Abstract
CLINICAL TRIALS HAVE DEMONSTRATED THE SUPERIORITY OF COORDINATED interdisciplinary stroke unit care over conventional treatment of stroke patients on general medical wards. The evidence is so strong that several national bodies have recommended that stroke unit care be widely implemented. Translation of these research findings and care guidelines into clinical practice, however, represents a challenge for health care systems unaccustomed to managing stroke in a coordinated manner. This report describes the organization, operation and outcomes of the Acute Stroke Unit at the Queen Elizabeth II Health Sciences Centre in Halifax. By replicating and adapting the core characteristics identified in the randomized trials, we have been able to demonstrate the effectiveness of stroke unit care in a routine clinical setting. Our experience may help facilitate the development of organized stroke care in Canada.
Randomized trials have shown that organized care of stroke patients by a coordinated multidisciplinary team — operating within a discrete stroke unit and capable of providing a substantial rehabilitation period, if required — is effective in reducing mortality and morbidity.1,2,3,4,5 A recent study6 showed that the significant factors in a stroke unit “treatment package” included earlier mobilization, earlier use of ASA, more frequent administration of parenteral fluid and more frequent use of antipyretic and antibiotic therapy. Organized stroke unit care appears to benefit a wide range of stroke patients in many ways, reducing death from secondary complications and decreasing the need for institutional care through reduced disability.7
Despite this evidence, organized stroke unit care is not widely available in Canada,8 probably because of logistical barriers. For example, in a 1998 survey of acute care hospitals in Ontario, only 4% had a dedicated stroke unit.9 The purpose of this paper is to describe how the Acute Stroke Unit at the Queen Elizabeth II Health Sciences Centre (QEII) in Halifax has been able to provide effective care through replication and adaptation of the core characteristics identified in randomized trials.
Program description
The objective of our Acute Stroke Program is to provide optimal care for stroke patients and their families using an interdisciplinary approach to assessment, treatment, education and research. The program model, goals and anticipated long-term effects are outlined in Fig. 1.
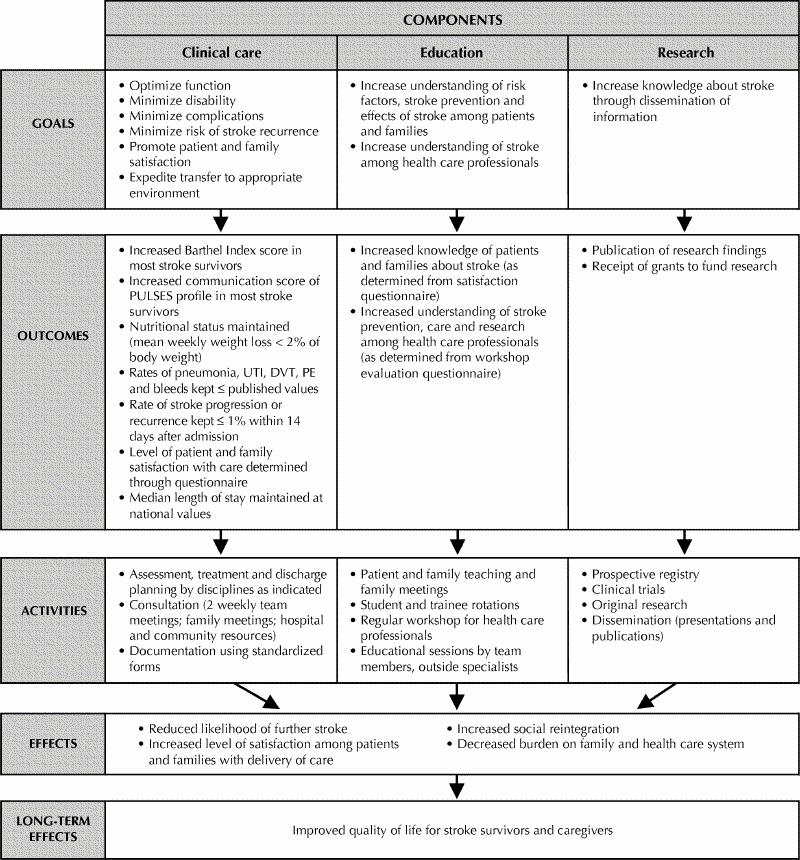
Fig. 1: Model of Acute Stroke Unit, illustrating connection between daily activities of interdisciplinary team and their long-term effects. PULSES = Physical condition, Upper limb functions, Lower limb functions, Sensory components (including communication), Excretory functions, Support factors;10 UTI = urinary tract infection; DVT = deep vein thrombosis; PE = pulmonary embolism.
