Highlights
-
•
Viral pneumonia in Southeast Asia (SEA) is understudied, impacting community-acquired pneumonia (CAP) morbidity and mortality.
-
•
Influenza, respiratory syncytial virus, and human rhinovirus/enterovirus are commonly tested pathogens for CAP in SEA.
-
•
Better microbiology capacity is needed to improve CAP surveillance in SEA.
Keywords: Viral community-acquired pneumonia, Southeast Asia
Abstract
Community-acquired pneumonia (CAP) remains a leading cause of morbidity and mortality worldwide, including in Southeast Asia (SEA). While bacterial causes are well studied, viral etiologies are less characterized. The COVID-19 pandemic has underscored the significance of viral pneumonia, alongside ongoing concerns from zoonotic influenza, human metapneumovirus, and other outbreaks. This review identified 16 studies from SEA, encompassing 8421 CAP patients (2012-2023), describing the viral etiology of CAP. Influenza virus (IV), respiratory syncytial virus (RSV), and human rhinovirus/enterovirus (hRV/EV) were the most frequently tested viral pathogens in 16, 13, and 12 studies, respectively. The pooled positivity rates were 9.02% (hRV/EV), 7.28% (IV), and 5.17% (RSV). While viral etiologies of CAP in SEA align with global trends, data remain limited. Enhancing microbiology capacity in SEA is essential to strengthen CAP surveillance, optimize treatment strategies, inform vaccination policies, and improve pandemic preparedness.
Introduction
Community-acquired pneumonia (CAP) is one of the most common infections encountered globally [1] and remains a leading cause of morbidity and mortality worldwide, including Southeast Asia (SEA) [2]. According to the Global Burden of Diseases, Injuries, and Risk Factors Study, an estimated 336.5 million cases of lower respiratory tract infections occurred in 2016, corresponding to a global incidence rate of 32.2 per 100,000 people [3]. Traditional diagnostic efforts and treatment guidelines have focused on bacterial pathogens such as Streptococcus pneumoniae, Mycoplasma pneumoniae, Haemophilus influenzae, and Legionella pneumophila.[[4], [5], [6]], often overlooking viral etiologies.
In high-income countries with well-established molecular microbiology facilities, viral pathogens were identified as a common etiology of CAP [[7], [8], [9], [10], [11]]. Similarly, the Pneumonia Etiology Research for Child Health (PERCH) study [12], a large multi-country investigation of severe childhood pneumonia, identified a substantial role of viral pathogens in pneumonia cases, especially in low- and middle-income country settings. These findings underscore the importance of comprehensive viral diagnostics, even in resource-limited environments. However, in many low- and middle-income countries, including in the SEA region, viral testing is often limited to a few key pathogens such as influenza and respiratory syncytial virus (RSV) [11,13,14], with tests for other respiratory viruses often being unavailable. The emergence of novel respiratory viruses, including SARS-CoV [15], pandemic influenza A (H1N1) 2009 [16], Middle East respiratory syndrome coronavirus (MERS-CoV) [17], avian influenza and the recent global pandemic COVID-19 caused by SARS- -[18] has underscored the need for a more comprehensive understanding of viral contributions to CAP.
Additionally, the identification of previously under-recognized respiratory viruses, such as human metapneumovirus (hMPV) [13,19,20], human bocavirus (BoV) [21], adenovirus (AdV) [13,22] and human seasonal coronaviruses (229E, OC43, NL63, HKU1) [23], highlights the complex landscape of respiratory infections [24]. Human rhinovirus/enterovirus (hRV/EV), once considered primarily a cause of upper respiratory infections, is now increasingly implicated in lower respiratory tract infections in children and adults [20,[25], [26], [27]]. These developments have emphasized the critical need to broaden our understanding of the viral causes of CAP by expanding the capacity of microbiology labs in the region to perform molecular testing. A potential benefit of testing for viral pathogens is a decrease in the use of antibiotics and potentially antimicrobial resistance in the region [28,29].
To our knowledge, no review has focused exclusively on the viral etiology of CAP in SEA. This study aims to synthesize recent literature on viral pathogens associated with CAP in the region to inform future research and guide evidence-based clinical management.
Methods
An electronic search was conducted in PubMed, SCOPUS, EBSCO (Medline), and Web of Science, for full-text research articles published up to 31 December 2023. The keywords used to search were (TITLE-ABS (("community acquired" OR "community-acquired") W/3 (pneumonia OR infection? OR alri))) AND (TITLE-ABS ("Southeastern Asia" OR "South-East* Asia" OR "South East* Asia" OR borneo OR brunei OR cambodia OR indochina OR indonesia* OR laos OR malaysia* OR "Mekong Valley" OR myanmar OR philippines OR singapore* OR thailand OR "Timor-Leste" OR vietnam*)) AND (LIMIT-TO (LANGUAGE, "English")). Two independent reviewers (YNH and HYT) screened the abstracts of all articles. Two other reviewers (SMH and YTW) then reviewed the short-listed full-text articles of the published articles for eligibility and relevance. Any disagreement was resolved by internal discussion within the team.
