Abstract
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial inflammation and joint destruction. Despite advances in biologic therapies targeting inflammatory mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, Janus kinase (JAK), and B cells, many patients do not respond adequately, emphasizing the need for deeper insights into RA pathogenesis. Research highlights the intricate interplay of genetic and epigenetic factors driving immune dysregulation. The breakdown of immune tolerance, often initiated in mucosal sites such as the gut, lung, and oral cavity, promotes the citrullination of antigens, leading to anti-citrullinated protein antibody production and subsequent immune activation. Single-cell and multi-omics approaches have shed light on underexplored immune cell types, such as T peripheral helper cells, CD4+/CD8+ cytotoxic T cells, and autoreactive B cells, broadening the understanding beyond traditionally studied Th17, Th1 cells, macrophages, and fibroblast-like synoviocytes. Future basic research in RA should prioritize elucidating the mechanisms behind peripheral tolerance breakdown, the pathogenesis of seronegative RA, and the molecular pathways driving refractory and recurrent disease. Moreover, leveraging multi-omics approaches to dissect disease heterogeneity will be pivotal for advancing personalized treatment strategies and improving long-term outcomes in RA patients.
Keywords: Rheumatoid arthritis, Anti-citrullinated protein antibody, Synovial inflammation, Immune dysregulation, Disease heterogeneity
INTRODUCTION
Rheumatoid arthritis (RA) affects approximately 0.5%~1.5% of the global population and imposes a significant burden on healthcare systems worldwide [1]. It is primarily characterized by synovitis, leading to joint destruction and systemic complications [2]. Although biologic therapies targeting key inflammatory pathways, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, Janus kinase (JAK), and B cells, have revolutionized RA treatment, there remains an unmet need to better understand the molecular and cellular mechanisms driving RA, particularly in patients who are unresponsive to current therapies [3].
Basic research into RA has significantly advanced our understanding of immune dysregulation, synovial biology, and the molecular pathways underlying chronic inflammation and joint damage [2]. However, RA pathogenesis is complex and multifactorial, involving genetic susceptibility, environmental triggers, epigenetic modifications, and various immune system abnormalities. A deeper exploration of RA pathogenesis is essential to address the unmet needs in its treatment. This paper explores the current state of basic research in RA and discusses promising directions for future investigation.
MAIN SUBJECTS
Current state of basic research in RA
1) Genetic and epigenetic regulation of RA
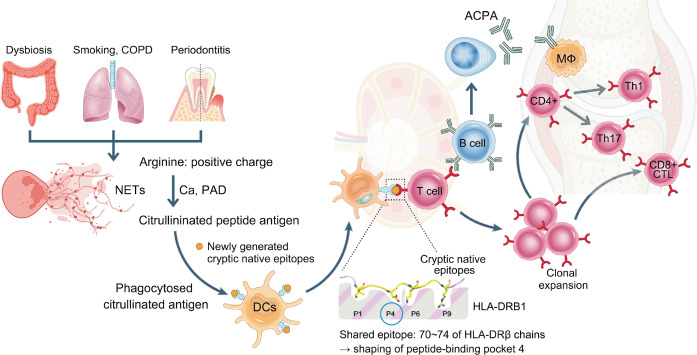
The heritability of RA has been estimated to be about 60%, with the strongest genetic association linked to a 5-amino acid sequence motif at positions 70~74 of HLA-DRB1 gene locus, known as the “Shared epitope” [4]. Genome-wide association studies (GWAS) data have refined the association signals for RA susceptibility in major histocompatiblity complex (MHC) region, pinpointing five key amino acid polymorphisms: three in HLA-DRB1 at positions 11, 71, and 74, and one each in HLA-B and HLA-DPβ1 at position 9, all of which are located in peptide-binding grooves suggesting their critical role in antigen presentation [4]. An expression quantitative trait loci analysis to dissect the link between genetic variants and gene expression revealed the significance of HLA-DPB2 in RA pathogenesis [5]. A single-nucleotide polymorphism, rs3128921, was identified as a key driver of HLA-DPB2 expression in synovial tissue, and both rs3128921 and HLA-DPB2 expression correlated with clinical severity and lympho-myeloid synovial pathotype, indicating a potential for aggressive treatment stratification [5]. Notably, 12.7% of phenotypic variance in RA can be attributed to these human leukocyte antigen (HLA) risk alleles, in contrast to 4% explained by non-HLA alleles (Figure 1) [4, 6].
Figure 1.
Loss of tolerance to citrullinated antigens at mucosal sites initiates the activation of adaptive immunity. NETs in the gut, lungs, and gingiva lead to increased protein citrullination in the presence of PAD enzymes and calcium. Dendritic cells that phagocytose these citrullinated antigens present them to T cells via HLA-DRB1 molecules containing the shared epitope. The weak binding between the citrullinated antigens and HLA-DRB1 enables T cells to escape the thymic selection, ultimately becoming autoreactive. These autoreactive T cells proliferate and migrate into synovial tissues, where they contribute to the development of synovitis. COPD: chronic obstructive pulmonary disease, NETs: neutrophil extracellular traps, PAD: peptidylarginine deiminase, DCs: dendritic cells, ACPA: anti-citrullinated protein antibodies, CD8+ CTL: CD8+ cytotoxic T lymphocyte.
Recent GWAS have expanded our knowledge of genetic risk loci beyond the HLA region. A large-scale GWAS involving 276,020 individuals from diverse ancestries identified 124 genetic loci associated with RA, including 34 novel loci [7]. Key genes such as TNIP2, TNFRSF11A, and WISP1 were linked to immune function and joint biology, while fine-mapping highlighted variants like rs58107865 at the LEF1 locus, emphasizing the role of regulatory T cells (Tregs) in RA [7]. Functional analyses revealed significant variants affecting gene expression and splicing, such as those in IL6R and peptidylarginine deiminase (PAD) 4, and underscored the importance of CD4+ T cells in RA pathogenesis [7].
DNA methylation is one of the most studied epigenetic modifications in RA. A genome-wide analysis identified 1,859 differentially methylated loci in RA fibroblast-like synoviocytes (FLSs) compared to osteoarthritis (OA) [8]. Genes such as CHI3L1, CASP1, STAT3, MAP3K5, MEFV, and WISP3 were found to be hypomethylated in RA FLSs, while hypermethylation was observed in genes like TGFBR2 and FOXO1 [8]. Hypomethylation was linked to increased gene expression, contributing to increased cell migration, inflammatory response, and synoviocyte invasion, highlighting the role of DNA methylation in the aggressive phenotype of RA synoviocytes [8].
Histone modifications, such as methylation, acetylation, and ubiquitination, play a critical role in regulating chromatin structure and accessibility, thereby controlling gene expression in RA. A comprehensive epigenetic study of FLSs identified 31,969 differentially modified epigenetic regions across 11 RA and 11 OA FLS samples [9]. Active enhancer regions were marked by significant changes in H3K27ac, while active promoters exhibited distinct modifications in H3K4me3 [9]. Transcription factor motifs, including AP-1 and STAT3, emphasized their roles in inflammation and matrix degradation [9]. The study also identified huntingtin-interacting protein 1 (HIP1) as a novel factor in RA pathogenesis, with functional validation showing its role in FLS invasion and joint damage [9]. A recent study highlighted the compelling role of histone modification in RA. Jumonji domain containing 1C (JMJD1C) functions as a histone demethylase that directly regulates STAT3 activity by demethylating its Lys140 residue, thereby facilitating dephosphorylation by the phosphatase PTPN6 [10]. The absence of JMJD1C leads to hypermethylation of STAT3, resulting in sustained phosphorylation and aberrant activation [10]. This dysregulated STAT3 signaling drives the overexpression of BLIMP1, a key regulator of plasma cell differentiation, thereby promoting excessive plasma cell production and autoantibody secretion [10]. The resulting disruption in germinal center dynamics impairs affinity maturation while amplifying plasma cell output, exacerbating RA severity [10].
Genome-wide open chromatin analysis of synovial tissue samples using single-cell assay for transposase-accessible chromatin with sequencing (ATAC-seq) and multi-modal snATAC-seq identified the presence of 24 distinct chromatin classes across five major cell types [11]. These chromatin classes, termed “superstates,” encapsulate multiple transcriptional cell states unified by shared chromatin accessibility patterns [11]. RA-specific chromatin classes were enriched in pathways critical to inflammation, such as JAK/STAT and TNF signaling, and prominently regulated by transcription factors including AP-1, STAT3, and TEAD1, particularly in inflammatory fibroblasts and T cells [11]. Additionally, genetic variants associated with RA were mapped to specific chromatin classes, providing a mechanistic link between these loci and their transcriptional activity in key pathogenic cell types [11].