The QEII is a 1073-bed teaching hospital in Halifax that provides primary and secondary care for about 300 000 people and tertiary care for about an additional 500 000. The Division of Neurology offers a 24-hour consultation service to the QEII Emergency Department. In general, patients who present with uncomplicated transient ischemic attacks (TIAs) or non-disabling strokes are not admitted to hospital. The Neurosurgery Service usually manages patients with subarachnoid hemorrhage. All other patients with suspected or confirmed acute stroke (including patients with intracranial hemorrhage not requiring surgery) are admitted to the Acute Stroke Unit. Patients receiving thrombolytic therapy11,12 are admitted to the intensive care unit and are transferred to the stroke unit after 24 hours. On any given day, the average number of patients in the Acute Stroke Unit is 17 (range 9–25). This includes patients in the acute phase of their illness, those receiving early rehabilitation, those awaiting transfer to an alternative level of care and those receiving palliative care.
The Acute Stroke Unit is located on an acute neurology ward that also houses a general neurology service managing patients with acute nonvascular neurological disorders. Different neurologists supervise the 2 services.
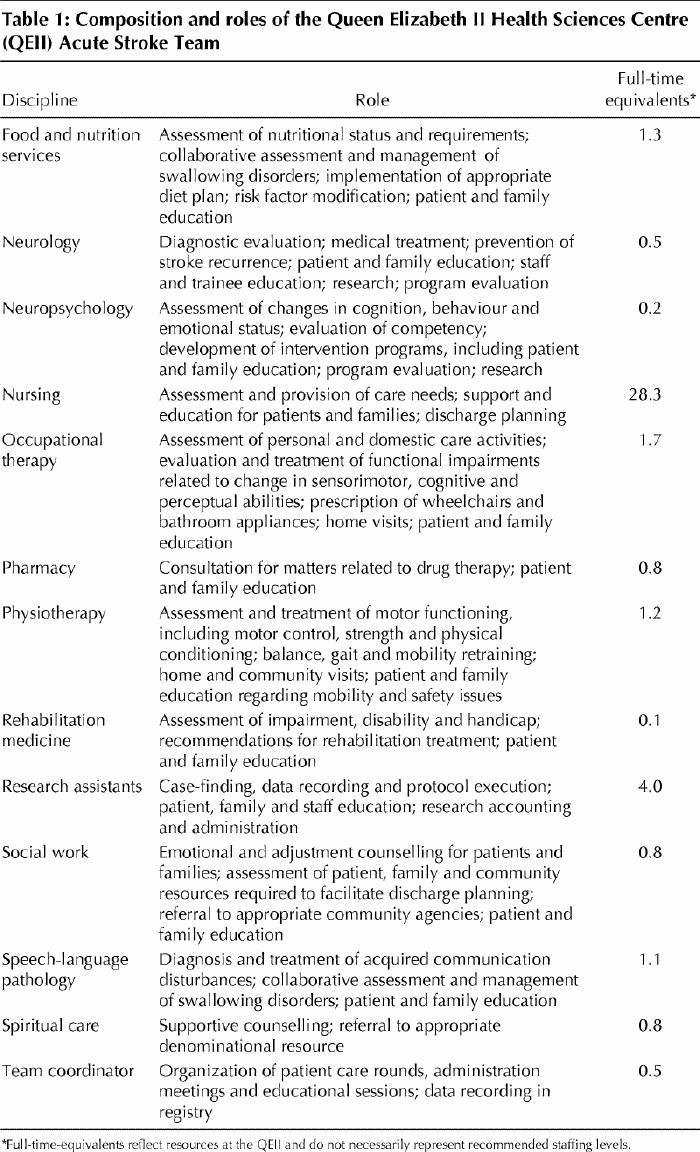
The composition and roles of our interdisciplinary stroke team are shown in Table 1. Other stroke units have reported similar staffing levels.13 Some team members also provide care for patients in the general neurology service. The nursing staff comprises 75% registered nurses (25% with national neuroscience certification or a post-RN course in neuroscience nursing) and 25% licensed practical nurses. Nurses function as active members of the interdisciplinary team, facilitating a patient-focused model of care delivery. The patient-to-nurse ratio is usually 4:1 during the day and 7:1 at night. Individual team members are responsible to their department heads for meeting standards of care delivery. The team coordinator reports to the manager of the Neurology Nursing Unit. The attending stroke neurologist reports to the head of the Division of Neurology.