Inclusion and exclusion criteria
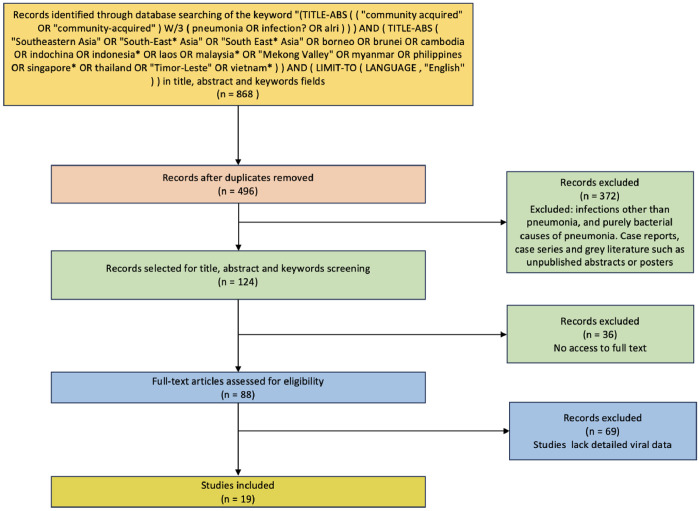
Articles published in English were selected. The titles and abstracts of all retrieved citations were thoroughly reviewed. Articles were included if they described cases of viral pneumonia in SEA countries, defined according to the United Nations geo scheme, which includes Brunei Darussalam, Cambodia, Indonesia, Lao People’s Democratic Republic, Malaysia, Myanmar, the Philippines, Singapore, Thailand, Timor-Leste, and Vietnam. Exclusion criteria included infections other than pneumonia and purely bacterial causes of pneumonia. Case reports, case series, and gray literature such as unpublished abstracts or posters were also excluded from the review (Figure 1).
Figure 1.
PRISMA flow diagram for the selection of articles for the review.
The review was prepared in accordance with the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [30].
The following data were extracted from the included studies: author(s), publication year, type of study, country, study year, sample size, age group, type of testing, specimens, number of viruses tested, and clinical setting. All the extracted data were entered and analyzed in a Microsoft Excel spreadsheet. Descriptive statistics were used for specific viral pathogens. A meta-analysis using a random-effects model with logit-transformed proportions was performed in R Studio using “meta” and “metafor” packages to estimate the pooled positivity rate with 95% confidence intervals (CIs), assessing heterogeneity using Cochran’s Q test and I² statistics.
Results
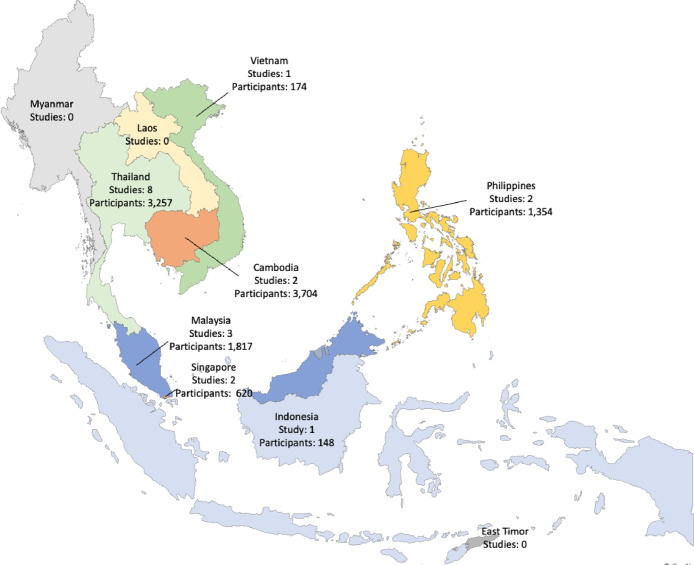
In total, 868 abstracts were identified, and 496 abstracts were screened after duplicates were removed. From these, 124 relevant English language articles were identified for retrieval after further screening of the titles and abstracts for eligibility. Of the 124 articles identified, 36 could not be accessed despite extensive efforts, including institutional access and inter-library loan requests, due to subscription barriers or incomplete information. Of the remaining 88 articles, 69 were excluded (lack of detailed viral data), leaving a total of 19 full-text articles included in the final review, comprising 11,074 patients (Table 1). The studies were from Cambodia [31,32], Indonesia [33], Malaysia [34,35], Philippines [37,38], Singapore [39,40], Thailand [12,[41], [42], [43], [44], [45], [46], [47]], and Vietnam [48] (Figure 2). Four were retrospective studies, 10 were prospective studies, two were cross-sectional studies, one was a case-control study, one was an active surveillance study, and the remaining one was a combined prospective and retrospective study. Of these 19 articles, a single study from Singapore included only patients with severe CAP (defined as either meeting the American Thoracic Society/Infectious Disease Society of America criteria for severe pneumonia or admission to the intensive care unit) [49], while the rest included mild, moderate, and severe CAP.
Table 1.
