Abstract
Chimeric Antigen Receptor (CAR) T-cell therapy, revolutionary in treating hematological malignancies, is emerging as a promising approach for systemic autoimmune rheumatic diseases (SARDs). This review examines the potential of CAR T-cell therapy in treating conditions such as systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and idiopathic inflammatory myopathies (IIMs). The evolution of CAR T cells technology, from first to fifth generation, has enhanced its efficacy and persistence. Early clinical studies in SARDs have shown encouraging results, with some patients achieving drug-free remission. CD19-targeted CAR T cells have demonstrated significant B-cell depletion and clinical improvement in patients with SLE, SSc, and IIMs. Despite promising outcomes, challenges remain, including cytokine release syndrome and the need for careful patient selection. Future directions include exploring dual-targeting CARs, chimeric autoantibody receptors (CAARs), and alternative cell sources like γδ T cells, regulatory T cells, natural killer cells. The integration of CAR-based cell therapy into treatment paradigms of patients with SARDs requires further research to optimize efficacy, mitigate side effects, and identify suitable target biomarkers. While hurdles exist CAR-based cell therapy holds the potential to revolutionize management of patients with SARDs, offering hope for long-term, drug-free remission in these complex autoimmune conditions.
Keywords: Chimeric Antigen Receptor T-cell therapy, Autoimmune diseases, Systemic lupus erythematosus, Systemic sclerosis, Idiopathic inflammatory myopathy
INTRODUCTION
Systemic autoimmune rheumatic diseases (SARDs) encompass a group of chronic and debilitating conditions characterized by dysregulation of the immune system, thereby leading to inflammation and damage across multiple organ systems. SARDs, such as systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and idiopathic inflammatory myopathies (IIMs), affect millions of people worldwide and pose significant challenges in terms of diagnosis, treatment, and long-term management [1].
Despite advances in conventional therapies and the introduction of biological disease-modifying antirheumatic drugs, a substantial proportion of patients with SARDs fail to achieve sustained remission or experience recurrent disease flares. The complex pathogenesis of these SARDs, involving multiple immune cell types and inflammatory pathways, underscores the need for novel therapeutic approaches to provide targeted and effective disease control [2].
Recently, the remarkable success of Chimeric Antigen Receptor (CAR) T-cell therapy in treating certain hematological malignancies has attracted interest in its potential applications to SARDs [3]. CAR T-cell are genetically engineered T lymphocytes that express a synthetic receptor capable of recognizing specific antigens and eliciting potent immune response [4]. The ability to redirect T-cell specificity towards chosen targets offers a promising avenue for addressing the underlying immunological aberrations in SARDs [5].
This review aims to provide a comprehensive analysis of the current landscape and future prospects of CAR T-cell therapy for SARDs. We examine the mechanistic basis of CAR T-cell function, evaluate ongoing studies and clinical trials, discuss potential cellular and molecular targets, and address the unique challenges and opportunities related to this innovative approach in the context of SARDs.
OVERVIEW OF CAR T-CELL THERAPY
Basic structure, production, and administration of CAR T cells
CAR T-cell therapy is a groundbreaking approach in cellular immunotherapy. At its core, the therapy involves genetic modification of a patient’s own T cells to express a CAR. This synthetic receptor combines the specificity of an antibody with the cytotoxic and proliferative capabilities of T cells [6].
CAR is a sophisticated fusion protein that forms the cornerstone of CAR T-cell therapy. Its structure typically comprises four main components, each crucial for the functionality of CAR T cells [7]. The extracellular antigen-binding domain, responsible for recognizing and binding to the target antigen, is most commonly derived from the single-chain variable fragment (scFv) of a monoclonal antibody (mAb). This scFv consists of variable regions of heavy and light chains of an antibody, connected by a flexible linker [8]. Connected to this is the hinge or spacer region, which is a flexible segment that links the antigen-binding domain to the transmembrane domain. Derived from molecules like CD8α, CD28, or IgG4-Fc, this hinge provides optimal spacing and flexibility, allowing the CAR to effectively engage with its target antigen. The length and composition of the hinge can significantly affect the function of CAR T cells and often needs to be optimized for each target antigen [9,10]. The transmembrane domain, typically a hydrophobic α-helix derived from type I membrane proteins, such as CD8, CD28, or CD3ζ, anchors the CAR in the T-cell membrane. The choice of the transmembrane domain can affect the stability and function of the CAR [11,12]. Finally, the intracellular signaling domains are crucial for activating T cells upon antigen recognition. They typically include an activation domain, usually the CD3ζ chain containing three immunoreceptor tyrosine-based activation motifs, and one or more costimulatory domains, most commonly CD28 or 4-1BB (CD137) [12,13]. The precise configuration of these components can be finetuned to optimize various aspects of CAR T-cell functions, including antigen sensitivity, T-cell activation threshold, persistence, and effector functions. This adaptability allows the design of each CAR to be tailored to a specific target antigen depending on the disease context, highlighting the versatility and potential of CAR T-cell therapy in addressing a wide range of medical conditions, including challenging autoimmune disorders such as SARDs.
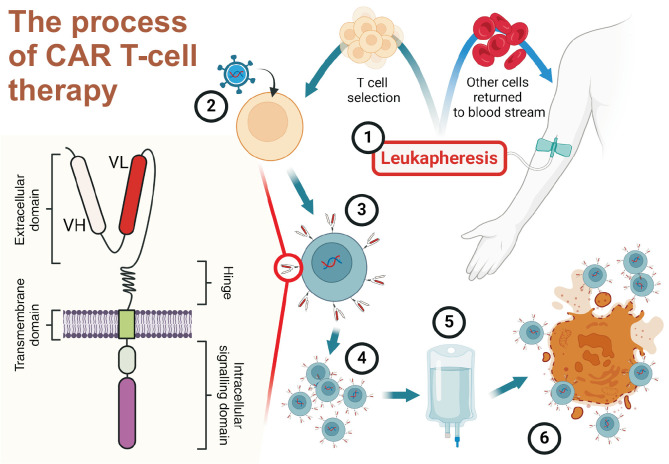
The processes of CAR T cells production and administration involve several key steps (Figure 1). Initially, the patient’s T cells are harvested by leukapheresis. These cells are genetically modified, usually through viral vector transduction, to express the CAR construct. The modified T cells undergo ex vivo expansion to achieve therapeutic numbers. Prior to CAR T cells infusion, patients typically undergo lymphodepleting chemotherapy to create a favorable environment for the engraftment of CAR T cells. Expanded CAR T cells are then reinfused into the patient, where they proliferate and exert their effector functions upon encountering target antigens [14].
