Abstract
Background
Insomnia is a major sleeping disorder that affects the quality of life and overall health in a tremendous way. Those pharmacological therapies of insomnia, which are typically applied, i.e., benzodiazepines, may result in side effects that are rather dangerous, i.e., dependency and the decline of the cognitive status. Chaihu plus Longgu Muli decoction is a traditional Chinese herbal formula (TCM) which has been used in the treatment of insomnia and can be used as a safer alternative or complementary approach in the treatment of this ailment.
Methos
PubMed, Embase, Cochrane library, CNKI, Wanfang, CQVIP and CBM databases have been searched in our meta-analysis since the establishment of the databases to July 2024. The studies containing the combination of Chaihu Longgu Muli decoction and Estazolam in treating insomnia were to be included in the proposed study without taking into consideration the published literatures, unpublished literatures, those which contain in-complete or inadequate information, animal experiments, literature synthesis and the systematic study. Data analysis was done on the STATA 15.1.
Results
The meta-analysis revealed that the blend of Chaihu plus Longgu Muli decoction and Estazolam used to be extra potent than Estazolam treatment in enhancing the quality of sleep, as indicated by higher effective rates (RR = 1.29, 95% CI: 1.20–1.38, P = 0.000) and lower Pittsburgh Sleep Quality Index (PSQI) scores (WMD=-2.98, 95% CI: -4.06 to -1.90, P = 0.000). Also, lesser Traditional Chinese Medicine (TCM) syndrome scores followed the mixture therapy (WMD=-4.04, 95% CI: -4.38 to -3.70, P = 0.000) and reduced incidence of adverse events (RR = 0.35, 95% CI: 0.16–0.76, P = 0.000).
Conclusion
The combination of Chaihu plus Longgu Muli decoction and Estazolam is more effective and safer than Estazolam alone in treating insomnia. This integrative approach could offer a promising alternative for patients seeking effective and safer treatment options for insomnia.
Keywords: Insomnia, Chaihu plus Longgu Muli decoction, Estazolam, Meta-analysis, Traditional Chinese medicine
Introduction
The sleeping disorder insomnia is a common disease that is explained by an inability to fall asleep or stay asleep, or have non- restorative sleep; impairment in daytime functioning would be the major identifying criterion as the component of the ICSD-3 [1]. Traditional pharmacological treatments, such as benzodiazepines and non-benzodiazepine hypnotics, are commonly prescribed to manage insomnia [2]. Nonetheless, these drugs produce some unpleasant side effects such as dependency, tolerance, and cognitive deviance. Consequently, there is an increasing demand to address the problem with alternative or complementary therapy which should be effective and safe [3].
Chaihu plus Longgu Muli decoction is a so-called traditional Chinese herbal compound that has been found to provide treatment against mental and emotional disorders, insomnia included, and has been employed hundreds of years ago [4]. The formula, which includes Bupleurum root (Chaihu), Dragon bone (Longgu), and Oyster shell (Muli), is believed to calm the mind, alleviate anxiety, and improve sleep quality. Recent studies have suggested that Chaihu plus Longgu Muli decoction, when combined with conventional medications, may enhance therapeutic outcomes in patients with insomnia [5].
Estazolam, a benzodiazepine derivative, is widely used for its hypnotic and anxiolytic properties [5]. It acts by providing further effects of the neurotransmitter gamma-aminobutyric acid (GABA) in the GABA-A receptor, thus causing the induction of sleep [6]. Although estazolam is initially effective, its long-term use is limited by the development of tolerance, reduced efficacy, and the risk of dependency, highlighting the need for combination therapies to enhance treatment outcomes and minimize adverse effects [7].
This meta-analysis will review the efficacy and safety of Chaihu plus Longgu Muli decoction combined with estazolam in treating insomnia in a systematic manner. By synthesizing data from multiple studies, we seek to determine whether this integrative approach offers superior benefits compared to conventional monotherapy. The findings of this analysis could provide valuable insights for clinicians and patients seeking effective and safer treatment options for insomnia.
Methods
Literature inclusion and exclusion criteria
Inclusion criteria:
Subjects: patients with insomnia.
Interventions: Composition of Chaihu Longgu Muli decoction and Estazolam with regard to insomnia.
Control: Estazolam.