2) Loss of immune tolerance at mucosal site
Evidence indicates that systemic autoimmunity in RA is initiated at mucosal sites, including the lungs, oral cavity, and gut, long before the onset of joint-specific symptoms (Figure 1). Recent research has revealed that individuals carrying RA genetic risk alleles, as measured by a polygenic risk score, displayed significant alterations in their gut microbiota composition even in the absence of clinical RA [12]. Notably, Prevotella species, particularly P. copri, is significantly more abundant in the gut of individuals with new-onset RA compared to healthy individuals, and its presence is linked to a cascade of proinflammatory immune responses, including the activation of Th17 cells [12]. In addition, evidence showed cross-reactivity between RA-relevant autoantigens and bacteria from the Lachnospiraceae and Ruminococcaceae families using dual IgA/IgG plasmablast-derived monoclonal antibodies from individuals at risk for RA [13]. Among these, when introduced into germ-free mice, colonization with Subdoligranulum isolate 7 led to joint swelling, increased serum autoantibodies, and histopathological changes resembling early-stage RA [13]. Mechanistically, Subdoligranulum isolate 7 also stimulated intestinal immune responses, including the formation of isolated lymphoid follicles, increased IgA levels in feces and serum, and Th17 cell expansion [13]. Notably, Subdoligranulum isolate 7 was detected in the feces of individuals at risk for RA and those with early RA, but not in healthy controls [13].
Another study demonstrated that dietary timing could modulate the composition and function of the gut microbiota, particularly affecting the bacterium Parabacteroides distasonis (P. distasonis) [14]. Disrupting the gut microbiota with antibiotics eliminated these circadian inflammatory rhythms of RA [14]. Mechanistic studies revealed that P. distasonis produces β-glucosidase to release glycitein, a bioactive compound with potent anti-inflammatory properties. Glycitein mitigates RA inflammation by inhibiting the SIRT5-NF-κB axis, thereby reducing inflammatory cytokine production and immune cell infiltration in affected tissues [14]. In addition, RA patients with Helicobacter pylori (H. pylori) infection exhibited significantly higher disease activity score-28 and anti-citrullinated protein antibody (ACPA) levels compared to uninfected patients, indicating that H. pylori infection exacerbates disease severity [15]. A recent study identified that H. pylori infection induces citrullination through upregulation of PAD4 via reactive oxygen species/hypoxia-inducible factor-1α signaling pathway [15]. PAD4 catalyzed the citrullination of keratin 1, and Cit-K1, in turn, triggered the production of anti-Cit-K1 autoantibodies, which were markedly elevated in the serum and synovial fluid of H. pylori-positive RA patients [15]. These autoantibodies correlated strongly with disease activity and ACPA levels, suggesting a significant role in the pathogenesis of RA [15].
Lung also may be one of critical sites of initiation of autoimmunity in RA (Figure 1). Single-cell study of bronchoalveolar lavage (BAL) fluid revealed that a significantly higher proportion of B cells was observed in the ACPA-positive individuals, both at risk for RA and with early RA, compared to ACPA-negative individuals [16]. These B cells from ACPA+ individuals displayed extensive somatic hypermutation and class-switching, and alongside higher levels of mutation-induced N-linked Fab glycosylation in the immunoglobulin variable regions, suggesting an antigen-driven selection process unique to ACPA+ individuals [16]. Monoclonal antibodies derived from BAL B cells exhibited reactivity to citrullinated peptides, and demonstrated binding to activated neutrophils, implicating their potential role in triggering inflammatory cascades [16]. These findings provide strong evidence that the lung acts as a site of active immune responses in RA, with local class switching and activation of citrulline-reactive B cells occurring even during the at-risk phase.
The oral cavity, particularly in the context of periodontal disease, has been implicated in the initiation of autoimmunity in RA (Figure 1). RA patients with periodontal disease exhibit frequent oral bacteremia, where citrullinated oral bacteria translocate into the bloodstream through mucosal break, triggering inflammatory responses [17]. These highly citrullinated oral bacteria play a pivotal role in immune activation, particularly by stimulating specific monocyte subsets, such as ISG15+HLADRhi and CD48hiS100A2pos monocytes, which are also enriched in inflamed synovial tissue during RA flares [17]. Furthermore, mass spectrometry analysis revealed that ACPAs, produced by RA plasmablasts, bind in vivo citrullinated bacterial peptides [17]. This cross-reactivity, driven by repeated exposure to citrullinated bacteria, likely promotes affinity maturation and epitope spreading, amplifying the autoimmune response in RA [17]. Another recent study profiled the tonsillar microbiota of RA patients and identified a notable dysbiosis, characterized by an enrichment of pathogenic Streptococcus species, including S. pyogenes, S. dysgalactiae, and S. agalactiae, alongside a notable reduction in commensal species like S. salivarius [18]. Colonization with these species exacerbated arthritis severity and promoted autoimmune responses in collagen-induced arthritis (CIA) models [18]. Conversely, the presence of commensal Streptococcus (S.) species, such as S. salivarius, was found to have protective effects, reducing arthritis progression and autoimmune responses [18]. Mechanistically, pathogenic Streptococcus (S.) species exhibit molecular mimicry with RA-associated autoantigens, such as citrullinated α-enolase (cit-ENO1), thereby driving autoimmune responses. However, S. salivarius exerts immunosuppressive effects by reducing pro-inflammatory T follicular helper (TFH) and germinal center B cells while modulating cytokine profiles [18].
3) ACPA formation and its function
Citrullination, a post-translational modification (PTM) catalyzed by PAD enzymes, converts arginine into citrulline within self-proteins. This process reshapes the peptide pool presented by MHC class II molecules by generating new peptides and destroying previously dominant ones. While it has been believed that citrulline-containing epitopes bind to HLA-DRB1, recent studies have revealed that most newly generated epitopes are native sequences rather than citrulline-containing peptides [19]. These native cryptic peptides are preferentially recognized by autoreactive CD4+ T cells in RA patients, contributing to immune tolerance breakdown [19]. This challenges the traditional paradigm that RA is primarily driven by citrulline-containing epitopes, instead highlighting the role of cryptic native epitopes as key triggers of autoimmunity (Figure 1) [19].
More than 100 patient-derived human monoclonal ACPAs have been generated from different B cell compartments, yet the precise disease-causing autoantigens remain unidentified [20]. Recent studies have focused on identifying FLS-derived autoantigens by isolating monoclonal antibodies from locally differentiated RA synovial B cells within ectopic lymphoid structures (ELSs). These antibodies have demonstrated specificity for neutrophil extracellular traps/citrullinated histones and RA-FLSs [21]. Monoclonal antibodies derived from ELS-residing synovial B cells target HSP60 in FLSs, exhibiting partial cross-reactivity with other stromal autoantigens such as calreticulin and vimentin [21]. Anti-FLS antibodies, including those specific to HSP60, have been shown to aggravate joint inflammation and destruction in CIA model [21]. Notably, HSP60 expression is significantly upregulated in RA synovial tissue, particularly in regions surrounding lymphocyte aggregates, and anti-HSP60 antibody levels have been correlated with disease activity in RA patients [21].
Despite the well-established pathogenic role of ACPAs, recent studies have identified subsets with protective effects [22]. For instance, the E4 ACPA clone, instead of inducing arthritis, provides protection by forming immune complexes with cit-ENO1 [22]. These complexes engage the inhibitory Fc receptor FCGR2B on macrophages, promoting IL-10 production, reducing osteoclastogenesis, and suppressing inflammation [22]. In addition, although complexes of citrullinated peptides and HLA-DR containing the shared epitope can serve as arthritis-initiating neo-antigens for CD4+ T cells, a recent study demonstrated that HLA-DR–bound citrullinated peptides can also induce immune tolerance, potentially impeding RA development [23]. In experimental models, immunization with the citrullinated peptides derived from cartilage intermediate layer protein or fibrinogen led to an expansion of Tregs and a reduction in pro-inflammatory Th1 cells [23]. This shift toward a tolerogenic immune profile indicates the potential of certain citrullinated peptides to modulate immune responses and maintain tissue homeostasis [23]. These findings suggest that different ACPA subsets may play distinct roles in RA, challenging the conventional view of ACPAs as solely pathogenic [24].
Citrullination is not the only PTM relevant to RA. The autoimmune response is further diversified by antibodies targeting proteins modified through carbamylation and acetylation. Anti-carbamylated protein (anti-CarP) antibodies recognize proteins altered through non-enzymatic conversion of lysine to homocitrulline, exhibiting broad reactivity to carbamylated antigens and cross-reactivity with citrullinated proteins [25]. Similarly, anti-acetylated protein antibodies (AAPA) target proteins modified by acetylation of lysine residues [26]. While AAPA also show cross-reactivity with citrullinated proteins, this interaction is less pronounced compared to that of anti-CarP antibodies [27]. Despite these advances, the precise roles of anti-CarP and AAPA in RA pathogenesis remain unclear.
4) T cell, B cell, and NK cell in RA
T cells play a key role in the pathogenesis of RA, orchestrating synovial inflammation through interactions with other immune cells and stromal components. Single-cell RNA sequencing combined with T-cell receptor (TCR)αβ sequencing in both ACPA+ and ACPA– RA patients, identified 12 distinct CD4+ T cell clusters, including naive, central memory, effector, Tregs, cytotoxic T cells, and peripheral helper T cells (TPH) [28]. Among them, GPR56+CXCL13high TPH cells are the most clonally expanded subset in ACPA+ synovial fluid, marked by tissue-resident memory markers like CXCR6 and CD69, and CXCL13, and PD-1, promoting B cell recruitment and activation [28]. RNA-velocity analysis revealed shared differentiation pathways between effector CD4+ T cells and CXCL13high TPH cells [28]. In addition, CD4+ cytotoxic T cells are significantly enriched in the synovial fluid of ACPA+ RA patients [28]. These cells express hallmark cytolytic molecules such as granzyme B (GZMB), perforin (PRF) 1, and natural killer cell granule protein 7 (NKG7), alongside transcription factors like HOBIT. The presence of these cytotoxic CD4+ T cells correlates with ACPA positivity, suggesting a role in RA pathogenesis (Table 1 and Figure 2) [28].
