Abstract
THIS ARTICLE DESCRIBES CONTEMPORARY UNDERSTANDING of fine-needle aspiration (FNA) biopsy of the thyroid gland, with particular reference to its triage role in the investigation of thyroid nodules. A team approach involving the pathologist and the clinician is crucial for its success. A nonsuction technique with a 25- or 27-gauge needle is recommended. Performance of FNA biopsy by physicians with faulty technique and interpretation of FNA specimens by pathologists without proper training are detrimental to patient outcome. These problems may lead to a high failure rate, which in turn might discourage use of FNA biopsy and could lead to use of other diagnostic methods that are more expensive or are associated with greater morbidity (or both). The size of the nodule may be important in determining the need for surgery in some patients. Decisions regarding surgery that are based on the size of the nodule and the specific cytologic features are discussed. A standardized terminology for reporting is proposed.
Thyroid nodules are a common clinical problem. This article describes contemporary understanding of fine-needle aspiration (FNA) biopsy of the thyroid. It stresses the team approach, the triage role of FNA in the investigation of thyroid nodules, use of a nonsuction technique and attention to nodule size, and recommends a standardized terminology for reporting to minimize ambiguity. The article is written primarily from a pathologist's perspective. However, if the FNA report is to be meaningful, it must be considered together with the patient's clinical features.
Role of FNA biopsy
Depending on the nature of the thyroid nodule, FNA biopsy can function as a diagnostic test or a triage tool.1,2,3 As a diagnostic test, FNA biopsy can be used to diagnose papillary carcinoma, poorly differentiated carcinoma, medullary carcinoma, anaplastic carcinoma, metastatic malignancy, thyroiditis, and most benign nodular goitres and cysts. However, follicular adenoma, well-differentiated carcinoma and some hypercellular goitres are indistinguishable on FNA biopsy.
As a triage tool, FNA biopsy can be used to distinguish thyroid nodules that might have a higher risk of malignancy (i.e., neoplasms), and would thus require surgical excision, from goitrous nodules or thyroiditis, which can be managed medically. Although FNA biopsy can reduce the number of diagnostic thyroidectomies by identifying benign lesions that need not be removed, it does not and cannot eliminate all diagnostic operations.1,2,3 Some patients with thyroid nodules who are referred for operation after FNA biopsy are actually found to have benign disease, because of our inability to distinguish accurately between follicular adenoma, well-differentiated carcinoma and some cellular goitres on FNA.
Indications for FNA biospy
Traditionally, the main indication for FNA biopsy of the thyroid has been the presence of a solitary nodule. It has been taught that the risk of cancer in a multinodular goitre is much less than that in a solitary nodule. However, recent literature indicates that if a nodule in a multinodular goitre has grown steadily, become distinctly dominant or changed in consistency, its risk of malignancy is the same as that for a solitary nodule.4,5 Under these circumstances, FNA investigation is indicated. In autoimmune thyroid diseases, such as Graves' disease and Hashimoto's thyroiditis, a dominant localized abnormality in the thyroid gland is an indication for FNA.6 FNA biopsy is also required for a diffuse, rapidly growing thyroid enlargement to rule out anaplastic carcinoma or lymphoma, especially in patients over 50 years of age.
Technical aspects
The FNA procedure need not be confined to large medical centres. Good results can be achieved in community hospital and rural settings,7 provided the procedure is carried out by a core group of dedicated physicians, regardless of whether they are endocrinologists, internists, surgeons, radiologists or pathologists. To maintain expertise, the number of aspirators and the number of interpreting cytopathologists should be kept small,8 and each individual aspirator must perform at least 1 to 5 aspiration procedures per month.9
Although both suction and nonsuction techniques have been described for thyroid FNA biopsy, I recommend the nonsuction technique with a 25- or 27-gauge fine needle10,11,12,13 (Fig. 1). The technique is simple, produces specimens that are less bloody, and is particularly effective for aspirating small lesions. However, the conventional suction technique sometimes yields more material than the nonsuction technique and vice versa, so it is unwise to use one technique to the exclusion of the other.