Outcome indicators: Effective rate, Syndrome score of Traditional Chinese Medicine (TCM) (a quantitative index related to TCM theory and used to describe the severity of syndromes according to symptoms, the appearance of the tongue, pulse reading etc.) [8]Pittsburgh Sleep Quality Index (PSQI) score (a self-reported questionnaire designed to measure the quality of sleep where the total scores vary between 0 and 21 where higher numbers reiterate adverse sleep quality) [9 and the occurrence of adverse events.
Study design: Randomized controlled trial (RCT).
Exclusion criteria:
Studies that had duplicate publication title; those that had no full-text data; those whose data could not be extracted; those that had been utilized by animal studies; reviews and systematic reviews.
Search strategy
The PubMed, Embase, Cochrane Library, CNKI, Wanfang, CQVIP and CBM databases were searched, and since the time of the databases, July 2024 of this meta-analysis is included in this meta-analysis. The mesh terms used were: ((insomnia[Title/Abstract]) AND (Chaihu Longgu Muli decoction[Title/Abstract])) AND ((western medicine[Title/Abstract]) OR (Estazolam[Title/Abstract])).
Literature screening and data extraction
Two researchers performed the study on the search of information, the screening of studies, and data extraction separately. The third party helped in solving all the problems or conflicts. The retrieved data were in terms of author, year of study, type of studies, cases and report of outcome.
Literature quality assessment
Two researchers assessed the quality of literature independently, and the contained literature was assessed with the help of the risk assessment tool of the Review manager 5.3 software upon the formation of the random sequence, the concealment of the allocation, the application of the blinding, the blinded treatment of the research outcome to the research, completeness of the outcome data, evaluation in accordance with the Cochrane Risk Assessment Scale, the gender and the choice of one reported outcome of the research, other biases, etc. and an opposing opinion is decided by means of discussion or consultating with a third party. A Meta-analysis was conducted on the basis of the relevant items of the Preferred Reporting Items of the Systematic Reviews and Meta Analysis (PRISMA) write-up [10].
Data synthesis and statistical analysis
The data were analysed with the help of STATA 15.1 [11]. A continuous variable weighted Mean difference (WMD) 95%CI was used. I [2] was used to assess cell heterogeneity. If the heterogeneity test was P ≥ 0.1 and I2 ≤ 50%, there was homogeneity of the studies and the studies were combined using a fixed effects model; if P < 0.1 and I2 > 50%, this group was not very homogenous; in case of a difference, the cause of the difference was determined through reliance on sensitivity analysis. Random-effects model was obligated to be used otherwise the data of the merged study would have been discarded and descriptive analysis may have been used in case they still remained large. A publication bias was evaluated using funnel plots and Eggers test.
Results
Literature search results
In this study, 546 articles have been gathered. The exact number of studies that was screened was 165, after the duplo records were removed. The 165 studies above were duly searched and screened according to the information on the titles and the abstracts and 112 full-text articles were matched to the eligibility. In the process of assessing these articles, 102 were omitted based on the following reasons; 67, the findings of interest were not reported, 28, no data was present, and 7 studies did not present RCTs. At last, 10 studies corresponding to the inclusion criteria were selected, and all of them were considered in the meta-analysis and also in the qualitative synthesis (Fig. 1).
Fig. 1.
PRISMA flow diagram for study selection
Baseline characteristics and quality assessment of the included studies
In this meta-analysis, ten RCTs had been reviewed. The sample size was between 40 and 148 and the total number of patients was 842 that was divided into the Experimental and Control groups, 421 respectively. The mean of the patient age in the experimental group was 38.30-59.25 and that of the experimental group patients was 36.99–58.65 and this demonstrates that there was not much difference in the age. Besides, reports in this study revealed that the adverse events were dizziness, fatigue, drowsiness, nausea, abdominal pain, dry mouth, headache, constipation, and edema. They were all mild in nature without any severe negative effects. All research involved in the current meta-analysis evaluated the quality of sleep through PSQI score. Figures 2 and 3 displayed the results of the quality assessment. The results indicated that 7 studies incorporated in this review were associated with the description of the formation of random sequences. Nevertheless, the studies mentioned nothing about so-called Allocation concealment whether there were any blind methods or not (Figs. 2 and 3)(Table 1).
Fig. 2.
Risk of bias graph
Fig. 3.

Risk of bias summary
Table 1.