Table 1.
The pathogenic roles of major cell types including T cells, B cells, NK cells, macrophages, dendritic cells, and fibroblasts involved in the pathogenesis of RA, based on this paper
| Cell type | Major roles in RA pathogenesis | Key features & molecules |
|---|---|---|
| T cells (CD4+, CD8+, etc.) | - Orchestrate chronic synovial inflammation by interacting with other immune and stromal cells - Promote B cell activation (especially, TPH via CXCL13) - Mediate direct cytotoxicity (CD4+ and CD8+ cytotoxic T cells secreting GZMB, PRF1) - Augment inflammation through IL-9 (Th9 cells), enhancing macrophage and FLS activation |
- Surface markers/subsets: GPR56+CXCL13high (TPH), CD4+ cytotoxic (GZMB+, PRF1+), CD8+ cytotoxic - Cytokines & transcription factors: IL-9, IFN-γ, PU.1 (Th9 cell regulation) - Antigen specificity: Reactivity to citrullinated antigens in ACPA+ RA (e.g., citrullinated vimentin) |
| B cells | - Produce autoantibodies (ACPA) driving immune complex formation and joint damage - Present antigens to T cells, further amplifying immune responses - Secrete proinflammatory cytokines (e.g., IL-8) that recruit neutrophils - Loss of regulatory B10 function contributes to persistent inflammation |
- Surface markers/subsets: Memory B cells (CD80+, CD86+, Ki-67+), plasmablasts (CXCR3+) - Autoantibody types: ACPA, other PTM-specific autoantibodies (e.g., anti-citrullinated, -homocitrullinated, -acetylated) - Regulatory B10 cells: Produce IL-10; function impaired by TNF-α |
| NK cells | - Modulate inflammation through cytotoxic activity and cytokine release - Contribute to remission via a “silent degranulation” phenotype (CD8+CD57+CD56dimKIR2DL1+) that limits excessive inflammation - Promote immune activation in active RA (CD56brightCD16− NK cells), supporting T/B cell responses in the synovium |
- Phenotypes: CD8+CD57+CD56dim (remission-associated), CD56brightCD16− (active disease) - Key properties: Silent degranulation vs. proinflammatory cytokine production - Receptors: KIR2DL1, NKG2A (inhibitory), modulating NK cell function and inflammatory output |
| FLSs | - Drive synovial inflammation and joint destruction by producing IL-6, CCL2, MMP3, etc. - Facilitate T and B cell interactions (ITGA5+ FLS promote TPH differentiation and B cell activation) - Exhibit tissue-specific behaviors (hand FLS show higher invasive potential; HOTAIR lncRNA differs by joint location) - Associated with flare-ups and therapy resistance (e.g., ITGA5+ FLS in refractory RA) |
- Key subsets: Lining vs. sublining FLS (activated vs. resting phenotypes) - Surface/integrin markers: ITGA5+, associated with TGF-β1 signaling and TPH induction - Regulatory molecules: HOTAIR (lncRNA) influencing PI3K-Akt, IL-6 pathways and osteoclastogenesis |
| Macrophages | - Maintain a balance between proinflammatory and anti-inflammatory states - Proinflammatory subsets (MerTK–CD206–) secrete TNF, IL-6, IL-1β, driving tissue damage - Anti-inflammatory subsets (MerTK+CD206+) facilitate resolution of inflammation via lipid mediators (resolvins) and promote tissue repair - Dysregulated ratio of these subsets correlates with disease activity and remission status |
- Key surface markers: MerTK, CD206 (anti-inflammatory macrophages) - Proinflammatory mediators: TNF, IL-6, IL-1β - Distinct gene signatures: TREM2+, FOLR2+, S100A12+ subsets identified by single-cell RNA sequencing - Clinical relevance: Higher MerTK+ macrophage fraction during remission reduces flare risk |
| Dendritic cells (cDCs) | - Activate T cells (especially Th1/Th17) and amplify synovial inflammation - Accumulate in RA synovial fluid, producing IL-1β, IL-8, and CCL3 and driving local immune responses - Trigger inflammasome pathways (e.g., NLRC4) when stimulated by immune complexes (IgG–dsDNA), exacerbating joint damage |
- Major subset: CD1c+ cDCs in synovial fluid (high CCR2, CD64) - Inflammasome activation: NLRC4 leads to increased IL-1β release - Crosstalk: Often localize near IL-17+ T cells, perpetuating inflammation - Reduced circulation: Decreased cDC levels in blood but enrichment in affected joints |
NK: natural killer, RA: rheumatoid arthritis, TPH: T peripheral helper cell, GZMB: granzyme B, IL: interleukin, FLSs: fibroblast-like synoviocytes, IFN: interferon, ACPA: anti-citrullinated protein antibody, PTM: post-translational modification, TNF: tumor necrosis factor, MMP: matrix metalloproteinase, lncRNA: long non-coding RNA, cDC: conventional dendritic cell, dsDNA: double strand deoxyribonucleic acid.
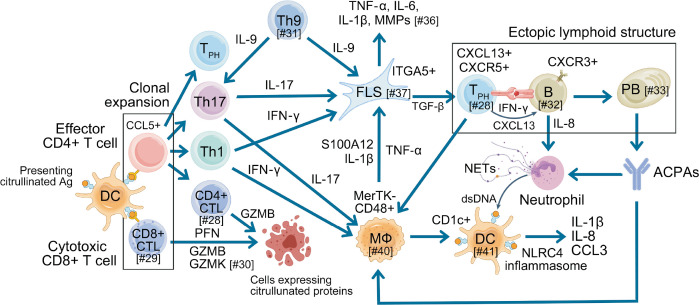
Figure 2.
Crosstalk among immune cells driving synovial inflammation in the pathogenesis of RA, as illustrated by the research findings discussed in this review. DCs phagocytose citrullinated antigens from the gut, lungs, and gingiva, and transport them to lymph nodes, where they present MHC-associated citrullinated antigens to naive T cells. This interaction activates T cells, prompting their proliferation and differentiation into effector and memory CD4+ T cells that migrate to the synovium. CD4+ effector T cells, particularly Th1 and Th17 subsets, recognize citrullinated antigens and produce IFN-γ and IL-17, respectively, which activate macrophages and FLSs to release inflammatory cytokines such as TNF-α, IL-1β, and IL-6. Cytotoxic GZMB+CD8+ T cells exhibit strong cytotoxic activity, while GZMK+CD8+ T cells have effector T cell transcriptional profiles. ITGA5+ FLSs enhance the differentiation of TPH through TGF-β1 signaling, promoting B cell activation and ACPA production. Neutrophils are recruited to the synovium by IL-8 from activated B cells and IL-17 from Th17 cells, where ACPAs, in combination with inflammatory cytokines, trigger NETosis, further amplifying the inflammatory response. The activation of the NLRC4 inflammasome in CD1c+ DCs by dsDNA enhances the production of IL-1β, IL-8, and CCL3, effectively activating Th1 and Th17 cells. This cascade drives synovitis and exacerbates tissue damage, highlighting the complex interplay of immune cells and cytokines in RA pathogenesis. The number following the # within the square brackets represents the reference number. RA: rheumatoid arthritis, DC: dendritic cell, MHC: major histocompatiblity complex, IFN: interferon IL: interleukin, FLSs: fibroblast-like synoviocytes, TNF: tumor necrosis factor, GZMB: granzyme B, TPH: T peripheral helper cell, ACPA: anti-citrullinated protein antibody, NLRC4: NOD-like receptor family caspase activation and recruitment domain containing 4, dsDNA: double strand deoxyribonucleic acid, CD8+ CTL: CD8+ cytotoxic T lymphocyte, PFN: perforin, GZMK: granzyme K, MMP: matrix metalloproteinase, PB: plasmablast, NETs: neutrophil extracellular traps.
