Abstract
Background
Early studies with desloratadine demonstrated efficacy in treating seasonal allergic rhinitis (SAR). A dose-ranging study was conducted to characterize its 24-hour efficacy in patients with SAR.
Methods
Patients (N = 1,026) were randomly assigned once-daily (QD) desloratadine (2.5, 5, 7.5, 10, or 20 mg) for 2 weeks in a placebo-controlled, double-blind study. The end point of 24-hour efficacy was assessed by the mean change from baseline in the average AM instantaneous total symptom score (TSS) over the treatment period. Day 2 data were assessed for efficacy of desloratadine following the first dose. Other efficacy variables included AM/PM previous total nasal and nonnasal symptom scores and individual symptom scores.
Results
Desloratadine 5–20 mg was significantly (P < .01) more effective than placebo in improving total AM instantaneous TSS and AM/PM previous total nasal and nonnasal symptom scores. This dosing range also was significantly (P < .01) more effective than placebo for reducing AM instantaneous TSS beginning with the first dose; thus, demonstrating the full 24-hour efficacy of desloratadine. AM/PM previous scores for all individual symptoms, including nasal congestion, were also significantly improved versus placebo (P < .05) with desloratadine at 5, 7.5, and 20 mg. All treatments were well tolerated. There were no clinically meaningful changes in electrocardiogram parameters.
Conclusion
Desloratadine 5–20 mg provided significant 24-hour relief of SAR signs and symptoms. There were no statistically significant differences between the 4 largest doses suggesting that desloratadine 5 mg QD offers the best therapeutic profile for patients with SAR.
Background
Second-generation oral antihistamines are among the most widely prescribed agents in the United States and Europe due to their effectiveness in the treatment of allergic diseases. Although newer long-acting antihistamine preparations permit once-daily dosing, many patients with seasonal allergic rhinitis (SAR) experience breakthrough symptoms and a diminution of clinical potency at the end of the dosing interval. Most antihistamines demonstrate a peak effect approximately 5 to 7 hours after oral administration, and the duration varies depending on the elimination half-life [1].
Desloratadine, a new nonsedating antihistamine, was recently approved by the Food and Drug Administration. This agent has a very high affinity for histamine1 (H1)-receptors and a very low affinity for H2 and muscarinic receptors [2]. Unlike other antihistamines, desloratadine does not have clinically significant pharmacokinetic or pharmacodynamic interactions with drugs that inhibit the cytochrome P-450 system or the p-glycoprotein transport system [3]. Desloratadine has a long half-life of approximately 27 hours [4,5]. Earlier reports suggested that desloratadine has 24-hour efficacy in patients with SAR [6,7] and in patients with chronic idiopathic urticaria [8]. This hypothesis was further explored by analysis of data from a large placebo-controlled dose-ranging study in subjects with SAR that characterized the efficacy of five once-daily desloratadine regimens (2.5 to 20 mg/d) at the end of the 24-hour dosing interval.
Methods
Participants
Subjects 12 years or older with a 2-year documented history of SAR and otherwise in good health were eligible for enrollment. All subjects had a positive skin test response to an appropriate tree and/or grass seasonal allergen within the 12 months before study initiation. Subjects were determined to be in good health based on medical history, physical examination, electrocardiogram (ECG), and routine laboratory tests (blood chemistry, complete blood count, and urinalysis). Women of childbearing potential agreed to use a medically accepted method of contraception during this study. All subjects, as well as a parent or guardian when appropriate, provided written informed consent.
At the screening and baseline visits, the signs and symptoms of allergic rhinitis were scored jointly by the investigator and subject (with assistance from the parent/guardian, if required). Evaluated nasal symptoms included rhinorrhea, nasal stuffiness/congestion, nasal itching, and sneezing. Evaluated nonnasal signs and symptoms included itching/burning eyes, tearing/watering eyes, redness of eyes, and itching of ears or palate. All symptoms were graded on a 4-point scale using the following system: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. For inclusion on the study, subjects had to have a nasal rhinorrhea score of at least 2, a total nasal symptom score of at least 6, and a total nonnasal score of at least 5.
