Abstract
Eradication and elimination strategies for lymphatic filariasis (LF) primarily rely on multiple rounds of annual mass drug administration (MDA), but also may benefit from vector control interventions conducted by malaria vector control programs. We aim to examine the overlap in LF prevalence and malaria vector control to identify potential gaps in program coverage. We used previously published geospatial estimates of LF prevalence from the Institute for Health Metrics and Evaluation, as well as publicly available insecticide-treated net (ITN) access (proportion of the total population with access to ITNs) and use (proportion of the total population that slept under an ITN) estimates among the total population and malaria Plasmodium falciparum parasite rates (PfPR) from the Malaria Atlas Project (MAP). We aggregated the 5x5 km2 estimates of LF prevalence estimates and ITN estimates to the implementation unit (IU) level using fractional aggregation, for 33 LF and malaria-endemic locations in Africa, and then overlaid the IU-level aggregates. In this analysis, ITN coverage was low in areas where LF is common, with 51.7% (90/174) of high-LF-prevalence-IUs having both access and use estimates under 40%. Most (67.8%; 61/90) of these low-ITN-coverage, high-LF-prevalence locations were also categorized as high- or highest-prevalence for malaria by PfPR, suggesting suboptimal ITN coverage even in some malaria-co-endemic locations. Even in IUs with high LF prevalence but low malaria prevalence, almost half (48.2%; 39/81) had high levels of access to ITNs. When accounting for population, however, gaps in ITN access in such areas were evident: more individuals lived in high-LF, low-malaria IUs with low ITN access (8.68 million) than lived in high-LF, low-malaria IUs with high ITN access (6.76 million). These results suggest that relying on current malaria vector control programs alone may not provide sufficient ITN coverage for high LF prevalence areas. Opportunities for coordinated vector control programs in places where LF and malaria prevalence are high but ITN coverage is low – or additional ITN distribution in high-LF, low-malaria locations - should be explored to help achieve elimination goals.
Author summary
Lymphatic filariasis is a vector-borne disease that can cause significant disability. There is evidence that insecticide-treated nets used by malaria programs can contribute to lymphatic filariasis elimination, but current lymphatic filariasis programs primarily focus on mass drug administration. As funding for programs has stalled and interventions have become more costly, there is a greater interest and need for vector management to be better integrated across sectors and diseases, with WHO promoting integrated vector management specifically for countries co-endemic with LF and malaria. We sought to review the overlap in lymphatic filariasis prevalence and malaria insecticide-treated nets across endemic African countries to identify areas where net distribution can be enhanced. We used previously published, publicly available lymphatic filariasis prevalence and malaria insecticide-treated net coverage results from the Institute for Health Metrics and Evaluation and the Malaria Atlas Project, respectively. Areas with high lymphatic filariasis prevalence were largely found to have low insecticide-treated net coverage. There is a need for disease programs to work together to maximize effective tools and methods to help achieve elimination goals. The impact of insecticide-treated nets on lymphatic filariasis prevalence will be location-specific and depend on a variety of epidemiological and programmatic factors.
Introduction
Lymphatic filariasis (LF) is a vector-borne disease caused by the filarial nematodes Wuchereria bancrofti, Brugia malayi, and Brugia timori, and is primarily transmitted by Anopheles, Aedes, Culex, and Mansonia mosquito species, varying geographically [1]. LF can lead to permanent disability, including that related to lymphedema and hydrocele, and causes significant mental, social, and financial burden to those afflicted. Under the World Health Organization (WHO)–established Global Programme to Eliminate Lymphatic Filariasis (GPELF), many countries have made significant progress: 17 countries have entered post-validation surveillance (ongoing transmission monitoring following GPELF certification recognizing elimination of LF as a public health problem), 11 have reached post–mass drug administration (MDA) surveillance, and all but two remaining countries have delivered MDA in some capacity [1]. To build upon these gains, the neglected tropical disease (NTD) Roadmap 2021–2030, in alignment with the Sustainable Development Goals, aims to eliminate LF as a public health problem in 58 countries by 2030 [2,3]. In 34 of the countries in the WHO Africa Region and Sudan, LF is a threat to approximately 406 million people [1,4]. In 2019, LF was estimated to have a prevalence rate of 1,472.22 cases per 100,000 (1,024.05 - 2,194.37) and contribute 432,679.92 Disability-adjusted life years (255,366.1 – 729,720.21) for the African Union alone [5]. Within this region, the countries with the highest prevalence include Nigeria, Côte d’Ivoire, the Democratic Republic of the Congo, and Mozambique, which made up approximately 57.6% of the region’s prevalent cases in 2019 [5].
Eradication and elimination strategies in endemic African countries primarily rely on multiple rounds of MDA and may additionally benefit from malaria vector control programs, since Anopheles species are one of the vectors of LF [6]. Malaria vector control initiatives, particularly insecticide-treated net (ITN) programs, have increased in recent years and contributed to ongoing success in combating malaria, while increasing evidence suggests secondary impacts on other vector-borne diseases [7]. However, although LF and malaria are largely co-endemic, areas with persistently high LF prevalence may not always coincide with areas where malaria prevalence or vector control is high [8,9]. In 2011, WHO released a statement promoting integrated vector management specifically for countries co-endemic with LF and malaria [10]. This statement was followed by the Global Vector Control Response 2017–2030, which aims to reduce mortality and incidence due to vector-borne diseases by at least 75% and 60% respectively, while also preventing epidemics by increasing capacity, enhancing surveillance, and improving coordination and integrated action across diseases and programs [11]. As global funding stalls and the cost of implementing interventions increases [7], it is more important than ever for vector control to be integrated across sectors and disease programs. To enhance cross-disease vector control management, it is crucial to identify where current vector control programs could be expanded to have the most impact. Here we aim to provide one of the first examinations of the overlap in LF prevalence and malaria vector control across endemic Africa to identify potential gaps in program coverage.