Baseline characteristics of the included studies
| Author | Year | Study design | Sample size | Sex | Age | During | Measurements | Treatment time scale (week) | Adverse events | Sleep quality assessment methods | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | Experimental | Control | Experimental | Control | Experimental | Control | Experimental | Control | |||||
| Qian [12] | 2019 | RCT | 20 | 20 | 13/7 | 12/8 | 44.53 ± 4.24 | 43.47 ± 4.18 | 5.08 ± 0.48 | 4.86 ± 0.42 | Longgu Muli decoction plus 1 mg Estazolam | 1 mg Estazolam | 3 | 3 | Dizziness, fatigue, and drowsiness | PSQI score |
| Yu [13] | 2019 | RCT | 42 | 41 | 22/20 | 22/19 | 46.58 ± 4.19 | 46.85 ± 4.32 | 4.20 ± 0.38 |
4.21 ± 0.40 |
Longgu Muli decoction plus 2 mg Estazolam | 2 mg Estazolam | 12 | 12 | Nausea, abdominal pain, dry mouth, fatigue and drowsiness | PSQI score |
| Huo [14] | 2020 | RCT | 52 | 52 | 20/32 | 23/29 | 43.71 ± 2.02 | 43.68 ± 1.94 | / | / | Longgu Muli decoction plus 1 mg Estazolam | 1 mg Estazolam | 4 | 4 | Nausea, dry mouth and fatigue | PSQI score |
| Sun [15] | 2020 | RCT | 74 | 74 | 38/36 | 33/41 | 46.88 ± 6.47 | 47.21 ± 6.53 |
2.54 ± 0.36 |
2.62 ± 0.33 |
Longgu Muli decoction plus 1 mg Estazolam | 1 mg Estazolam | 6 | 6 | / | PSQI score |
| Yang [16] | 2020 | RCT | 20 | 20 | 9/11 | 8/12 | 59.25 ± 10.04 | 58.65 ± 10.44 |
6.05 ± 2.35 |
5.95 ± 2.52 |
Longgu Muli decoction plus 1 mg Estazolam | 1 mg Estazolam | 4 | 4 | Dizziness, fatigue, and drowsiness | PSQI score |
| Lin [17] | 2022 | RCT | 40 | 40 | 22/18 | 25/15 | 38.30 ± 4.89 | 36.99 ± 5.27 | / | / | Longgu Muli decoction plus 2 mg Estazolam | 2 mg Estazolam | / | / | Dizziness, headache, dry mouth and constipation | PSQI score |
| Pan [18] | 2022 | RCT | 65 | 65 | / | / | 52.50 ± 0.26 | 52.50 ± 0.28 | 1.71 ± 0.09 | 1.70 ± 0.85 | Longgu Muli decoction plus 2 mg Estazolam | 2 mg Estazolam | 6 | 6 | / | PSQI score |
| Wang [19] | 2022 | RCT | 30 | 30 | / | / | 49.60 ± 2.10 | 49.80 ± 2.20 | / | / | Longgu Muli decoction plus 1 mg Estazolam | 1 mg Estazolam | 8 | 8 | / | PSQI score |
| Sun [20] | 2023 | RCT | 40 | 40 | / | / | 40.34 ± 3.23 | 41.41 ± 2.21 | 3.42 ± 0.12 | 2.31 ± 0.03 | Longgu Muli decoction plus 2 mg Estazolam | 2 mg Estazolam | 2 | 2 | Headache, drowsiness and edema | PSQI score |
| Zhu [21] | 2023 | RCT | 38 | 39 | 11/27 | 13/26 | 49.58 ± 13.66 | 50.49 ± 11.31 | / | / | Longgu Muli decoction plus 2 mg Estazolam | 2 mg Estazolam | 6 | 6 | / | PSQI score |
Results of meta-analysis
Effective rate
They had compared the effective rate between Chaihu Longgu Muli decoction plus Estazolam and Estazolam alone using them in other treatment of insomnia in nine studies. The meta-analysis has been conducted because there was no significant heterogeneity (I2 = 0.0%, P = 0.937) and that is why the fixed-effect model was used. These results showed that the effective rate of experimental group not only was far higher in the cheep of performance time of Estazolam alone, but also far higher in the cheep of control group (RR = 1.40, 95% CI:1.30–1.50, P = 0.000) (Fig. 4).
Fig. 4.
