Abstract
Bilateral perirenal lymphangiectasia is a rare condition characterized by the dilation of lymphatic ducts and the formation of fluid-filled cavities. Renal lymphangiectasia is rarely observed, affecting approximately 1% of cases, with only 34 bilateral cases reported in adults by 2025. This case report discusses a 55-year-old male with a history of recurrent pyelonephritis who was eventually diagnosed with bilateral acquired perirenal lymphangiectasia based on CT, MRI, and cytochemical analysis of perirenal fluid. The patient exhibited mild left flank pain without further complications, and given the disease's benign nature, an expectant management approach was adopted. Follow-up assessments confirmed an asymptomatic status. This report highlights the importance of considering lymphangiectasia in differential diagnoses for renal cystic lesions to avoid unnecessary procedures. Our case is the 35th documented instance of bilateral renal lymphangiectasia in the literature, underscoring the need for medical awareness of this condition to improve diagnosis and patient care.
Keywords: renal lymphangiectasia, bilateral, acquired, case report
Introduction
Lymphangiectasia is a rare condition characterized by the dilation of lymphatic ducts, which form cavities filled with lymphatic fluid [1]. Renal involvement is extremely rare (1%) and can be found in a perirenal, peripelvic, or intrarenal location. It has a similar incidence in men and women, affects both children and adults, and can be either congenital or acquired [2]. By 2025, 105 cases had been reported, 34 of which were adults with bilateral involvement [3] (See Table 2).
Table 2.
Demographics and treatment options of 35 cases of bilateral renal lymphangiectasia in adults.
| # | Author | Year published | Country | Sex | Age (years) | Treatment |
|---|---|---|---|---|---|---|
| 1 | Riehl et al. [4] | 1977 | Germany | Male | 22 | Conservative |
| 2 | De Maeyer et al. [5] | 1982 | Belgium | Male | 43 | Resection (lumbotomy) |
| 3 | Murakumo et al. [6] | 1986 | Japan | Female | 37 | Resection |
| 4 | Kutcher et al. [7] | 1987 | USA | Female | 74 | Nephrectomy |
| 5 | Meredith et al. [8] | 1988 | USA | Female | 23 | Nephrectomy |
| 6 | Meredith et al. [8] | 1988 | USA | Female | NS | Conservative |
| 7 | Murray et al. [9] | 1991 | USA | Male | 30 | Resection (Nephrectomy) |
| 8 | Schwarz et al. [10] | 1993 | Germany | Male | 42 | Conservative resection (Marsupialization) |
| 9 | Burton et al. [11] | 1994 | United Kingdom | Male | 35 | Percutaneous drainage |
| 10 | Leder et al. [12] | 1995 | USA | Female | NS | NS |
| 11 | Varela et al. [13] | 1998 | Spain | Female | 50 | NS |
| 12 | Ozmen et al. [14] | 2001 | Turkey | Female | 35 | Percutaneous drainage |
| 13 | Ramseyer [15] | 2001 | USA | Male | 28 | NS |
| 14 | Llorente et al. [16] | 2002 | Spain | Male | 30 | Conservative |
| 15 | Shaheen et al. [17] | 2003 | USA | Male | 36 | Conservative resection (Marsupialization) |
| 16 | Sarikaya et al. [18] | 2006 | Turkey | Female | 53 | Percutaneous drainage |
| 17 | Ashraf et al. [19] | 2007 | United Kingdom | Female | 23 | Conservative |
