neoplastic and neoplastic entities. Accurate differentiation is crucial for appropriate management and prognosis. To facilitate comparison and highlight key elements, Table 1 provides an overview of KD and its main differential diagnoses.
Table 1.
Key clinical and histopathologic elements of Kimura disease and its differential diagnoses
| Entity | Clinical Presentation | Histopathologic Features | Immunohistochemistry |
|---|---|---|---|
| Kimura Disease | Subcutaneous masses in head/neck, lymphadenopathy, pruritus, peripheral eosinophilia, elevated serum IgE | Follicular hyperplasia, eosinophils, eosinophilic folliculolysis and microabscesses, postcapillary venule proliferation, proteinaceous deposits, Warthin-Finkeldy polykaryocytes | IgE reticular staining within germinal centers |
| Epithelioid Hemangioma | Subcutaneous masses or papules in head/neck, pruritus, rare peripheral eosinophilia | Vascular proliferation with hobnailed endothelial cells, eosinophils | FOSB nuclear expression in endothelial cells |
| Nodal T Follicular Helper Cell Lymphoma, Follicular Type | Generalized lymphadenopathy, advanced-stage disease | Effacement of lymph node architecture, follicular pattern, intermediate-sized lymphocytes, eosinophils | CD3+, CD4+, strong PD1+, and one other T follicular helper cell marker |
| Classic Hodgkin Lymphoma | Nodal, supradiaphragmatic masses, B symptoms | Effacement of lymph node architecture, Hodgkin-Reed-Sternberg (HRS) cells, eosinophils | CD15+, CD30+, weak PAX5 + in HRS cells |
| Langerhans Cell Histiocytosis | Unifocal or multifocal, cutaneous lesions and lymphadenopathy in head/neck, often in children | Effacement of lymph node architecture, histiocytes with grooved nuclei, eosinophils | CD1a+, S100+, and CD207 + in histocytes |
| Reactive Lymphadenopathy | Lymphadenopathy, variable systemic symptoms | Variable histology (follicular hyperplasia, paracortical/interfollicular hyperplasia, sinus histiocytosis), variable eosinophils | Polyclonal B- and T-cells, no specific markers |
| IgG4-Related Disease | Multisystem involvement, lymphadenopathy, elevated serum IgG4 | Fibrosis, plasma cell infiltrates, follicular hyperplasia | Elevated IgG4 + plasma cells |
Among the differential diagnoses, EH is the closest mimic of KD. Clinically, both conditions can manifest as subcutaneous masses in the head and neck region with associated pruritus [57, 58]. Peripheral blood eosinophilia may be present, but elevation of serum IgE is uncommon [59]. While KD tends to present at a younger age compared to EH, the overall clinical presentation can be quite similar, especially for the subcutaneous presentation of EH, making biopsy and histologic evaluation critical [60].
Histologically, EH shows a proliferation of variably sized vessels lined by cuboidal endothelial cells described as “hobnailed” and epithelioid with abundant clear to eosinophilic and vacuolated cytoplasm (Fig. 3) [57]. FOSB nuclear positivity in the endothelial cells of EH has been reported [61], likely due to oncogenic FOS rearrangements [62, 63]. Mitotic activity and atypia are not usually present. The proliferation of vessels is accompanied by a dense inflammatory infiltrate composed of lymphocytes and eosinophils. The lymphocytes are mostly T-cells, but lymphoid follicles composed of B-cells can be seen. In early lesions, the vascular component with epithelioid endothelial cells is predominant, and the inflammatory infiltrate increases as the disease progresses [57, 64].
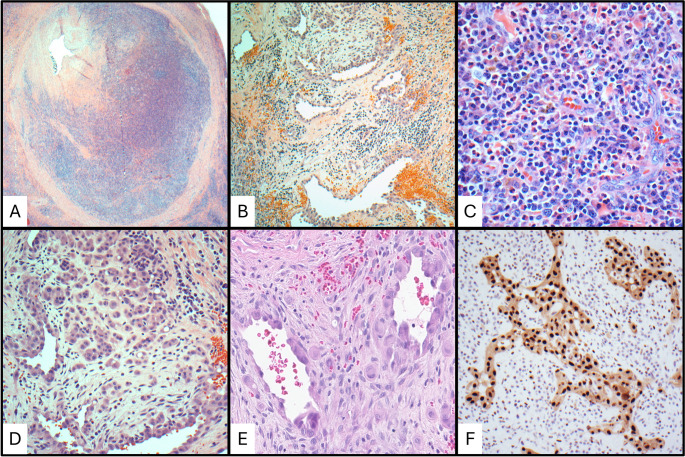
Fig. 3.