Characteristics of the available studies in SEA.
| No | Author, year of publication, ref | Type of study | Country, study year | Sample size | Age group | Type of testing | Specimens | Number of viruses tested | Clinical setting |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Rammaert et al. (2013) [31] | Prospective cohort | Cambodia (2007-2010) |
1800 | ≥15 years | PCR, culture | blood, sputum, throat, NPS | 18 | Community acquired ALRI surveillance (SISEA) |
| 2. | Vong et al. (2013) [32] | Prospective | Cambodia (Apr 2007-Dec 2009) |
1904 | ≥5 years | PCR, Culture | NPS and throat swabs | 18 | Hospital |
| 3. | Farida et al. (2015) [33] | Prospective, cohort | Indonesia (Oct 2007- Apr 2009) |
148 | Adults | Serology, PCR | Sputum, Blood, throat swab, paired sera | 16 | Hospital |
| 4. | Toh et al. (2019) [34] | Prospective | Malaysia (Jun 2017- May 2018) |
600 | >1 month | PCR | NPS | 13 | Hospital |
| 5. | Pang et al. (2021) [35] | Prospective study | Malaysia (Jul 2018-Aug 2019) |
1106 | ≥18 years | PCR | NPS, OPS | 1 | Active surveillance/ hospital |
| 6. | Ng et al (2022) [36] | Retrospective study | Malaysia (Apr-Oct 2021) |
111 | ≤12 years | PCR | NPA, ETA, endotracheal aspirate | 19 | Hospital |
| 7. | Suzuki et al. (2012) [37] | Prospective cohort | Philippines (2008-2009) |
819 | <13 years | Culture, PCR | NPS, blood | 15 | Hospital |
| 8. | Lupisan et al. (2019) [38] | Prospective | Philippines (2010-2012) |
535 | Adults | Culture, PCR | NPS, sputum, blood | 7 | Hospital |
| 9. | Siow et al. (2016) [39] | Prospective cohort | Singapore (Nov 2009-Sep 2011) |
100 | Adults | Culture, PCR | Respiratory specimen, blood, urine | 8 | Intensive care unit |
| 10. | Lim et al. (2022) [40] | Retrospective | Singapore (2020) |
520 | Adults | PCR | - | 7 | Hospital |
| 11. | Fry et al. (2007) [41] | Active surveillance | Thailand (Sep 2004- Aug 2005) |
1171 | All age group | Serology, PCR | NPS | 13 | Active surveillance/ hospital |
| 12. | Hara et al. (2011) [42] | Prospective, cohort | Thailand (2006-2008) |
119 | Adults | Culture, PCR | Blood, sputum | 10 | Hospital |
| 13. | Pratheepamornkull et al. (2015) [43] | Cross sectional | Thailand (Jun 2013- May 2014) |
102 | Children | PCR | NPS | 9 | Hospital |
| 14. | Kositpantawong et al. (2021) [44] | Retrospective | Thailand (2016-2020) |
143 | Adults | PCR | - | 1 | Hospital |
| 15. | Bunthi et al. (2021) [12] | Case-control | Thailand (Jan 2012- Feb 2014) |
882 | <5 years | PCR, culture | NPS/OPS | 33 | Hospital |
| 16. | Osman et al. (2021) [45] | Retrospective & prospective | Thailand (2015-2019) |
490 | ≥60 years | PCR | Throat, pleural fluid, tracheal aspirates | 3 | Hospital |
| 17. | Poovieng et al. (2022) [46] | Retrospective cohort | Thailand (2015-2019) |
250 | ≥15 years | PCR | Sputum, tracheal aspirates, NPS | >3? | Hospital |
| 18. | Wanlapakorn et al. (2023) [47] | Cross-sectional study | Thailand (2019-2020) |
100 | ≤5 years | PCR | NPA | 18 | Hospital |
| 19. | Takahashi et al. (2013) [48] | Prospective cohort | Vietnam (2009-2010) |
174 | ≥15 years old | Culture, PCR | NPS | 13 | Hospital |
NPS, nasopharyngeal swab; OPS, oropharyngeal swab; PCR, polymerase chain reaction; SEA, Southeast Asia.
Figure 2.
Number of studies available from Southeast Asia countries. Each country is color-coded.
The sample sizes varied widely, with the smallest study comprising 100 participants (Singapore, Thailand) and the largest encompassing 1904 participants (Cambodia). Twelve studies focused on adult populations, five studies targeted children (aged under 13 years or 5 years), and two studies included all age groups (Table 1). Polymerase chain reaction (PCR), often performed in multiplex, was the most common method for virus detection (84%), followed by culture (11%) and serology (5%) (Table 1, Supplementary Figure S-1). Nasopharyngeal swabs were the most commonly collected specimens (35%), followed by sputum (19%) and throat swabs (15%), with less frequent use of tracheal aspirates, pleural fluid, and blood (Supplementary Figure 2).
