Abstract
Objective
The aim of this study was to summarize the effects of amino acids (AA) on renal function and nutritional indices in patients with renal insufficiency (RI) after treatment and to analyze the development trend in this field.
Methods
The bibliometric evaluation of scholarly contributions in this field was conducted using the Web of Science database, with data analyzed via Bibliometrix and VOSviewer software. The randomized controlled trials (RCTs) published before January 13, 2025, were systematically retrieved from Embase, PubMed, and the Cochrane Library and meta-analyses were performed using Review Manager 5.4 software.
Results
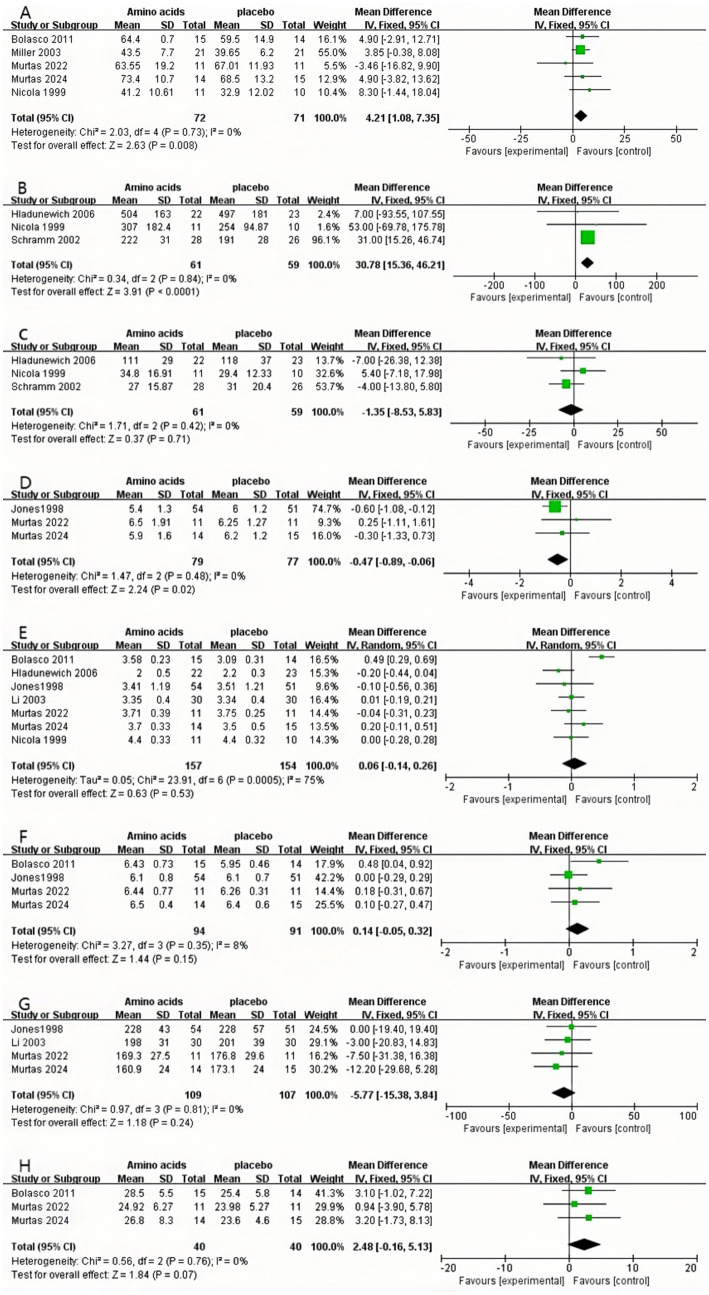
Key areas of focus included oxidative stress, chronic renal failure, hemodialysis, inflammation, chronic kidney disease, risk, plasma, progression, L-arginine, disease, and renal failure. Nine RCTs involving 407 participants were included, AA administration demonstrated significant effects compared to placebo: Increased blood urea nitrogen (MD: 4.21, 95% CI: 1.08 to 7.35, p = 0.008), elevated renal plasma flow (MD: 30.78, 95% CI: 15.36 to 46.21, p < 0.0001), and reduced uric acid levels (MD: −0.47, 95% CI: −0.89 to −0.06, p = 0.02).
Conclusion
These findings suggest that AA supplementation may partially improve renal function in RI patients. The progression and possible mechanisms of chronic kidney disease, as well as the search for new biomarkers, will be the trend of research and development in this field.
Keywords: renal insufficiency, amino acids, meta-analysis, bibliometrics, renal function, nutritional indicators
1. Introduction
Renal insufficiency (RI) is a clinical syndrome characterized by impaired kidney metabolic function resulting from diverse etiologies, leading to disruptions in the body’s metabolites, fluid balance, electrolytes, and acid–base homeostasis (1). RI is categorized into chronic renal insufficiency and acute renal insufficiency (2). However, inconsistent terminology in renal function and disease classification has prompted standardized nomenclature: chronic renal insufficiency is now termed chronic kidney disease (CKD) per Kidney Disease Improving Global Outcomes (KDIGO) conference consensus (3, 4), while acute renal insufficiency is defined as acute kidney injury (5–7). CKD encompasses pathologies such as glomerulonephritis, diabetic nephropathy, and end-stage renal disease (8, 9), and is staged 1–5 based on severity, progressing from mild functional impairment to renal failure requiring dialysis or transplantation. Projections indicate that by 2040, kidney disease will rank as the fifth leading cause of reduced life expectancy globally (4, 8).
Amino acids (AA) and their derivatives are ubiquitously utilized as dietary supplements in clinical and nutritional contexts, exerting profound influences on renal physiology and homeostasis. RI is associated with dysregulated serum AA concentrations, though evidence suggests moderate dietary AA intake may confer therapeutic benefits (1). Studies indicate that AA infusion protects renal function through mechanisms such as replenishing depleted AA, enhancing renal plasma flow (RPF), and improving estimated glomerular filtration rate (10–12). The kidneys also contribute significantly to protein metabolism: in healthy individuals, 97–98% of filtered AA and peptides undergo tubular reabsorption. In CKD patients, however, inflammatory states and metabolic acidosis disrupt AA and protein metabolism. Progressive CKD further alters filtration and reabsorption, exacerbating AA wasting and proteinuria (13, 14).
AA and their derivatives, as fundamental components of proteins and peptides, serve as critical dietary supplements with profound implications for renal health (15). AA exhibits multifaceted roles in nutrition, therapy, and medicine, frequently acting as a primary active ingredient in nutritional formulations (16). Biomarkers, such as albumin (ALB), total protein (TP), and transferrin (TRF), are indispensable for evaluating nutritional status in patients with RI, while also serving as predictors of disease progression and clinical outcomes (17). Despite their therapeutic potential, excessive intake of most AA supplements, particularly amino acids like glutamine and arginine, which are essential for tumor cell proliferation, may induce adverse effects (18). In RI patients, prolonged high-dose AA administration can disrupt amino acid homeostasis, leading to nitrogen accumulation and exacerbating renal damage (19).
Preclinical studies have demonstrated that AA supplementation improves glomerular filtration rate (GFR) in RI models, yet clinical trials report inconsistent outcomes. This discrepancy is compounded by substantial heterogeneity in study design, including variations in AA formulations, intervention durations, and patient baseline characteristics (20, 21). Notably, while AA metabolism is intricately linked to nutritional status, current research has underemphasized key nutritional parameters—such as ALB, TP, TRF, and body mass index (BMI)—which are central to assessing both nutritional adequacy and renal disease trajectories (17). Existing literature further highlights conflicting evidence regarding AA’s effects on these markers: divergent results have been reported for ALB, TP, and TRF in RI populations undergoing AA interventions. Although animal studies suggest renoprotective and nutritional benefits, the clinical evidence base remains fragmented, characterized by a lack of systematic quantitative synthesis, especially of high-quality randomized controlled trials (RCTs), hindering clarity on AA’s clinical applicability and optimal dosing strategies in RI (11, 22, 23). To address this gap, we conducted a bibliometric analysis to map research trends and a meta-analysis of RCTs to (1) evaluate AA effects on renal function: blood urea nitrogen (BUN), RPF, GFR, uric acid (UA), and nutritional parameters; (2) identify intervention modifiers through subgroup analyses; and (3) delineate unresolved evidence gaps for future inquiry. This integrated approach aims to quantify the clinical benefits of AA in renal protection and nutritional efficacy, providing robust evidence to inform clinical practice.