Figure 1.
Overview of Chimeric Antigen Receptor (CAR) T cells production and mechanism of action. A schematic illustration depicting the key steps in CAR T-cell therapy process and the structure of a CAR. The left figure shows the basic structure of a CAR, consisting of an extracellular domain with variable heavy (VH) and light (VL) chains, a hinge region, a transmembrane domain, and an intracellular signaling domain. The numbered steps illustrate the production and administration process: (1) Leukapheresis is performed to collect the patient’s T cells. (2) The collected T cells are genetically modified using viral vectors to express the CAR. (3) The engineered T cells expressing CAR undergo selection. (4) Selected CAR T cells are expanded ex vivo to achieve therapeutic numbers. (5) The expanded CAR T cells are formulated for infusion. (6) After administration, CAR T cells recognize and eliminate target cells expressing specific antigens. This process has shown promising results in treating various systemic autoimmune rheumatic diseases. Adapted from the article of Guffroy et al. (Joint Bone Spine 2024;91:105702) [14].
From the first to the fifth generation of CAR T cells
The evolution of CAR T-cell technology has been marked by significant advancements aimed at enhancing its efficacy, persistence, and safety. This progression can be categorized into five distinct generations, each based on the strengths of its predecessors while addressing their limitations.
First-generation CAR T cells, developed in the late 1980s, consisted of an extracellular antigen-binding domain derived from an scFv fused to the intracellular signaling domain of CD3ζ [13]. While these pioneering constructs demonstrated the feasibility of redirecting the specificity of T cells, they showed limited in vivo expansion and persistence. To address these shortcomings, second-generation CAR T cells were developed by incorporating a costimulatory domain. This crucial modification, usually involving CD28 or 4-1BB (CD137), markedly enhanced the functionalities of T cells, including improved proliferation, cytokine production, and persistence [15]. The success of this design has been exemplified by therapies approved by US Food and Drug Administration (FDA), such as tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta), for treating certain hematological malignancies [16].
Third-generation CAR T cells were built on this framework by integrating two costimulatory domains. Common combinations included CD28 with either 4-1BB or OX40, aiming to synergistically boost the activation and longevity of T cells [17]. Although promising in preclinical models, the clinical superiority of the third-generation CARs over their second-generation counterparts remains a subject of ongoing investigation [18].
The advent of fourth-generation CAR T cells, also known as T cells Redirected for Universal Cytokine Killing or armored CARs, has marked a significant leap in design complexity. These sophisticated constructs incorporate additional elements for enhancing their functionality and overcoming the limitations observed in treating solid tumors. These features may include inducible cytokine expression (e.g., interleukin [IL] 12 or IL-18) to modulate the tumor microenvironment, chemokine receptors to improve tumor homing, or safety switches for considerable control [19]. This generation aims to create relatively versatile and potent CAR T cells that are capable of overcoming immunosuppressive tumor environments.
The emerging fifth-generation CAR T cells represent the cutting edge of this technology. These designs typically include components that can activate constitutive signaling pathways, such as the Janus kinase–signal transducer and activator of transcription (STAT) pathway, in addition to the other features from earlier generations [20]. For instance, some constructs incorporate a truncated cytokine receptor domain, such as IL-2Rβ, to enhance the proliferation and survival of T cells through STAT3/5 signaling. This approach aims to create CAR T cells with enhanced antitumor efficacy and persistence, potentially expanding their applicability to a relatively broad range of malignancies and autoimmune diseases [21].
Each generation of CAR T cells has been built upon its predecessors and opened new avenues for research and clinical applications. For example, the principles for developing the relatively late-generation CARs are being explored in the context of other immune cells, such as natural killer (NK) cells and macrophages, for broadening the scope of cellular immunotherapy [22,23].
Current applications in hematology
Currently, anti-CD19 and anti-B-cell maturation antigen (BCMA) CAR T-cell therapies are authorized for use in patients with hematological malignancies in Europe and the US. The field of hematology has witnessed revolutionary advancements with the approval of several CAR T-cell therapies. These groundbreaking treatments have shown remarkable efficacy in various hematological malignancies, particularly relapsed or refractory B-cell leukemia and lymphomas [16]. The first CAR T-cell therapy to receive FDA approval was tisagenlecleucel (Kymriah) in 2017, indicated for treating pediatric and young adult patients with B-cell precursor acute lymphoblastic leukemia [24]. This was followed by the approval of axicabtagene ciloleucel (Yescarta) for use in adult patients with large B-cell lymphoma [25]. Both therapies target CD19, a surface protein expressed on B cells. In 2020, brexucabtagene autoleucel (Tecartus) was approved for treating mantle cell lymphoma, offering a new option for this aggressive form of non-Hodgkin lymphoma [26]. The following year, lisocabtagene maraleucel (Breyanzi) was approved for treating large B-cell lymphomas, adding to the armamentarium against these challenging diseases [27]. Recently, idecabtagene vicleucel (Abecma) became the first CAR T-cell therapy approved for treating multiple myeloma by targeting BCMA [28]. These approvals mark significant milestones in cancer immunotherapy and are hopeful for patients with limited treatment options. However, although these therapies have shown impressive response rates, they also have potentially serious side effects, including cytokine release syndrome (CRS) and neurotoxicity, thereby necessitating careful patient selection and management [29]. The success of CAR T-cell therapy in hematology, particularly its ability to induce deep and durable responses in refractory diseases, has paved the way for exploring its potential in other fields including SARDs [30].