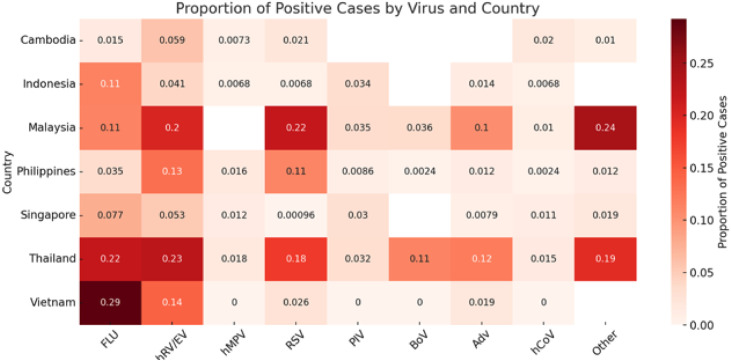
The studies reported multiple viral etiologies, ranging from a single virus to more than 19 different viral agents (Table 1, Table 2). Two studies [6,44] focused only on influenza. Specific individual viruses were identified in 4.2% to 93.0% of cases (Table 2). Influenza was the most tested virus (16/16 studies), followed by RSV (13/16 studies) and RV/EV (12/16 studies) (Table 2). Meta-analyses of studies on the viral etiology of CAP in SEA revealed a pooled positivity rate of approximately 7.28% (95% CI: 3.02-16.54%) for FLU based on 16 studies, 9.02% (95% CI: 4.64-16.81%), for hRV from 14 studies, and 5.17% (95% CI: 2.23-11.51%) for RSV from 14 studies. Considerable heterogeneity was observed across analyses, with some studies reporting markedly higher detection rates (Figure 2). Considerable heterogeneity in etiology was observed across countries. Detection rates for specific viruses varied by country; for instance, influenza and RSV were more frequently reported in Thailand and Vietnam, while studies from Singapore and Indonesia showed lower overall viral detection (Figure 3).
Table 2.
Virial etiology identification of CAP in the SEA region. Detection of pathogens is shown by actual number.
| Study | Total tested | Positive rate (%) | FLU | hRV/EV | hMPV | RSV | PIV(I-IV) | BoV | Adv | hCoV | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rammaert et al. [31] | 1270 | 11.4 | 17 | 60 | - | 24 | - | - | - | 26 | 18 |
| Vong et al. [32] | 959 | 14.3 | 16 | 68 | 7 | 23 | - | - | - | 18 | 6 |
| Farida et al. [33] | 148 | 22.3 | 17 | 6 | 1 | 1 | 5 | - | 2 | 1 | - |
| Toh et al. [34] | 599 | 48.1 | 75 | 25 | - | 122 | 21 | - | 62 | 6 | - |
| Pang et al. [35] | 1106 | 10.3 | 114 | - | - | - | - | - | - | - | - |
| Ng et al [36] | 111 | 87.4a | NR | 40 | NR | 26 | NR | 4 | NR | NR | 27 |
| Suzuki et al. [37] | 819 | 51.6 | 15 | 189 | 17 | 165 | 8 | 2 | 15 | 2 | 10 |
| Lupisan et al. [38] | 535 | 13.1 | 28 | 18b | 6 | 11 | 4 | - | 3 | - | - |
| Siow et al. [39] | 100 | 33.0 | 15 | 10 | 2 | 0 | 3 | - | 1 | 2 | - |
| Lim et al. [40] | 520 | 4.2 | 2 | 3 | 2 | 1 | - | - | 3 | 1 | 10 |
| Fry et al. (2007) [41] | 1168 | 4.5 | NR | NR | NR | NR | NR | 53 | NR | NR | NR |
| Hara et al. [42] | 119 | 9.2 | 7 | 2 | 0 | 2 | 0 | - | - | 0 | - |
| Pratheepamornkull et al. [43] | 91 | 46.2 | 0 | 11 | - | 22 | 0 | - | 9 | 0 | - |
| Kositpantawong et al. [44] | 1584 | 9.5 | 151 | - | - | - | - | - | - | - | - |
| Bunthi et al. [12] | 223 | 85.2 | 7 | 64 | 8 | 51 | 13 | 40 | 31 | 10 | 103 |
| Osman et al. [45] | 242 | 21.5 | 47 | - | - | 5 | - | - | - | - | - |
| Poovieng et al. [46] | 139 | 74.4 | 130 | - | - | - | - | - | - | - | 16 |
| Wanlapakorn et al. [47] | 100 | 93.0c | NR | 48 | NR | 38 | 7 | NR | NR | NR | NR |
| Takahashi et al. [48] | 154 | 48.1 | 45 | 22 | 0 | 4 | 0 | 0 | 3 | 0 | - |
Adv, adenovirus; BoV, boca virus; Entero, entero viruses; FLU, influenza viruses which include influenza A (H3N2, H1N1, pH1N1) and influenza B viruses; hCoV, human coronaviruses which includes 229E, OC43, NL63, HKU1, MERS-CoV, SARS-CoV, SARS-CoV-2 viruses; hRV/EV, human rhinovirus/enterovirus; hMPV, human metapneumovirus; NR, not reported; PIV(I-IV), parainfluenza virus type I to IV; RSV, respiratory syncytial virus; Others means those viruses not mentioned under individual viruses within the same study.
87.4 is the positive rate of four viruses (RV/EV, RSV, BoV, and SARS-CoV-2); the positive rate of the remaining individual viruses was not presented in the paper.