2. Materials and methods
2.1. Bibliometric analysis
Guided by PubMed Medical Subject Headings (MeSH) terminology and the KDIGO conference consensus statement, we systematically retrieved articles published between 1 January 2000 and 31 December 2024 from the Web of Science database on 1 January 2025, using RI and AA as primary search keywords (7, 24). The complete search strategy is provided in Supplementary material S1, and detailed eligibility criteria are outlined in Supplementary material S2. For bibliometric evaluation, the following data were extracted from the selected literature: author information, journal affiliations, citation metrics, and institutional collaborations. Text analysis and data visualization were performed using R software (v4.4.1) and VOSviewer (v1.6.20) (25, 26).
2.2. Meta-analysis
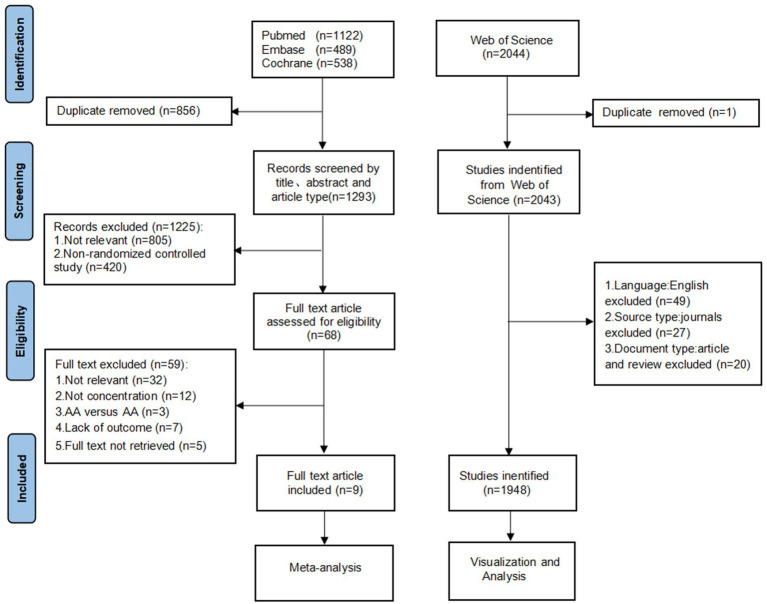
We registered the study on PROSPERO (CRD42024610476) in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (27). A systematic search was conducted across Embase, PubMed, and the Cochrane Library to identify English-language articles examining the effects of AA supplementation on renal function and nutritional indicators in patients with RI. The search encompassed all available records from database inception until January 13, 2025. The search strategy utilized three core terms combined with the AND operator and MeSH subject headings: RCTs, AA, and RI (Supplementary material S1). The screening process is summarized in Figure 1. Two independent investigators (Liu and Li) screened titles, keywords, and abstracts against predefined inclusion and exclusion criteria (Supplementary material S2). Full-text review was performed to extract the following data: (1) study characteristics: lead author, year of publication, sample size; (2) study participant characteristics: age, gender, total number of participants, number of intervention groups, number of control groups, intervention and control measures, and duration of the intervention; (3) type of patients with RI; and (4) data on study outcomes: BUN, RPF, GFR, UA, ALB, TP, TRF, BMI. Data extraction was conducted independently by both investigators using standardized forms, with discrepancies resolved through consultation with a third researcher (Zhang). Of the initial 1,293 identified records, 1,225 were excluded during title/abstract screening due to duplication (856), irrelevance (805), or non-RCT design (420), leaving 68 articles for full-text evaluation. Full-text exclusion criteria included off-topic content, missing outcome data, control groups receiving AA supplementation, or inaccessible full-text materials.
Figure 1.
Flowchart of study selection for meta-analysis. AA, amino acids.
Nine studies were ultimately included in the meta-analysis. For studies reporting standard errors instead of standard deviation (SD), conversions were performed using the formula (28):
Statistical analyses were conducted using Review Manager 5.4 (The Cochrane Collaboration, Oxford, United Kingdom) to generate forest plots and subgroup analyses. Publication bias and sensitivity analyses were performed using Stata 18.0 (StataCorp, College Station, TX, United States) (29, 30). Heterogeneity across studies was evaluated using the I2 value, with thresholds defined as follows: I2 ≤ 25% (low heterogeneity), 26–50% (moderate heterogeneity), and >50% (high heterogeneity). Sensitivity analyses were performed in cases of I2 > 50% or p < 0.05, and studies were excluded on a case-by-case basis to determine their impact on the overall estimate. Data were synthesized using either a fixed-effects or random-effects meta-analysis model, selected based on the degree of heterogeneity. A random-effects model was applied for I2 > 50%, while a fixed-effects model was used for I2 ≤ 50%. Continuous outcome measures were reported as Mean Difference (MD) and 95% confidence intervals (95% CI). Statistical significance was defined as p < 0.05 for all analyses.
2.3. Inclusion and exclusion criteria
2.3.1. Bibliometric inclusion and exclusion criteria
The included studies met the following criteria: (1) focus on patients with renal insufficiency undergoing interventions involving amino acids; (2) peer-reviewed articles and literature reviews; and (3) publications dated between January 1, 2000, and December 31, 2024.
Excluded studies encompassed (1) animal studies; (2) non-academic materials (e.g., conference proceedings, news reports, patents, calls for papers, newspaper abstracts); (3) non-English publications; (4) journals irrelevant to the topic; and (5) articles lacking accessible data.
2.3.2. Meta-analysis inclusion and exclusion criteria
To ensure precision, search results underwent manual screening during the meta-analysis, with post-screening studies validated twice and independently.
Inclusion criteria: (1) Population: Adult participants (≥18 years) diagnosed with renal impairment; (2) Study design: RCTs; (3) Language: English-language publications; (4) Intervention: Use of L-amino acids (individual or combined formulations) in the treatment group, compared to a placebo control; and (5) Outcomes: At least one renal function marker (BUN, RPF, GFR, UA) or nutritional indicator (ALB, TP, TRF, BMI).
Exclusion criteria: (1) animal or pediatric (<18 years) studies; (2) non-RCT designs (e.g., observational studies, case reports); (3) interventions involving non-L-amino acids, structurally modified L-amino acids (e.g., glutamine, acetylcysteine), or protein/amino acid-based nutritional supplements in control groups; and (4) insufficient outcome data or unavailable full-text articles.
2.4. Quality assessment
The risk of bias for each included trial was independently evaluated by two investigators using the Cochrane Risk of Bias Tool for Randomized Controlled Trials RoB 2 (31). This assessment focused on five domains: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in outcome measurement; and (5) bias in the selection of reported results. Study quality was categorized as follows: High-risk studies: Trials with two or more domains rated as high risk of bias; low-risk studies: 5 or more low-risk studies and no more than 1 high-risk study; Studies with some concerns: Trials that did not meet criteria for low-risk or high-risk classifications (32). Publication bias for primary outcomes was assessed using Stata 18.0 software to generate funnel plots or perform Egger’s regression test (33).
3. Results
3.1. Results of bibliometric analysis
3.1.1. General description of retrieved publications
To address source heterogeneity and language variability, inclusion criteria were restricted to English-language research articles. Further exclusions were applied based on document and publication type (Figure 1). A total of 1948 English-language publications on AA and RI patients’ renal function and nutrition were analyzed. Among these, original research articles predominated (1,530 articles, 75.54%), followed by reviews (418 articles, 21.46%).
