Abstract
Background
In recent years it has become evident that nonsteroidal anti-inflammatory drugs, in particular aspirin represent a potential class of cancer chemotherapeutic agents. Despite the wealth of knowledge gained from epidemiological, clinical and animal studies, the effectiveness of aspirin to treat established gastrointestinal cancer has not been determined. The present study examines the ability of aspirin to treat established polyposis in Min/+ mice.
Methods
Min/+ mice with established polyposis were treated orally once daily from 12–16 weeks of age with either drug vehicle or aspirin (25 mg/kg). Upon completion of treatment, the number, location and size of intestinal tumours was determined. Additional variables examined were the number of apoptotic cells within tumours and COX activity.
Results
Administration of aspirin for 4 weeks to Min/+ mice produce no effect on tumour number compared to vehicle-treated Min/+ mice (65 ± 8 vs. 63 ± 9, respectively). In addition, aspirin had no effect on tumour size or location. However, aspirin treatment produced a greater than 2-fold (p < 0.05) increase in the number of apoptotic positive cells within tumours and significantly decreased hepatic PGE2 content.
Conclusions
Aspirin was found to have no effect on tumour number and size when administered to Min/+ mice with established polyposis. The findings in the present study call in to question the utility of aspirin as a stand-alone treatment for established GI cancer. However, aspirin's ability to significantly promote apoptosis may render it suitable for use in combinatorial chemotherapy.
Background
Despite continuing decreases in incidence and mortality rates, cancers of the colon and rectum remain the third leading cause of cancer deaths in the North America [1,2]. The decline in incidence, and hence mortality, from colorectal cancers is most likely attributable to an increase in recommendations to perform routine screening on average risk individuals and to improved screening techniques [2]. In addition, there is ever advancing knowledge into the pathogenic mechanism of cancer and resulting strides in the development of more efficacious therapies.
In recent years it has become evident that nonsteroidal anti-inflammatory drugs (NSAIDs) represent a potential class of cancer chemotherapeutic agents. The utility of NSAIDs, in particular aspirin, in the treatment of colon cancer has stemmed from studies conducted both in animals [3-11] and humans [12-15]. Evidence from human studies has largely come from epidemiological data indicating that aspirin and other NSAIDs can reduce the relative risk of developing colorectal cancer by approximately 40–50% [13-16]. The utility of NSAIDs in the cancer therapeutics is not limited to prevention strategies. The NSAID sulindac has been shown to cause regression of colorectal adenomas of patients with hereditary forms of polyposis (Familial Adenomatous Polyposis (FAP) and Gardner's Syndrome) [17-19] and in sporadic colorectal adenomas [20]. In addition, case reports have demonstrated that indomethacin and sulindac were effective co-therapy agents in the treatment of desmoid tumours [21,22]. Finally, the inclusion either of indomethacin, naproxen or sulindac in the chemotherapy regiment of gastric carcinoma patients resulted in a significant increase in survival; 30 months versus 6 months when NSAIDs included in treatment regime [22].
The anti-neoplastic properties of NSAIDs can be duplicated in animal models (mainly rodent) of colon cancer. The two most commonly used are the multiple intestinal neoplasia (Min/+) mouse and the chemically (azoxymethane)-induced rat models. In both models, NSAID administration has been reported to significantly attenuate tumour number and size when provided either as a preventive [3-11] or treatment [3,6,11] agent. The Min/+ model has received great favour because it contains a mutation in the Apc gene, which is homologous to the human adenomatous polyposis coli (APC) gene [23]. Defects in the APC tumour suppressor gene are thought to play a role in greater than 70% of all colorectal cancers and is responsible for inherited syndromes of polyposis (FAP and Gardner's) [24,25]. Furthermore, in humans the majority of colorectal cancers arise from adenomatous polyps [26].
Polyposis in the Min/+ mouse differs slightly from the human condition of FAP. Polyps (multiple) are generally localized to the small intestine in Min/+ mice, opposed to the colons of FAP suffers [27]. In Min/+ mice adenoma formation is thought to occur within the first few weeks of life, with a full compliment of adenomas being attained by approximately 9 weeks of age (60–67 days of age) [6,28]. Following this time, adenoma multiplicity is thought not to change, however, adenoma size may still increase [6,28]. Min/+ mice are also normally found to develop anaemia by 60 days of age and possess a shortened life span of roughly 120 days (17 weeks) [29].
Aspirin most likely represents the best known and frequently purchased NSAID [26]. It is also the NSAID normally cited in epidemiological studies assessing the chemopreventive efficacy of NSAIDs [16], but yet the utility of aspirin in the treatment of established colorectal cancer has not been determined. We therefore wanted to determine if aspirin is able to reduce the number and size of adenomas in Min/+ mice with established polyposis.
Materials and methods
Animals
Min/+ (C57BL/6J-ApcMin) and wild type (C57BL/6J) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in a room with a 12 hour light-dark cycle and had free access to food (LabDiet,® Prolab® RMH 3500, Purina Mills, Inc., St. Louis, MO) and water during the entire protocol. All procedures were approved by the Albany Medical College Institutional Animal Care and Use Committee (IACUC).
