Abstract
ABSTRACT
Objectives
This systematic review investigated available evidence on the stand-alone and incremental predictive performance of ophthalmic artery Doppler (OAD) for pre-eclampsia.
Design
Systematic review.
Data sources
We conducted a literature search from PubMed (Medline), the Cochrane CENTRAL, EMBASE and Scopus from inception to 8 April 2025.
Eligibility criteria
Studies eligible for inclusion were prospective or retrospective cohort studies, case-control studies or randomised controlled trials that reported on the predictive performance of OAD for pre-eclampsia in singleton pregnancies; and conducted in either high-income country (HIC) or low- and middle-income country (LMIC).
Data extraction and synthesis
Two reviewers independently screened and assessed articles for inclusion. One reviewer then extracted data using a standardised data extraction sheet, and any uncertainties were discussed with a second reviewer. The Prediction model Risk of Bias Assessment Tool was used for quality and risk of bias assessment. Findings were summarised and reported according to the Preferred Reporting Items for Systematic Review and Meta-Analyses statement and synthesised qualitatively.
Results
We identified and included 11 observational studies (3 from HIC and 8 from LMICs) with a total of 12 150 singleton pregnancies, of which 517 (4.3%) were complicated by pre-eclampsia at end of follow-up. The included studies were of varied quality, with three at low risk of bias, four at unclear risk and four at high risk. No interventional study was identified. Three studies (27.3%) recruited high-risk pregnancies (defined according to the American College of Obstetricians and Gynecologists (ACOG) criteria as one or more of the following: chronic hypertension, personal or family history of pre-eclampsia, early (≤18 years) or late (≥40 years) first pregnancy, primipaternity, chronic kidney disease, increased body mass index >30 kg/m2, presence of diabetes mellitus prior to pregnancy, autoimmune disease and thrombophilia), while eight studies (72.7%) recruited undetermined risk pregnancies. Stand-alone performance of OAD (interpreted by area under the receiver operating curve at 95% CI) showed that in the first trimester, the peak systolic velocity (PSV) ratio demonstrated very good predictive ability (0.97, 95% CI 0.92 to 1.0) (n=1 study), and the second PSV (PSV2) demonstrated very good predictive ability (0.91, 95% CI 0.82 to 0.99) (n=1 study). Also, PSV2 demonstrated fair predictive ability (0.61, 95% CI 0.42 to 0.79; and 0.53, 95% CI 0.40 to 0.66) for early and late pre-eclampsia, respectively (n=1 study). In the second trimester, the PSV ratio demonstrated very good predictive ability (0.88, 95% CI 0.84 to 0.91) (n=1 study), and PSV2 demonstrated good predictive ability (0.73, 95% CI 0.66 to 0.81; and 0.76, 95% CI 0.71 to 0.81) for pre-eclampsia (n=2 studies). In the third trimester, the PSV ratio demonstrated good predictive ability (0.82, 95% CI 0.73 to 0.89; and 0.77, 95% CI 0.71 to 0.82) for preterm and term pre-eclampsia, respectively (n=1 study). Also, PSV2 demonstrated good predictive ability 0.70 (0.57 to 0.84) (n=1 study).
Subsequently, in the second trimester, PSV ratio demonstrated better incremental predictive performance than uterine artery pulsatility index for preterm pre-eclampsia, when added to maternal factors and mean arterial pressure (MAP) (56.1%–80.2% vs 56.1%–74.8% detection rate (DR) at 10% FPR) (n=1 study). Also in the third trimester, adding PSV ratio to maternal factors and MAP was superior to soluble fms-like tyrosine kinase-1/placental growth factor ratio in predicting pre-eclampsia at <3 weeks after screening (96.7% vs 70% DR, p value 0.027) (n=1 study).
Conclusion
The ophthalmic artery PSV ratio and PSV2 are potentially useful ultrasound markers for pre-eclampsia prediction. Particularly in the second trimester, adding PSV ratio to maternal factors and MAP significantly improved the prediction of preterm pre-eclampsia. Given the burden of early and preterm pre-eclampsia in low-resource settings, OAD appears promising for pre-eclampsia screening in these settings where serum biomarkers may be expensive and inaccessible, and where uterine artery Doppler may not be technically feasible. However, the extent to which this novel marker is implemented in routine antenatal care should be guided by larger and sufficiently powered validation studies.
PROSPERO registration number
CRD42022324569.
Keywords: OBSTETRICS, Prenatal diagnosis, Maternal medicine, Ultrasound
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This systematic review employed a comprehensive search across a wide range of databases, including studies from both high-income and low- and middle-income countries, with no restrictions on publication date.
We applied a systematic and rigorous methodology for study screening, selection and data extraction, along with the use of the Prediction model Risk of Bias Assessment Tool for quality and risk of bias assessment of included prognostic studies.
Although aimed for, large heterogeneity across the studies did not allow for a meta-analysis.
The inclusion of articles only published in the English language may be a limitation, although we do expect the majority of these types of studies would have been published in English.
Introduction
Pre-eclampsia, a hypertensive disorder in pregnancy, remains a major cause of maternal and perinatal mortality and morbidity worldwide.1 Annually, pre-eclampsia results in approximately 42 000 maternal deaths2 and approximately between 1 in 4 to 10 perinatal deaths.3 Other maternal and perinatal adverse outcomes of pre-eclampsia include eclampsia; cerebral bleeding; haemolysis, elevated liver enzymes and low platelets syndrome; multiorgan damage and failure; fetal growth restriction; prematurity; placental abruption; stillbirth and birth asphyxia. These complications are prevalent among women in low- and middle-income countries (LMICs) where inadequate resources and inaccessibility to quality maternal and newborn care remain a major public health concern.4 5
Early identification of women at risk of developing pre-eclampsia is necessary to ensure timely initiation of preventive interventions such as aspirin, calcium, intensified monitoring and timely delivery. The need for early diagnosis has led to the development of several prediction models.12 6,8 At present, pre-eclampsia prediction models depend on maternal risk factors such as maternal age, race, weight, parity, multiple gestation, body mass index (BMI), history of chronic hypertension, pre-existing diabetes, history of pre-eclampsia, family history of pre-eclampsia,9 mean arterial pressure (MAP)10 and uterine artery pulsatility index (PI)11 and several serum biomarkers such as placental growth factors (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1) and pregnancy-associated plasma protein A.12,15 However, it is not known if these prediction models do work well in women from LMICs since there are some genetic and comorbidity differences between them and those from high-income countries (HICs). Also, most of these models have been developed and externally validated in HICs, but not in LMICs.16 This is in contrast with the high burden of hypertensive disorders of pregnancies (HDPs) in LMICs. This also likely reflects a lack of research capacity and resources to carry out these studies in LMICs. Furthermore, due to logistical and financial constraints, not all prediction models developed from HICs which require expensive and difficult to access serum biomarkers can be fully implemented in LMICs.
As current prediction models comprising expensive serum biomarkers’ tests are expensive to be implemented in low-resource settings, the need to continually explore cheaper, accessible, non-invasive and effective approaches for pre-eclampsia prediction is of relevance. Interestingly, there is growing evidence that pre-eclampsia could be (also) a consequence of pre-existing cardiovascular system characteristics;17,19 hence, information on the maternal cardiovascular and peripheral circulation can be useful for predicting pre-eclampsia.
The ophthalmic artery (OA) is an easily accessible vessel, and ultrasound evaluation of this artery has the potential of enhancing the prediction of pre-eclampsia in a non-invasive, safe and cost-effective manner.20,22 This will particularly be useful in low-resource settings where accessing expensive serum biochemical tests for pre-eclampsia prediction is not readily feasible.