3.1.2. Trends in publication growth
Supplementary Figure S1 illustrates annual and cumulative publication trends from 2000 to 2024. Annual output ranged from 51 to 127 articles, with 973 publications from 2015 to 2024, representing a 45% increase compared to the preceding decade (672 publications from 2000 to 2014), indicating modest growth in research activity.
3.1.3. Bibliometric analysis of articles
Table 1 lists the top 10 most cited articles in the field over the past 24 years (citation range: 482–1898). The most cited work, “Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials,” published in Clinical Nutrition by Kondrup in 2003, was the most cited article (1898 citations). Notably, two of the top-cited articles appeared in the New England Journal of Medicine.
Table 1.
Top cited list of the top 10 highly cited papers related to renal function or nutritional indicators in patients with renal insufficiency treated with amino acids from 2000 to 2024.
| Ranking | Authors | Year | Source title | Cited by |
|---|---|---|---|---|
| 1st | Kondrup, J et al | 2003 | Clinical Nutrition | 1,898 |
| 2nd | Bauer J, et al | 2013 | Journal of the American Medical Directors Association | 1,573 |
| 3rd | Fanali G, et al | 2012 | Molecular Aspects Of Medicine | 1,385 |
| 4th | Vanholder R, et al | 2003 | Kidney International | 1,322 |
| 5th | Muscaritoli M, et al | 2010 | Clinical Nutrition | 1,198 |
| 6th | Tepel M, et al | 2000 | New England Journal of Medicine | 1,090 |
| 7th | Mangano DT, et al | 2006 | New England Journal of Medicine | 803 |
| 8th | Carrero JJ, et al | 2013 | Journal of Renal Nutrition | 602 |
| 9th | Gheorghiade M, et al | 2004 | Journal of the American Medical Association | 495 |
| 10th | Ikizler TA, et al | 2013 | Kidney International | 482 |
3.1.4. Bibliometric analysis of authors
The 1948 publications involved 11,354 authors, with the top 66 contributors (≥5 publications each) representing 0.58% of all authors. The top 10 authors with the most publications and the top 10 most cited authors in the field of AA on renal function and nutrition in patients with RI from 2000 to 2024 are shown in Table 2. Kamyar Kalantar-Zadeh led in both productivity (23 articles) and citations (2693).
Table 2.
Top 10 prolific authors and top 10 most cited authors on renal function or nutritional indicators in patients with renal insufficiency treated with amino acids from 2000 to 2024.
| Rank | Author | H-index | G-index | M-index | TC | NP | PY start |
|---|---|---|---|---|---|---|---|
| 1st | Kalantar-Zadeh K. | 19 | 23 | 1.000 | 2,693 | 23 | 2007 |
| 2nd | Kopple Joel D. | 14 | 15 | 0.700 | 1,031 | 15 | 2006 |
| 3rd | Talat Alp I. | 13 | 13 | 0.650 | 1,701 | 13 | 2006 |
| 4th | Stenvinkel Petter | 11 | 12 | 0.579 | 2,026 | 12 | 2007 |
| 5th | Bakker Stephan J. L. | 6 | 12 | 0.316 | 235 | 12 | 2007 |
| 6th | Garibotto Giacomo | 10 | 11 | 0.526 | 683 | 11 | 2007 |
| 7th | Cupisti Adamasco | 10 | 10 | 0.588 | 456 | 10 | 2009 |
| 8th | Garneata Liliana | 6 | 10 | 0.429 | 386 | 10 | 2012 |
| 9th | Fiaccadori Enrico | 9 | 9 | 0.500 | 410 | 9 | 2008 |
| 10th | Kovesdy Csaba P. | 9 | 9 | 0.500 | 969 | 9 | 2008 |
| 1st | Kalantar-Zadeh K. | 19 | 23 | 1.000 | 2,693 | 23 | 2007 |
| 2nd | Zidek Walter | 2 | 2 | 0.077 | 2,412 | 2 | 2000 |
| 3rd | Teta Daniel | 6 | 6 | 0.333 | 2,227 | 6 | 2008 |
| 4th | Stenvinkel Petter | 11 | 12 | 0.579 | 2,026 | 12 | 2007 |
| 5th | Kondrup Jens C. | 1 | 1 | 0.043 | 1,898 | 1 | 2003 |
| 6th | Talat Alp I. | 13 | 13 | 0.650 | 1,701 | 13 | 2006 |
| 7th | Passlick Deetjen J. | 2 | 2 | 0.087 | 1,628 | 2 | 2003 |
| 8th | Wanner C. | 4 | 4 | 0.154 | 1,614 | 4 | 2000 |
| 9th | Brunet Philippe | 2 | 2 | 0.087 | 1,594 | 2 | 2003 |
| 10th | Boirie Yves | 2 | 2 | 0.154 | 1,576 | 2 | 2013 |
TC, total cited; NP, number of publications; PY start, publication year start.
3.1.5. Bibliometric analysis of journals
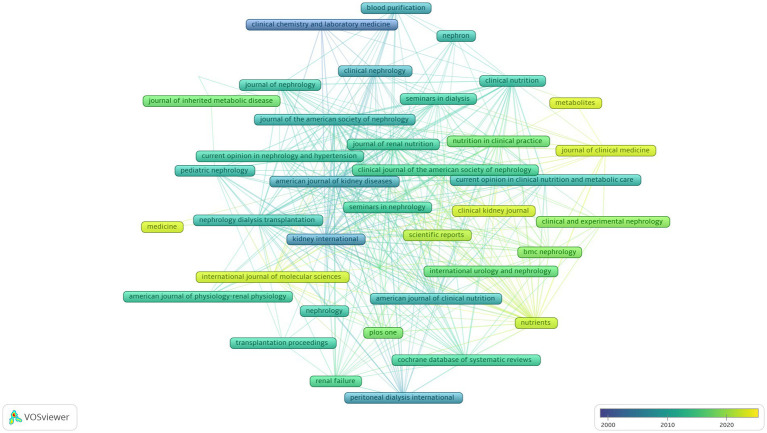
Table 3 lists the top 10 journals with the most publications related to the field of renal function and nutrition in patients with AA and RI. Kidney International ranked first (65 articles, 3.34%), followed by the Journal of Renal Nutrition (53 articles, 2.72%). Figure 2 shows a trend graph of the evolution of journals in this field over time.
Table 3.
List of the top 10 journals publishing research on renal function or nutritional indicators in patients with renal insufficiency treated with amino acids from 2000 to 2024.
| Ranking | Journal | Frequency | % | IFa |
|---|---|---|---|---|
| 1st | Kidney International | 65 | 3.34 | 14.8 |
| 2nd | Journal of Renal Nutrition | 53 | 2.72 | 3.4 |
| 3rd | American Journal of Kidney Diseases | 51 | 2.62 | 9.4 |
| 4th | Nephrology Dialysis Transplantation | 47 | 2.41 | 4.8 |
| 5th | Nutrients | 41 | 2.10 | 4.8 |
| 6th | Renal Failure | 34 | 1.75 | 3.1 |
| 7th | BMC Nephrology | 31 | 1.59 | 2.2 |
| 8th | PLoS ONE | 31 | 1.59 | 2.9 |
| 9th | Clinical Nephrology | 28 | 1.44 | 1.1 |
| 10th | Transplantation Proceedings | 28 | 1.44 | 0.8 |
SCR, standard competition ranking; IF, impact factor. An impact factors based on the 2023 journal citation reports 2023 from Clarivate analytics.
Figure 2.
Evolution of journals over time plotted with VOSviewer. In the visualization map, the color of the label reflects the sequence of the appearance of a journals. The yellower the color of a journals was, the later it appeared, and the bluer the color of a journals was, the earlier the journals appeared.