Treatment protocol
Min/+ mice were treated for a period of 28 days either with vehicle or aspirin (25 mg/kg/day). Dose of aspirin correlates to previous work conducted by Mahmoud et al. [9], which demonstrated that 0.5 mg of aspirin per day was chemopreventive in Min/+ mice. Aspirin was initially dissolved in dimethyl sulfoxide (DMSO; 5% by final volume) and then diluted to the desired concentration (5 mg/mL) with 0.5% carboxymethylcellulose (CMC). Both aspirin and drug vehicle were orally administered via a 22-gauge feeding needle (Kent Scientific Co., Litchfield, CT) attached to a 1 cc syringe. Compounds were delivered at a rate of 5 mL/kg body weight. A diluted concentration was used to aid in drug solubility and accuracy of drug administration.
Treatment began at 12 weeks (84 days) of age and concluded at 16 weeks (112 days) of age. Following completion of the treatment period, mice were euthanized with sodium pentobarbital (Nembutal®; 50 mg/kg i.m.) and the entire gastrointestinal (GI) tract was removed. The GI tract was segregated into stomach, small intestine and colon, and placed into 10% phosphate buffered formalin for fixation. A portion of liver tissue was also obtained to determine cyclooxygenase (COX) activity.
Assessment of polyp number and size
Following a 24 hour fixation period, the stomach and intestines were opened longitudinally and rinsed with phosphate buffered saline (PBS, pH 7.4). Tissues were then pinned flat on wax blocks and covered with trypan blue (0.4% solution) for 10 minutes and rinsed with PBS to improve polyp contrast. Polyp numbers were determined by manual count via use of a dissecting microscope (Bausch & Lomb, Rochester, NY) by an observer unaware of the treatment the mice had received. The entire GI tract for each animal was then photographed using a video camera (JVC, Wayne, NJ) outfitted with a Navitar Zoom 7000 macro lens (Technical Instruments, San Francisco, CA). Images were captured and analysed by use of computerised software (Scion Image, Scion Corporation, Frederick, MD). Individual polyp diameters were calculated from their measured areas.
Immunohistochemical detection of apoptosis
Sections of formalin-fixed small intestinal tissues, approximately 15 cm in length, were rolled upon themselves with the mucosal surface facing outwards. Tissue sections were then processed and embedded in paraffin using routine histological techniques. Small intestinal tissue was sectioned at 4 μm and immobilised on slides.
Apoptotic cells were labelled in situ by use of a commercially available detection kit (TACS 2 TdT DAB kit, Trevigen, Inc., Gaithersburg, MD). Samples were processed according to the manufacturer's instructions. Briefly, paraffin wax was removed from slides by heating at 57°C for 20 minutes, followed by two subsequent washes in 100% Xylene. Slides were then rehydrated by sequential washes in ethanol (100%, 95% and 70%, 1 × 5 min.), deionized water (2 × 2 min.) and PBS (1 × 10 min.). Following rehydration, tissue was proteolytically treated (Proteinase K, 20 μg/mL) for 15 minutes. Endogenous peroxidase activity was quenched by immersing slides in a 2% hydrogen peroxide (H2O2) solution. Excess peroxide solution was removed by tapping and slides were then immediately submersed in labelling buffer (50 mM Tris, pH 7.5; 5 mM MgCl2; 0.06 mM 2-Mercaptoethanesulfonic Acid; 0.05 mg/mL BSA) for 2 minutes. Slides were removed from labelling buffer and excess buffer surrounding the tissue was wiped away. Samples were covered with labelling reaction mixture (50 mM Tris, pH 7.5; 5 mM MgCl2; 0.06 mM 2-Mercaptoethanesulfonic Acid; 0.05 mg/mL BSA; 1 mM CoCl2; 0.008 mM TdT dNTP mix; 300 U/mL TdT) and incubated for 60 minutes in a humidified chamber (37°C). The labelling reaction was terminated by transferring the slides to 50 mL of stop buffer (10 mM EDTA, pH 8.0) for 5 minutes. Excess stop buffer was removed by washing the slide once in 50 mL of PBS for 2 minutes.
Detection of labelled cells was carried out via the conversion of diaminobenzidine (DAB) by streptavidin-horseradish peroxidase (strep-HRP). Labelled samples were covered with 100 μL strep-HRP solution and incubated for 10 minutes. Excess strep-HRP was removed by tapping prior to washing the slide twice in 50 mL of PBS. Slides were placed into 50 mL of DAB solution (0.5 mg/mL DAB and 0.03% H2O2 in PBS) for 5 to 10 minutes. Slides were briefly rinsed twice with deionized water, prior to counterstaining with methyl green.
Apoptotic cells were counted manually by an observer unaware of the treatment regime and were normalised based on polyp area. Polyp area was determined from captured images (Nikon, Labophot-2 microscope fitted with a JVC video camera) using the same computerised software stated above.