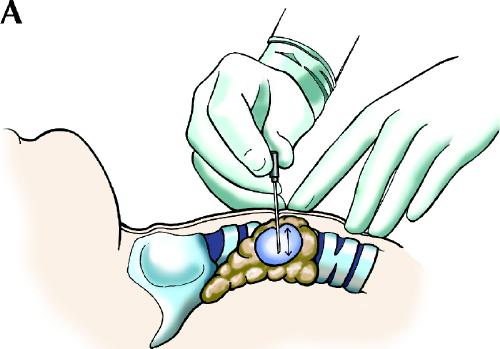
Fig. 1: Nonsuction fine-needle sampling with a 25- or 27-gauge needle. A: The needle is held directly between the thumb and the index finger. The nodule is immobilized with the index and middle fingers of the other hand. The needle is moved back and forth several times within the nodule with a rapid, gentle, stabbing motion. B: After the needle is withdrawn, an air-filled syringe with its plunger already retracted is immediately attached to the needle, and the needle contents are expelled onto clean glass slides. Thin, evenly spread smears are prepared from the ejected material. It is recommended that at least 2 or, preferably, 3 punctures be performed for each nodule.
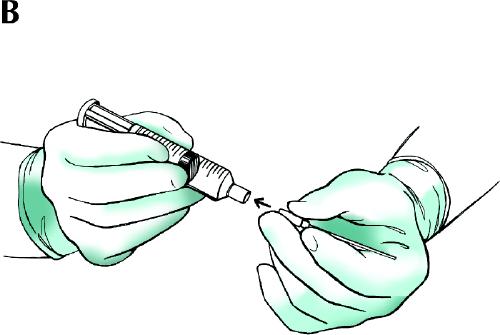
Fig. 1: Nonsuction fine-needle sampling with a 25- or 27-gauge needle. A: The needle is held directly between the thumb and the index finger. The nodule is immobilized with the index and middle fingers of the other hand. The needle is moved back and forth several times within the nodule with a rapid, gentle, stabbing motion. B: After the needle is withdrawn, an air-filled syringe with its plunger already retracted is immediately attached to the needle, and the needle contents are expelled onto clean glass slides. Thin, evenly spread smears are prepared from the ejected material. It is recommended that at least 2 or, preferably, 3 punctures be performed for each nodule.
If a cyst is encountered during nonsuction FNA, suction with a larger needle is recommended, to evacuate as much fluid as feasible. If there is a residual solid area, then a 25- or 27-gauge needle may be used for nonsuction sampling of this area.
Parallel preparation of alcohol-fixed and air-dried smears from the aspirated material is recommended. For alcohol fixation, the smears must be placed promptly in 95% alcohol, before any air drying occurs. Recently, ThinPrep processors (Cytyc, Boxborough, Mass.) have become available in many laboratories, allowing monolayer preparation. The major disadvantage of monolayer preparations is that the colloid, which is important for diagnosis, may not be fully preserved.
Because FNA of the thyroid is an invasive procedure, albeit minimally so, complications are possible although extremely rare.14,15 Bleeding complications occur only infrequently and are almost always self-limited if firm pressure is applied to the aspiration site. A more serious complication occurred in the early years of experience at our hospital, when a massive hematoma developed in a patient who had been discharged home. The hematoma led to tracheal compression, which necessitated emergency surgical ligation of a superficial thyroid artery. Since that unfortunate incident, we have advised all patients to report promptly to the emergency department if a hematoma develops that cannot be stopped by applying pressure to the bleeding site. We have now performed thousands of thyroid FNA procedures and have not encountered any other cases of serious bleeding.
Interpretation and reporting of results
Relevance of clinical information
The clinician and the pathologist must work closely together as a team. The pathologist who attempts to interpret FNA biopsy specimens without clinical information is doing a disservice to both the patient and the referring clinician.16 Therefore, pertinent history, clinical findings and imaging data, if any, should be communicated to the pathologist at the time of referral. Any of the following risk factors, if present, should alert the pathologist to the increased likelihood of the nodule being malignant: older male, history of head and neck irradiation, family history of multiple endocrine neoplasia syndrome type 2A or 2B, fixed nodule, enlarging mass, vocal cord paralysis (although large benign goitres may also be associated with such paralysis) and cervical lymphadenopathy (although this may be present in some cases of Hashimoto's thyroiditis).