Differential diagnosis of epithelioid hemangioma. (a) Low power showing a subcutaneous nodule. (b) Low power of vessels showing plump endothelial cells (epithelioid change). (c) High power of mixed inflammation with eosinophils and vascular hyperplasia. (d) High power of prominent plump endothelial cells. (e) ERG expression in the endothelial cells
Cases of EH with prominent eosinophilic infiltrate and lymphoid follicles can have significant histologic overlap with KD. While KD can have numerous post capillary venules, the endothelial cells do not appear hobnailed or epithelioid, like in EH [4]. Other frequently seen histologic features of KD, including eosinophilic folliculolysis, germinal center vascularization, germinal center proteinaceous deposits, and polykaryocytes, are absent [4, 7].
Nodal T follicular helper (TFH) cell lymphomas (nTFHL) are another important consideration with a lesser degree of clinical and histological overlap compared to EH. These are mature T-cell neoplasms with morphologic, immunophenotypic, and molecular features of TFH cells. TFH cells are a type of CD4 + T-cell that play a role in memory B-cell development and primarily reside in secondary follicles of normal lymph nodes. The clinical presentation does not mimic KD as closely as EH. Patients commonly have serum markers of inflammation, such as an increased erythrocyte sedimentation rate, and may show non-clonal hypergammaglobulinemia. IgE elevations are not seen, but peripheral eosinophilia and skin rashes can occur [65].
nTFHL, follicular type (nTFHL-F) demonstrates a follicular lymphoma-like pattern and has the most histopathologic overlap with KD. In nTFHL-F, the lymph node architecture is effaced by a nodular proliferation of intermediate-sized lymphocytes with moderate to abundant clear to eosinophilic cytoplasm with nuclei that are round to mildly irregular (Fig. 4) [65]. Eosinophils are often present. Assessment of a wide range of immunophenotypic markers for T-cell origin (CD2, CD3, CD4, CD5, CD7, and CD8) and TFH cell origin (CD10, ICOS, PD1, CXCL13) is useful. Expression of at least two markers of TFH origin, including strong PD1, is required for a diagnosis [65].
Fig. 4.
Differential diagnosis of nodal T follicular helper cell lymphoma. (a) Low power image of nodal T follicular helper cell lymphoma, NOS. (b) High power showing characteristic features of neoplastic T-cells with background eosinophils. (c) PD1 expression in the neoplastic T-cells
Compared to the expanded follicles in nTFHL-F, the follicles in KD are benign reactive follicles composed primarily of B-cells and have the typical expression of BCL-6 and CD10. Expression of BCL-6 and CD10 by neoplastic T-cells in nTFHL-F can occur, but should not be confused with normal follicular B-cells [65]. Additionally, a t(5, 9)(q33; q22), resulting in a ITK-SYK fusion transcript, has been identified in cases of nTFHL-F [66], as well as mutations in genes such as TET2, DNMT3A, IDH2, and RHOA [67, 68]. In cases of KD and nTFHL-F with a high degree of histopathologic overlap, the use of IHC and molecular studies can help resolve the differential.
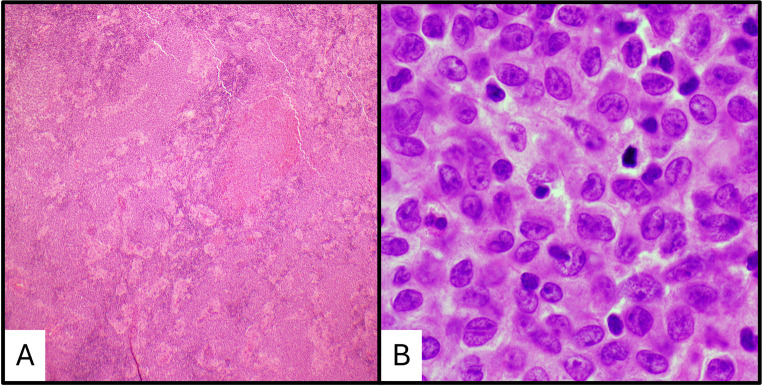
Other neoplastic entities characterized by an eosinophilic infiltrate on histology can occur in the head and neck, to include classic Hodgkin lymphoma (CHL) and Langerhans cell histiocytosis (LCH). While these entities do not pose a diagnostic challenge, we will discuss them briefly due to some overlapping morphologic features. CHL typically presents as a supradiaphragmatic nodal based disease, frequently involving the cervical lymph nodes, although primary extranodal disease can rarely occur [69, 70]. Peripheral blood eosinophilia is uncommon in CHL (15% of cases) and an elevated IgE is rarely seen [71, 72].