CURRENT STATUS OF CAR T-CELL THERAPY IN SARDS
Application of CAR T-cell therapy for systemic lupus erythematosus
SLE is a prototypic systemic autoimmune disease with a wide range of clinical manifestations including skin rashes, arthritis, nephritis, and neurological involvement [31]. B cells play a crucial role in the pathogenesis of SLE and are characterized by the production of autoantibodies against nuclear antigens [32]. The presence of autoantibodies, particularly anti-double-stranded DNA (anti-dsDNA) antibodies, often precedes the clinical manifestations of SLE, highlighting the central role of B cells in disease initiation and progression [33]. This understanding has led to developing B cell-targeted therapies, with B-cell depletion emerging as a promising therapeutic strategy [34]. B-cell depletion therapy with rituximab failed to meet primary endpoint in randomized controlled trial for SLE, despite some promising observations in case series [35]. Although rituximab has demonstrated effectiveness in some patients, particularly those with refractory disease, its use in SLE remains off-label in many countries owing to mixed results in large clinical trials [36]. Other B-cell-targeting biologics, including belimumab (which targets B-cell activating factor) and obinutuzumab (a second-generation anti-CD20 antibody), have also been studied in SLE with promising results [37,38].
The advent of CAR T-cell therapy has opened new avenues for highly targeted and potentially effective B-cell depletion in SLE. CAR T-cell therapy, which has shown remarkable success in hematological malignancies, is now being explored as a novel treatment approach for severe and refractory SLE. The most common target for CAR T cells in SLE is CD19, a surface protein expressed on B cells from the pro-B-cell stage through memory B cells but absent in long-lived plasma cells [30].
Preclinical studies on CAR T-cell therapy for SLE has provided crucial insights into its potential efficacy and mechanisms of action. CD19-targeted CAR T cells have demonstrated significant promise in murine models of lupus. Anti-CD19 CAR T cells effectively deplete B cells and plasma cells in MRL/lpr mice, leading to a marked reduction in autoantibodies and improvement in kidney function; This study has also reported long-term disease remission and prevention of lupus development in mice [39]. Second-generation CD19 CAR T cells with either CD28 or 4-1BB offer preventive and therapeutic effects in a murine model of SLE [40]. These preclinical studies have demonstrated the potential efficacy of CAR T-cell therapy in SLE and provided valuable insights into optimal targeting strategies and potential mechanisms of action, paving the way for clinical trials in humans.
Early case reports and small case series have provided encouraging results for CD19-targeted CAR T-cell therapy in patients with SLE. A landmark case report published in 2021 has described a patient with severe refractory SLE who achieved durable remission following treatment with CD19 CAR T cells; the patient experienced rapid and sustained depletion of CD19+ B cells, accompanied by a reduction in anti-dsDNA antibodies and improvement in clinical symptoms [30]. Following this initial report, a small case series of five patients with SLE treated with CD19 CAR T cells has further supported the potential of this approach; four of the five patients showed significant improvement in disease activity, with two achieving drug-free remission at the 12-month follow-up; the treatment was generally well tolerated, with manageable CRS in some patients [41]. These results suggest that CAR T-cell therapy may offer more profound and durable B-cell depletion than do conventional therapies, potentially leading to long-term disease modification in SLE. Several clinical trials (registered at ClinicalTrials.gov) are currently underway to further investigate the safety and efficacy of CAR T-cell therapy in patients with SLE (Table 1). These include a phase I trial of CD19-targeted CAR T cells (NCT03030976) and a study exploring BCMA-targeted CAR T cells in severe and refractory SLE (NCT04550910). Recently, results from a phase 1, single-arm, and open-label clinical trial have been published, focusing on patients of SLE with biopsy-confirmed lupus nephritis treated with BCMA and CD19 dual-targeting CAR T cells; dual-targeting CAR T cells were found to be effective in achieving drug-free remission and led to a significant reduction in SLE autoantibodies (NCT05030779) [42].
Table 1.
Representative clinical trials using CAR T-cell therapy for SARDs (registered at ClinicalTrials.gov)
| ClinicalTrials.gov ID | Phase | Target antigen | Target diseases | Distinctive properties of CAR T cells |
|---|---|---|---|---|
| NCT06585514 | 1 | CD19 | SLE | Not applicable |
| NCT06150651 | 1 | CD19 | SLE | Not applicable |
| NCT06056921 | 1 | CD19 | SLE, SSc, Sjögren’s syndrome, Dermatomyositis, AAV | Not applicable |
| NCT06106906 | 1/2 | CD19 | SLE | Not applicable |
| NCT06121297 | 1/2 | CD19 | SLE | Not applicable |
| NCT05859997 | Not applicable | CD19 | SLE, Sjögren’s syndrome, IIMs, AAV, Antiphospholipid syndrome | Allogenic |
| NCT05988216 | Not applicable | CD19 | SLE | Allogenic |
| NCT06294236 | 1 | CD19 | SLE, AAV | Allogenic |
| NCT06106893 | 1/2 | CD19 | SLE | Allogenic, CAR γδ T cell |
| NCT06544330 | 1 | CD19 | SLE | CAR T cell co-expressing an engineered IL-2 beta receptor. Followed by multiple subcutaneously engineered pegylated IL-2 cytokine. No conditioning chemotherapy. |
| NCT06308978 | 1 | CD19 | SLE | iPSC-derived CAR T cell |
| NCT06277427 | Not applicable | BCMA | SLE, AAV | Not applicable |
| NCT04561557 | 1 | BCMA | IMNM | Not applicable |
| NCT06497387 | 1 | BCMA | SLE,IgG4-related disease | Not applicable |
| NCT06038474 | 2 | BCMA | SLE | Not applicable |
| NCT06340750 | 1/2 | BAFF | SLE | Not applicable |
| NCT05030779 | 1 | CD19 and BCMA | SLE | Double antigen target |
| NCT05846347 | 1 | CD19 and BCMA | SLE | Double antigen target |
| NCT06249438 | 1 | CD20 and BCMA | SLE, IMNM | Double antigen target |
| NCT06530849 | 1/2 | CD19 and BCMA | SLE | Double antigen target |
| NCT05085444 | 1 | CD19 and BCMA | SSc | Double antigen target |
| NCT05085431 | 1 | CD19 and BCMA | Sjögren’s syndrome | Double antigen target |
| NCT06497361 | 1 | CD19 and BCMA | SLE,IgG4-related disease | Double antigen target |
| NCT06462144 | 1 | CD19 and CD20 | SLE, AAV, IIMs | Double antigen target |
| NCT06153095 | 1/2 | CD19 and CD20 | SLE | Double antigen target |
CAR: Chimeric Antigen Receptor, SARDs: systemic autoimmune rheumatic diseases, BCMA: B-cell maturation antigen, BAFF: B-cell activating factor, SLE: systemic lupus erythematosus, SSc: systemic sclerosis, AAV: anti-neutrophil cytoplasmic antibody-associated vasculitis, IIMs: idiopathic inflammatory myopathies, IMNM: immune-mediated necrotizing myopathy, IL: interleukin, iPSC: induced pluripotent stem cell.