Subjects who met the following criteria were excluded from participation: (1) chronic use of inhaled or systemic corticosteroids for treatment of asthma; (2) current or past history of clinically significant sinusitis or chronic purulent postnasal drip; (3) rhinitis medicamentosa; (4) a history of allergies to more than 2 classes of medications; (5) hypersensitivity to or intolerance of antihistamines; (6) an upper respiratory tract or sinus infection that required antibiotic treatment within the 14 days before study screening; (7) a viral upper respiratory infection within 7 days before study screening; (8) nasal structural abnormalities that significantly interfered with nasal air flow; (9) dependence on nasal, oral, or ocular decongestants, nasal topical antihistamines, or nasal corticosteroids; (10) use of immunotherapy unless on a regular maintenance schedule before screening and maintained on a schedule for the remainder of the study; (11) hypersensitivity to the study drug or its excipients; and (12) current pregnancy or lactation.
Study Design
This was a randomized, multicenter, parallel-group, placebo-controlled, double-blind study. The study was conducted at 29 medical centers across the United States. Following an approximately 1-week screening period, eligible subjects were randomly assigned in a 1:1:1:1:1:1 ratio to 2 weeks of treatment with desloratadine, 2.5, 5, 7.5, 10, or 20 mg, or placebo. Randomization was performed in blocks of 6 using a computer-generated randomization schedule. Each dose of study drug was administered orally in the morning, at approximately the same time each day. There were 5 scheduled study visits; a screening visit, a baseline visit on treatment day 1, and follow-up visits on days 4, 8, and 15. A central institutional review board and/or a local ethical committee approved the study before initiation.
To ensure blinding, the desloratadine and placebo tablets were identical in appearance and were packaged identically. Each daily dose consisted of 2 tablets. Desloratadine and placebo tablets were supplied by Schering Corporation, Kenilworth, NJ. Compliance with study medication was assessed at each study visit by questioning the subject, reviewing the diary cards, and examining the subject's drug supply.
Use of the following medications was prohibited during the study: nasal cromolyn sodium, nedocromil, saline, atropine, ipratropium bromide, azelastine, and corticosteroids; oral corticosteroids, antihistamines, and decongestants; ocular decongestants, corticosteroids, and saline; topical anti-inflammatory agents; and inhaled, intravenous, rectal, intramuscular, or intra-articular corticosteroids, as well as high-potency dermatologic corticosteroids. Use of systemic antibiotics was also prohibited, unless the patient was on a stable dose. There was a prespecified washout period for each of the prohibited medications, ranging from 12 hours for ocular and nasal saline to 3 months for intramuscular or intra-articular corticosteroids. Patients were able to take any medication that was not restricted by the protocol and that would not be expected to interfere with the study. Acetaminophen was permitted to be taken as needed for appropriate indications.
Clinical outcome assessment
Beginning at least 3 days before the baseline visit and throughout the remainder of the study, subjects recorded the severity of their nasal and nonnasal symptoms twice daily based upon how they felt the previous 12 hours (reflective) and also at the time of assessment (instantaneous). The nasal (rhinorrhea, nasal stuffiness/congestion, nasal itching, and sneezing) and nonnasal (itching/burning eyes, tearing/watering eyes, redness of eyes, and itching of ears or palate) symptoms were individually graded using a 4-point scale (0 = none to 3 = severe). The total symptom score was the sum of the scores for the 4 nasal and 4 nonnasal symptoms.
To characterize the efficacy of desloratadine at the end of the dosing interval, 24-hour efficacy of the study medications was evaluated using the AM instantaneous data set (before the next morning dose). The primary outcome of this analysis was the 2-week average AM instantaneous total symptom score expressed as a mean change from baseline. Secondary outcomes included the mean AM/PM previous total nasal and total nonnasal scores and individual symptom scores. In addition, day 2 AM instantaneous total symptom scores were analyzed to assess the effectiveness of desloratadine 24 hours after the first dose.