Reduced perfusion of certain targeted organs, cerebral autoregulation alteration and failure as a result of pre-eclampsia lead to overperfusion of the cerebral vasculature.23 This phenomenon is evident in the OA, the first branch of the internal carotid artery which provides information on the intracranial circulation. Earlier OA Doppler (OAD) studies have discovered a decrease in impedance to flow and increased waveform velocities in women with pre-eclampsia.24,26 Particularly, increased peak systolic velocity (PSV) ratio results from increased peripheral arterial resistance, a feature closely linked to HDPs.27 Maternal haemodynamic adaptations during the gestational period could therefore possibly explain the linkage between changes in the OAD indices and subsequent pre-eclampsia occurrence.18 28 Although a previous meta-analysis29 pointed to the usefulness of OAD for predicting pre-eclampsia, it is limited by the number of included studies (n=3), smaller sample size and geographical location (Brazil only), thereby affecting the generalisability of the study findings. Also, the incremental or added value of OAD on existing models such as maternal risk factors and serum biomarkers was not explored in that study.
This systematic review therefore aimed to evaluate the available evidence on the usefulness of OAD either as stand-alone or in combination with maternal factors and serum biomarkers for pre-eclampsia prediction from both HICs and LMICs. Findings from this review could further direct studies that will validate and possibly implement the use of OAD for pre-eclampsia prediction in low-resource settings, where pre-eclampsia remains a major public health challenge.
Methods
Protocol and registration
This systematic review was reported according to the Preferred Reporting Items for Systematic Review and Meta-Analyses statement30 and the review protocol was registered with PROSPERO (registration number: CRD42022324569).
Eligibility criteria
Any prospective or retrospective cohort study, case-control study and randomised-control trial which were carried out in either a HIC or a LMIC and reported on the performance of OAD for pre-eclampsia prediction were eligible for inclusion. Also, studies that explored the predictive performance of OAD at any trimester (ie, first, second or third trimester) in either low-risk, high-risk or undetermined risk women were considered for inclusion. A priori high-risk women definition was based on one or more of the following criteria: chronic hypertension, personal or family history of pre-eclampsia, early (≤18 years) or late (≥40 years) first pregnancy, primipaternity, chronic kidney disease, increased BMI >30 kg/m2, presence of diabetes mellitus prior to pregnancy, autoimmune disease and thrombophilia.31
OAD indices of interest for pre-eclampsia prediction were the first PSV (PSV1), second PSV (PSV2) also referred to as first diastolic peak velocity or peak mesodiastolic velocity, PSV ratio also known as peak ratio calculated as PSV2/PSV1, end-diastolic velocity (EDV), time-averaged mean velocity (MV), PI and resistive index (RI).
Outcome of interest was pre-eclampsia as defined by the publishing authors, and its subclassifications, namely early-onset pre-eclampsia (occurrence of pre-eclampsia with delivery at <34+0 weeks of gestation), late-onset pre-eclampsia (occurrence of pre-eclampsia with delivery at ≥34+0 weeks of gestation), preterm pre-eclampsia (occurrence of pre-eclampsia with delivery at <37+0 weeks of gestation) and term pre-eclampsia (occurrence of pre-eclampsia with delivery at ≥37+0 weeks of gestation).32
Case reports, editorials, letters and conference abstracts were excluded. Also, articles with missing full text and articles not translated into the English language were excluded. Even though this may be a limitation, we expect that the majority of these types of studies would have been published in English.
Information sources and search strategy
A literature search was conducted from the following electronic databases: PubMed (Medline), the Cochrane CENTRAL, EMBASE and Scopus for all available published studies until 8 April 2025. The search strategy and search blocks (online supplemental appendix A) were developed with the assistance of a librarian of Utrecht University.
No geographical or date restrictions were applied during the search, but a language restriction (English) was applied. The reference lists of eligible included full-text articles were further screened for eligible additional articles (snowballing).
Study selection
The retrieved records from the databases were exported to EndNote 21 for removal of duplicates. The remaining records after deduplication were then exported to Rayyan33 where title and abstract screening were independently assessed by two reviewers (JA and SA) for inclusion. In instances where disagreement existed in the inclusion of articles, the full text of these articles was assessed by the same two reviewers for resolution. A third member of the team (JLB) was consulted in case of persistent disagreement.
Full texts of the eligible studies were then retrieved and assessed by the same two reviewers (JA and SA) for final inclusion for qualitative synthesis. Selection was based on the following inclusion criteria: appropriate study population selection, the use of the appropriate predictors, measurement of intended outcomes and appropriate analysis.
Data extraction
With the use of a standardised34 and pre-piloted data extraction sheet agreed upon by the review team, JA extracted data for each article, and any uncertainties were discussed with a second reviewer (JLB) to identify the correct data. Data extracted included information on authors, year of publication, study country of origin, risk category of study participants, pre-eclampsia predictors (eg, OAD, maternal factors, MAP, serum biomarkers), gestational age at time of screening, incidence of outcome and predictive performance of OAD demonstrated by area under the receiver operating curve (AUROC) at 95% CI and DR at 10% false-positive rate.
Model discrimination by AUROC (95% CI) interpretation35
AUROC=1.0: excellent performance with perfect discriminatory capabilities.
0.8≤AUROC<0.9: very good performance with strong discriminatory capabilities.
0.7≤AUROC<0.8: good performance with reasonable discriminatory capabilities.
0.6≤AUROC<0.7: fair performance with some discriminatory capabilities but not very effective.
0.5<AUROC<0.6: poor performance indicating test no better than chance in its discriminatory capabilities.
Where missing data were encountered in any of the articles, the corresponding authors of the articles were contacted once by email.
Risk of bias assessment
Each eligible study was assessed for quality and risk of bias using the Prediction model Risk of Bias Assessment Tool (PROBAST)36 by JA. Any uncertainties were discussed and resolved with JLB. Domains included the following: Participants, Predictors, Outcome and Analysis. For this review, the application of appropriate predictors indicates the use of OAD, maternal and biochemical predictors for pre-eclampsia prediction. Furthermore, proper statistical handling of predictor data such as categorical and numerical data was assessed.
Each domain consisted of signalling questions such as “Were appropriate data sources used, for example, cohort, RCT or nested case-control study data?” under the Participants domain, “Were predictors defined and assessed in a similar way for all participants?” under the Predictors domain, “Was the outcome determined without knowledge of predictor information?” under the Outcome domain and “Were there a reasonable number of participants with the outcome?” under the Analysis domain. The signalling questions were answered with ‘Yes’, ‘Probably Yes’, ‘Probably No’, ‘No’ or ‘No information’ in order to assess the articles for any bias.
Patient and public involvement
No patient or public was involved in this study.
Results
Study selection
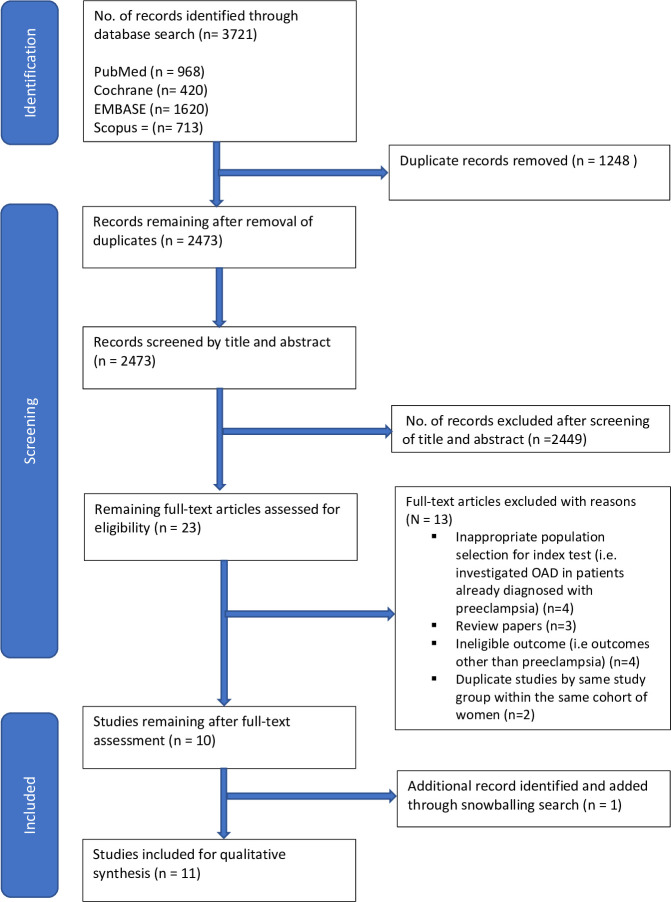
A total number of 3721 records were identified through the database search. After removal of duplicates, 2473 articles’ title/abstracts were screened, after which 23 articles were selected for full-text review. At full-text review, 13 articles were excluded. Reasons for exclusion included inappropriate patient selection (n=4), review papers (n=3) and ineligible outcome measures (n=4). Furthermore, three studies from the same study group22 28 37 which made use of the same cohort of women and similar outcomes were identified; hence, only the latest study37 with updated and comprehensive information was selected for inclusion. This was to prevent overlap and duplication of information. One article was identified through snowballing. Finally, a total of 11 articles remained eligible for this review (figure 1).