7 RV and one EV.
93.0% is based on the reported positive rate (only the top three common viruses were reported in the article.
Figure 3.
Country-level analysis of community-acquired pneumonia etiology of the included studies.
Adv, adenovirus; BoV, boca virus; Entero, entero viruses; FLU, influenza viruses which include influenza A (H3N2, H1N1,pH1N1) and influenza B viruses; hCoV, human coronaviruses which includes 229E, OC43, NL63, HKU1, MERS-CoV, SARS-CoV, SARS-CoV-2 viruses; hRV/EV, human rhinovirus/enterovirus; hMPV, human metapneumovirus; NR, not reported; PIV(I-IV), parainfluenza virus type I to IV; RSV, respiratory syncytial virus; Others means those viruses not mentioned under individual viruses within the same study.
Discussion
Respiratory viruses are thought to be responsible for 45-77% of CAP cases among children, and 20-55% of CAP cases [11,[50], [51], [52], [53], [54]] among adult populations worldwide. CAP poses a significant risk in children, accounting for an estimated 15.5% of all deaths and nearly a quarter (24.8%) of hospitalizations [55]. However, only a limited number of CAP studies focusing on the viral etiology of CAP have been conducted in SEA. Despite the availability of advanced diagnostic technology, such as multiplex PCR platforms, their use is still largely limited to research studies or private medical centers that do not often publish their data. Increased capacity to detect viral pathogens in patients with CAP will contribute to clinical management and provide data to advise policymakers on vaccine strategies and antimicrobial stewardships. This could come in the form of rapid diagnostic tests similar to the widely used SARS-CoV-2 antigen rapid tests.
Pooling data from available studies showed that RV, RSV, and influenza viruses were the most commonly detected in adults. This is consistent with studies from Europe, which reported influenza and hRV as the most detected viruses, with varying frequency of RSV, likely due to differences in the age group inclusion [11,53]. A recent case-control study of CAP among children in Africa and Asia reported RSV as the most commonly identified pathogen [56]. This may in part be because influenza, RV, and RSV were the most tested viral pathogens across the studies. The finding from the pooled positive rate of the three most tested pathogens (FLU, hRV, and RSV) underscores the variable viral landscape underlying CAP in SEA. The comparable contributions of hRV and RSV, alongside the variable detection of IV, suggest that multiple respiratory viruses significantly contribute to the pneumonia burden in the region. This also makes it difficult to determine the proportion of the most common viruses conclusively. The observed heterogeneity likely reflects differences in study design, diagnostic methods, and population demographics, highlighting the need for standardized testing protocols and coordinated regional surveillance to better inform targeted prevention and treatment strategies. A regional collaborative research initiative with a standardized pathogen list and provision of resources for molecular testing could be established to comprehensively identify and evaluate the epidemiology and viral etiology of CAP in the region. This initiative will provide robust baseline data on regional viral CAP incidence, helping to guide vaccine and therapeutic decisions and also prepare for emerging respiratory viral outbreaks.
The use of viral diagnostics is crucial in reducing unnecessary antibiotic use, thus combating AMR [29]. Multiplex reverse transcription (RT)-PCR platforms, which can differentiate between viral and bacterial infections within hours, help clinicians avoid antibiotics for viral infections, improving treatment specificity and possibly reducing antimicrobial resistance. A recent study [28] showed that multiplex RT-PCR of pneumonia has shown promise in clinical settings, leading to faster antibiotic adjustments (quicker escalations and timely de-escalations). However, the study focused on bacterial infections, with less attention to the role of viral etiologies. Additional studies to evaluate both viral and bacterial etiologies in guiding antibiotic escalation and de-escalation could potentially enhance our understanding of viral diagnostics' role in optimizing antiviral and antibiotic management.
Many of the studies (15 of 19) used nasopharyngeal swab samples to identify the viral pathogen. However, it is still unclear whether the rhinoviruses detected in the nasopharynx represent true infection or mere colonization. In contrast, negative PCR results from nasopharyngeal swab (NPS) samples may also underestimate lower respiratory tract infections [14,57]. As lower respiratory tract samples are difficult to obtain without contamination, it would be difficult to confirm this. However, viruses such as FLU, adenovirus, and RSV are not considered part of the normal human respiratory flora and thus detection is presumed indication of infection.
SEA is a major focal point for emerging respiratory viral infections. Apart from avian influenza and human metapneumovirus, its proximity and connections with East Asia make it an important sentinel site for novel strains of seasonal influenza. Adenovirus outbreaks have been reported in SEA presumably from East Asian sources [36]. SARS and SARS-CoV-2 were global public health emergencies. Improved detection and reporting from SEA will thus be critical for global health and potentially important to those concerned with pandemic preparedness internationally.
