Abstract
Background
Subcutaneous and sub rectus sheath wound drains are sometimes used in women who have undergone caesarean section. The indications for using drains vary by clinician.
Objectives
To compare the effects of using a wound drain with not using a wound drain at caesarean section, and of different types of drain, on maternal health and healthcare resource use.
Search methods
In November 2013, for this second update, we searched the Cochrane Wounds Group Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid Medline; Ovid Medline ‐ In‐Process & Other Non‐Indexed Citations; Ovid Embase; and EBSCO CINAHL. No date, language or publication status limits were applied
Selection criteria
Studies were included if they allocated women to groups at random and they compared any type of wound drain with no wound drainage, or with any other type of drain, in women undergoing caesarean section.
Data collection and analysis
Trials were evaluated for appropriateness for inclusion and methodological quality without consideration of their results. This was done by two reviewers according to pre‐stated eligibility criteria.
Main results
Ten trials that recruited 5248 women were included in the review. Meta‐analysis found no evidence of a difference in the risk of wound infection, other wound complications, febrile morbidity or pain in women who had wound drains compared with those who did not. There was some evidence from one trial that a subcutaneous drain may increase wound infection compared to a sub‐sheath drain (RR 5.42, 95% CI 1.28 to 22.98). No differences in outcomes were found between subcutaneous drainage and subcutaneous suturing in the three trials that made this comparison.
Authors' conclusions
Existing evidence suggests that the routine use of wound drains at caesarean section does not confer any substantial benefit to the women involved. However, neither moderate benefit nor harm are excluded.
Keywords: Female, Humans, Pregnancy, Cesarean Section, Cesarean Section/adverse effects, Drainage, Drainage/instrumentation, Drainage/methods, Randomized Controlled Trials as Topic
Plain language summary
Use of wound drains after caesarean section
Women who have a caesarean section operation (removing the baby by surgery through the mother's abdomen) sometimes have a wound drain put in place. A surgical drain is a tube used to remove pus, blood or other fluids from a wound. The review did not find any differences in the risk of wound infection or other post operative complications between women who had wound drains compared with those who did not.
Background
Caesarean section is probably the commonest major operation performed on women in the world. Essentially the operation involves exposing the uterus by entering the abdominal cavity through the abdominal wall. The peritoneal lining of the abdomen is opened and the peritoneum covering the uterus is usually also entered. The bladder is reflected away from the uterus to reduce the chance of damage to it during the operation. The uterus is then incised and the baby and placenta delivered. Adequate haemostasis is achieved by closure of the uterine muscle followed by closure of the abdominal wall.
There are many possible ways of performing a caesarean section operation, and operative techniques vary widely (Tully 2002). The techniques used may depend on many factors including the clinical situation and the preferences of the operator. For an overview of the techniques, indications for caesarean section and postoperative complications, see the Cochrane review 'Techniques for caesarean section' (Hofmeyr 2008).
Sub‐rectus sheath drains, or drains between the sheath and the skin (subcutaneous) are sometimes used after caesarean section operations. Drains are used routinely by fewer than 10% of obstetricians in the UK, but in a survey 52% used them 'when indicated at the time of operation' (Tully 2002).
The aim of siting a sub‐sheath drain is to remove as much blood as possible from the abdominal cavity after surgery. Blood and serous fluid can irritate the peritoneal lining of the abdomen and cause postoperative pain, and can also provide a medium in which bacterial infection may thrive. Theoretically, therefore, a drain can reduce pain and the risk of pelvic infection for the mother, while allowing fluid loss to be measured externally.
There are disadvantages of using a sub‐sheath drain. A drain may be ineffective if blood clots in the abdominal cavity. Women sometimes find the site of drainage tubes uncomfortable and an attached suction bottle inconvenient after surgery. Drains can be difficult or painful to remove and occasionally have to be removed under anaesthetic. The drainage tube itself can provide a focus for infection and can even lead to sinus formation between the skin and abdominal cavity. Some surgeons would argue that a drain is not necessary because the peritoneum heals extremely rapidly and reabsorbs blood as part of this process.
Types of drain that may be used at caesarean section include closed suction drains, in which the blood loss can be measured in a collection bottle. This can give an early indication of intra‐abdominal bleeding and has the advantage that the fluid loss is contained. Corrugated drains and wide‐bore tube drains are also sometimes used but their disadvantage is that any fluid drained needs to be soaked up by dressings.
This review incorporates the old Cochrane review "Closed suction wound drainage at caesarean section" (Enkin 1995) which incorporated two trials (Loong 1988; Saunders 1988). That review concluded that "The use of closed wound drainage may be of benefit when haemostasis is not adequate, but a benefit from the routine use of such drainage has not been established". This review includes trials which have been published since 1995.
Objectives
To compare the effects of using a wound drain with not using a wound drain at caesarean section, and the effects of different types of drain, on maternal health and healthcare resource use.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials comparing wound drainage with no drainage, or comparing different types of drain. Quasi‐randomised trials (for example those randomised by date of birth or hospital number) were excluded from the analysis. Studies reported only in abstract form were considered for inclusion in the review: if they did not report sufficient details of their methodology or results to allow inclusion, they were listed as studies awaiting classification, and they will be considered for inclusion in the review when a full publication is available.
Types of participants
Women undergoing a caesarean section.
Types of interventions
Any type of wound drain compared with no drain, or compared with any other type of drain. Types of drain that could be included were: suction drains, corrugated drains, wide‐bore tube drains. Drains inserted under the rectus sheath and those located between the skin and the sheath were included. Information on duration of drainage was recorded if available.
Types of outcome measures
Primary outcomes
Short‐term outcomes
Postoperative febrile morbidity (as defined by trial authors); Postoperative pain (measured by valid scoring system defined by trial authors); Wound infection (as defined by trial authors); Wound complications (as defined by trial authors; including any or all of wound infection, dehiscence, haematoma, seroma).
Secondary outcomes
Short‐term outcomes
Postoperative analgesia (as defined by trial authors); Blood transfusion; Postoperative anaemia (as defined by trial authors); Breast feeding (at discharge or as defined by trial authors); Voiding problems (as defined by trial authors); Duration of surgery; Thromboembolism; Need for re‐laparotomy; Maternal death or admission to intensive care unit.
Long term outcomes:
Long term wound complications (e.g. numbness, keloid formation, incisional hernia); Fertility problems (e.g. secondary infertility due to adhesion formation); Complications in future pregnancy (e.g. placenta praevia, uterine rupture); Complications at future surgery (e.g. adhesion formation).
Health service use:
Length of postoperative hospital stay; Readmission to hospital.
Only outcomes with available data appear in the analysis table. Outcome data that were not prespecified by the review authors, but which were reported by the trial authors are labelled as such in the analysis but are not used for the conclusions.
Search methods for identification of studies
The search methods for the first published version of this review can be found in Appendix 1
Electronic searches
In November 2013, for this second update, we searched the following databases:
The Cochrane Wounds Group Specialised Register (searched 14 November 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 10);
Ovid MEDLINE (1946 to November Week 1 2013);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, November 13, 2013);
Ovid EMBASE (1974 to 2013 November 13);
EBSCO CINAHL (1982 to 8 November 2013).