Assessment of cyclooxygenase activity
Cyclooxygenase activity was assessed ex vivo in hepatic tissue following a previously described method [30]. Briefly, mice were euthanized 3 hours following the final dose of vehicle or aspirin and a sample of liver tissue (~100 mg) was obtained. The samples were then placed into microcentrifuge tubes containing 1 mL of sodium phosphate buffer (10 mmol/L, pH 7.4) and finely minced with scissors for 15 seconds. Samples were then incubated for 20 minutes at 37°C in a shaking water bath. Following the incubation period, samples were centrifuged at 9 000 × g for 30 seconds and the supernatants collected. Supernatants were flash frozen in liquid nitrogen and stored at -80°C for subsequent determination of prostaglandin E2 (PGE2) content. PGE2 concentrations were determined using a commercially available ELISA assay (Cayman Chemical Company, Ann Arbor, MI).
Materials
Aspirin (acetylsalicylic acid), carboxymethylcellulose, dimethyl sulfoxide and hydrogen peroxide were obtained from Sigma Chemical Co. (St. Louis, MO). Histology supplies and reagents were purchased from Fisher Scientific (Springfield, NJ).
Statistical analysis
All data are expressed as the mean ± SEM. Comparison among groups of data were made using either an unpaired Student's t test or one-way analysis of variance (ANOVA), followed by a Bonferroni's post test. Differences between groups were considered significant with a p value of less than 0.05.
Results
Polyp number and size
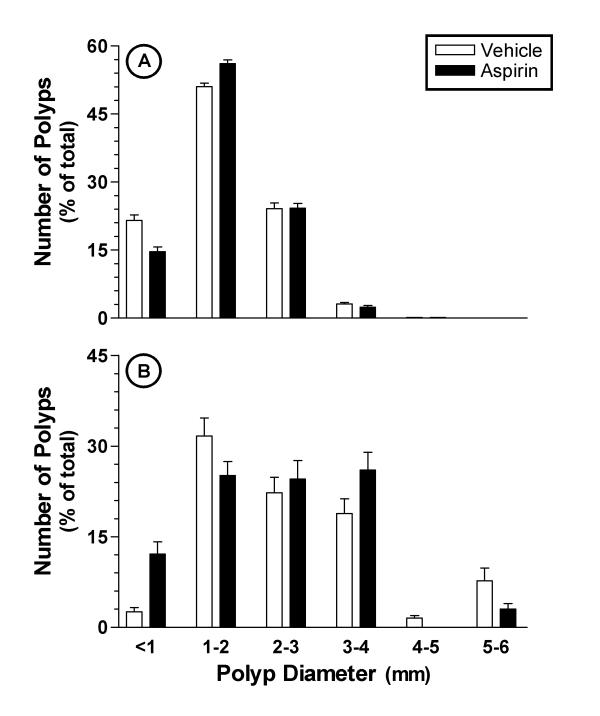
Sixteen week old Min/+ mice possessed approximately 63 ± 9 polyps (n = 13) within their small and large intestine, combined. Approximately 97% of the polyps present were located in the small intestine (table 1). Once daily oral administration of aspirin (25 mg/kg) for four weeks had no effect on the number of polyps in the intestinal tract of Min/+ mice. Aspirin-treated mice contained 62 ± 8 small intestinal polyps and 2.5 ± 0.4 large intestinal polyps (table 1). Concurrent with the above findings, no significant differences were seen in total adenoma area or average adenoma size. Adenomas in the small intestine had an average diameter of 1.64 ± 0.22 mm and 1.73 ± 0.14 mm for vehicle- and aspirin-treated Min/+ mice, respectively. Similar findings were seen within large intestine (table 1). Furthermore, no changes were seen in the distribution of adenomas when classified according to diameter (figure 1). Small intestinal adenomas were less than 4 mm in diameter, with the approximately 75% being 1–3 mm in diameter. Large intestinal adenomas as large as 5–6 mm in diameter were occasionally seen. Aspirin treatment had no effect on adenoma size distribution and both profiles were essentially identical to those of the vehicle-treated group (figure 1).
Table 1.
Effect of Aspirin Administration on Polyp Number and Area in Min/+ Mice.
| Vehicle-Treated (n = 13) | Aspirin-Treated (n = 11) | |||||
| Small | Large | Total | Small | Large | Total | |
| Polyp Number | 61 ± 9 | 1.9 ± 0.4 | 63 ± 9 | 62 ± 8 | 2.5 ± 0.4 | 65 ± 8 |
| Polyp Area (mm2) | 183 ± 70 | 8.7 ± 3.7 | 192 ± 73 | 159 ± 44 | 15.2 ± 5.5 | 174 ± 47 |
| Polyp Diameter (mm) | 1.6 ± 0.2 | 1.8 ± 0.5 | NA | 1.7 ± 0.1 | 2.4 ± 0.5 | NA |
Min/+ Mice were treated orally with either vehicle or aspirin (25 mg/kg) once daily for 28 days, commencing at 12 weeks of age. Data are expressed as the mean ± SEM. Groups of data were compared using an unpaired t test. Analysis revealed no statistically significant difference between the treatment groups. NA = Not Applicable
Figure 1.