Diary cards also were used to record use of study medication, use of concomitant medication, adverse events, and the daily number of hours the subject was exposed to outside air. The diary cards were collected and reviewed at each visit.
Statistical analysis
All randomized subjects who received at least 1 dose of study medication and had baseline and at least 1 postbaseline day of diary data were included in the primary intent-to-treat efficacy analyses. The safety analyses included all randomized subjects. Before combining data across centers, center-specific results were examined and the significance of the treatment-by-center interaction for the primary efficacy variable was examined. Data were analyzed using a two-way analysis of variance (ANOVA) that extracted sources of variation due to treatment and center. The primary comparison was based on the pairwise differences in least-squares means between desloratadine, 10 mg, and placebo (from the ANOVA) using a 5% significance level. If the desloratadine 10-mg treatment was significantly different from placebo, then all other doses of desloratadine were compared with placebo at the 5% (two-sided) level of significance without adjustment for the multiple comparisons. In addition, a test of trend for nondecreasing response with increasing desloratadine dose (0, 2.5, 5, 7.5, 10, and 20 mg) was evaluated using the same two-way ANOVA.
The study was designed to enroll 150 subjects per treatment group. The sample size was chosen to detect (with 90% power and a 5% significance level) a difference between treatment groups of 1.6 units or more in the mean change from baseline diary total symptom score, assuming a pooled SD of 4.25.
Safety assessment
Laboratory tests (complete blood count, blood chemistry, urinalysis) were performed and a 12-lead ECG was obtained at screening and at the conclusion of the study. Vital signs and adverse events were evaluated at each study visit.
Results
Patient disposition and demographics
Between April 1998 and June 1998, a total of 1,036 subjects was randomized to treatment; 10 subjects lacked baseline or postbaseline data and were excluded from the intent-to-treat analyses. For the 2.5-mg, 5-mg, 7.5-mg, 10-mg, 20-mg, and placebo groups, 2, 1, 1, 2, 3, and 1, patients were excluded, respectively. Fifty-eight subjects discontinued the study. There was no apparent differential incidence of discontinuation rates among the treatment groups. Treatment failure led to discontinuation among 3% of the placebo and desloratadine 2.5-mg groups and 1% to 2% of the remaining active treatment groups. Discontinuations due to adverse events were similar across all study groups, including placebo, and are discussed further next. The demographic and baseline disease characteristics of the treatment groups were similar (Table 1).
Table 1.
Summary of Demographic Data and Disease Characteristics at Baseline (all randomized subjects)
| Desloratadine | ||||||
| Characteristic |
