Summary
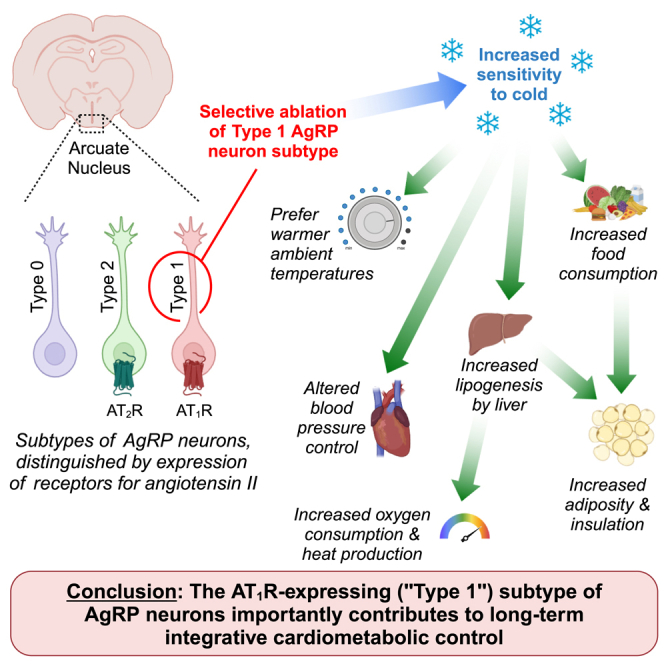
Subtypes of agouti-related peptide (AgRP) neurons can be distinguished based upon expression of angiotensin II receptors. The type 1 subtype, which expresses the angiotensin type 1 receptor (Agtr1a), was examined for its role in the integrative control of cardiometabolic functions. Mice expressing Cre-recombinase via the Agtr1a locus received bilateral microinjection of an AAV vector encoding Cre-dependent expression of caspase-3 into the arcuate nucleus of mice housed at 22°C or 30°C. Ablation of Agtr1a+ cells caused increased food intake, adiposity, resting metabolic rate and shifts in nutrient partitioning, elevated temperature preference, loss of core temperature defense, and changes in blood pressure and heart rate. Many phenotypes were ameliorated by housing animals at thermoneutrality. Thus, Agtr1a+ cells in the ventral hypothalamus (including the type 1 AgRP neuron) influence cardiometabolic control through modulation of thermoregulatory responses to cold. Some functions of these cells appear to oppose canonical roles of other AgRP neuron subtypes.
Subject areas: Molecular biology, Neuroscience, Omics
Graphical abstract
Highlights
-
•
A subset of agouti-related peptide (AgRP) neurons (type 1) express angiotensin AT1R
-
•
These cells were ablated by Cre-lox and viral targeting of a caspase-3 transgene
-
•
Ablation increased food intake, energy expenditure, and thermal place preference
-
•
Housing at thermoneutrality ameliorated energy balance effects of ablation
Molecular biology; Neuroscience; Omics
Introduction
Obesity commonly results from a small chronic imbalance between energy input and output, and such an imbalance may result from one or more behavioral, environmental, pharmacological, genetic, and epigenetic mechanisms.1 Although an array of interventions can induce weight loss (e.g., diet, exercise, drugs, and surgeries), most fail to cause durable long-term maintenance of a reduced body mass. Clinical studies support the concept that this failed long-term maintenance is due to the reduction of energy expenditure relative to body size and composition, termed “metabolic adaptation” (reviewed in the study by Oliveira et al.2).3,4 There remains a critical need to better understand the integrative control of energy expenditure, to develop effective approaches to long-term weight maintenance following weight loss.
Agouti-related peptide (AgRP) neurons within the arcuate nucleus of the hypothalamus (ARC) are implicated in the control of feeding behavior and energy expenditure. Multiple studies have examined the roles of AgRP neurons in long-term energy balance, using various methods and at different developmental stages to ablate these cells. Studies ablating AgRP-expressing cells throughout the body (including those within the ARC but also those in peripheral tissues, such as adrenal gland5) variably result in hypophagia and reduced body size,6 no major effects on feeding or growth,7 or altered nutrient partitioning, exaggerated growth, and obesity in adulthood.8 Ablation of AgRP-expressing cells in adulthood has been reported by several groups to cause hypophagia,7,9,10,11,12 yet others report that site-specific ablation of AgRP neurons in adult mice has minor or no lasting effects.13 Differences in ablation methods, site-specificity of ablation, efficiency of ablation, age at ablation, and other experimental variables complicate interpretation of these various studies.
While earlier studies considered AgRP neurons within the ARC a single homogeneous population of cells, more recent publications have demonstrated that subsets of AgRP neurons exhibit diverse projections, molecular profiles, and functions.14,15,16 Our team previously identified a major role for the angiotensin II type 1A receptor (Agtr1a or AT1R) within AgRP neurons in the control of resting metabolic rate (RMR) and thereby total energy expenditure (TEE).17,18 More recently, we discovered that AT1R is only expressed by a unique subtype of AgRP neurons, which we termed “type 1” AgRP neurons.19 This subtype accounts for approximately 1/3 of AgRP neurons within the ARC. Within these cells, AT1R signals via a cascade involving β-arrestin-1 and Gαi to cause cellular inhibition. During diet-induced obesity, however, AT1R in these cells exhibit a spontaneous G protein “signal switch,” such that the AT1R stops inhibiting the cell via the Gαi cascade and instead begins stimulating the cell via the Gαq cascade. This signal switching of the AT1R appears to contribute to obesity-associated metabolic rate adaptation, as chemogenetic activation of Gαi signaling within the type 1 AgRP neuron via the Gαi-coupled designer receptor exclusively activated by designer drugs (hM4Di DREADD) is sufficient to stimulate RMR in both lean and obese mice. Thus, we hypothesize an important role for the type 1 subtype of AgRP neurons in long-term energy balance through its actions to control RMR.
To evaluate the significance of the AT1R-positive type 1 AgRP neuron subtype in long-term energy balance, we examined the effects of selectively inducing apoptosis of AT1R-expressing cells of the adult ARC (which is essentially limited to this subtype of AgRP neurons17,18,19,20,21,22) via viral-mediated targeting of caspase-3. As hypothesized, ablation of these cells caused sustained, disproportionately large increases in RMR and behavioral preferences for warmer ambient temperatures. The ablation of these cells simultaneously caused increased food intake, increased body mass and adiposity, progressive loss of core temperature defense to cold challenge, altered blood pressure control, and altered hepatic function. Finally, the increases in RMR following ablation of type 1 AgRP cells were abolished when mice were housed at thermoneutral ambient temperatures, supporting the concept that these cells may control RMR through modulation of cold-sensitive thermoregulatory circuits.
Results
Ablation of AT1R+ cells
Adult (10–12 week old) mice that express Cre-recombinase via the Agtr1a promoter (“BAC-AT1A-Cre” mice19,23) underwent bilateral stereotaxic microinjection to deliver an AAV vector encoding Cre-dependent expression of caspase-3 (pAAV-flex-taCasp3-TEVp, Addgene) into the ARC (Figures 1A and S1). Combinations of BAC-AT1A-Cre+ mice or Cre-deficient littermate mice receiving anesthesia and a sham surgery or microinjection of an AAV vector encoding green fluorescent protein (GFP) instead of caspase-3 were used as controls throughout. Within the ARC, Agtr1a is only expressed within type 1 AgRP neurons,17,18,19,20,21,22 and therefore this approach resulted in the induction of apoptosis within type 1 AgRP neurons within 3–6 days after virus injection (Figures S2 and S3).
Figure 1.
Ablation of type 1 AgRP neurons causes sustained increases in food intake, weight gain and adiposity, and resting energy expenditure
(A) Cartoon illustrating approach to induce apoptosis in the Agtr1a-expressing (type 1) subtype of AgRP neurons.
(B) Average daily food intake during the first two weeks after virus injection. n = 6m + 8f control vs. 3m + 6f ablated.
(C) Change in body mass across first two weeks after virus injection. n = 15m + 18f control vs. 9m + 12f ablated.
(D–G) (D) Body mass, (E) fat-free mass (FFM), (F) fat mass, and (G) proportional fat mass.
(H) 24 h average aerobic heat production.
(I–K) (I) 24 h average heat production, (J) resting metabolic rate (RMR), and (K) activity-dependent heat production corrected for FFM and fat mass by GLM. For D–K, n = 21m + 10f control vs. 10m + 6f ablated. Summary data plotted as mean ± SEM (B–G) or estimated marginal mean ± SEM with FFM = 21.30 g and fat mass = 8.75 g (H–K). ∗p < 0.05 versus control group by 2-tailed independent t test (A and B), or Šídák multiple comparisons procedure (D–K).
Related data can be found in Tables S1–S4.