Size distribution of adenomas in the small (panel A) and large (panel B) intestines of Min/+ mice treated once daily with aspirin over a period of four weeks. Adenoma sizes were determined from captured images and categorised based on diameter (mm). Data are presented as the percent of total polyps.
Cyclooxygenase activity and apoptosis
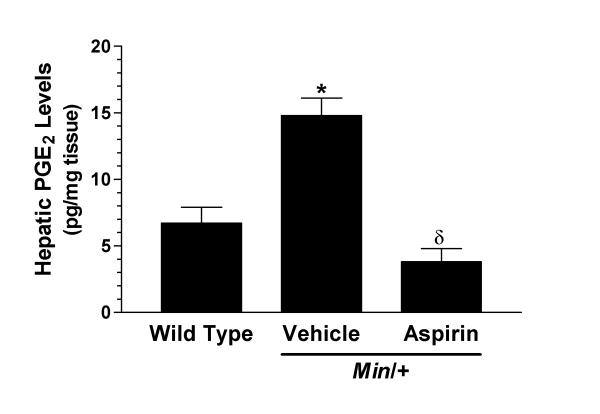
Vehicle-treated Min/+ mice displayed a 2-fold increase in their capacity to generate PGE2 when compared to wild type mice (figure 2). Once daily aspirin (25 mg/kg p.o.) administration to Min/+ mice caused a significant reduction in the ability of liver to generate PGE2 compared to vehicle-treated Min/+ mice (figure 2). PGE2 levels (3.8 ± 1.0 pg/mg tissue) in the aspirin-treated mice were comparable to those seen in wild type mice.
Figure 2.
Effect of aspirin on cyclooxygenase (COX) activity in Min/+ mice. COX activity was determined by measuring the ability of hepatic tissue to synthesise prostaglandin E2 (PGE2) ex vivo. Hepatic tissue was obtained 3 hours following the final dose of either vehicle (n = 13) or aspirin (25 mg/kg p.o., n = 11). A separate group of wild type mice (n = 6) were treated with vehicle once daily for 4 weeks to establish normal capacity of liver tissue to synthesise PGE2. Data are expressed as the mean ± SEM. * p < 0.05 versus wild type. δ p < 0.05 versus vehicle-treated Min/+ mice. Statistical analysis conducted using a one-way analysis of variance with a Bonferroni post test.
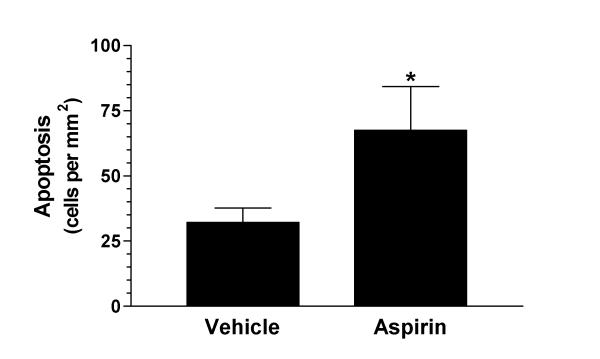
Aspirin administration also resulted in a significant increase in the number of apoptotic cells found in small intestinal adenomas (figure 3). Vehicle-treated Min/+ mice contained 32.2 ± 5.6 apoptotic cells per area (mm2) of adenoma. The number of apoptotic cells per adenoma area was twice (67.6 ± 16.7 cells per mm2; p = 0.05) as great in the aspirin-treated group.
Figure 3.
Assessment of apoptosis in small intestinal adenomas of vehicle- and aspirin-treated Min/+ mice. Apoptosis was detected in situ via the TUNEL principle. An average of 4 adenomas were counted per animal. Data are depicted as the number of apoptotic cells per adenoma area (mm2). * p = 0.05 versus vehicle-treated Min/+ mice.
Overall welfare
The quality of life of the Min/+ mice, as assessed by change in body weight, hematocrit level and overall survival, was not altered in the aspirin-treated group compared to the vehicle-treated group (table 2). Wild type animals treated with vehicle weighed initially 23.6 ± 1.6 g and gained approximately 2.9 ± 1.9 g over the 4-week period. Vehicle- and aspirin-treated Min/+ mice initially weighed 24.6 ± 1.1 g and 24.0 ± 1.1 g, respectively. Both Min/+ groups failed to gain weight, rather a modest decrease in body weight was noted over the study period (-0.9 ± 1.4 g and -0.1 ± 1.1 g, respectively). Both the vehicle- and aspirin-treated groups were found to be anemic based upon hematocrit values (table 2). Anaemia is characteristic of the model [29]. Both treatment groups displayed hematocrit levels 25–30% lower than expected values. Aspirin treatment did not improve or worsen the anaemia (table 2). Wild type (C57BL/6J, background strain of Min/+ mice) are referenced to have hematocrit levels of 44 ± 0.4 % [31].