The following search terms were used: #1 MeSH descriptor: [Cesarean Section] explode all trees 2211 #2 (caesarean or caesarian or cesarean or cesarian):ti,ab,kw 4870 #3 #1 or #2 4870 #4 MeSH descriptor: [Drainage] explode all trees 1986 #5 MeSH descriptor: [Suction] explode all trees 733 #6 drain*:ti,ab,kw 4040 #7 #4 or #5 or #6 4641 #8 #3 and #7 40 We have provided the search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL in; Appendix 2, Appendix 3 and Appendix 4 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Ovid EMBASE and EBSCO CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2009). There were no restrictions on the basis of date or language of publication. Trials registries were not searched for this update.
Searching other resources
We scrutinised the reference lists of relevant reviews and trials to identify additional studies.
Data collection and analysis
Selection of studies
We evaluated the identified trials independently according to the pre‐stated eligibility criteria without consideration of their results or the language in which they were published.
Data extraction and management
Data were extracted from the original publications onto data extraction forms by both review authors. Differences of opinion were resolved by discussion.
Assessment of risk of bias in included studies
The risk of bias for each study was assessed using the Cochrane bias risk assessment tool (Higgins 2011a). Both review authors assessed studies and resolved disagreements by discussion.
(1) Sequence generation
Random sequence generation methods were assessed as:
low risk of bias (any truly random process e.g. random number table; computer random number generator);
high risk of bias (any non random process e.g. odd or even date of birth; hospital or clinic record number);
unclear.
(2) Allocation concealment
Methods of allocation concealment were described for each included study, and assessed as at high risk of bias if a woman's allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
Methods were classified as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, tossing coin);
unclear.
(3) Blinding of participants and personnel
For each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received were described. Studies were judged at low risk of bias if they were securely blinded, or if the lack of blinding could not have affected the results.
The methods were assessed as:
low risk, high risk or unclear for participants;
low risk, high risk or unclear for personnel;
(4) Blinding of outcome assessment
For each outcome or class of outcomes, the methods used, if any, to ensure that outcome data were collected without knowledge of each participant's allocation.
The methods were assessed as low risk, high risk or unclear.
(5) Incomplete outcome data
For each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. High levels of missing outcome data, or imbalance between the groups in the amount of missing data, we regarded as potentially indicating bias.
(6) Selective reporting bias
We assessed whether there was any evidence in any studies that selective reporting bias had occurred. The methods were assessed as:
low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where it is clear that not all the study’s pre‐specified outcomes have been reported, or one or more reported primary outcomes were not pre‐specified);
unclear.
(7) Other sources of bias
We described any important concerns about other sources of bias in the included studies that we had.
(8) Overall risk of bias
We made a judgement about whether each study was at high risk of bias, according to the criteria given in the Handbook (Higgins 2011a). With reference to (1) to (7) above, we assessed the likely magnitude and direction of the bias and whether it was likely to impact on the findings. The impact of including studies at high risk of bias was assessed through undertaking sensitivity analyses ‐ see 'Sensitivity analysis'.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence interval.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods, but there were no data requiring this method.
Unit of analysis issues
We planned to include cluster randomised trials in the analysis alongside individually randomised trials, using the methods described in the Cochrane Handbook (Higgins 2011a) but no cluster randomised trials were identified. For studies that randomised women to more than two arms, we combined arms to create a comparison between drain and no‐drain groups. For example, if women were randomised to three groups, drain, subcutaneous closure or neither procedure, we combined the subcutaneous closure arm with the arm receiving neither procedure to make a comparison between drain and no drain. Combination of groups was performed for dichotomous outcomes by summing the events and denominators over the contributing groups, and for continuous outcomes by calculating the combined means and standard deviations.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention to treat basis i.e. as far as possible all participants who were randomised were included in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. We did not use any formal imputation methods.
Assessment of heterogeneity
Decisions about whether to combine studies in meta‐analyses were based primarily on consideration of their clinical and methodological similarity. Fixed effect meta‐analysis was used for combining study data if the trials were judged to be sufficiently similar. Heterogeneity was assessed by calculating I‐squared (I2) and tau‐squared (Tau2) statistics (Higgins 2002; Higgins 2009b). We considered that heterogeneity was substantial if I2 exceeded 30% and Tau2 was greater than zero. If a high level of heterogeneity was found among the trials included in an analysis, we considered whether it was appropriate and meaningful to produce an overall summary. If we judged that it was, we used random effects meta‐analysis to estimate the average treatment effect, and calculated a 95% prediction interval for the underlying treatment effect (Higgins 2009b).
Data synthesis
Data from different trials were combined if we judged them to be sufficiently similar for this to be reasonable. Fixed effect meta analysis (Mantel‐Haenszel method) was the primary method of analysis, and was used unless there was evidence of substantial heterogeneity (see above). If trials used different ways of measuring the same continuous outcome (for example pain), we planed to use standardised mean differences, but this method was not required.
Subgroup analysis and investigation of heterogeneity
Where high levels of heterogeneity were found we planned to explore this by the pre‐specified subgroup analyses and by sensitivity analyses excluding the trials most susceptible to bias based on the quality assessment: those with inadequate allocation concealment, high levels of post‐randomisation losses or exclusions or unblinded outcome assessment.
Planned subgroup analyses were:
First versus repeat caesarean section versus mixed/undefined;
Prelabour versus intrapartum caesarean section versus mixed/undefined;
Preterm versus term caesarean section versus mixed/undefined;
General versus regional anaesthesia versus mixed/undefined.
We planned to restrict subgroup analyses to primary outcomes only, and to use statistical interaction tests (available in RevMan) to evaluate whether treatment effects differed between subgroups (Deeks 2001). Subgroup analyses were not performed due to lack of data in the primary studies.
Because we found several trials that recruited only obese women, we carried out a non‐prespecified subgroup analysis classifying trials into those that recruited only obese women, and those that recruited an unselected population.
Results
Description of studies
Included studies
Comparisons and interventions
See Characteristics of included studies For this update one additional study met the inclusion criteria, therefore ten studies in total comparing wound drain with no drain were included in the review. Only three studies randomised women into two groups comparing a drain with no drainage (Al Inany 2002; Ramsey 2005; Saunders 1988). Al Inany 2002 and Ramsey 2005 used a subcutaneous drain, and Saunders 1988 used a sub rectus sheath drain. In Ramsey 2005 all women also had closure of the subcutaneous tissue. Two studies (Maharaj 2000 ; Ochsenbein‐Imhof 2001) each used two drains as the intervention, these were subcutaneous and sub rectus sheath drains which were closed suction in the Ochsenbein‐Imhof study and corrugated in the Maharaj study. Magann 2002; Allaire 2000 and Kumar 2004 undertook three‐armed studies, randomising women to a subcutaneous drain, suturing of subcutaneous fat, or neither. In these three trials, the suturing and no treatment groups were combined to form a "no drainage" control group for the comparison of drain with no drainage. Loong 1988 used a factorial design, carrying out separate randomisation for sub rectus sheath suction drain versus no drain and subcutaneous corrugated drain versus no drain. The results were reported as four groups: subcutaneous drain, rectus sheath drain, both drains and no drain. In the comparison of drain versus no drain in this review, the no drain group is compared with all of the women who received one or two drains. CAESAR 2010 was a large, international 2x2x2 factorial trial, evaluating single versus double layer closure of the uterine incision, closure versus non‐closure of the pelvic peritoneum and liberal versus restricted use of a sub‐sheath wound drain. The intervention was described as "liberal versus restricted use" as it was recognised that in some circumstances clinicians would be unwilling to insert a drain for women randomised to that group, and similarly, would want to use in drain in some women randomised to no drain.