3.1.6. Bibliometric analysis of countries
Over the past 24 years, at least 86 different countries have been involved in the publication of research in the field of renal function and nutrition in patients with AA and RI. The top 10 countries have authored a total of 1,387 articles, accounting for 71.2% of all studies in the relevant field. Table 4 shows the ranking of production and intercountry collaboration by author’s country, with the United States having the highest number of global publications in this field. Supplementary Figure S2 shows a map of national or regional collaborations in the field of renal function and nutrition in AA and RI between the major participating countries from 2000 to 2024. Table 5 shows the country citation analysis.
Table 4.
Top 10 countries ranked by collaborations based on corresponding authors.
| Rank | Country/Region | Articles | SCP | MCP | MCP% |
|---|---|---|---|---|---|
| 1st | USA | 385 | 297 | 88 | 22.9 |
| 2nd | China | 260 | 228 | 32 | 12.3 |
| 3rd | Italy | 171 | 142 | 29 | 17.0 |
| 4th | Japan | 143 | 136 | 7 | 4.9 |
| 5th | Germany | 125 | 77 | 48 | 38.4 |
| 6th | France | 78 | 56 | 22 | 28.2 |
| 7th | UK | 77 | 50 | 27 | 35.1 |
| 8th | Netherlands | 55 | 42 | 13 | 23.6 |
| 9th | Sweden | 47 | 34 | 13 | 27.7 |
| 10th | Turkey | 46 | 42 | 4 | 8.7 |
SCP, single country publication; MCP, multi-country publication. Country represent the affiliation of the corresponding author. Articles denote the number of publications per country based on the corresponding author’s affiliation.
Table 5.
Top 10 most cited countries on renal function or nutritional indicators in patients with renal insufficiency treated with amino acids from 2000 to 2024.
| Rank | Country/region | Total cited | Average article citations |
|---|---|---|---|
| 1st | USA | 20,201 | 52.5 |
| 2nd | Italy | 8,452 | 49.4 |
| 3rd | Germany | 7,330 | 58.6 |
| 4th | China | 4,092 | 15.7 |
| 5th | France | 3,192 | 40.9 |
| 6th | Japan | 3,084 | 21.6 |
| 7th | UK | 2,943 | 38.2 |
| 8th | Belgium | 2,924 | 132.9 |
| 9th | Denmark | 2,565 | 106.9 |
| 10th | Sweden | 2,194 | 46.7 |
3.1.7. Bibliometric analysis of institutions
The University of California System had the highest number of publication-related studies among institutions worldwide, with 139 articles, or 7.14% of all articles. Harvard University was the second most prolific research institution with 95 articles (4.88%), followed by the University of California Los Angeles with 89 (4.57%) articles (Table 6).
Table 6.
Top 10 prolific institutions in publishing papers on renal function or nutritional indicators in patients with renal insufficiency treated with amino acids from 2000 to 2024.
| Rank | Institution | Documents | Country | % N = 1948 |
|---|---|---|---|---|
| 1st | University of California System | 139 | USA | 7.14 |
| 2nd | Harvard University | 95 | USA | 4.88 |
| 3rd | University of California Los Angeles | 89 | USA | 4.57 |
| 4th | Karolinska Institutet | 87 | Sweden | 4.47 |
| 5th | Institut national Sante Recherche Medicale (INSERM) | 80 | France | 4.11 |
| 6th | University of London | 77 | UK | 3.95 |
| 7th | Universite Paris Cite | 72 | France | 3.70 |
| 8th | Egyptian Knowledge Bank (EKB) | 67 | Egypt | 3.44 |
| 9th | Assistance Publique Hopitaux Paris (APHP) | 65 | France | 3.34 |
| 10th | Vanderbilt University | 63 | USA | 3.23 |
3.1.8. Bibliometric analysis of keywords
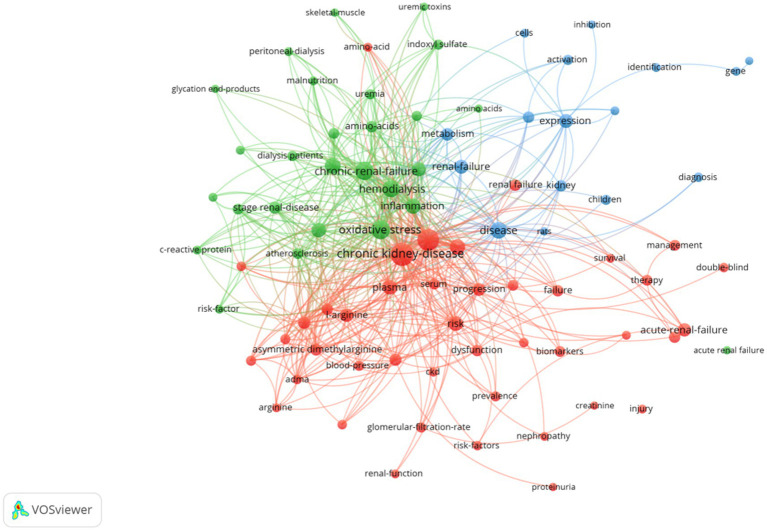
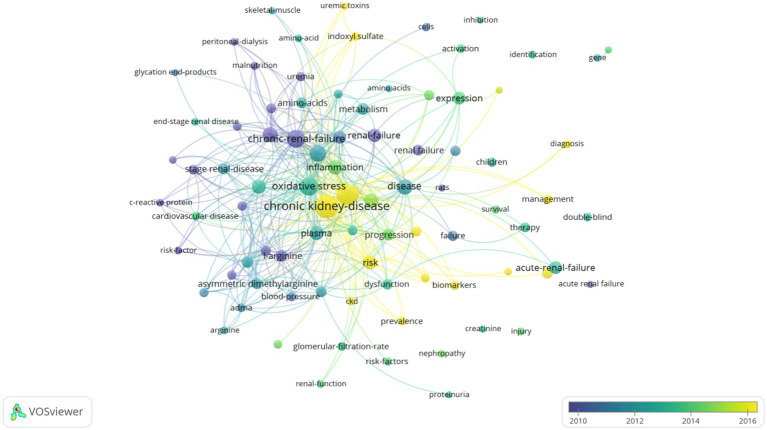
Terms with a minimum number of occurrences greater than 50 in all included publications were analyzed using the VOS viewer. Out of 8,497 terms, 85 terms reached this threshold, clustered into three thematic groups (Figure 3): Cluster 1 (Green): oxidative stress, chronic renal failure, hemodialysis, inflammation; Cluster 2 (Red): chronic kidney disease, risk, plasma, disease progression, L-arginine; Cluster 3 (Blue): renal failure, metabolism, kidney disease, biomarkers. Temporal keyword analysis revealed emerging foci on CKD, risk, biomarkers, and prevalence, suggesting future research directions (Figure 4).
Figure 3.
Network visualization map of terms in title/abstract fields of publications related to renal insufficiency and amino acids from 2000 to 2024. This visualized map of terms was developed when the minimum-term occurrences were placed at least 50 times. There are 85 terms that reach this threshold out of 8,497 in this field, which were divided into three clusters and colored differently. The size of the node indicates how many publications use that term.
Figure 4.
Network visualization map of terms in the title/abstract and their distribution according to the mean frequency of appearance. The blue terms emerged first, followed by the yellow and green terms that appeared later.
3.2. Meta-analysis results
3.2.1. Search results and study characteristics
Following systematic screening, 1,293 titles and abstracts of the literature were selected for further evaluation with 68 studies selected for full-text review. Ultimately, nine RCTs met the eligibility criteria and were included in the meta-analysis (11, 21–23, 34–38). Among the included studies: 7 RCTs reported ALB levels; 5 RCTs reported BUN; 4 RCTs provided TP data; 4 RCTs reported TRF; 3 RCTs included BMI, RPF, GFR and UA measurements. Table 7 provides a comprehensive overview of the specific details and population characteristics of the included studies. A total of 407 patients were included in the meta-analysis, of which 245 (60.2%) were dialysis patients (80 hemodialysis (HD) and 165 peritoneal dialysis (PD)), 63 (15.5%) chronic renal failure (CRF) patients, 54 (13.3%) renal transplant patients, and 45 (11%) glomerular injury patients. The cohort comprised 206 participants in intervention groups and 201 in control groups.