Methods
We used previously published geospatial LF prevalence estimates from the Institute for Health Metrics and Evaluation (IHME) [12] as well as publicly available ITN access and use estimates among the total population, indoor residual spraying (IRS) estimates among the total population, and malaria Plasmodium falciparum parasite rates (PfPR) from the Malaria Atlas Project (MAP) [13]. Briefly, the LF estimates were created using Bayesian model-based geostatistics and time-series methods to generate spatially continuous estimates of global, all-age LF prevalence as measured by immunochromatographic test (ICT) in 2000–2018 [12]. The ITN estimates used a Bayesian mixed modeling framework, and the IRS estimates were generated by collating IRS deployment data from various sources and converting to a standard proportion of households sprayed within the administrative division [13–15]. For PfPR, a cartographic approach was taken for 36 high-burden countries, while a surveillance approach was taken for other Pf-endemic countries [16]. Further details regarding the methodology used to create each set of estimates can be found in their respective publications [12–16]. For the purpose of this analysis, we chose to compare the most recent year available for each dataset at the time of analysis to present the most recent comparisons possible: 2018 for LF prevalence, 2019 for malaria prevalence, and 2020 for the vector control datasets. Unlike ITN access and use, which changes very rapidly year-to-year, LF epidemiology and elimination happen on longer time scales [4]. As such areas with high LF prevalence in 2018 are likely to be the same as in 2020, and therefore the maps presented below give the most up-to-date view of this overlap that is currently possible with available results. For a more direct comparison, figures using data from only 2018 have been provided in S4-S10 Figs.
Using population estimates from WorldPop [17], we aggregated the 5x5 km2 estimates of LF prevalence, ITN use, ITN access, malaria prevalence, IRS use, and population to the Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN) administrative implementing units (IU) level using fractional aggregation, for 33 LF and malaria-endemic locations in Africa, and then overlaid the IU-level aggregates [18]. IUs represent the administrative units designated by a country to be used for intervention implementation [19]. While these are typically at the district level, there may be variation between countries depending on the structure and objectives of each country’s control program [19]. To account for partial coverage of the 5 km2 grid by the IU boundaries and water bodies, we used fractional aggregation, whereby grid cells overlapping multiple IUs were proportionally distributed using the fraction of the cell lying within each IU. Our analyses included a total of 5,195 IUs and 162,868 overlapping 5x5 km2 grids.
We use two ITN metrics in this analysis: access, or the proportion of people among the total population who have access to an ITN; and use, or the proportion of the total population that use an ITN. Following Bertozzi-Villa et al., 2021 [14] we refer to specific metrics, like access and use, by name, and use coverage to more generally refer to combinations of metrics. This analysis focused on the proportion of the total population that had access to ITNs and the proportion of the total population that slept under an ITN, as the use of IRS has largely declined since 2010 [7]. However, maps of LF prevalence and IRS use have been included in S1 and S2 Figs [13]. All maps were created using ESPEN IU shapefiles as the base layer, which are made freely available under the Creative Commons Attribution 4.0 International License (CC BY 4.0) for academic use [18].
In this analysis, we defined IUs having LF prevalence ≥5% as high, while those with a prevalence <5% were considered low. In the absence of well-established standard classification thresholds, we categorized ITN access, use, and malaria prevalence using the following definitions based on the IU-level distributions of these metrics: lowest (<20%); low (20- < 40%); high (40%- < 60%); highest (≥60%).
Results
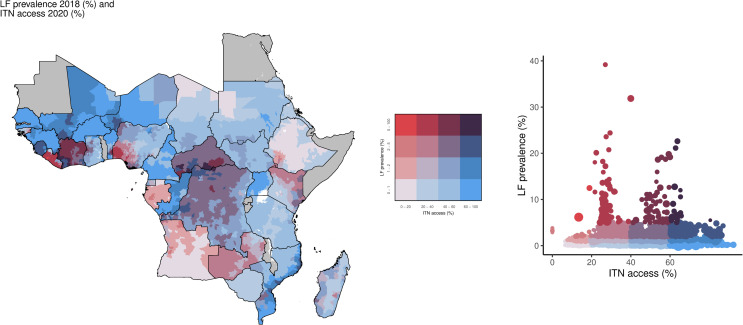
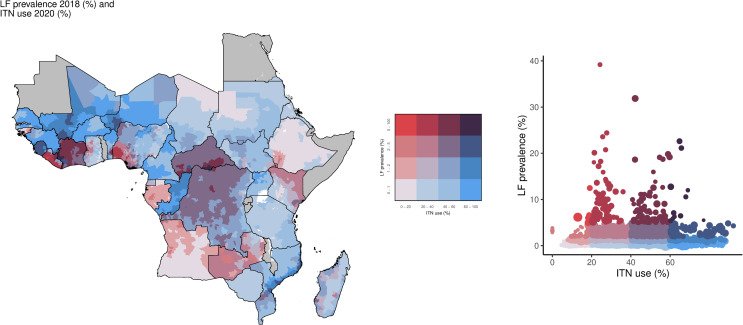
In this analysis, although only 3.4% (174/5,195) of IUs were categorized as having high LF prevalence (Table 1), a total of 38.2 million individuals lived in these locations, primarily located in Nigeria, Côte d’Ivoire, and Liberia. Of those living in these high prevalence areas, 21.9 million (57.3%) lived in IUs with low ITN access, and 1.66 million (4.3%) in IUs with the lowest ITN access, accounting for 51.7% (90/174) and 1.2% (2/174) IUs, respectively (Fig 1). In contrast, there were 10.6 million individuals (27.8%) living in 35.6% (62/174) of IUs with high ITN access and 13.5 million individuals (35.3%) in 42.0% (73/174) of IUs with high ITN use, while only 4.0 million (10.5%) lived in IUs (11.5%; 20/174) with the highest access and 1.8 million people (4.7%) in IUs (6.3%; 11/174) with the highest use (Fig 2).