CD8+ cytotoxic T cells have emerged as key players in RA pathogenesis (Figure 2). Recent findings revealed the presence of clonally expanded GZMB+GNLY+CD8+ T cells in both the blood and synovium of ACPA+ RA patients [29]. These cells exhibit cytotoxic and pro-inflammatory profiles, characterized by the expression of genes such as GZMB, GNLY, PRF1, and CCL4 [29]. Upon stimulation with citrullinated vimentin and histone H3, CD8+ T cells from ACPA+ RA patients displayed enhanced proliferation and cytotoxic responses, dependent on HLA class I presentation [29]. Importantly, these responses were specific to ACPA+ RA patients and absent in healthy controls or ACPA– RA patients. Functionally, citrullinated antigens activated CD8+ T cells to secrete cytotoxic mediators like GZMB and interferon (IFN)-γ, linking them to joint destruction [29]. Paired TCR analysis revealed shared clonotypes between blood and synovium, suggesting the migration and local expansion of citrulline-reactive CD8+ T cells [29]. Another single-cell RNA sequencing study further identified nine distinct CD8+ T cell populations in synovial tissue and blood, including clusters characterized by GZMK and GZMB expression, with the GZMK/B+ cluster being enriched in synovial tissue [30]. Clonal analysis revealed significant expansion in the GZMK/B+ memory cluster in synovial tissue and the GZMB+ TEMRA cluster in blood, suggesting distinct tissue-specific roles [30]. Limited clonal overlap between tissue compartments indicates that GZMK/B+ cells likely expand locally in the synovium rather than transitioning from blood-derived GZMB+ cells (Table 1) [30].
Th9 cells, a subset of CD4+ T cells, secrete IL-9 which promotes the hyperactivation of macrophages and FLSs, exacerbating inflammation and joint damage in RA (Figure 2). A recent study identified a positive feedback loop where PU.1 directly binds to the IL-9 promoter, enhancing its transcription [31]. IL-9, in turn, activates the IL-9R-JAK1/STAT3 pathway, further upregulating PU.1 expression and sustaining this feedback loop [31]. In animal models, PU.1 knockout in T cells or IL-9 significantly reduced joint inflammation, suppressed Th9 and Th17 cells, and increased Tregs [31]. The PU.1-IL9 axis drives pro-inflammatory macrophage polarization and enhances FLS proliferation and migration, ultimately contributing to cartilage and bone erosion [31].
Autoreactive B cells are essential contributors to the pathogenesis of RA, particularly through their ability to produce autoantibodies targeting proteins with PTMs as well as to participate in antigen presentation to T cells (Figure 2). Recent study has shown that the activation of ACPA-positive B cells is most prominent in patients with recent-onset RA and persists in those with established disease [32]. Notably, these cells maintain their activated phenotype even during clinical remission, indicating ongoing immunological activity despite the absence of overt symptoms [32]. In addition, ACPA-positive B cells significantly contribute significantly to inflammation by secreting IL-8, which promotes neutrophil migration into inflamed joints, alongside other proinflammatory cytokines that exacerbate joint damage and inflammation [32]. Other group showed that memory B cells specific to PTM antigens, including citrulline, homocitrulline, and acetyllysine, are predominantly found in RA synovium and exhibit cross-reactivity to multiple PTMs, suggesting broad activation driven by shared epitopes [33]. PTM-directed memory B cells display an activated phenotype, characterized by high expression of CD80, CD86, and Ki-67, alongside low levels of CD21 and CD24, indicative of recent activation [33]. Additionally, plasmablasts are prominent within this population and express high levels of CXCR3, facilitating their migration to inflamed tissues [33]. The continuous activation of PTM-directed B cells and their differentiation into plasmablasts may drive persistent inflammation, contributing to the chronicity of RA (Table 1) [33].
Dysfunction of regulatory B cells, particularly the IL-10-secreting B10 subset, is a common feature observed in the immune imbalance associated with RA. Studies have shown that B10 and B10pro cells, typically immunosuppressive through IL-10 secretion, are numerically decreased and functionally impaired in RA [34]. This dysfunction is characterized by reduced IL-10 production, with these cells instead adopting a proinflammatory phenotype marked by increased secretion of IFN-γ and IL-17A [34]. TNF-α has been identified as a key driver of this proinflammatory shift by inhibiting glycolysis, reducing cell proliferation, and disrupting SHIP-1 expression, a key regulator of B10 cell function [34]. Anti-TNF therapy was able to partially restore the frequency and function of these regulatory B cells, suggesting that targeting TNF-α may help rebalance the immune environment in RA [34].
Natural killer (NK) cells, key players in immune surveillance, have been shown to regulate inflammation in RA. Coyle et al. [35] identified a distinct subset of NK cells— CD8+CD57+CD56dimKIR2DL1+—associated with sustained remission in RA. This subset exhibited robust degranulation responses without producing pro-inflammatory cytokines, a phenomenon termed “silent degranulation,” which likely contributes to immune homeostasis [35]. In contrast, synovial fluid in active RA was enriched with CD56brightCD16– NK cells, distinct from the CD8+CD57+CD56dim NK cells prevalent in remission [35]. Functionally, remission-associated NK cells displayed reduced production of pro-inflammatory cytokines such as IFN-γ, while maintaining effective degranulation [35]. These cells also exhibited a balanced expression of inhibitory receptors, such as KIR2DL1 and NKG2A, reflecting a regulated and diverse phenotype [35]. This suggests their potential role in maintaining remission by modulating T cell activity and curbing autoimmunity while avoiding excessive inflammatory responses (Table 1) [35].
5) Fibroblast-like synoviocyte, macrophage, and dendritic cell in RA
FLSs are specialized cells in the synovium that maintain joint homeostasis under physiological conditions (Table 1 and Figure 2). However, FLSs undergo phenotypic changes in RA, transforming into aggressive, inflammation-promoting cells. Studies have shown that FLSs in RA can adopt distinct phenotypic states influenced by local cytokine environments and spatial interactions within the synovium [36]. Smith et al. [36] identified four major FLS states—activated and resting subsets within both the synovial lining and sublining compartments. Activated lining FLSs displayed enhanced pro-inflammatory and tissue-destructive pathways, including responses to TNF, IL-1β, and IFN-γ, and were enriched for matrix metalloproteinase (MMP) production and antigen presentation markers such as HLA-DR [36]. In contrast, resting sublining FLSs expressed extracellular matrix (ECM) homeostasis genes, such as PRG4 and ITGB8, and progenitor-like markers like CD34, indicating their role in tissue repair and maintenance. Cytokine signals, particularly TNF, IL-1β, and IFN-γ, induced transcriptional reprogramming in activated FLSs, driving the production of inflammatory mediators of IL-6 and CCL2, and remodeling enzymes, MMP3. Spatial mapping revealed that activated lining FLSs were primarily associated with cartilage and bone destruction, whereas sublining FLSs contributed to chronic inflammation by interacting with infiltrating immune cells (Table 1) [36].
Another interesting study identified a novel subset of ITGA5+ FLSs in the synovial sublining in active RA synovium (Figure 2) [37]. This population is characterized by a unique transcriptome enriched with genes involved in ECM remodeling, such as POSTN, and COL3A1, and immune modulation, such as TGFB1, and CCL5. Functionally, ITGA5+ fibroblasts drive the differentiation of CXCL13hiPD-1hi TPH through TGF-β1 signaling, thereby promoting B cell activation and lymphocyte aggregation [37]. ITGA5+ fibroblasts are particularly abundant in RA patients with refractory disease and are associated with resistance to biologic therapies, including rituximab and tocilizumab [37]. Their transcriptional signature overlaps with that of preinflammatory mesenchymal (PRIME) cells, which are implicated in RA flare-ups [37]. Intra-articular injection of ITGA5+ fibroblasts exacerbated joint inflammation, cartilage degradation, and bone erosion by upregulating inflammatory mediators such as IL-1β, MMP9, and remodeling factors like TGFB1 and vascular endothelial growth factor A [37].
FLSs from different anatomical locations exhibit distinct behaviors. Choi et al. [38] demonstrated that FLSs derived from hand joints have a more pronounced proinflammatory and migratory phenotype compared to those from hip or knee joints, which may contribute to the predilection for small joint involvement in RA. The long non-coding RNA HOTAIR (HOX transcript antisense RNA) has been identified as a critical regulator of joint-specific gene expression in RA. HOTAIR expression is predominantly detected in synovial fibroblasts from lower extremity joints and is significantly reduced in RA compared to OA [39]. Silencing HOTAIR in knee FLSs enhanced PI3K-Akt signaling and IL-6 production, promoting B cell recruitment and contributing to lymphoid histological patterns [39]. Furthermore, HOTAIR downregulation reduced osteoclast formation via cell-cell contact-dependent mechanisms and suppression of CXCL12 secretion [39]. HOTAIR appears to modulate the local inflammatory responses and shaping FLS subtypes, suggesting its potential as a target for joint-specific therapeutic strategies in RA [39].
Macrophages are central to the pathogenesis of RA, playing dual roles in driving inflammation and supporting tissue repair. Recent advances in single-cell technologies have revealed significant heterogeneity within synovial tissue macrophages (STMs) populations, identifying distinct clusters with diverse roles in RA (Figure 2) [40]. STMs in RA are divided into two main populations: MerTKposCD206pos STMs, which are anti-inflammatory and associated with joint homeostasis, and MerTKnegCD206neg STMs, which are pro-inflammatory and dominate in active disease states [40]. Single-cell RNA sequencing further identified nine STM clusters grouped into four functional subpopulations: TREM2pos, FOLR2high, HLApos, and CD48pos [40]. In active RA, pro-inflammatory clusters, such as osteopontin-producing SPP1pos and alarmin-producing S100A12pos subpopulations are enriched and drive synovitis and tissue destruction by secreting TNF, IL-6, and IL-1β [40]. During remission, MerTKposTREM2pos and LYVE1pos STMs are restored and actively promote resolution of inflammation and tissue repair by producing lipid mediators, such as resolvins, which enhance synovial fibroblast repair responses [40]. A reduced proportion of MerTKpos STMs in remission is associated with a higher risk of disease flare after treatment withdrawal (Table 1) [40].
