Abstract
Background: The increasing prevalence of dementia and mild cognitive impairment (MCI) among the elderly population is a global health issue. Information and Communication Technology (ICT)-based interventions hold promises for maintaining cognition, but their viability is affected by several challenges. Objectives: This study aimed to significantly assess the effectiveness of ICT-based cognitive and memory aid technology for individuals with MCI or dementia, identify adoption drivers, and develop an implementation model to inform practice. Methods: A PRISMA-based systematic literature review, with the protocol registered in PROSPERO (CRD420251051515), was conducted using seven electronic databases published between January 2015 and January 2025 following the PECOS framework. Random effects models were used for meta-analysis, and risk of bias was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklists. Results: A total of ten forms of ICT interventions that had proved effective to support older adults with MCI and dementia. Barriers to adoption included digital literacy differences, usability issues, privacy concerns, and the lack of caregiver support. Facilitators were individualized design, caregiver involvement, and culturally appropriate implementation. ICT-based interventions showed moderate improvements in cognitive outcomes (pooled Cohen’s d = 0.49, 95% CI: 0.14–1.03). A sensitivity analysis excluding high-risk studies yielded a comparable effect size (Cohen’s d = 0.50), indicating robust findings. However, trim-and-fill analysis suggested a slightly reduced corrected effect (Cohen’s d = 0.39, 95% CI: 0.28–0.49), reflecting potential small-study bias. Heterogeneity was moderate (I2 = 46%) and increased to 55% after excluding high-risk studies. Subgroup analysis showed that tablet-based interventions tended to produce higher effect sizes. Conclusions: ICT-based interventions considerably enhance cognition status, autonomy, and social interaction in older adults with MCI and dementia. To ensure long-term scalability, future initiatives must prioritize user-centered design, caregiver education, equitable access to technology, accessible infrastructure and supportive policy frameworks.
Keywords: dementia, mild cognitive impairment, digital health, cognitive support, technology adoption, digital literacy, ICT, healthcare technology, assistive technology
1. Introduction
A global increase in the aging population has accounted for the increased prevalence of age-associated cognitive impairments like mild cognitive impairment and dementia, with over 55 million individuals in the world recently estimated to have dementia [1,2]. As mental and functional abilities decline, many older adults express a strong preference to age in place and remain in their homes [3]. However, cognitive and functional impairment present enormous challenges to independence in daily living [4]. This new public health challenge has sparked interest in large-scale, non-pharmacological interventions to facilitate care and improve quality of life. Among these approaches, Information and Communication Technology (ICT) has been viewed as a promising solution. In response to this emerging public health concern, attention has increasingly turned toward large-scale, non-pharmacological strategies aimed at enhancing care and improving quality of life. Among these, ICTs have enhanced the quality of life of older adults with dementia. ICTs range from digital devices, such as smartphones, tablets, and wearables, to telemedicine equipment, intending to facilitate communication, stimulate cognition, and assist activities of daily living (ADLs) among older adults [5,6,7]. These technologies encompass a wide array of digital devices, including smartphones, tablets, wearables, and telemedicine systems, designed to stimulate cognition and bridge communication gaps.
While awareness of gerontechnology has increased since 2014 [8], particularly in high-income nations, its actual adoption among people with dementia has been hindered by barriers such as low digital literacy, usability challenges, and inadequate caregiving support [9,10]. These factors hinder the practical implementation of digital interventions in real-world settings.
The COVID-19 pandemic significantly accelerated the adoption of digital health interventions, transforming the way older adults and their caregivers interact with technology. During that period, digital platforms enabled the continuation of remote clinical assessments, mHealth communication, virtual therapy, and caregiver-mediated support [11,12,13]. However, recent evidence from 2022 to 2023 has revealed persistent inequalities in access to digital tools, highlighting the need for inclusive digital health strategies for aging populations [14,15,16].
Even as ICT use becomes more widespread during and after the pandemic, data indicate that the actual adoption of cognitive-specific technologies remains suboptimal. For instance, although more than half of older adults with dementia or MCI do utilize smartphones or tablets, a small minority (9.76%) utilize programs or apps aimed explicitly at memory or cognitive assistance [17,18]. Barriers include cognitive limitations, a lack of familiarity with ICT systems, and inadequate support from caregivers or health professionals [19,20,21]. On the other hand, individuals with early-stage cognitive decline (MCI) have shown interest in self-management tools and ICT-supported social interaction [22,23].
In this context, caregivers serve as essential facilitators in helping older people with dementia integrate such tools into their daily routines and overcome usage barriers [24,25,26]. Recent studies merge conventional assistive technologies with those designed to support cognitive processes, such as attention, memory, and executive function. Although digital assistive technologies (DATs), such as GPS trackers and fall detectors, support mobility and ensure data security and safety [27], the use and effectiveness of ICT-based cognitive support interventions remain less explored, particularly in everyday life and community-based settings [28].
Moreover, usability problems, cultural insensitivity, and the lack of incorporation of caregiver experience in studies are among the other barriers to implementation [29,30,31]. Addressing these challenges requires a nuanced understanding of the technologies involved, the support systems surrounding their use, and the lived experiences of users and caregivers. Several studies have investigated how caregivers’ perceptions, digital literacy, and the sociotechnical context, such as Facebook and Twitter, significantly influence the adoption of technology among older adults [32]. At the same time, some caregivers and healthcare professionals remain skeptical, assuming older adults with dementia or MCI cannot effectively use such tools, which further hinders their adoption [7,33]. These misconceptions emphasize the importance of tailoring ICT solutions to users’ needs, capabilities, and preferences.
This review aims to systematically examine ICT-based cognitive support interventions that differ from general care or safety technologies in enhancing memory, attention, problem-solving, and day-to-day functioning among older adults with mild cognitive impairment (MCI) or dementia. The interventions evaluated in this review include mobile memory aids, tablet-based cognitive training, telehealth-delivered cognitive behavioral therapy (CBT), wearable cognitive monitoring devices, and AI-based conversational agents. By focusing exclusively on cognitive support functions, this study addresses a significant gap in the literature and contributes differentiated, post-pandemic insights into the evolving field of dementia care technology.
Since previous review studies have broadly assessed digital health or assistive technologies, this study reviews ICT interventions that support cognitive functioning in older adults with dementia or mild cognitive impairment (MCI). Covering literature from 2015 to 2025, it incorporates the most recent trends in telehealth, mHealth, and caregiver-facilitated digital care. The study also aims to explore usability, adoption barriers, and caregiver roles, providing a framework to design scalable, inclusive, and demand-driven, user-centered ICT solutions to support cognitive aging.
The research questions are as follows:
What types of ICT technologies are most used in cognitive support interventions?
How do ICT-based cognitive and memory support tools affect the mental health, daily activities, and independence of older adults?
What are the long-term sustainability and implementation challenges of these interventions in real-world settings?
What are the key enablers and barriers that influence the usability and adoption of technology-based cognitive support tools among older adults?
The systematic review and meta-analysis aim to address the above questions and provide a comprehensive understanding of how ICT can facilitate the management of dementia-related decline. The aim will be to direct the development of evidence-based practice that enhances the lives of older people with dementia and MCI.
2. Materials and Methods
The systematic literature review followed the PRISMA guidelines [34]. The PECOS framework defines inclusion criteria [35]. The eligibility criteria for systematic reviews and meta-analyses are based on the use of the Population, Exposure, Comparator, Outcome, and Study design (PECOS) framework to ensure methodological rigor and relevance, as follows.
Inclusion and exclusion criteria were defined based on the PECOS framework. Studies were eligible if they examined the use of ICT-based interventions targeting cognitive or psychosocial outcomes in older adults (aged ≥55) diagnosed with mild cognitive impairment (MCI), dementia, or Alzheimer’s disease. Acceptable study designs included randomized controlled trials (RCTs), cohort studies, cross-sectional analyses, mixed-methods studies, and systematic reviews published between 1 January 2015, and 31 January 2025. Eligible exposures included digital cognitive training tools, telehealth platforms, AI-based diagnostic systems, assistive technologies (e.g., GPS tracking, wearables), mHealth applications, and serious games. Comparators involved standard care, paper-based cognitive training, or no intervention. The primary outcomes of interest included changes in cognitive performance (e.g., memory recall, executive function), behavioral and psychological symptoms of dementia (e.g., depression, anxiety), activities of daily living (ADLs) and instrumental activities of daily living (IADLs), and social engagement through technology-mediated interaction. Secondary outcomes involved usability, acceptability, and adherence to ICT interventions.
Studies were excluded if they involved participants aged <55 years, focused on non-ICT interventions (e.g., pharmacological treatment, diet modification, conventional caregiving), or did not evaluate cognitive, functional, or psychosocial outcomes. Editorials, preprints, conference abstracts, and studies published before 2015 were also excluded due to limited data quality or because they were of historical relevance only.
2.1. Summary of Inclusion and Exclusion Criteria
A summary of the detailed inclusion and exclusion criteria, organized by the PECOS framework, is summarized in Table 1:
Table 1.
Inclusion and exclusion criteria (based on PECOS framework).
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population |
|
|
| Exposure |
|
|
| Comparator |
|
|
| Outcome |
|
|
| Study Design |
|
|
Note: ICT = Information and Communication Technology; ADLs = activities of daily living; iADLs = instrumental activities of daily living.
2.2. Study Selection Process
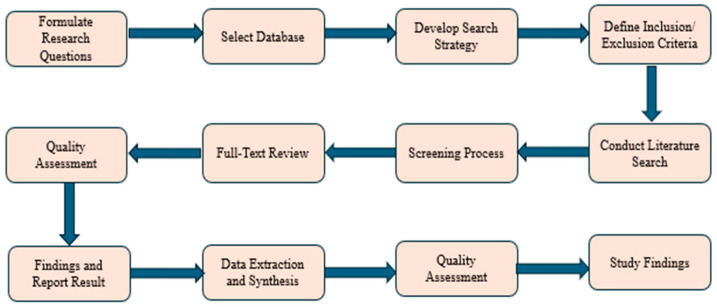
The included studies were conducted over the last 10 years (1 January 2015 to 31 January 2025) using the electronic databases PubMed, Web of Science, PsycINFO, Scopus, ProQuest, IEEE, and EBSCOhost. Establishing the study aim, systematically retrieving and assessing pertinent material, and synthesizing and analyzing data are the three primary steps that comprise the systematic literature review method. A standardized search procedure was employed across the databases to identify, review, and evaluate studies on the implementation of ICT in dementia care management. The study questions corresponded with the data acquisition and analysis. The PRISMA checklist guarantees consistency and reliability in obtaining an in-depth analysis. The whole selection process of the study papers in this systematic review is shown in Figure 1 as follows:
Figure 1.
Systematic literature review (SLR) process for article selection.
The search term (Table 2) was created using the keywords and the Boolean operators “AND” and “OR”:
Table 2.
Search terms applied in the SLR procedure.
| Search Term | Databases | Results |
|---|---|---|
|
(“ICT” OR “Digital Health” OR “mHealth” OR “eHealth” OR “Telehealth” OR “Telemedicine” OR “Wearable Technology” OR “Mobile Health Applications” OR “Device Ownership” OR “Technology Adoption” OR “Digital Literacy”)
AND (“Dementia” OR “Cognitive Impairment” OR “Cognitive Decline” OR “Alzheimer’s Disease” OR “Neurodegenerative Disorders” OR “Memory Loss”) AND (“Older Adults” OR “Aging Population” OR “Elderly” OR “Seniors” OR “Medicare Beneficiaries”)) |
|
67 64 799 1121 901 123 475 |
2.3. Data Extraction
Data was retrieved and extracted from multiple databases, including PsycINFO, PubMed, Scopus, Web of Science, ProQuest, IEEE, and EBSCOhost. Firstly, the records were imported into Zotero, and duplicate articles were removed. Two authors worked independently to screen the titles and abstracts, identifying studies based on the established inclusion and exclusion criteria. The third reviewer was responsible for resolving any discrepancies that arose. Thus, the final selections were confirmed.
2.4. Selected Study
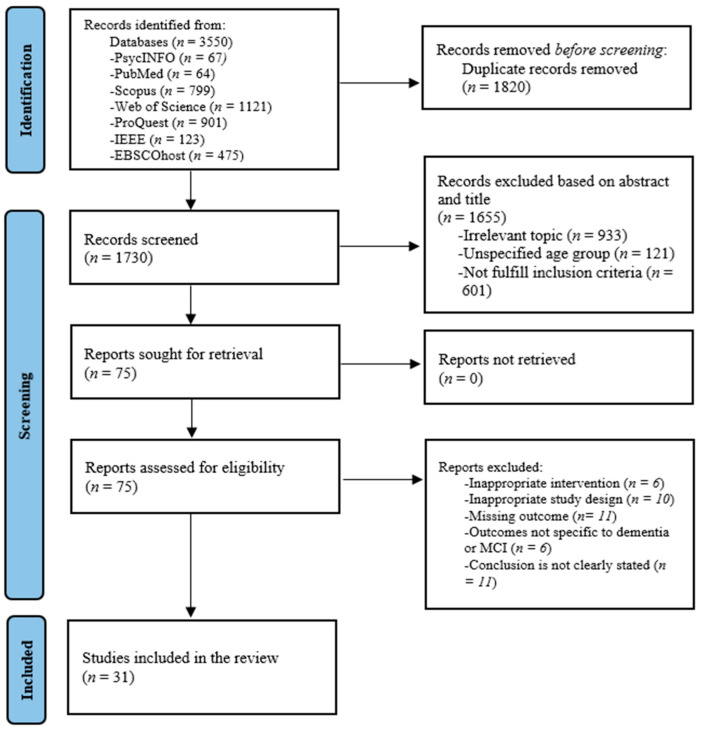
The systematic literature review initially generated 3350 records from specified databases. Then, the removal of 1820 duplicate records and 1730 unique records was screened based on titles and abstracts, resulting in the exclusion of 1655 studies, which were deemed to be irrelevant or failed to meet the inclusion criteria. Full texts were retrieved for 75 studies. Upon full-text review, 21 studies were excluded, and 61 were retrieved and examined for eligibility. Thirty-one studies were included in the final evaluation. The reasons for exclusion at this point were an irrelevant study population (n = 6), inappropriate study design (n = 10), missing outcome data (n = 11), outcomes not specifically related to dementia or mild cognitive impairment (n = 6), or a lack of clearly stated conclusions (n = 11).