Food intake and body composition
Within two weeks of virus injection, ablation of type 1 AgRP neurons resulted in significant increases in food intake and weight gain, regardless of sex (Figures 1B and 1C). Body mass, fat-free mass (FFM), fat mass, and proportional fat mass all continued to increase for months after ablation in mice of each sex (Figures 1D–1G, Tables S1–S4). Although various adipose tissues increased in mass after ablation of type 1 AgRP neurons (in part via adipocyte hypertrophy, Figure S4), masses of the heart, kidneys, and liver were not different from control littermates through 17–18 weeks after ablation. As expected, ablation of type 1 AgRP neurons reduced mRNA for Agtr1a and Agrp within the ARC (Tables S5 and S6). Consistent with previous demonstrations that type 1 AgRP neurons express somatostatin,20,21,22 mRNA for somatostatin (Sst) was also significantly suppressed. No significant change in proopiomelanocortin (Pomc) expression was detected.
Energy expenditure
Without correction for body size or composition, aerobic TEE was significantly increased at 2 weeks after ablation and continued to increase through 13 weeks after ablation (Figure 1H). Correction of TEE for FFM and fat mass covariates by general linear modeling (GLM) showed that body size and composition did not account for the increase in TEE (Figure 1I). The increase in TEE after ablation was completely attributable to increases in RMR, as no change in activity-dependent EE was observed (Figures 1J and 1K). Bomb calorimetric analysis of feces collected from a subset of animals 14 weeks after virus injection indicated that ablation had no effect upon digestive efficiency (Table S7). Thus, ablation of type 1 AgRP neurons increased food intake, body mass, and adiposity, and simultaneously increased RMR more than would be anticipated based solely upon mass gains.
Sensitivity to cold ambient temperature
Ambient temperature preferences were assessed during the light phase in a subset of mice 12 weeks after ablation using a linear gradient (Figure S5). Control mice exhibited an average place preference for 29.4°C, consistent with previous studies,24 yet ablation caused mice to localize to a warmer temperature of 30.9°C (Figure 2A). A separate cohort of mice then underwent the ablation procedure but was housed at a thermoneutral (30°C) ambient temperature (TAMB) thereafter, and energy expenditure two weeks after ablation was compared to the previous cohort that had been housed at 22°C. Whereas TEE and RMR were elevated after ablation in mice housed at 22°C, this increase in energy expenditure was not seen in mice housed at 30°C (Figures 2B–2D, Table S8). Ablation had no effect on the masses of tissues in mice housed at 30°C (Table S9). Similarly, respiratory exchange ratio (RER) tended to be higher in the dark phase and lower in the light phase 2 weeks after ablation when mice were housed at 22°C, but this effect of ablation was attenuated by housing mice at 30°C (Figure S6). A comparison of tissue masses assessed at 3 weeks after virus injection in this cohort housed at 30°C versus a cohort housed at 22°C revealed that ablation of type 1 AgRP neurons caused TAMB-dependent effects upon perigonadal adipose tissue gains (Tables S3, S9, and S10). In contrast, TAMB modified growth of other tissues (heart, interscapular brown adipose tissue [BAT]) similarly regardless of ablation. These data collectively support the concept that type 1 AgRP neurons contribute to the control of RMR and adiposity through the modulation of cold-responsive circuits.
Figure 2.
Ablation of type 1 AgRP neurons alters thermoregulatory behavior, metabolic responses to cold stimuli, and defense of core temperature (TC)
(A) Preferred ambient temperature, assessed by a thermal place preference test. n = 10m vs. 6m.
(B–D) (B) 24 h average aerobic heat production, (C) resting metabolic rate (RMR), and (D) activity-dependent heat production corrected for FFM and fat mass by GLM.
(E) Body masses before and after radiotelemeter implantation and viral vector injections into the ARC.
(F) 24 h average TC before and after virus injections.
(G) 24 h average TC before and 12 weeks after virus injections at 22°C, and during brief sequential 3 days exposures to 30°C and 16°C.
(H) Hourly average TC throughout the light cycle before and 12 weeks after virus injections, when mice were housed at 22°C. For B–D, n = 21m + 10f control and 10m + 6f ablated at 22°C versus 4m + 5f control and 4m + 3f at 30°C. For E–H, n = 6m. Summary data plotted as mean ± SEM (A and E–H), or estimated marginal mean ± SEM with FFM = 18.94 and fat mass = 5.71 g (B–D). ∗p < 0.05 versus control group (A–D and G–H) or against pre-ablation baseline (F) by 2-tailed independent t test (A), or Šídák multiple comparisons procedure (B–H).
Related data can be found in Figure S4 and Tables S8 and S9.
Core temperature
Additional mice individually housed at 22°C were instrumented with radiotelemetric transducers to assess core body temperature (TC). Four weeks later, type 1 AgRP neurons were ablated. Body mass trajectories were similar to mice from the previous ablated groups (Figure 2E). Over the following 20 weeks, 24 h average TC progressively decreased (Figure 2F). In the week before ablation, and again 12 weeks after ablation, TC was assessed while mice were housed with bedding but no nesting materials at 30°C, 22°C, and 16°C for three days each. Before ablation, mice defended TC when exposed to colder temperatures. After ablation, however, mice exhibited reduced TC defense when exposed to temperatures below thermoneutrality (Figures 2G and S7). At 22°C, for example, this reduction in TC appeared to occur throughout the light cycle but differences were more pronounced during the dark phase (Figure 2H). Consistent with the loss of TC defense after ablation, the expression of genes associated with adaptive thermogenesis in interscapular and inguinal adiposes (e.g., Ucp1, Dio2, Cidea, Pgc1a, etc.) were variably suppressed at 3 and 17–18 weeks after ablation in mice that had been housed at 22°C (Figures 3A and 3B, Tables S11–S14). These data illustrate the important role that type 1 AgRP neurons play in long-term thermoregulation and indicate that the long-term elevations in RMR induced by ablation of these cells is not mediated by adipose thermogenic mechanisms.
Figure 3.
Ablation of type 1 AgRP neurons alters hepatic lipid production pathways
(A and B) Expression of Ucp1, Dio2, and Ppargc1a genes in (A) interscapular brown adipose (control n = 6m + 7f vs. ablated n = 4m + 5m) and (B) inguinal white adipose (control n = 5m + 8f vs. ablated n = 4m + 4m) 3 weeks after ablation, in mice housed at 22°C vs. 30°C.
(C) Differentially expressed genes in liver, 3 weeks after ablation (n = 3m + 3f) or control (n = 3m + 3f), in mice housed at 22°C.
(D) Differentially expressed genes in liver, three weeks after ablation in mice housed at 22°C (n = 3m + 3f) versus 30°C (n = 3m + 3f).
(E) Venn diagram identifying genes that were differentially expressed following ablation only when mice were housed at 22°C.
(F) Enriched molecular functions of genes identified in D, identified by ShinyGo 0.81.
(G) Expression of Scd2 in liver 3 weeks after ablation, in mice housed at 22°C (control n = 5m + 9f; ablated n = 4m + 5f) or 30°C (control n = 2m + 5f; ablated n = 4m + 3f). ∗p < 0.05 by Šídák multiple comparisons procedure (A and F).
Related data can be found in Tables S15, S16, S17, S18, S19, S20, S21, and S22.
Hepatic lipogenesis
Although adipose thermogenesis was suppressed after ablation of type 1 AgRP neurons, this ablation induced changes in hepatic functions that likely explain the elevated RMR in these animals. RNA sequencing was performed on livers from a subset of animals 3 weeks after ablation, housed at 22°C or 30°C, after a 6 h fast (Tables S15, S16, S17, S18, S19, S20, and S21). At 22°C, ablation induced differential expression of 71 genes (Figure 3C). In contrast, 164 genes were differentially expressed between mice with ablation and housed at 22°C versus 30°C (Figure 3D). Comparison of these outputs identified a subset of 11 genes that were differentially expressed in response to ablation of type 1 AgRP neurons only when mice were housed at 22°C (Figure 3E). Network analysis of these 11 genes using ShinyGo 0.81 identified multiple canonical molecular function pathways that were enriched, including those mediating lipid desaturation, glutathione transferase activities, and growth factor signaling (Figure 3F). Ambient temperature-dependent induction of stearoyl-coenzyme A desaturase 2 (Scd2, which catalyzes an oxygen-consuming desaturation of various lipids) in the liver 3 weeks after ablation of type 1 cells was confirmed by quantitative PCR in a larger cohort of samples (Figure 3G, Table S22). Together, these data support a role for altered lipid synthesis by the liver as a mechanism that helps explain the increased aerobic metabolism induced by ablation of type 1 AgRP neurons when housed at 22°C.
Glycemic control
At 17–18 weeks after ablation in mice housed at 22°C and fasted 5 h, blood glucose concentration was not altered, but plasma insulin was increased relative to controls (Table S23). Indices of beta-cell function (e.g., HOMA-β) and insulin sensitivity (e.g., HOMA-IR, HOMA-S, and QUICKI) suggest changes in responsiveness to insulin, but not beta-cell function, after ablation. Thus, ablation of type 1 AgRP neurons may modulate insulin sensitivity, though it is unclear whether this is secondary to the excess weight gain in these animals.