Dendritic cells (DCs) also interact closely with other immune cells in the synovium (Figure 2). Delgado-Arévalo et al. [41] demonstrated that CD1c+ cDCs are reduced in the blood but enriched in the RA synovial fluid, where they express high levels of CD64 and CCR2, markers of activation and migration, respectively. These cells produce pro-inflammatory cytokines, such as IL-1β, IL-8, and CCL3, and efficiently activate Th1/Th17 T cells, driving synovitis and tissue damage [41]. The NLRC4 inflammasome is a key innate sensing pathway in CD1c+ cDCs, activated by immune complexes containing dsDNA and IgG, leading to increased inflammatory cytokine production and exacerbating RA-like profiles [41]. Functionally, CD1c+ cDCs in the synovial fluid were observed in close proximity to IL-17+ T cells within RA synovial membranes, implicating their direct role in sustaining inflammation and promoting tissue damage (Table 1) [41].
Future directions of basic research in RA
1) Investigating the mechanisms of peripheral tolerance breakdown in RA
ACPA positivity reflects a breakdown of central tolerance to citrullinated antigens. However, only 20%~30% of individuals with ACPA positivity progress to develop RA, highlighting the critical role of peripheral tolerance breakdown in RA pathogenesis [42]. The disruption of peripheral tolerance permits autoreactive immune cells to escape regulatory mechanisms, leading to attacks on the body’s own tissues, particularly the synovium in RA. A key mechanism implicated in the peripheral tolerance breakdown in RA is the impairment of Tregs function [43]. Tregs, although numerically increased in RA synovium, show impaired suppressive capacity with reduced regulatory and elevated pro-inflammatory markers [44]. This impairment is associated with factors such as IL-6 overexpression and hypoxic conditions in the synovium, which destabilize Foxp3 expression and drive the conversion of Tregs into pathogenic Th17 cells [45,46]. The reduced ability of Tregs to suppress effector T cell activity, combined with an imbalance between Tregs and pro-inflammatory Th17 cells, significantly contributes to the breakdown of peripheral tolerance in RA.
In addition, recent studies have identified a pathologically expanded subset of TPH cells in RA patients, which plays a significant role in promoting B cell activation and differentiation [47]. In ACPA+ individuals, particularly those progressing to RA, a significant expansion of HLA-DR+ TPH cells has been observed [47]. These cells are characterized by high expression of PD-1, Inducible T-cell COStimulator (ICOS), and T Cell Immunoreceptor With Ig And ITIM Domains (TIGIT), enabling them to efficiently activate B cells and drive autoantibody production. Longitudinal studies have revealed persistently high levels of HLA-DR+ TPH cells and CXCR5−CD11c−CD38+ naive B cells in individuals transitioning from pre-RA to classified RA, implicating these immune cell subsets in disease progression [47]. Unlike TFH cells, which typically support B cell maturation in lymphoid organs, TPH cells are localized in inflamed synovial tissues and are distinguished by high PD-1 and CXCR5 expression [47]. The expansion of TPH cells may contribute to the breakdown of peripheral tolerance and serve as potential biomarkers for predicting the development of RA.
Along with TPH cells, B cell tolerance is another critical aspect in peripheral tolerance breakdown. Mature naive B cells from RA patients show marked defects in immune tolerance, as evidenced by a higher prevalence of autoreactive antibodies compared to healthy individuals [48]. The antibody repertoire in RA is further distinguished by the presence of unusually long complementarity-determining region 3 (CDR3) regions, which are strongly associated with autoreactivity [48]. These observations suggest anomalies in B-cell receptor (BCR) signaling and selection processes during B cell development, fostering the persistence of autoreactive clones [48]. Mechanistic insights suggest that elevated levels of serum B-cell activating factor (BAFF) in RA patients have been implicated in the survival and maturation of self-reactive B cells [48]. In addition, impairments in BCR signaling and receptor editing exacerbate the failure of tolerance checkpoints [48]. A recent study highlighted the dynamic involvement of B cells in RA flares, revealing that B cell activation is detectable approximately two weeks prior to flare onset [49]. This activation is thought to trigger a cascade of immune responses, including the activation of other immune cells, particularly mesenchymal-origin PRIME cells, which then contribute directly to joint inflammation [49]. However, the mechanisms driving the activation of B cells preceding flares remain unclear.
The last key player in peripheral immune tolerance is lymph node stromal cells (LNSCs). LNSCs, including fibroblastic reticular cells and lymphatic endothelial cells, present peripheral tissue-restricted antigens (PTAs) on their MHC molecules to T cells, leading to inducing anergy or promoting the differentiation of Treg cells [50]. LNSCs also exhibit the capacity for MHC class II expression, particularly when stimulated with IFNγ, enabling them to present antigens to T cells [51]. Furthermore, they express immunomodulatory molecules, such as PD-L1 and CD40, though at lower levels than dendritic cells, indicating a role in shaping immune responses and maintaining immune tolerance [51]. In RA, dysregulation of LNSCs is evident. RA patients and at-risk individuals exhibit variability in PTAs expression, characterized by decreased Ras related glycolysis inhibitor and calcium channel regulator (RRAD) and increased glutamate decarboxylase 1 (GAD1) transcript levels in RA-risk individuals [51]. In addition, reduced HLA-DR and PD-L1 expression in RA patients compared to healthy individuals indicates a disruption of tolerance mechanisms (Table 2) [51].
Table 2.
Future directions in basic research on RA, based on this paper
| Focus | Knowledge gaps | Proposed future directions | Potential clinical/translational relevance |
|---|---|---|---|
| Mechanisms of peripheral tolerance breakdown | - How Tregs become functionally impaired (e.g., IL-6 exposure, hypoxia, Th17 conversion) - Factors driving TPH cell expansion in pre-RA vs. established RA - Mechanisms of defective B cell tolerance and abnormal BCR signaling - LNSC dysfunction in RA |
- Restore Treg function: Identify and target pathways (e.g., Foxp3 destabilization) that undermine Treg suppressive capacity - Characterize TPH cells: Determine how HLA-DR+ TPH subsets expand and initiate autoantibody production - Investigate B cell tolerance: Examine BAFF-mediated survival of autoreactive B cells and receptor editing failures - Assess LNSC dysregulation: Uncover how altered antigen presentation (PD-L1, HLA-DR) affects T cell anergy |
- Targeted immunomodulators to preserve or re-establish Treg phenotype - Predictive biomarkers (e.g., TPH cell frequencies) for progression from pre-RA to classified RA - B cell–targeted therapies (e.g., BAFF inhibitors) to rescue tolerance - Restoring LNSC function to maintain peripheral tolerance and prevent synovial damage |
| Pathogenesis of seronegative RA | - Weaker linkage to HLA-DRB1 shared epitope - Distinct genetic risk factors (HLA-B position 9, HLA-DRB1 position 11, CLEC16A, IRF5) - Greater emphasis on innate immune pathways - Unique synovial inflammatory patterns (e.g., macrophage-driven) |
- Expand genetic/epigenetic profiling: Identify seronegative-specific variants via GWAS and single-cell techniques - Focus on innate immunity: Characterize macrophage, DC, and neutrophil roles in seronegative synovitis - Comparative single-cell analyses: Contrast cellular subsets in ACPA– vs. ACPA+ RA - Optimized treatments: Develop therapies addressing innate-driven inflammation (e.g., IL-1 or CCL2 inhibitors) |
- Personalized approaches: Tailor interventions for seronegative RA using innate immunity–targeted biologics - Biomarker development: Detect early innate immune activation patterns, facilitating differential diagnosis - Better disease control: Reduce reliance on autoantibody-targeting strategies (e.g., B cell depletion), which may be less effective in seronegative RA |
| Molecular mechanisms in refractory & recurrent RA | - PIRRA vs. NIRRA - Epigenetic and somatic mutations driving therapy resistance - Role of Treg/B cell dysfunction in flares or refractory states - Synovial fibroblast heterogeneity sustaining chronic inflammation |
- Molecular stratification: Use multi-omics (RNA-seq, methylation, proteomics) to differentiate PIRRA vs. NIRRA - Novel immunoregulatory targets: Address pathways beyond TNF/IL-6 (e.g., GM-CSF, JAK-STAT, Treg reconstitution) - B cell–focused strategies: Investigate extended/alternate B cell depletion (e.g., rituximab) for treatment resistance - Fibroblast subtyping: Identify disease-promoting FLS subsets that maintain chronic synovitis |
- Improved remission rates: Tailored therapies based on refractory RA subtype - Reduced trial-and-error: Molecular profiling guides selection of alternative cytokine inhibitors - Predictive biomarkers: Epigenetic or transcriptomic signatures to foresee flares or therapy failure - Fibroblast-targeting agents: New drug classes aimed at destructive and proinflammatory FLS populations |
| Multi-omics for personalized therapy | - Complexity of RA heterogeneity complicates one-size-fits-all treatments - Limited integration of transcriptomic, proteomic, metabolomic, and microbiomic data - Early-stage machine learning models for predicting drug response |
- Machine learning integration: Merge multi-omics datasets (genomic, proteomic, microbiomic) to build high-accuracy models for therapy response (e.g., TNF inhibitor vs. IL-6 inhibitor) - Longitudinal patient tracking: Monitor molecular changes pre- and post-treatment to assess “molecular remission” - Microbiome-directed interventions: Investigate how modifying gut flora impacts RA outcomes |
- Precision medicine: Personalized therapy aligned with a patient’s unique molecular profile - Reduced non-responder rates: Pre-treatment identification of likely responders - Adaptive treatment strategies: Real-time molecular data to guide medication adjustments and achieve sustained remission - New biomarkers: Metabolomic or microbiomic signatures for monitoring disease activity and optimizing dosing |
RA: rheumatoid arthritis, Tregs: regulatory T cells, IL: interleukin, TPH: peripheral helper T cells, LNSC: lymph node stromal cell, GWAS: genome-wide association studies, DC: dendritic cell, ACPA: anti-citrullinated protein antibody, PIRRA: persistent inflammatory refractory RA, NIRRA: non-inflammatory refractory RA, RNA-seq: RNA sequencing, TNF: tumor necrosis factor, GM-CSF: granulocyte-macrophage colony-stimulating factor, JAK-STAT: Janus kinase-signal transducer and activator of transcription.