These trials aimed to provide highly robust data on the potential of CAR T-cell therapy in SLE and to help define the optimal patient population for this treatment approach.
However, the application of CAR T-cell therapy in SLE is challenging. The therapy is associated with significant potential side effects, including CRS and neurotoxicity, which require careful management [29]. Additionally, the long-term consequences of profound B-cell depletion in patients with SLE, including the risk of infections and potential for the emergence of new autoimmune phenomena, remain to be fully elucidated.
In summary, although CAR T-cell therapy shows promise as a potentially transformative treatment strategy for severe and refractory SLE, further studies are needed to fully understand its long-term efficacy, safety profile, and optimal position in the SLE treatment paradigm. Ongoing clinical trials and accumulating real-world experiences will be crucial for shaping the future role of CAR T-cell therapy in SLE and other autoimmune diseases.
Application of CAR T-cell therapy for systemic sclerosis
SSc is a complex autoimmune disease, which is characterized by extensive fibrosis of the skin and internal organs, vascular abnormalities, and immune dysregulation [43]. The pathogenesis of SSc involves an intricate interplay among fibroblast activation, vascular dysfunction, and aberrant immune responses [44]. Recent studies have shed light on the potential role of B cells in the pathogenesis of SSc. The presence of SSc-specific autoantibodies, such as anti-topoisomerase I and anti-centromere antibodies, often precedes clinical manifestations, highlighting the potential importance of B cells in disease initiation and progression [45]. Further supporting this hypothesis, experimental studies using mouse models of SSc have demonstrated that the removal of B cells can lead to a reduction in fibrosis, a hallmark of the disease [46]. These findings collectively indicate that B cells play important roles in the complex pathophysiology of SSc and open new avenues for therapeutic interventions. Several studies have investigated the efficacy of rituximab in managing various aspects of SSc, including skin fibrosis, lung involvement, and overall disease activity [47]. A systematic review of observational studies and case series has suggested that rituximab may improve skin fibrosis and stabilize lung function in patients with SSc [48]. Despite these promising results, the use of rituximab for treating SSc faces several limitations. The heterogeneity of SSc presentation and variable responses to treatment among patients pose challenges in predicting treatment outcomes. Additionally, the optimal dosing regimen and treatment duration remain unclear, despite studies using different protocols [49,50]. Moreover, the effect of rituximab on specific SSc manifestations, such as digital ulcers and gastrointestinal involvement, is not well-established [51]. Although autologous hematopoietic stem cell transplantation has demonstrated significant therapeutic efficacy in severe SSc by surpassing the limitations of conventional treatments, it is associated with considerable transplant-related mortality [52]. Therefore, a therapeutic approach that achieves profound and comprehensive depletion of CD19+ B cells may offer a highly favorable safety profile while potentially maintaining high efficacy. Such an approach could strike a balance between treatment potency and tolerability in managing severe SSc.
The first case report detailing the administration of autologous CD19 CAR T-cell therapy to a patient with SSc was published in 2023 [53]. This landmark publication provides initial insights into the potential application of this innovative therapeutic approach for SSc treatment. According to a subsequently published case series, four patients with SSc treated with CD19 CAR T-cell therapy have shown improved modified Rodnan skin scores and European Scleroderma Trials and Research Group activity indices during a 1-year follow-up with drug-free remission [54]. A recent study has reported the outcomes of allogeneic CD19 CAR T-cell therapy, genetically engineered using the CRISPR-Cas9 technology, in two patients with diffuse cutaneous SSc. Remarkably, complete B-cell depletion has been observed in all patients within two weeks post-infusion, and extensive fibrosis in major organs, previously considered irreversible, has shown significant improvement [55]. These clinical studies demonstrate significant improvements in fibrosis and disease activity, and seroconversion of SSc-specific autoantibodies, thereby providing compelling evidence for the potential efficacy of CAR T-cell therapy in SSc.
Application of CAR T-cell therapy for idiopathic inflammatory myopathies
IIMs are rare autoimmune diseases characterized by muscle inflammation, weakness, and various systemic manifestations. The pathogenesis of IIMs involves a complex interplay among genetic susceptibility, environmental factors, and dysregulated immune responses [56]. The presence of myositis-specific and myositis-associated autoantibodies is a hallmark of IIMs and often correlates with distinct clinical phenotypes and disease courses [57]. These autoantibodies, produced by B cells, can be detected in patient sera even before the onset of clinical symptoms, suggesting a potential role of B cells in disease initiation.