Table 2.
Effect of Aspirin Administration on Animal Welfare.
| Genotype | Treatment | Body Wt (% Change) | Hematocrit (%) | Pre-Mature Deaths |
| Wild Type | Vehicle | 2.9 ± 1.9 (6) | 44 ± 0.4† (10)† | 0/6 |
| Min/+ | Vehicle | -0.9 ± 1.4 (13) | 31 ± 3 (8) | 1/14 |
| Min/+ | ASA | -0.1 ± 1.1 (11) | 34 ± 3 (5) | 0/11 |
Data are presented as mean ± SEM and n values are shown in parentheses. † Reference standard for normal hematocrit value for C57BL/6J mice (cited from Ref. No. [31])
Survival rate of the Min/+ mice was greater than 95%. One animal had to be sacrificed prior (2 days) to the completion of the study and was in the vehicle treatment group (table 2). Post-mortem examination revealed a total of 87 polyps (82 small and 5 large intestinal). Data obtained from this animal was not included in the analysis. In addition, one animal was found to possess no small or large intestinal polyps. The animal had been treated with a full regiment of aspirin, however, it was assumed not to possess the Min/+ genotype and was therefore not included in data analysis. A wild type genotype was assumed, since no existing data suggests or indicates that aspirin is able to cause complete elimination of adenomas in this model. The animal also possessed a normal hematocrit value of 44% [31].
Discussion
The American Cancer Society has indicated that cancer incidence rates are on the decline, however, they estimate that greater than 1.2 million new cases will be diagnosed this year [1]. Of these new cases, it is expected that colorectal cancers will comprise approximately 10–11% [1]. With staggering numbers as these, it is not surprising that chemoprevention has been proposed as a viable strategy to fight cancer. Among the leading therapeutic agents in colorectal cancer prevention are NSAIDs, especially aspirin. Epidemiological studies have indicated that the frequent, long term use of aspirin could reduce the relative risk of developing colorectal cancer by 40–50% [13-15,32]. Despite the wealth of information regarding the chemopreventive ability of aspirin, information to indicate aspirin's utility in a therapeutic modality is lacking. To date no studies or case reports have examined the use of aspirin in the treatment of FAP, although various clinical trials are under way [33]. In accordance with the above data, studies conducted in animals have always employed a prevention strategy as well [4,7,9]. To our knowledge, this is the first study to examine the therapeutic potential of aspirin in a rodent model of colon carcinogenesis, in particular, the Min/+ murine model of polyposis. The study revealed that treatment of Min/+ mice with established polyposis with aspirin for 4 weeks produce no appreciable therapeutic effect.
While the evaluation of aspirin in the Min/+ model utilising a therapeutic regime is lacking, other NSAIDs (sulindac, flurbiprofen, piroxicam and celecoxib) have been shown to significantly reduce polyp numbers when administered using similar protocols [6,11,34,35]. In addition, three recent reports have demonstrated a lack of effect of aspirin as a chemopreventive agent in Min/+ mice [36-38]. Combined, these studies indicate that the Min/+ model is responsive to NSAIDs other than aspirin when provided as a treatment. They also raise into question the efficacy of aspirin as a chemotherapeutic for colon cancer, and perhaps more specifically, familial adenomatous polyposis.
With the exemplary track record of NSAIDs in this model of colon cancer, why was a lack of effect seen in this model? Was it a consequence of the dose of aspirin and/or duration of treatment used? In the current study, mice received approximately 500 μg of aspirin per day. This amount of aspirin was found by Mahmoud et al.[9] to be sufficient (significant) to reduce both polyp number and intestinal prostaglandin (PGE2) content by greater than 45% when provided from 5–6 weeks of age to 16 weeks of age (i.e., chemopreventive regime). Barnes and Lee [7] have also suggested that the maximal tumour suppression for aspirin may be in the 50% range. The above authors followed a similar protocol to Mahmoud et al. [9], but employed far greater doses of aspirin (45 and 90 mg/kg/day) and reported no dose-response relationship and a maximal suppression of 55% [7]. Furthermore, in the rat model of azoxymethane-induced colon carcinogenesis, aspirin has also only been found to inhibit aberrant crypt foci and tumour development by no more than 65% [4,39]. Coincidentally, epidemiological data also report reductions in the 40–50% range [13-15,32]. However, whether this can be used as an argument to support the notion that aspirin is only able to suppress tumour formation by 50% is difficult to extrapolate because two different end points are being examined (i.e., relative risk versus tumour number).
Finally, as indicated earlier, other studies have demonstrated that doses of NSAIDs other than aspirin which were found to be chemopreventive were able to produce similar reductions in polyp numbers when used in a treatment regime [6,11,34,35]. Chiu et al.[6] demonstrated that Min/+ mice at approximately 11 weeks of age possessed roughly 35–40 polyps and that treatment (starting at 11 weeks of age) for as little as 4 days with sulindac produced a 75% reduction in polyp number. Similarly, Ritland and Gendler [34] reported that administration of piroxicam (200 ppm in diet) to 10–13 week old Min/+ mice for 6 or more days resulted in maximal tumour number suppression (>90%). Finally, R-flurbiprofen (10 mg/kg/day) orally administered to Min/+ for 42 days starting at 10 weeks of age was also able to decrease tumour number by greater than 58% [11].