2) Pathogenesis of seronegative RA
To date, most research on RA has centered on seropositive RA, which is associated with the presence of ACPA. However, seronegative RA—affecting approximately 30% of RA patients—lacks autoantibodies such as rheumatoid factor and ACPA and differs significantly from seropositive RA in terms of genetic predisposition, immunopathology, and clinical features [52]. The genetic basis of seronegative RA diverges markedly from that of seropositive RA, which is strongly linked to HLA-DRB1 alleles containing the shared epitope. In contrast, seronegative RA shows weaker associations with these alleles and is instead linked to variations at HLA-B position 9 and HLA-DRB1 position 11, as highlighted by genome-wide studies [53]. Furthermore, GWAS have identified other non-HLA genes, such as CLEC16A, IRF5, ANKRD55, BLK, and CLYBL as contributors to seronegative RA susceptibility but not seropositive RA [53,54]. These genetic distinctions likely reflect the different immune pathways driving the two RA subtypes: seropositive RA is dominated by adaptive immune mechanisms involving autoantibody production, whereas seronegative RA relies more heavily on innate immune pathways.
The immunopathology of seropositive and seronegative RA also presents notable differences, particularly in terms of immune cell involvement and synovial inflammation. Single-cell sequencing has helped to delineate the immunological differences between ACPA-positive and ACPA-negative RA subtypes [55]. ACPA+ RA is marked by increased cytotoxic T cells and exhausted T cells in synovial tissue and peripheral blood, indicating a more cytotoxic immune environment. In contrast, ACPA– RA is characterized by upregulation of proinflammatory genes such as CCL13, CCL18, and MMP3 in myeloid cells, DCs, and T cells, coupled with reduced HLA-DRB5 expression in B cells, macrophages, and DCs [55]. In B cell subsets, ACPA– RA shows reduced HLA-DRB5+ plasma and memory B cells in synovial tissue, while ACPA+ RA features an enrichment of these subsets, suggesting a stronger role for antigen presentation and adaptive immunity in ACPA+ disease [55]. In myeloid cells, ACPA– RA is associated with macrophages exhibiting heightened proinflammatory markers, such as IL1B, but impaired phagocytic function due to reduced CD36 expression [55]. In T cells, ACPA+ RA is characterized by higher expression of cytotoxic markers like GZMB and NKG7, while ACPA– RA lacks strong cytotoxic signatures but shows distinct dendritic cell-T cell interactions mediated by chemokine signaling [55].
Understanding these differences is vital for the personalized treatment of RA. Seropositive RA patients may benefit from therapies targeting B cells or specific cytokines such as TNF-α, which play significant roles in adaptive immunity. In contrast, seronegative RA patients might respond better to treatments targeting innate immune components or pathways, which address the myeloid-driven inflammation characteristic of this subgroup [55]. Despite these advances, much remains unclear about the pathogenesis of seronegative RA, highlighting the need for further research to elucidate its mechanisms and optimize therapeutic strategies (Table 2).
3) Molecular mechanisms in the refractory and recurrent RA
Refractory RA represents a subset of patients who fail to achieve adequate disease control despite treatment with biologic and targeted synthetic disease-modifying antirheumatic drugs (DMARDs). This treatment-resistant state can be broadly categorized into two phenotypes: persistent inflammatory refractory RA (PIRRA), characterized by ongoing synovitis, and non-inflammatory refractory RA (NIRRA), marked by persistent pain in the absence of objective inflammation [56]. While TNF and IL-6 inhibitors are effective for many RA patients, they often fail in PIRRA due to the redundancy of inflammatory pathways and the involvement of cytokines not targeted by current therapies [56].
The joint microenvironment in PIRRA is notable for aberrant immune cell infiltration, including macrophages, T cells, and B cells, which perpetuate chronic inflammation [57]. De novo somatic mutations that influence epigenetic modifications, such as altered DNA methylation and microRNA expression, may further affect both adaptive and innate immune cells, thereby driving the immunopathogenesis of refractory RA [58]. Dysfunction of Treg undermines immune tolerance, further contributing to the refractory nature of the disease [59]. The fact that the rituximab that deplete B cell is effective for refractory RA highlights the critical role of B cell abnormalities in sustaining inflammation and mediating resistance to biologic and targeted synthetic DMARDs [60]. In addition, synovial fibroblast are not merely passive structural components but active players in the persistence of chronic inflammation [61].
Recurrent RA, along with refractory cases, poses a significant challenge in the clinical management of RA. A recent single-cell sequencing study has provided molecular insights into the immune dysregulation observed during RA flares as compared to drug-free remission states [44]. During RA flares, immune dysregulation is marked by increased memory T and B cells, including dysfunctional CD4+PD1hi Tregs, and heightened CD4+ cytotoxic T cells, IgA+ plasma cells, CD8+CXCR5+ T cells, and CXCR3+ B cells show activation. Clonal expansion occurs prominently in CD8+ memory T cells, while cytotoxic CD4+ T cell expansion is limited to GZM-expressing subsets [44]. Despite increased IgA+ plasma cell activity, B cell clonal changes are minimal [44]. Tregs, although numerically increased, show impaired suppressive capacity with reduced regulatory and elevated pro-inflammatory markers [44]. The study proposes that DMARDs and functional Tregs maintain immune balance in remission, but DMARD cessation activates effector memory lymphocytes, overwhelming Tregs and triggering flares. These findings highlight specific T and B cell subsets as biomarkers for flare prediction and the need for strategies to manage immune dysregulation during DMARD tapering (Table 2).
4) Integration of multi-omics approaches for personalized therapy: understanding disease heterogeneity
Recently, multi-omics technologies, integrating genomics, transcriptomics, proteomics, metabolomics, and microbiomics, have emerged as valuable tools for elucidating disease mechanisms and identifying biomarkers that can guide personalized treatment strategies. The integration of multi-omics technologies has significantly deepened our understanding of RA pathogenesis, revealing complex interactions between immune, genetic, metabolic, and microbial factors.
The use of multi-omics data in conjunction with machine learning techniques has shown promise in predicting treatment response to anti-TNF therapy in RA [62]. Machine learning models based on this multi-omics data achieved high accuracy in classifying non-responders before the initiation of treatment. Specifically, the expression of the CHI3L1 gene and its protein YKL-40, which regulates T cell activation, was suppressed in anti-TNF treatment responders [62]. Similarly, Tao et al. [63] investigated treatment responses to two widely used TNF inhibitors, adalimumab and etanercept. They used differential gene expression and DNA methylation profiling in peripheral blood mononuclear cell, and etanercept responders have strongly hypermethylated differentially methylated positions, but not adalimumab [63]. Their machine learning models demonstrated that baseline molecular signatures could accurately predict which patients would respond to either adalimumab or etanercept, paving the way for personalized anti-TNF therapy in RA [63].
The ultimate goal of integrating multi-omics approaches is to achieve precision medicine, where treatment is tailored to each patient’s unique molecular profile. A longitudinal multi-omics monitoring to assess drug response in RA patients found that molecular profiles, including transcriptomic, serum proteomic, and immunophenotypic data, could be used to assess the proximity of treated patients to a “molecular remission” state, similar to healthy controls [64]. This high-dimensional phenotyping allows for a quantitative measure of treatment efficacy and aids in optimizing therapy to achieve sustained remission [64]. Chen et al. [65] explored the relationship between disease activity and changes in multi-omics profiles in RA patients. By integrating plasma metabolites and gut microbiota data, they observed that RA patients with different levels of disease activity exhibited significant changes in both their gut microbiota and plasma metabolites, offering a promising tool for guiding treatment adjustments in clinical practice [65].