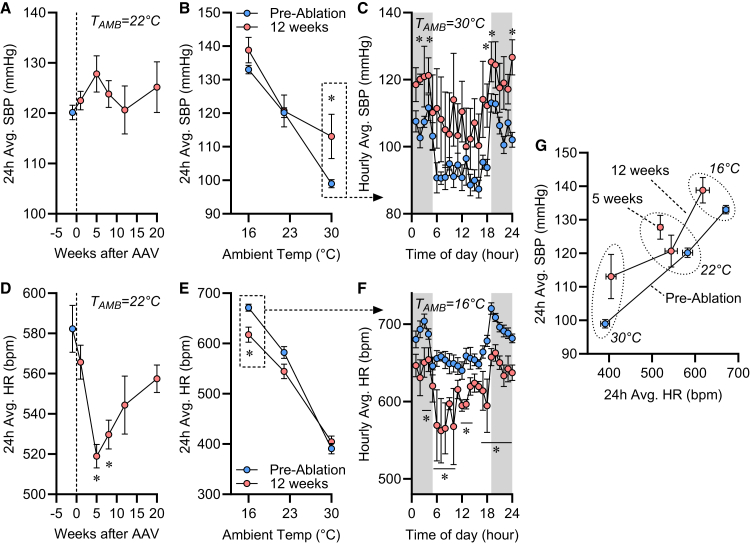
Blood pressure and heart rate
Finally, we probed a role for type 1 AgRP neurons in blood pressure control, using the same radiotelemetric devices used to evaluate TC (Figures 2E–2H). When housed at 22°C, systolic blood pressure (SBP) was essentially unchanged through 20 weeks after ablation (Figure 4A). Ablation caused increased SBP during exposure to 30°C at 12 weeks after the ablation, however, and this was largely driven by increases during the dark phase (Figures 4B and 4C). In contrast to SBP, heart rate (HR) was reduced after virus injection in a biphasic manner, reaching a maximum reduction of −63 bpm (−11%) after 5 weeks (Figure 4D). Ablation reduced HR during exposure to 16°C at 12 weeks after the ablation, and this occurred through suppression of HR essentially throughout the entire light/dark cycle (Figures 4E and 4F). Comparison of 24 h average SBP versus HR at various timepoints after ablation revealed a significant shift in the relationship between SBP and HR such that following ablation, any given HR was associated with a greater SBP (Figure 4G). Alternatively, for any given SBP, ablation was associated with a significantly lower HR. In a cohort of mice euthanized at 3 weeks after virus injections, ablation of type 1 AgRP neurons significantly increased renin (Ren1) and Agtr1a expression in the kidney, but mRNA encoding the thiazide-sensitive sodium-chloride cotransporter (Slc12a3), sodium/hydrogen exchanger-3 (Slc9a3), sodium/potassium-ATPase (Atp1a1), sodium-potassium-chloride cotransporter (Slc12a1), and the epithelial sodium channel alpha subunit (Scnn1a) were unchanged (Table S24). In the cohort of mice that underwent serial metabolic phenotyping, rates of aldosterone and corticosterone elimination to urine were unchanged at 14 weeks after virus injection (Table S7). In contrast, elimination of copeptin (a marker of arginine vasopressin secretion) to the urine was significantly increased in the ablated mice. Together, these data support a potential role for the type 1 AgRP neuron in hemodynamic control, likely through mechanisms involving autonomic activity, the renin-angiotensin system, and arginine vasopressin.
Figure 4.
Ablation of Type 1 AgRP neurons alters blood pressure control
(A) 24 h average systolic blood pressure (SBP) before and after virus injections.
(B) 24 h average SBP before and 12 weeks after virus injections, at 22°C and during brief sequential 3 days exposures to 30°C and 16°C.
(C) Hourly average SBP throughout the light cycle before and 12 weeks after virus injections, when mice were housed at 30°C.
(D) 24 h average heart rate (HR) before and after virus injections.
(E) 24 h average HR before and 12 weeks after virus injections, at 22°C and during brief sequential 3 days exposures to 30°C and 16°C.
(F) Hourly average HR throughout the light cycle before and 12 weeks after virus injections, when mice were housed at 16°C.
(G) Association of SBP to HR data, replotted from A–B and D–E. Deming regression comparison of lines p < 0.001. For all , n = 6m. Summary data plotted as mean ± SEM. ∗p < 0.05 against pre-ablation baseline (A and D) or versus control group (B–C and E–F) by Šídák multiple comparisons procedure.
Discussion
AgRP neurons in the ARC are well recognized to participate in the initiation of feeding, glycemic control, and energy expenditure via projections to an array of structures throughout the brain, using AgRP, neuropeptide Y (NPY), and γ-aminobutyric acid (GABA) to communicate with postsynaptic cells.25 The identification of multiple unique subtypes of AgRP neurons prompts questions regarding the distribution of projections from each subtype, and the neurotransmitter use of each projection. We previously determined that the type 1 subtype predominantly projects only to a small number of these targets, including the medial preoptic area (MPA) and the paraventricular nucleus,19 both of which are implicated in the control of feeding, thermoregulatory behaviors, autonomic activity, and energy expenditure. Further, we previously demonstrated that the thermogenic effects of angiotensin II within the brain, but not its pressor effects, require type 4 melanocortin receptors (MC4R).26 Shi et al. have also demonstrated that AT1R activation in the ARC of rats stimulates cardiovascular sympathetic nervous activity and blood pressure through a mechanism that appears to involve NPY signaling to its Y1R receptor within the paraventricular nucleus.27,28,29,30 Collectively, these findings have led us to propose that type 1 AgRP neurons utilize distinct anatomical projections, and distinct neurotransmitters, to separately control thermoregulatory behaviors and metabolism versus blood pressure.31
In contrast to the hypophagia and weight loss commonly reported in response to ablation of AgRP neurons of adult mice,7,9,10,11,12 ablation of the AT1R-expressing type 1 subset of AgRP neurons rapidly induced hyperphagia and weight gain. These findings parallel a previous study from Joly-Amado et al., which demonstrated that ablating AgRP-expressing cells soon after birth similarly caused increased adiposity, increased aerobic energy expenditure, and reduced TC in adulthood, and this was associated with a shift in nutrient partitioning through mechanisms involving increased hepatic lipid production and peripheral lipid disposal.8 The authors noted a disproportionate increase in oxygen consumption after AgRP neuron ablation and increased hepatic expression of stearoyl-coenzyme A desaturase, paralleling effects observed in the current study after specifically ablating only the type 1 subtype in adulthood. Notably, Joly-Amado et al. similarly found no major role for canonical non-shivering adaptive thermogenic mechanisms, such as the induction of adipose Ucp1, in the thermogenic responses to ablating AgRP neurons. The present study builds upon their findings by documenting a critical role for the type 1 AgRP neuron subtype in the control of hepatic lipid handling, and by implicating the modulation of cold-sensitive circuits in this mechanism.
It is important to note that multiple mechanisms contributing to total body energy balance were not assessed in the current study, and these mechanisms may explain the paradoxical reduction in core body temperature despite an increase in aerobic metabolism. First, gas respirometric (indirect) calorimetry methods, such as those used herein are blind to anaerobic metabolism. We and others have demonstrated that anaerobic metabolism contributes up to 10% of energy expenditure in rodents and other species, and that this contribution is sensitive to genetics, diet, and body composition.32,33,34,35,36,37,38,39 For example, we demonstrated that high fat diet feeding and associated weight gain in C57BL/6J mice causes rapid increases in aerobic metabolism but simultaneously reduces anaerobic metabolism.34 It is therefore possible that type 1 AgRP neurons may normally promote anaerobic forms of energy expenditure, and their experimental destruction might result in reduced anaerobic expenditure. Second, although increased aerobic energy expenditure was documented, typical non-shivering thermogenic mechanisms (such as adaptive thermogenic responses via adipose thermogenesis) and locomotive behaviors were not engaged after ablation of type 1 AgRP neurons. These findings prompt questions about other thermogenic mechanisms, including non-exercise activity thermogenesis (NEAT), the thermic effect of food (TEF), shivering thermogenesis, and various other molecular mechanisms that contribute to resting (RMR) and basal (BMR) metabolic rates. Future studies to probe possible contributions of type 1 AgRP neurons to these various forms of heat production are appropriate. Third, core body temperature results from the balance of heat production versus heat loss to the environment. It is possible that type 1 AgRP neurons may influence skin blood flow, ventilation, and saliva spreading behaviors to alter thermal conductance and water loss, ultimately resulting in changes in radiative, conductive, convective, and evaporative forms of heat loss. Future studies to evaluate the contribution of type 1 AgRP neurons to the control of anaerobic heat production, various mechanisms of aerobic heat production beyond RMR, and the many mechanisms influencing rates of heat dissipation are required. Regardless of such possible additional functions, the data presented herein positively implicate type 1 AgRP cells in the control of RMR responses to cold TAMB.