Comparing the differences in effective rate between Chaihu Longgu Muli decoction combined with Estazolam and Estazolam alone
TCM syndrome score
Two studies complied the TCM syndrome score of Chaihu Longgu Muli decoction plus Estazolam with Estazolam in insomnia treatment. Because of no noticeable heterogeneity (I2 = 12.9%, P = 0.284) the meta-analysis was conducted in fixed-effect model. Totals indicated that the score of TCM syndrome belonging to experimental group was quite explicitly less than the score of Estazolam alone (WMD=-4.04, 95% CI:-4.38~-3.70, P = 0.000) (Fig. 5).
Fig. 5.
Comparing the differences in TCM syndrome score between Chaihu Longgu Muli decoction combined with Estazolam and Estazolam alone
PSQI score
Eight studies examined PSQI score of Chaihu Longgu Muli decoction combined with Estazolam and Estazolam alone in terms of treatment of insomnia. Because of a high heterogeneity (I2 = 94.9% and, P = 0.000), A random-effect model was used to make meta-analysis. The result shows that PSQI of experimental group was significantly lower than Estazolam monotherapies (WMD=-2.21, 95% CI:-3.05~-1.37, P = 0.000) (Fig. 6).
Fig. 6.
Comparing the differences in PSQI score between Chaihu Longgu Muli decoction combined with Estazolam and Estazolam alone
Incidence of adverse events
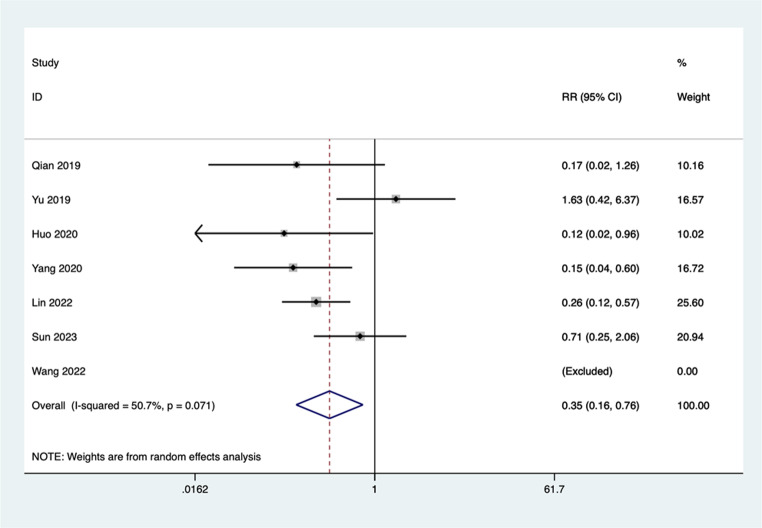
There were seven studies comparing that of Chaihu Longgu Muli decoction combined with Estazolam and Estazolam in the incidence wort off adverse event with respect to insomnia. The meta-analysis technique used in our case was random-effect model because the heterogeneity was to a large extent (I2 = 50.7%, P = 0.071). These results were, the occurrence of adverse events of experimental group was very much reduced compared to the occurrence of adverse events of the Estazolam alone (RR = 0.35, 95% CI:0.16–0.76, P = 0.000) (Fig. 7).
Fig. 7.
Comparing the differences in incidence of adverse events between Chaihu Longgu Muli decoction combined with Estazolam and Estazolam alone
Subgroup analysis
Subgroup analysis was also conducted in relation to the different types of adverse reaction so that the occurrence of the adverse events could be examined further. The Immersed findings revealed that the rates of incidences of dizziness were reported as (RR = 0.25, 95% CI:0.07–0.85, P = 0.026) and fatigue (RR = 0.22, 95% CI:0.06–0.83, P = 0.026) were significantly lower in the Chaihu Longgu Muli decoction combined with Estazolam group compared to the Estazolam alone group. But their results did not show a great variance in both groups regarding occurrence of drowsiness (RR = 0.40, 95% CI:0.13–1.23, P = 0.110), nausea (RR = 0.99, 95% CI:0.20–4.86, P = 0.988), dry mouth (RR = 0.40, 95% CI:0.13–1.23, P = 0.110), and headache (RR = 0.40, 95% CI:0.08-2.00, P = 0.265) (Fig. 8).
Fig. 8.