Table 1. Table of number of IUs by LF prevalence, ITN access, and ITN use stratified by level of malaria PfPR. LF: Lymphatic filariasis; ITN: Insecticide-treated bednets; PfPR: Plasmodium falciparum parasite rate.
| LF prevalence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | All | ||||||||
| Lowest malaria PfPR | Low malaria PfPR | High malaria PfPR | Highest malaria PfPR | Lowest malaria PfPR | Low malaria PfPR | High malaria PfPR | Highest malaria PfPR | Total | ||
| ITN Access | Lowest | 889 | 115 | 19 | 0 | 0 | 2 | 0 | 0 | 1,025 |
| Low | 916 | 188 | 82 | 0 | 4 | 24 | 57 | 5 | 1,276 | |
| High | 890 | 695 | 236 | 30 | 6 | 33 | 17 | 6 | 1,913 | |
| Highest | 521 | 323 | 115 | 2 | 1 | 11 | 8 | 0 | 981 | |
| ITN Access Total | 3,216 | 1,321 | 452 | 32 | 11 | 70 | 82 | 11 | 5,195 | |
|
ITN
Use |
Lowest | 1,225 | 130 | 19 | 0 | 2 | 4 | 0 | 0 | 1,380 |
| Low | 811 | 294 | 104 | 1 | 2 | 21 | 56 | 5 | 1,294 | |
| High | 836 | 673 | 265 | 31 | 6 | 38 | 23 | 6 | 1,878 | |
| Highest | 344 | 224 | 64 | 0 | 1 | 7 | 3 | 0 | 643 | |
| ITN Use Total | 3,216 | 1,321 | 452 | 32 | 11 | 70 | 82 | 11 | 5,195 | |
Fig 1. Overlay map of LF prevalence (counts; 2018) and ITN access among the total population (%; 2020).
The bivariate choropleth map and scatter plot color key in the center show the degree to which LF prevalence (vertical axis, white to red) and ITN access (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; ITN: insecticide-treated net. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
Fig 2. Overlay map of LF prevalence (counts; 2018) and ITN use among the total population (%; 2020).
The bivariate choropleth map and scatter plot color key indicate the degree to which LF prevalence (vertical axis, white to red) and ITN use (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; ITN: insecticide-treated net. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
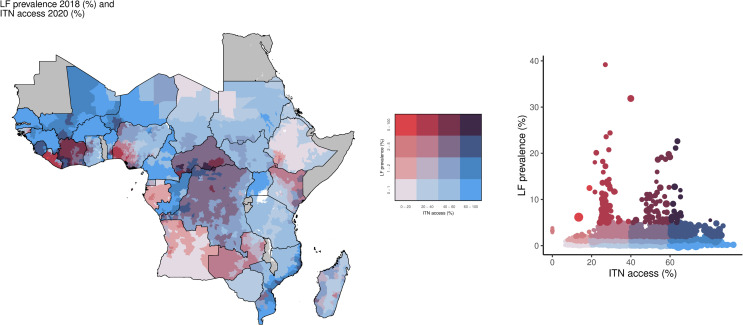
Just under half of the high LF prevalence locations had at least 40% ITN access (47.1%; 82/174 of IUs) and use (48.3%; 84/174) (Figs 3 and 4). For high LF prevalence areas, 51.7% (90/174) had both ITN access and use in the low or lowest categories. Geographically, areas with high LF prevalence and low ITN use were concentrated in Liberia, Zambia, Kenya, Angola, and Nigeria, whereas large parts of the Central African Republic, Democratic Republic of the Congo, Côte d’Ivoire, Mali, and Sierra Leone had high LF prevalence and high ITN use (Fig 4).
Fig 3. Overlay map and scatter plot of LF prevalence by (%; 2018) and ITN access among the total population (%; 2020).
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and ITN access (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; ITN: insecticide-treated net. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
Fig 4. Overlay map of LF prevalence (%; 2018) and ITN use among the total population (%; 2020).
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and ITN use (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; ITN: insecticide-treated net. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
Approximately 53.5% (93/174) of high LF prevalence areas had high or highest prevalence of malaria, representing 18.4 million (48.2%) individuals. Of these areas, 33.3% (31/93) of IUs had both ITN access and use ≥ 40%, of which only 9.7% (3/31) had the highest levels of both access and use. IUs with high prevalence of both LF and malaria most commonly had low access (66.7% [62/93] of IUs, containing 13.3 million [72.3%] people) and low use (65.6% [61/93] of IUs, containing 12.7 million [69.0%] people). None of the areas with high prevalence of both LF and malaria had both access and use in the lowest categories, however.
Of the IUs with high LF prevalence but low malaria prevalence, almost half had high ITN access (48.2%; 39/81) and over half had high ITN use (54.3%; 44/81) representing 6.76 million and 8.08 million individuals respectively. Even though only around one third of these IUs (34.6%; 28/81) had low ITN access, there were more people living in these IUs (8.68 million) than in those with high ITN access (6.76 million). These low access IUs were primarily located in Nigeria.
Of the 903 million individuals living in the remaining 96.7% (5,021/5,195) of IUs with low LF prevalence, 200 million (22.2%) lived in IUs with low ITN access (23.6% of IUs; 1,186/5,021) and 114 million (12.6%) in IUs with the lowest ITN access (20.4%; 1,023/5,021). There were 509 million (56.4%) individuals living in IUs with low LF prevalence but with ITN access ≥40% (56.0% of IUs; 2,812/5,021), compared to 124 million people (13.7%) living in IUs with the highest ITN access (19.1%; 961/5,021 IUs). Over half of these low LF prevalence areas had ITN use < 40% (51.5%; 2,584/5,021). Of these, there were fewer areas with low ITN use (24.1%; 1,210/5,021) than the lowest use (27.4%; 1,374/5,021) (Fig 4).