Studies varied in the duration of wound drainage, from 6 to 36 hours in Loong 1988 to 72 hours in Allaire 2000. Three studies (CAESAR 2010; Magann 2002; Ochsenbein‐Imhof 2001) did not document when the drains were removed.
Populations
All studies specified the inclusion of women having either first or repeat caesarean sections, except Ochsenbein‐Imhof 2001 and Maharaj 2000, where this was not specified. Eight studies included both pre labour and intrapartum caesarean sections; Al Inany 2002 restricted the eligibility to intrapartum operations, and Maharaj 2000 included only "emergency" caesareans but did not specify whether these were intrapartum or not.
Five trials were restricted to obese women, with either more than 2cm thickness of subcutaneous fat (Al Inany 2002; Allaire 2000; Kumar 2004; Magann 2002), or more than 4cm of subcutaneous fat (Ramsey 2005). Six trials used transverse skin incisions in all (or virtually all) participants (Al Inany 2002; CAESAR 2010; Kumar 2004; Loong 1988; Maharaj 2000; Saunders 1988), two used a mixture of transverse and vertical incisions (Magann 2002; Ramsey 2005), and two did not specify the types of skin incision used (Allaire 2000; Ochsenbein‐Imhof 2001).
Outcome assessment
The timing of outcome assessment varied. Only four studies (Al Inany 2002; CAESAR 2010; Magann 2002; Ramsey 2005) attempted comprehensive follow up of all women several weeks after discharge. The remainder recorded outcomes up to discharge or the time of skin staple removal, which was a maximum of 10 days. Allaire 2000 performed a chart review to identify any outcomes that occurred later, but this would not have detected any outcomes that occurred after discharge and were treated in a different hospital.
Studies awaiting assessment
Two studies are classified as awaiting assessment, both are abstracts reporting insufficient detail of their methodology, full text publication is awaited (Bose 2006a; Imhof 1999a).
Excluded studies
One study which had been classified as 'awaiting assessment' in the original review has now been moved to the Excluded Studies table. Bose 2006 is an abstract and although attempts have been made to identify if the study has been published in full and the authors contacted for further information, at this time there is insufficient information to make a judgement on inclusion.
Risk of bias in included studies
Randomisation and allocation concealment
Six studies (Allaire 2000; CAESAR 2010; Kumar 2004; Magann 2002; Maharaj 2000; Ramsey 2005) used computer generated random numbers to ensure random assignment to the groups. The other four studies gave no information on the generation of the allocation sequence.
One study used a telephone randomisation system to allocate women to groups (CAESAR 2010). Eight studies used sealed opaque envelopes, although only one of them mentioned numbering of the envelopes or any other measures to ensure that all envelopes could be accounted for (Ramsey 2005). One study (Loong 1988) stated that randomisation was by "sets of random numbers used by nursing staff". This suggests that the randomisation schedule may have been open, with no allocation concealment.
Magann 2002 randomised women before surgery, and excluded them from the analysis if subcutaneous fat thickness was found during surgery to be less than 2cm. This procedure could cause several problems. Firstly, a strict intention to treat analysis was not done because a large proportion of those randomised were found not to be eligible (374/964). Secondly, consent was obtained from 374 women who were not eligible, which may be ethically questionable. Thirdly, assessment of eligibility once the woman's randomised group was already known could introduce bias. Surgeons' decisions about eligibility in cases where the subcutaneous fat thickness was close to 2cm may have been influenced, consciously or unconsciously, by the random assignment. However, no differences between the groups in baseline characteristics were apparent. The other studies designed to look at drain use in obese women randomised during the operation, after measurement of the subcutaneous fat.
Blinding
Blinding of participants would be difficult, and blinding of surgeons impossible when drains are being used. We have assumed that neither of these were done. No study mentioned any attempt at blinding the people who were assessing outcomes. Blinding of outcome assessment is possible and it is important to achieve this especially when the assessment of outcomes can be subjective, for example if infection is judged to be present on appearance alone, or if a postoperative complication is viewed as relevant.
Exclusions and ITT analysis
The Magann study had a high level of post‐randomisation exclusions, as noted above. These were not reported by randomised group, but the groups as analysed were of similar size, so it seems unlikely that the number of exclusions was substantially different between the groups.
There was no report of any post‐randomisation exclusions in five studies, and only small numbers were excluded in another three (Al Inany 2002, 2 women; Maharaj 2000, 3 women; Ramsey 2005 12 women). No outcome information for these women was given by Al Inany 2002 or Ramsey 2005, so they could not be included in the review. It was unclear for Maharaj 2000 whether the three excluded women (who were all allocated to no drainage but received drains) were in fact included in the results or not. For this review we have used the data as published, assuming that data on the three "exclusions" are reported, pending further investigation.
CAESAR 2010 randomised women before the start of surgery, and a small number of women delivered vaginally (30/3033). Two women also withdrew consent before caesarean section. These 32 women (1%) were excluded from the analysis.
Magann 2002 followed up all women at 2 to 6 weeks, but suffered from a high rate of loss to follow up at this point (24%). However, the results reported in the paper did not refer only to those women who were successfully followed up at 2 weeks, but included data from hospital discharge, when all women were examined. Thus, it would not be correct to use the number of women followed up as the denominator, as some of those who had outcomes may not have had a follow‐up examination. The numerator used in the review is therefore the number of each outcome reported. The denominator used is the number of women for whom outcome information was available (in this case all the women randomised). This is likely to lead to an underestimate of the true risk of the outcomes.
Overall risk of bias
The overall methodological quality of most studies was moderate or unclear. One large study (CAESAR 2010) was of good methodological quality. Two studies had specific bias risks: Loong 1988 did not appear to have used an adequate method of allocation concealment, and Magann 2002 may have been subject to bias introduced by differential withdrawal or ineligibility. No studies performed blinded outcome assessment so there is potential for bias to be introduced in all (Figure 1; Figure 2).
1.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
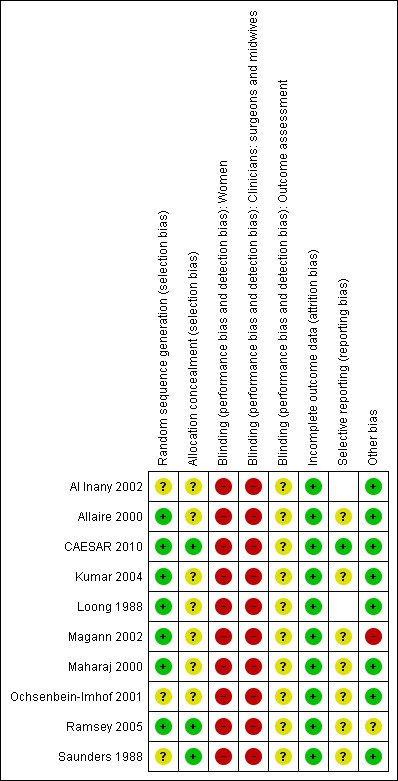
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Comparison 1: Wound drain compared with no drain
Primary outcomes
Wound drainage did not appear to reduce the incidence of wound infections (Analysis 1.1; RR 1.02, 95% CI 0.85 to 1.21); 7 trials, 4377 women), or febrile morbidity (Analysis 1.3; RR 0.87, 95% CI 0.66 to 1.15); 6 studies, 3829 women). The incidence of febrile morbidity varied widely between the trials, from less than 1% (CAESAR 2010; Ochsenbein‐Imhof 2001) to 27% (Loong 1988; Saunders 1988). This may reflect different incidence of the outcome in different populations, but also different definitions of febrile morbidity. CAESAR 2010 reported maternal fever in several ways; temperature >390 C on any occasion, temperature >380 C on two or more days, and antibiotics given for febrile morbidity. We have used antibiotics given for febrile morbidity as the outcome in the review; the numbers with temperature > 380 C on two or more days were very similar, and the incidence of temperature > 390 C was very low.