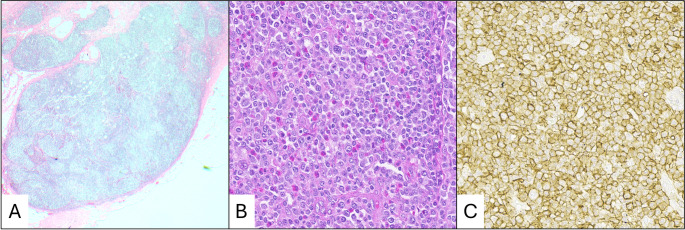
CHL is characterized by the presence of Hodgkin-Reed-Sternberg (HRS) cells (multinucleate) and Hodgkin cells (mononuclear) with a background inflammatory infiltrate of histiocytes, plasma cells, and eosinophils (Fig. 5). Immunoblasts in KD can mimic Hodgkin cells, but KD immunoblasts are typically smaller and do not have the prominent red nucleolus seen in Hodgkin cells. Further, immunoblasts will not be multinucleated, helping to differentiate from HRS cells. While sclerosis is often present in KD, sclerotic bands like those seen in nodular sclerosis CHL should not be [4].
Fig. 5.
Differential diagnosis of classic Hodgkin lymphoma. (a) Low power showing effacement of lymph node architecture. (b) Intermediate power showing Hodgkin-Reed-Sternberg cells and numerous eosinophils
LCH is a monoclonal neoplasm of myeloid dendritic cells that primarily occurs in children, but can occur at any age [69, 73]. LCH can be unifocal or multifocal and can involve bone, skin, lymph nodes, and multiple organs. In the head and neck, LCH commonly presents as cutaneous lesions or as cervical lymphadenopathy [69]. Cutaneous lesions can present as seborrheic dermatitis or eczematous eruptions, most commonly on the scalp and trunk [73].
Lymph nodes involved by LCH show effacement of normal lymph node architecture with increased eosinophils (Fig. 6a). Follicular hyperplasia is not a common feature. The histiocytes in LCH have moderate to abundant pale pink cytoplasm and round to oval nuclei with complex contours and longitudinal grooves (Fig. 6b) and are immunoreactive for CD1a, S100, CD207 (langerin) and CD68 [73]. The Warthin-Finkeldy polykaryocytes seen in KD have morphologic similarities with the histiocytes in LCH, but these polykaryocytes demonstrate a T-cell phenotype, expressing CD43 and CD3. A BRAF V600E mutation is seen in approximately 36–70% of LCH cases [74], which is not seen in KD.
Fig. 6.
Differential diagnosis of Langerhans histiocytosis. (a) Low power showing effacement of lymph node architecture. (b) High power showing Langerhans cells with characteristic nuclear grooves and eosinophils
Among non-neoplastic conditions, reactive lymphadenopathies can present with follicular hyperplasia and varying degrees of eosinophilic infiltration. However, these are generally associated with identifiable etiologies such as infections, autoimmune disorders, or allergies [75], and lack the specific histopathologic hallmarks of KD, such as eosinophilic folliculolysis and Warthin-Finkeldy polykaryocytes.
Allergies can lead to lymphadenopathy and peripheral eosinophilia due to a Th2-mediated immune response [76]. This specific etiology of reactive lymphadenopathy can have significant clinicopathologic overlap with KD. Clinical history and allergy testing may be prudent in such cases before initiating treatment. Other specific etiologies of follicular hyperplasia, such as Castleman disease, toxoplasmosis, syphilis, and HIV-associated lymphadenopathy, have well-characterized features [77]. These diseases are not likely to be in the differential diagnosis of KD as they lack the other classic features of KD.
