Abstract
Abstract
For over 20 years, the two key tenets of adrenal incidentaloma (AI) evaluation have been the upper threshold of 10 Hounsfield units (HU) on noncontrast CT (ncCT) to delineate benignity, and the utilisation of adrenal washout CT (AWCT) to evaluate those above this cutoff. In light of growing recent evidence that challenges these two traditional principles, as well as re-evaluation of the data that led to their acceptance, we conclude that neither of these mainstays of adrenal CT remains relevant in modern AI diagnostic workup. With an appropriate definition of an incidentaloma and endocrine assessment for the majority of adrenal lesions, our analysis establishes that the use of AWCT should be ceased in the assessment of AIs, and that a 20 HU attenuation threshold for lesions < 4 cm should replace the traditional 10 HU threshold to exclude malignancy in this patient population. We therefore propose new recommendations for the management of AIs based primarily on CT attenuation and lesion size on ncCT.
Critical relevance statement
Increasing the CT attenuation threshold to 20 HU for lesions < 4 cm and eliminating washout CT for true adrenal incidentalomas, together with recommendations for endocrine assessment, will significantly decrease the over-investigation of overwhelmingly benign adrenal lesions, whilst confidently excluding malignancy.
Key Points
True incidentalomas exclude current or prior extra-adrenal malignancy and clinically suspected adrenal disease.
Adrenal washout CT was never proven in the malignancy-sparse true incidentaloma population.
Hormonal correlation in parallel with < 20 HU and < 4 cm thresholds of homogeneous lesions on noncontrast CT excludes malignancy.
Graphical Abstract
Keywords: Adrenal incidentaloma, Adrenal gland neoplasms, Tomography (x-ray computed)
Introduction
A 2002 paper encapsulated the prevailing views at the time regarding the two key principles of adrenal CT: a 10 Hounsfield unit (HU) upper threshold for lipid-rich adenomas (LRA), and adrenal washout calculation for the remainder. The authors stated, “with a combination of unenhanced and delayed enhanced CT, nearly all adrenal masses can be correctly categorised as adenomas or nonadenomas” [1].
Over 20 years later, this practice remains unchanged in many institutions and international guidelines [2–5]. However, on review of the interval published evidence and reanalysis of the foundational literature, we believe it is time to re-evaluate these tenets of adrenal incidentaloma (AI) imaging, proposing that the use of adrenal washout CT (AWCT) be ceased, and that the 10 HU threshold be revised upward.
In this paper, we revisit the history, established principles and international guidelines pertaining to adrenal imaging with a focus on CT and reinforce the necessity of endocrine assessment for the majority of AIs. We subsequently challenge the evidence behind AWCT, demonstrating its inherent inaccuracy, and highlight that the technique was never proven in the malignancy-sparse true AI population. We then provide substantiation of 20 HU as a new threshold for benign AIs measuring < 4 cm.
Methodology
This narrative review specifically targeted the evidence behind AWCT and attenuation thresholds on noncontrast CT (ncCT) by identifying the seminal papers that described their establishment and evaluating the cited literature within the major historic and current AI international guidelines (J.S., D.S.). Additional literature was retrieved with library assistance (South & East Metropolitan Health Service Library and Information Service) using Medline, PubMed, Web of Science, Trip Medical Database, Embase and cross-correlation with Google Scholar databases from 2023 to September 2024. Search criteria included “adrenal”, “nodule”, “lesion”, “adenoma”, “lipid poor”, “lipid rich”, “nonadenoma”, “wash-in”, “washout”, “phaeochromocytoma”, “carcinoma”, “adrenal incidentaloma*” (J.S., C.W.). The search was constrained to title, abstract, keyword, key field, and English. Acceptance for inclusion was based on consensus discussion between all authors.
Definition of adrenal incidentalomas
Adrenal lesions (AL) are identified in up to 4–5% of CT examinations [6–8] and encountered in three main clinical scenarios. First, ALs may result in clinically evident symptoms, primarily endocrine in nature. Second, ALs may be identified in patients with extra-adrenal malignancy, although unless there are also other metastases, most ALs are still benign in this setting [9].
However, third and most frequently, ALs are incidental findings, though what constitutes an incidentaloma varies markedly in the literature. For example, a definition of “an adrenal mass detected on imaging not performed for a suspected adrenal disease” [3] excludes suspected functional lesions, but without further qualifiers, classifies unsuspected adrenal metastases in those with known malignancy as incidentalomas.
Alternatively, the Society of Abdominal Radiology’s AI definition is “an incidentally detected adrenal nodule or mass that is unrelated to the clinical indication for the imaging examination performed”, thereby excluding adrenal metastases detected in cancer staging [10]. However, imaging performed to confirm diverticulitis in someone with recent lung cancer bypasses this definition. Furthermore, both definitions may include those with no previously known malignancy, but with unequivocal extra-adrenal malignancy on current imaging.
We therefore propose that an optimal AI definition is one that excludes patients with current or prior extra-adrenal malignancy or clinically suspected adrenal disease. Additionally, we conform to published standards that AIs measure ≥ 1 cm [2, 3, 10], with a recent study finding this to be a clinically appropriate threshold [11].
The history of adrenal imaging
Adrenal imaging began over a century ago with air-insufflation radiography in the 1920s [12] and then incorporated tomography in the 1940s [13]. CT provided the first published cross-sectional image of an adrenal gland in 1976 [14], and the first specific lesion in 1977—a macroscopic fat-containing myelolipoma [15].
Reports of hypoattenuating ALs (without macroscopic fat) followed, with recognition they correlated with benign adrenocortical adenomas [16, 17]. By 1991, a 10 HU cutoff had been proposed for differentiating benign and malignant lesions [18], and whilst alternative levels were suggested [19], the 10 HU threshold became widely accepted in international radiological and clinical guidelines [2, 4, 5, 20–23].
Dynamic contrast-enhanced evaluation of ALs began with MRI in the early 1990s [24–26], whilst the development of AWCT appeared less intentional. Routine post-contrast lesion attenuation was deemed unhelpful, therefore requiring performance of ncCT on separate days [27]. Publications subsequently evaluated the utility of lesion attenuation assessment after diminishing 60-, 30-, and 12–18-min delays [28–30].
Key papers by Korobkin et al and Szolar et al in 1998 then firmly established the AWCT technique, leading Caoili et al to conclude that nearly all adenomas and non-adenomas could be differentiated [1, 31, 32]. Whilst there was some technique variation, the dominating protocol involved portal venous (PV) and 15-min phases, and with Absolute Percentage Washout (APW) > 60% and Relative Percentage Washout (RPW) > 40% considered indicative of adenomas [10, 21].
These principles of adrenal imaging: a 10 HU threshold and AWCT, have essentially remained unchanged over the last two decades, as reinforced by current international guidelines [2–5].
Adrenal incidentaloma guidelines and endocrine evaluation
Initial AI guidelines were conservative, with the combined American Associations of Clinical Endocrinologists and Endocrine Surgeons 2009 publication recommending re-imaging of radiologically benign lesions (< 4 cm and < 10 HU), at 3–6 months, then annually for 1–2 years [20], a stance that has perpetuated as recently as 2017 [33].
The American College of Radiology 2010 Incidental Findings White Paper and 2017 update, however, proposed that radiologically benign lesions: myelolipomas, non-enhancing lesions ± calcification, those ≤ 10 HU (or with MRI opposed-phase signal loss), and lesions with ≥ 1 year stability did not require imaging follow-up. Notably, they also recommended AWCT for lesions > 10 HU [2, 21].
Similarly, AWCT and the 10 HU threshold were incorporated into the 2011 & 2023 Canadian Urological Association, 2016 & 2023 European Society of Endocrinology (ESE)/European Network for the Study of Adrenal Tumors (ENSAT), 2017 Korean Endocrinology Society and 2023 BMJ Best Practice guidelines [3–5, 22, 23, 34, 35].
Additionally, these guidelines all recommend initial hormone testing for AIs, as approximately 15% are hormonally active [36, 37], and 20–50% of adenomas may cause mild autonomous cortisol secretion (MACS) [3]. Whilst the details lie beyond this article’s scope, the recommended initial endocrine assessment includes: clinical examination for hormone excess; testing for cortisol secretion and metanephrines; and aldosterone/renin ratios, sex-hormones and steroid precursors in select populations (Table S1) [3]. In the 2023 ESE revision [3], metanephrine assessment for lesions < 10 HU was removed, following studies demonstrating phaeochromocytomas (PCCs) were consistently above this threshold [38–44].