1.1. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 1 Wound infection.
1.3. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 3 Febrile morbidity.
The results for wound complications were heterogeneous (Analysis 1.2; I2 = 57% and Tau2 = 0.15, 6 studies, 1640 women), which may have been partly due to differing definitions of "wound complications" between studies. This outcome was summarised with a random effects analysis. The average treatment effect was RR 0.85 (95% CI 0.55 to 1.32) and the 95% prediction interval was 0.25 to 2.94. This analysis suggests that there is no evidence of a reduction in wound complications with drainage, but individual studies may find widely differing effects. The width of the prediction interval reflects the relatively small number of studies and the inconsistency between them. The largest study, CAESAR 2010, did not report the composite outcome wound complications, but did report operative procedures on the wound 12/1398 in the drain group versus 5/1398 in the no drain group; RR 2.40 (95% CI 0.85 to 6.79).
1.2. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 2 Wound complications.
Postoperative pain was reported by two studies (CAESAR 2010; Kumar 2004). Both used a visual analogue scale (VAS). Kumar 2004 (using a 10cm VAS) found no difference between the drain and no drain groups (mean difference ‐0.15, 95% CI ‐0.36 to 0.06; Analysis 1.4). CAESAR 2010 reported medians and IQRs for pain at hospital discharge and at 6 weeks post‐partum (Table 1). No differences were found between the liberal and restricted drain use groups.
1.4. Analysis.
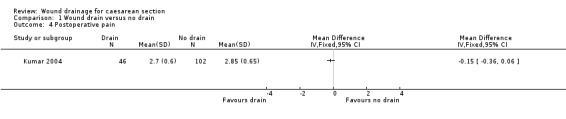
Comparison 1 Wound drain versus no drain, Outcome 4 Postoperative pain.
1. Pain outcomes in CAESAR 2010.
| Drain (liberal use) |
No drain (restricted use) |
P‐value (rank sum test) | |
| Hospital discharge | 20 (9‐37) | 19 (8‐34) | 0.2 |
| 6 weeks | 3 (0‐11) | 2 (0‐10) | 0.1 |
Secondary outcomes
Two trials analysed the difference in mean blood loss between the groups. The meta‐analysis showed an average of 23 ml more blood loss in the drain group (95% CI ‐1.93 to 48.74) (Analysis 1.5). This suggests that if there is an advantage to wound drainage, it is small and probably not clinically significant. CAESAR 2010 reported blood transfusion (Analysis 1.6); there was no evidence of a difference between the groups (RR 1.02, 95% CI 0.70 to 1.48).
1.5. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 5 Blood loss (ml).
1.6. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 6 Blood transfusion.
Four trials reported the duration of surgery. There was substantial heterogeneity in this outcome (Tau2 = 16.21 and I2 = 93%) and we judged that the results were too different to combine, hence no meta‐analysis was performed. Two trials found no difference, one found that duration of surgery was 3.4 minutes longer in the drain group, and the third found that it was 10 minutes longer in the drain group (Analysis 1.7). It would be expected that an operation including the insertion of a drain would take longer, and it is not clear whether differences in the duration of operation of a few minutes have any impact on women's health.
1.7. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 7 Duration of surgery (mins).
Five studies reported the effects on postnatal hospital stay between the drain and no drain groups. The results were heterogeneous, with four studies finding no difference but one (Ochsenbein‐Imhof 2001) finding that hospital stay was 0.9 days shorter in the no drain group. These results were not combined because of the high heterogeneity (Analysis 1.8).
1.8. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 8 Duration of postnatal hospital stay (days).
Two studies (Ramsey 2005; CAESAR 2010) reported readmission of women to hospital (Analysis 1.9). There was no evidence of a difference between the groups (RR 1.08, 95% CI 0.70 to 1.66).
1.9. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 9 Readmission to hospital.
Non‐prespecified outcomes
Two studies (CAESAR 2010; Magann 2002) reported the effects of wound drainage on endometritis (RR 1.20, 95% CI 0.90 to 1.59) (Analysis 1.12).
1.12. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 12 Endometritis.
CAESAR 2010 reported a composite outcome, infectious morbidity, as its primary outcome. This was defined as one or more of the following: (i) antibiotic use for maternal febrile morbidity during the postnatal hospital stay; (ii) endometritis; (iii) wound infection treated with antibiotics. This outcome occurred in 247/1398 women in the liberal drain use group and 228/1398 in the restricted drain use group (RR 1.08, 95% CI 0.92, 1.27).
Comparison 2: Subcutaneous drain compared with sub‐sheath drain
One small trial provided a comparison between two different types of drains (Loong 1988). The trial found significantly more wound infections when a subcutaneous drain was used, compared with a sub‐sheath drain (RR 5.42, 95% CI 1.28 to 22.98, Analysis 2.1). However, there were only 121 women in this comparison, and the trial used a method of allocation concealment that was probably insecure. The same trial did not demonstrate a difference in the risk of febrile morbidity between the groups (RR 1.28, 95% CI 0.70 to 2.34, Analysis 2.2), although the difference was in the same direction.
2.1. Analysis.

Comparison 2 Subcutaneous drain versus sub‐sheath drain, Outcome 1 Wound infection.
2.2. Analysis.

Comparison 2 Subcutaneous drain versus sub‐sheath drain, Outcome 2 Febrile morbidity.
Comparison 3: Drain compared with subcutaneous suture
Three trials compared drainage with subcutaneous suturing (Allaire 2000; Kumar 2004; Magann 2002). there was no evidence of a difference between the interventions in terms of wound infection (RR 0.77, 95% CI 0.42 to 1.44, Analysis 3.1) or wound complications (random effects analysis, RR 0.56 95% CI 0.17 to 1.87, Analysis 3.2; I2= 44%, Tau2=0.51). The 95% prediction interval for this analysis was extremely wide and encompassed all plausible values; hence there is little evidence about the likely range of treatment effects, due to the small number of trials in this analysis. Febrile morbidity, blood loss, duration of surgery, duration of postnatal hospital stay, postoperative pain and endometritis were reported by only one trial, and none showed any evidence of a difference between the interventions (Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8).
3.1. Analysis.

Comparison 3 Wound drain versus subcutaneous suture, Outcome 1 Wound infection.
3.2. Analysis.

Comparison 3 Wound drain versus subcutaneous suture, Outcome 2 Wound complications.
3.3. Analysis.

Comparison 3 Wound drain versus subcutaneous suture, Outcome 3 Febrile morbidity.
3.4. Analysis.

Comparison 3 Wound drain versus subcutaneous suture, Outcome 4 Blood loss.
3.5. Analysis.
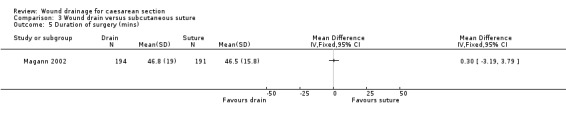
Comparison 3 Wound drain versus subcutaneous suture, Outcome 5 Duration of surgery (mins).
3.6. Analysis.

Comparison 3 Wound drain versus subcutaneous suture, Outcome 6 Duration of postnatal hospital (days).