2.5 mg (n = 173) |
5 mg (n = 172) |
7.5 mg (n = 173) |
10 mg (n = 172) |
20 mg (n = 172) |
Placebo (n = 174) |
| Mean (range) age, years | 34 (13–73) | 34 (12–75) | 34 (12–73) | 34 (12–70) | 34 (12–72) | 35 (12–63) |
| Age group, years | ||||||
| 12–17 | 22 | 26 | 25 | 28 | 25 | 22 |
| 18–65 | 150 | 143 | 145 | 143 | 145 | 152 |
| >65 | 1 | 3 | 3 | 1 | 2 | 0 |
| Sex | ||||||
| Male | 73 | 68 | 68 | 71 | 62 | 81 |
| Female | 100 | 104 | 105 | 101 | 110 | 93 |
| Mean duration (range) of seasonal allergic rhinitis, years | 18 (2–60) | 18 (2–55) | 16 (2–50) | 17 (2–49) | 16 (2–48) | 17 (2–50) |
Efficacy analyses
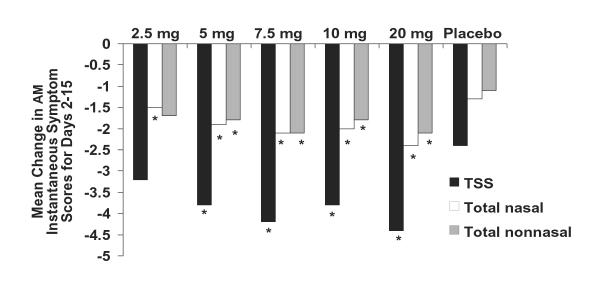
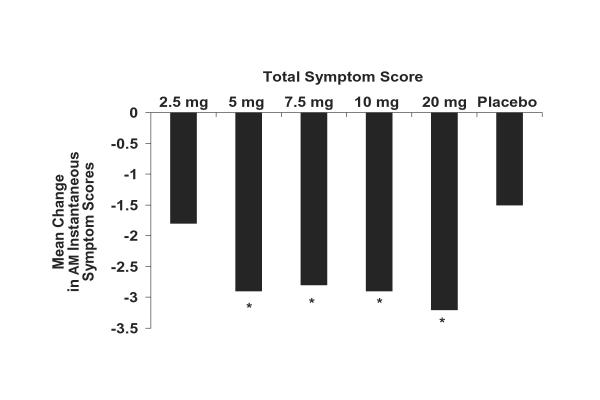
The changes from baseline in subject-evaluated AM instantaneous total symptom scores averaged over the 2-week treatment period for once-daily desloratadine doses of 5, 7.5, 10, and 20 mg were all superior to placebo (P < .01; Figure 1); the 2.5-mg dose was numerically superior to placebo (P = not significant). Efficacy was maintained 24 hours after administration of the first dose (day 2 assessment) when once-daily desloratadine doses of 5, 7.5, 10, and 20 mg significantly reduced AM instantaneous total symptom scores compared with placebo (P < .01; Figure 2). No statistically significant differences were found when comparing desloratadine 5-, 7.5-, 10-, and 20-mg doses with each other.
Figure 1.
Mean change in AM instantaneous symptom scores for desloratadine 2.5-, 5-, 7.5-, 10-, and 20-mg tablets compared with placebo for days 2–15. Mean changes from baseline are least-square means based on ANOVA model with treatment and center effects. TSS = total symptom score is nasal + nonnasal scores. (*P < .01 versus placebo.)
Figure 2.
Mean change in AM instantaneous total symptom scores for desloratadine 2.5-, 5-, 7.5-, 10-, and 20-mg tablets compared with placebo after the first dose (day 2). Mean changes from baseline are least-square means based on ANOVA model with treatment and center effects. Total symptom score is nasal + nonnasal scores. (*P < .01 versus placebo.)
Upon examination of the mean change from baseline in subject-evaluated AM/PM previous total nasal and nonnasal symptom scores averaged over the 2-week treatment period, desloratadine doses of 5, 7.5, 10, and 20 mg were all superior to placebo (P < .01). During this interval, mean reductions in AM/PM previous total nasal symptom scores for the desloratadine groups were 1.6 for 2.5 mg, 2.2 for 5 mg, 2.3 for 7.5 mg, 2.0 for 10 mg, and 2.5 for 20 mg; the placebo group had a 1.4 reduction. The 5-, 7.5-, and 20-mg doses of desloratadine were all significantly (P < .01) more effective than the placebo in reducing subject-evaluated total nasal symptom scores. Mean reductions in AM/PM previous total nonnasal symptoms averaged over the 2-week treatment period were 1.6 with desloratadine 2.5 mg, 2.1 with desloratadine 5 and 7.5 mg, 1.9 with desloratadine 10 mg, 2.3 with desloratadine 20 mg, and 1.2 with placebo.