Figure 1. Preferred Reporting Items for Systematic Review and Meta-Analyses flow diagram. OAD, ophthalmic artery Doppler.
Study characteristics
The 11 studies included a total of 12 150 women with singleton pregnancies in whom 517 (4.3%) diagnoses of pre-eclampsia were reported. Among the 11 studies, 3 studies (27.3%) were carried out in a HIC (UK) with a total study population of 9257 women. Eight studies (72.7%) were carried out in LMICs (Brazil, India, Iran and Egypt) with a total study population of 2893 women. Three studies (27.3%) recruited high-risk women to develop pre-eclampsia as their cohort; the other (n=8, 72.7%) recruited women presenting for routine antenatal care with undetermined risk factors for developing pre-eclampsia. Characteristics of the included studies are presented in table 1.
Table 1. Characteristics of studies included in the systematic review.
| Author (year) | Country of study | Study design | Study period | Population size | Gestational period of screening | OAD predictors investigated | Incidence of PE (%) with (subclassification) | Predictive performance measurement |
|---|---|---|---|---|---|---|---|---|
| Gana et al (2022)45 | UK | Cohort (prospective) | June to August 2019 and May 2020 to Feb. 2021 | 4066 | First trimester | PSV1, PSV2, PSV ratio | 114/4066 (2.8)(preterm PE (0.6%), term PE (2.2%)) | DR (at 10% FPR) |
| Selima et al (2022)38 | Egypt | Cohort (prospective) | June 2020 to Sept 2021 | 120 | First trimester | PSV1, PSV2, PSV ratio, EDV, PI, RI | 24/120 (20)(mild PE (13.3%), severe PE (6.7%)) | AUROC (95% CI) |
| Gurgel Alves et al (2014)39 | Brazil | Cohort (prospective) | August 2009 to Feb. 2011 | 440 | First trimester | PI, RI, PD1, PR, PSV1 | 31/440 (7)(early-onset PE (2%), late-onset PE (5%)) | AUROC (95% CI),DR (at 10% FPR) |
| Aquino et al (2014)43 | Brazil | Cohort (prospective) | NG | 73 | Second trimester | RI | 14/73 (19)(subclassification NG) | AUROC (95% CI) |
| Matias et al (2014)40 | Brazil | Cohort (prospective) | March 2010 to June 2012 | 347 | Second trimester | PSV, EDV, PMDV, MV, RI, PI, PSV ratio | 40/347 (11.5)(early-onset PE (1.2%), late-onset PE (8.1%)) | AUROC (95% CI) |
| Matias et al (2020)41 | Brazil | Cohort (prospective) | NG | 305 | Second trimester | PSV1, PSV2, PI, PSV ratio, RI, MV, EDV | 31/305 (10.2)(subclassification NG) | AUROC (95% CI) |
| Praciano de Souza et al (2018)21 | Brazil | Cohort (prospective) | Feb. 2011 to October 2014 | 415 | Second trimester | PI, PD1, PR | 40/415 (9.6)(early-onset PE (0.9%), late-onset PE (8.7%)) | DR (at 10% FPR) |
| Shah et al (2023)42 | India | Cohort (prospective) | June 2021 to December 2022 | 398 | Second trimester | PSV ratio | 24/398 (6)(early-onset PE (2%), late-onset PE (4%)) | AUROC (95% CI),DR (at 10% FPR) |
| Sapantzoglou et al (2021)46 | UK | Cohort (prospective) | August 2019 to April 2020 | 2853 | Second trimester | PSV1, PSV2, PI, PSV ratio | 76/2853 (2.7)(preterm PE (0.7%), term PE (2%)) | DR (at 10% FPR) |
| Lau et al (2022)37 | UK | Cohort (prospective) | June 2019 to Sept. 2021 and Sept. 2020 to Sept. 2021 | 2338 | Third trimester | PSV1, PSV2, PSV ratio | 75/2338 (3.2)(late-onset PE) | DR (at 10% FPR) |
| Saleh et al (2022)44 | Iran | Cohort (prospective) | January 2020 to March 2021 | 795 | Third trimester | PSV1, PSV2, PI, PSV ratio | 48/795 (6)(mild PE (5.3%), severe PE (0.7%)) | AUROC (95% CI) |
AUROC, area under the receiver operator curve; DR, detection rate; EDV, end-diastolic velocity; FPR, false-positive rate; MV, time-averaged mean velocity; NG, not given; PD1, first diastolic peak velocity; PE, pre-eclampsia; PI, pulsatility index; PMDV, peak mesodiastolic velocity; PR, peak ratio; PSV1, first peak systolic velocity; PSV, peak systolic velocity; PSV2, second peak systolic velocity; RI, resistive index.
Quality assessment of included studies
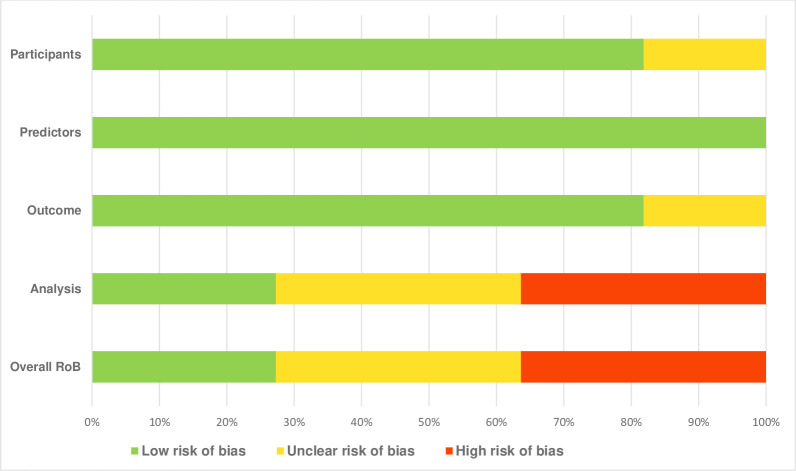
Result of risk of bias assessment and individual studies risk assessment carried out with PROBAST is available in figure 2 and online supplemental appendix B, respectively.
Figure 2. Results of risk of bias assessment. ROB, risk of bias.
After assessment, the application of the appropriate predictors across all the studies (n=11) was seen to have the lowest risk of bias. Nine studies (n=9, 81.8%) demonstrated low risk of bias in participants’ selection and outcome measurement, while two studies (n=2, 18.2%) demonstrated unclear risk of bias in participants’ selection and outcome measurement. High risk of bias (n=4) and unclear risk of bias (n=4) were also identified under the Analysis domain, mainly due to reasons such as the following: failure to report and demonstrate that all the recruited participants were included in the final analysis, inappropriate handling of patients with missing data, model overfitting and optimisation of model performance not accounted for; and inappropriate handling of continuous and categorical predictors. Overall, of the 11 included studies, three (27.2%) were assessed as having a low risk of bias, four (36.4%) had an unclear risk and the remaining four (36.4%) were judged to have a high risk of bias (online supplemental appendix B).