Table 7.
Baseline demographics and characteristics of included RCTs.
| Author, year | Countries | Participants (intervention/control) | Patients type | Mean age | Sex (F/M) | AA formulation | Comparator | Intervention time | Duration of intervention | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Murtas, 2024 (22) | Italy | 29 (14/15) | Hemodialysis | 73.2 | 15/14 | AminoDyal | Placebo | Novel mixture three times a week containing 5.4 g of AAs | 3 months | BUN; UA; ALB; TP; TRF; BMI |
| Murtas, 2022 (11) | Italy | 22 (11/11) | Hemodialysis | 67.7 | 13/9 | Amino-Ther-PRO | Placebo | A weekly total of 31.5 g AAs was given | 6 months | BUN; UA; ALB; TP; TRF; BMI |
| Bolasco, 2011 (23) | Italy | 29 (15/14) | Hemodialysis | 73.9 | 19/10 | Aminotrophic | Standard care | oral amino acid supplementation (4 g thrice a day) | 3 months | BUN; ALB; TP; BMI |
| Hladunewich, 2006 (21) | Canada | 45 (22/23) | Glomerular injury | 28.5 | 45/0 | L-arginine | Placebo | (3.5 g every 6 h), or intravenously (10 g every 8 h) | 10 days | RPF; GFR; ALB |
| Miller, 2003 (35) | Israel | 42 (21/21) | Chronic renal failure | 70.5 | 11/31 | L-arginine | Placebo | Prior to the angiographic examination (20 g) | Over20–30 min | BUN |
| Li, 2003 (37) | China | 60 (30/30) | Peritoneal dialysis | 46.0 | 28/32 | Nutrineal | Glucose | received one 2-L bag of Nutrineal (1.1% Total Amino Acids) every morning | 3 years | ALB; TRF |
| Schramm, 2002 (34) | Germany | 54 (28/26) | Kidney transplantation | 47.4 | 19/35 | L-arginine | Placebo (saline) | 0.75 g/kg body weight/day | 3 days | GFR; RPF |
| Nicola, 1999 (36) | Italy | 21 (11/10) | Chronic renal failure | 47.9 | 6/15 | L-arginine | Placebo | 0.2 g/kg body weight/day | 6 months | BUN; RPF; GFR; ALB |
| Jones, 1998 (38) | United States | 105 (54/51) | Peritoneal dialysis | 53.5 | 56/49 | Nutrineal | Glucose | Received one 2-L bag of Nutrineal (1.1% Total Amino Acids) every morning | 3 months | UA; ALB; TP; TRF |
AA, amino acids; BUN, blood urea nitrogen; RPF, renal plasma flow; GFR, glomerular filtration rate; UA, uric acid; ALB, albumin; TP, total protein; TRF, transferrin; BMI, body mass index.
3.2.2. Quality of enrolled trials
All 9 studies described random allocation methods, with 7 explicitly implementing allocation concealment and blinding protocols. Completeness of outcome data was reported in all studies, and none exhibited selective reporting. Based on the Cochrane RoB 2 tool, 7 studies were classified as low-risk and 2 as high-risk for bias (Supplementary Figure S3).
3.2.3. Publication bias
Publication bias was assessed via funnel plots and Egger’s linear regression test. The funnel plot indicated no significant bias for ALB and BUN outcomes (Supplementary Figures S4, S5). The results of the remaining studies were assessed using the Egger test, and no significant bias was found either.
3.2.4. BUN
The pooled estimates of the included trials demonstrated a statistically significant difference in BUN between the AA group and the control group (MD: 4.21, 95% CI: 1.08 to 7.35, p = 0.008, I2 = 0%, N = 5, fixed-effects model) (Figure 5A).
Figure 5.
Forest plots of included RCTs. (A) Pooled analysis of RCTs evaluating the effect of amino acid therapy on BUN.·(B) Pooled analysis of RCTs evaluating the effect of amino acid therapy on RPF. (C) Pooled analysis of RCTs evaluating the effect of amino acid therapy on GFR. (D) Pooled analysis of RCTs evaluating the effect of amino acid therapy on UA. (E) Pooled analysis of RCTs evaluating the effect of amino acid therapy on ALB.·(F) Pooled analysis of RCTs evaluating the effect of amino acid therapy on TP. (G) Pooled analysis of RCTs evaluating the effect of amino acid therapy on TRF. (H) Pooled analysis of RCTs evaluating the effect of amino acid therapy on BMI.
3.2.5. RPF
The findings of the pooled estimates from the included trials demonstrated a statistically significant difference in RPF in the AA group compared with the control group (MD: 30.78, 95% CI: 15.36 to 46.21, p < 0.0001, I2 = 0%, N = 3, fixed-effects model) (Figure 5B).
3.2.6. GFR
The findings of the pooled estimates from the included trials did not demonstrate a statistically significant difference in GFR in the AA group compared with the control group (MD: −1.35, 95% CI: −8.53 to 5.83, p = 0.71, I2 = 0%, N = 3, fixed-effects model) (Figure 5C).
3.2.7. UA
The pooled estimates from the included trials demonstrated a statistically significant difference in UA in the AA group compared with the control group (MD: −0.47, 95% CI: −0.89 to −0.06, p = 0.02, I2 = 0%, N = 3, fixed-effects model) (Figure 5D).
3.2.8. ALB
The results of the meta-analysis of the included trials revealed no statistically significant difference in ALB in the AA group in comparison with the control group (MD: 0.06, 95% CI: −0.14 to 0.26, p = 0.53, I2 = 75%, N = 7, random effects model) (Figure 5E).
3.2.9. TP
The findings of the pooled estimates from the included trials indicated that there was no statistically significant difference in TP in the AA group compared with the control group (MD: 0.14, 95% CI: −0.05 to 0.32, p = 0.15, I2 = 8%, N = 4, fixed-effects model) (Figure 5F).
3.2.10. TRF
The pooled estimates of the included trials demonstrated that there was no statistically significant difference in TRF in the AA group compared to the control group (MD: −5.77, 95% CI: −15.38 to 3.84, p = 0.24, I2 = 0%, N = 4, fixed-effects model) (Figure 5G).
3.2.11. BMI
The pooled estimates from the included trials demonstrated that there was no statistically significant difference in BMI in the AA group compared with the control group (MD: 2.48, 95% CI: −0.16 to 5.13, p = 0.07, I2 = 0%, N = 3, fixed-effects model) (Figure 5H).
3.2.12. Subgroup analysis
Given the substantial heterogeneity observed in ALB outcomes, subgroup analyses were conducted to evaluate potential confounding factors, including AA formulation type, RI subtype, and intervention duration (Supplementary Figure S6). These analyses revealed no statistically significant effect of AA formulation, RI classification, or treatment duration on ALB levels (p > 0.05).
3.2.13. Sensitivity analysis
Sensitivity analyses of single studies were performed by exclusion, combining the remaining studies after excluding one study at a time to assess the impact of single studies on the combined results. The results showed that after the sequential exclusion of individual studies, there was no significant change in the confidence level among the remaining studies, indicating that the results of the meta-analysis were reliable (Supplementary material 3).
4. Discussion
Researchers with high citation rates or prolific publication outputs exert significant influence on the trajectory of scientific inquiry within a field, making their identification critical to understanding its leadership (39). This analysis identifies Kalantar-Zadeh Kamyar and Zidek Walter as pivotal contributors to advancing knowledge on AA interventions in RI. Understanding national and institutional contributions to research in this field will help researchers to seek collaborations between universities or international institutions to advance the field (40). For instance, the University of California System emerged as the most productive institution in this domain, underscoring its leadership in AA-related renal research.