The PRISMA flow diagram illustrates this systematic process, ensuring that the final list of studies selected for review met predefined inclusion and exclusion criteria, thereby enhancing the validity and reliability of the findings, as shown in Figure 2.
Figure 2.
PRISMA flow diagram.
2.5. Quality Assessment (Risk of Bias)
The assessment of risk of bias and methodological quality was conducted using the Joanna Briggs Institute (JBI) Critical Appraisal Checklists, which were selected based on the study design (e.g., randomized controlled trials, cross-sectional studies, qualitative research, mixed-methods studies, and systematic reviews) [36]. The JBI consists of several study questions, resulting in an overall appraisal decision for (1) inclusion, (2) exclusion, or (3) seeking further information (Appendix A). Only studies which met the minimum quality threshold for inclusion were retained.
The results of the assessment were used to inform data extraction and synthesis, as well as to guide the overall evaluation of each study’s contribution to the evidence base. Two reviewers (AA and MGR) independently conducted the quality assessments. Disagreements were then resolved through discussion, and when consensus could not be reached, a senior reviewer (VP) was consulted to resolve discrepancies. For each study, the JBI score was calculated by dividing the number of “Yes” responses by the total number of applicable checklist items (excluding “Not Applicable” responses) and then converting the result into a percentage. In the next stage, studies were categorized based on identified JBI scores, as follows: high quality (≥75%), moderate quality (50–74%), and low quality (<50%) [36,37].
Among the 31 included studies, 27 (87%) were rated as high-quality, while 4 (13%) were rated as moderate-quality. This distribution generally indicates a high level of methodological rigor, with some variability. High-quality studies frequently reported clearly defined objectives, appropriate and transparent study designs, and the use of valid and reliable outcome measures. In contrast, moderate-quality studies often lacked detail regarding sampling strategies, data analysis procedures, or reporting of study limitations.
3. Analytical Process
The systematic review and meta-analysis (using Stata 18) revealed a relationship between greater ownership and utilization of ICT devices and better cognitive reserve, accompanied by less subjective cognitive distress, among older adults. Most studies have shown that the repetitive use of electronic devices (such as tablets and mobile phones) helps improve memory and executive function and protects against cognitive decline [38].
The final study investigated the trends in the use of ICT devices among older people with dementia. There is a remarkable rise in the number of papers published in 2022 regarding ICT usages and interventions, and this peaked in 2024, indicating that there is increased research interest in using ICTs in cognitive health management. This shows an increasing awareness of technology in dementia care, accompanied by growing research evidence supporting the development of new assistive technologies and e-healthcare solutions.
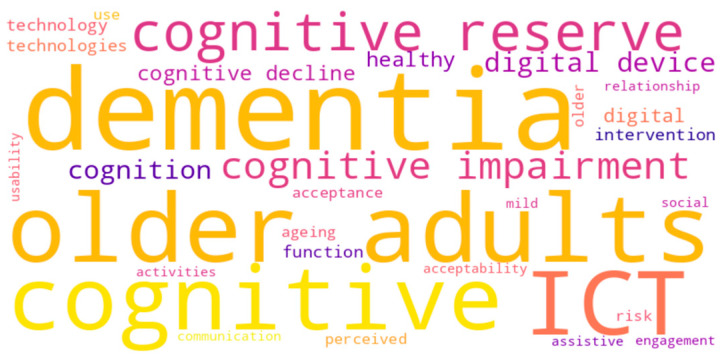
3.1. Word Cloud Visualization of Key Terms
A word cloud (Figure 3) is a robust method for extracting knowledge from text corpora by representing term frequency in a weighted visual matrix. For this review, we examined the 38 abstracts of the systematic literature review (SLR) using atlas.ai, excluding general words such as “study,” “category,” and “topic,” as well as other stop words, to avoid missing significant content [39]. While some broad terms were repeated, the resulting map highlighted nuanced yet essential differences, emphasizing the field’s focus on cognitive, digital, and usability issues in interventions for older adults with mild cognitive impairment or dementia.
Figure 3.
Word cloud visualization of key terms.
3.2. Key Findings
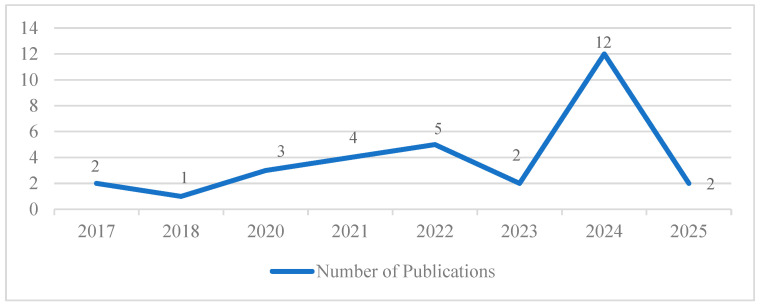
Figure 4 illustrates the publication trend from 2015 to 2025, which indicates a growing academic interest in the adoption and appropriation of ICT devices among older adults. The line chart represents the number of publications from 2017 to 2025, with a fluctuating trend over the years. It was two in 2017, which dropped slightly to one in 2018. It followed a steady increase to three in 2020, four in 2021, and five in 2022. It fell to 2 in 2023 before jumping to 12 publications in 2024, which was the highest number of papers published during the period focused on in this study. In 2025, the number of publications decreased again to two. Overall, the data demonstrates an irregular growth pattern with two high peaks in 2022 and 2024. The rise in research in 2024 indicates a higher ICT adoption rate, increased digital literacy, and the potential to reach older people [40]. The pandemic accelerated the adoption of ICT among elderly people with dementia, enhancing their use of telemedicine, online banking, and social networking platforms. Studies on digital inclusion also explored this group, including the adoption barriers of this population. This smart home and health technology, which utilizes AI, has also triggered additional studies on the use of new technology among older people [41]. In 2025, two journal articles were recapitulated in a monthly timeline. The study reveals an increasing research interest in utilizing ICT in web activities, cognitive functions, and healthcare access.
Figure 4.
Yearly distribution of publications.
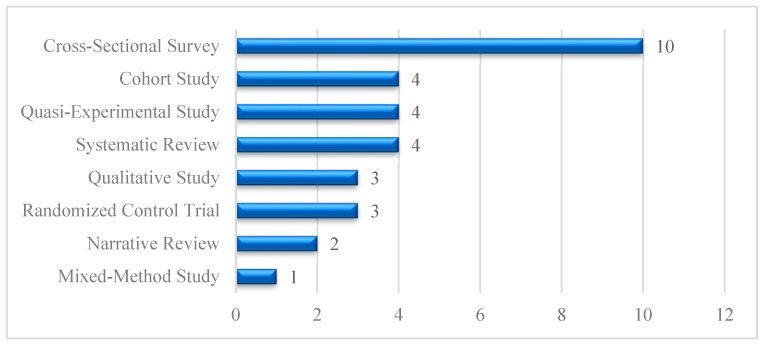
Figure 5 illustrates the prevalence of study types in ICT adoption research among older adults. Cross-sectional studies (n = 10) predominate, focusing on the statistical examination of technology use, while cohort studies, quasi-experimental studies, and systematic review studies (n = 4) investigate users’ experiences and perceptions. Qualitative and randomized controlled studies each contributed (n = 3), demonstrating a balanced presence of evidence synthesis, longitudinal assessment, and intervention-based approaches. Additionally, narrative studies (n = 2) and mixed-method studies (n = 1) provide longitudinal insights, by synthesizing existing literature and providing critical perspectives on digital inclusion, usability challenges, and the role of assistive technologies in aging populations.
Figure 5.
Distribution of study designs.
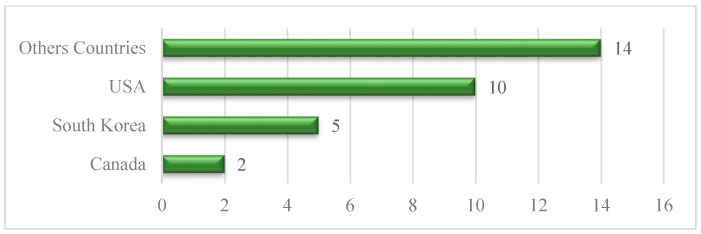
Figure 6 illustrates the countrywide distribution, where the largest group, “Other Countries,” comprises 14 studies, indicating several international contributions. The USA follows with 10 studies, while South Korea accounts for 5 articles, and Canada accounts for 2. The chart reflects a notable contribution from the USA, with significant contributions from other countries, reflecting higher but not equal global interest in ICT adoption research among older adults.
Figure 6.
Geographical distribution.
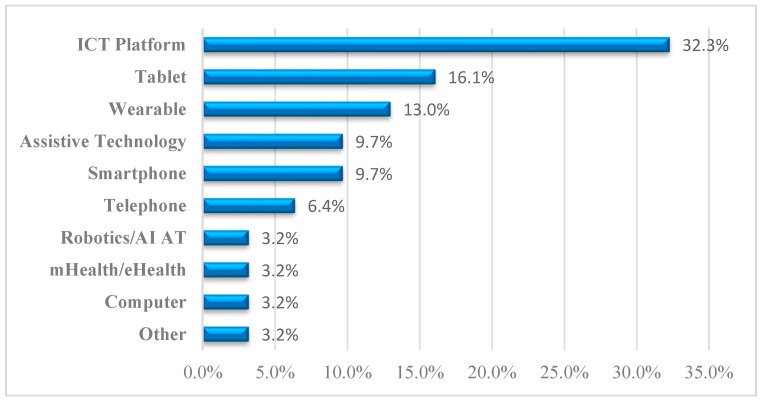
Figure 7 presents the distribution pattern of ICT devices used across studies targeting cognitive health interventions for older adults with mild cognitive impairment (MCI) and dementia. Among the various technologies, the highest percentage (32.3%) involved the use of ICT platforms, indicating a general adoption of digital tools. The second-largest category was tablet-based interventions, which were observed in 16.1% of studies, reflecting their popularity due to their ease of use and portability. Next, wearable devices accounted for 13.0%, and assistive technologies and smartphones each represented 9.7%, indicating balanced adoption across these user-friendly tools. Meanwhile, telephone-based interventions made up 6.4%. Less commonly employed tools included robotics/artificial intelligence (AI) assistive technologies, mHealth/eHealth tools, and computers, each comprising 3.2% of the total. Furthermore, other technologies were found to account for 3.2%, indicating moderate use of support equipment and miscellaneous, unclassified computerized solutions. Notably, very few respondents reported daily technology use, and those who did engaged with a diverse range of devices, suggesting an underutilization of familiar technologies within formal interventions.
Figure 7.
Distribution of device types used in ICT-based interventions for older adults with cognitive impairment.
4. Results
Table 3 provides a summary of recent studies on the adoption and use of technology among older adults with dementia or cognitive impairment. It represents key study characteristics, including study design, study settings, device types, type of intervention, and findings on the usability, effectiveness, and accessibility of digital and cognitive effects tools for managing dementia.
Table 3.
Study Characteristics of the selected articles on Technology Use for Dementia/Cognitive Impairment.