3.7. Analysis.

Comparison 3 Wound drain versus subcutaneous suture, Outcome 7 Postoperative pain.
3.8. Analysis.

Comparison 3 Wound drain versus subcutaneous suture, Outcome 8 Endometritis.
Sub‐group analyses
The prespecified sub‐group analyses were not performed because insufficient data were available. CAESAR 2010 reported that there was no evidence of a difference in outcomes between caesarean sections performed in labour and not in labour, but did not present the relevant data.
The non prespecified subgroup analysis of obese women compared with unselected populations showed no differences in wound infection (obese, 3 trials: RR 0.86, 95% CI 0.50 to 1.51; unselected, 3 trials: RR 0.95, 95% CI 0.48 to 1.87), wound complications or febrile morbidity.
Sensitivity analyses
Exclusion of the study with inadequate allocation concealment (Loong 1988) from Comparison 1 made no substantial difference to the results.
Discussion
Summary of main results
For the comparison of drain with no drain, there were no clear differences in any of the outcomes measured, though the point estimates for wound complications and febrile morbidity were in the direction of benefit to wound drainage. The point estimates for blood loss, endometritis and breastfeeding were in the opposite direction, but were based on only one or two trials. There were inconsistent results on duration of postnatal hospital stay and duration of surgery.
Data comparing sub‐sheath with subcutaneous drains were available from only one trial, and favoured sub‐sheath drainage for wound infection.
Comparison of wound drainage with subcutaneous suturing found no clear differences in outcome between the groups.
Overall completeness and applicability of evidence
The trials included in the review were conducted in a variety of different countries with different health care systems, and consequently differences in the care provided outside the trial and in the surgical techniques used during the caesarean section operation. There were also clear differences between the populations included in the trials, with five of them restricted to obese women. This also may have introduced heterogeneity, as it is possible that the interventions studies may be either more or less effective in women with a thick layer of subcutaneous fat. We did not find any differences in results between studies that recruited obese women and those that recruited unselected populations, but there were few studies and these analyses lacked power.
The analysis of CAESAR 2010 explored interactions between the pairs of randomised interventions in this 2x2x2 factorial trial. It found that there was evidence of an interaction between closure of the peritoneum and use of a sub rectus sheath drain for the primary outcome, infectious morbidity. In women allocated liberal drain use, there was a higher incidence of infectious morbidity in those who had non‐closure of the pelvic peritoneum (20.8% versus 14.8% in the group with closure of the pelvic peritoneum), but in women allocated to restricted drain use, the incidence of infectious morbidity was lower among those allocated to peritoneal closure (15.6% versus 17.7%; interaction test P=0.006). Similar effects were apparent for wound infection (P=0.01) and duration of surgery (P=0.005). These results suggest that the treatment effect of drainage versus no drainage may depend on other surgical techniques that are used. This may introduce heterogeneity into the results of trials, and mean that evaluation of individual aspects of the operation may not identify the most effective techniques.
Quality of the evidence
Most of the included studies had small to moderate sample sizes, which were inadequate to detect clinically important differences in relatively rare outcomes. Only two trials (Loong 1988 and Maharaj 2000) documented having carried out a sample size calculation before the trial. One study was much larger (CAESAR 2010), recruiting over 2800 women, and had the fewest methodological issues, so this trial is likely to provide the best quality evidence.
Methodological quality was generally moderate or unclear. Information about allocation concealment and blinding was limited, and no studies reported measures to blind outcome assessment. Blinding of outcome assessment is important to achieve especially when the assessment of outcomes can be subjective, for example if infection is judged to be present on appearance alone.One study did not use a secure method of allocation concealment, and another study had a query over post‐randomisation exclusions.
Studies also reported different outcome measures, so that although ten trials compared wound drainage with no drainage, no analysis contained data from more than seven trials. Follow‐up beyond hospital discharge was very limited, and no studies have yet reported long‐term follow‐up. The effects of these techniques for subsequent pregnancies and long‐term morbidity remain unknown.
Authors' conclusions
Implications for practice.
The existing evidence from ten trials does not show benefit from wound drainage at caesarean section, and is compatible with modest benefit or harm. The trials did not address the question of whether wound drainage is of benefit when haemostasis is not felt to be adequate.
Implications for research.
Assessment of interventions' effects on longer term outcomes is needed. It may also be of interest to explore the role of different types and sites of wound drain, the effects of different degrees of obesity, first or repeat caesarean section, and whether the caesarean section is performed intrapartum or pre labour, and the interactions of wound drainage with other aspects of the caesarean section operation.
What's new
| Date | Event | Description |
|---|---|---|
| 14 November 2013 | New citation required and conclusions have changed | Second update, new search. Conclusions strengthened. |
| 14 November 2013 | New search has been performed | One trial added (CAESAR 2010). Risk of bias assessments, analyses and text updated. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 3 August 2010 | New search has been performed | Updated search and analyses, two trials added: Kumar 2004, Ramsey 2005, risk of bias completed and conclusions remain unchanged. |
| 11 November 2008 | Amended | Contact details updated |
| 8 August 2008 | Amended | Converted to new review format. |
| 21 September 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the following referees for their comments on the review: Cochrane Wounds Group Editors (Nicky Cullum, Andrew Jull, Mieke Flour, Seokyung Hahn); Referees (Mary Steen, Saba Balasubramanian, Sheila Benton‐Jones, Jodie Dodd). M. Enkin and C. Williamson conducted the first version of this Cochrane review, on which these updates are based.
Appendices
Appendix 1. Search methods used in the first review update (2010)
Electronic searches
For this first update we searched the following databases:
Cochrane Wounds Group Specialised Register (Searched 3/8/10); The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010 Issue 3); Ovid MEDLINE ‐ 1950 to July Week 3 2010; Ovid MEDLINE ‐ In‐Process & Other Non‐Indexed Citations August 02, 2010; Ovid EMBASE ‐ 1980 to 2010 Week 30; EBSCO CINAHL ‐ 1982 to July 30 2010 Cochrane Wounds Group Specialised Register (Searched 24/2/10).
The following search terms were used: #1 MeSH descriptor Cesarean Section explode all trees #2 caesarean or caesarian or cesarean or cesarian:ti,ab,kw #3 (#1 OR #2) #4 MeSH descriptor Drainage explode all trees #5 MeSH descriptor Suction explode all trees #6 drain*:ti,ab,kw #7 (#4 OR #5 OR #6) #8 (#3 AND #7) We have provided the search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL in; Appendix 2, Appendix 3 and Appendix 4 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2009). We combined the Ovid EMBASE and EBSCO CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2009).¬There were no restrictions on the basis of date or language of publication.
Searching other resources
We scrutinised the reference lists of relevant reviews and trials to identify additional studies.