| 18 | Rastogi et al. [20] | 2008 | India | Male | 20 | Percutaneous drainage |
| 19 | Chen et al. [21] | 2009 | China | Female | 34 | Conservative |
| 20 | Bagheri et al. [22] | 2009 | Iran | Male | 21 | NS |
| 21 | Ali Al-Dofri [23] | 2009 | Yemen | Male | 22 | Percutaneous drainage |
| 22 | P. Antonopoulos [24] | 2010 | Greece | Female | 39 | Conservative |
| 23 | Rastogi et al. [25] | 2010 | India | Male | 60 | Conservative |
| 24 | Bazari et al. [26] | 2010 | USA | Male | 49 | Conservative resection (Marsupialization) |
| 25 | Magu et al. [27] | 2010 | India | Male | 28 | Percutaneous drainage |
| 26 | Hakeem et al. [28] | 2010 | India | Female | 50 | NS |
| 27 | Viglietti et al. [29] | 2012 | France | Male | 49 | Percutaneous drainage |
| 28 | Karkouche et al. [30] | 2013 | France | Female | 22 | Conservative resection (Marsupialization) |
| 29 | Elbanna et al. [31] | 2015 | Saudi Arabia | Male | 38 | Conservative |
| 30 | Renacci et al. [32] | 2017 | USA | Male | 30 | Conservative |
| 31 | Umapathy et al. [33] | 2020 | India | Female | 69 | Conservative |
| 32 | Umapathy et al. [33] | 2020 | India | Male | 43 | Conservative |
| 33 | Alzahrani et al. [34] | 2021 | Saudi Arabia | Female | 39 | Conservative |
| 34 | Ayed et al. [2] | 2024 | Saudi Arabia | Female | 55 | Conservative |
| 35 | Present case | 2025 | Ecuador | Male | 55 | Conservative |
NS: Non specified
This case report is particularly relevant because of the condition's exceptionally low frequency, unusual location, and evidenced acquired nature based on imaging findings.
Case report
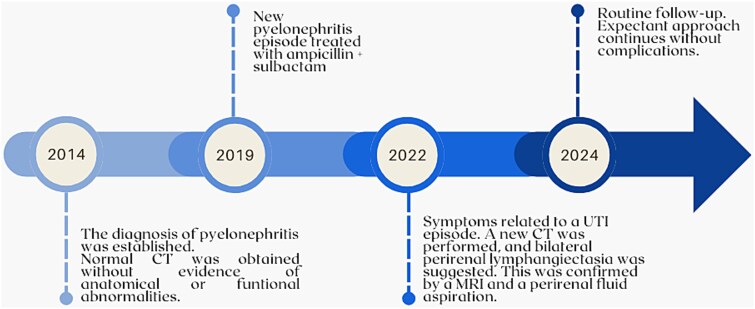
The patient is a 55-year-old mestizo male with no relevant family or medical history who, in 2014, presented fever, chills, and tenderness on palpation of the left flank. Regarding complementary findings, neutrophilic leukocytosis was observed and creatinine levels were normal (See Table 1). Urinalysis revealed bacteriuria, which led to a diagnosis of pyelonephritis. The patient was treated with oral ciprofloxacin 500 mg twice daily for 10 days, with partial improvement. Due to persistent symptoms, he was hospitalized and treated with IV ceftriaxone 1 g twice daily for 7 days before being discharged with prophylactic antibiotics. Follow-up evaluations showed persistent bacteriuria but normal findings on contrast-enhanced computed tomography (CT), as confirmed by a radiologist's report.
Table 1.
Baseline serum parameters levels.
| Serum paramethers | Reference values | Patient’s results |
|---|---|---|
| White Blood Cell Count | 4000–11 000/μl | 13 500/μl |
| Neutrophils (%) | 40%–70% | 85% |
| Lymphocytes (%) | 20%–45% | 12% |
| Creatinine | 0.5–1.2 mg/dl | 0.9 mg/dl |
| Urea | 15–40 mg/dl | 27 mg/dl |
| Blood Urea Nitrogen | 7–20 mg/dl | 18 mg/dl |
| Glucose | 70–100 mg/dl | 89 mg/dl |
In 2019, the patient returned with stabbing left flank pain and foul-smelling urine. Urinalysis confirmed bacteriuria, and a urinary tract infection (UTI) was diagnosed. Treatment with ampicillin-sulbactam 375 mg orally twice daily for 10 days resolved symptoms, but the patient did not attend follow-up.