| Study | Study Year | Study Design | Country | Study Setting | Technology Type | Groups in the Study | Device Used | Type of Intervention and Study Type | Objective | Main Findings | Cognitive Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Martinez et al. [42] | 2025 | Cross-sectional study | USA | Home-based | ICT devices (Smartphones, tablets, computers, the internet, social media, or other applications) | Older Asian Americans in affordable housing | ICT Platforms/Devices | Training cognitive function with a computer game | To investigate the relationships between perceived usefulness (PU), perceived ease of use (PEOU), ICT use, and loneliness among low-income, older Asian Americans. | 1. ICT acceptance and use reduce loneliness 2. Ease of use influences ICT adoption 3. Digital literacy training is essential. 4. Policy support is needed for access to technology |
Subjective cognitive decline |
| Kwek et al. [43] | 2025 | Qualitative study | Singapore | Lab-based playtesting and interview sessions | Tablet-based CURATE.DTx system | Community-dwelling older adults | Tablet-based | Digital therapeutic cognitive training | To evaluate the acceptability and user experience of CURATE.DTx, a multitasking-based DTx platform for cognitive training | 1. Digital therapy is acceptable. 2. Users need more training 3. Customization improves engagement 4. Future improvements should target accessibility |
Improved attention, engagement, and executive functioning |
| Yan et al. [44] | 2024 | Cohort study | Canada | Remote preoperative assessments | Telemedicine-based cognitive assessment tools (Telephone Montreal Cognitive Assessment) | Patients were assessed using four cognitive screening tools | Telephone | Remote cognitive screening tools are used through telemedicine | To determine the prevalence of suspected cognitive impairment using multiple screening tools | 1. Screening tools detect cognitive impairment 2. Different tools yield varying prevalence rates 3. Remote assessments improve risk stratification. 4. Further validation needed |
Detected impairment; varied by screening tool |
| Peng et al. [45] | 2024 | Cross-sectional study | USA | Data analysis from the National Health and Aging Trends Study (NHATS) | ICT devices, everyday technology, digital health technology | Homebound, semi-homebound, and non-homebound groups | ICT platforms/devices | Assessment of digital technology usage | To examine 1. The prevalence of digital technology use among community-dwelling older adults with or without homebound status 2. The association between digital technology use and homebound status. |
1. Homebound seniors tend to have lower rates of technology adoption 2. Physical and cognitive limitations impact digital engagement 3. Accessibility remains a key barrier for homebound individuals. |
Digital exclusion accelerates cognitive decline |
| Nakahara and Yokoi [46] | 2024 | Cross-sectional study | Japan | Community gathering places in Osaka Prefecture, Japan | ICT devices (including the internet, communication tools, etc.) | Older adults participating in social activities | ICT platforms/devices | ICT-facilitated social participation | To quantify how ICT use, participation frequency, and social networks influence cognitive function and loneliness among socially active people | 1. ICT use strengthens social interaction, improving the quantity and quality of social participation among older adults 2. Frequent use of ICT improves cognitive function 3. ICT-driven engagement reduces feelings of loneliness |
Improved cognitive function indirectly |
| McMurray et al. [47] | 2024 | Mixed-methods study | Canada | Primary care settings (FHTs) | Tablet-based digital screening tool (BrainFx SCREEN) | Older adults diagnosed with dementia | Tablet-based | Tablet-based cognitive impairment screening | To assess the validity, reliability, and applicability of the BrainFx SCREEN tool for MCI screening in a primary care context | 1. Tablet-based screening shows promise 2. Accessibility improvements needed. 3. Large-scale testing is required 4. Integration with healthcare workflows is beneficial |
Moderate sensitivity; limited reliability |
| Hackett et al. [48] | 2024 | Cross-sectional study | USA | Home-based and real-world mobility tracking | Smartphone with the mindLAMP app for passive GPS tracking | Community-dwelling older adults | Smartphones | Smartphone-based digital phenotyping | To assess the feasibility, acceptability, and validity of using smartphone-based GPS tracking to infer cognition, function, and mood | 1. Mobility patterns link to cognition 2. GPS-based tracking shows cognitive decline patterns 3. Future studies should refine model accuracy |
Greater mobility is linked to cognition |
| Martinez et al. [49] | 2024 | Cross-sectional study | USA | Survey-based data collection | ICTs (general technology use, internet, computers, mobile devices) | Low-income Asian American older adults | ICT platforms/devices | Technology acceptance modeling | To examine the role of self-rated health and subjective cognitive decline in ICT use | 1. ICT use is linked to self-rated health perceptions 2. Digital skills training improves adoption 3. Socioeconomic factors influence engagement 4. Tailored policies can improve participation |
Subjective decline moderated ICT use. |
| Choi et al. [50] | 2024 | Cross-sectional study | USA | Survey-based data collection | ICT and ICT-based communication and internet access (focused on cellphones, email, texting, and the internet) | Homebound and semi-homebound older adults | ICT platforms/devices | Assessment of the digital divide | To explore the digital divide between homebound and semi-homebound older adults using ICT device ownership and usage data | 1. Homebound older adults face severe digital inequities. 2. Socioeconomic factors have a significant impact on access to ICT 3. Policy interventions can help bridge the gap 4. More targeted interventions are needed |
Dementia is linked to reduced ICT use |
| Chae and Lee [51] | 2024 | Randomized controlled trial | South Korea | Participant’s home | Tablet-based training (Smart Brain program) | Community-dwelling older adults | Tablet-based | Digital Therapeutic Cognitive Training | To examine the effects of Smart Brain, an ICT-based cognitive training program, on multi-domain function in older adults with dementia | 1. Cognitive function improved significantly in the intervention group. 2. Depression levels decreased among Smart Brain users. 3. Physical and nutritional health showed positive changes. 4. Participants reported high adherence and satisfaction. |
Improved cognition, mood, physical, and nutritional status |
| Cay et al. [52] | 2024 | Cross-sectional study | USA | Lab-based reading task with speech recording | Wearable microphone to derive digital biomarkers (machine learning) | Cognitively impaired vs. cognitively intact groups | Wearable device | Speech-based digital biomarker analysis | To evaluate the effectiveness of speech-based digital biomarkers in detecting cognitive impairment severity | 1. Speech biomarkers offer a viable screening method 2. Strong correlation with cognitive test results 3. May serve as an early diagnostic tool |
Accurately detected and predicted cognitive impairment |
| Anaraky et al. [53] | 2024 | Cohort study | USA | Observational study using survey data | Internet, computers, tablets, texting, and emails | Community-dwelling older adults | ICT platforms/devices | Monitoring technology use for cognitive change | To determine whether technology use patterns could serve as an indicator of cognitive change in older adults | 1. Tech usage changes can indicate early dementia 2. Monitoring online activity is useful 3. Declining engagement predicts cognitive issues |
Technology discontinuation is linked to decline |
| Addae et al. [54] | 2024 | Narrative review | Various countries | Not specified (focuses on technological interventions) | IoT devices, wearable technologies, and machine learning algorithms | Older adults with dementia. | Wearable and IoT | Monitoring dementia | To explore smart and innovative solutions for early detection, prediction, monitoring, and management of dementia for the advancement of IOT | 1. Wearable tech improves dementia management 2. AI-driven models help with early detection 3. Future research should integrate solutions |
Supports early detection and monitoring |
| Kim et al. [55] | 2024 | Quasi-experimental study | South Korea | Older adults living alone | ICT-based smart care services for physical and cognitive functions | Older adults living alone in the community | ICT platforms/devices | ICT-based smart care services | To examine how ICT-based innovative care services affect physical and cognitive functions in older adults living alone | 1. ICT-based innovative care services are effective at enhancing the physical function of the lower limb 2. Improvements observed in working memory and attention 3. Mixed results in global cognition (decreased K-MMSE score) |
Improved working memory function |
| Heponiemi et al. [56] | 2023 | Cohort study | Finland | Community-dwelling adults | Internet use (not device-specific; focused on performance tests and self-reported digital use) | Community-dwelling adults | Telephone | Digital access competence prediction | To examine how impairments in visual, physical, and cognitive functioning predict internet use and digital competence | 1. Older adults with physical and cognitive limitations are more likely to experience digital exclusion 2. Vision and physical functioning affect digital skill levels 3. Memory performance is a key indicator of digital competence |
Smart technologies support mitigating cognitive decline. |
| Benge et al. [57] | 2023 | Cross-sectional study | USA | Observational research setting | Smartphones, social media, texting, and video calls | People having internet access | Smartphones and social media | Technology use and subjective cognition | To evaluate whether the frequency of digital device use is associated with greater or lesser subjective cognitive concerns (SCCs) in older adults. Cross-sectional study using hierarchical multiple | 1. Increased device use is associated with fewer symptoms of cognitive control (SCC), especially in terms of executive function 2. General device usage matters more than use of social media or texting 3. Digital engagement may protect cognitive function with age |
More use is linked to fewer concerns |
| Park et al. [58] | 2022 | Quasi-experimental study | USA | Sensor-based in-home interactive exercise system (tele-exergame) | Home-based remote intervention | Sensor-based tele-exergame system with a telemedicine interface | Computer | Telemedicine-based exergame training | To assess the feasibility, acceptability, and effectiveness of a sensor-based in-home tele-Exergame system for cognitive and motor function improvement in older adults with MCI/dementia | 1. Tele-exergames are feasible. 2. Engagement was high 3. The long-term impact requires further study 4. Technology accessibility needs improvement |
Cognition improved with the tele-exergame |
| König et al. [59] | 2022 | Qualitative study | Various countries | Home-based intervention | MEMENTO, a system of two e-ink tablets and a smartwatch | EG assistive intervention with MEMENTO | Assistive Technology | Assistive digital device system | To evaluate the usability, acceptance, and impact of the MEMENTO assistive system for dementia patients and their caregivers | 1. Assistive devices help with daily functions 2. Setup complexity is a challenge 3. Caregivers provide essential support 4. Personalization enhances use |
No measurable cognitive improvement observed |
| Holthe et al. [60] | 2022 | Systematic review | Norway | Community-dwelling older adults with MCI and dementia | Digital assistive technology | Older community adults with MCI and dementia | Assistive technology | Wearable and assistive technologies to support older adults with MCI and dementia. | To assess advancements in technology use for older adults with mild cognitive impairment | 1. Wearables enhance independence by providing reminders for individuals with dementia 2. Apps help with managing daily activities 3. Adoption is rising, but still limited |
Supportive role in daily cognition |
| Eun et al. [61] | 2022 | Quasi-experimental study | South Korea | Older adults with varying cognitive impairments | AI-driven serious game | Older adults with varying cognitive impairments | Wearables (or motion-sensing gaming devices) | AI-personalized therapeutic exercise serious game | To assess the effectiveness of an AI-based personalized serious game in enhancing cognitive and physical abilities | 1. AI-based serious games were found to be acceptable, interesting, and motivating for elderly participants. Post-intervention assessments revealed improvements in cognitive function, a reduction in depression levels, and an enhanced quality of life. | Improved cognition and motivation |
| Coley et al. [62] | 2022 | Randomized controlled trial | Various countries | Online (web-based) | eHealth (web-based platform) | High-, moderate-, and low-engagement groups | ICT platforms/devices | Web-based eHealth intervention with personalized coaching and health tracking features | 1. Identifying factors influencing older adults’ engagement with an eHealth intervention 2. Examining its impact on cardiovascular and dementia risk factors |
1. Participants who engaged more showed better improvements in cardiovascular and dementia risk factors, including blood pressure, body mass index (BMI), and cholesterol levels 2. Greater interaction (logins, goal setting, coach messages) led to significantly better health |
Higher engagement is linked to improvement |
| Manca et al. [63] | 2021 | Quasi-experimental study | Italy | Controlled laboratory or training setting | Humanoid robots in supporting serious games | Local Train the Brain program. | Robotics/AI-assistive technology | Humanoid robot-based serious games | This study aims to explore the effect of utilizing humanoid robots in supporting serious games for older adults with mild cognitive impairment (MCI) | 1. Robot users showed higher levels of engagement and emotional connection 2. Tablet users achieved higher accuracy in correct responses 3. Both groups improved over time, but the robot’s empathic cues appeared to boost motivation |
Improved engagement and cognitive training |
| Kim et al. [64] | 2021 | Randomized controlled trial | South Korea | Community-dwelling or institutionalized | ICT-based Training platform | Community-dwelling or institutionalized | ICT-based system | ICT-based cognitive-physical training | ICT-based training devices, including a virtual reality (VR) bicycle integrated with cognitive training modules such as arithmetic operations, fruit-picking tasks, and puzzle-solving activities | 1. Personalized serious games are effective for elderly care 2. Integration of AI enhances therapeutic impact 3. High usability and acceptance among older users 4. Supports dual-target interventions (physical and cognitive) |
Improved ADAS–Cog cognitive score (better cognitive performance) |
| Kelleher et al. [65] | 2021 | Cohort study | USA | MapHabit mHealth app, which assists with cognitive impairments | Tablet-based MapHabit app | MapHabit mHealth app in improving ADL recall | mHealth/eHealth | Assistive technology app featuring personalized visual mapping templates for supporting activities of daily living | To assess the feasibility and preliminary impact of a mHealth assistive technology app in supporting individuals with cognitive impairment in performing ADLs | 1. Older adults with cognitive impairments were willing to use the MapHabit mHealth app to assist with activities of daily living (ADLs) 2. After using the app, participants perceived positive effects on functional abilities, social engagement, mood, and memory |
Improved memory and ADL performance |
| Jung et al. [66] | 2021 | Systematic review | South Korea | Home-based or familiar environments | ICT devices (PCs, desk-tops, laptops, handheld devices, and wireless or other devices) | Patients undergoing cognitive assessment | ICT platforms/devices | ICT-based cognitive interventions | To analyze the effectiveness of ICT interventions for older adults with MCI using a systematic review and meta-analysis | 1. ICT-based interventions have been shown to significantly improve cognitive function in older adults with MCI 2. The findings support their feasibility, with a call for more rigorous and long-term studies |
Significant improvement in cognition |
| Diaz-Orueta et al. [67] | 2020 | Narrative review | European Union | Not applicable (discussion and review) | Various ICT devices (e.g., robotics, serious games, AR, VR, Smart home systems) | Older adults | Assistive technology | Shaping tech for older adults ethically | To explore the ethical implications, privacy concerns, and autonomy considerations associated with the development and implementation of ICT | 1. Ethical considerations are lacking in tech design 2. Older adults prioritize autonomy 3. Privacy and data security concerns |
Focus on ethical cognitive support |
| Contreras-Somoza et al. [68] | 2020 | Cross-sectional study | Eight European countries | Older adults with MCI, informal caregivers, formal caregivers, and administrative staff | EhcoBUTLER ICT platform (tablet-based) | Older adults with MCI, informal caregivers, and formal caregivers | Tablet-based | Feasibility of ICT-based cognitive tools | To assess the acceptability of the EchoBUTLER ICT platform among older adults with MCI and stakeholders involved in their care | 1. The EchoBUTLER platform is generally acceptable to older adults with MCI and their caregivers 2. End users find social functionalities particularly valuable |
Potential support for cognitive training |
| Blok et al. [69] | 2020 | Qualitative study | Netherlands | Home and daily life settings | Various ICT devices in everyday use (smartphones, computers, tablets) | Older people with cognitive impairments | ICT platforms/devices | ICT use experience analysis | 1. To address how older adults with cognitive impairments use ICT daily 2. To identify the perceived barriers and benefits of ICT usage by older adults |
1. ICT use helps enhance social and emotional well-being, facilitating engagement in daily activities 2. Social networks influence ICT use by assisting, initiating, restricting, or enabling the use of shared devices |
Supports social and emotional engagement |
| Holthe et al. [70] | 2018 | Systematic review | Various countries | Home-based and research settings | Various (assistive devices, smart home tech, entertainment, and social tech) | Community-dwelling with MCI and dementia | Wide range/not device-specific | Assistive technology for everyday support | To review technologies explored for older people with MCI/D, usability and acceptability, and involvement of family carers | 1. Usability issues impact adoption among older adults 2. User participation enhances the design of technology 3. Standardized assessments are needed for evaluation 4. Accessibility remains a critical concern |
Support for aging in place |
| Pinto-Bruno et al. [71] | 2017 | Systematic review | Various countries | Mixed (community and institutionalized) | ICT-based application in daily living | 10 different interventions identified | Smartphone | Digital phenotyping to monitor cognition, mood, and life-space | To assess the validity and efficacy of ICT-based interventions in promoting social health and active aging among people with dementia | 1. ICT interventions enhance social participation 2. The lack of standard measures limits the effectiveness of the impact assessment 3. Personalized interventions yield better results |
Greater mobility is associated with improved cognitive function |
| Malinowsky et al. [72] | 2017 | Cross-sectional study | Sweden | Interview-based assessment | Various ET devices for everyday use (unspecified) | Older adults with SCI and MCI | Everyday technologies such as Wearables, computer, phone | Comparing technology use with varying cognitive status. | To investigate and compare self-perceived ability in ET use and the number of ETs used among older adults with SCI, MCI, and controls | 1. MCI patients tend to engage less with technology 2. Self-reported usage predicts decline 3. Support programs needed for accessibility |
Supports memory and daily functioning |
4.1. ICT Intervention Types in Dementia Care
The following section outlines key categories of ICT interventions commonly employed in dementia care, highlighting the unique features, benefits, and limitations associated with each device type.