Subgroup analysis of differences in the incidence of specific adverse events between the Chaihu Longgu Muli decoction combined with estazolam group and the estazolam alone group
Sensitivity analysis
To get a sensitivity analysis, the individual studies were sequentially removed and their data re-analyzed using the others to determine the impact of each study. To our surprise, the issue of removal of any individual study was not of much consequence on the overall results, signifying that the meta-analysis results were stable and well-grounded.
Publication bias
The funnel plot in this experiment as shown below indicates the overall symmetrical pattern. P-value of 0.634 was found after conducting Egger test, which indicated that there is no serious publication bias in the research under consideration (Fig. 9).
Fig. 9.
Funnel plot for evaluating the publication bias of this meta-analysis
Discussion
In this meta-analysis, the effect of Chaihu plus Longgu Muli decoction and Estazolam, in the management of insomnia was assessed. These findings revealed that the combination treatment was much better than the single individual Estazolam in enhancing the quality of sleep in terms of effectiveness and reduction of the PSQI scores. Moreover, the combination therapy also resulted in lower TCM syndrome scores and a reduced incidence of adverse events.
The findings align with the hypothesis that Chaihu plus Longgu Muli decoction, a traditional Chinese herbal formula, can enhance the therapeutic outcomes of conventional pharmacological treatments. Chaihu plus Longgu Muli decoction, which includes Bupleurum root (Chaihu), Dragon bone (Longgu), and Oyster shell (Muli), is traditionally used to calm the mind and alleviate anxiety, contributing to improved sleep quality [22]. These are thought to supplement the hypnotic and anxiolytic effects of Estazolam, and make a more comprehensive treatment of insomnia.
The significance of this study lies in its demonstration of the potential benefits of integrating TCM with conventional pharmacological treatments. The use of Chaihu together with Longgu Muli decoction and Estazolam meets the demand in regard to more beneficial and safer medicinal means of insomnia, which is a common disorder triggering impairments in the quality of life and general health of a suffering person. By providing evidence that Chaihu plus Longgu Muli decoction can enhance the efficacy and safety of Estazolam, this study suggests a potentially beneficial integrative approach for clinical practice. Several mechanisms may underlie the observed benefits of the combination therapy. The components of Chaihu plus Longgu Muli decoction have been shown to possess various pharmacological properties that can enhance sleep quality. The use of Bupleurum root (Chaihu) is said to have anti-inflammatory and anxiolytic properties by regulation of the HPA axis and inhibition of pro-inflammatory cytokines [23]. Longgu and Muli, all-calcium and other trace elements, are supposed to have a sedative effect and tranquillizing properties stabilization of the neuronal excitability as well as the calcium signalling pathways [24]. Moreover, preclinical evidence showed that the interaction of these herbs may control neurotransmitters related to sleep, including increasing the level of serotonin (5-HT) and melatonin, and consequently, ensuring circadian rhythm balance and sleep homeostasis [25]. Estazolam increases the activity of GABA at the GABA-A receptor stimulating sleep by inhibitory neurotransmission. The synergy between Estazolam and the herbal decoction can be explained by the fact that these drugs have complementary effects based on the several neurochemical pathways. While Estazolam primarily targets GABAergic signaling, Chaihu plus Longgu Muli decoction may act on serotonergic and melatonergic systems [26]thereby improving sleep initiation, maintenance, and overall sleep architecture.
The lower PSQI scores in the combination therapy group suggest that Chaihu plus Longgu Muli decoction may enhance the sleep-promoting effects of Estazolam, potentially allowing for reduced dosages and improved tolerability. Moreover, the reduction in TCM syndrome scores within the combination therapy group is considerably illustrated by improving overall health and well-being in accordance with the traditional Chinese medicine standards.
Among the most interesting results of this research is the low number of adverse events seen in the combination therapy category. This suggests that Chaihu plus Longgu Muli decoction may help alleviate some of the side effects associated with estazolam during treatment. The reduction in side effects may be related to the pharmacological actions of the herbal components, although the mechanisms are not yet fully understood. The observed reduction in specific side effects, such as dizziness and fatigue, may reflect improved short-term tolerability, although broader safety benefits require further investigation.