Quantitative data synthesis
A meta-analysis was not possible due to heterogeneity in the study population (risk categorisation), timing of screening, type of OAD index used, OAD thresholds for predicting pre-eclampsia and type of pre-eclampsia investigated. Therefore, and after consultation with a methodological expert in prognostic research, results for this study were narratively summarised and presented in tables.
Stand-alone predictive performance of ophthalmic artery Doppler (OAD)
The predictive performance of OAD for pre-eclampsia with respective DRs (at 10% FPR) and AUROC (at 95% CI) with cut-offs are reported as follows:
First trimester OAD performance
In the study by Selima et al38 involving a small cohort of 120 women with undetermined risk in Egypt, the PSV ratio demonstrated an AUROC of 0.97 (0.92 to 1.0) at a cut-off >0.59 (sensitivity 77.3%, specificity 65.8%) for predicting pre-eclampsia. In this study also, the PSV2 demonstrated an AUROC of 0.91 (0.82 to 0.99) at a cut-off >20.1 cm/s (sensitivity 91.7%, specificity 61.7%), and the PI also demonstrated an AUROC of 0.60 (0.50 to 1.27) at cut-off <1.76 (sensitivity 89.7%, specificity 65.8%).
In another study by Alves et al39 involving a cohort of 440 women with undetermined risk in Brazil, the PSV2 depicted an AUROC of 0.61 (0.42 to 0.79) at cut-off >21.1 cm/s for predicting early pre-eclampsia and AUROC of 0.53 (0.40 to 0.66) in predicting late pre-eclampsia. Also, for this study, the PSV2 depicted an AUROC of 0.56 (95% CI, 0.45 to 0.66) for predicting pre-eclampsia at any time.
Second trimester OAD performance
In the study by Matias et al40 involving a cohort of 347 high-risk women in Brazil, the PSV2 demonstrated an AUROC of 0.73 (0.66 to 0.81) at cut-off >22.1 cm/s (sensitivity 70%, specificity 75%) for predicting pre-eclampsia.
In another study by Matias et al41 involving a cohort of 305 high-risk women in Brazil, the PSV2 demonstrated an AUROC of 0.76 (0.71 to 0.81) at cut-off ≥21.4 cm/s (sensitivity 69%, specificity 78%) for predicting pre-eclampsia.
In the study by Shah et al42 involving a cohort of 398 women with undetermined risk in India, the PSV ratio demonstrated a sensitivity, specificity and DR of 100%, 83.3% and 83.7%, respectively, for early pre-eclampsia. Also in this study, the PSV ratio demonstrated an AUROC of 0.88 (0.84 to 0.91) at a cut-off ≥0.65 (sensitivity 86.05%, specificity 89.68%) for predicting pre-eclampsia at any stage.
In the study by Aquino et al43 involving a small cohort of 73 high-risk women in Brazil, the OAD RI, which was the only OAD index investigated, demonstrated an AUROC of 0.69 (0.54 to 0.85).
Third trimester OAD performance
In the study by Lau et al37 involving a large cohort of 2338 women with undetermined risk in the UK, the PSV ratio demonstrated an AUROC of 0.77 (0.71 to 0.82) at cut-off >0.76 and DR of 41.3% for prediction of late-onset pre-eclampsia. In this study, the PSV ratio depicted an AUROC of 0.82 (0.73 to 0.89) and DR of 56.7% for prediction of pre-eclampsia less than 3 weeks after OAD assessment.
In another study by Saleh et al44 involving a cohort of 795 women with undetermined risk in Iran, the PSV ratio demonstrated an AUROC of 0.97 (0.94 to 0.99) at cut-off 0.07 (sensitivity 100%, specificity 90%), and the PSV2 demonstrated an AUROC of 0.70 (0.57 to 0.84) (sensitivity 78%, specificity 69%) for pre-eclampsia prediction. In this study, the PI demonstrated an AUROC of 0.88 (0.78 to 0.97) at cut-off 0.07 (sensitivity 94%, specificity 79%) for pre-eclampsia.
Combining ophthalmic artery Doppler with maternal risk factors and other prediction models
Studies in first trimester
In the study by Alves et al39 involving a cohort of 440 women with undetermined risk, the DRs for early pre-eclampsia and pre-eclampsia at any stage increased from 63% to 67% and from 45% to 48%, respectively, when PSV2 was added to maternal factors, namely nulliparity, BMI >30 kg/m2, history of pre-eclampsia and family history of pre-eclampsia. The addition of uterine artery pulsatility index (UtA-PI) to these maternal factors increased the DRs for early pre-eclampsia and pre-eclampsia at any stage from 63% to 67% and from 45% to 58%, respectively. Subsequently, when PSV2 and UtA-PI were added to these maternal factors, the DRs for early pre-eclampsia and pre-eclampsia at any stage increased from 63% to 68% and from 45% to 54%, respectively.
In another study by Gana et al45 involving a large cohort of 4066 women with undetermined risk, the DRs for preterm and term pre-eclampsia increased from 57.3% to 63.8% and from 44.5% to 44.6%, respectively, when PSV ratio was added to MAP and maternal factors, namely mean weight of 73 kg, nulliparity, BMI >30 kg/m2, history of chronic hypertension, history of pre-eclampsia, family history of pre-eclampsia and mean interpregnancy interval of 3.4 years. The addition of UtA-PI to MAP and these maternal factors increased the DRs for preterm and term pre-eclampsia from 57.3% to 65.9% and from 44.5% to 44.8%, respectively. Subsequently, adding PSV ratio to these maternal factors, MAP, UtA-PI and PlGF increased the DRs for preterm and term pre-eclampsia from 74.6% to 76.7% and from 46.7% to 47%, respectively.
Studies in the second trimester
In the study by Matias et al40 involving a cohort of 347 high-risk women, model performance for pre-eclampsia prediction by AUROC moved from 0.72 (0.67 to 0.77) to 0.78 (0.73 to 0.82) when PSV2 was added to maternal factors, namely advancing age (>36 years), primigravida ≤18 years, BMI >30 kg/m2, prior hypertension, history of pre-eclampsia and family history of pre-eclampsia. Subsequently, when PSV2 was added to a model that contained these maternal factors and UtA-PI, the model’s performance for pre-eclampsia prediction by AUROC moved from 0.82 (0.77 to 0.86) to 0.88 (0.83 to 0.91).
In another study by Matias et al41 involving a cohort of 305 high-risk women, model performance for pre-eclampsia prediction by AUROC moved from 0.77 (0.72 to 0.82) to 0.84 (0.79 to 0.88) when PSV2 was added to maternal factors, namely primigravida ≤18 years, BMI >30 kg/m2, nulliparity, prior hypertension, history of pre-eclampsia and family history of pre-eclampsia.
In the study by Shah et al42 involving a cohort of 398 women with undetermined risk factor, DR for preterm pre-eclampsia moved from 90% to 100% when PSV ratio was added to an HDP-Gestosis score ≥3.
In the study by Sapantzoglou et al46 involving a large cohort of 2853 women with undetermined risk, the DRs for preterm and term pre-eclampsia increased from 56.1% to 80.2% and from 33.8% to 46.0%, respectively, when PSV ratio was added to maternal factors, namely mean weight of 75 kg, nulliparity, BMI >30 kg/m2, chronic hypertension, diabetes mellitus, history of pre-eclampsia and family history of pre-eclampsia. Adding UtA-PI to these maternal factors, the DRs for preterm and term pre-eclampsia increased from 56.1% to 74.8% and from 33.8% to 41.1%, respectively. Also, the DR for preterm and term pre-eclampsia increased from 69.1% to 83.0% and 41.8% to 50.5%, respectively, when PSV ratio was added to MAP and these maternal factors. Adding UtA-PI to MAP and these maternal factors, the DRs for preterm and term pre-eclampsia increased from 69.1% to 80.7% and 41.8% to 46.6%, respectively. Subsequently, the DRs for preterm and term pre-eclampsia increased from 84.9% to 89.9% and from 43.0% and 51.2%, respectively, when PSV ratio was added to these maternal factors, namely MAP, UtA-PI, PlGF and sFlt-1.