Over the 2-week study period, mean AM/PM previous scores for all 8 individual symptoms, including nasal congestion/stuffiness, showed statistically significant improvements versus placebo (P < .05 to P ≤ .01) with desloratadine doses of 5, 7.5, and 20 mg. The 10-mg dose of desloratadine showed numerical improvement versus placebo in all 8 individual symptom scores and significantly reduced (P < .05 versus placebo) nasal itching and sneezing, itching/burning eyes, tearing/watering eyes, and itching of ears or palate.
Safety evaluation
All treatments were well tolerated; there were no unusual or unexpected adverse events. Treatment-related adverse events were reported by approximately 15% to 22% of subjects in the desloratadine group compared with 14% for the placebo group. The overall incidence of adverse events was similar among all 6 treatment groups (including placebo); there were no apparent dose-related trends in the adverse events. The majority of adverse events were mild or moderate in severity. Headache, somnolence, and fatigue were the most frequently reported adverse events. The incidence of headache in the 2.5-mg, 5-mg, 7.5-mg, 10-mg, and 20-mg desloratadine groups was 9%, 6%, 7%, 7%, and 8%, respectively; the incidence with placebo was 5%; somnolence was 3%, 2%, 4%, 3%, and 6%, respectively for desloratadine and 2% with placebo. Treatment-related fatigue was reported in 2%, 2%, 3%, 4%, and 4%, respectively for the desloratadine group and <1% for the placebo group.
Twenty subjects did not complete the study because of adverse events. There was no apparent pattern of occurrence with respect to treatment group, and no particular risk was associated with active treatment at any dosage. Approximately half the adverse events resulting in study discontinuation were due to concurrent illness and were considered unrelated to study treatment by the investigator. The other discontinuations were due to a wide variety of adverse events that were considered at least possibly related to treatment by the investigator; none of these events was reported by more than 3 subjects who discontinued treatment. There were no clinically relevant changes in median laboratory test values or vital signs and no clinically significant changes in mean ECG parameters, including QTc intervals, with any dose of desloratadine.
Discussion
This study demonstrates that once-daily desloratadine therapy at the approved 5-mg dose is significantly more effective than placebo in relieving the symptoms of SAR and retains its effectiveness to the end of the once-daily dosing interval. Moreover, full 24-hour efficacy was apparent after the first dose. Desloratadine doses of 5, 7.5, 10, and 20 mg were similarly effective, significantly reducing the nasal and nonnasal symptoms of SAR for a full 24 hours.
Previous studies have attempted to evaluate the efficacy of antihistamines at the end of the dosing interval by assessing nasal airway resistance following nasal challenge with histamine,[9] the wheal-and-flare response following epicutaneous administration of histamine,[10,11] and subjective and objective responses in patients exposed to pollen in the Vienna Challenge Chamber.[12] Although these studies have demonstrated some effects at the end of the dosing interval, the clinical relevance of the employed methods has not been established. By contrast, this is the first dose-ranging study that demonstrates the efficacy of an antihistamine at the end of the dosing interval. The parameters used to assess efficacy at the end of the dosing interval in this study provide clinically meaningful information that supports the 24-hour control of desloratadine. The overall incidence of adverse events reported with desloratadine during this study was comparable to that with placebo. Use of desloratadine was not associated with any clinically meaningful changes in ECG results, including the critical QTc interval.
Conclusions
In conclusion, desloratadine provided 24-hour relief of the signs and symptoms of SAR, with no statistically significant differences between the 5-, 7.5-, 10-, and 20-mg doses. All desloratadine doses were well tolerated. Because there were no significant differences in efficacy or safety, a 5-mg once-daily dose of desloratadine appears to be optimal for the treatment of SAR, providing full 24-hour efficacy beginning with the first dose and maintained over the 2-week study period.
Abbreviations
SAR = seasonal allergic rhinitis; H1 = histamine1; ECG = electrocardiogram; ANOVA = analysis of variance; SD = standard deviation.
Competing interests
Luis Salmun, MD, and Richard Lorber, MD, are employees of Schering-Plough Research Institute.