The lower critical temperature and thermoneutral zone for mice are higher than for humans, and as a result, mice housed at 22°C exhibit chronic activation of thermogenic mechanisms and actively engage in thermoregulatory behaviors (e.g., nesting, burrowing, and huddling).24,40 As reviewed by Gordon,24 classic studies by Herrington,41 Pennycuick,42 Heldmaier,43 and Yamauchi44 demonstrated that when mice are chronically housed at temperatures below 30°C, they exhibit increased body size, food intake, heat production, and disproportionately increased masses of tissues including interscapular brown fat, liver, kidney, and heart. In the current study we similarly documented increased body, heart, and kidney masses in mice housed at 22°C versus 30°C, and these effects were not altered by ablation of type 1 AgRP neurons. In contrast, ablation exaggerated the effect of 22°C housing to increase interscapular brown and perigonadal white fat pad masses, RMR, and TEE. It is therefore tempting to speculate that these increases are directly related to increased lipid accumulation and utilization as a fuel source, supported by the increased production of unsaturated fatty acids by the liver in these animals and progressively decreased RER. Additional future work is warranted to explore whether type 1 AgRP neurons and their context-dependent control of nutrient partitioning can be targeted as a therapeutic approach for disorders of lipid accumulation, such as chronic obesity.
Although the current study design cannot conclusively distinguish primary versus secondary consequences of ablation upon energy intake (e.g., rates of food intake) versus expenditure, some data support the contention that these effects are dissociable. First, although food intake was accelerated during the first two weeks after ablation, this increase in food intake was no longer observed at later time points when animals were housed in the Promethion multiplexed phenotyping system. In contrast, RMR and TEE were significantly elevated at these time points after ablation even after correction for body composition. Thus, it appears that the longer-term energy expenditure effects of ablating type 1 AgRP neurons are dissociable from the transiently-increased food intake behavior induced by this ablation.
It is conceivable that altered food intake patterns may influence the observed changes in hepatic gene expression. Various groups (reviewed in the study by Hu et al.45) have previously shown that stearoyl-coenzyme A desaturase expression is increased by high carbohydrate or cholesterol diets, iron, insulin, PPAR, and glucocorticoids, but suppressed by glucagon, polyunsaturated fatty acids, thyroid hormone, vitamin A, retinoic acid, and thiazolidinedione drugs. Increased activity is observed during obesity, atherosclerosis, diabetes, and cancer. Importantly, high fat diet simultaneously causes increased caloric intake, adiposity, and stearoyl-coenzyme A desaturase in diet-sensitive C57BL/6J mice, while in diet-resistant FVB/N mice there were no changes in adiposity or stearoyl-coenzyme A desaturase despite the increase in caloric intake. These findings hint that diet composition and total caloric intake per se can be dissociated from hepatic functions. Consistent with this idea, Rojas previously demonstrated that ICV injection of neuropeptide-Y (NPY) or an agonist of its Y1 receptor into fasted obese ZF rats stimulated hepatic stearoyl-coenzyme A desaturase.46 Critically, this effect was abolished by hepatic sympathectomy. Those results support the idea that autonomic innervation of the liver contributes to the control of stearoyl-coenzyme A desaturase. Future characterization of hepatic autonomic activity, and the role of such projections in the control of hepatic functions by type 1 AgRP neurons, is obviously warranted. Additional studies of pair-fed animals may also inform the dissection of these mechanisms.
Mice of both sexes were studied for the majority of endpoints investigated here, and although the magnitude of effects differed between males and females for selected endpoints, the qualitative effects of ablating type 1 AgRP neurons were similar regardless of sex. Various studies have documented sex-dependent and estrogenic modulation of selected functions of AgRP neurons.47,48,49,50 Importantly, however, those studies have not dissected the interactions and modulatory effects of estrogenic signaling within subtypes of AgRP neurons. Thus, it remains unclear how sex and estrogenic signaling specifically modulate type 1 AgRP neurons. Our previous work hints that while the functional significance of these cells to control RMR in lean adult mice is independent of sex, the plasticity of these functions during prolonged diet-induced obesity is sex-dependent.19 It is worth noting that the current study was performed using mice maintained on soy protein-free (and thereby phytoestrogen-free) diets, which may also modify interactions among food intake, sex, estrous stage, estrogenic signaling, and energy balance.51 Many additional studies to explore potential modulatory effects of sex and estrogenic signaling specifically in type 1 AgRP neurons are required.
The mechanism by which ablation of type 1 AgRP neurons influences blood pressure control remains unclear. Based on the work of Shi et al. demonstrating a stimulatory effect of acute AT1R activation within the ARC upon autonomic activity and arginine vasopressin release in rats,27,28,29,30 we hypothesize that these mechanisms may contribute. Consistent with this hypothesis, we note that ablation of type 1 cells caused increased SBP for any given HR (possibly implicating changes in baroreflex control). As heart mass did not change even months after ablation of the type 1 neuron, this may indicate that stroke volume and thereby cardiac output is not substantially increased across this time frame. As pressure is proportional to stroke volume, HR, and peripheral resistance, we propose that peripheral resistance more likely accounts for the observed changes in SBP and HR. This increase in resistance may result from sympathetic stimulation of vascular constriction, and the observed increases in renal renin expression and arginine vasopressin release may also contribute. Simultaneously, it is likely that the rapid expansion of adiposity may contribute to increased peripheral resistance. However, it is unclear why the HR changes were biphasic (i.e., progressively decreasing up to five weeks after ablation, and then rebounding thereafter) while adiposity continued to increase across this same time frame. Future studies assessing the effects of autonomic modulators, and renin-angiotensin system and arginine vasopressin blockers, along with manipulations of nutrient supply are needed to dissect this mechanism.
Previous work by our group and others (reviewed in the study by Lawton et al.31), along with the current study, support the concept that AT1R-expressing cells in the ventral hypothalamus exhibit high levels of expression of somatostatin (Sst) in addition to Agrp, Npy, and genes involved in the synthesis of GABA. This is in contrast to other subtypes of AgRP neuron (i.e., types 0 and 2), which do not express Sst.20 The selective expression of Sst within the Agtr1a-expressing type 1 subtype, and high levels of expression of this transcript that encodes a relatively large (14 or 28 amino acid) peptide product, prompt an array of future questions regarding its possible use by the type 1 AgRP neuron as a neurotransmitter or neuromodulator. Indeed, several of its receptors have been documented within brain regions that are targeted by type 1 AgRP neuron projections, including the MPA and paraventricular nucleus (PVN).52 Studies examining the functional significance of these receptors in RMR (and other) responses to TAMB fluctuations are needed.
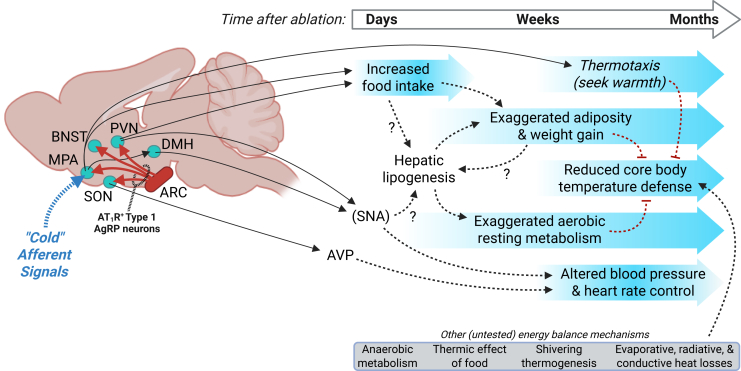
Based upon ongoing work from our team and others examining the functional significance of AT1R within the ARC (reviewed in the study by Lawton et al31), we propose a working model to explain the consequences of ablating type 1 AgRP neurons as observed here (Figure 5). Briefly, our work implicates melanocortin type 4 receptor (Mc4r) signaling in RMR but not BP control by angiotensin II within the brain,26 while work from the Brooks group has implicated NPY signaling within the PVN in cardiovascular autonomic and BP responses to acute microinjection of angiotensin II within the ARC.27,28 We have documented that type 1 AgRP neurons primarily project to four brain regions, including the MPA, PVN, supraoptic nucleus (SON), and the bed nucleus of the tractus solitarius (BNST).19 The literature identifies the MPA as a primary site integrating afferent signals to coordinate behavioral, feeding, and thermogenic responses to changes in TAMB53; a subset of glutamatergic neurons in the MPA express Mc4r54; and pharmacological stimulation of melanocortin receptors in this preoptic area stimulate resting energy expenditure via projections to the dorsomedial hypothalamus (DMH).55 Collectively these findings prompt the concept that type 1 AgRP neurons of the ARC contribute to the control of energy balance via modulation of cold-sensitive Mc4r-expressing circuits within the MPA, and contribute to the control of cardiovascular function via modulation of pre-autonomic circuits within the PVN. Critically, it remains unclear how type 1 neurons modulate cardiovascular responses to changes in TAMB, and whether these effects rely upon circuits in the MPA, PVN, or both.
Figure 5.