In a study by Praciano de Souza et al21 involving a cohort of 415 women with undetermined risk, the DR for pre-eclampsia at any stage moved from 42.5% to 45% when PSV ratio was added to maternal factors, namely BMI >30 kg/m2 and nulliparity, MAP and UtA-PI.
Study in the third trimester
In the study by Lau et al37 involving a large cohort of 2338 women with undetermined risk, the addition of PSV ratio to maternal factors, namely mean weight of 85 kg, nulliparity, BMI >30 kg/m2, history of chronic hypertension, history of pre-eclampsia, family history of pre-eclampsia, mean interpregnancy interval of 3.0 years and MAP, was superior to sFlt-1/PlGF ratio in predicting pre-eclampsia <3 weeks (96.7% vs 70% DR) and predicting pre-eclampsia at any time (78.7% vs 62.7% DR).
A summary of the incremental predictive value of OAD is available in table 2.
Table 2. Incremental predictive value of ophthalmic artery PSV ratio and PSV2 for pre-eclampsia.
| Incremental predictive performance by DR (at 10% FPR) | |||||
|---|---|---|---|---|---|
| First author/year | DR (%) for preterm PE | DR (%) for term PE | |||
| Method of screening | PSV ratio | PSV ratio | |||
| Excluded | Included | Excluded | Included | ||
| Gana 202245 | MF | 46.3 | 58.4 | 36.1 | 37.7 |
| MF+MAP | 57.3 | 63.8 | 44.5 | 44.6 | |
| MF+MAP+UtA-PI | 65.9 | 70.6 | 44.8 | 45.1 | |
| MF+MAP+UtA-PI+PlGF | 74.6 | 76.7 | 46.7 | 47 | |
| MF+PAPP-A | 50.7 | 60.5 | 37.2 | 38.6 | |
| MF+MAP+UtA-PI+PAPP-A | 67.6 | 71.8 | 45.4 | 45.7 | |
| DR (%) for early PE | DR (%) for PE at any stage | ||||
| Method of screening | PSV2 | PSV2 | |||
| Excluded | Included | Excluded | Included | ||
| G. Alves 201439 | MF | 63 | 67 | 45 | 48 |
| MF+UtA-PI | 67 | 68 | 58 | 54 | |
| DR (%) for preterm PE | DR (%) for term PE | ||||
| Method of screening | PSV ratio | PSV ratio | |||
| Excluded | Included | Excluded | Included | ||
| Shah 202342 | HDP-Gestosis | 90 | 100 | 57.6 | 84.9 |
| HDP-Gestosis+MAP | 100 | 100 | 93.9 | 96.9 | |
| HDP-Gestosis+UtA-PI | 90 | 100 | 57.6 | 84.9 | |
| HDP-Gestosis+MAP+UtA-PI | 100 | 100 | 93.9 | 96.9 | |
| DR (%) for preterm PE | DR (%) for term PE | ||||
| Method of screening | PSV ratio | PSV ratio | |||
| Excluded | Included | Excluded | Included | ||
| Sapantzoglou 202146 | MF | 56 | 80 | 34 | 46 |
| MF+MAP | 69 | 83 | 42 | 51 | |
| MF+UtA-PI | 75 | 86 | 41 | 51 | |
| MF+PlGF | 76 | 86 | 31 | 44 | |
| MF+sFlt-1 | 60 | 81 | 31 | 45 | |
| MF+MAP+UtA-PI | 81 | 88 | 47 | 54 | |
| MF+MAP+UtA-PI+PlGF | 86 | 90 | 45 | 53 | |
| MF+MAP+UtA-PI+PlGF+sFlt-1 | 85 | 90 | 43 | 51 | |
| DR (%) for PE at any stage | DR (%) for term PE | ||||
| PSV ratio | PSV ratio | ||||
| Method of screening | Excluded | Included | Excluded | Included | |
| Praciano de Souza 201821 | MF | 28 | 45 | n/a | n/a |
| MF+MAP+UtA-PI | 43 | 45 | n/a | n/a | |
| Incremental predictive performance by AUROC (at 95% CI) | |||||
| AUROC (95% CI) for PE at any stage | AUROC (95% CI) for term PE | ||||
| Method of screening | PSV2 | PSV2 | |||
| Excluded | Included | Excluded | Included | ||
| Matias 201440 | MF | 0.72 (0.67 to 0.77) | 0.78 (0.73 to 0.82) | n/a | n/a |
| MF+UtA-PI | 0.82 (0.77 to 0.86) | 0.88 (0.83 to 0.91) | n/a | n/a | |
| AUROC (95% CI) for PE at any stage | AUROC (95% CI) for term PE | ||||
| Method of screening | PSV2 | PSV2 | |||
| Excluded | Included | Excluded | Included | ||
| Matias 202041 | MF | 0.77 (0.72 to 0.82) | 0.84 (0.79 to 0.88) | n/a | n/a |
AUROC, area under the receiver operator curve; DR, detection rate; FPR, false-positive rate; HDP, hypertensive disorders of pregnancy; MAP, mean arterial pressure; MF, maternal factors; n/a, information not available; PAPP-A, pregnancy-associated plasma protein A; PE, pre-eclampsia; PlGF, placental growth factor; PSV2, second peak systolic velocity; PSV, peak systolic velocity; sFlt-1, soluble fms-like tyrosine kinase-1; UtA-PI, uterine artery pulsatility index.
Sensitivity analyses based on study quality
In order to assess the robustness of our study findings, we conducted a sensitivity analysis by excluding studies with high risk of bias (n=4). The remaining seven studies (three with low risk and four with unclear risk) were re-evaluated to determine whether the inclusion of lower quality evidence had any influence on the overall conclusions.
The sensitivity analysis indicated that the main patterns remained largely consistent, supporting the robustness of the overall synthesis. For instance, regarding the incremental predictive performance of OAD when added to maternal factors and MAP, excluding the two high-risk studies that investigated the incremental predictive performance of OAD did not affect the overall conclusion. However, a stand-alone predictive performance of OAD in the first trimester was primarily supported by a study with high risk of bias. Excluding the other three high risk of bias studies carried out in the second trimester did not affect the stand-alone predictive performance of OAD.
Discussion
This systematic review assessed the usefulness of OAD for prediction of pre-eclampsia and included a large cohort of 12 150 women from 11 studies. Two main significant findings were determined from this systematic review.
First, we identify the PSV ratio and the PSV2 as the most reliable stand-alone OAD indices for pre-eclampsia prediction at any gestational age. Our findings are comparable to those from a narrative review of five articles47 and meta-analysis29 where the authors reported these indices as the most statistically relevant for pre-eclampsia prediction. From all the included studies in our review, either the PSV ratio or PSV2 or both were significantly increased in women who developed early-onset, late-onset, preterm or term pre-eclampsia as compared with normotensive women. The PI, PSV1, EDV, time-averaged MV and RI did not contribute much to the prediction of pre-eclampsia.