Table 1
The physician who assesses the patient upon admission records the clinical findings on a standardized history and physical form adapted from the Royal College of Physicians stroke audit package.14 This form minimizes the amount of writing required and prompts the physician to consider the salient features of the clinical assessment. The use of such a form improves the recording of information15 and, in our experience, makes information more accessible to the stroke team.
A standardized admission orders form prompts the physician to consider all aspects of care and to select an appropriate course of action from a menu of options. For patients assessed within the first 3 hours after ischemic stroke, an eligibility checklist and standing orders form facilitate thrombolysis treatment.
By completing a single requisition, the physician can initiate interdisciplinary assessment and treatment by the team's dietitian, neuropsychologist, occupational therapist, physiotherapist, social worker and speech-language pathologist (Table 1). Pharmacy support is provided routinely. Pastoral care services are also available. Other disciplines such as physical medicine and rehabilitation, neurosurgery, psychiatry and clinical psychology are consulted as needed. The team meets twice weekly to discuss the status of each patient and to update the plan of care.
We maintain a prospective computerized registry of all cases of stroke and TIA admitted to the Acute Stroke Unit. Data summarizing demographic details, stroke type, lesion localization, stroke severity, functional status, complications, investigations and other information are recorded during the hospital stay by the team coordinator. At discharge the data collection form is removed from the patient's chart by a research assistant who checks the accuracy of the information and enters it into the database. The registry is a useful clinical tool for program planning, research and quality assurance and audit purposes.
The stroke team has developed educational materials for patients and families to complement those published by organizations such as the Heart and Stroke Foundation. In-service training sessions concerning various aspects of the care of stroke patients are held regularly. For the past several years we have organized annual workshops attended by health care professionals from the Maritime provinces. Revenues from the workshops are used to support the professional education of team members. Trainees from the various team disciplines rotate through the program.
We have participated in several clinical trials16,17,18,19,20,21 and have used our registry for original projects22,23,24,25,26 as well as for quality control and audit purposes. Our research is clinically oriented, focusing on treatment and outcomes. Our philosophy is to integrate patient care and research as much as possible. Research personnel contribute to the clinical, educational and administrative activities of the team, and team members participate in research projects.
Administration meetings are held every 2 to 4 weeks and provide team members with an equal opportunity to participate in the decision-making related to the functioning of the program. An annual 1-day retreat provides a forum for in-depth review of the program (including the registry data) and allows for strategic planning.