Keyword and term analyses derived from article titles and abstracts reveal thematic priorities (41). Recurrent terms such as chronic kidney disease risk progression oxidative stress chronic renal failure inflammation renal failure expression and metabolism highlight a sustained focus on elucidating mechanisms underlying renal injury and failure progression. Density visualization emphasized chronic kidney disease and oxidative stress as dominant themes, while temporal trends identified biomarkers as an emerging keyword, reflecting intensified efforts to discover novel CKD biomarkers in recent years.
In terms of the development of research, the first study evaluating AA in RI was published in 1973, which showed that intravenous AA produced favorable effects in patients with acute renal insufficiency (42). Subsequent milestones include an RCT in 1982 comparing standard AA with essential amino acids (EAA) in renal impairment (43, 44), which solidified EAA supplementation as a sustained research priority in RI through 2000. Post-2000, investigations increasingly targeted CKD management, with recent emphasis on AA formulations to improve renal function and nutritional status in CKD and dialysis populations (11, 18). Notably, the role of AA in preventing cardiac surgery-associated acute kidney injury has emerged as a prominent focus, supported by high-quality clinical trials (10, 12, 45, 46).
Renal function indices were altered to varying degrees in patients with different types of RI compared to normal subjects. In normal physiology, intravenous AA administration elevates GFR via activation of renal functional reserve. However, this compensatory mechanism diminishes as CKD progresses to end-stage renal disease (47). Stevens et al. identified a sustained decline in GFR as a predictor of adverse outcomes in CKD (48). Seki et al. linked elevated BUN to accelerated renal deterioration in advanced CKD (stages 3–5) (49). Jiang et al. postulated that AA supplementation enhances RPF, thereby exerting renoprotective effects (50). Hyperuricemia and urinary stone formation, driven by elevated UA, are recognized risk factors for CKD progression (51, 52). Dysregulated AA metabolism contributes to hyperuricemia in CKD patients; AA supplementation may correct this imbalance and reduce UA levels, even in advanced disease (11, 53). This meta-analysis synthesizes evidence supporting AA’s potential renal benefits. For instance, there was a numerical increase in RPF in 3 of the 3 studies (11, 21, 36), and UA levels were reduced in 2 of the 3 studies (11, 38). Consistent with preliminary results and preclinical studies. However, BUN levels were elevated in 4 of 5 studies (22, 23, 35, 36). Singer et al. noted that short-term AA infusion (75 g/day) in acute kidney injury patients significantly raised BUN by days 2–4 (p < 0.04) (50), likely due to increased protein catabolism. In stable CKD, elevated BUN is primarily attributed to excessive protein intake (51), and low-protein diets effectively mitigate this effect (52). Therapeutic strategies may thus require tailored approaches: high-dose AA infusion (150 g/day) for short-term acute kidney injury management versus long-term protein restriction in CKD to counteract BUN elevation (54, 55).
Nutrition serves as a cornerstone in CKD management (56). The kidneys are central to maintaining nutritional homeostasis, regulating AA balance partially through synthesis and catabolism (57). In therapeutic contexts, AA is primarily utilized in drug therapy to support protein synthesis (58). For patients with kidney injury, 9AA is generally used, which contains eight EAA and histidine in its composition, and the intervention in this study contained 9AA in all AA mixtures except arginine (59). EAA supplementation in CKD patients aids in preserving protein homeostasis (19), while biomarkers such as ALB, TP, TRF, and BMI are widely employed to assess nutritional status in CKD and HD populations (60). Protein depletion and malnutrition are prevalent in HD-treated CKD patients due to AA losses during dialysis (17). Murtas et al. demonstrated that CKD disrupts AA kinetic metabolism, impairing protein anabolism, a phenomenon exacerbated by HD-induced AA depletion (11). Conversely, Erkan et al.’s retrospective study found no significant improvement in ALB levels with daily 1.1% AA solution use in PD patients over 12 months (61). This meta-analysis corroborates prior findings, revealing no statistically significant impact of AA therapy on ALB, TP, TRF, or BMI in RI patients (17, 62). The lack of significant nutritional parameter changes may be attributed to (1) Intervention heterogeneity: Included studies utilized diverse AA formulations (e.g., L-arginine, variable EAA compositions) and short intervention durations (7/9 studies ≤3 months), potentially blunting effects on slow-turnover biomarkers like ALB (half-life: 2–3 weeks) (63). (2) CKD-associated catabolic states: Uremic toxin accumulation, chronic inflammation, and dialysis-induced AA losses (up to 800 g/year in HD patients) counteract the anabolic effects of supplementation, with 60.2% of participants in the population included in this article receiving dialysis treatment (22, 64). These findings underscore a critical limitation: standalone nutritional parameters may not capture AA efficacy in complex RI populations, necessitating integrated assessments of inflammation, metabolic markers, and patient-centered outcomes. For CKD patients, especially those who need dialysis, supplemental AA therapy should be supplemented with attention to the nutritional status of the patients and appropriate nutritional supplements, while focusing on the changes in renal function indices for a protein-restricted diet or other effective measures. Future studies should further explore the effects of different AA types, dosages, and treatment durations on nutritional indicators to optimize the treatment regimen.
This study has several limitations. In the bibliometric analysis, reliance solely on the Web of Science database may introduce selection bias, as inclusion of additional repositories (e.g., Scopus, MEDLINE) could yield divergent results. Furthermore, the search strategy encompassed studies involving AA structural derivatives, potentially capturing marginally relevant literature. For the meta-analysis, heterogeneity in eligibility criteria, control interventions, AA treatment components (e.g., dosage, formulation), and patient subtypes (e.g., CKD stages, dialysis modalities) limits the generalizability of pooled results. Such variability underscores the need for standardized protocols in future trials to enhance comparability.
5. Conclusion
Bibliometric analysis suggests that the keywords oxidative stress, inflammation, risk, and progression aim to shed light on possible mechanisms of RI. Keyword trends focused on CKD and new biomarkers, findings that reiterated the importance of chronic kidney disease progression and mechanisms. The meta-analysis yielded a multifaceted therapeutic landscape for AA supplementation: while it exhibited promising effects by enhancing RPF and reducing UA levels, these advantages were counterbalanced by sustained elevation in BUN and lack of significant alterations in nutritional parameters. This suggests that the renal impacts of AA are context-dependent, influenced by factors such as patient population, intervention duration, and formulation composition, and thus warrant nuanced interpretation and judicious clinical consideration. Clinically, these findings underscore the need to balance observed improvements in GFR and UA against potential risks associated with BUN elevation when integrating AA supplementation into therapeutic regimens.
5.1. Future research priorities
Priority areas include the following to address existing evidence gaps: (1) Dose–response characterization: Conduct dose-ranging studies to define thresholds for optimizing renal protection while minimizing BUN elevation; (2) Mechanistic investigations: Elucidate interactions between AA formulations, uremic metabolism, and inflammatory pathways influencing renal and nutritional outcomes; (3) Stratified trial designs: Perform subgroup analyses by CKD stage (pre-dialysis vs. HD/PD) and AA subtype, complemented by adaptive dose-finding trials; and (4) Long-term outcome evaluation: Implement RCTs with ≥1-year follow-up to characterize AA therapy’s impact on end-stage renal disease progression and survival.
Funding Statement
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by a grant from (1) Macao Polytechnic University (RP/FCA-14/2023) and a Joint Research Funding Program between the Macau Science and Technology Development Fund (FDCT); (2) the Department of Science and Technology of Guangdong Province (FDCT-GDST) (Grant No. 0009/2024/AGJ); (3) Guangxi Natural Science Foundation (No. 2023JJA141291); and (4) Wu Jieping Medical Foundation (No. 320.6750.2024-25-8).