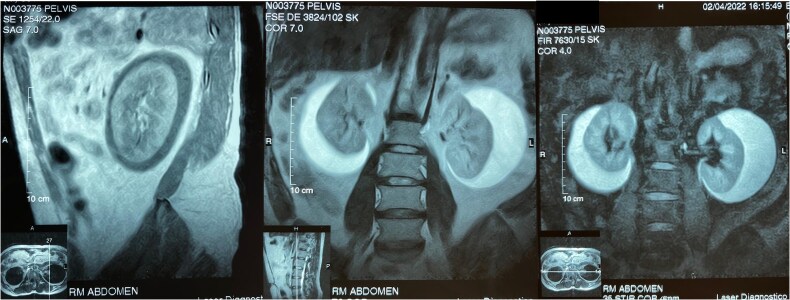
In 2022, the patient presented fever, anorexia, and general malaise. Considering his history of recurrent pyelonephritis, a CT was performed and revealed bilateral perirenal fluid with apparent septations. A subsequent magnetic resonance imaging (MRI) demonstrated subcapsular fluid encasing and displacing the renal parenchyma and adjacent structures, hypointense on T1, and hyperintense on T2. The right kidney contained approximately 332 ml of fluid, and the left 339 ml (Fig. 1). A perirenal fluid aspiration on the left flank showed yellow fluid with sparse lymphocytes, glucose 39.5 mg/dL, cholesterol 19.9 mg/dl, triglycerides 18.3 mg/dl, proteins 2.56 g/dl, albumin 1.85 g/dl, LDH 177 U/l, lipase 3.7 U/l, amylase 26 U/l, cells 0–1 per field, uncountable erythrocytes, and negative for Gram staining and culture, confirming the diagnosis of bilateral perirenal lymphangiectasia. Given the absence of complications, an expectant approach was chosen.
Figure 1.
Magnetic resonance imaging, T1, T2, and STIR sequences. The presence of subcapsular bilateral perirenal fluid envelopes and displaces the renal parenchyma and adjacent structures with apparent septa inside.
At routine follow-up in April 2024, the patient remained asymptomatic. No problems related to lymphangiectasia were observed on CT (Fig. 2), so expectant management continued (See the patient’s timeline in Fig. 3).
Figure 2.
Follow-up computed tomography, coronal and axial planes. No differences or complications were observed.
Figure 3.
Timeline of relevant events.
Patient perspective
I was highly concerned about my health because a doctor, upon reviewing the imaging tests, suggested a total nephrectomy and dialysis. I sought a second opinion at the UROMEDIK center, where I received a more detailed explanation of my condition, learning that I could keep my kidneys.
Discussion
This rare benign malformation of the renal lymphatic vessels has been documented under several terms, such as renal lymphangiectasia, renal lymphangiomatosis, and renal lymphangioma. The condition probably arises from a lack of communication within the renal and retroperitoneal lymphatic vessels, leading to lymphatic vessel dilation and cyst formation in and around the kidneys [2]. It affects both sexes equally and can occur at any age. Most cases are asymptomatic and 16% are identified incidentally [3].
This report shows evidence of an acquired bilateral perirenal lymphangiectasia in an adult, as the patient presented normal renal morphology on previous imaging examinations. The diagnosis was established through characteristic radiological findings supplemented by cytochemical fluid analysis. Nevertheless, the speed and accuracy were limited, probably due to the lack of clinical guidelines and the medical community’s lack of awareness of this condition.
Complications are rare but may include hypertension, ascites, and pleural effusion (the latter two of which should be treated immediately) [1, 3, 35, 36], and the percentage of different renal lymphatic vessels affected is unclear [1–3]. As there is no consensus for treating and supervising this condition, appropriate timing for follow-up should be determined based on the clinician’s criterion (annually in our case) and the potential complications’ usual standard of care (e.g. monthly or bimonthly in hypertension). In our case, the patient experienced mild pain in the left flank with no additional complications. Patient management may be expectant or include percutaneous drainage, medications, or, in severe cases, surgery, depending on the clinical presentation and complications [14, 19, 20]. Asymptomatic patients, like our case, usually require follow-up without invasive interventions. Hypertension, ascites, and pain can be controlled with antihypertensives, diuretics, and analgesics, respectively. Regarding the antihypertensives, the drug choice should follow the regular hypertension guidelines-guided standard of care. Ascites’ management should include loop or potassium sparing diuretics [37], and the analgesia should be personalized based on the patient’s needs.