Among all low-LF-prevalence areas, 9.6% (484/5,021) were high-or-highest-prevalence for malaria, accounting for 84.6 million (9.4%) individuals (S3 Fig). Within these, 18.4 million (21.8%) individuals lived in IUs with low ITN use (21.7%; 105/484 IUs) and 14.0 million individuals in IUs with low ITN access (16.9%; 82/484 IUs). Only 3.9% (19/484) of these low-LF prevalence, but high-or-highest malaria prevalence IUs, containing 1.6 million people, had the lowest ITN access and use. In comparison, 50.0% (242/484) of these IUs had high ITN access and use, and 13.2% (64/484) the highest.
Discussion
This analysis found that most individuals living in high-LF-prevalence areas live in places with low ITN coverage, with the majority falling in the low coverage range. Conversely, the majority of individuals living in areas of low LF prevalence had high ITN coverage. Over half of the areas with high LF prevalence also had high malaria prevalence, but only around one third of these areas had high ITN coverage. For the high LF prevalence areas with low malaria prevalence, despite almost half of IUs having high ITN access, more individuals lived in IUs with low ITN access, suggesting that relying on current malaria vector control programs may not be sufficient for some high LF prevalence areas.
Previous studies evaluating the intersection of LF prevalence, ITN coverage, and malaria prevalence have primarily been limited to a subset of endemic African countries [20–24]. Several of the findings from this analysis, such as ITN coverage being generally low in areas where LF prevalence was high and partial overlap of high LF and malaria prevalence areas, echo the results of these other studies [21,23]. There are likely several factors contributing to the low coverage of ITNs seen in high-LF areas in this analysis. ITN access has been closely linked to development assistance for health funding (DAH), with global organizations playing an important role in deciding resource and program priorities between and within countries [25,26]. Funding for ITNs from organizations such as the Global Fund to Fight AIDS, Tuberculosis and Malaria focuses primarily on high malaria burden areas rather than on areas with high LF prevalence, which likely contributes to some of the low ITN coverage seen for these locations in this analysis [27–29]. Furthermore, many of the countries that initially benefitted from DAH support, much of which was from the Global Fund, tended to be from the same region, such as Eastern Africa [30,31], including some areas where LF prevalence was considered relatively low or non-endemic due to the historical use of dichlorodiphenyltrichloroethane (DDT) spraying against human African trypanosomiasis (HAT) [32]. Importantly, our analysis identified areas with both high malaria prevalence and high LF prevalence but low ITN coverage, suggesting an opportunity for coordinated vector control activities between programs.
In countries affected by war and civil unrest, disruptions to health services and support for ongoing disease programs are likely to further contribute to the observed ITN coverage patterns [33]. Countries also differ in their utilization of ITNs as well as MDA depending on political commitments, competing priorities, and health system structures. In the past, some malaria ITN programs primarily distributed ITNs via antenatal clinics and Expanded Programme for Immunization (EPI) visits, with pregnant persons and children serving as the main target populations due to high health burdens in these groups [34]. For LF, these strategies may have been suboptimal, given that preventing infection across the lifespan is of particular importance to prevent the disabling sequelae of chronic and repeated parasite exposure [35,36]. Although antenatal and EPI visits still play an important role in some countries for continuous ITN distribution [37], since 2007 the WHO has recommended a shift in distribution strategy towards universal coverage [10,37], and more recently towards the subnational tailoring of interventions [38]. In alignment with these recommendations, collaboration to increase the access and use of nets in the highest priority areas could be considered to extend benefits to LF control programs.
The partial overlap seen in this analysis of areas with high LF prevalence and high malaria prevalence could indicate that LF programs may need to consider alternative ITN distribution methods or special net programs for high-LF but low-malaria locations with low ITN coverage, such as some of those outlined in the WHO’s document on scaling up ITNs [34]. Despite ITN use among those with nets usually being high [14,39], there are known factors contributing to ongoing ITN non-use, including decreased risk perception during dry seasons for areas where malaria is seasonal [30]. Seasonal trends in LF and malaria may be similar in settings where the same Anopheles vectors account for most of the transmission for both diseases [6,8], but due to the chronic nature of LF, these extended periods of ITN non-use could be particularly harmful for those living in LF-endemic areas [4].
While vector control is not currently required for validation of EPHP, there is evidence that it helps to greatly reduce LF prevalence in some settings and could accelerate elimination and eradication programs [8,22,40–46]. A study in Papua New Guinea found that the introduction of ITNs directly led to a decrease in the annual infective biting rate [42], and in The Gambia, LF elimination was reached in the absence of MDA while scaling up ITNs [47]. However, as LF can be transmitted by multiple vector species, the impact of ITNs on LF prevalence is likely to vary depending on the predominant vector species and their habits, such as whether the species tends to feed outdoors (exophagic) or indoors (endophagic) and when peak biting times occur [8,45,48,49]. Encouragingly, a study conducted in an area of Southeastern Nigeria endemic for both malaria and LF that had not undergone MDA due to co-endemicity with Loa loa showed that, even in the presence of multiple LF vectors and high transmission, LF transmission could be halted with the use of ITNs alone – though coverage of 1 net per 2 people in each household was required to do so [46]. Vector control programs also have the potential to drive vector behavior modification which may lead to decreased ITN impact over time [50–52]. Furthermore, location differences in MDA coverage, ITN implementation, IRS use, and other factors will also affect ITN impact.