We therefore reiterate that radiology reports for all AIs (excluding non-adenomatous benign aetiologies, such as myelolipomas and cysts) include a generalised recommendation for hormonal evaluation. In one study, this improved rates of endocrine testing from 13.9 to 48.0%, whilst 100% were evaluated if seen by endocrinologists, implying additional benefits of recommending endocrinologist referral [45].
The problem with phaeochromocytomas
The first challenge to the tenets of adrenal imaging arose with reports of washout in PCCs mimicking adenomas, culminating in a 2018 meta-analysis evaluating 10 studies, 1017 patients and 114 PCCs [46]. Of the studies with “strict” AWCT technique, 47% of PCCs met adenoma washout criteria. A 2019 study of pathologically proven PCCs similarly found that 50% would be misdiagnosed on AWCT overall, and 80% of those < 3 cm [47]. A further international ENSAT study, although reporting a lower 28.9% rate, still concluded that AWCT was unreliable for excluding PCCs [48].
Consequently, additional CT criteria for PCCs have been evaluated, including: noncontrast, arterial, PV and delayed attenuation thresholds, and enhancement ratios [40, 49–56]. However, several criteria significantly traded off specificity for sensitivity, others reported conflicting results, some relied on AWCT for diagnosis, whilst others artificially excluded alternative pathologies. A revealing finding in one study was that although size and noncontrast attenuation thresholds had limited performance in differentiating PCC from lipid-poor adenomas (LPA) (AUC 0.781 and 0.845, respectively), they both outperformed all enhancement criteria (AUC range 0.600–0.727), therefore questioning any utility of contrast assessment [54].
MRI diagnosis is also unreliable, as 35% of PCC may lack classical high T2-signal [57], and in a recent small study, > 50% had ‘atypical’ MRI appearances [58]. A proposed quantitative T2-signal and entropy calculator has also had overall mixed results [59–62].
Despite these challenges of differentiating between PCCs and adenomas with CT/MRI, importantly, only 10–20% of PCCs are clinically silent, and urinary and plasma metanephrine testing have reported 97–99% sensitivity [63, 64]. Given that all AIs > 10 HU require metanephrine testing, the real-world consequences are therefore limited.
Is adrenal washout CT washed up?
Beyond PCC, AWCT has even poorer diagnostic performance with other classically hypervascular lesions, such as renal-cell and hepatocellular carcinoma metastases, with up to 95% meeting adenoma APW criteria [65]. Further confounding their differentiation is that these may also contain metastatic lipid, which does not necessarily correlate with primary tumour adiposity [66].
However, even when including non-hypervascular lesions, in a study evaluating 142 mixed tumours > 10 HU, 43% of benign lesions did not wash out, and 22% of malignancies did wash out, resulting in AWCT misclassifying 36% of all masses [67]. They concluded, AWCT “with the established thresholds for APW and RPW is insufficient to reliably diagnose adrenal masses”.
Additionally, studies have largely evaluated AWCT in artificially enriched populations, partly to achieve statistical significance. However, as ALs are most frequently encountered incidentally, a landmark paper by Corwin et al evaluated AWCT in a pure AI population [68]. In this multi-institution study, utilising reference standards of histopathology (n = 54), imaging follow-up > 1 year (n = 269; x̄ = 4.6 years) or clinical follow-up > 5 years (n = 13; x̄ = 8.0 years), 336 AIs > 10 HU and > 10 mm (x̄ = 22 mm) were identified. The vast majority were benign (95.8%, n = 322), with 2.7% PCCs (n = 9), and only 1.5% malignant (3 adrenocortical carcinomas (ACCs), 3 metastases). Furthermore, in those < 4 cm, the malignancy prevalence was only 0.3% (n = 1), despite the inherently higher risk > 10 HU population.
Acknowledging the high sensitivity of metanephrines for PCC, the prevalence of non-PCC malignancy was not statistically different in nodules < 4 cm with APW > 60% (0%, n = 0) versus with APW < 60% (1.3%, n = 1). Additionally, even in masses > 4 cm with an inherently higher malignancy rate, there was no significant difference between those with (16.7%) and without washout (23.1%). Importantly, when excluding PCC, for nodules < 4 cm, the negative predictive value (NPV) of washout (for benignity) was only 1.4%, meaning that with a supposed “positive” malignancy result, 98.6% of lesions were still benign.
The utility of AWCT for AIs therefore appears negligible, due to both its inherent limited accuracy and the extremely low malignancy prevalence in true AI populations [68–70]. Notably, this low malignancy prevalence was established as early as 2008 by Song et al, who found no malignant lesions in 1049 consecutive true AIs [8]. Whilst these findings appear disparate to the seminal studies that established AWCT, re-evaluation of these reveals each had highly enriched proportions of malignancies, and furthermore were dominated by LRAs, for which AWCT is no longer recommended. In fact, disproportionately, each had more malignancies than LPAs (malignancy:LPA ratios in Szolar et al, Korobkin et al and Caoili et al, respectively 54:33, 22:7, 36:22) [1, 31, 32]. AWCT was therefore never proven in the true malignancy-sparse AI population.
Furthermore, this low malignancy rate is not unexpected, considering primary adrenal malignancy (ACC) is extremely rare (annual incidence 0.5–2.0 cases/million) [71, 72], but moreover up to 60% are hormonally active [71, 72], and in two studies, 81.9–89.4% were symptomatic (with median size 8.5–12 cm) [71, 73], and therefore few are clinically incidental. Additionally, secondary malignancy (metastases), although much more common, is extremely unlikely to present as isolated adrenal disease. Specifically, in 1715 patients with unknown primary cancers, whilst 5.8% had adrenal metastases, only 0.2% had isolated adrenal involvement, and these were all > 6 cm, mostly bilateral, and retrospectively all symptomatic [74]. Truly incidental isolated adrenal metastases therefore are essentially non-existent, and combined with the rarity of asymptomatic ACC, result in a minimal AI malignancy rate.
In light of these findings, we recommend that the use of AWCT in the true AI population be ceased.
CT attenuation threshold: 20 is the new 10
With AWCT’s credibility doubtful, we question the second tenet of adrenal CT: the 10 HU threshold for LRAs and benignity in general. The 2023 ESE guidelines reported pooled sensitivities and specificities for a 20 HU threshold for malignancy of 96.8% and 76.7%, respectively, versus 100% and 57.5% for 10 HU, based on five key papers [3]. In addition, through expansion of the ESE search strategy to cover more recent studies, we identified one additional paper with data relevant to the 20 HU threshold [37]. These six papers (Tables 1, 2) are individually readdressed here for two reasons.
Table 1.