Although glucocorticoids in high doses remain the first-line treatment for IIMs, their long-term use is associated with significant adverse effects. Other immunosuppressive agents, such as methotrexate, azathioprine, and mycophenolate mofetil, are often used as steroid-sparing agents or in refractory cases; however, their efficacy varies [58]. Several studies have investigated the efficacy of B-cell depletion therapies, particularly rituximab, in managing various aspects of IIMs. A large randomized controlled trial has indicated that rituximab in myositis study does not meet its primary endpoint in treating patients with refractory adult and juvenile myositis [59]. Subsequent analyses and case series have suggested that rituximab may be particularly effective in certain subgroups of patients with IIMs, particularly those with anti-synthetase syndrome (ASS) or dermatomyositis [60].
CAR T-cell therapy has shown remarkable success in SLE; however, its application in IIMs is still in the early stages (Table 1). As of 2024, a few pioneering studies have emerged, offering initial insights into the potential of this approach. Two case reports published in 2023 have described the treatment of a patient with refractory ASS using autologous CD19 CAR T cells, resulting in a significant improvement in muscle strength and reduction in muscle enzyme and anti-Jo-1 antibody levels, with a favorable safety profile [61,62]. This was followed by a small case series by Müller et al. [54], which included three patients with severe idiopathic inflammatory myositis treated with CD19 CAR T cells, showing complete remission of clinical manifestations and serological markers. Unlike previous CAR T cells that were derived from autologous sources, a recent case series has demonstrated that allogenic CD19 CAR T cells led complete remission and alleviate muscle damage in a patient with refractory immune-mediated necrotizing myopathy [40].
Although these early findings are encouraging, they also highlight the need for relatively large and controlled studies to fully elucidate the efficacy, safety, and long-term outcomes of CAR T-cell therapy in IIMs.
FUTURE DIRECTIONS OF CAR T-CELL THERAPY IN SARDS
CAR T-cell therapy and mAbs are two innovative approaches for treating SARDs, each with distinct advantages and limitations (Table 2). MAbs offer targeted immunosuppression with a well-established safety profile and are easily administered. Furthermore, mAbs are readily available and have relatively broad applicability at low costs across various autoimmune conditions. However, their effects are often transient and require repeated dosing [63]. In contrast, CAR T cells have the potential for long-lasting remission through persistent immunomodulation, as demonstrated in recent trials of SLE, SSc, and IIMs. A fundamental characteristic distinguishing CAR T-cell therapy from conventional B-cell depleting approaches lies in its superior capacity to achieve deep tissue B-cell depletion. Recent investigations have revealed that CAR T cells possess the unique ability to effectively penetrate and eliminate B cells within secondary lymphoid organs, particularly lymph nodes, in patients with SARDs- a capability not observed with rituximab treatment [64]. This enhanced tissue penetration may explain the profound and durable clinical responses observed in CAR T-cell therapy recipients. Nevertheless, CAR T-cell therapy faces challenges, including the risk of severe CRS, neurotoxicity, and complexity of production [16]. Recent clinical trials on SARDs are advancing towards maximizing the advantages of CAR T cells while minimizing their limitations, with the scope of research now expanding to include diseases such as anti-neutrophil cytoplasmic antibody-associated vasculitis and IgG4-related disease (Table 1). Furthermore, innovative technologies are being explored for enhancing the efficacy and safety of CAR-based cell therapy for SARDs (Figure 2).
Table 2.
Comparison between autologous CAR T-cell therapy and monoclonal antibodies in treatment of patients with SARDs
| Autologous CAR T cells | Monoclonal antibodies | |
|---|---|---|
| Advantage | Drug-free remission achieved through a single infusion Deep tissue depletion efficacy |
Relatively safe Ready access without delay Standardized output across multiple productions Industrial-level production output |
| Disadvantage | Serious side effects Cytokine release syndrome Immune effector cell-associated neurotoxicity syndrome Severe infection associated with lymphodepletion pretreatment High costs Manufacturing complexity and time-consuming nature of production |
Inconvenience of repeated administration Persistent medication uses to sustain disease remission Restricted therapeutic penetration beyond peripheral circulation |
CAR: Chimeric Antigen Receptor, SARDs: systemic autoimmune rheumatic diseases.
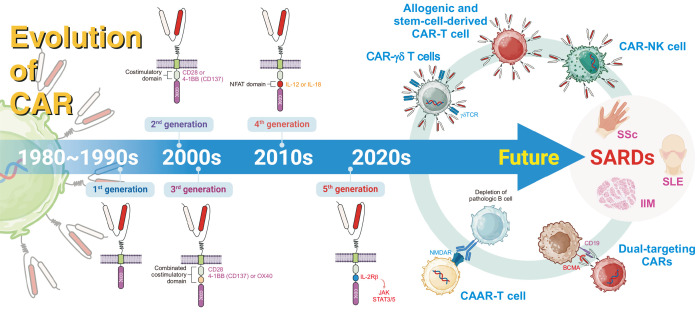
Figure 2.
The evolution of Chimeric Antigen Receptor (CAR) technology for systemic autoimmune rheumatic diseases (SARDs). This figure illustrates the evolution of CAR technology from its inception in the 1980s to future directions. Early CARs in the 1980~1990s featured a basic design with a CD3ζ signaling domain but limited efficacy. The 2000s saw the introduction of second-generation CARs with costimulatory domains (e.g., CD28 or 4-1BB) to enhance T-cell activation, and third-generation CARs combined multiple costimulatory domains for improved antitumor activity. In the 2010s, fourth-generation CARs incorporated transcriptional activation domains like NFAT to induce cytokine release (e.g., IL-12 or IL-18), while fifth-generation CARs added cytokine receptor signaling pathways (e.g., IL-2Rβ and JAK-STAT) for enhanced functionality. The 2020s brought advanced CAR variants such as CAAR-T cells for pathological B-cell depletion, dual-targeting CARs, CAR-γδ T cells, CAR-NK cells, allogeneic CAR T cells derived from stem cells. Looking ahead, CAR technologies are being explored for SARDs like systemic sclerosis (SSc), systemic lupus erythematosus (SLE), and idiopathic inflammatory myopathy (IIM), with innovations targeting diverse immune subsets and enhancing therapeutic efficacy while reducing toxicity. CD: cluster of differentiation, IL: interleukin, JAK: Janus kinase, STAT: signal transducer and activator of transcription, NK cells: natural killer cells, NMDAR: N-methyl-D-aspartate receptor, BCMA, B-cell maturation antigen, NFAT: nuclear factor of activated T cells, TCR: T-cell receptor, CAAR: chimeric autoantibody receptor.