Rare cases of KD have been reported with IgG4-related disease (IgG4-RD), but the relationship between the diseases is uncertain [62]. IgG4-RD is a non-neoplastic fibroinflammatory multisystem disease that involves lymph nodes with follicular hyperplasia and fibrosis [77]. However, lesions in IgG4-RD are distinguished by dense plasma cell infiltrates, with a significant proportion of IgG4-positive plasma cells (typically > 10 per high-power field or an IgG4/IgG ratio ≥ 40%) [78]. In contrast, KD does not show a predominance of IgG4-positive plasma cells and lacks the extensive fibrosis characteristic of IgG4-RD.
Conclusion
Kimura disease is a unique inflammatory condition that poses diagnostic and therapeutic challenges due to its rarity and overlapping features with other disorders. A thorough understanding of its clinical, histologic, and immunologic characteristics is essential for accurate diagnosis and management. While the prognosis remains favorable, further research into its pathogenesis and novel therapies may enhance patient outcomes.
Author Contributions
I.L., D.D., and J.M. wrote the main manuscript. N.A., I.L., D.D., and R.F. prepared the figures. R.F. contributed to the Clinical Presentation and Laboratory Findings section. A.A. and N.A. provided the majority of revisions. All authors reviewed the manuscript.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This study was not supported by any funding.
Data Availability
No datasets were generated or analysed during the current study.
Code Availability
Not applicable.
Declarations
Ethics Approval
This article does not contain any studies with human participants are animals performed by any of the authors.
Consent to Participate
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent for publication is not required.
Statistical Declaration
No statistical analysis was performed for this manuscript.
Disclaimer
The views expressed herein are those of the authors and do not necessarily reflect the official policy or position of Walter Reed National Military Medical Center, Fort Belvoir Community Hospital, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Air Force, the Department of the Army, Department of Defense, the Uniformed Services University of the Health Sciences, or any other agency of the U.S. Government. The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the authors, DoD, or any component agency.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee C-C, Yu K-H, Chan T-M (2022) Kimura’s disease: A clinicopathological study of 23 cases. Front Med 9:1069102. 10.3389/fmed.2022.1069102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H Eosinophilic hyperplastic lymphogranuloma, comparison with mikulicz’s disease. Chi Med J 1937:699–670
- 3.Kimura T On the unusual granulations combined with hyperplastic changes of lymphatic tissue. Trans Soc Pathol Jpn 1948:179–180
- 4.Chen H, Thompson LDR, Aguilera NSI, Abbondanzo SL (2004) Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol 28:505–513. 10.1097/00000478-200404000-00010 [DOI] [PubMed] [Google Scholar]
- 5.Wells GC, Whimster IW (1969) Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol 81:1–14. 10.1111/j.1365-2133.1969.tb15914.x [DOI] [PubMed] [Google Scholar]
- 6.Kuo TT, Shih LY, Chan HL (1988) Kimura’s disease. Involvement of regional lymph nodes and distinction from angiolymphoid hyperplasia with eosinophilia. Am J Surg Pathol 12:843–854 [PubMed] [Google Scholar]
- 7.Googe PB, Harris NL, Mihm MC (1987) Kimura’s disease and angiolymphoid hyperplasia with eosinophilia: two distinct histopathological entities. J Cutan Pathol 14:263–271. 10.1111/j.1600-0560.1987.tb00498.x [DOI] [PubMed] [Google Scholar]
- 8.Kung IT, Gibson JB, Bannatyne PM (1984) Kimura’s disease: a clinico-pathological study of 21 cases and its distinction from angiolymphoid hyperplasia with eosinophilia. Pathology 16:39–44. 10.3109/00313028409067909 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Bao H, Zhang X, Yang F, Liu Y, Li H et al (2022) Kimura’s disease: clinical characteristics, management and outcome of 20 cases from China. Clin Exp Rheumatol 40:532–538. 10.55563/clinexprheumatol/lahgfo [DOI] [PubMed] [Google Scholar]