The administration and funding of the component parts of the are organized on a departmental basis. Research contracts and grants support our research personnel and registry. An economic analysis using data from the Stroke Unit Trialists' Collaboration indicated that stroke units are unlikely to consume extra resources and may even release some for use elsewhere.27
Preliminary evaluation
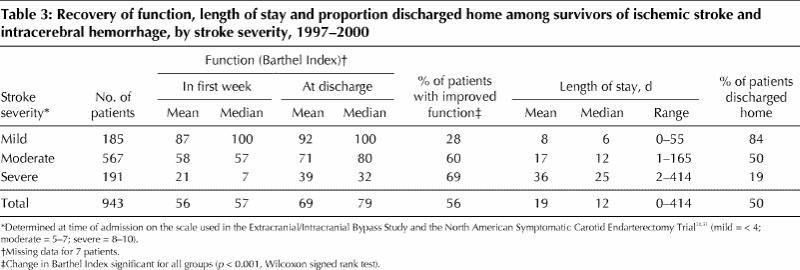
Table 2 provides a summary description of the patients admitted to the Acute Stroke Unit between Jan. 1, 1997, and Dec. 31, 2000. During this time the in-hospital case-fatality rate was 13% (95% confidence interval [CI] 11%–15%). Table 3 summarizes stroke survivors' recovery of function and minimization of disability (as measured by change in Barthel Index scores10,32 between admission and discharge), length of stay in the Acute Stroke Unit and the proportion of patients discharged home.
Table 2
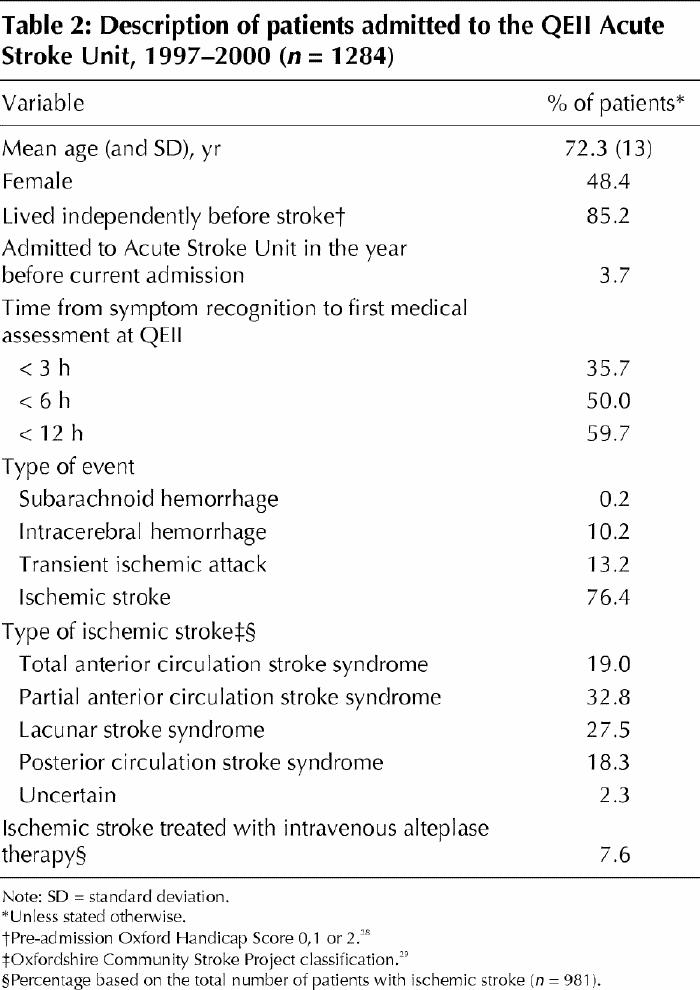
Table 3
We evaluated patient satisfaction by questionnaire during the autumn of 1999. The questions were constructed to obtain feedback related to program goals. The questionnaire was given to 34 consecutive patients just before discharge from the stroke unit. If required, a member of the family or the QEII volunteer service assisted in completing the questionnaire. Thirty-two patients (94%) returned the questionnaire. On a scale of 1 (very dissatisfied) to 5 (very satisfied), the mean rating for items related to care delivery was 4.6.
Length of stay, complications, and death or need for long-term institutional care for patients admitted to the Acute Stroke Unit were compared with these outcomes for TIA and stroke patients admitted during 1993–1996, before the stroke unit was established (Table 4). Despite the methodological limitations of a before–after comparison, the data show that patients managed in the stroke unit had a shorter median length of stay than those managed beforehand. With a cost of $1066 per day at the QEII, a 2-day reduction in median length of stay per stroke unit patient translates into a saving of more than $2.1 million per 1000 patients treated.