Various studies have reported increased levels of prostaglandins and COX-2 expression in colorectal adenomas and adenocarcinomas obtained both from animals [40,41] and humans [42-44]. These findings have lead to the implication that COX-2 plays an important role in intestinal neoplasia and that the anti-neoplastic effects of NSAIDs are attributable to their ability to inhibit COX-2. In light of this information, the mechanism by which aspirin and other NSAIDs inhibit colorectal cancer progression has not been fully elucidated and cannot simply be equated to the suppression of COX-2 activity. Shiff and Rigas [45] indicate that NSAIDs may to affect colorectal cancer development via one or more of the following four areas: (1) COX-mediated carcinogen activation, (2) cell proliferation, (3) apoptosis and (4) immune surveillance. Within these potential mechanisms exists the possibility for both COX-dependent and -independent mechanisms. In the current study we also examined COX-activity and apoptosis. The treatment regime employed was sufficient to attenuate COX activity by 75% and increased the number of apoptotic bodies within tumours by greater than 2-fold. Similar findings were reported by Mahmoud et al. [9] using an equivalent dose of aspirin in a prevention strategy. These authors demonstrated a >50% reduction in small intestinal tumour number and PGE2 content and a >4-fold increase in apoptosis. Contrary to the similarities seen in COX-inhibition and increased apoptosis, the present study saw no effect on tumour number or size. The discordant effect seen between the current study and that of Mahmoud et al. [9] are difficult to explain. The duration and timing of treatment (i.e., prevention vs. treatment) would seem the most likely explanation. This possibility has also been eluded in epidemiological studies. The Physicians' Health Study found no significant reduction in the relative risk of developing colorectal cancer in individuals consuming 325 mg of aspirin every other day for 5 years [46]. The Nurses' Health Study reported that a significant decrease in risk was only seen in women who took aspirin on a regular basis for 10 or more years [13]. Whether this simple explanation accounts for the difference in results reported here and by Mahmoud et al. [9] does not seem likely. As stated earlier, three previous studies employing prevention strategies and higher doses of aspirin also found no effect on tumour burden [36-38]. Perhaps a similar discordant effect will appear in human studies as results are released from the currently ongoing clinical trials [33]. If such a situation does arise, it could indicate that aspirin might not be the NSAID of choice for colorectal cancer therapy. In contrast, to date no conflicting data have been published regarding the use of sulindac. Sulindac has been shown to be efficacious both in rodent models of FAP and in human FAP patients.
Although no change was seen in tumour number or size, the significant increase seen in apoptosis might imply that aspirin could still hold utility in treating colorectal cancer. The present study would suggest that aspirin has little or no use as a stand-alone therapy, but could present a viable option in combination chemotherapy. Recent reports by Torrance et al.[47] and Mann et al.[48] demonstrate that combinatorial therapy comprised of a COX inhibitor and growth factor receptor inhibitors resulted in synergistic anti-tumour activity. In fact, the study conducted by Torrance and colleagues [47] demonstrated that doses of sulindac (5 mg/kg) found to be ineffective as stand-alone chemotherapy in the Min/+ model became extremely potent when combined with an epidermal growth factor receptor kinase inhibitor. The combination therapy was able to completely abolish all macroscopically visible tumours in 47% of the animals treated [47]. This could prove to be very advantageous since it may allow for lower doses of NSAIDs to be used, thereby limiting the adverse effects associated with long term NSAID use. Pursuing future research into the combinatorial chemopreventive/chemotherapeutic potential of aspirin may be of greater value than trying to account for the discordant effect.
Conclusions
Despite abundant evidence from epidemiological, clinical and animal studies regarding the potential utility of aspirin as a chemotherapeutic agent for colorectal cancer, the current study found no change in tumour burden in the extensively used Min/+ murine model of colon cancer. The findings that aspirin was still able to significantly increase the number of apoptotic cells within tumours and lower prostaglandin levels suggests that it may be better suited to combinatorial therapy, but its utility as a stand-alone modality in established GI cancer is questionable. Promising results have already been attained in animal models using COX inhibitors as co-therapies in the treatment of colorectal cancer and present a valuable future research direction.
Competing interests
None declared.
Author's contributions
BKR performed the animal studies, immunoassays and drafted the manuscript. XJZ carried out the immunohistochemistry and aided with tissue collection. MJS participated in study design, coordination and manuscript preparation.
All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors wish to extend our gratitude to NicOx, Sophia-Antipolis, France for graciously providing funding for Dr. Reuter and the research project.
Contributor Information
Brian K Reuter, Email: ok_go@hotmail.com.
Xiao-Jing Zhang, Email: xiaojingz@hotmail.com.