The selection of appropriate targets is crucial for the success and safety of CAR T-cell therapy for SARDs. A promising strategy involves the use of dual-targeting CAR T cells designed to recognize two distinct antigens (e.g., CD19/BCMA and CD19/CD20) of B cells, which potentially improves specificity and reduces the risk of antigen escape [65]. To more selectively target specific autoreactive B cells, chimeric autoantibody receptor (CAAR)-based T-cell therapy has been developed [66]. This approach is expected to reduce toxicity by minimizing the depletion of normal B cells. A phase I clinical trial is currently underway for anti-Muscle-Specific Tyrosine Kinase (MuSK) CAAR-T cell therapy in patients with MuSK-associated myasthenia gravis (NCT05451212). The development of CAAR-T cell therapy is anticipated for other SARDs where disease mechanisms associated with specific autoantibodies are well understood.
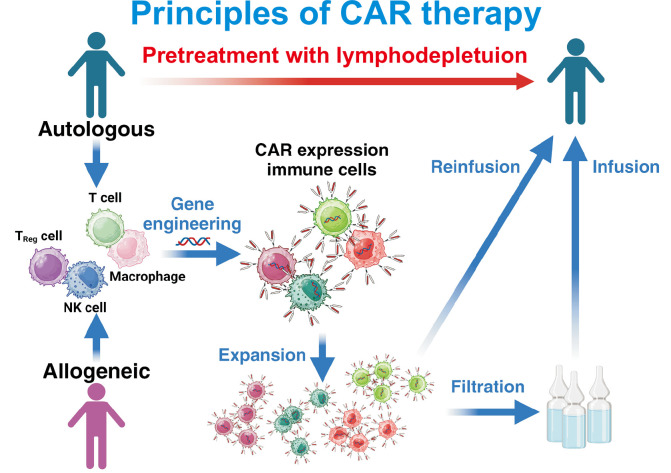
Besides conventional T cells, other cellular sources such as γδ T cells, regulatory T cells, and NK cells could also be effective therapeutic strategies for CAR-based cell therapy in SARDs (Figure 3). The innate ability of γδ T cells to distinguish healthy and pathogenic tissues may offer improved safety profiles in autoimmune settings [67]. Furthermore, although CAR-T regulatory cells exhibit a short survival period in vivo, they were able to modulate the autoimmunity in a lupus mouse model [68]. Additionally, the innate immune response and cytotoxic effects mediated by NK cells could make CAR-NK cells a promising therapeutic strategy for SARDs [69]. Allogeneic CAR T-cell therapies are being investigated as off-the-shelf options that could ensure immediate availability and reduce production complexities associated with autologous products [70]. Building on this, the advent of induced pluripotent stem cell-derived CAR T cells presents an opportunity for relatively standardized and scalable production, thereby potentially addressing manufacturing challenges and reducing variability among batches [71].
Figure 3.
Fundamental principles and new approaches of Chimeric Antigen Receptor (CAR)-based cell therapy. The figure illustrates the two main approaches to CAR-based cell therapy: autologous and allogeneic. In autologous therapy, immune cells are collected from the patient, while allogeneic therapy utilizes cells from healthy donors. Various immune cell types can be engineered to express CARs, including conventional T cells, regulatory T cells (T Reg cells), natural killer (NK) cells, and macrophages. Before CAR-cell infusion, patients typically undergo lymphodepletion pretreatment to create a favorable environment for the engineered cells. This comprehensive diagram showcases the versatility of CAR-based therapy platforms and their potential applications in treating systemic autoimmune rheumatic diseases (SARDs). The ability to utilize different cell types and sources provides multiple therapeutic strategies that can be tailored to individual patient needs. Adapted from the article of Capsomidis et al. (Mol Ther 2018;26:354-65) [67], Doglio et al. (Nat Commun 2024;15:2542) [68], Hassan et al. (Med Oncol 2024;41:127) [69], Aparicio et al. (Exp Hematol Oncol 2023;12:73) [70], and Sadeqi Nezhad et al. (Pharm Res 2021;38:931-45) [71].
While advancing CAR-based cell therapy technology is crucial, it is equally important to carefully select SARDs patients who are most likely to benefit. CAR-based cell therapy may be more effective in patients with active, progressive inflammation driven by excessive B-cell-mediated autoantibody production, rather than those in the advanced stages of tissue damage. Furthermore, as cytotoxic agents such as fludarabine and cyclophosphamide are administered as preconditioning regimens for CAR-based cell therapy, careful consideration must be given when deciding on treatment for patients at higher risk of complications, such as the elderly. In this context, further clinical studies are needed to determine the necessity and optimal dosing of preconditioning agents in SARDs [72].
The impact of CAR T-cell therapy on vaccination efficacy, both before and after treatment, represents a critical safety consideration in clinical practice. A recently published study evaluated immune responses to SARS-CoV-2 vaccination in patients who underwent CAR T-cell therapy, providing valuable insights into this important clinical question [73]. The investigation revealed that patients who received vaccination prior to CAR T-cell therapy demonstrated sustained humoral and cellular responses over an extended period. Also, the study demonstrated that even in patients vaccinated after CAR T-cell therapy, despite the absence of neutralizing antibody production, vaccine effectiveness was maintained through the development of T-cell responses. Based on these findings, there is an urgent need to establish comprehensive vaccination guidelines for the pre- and post-CAR T-cell therapy period, with particular emphasis on infection-related safety considerations.
The integration of CAR T-cell therapy into the treatment paradigm for SARDs requires a multidisciplinary approach involving rheumatologists, immunologists, and experts in cellular therapy. As our understanding of the complex interplay between CAR T cells and the autoimmune environment grows, we may see the emergence of highly personalized approaches, tailoring CAR designs and treatment protocols to individual patient characteristics and disease manifestations.