4.1.1. ICT Platforms
ICT platforms and devices have also emerged as an essential assistant in the care of dementia patients due to their wide-ranging ability to deal with cognitive impairments and promote social interaction among older adults with dementia or MCI [42,45,46]. These technologies enhance daily life, mental stimulation, and social interaction, significantly improving the quality of life for individuals who age independently [42,46,53]. Studies have depicted significant cognitive improvement through interactive digital media that involves people in frequent cognitive stimulation [45]. Furthermore, ICT interventions virtually eliminate social isolation by providing frequent opportunities for social interaction, which benefits dementia patients living in isolated settings [46].
Empirical data consistently shows substantial improvements in cognitive and daily functional capacity among older adults who use these ICT platforms, which legitimize their inclusion within dementia care plans [46,49,50,62]. Likewise, social connectivity has emerged as a crucial protective factor for cognitive health; several studies have focused on using digital communication platforms to promote engagement [45,46]. Video conference applications, social media groups, community network sites, and online forums allow older adults to maintain interpersonal relationships, participate in group activities, and access support services despite their cognitive or physical limitations. The use of these platforms in daily life was associated with improved mood, reduced loneliness, and increased mental stimulation [45,46]. However, problems such as insufficient trust in online platforms, a lack of digital literacy, difficulty in managing passwords, and fears of online fraud were prevalent among older users [69]. Therefore, customized, tailored digital training programs and user-friendly platform designs were identified as acute facilitators for effective social ICT engagement among this population. Social interaction websites, such as video conferencing platforms and web chats, have helped alleviate loneliness and stimulate cognitive activity [49,50]. However, they have also presented issues of digital literacy and cybersecurity threats that require regular attention [45,46]. Innovative care services based on ICT have been explicitly highlighted as significantly improving physical and cognitive functionality, as well as addressing the loneliness and inactivity problems commonly experienced by older populations [55]. After experiencing initial use issues, the technologies provide significant long-term benefits, suggesting flexibility and sustained use [66,69].
4.1.2. Tablet-Based Devices
Tablet-based interventions have become highly effective among older adults due to their simplicity, portability, interactive properties, and user-friendly interfaces. The general acceptability and user satisfaction of a tablet-based multitasking platform in Singapore are notable, as digital therapy is not only acceptable but also benefits from customization and training support to enhance user engagement [43,59]. Likewise, a tablet-based screening tool for cognitive impairment emphasizes reliability and practical use in primary care settings [47]. Additionally, tablet-based “Smart Brain” application significantly improved mental function and reduced depression symptoms among older adults with dementia [51]. Further supporting these outcomes, the study demonstrated that an ICT-based multicomponent program delivered via tablets effectively enhanced both body composition and cognitive performance [65,68]
4.1.3. Assistive Technologies
Dementia-specific assistive technologies have also been crucial in enhancing the daily functioning, independence, and quality of life of individuals with dementia [59,60]. Numerous studies have reported that these interventions enhance users’ self-management, autonomy, and functional performance capacity, accompanied by high levels of user satisfaction and significant practical benefits [59,60]. Personalization and ethics are emphasized as crucial for the optimal effectiveness and usability of assistive technologies in providing personalized care for individuals with dementia [67]. This approach maximally enhances user experience and effectiveness in real-world dementia management.
4.1.4. Wearable Devices
Wearable devices continuously track physical and mental health, providing timely and proactive interventions tailored to individuals’ needs. Wearables’ capability to perform continuous tracking enormously enhances their potential in preventive and personalized healthcare intervention among dementia patients [52,61]. Integrating wearable technology with exergaming further enhances these benefits, providing both cognitive and physical health benefits simultaneously in a fun and interactive manner, thereby supplementing the overall effectiveness of wearables in comprehensive dementia management interventions. Additionally, random Forest models achieved 92.8% accuracy in classifying people with Alzheimer’s disease separately from healthy controls using behavioral and physiological data. In comparison, Support Vector Machines (SVMs) achieved up to 88.5% accuracy in distinguishing mild cognitive impairment (MCI) cases from normal aging populations [54]. These algorithms are commonly embedded in AI-driven cognitive assessment platforms, enabling real-time monitoring, risk stratification, and adaptive feedback for older adults with MCI or dementia [54].
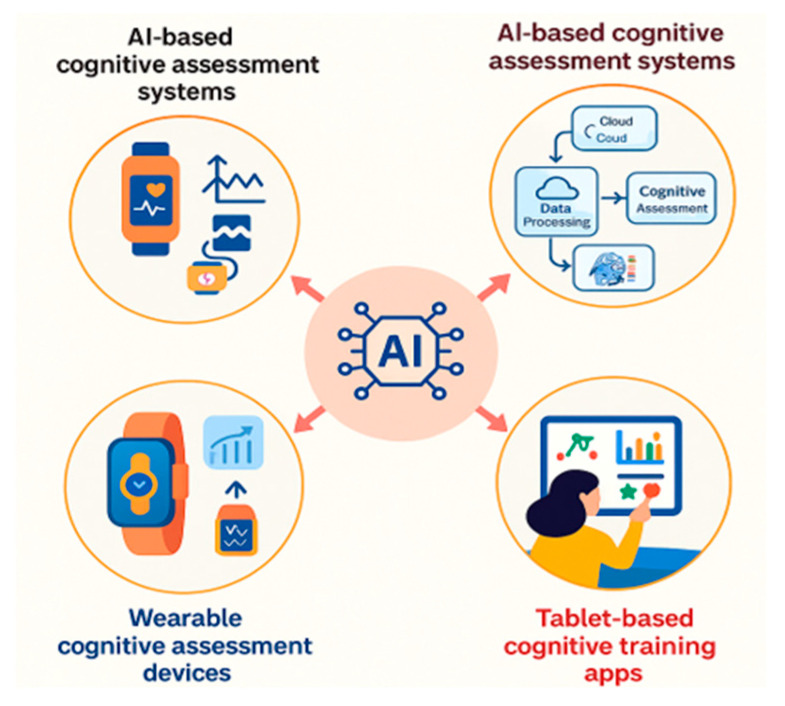
This Figure presents an AI-enabled cognitive care framework. It integrates three core technologies: AI-based cognitive assessment systems for structured testing, wearable devices for continuous physiological and behavioral monitoring, and tablet-based apps delivering interactive cognitive training (Figure 8). A centralized AI engine processes multimodal inputs to generate real-time, personalized interventions. The framework supports early detection, user engagement, and scalable delivery of tailored cognitive health solutions. It reflects key principles of digital precision health and is well-suited to support aging populations and dementia care in remote and community-based settings.
Figure 8.
AI-integrated cognitive assessment and training systems.
4.1.5. Smartphone Devices
Smartphones offer enhanced cognitive and social interaction capabilities through various apps, which help reduce loneliness considerably and enable regular, high-quality social interactions [48]. Earlier studies also highlight the promising potential of telephones for substantially enhancing social activity, building formative evidence for long-term use and adoption in dementia care [71]. In addition, frequent use of smartphones among older adults was associated with fewer subjective cognitive concerns, particularly in executive functioning, reinforcing the role of everyday digital engagement in supporting cognitive well-being [57].
4.1.6. Telephone
Telephone technology is the prime tool for facilitating social connectedness, psychological well-being, and mental stimulation among people with dementia [44,56]. Telephonic interventions are crucial for addressing the critical need for frequent social interaction and emotional support, particularly among older populations who are geographically remote or socially isolated [44].
4.1.7. Robotics and AI Assistive Devices
Advanced robotic and AI-based technologies provide excellent cognitive stimulation and emotional support, offering highly interactive therapeutic benefits for individuals with dementia [63]. Robot-based interventions, particularly those involving humanoid robots, significantly enhance cognitive engagement, emotional interaction, and overall cognitive functioning, highlighting new prospects for active and engaging interventions for individuals with dementia [63].
4.1.8. mHealth and eHealth Interventions
Both eHealth and mHealth interventions significantly contribute to improving the functional status, autonomy, and quality of life of patients with dementia [66]. Personalized visual mapping technologies and other digital solutions allow control over tasks of daily living, significantly enhancing independence and daily competence in older patients with dementia [66].
4.1.9. Computer
A computer-based tele-exergame system that combines sensor-driven balance training with cognitive tasks for older adults with MCI or dementia [58]. Delivered via a telemedicine interface, the intervention allowed users to self-administer interactive exercises at home. The study demonstrated strong feasibility and acceptability, with participants reporting positive attitudes and improved cognitive function [58].
4.1.10. Other Devices
Other notable technological interventions include computer-based applications, broad-spectrum, internet-enabled devices, and a variety of general, non-device-specific technological solutions [70]. Innovative technology solutions continue to expand in scope, offering innovative pathways for detecting, predicting, monitoring, and managing dementia. Several studies have provided a comprehensive overview of innovative systems ranging from sensor-based networks to AI-enabled monitoring platforms that contribute to real-time, data-driven dementia care strategies. These integrated approaches support early intervention and continuous health monitoring, emphasizing the growing relevance of ubiquitous computing in personalized dementia management.
Similarly, research has investigated the usability and acceptability of various technological solutions among community-dwelling older adults with mild cognitive impairment or dementia. Their findings underscore the value of involving older adults and caregivers in the design and implementation process to ensure that systems are effective and user-friendly. This review categorizes technologies by purpose, such as safe walking, independent living, and social engagement, and reinforces the need for ongoing participatory development to increase adoption and positive outcomes in dementia care [70].
4.2. ICT Adoption Enablers and Barriers
Table 4 presents a structured synthesis of adoption enablers and barriers across ten categories of ICT used in dementia care. The matrix reflects insights from 31 peer-reviewed studies, highlighting both transformative enablers and ongoing obstacles of ICT solutions for older adults with MCI or dementia.
Table 4.
ICT adoption enabler–barrier matrix.
| Technology | Adoption Enablers (with References) | Adoption Barriers (with References) |
|---|---|---|
| ICT Platform | ||
| Tablet | ||
| Assistive Technology | ||
| Wearable | ||
| Smartphone | ||
| Telephone | ||
| Robotics/AI Assistive | ||
| mHealth/eHealth | ||
| Computer | ||
| Other Devices |
|
4.2.1. ICT Platforms
ICT platforms (such as web-based portals, videoconferencing systems, and virtual social spaces) excel in their cognitive stimulation capabilities [42,45,46,53] and ability to facilitate social interaction [47,50,70], promote their independence [50,62], and support remote healthcare delivery and services [55,69]. However, there are several significant barriers hindering the adoption of ICTs, including limited digital literacy [45,69], security concerns [46], and a lack of trust in platforms among older adults [53,69]. Additional barriers include poor password management, difficulty navigating portals with small fonts or confusing layouts, and inconsistent access to high-speed internet, which is vital for video functionality. Many users also express anxiety over teleconsultations due to a lack of privacy in shared living environments.
4.2.2. Tablet
Tablets are widely accepted due to their interactive interfaces [43,47], intuitive usability [47,51], and portability [65,68], mainly when they are used for cognitive training or mental health applications. Despite these enabling factors, barriers persist, including training requirements [51], user fatigue [65], and limited customization [68], which continue to hinder sustained use. Older adults also cite small on-screen keyboards, difficulty adjusting brightness and contrast due to vision impairments, and a lack of physical dexterity support as additional challenges.
4.2.3. Assistive Technologies
Assistive technologies (such as memory aids, medication reminders, and smart home tools) are recognized for promoting autonomy [59,60], supporting daily functioning [60], and enabling personalized care [67]. However, ethical concerns [68], limited personalization [59], and a lack of flexible design [60] demonstrate obstacles to broader integration. Privacy issues arise when sensors are placed in bedrooms or bathrooms. Furthermore, some systems generate excessive alerts or false alarms, which can be frustrating for both users and caregivers.
4.2.4. Wearables
Wearables provide real-time, continuous monitoring of health and cognitive status [52,61], enabling early detection of decline [52] and supporting preventive strategies [72]. These benefits are often offset by discomfort [61], sensor inaccuracy [52], and concerns about privacy [72]. Additional challenges include frequent charging requirements, overly complex interfaces on companion apps, and concerns about GPS tracking being conducted by third parties without users’ consent.
4.2.5. Smartphones
Smartphones are praised for enhancing social connectivity [48,71], facilitating communication [72], and supporting routine cognitive tasks [57]. Yet, barriers remain, including interface complexity [58], cybersecurity concerns [48], and low technology confidence among older adults [71]. Specific issues include app overload, difficulty managing updates, stress caused by spam or scam calls, accidental app deletions, vision and fine motor limitations, which further compound navigation difficulties.
4.2.6. Telephone
Telephone-based interventions, though low-tech, are consistently cited for their accessibility [45] and emotional support value for isolated older adults [56]. Still, their limited interactivity restricts deeper cognitive or therapeutic engagement [44,56]. Additional barriers include hearing impairments, the absence of visual cues, and difficulty using automated phone trees.
4.2.7. Robotics and AI
Robotics and AI-assisted systems provide engaging and emotionally intelligent therapeutic interventions [63]. Despite their potential, steep learning curves and user skepticism about AI remain serious deterrents to implementation [63]. Older adults report that robots can be perceived as impersonal, confusing, and intimidating at times. Some fear dependency on machines and express frustration when voice recognition fails due to dialects or speech impairments.
4.2.8. mHealth and eHealth
mHealth and eHealth tools, such as mobile health apps and web-based health platforms, are shown to improve personalized care [66] and overall health outcomes [66]. Nonetheless, these devices often face issues related to a lack of awareness and usability issues that are primarily prevalent among older individuals [66]. Barriers include overly medical language in content, lack of bilingual or audio support, and complex login authentication processes. Many apps also lack offline functionality, which limits access for users in rural areas.