Limitations
There were several limitations to this review. Firstly, data from several countries in SEA, including Brunei, Laos, Myanmar, and Timor-Leste, was not readily available and thus was not adequately represented. Two studies from Malaysia [58,59] investigated the viral etiology of pneumonia, bronchiolitis, and lower respiratory tract infections. However, these studies did not provide a specific breakdown of the number of pneumonia cases, and therefore, they were excluded from the analysis in the current study. Secondly, a large proportion of data sets obtained from referenced papers were incomplete and heterogeneous, and we did not write to the authors of the papers. A limitation of the methodology is that only studies published in English were included. Hence, other relevant studies published in other languages may not have been included. In addition, most available data were also from hospitals in urban areas, which have more resources to carry out identification of etiology and studies to investigate the etiology of CAP, which has a disproportionate mortality impact in rural areas.
Conclusion
In conclusion, data on viral CAP in SEA remains limited. Based on limited available data, hRV/EV, RSV, and influenza are the most common viral etiology of CAP in SEA, however, there was very limited testing for other pathogens. This review highlights the need to increase the diagnostic capacity of CAP in SEA. A wider use of multiplex RT-PCR platforms in clinical settings would allow faster etiology diagnosis, tailored relevant therapeutic treatment, and vaccine strategies, and aid in pandemic preparedness.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This study is funded by the National Medical Research Council (NMRC) Open Fund Large Collaborative Grant (Award ID: OFLCG19May-0034). It has no role in the conceptualization, design, data collection, analysis, or decision to publish, and in preparation of the manuscript.
Ethical approval statement
Not applicable.
Author contributors
SMH: Conceptualization, Methodology, Data curation, Formal analysis, Writing-original draft, Writing-review & editing; PSH: Conceptualization, Writing-review & editing; HYN & TTHY: Data extraction, Validation; PAT: Conceptualization, Project administration, Resources, Funding, Supervision, Writing-review & editing. YTW: Conceptualization, Methodology, Writing-review & editing, Resources, Funding, Supervision.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2025.100672.
Appendix. Supplementary materials
References
- 1.Ferreira-Coimbra J., Sarda C., Rello J. Burden of community-acquired pneumonia and unmet clinical needs. Adv Ther. 2020;37:1302–1318. doi: 10.1007/s12325-020-01248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi R., Komazawa O., Team ES. ERIA; Jakarta: 2022. Cause of death statistics in ASEAN+3 countries’. Health and long-term care information in ageing Asia. [Google Scholar]
- 3.GBD 2016 Lower Respiratory Infections Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandell LA. Community-acquired pneumonia: an overview. Postgrad Med. 2015;127:607–615. doi: 10.1080/00325481.2015.1074030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen K., Yamba Yamba L., Wasserstrom L., Rünow E., Göransson T., Nilsson A., et al. Exploring the microbial landscape: uncovering the pathogens associated with community-acquired pneumonia in hospitalized patients. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1258981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song J.H., Huh K., Chung DR. Community-acquired pneumonia in the Asia-Pacific region. Semin Respir Crit Care Med. 2016;37:839–854. doi: 10.1055/s-0036-1592075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Chen B., Zhang S., Li X., Chang J., Tang Y., et al. Rapid detection of respiratory pathogens for community-acquired pneumonia by capillary electrophoresis-based multiplex PCR. SLAS Technol. 2019;24:105–116. doi: 10.1177/2472630318787452. [DOI] [PubMed] [Google Scholar]
- 8.Tao R.J., Luo X.L., Xu W., Mao B., Dai R.X., Li C.W., et al. Viral infection in community acquired pneumonia patients with fever: a prospective observational study. J Thorac Dis. 2018;10:4387–4395. doi: 10.21037/jtd.2018.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burk M., El-Kersh K., Saad M., Wiemken T., Ramirez J., Cavallazzi R. Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur Respir Rev. 2016;25:178–188. doi: 10.1183/16000617.0076-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu J.X., Gu L., Pu Z.H., Yu X.M., Liu Y.M., Li R., et al. Viral etiology of community-acquired pneumonia among adolescents and adults with mild or moderate severity and its relation to age and severity. BMC Infect Dis. 2015;15:89. doi: 10.1186/s12879-015-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alimi Y., Lim W.S., Lansbury L., Leonardi-Bee J., Nguyen-Van-Tam JS. Systematic review of respiratory viral pathogens identified in adults with community-acquired pneumonia in Europe. J Clin Virol. 2017;95:26–35. doi: 10.1016/j.jcv.