Study population data for studies reporting 20 HU attenuation and 4 cm size thresholds for discrimination between benign and malignant adrenal lesions
| Study (author [ref.]) |
Study population selection criteria | Non-incidental and functional subpopulations (if known) |
Estimated true AI subpopulation | Histopathology reference standard (%) | PCC (%) | Non-PCC malignancy (%) |
|---|---|---|---|---|---|---|
| Marty et al [77] |
233 patients with 253 “AIs” ≥ 1 cm referred to endocrinology (after exclusion of 184 patients without histopathology or adequate follow-up). |
10.7% patients (n = 22) had a history of cancer considered in remission, including 1 prior contralateral ACCa At least 27.5% patients (n = 64) had hormone production (62 requiring surgery/biopsy + 2 biochemically diagnosed PCC)b |
Unknown—endocrinology referred, surgically enriched population |
50.6% (n = 118; 115 surgery, 3 biopsy) |
13.0% (n = 33/253) |
11.1% (n = 28/253; 23 ACC, 1 mets, 1 bilateral lymphoma, 2 other) |
| Schloetelburg et al [67] |
216 patients with 252 “adrenal lesions” ≥ 1 cm with washout CT (after exclusion of 48 patients without histopathology or adequate follow-up). |
18.7% (n = 47) lesions in patients with current extra-adrenal malignancya 12.3% (n = 31) lesions in patients with suspected adrenal diseasea |
At most, 69.0%a |
36.5% (n = 92) |
4.4% (n = 11/252) |
15.1% (n = 38/252; 9 ACC, 25 mets, 4 lymphoma) |
| Ebbehoj et al [75] | 1287 patients with an “adrenal tumour” (any size) from population health records (660 with unenhanced CT). |
12.1% (n = 156) detected in cancer staging or follow-upa 4.8% (n = 62) in investigation of hormone excess symptomsa 1.5% (n = 19) “other”, e.g., postmortem, in surgery, other symptomsa |
At most, 81.6%a |
43.2% (n = 48/111) of malignant lesions (as per supplementary appendix)b Not stated for benign lesions |
1.1% (n = 14/1287) |
8.6% (n = 111/1287; 4 ACC, 96 mets, 4 lymphoma, 7 neuroblastoma) In “AI” subgroup, 3.3% (n = 35/1050; 1 ACC; 30 mets; 1 lymphoma, 3 neuroblastoma) In “cancer” subgroup, 42.9% (n = 67/156; 0 ACC, 62 mets, 3 lymphoma, 2 neuroblastoma) |
| Bancos et al [76] |
2017 patients from specialist centres with a “newly identified adrenal mass” > 1 cm. Excluded patients with biochemical evidence of PCC, pregnancy, lactation, steroid affecting drugs. Excluded CTs for cancer staging/monitoring. |
16.4% (n = 331) “non-incidental”—imaging for clinical signs and symptoms of steroid excess or a tumoura | At most, 83.6%a |
29.0% (n = 585/2017)b |
0.5% (n = 10/2017) |
8.1% (n = 163/2017; 98 ACC, 39 mets, 8 lymphoma, 18 other) |
| Hong et al [78] |
1149 patients with “newly diagnosed AIs”. Excluded suspected adrenal pathology and history of extra-adrenal malignancy. |
30.5% functional (benign + ACC; n = (348 + 2)/1149)b including 4.5% with overt Cushing with Cushingoid features (benign + ACC; n = (50 + 2)/1149) 11.5% had primary aldosteronism (n = 132) and 7.1% MACS (n = 82)a |
At most 95.5%b | Not stated |
7.3% (n = 84/1149) |
1.7% (n = 20/1149; 14 ACC, 3 lymphoma, 2 leiomyosarcoma, 1 neuroblastoma, 0 mets) |
| Kahramangil et al [37] |
2219 patients with “AIs” ≥ 1 cm. Excluded known active malignancy, signs and symptoms of hormonal hyperfunction, pain due to an adrenal mass. |
15.7% functional (of those worked up); no overt Cushing | Possibly all |
At least 25.9% (n = 574; 382 immediate and 192 delayed surgery)b |
4.4% (n = 97/2219) |
2.2% (n = 49/2219; 38 ACC, 9 mets, 2 lymphoma, as per supplementary table) |
Studies ordered from lowest to highest estimated population of true AIs
AI adrenal incidentaloma, ACC adrenocortical carcinoma, PCC phaeochromocytoma, MACS mild autonomous cortisol secretion, mets metastases
a Provided data
b Calculated from data
Table 2.
Threshold data for studies reporting 20 HU attenuation and 4 cm size thresholds for discrimination between benign and malignant adrenal lesions
| Study (author [ref.]) |
Estimated true AI subpopulation | HU thresholds | Size thresholds | Combined thresholds |
|---|---|---|---|---|
| Marty et al [77] | Unknown—endocrinology referred, surgically enriched population |
≤ 15 HU = 100% PPV for ‘adenoma’a,c ≤ 20 HU = 96.2% PPV for ‘adenoma’a,c (Note PCC included in ‘non-adenomas’) |
≤ 3 cm = 93.4% PPV for ‘adenoma’a,c ≤ 4 cm = 89.4% PPV for ‘adenoma’a,c (Note PCC included in ‘non-adenomas’) |
≤ 3 cm and ≤ 20 HU = 100% PPV for ‘adenoma’a,c ≤ 4 cm and ≤ 15 HU = 100% PPV for ‘adenoma’a,c ≤ 4 cm and ≤ 20 HU = 98.6% PPV for ‘adenoma’a,c (Note PCC included in ‘non-adenomas’) |
| Schloetelburg et al [67] | At most, 69.0%a |
≤ 20 HU = 99.0% PPV for benignitya (Note PCC considered malignant: 1x PCC and 1x metastasis < 20 HU) |
< 4 cm = 87.9% PPV for benignitya (Note PCC considered malignant) |
None (but ≤ 20 HU alone = 99.0% PPV for benignitya) |
| Ebbehoj et al [75] | At most, 81.6%a |
< 20 HU = 99.4% PPV for benignityb (3 of 518 malignant, all 10–20 HU) 10–19 HU = 98.1% PPV for benignityb (3 of 157 malignant) (Note PCC considered benign) |
< 2 cm = 94.1% PPV for benignityb (46 of 784 malignant) ≤ 4 cm = 93.1% PPV for benignityb (82 of 1190 malignant) 2–4 cm = 91.1% PPV for benignityb (36 of 406 malignant) (Note PCC considered benign) |
None (but < 20 HU alone = 99.4% PPV for benignityb) |
| Bancos et al [76] | At most, 83.6%a |
≤ 20 HU = 99.8% PPV for benignityb (1 ACC + 1 OM in 1162 < 20 HU, both 10–20 HU) 10–20 HU = 99.1% PPV for benignityb (Note PCC considered benign) |
< 2 cm = 99.0% PPV for benignityb (6 of 576 malignant—all OM) < 4 cm = 98.6% PPV for benignityb (21 of 1529 malignant) 2 to < 4 cm = 98.4% PPV for benignityb (Note PCC considered benign) |
< 4 cm and ≤ 20 HU = 100% PPV for non-ACCb (possibly also 100% PPV for benignity, as 1 x OM 10–20 HU, but size not stated) |
| Hong et al [78] | At most 95.5%b |
< 19.9 HU = 100% PPV for benignityb (1 ACC = 19.9 HU, ≥ 4 cm) (Note after excluding PCC, myelolipomas, cysts and other benign) |
< 3.5 cm = 100% PPV for benignityb < 4 cm = 99.9% PPV for benignityb (1 neuroblastoma 3.5 cm, 38.2 HU; 1 ACC 4.0 cm) (Note after excluding PCC, myelolipomas, cysts and other benign) |
< 20 HU and < 4 cm = 100% PPV for benignityb |
| Kahramangil et al [37] | Possibly all |
≤ 20 HU = 99.8% PPV for non-ACCb 10–20 HU = 99.5% PPV for non-ACCb (Note no figures for OM) |
< 4 cm = 99.9% PPV for non-ACCb 4–6 cm = 97.6% PPV for non-ACCb > 6 cm = 80.5% PPV for non-ACCb (Note no figures for OM) |
None (but < 4 cm alone = 99.9% PPV for non-ACCb) (and ≤ 20 HU alone = 99.8% PPV for non-ACCb) |
Bold typeface indicates PPVs closest to 20 HU and 4 cm threshold criteria
Studies ordered by increasing estimated rates of true AIs
AI adrenal incidentaloma, ACC adrenocortical carcinoma, PCC phaeochromocytoma, HU Hounsfield unit, OM other malignancy, PPV positive predictive value
a Provided data
b Calculated from data
c ‘Adenoma’ was defined by size stability ≥ 1 year
First, there are data variances that likely arise from differing statistical reporting—some providing sensitivities of > 20 HU for all malignancy, or only ACC; and conversely, some reporting specificities of < 20 HU for benignity, or solely adenomas. However, where provided or calculable, we report the positive predictive value (PPV) for benignity, given it factors in disease prevalence.
Second, the ESE analysis presumed each study comprised incidentaloma populations; however, upon interrogation, the true AI proportions varied greatly (Table 1). In Ebbehoj et al, 18.4% were identified non-incidentally (e.g., cancer/hormonal workup) [75]; in the EURINE-ACT study, 16.4% had clinically suspected steroid excess or tumours [76]; and in Schloetelburg et al, 31% had extra-adrenal malignancy or suspected adrenal disease [67]. In Marty et al, > 50% either had adrenal biopsy or surgery, highly atypical for an incidentaloma population [77]. Accordingly, in these four studies, the non-PCC malignancy rates reached up to 15.1%.