4.2.9. Computer
Computer-based interventions, such as those used for home-based cognitive training and exercise (e.g., tele-exergames), have been shown to have positive effects on digital engagement and cognition [58]. However, they may present usability challenges due to maintenance requirements and complexity for older adults [58]. Specific issues include long loading times, accidental file deletion, and difficulty managing device settings or updates. Caregivers often need to provide recurring technical support.
4.2.10. Other ICT
Other ICT applications, such as general-purpose sensor networks and integration tools, offer a broad spectrum of utility for detection, monitoring, and care coordination [70]. The primary challenges of this group stem from poor personalization and a lack of user involvement in design processes [70]. Additionally, many of these tools are integrated into commercial packages without transparency in pricing, raising concerns about their affordability. A lack of multilingual interface options and a lack of cultural relevance also hinder their uptake among diverse aging populations.
4.3. Effectiveness of ICT-Based Interventions Regarding Cognitive Outcomes
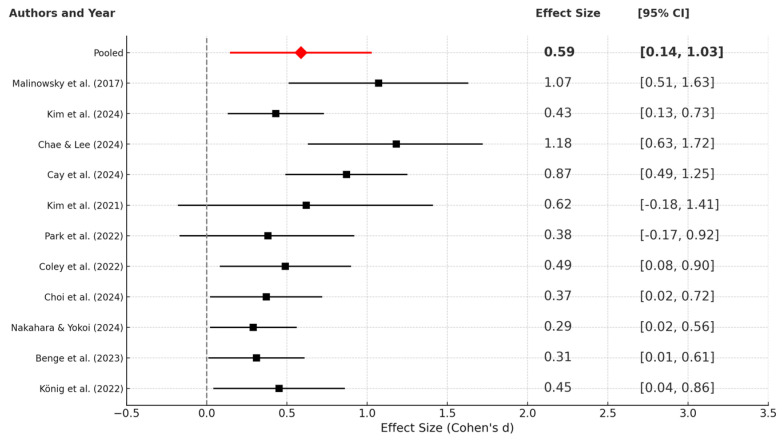
The forest plot displayed (Figure 9) presents the standardized mean differences (Cohen’s d) from 11 studies that estimate the effect of ICT-based interventions on cognitive outcomes among older adults. Each study’s effect size is denoted by a square, with horizontal lines indicating the 95% confidence interval (CI). The pooled effect size, represented by a red diamond at the top, is 0.59 [0.14, 1.03], indicating a moderate and statistically significant overall effect.
Figure 9.
Forest plot showing random effects [46,50,51,52,55,57,58,59,62,64,72].
Several individual studies, including those by Kim et al. [55] (d = 0.43, CI: [0.13, 0.73]), Coley et al. [62] (d = 0.49, CI: [0.08, 0.90]), and Choi et al. [50] (d = 0.37, CI: [0.02, 0.72]), reported significant small-to-moderate effects. Other studies, such as those by Malinowsky et al. [72] and Chae & Lee [51], demonstrated larger effect sizes (d = 1.07 and 1.18, respectively), although their wider confidence intervals (CIs) indicate variability and possible heterogeneity. Some studies have reported large effect sizes, such as those by Malinowsky et al. [72] (d = 1.07) and Chae & Lee [51] (d = 1.18), with wider confidence intervals, indicating greater uncertainty and potential heterogeneity.
In contrast, Kim et al. [55] and Park et al. [58] reported non-significant effects with confidence intervals crossing zero, suggesting greater uncertainty in the intervention effect. Finally, the pooled effect size of 0.59 indicates that ICT-based interventions exert a moderate positive influence on cognitive function in older adults. While there is some variation across these studies, the direction of effect is consistently positive, and the confidence interval does not include zero, indicating statistical significance. These findings collectively support the efficacy of ICT tools in enhancing cognitive health and mitigating age-related cognitive decline.
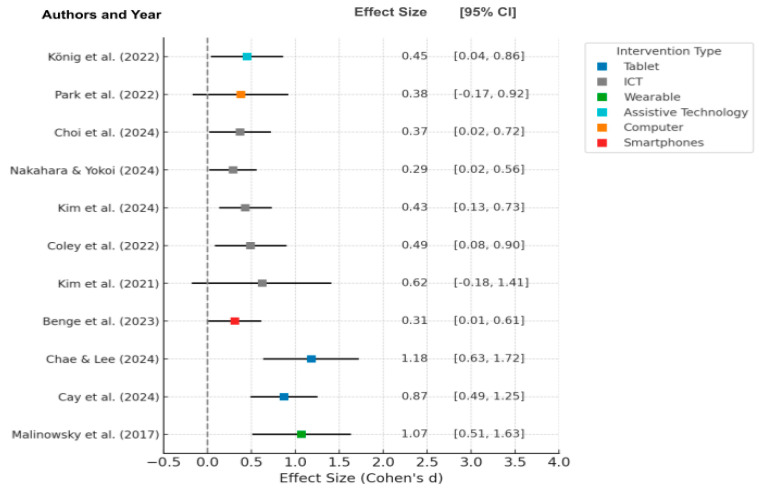
For further analysis of the sources of heterogeneity observed in the meta-analysis, the researchers also conducted a structured subgroup analysis, categorizing studies by the mode of intervention (such as tablets, ICT platforms, wearable devices, assistive technology, computers, and smartphones), as shown in Figure 10. This subgroup analysis uncovered variability in effect sizes across different intervention types, with tablet-based interventions generally yielding larger effect sizes (e.g., Chae & Lee [51], d = 1.18 and Cay et al. [52], d = 0.87) compared to ICT platforms and other modalities, which demonstrated more minor, but still positive effects.
Figure 10.
Forest plot of effect sizes (Cohen’s d) by intervention type [46,50,51,52,55,57,58,59,62,64,72].
Heterogeneity, as measured by I2, increased from 46% to 55% after excluding high-risk studies, indicating that the removal of lower-quality studies may have overstated the variability among the remaining studies. This suggests that study quality and intervention type are essential determinants of heterogeneity. Meta-regression for the severity of dementia was not possible due to the paucity of reporting dementia severity in studies, but this is a critical area for future research.
This means that study quality and intervention type are essential predictors of heterogeneity. Unfortunately, meta-regression by severity of dementia could not be undertaken, since reporting across studies of severity of dementia was poor, but this is something that could be addressed in future studies.
4.4. Heterogeneity and Publication Bias Assessment
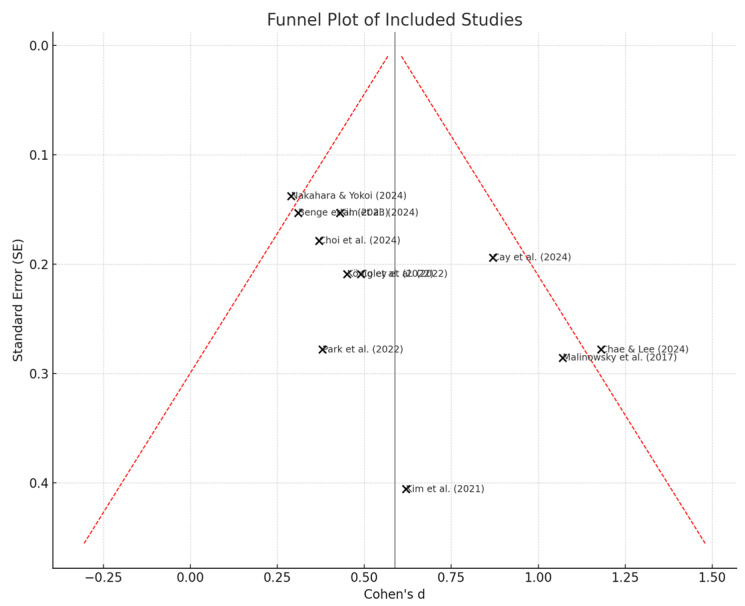
The funnel plot illustrates the reliability and robustness of the meta-analysis findings, where the studies (Figure 11) provide a visual analysis of publication bias by plotting effect sizes (Cohen’s d) against their standard errors. Although the overall distribution appears relatively symmetrical, minor asymmetries are present, particularly at the lower-precision end. This asymmetry suggests publication bias because very few studies with non-significant effects appear to be underrepresented. Additionally, the lack of studies in the lower-left quadrant of the plot, along with the concentration of small studies that report moderate-to-significant positive effects, has likely inflated the overall pooled estimate. The trend is suspicious, suggesting that more positive results were published and considered, while null results may have been excluded. As a result, the meta-analysis slightly overestimates the efficacy of ICT-based interventions regarding cognitive and behavioral outcomes.
Figure 11.
Funnel plot of meta-analysis [46,50,51,52,55,57,58,59,62,64,72].
Table 5 shows that Egger’s regression test indicated significant funnel plot asymmetry, with an intercept of 1.83 (SE = 0.30; 95% CI: 1.16 to 2.51; Z = 6.14; p < 0.001) and a negative slope of −0.25 (SE = 0.12; 95% CI: −0.49 to −0.01; p = 0.04), suggesting the presence of small-study effects. To further assess potential bias, we performed a Duval and Tweedie trim-and-fill analysis. The unadjusted pooled effect size was Cohen’s d = 0.46 (95% CI: 0.35 to 0.57), which decreased slightly to d = 0.39 (95% CI: 0.28 to 0.49) after adjusting for missing studies.
Table 5.
Publication bias analysis.
| Publication Bias | Coefficient | SE | 95% CI | Z | p | ||
| Lower Limit | Upper Limit | ||||||
| Egger’s regression test | intercept | 1.83 | 0.30 | 1.16 | 2.51 | 6.14 | <0.001 |
| slope | −0.25 | 0.12 | −0.49 | −0.01 | −2.06 | 0.04 | |
| Hedge’s | 95% CI | ||||||
| Lower Limit | Upper Limit | ||||||
| Trim and fill | original | 0.46 | 0.35 | 0.57 | - | - | |
| corrected | 0.39 | 0.28 | 0.49 | - | - | ||
4.5. Interpretation of Heterogeneity (I2)
The overall heterogeneity among the 11 studies included in the meta-analysis was found to be moderate (I2 = 46.0%), indicating some variability in the study outcomes. After excluding two high-risk studies (Kim et al. [55] and Choi et al. [50]), the heterogeneity increased slightly (I2 = 55.4%), indicating substantial heterogeneity. This level of heterogeneity reflects differences in intervention modalities, population characteristics, and study design quality across the included studies.
4.6. Sensitivity Analyses
To examine the robustness of the meta-analytic findings, we employed a leave-one-out approach, and sensitivity analyses were conducted by excluding studies considered to have a high risk of bias. The overall meta-analysis, which included all studies, yielded a pooled effect size of Cohen’s d = 0.49 with moderate heterogeneity (I2 = 46.0%). After eliminating two high-risk studies (Kim et al. [55]; Choi et al. [50]), the effect size increased only slightly to d = 0.50, while heterogeneity rose slightly to I2 = 55.4%. This minor variation in the pooled effect size suggests that the results are stable and not unduly influenced by the presence of high-risk studies, thereby reinforcing the reliability and robustness of the overall findings (Table 6).
Table 6.
Sensitivity analysis of the pooled effect size.
| Sensitivity Analysis | Pooled Effect Size (Cohen’s d) | I2 (%) | Impact on Overall Result |
|---|---|---|---|
| All studies included | 0.49 | 46.0% | -- |
| Excluding high-risk bias studies | 0.50 | 55.4% | Minimal, robust findings |
5. Discussion
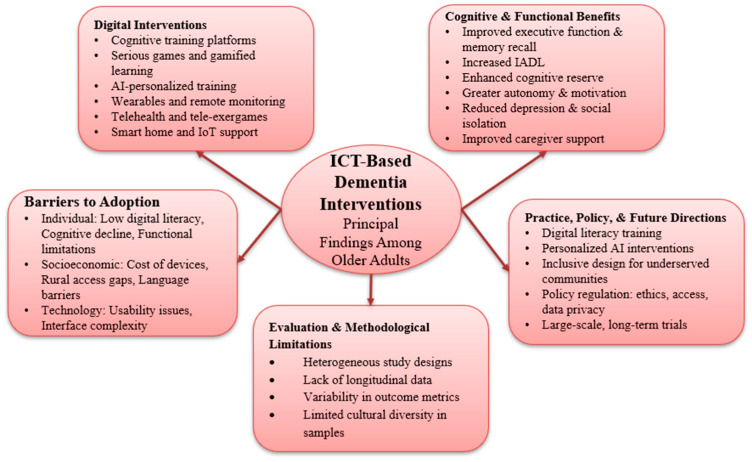
This systematic review revealed the increasing importance of ICT-based interventions in facilitating cognitive ability and mental well-being of older adults with MCI and dementia. A wide range of devices, including wearable sensors, AI platforms, smartphones, and tablets, promise cognitive stimulation, early detection of deteriorating cognitive function, and facilitation of social activity. This systematic review summarizes the key findings regarding the contribution of ICT devices to dementia care and cognitive functioning in older people (Figure 12).
Figure 12.
Overview of principal findings: ICT-based dementia interventions among older adults.
The COVID-19 pandemic accelerated the quick normalization and adoption of ICT-based interventions among older adults, especially in the areas of telehealth, mHealth/eHealth, and social technologies. Virtual check-ins, remote care models, and digital social platforms became crucial for maintaining cognitive engagement and access to services [42,44]. This shift increased digital inclusion among populations that were previously underserved by technology, although disparities in access and literacy persisted [45,46]. Studies from 2022 to 2023 demonstrate the increasing prevalence and acceptance of caregiver-mediated digital tools [45,46], remote cognitive screening [56], and mobile health monitoring [56] following the pandemic. Moreover, pandemic-induced restrictions highlighted the importance of flexible, remote health support systems, prompting innovations in usability, personalization, and caregiver engagement [46]. These changes have prompted a reevaluation of the role of ICT in resilient aging care infrastructure.
Notably, smartphones and tablets were ubiquitous and adept venues for cognitive training and communication; however, a chief hindrance to equal access was the digital literacy gap. Wearable technology was the technological leader in facilitating passive monitoring of cognition and health status in everyday settings, but maintenance requirements and privacy threats deterred its traction. The intersection of AI technologies has brought together personalization and prediction capabilities, but it has also raised new ethical concerns, including concerns about data transparency and algorithmic fairness. By contrast, tele-exergames and robot systems can effectively combine physical and mental exercise, but they face hurdles in terms of cost and usability complexity.