In Africa, Anopheles are the most widespread species, whereas Culex and Mansonia are more localized in east Africa and west Africa, respectively [53–55]. Elimination of LF by MDA alone may be more likely in areas where Anopheles are the primary vector than for other species [6,48,55], but others have argued that adding vector control to MDA in Anopheles-dominant areas would be advantageous [20,45,56,57], and WHO specifically recommends the use of ITN in areas where Anopheles is the primary vector for LF [4]. This is even more important in urban areas where it is costly and challenging to implement MDA [58], as well as areas that are co-endemic with Loa loa where the combination drug approach of MDA drugs for LF (in Africa, albendazole with either ivermectin or diethylcarbamazine), is not recommended, and instead a combination of albendazole-only MDA and vector control is preferred [1].
Differential insecticide resistance patterns should also be considered in areas where the primary vectors are Anopheles or Culex [59–61] but should not deter the use of ITNs [49,62–65]. Importantly, with the increased concern regarding the spread of the urban-dwelling Anopheles stephensi [66], malaria vector control programs may begin shifting from their historically rural focus to include more urban areas, which could increase the potential for overlap between malaria vector control efforts and LF-prevalent locations. Furthermore, Anopheles stephensi has been found to coexist with others such as Aedes and Culex [66–69], presenting an opportunity for broader vector control collaboration to combat not only LF and malaria, but also other mosquito-borne diseases such as dengue.
This analysis carries some limitations. We chose to compare the most recent available geospatial estimates for LF prevalence, ITN coverage, and malaria prevalence, in order to produce the most up-to-date comparisons possible at the time of analysis. As LF results were only available through the year 2018, however, these results do not fully reflect any recent changes in the current spread and level of LF prevalence. Furthermore, both LF and malaria estimates may be subject to accuracy limitations where data is sparse. As mentioned, this analysis used malaria ITN estimates among the total population and does not account for any ITN distribution that may have happened outside of malaria vector control initiatives. An analysis of ITN coverage and LF prevalence over time was outside the scope of this paper, though has been examined in previous country-specific analyses [20].
To the authors’ knowledge this is one of the first papers looking at the overlap of LF and ITNs across endemic Africa. These results illustrate the degree to which malaria control programs have achieved access to and use of ITNs in LF-endemic areas. Where the predominant vector species distributions and the context of MDA and other control efforts suggest a role for ITNs in LF control and elimination, these results help to identify locations where additional ITN coverage may be of the most benefit for both diseases. In high-LF, low-malaria locations with low ITN coverage, LF-driven programs to enhance ITN coverage may be needed. Spatial analyses like these can be combined with other context-specific knowledge to help inform future elimination and control strategies.
Supporting information
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and IRS use (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; IRS: indoor residual spraying. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and IRS use (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; IRS: indoor residual spraying. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and malaria Pf prevalence (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; Pf: Plasmodium falciparum. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and ITN access (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; ITN: insecticide-treated nets. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and ITN access (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; ITN: insecticide-treated nets. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and ITN use (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; ITN: insecticide-treated nets. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and ITN use (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; ITN: insecticide-treated nets. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and IRS use (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; IRS: indoor residual spraying. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and IRS use (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; IRS: indoor residual spraying. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
The bivariate choropleth map and scatter plot color key in the center indicate the degree to which LF prevalence (vertical axis, white to red) and malaria Pf prevalence (horizontal axis, white to blue) overlap. Grey indicates areas considered to be non-endemic. LF: lymphatic filariasis; Pf: Plasmodium falciparum. Map base layer shapefile is from ESPEN, available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [18].
(PDF)
(XLSX)
(CSV)
Data Availability
The results dataset has been included in the Supporting Information (S1 Data and S2 Data). The code is publicly available via GIT repository (https://github.com/ihmeuw/lf-malaria-overlap). All input estimates used to produce the results dataset are publicly available as indicted in their respective cited publications and at the following URLs: Lymphatic filariasis prevalence https://doi.org/10.1016/S2214-109X(20)30286-2 https://vizhub.healthdata.org/lbd/lf Insecticide-treated net access https://doi.org/10.1038/s41467-021-23707-7 https://data.malariaatlas.org/maps?layers=Interventions:202106_Africa_Insecticide_Treated_Net_Access Insecticide-treated net use https://doi.org/10.1038/s41467-021-23707-7 https://data.malariaatlas.org/maps?layers=Interventions:202106_Africa_Insecticide_Treated_Net_Use Indoor Residual Spraying https://doi.org/10.1186/s12936-020-03216-6 https://data.malariaatlas.org/maps?layers=Interventions:202106_Africa_IRS_Coverage Malaria PfPR prevalence https://doi.org/10.1016/S0140-6736(19)31097-9 https://data.malariaatlas.org/maps?layers=Malaria:202206_Global_Pf_Parasite_Rate Population estimates https://doi.org/10.1080/20964471.2019.1625151 https://hub.worldpop.org/project/categories?id=3 Shapefile base layer https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database.