Nonetheless, despite their heavily surgically enriched population, Marty et al found that < 20 HU maintained a 96.2% PPV for adenomas, improving to 98.6% when combined with a 4 cm cutoff (Table 2) [77]. Schloetelburg et al and Ebbehoj et al had PPVs for benignity for 20 HU of 99.0% and 99.4%, respectively [67, 75]. Even more convincingly, the EURINE-ACT study reported 99.8% PPV for 20 HU, and independently, 98.6% PPV for size < 4 cm [76]. When combined, 20 HU and 4 cm excluded 100% of ACC, and there was only one non-ACC malignancy < 20 HU, with size not stated.
Hong et al had a more appropriate 1.7% malignancy rate, though with 30.5% functional lesions (benign + ACC), including 4.5% with overt Cushing syndrome, and therefore not incidental [78]. Nonetheless, a 19.9 HU threshold was 100% sensitive for malignancy, with a single ACC of that attenuation in 1149 patients, but notably > 4 cm, giving 100% PPV for benignity for 20 HU and 4 cm combined.
Most recently, in the study not reviewed in the 2023 ESE Guidelines, Kahramangil et al found a malignancy rate of 2.2% in 2219 AIs. The ACC risk was only 0.16% for < 20 HU, and independently 0.1% for < 4 cm, and we theorise these combined parameters would approach, if not reach 100% PPV for benignity [37]. Additionally, in a study of exclusively large (> 4 cm) tumours, comprising only 67% true AIs, a 20 HU cutoff still had 98.5% NPV for malignancy [79].
Accordingly, we propose that in true AIs, combined 20 HU and 4 cm cut-offs exclude malignancy, and replace the established 10 HU threshold. We also highlight that even in those > 20 HU but < 4 cm, or > 4 cm but < 20 HU, malignancy rates are also extremely low.
Where should the focus of future adrenal incidentaloma research be?
Whilst we believe there is sufficient data to support our recommendations thus far, we acknowledge other aspects of AI workup presently lack strong evidence. We therefore encourage further research into these areas to assist in refining an algorithmic approach to AI management.
Further attenuation and size subcategorisation
Further stratification of subgroups within and beyond the stated 20 HU and 4 cm thresholds (e.g., combinations of 10–20 HU, 20–40 HU; 1–2 cm, 2–4 cm, 4–6 cm, > 6 cm) may enable reduced imaging for additional benign lesions. Some evidence suggests a more cautious approach may be appropriate for all lesions > 40 HU on ncCT [40, 49, 54] given the relative infrequency of adenomas in this range; however, this data was obtained primarily in the context of PCC determination. Additionally, some studies indicate lesions > 6 cm have a significantly increased risk of malignancy, independent of attenuation [36, 37, 79, 80]. Conversely, there is early data that AIs below a size threshold of 1.5 cm may not require cortisol testing; however, larger validation studies are required [81].
Post-contrast CT detected adrenal incidentalomas
For AIs first detected on post-contrast CT with indeterminate imaging features, ncCT may be performed. However, given the established low ~0.1% risk of malignancy of true AIs < 4 cm of any attenuation [37, 78], it may be possible that ncCT could be delayed 6–12 months in this subgroup to simultaneously allow growth assessment. Additionally, further research may identify subgroups where ncCT may not be required at all.
Stability and growth
Current guidelines indicate a variable range of 6–12-month stability as evidence of benignity [2, 3, 5], and there is currently only limited data assessing adenoma versus ACC growth rates [82, 83]. Further research into both these areas is therefore warranted.
Spectral CT attenuation
Historic studies have been based on conventional 120 kVp imaging data; however, attenuation values derived from spectral CT, including virtual noncontrast measurements, may differ [84–87]. With the expected proliferation of photon-counting CT, it will be important to establish how spectral CT attenuation measurements can be used in AL assessment.
Heterogeneous lesions and morphology
A recent study indicated that attenuation measurement in heterogeneous lesions has poor diagnostic performance [88]. Furthermore, heterogeneity and other morphologic features in some studies have shown high specificity but poor sensitivity for indicating malignancy [89–91]. Further investigation into the management of heterogeneous nodules is therefore warranted, noting recent disappointing results also for MRI in differentiating adenomas and metastases by heterogeneity and T2-weighted signal [59–62].
Chemical-shift MRI
Chemical-shift (CS) MRI is long-established as an alternative method for detecting microscopic fat, largely assessed qualitatively, though with the most cited quantitative cutoff being a signal intensity index [(in-phase signal − opposed-phase signal)/in-phase signal] ≥ 16.5% [10, 92, 93]. The utility of CS MRI, however, not unexpectedly declines with increasing lesion CT attenuation, particularly ≥ 40 HU [94–97]. Given the availability and cost considerations of MRI, feasibility and efficacy studies into its role in lesions 20–40 HU are therefore suggested, as well as head-to-head comparisons to spectral-CT fat quantification as an alternative CT-based modality [98, 99].
PET-CT
Data demonstrating higher specificity of 18F-FDG PET-CT than CT attenuation for malignancy has been largely based on cancer populations [100–102] and therefore not applicable to AIs. Furthermore, functional (especially cortisol-secreting) adenomas have been associated with higher FDG uptake [103], as well as those of higher attenuation, reducing specificity in the group that it would be of most potential gain [104]. Nonetheless, there may be a future role of PET-CT, with research into other tracer candidates such as 11C-metomidate, which has specific adrenal cortical uptake [105–107].
Artificial intelligence
There are multiple preliminary studies describing the use of artificial intelligence in adrenal incidentalomas, primarily in the areas of lesion detection, segmentation and characterisation, with the latter utilising radiomics and machine learning or specifically deep learning; however, most studies are of small populations, and with current limited clinical usage [108]. Larger studies are therefore recommended, particularly those that integrate clinical information, including endocrine laboratory results, with the imaging findings.
Non-incidental adrenal lesions
We reiterate that our recommendation to cease the use of AWCT applies only to the true AI patient population. Outside of this, further delineation of any remaining role of AWCT is recommended, acknowledging that for those with extra-adrenal malignancy, this may depend on the nature of the primary tumour. It is noted that in the 2023 ESE Guidelines, the diagnostic algorithm for ALs in this clinical context includes biopsy, FDG PET-CT and resection, with no current reference to AWCT [3]. It is also unclear if there is any role for AWCT in patients with functional lesions, given that these are more likely to be managed surgically. Nonetheless, where biopsy and PET-CT are unavailable, it is possible AWCT may still have a contributory role in non-incidental adrenal lesions.
Summarised recommendations for adrenal incidentaloma management
Based on the presented evidence, and with acknowledgement of areas requiring further research, the following AI management principles are provided:
Definition of adrenal incidentalomas
A robust definition of an AI inherently delineates a population with an extremely low prevalence of malignancy. To reiterate, we define AIs as ≥ 1 cm lesions in patients without current or prior extra-adrenal malignancy, or clinically suspected adrenal disease. AIs with overtly benign imaging features, such as myelolipomas or cysts, or those with long-term stability (for now, defined as 6–12 months) do not require further evaluation.
Recommendation for hormonal correlation
Radiology reports should include a recommendation to perform a hormonal evaluation for all remaining AIs, which will identify nearly all PCC, as well as most ACC. Additionally, endocrine correlation identifies subclinically functional adenomas (including MACS), where clinical management may take precedence. Recommending additional endocrinologist referral may depend on local preferences, as in some regions, to manage cost and access issues, endocrine testing can be performed by primary care physicians, with endocrinologist referral limited to those with abnormal results.
Elimination of adrenal washout CT
In the defined AI population, and with application of endocrine assessment, current evidence indicates no role for AWCT.
Categorisation on noncontrast CT
On ncCT (Fig. 1), homogeneous AIs ≤ 20 HU and ≤ 4 cm, or as per existing guidelines, < 10 HU and any size, are considered benign (Category-1), with no imaging follow-up required. AIs which are > 20 HU and 1–4 cm, OR 10–20 HU but > 4 cm, are highly likely benign (Category-2), and therefore 6–12 month ncCT is currently suggested as supported by most AI guidelines, purely to identify growth or stability.