Table 4
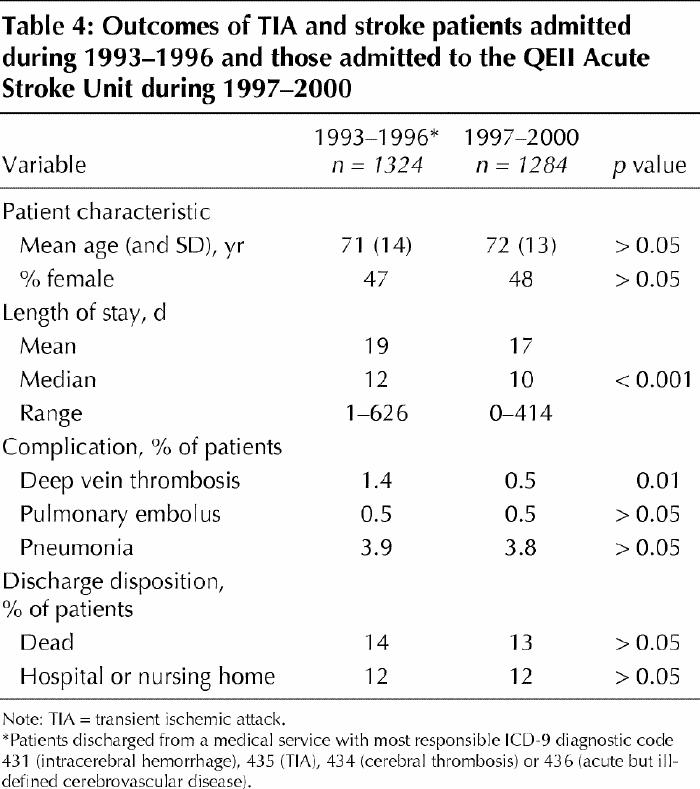
In the Acute Stroke Unit the odds of symptomatic deep vein thrombosis occurring were reduced by 68% (95% CI 16%–89%) (Table 4). The odds of death or needing long-term institutional care after a median follow-up of 10 days was reduced by 3% (n = 943; 95% CI 19% reduction to 16% increase, p = 0.12). This trend is in keeping with the results of the meta-analysis of randomized trials (n = 3812), which showed an odds reduction of 23% (95% CI 12%–32%, p < 0.001) after a median follow-up of 1 year.1
Comments
The Acute Stroke Program at the QEII integrates clinical care, education and research to maintain a high standard of care and education for patients and families, while developing the knowledge and various interests of the disciplines contributing to the program. Patients report a high level of satisfaction with the care they receive. The before–after analysis indicated that stroke unit care at our hospital has been associated with a statistically and economically significant reduction in length of stay and a reduction in the incidence of deep vein thrombosis. The program provides an ideal training environment for health care professionals from a broad range of disciplines. An organized approach also facilitates clinical research, including participation in treatment trials.
Areas needing growth and development include our neurovascular ambulatory care program and training in stroke medicine for postgraduate medical trainees. Also, in our aspirations for smooth and continuous patient care, we would like to improve the transition between acute stroke care and long-term rehabilitation, and between hospital care and community living.
Recommendations for stroke-unit care have been published in Europe,33 the United States34 and Canada.35 We hope that this description of our experience helps facilitate the development of a more organized approach to stroke care in Canada.
β See related article page 649
Acknowledgments
The Acute Stroke Unit registry has been supported by funds from the Heart and Stroke Foundation of Nova Scotia, GlaxoSmithkline Inc., AstraZeneca Canada Inc., Leo Pharma Inc., Bayer Health Care Division, Sanofi Canada Inc., Hoffmann–La Roche Ltd., Aventis Pharma Inc. and Servier Canada Inc.
Footnotes
The clinical care forms described in the text are available from the corresponding author upon request.
This article has been peer reviewed.