Working model
Within the ventral hypothalamus, angiotensin II type 1 receptor (AT1R/Agtr1a) expression is largely restricted to type 1 agouti-related peptide (AgRP) neurons of the hypothalamic arcuate nucleus (ARC). These neurons project to the paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BNST), medial preoptic area (MPA), and supraoptic nucleus (SON). The current study demonstrates that conditional ablation of AT1R-expressing cells of the ventral hypothalamus results in progressive alterations of an array of energy balance phenotypes. Within days after ablation, food intake is exaggerated, increasing body mass and adiposity. This is rapidly followed by increased aerobic energy expenditure due to increased resting metabolic rate, which appears to involve hepatic lipogenesis but not canonical adipose non-shivering thermogenic mechanisms. All of these phenotypes were prevented by housing animals at thermoneutral (30°C) ambient temperatures, prompting the hypothesis that the type 1 AgRP neuron projections to the MPA mediate some combination of these phenotypes, as glutamatergic MPA projections to the dorsomedial hypothalamus (DMH) are understood to coordinate behavioral and thermogenic responses to cold ambient temperatures through control of sympathetic nervous activity (SNA) to various peripheral tissues. Finally, ablation of AT1R-expressing cells in the ventral hypothalamus also resulted in progressive reductions in core body temperature defense during cold (22°C or 16°C) exposures, along with multiphasic ambient temperature-dependent alterations in blood pressure and heart rate in the weeks and months following ablation. Various additional mechanisms contributing to heat production and retention kinetics remain untested, but may contribute to the observed phenotypes. AVP, arginine vasopressin.
Limitations of the study
The primary drawback of the current study design is 2-fold. First, it remains possible that a small number of Agtr1a-expressing cells outside of the ARC may also conceivably be ablated by this method. Sporadic cells expressing Agtr1a are found outside the ARC but within a ≈1 mm radius of our injection coordinates (including the ventral portion of the DMH, the medial portion of the ventromedial hypothalamus, and the anterior portion of the mammillary nucleus). Further, our injection needle tracts were adjacent to the posterior regions of the PVN, which is well established to express Agtr1a in selected neuron populations.56,57 Thus, off-target ablation of a subset of Agtr1a-expressing cells in these various regions may have occurred. Second, it is likely that not all type 1 AgRP neurons were ablated in the current study. The ARC is a relatively long structure in its anterior-posterior dimension, yet only a single injection was performed on each side of the brain. Thus, it is likely that some type 1 cells remained outside the range of virus spread. Despite these complementary concerns resulting from our use of viral targeting methods, the current study robustly implicates Agtr1a-expressing cells of the ventral hypothalamus (predominantly representing type 1 AgRP neurons of the ARC) in the control of an array of cardiometabolic phenotypes. Future studies utilizing more sophisticated intersectional genetics approaches to limit manipulations to cells that express both Agtr1a and Agrp, for example, are therefore needed to confirm and extend these findings.
In summary, we have documented major roles for Agtr1a-expressing cells of the ventral hypothalamus (largely representing the type 1 subset of AgRP neurons within the ARC) in the regulation of feeding and thermoregulatory behaviors, RMR and TEE, hepatic lipogenesis, arginine vasopressin release, and blood pressure control. Many of these functions were sensitive to TAMB, supporting the working hypothesis that type 1 neurons achieve such control through modulation of the detection and integration of cold stimuli. Future work is needed to clarify roles of type 1 neuron projections from the ARC to individual second-order nuclei, and the use of various neurotransmitters (AgRP, NPY, GABA, and somatostatin) by these projections.
Resource availability
Lead contact
Requests for further information and resources should be directed to Justin L. Grobe, PhD (jgrobe@mcw.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
RNA sequencing data have been deposited at NCBI GEO as GSE284096 and are publicly available as of the date of publication.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
The authors gratefully acknowledge assistance provided by the Medical College of Wisconsin Comprehensive Rodent Metabolic Phenotyping Core, Engineering Core, Biomedical Resource Center, and Genotyping Service Center. The authors also wish to acknowledge technical support from Patricia C. Muskus, Kelsey K. Wackman, and Ko-Ting Lu. Schematics were made using Biorender.com. This work and the authors were supported by the National Institutes of Health (DK133121, GM080202, HL084207, HL134850, HL153101, HL177123, and OD032136), the Medical College of Wisconsin Clinical and Translational Science Institute (UL1TR001436), the University of Chicago Diabetes Research and Training Center Pilot and Feasibility Award (DK020595), the Children’s Research Institute, endowments from the Butenhoff, Smith, and Mellowes families, and the Advancing a Healthier Wisconsin Endowment to the Medical College of Wisconsin.
Author contributions
J.P., V.A.W., S.B.R.L., L.L.M., J.L.S., C.D.S., P.N., and J.L.G. designed studies. J.P.., V.A.W., S.B.R.L., A.M.M., K.N., N.M.M., C.C.G., J.J.R., D.T.B., J.L.S., and J.L.G. performed experiments. C.C.G., B.P.F., A.J.S., C.M.L.B., and J.L.G. developed equipment and assays required for the project. L.L.M., J.L.S., C.D.S., P.N., and J.L.G. provided project oversight and secured funding.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| pAAV-flex-taCasp3-TEVp | Addgene | 45580-AAV5 |
| AAV-GFP | University of Iowa Viral Vector Core | VVC-U of Iowa-480 |
| Critical commercial assays | ||
| ApopTag Red In Situ Apoptosis Detection Kit | EMD Millipore | 57165 |
| Glucose assay | Crystal Chem | 81692 |
| Insulin assay | Crystal Chem | 90080 |
| Triglyceride assay | BioAssay Systems | ETGA-200 |
| Deposited data | ||
| RNAsequencing results from liver samples | NCBI GEO | GSE284096 |
| Experimental models: Organisms/strains | ||
| BAC-AT1A-Cre (Ritter et al.17 and Balapattabi et al;16) | Curt D. Sigmund, PhD | BAC-AT1A-Cre |
| Ai39 (B6; 129S-Gt(ROSA)26Sortm39(CAG-hop/EYFP)Hze/J) | Jackson Laboratories | 014539 |
| Oligonucleotides | ||
| Please see Table S6 for complete list of qPCR assays | Thermo Fisher | (various) |
Experimental model and study participant details
Ethical approval
All studies complied with the American Physiological Society’s Guiding Principles in the Care and Use of Laboratory Animals, the National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the Medical College of Wisconsin (MCW) Institutional Animal Care and Use Committee (AUA00006720).
Animals
Mice expressing Cre-recombinase via the Agtr1a locus within a large BAC transgene (BAC-AT1A-Cre mice) were previously developed by Curt D. Sigmund, PhD.23 A subset of mice also carried the Ai39 construct, encoding Cre-dependent activation of halorhodopsin plus a yellow fluorescent protein (YFP) reporter.58 All mice were housed in ventilated microisolation caging on commercial racks (Allentown, Allentown, NJ) with hardwood chip bedding (Sani-Chips, P.J. Murphy, Montville, NJ) and nesting material (Enviropak, W.F. Fisher and Son, Branchburg, NJ). Mice were supplied soy protein-free (and thereby, phytoestrogen-free) natural ingredient Teklad/Envigo 2920x irradiated diet (Envigo, Indianapolis, IN) and water that was filtered by reverse osmosis (Avidity Science, Waterford, WI) and subsequently chlorinated to maintain sterility (approximately 1.4 ppm, pH 6.5). Lights were maintained on a 14:10 light:dark cycle at approximately 325 lux, and housing rooms were maintained at 20°C–22°C, between 35 and 75% relative humidity.
An institutional surveillance program was employed to ensure the health of the mice. Briefly, excluded murine agents include Clostridium piliforme, Corynebacterium bovis, cilia-associated respiratory bacillus, ectromelia virus, Encephalitozoon caniculi, lymphocytic choriomeningitis virus, minute virus of mice, mouse adenovirus, mouse cytomegalovirus, mouse hepatitis virus, mouse parvovirus, mouse rotavirus, mouse thymic virus, Mycoplasma pulmonis, pneumonia virus of mice, polyoma virus, reovirus, Sendai virus, Theiler murine encephalomyelitis virus, pinworms, and fur mites. Murine norovirus and Helicobacter spp. were not specifically excluded.
Viral-mediated ablation of Cre+ cells
Under isoflurane anesthesia, mice underwent stereotaxic (David Kopf Instruments, Tujunga, CA; Model 1900) bilateral microinjection of viral vectors into the arcuate nucleus of the hypothalamus (ARC: −0.30 lateral, −1.55 posterior, −6.40 mm ventral, relative to bregma). One subset of BAC-AT1A-Cre+ mice received bilateral injection of viruses encoding Cre-dependent expression of caspase-3 (Casp3), resulting in cell-type specific ablation of Type 1 AgRP neurons (Addgene, Watertown, MA; pAAV-flex-taCasp3-TEVp; catalog #45580-AAV5; Lot #V143243, titer: 1.1x1013 vg/mL). Multiple other subsets of BAC-AT1A-Cre mice served as controls, including animals that underwent isoflurane anesthesia and a sham surgical preparation, or bilateral microinjection of AAV vectors encoding green fluorescent protein (GFP) (University of Iowa Viral Vector Core; catalog #VVC-U of Iowa-480; Lot #AAV4069, titer: 2.67x1012 vg/mL). Additionally, littermates deficient for the BAC-AT1A-Cre transgene received sham surgery, or GFP or Casp3 viruses and served as additional control groups. Animals were administered meloxicam (5 mg/kg, sc) on the day of surgery and again on the following day.