However, none of these historical or clinical features can be used to independently and reliably determine the likelihood of cancer in a thyroid nodule. Interestingly, a number of studies17,18,19,20,21 have found that for hypercellular, nonpapillary follicular lesions revealed by FNA biopsy, increasing size of the lesion is associated with increasing risk of malignancy; therefore, nodule size is an important consideration in selecting treatment (surgical or nonsurgical) (see the discussion of hypercellular follicular nodules, below). This size consideration does not apply to papillary carcinomas, which can be quite small. A small papillary carcinoma manifesting initially as nodal metastasis in the neck is not unusual.22
Recommendations for reporting
The pathologist's report must be clinically relevant and readily understood by clinicians. Some medical centres have used ambiguous terms, such as “atypical” and “indeterminate,” with no reference to the specific histologic type or the probability of cancer. A plea has been made for reporting thyroid FNA diagnoses in standardized histopathologic terminology,3,21 which is universally understood, can be related to the probability of cancer and therefore can guide management decisions (Table 1). The diagnostic groups as seen on fine needle aspirates are outlined below.
Table 1
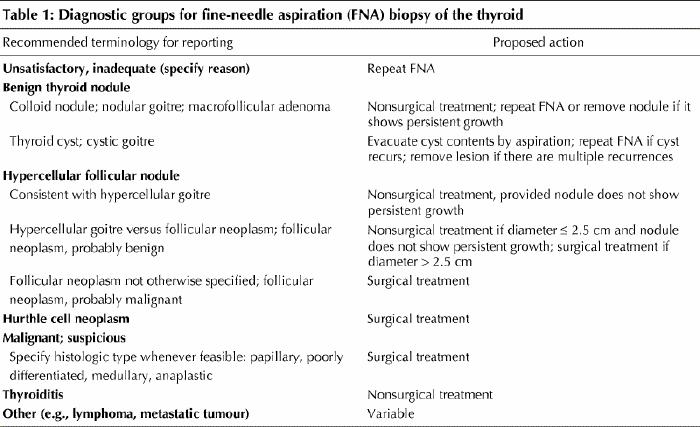
Diagnostic groups
Unsatisfactory or inadequate
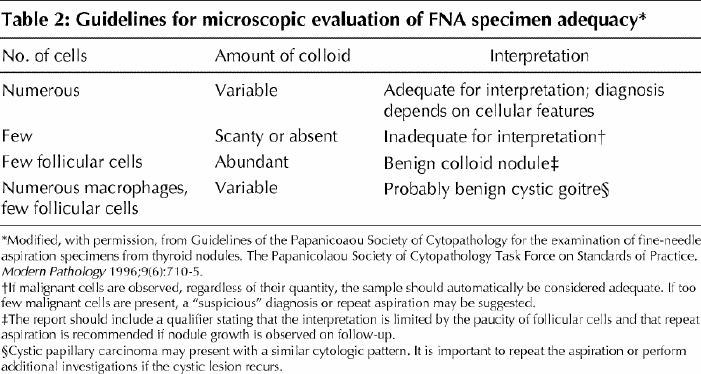
Many pathologists define an aspirate as adequate only if it contains a certain minimal number of follicular cell groups (typically from 6 to 10 groups).15,23,24 More recently, the Papanicolaou Society of Cytopathology (PSC) has published guidelines3 that do not specify a certain minimal number of follicular cells, but instead stress the importance of assessing the amount of colloid in determining specimen adequacy (Table 2).
Table 2
Benign thyroid nodules
The benign nodules include nodular goitre, macrofollicular adenoma and cystic goitre. No surgical treatment is required for any nodules in this group, so long as they do not grow.
In cystic nodules, follicular epithelial cells may be absent or too few for evaluation. The paucity of cells is due to the dilution effect of the fluid and does not reflect poor aspiration skill. At our institution we follow the PSC guidelines (Table 2) and do not classify these samples as inadequate. In the FNA report we say that “the cytologic findings are consistent with benign thyroid cyst; however, no follicular cells are present for assessment. If the cyst recurs or shows progressive growth on follow-up, a repeat aspiration or other investigation is advised.” Most thyroid cysts are benign, but a small number of papillary carcinomas are also cystic. About 50% to 70% of benign cysts do not recur after evacuation; in contrast, all cystic papillary carcinomas recur.
Hypercellular follicular nodules
In the early days of FNA, many pathologists equated smear hypercellularity with thyroid neoplasm and, as a result, many patients with benign hypercellular goitre were referred for surgery. More recently, the full spectrum of hypercellular follicular lesions has been recognized, and this spectrum can be arbitrarily divided into 3 subsets. This stratification has aided clinicians in determining the need for surgery in individual patients.
For the first subset, the diagnosis is “hypercellular follicular nodule consistent with hypercellular goitre.” The aspirates are characterized by hypercellularity, with uniform follicular cells in a regular honeycomb arrangement. The treatment of choice is nonsurgical, provided the nodule does not grow.