CONCLUSION
Despite significant challenges, the potential of CAR T-cell therapy for inducing profound and sustained remission in SARDs warrants further investigation and optimization. Early clinical success, particularly in refractory cases of SLE, SSc, and IIMs, highlights the transformative potential of this approach. As research progresses, the key areas for improvement include enhancing CAR specificity, mitigating adverse events, and identifying target biomarkers. Achieving drug-free remission, long considered elusive in rheumatology, may now be possible thanks to the precise mechanism of action of CAR T-cell therapy. This innovative approach could potentially reset a dysregulated immune system and address the root causes of complex autoimmune conditions. Although various challenges are recognized, the scientific community remains cautiously optimistic. As our understanding deepens, CAR-based cell therapy may offer new hope for patients with severe and refractory autoimmune diseases, and fundamentally transform SARD management, bringing us closer to the goal of long-term and drug-free remission.
ACKNOWLEDGMENTS
None.
Footnotes
FUNDING
None.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the manuscript or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors have read and agreed to the published version of the manuscript.
REFERENCES
- 1.Moutsopoulos HM. Autoimmune rheumatic diseases: one or many diseases? J Transl Autoimmun. 2021;4:100129. doi: 10.1016/j.jtauto.2021.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian X, Li M, Zeng X. The current status and challenges in the diagnosis and treatment of rheumatoid arthritis in China: an annual report of 2019. Rheumatol Immunol Res. 2021;2:49–56. doi: 10.2478/rir-2021-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohno R, Nakamura A. Advancing autoimmune rheumatic disease treatment: CAR-T cell therapies - evidence, safety, and future directions. Semin Arthritis Rheum. 2024;67:152479. doi: 10.1016/j.semarthrit.2024.152479. [DOI] [PubMed] [Google Scholar]
- 4.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari S, Bhandari S, Bhandari S. Chimeric antigen receptor T cell therapy for the treatment of systemic rheumatic diseases: a comprehensive review of recent literature. Ann Med Surg (Lond) 2023;85:3512–8. doi: 10.1097/MS9.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–98. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48. doi: 10.1016/j.annonc.2020.10.478. [DOI] [PubMed] [Google Scholar]
- 8.Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36:847–56. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–35. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen MC, Riddell SR. Designing chimeric antigen receptors to effectively and safely target tumors. Curr Opin Immunol. 2015;33:9–15. doi: 10.1016/j.coi.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol. 2010;184:6938–49. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 12.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–26. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–8. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guffroy A, Jacquel L, Guffroy B, Martin T. CAR-T cells for treating systemic lupus erythematosus: a promising emerging therapy. Joint Bone Spine. 2024;91:105702. doi: 10.1016/j.jbspin.2024.105702. [DOI] [PubMed] [Google Scholar]
- 15.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos CA, Rouce R, Robertson CS, Reyna A, Narala N, Vyas G, et al. Invivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin's lymphomas. Mol Ther. 2018;26:2727–37. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep. 2017;7:10541. doi: 10.1038/s41598-017-10940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147–67. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwivedi A, Karulkar A, Ghosh S, Rafiq A, Purwar R. Lymphocytes in cellular therapy: functional regulation of CAR T cells. Front Immunol. 2019;9:3180. doi: 10.3389/fimmu.2018.03180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–53. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Yang M, Zhang W, Liu N, Wang D, Jing L, et al. Chimeric antigen receptor-based natural killer cell immunotherapy in cancer: from bench to bedside. Cell Death Dis. 2024;15:50. doi: 10.1038/s41419-024-06438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 28.Munshi NC, Anderson LD, Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–16. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 29.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385:567–9. doi: 10.1056/NEJMc2107725. [DOI] [PubMed] [Google Scholar]
- 31.Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 33.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann K, Clauder AK, Manz RA. Targeting B cells and plasma cells in autoimmune diseases. Front Immunol. 2018;9:835. doi: 10.3389/fimmu.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–45. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 37.Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383:1117–28. doi: 10.1056/NEJMoa2001180. [DOI] [PubMed] [Google Scholar]
- 38.Arnold J, Dass S, Twigg S, Jones CH, Rhodes B, Hewins P, et al. Efficacy and safety of obinutuzumab in systemic lupus erythematosus patients with secondary non-response to rituximab. Rheumatology (Oxford) 2022;61:4905–9. doi: 10.1093/rheumatology/keac150. [DOI] [PubMed] [Google Scholar]
- 39.Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019;11:eaav1648. doi: 10.1126/scitranslmed.aav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin X, Xu Q, Pu C, Zhu K, Lu C, Jiang Y, et al. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2021;18:1896–903. doi: 10.1038/s41423-020-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackensen A, Müller F, Mougiakakos D, Böltz S, Wilhelm A, Aigner M, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022;28:2124–32. doi: 10.1038/s41591-022-02017-5. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, He S, Zhang W, Zhang H, DeStefano VM, Wada M, et al. BCMA-CD19 compound CAR T cells for systemic lupus erythematosus: a phase 1 open-label clinical trial. Ann Rheum Dis. 2024;83:1304–14. doi: 10.1136/ard-2024-225785. [DOI] [PubMed] [Google Scholar]