Fig. 1.
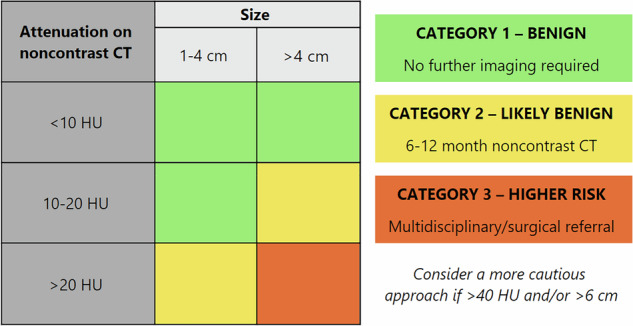
Management of adrenal incidentalomas on noncontrast CTa. a Table only to be used in conjunction with summarised recommendations. HU, Hounsfield unit
Those > 20 HU AND > 4 cm are considered higher risk (Category-3), with multidisciplinary meeting or surgical referral recommended, albeit acknowledging that most will still be of benign aetiology. As discussed prior, a more cautious approach is advised if AIs are > 40 HU and/or > 6 cm.
Conclusion
Whilst the 10 HU threshold and AWCT have been ingrained in the radiology mindset over the last 2–3 decades, we believe it is now a timely end of an era for both these tenets of AI imaging. First, there is now sufficient evidence that a 20 HU threshold in AIs < 4 cm can safely replace the prior 10 HU limit. Second, due to its inherent inaccuracy and the extremely low incidence of malignancy in true AIs, AWCT has no role in the evaluation of ALs in patients without prior/current malignancy or suspected adrenal disease. Instead, AIs can be managed with ncCT, in parallel with endocrine testing, which complementarily improves detection of benign (and rarely malignant) hormonally active lesions.
We believe these measures will be of significant benefit to patients, radiology departments and healthcare systems in reducing the volume of unnecessary imaging for adrenal lesions. We also encourage further research to identify additional lower-risk subgroups where imaging follow-up or clinical escalation may be unnecessary.
Supplementary information
Acknowledgements
We appreciate Dr Sarah Wong, who assisted with reference management, and the South & East Metropolitan Health Service Library and Information Service for providing literature access.
Abbreviations
- ACC
Adrenocortical carcinoma
- AI
Adrenal incidentaloma
- AL
Adrenal lesion
- APW
Absolute percentage washout
- AWCT
Adrenal washout CT
- CS
Chemical shift
- ENSAT
European Network for the Study of Adrenal Tumors
- ESE
European Society of Endocrinologists
- HU
Hounsfield units
- LPA
Lipid-poor adenoma
- LRA
Lipid-rich adenoma
- MACS
Mild autonomous cortisol secretion
- ncCT
Noncontrast CT
- NPV
Negative predictive value
- PCC
Phaeochromocytoma
- PPV
Positive predictive value
- PV
Portal venous
- RPW
Relative percentage washout
Author contributions
J.S. conceptualised the manuscript, was lead writer of the original draft and lead editor. D.S., C.W., A.S. and J.G. extensively reviewed, fact-checked and edited the manuscript. All authors read and approved the final manuscript.
Funding
The authors state that this work has not received any funding.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
James H. Seow, Email: james.seow@health.wa.gov.au
Damien L. Stella, Email: ds@unimelb.edu.au
Supplementary information
The online version contains supplementary material available at 10.1186/s13244-025-02015-4.
References
- 1.Caoili EM, Korobkin M, Francis IR et al (2002) Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology 222:629–633. 10.1148/radiol.2223010766 [DOI] [PubMed] [Google Scholar]
- 2.Mayo-Smith WW, Song JH, Boland GL et al (2017) Management of incidental adrenal masses: a white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 14:1038–1044. 10.1016/j.jacr.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 3.Fassnacht M, Tsagarakis S, Terzolo M (2023) European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 189:G1–G42. 10.1093/ejendo/lvad066 [DOI] [PubMed] [Google Scholar]
- 4.Lee JM, Kim MK, Ko SH et al (2017) Clinical guidelines for the management of adrenal incidentaloma. Endocrinol Metab 32:200–218. 10.3803/EnM.2017.32.2.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowe NE, Kumar RM, Schieda N et al (2023) Canadian Urological Association guideline: diagnosis, management, and followup of the incidentally discovered adrenal mass. Can Urol Assoc J 17:12–24. 10.5489/cuaj.8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bovio S, Cataldi A, Reimondo G et al (2006) Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 29:298–302. 10.1007/BF03344099 [DOI] [PubMed] [Google Scholar]
- 7.Hammarstedt L, Muth A, Wängberg B et al (2010) Adrenal lesion frequency: a prospective, cross-sectional CT study in a defined region, including systematic re-evaluation. Acta Radiol 51:1149–1156. 10.3109/02841851.2010.516016 [DOI] [PubMed] [Google Scholar]
- 8.Song JH, Chaudhry FS, Mayo-Smith WW (2008) The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol 190:1163–1168. 10.2214/AJR.07.2799 [DOI] [PubMed] [Google Scholar]
- 9.Hammarstedt L, Muth A, Sigurjónsdóttir HÁ, Almqvist E, Wängberg B, Hellström M (2012) Adrenal lesions in patients with extra-adrenal malignancy—benign or malignant? Acta Oncol 51:215–221. 10.3109/0284186X.2011.608084 [DOI] [PubMed] [Google Scholar]
- 10.Glazer DI, Mayo-Smith WW, Remer EM et al (2023) Lexicon for adrenal terms at CT and MRI: a consensus of the Society of Abdominal Radiology adrenal neoplasm disease-focused panel. Abdom Radiol (NY) 48:952–975. 10.1007/s00261-022-03729-5 [DOI] [PubMed] [Google Scholar]
- 11.Kim MK, Kang KA, Park SY (2022) Clinical significance of a 10-mm cutoff size for adrenal lesions: a retrospective study with 547 non-oncologic patients undergoing adrenal computed tomography. Abdom Radiol (NY) 47:1091–1097. 10.1007/s00261-021-03405-0 [DOI] [PubMed] [Google Scholar]
- 12.Cahill GF (1935) Air injections to demonstrate the adrenals by X-ray. J Urol 34:238–243. 10.1016/S0022-5347(17)76948-0 [Google Scholar]
- 13.Watt-Wyness JN (1949) Adrenal gland investigation by air-insufflation tomography. Radiography 15:66 [PubMed] [Google Scholar]
- 14.Sheedy PF 2nd, Stephens DH, Hattery RR, Muhm JR, Hartman GW (1976) Computed tomography of the body: initial clinical trial with the EMI prototype. AJR Am J Roentgenol 127:23–51. 10.2214/ajr.127.1.23 [DOI] [PubMed] [Google Scholar]
- 15.Behan M, Martin EC, Muecke EC, Kazam E (1977) Myelolipoma of the adrenal: two cases with ultrasound and CT findings. AJR Am J Roentgenol 129:993–996. 10.2214/ajr.129.6.993 [DOI] [PubMed] [Google Scholar]
- 16.Schaner EG, Dunnick NR, Doppman JL, Strott CA, Gill JR Jr, Javadpour N (1978) Adrenal cortical tumors with low attenuation coefficients: a pitfall in computed tomography diagnosis. J Comput Assist Tomogr 2:11–15. 10.1097/00004728-197801000-00002 [DOI] [PubMed] [Google Scholar]
- 17.Miyake H, Maeda H, Tashiro M (1989) CT of adrenal tumors: frequency and clinical significance of low-attenuation lesions. AJR Am J Roentgenol 152:1005–1007. 10.2214/ajr.152.5.1005 [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Hahn PF, Papanicolaou N (1991) Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology 179:415–418. 10.1148/radiology.179.2.2014283 [DOI] [PubMed] [Google Scholar]
- 19.Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR (1998) Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol 171:201–204. 10.2214/ajr.171.1.9648789 [DOI] [PubMed] [Google Scholar]
- 20.Zeiger MA, Thompson GB, Duh QY et al (2009) American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas: executive summary of recommendations. Endocr Pract 15:450–453. 10.4158/EP.15.5.450 [DOI] [PubMed] [Google Scholar]