This case contributes to the medical literature as the 35th documented instance of bilateral renal lymphangiectasia in adults, emphasizing the importance of considering this condition in differential diagnoses for renal cystic lesions.
Conclusion
Renal lymphangiectasia is a rare disease that usually remains asymptomatic. It is diagnosed through imaging studies and should be considered in the differential diagnosis for other renal cystic lesions. Radiologists and physicians must be familiar with this rare condition to ensure accurate diagnosis and treatment and avoid unnecessary invasive diagnostic and therapeutic procedures.
Acknowledgements
None.
Contributor Information
Daniel Peñaherrera-Vásquez, Facultad de Ciencias Médicas, Universidad Central del Ecuador, Iquique and Sodiro St - Quito 170403, Ecuador.
Diego Peñaherrera, UROMEDIK Urology Center, Belisario Quevedo Ave and Marqués de Maenza St - Latacunga 050101, Ecuador.
Luis Fuenmayor-González, Facultad de Ciencias Médicas, Universidad Central del Ecuador, Iquique and Sodiro St - Quito 170403, Ecuador; Unidad de Revisiones Sistemáticas y Metaanálisis-URMA, Facultad de Ciencias Médicas, Universidad Central del Ecuador, Iquique and Sodiro St - Quito 170403, Ecuador.
Ethics approval
Not applicable.
Informed consent
Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Conflict of interest
The authors declared no potential conflicts of interest regarding this article's research, authorship, and publication.
Funding
The authors received no financial support for this article's research, authorship, and publication.
Guarantor
Luis Fuenmayor-González, corresponding author.
CRediT statement
P-V. D. contributed to data collection, informed consent acquisition, writing the original draft, and writing, reviewing, and editing the final draft. P.D. contributed to data collection writing the original draft, and writing, reviewing, and editing the final draft. F-G. L. contributed to the conceptualization, methodology, project administration, validation, visualization, and writing, reviewing, and editing of the final draft.
References
- 1. Pereira LC, Gómez OMDLC, Batista RR. et al. Linfangiectasia renal bilateral. Revista Cubana de Urología 2019;8:61–5. Available from: https://revurologia.sld.cu/index.php/rcu/article/view/470 [Google Scholar]
- 2. Ayed A, Sohail SK, Rizvi SF. et al. Bilateral renal lymphangiectasia: literature review of a rare entity. Saudi Med J 2024;45:537–40. 10.15537/smj.2024.45.5.20231019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alshanafey S, Alkhani A, Alkibsi A. Renal lymphangiectasia in pediatric population: case series and review of literature. Ann Saudi Med 2022;42:139–44. 10.5144/0256-4947.2022.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riehl J, Schmitt H, Schäfer L. et al. Retroperitoneal lymphangiectasia associated with bilateral renal vein thrombosis. Nephrol Dial Transplant 1997;12:1701–3. 10.1093/ndt/12.8.1701 [DOI] [PubMed] [Google Scholar]
- 5. De Maeyer P, Baert AL, Usewils R. et al. CT demonstration of perirenal lymphatic cysts. Urol Radiol 1982;4:29–31. 10.1007/BF02924021 [DOI] [PubMed] [Google Scholar]
- 6. Murakumo M, Sakashita S. A case report of bilateral perirenal lymphatic cysts. Rinsho Hinyokika 1986;40:653–5. Available from: 10.11477/mf.1413204325 [DOI] [Google Scholar]