Method details
TUNEL assay
Animals were anesthetized with isoflurane (Isospire, Dechra, Overland Park, KS) and transcardially perfused with 16 mL of ice-cold 4% paraformaldehyde. Brains were dissected and post-fixed in 4% paraformaldehyde overnight at 4°C. Brains were transferred to 70% EtOH for 48 h at 4°C. Samples were sent to the Children’s Research Institute Histology Core at the Medical College of Wisconsin for paraffin-embedding. TUNEL was performed using an indirect ApopTag Red In Situ Apoptosis Detection Kit (Cat: 57165, EMD Millipore, Burlington, MA) according to the manufacturer’s protocol. Briefly, deparaffinized 30 μm sections were permeabilized for 15 min at room temperature in proteinase K solution (20 mg/mL, Thermo Fisher Scientific, Waltham, MA), treated with equilibration buffer, and then incubated for one hour at 37°C in a humidified atmosphere with terminal deoxynucleotidyl transferase (TdT). Sections were then incubated under agitation in stop/wash buffer for ten minutes and washed in 3 changes of PBS. Sections were incubated for 30 min at room temperature with rhodamine-conjugated anti-digoxigenin antibody. Negative control tissue was treated as above without active TdT enzyme. A section was used to prepare a positive control by pretreatment with DNase-I. After permeabilization, the section with was incubated in DN buffer for 5 min at room temperature. The section was treated with 0.5 μg/mL DNase-I (Roche, Basel, Switzerland) for ten minutes at room temperature. The section was rinsed with six changes of deionized water and continued with the protocol wit incubation in equilibration buffer. Completed sections were mounted using glass coverslips and VECTASHIELD Hardset Antifade Mounting Medium (Vector Laboratories, Newwark, CA) and imaged on a Keyence BZ-X810 microscope (Keyence Corporation of America, Elmwood Park, NJ).
Multiplexed metabolic phenotyping
Food and water intake and patterning, aerobic energy expenditure, and locomotion were analyzed using a Promethion multiplexed metabolic phenotyping system (Sable Systems International, Las Vegas, NV) enclosed within environmental cabinets allowing control of ambient temperature conditions.59 Nesting materials were not provided within the Promethion cages.
Body composition
Body composition was determined using time-domain nuclear magnetic resonance (NMR) (Bruker, Billerica, MA; model LF-110).59
Metabolic caging
A subset of mice was studied using metabolic caging (Tecniplast, Buguggiate, Italy; model 3600M021).59 Animals were singly housed and offered ad libitum access to drinking water and a powdered version of the Teklad 2920x diet. Urine and feces were quantitatively collected daily, and frozen at −80°C before further analyses. Aldosterone (Arbor Assays; K052-H1, Lot#: 24AL001a), corticosterone (Arbor Assays; K014-H1, Lot#: 24CS002d) and copeptin (Cloud-Clone Corp; CEA365Mu, Lot#: L240108437) were analyzed in urine using EIA kits, and caloric contents of food and fecal samples were determined using semi-micro bomb calorimetry (Parr Instrument Company, Moline, IL).59 Digestive efficiency was determined as the ratio of calories absorbed to the calories ingested.
Thermal gradient
A thermal gradient system was fabricated to include an aluminum baseplate (1/8 × 24 × 21 inches/0.32 × 60.96 × 53.34 cm) resting upon two heating/cooling water blocks at the opposite ends of the long axis (Figure S4). A superstructure of opaque white polycarbonate rested on the aluminum plate, which defined four parallel testing lanes. Each lane had interior dimensions of 58.4 cm long, 7.5 cm wide, and 20.5 cm tall. The polycarbonate superstructure was not adhered to the baseplate, allowing disassembly and thereby thorough cleaning between test sessions. One of the opposing water blocks was supplied water heated by a circulating water bath (set at 50°C), and the other water block was supplied water cooled by a second circulating water bath (set at 10°C). Above the structure, a digital CMOS camera was mounted in a fixed position at its optimal focal length to visualize the four testing lanes. This camera was interfaced with the AnyMaze software system (Stoelting), which enabled continuous tracking and quantification of animal position and motion throughout recording sessions. Above the animal-tracking camera, a digital infrared camera (Teledyne FLIR A700) was mounted at its optimal focal length to visualize the four testing lanes plus the two exterior lanes. This camera was interfaced with the FLIR Research Studio software package to visualize and quantify temperatures along the gradient. On the day of testing, naive mice were randomized to one of the four parallel lanes, and released into the center of that lane at approximately 10:00 a.m. (4 h into the 14h light phase). Animal positions within the lane were recorded and assessed continuously via AnyMaze for four hours.
Blood pressure and core temperature
A subset of mice underwent surgical implantation of radiotelemetric blood pressure/core temperature transducers (Data Sciences International, New Brighton, MN; model HD-X10) under ketamine/xylazine anesthesia (100/10 mg/kg, ip). Animals were subsequently singly-housed and administered meloxicam (5 mg/kg, sc) on the day of surgery and again on the following day. After 7–10 days of recovery, blood pressure and core temperatures were recorded at 2,000 Hz for 10 min every hour for several days each at standard room temperature (22°C), thermoneutrality (30°C), and cold challenge (16°C). Mice then underwent stereotaxic microinjection surgeries as described above. Blood pressures and core temperatures at various ambient temperatures were then serially assessed at specific timepoints following microinjections.
Plasma insulin, glucose, and triglycerides
All assays analyzed 5h fasted, terminal plasma samples (BD Microtainer, Tubes with K2E, Franklin Lakes, NJ) obtained at tissue collection from the trunk (a mixture of arterial and venous blood). Glucose (mg/dL) was measured by colorimetric assay in neat samples following the manufacturer’s protocol (Cat: 81692, Crystal Chem, Elk Grove Village, IL). Insulin (ng/mL) was measured by ELISA in neat samples following the manufacturer’s protocol (Cat: 90080, Crystal Chem, Elk Grove Village, IL). Circulating triglycerides (mmol/L) were measured in samples diluted 1:5 in water (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol (Cat: ETGA-200, BioAssay Systems, Hayward, CA).
Hepatic triglycerides
Triglycerides were extracted from 20 to 25 mg of snap frozen liver tissue in extraction buffer (5 volumes isopropanol: 2 volumes water: 2 volumes Triton X-100; 50 μL buffer/mg tissue) via bead beating on a BeadBlaster 24R Refrigerated Microtube Homogenizer (Benchmark Scientific, Sayreville, NJ) and centrifuged at room temperature at 13,000 rpm for 3 min. Liver homogenates were diluted 1:5 in water (Invitrogen, Carlsbad, CA), and the assay (Cat: ETGA-200, BioAssay Systems, Hayward, CA) was run following the manufacturers protocol.
RNA isolation and gene expression by qPCR
Total RNA from the ARC, brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), liver, and kidney were isolated using TRIzol and isopropanol precipitation. To prepare ARC samples, brains were removed and cut into 1 mm coronal sections using a commercially available brain matrix using a blunt tip 0.75mm punch set. Briefly, tissue samples were homogenized in TRIzol Reagent (Invitrogen, Carlsbad, CA) on a BeadBlaster 24R Refrigerated Microtube Homogenizer for BAT, iWAT, liver, and kidney samples or using a Bio-vortexer Homogenizer (Weber Scientific, Hamilton, NJ) for ARC samples. The homogenate was combined with chloroform (ThermoFisher Scientific, Waltham, MA), vortexed, incubated at room temperature for ∼3 min, and centrifuged at 4°C at 12,000xg for 15 min. Total RNA was isolated from the aqueous layer by mixing with GlyoBlue (Invitrogen, Carlsbad, CA) and 2-propanol (Millipore Sigma, Darmstadt, Germany), freezing at −80°C, and centrifuging 4°C at 12,000xg for 20 min. Samples were washed twice with 70% EtOH and centrifuged at 4°C at 12,000xg for 20 min. Samples were resuspended in Rnase-free water. RNA was quantified by a NanoDrop 1000 Spectrophotometer (ThermoFisher Scientific, Waltham, MA) and stored at −80°C. Purified RNA (∼1 μg) was reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). All RT-qPCR assays were prepared following the manufacturer protocol using TaqMan Master Mix (Applied Biosystems, Foster City, CA). Experiments were conducted on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA). See Table S6 for gene expression assay information.