Funding Statement
EC, TG, CK, JBK, AS, JFM, and JW report support for the present manuscript from the Bill and Melinda Gates Foundation, worktag GR024212. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2021 [Internet]. WHO; 2022. [cited 2023 Sep 14]. Available from: https://www.who.int/publications/i/item/who-wer9741-513-524 [Google Scholar]
- 2.World Health Organization. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030 [Internet]. WHO; 2021. [cited 2023 Sep 14]. Available from: https://www.who.int/publications/i/item/9789240010352 [Google Scholar]
- 3.United Nations Department of Economic and Social Affairs. The Sustainable Development Goals Report 2023: Special Edition [Internet]. United Nations; 2023. Jul [cited 2023 Sep 18]. Available from: https://www.un-ilibrary.org/content/books/9789210024914 [Google Scholar]
- 4.World Health Organization. Lymphatic filariasis. [cited 2023 September 18]. Available from: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis [Google Scholar]
- 5.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza DK, Koudou B, Kelly-Hope LA, Wilson MD, Bockarie MJ, Boakye DA. Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit Vectors. 2012;5:259. doi: 10.1186/1756-3305-5-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. World malaria report 2022 [Internet]. WHO; 2022. [cited 2023 Sep 14]. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 [Google Scholar]
- 8.van den Berg H, Kelly-Hope LA, Lindsay SW. Malaria and lymphatic filariasis: the case for integrated vector management. Lancet Infect Dis. 2013;13(1):89–94. doi: 10.1016/S1473-3099(12)70148-2 [DOI] [PubMed] [Google Scholar]
- 9.Manguin S, Bangs MJ, Pothikasikorn J, Chareonviriyaphap T. Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infect Genet Evol. 2010;10(2):159–77. doi: 10.1016/j.meegid.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO position statement on integrated vector management to control malaria and lymphatic filariasis. WHO. 2011. Available from: https://www.who.int/publications-detail-redirect/who-wer8613-121-127 [PubMed] [Google Scholar]
- 11.World Health Organization. Global vector control response 2017–2030. WHO. 2017. Available from: https://www.who.int/publications/i/item/9789241512978 [Google Scholar]
- 12.Cromwell EA, Schmidt CA, Kwong KT, Pigott DM, Mupfasoni D, Biswas G. The global distribution of lymphatic filariasis, 2000–18: a geospatial analysis. Lancet Glob Health. 2020;8(9):e1186-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaria Atlas Project. Malaria Atlas Project | Data. [cited 2023 September 14]. Available from: https://data.malariaatlas.org/maps?layers=Malaria:202406_Global_Pf_Parasite_Rate [Google Scholar]
- 14.Bertozzi-Villa A, Bever CA, Koenker H, Weiss DJ, Vargas-Ruiz C, Nandi AK, et al. Maps and metrics of insecticide-treated net access, use, and nets-per-capita in Africa from 2000-2020. Nat Commun. 2021;12(1):3589. doi: 10.1038/s41467-021-23707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangena J-AA, Hendriks CMJ, Devine M, Tammaro M, Trett AE, Williams I, et al. Indoor residual spraying for malaria control in sub-Saharan Africa 1997 to 2017: an adjusted retrospective analysis. Malar J. 2020;19(1):150. doi: 10.1186/s12936-020-03216-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss DJ, Lucas TCD, Nguyen M, Nandi AK, Bisanzio D, Battle KE, et al. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000–17: a spatial and temporal modelling study. Lancet Lond Engl. 2019;394(10195):322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd CT, Chamberlain H, Kerr D, Yetman G, Pistolesi L, Stevens FR, et al. Global spatio-temporally harmonised datasets for producing high-resolution gridded population distribution datasets. Big Earth Data. 2019;3(2):108–39. doi: 10.1080/20964471.2019.1625151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Expanded Special Project for Elimination of Neglected Tropical Diseases. Cartography database | ESPEN [Internet]. [cited 2023 Sep 14]. Available from: https://espen.afro.who.int/tools-resources/data-query-tools/cartography-database [Google Scholar]
- 19.WHO & GPELF. Monitoring and epidemiological assessment of the programme to eliminate lymphatic filariasis at implementation unit level [Internet]. WHO; 2005. [cited 2025 Jan 27]. Report No.: WHO/CDS/CPE/CEE/2005.50. Available from: https://iris.who.int/handle/10665/69172 [Google Scholar]
- 20.Nsakashalo-Senkwe M, Mwase E, Chizema-Kawesha E, Mukonka V, Songolo P, Masaninga F. Significant decline in lymphatic filariasis associated with nationwide scale-up of insecticide-treated nets in Zambia. Parasite Epidemiol Control. 2017;2(4):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eneanya OA, Reimer LJ, Fischer PU, Weil GJ. Geospatial modelling of lymphatic filariasis and malaria co-endemicity in Nigeria. Int Health. 2023;15(5):566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanton MC, Mkwanda S, Mzilahowa T, Bockarie MJ, Kelly-Hope LA. Quantifying filariasis and malaria control activities in relation to lymphatic filariasis elimination: a multiple intervention score map (MISM) for Malawi. Trop Med Int Health. 2014;19(2):224–35. doi: 10.1111/tmi.12266 [DOI] [PubMed] [Google Scholar]