Author contributions
XL: Writing – original draft, Formal analysis, Methodology, Visualization, Project administration, Data curation, Validation, Investigation, Resources, Software, Funding acquisition, Writing – review & editing, Conceptualization, Supervision. QL: Resources, Visualization, Data curation, Investigation, Project administration, Validation, Conceptualization, Writing – original draft, Methodology, Writing – review & editing, Software. LZ: Writing – review & editing, Methodology, Validation, Data curation, Software, Investigation, Resources, Visualization, Conceptualization. YH: Software, Resources, Writing – review & editing, Investigation, Visualization, Data curation, Methodology, Conceptualization, Validation. ST: Resources, Investigation, Software, Visualization, Conceptualization, Data curation, Validation, Writing – review & editing, Methodology. XC: Validation, Project administration, Supervision, Writing – review & editing, Methodology, Writing – original draft, Resources, Funding acquisition. KL: Validation, Writing – original draft, Methodology, Conceptualization, Project administration, Supervision, Resources, Funding acquisition, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1594507/full#supplementary-material
References
- 1.Li X, Zheng S, Wu G. Amino acid metabolism in the kidneys: nutritional and physiological significance. Adv Exp Med Biol. (2020) 1265:71–95. doi: 10.1007/978-3-030-45328-2_5, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Stein G. Infusionstherapie und parenterale Ernährung bei akutem Nierenversagen und chronischer Niereninsuffizienz [infusion therapy and parenteral nutrition in acute renal failure and chronic renal insufficiency]. Z Gesamte Inn Med. (1980) 35:758–62. [PubMed] [Google Scholar]
- 3.Martínez-Hernández SL, Muñoz-Ortega MH, Ávila-Blanco ME, Medina-Pizaño MY, Ventura-Juárez J. Novel approaches in chronic renal failure without renal replacement therapy: a review. Biomedicines. (2023) 11:2828. doi: 10.3390/biomedicines11102828, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn KM, Strutz F. The early diagnosis and treatment of chronic renal insufficiency. Dtsch Arztebl Int. (2024) 121:428–35. doi: 10.3238/arztebl.m2024.0072, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostermann M, Chang RW. Challenges of defining acute kidney injury. QJM. (2011) 104:237–43. doi: 10.1093/qjmed/hcq185, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Btaiche IF, Mohammad RA, Alaniz C, Mueller BA. Amino acid requirements in critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy. (2008) 28:600–13. doi: 10.1592/phco.28.5.600, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR, et al. Nomenclature for kidney function and disease: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. (2020) 97:1117–29. doi: 10.1016/j.kint.2020.02.010, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. (2021) 398:786–802. doi: 10.1016/S0140-6736(21)00519-5, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Kang J, Guo X, Peng H, Deng Y, Lai J, Tang L, et al. Metabolic implications of amino acid metabolites in chronic kidney disease progression: a metabolomics analysis using OPLS-DA and MBRole2.0 database. Int Urol Nephrol. (2024) 56:1173–84. doi: 10.1007/s11255-023-03779-8, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Landoni G, Monaco F, Ti LK, Baiardo Redaelli M, Bradic N, Comis M, et al. A randomized trial of intravenous amino acids for kidney protection. N Engl J Med. (2024) 391:687–98. doi: 10.1056/NEJMoa2403769, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Murtas S, Aquilani R, Fiori G, Maestri R, Iadarola P, Graccione C, et al. Effects of a novel amino acid formula on nutritional and metabolic status, Anemia and myocardial function in thrice-weekly hemodialysis patients: results of a six-month randomized double-blind placebo-controlled pilot study. Nutrients. (2022) 14:3492. doi: 10.3390/nu14173492, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazawa M, Kabata D, Yoshida H, Minami K, Maeda T, Yoshitani K, et al. Amino acids to prevent cardiac surgery-associated acute kidney injury: a randomized controlled trial. JA Clin Rep. (2024) 10:19. doi: 10.1186/s40981-024-00703-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garibotto G, Sofia A, Saffioti S, Bonanni A, Mannucci I, Verzola D. Amino acid and protein metabolism in the human kidney and in patients with chronic kidney disease. Clin Nutr. (2010) 29:424–33. doi: 10.1016/j.clnu.2010.02.005, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Duranton F, Lundin U, Gayrard N, Mischak H, Aparicio M, Mourad G, et al. Plasma and urinary amino acid metabolomic profiling in patients with different levels of kidney function. Clin J Am Soc Nephrol. (2014) 9:37–45. doi: 10.2215/CJN.06000613, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. (2009) 37:1–17. doi: 10.1007/s00726-009-0269-0, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Pukleš I, Páger C, Sakač N, Šarkanj B, Marković D, Sakač MK, et al. Green microfluidic method for sustainable and high-speed analysis of basic amino acids in nutritional supplements. Molecules. (2024) 29:5554. doi: 10.3390/molecules29235554, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Xiao X, Qin DP, Tan RS, Zhong XS, Zhou DY, et al. Comparison of intradialytic parenteral nutrition with glucose or amino acid mixtures in maintenance hemodialysis patients. Nutrients. (2016) 8:220. doi: 10.3390/nu8060220, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holeček M. Side effects of amino acid supplements. Physiol Res. (2022) 71:29–45. doi: 10.33549/physiolres.934790, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling ZN, Jiang YF, Ru JN, Lu JH, Ding B, Wu J. Amino acid metabolism in health and disease. Signal Transduct Target Ther. (2023) 8:345. doi: 10.1038/s41392-023-01569-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jufar AH, Evans RG, May CN, Hood SG, Betrie AH, Trask-Marino A, et al. The effects of recruitment of renal functional reserve on renal cortical and medullary oxygenation in non-anesthetized sheep. Acta Physiol (Oxf). (2023) 237:e13919. doi: 10.1111/apha.13919, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hladunewich MA, Derby GC, Lafayette RA, Blouch KL, Druzin ML, Myers BD. Effect of L-arginine therapy on the glomerular injury of preeclampsia: a randomized controlled trial. Obstet Gynecol. (2006) 107:886–95. doi: 10.1097/01.AOG.0000207637.01824.fe, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Murtas S, Reggiardo G, Contu R, Cadeddu M, Secci R, Putzu P, et al. Replacement of the massive amino acid losses induced by hemodialysis: a new treatment option proposal for a largely underestimated issue. Clin Nutr ESPEN. (2024) 63:354–63. doi: 10.1016/j.clnesp.2024.06.025, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Bolasco P, Caria S, Cupisti A, Secci R, Dioguardi SF. A novel amino acids oral supplementation in hemodialysis patients: a pilot study. Ren Fail. (2011) 33:1–5. doi: 10.3109/0886022X.2010.536289 [DOI] [PubMed] [Google Scholar]
- 24.Humphrey SM. File maintenance of MeSH headings in MEDLINE. J Am Soc Inf Sci. (1984) 35:34–44. doi: 10.1002/asi.4630350106, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA. (2004) 101 Suppl 1:5303–10. doi: 10.1073/pnas.0307513100, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informet. (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007, PMID: 40433219 [DOI] [Google Scholar]
- 27.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi KY, Li MY, Chen C, Kang E, Cochrane Taiwan . Ten circumstances and solutions for finding the sample mean and standard deviation for meta-analysis. Syst Rev. (2023) 12:62. doi: 10.1186/s13643-023-02217-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.5 The Cochrane Collaboration; (2024). [Google Scholar]
- 30.Review manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration (2020).