The integration of multi-omics technologies in RA research has provided valuable insights into the molecular mechanisms driving disease heterogeneity and treatment response. Machine learning models utilizing multi-omics data further enhance the potential for predicting treatment outcomes, ultimately leading towards precision medicine in RA. Future research should focus on refining these models, validating biomarkers in larger cohorts, and integrating multi-omics data into routine clinical practice to optimize treatment strategies and improve patient outcomes (Table 2).
CONCLUSION
Basic research in RA has made tremendous progress in uncovering the genetic, immunological, and cellular mechanisms underlying the disease. However, much remains to be understood, particularly in the context of refractory disease and patient heterogeneity. The future of RA research lies in integrating advanced technologies, such as multi-omics, single-cell analysis, and microbiome studies, to develop more personalized and effective treatments. By targeting the diverse pathways involved in RA pathogenesis, we can move closer to a cure for this debilitating disease.
ACKNOWLEDGMENTS
This paper was conducted as part of the Research Committee Project of the Korean Colleague of Rheumatology (KCR). We sincerely thank Professor Yoon-Kyung Sung for his invaluable contributions to the planning and organization of this work.
Footnotes
FUNDING
This research was supported by the National Research Foundation of Korea (NRF) grant to S.W.H., funded by the Korea government (Ministry of Education) (grant numbers RS-2023-00243140).
CONFLICT OF INTEREST
J.K.P. and S.W.H. have been an editorial board member since June 2020 and May 2024, respectively, but have no role in the decision to publish this article. The rest of the authors have no potential conflicts of interest relevant to this article.
AUTHOR CONTRIBUTIONS
The authors confirm their contributions to the paper as follows: study conception and design: JKP and SWH; data collection: BZG, JKP, JHJ, and SWH; analysis and interpretation of results: BZG, JHJ, and SWH; draft manuscript preparation: BZG and SWH; drawing scientific concepts: SWH. All authors reviewed the results and approved the final version of the manuscript.
REFERENCES
- 1.Finckh A, Gilbert B, Hodkinson B, Bae SC, Thomas R, Deane KD, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18:591–602. doi: 10.1038/s41584-022-00827-y. [DOI] [PubMed] [Google Scholar]
- 2.Gravallese EM, Firestein GS. Rheumatoid arthritis - common origins, divergent mechanisms. N Engl J Med. 2023;388:529–42. doi: 10.1056/NEJMra2103726. [DOI] [PubMed] [Google Scholar]
- 3.Konzett V, Aletaha D. Management strategies in rheumatoid arthritis. Nat Rev Rheumatol. 2024;20:760–9. doi: 10.1038/s41584-024-01169-7. [DOI] [PubMed] [Google Scholar]
- 4.Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, Jia X, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012;44:291–6. doi: 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldmann K, Spiliopoulou A, Iakovliev A, Plant D, Nair N, Cubuk C, et al. Expression quantitative trait loci analysis in rheumatoid arthritis identifies tissue specific variants associated with severity and outcome. Ann Rheum Dis. 2024;83:288–99. doi: 10.1136/ard-2023-224540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42:508–14. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishigaki K, Sakaue S, Terao C, Luo Y, Sonehara K, Yamaguchi K, et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. Nat Genet. 2022;54:1640–51. doi: 10.1038/s41588-022-01213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakano K, Whitaker JW, Boyle DL, Wang W, Firestein GS. DNA methylome signature in rheumatoid arthritis. Ann Rheum Dis. 2013;72:110–7. doi: 10.1136/annrheumdis-2012-201526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai R, Laragione T, Hammaker D, Boyle DL, Wildberg A, Maeshima K, et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat Commun. 2018;9:1921. doi: 10.1038/s41467-018-04310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Y, Yang X, Wu S, Ding X, Zhu H, Long X, et al. Jmjd1c demethylates STAT3 to restrain plasma cell differentiation and rheumatoid arthritis. Nat Immunol. 2022;23:1342–54. doi: 10.1038/s41590-022-01287-y. [DOI] [PubMed] [Google Scholar]
- 11.Weinand K, Sakaue S, Nathan A, Jonsson AH, Zhang F, Watts GFM, et al. The chromatin landscape of pathogenic transcriptional cell states in rheumatoid arthritis. Nat Commun. 2024;15:4650. doi: 10.1038/s41467-024-48620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells PM, Adebayo AS, Bowyer RCE, Freidin MB, Finckh A, Strowig T, et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: a cross-sectional study. Lancet Rheumatol. 2020;2:e418–27. doi: 10.1016/S2665-9913(20)30064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chriswell ME, Lefferts AR, Clay MR, Hsu AR, Seifert J, Feser ML, et al. Clonal IgA and IgG autoantibodies from individuals at risk for rheumatoid arthritis identify an arthritogenic strain of Subdoligranulum. Sci Transl Med. 2022;14:eabn5166. doi: 10.1126/scitranslmed.abn5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma F, Li Z, Liu H, Chen S, Zheng S, Zhu J, et al. Dietary-timing-induced gut microbiota diurnal oscillations modulate inflammatory rhythms in rheumatoid arthritis. Cell Metab. 2024;36:2367–82.e5. doi: 10.1016/j.cmet.2024.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Yuan H, Zhang J, He T, Deng Y, Chen Y, et al. Helicobacter pylori upregulates PAD4 expression via stabilising HIF-1α to exacerbate rheumatoid arthritis. Ann Rheum Dis. 2024;83:1666–76. doi: 10.1136/ard-2023-225306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshua V, Loberg Haarhaus M, Hensvold A, Wähämaa H, Gerstner C, Hansson M, et al. Rheumatoid arthritis-specific autoimmunity in the lung before and at the onset of disease. Arthritis Rheumatol. 2023;75:1910–22. doi: 10.1002/art.42549. [DOI] [PubMed] [Google Scholar]
- 17.Brewer RC, Lanz TV, Hale CR, Sepich-Poore GD, Martino C, Swafford AD, et al. Oral mucosal breaks trigger anti-citrullinated bacterial and human protein antibody responses in rheumatoid arthritis. Sci Transl Med. 2023;15:eabq8476. doi: 10.1126/scitranslmed.abq8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Li S, Jin J, Guo R, Jin Y, Cao L, et al. The aberrant tonsillar microbiota modulates autoimmune responses in rheumatoid arthritis. JCI Insight. 2024;9:e175916. doi: 10.1172/jci.insight.175916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran AM, Girgis AA, Jang Y, Crawford JD, Thomas MA, Kawalerski R, et al. Citrullination modulates antigen processing and presentation by revealing cryptic epitopes in rheumatoid arthritis. Nat Commun. 2023;14:1061. doi: 10.1038/s41467-023-36620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raposo B, Klareskog L, Robinson WH, Malmström V, Grönwall C. The peculiar features, diversity and impact of citrulline-reactive autoantibodies. Nat Rev Rheumatol. 2024;20:399–416. doi: 10.1038/s41584-024-01124-6. [DOI] [PubMed] [Google Scholar]
- 21.Corsiero E, Caliste M, Jagemann L, Fossati-Jimack L, Goldmann K, Cubuk C, et al. Autoimmunity to stromal-derived autoantigens in rheumatoid ectopic germinal centers exacerbates arthritis and affects clinical response. J Clin Invest. 2024;134:e169754. doi: 10.1172/JCI169754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y, Ge C, Moreno-Giró À, Xu B, Beusch CM, Sandor K, et al. A subset of antibodies targeting citrullinated proteins confers protection from rheumatoid arthritis. Nat Commun. 2023;14:691. doi: 10.1038/s41467-023-36257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McElwee MK, Dileepan T, Mahmud SA, Jenkins MK. The CD4+ T cell repertoire specific for citrullinated peptides shows evidence of immune tolerance. J Exp Med. 2023;220:e20230209. doi: 10.1084/jem.20230209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y, Aoun M, Xu Z, Holmdahl R. Shift in perspective: autoimmunity protecting against rheumatoid arthritis. Ann Rheum Dis. 2024;83:550–5. doi: 10.1136/ard-2023-225237. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A. 2011;108:17372–7. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juarez M, Bang H, Hammar F, Reimer U, Dyke B, Sahbudin I, et al. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann Rheum Dis. 2016;75:1099–107. doi: 10.1136/annrheumdis-2014-206785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Wesemael TJ, Reijm S, Kawakami A, Dorjée AL, Stoeken G, Maeda T, et al. IgM antibodies against acetylated proteins as a possible starting point of the anti-modified protein antibody response in rheumatoid arthritis. Ann Rheum Dis. 2024;83:267–70. doi: 10.1136/ard-2023-224553. [DOI] [PubMed] [Google Scholar]