Contributors: Stephen Phillips, Gordon Gubitz, and Gail Eskes have all made major contributions to the development and operation of the Acute Stroke Unit at Queen Elizabeth II Health Sciences Centre. Dr. Eskes provided the impetus to report our experience. Dr. Phillips wrote the first draft of the manuscript. Drs. Gubitz and Eskes provided essential input into the analyses of our registry database, and into all revisions of the manuscript. All three authors gave final approval of the version submitted for publication.
Competing interests: None declared.
Correspondence to: Dr. Stephen J. Phillips, Division of Neurology, Rm. 3831, Halifax Infirmary, 1796 Summer St., Halifax NS B3H 3A7; fax 902 473-4438; stephill@is.dal.ca
References
- 1.Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke [review]. Cochrane Database Syst Rev 2000;(2):CD000197. [DOI] [PubMed]
- 2.Langhorne P, Dennis M, editors. Stroke units: an evidence based approach. London: BMJ Books; 1998.
- 3.Hankey GJ, Warlow CP. Treatment and secondary prevention of stroke: evidence, costs, and effects on individuals and populations. Lancet 1999;354:1457-63. [DOI] [PubMed]
- 4.Indredavik B, Bakke F, Slørdahl SA, Rokseth R, Håheim LL. Stroke unit treatment: 10-year follow-up. Stroke 1999;30:1524-7. [DOI] [PubMed]
- 5.Stegmayr B, Asplund K, Hulter-Asberg K, Norrving B, Peltonen M, Terent A, et al, for the Riks-Stroke Collaboration. Stroke units in their natural habitat. Can results of randomized trials be reproduced in routine clinical practice? Stroke 1999;30:709-14. [DOI] [PubMed]
- 6.Rønning OM, Guldvog B. Stroke unit versus general medical wards, II: neurological deficits and activities of daily living. A quasi-randomized controlled trial. Stroke 1998;29:586-90. [DOI] [PubMed]
- 7.Stroke Unit Trialists' Collaboration. How do stroke units improve patient outcomes? A collaborative systematic review of the randomized trials. Stroke 1997;28:2139-44. [DOI] [PubMed]
- 8.Hakim AM, Silver F, Hodgson C. Is Canada falling behind international standards for stroke care? CMAJ 1998;159:671-3. [PMC free article] [PubMed]
- 9.Tu JV, Porter J. Stroke care in Ontario: hospital survey results. Toronto: Heart and Stroke Foundation of Ontario; Goverment of Ontario; Institute for Clinical Evaluative Sciences; and Ontario Hospital Association; 1999. Available: www.ices.on.ca/PDFs/TechnicalReports/99-05-TR.pdf (accessed 2002 Aug 14).
- 10.Granger CV, Albrecht GL, Hamilton BB. Outcome of comprehensive medical rehabilitation: measurement by PULSES profile and the Barthel Index. Arch Phys Med Rehabil 1979;60:145-54. [PubMed]
- 11.Norris JW, Buchan A, Côté R, Hachinski V, Phillips SJ, Shuaib A, et al, on behalf of the Canadian Stroke Consortium. Canadian guidelines for intravenous thrombolytic treatment in acute stroke. Can J Neurol Sci 1998;25:257-9. [DOI] [PubMed]
- 12.CAEP Committee on Thrombolytic Therapy for Acute Ischemic Stroke. Thrombolytic therapy for acute ischemic stroke [position statement]. CJEM 2001;3:8-12.
- 13.Berman P, Hankey G, Indredavik B, Kalra L. Descriptions of stroke unit care. In: Langhorne P, Dennis M, editors. Stroke units: an evidence based approach. London: BMJ Books; 1998. p. 84-92.
- 14.Royal College of Physicians. Stroke audit package. London: Royal College of Physicians; 1994.