- 21.Berland LL, Silverman SG, Gore RM et al (2010) Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 7:754–773. 10.1016/j.jacr.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 22.Kapoor A, Morris T, Rebello R (2012) Guidelines for the management of the incidentally discovered adrenal mass. Can Urol Assoc J 5:241–247. 10.5489/cuaj.11135. Erratum in: Can Urol Assoc J 6:244. doi: 10.5489/cuaj.12208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fassnacht M, Arlt W, Bancos I et al (2016) Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 175:G1–G34. 10.1530/EJE-16-0467 [DOI] [PubMed] [Google Scholar]
- 24.Krestin GP, Steinbrich W, Friedmann G (1989) Adrenal masses: evaluation with fast gradient-echo MR imaging and Gd-DTPA-enhanced dynamic studies. Radiology 171:675–680. 10.1148/radiology.171.3.2717737 [DOI] [PubMed] [Google Scholar]
- 25.Krestin GP, Freidmann G, Fishbach R, Neufang KF, Allolio B (1991) Evaluation of adrenal masses in oncologic patients: dynamic contrast-enhanced MR vs CT. J Comput Assist Tomogr 15:104–110. 10.1097/00004728-199101000-00016 [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa T, Ohtomo K, Uchiyama G, Fujimoto H, Nasu K (1995) Contrast-enhanced dynamic MRI of adrenal masses: classification of characteristic enhancement patterns. Clin Radiol 50:295–300. 10.1016/s0009-9260(05)83419-1 [DOI] [PubMed] [Google Scholar]
- 27.Korobkin M, Brodeur FJ, Yutzy GG et al (1996) Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. AJR Am J Roentgenol 166:531–536. 10.2214/ajr.166.3.8623622 [DOI] [PubMed] [Google Scholar]
- 28.Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Goodsitt M (1996) Delayed enhanced CT for differentiation of benign from malignant adrenal masses. Radiology 200:737–742. 10.1148/radiology.200.3.8756924 [DOI] [PubMed] [Google Scholar]
- 29.Szolar DH, Kammerhuber F (1997) Quantitative CT evaluation of adrenal gland masses: a step forward in the differentiation between adenomas and nonadenomas? Radiology 202:517–521. 10.1148/radiology.202.2.9015083 [DOI] [PubMed] [Google Scholar]
- 30.Boland GW, Hahn PF, Peña C, Mueller PR (1997) Adrenal masses: characterization with delayed contrast-enhanced CT. Radiology 202:693–696. 10.1148/radiology.202.3.9051018 [DOI] [PubMed] [Google Scholar]
- 31.Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Londy F (1998) CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol 170:747–752. 10.2214/ajr.170.3.9490968 [DOI] [PubMed] [Google Scholar]
- 32.Szolar DH, Kammerhuber FH (1998) Adrenal adenomas and nonadenomas: assessment of washout at delayed contrast-enhanced CT. Radiology 207:369–375. 10.1148/radiology.207.2.9577483 [DOI] [PubMed] [Google Scholar]
- 33.Gendy R, Rashid P (2017) Incidental adrenal masses—a primary care approach. Aust Fam Physician 46:385–390 [PubMed] [Google Scholar]
- 34.Maas M, Nassiri N, Bhanvadia S, Carmichael JD, Duddalwar V, Daneshmand S (2021) Discrepancies in the recommended management of adrenal incidentalomas by various guidelines. J Urol 205:52–59. 10.1097/JU.0000000000001342. Erratum in: J Urol 205:942 [DOI] [PubMed] [Google Scholar]
- 35.BMJ Best Practice (2023) Assessment of incidental adrenal mass. Available via https://bestpractice.bmj.com/topics/en-gb/518/pdf/518/Assessment%20of%20incidental%20adrenal%20mass.pdf. Accessed 4 Dec 2024
- 36.Mantero F, Terzolo M, Arnaldi G et al (2000) A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab 85:637–644. 10.1210/jcem.85.2.6372 [DOI] [PubMed] [Google Scholar]
- 37.Kahramangil B, Kose E, Remer EM et al (2022) A modern assessment of cancer risk in adrenal incidentalomas: analysis of 2219 patients. Ann Surg 275:e238–e244. 10.1097/SLA.0000000000004048 [DOI] [PubMed] [Google Scholar]
- 38.Buitenwerf E, Korteweg T, Visser A (2018) Unenhanced CT imaging is highly sensitive to exclude pheochromocytoma: a multicenter study. Eur J Endocrinol 178:431–437. 10.1530/EJE-18-0006 [DOI] [PubMed] [Google Scholar]
- 39.Sane T, Schalin-Jäntti C, Raade M (2012) Is biochemical screening for pheochromocytoma in adrenal incidentalomas expressing low unenhanced attenuation on computed tomography necessary? J Clin Endocrinol Metab 97:2077–2083. 10.1210/jc.2012-1061 [DOI] [PubMed] [Google Scholar]
- 40.Patel J, Davenport MS, Cohan RH, Caoili EM (2013) Can established CT attenuation and washout criteria for adrenal adenoma accurately exclude pheochromocytoma? AJR Am J Roentgenol 201:122–127. 10.2214/AJR.12.9620 [DOI] [PubMed] [Google Scholar]
- 41.Schalin-Jäntti C, Raade M, Hämäläinen E, Sane T (2015) A 5-year prospective follow-up study of lipid-rich adrenal incidentalomas: no tumor growth or development of hormonal hypersecretion. Endocrinol Metab 30:481–487. 10.3803/EnM.2015.30.4.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jun JH, Ahn HJ, Lee SM et al (2015) Is preoperative biochemical testing for pheochromocytoma necessary for all adrenal incidentalomas? Medicine (Baltimore) 94:e1948. 10.1097/MD.0000000000001948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buitenwerf E, Berends AMA, van Asselt ADI et al (2019) Diagnostic accuracy of computed tomography to exclude pheochromocytoma: a systematic review, meta-analysis, and cost analysis. Mayo Clin Proc 94:2040–2052. 10.1016/j.mayocp.2019.03.030 [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal S, Prete A, Chortis V et al (2023) Pheochromocytomas most commonly present as adrenal incidentalomas: a large tertiary center experience. J Clin Endocrinol Metab 109:e389–e396. 10.1210/clinem/dgad401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corwin MT, Arora A, Loehfelm TW, Fananapazir G, Campbell MJ (2020) Adherence to guidelines for hormonal evaluation in patients with incidentally detected adrenal nodules: effects of radiology report wording and standardized reporting. Abdom Radiol (NY) 45:2910–2915. 10.1007/s00261-020-02517-3 [DOI] [PubMed] [Google Scholar]
- 46.Woo S, Suh CH, Kim SY, Cho JY, Kim SH (2018) Pheochromocytoma as a frequent false-positive in adrenal washout CT: a systematic review and meta-analysis. Eur Radiol Mar 28:1027–1036. 10.1007/s00330-017-5076-5 [DOI] [PubMed] [Google Scholar]
- 47.Altinmakas E, Perrier ND, Grubbs EG, Lee JE, Prieto VG, Ng CS (2020) Diagnostic performance of adrenal CT in the differentiation of adenoma and pheochromocytoma. Acta Radiol 61:1080–1086. 10.1177/0284185119889568 [DOI] [PubMed] [Google Scholar]
- 48.Canu L, Van Hemert JAW, Kerstens MN et al (2019) CT characteristics of pheochromocytoma: relevance for the evaluation of adrenal incidentaloma. J Clin Endocrinol Metab 104:312–318. 10.1210/jc.2018-01532 [DOI] [PubMed] [Google Scholar]
- 49.Kang S, Oh YL, Park SY (2021) Distinguishing pheochromocytoma from adrenal adenoma by using modified computed tomography criteria. Abdom Radiol (NY) 46:1082–1090. 10.1007/s00261-020-02764-4 [DOI] [PubMed] [Google Scholar]
- 50.Mohammed MF, ElBanna KY, Ferguson D, Harris A, Khosa F (2018) Pheochromocytomas versus adenoma: role of venous phase CT enhancement. AJR Am J Roentgenol 210:1073–1078. 10.2214/AJR.17.18472 [DOI] [PubMed] [Google Scholar]