Despite the promise of these technologies, digital exclusion remains a reality. Financial, infrastructural, and educational obstacles disproportionately affect minority and low-income older adult populations. Consistent with previous research [73,74], interventions must focus on user-centered design, digital literacy assistance, and culturally tailored adjustments to make technological advancements accessible to all individuals at risk of cognitive decline. Furthermore, the temporal development of ICT interventions from initial positions as plain communication centers into sophisticated prediction and monitoring systems reflect enormous technological changes. Still, it must also evolve in response to similar interests in ethical standards, policy frameworks, and caregiver training. The long-term effectiveness of multimodal, integrative ICT solutions that combine cognitive support, health surveillance, social activity, and individualized risk avoidance in a unified, easy-to-navigate platform warrants further investigation.
While several ICT interventions report promising short-term outcomes, a significant gap remains in understanding the long-term sustainability of ICT usage. Few studies are constrained by focusing on follow-up durations (23% of the included studies provided follow-up data beyond six months, highlighting a significant gap in understanding long-term sustainability), pilot-scale implementations, or inadequate funding, which limits their ability to evaluate long-term impacts [51,52]. This lack of extended assessment hampers our understanding of how sustained ICT engagement affects cognitive, physical, and emotional health among older adults. Furthermore, the absence of structured integration into routine care workflows reduces the potential scalability of these technologies [59,61].
To bridge this gap, future research should implement long-duration, randomized controlled trials (RCTs) [64], conduct post-implementation follow-ups, and conduct real-world evidence studies [60]. These strategies would enable a greater understanding of cost-effectiveness, user engagement, and the evolution of interventions over time [64]. Continual monitoring will also provide valuable insights into the extent to which technology fatigue, changing user requirements, or caregiver reliance affect the long-term uptake of ICT among aging populations. Although numerous interventions were possible and initially practical, long-term compliance, impacts on various cultural and socioeconomic populations, and scaling ICT innovations to real-world community settings are gaps that need to be addressed. Addressing these gaps will be crucial to realizing ICT’s full potential for enhancing the quality of life and cognitive capacity of older adults globally. Therefore, Table 7 summarizes the key distinctions between this research and the previous research.
Table 7.
Comparison of the current study with existing studies on ICT use in dementia care.
| Dimension | Focus on the Current Study | Typical Approaches in Prior Studies |
|---|---|---|
| Study Scope |
|
|
| Primary Focus |
|
|
| Key Focuses |
|
|
| Research Gaps |
|
|
| Study Contributions |
|
|
| Data Confidentiality |
|
|
| User Experience and Accessibility |
|
|
| Integration with Care Systems |
|
|
| Future Directions |
|
|
5.1. Ethical Considerations and Implications
With the increasing adoption of ICT-based interventions in dementia care and ethical concerns regarding data privacy, informed consent, autonomy, and equity of access, it is essential to establish digital interventions in an ethically configured manner. To achieve this, effective policy mechanisms are needed to advance cybersecurity, data protection, and accountability. Policymakers must enact congruent legislation to govern the ethical application of AI, telehealth, and remote monitoring technology in dementia care, a field that is becoming increasingly relevant. AI systems and remote monitoring technologies raise advanced questions regarding transparency, algorithmic justice, and user control over personal health information [59,67]. In addition, developing digital interventions ethically requires robust policy frameworks that prioritize data protection, cybersecurity, and accountability. Policymakers must establish harmonized legislation to govern the ethical use of AI, telehealth, and remote monitoring technologies in dementia care [67,69].
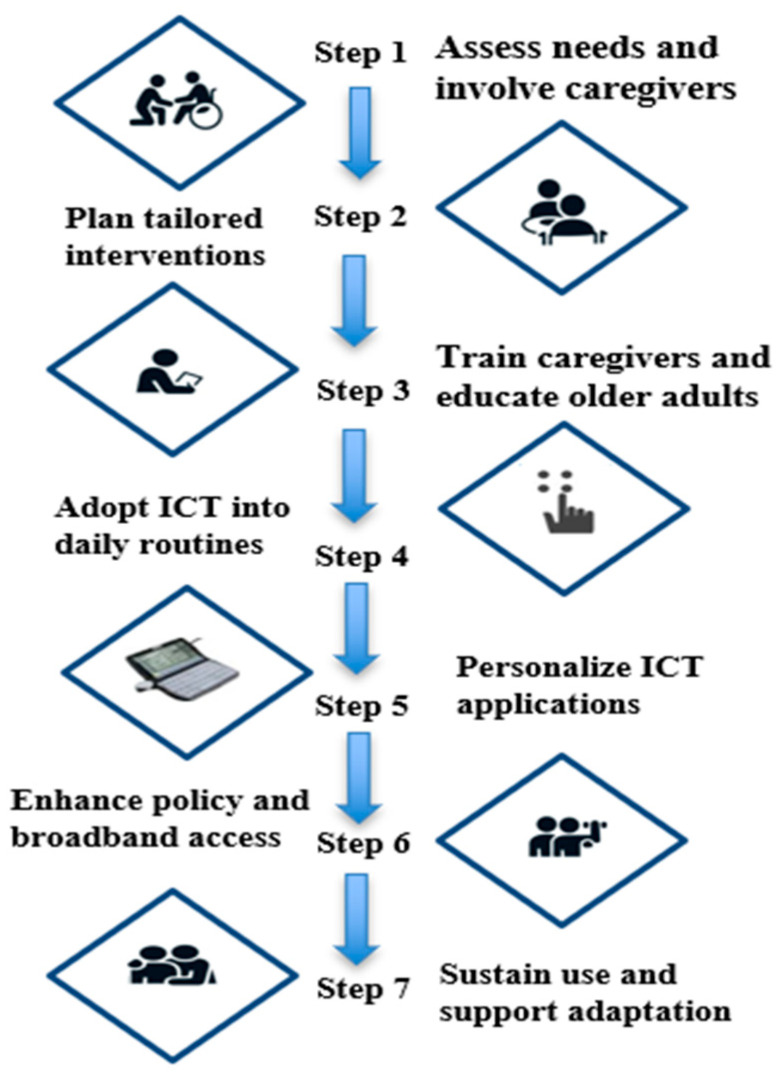
This review’s findings also include several actionable implications for care settings and policy-level adoption. First, ICT skills training should be integrated into caregiver and geriatric care education to enhance frontline workers’ digital competency and confidence [62]. Second, subsidies for devices and the internet should be provided under community health programs to address issues related to affordability, particularly for vulnerable populations [50,54]. Third, the design of intervention needs to prioritize participatory, user-centered methods that are culturally reflective and address the personalization requirements of diverse aging populations [67]. At the policy level, the more widespread implementation can be enabled by incentives for age-friendly ICT design innovation [70], public–private partnerships for investment in infrastructure [71], and policies that enforce accessibility and fairness in digital health access [60]. Robust guidelines must be formulated to achieve user control, safeguard vulnerable groups, and promote ethical innovation. Policies must also ensure parity of reimbursement and access to digital therapeutics so that ICT-based cognitive interventions become mainstream in formal health systems and are accessible to different patient populations [71]. Otherwise, the technologies will reinforce current health inequities rather than mitigate them.
5.2. Policy Direction
Policy interventions are necessary to overcome structural barriers to the adoption of ICT in dementia care. Actions must target growing digital literacy training programs for older individuals and carers, subsidizing the acquisition of devices and broadband services, and technologically appropriate technical support [42,62]. Overcoming cost barriers and rural access inequalities is critical to achieving equitable uptake of these technologies [46]. Furthermore, the government must implement rigorous regulatory measures to protect data, ensure privacy, and promote the ethical use of AI-based platforms [67,69]. Digital health tools must be integrated into national health policies, including sufficient reimbursement mechanisms and access guarantees, to facilitate the large-scale implementation and sustainability of ICT-based cognitive interventions.
Recent economic evaluations indicate that investing in cognitive-supportive ICT is not only socially beneficial but also financially prudent. A 2024 review found that most technology-based interventions, such as telecare, wearables, and apps, are cost-effective when used as a form of dementia care [75]. For instance, Maintenance Cognitive Stimulation Therapy (MCST), a digital training tool designed to enhance daily cognitive engagement, was identified as particularly cost-effective, yielding meaningful improvements in cognitive function at a relatively low cost [76]. Likewise, supportive care models (cognitive therapy, caregiver training, and multicomponent interventions) resulted in significant health system savings [77]. Notably, a rural digital literacy program in South Korea reported measurable social returns, including improved well-being (+3.7 points) and better cognition (+1.1 points), which suggests that subsidy-funded training can deliver psychosocial dividends [78]. Supporting these initiatives with broadband investments, subsidized devices, and training may thus yield proportional reductions in health and social care expenditures, while also reducing equity gaps.
6. Future Research
Although encouraging initial results exist, significant gaps remain that future research must address.
Large-scale, long-term follow-up studies are necessary to compare the long-term cognitive, affective, and functional impact of digital interventions [66]. Multi-stakeholder funding models integrate research grants with investments in the healthcare system.
Future trials must examine the impact of early prolonged digital activity on the postponement of cognitive decline onset and progression of dementia [57].
Evaluations of the cost-effectiveness, feasibility, and real-world utility of customized digital interventions, remote cognitive testing, and serious games are necessary to optimize intervention designs [43,60]. Therefore, it is necessary to integrate the collection of real-world evidence through routine healthcare data.
Moreover, it is essential to extend research into heterogeneous cultural and socioeconomic populations to strengthen global applicability and inclusiveness [42,69].
Emerging technologies, such as voice-controlled assistants, socially assistive robots, and cognitive prediction tools based on AI, must be extensively validated for usability, ethical integration, and clinical utility [67].
Future research should prioritize pragmatic trial designs with embedded sustainability assessments and stakeholder engagement throughout the implementation process.
7. Strengths and Limitations
To provide a more straightforward overview of our review’s methodological context, Table 8 presents the key limitations and strengths of this study in a simplified and easy-to-understand format.
Table 8.
Summary of limitations and strengths.
| Strengths | Limitations |
|---|---|
| The review followed PRISMA guidelines to ensure a transparent and reproducible methodology. | Many studies used self-reported cognitive data, which may miss subtle impairments and introduce personal bias [47,57]. |
| Covered a broad range of ICT interventions (e.g., tablets, smartphones, mHealth, AI, wearables), showing real-world applicability. | Few studies included long-term follow-up, making it difficult to assess the lasting impact of ICT interventions [43,60]. |
| Focused on diverse user groups, including those with MCI and dementia, to capture varying stages of cognitive decline [42,43,46]. | Digital literacy challenges were not thoroughly addressed, particularly among low-income or minority groups [42,46]. |
| Highlighted the need for user-centered and ethically grounded digital solutions, especially in AI-based dementia care technologies. | Ethical issues such as data privacy, transparency, and accountability) In AI-based care, these issues were rarely discussed [58,68]. |
| Emphasized the central role of caregivers in enabling and sustaining ICT use among individuals with cognitive impairment [25,26]. | Limited research exists on caregiver support and technological readiness, despite these factors being vital for successful adoption [25,26,59]. |
8. Recommendation
Based on the systematic review and the framework shown in Figure 13, the following recommendations are provided to guide the integration of ICT interventions into dementia care.
Figure 13.
Framework for Integrating ICT into Dementia Care.
Step 1. Assess Needs and Involve Caregivers
Educational strategies require systematic and continuous development to engage caregivers and older adults, promoting the acceptability of technology and enabling the integration of technology-enabled activities into daily life [18]. Caregiver involvement is necessary for both the initial adoption and long-term maintenance of ICT-based interventions.
Step 2. Tailored Interventions
ICT interventions should be advanced to accommodate people with dementia’s cognitive, physical, and emotional needs. Adapted AI-facilitated cognitive training is a promising approach to enhancing intervention quality and warrants further examination in practice [79].
Step 3. Educating Older Adults and Training Caregivers
Formal training should include the aging population and their carers to ensure maximum digital literacy for enhanced exploitation and use of ICT equipment [80]. Training would be necessary to focus not only on working with machinery but also on self-confidence and problem-solving abilities.
Step 4. Incorporate ICT into Daily Practice
Incorporation of ICT into daily life activities can also enhance independence and quality of life in people with dementia. Empirical evidence demonstrates that assistive technology, when incorporated into day-to-day practices without challenge, greatly improves functional outcomes and accelerates the development of person-centered care environments [59].
Step 5. Personalize ICT Applications
Technology design must be sensitive to the cognitive functions of older adults. Interfaces must incorporate large print, voice control, minimal navigation, and multifunctionality to improve usability and lower cognitive burden [81]. Individualized adaptations enhance patient satisfaction and medication adherence.
Step 6. Enhance Policy and Broadband Access
Policy intervention is urgently needed to bridge the digital divide. The broadband infrastructures must be subsidized, and the internet must be made affordable, with subsidies for ICT devices, particularly for low-income people and rural areas [42]. Inclusive deployment strategies are necessary to guarantee equitable access to digital dementia care innovations.
To mitigate digital disparities, which necessitate policy intervention in the short term, government investment in expanding broadband infrastructure, with a focus on making internet access affordable and supporting ICT devices for low-income and underserved communities, is essential [42]. There is a need for inclusive deployment strategies to ensure that innovations in digital dementia care are accessible to all.
Step 7. Sustain Use and Support Adaptation
Sustainable ICT adoption of interventions relies on ongoing digital skill development, community reinforcement programs, and ethical governance principles. As AI-powered solutions become increasingly prominent in dementia care [67], concerns regarding data confidentiality, patient autonomy, and platform transparency must be addressed. Sustainability initiatives should involve systematic digital skills refreshments and technology sustenance for older adults.
9. Conclusions
This systematic review and meta-analysis, conducted in accordance with the PRISMA guidelines, further strengthens the emerging role of ICT-supported interventions in enhancing cognitive function, independence, and social engagement in older adults with mild cognitive impairment (MCI) and dementia. All digital interventions have been proven effective in improving memory, executive function, well-being, and reducing symptoms of depression, regardless of the device used, including tablets, smartphones, wearables, AI applications, tele-exergame technology, or assistive robots [43,59,67].