For the second subset, the diagnosis is “hypercellular goitre versus follicular neoplasm” or “follicular neoplasm, probably benign.” These specimens often show overlapping cytologic features of follicular neoplasm and goitre. If the pathologist is unsure as to whether the aspirate represents follicular neoplasm or goitre, more information is needed in order to offer the patient the best possible advice regarding treatment options. Some medical centres recommend surgical excision in such cases, for fear of missing a malignant lesion. Over the years, many workers have reported that the size of a follicular neoplasm is a useful clinical predictor of malignancy.17,18,19,20,21,25 For nonpapillary follicular nodules with diameter 2.5 cm or less and after follicular variant of papillary carcinoma has been excluded to the satisfaction of the pathologist, the policy at our institution is to recommend conservative management with follow-up on nodule size. For nodules with diameter greater than 2.5 cm, we generally recommend surgical removal, unless the nodule is shown to be “hot” on scanning with iodine-123.
For the third subset, the diagnosis is “follicular neoplasm not otherwise specified” or “follicular neoplasm, probably malignant.” These aspirates show hypercellularity and many disorganized, atypical microfollicles. Surgical excision is recommended, regardless of nodule size.
Malignant, specify histologic type whenever feasible
Tumours that are diagnosed as malignant or suspicious are usually carcinomas of the papillary, poorly differentiated, medullary or anaplastic variety. An experienced pathologist will be able to diagnose most of these carcinomas without equivocation, but some less-than-perfect, alternative diagnoses rendered by less experienced pathologists, such as “neoplasm, probably carcinoma” or “neoplasm suspicious of carcinoma,” are also acceptable, provided there is a clear understanding that such diagnoses should lead to further investigation or surgical excision of the nodule.
Thyroiditis
In some cases, Hashimoto's thyroiditis presents as a nodular lesion, mimicking thyroid neoplasm. The pathologist must rule out lymphoma and papillary carcinoma, either of which can coexist with Hashimoto's thyroiditis.
Follow-up strategies
Thyroid nodules diagnosed as benign on the basis of adequate FNA specimens can be managed conservatively if they do not grow. The following circumstances necessitate repeat FNA: unsatisfactory or inadequate FNA sample, an enlarging nodule, a recurrent cyst or a clinically suspicious nodule for which the FNA findings are reported as benign.
Some workers recommend repeating FNA biopsy routinely during follow-up of all benign nodules to detect initial false-negative results. However, it must be kept in mind that the lower the false-negative rate on first biopsy, the less likely a false-negative diagnosis will be detected on re-aspiration. Therefore, routinely repeating FNA more than once in cases of nodules diagnosed as benign may result in a very low cancer yield and may be counterproductive (i.e., increase in false-positive results, increase in patient anxiety and decrease in cost-effectiveness).26 In these patients, attention to clinical findings (e.g., persistent growth of the nodule or pressure symptoms) is as useful as repeating the FNA.
Finally, the clinician following the patient must be knowledgeable about the biologic behaviour of various neoplastic and non-neoplastic diseases of the thyroid. He or she must also be fully aware of the utility and limitations of thyroid FNA biopsy.