- 10.Sangwan A, Goyal A, Bhalla AS, Kumar A, Sharma R, Arava S et al (2022) Kimura disease: A case series and systematic review of Clinico-radiological features. Curr Probl Diagn Radiol 51:130–142. 10.1067/j.cpradiol.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 11.Lee C-C, Feng I-J, Chen Y-T, Weng S-F, Chan L-P, Lai C-S et al (2022) Treatment algorithm for kimura’s disease: A systematic review and meta-analysis of treatment modalities and prognostic predictors. Int J Surg 100:106591. 10.1016/j.ijsu.2022.106591 [DOI] [PubMed] [Google Scholar]
- 12.Varshney MK, Kumar A, Khan SA, Yadav CS (2008) Kimura disease of extremity: unusual manifestation in a long bone. Joint Bone Spine 75:492–494. 10.1016/j.jbspin.2007.07.020 [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Jiao Y (2019) The clinicopathological characteristics of Kimura disease in Chinese patients. Clin Rheumatol 38:3661–3667. 10.1007/s10067-019-04752-6 [DOI] [PubMed] [Google Scholar]
- 14.Molla YD, Alemu HT, Zegeye KB, Bekele T, Tadesse AK, Answar IO (2024) Kimura disease, a rare Ethiopian case report. Heliyon 10:e39651. 10.1016/j.heliyon.2024.e39651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsmoudi H, Sleiay M, Almohamed A, Hamsho S, Alhadla A, Alqreea M et al (2024) A 23-year-old male patient with kimura’s disease without renal transplantation: a rare case report from Syria. Ann Med Surg (Lond) 86:4927–4931. 10.1097/MS9.0000000000002341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo R, Yang G, Shi H, He Y, Han Y, Tian Z et al (2025) A Stepwise decision tree model for differential diagnosis of kimura’s disease in the head and neck. BMC Med Imaging 25:90. 10.1186/s12880-025-01618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S-W, Kim H-J, Sung KJ, Lee JH, Park IS (2012) Kimura disease: CT and MR imaging findings. AJNR Am J Neuroradiol 33:784–788. 10.3174/ajnr.A2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Liu S, Xu J, Xu X, Wang M (2023) An unusual case of systemic Lymphadenopathy - Kimura’s disease. J Inflamm Res 16:701–705. 10.2147/JIR.S397470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong Y, Gu J-Y, Labh S, Shi Y-L (2015) Kimura disease accompanied with nephrotic syndrome in a 45-year-old male. Diagn Pathol 10:43. 10.1186/s13000-015-0277-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu B, Yang Z, Song D, Wang Z, Xu D, Wang S et al (2020) Eosinophilic peritonitis and nephrotic syndrome in kimura’s disease: a case report and literature review: eosinophilic peritonitis in kimura’s disease. BMC Nephrol 21:138. 10.1186/s12882-020-01791-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majumder A, Sen D (2019) Kimura disease with nephrotic syndrome in a child- A rare association. Indian J Pathol Microbiol 62:437–440. 10.4103/IJPM.IJPM_517_18 [DOI] [PubMed] [Google Scholar]
- 22.Fouda MA, Gheith O, Refaie A, El-Saeed M, Bakr A, Wafa E et al (2011) Kimura disease: a case report and review of the literature with a new management protocol. Int J Nephrol 2010:673908. 10.4061/2010/673908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui PK, Chan JK, Ng CS, Kung IT, Gwi E (1989) Lymphadenopathy of kimura’s disease. Am J Surg Pathol 13:177–186. 10.1097/00000478-198903000-00001 [DOI] [PubMed] [Google Scholar]