Despite these advancements, barriers such as digital learning, usability, socioeconomic disparities, infrastructure gaps, and ethical concerns regarding data privacy and transparency continue to pose hurdles, which contribute to the slow adoption [42,46,49,69]. Furthermore, the current evidence base is limited by heterogeneity in study designs, a lack of long-term outcome data, and inadequate representation of diverse communities.
Interventions would need to adopt user-centered design approaches, address digital literacy gaps through training for caregivers and patients, and implement an equitable approach through policy improvements to achieve the maximum penetration of ICT in dementia care. Additionally, ethical standards must be integrated into the development and use of AI-driven technologies to ensure user independence and confidentiality [67,71].
Lastly, future research is required, particularly in the form of realistic and culturally compliant longitudinal studies, to prevent potential ICT applications for dementia from becoming mere technological interventions that are feasible, sustainable, accessible, and ethically responsible on a global scale. Such innovations have the potential to bridge gaps in ICT-first interventions, significantly promoting cognitive reserve, independence, and improved quality of life for millions of older adults worldwide.
Abbreviations
| MCI | Mild Cognitive Impairment. |
| ICT | Information and Communication Technology. |
| AT | Assistive Technology. |
| ADL | Activities of Daily Living. |
| ADAS | Alzheimer’s Disease Assessment Scale. |
Appendix A. JBI Quality Assessment
| Study Type | Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Total | JBI Quality Rating | Total Overall Appraisal |
| Randomized Control Trial | [51] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13 | High | include |
| [62] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 11 | High | include | |
| [64] | Y | N | Y | N | N | N | Y | Y | Y | Y | Y | Y | Y | 9 | Moderate | include | |
| Systematic Review | [60] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10 | High | include | ||
| [66] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10 | High | include | |||
| [70] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10 | High | include | |||
| [71] | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | 8 | Moderate | include | |||
| Cohort Study | [44] | Y | Y | Y | Y | Y | U | Y | N | N | N | Y | 7 | Moderate | include | ||
| [53] | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | 9 | High | ||||
| [56] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 | High | include | |||
| [65] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | 10 | High | include | |||
| Qualitative Study | [43] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | 8 | |||||
| [59] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | 8 | High | include | ||||
| [69] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | 8 | High | include | ||||
| Mixed-Method Study | [47] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8 | High | include | |||
| Y | Y | N | Y | Y | Y | N | Y | 6 | |||||||||
| Quasi-Experimental Study | [55] | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 | High | include | ||||
| [58] | Y | Y | Y | N | Y | Y | Y | Y | Y | 8 | High | include | |||||
| [61] | Y | Y | Y | N | Y | Y | Y | Y | Y | 8 | High | include | |||||
| [63] | Y | Y | Y | N | N | Y | Y | Y | Y | 7 | High | include | |||||
| Analytical Cross-Sectional Survey | [42] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | High | include | |||||
| [45] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | High | include | ||||||
| [46] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | High | include | ||||||
| [48] | Y | Y | Y | Y | N | N | Y | Y | 6 | Moderate | include | ||||||
| [49] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | High | include | ||||||
| [50] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | High | include | ||||||
| [52] | Y | Y | Y | Y | N | N | Y | Y | 6 | Moderate | include | ||||||
| [57] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | High | include | ||||||
| [68] | Y | Y | Y | N | N | Y | Y | Y | 6 | High | include | ||||||
| [72] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | High | include | ||||||
| Narrative Review | [54] | Y | Y | Y | Y | Y | Y | 6 | High | include | |||||||
| [67] | Y | Y | Y | Y | Y | Y | 6 | High | include |
JBI critical assessment: Q: question; Y: yes; N: no; U: unclear; NA: not applicable. JBI score: high-quality (≥70%); moderate-quality (50–69%); low-quality (<50%).
Author Contributions
Conceptualization, A.A.; methodology, A.A.; software, A.A.; validation, A.A., M.G.R. and V.R.P.; formal analysis, A.A.; investigation, A.A. and M.G.R.; resources, A.A. and M.G.R.; data curation, A.A. and M.G.R.; writing—original draft preparation, A.A.; writing—review and editing, A.A. and M.G.R.; visualization, A.A.; supervision, V.R.P.; project administration, A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Long S., Benoist C., Weidner W. World Alzheimer Report 2023: Reducing Dementia Risk: Never Too Early, Never Too Late. Alzheimer’s Disease International; London, UK: 2023. [(accessed on 4 May 2025)]. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2023.pdf. [Google Scholar]
- 2.Evans-Lacko S., Aguzzoli E., Read S., Comas-Herrera A., Farina N. World Alzheimer Report 2024: Global Changes in Attitudes to Dementia. Alzheimer’s Disease International; London, UK: 2024. [(accessed on 4 May 2025)]. Available online: https://www.alzint.org/resource/world-alzheimer-report-2024/ [Google Scholar]
- 3.Rapaport P., Burton A., Leverton M., Herat-Gunaratne R., Beresford-Dent J., Lord K., Downs M., Boex S., Horsley R., Giebel C., et al. “I just keep thinking that I don’t want to rely on people.” a qualitative study of how people living with dementia achieve and maintain independence at home: Stakeholder perspectives. BMC Geriatr. 2020;20:5. doi: 10.1186/s12877-019-1406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy L., Markey K., O’Donnell C., Moloney M., Doody O. The impact of the COVID-19 pandemic and its related restrictions on people with pre-existent mental health conditions: A scoping review. Arch. Psychiatr. Nurs. 2021;35:375–394. doi: 10.1016/j.apnu.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee M., Mishra R.K., Momin A., El-Refaei N., Bagheri A.B., York M.K., Kunik M.E., Derhammer M., Fatehi B., Lim J., et al. Smart-home concept for remote monitoring of instrumental activities of daily living (IADL) in older adults with cognitive impairment: A proof of concept and feasibility study. Sensors. 2022;22:6745. doi: 10.3390/s22186745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H., Koh S.B., Jo H.S., Lee T.H., Nam H.K., Zhao B., Lim S., Lim J.A., Lee H.H., Hwang Y.S., et al. Global trends in social prescribing: Web-based crawling approach. J. Med. Internet Res. 2023;25:e46537. doi: 10.2196/46537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorrentino M., Fiorilla C., Mercogliano M., Esposito F., Stilo I., Affinito G., Moccia M., Lavorgna L., Salvatore E., Maida E., et al. Technological interventions in European dementia care: A systematic review of acceptance and attitudes among people living with dementia, caregivers, and healthcare workers. Front. Neurol. 2024;15:1474336. doi: 10.3389/fneur.2024.1474336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J., Lee H.Y., Christensen M.C., Merighi J.R. Technology access and use, and their associations with social engagement among older adults: Do women and men differ? J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2017;72:836–845. doi: 10.1093/geronb/gbw123. [DOI] [PubMed] [Google Scholar]
- 9.Álvarez-Dardet S.M., Lara B.L., Pérez-Padilla J. Older adults and ICT adoption: Analysis of the use and attitudes toward computers in elderly Spanish people. Comput. Hum. Behav. 2020;110:106377. doi: 10.1016/j.chb.2020.106377. [DOI] [Google Scholar]
- 10.Zuppo C.M. Defining ICT in a boundaryless world: The development of a working hierarchy. Int. J. Manag. Inf. Technol. 2012;4:13. doi: 10.5121/ijmit.2012.4302. [DOI] [Google Scholar]
- 11.Daly Lynn J., Rondón-Sulbarán J., Quinn E., Ryan A., McCormack B., Martin S. A systematic review of electronic assistive technology within supporting living environments for people with dementia. Dementia. 2019;18:2371–2435. doi: 10.1177/1471301217733649. [DOI] [PubMed] [Google Scholar]
- 12.Cohen-Mansfield J., Creedon M.A., Malone T.B., Kirkpatrick I.I.I.M.J., Dutra L.A., Herman R.P. Electronic memory aids for community-dwelling elderly persons: Attitudes, preferences, and potential utilization. J. Appl. Gerontol. 2005;24:3–20. doi: 10.1177/0733464804271277. [DOI] [Google Scholar]
- 13.Thorpe J.R., R⊘nn-Andersen K.V.H., Bień P., Özkil A.G., Forchhammer B.H., Maier A.M. Pervasive assistive technology for people with dementia: A UCD case. Healthc. Technol. Lett. 2016;3:297–302. doi: 10.1049/htl.2016.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neubauer N.A., Azad-Khaneghah P., Miguel-Cruz A., Liu L. What do we know about strategies to manage dementia-related wandering? A scoping review. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018;10:615–628. doi: 10.1016/j.dadm.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraillon J., Ainley J., Schulz W., Friedman T., Duckworth D. Preparing for Life in a Digital World: IEA International Computer and Information Literacy Study 2018 International Report. Springer; Berlin/Heidelberg, Germany: 2020. [Google Scholar]
- 16.Pot A.M., Willemse B.M., Horjus S. A pilot study on the use of tracking technology: Feasibility, acceptability, and benefits for people in early stages of dementia and their informal caregivers. Aging Ment. Health. 2012;16:127–134. doi: 10.1080/13607863.2011.596810. [DOI] [PubMed] [Google Scholar]
- 17.Liu L., Stroulia E., Nikolaidis I., Miguel-Cruz A., Rincon A.R. Smart homes and home health monitoring technologies for older adults: A systematic review. Int. J. Med. Inform. 2016;91:44–59. doi: 10.1016/j.ijmedinf.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Guzman-Parra J., Barnestein-Fonseca P., Guerrero-Pertiñez G., Anderberg P., Jimenez-Fernandez L., Valero-Moreno E., Goodman-Casanova J.M., Cuesta-Vargas A., Garolera M., Quintana M., et al. Attitudes and use of information and communication technologies in older adults with mild cognitive impairment or early stages of dementia and their caregivers: Cross-sectional study. J. Med. Internet Res. 2020;22:e17253. doi: 10.2196/17253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedman A., Nygård L., Almkvist O., Kottorp A. Amount and type of everyday technology use over time in older adults with cognitive impairment. Scand. J. Occup. Ther. 2015;22:196–206. doi: 10.3109/11038128.2014.982172. [DOI] [PubMed] [Google Scholar]
- 20.Nygård L., Starkhammar S. The use of everyday technology by people with dementia living alone: Mapping out the difficulties. Aging Ment. Health. 2007;11:144–155. doi: 10.1080/13607860600844168. [DOI] [PubMed] [Google Scholar]
- 21.Osvath P., Kovacs A., Boda-Jorg A., Tenyi T., Fekete S., Voros V. The use of information and communication technology in elderly and patients with dementia. J. Gerontol. Geriatr. Res. 2018;7:475. doi: 10.4172/2167-7182.1000475. [DOI] [Google Scholar]
- 22.LaMonica H.M., English A., Hickie I.B., Ip J., Ireland C., West S., Shaw T., Mowszowski L., Glozier N., Duffy S., et al. Examining internet and eHealth practices and preferences: Survey study of Australian older adults with subjective memory complaints, mild cognitive impairment, or dementia. J. Med. Internet Res. 2017;19:e358. doi: 10.2196/jmir.7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee A.R., McDermott O., Orrell M. Understanding barriers and facilitators to online and app activities for people living with dementia and their supporters. J. Geriatr. Psychiatry Neurol. 2023;36:366–375. doi: 10.1177/08919887221149139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidoni E.D., Watts A.S., Burns J.M., Greer C.S., Graves R.S., Van Sciver A., Black J.R., Cooper S.K., Nagely A.C., Uphoff E., et al. Feasibility of a memory clinic-based physical activity prescription program. J. Alzheimer’s Dis. 2016;53:161–170. doi: 10.3233/JAD-160158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damant J., Freddolino P., Dangoor M., Hu B., King D., Wittenberg R. Unpaid carers of people with dementia and information communication technology: Use, impact and ideas for the future. Dementia. 2024;23:779–799. doi: 10.1177/14713012241249793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sriram V., Jenkinson C., Peters M. Carers’ experience of using assistive technology for dementia care at home: A qualitative study. BMJ Open. 2020;10:e034460. doi: 10.1136/bmjopen-2019-034460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert A., Cotten S.R., Xie B. A double burden of exclusion? Digital and social exclusion of older adults in times of COVID-19. J. Gerontol. Ser. B. 2021;76:e99–e103. doi: 10.1093/geronb/gbaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schepens Niemiec S.L., Granados G., Diaz J., Blanchard J., Pyatak E., Padilla Vega D. Practice-Based Evidence of Lifestyle Redesign® for Hypertension and Diabetes in a Safety-Net Primary Care Setting. Am. J. Occup. Ther. 2022;76:1. doi: 10.5014/ajot.2022.76S1-RP15. [DOI] [Google Scholar]
- 29.Tsai Y.I., Beh J., Ganderton C., Pranata A. Digital interventions for healthy ageing and cognitive health in older adults: A systematic review of mixed method studies and meta-analysis. BMC Geriatr. 2024;24:217. doi: 10.1186/s12877-023-04617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takano E., Maruyama H., Takahashi T., Mori K., Nishiyori K., Morita Y., Fukuda T., Kondo I., Ishibashi Y. User Experience of Older People While Using Digital Health Technologies: A Systematic Review. Appl. Sci. 2023;13:12815. doi: 10.3390/app132312815. [DOI] [Google Scholar]
- 31.Wilson S.A., Byrne P., Rodgers S.E., Maden M. A systematic review of smartphone and tablet use by older adults with and without cognitive impairment. Innov. Aging. 2022;6:igac002. doi: 10.1093/geroni/igac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J.W., Byun M.S., Yi D., Lee J.H., Sung K., Han D., Byeon G., Kim M.J., Jung J.H., Chang Y.Y., et al. Association of low meal frequency with decreased in vivo Alzheimer’s pathology. Iscience. 2022;25:105422. doi: 10.1016/j.isci.2022.105422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsson A., Engström M., Skovdahl K., Lampic C. My, your and our needs for safety and security: Relatives’ reflections on using information and communication technology in dementia care. Scand. J. Caring Sci. 2012;26:104–112. doi: 10.1111/j.1471-6712.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 34.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviewsDeclaracion PRISMA 2020: Una guia actualizada para la publicacion de revisiones sistematicas. Rev. Esp. Cardiol. 2022;46:e112. doi: 10.26633/RPSP.2022.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan R.L., Whaley P., Thayer K.A., Schünemann H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Pt 1Environ. Int. 2018;121:1027. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aromataris E., Lockwood C., Porritt K., Pilla B., Jordan Z., JBI Manual for Evidence Synthesis JBI. 2024. [(accessed on 4 May 2025)]. Available online: https://synthesismanual.jbi.global.