Mark JS Miller, Email: millermj@mail.amc.edu.
References
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- McLeod RS, am CT. Screening strategies for colorectal cancer: A systematic review of the evidence. Can J Gastroenterol. 2001;15:647–660. doi: 10.1155/2001/284746. [DOI] [PubMed] [Google Scholar]
- Pollard M, Luckert PH. Effect of indomethacin on intestinal tumors induced in rats by the acetate derivative of dimethylnitrosamine. Science. 1981;214:558–559. doi: 10.1126/science.7291992. [DOI] [PubMed] [Google Scholar]
- Bak AW, McKnight W, Li P, Del Soldato P, Calignano A, Cirino G, et al. Cyclooxygenase-independent chemoprevention with an aspirin derivative in a rat model of colonic adenocarcinoma. Life Sci. 1998;62:L-73. doi: 10.1016/S0024-3205(98)00191-X. [DOI] [PubMed] [Google Scholar]
- Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. [PubMed] [Google Scholar]
- Chiu CH, McEntee MF, Whelan J. Sulindac causes rapid regression of preexisting tumors in Min/+ mice independent of prostaglandin biosynthesis. Cancer Res. 1997;57:4267–4273. [PubMed] [Google Scholar]
- Barnes CJ, Lee M. Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology. 1998;114:873–877. doi: 10.1016/s0016-5085(98)70305-1. [DOI] [PubMed] [Google Scholar]
- Jacoby RF, Marshall DJ, Newton MA, Novakovic K, Tutsch K, Cole CE, et al. Chemoprevention of spontaneous intestinal adenomas in the Apc Min mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res. 1996;56:710–714. [PubMed] [Google Scholar]
- Mahmoud NN, Dannenberg AJ, Mestre J, Bilinski RT, Churchill MR, Martucci C, et al. Aspirin prevents tumors in a murine model of familial adenomatous polyposis. Surgery. 1998;124:225–231. doi: 10.1067/msy.1998.90369. [DOI] [PubMed] [Google Scholar]
- Mahmoud NN, Boolbol SK, Dannenberg AJ, Mestre JR, Bilinski RT, Martucci C, et al. The sulfide metabolite of sulindac prevents tumors and restores enterocyte apoptosis in a murine model of familial adenomatous polyposis. Carcinogenesis. 1998;19:87–91. doi: 10.1093/carcin/19.1.87. [DOI] [PubMed] [Google Scholar]
- Wechter WJ, Kantoci D, Murray ED, Jr, Quiggle DD, Leipold DD, Gibson KM, et al. R-flurbiprofen chemoprevention and treatment of intestinal adenomas in the APC(Min)/+ mouse model: implications for prophylaxis and treatment of colon cancer. Cancer Res. 1997;57:4316–4324. [PubMed] [Google Scholar]
- Peleg II, Lubin MF, Cotsonis GA, Clark WS, Wilcox CM. Long-term use of nonsteroidal antiinflammatory drugs and other chemopreventors and risk of subsequent colorectal neoplasia. Dig Dis Sci. 1996;41:1319–1326. doi: 10.1007/BF02088554. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399–4404. [PubMed] [Google Scholar]
- Logan RF, Little J, Hawtin PG, Hardcastle JD. Effect of aspirin and non-steroidal anti-inflammatory drugs on colorectal adenomas: case-control study of subjects participating in the Nottingham faecal occult blood screening programme. BMJ. 1993;307:285–289. doi: 10.1136/bmj.307.6899.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ. NSAID use and decreased risk of gastrointestinal cancers. Gastroenterol Clin North Am. 1996;25:333–348. doi: 10.1016/s0889-8553(05)70250-8. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Guldenschuh I, Hurlimann R, Muller A, Ammann R, Mullhaupt B, Dobbie Z, et al. Relationship between APC genotype, polyp distribution, and oral sulindac treatment in the colon and rectum of patients with familial adenomatous polyposis. Dis Colon Rectum. 2001;44:1090–1097. doi: 10.1007/BF02234627. [DOI] [PubMed] [Google Scholar]
- Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- DiSario JA, Alberts DS, Tietze CC, Khullar SK, Bohman VD, Larsen BR, et al. Sulindac induces regression and prevents progression of sporadiac colorectal adenomas. Gastroenterology. 1997;112:A555. [Google Scholar]
- Waddell WR, Gerner RE. Indomethacin and ascorbate inhibit desmoid tumors. J Surg Oncol. 1980;15:85–90. doi: 10.1002/jso.2930150113. [DOI] [PubMed] [Google Scholar]
- Waddell WR, Gerner RE, Reich MP. Nonsteroid antiinflammatory drugs and tamoxifen for desmoid tumors and carcinoma of the stomach. J Surg Oncol. 1983;22:197–211. doi: 10.1002/jso.2930220314. [DOI] [PubMed] [Google Scholar]