Authors' Contributions
LS drafted the manuscript. RL participated in the design of the study. Both authors reviewed and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgments
Supported by an educational grant from Schering-Plough Research Institute.
Contributor Information
Luis M Salmun, Email: luis.salmun@spcorp.com.
Richard Lorber, Email: richard.lorber@spcorp.com.
References
- Brunton SA. Allergy management strategies: An update. Patient Care. 2002;Spring:16–25. [Google Scholar]
- Kreutner W, Hey JA, Anthes J, Barnett A, Young S, Tozzi S. Preclinical pharmacology of desloratadine, a selective and nonsedating histamine H1 receptor antagonist. 1st communication: receptor selectivity, antihistaminic activity, and antiallergenic effects. Arzneimittelforschung. 2000;50:345–352. doi: 10.1055/s-0031-1300213. [DOI] [PubMed] [Google Scholar]
- McClellan K, Jarvis B. Desloratadine. Drugs. 2001;61:789–796. doi: 10.2165/00003495-200161060-00007. [DOI] [PubMed] [Google Scholar]
- Gupta S, Banfield C, Affrime M, Marbury T, Padhi D, Glue P. Oral bioavailability of desloratadine is unaffected by food. Clin Pharmacokinet. 2002;41:7–12. doi: 10.2165/00003088-200241001-00002. [DOI] [PubMed] [Google Scholar]
- Gupta S, Banfield C, Affrime M, Marco A, Cayen M, Herron J, Padhi D. Desloratadine demonstrates dose proportionality in healthy adults after single doses. Clin Pharmacokinet. 2002;41:1–6. doi: 10.2165/00003088-200241001-00001. [DOI] [PubMed] [Google Scholar]
- Meltzer E, Prenner B, Nayak A, Desloratadine Study Group Efficacy and tolerability of once-daily 5 mg desloratadine, an H1-receptor antagonist, in patients with seasonal allergic rhinitis: Assessment during the spring and fall allergy seasons. Clin Drug Invest. 2001;21:25–32. [Google Scholar]
- Geha RS, Meltzer EO. Desloratadine: a new, nonsedating, oral antihistamine. J Allergy Clin Immunol. 2001;107:751–762. doi: 10.1067/mai.2001.114239. [DOI] [PubMed] [Google Scholar]
- Ring J, Hein R, Gauger A, Bronsky E, Miller B, The Desloratadine Study Group Once-daily desloratadine improves the signs and symptoms of chronic idiopathic urticaria: a randomized, double-blind, placebo-controlled study. Int J Dermatol. 2001;40:1–5. doi: 10.1046/j.1365-4362.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- Frossard N, Benabdesselam O, Melac M, Glasser N, Lacronique J, Pauli G. Nasal effect of cetirizine and loratadine at 24 hours in patients with allergic rhinitis. Am J Ther. 1998;5:307–311. doi: 10.1097/00045391-199809000-00006. [DOI] [PubMed] [Google Scholar]
- Grant JA, Riethuisen JM, Moulaert B, DeVos C. A double-blind, randomized, single-dose, crossover comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjects. Ann Allergy Asthma Immunol. 2002;88:190–197. doi: 10.1016/S1081-1206(10)61995-3. [DOI] [PubMed] [Google Scholar]
- Purohit A, Duvernelle C, Melac M, Pauli G, Frossard N. Twenty-four hours of activity of cetirizine and fexofenadine in the skin. Ann Allergy Asthma Immunol. 2001;86:387–392. doi: 10.1016/S1081-1206(10)62483-0. [DOI] [PubMed] [Google Scholar]
- Horak F, Stubner P, Zieglmayer R, Kavina A, De Vos C, Burtin B, Donnelly F. Controlled comparison of the efficacy and safety of cetirizine 10 mg o.d. and fexofenadine 120 mg o.d. in reducing symptoms of seasonal allergic rhinitis. Int Arch Allergy Immunol. 2001;125:73–79. doi: 10.1159/000053799. [DOI] [PubMed] [Google Scholar]