- 51.Northcutt BG, Trakhtenbroit MA, Gomez EN, Fishman EK, Johnson PT (2016) Adrenal adenoma and pheochromocytoma: comparison of multidetector CT venous enhancement levels and washout characteristics. J Comput Assist Tomogr 40:194–200. 10.1097/RCT.0000000000000343 [DOI] [PubMed] [Google Scholar]
- 52.Northcutt BG, Raman SP, Long C et al (2013) MDCT of adrenal masses: can dual-phase enhancement patterns be used to differentiate adenoma and pheochromocytoma? AJR Am J Roentgenol 201:834–839. 10.2214/AJR.12.9753 [DOI] [PubMed] [Google Scholar]
- 53.Goroshi M, Jadhav SS, Sarathi V et al (2019) Radiological differentiation of phaeochromocytoma from other malignant adrenal masses: importance of wash-in characteristics on multiphase CECT. Endocr Connect 8:898–905. 10.1530/EC-19-0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.An YY, Yang GZ, Lin B et al (2021) Differentiation of lipid-poor adenoma from pheochromocytoma on biphasic contrast-enhanced CT. Abdom Radiol (NY) 46:4353–4361. 10.1007/s00261-021-03121-9 [DOI] [PubMed] [Google Scholar]
- 55.Nagayama Y, Inoue T, Kato Y et al (2021) Relative enhancement ratio of portal venous phase to unenhanced CT in the diagnosis of lipid-poor adrenal adenomas. Radiology 301:360–368. 10.1148/radiol.2021210231 [DOI] [PubMed] [Google Scholar]
- 56.Jin S, Zhang H, Pan W et al (2022) Diagnostic value of the relative enhancement ratio of the portal venous phase to unenhanced CT in the identification of lipid-poor adrenal tumors. Abdom Radiol (NY) 47:3308–3317. 10.1007/s00261-022-03593-3 [DOI] [PubMed] [Google Scholar]
- 57.Varghese JC, Hahn PF, Papanicolaou N, Mayo-Smith WW, Gaa JA, Lee MJ (1997) MR differentiation of phaeochromocytoma from other adrenal lesions based on qualitative analysis of T2 relaxation times. Clin Radiol 52:603–606. 10.1016/s0009-9260(97)80252-8 [DOI] [PubMed] [Google Scholar]
- 58.Maurea S, Attanasio L, Galatola R et al (2024) MR imaging characterization of pheochromocytoma: a comparison between typical and atypical tumor lesions. Clin Transl Imaging 12:337–346. 10.1007/s40336-023-00608-x [Google Scholar]
- 59.Schieda N, Siegelman ES (2017) Update on CT and MRI of adrenal nodules. AJR Am J Roentgenol 208:1206–1217. 10.2214/AJR.16.17758 [DOI] [PubMed] [Google Scholar]
- 60.Gerson R, Tu W, Abreu-Gomez J et al (2022) Evaluation of the T2-weighted (T2W) adrenal MRI calculator to differentiate adrenal pheochromocytoma from lipid-poor adrenal adenoma. Eur Radiol 32:8247–8255. 10.1007/s00330-022-08867-4 [DOI] [PubMed] [Google Scholar]
- 61.Tu W, Abreu-Gomez J, Udare A, Alrashed A, Schieda N (2020) Utility of T2-weighted MRI to differentiate adrenal metastases from lipid-poor adrenal adenomas. Radiol Imaging Cancer 2:e200011. 10.1148/rycan.2020200011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tu W, Badawy M, Carney BW et al (2024) Multicenter validation of a T2-weighted MRI calculator to differentiate adrenal adenoma from adrenal metastases. AJR Am J Roentgenol 222:e2329727. 10.2214/AJR.23.29727 [DOI] [PubMed] [Google Scholar]
- 63.Berry R, Busireddy K, Chu LC, Johnson PT, Fishman EK (2022) The good, the bad, and the ugly: uncommon CT appearances of pheochromocytoma. Abdom Radiol (NY) 47:1406–1413. 10.1007/s00261-022-03447-y [DOI] [PubMed] [Google Scholar]
- 64.Mazzaglia PJ, Varghese J, Habra MA (2020) Evaluation and management of adrenal neoplasms: endocrinologist and endocrine surgeon perspectives. Abdom Radiol (NY) 45:1001–1010. 10.1007/s00261-020-02464-z [DOI] [PubMed] [Google Scholar]
- 65.Choi YA, Kim CK, Park BK, Kim B (2013) Evaluation of adrenal metastases from renal cell carcinoma and hepatocellular carcinoma: use of delayed contrast-enhanced CT. Radiology 266:514–520. 10.1148/radiol.12120110 [DOI] [PubMed] [Google Scholar]
- 66.Moosavi B, Shabana WM, El-Khodary M et al (2016) Intracellular lipid in clear cell renal cell carcinoma tumor thrombus and metastases detected by chemical shift (in and opposed phase) MRI: radiologic-pathologic correlation. Acta Radiol 57:241–248. 10.1177/0284185115572207 [DOI] [PubMed] [Google Scholar]
- 67.Schloetelburg W, Ebert I, Petritsch B et al (2021) Adrenal wash-out CT: moderate diagnostic value in distinguishing benign from malignant adrenal masses. Eur J Endocrinol 186:183–193. 10.1530/EJE-21-0650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corwin MT, Badawy M, Caoili EM (2022) Incidental adrenal nodules in patients without known malignancy: prevalence of malignancy and utility of washout CT for characterization—a multiinstitutional study. AJR Am J Roentgenol 219:804–812. 10.2214/AJR.22.27901 [DOI] [PubMed] [Google Scholar]
- 69.Corwin MT, Remer EM (2021) Adrenal washout CT: point-not useful for characterizing incidentally discovered adrenal nodules. AJR Am J Roentgenol 216:1166–1167. 10.2214/AJR.20.24417 [DOI] [PubMed] [Google Scholar]
- 70.van Aswegen T, Trinh B, Jacques A, Lo G (2024) Adrenal washout CT in patients with no history of cancer: a waste of time? Abdom Radiol (NY) 49:3117–3126. 10.1007/s00261-024-04333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim JS, Lee SE, Kim JH, Kim JH (2020) Characteristics of adrenocortical carcinoma in South Korea: a registry-based nationwide survey. Endocr Connect 9:519–529. 10.1530/EC-20-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmed AA, Thomas AJ, Ganeshan DM et al (2020) Adrenal cortical carcinoma: pathology, genomics, prognosis, imaging features, and mimics with impact on management. Abdom Radiol (NY) 45:945–963. 10.1007/s00261-019-02371-y [DOI] [PubMed] [Google Scholar]
- 73.Yasar HA, Aktas BY, Ucar G (2024) Adrenocortical cancer in the real world: a comprehensive analysis of clinical features and management from the Turkish Oncology Group (TOG). Clin Genitourin Cancer 22:102077. 10.1016/j.clgc.2024.102077 [DOI] [PubMed] [Google Scholar]
- 74.Lee JE, Evans DB, Hickey RC et al (1998) Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery 124:1115–1122. 10.1067/msy.1998.92009 [DOI] [PubMed] [Google Scholar]
- 75.Ebbehoj A, Li D, Kaur RJ et al (2020) Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol 8:894–902. 10.1016/S2213-8587(20)30314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bancos I, Taylor AE, Chortis V et al (2020) Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol 8:773–781. 10.1016/S2213-8587(20)30218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marty M, Gaye D, Perez P et al (2018) Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol 178:439–446. 10.1530/EJE-17-1056 [DOI] [PubMed] [Google Scholar]
- 78.Hong AR, Kim JH, Park KS et al (2017) Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice. Eur J Endocrinol 177:475–483. 10.1530/EJE-17-0372 [DOI] [PubMed] [Google Scholar]
- 79.Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA et al (2017) Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes 2:30–39. 10.1016/j.mayocpiqo.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corwin MT, Lan C, Wilson M, Loehfelm TW, Campbell MJ (2021) Can abdominal CT features predict autonomous cortisol secretion in patients with adrenal nodules? Abdom Radiol (NY) 46:4338–4344. 10.1007/s00261-021-03110- [DOI] [PubMed] [Google Scholar]