- 24.Chan JKC, Hui PK, Ng CS, Yuen NWF, Kung ITM, Gwi E (1989) Epithelioid haemangioma (angiolymphoid hyperplasia with eosinophilia) and kimura’s disease in Chinese. Histopathology 15:557–574. 10.1111/j.1365-2559.1989.tb01622.x [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Zhang W, Huang M, Li K, Zhu L, Ke L et al (2024) The clinical and pathological features of Kimura disease in pediatric patients. Front Med (Lausanne) 11:1352206. 10.3389/fmed.2024.1352206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundaresan M, Rhodes T, Akosa AB (1991) Immunoglobulin heavy chain patterns in reactive lymphadenopathy. J Clin Pathol 44:753–755. 10.1136/jcp.44.9.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raphael I, Nalawade S, Eagar TN, Forsthuber TG (2015) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74:5–17. 10.1016/j.cyto.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulkerson PC, Schollaert KL, Bouffi C, Rothenberg ME (2014) IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J Immunol 193:4043–4052. 10.4049/jimmunol.1400732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujisawa T, Kato Y, Atsuta J, Terada A, Iguchi K, Kamiya H et al (2000) Chemokine production by the BEAS-2B human bronchial epithelial cells: differential regulation of eotaxin, IL-8, and RANTES by TH2- and TH1-derived cytokines. J Allergy Clin Immunol 105:126–133. 10.1016/s0091-6749(00)90187-8 [DOI] [PubMed] [Google Scholar]
- 30.Peng C, Zhang H, Yang J, Xu J, Guan S, Xia J et al (2022) Regulation of eotaxin expression in skin allergic diseases. Postepy Dermatol Alergol 39:565–579. 10.5114/ada.2022.117739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer-Hoffert U, Lezcano-Meza D, Bartels J, Montes-Vizuet AR, Schröder J-M, Teran LM (2003) Th2- and to a lesser extent Th1-Type cytokines upregulate the production of both CXC (IL-8 and Gro-Alpha) and CC (RANTES, eotaxin, Eotaxin-2, MCP-3 and MCP-4) chemokines in human airway epithelial cells. Int Arch Allergy Immunol 131:264–271. 10.1159/000072138 [DOI] [PubMed] [Google Scholar]
- 32.Marques RE, Guabiraba R, Russo RC, Teixeira MM (2013) Targeting CCL5 in inflammation. Expert Opin Ther Targets 17:1439–1460. 10.1517/14728222.2013.837886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohta N, Fukase S, Suzuki Y, Ito T, Yoshitake H, Aoyagi M (2011) Increase of Th2 and Tc1 cells in patients with kimura’s disease. Auris Nasus Larynx 38:77–82. 10.1016/j.anl.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki K, Kawashima H, Sato S, Tsunoda H, Yoshimura Y, Higuchi M et al (2013) Increased CD45RO + CD62L + CD4 + T-cell subpopulation responsible for Th2 response in kimura’s disease. Hum Immunol 74:1097–1102. 10.1016/j.humimm.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 35.Maehara T, Munemura R, Shimizu M, Kakizoe N, Kaneko N, Murakami Y et al (2019) Tissue-infiltrating immune cells contribute to Understanding the pathogenesis of Kimura disease: A case report. Med (Baltim) 98:e18300. 10.1097/MD.0000000000018300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carli MD, D’elios MM, Zancuoghi G, Romagnani S, Prete GD (1994) Review human Th1 and Th2 cells: functional properties, regulation of development and role in autoimmunity. Autoimmunity 18:301–308. 10.3109/08916939409009532 [DOI] [PubMed] [Google Scholar]
- 37.Ko H, Kim CJ, Im S-HT (2022) Helper 2-Associated immunity in the pathogenesis of systemic lupus erythematosus. Front Immunol 13. 10.3389/fimmu.2022.866549 [DOI] [PMC free article] [PubMed]
- 38.Crane IJ, Forrester JV Th1 and Th2 lymphocytes in autoimmune disease. CRI 2005;25. 10.1615/CritRevImmunol.v25.i2.10 [DOI] [PubMed]
- 39.Luo P, Wang P, Xu J, Hou W, Xu P, Xu K et al (2022) Immunomodulatory role of T helper cells in rheumatoid arthritis: a comprehensive research review. Bone Joint Res 11:426–438. 10.1302/2046-3758.117.BJR-2021-0594.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J (2010) Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med 16:701–707. 10.1038/nm.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abuel-Haija M, Hurford MT, Kimura, Disease (2007) Arch Pathol Lab Med 131:650–651. 10.5858/2007-131-650-KD [DOI] [PubMed] [Google Scholar]
- 42.Ye P, Ma D-Q, Yu G-Y, Gao Y, Peng X (2017) Comparison of the efficacy of different treatment modalities for kimura’s disease. Int J Oral Maxillofac Surg 46:350–354. 10.1016/j.ijom.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 43.Ye P, Wei T, Yu G-Y, Wu L-L, Peng X (2016) Comparison of local recurrence rate of three treatment modalities for Kimura disease. J Craniofac Surg 27:170. 10.1097/SCS.0000000000002337 [DOI] [PubMed] [Google Scholar]