Second, this review highlights the incremental predictive value of OAD for pre-eclampsia when it is added to maternal risk factors, MAP, uterine artery Doppler or biochemical markers. The common maternal risk factors identified from the included studies were nulliparity, BMI >30 kg/m2, chronic hypertension, history of pre-eclampsia and family history of pre-eclampsia. The addition of OAD to these maternal risk factors, MAP and biochemical markers in the first and second trimesters significantly improved the prediction of early pre-eclampsia and preterm pre-eclampsia, than for late pre-eclampsia and term pre-eclampsia. For instance, in the first trimester, when PSV ratio was added to maternal factors and MAP, the DR significantly improved from 57.7% to 63.8% for preterm pre-eclampsia, in comparison with a very slight increment in the DR from 44.5% to 44.6% for term pre-eclampsia.45 In another first trimester study, when the PSV2 was added to maternal risk factors, the DR for early pre-eclampsia increased from 63% to 67%, while the DR for pre-eclampsia at any stage was slightly increased from 45% to 48%.39 Similarly, in the second trimester, when PSV ratio was added to maternal factors, the DR was significantly increased from 56.1% to 80.2% for preterm pre-eclampsia, while the DR for term pre-eclampsia showed a slight increase from 33.8% to 46.0%. Furthermore, when the PSV ratio was added to maternal factors and MAP, the DR was significantly increased from 69.1% to 83.0% for preterm pre-eclampsia, while for term pre-eclampsia, there was a slight increase in DR from 41.8% to 50.5%.46
For the incremental predictive performance of OAD compared with UtA-PI in predicting pre-eclampsia, we identified that OAD demonstrated better incremental predictive performance than UtA-PI in the second trimester for both preterm and term pre-eclampsia. In the second trimester study by Sapantzoglou et al,46 the addition of PSV ratio to maternal factors resulted in better incremental predictive performance for preterm pre-eclampsia as compared with the addition of UtA-PI to these maternal factors (56.1% to 80.2% vs 56.1% to 74.8%). Also, for term pre-eclampsia, the addition of PSV ratio to maternal factors resulted in better incremental predictive performance as compared with the addition of UtA-PI to these maternal factors (33.8% to 46.0% vs 33.8% to 41.1%). Similarly, the model that added PSV ratio to maternal factors and MAP resulted in better incremental predictive performance for preterm pre-eclampsia than the model that added UtA-PI to these maternal factors and MAP (69.1% to 83.0% vs 69.1% to 80.7%). Also, for term pre-eclampsia, the model that added PSV ratio to maternal factors and MAP resulted in better incremental predictive performance than the model that added UtA-PI to these maternal factors and MAP (41.8% to 50.5% vs 41.8% to 46.6%).
However, in the first trimester study by Alves et al,39 the addition of either PSV2 or UtA-PI to maternal factors resulted in the same incremental predictive performance, with each model resulting in increased DR from 63% to 67% for early pre-eclampsia. Also, for pre-eclampsia at any stage, the addition of PSV2 to maternal factors did not perform better than when UtA-PI was added to maternal factors (45% to 48% vs 45% to 58%). In another first trimester study by Gana et al,45 adding UtA-PI to maternal factors and MAP resulted in better incremental predictive performance for preterm pre-eclampsia as compared with adding PSV ratio to maternal factors and MAP (57.3% to 65.9% vs 57.3% to 63.8%). For term pre-eclampsia, the incremental predictive performance for the model containing PSV ratio was similar to the model containing UtA-PI (44.5% to 44.6% vs 44.5% to 44.8%).
Strengths and limitations of review
This systematic review on the predictive performance of OAD for pre-eclampsia involves a relatively large number of women (n=12 150) from both HIC and LMICs with either high risk or undetermined risk for pre-eclampsia. We comprehensively synthesised evidence on the predictive ability of OAD for pre-eclampsia in the first, second and third trimesters, and also evaluated the incremental predictive value of OAD to pre-eclampsia prediction models comprising maternal factors, MAP and biochemical markers. Although a previous meta-analysis29 on the usefulness of OAD for pre-eclampsia prediction has been carried out, it is limited by the number of included studies (n=3), smaller sample size (n=1199) and geographical location (Brazil only), thereby affecting the generalisability of the study findings. Also, the incremental or added value of OAD on existing models such as maternal risk factors, MAP and biomarkers was not explored in that study. Our study therefore bridged this existing gap by providing a comprehensive review on the available evidence on the stand-alone and incremental predictive performance of OAD for pre-eclampsia from different geographical locations.
A limitation of this review is the lack of uniformity in how pre-eclampsia was defined across the included studies. This therefore highlights the need for pre-eclampsia studies to adopt a standardised definition of the condition. Also, although aimed for, we were not able to conduct a meta-analysis due to heterogeneity in the study population (risk categorisation), timing of screening, type of OAD index used, OAD thresholds and type of pre-eclampsia investigated among the individual prognostic studies. We also acknowledge that interpretation and clinical application of our review findings should be carried out in light of the methodological quality of included studies. Although the sensitivity analysis indicated that the four high-risk studies among the 11 included studies did not alter the overall patterns of the review, the stand-alone predictive performance of OAD particularly in the first trimester should be interpreted with caution, as this specific finding was influenced by a study with high risk of bias. Few studies (n=3) screened a relatively smaller cohort of 725 high-risk women, while a majority of the studies (n=8) screened a much larger cohort of 11 425 women with undetermined risk for pre-eclampsia. This implies that we have to be careful in generalising this review’s findings to all women of different risk categories. Hence, our findings may be more applicable to women presenting for routine antenatal care with undetermined risk factor for pre-eclampsia. Furthermore, the three studies that enrolled high-risk women did not report on the number of participants who were receiving aspirin prophylaxis. Given the established role of low-dose aspirin in preventing pre-eclampsia among high-risk pregnant women, this omission limits the interpretability of findings, as it introduces potential bias that was not accounted for. Future research should therefore prioritise transparent reporting of such co-interventions to improve the quality and validity of results.
The inclusion of articles only published in the English language may be a limitation, although we do expect that the majority of these types of studies would have been published in English.
Practical and research implications
Potentials of OAD screening for pre-eclampsia in low-resource setting
Given the global burden of hypertensive disorders of pregnancy with its associated morbidity and mortality, particularly in low(er)-resource setting,48 the performance of OAD among studies conducted in these settings is encouraging. In the first trimester, the OAD performed well in pre-eclampsia prediction in a low-resource setting with sensitivity of 91.7% and specificity of 61.7%,38 which is similar to a first trimester uterine artery Doppler performance for pre-eclampsia prediction also carried out in a low-resource setting with sensitivity of 61.5% and specificity of 63.8%.49 In the second trimester, three studies depicted moderate performance for OAD in predicting pre-eclampsia in a low-resource setting with sensitivity of 69.0% and specificity of 78.0%,40 sensitivity of 70.0% and specificity of 75.0%,41 and sensitivity of 86.0% and specificity of 89.8%,42 which are similar to the performance of uterine artery Doppler for pre-eclampsia in a low-resource setting with sensitivity of 61.9% and specificity of 86.8%.50 Furthermore, just like the uterine artery Doppler where studies have identified incremental improvement in the prediction of pre-eclampsia when it is combined with maternal factors, MAP and serum biomarkers,51,53 we also encourage the addition of OAD to maternal factors, MAP and serum biomarkers since per this review it has proven to be effective for prediction of early and preterm pre-eclampsia.37 39 45 46
Second, given the significant morbidity and mortality associated with early and preterm pre-eclampsia in low-resource settings,48 the incremental predictive performance of OAD when added to maternal factors and MAP for preterm pre-eclampsia is promising. For instance, in the second trimester study by Sapantzoglou et al46 involving a large cohort of 2853 women, when the PSV ratio was added to maternal factors and MAP, it demonstrated better incremental predictive performance than uterine artery Doppler for preterm pre-eclampsia (56.1% to 80.2% vs 56.1% to 74.8% DR). Also in this study, the PSV ratio enhanced the predictive performance of the PlGF and sFlt-1 for preterm pre-eclampsia. Furthermore, in the third trimester study by Lau et al37 also involving a large cohort of 2338 women in the third trimester (35+0 to 36+6 weeks), a prediction model which combined PSV ratio, maternal factors and MAP was superior to sFlt-1/PlGF ratio in predicting pre-eclampsia at <3 weeks after screening (96.7% vs 70% DR, p value 0.027) and pre-eclampsia at any time (78.7% vs 62.7% DR, p value 0.025). Therefore, in low-resource settings where sFlt-1 and PlGF tests may be expensive or unavailable, the addition of PSV ratio to maternal risk factors and MAP will potentially be beneficial in predicting preterm pre-eclampsia in a cost-effective manner. Additionally, OAD is a promising novel ultrasound marker for inclusion in preterm pre-eclampsia prediction models in low-resource settings, particularly in situations where uterine artery Doppler may not be technically feasible.