- 81.Cyranska-Chyrek E, Szczepanek-Parulska E, Olejarz M, Ruchala M (2019) Malignancy risk and hormonal activity of adrenal incidentalomas in a large cohort of patients from a single tertiary reference center. Int J Environ Res Public Health 16:1872. 10.3390/ijerph16101872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corwin MT, Navarro SM, Malik DG et al (2019) Differences in growth rate on CT of adrenal adenomas and malignant adrenal nodules. AJR Am J Roentgenol 213:632–636. 10.2214/AJR.19.21342 [DOI] [PubMed] [Google Scholar]
- 83.Ceccato F, Tizianel I, Voltan G et al (2021) Attenuation value in adrenal incidentalomas: a longitudinal study. Front Endocrinol 12:794197. 10.3389/fendo.2021.794197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeh BM, Shepherd JA, Wang ZJ, Teh HS, Hartman RP, Prevrhal S (2009) Dual-energy and low-kVp CT in the abdomen. AJR Am J Roentgenol 193:47–54. 10.2214/AJR.09.2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao J, Lennartz S, Parakh A et al (2021) Dual-layer dual-energy CT for characterization of adrenal nodules: can virtual unenhanced images replace true unenhanced acquisitions? Abdom Radiol (NY) 46:4345–4352. 10.1007/s00261-021-03062-3 [DOI] [PubMed] [Google Scholar]
- 86.Nagayama Y, Inoue T, Oda S et al (2020) Adrenal adenomas versus metastases: diagnostic performance of dual-energy spectral CT virtual noncontrast imaging and iodine maps. Radiology 296:324–332. 10.1148/radiol.2020192227 [DOI] [PubMed] [Google Scholar]
- 87.Bette S, Risch F, Canalini L et al (2024) Diagnostic performance of photon-counting detector CT for differentiation between adrenal adenomas and metastases. Eur Radiol 34:5944–5953. 10.1007/s00330-024-10675-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corwin MT, Caoili EM, Elsayes KM et al (2024) Performance of CT with adrenal-washout protocol in heterogeneous adrenal nodules: a multiinstitutional study. AJR Am J Roentgenol 222:e2330769. 10.2214/AJR.23.30769 [DOI] [PubMed] [Google Scholar]
- 89.Song JH, Grand DJ, Beland MD, Chang KJ, Machan JT, Mayo-Smith WW (2013) Morphologic features of 211 adrenal masses at initial contrast-enhanced CT: can we differentiate benign from malignant lesions using imaging features alone? AJR Am J Roentgenol 201:1248–1253. 10.2214/AJR.12.10302 [DOI] [PubMed] [Google Scholar]
- 90.Tu W, Verma R, Krishna S, McInnes MDF, Flood TA, Schieda N (2018) Can adrenal adenomas be differentiated from adrenal metastases at single-phase contrast-enhanced CT? AJR Am J Roentgenol 211:1044–1050. 10.2214/AJR.17.19276 [DOI] [PubMed] [Google Scholar]
- 91.Corwin MT, Mitchell AS, Wilson M, Campbell MJ, Fananapazir G, Loehfelm TW (2021) Accuracy of focal cystic appearance within adrenal nodules on contrast-enhanced CT to distinguish pheochromocytoma and malignant adrenal tumors from adenomas. Abdom Radiol (NY) 46:2683–2689. 10.1007/s00261-020-02925-5 [DOI] [PubMed] [Google Scholar]
- 92.Fujiyoshi F, Nakajo M, Fukukura Y, Tsuchimochi S (2003) Characterization of adrenal tumors by chemical shift fast low-angle shot MR imaging: comparison of four methods of quantitative evaluation. AJR Am J Roentgenol 180:1649–1657. 10.2214/ajr.180.6.1801649 [DOI] [PubMed] [Google Scholar]
- 93.Platzek I, Sieron D, Plodeck V et al (2019) Chemical shift imaging for evaluation of adrenal masses: a systematic review and meta-analysis. Eur Radiol 29:806–817. 10.1007/s00330-018-5626-5 [DOI] [PubMed] [Google Scholar]
- 94.Haider MA, Ghai S, Jhaveri K, Lockwood G (2004) Chemical shift MR imaging of hyperattenuating (> 10 HU) adrenal masses: does it still have a role? Radiology 231:711–716. 10.1148/radiol.2313030676 [DOI] [PubMed] [Google Scholar]
- 95.Park BK, Kim CK, Kim B, Lee JH (2007) Comparison of delayed enhanced CT and chemical shift MR for evaluating hyperattenuating incidental adrenal masses. Radiology 243:760–765. 10.1148/radiol.2433051978 [DOI] [PubMed] [Google Scholar]
- 96.Koo HJ, Choi HJ, Kim HJ, Kim SO, Cho KS (2014) The value of 15-minute delayed contrast-enhanced CT to differentiate hyperattenuating adrenal masses compared with chemical shift MR imaging. Eur Radiol 24:1410–1420. 10.1007/s00330-013-3084-7 [DOI] [PubMed] [Google Scholar]
- 97.Seo JM, Park BK, Park SY, Kim CK (2014) Characterization of lipid-poor adrenal adenoma: chemical-shift MRI and washout CT. AJR Am J Roentgenol 202:1043–1050. 10.2214/AJR.13.11389. Erratum in: AJR Am J Roentgenol 212:232 [DOI] [PubMed] [Google Scholar]
- 98.Nagayama Y, Inoue T, Oda S et al (2021) Unenhanced dual-layer spectral-detector CT for characterizing indeterminate adrenal lesions. Radiology 301:369–378. 10.1148/radiol.2021202435 [DOI] [PubMed] [Google Scholar]
- 99.Nagayama Y, Uchimura R, Maruyama N et al (2025) Non-contrast spectral CT vs chemical-shift MRI in discriminating lipid-poor adrenal lesions. Eur Radiol 35:370–380. 10.1007/s00330-024-10929-8 [DOI] [PubMed] [Google Scholar]
- 100.Boland GW, Dwamena BA, Jagtiani Sangwaiya M et al (2011) Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology 259:117–126. 10.1148/radiol.11100569 [DOI] [PubMed] [Google Scholar]
- 101.Delivanis DA, Bancos I, Atwell TD et al (2018) Diagnostic performance of unenhanced computed tomography and 18F-fluorodeoxyglucose positron emission tomography in indeterminate adrenal tumours. Clin Endocrinol 88:30–36. 10.1111/cen.13448 [DOI] [PubMed] [Google Scholar]
- 102.Schaafsma M, Berends AMA, Links TP, Brouwers AH, Kerstens MN (2023) The diagnostic value of 18F-FDG PET/CT scan in characterizing adrenal tumors. J Clin Endocrinol Metab 108:2435–2445. 10.1210/clinem/dgad138 [DOI] [PubMed] [Google Scholar]
- 103.Akkuş G, Güney IB, Ok F et al (2019) Diagnostic efficacy of 18F-FDG PET/CT in patients with adrenal incidentaloma. Endocr Connect 8:838–845. 10.1530/EC-19-0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murayama R, Nishie A, Hida T et al (2019) Uptake of 18F-FDG in adrenal adenomas is associated with unenhanced CT value and constituent cells. Clin Nucl Med 44:943–948. 10.1097/RLU.0000000000002759 [DOI] [PubMed] [Google Scholar]
- 105.Minn H, Salonen A, Friberg J et al (2004) Imaging of adrenal incidentalomas with PET using 11C-metomidate and 18F-FDG. J Nucl Med 45:972–979 [PubMed] [Google Scholar]
- 106.Chen Cardenas SM, Santhanam P (2020) 11C-metomidate PET in the diagnosis of adrenal masses and primary aldosteronism: a review of the literature. Endocrine 70:479–487. 10.1007/s12020-020-02474-3 [DOI] [PubMed] [Google Scholar]
- 107.Wu X, Senanayake R, Goodchild E et al (2023) [11C]metomidate PET-CT versus adrenal vein sampling for diagnosing surgically curable primary aldosteronism: a prospective, within-patient trial. Nat Med 29:190–202. 10.1038/s41591-022-02114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barat M, Gaillard M, Cottereau AS et al (2023) Artificial intelligence in adrenal imaging: a critical review of current applications. Diagn Interv Imaging 104:37–42. 10.1016/j.diii.2022.09.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