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunthi C., Rhodes J., Thamthitiwat S., Higdon M.M., Chuananon S., Amorninthapichet T., et al. Etiology and clinical characteristics of severe pneumonia among young children in Thailand: Pneumonia Etiology Research for Child Health (PERCH) case-control study findings, 2012–2013. Pediatr Infect Dis J. 2021;40:S91–S100. doi: 10.1097/INF.0000000000002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain S., Williams D.J., Arnold S.R., Ampofo K., Bramley A.M., Reed C., et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi S.H., Hong S.B., Ko G.B., Lee Y., Park H.J., Park S.Y., et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186:325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 15.Peiris J.S.M., Guan Y., Yuen K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Padilla R., De La Rosa-zamboni D., Ponce de Leon S., Hernandez M., Quiñones-Falconi F., Bautista E., et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 17.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 18.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A., et al. A newly discovered human Pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamelin M.E., Côtù S., Laforge J., Lampron N., Bourbeau J., Weiss K., et al. Human Metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41:498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 21.Choi S.H., Huh J.W., Hong S.B., Jung J., Kim M.J., Chong Y.P., et al. Severe human bocavirus-associated pneumonia in adults at a referral hospital, Seoul, South Korea. Emerg Infect Dis. 2021;27:226–228. doi: 10.3201/eid2701.202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masaki K., Asakura T., Sakurai K., Ohkusu K. Adenovirus type 5 community-acquired pneumonia in an immunocompetent patient. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2018-228914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson P.P., Papadopoulos N.G., Skevaki C. Janes SM, Encyclopedia of Respiratory Medicine. Elsevier; Amsterdam: 2021. Respiratory viral pathogens; pp. 129–137. [Google Scholar]
- 25.Wang K., Xi W., Yang D., Zheng Y., Zhang Y., Chen Y., et al. Rhinovirus is associated with severe adult community-acquired pneumonia in China. J Thorac Dis. 2017;9:4502–4511. doi: 10.21037/jtd.2017.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira HLDS, Costa K.L., Cariolano M.S., Oliveira G.S., Felipe K.K., Silva E.S., et al. High incidence of rhinovirus infection in children with community-acquired pneumonia from a city in the Brazilian pre-Amazon region. J Med Virol. 2019;91:1751–1758. doi: 10.1002/jmv.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imakita M., Shiraki K., Yutani C., Ishibashi-Ueda H. Pneumonia caused by rhinovirus. Clin Infect Dis. 2000;30:611–612. doi: 10.1086/313723. [DOI] [PubMed] [Google Scholar]
- 28.Virk A., Strasburg A.P., Kies K.D., Donadio A.D., Mandrekar J., Harmsen W.S., et al. Rapid multiplex PCR panel for pneumonia in hospitalised patients with suspected pneumonia in the USA: a single-centre, open-label, pragmatic, randomised controlled trial. Lancet Microbe. 2024;5 doi: 10.1016/S2666-5247(24)00170-8. [DOI] [PubMed] [Google Scholar]
- 29.Erdem G., Watson J.R., Tomatis C., Ceyhan K., Barson W. Impact of viral testing on duration of antibiotic treatment and hospitalisation of febrile infants. Acta Paediatr. 2025;114:116–121. doi: 10.1111/apa.17413. [DOI] [PubMed] [Google Scholar]
- 30.LMP U. What Is PRISMA Guideline & what's new in the [2020 guideline]? 2020. https://www.covidence.org/blog/what-is-prisma-whats-new-in-the-2020-guideline-2/ [accessed 13 January 2025]
- 31.Rammaert B., Goyet S., Tarantola A., Hem S., Rith S., Cheng S., et al. Acute lower respiratory infections on lung sequelae in Cambodia, a neglected disease in highly tuberculosis-endemic country. Respir Med. 2013;107:1625–1632. doi: 10.1016/j.rmed.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vong S., Guillard B., Borand L., Rammaert B., Goyet S., Te V., et al. Acute lower respiratory infections in ≥5 year -old hospitalized patients in Cambodia, a low-income tropical country: clinical characteristics and pathogenic etiology. BMC Infect Dis. 2013;13:97. doi: 10.1186/1471-2334-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farida H., Gasem M.H., Suryanto A., Keuter M., Zulkarnain N., Satoto B., et al. Viruses and Gram-negative bacilli dominate the etiology of community-acquired pneumonia in Indonesia, a cohort study. Int J Infect Dis. 2015;38:101–107. doi: 10.1016/j.ijid.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toh T.H., Hii K.C., Fieldhouse J.K., Ting J., Berita A., Nguyen T.T., et al. High prevalence of viral infections among hospitalized pneumonia patients in equatorial Sarawak. Malaysia. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz074. ofz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang Y.K., Ismail A.I., Chan Y.F., Cheong A., Chong Y.M., Doshi P., et al. Influenza in Malaysian adult patients hospitalized with community-acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease or asthma: a multicenter, active surveillance study. BMC Infect Dis. 2021;21:644. doi: 10.1186/s12879-021-06360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng O.T., Thoon K.C., Chua H.Y., Tan N.W., Chong C.Y., Tee N.W., et al. Severe pediatric adenovirus 7 disease in Singapore linked to recent outbreaks across Asia. Emerg Infect Dis. 2015;21:1192–1196. doi: 10.3201/eid2107.141443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki A., Lupisan S., Furuse Y., Fuji N., Saito M., Tamaki R., et al. Respiratory viruses from hospitalized children with severe pneumonia in the Philippines. BMC Infect Dis. 2012;12:267. doi: 10.1186/1471-2334-12-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupisan S., Suzuki A., Macalalad N., Egos R., Sombrero L., Okamoto M., et al. Etiology and epidemiology of community-acquired pneumonia in adults requiring hospital admission: a prospective study in rural Central Philippines. Int J Infect Dis. 2019;80:46–53. doi: 10.1016/j.ijid.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Siow W.T., Koay E.S., Lee C.K., Lee H.K., Ong V., Ngerng W.J., et al. The use of polymerase chain reaction amplification for the detection of viruses and bacteria in severe community-acquired pneumonia. Respiration. 2016;92:286–294. doi: 10.1159/000448555. [DOI] [PubMed] [Google Scholar]