Appendix 2. Search strategy Ovid MEDLINE
1 exp Cesarean Section/ (34780) 2 (caesarean or caesarian or cesarean or cesarian).ti,ab. (39971) 3 or/1‐2 (50972) 4 exp Drainage/ (44586) 5 exp Suction/ (10337) 6 drain$.ti,ab. (87148) 7 or/4‐6 (111614) 8 3 and 7 (372) 9 randomized controlled trial.pt. (389866) 10 controlled clinical trial.pt. (89904) 11 randomized.ab. (287333) 12 placebo.ab. (156850) 13 clinical trials as topic.sh. (175428) 14 randomly.ab. (199448) 15 trial.ti. (124434) 16 or/9‐15 (894315) 17 Animals/ (5502996) 18 Humans/ (13679581) 19 17 not 18 (3968230) 20 16 not 19 (823783) 21 8 and 20 (36)
Appendix 3. Search strategy Ovid EMBASE
1 exp Cesarean Section/ (60871) 2 (caesarean or caesarian or cesarean or cesarian).ti,ab. (55695) 3 or/1‐2 (74109) 4 exp Wound Drainage/ (18262) 5 exp Suction Drainage/ (1499) 6 drain$.ti,ab. (120334) 7 or/4‐6 (130285) 8 3 and 7 (484) 9 exp Clinical trial/ (1010415) 10 Randomized controlled trial/ (362324) 11 Randomization/ (63888) 12 Single blind procedure/ (18503) 13 Double blind procedure/ (121099) 14 Crossover procedure/ (38963) 15 Placebo/ (241295) 16 Randomi?ed controlled trial$.tw. (96312) 17 RCT.tw. (12994) 18 Random allocation.tw. (1349) 19 Randomly allocated.tw. (20202) 20 Allocated randomly.tw. (1957) 21 (allocated adj2 random).tw. (817) 22 Single blind$.tw. (14347) 23 Double blind$.tw. (148182) 24 ((treble or triple) adj blind$).tw. (362) 25 Placebo$.tw. (202745) 26 Prospective study/ (254703) 27 or/9‐26 (1450393) 28 Case study/ (22250) 29 Case report.tw. (265341) 30 Abstract report/ or letter/ (911048) 31 or/28‐30 (1193166) 32 27 not 31 (1412063) 33 animal/ (1893976) 34 human/ (14948350) 35 33 not 34 (1410270) 36 32 not 35 (1384081) 37 8 and 36 (32)
Appendix 4. Search strategy EBSCO CINAHL
S8 S7 and S3 S7 S6 or S5 or S4 S6 drain* S5 (MH "Suction+") S4 (MH "Drainage+") S3 (S2 or S1) S2 caesarean or caesarian or cesarean or cesarian S1 (MH "Cesarean Section+")
Data and analyses
Comparison 1. Wound drain versus no drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 7 | 4377 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.21] |
| 2 Wound complications | 6 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.32] |
| 3 Febrile morbidity | 6 | 3829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.66, 1.15] |
| 4 Postoperative pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Blood loss (ml) | 2 | 1030 | Mean Difference (IV, Fixed, 95% CI) | 23.41 [‐1.93, 48.74] |
| 6 Blood transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Duration of surgery (mins) | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Duration of postnatal hospital stay (days) | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Readmission to hospital | 2 | 3064 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.70, 1.66] |
| 10 Breastfeeding at hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Postoperative analgesia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Endometritis | 2 | 3386 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.90, 1.59] |
| 13 Operative procedures on wound | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.10. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 10 Breastfeeding at hospital discharge.
1.11. Analysis.
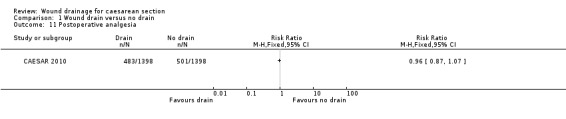
Comparison 1 Wound drain versus no drain, Outcome 11 Postoperative analgesia.
1.13. Analysis.

Comparison 1 Wound drain versus no drain, Outcome 13 Operative procedures on wound.
Comparison 2. Subcutaneous drain versus sub‐sheath drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Febrile morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Wound drain versus subcutaneous suture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 3 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.44] |
| 2 Wound complications | 3 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.17, 1.87] |
| 3 Febrile morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Blood loss | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Duration of surgery (mins) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Duration of postnatal hospital (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Postoperative pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Endometritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al Inany 2002.
| Methods | Randomised controlled trial. 2:1 randomisation (drain: no drain). | |
| Participants | 120 obese women undergoing caesarean section with a body mass index of more than 32.
80 women in the drain group and 40 women in the 'no drain' group.
2 women were excluded because they left the hospital after two days and did not complete follow‐up. Excluded if: pre‐labour caesarean section; prolonged pre‐labour rupture of membranes; prolonged labour; "long pre‐operative hospitalisation"; chronic illness. Included if: first or repeat caesarean; intrapartum caesarean. All skin incisions were Pfannenstiel incisions. No subcutaneous sutures were used. |
|
| Interventions | Treatment group: subcutaneous suction drain.
Control group: no subcutaneous drain. Drains were left in for 24 hours or until the drainage was <50 ml (time period not specified). |
|
| Outcomes | Wound breakdown, wound haematoma and post‐operative febrile morbidity (> 38.5C, >24 hours and < 5 days) incidence available on 118 women. Wounds assessed prior to discharge, 5‐7 days post‐operatively and at 3 weeks. | |
| Notes | Trial conducted in Egypt 1999 to 2000. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | "Participants were allocated using sealed envelopes" ‐ numbering, opacity or method of using sealed envelopes not mentioned, not whether all envelopes were accounted for. |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed not done. |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | Not possible. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We have assumed that the two women "excluded from the study" had been randomised. 120 randomised, outcome data for 118. |
| Other bias | Low risk | |
Allaire 2000.
| Methods | Randomised controlled trial. Randomisation during surgery after fat depth had been measured. | |
| Participants | 76 women undergoing caesarean section with more than 2cm of subcutaneous fat.
24 women in the drain group and 52 women in the 'no drain' group. Excluded if no time for adequate consent. Included if: first or repeat caesarean; pre labour or intrapartum caesarean. The type of skin incision was not described. |
|
| Interventions | Treatment group: subcutaneous drain.
Control group for this review: no subcutaneous drain (with or without closure of the subcutaneous tissue). Three randomised groups: subcutaneous drain used; closure of subcutaneous tissue; no closure of subcutaneous tissue or use of subcutaneous drain. Drains were "removed in 72 hours" or when drainage was <50 ml in 24 hours. |
|
| Outcomes | Wound infection and wound complication incidence available on all randomised women.
No loss to follow‐up documented. Wounds assessed prior to discharge and at staple removal (7 to 10 days post‐partum). Further complications identified by retrospective chart review (timing not stated). |
|
| Notes | Trial conducted in USA 1995 to 1997. 1109 women delivered by caesarean section; 76 enrolled in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Unclear risk | Numbers kept in opaque sealed envelopes. No information on whether all envelopes were accounted for. |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed not done. |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | Not possible. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses documented. |
| Selective reporting (reporting bias) | Unclear risk | No information. |
| Other bias | Low risk | |
CAESAR 2010.