- Dove WF, Cormier RT, Gould KA, Halberg RB, Merritt AJ, Newton MA, et al. The intestinal epithelium and its neoplasms: genetic, cellular and tissue interactions. Philos Trans R Soc Lond B Biol Sci. 1998;353:915–923. doi: 10.1098/rstb.1998.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology. 2000;119:854–865. doi: 10.1053/gast.2000.16507. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Tsukita S. Where is APC going? J Cell Biol. 2001;154:1105–1109. doi: 10.1083/jcb.200106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman S, Rex DK, Sonnenberg A. Chemoprevention of colorectal cancer by aspirin: a cost-effectiveness analysis. Gastroenterology. 2002;1222:78–84. doi: 10.1053/gast.2002.29689. [DOI] [PubMed] [Google Scholar]
- Hursting SD. Experimental models of gene-environment interaction for cancer chemoprevention studies. Curr Opin Oncol. 1997;9:487–491. doi: 10.1097/00001622-199709050-00015. [DOI] [PubMed] [Google Scholar]
- Shoemaker AR, Moser AR, Dove WF. N-ethyl-N-nitrosourea treatment of multiple intestinal neoplasia (Min) mice: age-related effects on the formation of intestinal adenomas, cystic crypts, and epidermoid cysts. Cancer Res. 1995;55:4479–4485. [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Ferraz JG, Sharkey KA, Reuter BK, Asfaha S, Tigley AW, Brown ML, et al. Induction of cyclooxygenase 1 and 2 in the rat stomach during endotoxemia: role in resistance to damage. Gastroenterology. 1997;113:195–204. doi: 10.1016/s0016-5085(97)70095-7. [DOI] [PubMed] [Google Scholar]
- Russell E, Neufeld E, Higgins C. Comparison of normal blood picture of young adults from 18 inbred strains of mice. Proc Soc Exper Biol Med. 1951;78:761–766. doi: 10.3181/00379727-78-19210. [DOI] [PubMed] [Google Scholar]
- Greenberg ER, Baron JA, Freeman DH, Jr, Mandel JS, Haile R. Reduced risk of large-bowel adenomas among aspirin users. The Polyp Prevention Study Group. J Natl Cancer Inst. 1993;85:912–916. doi: 10.1093/jnci/85.11.912. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- Ritland SR, Gendler SJ. Chemoprevention of intestinal adenomas in the ApcMin mouse by piroxicam: kinetics, strain effects and resistance to chemosuppression. Carcinogenesis. 1999;20:51–58. doi: 10.1093/carcin/20.1.51. [DOI] [PubMed] [Google Scholar]
- Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040–5044. [PubMed] [Google Scholar]
- Chiu CH, McEntee MF, Whelan J. Discordant effect of aspirin and indomethacin on intestinal tumor burden in Apc(Min/+)mice. Prostaglandins Leukot Essent Fatty Acids. 2000;62:269–275. doi: 10.1054/plef.2000.0154. [DOI] [PubMed] [Google Scholar]
- Williamson SL, Kartheuser A, Coaker J, Kooshkghazi MD, Fodde R, Burn J, et al. Intestinal tumorigenesis in the Apc1638N mouse treated with aspirin and resistant starch for up to 5 months. Carcinogenesis. 1999;20:805–810. doi: 10.1093/carcin/20.5.805. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Stark LA, Dunlop MG, Clarke AR. Suppression of intestinal and mammary neoplasia by lifetime administration of aspirin in Apc(Min/+) and Apc(Min/+), Msh2(-/-) mice. Cancer Res. 2001;61:7060–7064. [PubMed] [Google Scholar]
- Reddy BS, Rao CV, Rivenson A, Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993;14:1493–1497. doi: 10.1093/carcin/14.8.1493. [DOI] [PubMed] [Google Scholar]
- Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- Williams CS, Luongo C, Radhika A, Zhang T, Lamps LW, Nanney LB, et al. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology. 1996;111:1134–1140. doi: 10.1016/s0016-5085(96)70083-5. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Ruffin MT, Normolle D, Shureiqi I, Burney K, Bailey J, et al. Colonic mucosal prostaglandin E2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:447–453. [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Tomozawa S, Tsuno NH, Sunami E, Hatano K, Kitayama J, Osada T, et al. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br J Cancer. 2000;83:324–328. doi: 10.1054/bjoc.2000.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff SJ, Rigas B. Nonsteroidal anti-inflammatory drugs and colorectal cancer: evolving concepts of their chemopreventive actions. Gastroenterology. 1997;113:1992–1998. doi: 10.1016/s0016-5085(97)99999-6. [DOI] [PubMed] [Google Scholar]
- Gann PH, Manson JE, Glynn RJ, Buring JE, Hennekens CH. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85:1220–1224. doi: 10.1093/jnci/85.15.1220. [DOI] [PubMed] [Google Scholar]
- Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, et al. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–1028. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- Mann M, Sheng H, Shao J, Williams CS, Pisacane PI, Sliwkowski MX, et al. Targeting cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal carcinoma growth. Gastroenterology. 2001;120:1713–1719. doi: 10.1053/gast.2001.24844. [DOI] [PubMed] [Google Scholar]