Research implications
Although this review points out some potentials for OAD in routine clinical use for pre-eclampsia prediction, to the best of our knowledge, no study within the literature has further carried out external validation studies on the predictive potentials of OAD for pre-eclampsia in low-resource settings. Even with the widely used prediction models, most externally validated studies have been carried out in HICs and not in LMICs, as identified in a recent meta-analysis on externally validated pre-eclampsia prediction models.16 This could be as a result of a lack of resources involved in carrying out these studies that require expensive serum biomarkers’ testing. It is therefore imperative that accessible and cheaper prediction models continue to be explored, developed and (externally) validated within low-resource settings which use simple low-cost techniques, such as OAD that can be integrated into routine antenatal ultrasound examinations. These future studies should also evaluate the potential of OAD in the prediction of adverse perinatal outcomes such as intrauterine growth restriction, eclampsia, placental abruption and perinatal death. As is heterogeneity in cut-off thresholds for prediction of pre-eclampsia by PSV ratio and PSV2 identified from this review, these studies should be large enough to establish thresholds for PSV ratio and PSV2 for pre-eclampsia prediction and reference curves. Also, since OAD provides a simple ultrasound technique for screening women, studies that evaluate the efficacy of handheld portable Doppler ultrasound devices which run on batteries or solar instead of electricity are worth exploring, as this will enhance access to OAD screening in low-resource regions where electricity may be lacking.
Practicality in obtaining OAD measurements
Five studies from this review obtained OAD measurements from both eyes and calculated the mean values of the indices obtained,3742 44,46 while six studies carried out the measurements from the right eye only.2138,41 43 Although previous studies suggest that there is no statistical difference between one eye and both eyes Doppler measurements,54 55 we suggest large cohort studies that establish the best method for obtaining OAD measurement. Also, further studies that look into intra-operator and inter-operator reliability assessment of OAD measurement are needed.
Conclusion
The ophthalmic artery PSV ratio and PSV2 are potentially useful ultrasound markers for pre-eclampsia prediction. Particularly in the second trimester, adding PSV ratio to maternal factors and MAP significantly improved the prediction of preterm pre-eclampsia. Given the burden of early and preterm pre-eclampsia in low-resource settings, OAD appears promising for pre-eclampsia screening in these settings where serum biomarkers may be expensive and inaccessible, and where uterine artery Doppler may not be technically feasible. However, the extent to which this novel marker is implemented in routine antenatal care should be guided by larger and sufficiently powered validation studies.
Supplementary material
Acknowledgements
We are grateful to Prof Carl Moons of University Medical Center, Utrecht, for his valuable advice during the methodological planning phase of this systematic review. Thanks also go to Dr Marcus Rijken of the Netherlands Cancer Institute-Antoni van Leeuwenhoek hospital, Amsterdam, for his contributions to this review. Many thanks also to Paulien Wiersma, a librarian at Utrecht University, for her guidance in planning the literature search strategy.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-094348).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Data availability free text: n/a.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Brown MA, Magee LA, Kenny LC, et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 2.Chappell LC, Cluver CA, Kingdom J, et al. Pre-eclampsia. Lancet. 2021;398:341–54. doi: 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- 3.Hodgins S. Pre-eclampsia as Underlying Cause for Perinatal Deaths: Time for Action. Glob Health Sci Pract. 2015;3:525–7. doi: 10.9745/GHSP-D-15-00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salam RA, Das JK, Ali A, et al. Diagnosis and management of preeclampsia in community settings in low and middle-income countries. J Family Med Prim Care. 2015;4:501–6. doi: 10.4103/2249-4863.174265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokslag A, van Weissenbruch M, Mol BW, et al. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102:47–50. doi: 10.1016/j.earlhumdev.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Henderson JT, et al. Screening for Preeclampsia: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville (MD): 2017. [PubMed] [Google Scholar]
- 7.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377:613–22. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 8.WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. 2023. https://www.who.int/publications-detail-redirect/9789241548335 Available. [PubMed]
- 9.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayrink J, Souza RT, Feitosa FE, et al. Mean arterial blood pressure: potential predictive tool for preeclampsia in a cohort of healthy nulliparous pregnant women. BMC Pregnancy Childbirth. 2019;19:460. doi: 10.1186/s12884-019-2580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedroso MA, Palmer KR, Hodges RJ, et al. Uterine Artery Doppler in Screening for Preeclampsia and Fetal Growth Restriction. Rev Bras Ginecol Obstet. 2018;40:287–93. doi: 10.1055/s-0038-1660777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill S, Rusterholz C, Zanetti-Dällenbach R, et al. Potential markers of preeclampsia--a review. Reprod Biol Endocrinol. 2009;7:70. doi: 10.1186/1477-7827-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HJ, Kim SH, Jung YW, et al. Screening models using multiple markers for early detection of late-onset preeclampsia in low-risk pregnancy. BMC Pregnancy Childbirth. 2014;14:35. doi: 10.1186/1471-2393-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Gorman N, Wright D, Poon LC, et al. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;49:751–5. doi: 10.1002/uog.17399. [DOI] [PubMed] [Google Scholar]
- 15.Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am J Obstet Gynecol. 2022;226:S1071–97. doi: 10.1016/j.ajog.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Tiruneh SA, Vu TTT, Moran LJ, et al. Externally validated prediction models for pre‐eclampsia: systematic review and meta‐analysis. Ultrasound in Obstet & Gyne . 2024;63:592–604. doi: 10.1002/uog.27490. [DOI] [PubMed] [Google Scholar]
- 17.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703–14. doi: 10.1161/CIRCULATIONAHA.113.003664. [DOI] [PubMed] [Google Scholar]
- 18.Perry H, Khalil A, Thilaganathan B. Preeclampsia and the cardiovascular system: An update. Trends Cardiovasc Med. 2018;28:505–13. doi: 10.1016/j.tcm.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Thilaganathan B. Pre-eclampsia and the cardiovascular-placental axis. Ultrasound Obstet Gynecol. 2018;51:714–7. doi: 10.1002/uog.19081. [DOI] [PubMed] [Google Scholar]
- 20.Al D, et al. Ophthalmic artery Doppler as a measure of severe pre-eclampsia. Int J Gynaecol Obstet. 2008;100 doi: 10.1016/j.ijgo.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Praciano de Souza P, Gurgel Alves J, Bezerra Maia e Holanda Moura S, et al. Second Trimester Screening of Preeclampsia Using Maternal Characteristics and Uterine and Ophthalmic Artery Doppler. Ultraschall in Med. 2018;39:190–7. doi: 10.1055/s-0042-104649. [DOI] [PubMed] [Google Scholar]