- 44.Chang AR, Kim K, Kim HJ, Kim IH, Park CI, Jun YK (2006) Outcomes of kimura’s disease after radiotherapy or nonradiotherapeutic treatment modalities. Int J Radiation Oncology*Biology*Physics 65:1233–1239. 10.1016/j.ijrobp.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 45.Rajpoot DK, Pahl M, Clark J (2000) Nephrotic syndrome associated with Kimura disease. Pediatr Nephrol 14:486–488. 10.1007/s004670050799 [DOI] [PubMed] [Google Scholar]
- 46.Senel MF, Van Buren CT, Etheridge WB, Barcenas C, Jammal C, Kahan BD (1996) Effects of cyclosporine, azathioprine and prednisone on kimura’s disease and focal segmental glomerulosclerosis in renal transplant patients. Clin Nephrol 45:18–21 [PubMed] [Google Scholar]
- 47.Zhu S, Wei P, Chen J, Zhao Z, Xu Q, Ye L (2015) Diagnosis and treatment of a patient with kimura’s disease associated with nephrotic syndrome and lymphadenopathy of the epitrochlear nodes. BMC Nephrol 16:10. 10.1186/s12882-015-0007-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battesti G, Jachiet M, Lepelletier C, Petit A, Vignon-Pennamen M-D, Cassius C et al (2024) Two cases of dupilumab-responsive Kimura disease. Clin Exp Dermatol 49:502–506. 10.1093/ced/llad455 [DOI] [PubMed] [Google Scholar]
- 49.Luo S-Y, Zhou K-Y, Wang Q-X, Deng L-J, Fang S (2024) Kimura’s disease treated with dupilumab: A case report and literature review. Int Immunopharmacol 131:111895. 10.1016/j.intimp.2024.111895 [DOI] [PubMed] [Google Scholar]
- 50.Lyu Y, Cui Y, Ma L, Guan L, Wen Z, Huang J et al (2024) Dupilumab combined with corticosteroid therapy for Kimura disease with multiple systemic masses: a case report and literature review. Front Immunol 15:1492547. 10.3389/fimmu.2024.1492547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teraki Y, Terao A (2022) Treatment of Kimura disease with dupilumab. JAMA Dermatology 158:329–330. 10.1001/jamadermatol.2021.5885 [DOI] [PubMed] [Google Scholar]
- 52.Zheng Y, Luo S (2025) Successful treatment of kimura’s disease with dupilumab and review of dupilumab in treating eosinophilic dermatoses. J Dermatological Treat 36:2449153. 10.1080/09546634.2024.2449153 [DOI] [PubMed] [Google Scholar]
- 53.Harb H, Chatila TA (2020) Mechanisms of dupilumab. Clin Experimental Allergy 50:5–14. 10.1111/cea.13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X, Wang A, Zhang S, Wang X, Guo D, Zhu W et al (2023) Multiomic landscape of immune pathogenesis in kimura’s disease. iScience 26:106559. 10.1016/j.isci.2023.106559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P et al (1999) Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J Immunol 163:1611–1618 [PubMed] [Google Scholar]
- 56.Pazdrak K, Olszewska-Pazdrak B, Stafford S, Garofalo RP, Alam R, Lyn (1998) Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of Interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. J Exp Med 188:421–429. 10.1084/jem.188.3.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen TG, Helwig EB (1985) Angiolymphoid hyperplasia with eosinophilia: A clinicopathologic study of 116 patients. J Am Acad Dermatol 12:781–796. 10.1016/S0190-9622(85)70098-9 [DOI] [PubMed] [Google Scholar]
- 58.Adler BL, Krausz AE, Minuti A, Silverberg JI, Lev-Tov H (2016) Epidemiology and treatment of angiolymphoid hyperplasia with eosinophilia (ALHE): A systematic review. J Am Acad Dermatol 74:506–512e11. 10.1016/j.jaad.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 59.Buder K, Ruppert S, Trautmann A, Bröcker E-B, Goebeler M, Kerstan A (2014) Angiolymphoid hyperplasia with eosinophilia and kimura’s disease - a clinical and histopathological comparison. J Dtsch Dermatol Ges 12:224–228. 10.1111/ddg.12257_suppl [DOI] [PubMed] [Google Scholar]
- 60.Ahn HJ, Lee KG (1990) A clinicopathological study of kimura’s disease and epithelioid hemangioma. Yonsei Med J 31:205–211. 10.3349/ymj.1990.31.3.205 [DOI] [PubMed] [Google Scholar]
- 61.Ortins-Pina A, Llamas-Velasco M, Turpin S, Soares-de-Almeida L, Filipe P, Kutzner H (2018) FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol 45:395–402. 10.1111/cup.13141 [DOI] [PubMed] [Google Scholar]