Although the result is positive, this research is associated with several limitations. First, no trials were placebo-controlled, and most of them were inadequately blinded and did not adequately conceal the allocation, which provokes a risk of bias and influences the internal validity. Therefore, future studies should adopt rigorous randomized, double-blind, placebo-controlled designs to improve methodological quality. Secondly, all included studies assessed sleep quality using subjective tools such as the PSQI, and none employed objective methods like polysomnography, which reduces the reliability of the outcomes. Third, the investigation of PSQI score has a high heterogeneity that leads to decreasing of the objectivity of research findings. Heterogeneity can be introduced by the fact that the participants of the research can possess different initial parameters like age, gender, medical history, etc. The differences could be subjected to disparity in study findings. Meta-analysis in the future may strive to include more studies and also carry out various sub-group analysis depending on baseline properties of the patients to minimize occurrence of heterogeneity. It will help in having a better comprehension of variation between different subgroups.
Conclusion
In conclusion, the combination of Chaihu plus Longgu Muli decoction and estazolam showed potential benefits in improving subjective sleep quality and reducing adverse events compared to estazolam alone. Yet these findings must be taken with a grain of salt owing to the limitations of the studies included, including the weakness to measure outcomes objectively and the absence of placebo control. More stringent clinical trials that further prove the performance and safety of this combination therapy in treatment of insomnia are to be carried out.
Acknowledgements
Not applicable.
Author contributions
Mengfan Li wrote the manuscript and created the figures. Zhaolian Cai, Xiaoyong Zhong, Bin Chen and Haiyan Zheng participated in literature screening, Shangzhong Chen, Li Lin, Hui Liang and Zhizhen Liu participated in data extraction. Jing Luo conceived the final approval of the version to be submitted and provided the funding. All authors read and approved the final manuscript.
Funding
The Fujian Provincial Department of Science and Technology Health Joint Funding Project provided financial support in the form of grant No.2023J01833; The Fujian Provincial Health Commission Youth Research Project provided financial support in the form of grant No.2022QNA077; The Fujian Provincial Department of Education Youth Project provided financial support in the form of grant No.JAT220117.
Data availability
Data available on request from the authors.
Declarations
Ethical approval
For this type of study formal consent is not required.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
All of the authors are aware of and agree to the content of the paper and their being listed as a co-author of the paper.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bresser T, Blanken TF, de Lange SC, Leerssen J, Foster-Dingley JC, Lakbila-Kamal O et al (2024) Insomnia subtypes have differentiating deviations in brain structural connectivity. Biol Psychiatry [DOI] [PubMed]
- 2.Roepke S, Schellong J, Bergemann N, Frommberger U, Schmidt U (2024) [Pharmacological treatment of posttraumatic stress disorder]. Nervenarzt [DOI] [PubMed]
- 3.Van Dyk TR, Simmons DM, Durracio K, Becker SP, Byars KC (2024) The role of psychiatric symptoms, sociodemographic factors, and baseline sleep variables on pediatric insomnia treatment outcomes in a clinically referred population. J Clin Sleep Med [DOI] [PMC free article] [PubMed]
- 4.Huang X, Xiong Y, Jiang S, Tang L, Lin X, Fang X et al (2023) Chaihu Longgu Muli Decoction for post-stroke insomnia: A protocol for systematic review and meta-analysis. Med (Baltim) 102(15):e33376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Ju J, Li J, Fan Y, Xu H (2020) Chaihu Longgu Muli decoction, a Chinese herbal formula, for the treatment of insomnia: A systematic review and meta-analysis. Med (Baltim) 99(40):e22462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du H, Lin R, Xiao S, Zhao Y, Wu M, Chen W et al (2023) Improved sleep affects epigastric pain in functional dyspepsia by reducing the levels of inflammatory mediators. Dig Dis 41(6):835–844 [DOI] [PubMed] [Google Scholar]