- 7. Kutcher R, Mahadevia P, Nussbaum MK. et al. Renal peripelvic multicystic lymphangiectasia. Urology 1987;30:177–9. 10.1016/0090-4295(87)90191-9 [DOI] [PubMed] [Google Scholar]
- 8. Meredith WT, Levine E, Ahlstrom NG. et al. Exacerbation of familial renal lymphangiomatosis during pregnancy. AJR Am J Roentgenol 1988;151:965–6. 10.2214/ajr.151.5.965 [DOI] [PubMed] [Google Scholar]
- 9. Murray KK, McLellan GL. Renal peripelvic lymphangiectasia: appearance at CT. Radiology 1991;180:455–6. 10.1148/radiology.180.2.2068311 [DOI] [PubMed] [Google Scholar]
- 10. Schwarz A, Lenz T, Klaen R. et al. Hygroma renale: pararenal lymphatic cysts associated with renin-dependent hypertension (page kidney). Case report on bilateral cysts and successful therapy by marsupialization. J Urol 1993;150:953–7. 10.1016/s0022-5347(17)35660-4 [DOI] [PubMed] [Google Scholar]
- 11. Burton IE, Sambrook P, McWilliam LJ. Secondary polycythaemia associated with bilateral renal lymphocoeles. Postgrad Med J 1994;70:515. 10.1136/pgmj.70.825.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leder RA, Frederick MG, Hall BP. et al. Genitourinary case of the day. Renal lymphangiomatosis AJR Am J Roentgenol 1995;165:197–200. 10.2214/ajr.165.1.7785592 [DOI] [PubMed] [Google Scholar]
- 13. Varela JR, Bargiela A, Requejo I. et al. Bilateral renal lymphangiomatosis: US and CT findings. Eur Radiol 1998;8:230–1. 10.1007/s003300050368 [DOI] [PubMed] [Google Scholar]
- 14. Özmen M, Deren Ö, Akata D. et al. Renal lymphangiomatosis during pregnancy: management with percutaneous drainage. Eur Radiol 2001;11:37–40. 10.1007/s003300000550 [DOI] [PubMed] [Google Scholar]
- 15. Ramseyer LT. Case 34: renal lymphangiectasia. Radiology 2001;219:442–4. 10.1148/radiology.219.2.r01ma17442 [DOI] [PubMed] [Google Scholar]
- 16. Llorente JG, García AD, Sacristan JS. et al. Renal lymphangiectasia: radiologic diagnosis and evolution. Abdom Imaging 2002;27:637–9. 10.1007/s00261-001-0147-z [DOI] [PubMed] [Google Scholar]
- 17. Shaheen M, Hilgarth KA, Hawes D. et al. A Mexican man with “too much blood”. Lancet 2003;362:806. 10.1016/s0140-6736(03)14291-2 [DOI] [PubMed] [Google Scholar]
- 18. Sarikaya B, Akturk Y, Bekar U. et al. Bilateral renal lymphangiomatosis mimicking hydronephrosis: multidetector CT urographic findings. Abdom Imaging 2006;31:732–4. 10.1007/s00261-005-8014-y [DOI] [PubMed] [Google Scholar]
- 19. Ashraf K, Raza SS, Ashraf O. et al. Renal lymphangiectasia. Br J Radiol 2007;80:e117–8. 10.1259/bjr/16931054 [DOI] [PubMed] [Google Scholar]
- 20. Rastogi R, Rastogi V. Computed tomographic scan in the diagnosis of bilateral renal lymphangiectasia. Saudi J Kidney Dis Transpl 2008;19:976–9. Available from: https://www.researchgate.net/publication/23442152 [PubMed] [Google Scholar]
- 21. Chen Z, Qi L, Tang Z. et al. Renal lymphangiectasia. Scand J Urol Nephrol 2009;43:428–30. 10.3109/00365590902930857 [DOI] [PubMed] [Google Scholar]
- 22. Bagheri MH, Zare Z, Sefidbakht S. et al. Bilateral renal lymphangiomatosis: sonographic findings. J Clin Ultrasound 2009;37:115–8. 10.1002/jcu.20488 [DOI] [PubMed] [Google Scholar]