| Methods | Randomised controlled 2x2x2 factorial trial. Comparisons were: 1. single‐ versus double‐layer closure of the uterine incision; 2. closure versus nonclosure of the pelvic peritoneum; 3. liberal versus restricted use of a sub rectus sheath drain. 2827 women randomised to the liberal versus restricted drainage comparison. |
|
| Participants | Women having first caesarean section; uterine incision via lower segment; age 16 years or over. 1414 randomised to liberal use of sub‐sheath drain. 1413 randomised to restricted use of sub‐‐sheath drain. |
|
| Interventions | liberal use of a sub‐sheath drain; drain should be used unless there was an overriding clinical reason not to use it. restricted use of a sub‐sheath drain; drain should not be used unless there was an overriding reason to use it. |
|
| Outcomes | Primary outcome: maternal infectious morbidity, defined as having one or more of the following: (i) antibiotic use for maternal febrile morbidity during the postnatal hospital stay; (ii) endometritis; (iii) wound infection treated with antibiotics Secondary outcomes: antibiotic use, endometritis, wound infection treated with antibiotics, further operative procedures on the wound, pain, blood transfusion, breastfeeding at hospital discharge and at 6 weeks, other severe or unexpected maternal morbidity. |
|
| Notes | Wound drain was used in 63% of women allocated to liberal use, and 6% of women allocated to restricted use. Primary outcome was changed during recruitment from febrile morbidity/endometritis to infectious morbidity, because of low incidence of the original primary outcome. Trial conducted in UK and Italy, 2000 to 2006. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence. |
| Allocation concealment (selection bias) | Low risk | Remote telephone randomisation system with minimisation for participating centre; ‘in labour’ or ‘not in labour’; single or multiple pregnancy. |
| Blinding (performance bias and detection bias) Women | High risk | Although trial report states that women were unaware of the allocations used, they would probably have been aware of whether they had a drain or not. |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | Not possible to blind clinicians. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Outcomes recorded from hospital notes; therefore likely that they could have been recorded with knowledge of allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomisation before the start of surgery, hence 30 women excluded because they did not deliver by caesarean section, and 2 excluded because they withdrew consent prior to surgery.. Approx 10% loss to follow‐up for outcomes at 6 weeks. |
| Selective reporting (reporting bias) | Low risk | OUtcomes reported are specified in the study protocol. |
| Other bias | Low risk | |
Kumar 2004.
| Methods | Randomised controlled trial. Randomisation during surgery. Outcomes measured at 2 and 6 weeks. | |
| Participants | 148 women requiring caesarean section with >2cm subcutaneous fat (measured during surgery). 46 in the drain group and 102 in the no drain group. No exclusions mentioned. |
|
| Interventions | Treatment group: subcutaneous drain.
Control group for this review: no subcutaneous drain (with or without closure of the subcutaneous tissue). Randomisation was to three groups: 1. subcutaneous drain; 2. subcutaneous stitch; 3. neither drain nor stitch. Groups 2 and 3 combined for the review to give a comparison of drain versus no drain. |
|
| Outcomes | Post‐operative febrile morbidity, wound infection, wound complications. operative procedures on wound, duration of postnatal hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes opened in operating theatre after closure of rectus sheath. No mention of numbering or accounting for all envelopes. |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed no blinding. |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | Not possible. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Unclear risk | No information. |
| Other bias | Low risk | |
Loong 1988.
| Methods | Randomised controlled trial. Randomisation during surgery. | |
| Participants | 400 women undergoing caesarean section; unclear how many randomised.
262 women included, 138 women excluded.
193 women in the drain group and 69 women in the 'no drain' group. Excluded if: pre‐existing pyrexia; prophylactic antibiotics. Included if: first or repeat caesarean; pre labour or intrapartum caesarean. Obesity unspecified. All skin incisions were Pfannenstiel incisions. |
|
| Interventions | Treatment group: drain used (subcutaneous or sub‐rectus).
Control group: no drain. Four randomised groups: subcutaneous drain used; sub‐rectus sheath drain used; both drains used; no drain used. Drains left in for >6 hours and <36 hours. |
|
| Outcomes | Wound infection and febrile morbidity incidence available on 262 women.
No loss to follow‐up documented. Timing of wound assessment not recorded. |
|
| Notes | Trial conducted in Hong Kong 1986. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information on how random numbers were used to perform allocations |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed not done |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | Not possible |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up mentioned. |
| Other bias | Low risk | |
Magann 2002.
| Methods | Randomised controlled trial. Randomisation by computer generated random number sequence. | |
| Participants | 590 women undergoing caesarean section were deemed eligible intraoperatively because they had more than 2cm of subcutaneous fat.
964 women undergoing caesarean section were however randomised preoperatively.
194 women in the drain group and 396 women in the 'no drain' group Excluded if: no time for adequate consent; less than 2cm of subcutaneous fat (when measured intraoperatively). Included if: first or repeat caesarean; pre labour or intrapartum caesarean; vertical or transverse skin incision. |
|
| Interventions | Treatment group: subcutaneous drain used.
Control group for this review: no subcutaneous drain (with or without subcutaneous tissue closure). Three randomised groups: subcutaneous drain used; closure of subcutaneous tissue; no closure of subcutaneous tissue or use of subcutaneous drain. The timing of drain removal was not documented. |
|
| Outcomes | Wound infection, wound complication and endometritis incidence available on all randomised women at staple removal (7 to 10 days post‐partum).
Mean blood loss and mean duration of operation also available. Unclear at which point in the follow‐up period the recorded outcomes were diagnosed. |
|
| Notes | Trial conducted in USA 1998 to 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Numbers kept in opaque envelopes. No information on whether all envelopes were accounted for. |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed not done |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | Not possible |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up documented up to discharge from hospital. |
| Selective reporting (reporting bias) | Unclear risk | No information. |
| Other bias | High risk | Pre‐operative randomisation means that 374 women were excluded post‐randomisation because they did not fulfil the eligibility criteria (depth of subcutaneous tissue <2cm). Possible for this to introduce bias. |
Maharaj 2000.
| Methods | Randomised controlled trial. | |
| Participants | 440 women undergoing 'emergency' caesarean section.
217 women in the drain group and 223 women in the 'no drain' group
3 women from the 'no drain' group excluded because they required a drain. It is not clear whether these were included in the 223 or not. Excluded if: elective caesarean section; vertical skin incision; existing intra‐uterine infection; drain definitely required; Included if: Pfannenstiel incision. Not specified if first or repeat caesarean, or pre labour or intrapartum caesarean. |
|
| Interventions | Treatment group: subcutaneous drain and sub‐rectus sheath drain.
Control group: no drains. Drains removed at 48 hours or when drainage stopped. |
|
| Outcomes | Wound 'morbidity', haematoma, purulent discharge and wound dehiscence available on all randomised women at discharge or day 7. Mean blood loss, mean duration of surgery and mean post‐natal stay also recorded. | |
| Notes | Trial conducted in South Africa 1996 to 1997 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Numbers kept in opaque envelopes. No information on whether all envelopes were accounted for. |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed not done |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up documented at discharge from hospital |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Low risk | |
Ochsenbein‐Imhof 2001.
| Methods | Randomised controlled trial. | |
| Participants | 305 women undergoing caesarean section.
151 women in the drain group and 154 women in the 'no drain' group. Excluded if: 'emergency' caesarean section; pre‐eclampsia; bleeding diathesis; increased risk of bleeding; severe fetal deformity. Included if: elective or intrapartum caesarean section. Not specified if: first or repeat caesarean; obese or non‐obese; transverse or vertical skin incision. |
|
| Interventions | Treatment group: subcutaneous and sub‐rectus sheath suction drains.