- 23.Okorie PN, Ademowo GO, Saka Y, Davies E, Okoronkwo C, Bockarie MJ, et al. Lymphatic filariasis in Nigeria; micro-stratification overlap mapping (MOM) as a prerequisite for cost-effective resource utilization in control and surveillance. PLoS Negl Trop Dis. 2013;7(9):e2416. doi: 10.1371/journal.pntd.0002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly-Hope LA, Thomas BC, Bockarie MJ, Molyneux DH. Lymphatic filariasis in the Democratic Republic of Congo; micro-stratification overlap mapping (MOM) as a prerequisite for control and surveillance. Parasit Vectors. 2011;4:178. doi: 10.1186/1756-3305-4-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flaxman AD, Fullman N, Otten MW Jr, Menon M, Cibulskis RE, Ng M, et al. Rapid scaling up of insecticide-treated bed net coverage in Africa and its relationship with development assistance for health: a systematic synthesis of supply, distribution, and household survey data. PLoS Med. 2010;7(8):e1000328. doi: 10.1371/journal.pmed.1000328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snow RW, Guerra CA, Mutheu JJ, Hay SI. International Funding for Malaria Control in Relation to Populations at Risk of Stable Plasmodium falciparum Transmission. PLoS Med. 2008. Jul;5(7):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang XX, Toure H, Biswas G. Resource tracking for neglected tropical disease programmes: the first step for developing a sustainable financing strategy. Trans R Soc Trop Med Hyg. 2021;115(2):179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute for Health Metrics and Evaluation (IHME). Financing Global Health 2021: Global Health Priorities in a Time of Change [Internet]. Seattle, WA: IHME; 2023. [cited 2023 Nov 29]. Available from: https://www.healthdata.org/research-analysis/library/financing-global-health-2021-global-health-priorities-time-change [Google Scholar]
- 29.Neglected Tropical Diseases Program. NTD Donor Landscape 2017 [Internet]. USAID; 2020. [cited 2023 Dec 4]. Available from: https://www.neglecteddiseases.gov/ntd-donor-landscape-2017/. [Google Scholar]
- 30.Korenromp EL, Hosseini M, Newman RD, Cibulskis RE. Progress towards malaria control targets in relation to national malaria programme funding. Malar J. 2013;12:18. doi: 10.1186/1475-2875-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IHME. Institute for Health Metrics and Evaluation. [cited 2023 Dec 5]. Financing Global Health. Available from: http://vizhub.healthdata.org/fgh/. [Google Scholar]
- 32.Bockarie MJ, Rebollo MP. Reducing the population requiring interventions against lymphatic filariasis in Africa. Lancet Glob Health. 2016;4(3):e154-5. doi: 10.1016/S2214-109X(15)00292-2 [DOI] [PubMed] [Google Scholar]
- 33.Goniewicz K, Burkle FM, Horne S, Borowska-Stefańska M, Wiśniewski S, Khorram-Manesh A. The Influence of War and Conflict on Infectious Disease: A Rapid Review of Historical Lessons We Have Yet to Learn. Sustainability. 2021;13(19):10783. doi: 10.3390/su131910783 [DOI] [Google Scholar]
- 34.World Health Organization. Scaling up insecticide-treated netting programmes in Africa [Internet]. WHO; 2005. [cited 2023 Nov 29]. Available from: https://www.who.int/publications/m/item/scaling-up-insecticide-treated-netting-programmes-in-africa [Google Scholar]
- 35.Chesnais CB, Awaca-Uvon N-P, Vlaminck J, Tambwe J-P, Weil GJ, Pion SD, et al. Risk factors for lymphatic filariasis in two villages of the Democratic Republic of the Congo. Parasit Vectors. 2019;12(1):162. doi: 10.1186/s13071-019-3428-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesnais CB, Missamou F, Pion SD, Bopda J, Louya F, Majewski AC, et al. A case study of risk factors for lymphatic filariasis in the Republic of Congo. Parasit Vectors. 2014;7:300. doi: 10.1186/1756-3305-7-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. WHO guidelines for malaria [Internet].2024. Nov [cited 2025 Mar 26]. Available from: https://www.who.int/publications/i/item/guidelines-for-malaria [Google Scholar]
- 38.World Health Organization. Malaria Policy Advisory Committee meeting report (October 2019) [Internet]. 2019. Oct [cited 2025 Mar 26]. Available from: https://www.who.int/publications/i/item/WHO-CDS-GMP-2019.12 [Google Scholar]
- 39.Koenker H, Kumoji EK, Erskine M, Opoku R, Sternberg E, Taylor C. Reported reasons for non-use of insecticide-treated nets in large national household surveys, 2009–2021. Malar J. 2023;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis EL, Prada J, Reimer LJ, Hollingsworth TD. Modelling the impact of vector control on lymphatic filariasis programs: current approaches and limitations. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;72(Suppl 3):S152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly-Hope LA, Molyneux DH, Bockarie MJ. Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; capturing a window of opportunity?. Parasite Vectors. 2013;6(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reimer LJ, Thomsen EK, Tisch DJ, Henry-Halldin CN, Zimmerman PA, Baea ME, et al. Insecticidal bed nets and filariasis transmission in Papua New Guinea. N Engl J Med. 2013;369(8):745–53. doi: 10.1056/NEJMoa1207594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens ER, Aldridge A, Degbey Y, Pignandi A, Dorkenoo MA, Hugelen-Padin J. Evaluation of the 2011 long-lasting, insecticide-treated net distribution for universal coverage in Togo. Malar J. 2013;12:162. doi: 10.1186/1475-2875-12-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moraga P, Cano J, Baggaley RF, Gyapong JO, Njenga SM, Nikolay B, et al. Modelling the distribution and transmission intensity of lymphatic filariasis in sub-Saharan Africa prior to scaling up interventions: integrated use of geostatistical and mathematical modelling. Parasit Vectors. 2015;8:560. doi: 10.1186/s13071-015-1166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bockarie MJ, Pedersen EM, White GB, Michael E. Role of vector control in the global program to eliminate lymphatic filariasis. Annu Rev Entomol. 2009;54:469–87. doi: 10.1146/annurev.ento.54.110807.090626 [DOI] [PubMed] [Google Scholar]
- 46.Richards FO, Emukah E, Graves PM, Nkwocha O, Nwankwo L, Rakers L. Community-wide distribution of long-lasting insecticidal nets can halt transmission of lymphatic filariasis in southeastern Nigeria. Am J Trop Med Hyg. 2013;89(3):578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rebollo MP, Sambou SM, Thomas B, Biritwum N-K, Jaye MC, Kelly-Hope L, et al. Elimination of lymphatic filariasis in the Gambia. PLoS Negl Trop Dis. 2015;9(3):e0003642. doi: 10.1371/journal.pntd.0003642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duerr HP, Dietz K, Eichner M. Determinants of the eradicability of filarial infections: a conceptual approach. Trends Parasitol. 2005. Feb;21(2):88–96. [DOI] [PubMed] [Google Scholar]