- 23. Al-Dofri SAA. Renal lymphangiectasia presented by pleural effusion and ascites. J Radiol Case Rep 2009;3:5–10. 10.3941/jrcr.v3i10.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antonopoulos P, Charalampopoulos G, Constantinidis F. et al. Familial renal retroperitoneal lymphangiomatosis: personal experience and review of literature. JBR-BTR 2010;93:258–61. 10.5334/jbr-btr.331 [DOI] [PubMed] [Google Scholar]
- 25. Rastogi R, Rastogi U, Sarikwal A. et al. Renal lymphangiectasia associated with chronic myeloid leukemia. Saudi J Kidney Dis Transpl 2010;21:724–7. Available from: https://www.researchgate.net/publication/44851876 [PubMed] [Google Scholar]
- 26. Bazari H, Attar EC, Dahl DM. et al. Case records of the Massachusetts General Hospital. Case 23-2010. A 49-year-old man with erythrocytosis, perinephric fluid collections, and renal failure. N Engl J Med 2010;363:463–75. 10.1056/NEJMcpc1004086 [DOI] [PubMed] [Google Scholar]
- 27. Magu S, Agarwal S, Dalaal SK. Bilateral renal lymphangioma - an incidental finding. Indian J Nephrol 2010;20:114. 10.4103/0971-4065.65309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hakeem A, Gojwari T, Reyaz S. et al. Computed tomography findings in bilateral perinephric lymphangiomatosis. Urol Ann 2010;2:26. 10.4103/0974-7796.62922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Viglietti D, Sverzut JM, Peraldi MN. Perirenal fluid collections and monoclonal gammopathy. Nephrol Dialysis Transplant 2012;27:448–9. 10.1093/ndt/gfr433 [DOI] [PubMed] [Google Scholar]
- 30. Karkouche R, Rocher L, Guettier C. et al. Bilateral renal lymphangiomatosis: imaging and histopathologic findings. Abdom Imaging 2013;38:858–62. 10.1007/s00261-012-9977-0 [DOI] [PubMed] [Google Scholar]
- 31. Elbanna K, Almutairi B, Zidan A. Bilateral renal lymphangiectasia: radiological findings by ultrasound, computed tomography, and magnetic resonance imaging. J Clin Imaging Sci 2015;5:1–3. 10.4103/2156-7514.150449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Renacci RM, Bartolotta RJ. Gorham disease: lymphangiomatosis with massive osteolysis. J Clin Imaging 2017;41:83–5. 10.1016/j.clinimag.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 33. Umapathy S, Alavandar E, Renganathan R. et al. Renal Lymphangiectasia: an unusual mimicker of cystic renal disease – a case series and literature review. Cureus 2020;12:1–9. 10.7759/cureus.10849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alzahrani AM, Khamis AA, Barakat AE. et al. Bilateral renal Lymphangiectasia with No significant morbidity for over 25 years: a case report. Am J Case Rep 2021;22:e933934–1. 10.12659/AJCR.933934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeong KK, Han JA, Kim KR. et al. Renal Lymphangioma manifested As a solid mass on ultrasonography and computed tomography. J Ultrasound Med 2002;21:203–6. 10.7863/jum.2002.21.2.203 [DOI] [PubMed] [Google Scholar]
- 36. Pandya VK, Shah MK, Gandhi SP. et al. Bilateral renal Lymphangiectasia. J Clin Diagn Res 2016;10:TD01–2. 10.7860/JCDR/2016/19475.8409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flores Cruz G, Aguila Gómez MV, Lazo Vargas A. et al. Ascitis quilosa o quiloperitoneo, un desafío diagnóstico y un reto en el tratamiento conservador Para el cirujano general: presentación de un Caso y revisión de la literatura. Revista Médica La Paz 2021;27:47–53. Available from: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1726-89582021000100008&lng=es&nrm=iso&tlng=es [Google Scholar]