RNAsequencing
Snap-frozen liver samples were homogenized in TRIzol Reagent on a BeadBlaster 24R Refrigerated Microtube Homogenizer. Samples were centrifuged at 21,100xg for 3 min to pellet unhomogenized tissue. The supernatant was combined equally with chloroform, vortexed, incubated at room temperature for ∼3 min, and centrifuged at 4°C at 12,000xg for 15 min. Total RNA was purified from the aqueous layer following the PureLink RNA Mini Kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol. Samples were treated with DNase I from the PureLink DNase set (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol to remove gDNA. Samples were shipped to Novogene Corporation Inc. for quality control and sequencing. In brief, RNA samples were checked for quality using Qubit and real-time PCR for quantification and by bioanalyzer for size distribution detection. mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Libraries were prepared with poly-A enrichment and were sequenced on a NovaSeqX Plus instrument using 150 bp paired-end reads. The original image data was transformed to sequenced reads by CASAVA base recognition. FASTQ sequencing files (publicly available at GSE284096) were aligned to the mouse reference genome (GRCm39/mm39) using HISAT2.
Only protein coding genes were considered for differential gene expression (DEG) and enrichment analyses. For the combined sex comparisons, genes continued in our analysis pipeline if there were three or more non-zero read counts per group. For individual sex comparisons, genes continued in our analysis if there were two or more non-zero read counts per group. DEG analysis was completed using DESeq2 in R 4.3.1 (absolute Fold Change ≥1.1, Benjamini-Hochberg False Discovery Rate <5%).60,61 Pairwise comparisons between treatment and temperature groups were done by the Wald test, and LFC estimates were shrunk using the "apeglm" option. DEGs were submitted to ShinyGO 0.81 for gene enrichment analysis using Gene Ontology Biological Processes, GO Molecular Function, KEGG, and Reactome.62
Adipose histology
Perigonadal and inguinal white adipose tissues were fixed in 10% neutral buffered formalin and paraffin-embedded, sectioned (4 μm) and H&E stained (Children’s Research Institute Histology Core, Medical College of Wisconsin). Sections were scanned with a Hamamatsu slide scanner (Children’s Research Institute Imaging Core, Medical College of Wisconsin), and.ndpi image files were analyzed using Nanozoomer software (Hamamatsu Photonics, Hamamatsu, Japan).
Quantification and statistical analysis
Statistics
Blinding and randomization were utilized as possible throughout all studies. All data were analyzed using parametric approaches including 2-tailed independent, paired, or one-sample t-tests, ANOVA or mixed-effects models, or General Linear Modeling (GLM), followed by Šídák multiple comparison procedures. Throughout, statistical significance was defined as p < 0.05. All calculations were performed using GraphPad Prism v10 and IBM SPSS v24. Details of analytical tests and outcomes are reported within each figure, figure legend, and table. Throughout, reported n’s represent numbers of individual mice, and summary data are presented as mean ± SEM, unless otherwise indicated.
Published: May 30, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.112797.
Supplemental information
References
- 1.Hall K.D., Sacks G., Chandramohan D., Chow C.C., Wang Y.C., Gortmaker S.L., Swinburn B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet (London, England) 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira V., Kwitek A.E., Sigmund C.D., Morselli L.L., Grobe J.L. Recent Advances in Hypertension: Intersection of Metabolic and Blood Pressure Regulatory Circuits in the Central Nervous System. Hypertension. 2021;77:1061–1068. doi: 10.1161/HYPERTENSIONAHA.120.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fothergill E., Guo J., Howard L., Kerns J.C., Knuth N.D., Brychta R., Chen K.Y., Skarulis M.C., Walter M., Walter P.J., Hall K.D. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring, Md.) 2016;24:1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall K.D., Kahan S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. North Am. 2018;102:183–197. doi: 10.1016/j.mcna.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sapouckey S.A., Morselli L.L., Deng G., Patil C.N., Balapattabi K., Oliveira V., Claflin K.E., Gomez J., Pearson N.A., Potthoff M.J., et al. Exploration of cardiometabolic and developmental significance of angiotensinogen expression by cells expressing the leptin receptor or agouti-related peptide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318:R855–R869. doi: 10.1152/ajpregu.00297.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bewick G.A., Gardiner J.V., Dhillo W.S., Kent A.S., White N.E., Webster Z., Ghatei M.A., Bloom S.R. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. Faseb J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 7.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Sci. Technol. Humanit. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 8.Joly-Amado A., Denis R.G.P., Castel J., Lacombe A., Cansell C., Rouch C., Kassis N., Dairou J., Cani P.D., Ventura-Clapier R., et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 2012;31:4276–4288. doi: 10.1038/emboj.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gropp E., Shanabrough M., Borok E., Xu A.W., Janoschek R., Buch T., Plum L., Balthasar N., Hampel B., Waisman A., et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 10.Phillips C.T., Palmiter R.D. Role of agouti-related protein-expressing neurons in lactation. Endocrinology. 2008;149:544–550. doi: 10.1210/en.2007-1153. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q., Howell M.P., Cowley M.A., Palmiter R.D. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc. Natl. Acad. Sci. USA. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Q., Boyle M.P., Palmiter R.D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai J., Chen J., Ortiz-Guzman J., Huang J., Arenkiel B.R., Wang Y., Zhang Y., Shi Y., Tong Q., Zhan C. AgRP neurons are not indispensable for body weight maintenance in adult mice. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betley J.N., Cao Z.F.H., Ritola K.D., Sternson S.M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyward F.D., Liu N., Jacobs C., Machado N.L.S., Ivison R., Uner A., Srinivasan H., Patel S.J., Gulko A., Sermersheim T., et al. AgRP neuron cis-regulatory analysis across hunger states reveals that IRF3 mediates leptin's acute effects. Nat. Commun. 2024;15:4646. doi: 10.1038/s41467-024-48885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich M.O., Zimmer M.R., Bober J., Horvath T.L. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015;160:1222–1232. doi: 10.1016/j.cell.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morselli L.L., Claflin K.E., Cui H., Grobe J.L. Control of Energy Expenditure by AgRP Neurons of the Arcuate Nucleus: Neurocircuitry, Signaling Pathways, and Angiotensin. Curr. Hypertens. Rep. 2018;20:25. doi: 10.1007/s11906-018-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claflin K.E., Sandgren J.A., Lambertz A.M., Weidemann B.J., Littlejohn N.K., Burnett C.M.L., Pearson N.A., Morgan D.A., Gibson-Corley K.N., Rahmouni K., Grobe J.L. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J. Clin. Investig. 2017;127:1414–1424. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balapattabi K., Yavuz Y., Jiang J., Deng G., Mathieu N.M., Ritter M.L., Opichka M.A., Reho J.J., McCorvy J.D., Nakagawa P., et al. Angiotensin AT(1A) receptor signal switching in Agouti-related peptide neurons mediates metabolic rate adaptation during obesity. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner V.A., Deng G., Claflin K.E., Ritter M.L., Cui H., Nakagawa P., Sigmund C.D., Morselli L.L., Grobe J.L., Kwitek A.E. Cell-specific transcriptome changes in the hypothalamic arcuate nucleus in a mouse deoxycorticosterone acetate-salt model of hypertension. Front. Cell. Neurosci. 2023;17 doi: 10.3389/fncel.2023.1207350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapouckey S.A., Deng G., Sigmund C.D., Grobe J.L. Potential mechanisms of hypothalamic renin-angiotensin system activation by leptin and DOCA-salt for the control of resting metabolism. Physiol. Genomics. 2017;49:722–732. doi: 10.1152/physiolgenomics.00087.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romanov R.A., Zeisel A., Bakker J., Girach F., Hellysaz A., Tomer R., Alpár A., Mulder J., Clotman F., Keimpema E., et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat. Neurosci. 2017;20:176–188. doi: 10.1038/nn.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritter M.L., Deng G., Reho J.J., Deng Y., Sapouckey S.A., Opichka M.A., Balapattabi K., Wackman K.K., Brozoski D.T., Lu K.T., et al. Cardiometabolic Consequences of Deleting the Regulator of G protein Signaling-2 (Rgs2) From Cells Expressing Agouti-Related Peptide or the ANG (Angiotensin) II Type 1A Receptor in Mice. Hypertension. 2022;79:2843–2853. doi: 10.1161/HYPERTENSIONAHA.122.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon C.J. Thermal physiology of laboratory mice: Defining thermoneutrality. J. Therm. Biol. 2012;37:654–685. doi: 10.1016/j.jtherbio.2012.08.004. [DOI] [Google Scholar]