Control group: no drains. The timing of drain removal was not documented. |
|
| Outcomes | Wound haematoma, need for further surgery, mean amount of opiate analgesia, mean duration of surgery and mean duration of post‐natal stay results available on all randomised women at discharge. Wounds assessed at discharge (time‐period not specified). |
|
| Notes | Trial conducted in Switzerland 1998 to 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Numbers kept in opaque envelopes. No information on whether all envelopes were accounted for. |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed not done |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up documented at discharge from hospital |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Low risk | |
Ramsey 2005.
| Methods | Randomised controlled trial. Randomisation during surgery. Outcomes measured at 7‐14 days post discharge and 4‐6 weeks. | |
| Participants | 280 women. Delivery by caesarean section, BMI >= 30 at admission, subcutaneous fat >=4cm (measured during surgery). Exclusion criteria: 1. no consent; 2. moribund caesarean delivery; 3. no plan for postpartum care in recruiting centre. | |
| Interventions | Treatment group: Subcutaneous suture plus drain (131 women) Control group: subcutaneous suture only (149 women). | |
| Outcomes | Wound complications, readmission to hospital. | |
| Notes | Report states that randomisation block size was 20 but there is a discrepancy of 18 between the numbers randomised to the two arms; this would be impossible with a block size of 20 unless there were patients missing from the analysis. Therefore we suspect that there are unreported post‐randomisation exclusions. Outcome data given as percentages not numbers, so there is doubt about exact numbers in some cases. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated, block size = 20. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed not blinded. |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | Not possible. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 women (4.3%) did not have follow‐up wound assessment (reasons not given). |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Discrepancy of 18 in numbers randomised to each group despite block size of 20. Suggests that there may be women randomised to drain group missing. |
Saunders 1988.
| Methods | Randomised controlled trial. | |
| Participants | 200 women undergoing lower‐segment caesarean section.
100 women in the drain group and 100 women in the 'no drain' group Excluded if: drain definitely required; Included if: first or repeat caesarean; pre labour or intrapartum caesarean. All skin incisions were Pfannenstiel incisions. Not specified if: obese or non‐obese. |
|
| Interventions | Treatment group: sub‐rectus sheath suction drain.
Control group: no drain. Drains removed at 48 hours or when drainage stopped. |
|
| Outcomes | Wound haematoma, wound infection, mean number of analgesic doses and mean length of hospital stay available on all randomised women at discharge. Wounds assessed at 2, 4 and 6 days post‐operatively. |
|
| Notes | Trial conducted in UK. Published in 1988. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | 200 opaque sealed envelopes shuffled and stored in the operating theatre. All envelopes accounted for. |
| Blinding (performance bias and detection bias) Women | High risk | Not mentioned, assumed not done. |
| Blinding (performance bias and detection bias) Clinicians: surgeons and midwives | High risk | Not possible |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up documented at discharge from hospital |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bose 2006 | abstract only, author contacted for further information, no response. |
Contributions of authors
Both review authors contributed to writing the protocol, assessing studies and extracting data, writing the review and undertaking the updates.
Contributions of editorial base:
Nicky Cullum: edited the review, advised on methodology, interpretation and review content. Approved the final review and review update prior to submission. Joan Webster, Editor: approved the second review update prior to submission. Sally Bell‐Syer: coordinated the editorial process. Advised on methodology, interpretation and content. Edited and copy edited the review and the updated reviews. Ruth Foxlee: designed the search strategy, ran the searches and edited the search methods section for the updates.
Sources of support
Internal sources
Department of Health, UK.
External sources
No sources of support supplied
Declarations of interest
Both review authors were involved in the conduct of the CAESAR trial, which is included in this review, while they were employed at the National Perinatal Epidemiology Unit, University of Oxford, UK. Neither of the authors was involved in the analysis or reporting of this trial.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Al Inany 2002 {published data only}
- Al Inany H, Youssef G, Maguid AA, Hamid MA, Naguib A. Value of subcutaneous drainage system in obese females undergoing cesarean section using Pfannenstiel incision. Gynecologic and Obstetric Investigation 2002;53(2):75‐8. [DOI] [PubMed] [Google Scholar]
Allaire 2000 {published data only}
- Allaire A, Fisch J, McMahon M. A prospective randomized trial of subcutaneous drain versus subcutaneous suture in obese women undergoing cesarean section. American Journal of Obstetrics and Gynecology 1998;178(1 (Pt 2)):S78. [Google Scholar]
- Allaire AD, Fisch J, McMahon MJ. Subcutaneous drain vs. suture in obese women undergoing cesarean delivery: A prospective, randomized trial. Journal of Reproductive Medicine 2000;45(4):327‐31. [PubMed] [Google Scholar]
CAESAR 2010 {published data only}
- CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG 2010;117(11):1366‐76. [DOI] [PubMed] [Google Scholar]
Kumar 2004 {published data only}
- Kumar SA. Subcutaneous drain versus subcutaneous stitch closure to prevent wound disruption after cesarean section. Journal of Obstetrics and Gynecology of India 2004;54(3):237‐42. [Google Scholar]
Loong 1988 {published data only}
- Loong R, Rogers M, Chang A. A controlled trial on wound drainage in Caesarean section. Australian and New Zealand Journal of Obstetrics and Gynaecology 1988;28(4):266‐9. [DOI] [PubMed] [Google Scholar]
Magann 2002 {published data only}
- Magann E, Chauhan S, Rodts Palenik S, Bufkin L, Martin JN Jr, et al. Subcutaneous stitch closure versus subcutaneous drain to prevent wound disruption after cesarean delivery: A randomized clinical trial. American Journal of Obstetrics and Gynecology 2002;186(6):1119‐23. [DOI] [PubMed] [Google Scholar]
Maharaj 2000 {published data only}
- Maharaj D, Bagratee JS, Moodley J. Drainage at caesarean section ‐ A randomised prospective study. South African Journal of Surgery 2000;38(1):9‐12. [PubMed] [Google Scholar]
Ochsenbein‐Imhof 2001 {published data only}
- Imhof N, Hebisch G, Huch A, Huch R, Zimmerman R. Use of drainage vs no drain for caesarean section. Gynäkologisch‐geburtshilfliche Rundschau 1999;39:164. [Google Scholar]
- Ochsenbein‐Imhof N, Huch A, Huch R, Zimmermann R. No benefit from post‐caesarean wound drainage. Swiss Medical Weekly 2001;131(17‐18):248‐50. [DOI] [PubMed] [Google Scholar]
Ramsey 2005 {published data only}
- Ramsey PS, White AM, Duinn DA, Lu GC, Ramin SM, Davies JK, et al. Subcutaneous tissue re approximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstetrics and Gynecology 2005;105(5 Part 1):967‐73. [DOI] [PubMed] [Google Scholar]
Saunders 1988 {published data only}
- Saunders NJ, Barclay C. Closed suction wound drainage and lower‐segment caesarean section. British Journal of Obstetrics and Gynaecology 1988;95(10):1060‐2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bose 2006 {published data only}
- Bose M, Pannigrahi R, Mohapatra K, Patel O, Sahoo LN. Subcutaneous drain versus subcutaneous stitch closure to reduce postoperative morbidity following cesarean section [abstract]. 49th All India Congress of Obstetrics and Gynaecology; Jan 6‐9 2006; Cochin, Kerala, India. 2006.
Additional references
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001. [Google Scholar]
Enkin 1995
- Enkin MW. Closed suction wound drainage at caesarean section. Cochrane Database of Systematic Reviews 1995, Issue Disk Issue 2. [Google Scholar]
Higgins 2002
- Higgins J, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2009b
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society, Series A 2009;172:137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT and Altman DG on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2008
- Hofmeyr GJ, Mathai M, Shah AN, Novikova N. Techniques for caesarean section. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004662.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SIGN 2009
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 9 June 2009).
Tully 2002
- Tully L, Gates S, Brocklehurst P, McKenzie‐McHarg K, Ayers S. Surgical techniques used during caesarean section operations: results of a national survey of practice in the UK. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;102(2):120‐6. [DOI] [PubMed] [Google Scholar]