- 22.Sarno M, Wright A, Vieira N, et al. Ophthalmic artery Doppler in combination with other biomarkers in prediction of pre-eclampsia at 35-37 weeks’ gestation. Ultrasound Obstet Gynecol. 2021;57:600–6. doi: 10.1002/uog.23517. [DOI] [PubMed] [Google Scholar]
- 23.Jones-Muhammad M, Warrington JP. Cerebral Blood Flow Regulation in Pregnancy, Hypertension, and Hypertensive Disorders of Pregnancy. Brain Sci. 2019;9:224. doi: 10.3390/brainsci9090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belfort MA, Giannina G, Herd JA. Transcranial and orbital Doppler ultrasound in normal pregnancy and preeclampsia. Clin Obstet Gynecol. 1999;42:479–506. doi: 10.1097/00003081-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Riskin-Mashiah S, Belfort MA, Saade GR, et al. Transcranial doppler measurement of cerebral velocity indices as a predictor of preeclampsia. Am J Obstet Gynecol. 2002;187:1667–72. doi: 10.1067/mob.2002.127594. [DOI] [PubMed] [Google Scholar]
- 26.Adekanmi A, Olatunji R, Obajimi M, et al. Maternal ophthalmic artery Doppler velocimetry in pre-eclampsia in Southwestern Nigeria. IJWH . 2015;7:723. doi: 10.2147/IJWH.S86314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbone E, Sapantzoglou I, Nuñez-Cerrato ME, et al. Relationship between ophthalmic artery Doppler and maternal cardiovascular function. Ultrasound Obstet Gynecol. 2021;57:733–8. doi: 10.1002/uog.23601. [DOI] [PubMed] [Google Scholar]
- 28.Sarno M, Wright A, Vieira N, et al. Ophthalmic artery Doppler in prediction of pre-eclampsia at 35-37 weeks’ gestation. Ultrasound Obstet Gynecol. 2020;56:717–24. doi: 10.1002/uog.22184. [DOI] [PubMed] [Google Scholar]
- 29.Kalafat E, Laoreti A, Khalil A, et al. Ophthalmic artery Doppler for prediction of pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51:731–7. doi: 10.1002/uog.19002. [DOI] [PubMed] [Google Scholar]
- 30.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gestational Hypertension and Preeclampsia. Obstetrics & Gynecology. 2020;135:e237–60. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 32.Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145 Suppl 1:1–33. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moons KGM, de Groot JAH, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11:e1001744. doi: 10.1371/journal.pmed.1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Çorbacıoğlu ŞK, Aksel G. Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turk J Emerg Med. 2023;23:195–8. doi: 10.4103/tjem.tjem_182_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolff RF, Moons KGM, Riley RD, et al. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann Intern Med. 2019;170:51–8. doi: 10.7326/M18-1376. [DOI] [PubMed] [Google Scholar]
- 37.Lau K, Wright A, Sarno M, et al. Comparison of ophthalmic artery Doppler with PlGF and sFlt-1/PlGF ratio at 35-37 weeks’ gestation in prediction of imminent pre-eclampsia. Ultrasound Obstet Gynecol. 2022;59:606–12. doi: 10.1002/uog.24874. [DOI] [PubMed] [Google Scholar]
- 38.Selima ER, Abar AM, Dessouky BAE. Role of Ophthalmic Artery Doppler in Prediction of Preeclampsia. The Egyptian Journal of Hospital Medicine . 2022;87:1944–52. doi: 10.21608/ejhm.2022.231664. [DOI] [Google Scholar]
- 39.Gurgel Alves JA, Praciano de Sousa PC, Bezerra Maia e Holanda Moura S, et al. First‐trimester maternal ophthalmic artery Doppler analysis for prediction of pre‐eclampsia. Ultrasound in Obstet & Gyne. 2014;44:411–8. doi: 10.1002/uog.13338. [DOI] [PubMed] [Google Scholar]
- 40.Matias DS, Costa RF, Matias BS, et al. Predictive value of ophthalmic artery Doppler velocimetry in relation to development of pre-eclampsia. Ultrasound Obstet Gynecol. 2014;44:419–26. doi: 10.1002/uog.13313. [DOI] [PubMed] [Google Scholar]
- 41.Matias DS, Santos R, Ferreira T, et al. Predictive value of ophthalmic artery Doppler velocimetry in relation to hypertensive disorders of pregnancy. J Clin Ultrasound. 2020;48:388–95. doi: 10.1002/jcu.22823. [DOI] [PubMed] [Google Scholar]
- 42.Shah P, Maitra N, Vaishnav P, et al. Performance of HDP-Gestosis Score and Ophthalmic Artery Doppler in Prediction of Pre-eclampsia. J Obstet Gynaecol India. 2023;73:43–50. doi: 10.1007/s13224-023-01799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aquino LO de, Leite HV, Cabral ACV, et al. Doppler flowmetry of ophthalmic arteries for prediction of pre-eclampsia. Rev Assoc Med Bras (1992) 2014;60:538–41. doi: 10.1590/1806-9282.60.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Saleh M, Naemi M, Aghajanian S, et al. Diagnostic value of ophthalmic artery Doppler indices for prediction of preeclampsia at 28–32 weeks of gestation. Intl J Gynecology & Obste . 2023;160:120–30. doi: 10.1002/ijgo.14305. [DOI] [PubMed] [Google Scholar]
- 45.Gana N, Sarno M, Vieira N, et al. Ophthalmic artery Doppler at 11-13 weeks’ gestation in prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2022;59:731–6. doi: 10.1002/uog.24914. [DOI] [PubMed] [Google Scholar]
- 46.Sapantzoglou I, Wright A, Arozena MG, et al. Ophthalmic artery Doppler in combination with other biomarkers in prediction of pre-eclampsia at 19-23 weeks’ gestation. Ultrasound Obstet Gynecol. 2021;57:75–83. doi: 10.1002/uog.23528. [DOI] [PubMed] [Google Scholar]
- 47.Nicolaides KH, Sarno M, Wright A. Ophthalmic artery Doppler in the prediction of preeclampsia. Am J Obstet Gynecol. 2022;226:S1098–101. doi: 10.1016/j.ajog.2020.11.039. [DOI] [PubMed] [Google Scholar]
- 48.Teka H, Yemane A, Abraha HE, et al. Clinical presentation, maternal-fetal, and neonatal outcomes of early-onset versus late onset preeclampsia-eclampsia syndrome in a teaching hospital in a low-resource setting: A retrospective cohort study. PLoS One. 2023;18:e0281952. doi: 10.1371/journal.pone.0281952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oancea M, Grigore M, Ciortea R, et al. Uterine Artery Doppler Ultrasonography for First Trimester Prediction of Preeclampsia in Individuals at Risk from Low-Resource Settings. Medicina (Kaunas) 2020;56:428. doi: 10.3390/medicina56090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verma D, Gupta S. Prediction of adverse pregnancy outcomes using uterine artery Doppler imaging at 22-24 weeks of pregnancy: A North Indian experience. Turk J Obstet Gynecol . 2016;13:80–4. doi: 10.4274/tjod.55632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salem MAA, Ammar IMM. First-Trimester Uterine Artery Pulsatility Index and Maternal Serum PAPP-A and PlGF in Prediction of Preeclampsia in Primigravida. J Obstet Gynaecol India. 2018;68:192–6. doi: 10.1007/s13224-017-1012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mönckeberg M, Arias V, Fuenzalida R, et al. Diagnostic Performance of First Trimester Screening of Preeclampsia Based on Uterine Artery Pulsatility Index and Maternal Risk Factors in Routine Clinical Use. Diagnostics (Basel) 2020;10:182. doi: 10.3390/diagnostics10040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Zhang Y, Zhao L, et al. Plasma SerpinA5 in conjunction with uterine artery pulsatility index and clinical risk factor for the early prediction of preeclampsia. PLoS ONE. 2021;16:e0258541. doi: 10.1371/journal.pone.0258541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erickson SJ, Hendrix LE, Massaro BM, et al. Color Doppler flow imaging of the normal and abnormal orbit. Radiology. 1989;173:511–6. doi: 10.1148/radiology.173.2.2678264. [DOI] [PubMed] [Google Scholar]
- 55.Lieb WE, Cohen SM, Merton DA, et al. Color Doppler imaging of the eye and orbit. Technique and normal vascular anatomy. Arch Ophthalmol . 1991;109:527–31. doi: 10.1001/archopht.1991.01080040095036. [DOI] [PubMed] [Google Scholar]