- 31.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. (2006) 295:676–80. doi: 10.1001/jama.295.6.676, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Schramm L, La M, Heidbreder E, Hecker M, Beckman JS, Lopau K, et al. L-arginine deficiency and supplementation in experimental acute renal failure and in human kidney transplantation. Kidney Int. (2002) 61:1423–32. doi: 10.1046/j.1523-1755.2002.00268.x, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Miller HI, Dascalu A, Rassin TA, Wollman Y, Chernichowsky T, Iaina A. Effects of an acute dose of L-arginine during coronary angiography in patients with chronic renal failure: a randomized, parallel, double-blind clinical trial. Am J Nephrol. (2003) 23:91–5. doi: 10.1159/000068036, PMID: [DOI] [PubMed] [Google Scholar]
- 36.De Nicola L, Bellizzi V, Minutolo R, Andreucci M, Capuano A, Garibotto G, et al. Randomized, double-blind, placebo-controlled study of arginine supplementation in chronic renal failure. Kidney Int. (1999) 56:674–84. doi: 10.1046/j.1523-1755.1999.00582.x, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Li FK, Chan LY, Woo JC, Ho SK, Lo WK, Lai KN, et al. A 3-year, prospective, randomized, controlled study on amino acid dialysate in patients on CAPD. Am J Kidney Dis. (2003) 42:173–83. doi: 10.1016/s0272-6386(03)00421-9, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Jones M, Hagen T, Boyle CA, Vonesh E, Hamburger R, Charytan C, et al. Treatment of malnutrition with 1.1% amino acid peritoneal dialysis solution: results of a multicenter outpatient study. Am J Kidney Dis. (1998) 32:761–9. doi: 10.1016/s0272-6386(98)70131-3, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Chen Y, Gou Y, Yang D, Xiong L. Chronic kidney diseases and inflammation research: a bibliometric analysis. Front Med (Lausanne). (2024) 11:1388665. doi: 10.3389/fmed.2024.1388665, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao Y, Xu S, Ge X. Trends in research on acute kidney injury: a bibliometric analysis of academic journals published between the years 2000 and 2022. Iran J Kidney Dis. (2024) 18:137–49. doi: 10.52547/tvy8jz17 [DOI] [PubMed] [Google Scholar]
- 41.Li J, Gong X. Bibliometric and visualization analysis of kidney repair associated with acute kidney injury from 2002 to 2022. Front Pharmacol. (2023) 14:1101036. doi: 10.3389/fphar.2023.1101036, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abel RM, Beck CH, Jr, Abbott WM, Ryan JA, Jr, Barnett GO, Fischer JE. Improved survival from acute renal failure after treatment with intravenous essential L-amino acids and glucose. Results of a prospective, double-blind study. N Engl J Med. (1973) 288:695–9. doi: 10.1056/NEJM197304052881401, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Mirtallo JM, Schneider PJ, Mavko K, Ruberg RL, Fabri PJ. A comparison of essential and general amino acid infusions in the nutritional support of patients with compromised renal function. JPEN J Parenter Enteral Nutr. (1982) 6:109–13. doi: 10.1177/0148607182006002109, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Akhtar N, Fayyaz T. The role of essential amino acids in patients on chronic haemodialysis. Specialist. (1994) 10:307–10. [Google Scholar]
- 45.Holm J, Vanky F, Svedjeholm R. Association of glutamate infusion with risk of acute kidney injury after coronary artery bypass surgery: a pooled analysis of 2 randomized clinical trials. JAMA Netw Open. (2024) 7:e2351743. doi: 10.1001/jamanetworkopen.2023.51743, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss R, Meersch M, Gerke M, Wempe C, Schäfers M, Kellum JA, et al. Effect of glutamine administration after cardiac surgery on kidney damage in patients at high risk for acute kidney injury: a randomized controlled trial. Anesth Analg. (2023) 137:1029–38. doi: 10.1213/ANE.0000000000006288, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Kotani Y, Baiardo Redaelli M, Pruna A, Losiggio R, Cocozza S, Ti LK, et al. Intravenous amino acid for kidney protection: current understanding and future perspectives. Clin Kidney J. (2024) 18:sfae409. doi: 10.1093/ckj/sfae409, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. (2006) 354:2473–83. doi: 10.1056/NEJMra054415, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Seki M, Nakayama M, Sakoh T, Yoshitomi R, Fukui A, Katafuchi E, et al. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3-5 chronic kidney disease: a prospective observational study. BMC Nephrol. (2019) 20:115. doi: 10.1186/s12882-019-1306-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang W, Shi K, Shao J, Song L, Shi Y, Wang H, et al. Protective effect of intravenous amino acid on kidney function: a systematic review and meta-analysis of randomized controlled trials. J Crit Care. (2025) 85:154937. doi: 10.1016/j.jcrc.2024.154937, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Chien TM, Lu YM, Li CC, Wu WJ, Chang HW, Chou YH. A retrospective study on sex difference in patients with urolithiasis: who is more vulnerable to chronic kidney disease? Biol Sex Differ. (2021) 12:40. doi: 10.1186/s13293-021-00382-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li K, Xia X, Fu T, Ma Y, Wang Y, Fan M, et al. Research progress on the mechanism of hyperuricemic nephropathy based on multi-omics technique: a review. Medicine (Baltimore). (2024) 103:e40975. doi: 10.1097/MD.0000000000040975, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. (2009) 53:796–803. doi: 10.1053/j.ajkd.2008.12.021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singer P. High-dose amino acid infusion preserves diuresis and improves nitrogen balance in non-oliguric acute renal failure. Wien Klin Wochenschr. (2007) 119:218–22. doi: 10.1007/s00508-007-0794-3, PMID: [DOI] [PubMed] [Google Scholar]
- 55.Kim HJ, Kim TE, Han M, Yi Y, Jeong JC, Chin HJ, et al. Effects of blood urea nitrogen independent of the estimated glomerular filtration rate on the development of anemia in non-dialysis chronic kidney disease: the results of the KNOW-CKD study. PLoS One. (2021) 16:e0257305. doi: 10.1371/journal.pone.0257305, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amiri Khosroshahi R, Zare M, Zeraattalab-Motlagh S, Kiany F, Talebi S, Mohammadi H. Effects of a low-protein diet on kidney function in patients with chronic kidney disease: an umbrella review of systematic reviews and Meta-analyses of randomized controlled trials. Nutr Rev. (2024):1–12. nuae178. doi: 10.1093/nutrit/nuae178, PMID: [DOI] [PubMed] [Google Scholar]
- 57.Letourneau P, Bataille S, Chauveau P, Fouque D, Koppe L. Source and composition in amino acid of dietary proteins in the primary prevention and treatment of CKD. Nutrients. (2020) 12:3892. doi: 10.3390/nu12123892, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Song H, Liu J, Lin J, Fang L. Comprehensive evaluation of clinical application of balanced compound amino acid injection. Front Nutr. (2022) 9:880256. doi: 10.3389/fnut.2022.880256, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Y, Song H, Fang L, Wu F, Qiu B, Tian D, et al. Is human albumin injection the best choice to treat hypoalbuminemia? PLoS One. (2024) 19:e0311949. doi: 10.1371/journal.pone.0311949, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolasco P. Hemodialysis-nutritional flaws in diagnosis and prescriptions. Could amino acid losses be the sharpest "sword of Damocles"? Nutrients. (2020) 12:1773. doi: 10.3390/nu12061773, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dervisoglu E, Ozdemir O, Yilmaz A. Commencing peritoneal dialysis with 1.1% amino acid solution does not influence biochemical nutritional parameters in incident CAPD patients. Ren Fail. (2010) 32:653–8. doi: 10.3109/0886022X.2010.485287, PMID: [DOI] [PubMed] [Google Scholar]
- 62.Sukkar SG, Gallo F, Borrini C, Vaccaro A, Marchello C, Boicelli R, et al. Effects of a new mixture of essential amino acids (Aminotrofic (®)) in malnourished haemodialysis patients. Med J Nutrition Metab. (2012) 5:259–66. doi: 10.1007/s12349-012-0098-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43:181–93. doi: 10.1002/jpen.1451, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI. Oxidative stress mitigation by antioxidants – an overview on their chemistry and influences on health status. Eur J Med Chem. (2021) 209:112891. doi: 10.1016/j.ejmech.2020.112891, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.