- 40.Lim S.Y.C., Zhou Y.P., Yii D., Chin D.Z., Hung K.C., Lee L.W., et al. Stemming the rise of antibiotic use for community-acquired acute respiratory infections during COVID-19 pandemic. Antibiotics (Basel) 2022;11:846. doi: 10.3390/antibiotics11070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fry A.M., Lu X., Chittaganpitch M., Peret T., Fischer J., Dowell S.F., et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hara K., Yahara K., Gotoh K., Nakazono Y., Kashiwagi T., Imamura Y., et al. Clinical study concerning the relationship between community-acquired pneumonia and viral infection in northern Thailand. Intern Med. 2011;50:991–998. doi: 10.2169/internalmedicine.50.4738. [DOI] [PubMed] [Google Scholar]
- 43.Pratheepamornkull T., Ratanakorn W., Samransamruajkit R., Poovorawan Y. Causative agents of severe community acquired viral pneumonia among children in Eastern Thailand. Southeast Asian J Trop Med Public Health. 2015;46:650–656. [PubMed] [Google Scholar]
- 44.Kositpantawong N., Surasombatpattana S., Siripaitoon P., Kanchanasuwan S., Hortiwakul T., Charernmak B., et al. Outcomes of early oseltamivir treatment for hospitalized adult patients with community-acquired influenza pneumonia. PLoS One. 2021;16 doi: 10.1371/journal.pone.0261411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osman M., Manosuthi W., Kaewkungwal J., Silachamroon U., Mansanguan C., Kamolratanakul S., et al. Etiology, clinical course, and outcomes of pneumonia in the elderly: a retrospective and prospective cohort study in Thailand. Am J Trop Med Hyg. 2021;104:2009–2016. doi: 10.4269/ajtmh.20-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poovieng J., Sakboonyarat B., Nasomsong W. Bacterial etiology and mortality rate in community-acquired pneumonia, healthcare-associated pneumonia and hospital-acquired pneumonia in Thai university hospital. Sci Rep. 2022;12:9004. doi: 10.1038/s41598-022-12904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wanlapakorn N., Thongpan I., Sarawanangkoor N., Vichaiwattana P., Auphimai C., Srimuan D., et al. Epidemiology and clinical characteristics of severe acute respiratory infections among hospitalized children under 5 years of age in a tertiary care center in Bangkok, Thailand, 2019–2020. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi K., Suzuki M., Minh L.N., Anh N.H., Huong L.T., Son T.V., et al. The incidence and aetiology of hospitalised community-acquired pneumonia among Vietnamese adults: a prospective surveillance in Central Vietnam. BMC Infect Dis. 2013;13:296. doi: 10.1186/1471-2334-13-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim K.J., Kim DH. The frequency and seasonal distribution of viral infection in patients with community-acquired pneumonia and its impact on the prognosis. Acute Crit Care. 2022;37:550–560. doi: 10.4266/acc.2022.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan Y., Yang Z., Chen R., Wang Y., Guan W., Zhao S. Respiratory virus is a real pathogen in immunocompetent community-acquired pneumonia: comparing to influenza like illness and volunteer controls. BMC Pulm Med. 2014;14:144. doi: 10.1186/1471-2466-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das D., Le Floch H., Houhou N., Epelboin L., Hausfater P., Khalil A., et al. Viruses detected by systematic multiplex polymerase chain reaction in adults with suspected community-acquired pneumonia attending emergency departments in France. Clin Microbiol Infect. 2015;21:608. doi: 10.1016/j.cmi.2015.02.014. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piralla A., Mariani B., Rovida F., Baldanti F. Frequency of respiratory viruses among patients admitted to 26 intensive care units in seven consecutive winter-spring seasons (2009–2016) in Northern Italy. J Clin Virol. 2017;92:48–51. doi: 10.1016/j.jcv.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X., Wang Q., Wang M., Su X., Xing Z., Zhang W., et al. Incidence of respiratory viral infections detected by PCR and real-time PCR in adult patients with community-acquired pneumonia: a meta-analysis. Respiration. 2015;89:343–352. doi: 10.1159/000369561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J., et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pneumonia Etiology Research for Child Health (PERCH) Study Group Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394:757–779. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karhu J., Ala-Kokko T.I., Vuorinen T., Ohtonen P., Syrjälä H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis. 2014;59:62–70. doi: 10.1093/cid/ciu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamberi S., Zulkifli I., Ilina I. Respiratory viruses detected in hospitalised paediatric patients with respiratory infections. Med J Malaysia. 2003;58:681–687. [PubMed] [Google Scholar]
- 59.Chan P.W., Goh A.Y., Chua K.B., Kharullah N.S., Hooi PS. Viral aetiology of lower respiratory tract infection in young Malaysian children. J Paediatr Child Health. 1999;35:287–290. doi: 10.1046/j.1440-1754.1999.00359.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.