- 37.JBI JBI Critical Appraisal Tools|JBI. [(accessed on 1 June 2025)]. Available online: https://jbi.global/critical-appraisal-tools.
- 38.Liang C., Subramaniam P., Mohd Ridzwan Goh N.S., Kok Wai T., Moustafa A.A. Digital device use, risk of cognitive impairment, and cognition in healthy older adults: The role of cognitive reserve. Healthcare. 2023;11:2822. doi: 10.3390/healthcare11212822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heimerl F., Lohmann S., Lange S., Ertl T. Word cloud explorer: Text analytics based on word clouds; Proceedings of the 2014 47th Hawaii International Conference on System Sciences; Waikoloa, HI, USA. 6–9 January 2014; New York, NY, USA: IEEE; pp. 1833–1842. [Google Scholar]
- 40.Haase K.R., Cosco T., Kervin L., Riadi I., O’Connell M.E. Older adults’ experiences with using technology for socialization during the COVID-19 pandemic: Cross-sectional survey study. JMIR Aging. 2021;4:e28010. doi: 10.2196/28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou C., Qian Y., Kaner J. A study on smart home use intention of elderly consumers based on technology acceptance models. PLoS ONE. 2024;19:e0300574. doi: 10.1371/journal.pone.0300574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez P.D., Tancredi D., Pavel M., Garcia L., Young H.M. Adapting the Technology Acceptance Model to Examine the Use of Information Communication Technologies and Loneliness Among Low-Income, Older Asian Americans: Cross-Sectional Survey Analysis. JMIR Aging. 2025;8:e63856. doi: 10.2196/63856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwek S.P., Leong Q.Y., Lee V.V., Lau N.Y., Vijayakumar S., Ng W.Y., Rai B., Raczkowska M.N., Asplund C.L., Remus A., et al. Exploring the General Acceptability and User Experience of a Digital Therapeutic for Cognitive Training in a Singaporean Older Adult Population: Qualitative Study. JMIR Form. Res. 2025;9:e63568. doi: 10.2196/63568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan E., Butris N., Alhamdah Y., Kapoor P., Lovblom L.E., Islam S., Saripella A., Wong J., Tang-Wai D.F., Mah L., et al. The utility of remote cognitive screening tools in identifying cognitive impairment in older surgical patients: An observational cohort study. J. Clin. Anesth. 2024;97:111557. doi: 10.1016/j.jclinane.2024.111557. [DOI] [PubMed] [Google Scholar]
- 45.Peng W., Zhu G., Chen Z., Hou T., Luo Y., Huang L., Qiao J., Li Y. Digital technology use in US community-dwelling seniors with and without homebound status. J. Am. Med. Dir. Assoc. 2024;25:105284. doi: 10.1016/j.jamda.2024.105284. [DOI] [PubMed] [Google Scholar]
- 46.Nakahara K., Yokoi K. Role of Meaningful Social Participation and Technology Use in Mitigating Loneliness and Cognitive Decline Among Older Adults. Am. J. Occup. Ther. 2024;78:7806205150. doi: 10.5014/ajot.2024.050794. [DOI] [PubMed] [Google Scholar]
- 47.McMurray J., Levy A., Pang W., Holyoke P. Psychometric evaluation of a tablet-based tool to detect mild cognitive impairment in older adults: Mixed methods study. J. Med. Internet Res. 2024;26:e56883. doi: 10.2196/56883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hackett K., Xu S., McKniff M., Paglia L., Barnett I., Giovannetti T. Mobility-Based Smartphone Digital Phenotypes for Unobtrusively Capturing Everyday Cognition, Mood, and Community Life-Space in Older Adults: Feasibility, Acceptability, and Preliminary Validity Study. JMIR Hum. Factors. 2024;11:e59974. doi: 10.2196/59974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeLange Martinez P., Tancredi D., Pavel M., Garcia L., Young H.M. The Role of Health in the Technology Acceptance Model Among Low-Income Asian American Older Adults: Cross-Sectional Survey Analysis. JMIR Form. Res. 2024;8:e57009. doi: 10.2196/57009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi N.G., Choi B.Y., Marti C.N. Digital Divide Among Homebound and Semi-Homebound Older Adults. J. Appl. Gerontol. 2024;44:07334648241292971. doi: 10.1177/07334648241292971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chae H.J., Lee S.H. Effectiveness of the online-based comprehensive cognitive training application, Smart Brain, for community-dwelling older adults with dementia: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2024;60:423–432. doi: 10.23736/S1973-9087.24.08043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cay G., Pfeifer V.A., Lee M., Rouzi M.D., Nunes A.S., El-Refaei N., Momin A.S., Atique M.M., Mehl M.R., Vaziri A., et al. Harnessing Speech-Derived Digital Biomarkers to Detect and Quantify Cognitive Decline Severity in Older Adults. Gerontology. 2024;70:429–438. doi: 10.1159/000536250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghaiumy Anaraky R., Schuster A.M., Cotten S.R. Can Changes in Older Adults’ Technology Use Patterns Be Used to Detect Cognitive Decline? Gerontologist. 2024;64:gnad158. doi: 10.1093/geront/gnad158. [DOI] [PubMed] [Google Scholar]
- 54.Addae S., Kim J., Smith A., Kang M., Rajana P. Smart solutions for detecting, predicting, monitoring, and managing dementia in the elderly: A survey. IEEE Access. 2024;12:100026–100056. doi: 10.1109/ACCESS.2024.3421966. [DOI] [Google Scholar]
- 55.Kim D.R., Lai T.F., Sung M., Jang M., Shin Y.K., jin Ra Y., Liao Y., Park J.H., Shin M.J. Effect of information and communication technology-based smart care services for physical and cognitive functions in older adults living alone: A quasi-experimental study. J. Nutr. Health Aging. 2024;28:100318. doi: 10.1016/j.jnha.2024.100318. [DOI] [PubMed] [Google Scholar]
- 56.Heponiemi T., Kainiemi E., Virtanen L., Saukkonen P., Sainio P., Koponen P., Koskinen S. Predicting internet use and digital competence among older adults using performance tests of visual, physical, and cognitive functioning: Longitudinal population-based study. J. Med. Internet Res. 2023;25:e42287. doi: 10.2196/42287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benge J.F., Kiselica A.M., Aguirre A., Hilsabeck R.C., Douglas M., Paydarfar D., Scullin M.K. Technology use and subjective cognitive concerns in older adults. Arch. Gerontol. Geriatr. 2023;106:104877. doi: 10.1016/j.archger.2022.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park C., Mishra R.K., York M.K., Enriquez A., Lindsay A., Barchard G., Vaziri A., Najafi B. Tele-medicine based and self-administered interactive exercise program (Tele-exergame) to improve cognition in older adults with mild cognitive impairment or dementia: A feasibility, acceptability, and proof-of-concept study. Int. J. Environ. Res. Public Health. 2022;19:16361. doi: 10.3390/ijerph192316361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.König T., Pigliautile M., Águila O., Arambarri J., Christophorou C., Colombo M., Constantinides A., Curia R., Dankl K., Hanke S., et al. User experience and acceptance of a device assisting persons with dementia in daily life: A multicenter field study. Aging Clin. Exp. Res. 2022;34:869–879. doi: 10.1007/s40520-021-02013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holthe T., Halvorsrud L., Lund A. Digital assistive technology to support everyday living in community-dwelling older adults with mild cognitive impairment and dementia. Clin. Interv. Aging. 2022;17:519–544. doi: 10.2147/CIA.S357860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eun S.J., Kim E.J., Kim J. Artificial intelligence-based personalized serious game for enhancing the physical and cognitive abilities of the elderly. Future Gener. Comput. Syst. 2023;141:713–722. doi: 10.1016/j.future.2022.12.017. [DOI] [Google Scholar]
- 62.Coley N., Andre L., Hoevenaar-Blom M.P., Ngandu T., Beishuizen C., Barbera M., van Wanrooij L., Kivipelto M., Soininen H., van Gool W., et al. Factors predicting engagement of older adults with a coach-supported eHealth intervention promoting lifestyle change and associations between engagement and changes in cardiovascular and dementia risk: Secondary analysis of an 18-month multinational randomized controlled trial. J. Med. Internet Res. 2022;24:e32006. doi: 10.2196/32006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manca M., Paternò F., Santoro C., Zedda E., Braschi C., Franco R., Sale A. The impact of serious games with humanoid robots on mild cognitive impairment older adults. Int. J. Hum. Comput. Stud. 2021;145:102509. doi: 10.1016/j.ijhcs.2020.102509. [DOI] [Google Scholar]
- 64.Kim D.R., Song S., Kim G.M., Chang J.H., Tak Y.J., Huh U., Cho J.S., Liao Y., Han K.S., Ko M.H., et al. Effects of ICT-based multicomponent program on body composition and cognitive function in older adults: A randomized controlled clinical study. Clin. Interv. Aging. 2021;16:1161–1171. doi: 10.2147/CIA.S306894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelleher J., Zola S., Cui X., Chen S., Gerber C., Parker M.W., Davis C., Law S., Golden M., Vaughan C.P. Personalized visual mapping assistive technology to improve functional ability in persons with dementia: Feasibility cohort study. JMIR Aging. 2021;4:e28165. doi: 10.2196/28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung A.R., Kim D., Park E.A. Cognitive intervention using information and communication technology for older adults with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2021;18:11535. doi: 10.3390/ijerph182111535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diaz-Orueta U., Hopper L., Konstantinidis E. Shaping technologies for older adults with and without dementia: Reflections on ethics and preferences. Health Inform. J. 2020;26:3215–3230. doi: 10.1177/1460458219899590. [DOI] [PubMed] [Google Scholar]
- 68.Contreras-Somoza L.M., Irazoki E., Castilla D., Botella C., Toribio-Guzmán J.M., Parra-Vidales E., Suso-Ribera C., Suárez-López P., Perea-Bartolomé M.V., Franco-Martín M.Á. Study on the acceptability of an ICT platform for older adults with mild cognitive impairment. J. Med. Syst. 2020;44:1–2. doi: 10.1007/s10916-020-01566-x. [DOI] [PubMed] [Google Scholar]
- 69.Blok M., van Ingen E., de Boer A.H., Slootman M. The use of information and communication technologies by older people with cognitive impairments: From barriers to benefits. Comput. Hum. Behav. 2020;104:106173. doi: 10.1016/j.chb.2019.106173. [DOI] [Google Scholar]
- 70.Holthe T., Halvorsrud L., Karterud D., Hoel K.A., Lund A. Usability and acceptability of technology for community-dwelling older adults with mild cognitive impairment and dementia: A systematic literature review. Clin. Interv. Aging. 2018;13:863–886. doi: 10.2147/CIA.S154717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinto-Bruno Á.C., García-Casal J.A., Csipke E., Jenaro-Río C., Franco-Martín M. ICT-based applications to improve social health and social participation in older adults with dementia. A systematic literature review. Aging Ment. Health. 2017;21:58–65. doi: 10.1080/13607863.2016.1262818. [DOI] [PubMed] [Google Scholar]
- 72.Malinowsky C., Kottorp A., Wallin A., Nordlund A., Björklund E., Melin I., Pernevik A., Rosenberg L., Nygård L. Differences in the use of everyday technology among persons with MCI, SCI and older adults without known cognitive impairment. Int. Psychogeriatr. 2017;29:1193–1200. doi: 10.1017/S1041610217000643. [DOI] [PubMed] [Google Scholar]
- 73.Czaja S.J. Long-term care services and support systems for older adults: The role of technology. Am. Psychol. 2016;71:294. doi: 10.1037/a0040258. [DOI] [PubMed] [Google Scholar]
- 74.Charness N., Boot W.R. A grand challenge for psychology: Reducing the age-related digital divide. Curr. Dir. Psychol. Sci. 2022;31:187–193. doi: 10.1177/09637214211068144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vargas-Martínez A.M. Economic evaluations of technology-based interventions used to provide care support for people with mild dementia or mild cognitive impairment and their caregivers: A systematic review. J. Alzheimer’s Dis. 2024;102:597–616. doi: 10.1177/13872877241291070. [DOI] [PubMed] [Google Scholar]
- 76.Eaglestone G., Gkaintatzi E., Jiang H., Stoner C., Pacella R., McCrone P. Cost-effectiveness of non-pharmacological interventions for mild cognitive impairment and dementia: A systematic review of economic evaluations and a review of reviews. PharmacoEconomics-Open. 2023;7:887–914. doi: 10.1007/s41669-023-00440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guzzon A., Rebba V., Paccagnella O., Rigon M., Boniolo G. The value of supportive care: A systematic review of cost-effectiveness of non-pharmacological interventions for dementia. PLoS ONE. 2023;18:e0285305. doi: 10.1371/journal.pone.0285305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee H., Lim J.A., Nam H.K. Effect of a digital literacy program on older adults’ digital social behavior: A quasi-experimental study. Int. J. Environ. Res. Public Health. 2022;19:12404. doi: 10.3390/ijerph191912404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knapp M., Shehaj X., Wong G. Digital interventions for people with dementia and carers: Effective, cost-effective and equitable? Neurodegener. Dis. Manag. 2022;12:215–219. doi: 10.2217/nmt-2022-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali Z., Janarthanan J., Mohan P. Understanding digital dementia and cognitive impact in the current era of the internet: A review. Cureus. 2024;16:e70029. doi: 10.7759/cureus.70029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson S.A., Byrne P., Rodgers S.E. ‘I’d be lost without my smartphone’: A qualitative analysis of the use of smartphones and tablets by people living with dementia, mild cognitive impairment, and their caregivers. Aging Ment. Health. 2024;28:595–603. doi: 10.1080/13607863.2023.2205585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.