- 43.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–99. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 44.Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, et al. Systemic sclerosis. Nat Rev Dis Primers. 2015;1:15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 45.Didier K, Bolko L, Giusti D, Toquet S, Robbins A, Antonicelli F, et al. Autoantibodies associated with connective tissue diseases: what meaning for clinicians? Front Immunol. 2018;9:541. doi: 10.3389/fimmu.2018.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakkas LI, Bogdanos DP. Systemic sclerosis: new evidence re-enforces the role of B cells. Autoimmun Rev. 2016;15:155–61. doi: 10.1016/j.autrev.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015;74:1188–94. doi: 10.1136/annrheumdis-2013-204522. [DOI] [PubMed] [Google Scholar]
- 48.Thiebaut M, Launay D, Rivière S, Mahévas T, Bellakhal S, Hachulla E, et al. Efficacy and safety of rituximab in systemic sclerosis: French retrospective study and literature review. Autoimmun Rev. 2018;17:582–7. doi: 10.1016/j.autrev.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Elhai M, Boubaya M, Distler O, Smith V, Matucci-Cerinic M, Alegre Sancho JJ, et al. Outcomes of patients with systemic sclerosis treated with rituximab in contemporary practice: a prospective cohort study. Ann Rheum Dis. 2019;78:979–87. doi: 10.1136/annrheumdis-2018-214816. [DOI] [PubMed] [Google Scholar]
- 50.Daoussis D, Melissaropoulos K, Sakellaropoulos G, Antonopoulos I, Markatseli TE, Simopoulou T, et al. A multicenter, open-label, comparative study of B-cell depletion therapy with rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis Rheum. 2017;46:625–31. doi: 10.1016/j.semarthrit.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Bosello SL, De Luca G, Rucco M, Berardi G, Falcione M, Danza FM, et al. Long-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosis. Semin Arthritis Rheum. 2015;44:428–36. doi: 10.1016/j.semarthrit.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 52.van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311:2490–8. doi: 10.1001/jama.2014.6368. [DOI] [PubMed] [Google Scholar]
- 53.Bergmann C, Müller F, Distler JHW, Györfi AH, Völkl S, Aigner M, et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann Rheum Dis. 2023;82:1117–20. doi: 10.1136/ard-2023-223952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Völkl S, et al. CD19 CAR T-cell therapy in autoimmune disease - a case series with follow-up. N Engl J Med. 2024;390:687–700. doi: 10.1056/NEJMoa2308917. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Wu X, Tan B, Zhu L, Zhang Y, Lin L, et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell. 2024;187:4890–904.e9. doi: 10.1016/j.cell.2024.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372:1734–47. doi: 10.1056/NEJMra1402225. [DOI] [PubMed] [Google Scholar]
- 57.Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med. 2016;280:8–23. doi: 10.1111/joim.12451. [DOI] [PubMed] [Google Scholar]
- 58.Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, Visser M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76:1955–64. doi: 10.1136/annrheumdis-2017-211468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314–24. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aggarwal R, Bandos A, Reed AM, Ascherman DP, Barohn RJ, Feldman BM, et al. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 2014;66:740–9. doi: 10.1002/art.38270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Müller F, Boeltz S, Knitza J, Aigner M, Völkl S, Kharboutli S, et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet. 2023;401:815–8. doi: 10.1016/S0140-6736(23)00023-5. [DOI] [PubMed] [Google Scholar]
- 62.Pecher AC, Hensen L, Klein R, Schairer R, Lutz K, Atar D, et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA. 2023;329:2154–62. doi: 10.1001/jama.2023.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013;11:88. doi: 10.1186/1741-7015-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tur C, Eckstein M, Velden J, Rauber S, Bergmann C, Auth J, et al. CD19-CAR T-cell therapy induces deep tissue depletion of B cells. Ann Rheum Dis. 2024 Sep 11; doi: 10.1136/ard-2024-226142. [Epub]. doi: 10.1136/ard-2024-226142. [DOI] [PubMed] [Google Scholar]
- 65.Feng J, Hu Y, Chang AH, Huang H. CD19/BCMA CAR-T cell therapy for refractory systemic lupus erythematosus - safety and preliminary efficacy data from a phase I clinical study. Blood. 2023;142(Suppl 1):4835. doi: 10.1182/blood-2023-186669. [DOI] [Google Scholar]
- 66.Oh S, Mao X, Manfredo-Vieira S, Lee J, Patel D, Choi EJ, et al. Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells. Nat Biotechnol. 2023;41:1229–38. doi: 10.1038/s41587-022-01637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Capsomidis A, Benthall G, Van Acker HH, Fisher J, Kramer AM, Abeln Z, et al. Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol Ther. 2018;26:354–65. doi: 10.1016/j.ymthe.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doglio M, Ugolini A, Bercher-Brayer C, Camisa B, Toma C, Norata R, et al. Regulatory T cells expressing CD19-targeted chimeric antigen receptor restore homeostasis in Systemic Lupus Erythematosus. Nat Commun. 2024;15:2542. doi: 10.1038/s41467-024-46448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassan SH, Alshahrani MY, Saleh RO, Mohammed BA, Kumar A, Almalki SG, et al. A new vision of the efficacy of both CAR-NK and CAR-T cells in treating cancers and autoimmune diseases. Med Oncol. 2024;41:127. doi: 10.1007/s12032-024-02362-0. [DOI] [PubMed] [Google Scholar]
- 70.Aparicio C, Acebal C, González-Vallinas M. Current approaches to develop "off-the-shelf" chimeric antigen receptor (CAR)-T cells for cancer treatment: a systematic review. Exp Hematol Oncol. 2023;12:73. doi: 10.1186/s40164-023-00435-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sadeqi Nezhad M, Abdollahpour-Alitappeh M, Rezaei B, Yazdanifar M, Seifalian AM. Induced pluripotent stem cells (iPSCs) provide a potentially unlimited T cell source for CAR-T cell development and off-the-shelf products. Pharm Res. 2021;38:931–45. doi: 10.1007/s11095-021-03067-z. [DOI] [PubMed] [Google Scholar]
- 72.Schett G, Müller F, Taubmann J, Mackensen A, Wang W, Furie RA, et al. Advancements and challenges in CAR T cell therapy in autoimmune diseases. Nat Rev Rheumatol. 2024;20:531–44. doi: 10.1038/s41584-024-01139-z. [DOI] [PubMed] [Google Scholar]
- 73.Reimann H, Kremer AN, Blumenberg V, Schmidt K, Aigner M, Jacobs B, et al. Cellular and humoral immune responses to SARS-CoV-2 vaccination in patients after CD19.CAR T-cell therapy. Blood Adv. 2023;7:2066–9. doi: 10.1182/bloodadvances.2022007806. [DOI] [PMC free article] [PubMed] [Google Scholar]