- 49.Lindsay SW, Thomas MB, Kleinschmidt I. Threats to the effectiveness of insecticide-treated bednets for malaria control: thinking beyond insecticide resistance. Lancet Glob Health. 2021;9(9):e1325–31. doi: 10.1016/S2214-109X(21)00216-3 [DOI] [PubMed] [Google Scholar]
- 50.Kreppel KS, Viana M, Main BJ, Johnson PCD, Govella NJ, Lee Y. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci Rep. 2020;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sokhna C, Ndiath MO, Rogier C. The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria. Clin Microbiol Infect. 2013;19(10):902–7. doi: 10.1111/1469-0691.12314 [DOI] [PubMed] [Google Scholar]
- 52.Sanou A, Nelli L, Guelbéogo WM, Cissé F, Tapsoba M, Ouédraogo P, et al. Insecticide resistance and behavioural adaptation as a response to long-lasting insecticidal net deployment in malaria vectors in the Cascades region of Burkina Faso. Sci Rep. 2021;11(1):17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brengues J, Bain O. Passage des microfilaires de l’estomac vers l’hémocèle du vecteur, dans les couples Wuchereria bancrofti - Anopheles gambiae A, W. bancrofti - Aedes aegypti et Setaria labiatopapillosa - A. aegypti. In 1972. [cited 2023 Nov 29]. Available from: https://www.semanticscholar.org/paper/Passage-des-microfilaires-de-l’estomac-vers-du-dans-Brengues-Bain/d80e1f649c998a607ce30081aedbf97898ec96c6 [Google Scholar]
- 54.Subra R. Biology and control of Culex pipiens quinquefasciatus Say, 1823 (Diptera, Culicidae) with special reference to Africa. Int J Trop Insect Sci. 1981;1(4):319–38. [Google Scholar]
- 55.Ughasi J, Bekard HE, Coulibaly M, Adabie-Gomez D, Gyapong J, Appawu M, et al. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasit Vectors. 2012;5:89. doi: 10.1186/1756-3305-5-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amuzu H, Wilson MD, Boakye DA. Studies of Anopheles gambiae s.l (Diptera: Culicidae) exhibiting different vectorial capacities in lymphatic filariasis transmission in the Gomoa district, Ghana. Parasit Vectors. 2010;3:85. doi: 10.1186/1756-3305-3-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zagaria N, Savioli L. Elimination of lymphatic filariasis: a public-health challenge. Ann Trop Med Parasitol. 2002;96 Suppl 2:S3-13. doi: 10.1179/00034980215002347 [DOI] [PubMed] [Google Scholar]
- 58.Koudou BG, de Souza DK, Biritwum N-K, Bougma R, Aboulaye M, Elhassan E, et al. Elimination of lymphatic filariasis in west African urban areas: is implementation of mass drug administration necessary?. Lancet Infect Dis. 2018;18(6):e214–20. doi: 10.1016/S1473-3099(18)30069-0 [DOI] [PubMed] [Google Scholar]
- 59.Matowo NS, Abbasi S, Munhenga G, Tanner M, Mapua SA, Oullo D, et al. Fine-scale spatial and temporal variations in insecticide resistance in Culex pipiens complex mosquitoes in rural south-eastern Tanzania. Parasit Vectors. 2019;12(1):413. doi: 10.1186/s13071-019-3676-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rai P, Bharati M, Subba A, Saha D. Insecticide resistance mapping in the vector of lymphatic filariasis, Culex quinquefasciatus Say from northern region of West Bengal, India. PLoS One. 2019;14(5):e0217706. doi: 10.1371/journal.pone.0217706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bradley J, Ogouyèmi-Hounto A, Cornélie S, Fassinou J, de Tove YSS, Adéothy AA, et al. Insecticide-treated nets provide protection against malaria to children in an area of insecticide resistance in Southern Benin. Malar J. 2017;16(1):225. doi: 10.1186/s12936-017-1873-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henry MC, Assi S, Rogier C, Dossou-Yovo J, Chandre F, Guillet P. Protective efficacy of lambda-cyhalothrin treated nets in Anopheles gambiae pyrethroid resistance areas of Côte d’Ivoire. ResearchGate. 2005;73(5):859–64. [PubMed] [Google Scholar]
- 63.Lindblade KA, Mwandama D, Mzilahowa T, Steinhardt L, Gimnig J, Shah M. A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance, Malawi. Malar J. 2015;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okoyo C, Mwandawiro C, Kihara J, Simiyu E, Gitonga CW, Noor AM, et al. Comparing insecticide-treated bed net use to Plasmodium falciparum infection among schoolchildren living near Lake Victoria, Kenya. Malar J. 2015;14:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uttah E, Wokem GN, Okonofua C. The abundance and biting patterns of Culex quinquefasciatus Say (Culicidae) in the coastal region of Nigeria. ResearchGate. 2013;2013(5):1–7. [Google Scholar]
- 66.Al-Eryani SM, Irish SR, Carter TE, Lenhart A, Aljasari A, Montoya LF. Public health impact of the spread of Anopheles stephensi in the WHO Eastern Mediterranean Region countries in Horn of Africa and Yemen: need for integrated vector surveillance and control. Malar J. 2023;22(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chakraborty S, Ray S, Tandon N. Seasonal prevalence of Anopheles stephensi larvae and existence of two forms of the species in an urban garden in Calcutta City. Indian J Malariol. 1998;35(1):8–14. [PubMed] [Google Scholar]
- 68.Mariappan T, Thenmozhi V, Udayakumar P, Bhavaniumadevi V, Tyagi B. An observation on breeding behaviour of three different vector species (Aedes aegypti Linnaeus 1762, Anopheles stephensi Liston 1901 and Culex quinquefasciatus Say 1823) in wells in the coastal region of Ramanathapuram district, Tamil Nadu, India. Int J Mosq Res [Internet]. 2015. Jun 1 [cited 2023 Nov 29]; Available from: https://www.semanticscholar.org/paper/An-observation-on-breeding-behaviour-of-three-(-and-Mariappan-Thenmozhi/a4670bbbf5fd44745425859e313e8a6afb8ead5e [Google Scholar]
- 69.Weetman D, Kamgang B, Badolo A, Moyes CL, Shearer FM, Coulibaly M, et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int J Environ Res Public Health. 2018;15(2):220. doi: 10.3390/ijerph15020220 [DOI] [PMC free article] [PubMed] [Google Scholar]