Acknowledgments
This article is dedicated to the memory of the late Hamish W. McIntosh, formerly Professor of Medicine and Head of the Division of Endocrinology and Metabolism, University of British Columbia, Vancouver.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. Kenneth C. Suen, Department of Pathology, Vancouver General Hospital, 855 W 12th Ave., Vancouver BC V5Z 1M9; fax 604 875-4035; ksuen@vanhosp.bc.ca
References
- 1.Suen KC, Quenville N. Fine needle aspiration biopsy of the thyroid gland: a study of 304 cases. J Clin Pathol 1983;36:1036-45. [DOI] [PMC free article] [PubMed]
- 2.Suen KC. Limitations of aspiration cytology in the diagnosis of primary neoplasms [letter]. Acta Cytol 1985;9:488-9. [PubMed]
- 3.Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Mod Pathol 1996; 9(6):710-5. [PubMed]
- 4.Franklyn JA, Daykin J, Young J, Oates GD, Sheppard MC. Fine needle aspiration cytology in diffuse or multinodular goitre compared with solitary thyroid nodules. BMJ 1993;307:240. [DOI] [PMC free article] [PubMed]
- 5.Tollin SR, Mery GM, Jelveh N, Fallon EF, Mikhail M, Blumenfeld W, et al. The use of fine-needle aspiration biopsy under ultrasound guidance to assess the risk of malignancy in patients with a multinodular goiter. Thyroid 2000; 10:235-41. [DOI] [PubMed]
- 6.Joseph UA, Jhingran SG. Graves' disease and concurrent thyroid carcinoma. The importance of thyroid scintigraphy in Graves' disease. Clin Nucl Med 1995; 20:416-8. [DOI] [PubMed]
- 7.Pepper GM, Zwickler D, Rosen Y. Fine-needle aspiration biopsy of the thyroid nodule. Arch Intern Med 1989;149:594-6. [PubMed]
- 8.Cramer H. Fine-needle aspiration cytology of the thyroid. An appraisal. Cancer Cytopathol 2000;90:325-9. [PubMed]
- 9.Burch HB. Evaluation and management of the solid thyroid nodule. Endocrinol Metab Clin North Am 1995;24:663-704. [PubMed]
- 10.Dey P, Ray R. Comparison of fine needle sampling by capillary action and fine needle aspiration. Cytopathology 1993;4:299-303. [DOI] [PubMed]
- 11.Santos JE, Leiman G. Nonaspiration fine needle cytology. Application of a new technique to nodular thyroid disease. Acta Cytol 1988;32:353-6. [PubMed]
- 12.Yang GC, Liebeskind D, Messina AV. Ultrasound-guided fine-needle aspiration of the thyroid assessed by ultrafast Papanicolaou stain: data from 1135 biopsies with a two- to six-year follow-up. Thyroid 2001;11:581-9. [DOI] [PubMed]
- 13.Zajdela A. Cancer cytological diagnosis by fine needle sampling without aspiration. Cancer 1987;59:1201-5. [DOI] [PubMed]
- 14.Kini SR. Techniques of fine-needle aspiration biopsy. In: Guide to clinical aspiration cytology: thyroid. New York: Igaku-Shoin; 1987. p. 5-12.
- 15.Nguyen GK, Ginsberg J, Crockford PM. Fine-needle aspiration biopsy cytology of the thyroid. Its value and limitations in the diagnosis and management of solitary thyroid nodules. Pathol Annu 1991;26 Pt 1:63-91. [PubMed]
- 16.Abele JS, Miller TR. Fine-needle aspiration of the thyroid nodule: clinical application. In: Clarke OH, editor. Endocrine surgery of the thyroid and parathyroids. St. Louis: Mosby; 1985. p. 293-366.
- 17.Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration biopsy. Diagn Cytopathol 2002;26:41-4. [DOI] [PubMed]
- 18.Carrillo JF, Frias-Mendivil M, Ochoa-Carrillo FJ, Ibarra M. Accuracy of fine-needle aspiration biopsy of the thyroid combined with an evaluation of clinical and radiologic factors. Otolaryngol Head Neck Surg 2000;122:917-21. [DOI] [PubMed]
- 19.Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid 1998;8:377-83. [DOI] [PubMed]
- 20.Schlinkert RT, van Heerden JA, Goellner JR, Gharib H, Smith SL, Rosales RF, et al. Factors that predict malignant thyroid lesion when fine-needle aspiration is “suspicious for follicular neoplasm.” Mayo Clin Proc 1997;72:913-6. [DOI] [PubMed]
- 21.Hamburger JI. Diagnosis of thyroid nodules by fine needle biopsy: use and abuse. J Clin Endocrinol Metab 1994;79:335-9. [DOI] [PubMed]
- 22.Rosai J, Carcangiu ML, DeLellis RA. Tumors of the thyroid gland. Washington: Armed Forces Institute of Pathology; 1990. p. 96-100.
- 23.Goellner JR, Gharib H, Grant CS, Johnson DS. Fine needle aspiration of the thyroid, 1980 to 1986. Acta Cytol 1987;31:587-90. [PubMed]
- 24.Hamburger JI, Husain M. Semiquantitative criteria for fine-needle biopsy diagnosis: reduced false-negative diagnosis. Diagn Cytopathol 1988;4:14-7. [DOI] [PubMed]
- 25.Davis NL, Gordon M, Germann E, Robins RE, McGregor GI. Clinical parameters predictive of malignancy of thyroid follicular neoplasms. Am J Surg 1991; 161:567-9. [DOI] [PubMed]
- 26.Belfiore A, La Rosa GL. Fine-needle aspiration biopsy of the thyroid. Endocrinol Metab Clin North Am 2001;30:361-400. [DOI] [PubMed]