- 15.Davenport RJ, Dennis MS, Warlow CP. Improving the recording of the clinical assessment of stroke patients using a clerking pro forma. Age Ageing 1995; 24:43-8. [DOI] [PubMed]
- 16.Halliday AW. The Asymptomatic Carotid Surgery Trial (ACST): rationale and design. Eur J Vasc Surg 1994;8:703-10. [DOI] [PubMed]
- 17.CAPRIE Steering Committee. A randomized, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348: 1329-39. [DOI] [PubMed]
- 18.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19 435 patients with acute ischaemic stroke. Lancet 1997;349: 1569-81. [PubMed]
- 19.Barnett HJM, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al, for the North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998;339:1415-25. [DOI] [PubMed]
- 20.Wahlgren NG, Ranasinha KW, Rosolacci T, Franke CL, van Erven PMM, Ashwood T, et al, for the CLASS Study Group. Clomethiazole Acute Stroke Study (CLASS). Results of a randomized, controlled trial of clomethiazole versus placebo in 1360 acute stroke patients. Stroke 1999;30:21-8. [DOI] [PubMed]
- 21.Lyden PD, Ashwood T, Claesson L, Odergren T, Friday GH, Martin-Munley S. The Clomethiazole Acute Stroke Study in ischemic, hemorrhagic, and t-PA treated stroke: Design of a phase III trial in the United States and Canada. J Stroke Cerebrovasc Dis 1998;7:435-41. [DOI] [PubMed]
- 22.Al-Buhairi AR, Phillips SJ, Llewellyn G, Jan MMS. Prediction of infarct topography using the Oxfordshire Community Stroke Project classification of stroke subtypes. J Stroke Cerebrovasc Dis 1998;7:1-6. [DOI] [PubMed]
- 23.Gubitz G, Phillips S, Dwyer V. What is the cost of admitting patients with transient ischemic attacks to hospital? Cerebrovasc Dis 1999;9:210-4. [DOI] [PubMed]
- 24.Ingles JL, Eskes GA, Phillips SJ. Fatigue after stroke. Arch Phys Med Rehabil 1999; 80:173-8. [DOI] [PubMed]
- 25.Gubitz G, Phillips S, Aguilar E. Discharge disposition of patients on an acute stroke unit. J Stroke Cerebrovasc Dis 1999;8:330-5. [DOI] [PubMed]
- 26.Leckey R, Aguilar EG, Phillips SJ. Atrial fibrillation and the use of warfarin in patients admitted to an acute stroke unit. Can J Cardiol 2000;16:481-5. [PubMed]
- 27.Major K, Walker A. Economics of stroke unit care. In: Langhorne P, Dennis M, editors. Stroke units: an evidence based approach. London: BMJ Books; 1998. p. 56-65.
- 28.Bamford JM, Sandercock PAG, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients [letter]. Stroke 1989;20:828. [DOI] [PubMed]
- 29.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. [DOI] [PubMed]
- 30.The EC/IC Bypass Study Group. The International Cooperative Study of Extracranial/Intracranial Arterial Bypass (EC/IC Bypass Study): methodology and entry characteristics. Stroke 1985;16:397-406. [DOI] [PubMed]
- 31.Haynes RB, Mukherjee J, Sackett DL, Taylor W, Barnett HJM, Peerless SJ, for the EC/IC Bypass Study Group. Functional status changes following medical or surgical treatment for cerebral ischemia. JAMA 1987;257:2043-6. [PubMed]
- 32.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5. [PubMed]
- 33.Kaste M, Skyhoj Olsen T, Orgogozo JM, Bogousslavsky J, Hacke W, for the EUSI Executive Committee. Organization of stroke care: education, stroke units and rehabilitation. Cerebrovasc Dis 2000;10(Suppl 3):1-11. Available: www.eusi-stroke.com/recommendations/rc_organization1.shtml (accessed 2002 Aug 14). [DOI] [PubMed]
- 34.Alberts MJ, Hademenos G, Latchaw RE, Jagoda A, Marler JR, Mayberg MR, et al, for the Brain Attack Coalition. Recommendations for the establishment of primary stroke centres. JAMA 2000;283:3102-9. [DOI] [PubMed]
- 35.Wilson E, Taylor G, Phillips S, Stewart PJ, Dickinson G, Ramsden VR, et al, and the Canadian Stroke Systems Coalition. Creating a Canadian stroke system [editorial]. CMAJ 2001;164(13):1853-5. [PMC free article] [PubMed]