- 28.Argyriou A, Wadsworth MH, 2nd, Lendvai A, Christensen SM, Hensvold AH, Gerstner C, et al. Single cell sequencing identifies clonally expanded synovial CD4+ TPH cells expressing GPR56 in rheumatoid arthritis. Nat Commun. 2022;13:4046. doi: 10.1038/s41467-022-31519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon JS, Younis S, Ramadoss NS, Iyer R, Sheth K, Sharpe O, et al. Cytotoxic CD8+ T cells target citrullinated antigens in rheumatoid arthritis. Nat Commun. 2023;14:319. doi: 10.1038/s41467-022-35264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunlap G, Wagner A, Meednu N, Wang R, Zhang F, Ekabe JC, et al. Clonal associations between lymphocyte subsets and functional states in rheumatoid arthritis synovium. Nat Commun. 2024;15:4991. doi: 10.1038/s41467-024-49186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu J, Chen W, Huang W, Wang X, Fang Y, Wu X, et al. Positive feedback loop PU.1-IL9 in Th9 promotes rheumatoid arthritis development. Ann Rheum Dis. 2024;83:1707–21. doi: 10.1136/ard-2024-226067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristyanto H, Blomberg NJ, Slot LM, van der Voort EIH, Kerkman PF, Bakker A, et al. Persistently activated, proliferative memory autoreactive B cells promote inflammation in rheumatoid arthritis. Sci Transl Med. 2020;12:eaaz5327. doi: 10.1126/scitranslmed.aaz5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reijm S, Kwekkeboom JC, Blomberg NJ, Suurmond J, van der Woude D, Toes RE, et al. Autoreactive B cells in rheumatoid arthritis include mainly activated CXCR3+ memory B cells and plasmablasts. JCI Insight. 2023;8:e172006. doi: 10.1172/jci.insight.172006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu F, Shi L, Liu X, Chen Y, Zhang X, Jia Y, et al. Proinflammatory phenotype of B10 and B10pro cells elicited by TNF-α in rheumatoid arthritis. Ann Rheum Dis. 2024;83:576–88. doi: 10.1136/ard-2023-224878. [DOI] [PubMed] [Google Scholar]
- 35.Coyle C, Ma M, Abraham Y, Mahony CB, Steel K, Simpson C, et al. NK cell subsets define sustained remission in rheumatoid arthritis. JCI Insight. 2024;9:e182390. doi: 10.1172/jci.insight.182390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MH, Gao VR, Periyakoil PK, Kochen A, DiCarlo EF, Goodman SM, et al. Drivers of heterogeneity in synovial fibroblasts in rheumatoid arthritis. Nat Immunol. 2023;24:1200–10. doi: 10.1038/s41590-023-01527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng L, Gu M, Li X, Hu X, Chen C, Kang Y, et al. ITGA5+ synovial fibroblasts orchestrate proinflammatory niche formation by remodelling the local immune microenvironment in rheumatoid arthritis. Ann Rheum Dis. 2024 Nov 1; doi: 10.1136/ard-2024-225778. [Epub]. DOI: 10.1136/ard-2024-225778. [DOI] [PubMed] [Google Scholar]
- 38.Choi E, Machado CR, Okano T, Boyle D, Wang W, Firestein GS. Joint-specific rheumatoid arthritis fibroblast-like synoviocyte regulation identified by integration of chromatin access and transcriptional activity. JCI Insight. 2024;9:e179392. doi: 10.1172/jci.insight.179392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elhai M, Micheroli R, Houtman M, Mirrahimi M, Moser L, Pauli C, et al. The long non-coding RNA HOTAIR contributes to joint-specific gene expression in rheumatoid arthritis. Nat Commun. 2023;14:8172. doi: 10.1038/s41467-023-44053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alivernini S, MacDonald L, Elmesmari A, Finlay S, Tolusso B, Gigante MR, et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat Med. 2020;26:1295–306. doi: 10.1038/s41591-020-0939-8. [DOI] [PubMed] [Google Scholar]
- 41.Delgado-Arévalo C, Calvet-Mirabent M, Triguero-Martínez A, Vázquez de Luis E, Benguría-Filippini A, Largo R, et al. NLRC4-mediated activation of CD1c+ DC contributes to perpetuation of synovitis in rheumatoid arthritis. JCI Insight. 2022;7:e152886. doi: 10.1172/jci.insight.152886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hensvold AH, Frisell T, Magnusson PK, Holmdahl R, Askling J, Catrina AI. How well do ACPA discriminate and predict RA in the general population: a study based on 12 590 population-representative Swedish twins. Ann Rheum Dis. 2017;76:119–25. doi: 10.1136/annrheumdis-2015-208980. [DOI] [PubMed] [Google Scholar]
- 43.Ceeraz S, Hall C, Choy EH, Spencer J, Corrigall VM. Defective CD8+CD28+ regulatory T cell suppressor function in rheumatoid arthritis is restored by tumour necrosis factor inhibitor therapy. Clin Exp Immunol. 2013;174:18–26. doi: 10.1111/cei.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker KF, McDonald D, Hulme G, Hussain R, Coxhead J, Swan D, et al. Single-cell insights into immune dysregulation in rheumatoid arthritis flare versus drug-free remission. Nat Commun. 2024;15:1063. doi: 10.1038/s41467-024-45213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–8. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 46.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105:19396–401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takada H, Demoruelle MK, Deane KD, Nakamura S, Katsumata Y, Ikari K, et al. Expansion of HLA-DR positive peripheral helper T and naive B cells in anticitrullinated protein antibody-positive individuals at risk for rheumatoid arthritis. Arthritis Rheumatol. 2024;76:1023–35. doi: 10.1002/art.42839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201:1659–67. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orange DE, Yao V, Sawicka K, Fak J, Frank MO, Parveen S, et al. RNA identification of PRIME cells predicting rheumatoid arthritis flares. N Engl J Med. 2020;383:218–28. doi: 10.1056/NEJMoa2004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grasso C, Pierie C, Mebius RE, van Baarsen LGM. Lymph node stromal cells: subsets and functions in health and disease. Trends Immunol. 2021;42:920–36. doi: 10.1016/j.it.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Hähnlein JS, Nadafi R, Jong TA, Semmelink JF, Remmerswaal EBM, Safy M, et al. Human lymph node stromal cells have the machinery to regulate peripheral tolerance during health and rheumatoid arthritis. Int J Mol Sci. 2020;21:5713. doi: 10.3390/ijms21165713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong LE, Wechalekar MD, Kutyna M, Small A, Lim K, Thompson-Peach C, et al. IDH-mutant myeloid neoplasms are associated with seronegative rheumatoid arthritis and innate immune activation. Blood. 2024;143:1873–7. doi: 10.1182/blood.2023023593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bossini-Castillo L, de Kovel C, Kallberg H, van 't Slot R, Italiaander A, Coenen M, et al. A genome-wide association study of rheumatoid arthritis without antibodies against citrullinated peptides. Ann Rheum Dis. 2015;74:e15. doi: 10.1136/annrheumdis-2013-204591. [DOI] [PubMed] [Google Scholar]
- 54.Padyukov L, Seielstad M, Ong RT, Ding B, Rönnelid J, Seddighzadeh M, et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis. 2011;70:259–65. doi: 10.1136/ard.2009.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, Liu Y, Jin S, Wang M, Jiao Y, Yang B, et al. Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat Commun. 2021;12:4977. doi: 10.1038/s41467-021-25246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDermott GC, DiIorio M, Kawano Y, Jeffway M, MacVicar M, Dahal K, et al. Reasons for multiple biologic and targeted synthetic DMARD switching and characteristics of treatment refractory rheumatoid arthritis. Semin Arthritis Rheum. 2024;66:152421. doi: 10.1016/j.semarthrit.2024.152421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dennis G, Jr, Holweg CT, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA, et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther. 2014;16:R90. doi: 10.1186/ar4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savola P, Kelkka T, Rajala HL, Kuuliala A, Kuuliala K, Eldfors S, et al. Somatic mutations in clonally expanded cytotoxic T lymphocytes in patients with newly diagnosed rheumatoid arthritis. Nat Commun. 2017;8:15869. doi: 10.1038/ncomms15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esensten JH, Wofsy D, Bluestone JA. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:560–5. doi: 10.1038/nrrheum.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 61.Naylor AJ, Filer A, Buckley CD. The role of stromal cells in the persistence of chronic inflammation. Clin Exp Immunol. 2013;171:30–5. doi: 10.1111/j.1365-2249.2012.04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoosuf N, Maciejewski M, Ziemek D, Jelinsky SA, Folkersen L, Müller M, et al. Early prediction of clinical response to anti-TNF treatment using multi-omics and machine learning in rheumatoid arthritis. Rheumatology (Oxford) 2022;61:1680–9. doi: 10.1093/rheumatology/keab521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tao W, Concepcion AN, Vianen M, Marijnissen ACA, Lafeber FPGJ, Radstake TRDJ, et al. Multiomics and machine learning accurately predict clinical response to adalimumab and etanercept therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2021;73:212–22. doi: 10.1002/art.41516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tasaki S, Suzuki K, Kassai Y, Takeshita M, Murota A, Kondo Y, et al. Multi-omics monitoring of drug response in rheumatoid arthritis in pursuit of molecular remission. Nat Commun. 2018;9:2755. doi: 10.1038/s41467-018-05044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Li S, Zhu J, Su W, Jian C, Zhang J, et al. Multi-omics profiling reveals potential alterations in rheumatoid arthritis with different disease activity levels. Arthritis Res Ther. 2023;25:74. doi: 10.1186/s13075-023-03049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]