- 62.Huang S-C, Zhang L, Sung Y-S, Chen C-L, Krausz T, Dickson BC et al (2015) Frequent FOS gene rearrangements in epithelioid hemangioma: A molecular study of 58 cases with morphologic reappraisal. Am J Surg Pathol 39:1313–1321. 10.1097/PAS.0000000000000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Llamas-Velasco M, Kempf W, Cota C, Fernández-Figueras MT, Lee J, Ferrara G et al (2019) Multiple eruptive epithelioid hemangiomas: A subset of cutaneous cellular epithelioid hemangioma with expression of FOS-B. Am J Surg Pathol 43:26–34. 10.1097/PAS.0000000000001003 [DOI] [PubMed] [Google Scholar]
- 64.Guo R, Gavino ACP (2015) Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med 139:683–686. 10.5858/arpa.2013-0334-RS [DOI] [PubMed] [Google Scholar]
- 65.Huang Y, Moreau A, Dupuis J, Streubel B, Petit B, Le Gouill S et al (2009) Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and May show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol 33:682–690. 10.1097/PAS.0b013e3181971591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Streubel B, Vinatzer U, Willheim M, Raderer M, Chott A (2006) Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 20:313–318. 10.1038/sj.leu.2404045 [DOI] [PubMed] [Google Scholar]
- 67.Wang J, Tang W, Zhang W, Wang J, Chen F, Zhong L et al (2021) Clinicopathological characterization of follicular helper T-cell-derived peripheral T-cell lymphoma involving waldeyer’s ring. Virchows Arch 479:355–363. 10.1007/s00428-021-03071-z [DOI] [PubMed] [Google Scholar]
- 68.Dobay MP, Lemonnier F, Missiaglia E, Bastard C, Vallois D, Jais J-P et al (2017) Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica 102:e148–e151. 10.3324/haematol.2016.158428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danielson DT, Aguilera NS, Auerbach A (2025) Head and neck classic hodgkin, T and NK lymphomas with eosinophilia. Head Neck Pathol 19:10. 10.1007/s12105-025-01751-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Storck K, Brandstetter M, Keller U, Knopf A (2019) Clinical presentation and characteristics of lymphoma in the head and neck region. Head Face Med 15:1. 10.1186/s13005-018-0186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaughan Hudson B, Linch DC, Macintyre EA, Bennett MH, MacLennan KA, Vaughan Hudson G et al (1987) Selective peripheral blood eosinophilia associated with survival advantage in hodgkin’s disease (BNLI report 31). British National lymphoma investigation. J Clin Pathol 40:247–250. 10.1136/jcp.40.3.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellis AK, Waserman S (2009) Hodgkin’s lymphoma presenting with markedly elevated ige: a case report. Allergy Asthma Clin Immunol 5:12. 10.1186/1710-1492-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Danielson DT, Lagerstrom I, Wary Z, Auerbach A, Cassarino DS (2025) Dermatologic lesions with eosinophilia in the head and neck. Head Neck Pathol 19:27. 10.1007/s12105-025-01757-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alayed K, Medeiros LJ, Patel KP, Zuo Z, Li S, Verma S et al (2016) BRAF and MAP2K1 mutations in Langerhans cell histiocytosis: a study of 50 cases. Hum Pathol 52:61–67. 10.1016/j.humpath.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 75.Tzankov A, Dirnhofer S (2018) A pattern-based approach to reactive lymphadenopathies. Semin Diagn Pathol 35:4–19. 10.1053/j.semdp.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi T, Iijima K, Dent AL, Kita H (2017) Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol 139:300–313e7. 10.1016/j.jaci.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss LM, O’Malley D (2013) Benign lymphadenopathies. Mod Pathol 26:S88–96. 10.1038/modpathol.2012.176 [DOI] [PubMed] [Google Scholar]
- 78.Wick MR, O’Malley DP (2018) Lymphadenopathy associated with IgG4-related disease: diagnosis & differential diagnosis. Semin Diagn Pathol 35:61–66. 10.1053/j.semdp.2017.11.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.
Not applicable.