- 25.Deem J.D., Faber C.L., Morton G.J. AgRP neurons: Regulators of feeding, energy expenditure, and behavior. FEBS J. 2022;289:2362–2381. doi: 10.1111/febs.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira V., Riedl R.A., Claflin K.E., Mathieu N.M., Ritter M.L., Balapattabi K., Wackman K.K., Reho J.J., Brozoski D.T., Morgan D.A., et al. Melanocortin MC(4)R receptor is required for energy expenditure but not blood pressure effects of angiotensin II within the mouse brain. Physiol. Genomics. 2022;54:196–205. doi: 10.1152/physiolgenomics.00015.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Z., Stornetta D.S., Stornetta R.L., Brooks V.L. Arcuate Angiotensin II Increases Arterial Pressure via Coordinated Increases in Sympathetic Nerve Activity and Vasopressin Secretion. eNeuro. 2022;9:1. doi: 10.1523/ENEURO.0404-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Z., Bonillas A.C., Wong J., Padilla S.L., Brooks V.L. Neuropeptide Y suppresses thermogenic and cardiovascular sympathetic nerve activity via Y1 receptors in the paraventricular nucleus and dorsomedial hypothalamus. J. Neuroendocrinol. 2021;33 doi: 10.1111/jne.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Z., Zhao D., Cassaglia P.A., Brooks V.L. Sites and sources of sympathoexcitation in obese male rats: role of brain insulin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318:R634–R648. doi: 10.1152/ajpregu.00317.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z., Madden C.J., Brooks V.L. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. J. Clin. Investig. 2017;127:2868–2880. doi: 10.1172/JCI92008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawton S.B.R., Wagner V.A., Nakagawa P., Segar J.L., Sigmund C.D., Morselli L.L., Grobe J.L. Angiotensin in the Arcuate: Mechanisms Integrating Cardiometabolic Control: The 2022 COH Mid-Career Award for Research Excellence. Hypertension. 2024;81:2209–2217. doi: 10.1161/HYPERTENSIONAHA.124.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedl R.A., Burnett C.M.L., Pearson N.A., Reho J.J., Mokadem M., Edwards R.A., Kindel T.L., Kirby J.R., Grobe J.L. Gut Microbiota Represent a Major Thermogenic Biomass. Function (Oxf) 2021;2 doi: 10.1093/function/zqab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soto J.E., Burnett C.M.L., Ten Eyck P., Abel E.D., Grobe J.L. Comparison of the Effects of High-Fat Diet on Energy Flux in Mice Using Two Multiplexed Metabolic Phenotyping Systems. Obesity (Silver Spring, Md.) 2019;27:793–802. doi: 10.1002/oby.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnett C.M.L., Grobe J.L. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Mol. Metab. 2014;3:460–464. doi: 10.1016/j.molmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnett C.M.L., Grobe J.L. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. Am. J. Physiol. Endocrinol. Metab. 2013;305:E916–E924. doi: 10.1152/ajpendo.00387.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsberg G.E., Hoffman T.C.M. Using direct calorimetry to test the accuracy of indirect calorimetry in an ectotherm. Physiol. Biochem. Zool. 2006;79:830–835. doi: 10.1086/505514. [DOI] [PubMed] [Google Scholar]
- 37.Walsberg G.E., Hoffman T.C.M. Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. J. Exp. Biol. 2005;208:1035–1043. doi: 10.1242/jeb.01477. [DOI] [PubMed] [Google Scholar]
- 38.Pittet P., Chappuis P., Acheson K., De Techtermann F., Jéquier E. Thermic effect of glucose in obese subjects studied by direct and indirect calorimetry. Br. J. Nutr. 1976;35:281–292. doi: 10.1079/bjn19760033. [DOI] [PubMed] [Google Scholar]
- 39.Pittet P., Gygax P.H., Jéquier E. Thermic effect of glucose and amino acids in man studied by direct and indirect calorimetry. Br. J. Nutr. 1974;31:343–349. doi: 10.1079/bjn19740042. [DOI] [PubMed] [Google Scholar]
- 40.Lawton S.B., Grobe C.C., Reho J.J., Raff H., Thulin J.D., Jensen E.S., Burnett C.M., Segar J.L., Grobe J.L. Differences in Fluid, Electrolyte, and Energy Balance in C57BL/6J Mice (Mus musculus) in Metabolic Caging at Thermoneutral or Standard Room Temperatures. J. Am. Assoc. Lab. Anim. Sci. 2024;63:190–200. doi: 10.30802/AALAS-JAALAS-23-000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrington L.P. The heat regulation of small laboratory animals at various environmental temperatures. Am. J. Physiol. 1940;129:123–139. doi: 10.1152/ajplegacy.1940.129.1.123. [DOI] [Google Scholar]
- 42.Pennycuik P.R. A comparison of the effects of a variety of factors on the metabolic rate of the mouse. Aust. J. Exp. Biol. Med. Sci. 1967;45:331–346. doi: 10.1038/icb.1967.33. [DOI] [PubMed] [Google Scholar]
- 43.Heldmaier G. Temperature adaptation and brown adipose tissue in hairless and albino mice. J. Comp. Physiol. 1974;92:281–292. doi: 10.1007/BF00696616. [DOI] [Google Scholar]
- 44.Yamauchi C., Fujita S., Obara T., Ueda T. Effects of room temperature on reproduction, body and organ weights, food and water intakes, and hematology in mice. Jikken Dobutsu. 1983;32:1–11. doi: 10.1538/expanim1978.32.1_1. [DOI] [PubMed] [Google Scholar]
- 45.Hu C.C., Qing K., Chen Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes. Res. 2004;12:1264–1270. doi: 10.1038/oby.2004.160. [DOI] [PubMed] [Google Scholar]
- 46.Rojas J.M., Bruinstroop E., Printz R.L., Alijagic-Boers A., Foppen E., Turney M.K., George L., Beck-Sickinger A.G., Kalsbeek A., Niswender K.D. Central nervous system neuropeptide Y regulates mediators of hepatic phospholipid remodeling and very low-density lipoprotein triglyceride secretion via sympathetic innervation. Mol. Metab. 2015;4:210–221. doi: 10.1016/j.molmet.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada C., Saegusa Y., Nahata M., Sadakane C., Hattori T., Takeda H. Influence of Aging and Gender Differences on Feeding Behavior and Ghrelin-Related Factors during Social Isolation in Mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheffer-Babila S., Sun Y., Israel D.D., Liu S.M., Neal-Perry G., Chua S.C., Jr. Agouti-related peptide plays a critical role in leptin's effects on female puberty and reproduction. Am. J. Physiol. Endocrinol. Metab. 2013;305:E1512–E1520. doi: 10.1152/ajpendo.00241.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asarian L., Geary N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodin S.Z., Kiechler A.R., Smith M., Wendt D., Strader A.D. Effect of gonadectomy on AgRP-induced weight gain in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1747–R1753. doi: 10.1152/ajpregu.90345.2008. [DOI] [PubMed] [Google Scholar]
- 51.Reho J.J., Muskus P.C., Bennett D.M., Grobe C.C., Burnett C.M.L., Nakagawa P., Segar J.L., Sigmund C.D., Grobe J.L. Modulatory effects of estrous cycle on ingestive behaviors and energy balance in young adult C57BL/6J mice maintained on a phytoestrogen-free diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024;326:R242–R253. doi: 10.1152/ajpregu.00273.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beaudet A., Greenspun D., Raelson J., Tannenbaum G.S. Patterns of expression of SSTR1 and SSTR2 somatostatin receptor subtypes in the hypothalamus of the adult rat: relationship to neuroendocrine function. Neuroscience. 1995;65:551–561. doi: 10.1016/0306-4522(94)00486-O. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura K., Nakamura Y., Kataoka N. A hypothalamomedullary network for physiological responses to environmental stresses. Nat. Rev. Neurosci. 2022;23:35–52. doi: 10.1038/s41583-021-00532-x. [DOI] [PubMed] [Google Scholar]
- 54.Moffitt J.R., Bambah-Mukku D., Eichhorn S.W., Vaughn E., Shekhar K., Perez J.D., Rubinstein N.D., Hao J., Regev A., Dulac C., Zhuang X. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Sci. Technol. Humanit. 2018;362 doi: 10.1126/science.aau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monge-Roffarello B., Labbe S.M., Lenglos C., Caron A., Lanfray D., Samson P., Richard D. The medial preoptic nucleus as a site of the thermogenic and metabolic actions of melanotan II in male rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R158–R166. doi: 10.1152/ajpregu.00059.2014. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez A.D., Wang G., Waters E.M., Gonzales K.L., Speth R.C., Van Kempen T.A., Marques-Lopes J., Young C.N., Butler S.D., Davisson R.L., et al. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience. 2012;226:489–509. doi: 10.1016/j.neuroscience.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sumners C., Alleyne A., Rodríguez V., Pioquinto D.J., Ludin J.A., Kar S., Winder Z., Ortiz Y., Liu M., Krause E.G., de Kloet A.D. Brain angiotensin type-1 and type-2 receptors: cellular locations under normal and hypertensive conditions. Hypertens. Res. 2020;43:281–295. doi: 10.1038/s41440-019-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madisen L., Mao T., Koch H., Zhuo J.M., Berenyi A., Fujisawa S., Hsu Y.W.A., Garcia A.J., Gu X., Zanella S., et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reho J.J., Nakagawa P., Mouradian G.C., Jr., Grobe C.C., Saravia F.L., Burnett C.M.L., Kwitek A.E., Kirby J.R., Segar J.L., Hodges M.R., et al. Methods for the Comprehensive in vivo Analysis of Energy Flux, Fluid Homeostasis, Blood Pressure, and Ventilatory Function in Rodents. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.855054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.R Development Core Team . R Foundation for Statistical Computing; 2023. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 62.Ge S.X., Jung D., Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics (Oxford, England) 2020;36:2628–2629. doi: 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
RNA sequencing data have been deposited at NCBI GEO as GSE284096 and are publicly available as of the date of publication.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.