- 7.Zheng Q, Li S, Wen F, Lin Z, Feng K, Sun Y et al (2022) The association between sleep disorders and incidence of dry eye disease in ningbo: data from an integrated health care network. Front Med (Lausanne) 9:832851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian ZX, Xu H, Lu AP, Lee MS, Cheung H (2014) Insights of Chinese medicine syndrome study: from current status to future prospects. Chin J Integr Med 20(5):326–331 [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213 [DOI] [PubMed]
- 10.Sarkis-Onofre R, Catala-Lopez F, Aromataris E, Lockwood C (2021) How to properly use the PRISMA statement. Syst Rev 10(1):117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazlik MO, Mustak IB, Ozkan H, Vural SA, Kaya U, Ozoner O et al (2024) The presence of virulence factor genes downregulates uterine AQP3 and alters glutathione peroxidase activity and uterine histopathology in canine pyometra. Reprod Domest Anim 59(5):e14615 [DOI] [PubMed] [Google Scholar]
- 12.Qian HY, Xia J, Zhang JM, Chen ZM (2019) Clinical efficacy of modified Chaihu Longgu Muli Decoction combined with Western medicine in the treatment of insomnia. Clin Med Res Pract 4(30):152–153 [Google Scholar]
- 13.Yu LH (2019) Efficacy analysis of syndrome-based modified Chaihu Longgu Muli Decoction in treating refractory insomnia (syndrome of phlegm-heat disturbing the interior). Chin Med Recuper Med 28(6):670–672 [Google Scholar]
- 14.Huo SY (2020) Clinical observation of modified Chaihu Longgu Muli Decoction in treating insomnia of liver depression transforming into fire type. Electron J Clin Med Lit 7(A2):149 [Google Scholar]
- 15.Sun XT (2020) Therapeutic effect of Chaihu Longgu Muli Decoction on insomnia in patients with Qi stagnation constitution and its influence on anxiety and depression. J Liaoning Univ Tradit Chin Med 22(1):89–92 [Google Scholar]
- 16.Yang SQ, Gao F, Wang MH (2020) Clinical observation of modified Chaihu Longgu Muli Decoction combined with Western medicine in the treatment of insomnia with phlegm-heat disturbing the interior. World Sleep Med J 7(7):1159–1161 [Google Scholar]
- 17.Lin LC, Lin JM, Yao BN (2022) Clinical efficacy analysis of modified Chaihu Longgu Muli Decoction in treating insomnia. Online Doctor 12(5):35–37 [Google Scholar]
- 18.Pan X, Sun T, Yang JD, Liu W (2022) Observation on the effect of modified Chaihu Longgu Muli Decoction in treating liver Qi stagnation type perimenopausal insomnia. Mod Med Health Res (Electronic Edition) 6(4):95–98 [Google Scholar]
- 19.Wang JF, Wang DH (2022) Effect of modified Chaihu Longgu Muli Decoction on sleep quality, negative emotions, and endocrine hormones in patients with perimenopausal insomnia. Mod J Integr Tradit Chin West Med 31(13):1842–1845 [Google Scholar]
- 20.Sun T (2023) Clinical observation on Chaihu Longgu Muli Decoction in the treatment of insomnia due to liver depression transforming into fire. Mod Distance Educ Chin Tradit Med 21(18):89–91 [Google Scholar]
- 21.Zhu KH, Wei J, Gu YY, Peng JW, Zhu CL (2023) Analysis of the effect of Chaihu Longgu Muli Decoction on sleep quality and clinical efficacy in patients with chronic insomnia of Qi stagnation constitution. World Sleep Med J 10(8):1768–1770 [Google Scholar]
- 22.Cao XL, Peng XM, Li GB, Ding WS, Wang KZ, Wang XL et al (2023) Chaihu-Longgu-Muli Decoction improves sleep disorders by restoring orexin-A function in CKD mice. Front Endocrinol (Lausanne) 14:1206353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li DQ, Wu J, Liu LY, Wu YY, Li LZ, Huang XX et al (2015) Cytotoxic triterpenoid glycosides (saikosaponins) from the roots of Bupleurum Chinense. Bioorg Med Chem Lett 25(18):3887–3892 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Li P, Hu J, Yang Y, Yan Q, Liu H et al (2023) Guizhi Longgu Muli Decoction ameliorates pathological changes in heart and bone in ovariectomized rats. Pak J Pharm Sci 36(6Special):1891–1899 [PubMed] [Google Scholar]
- 25.Liu WL, Wu BF, Shang JH, Wang XF, Zhao YL, Huang AX (2022) Moringa oleifera seed ethanol extract and its active component Kaempferol potentiate pentobarbital-induced sleeping behaviours in mice via a GABAergic mechanism. Pharm Biol 60(1):810–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CT, Lin HY, Peng WH, Wu LY (2024) Multi-Target mechanisms of Si-Ni-San on anxious insomnia: an example of Network-pharmacology and molecular Docking analysis. Curr Med Chem [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.