Abstract
Background
Shorter-course treatment for group A streptococcal (GAS) pharyngitis may be equivalent to 10 days for clinical cure but effectiveness for pharyngeal GAS eradication is uncertain. The effect on household transmission has not been examined directly. In 2022, a laboratory antimicrobial stewardship initiative drove an abrupt reduction in treatment durations for GAS pharyngitis locally. This study aimed to assess whether this had any negative effect on key treatment outcomes: clinical failure, microbiological failure, immune sequelae, and household GAS transmission.
Methods
Positive throat swab cultures for GAS 2 years prechange until 25 months postchange were matched to antibiotic dispensing data. Logistic models were fitted to examine associations between treatment duration and 30-day repeat antibiotic treatment, repeat GAS-positive throat swab cultures, hospitalization, incident household cases, and 90-day incidence of rheumatic fever.
Results
A total of 851 patients prechange and 1746 postchange were included; 31.3% prechange received ≤7 days’ treatment versus 59.0% postchange (P < .01). There were no significant differences across any outcome measure between periods. When the postchange period was examined specifically, no significant increases occurred for any outcome measure for patients receiving 0, 5, or 7 days of antibiotics versus 10 days, except higher 30-day antibiotic treatment in those initially receiving no antibiotics (15.6% vs 11.4%, P < .01).
Conclusions
Shorter-course treatment had no detectable negative effect on GAS pharyngitis outcomes in this population, including patients receiving no antibiotics. Short-course therapy for GAS pharyngitis has been associated with lower pharyngeal eradication; this did not translate to a detectable increase in household transmission in this real-world setting, which to our knowledge is a novel finding.
Keywords: antibiotics, diagnostic stewardship, duration(s), pharyngitis, streptococcus pyogenes
In this large cohort of patients with group A streptococcal–positive throat swabs, there was no increase in repeat antibiotic treatment, repeat positive throat swab culture, hospitalization, rheumatic fever, or household transmission after a widespread reduction in antibiotic treatment durations.
Sore throat is a common presentation to general practice. It is often treated with antibiotics, despite most sore throats being viral in origin. This overprescribing has been an area of concern for antimicrobial stewardship (AMS). When Streptococcus pyogenes (group A streptococcus [GAS]) is isolated, many guidelines recommend 10 days of antibiotics, rather than shorter courses; however, the optimal duration is debated [1–3]. A number of systematic reviews have found shorter courses to be noninferior for clinical cure, although most included studies use more broad-spectrum agents such as cephalosporins or macrolides as short-course therapy [4–9]. However, a systematic review found that microbiological failure (ie, failure to eradicate pharyngeal GAS) is more common with shorter courses [10]. This is consistent with a more recent randomized trial finding similar treatment success with 5 versus 10 days of oral penicillin but lower bacterial eradication with the shorter duration; however, differences in agents and dosing schedules across studies mean uncertainly remains [10, 11]. GAS is well-known to spread in households and other close-contact settings [12], which is why importance has been placed on bacterial eradication, with the reasoning being persistence likely increases the risk of transmission. However, to our knowledge, the effect of shorter course therapy for GAS pharyngitis on household transmission has never been examined directly.
Unlike most other developed nations Aotearoa New Zealand (NZ) has a high incidence of acute rheumatic fever (ARF) [13]. Ethnicity is an independent risk factor for ARF in NZ, and ARF occurs almost exclusively in people of Māori or Pacific ethnicity who consequently are targeted for prompt diagnosis and treatment of GAS pharyngitis as part of a national program [13]. Conversely, in those without ethnicity risk factors, ARF is rare and regional and national guidelines recommend minimizing throat swabbing and antibiotic treatment [14, 15]. As an AMS initiative, and to encourage prescribing according to local guidelines, our laboratory altered the reporting of throat swab cultures (TSCs) isolating GAS in 2022 to encourage less antibiotic use and shorter treatment durations for those without ARF risk factors. This had an immediate and widespread effect in our region, with the proportion of patients being dispensed 10 days of antibiotics following GAS-positive TSC dropping from 63% to 37%, with shorter durations becoming more common [16]. The aim of this before-and-after retrospective cohort study was to determine whether this abrupt reduction in treatment durations had any negative effect on key outcome measures for GAS pharyngitis treatment.
MATERIALS AND METHODS
Setting
Awanui Labs Wellington is the single provider of clinical microbiology testing for the greater Wellington region of NZ, serving a population of approximately 500 000. The population is predominantly suburban and mostly identifies as NZ European ethnicity (72.6%), with 15.5% and 9.1%, respectively, identifying as Māori or Pacific, which is similar to national averages [17]. The incidence of ARF in 2023–2024 was approximately 4 per 100,000, which is similar to other metropolitan centers in NZ [18]. TSC in our laboratory is on nonselective 5% sheep blood agar, with β-hemolytic colonies followed up. The target organisms are GAS and Streptococcus dysgalactiae subspecies equisimilis. Other organisms are rarely reported. Reporting of scanty growths is discouraged. All results require electronic signoff by requesters, and results for community samples are not reported to requesters until all testing is finalized (ie, microscopy, culture, and susceptibilities, if required). The change to laboratory reporting of TSC came into effect on 21 September 2022 and involved a change to the comment attached to the report when GAS was isolated. Prior to the change the comment used was: “Beta-hemolytic streptococci are universally susceptible to penicillin”; which was changed to: “For patients WITHOUT risk factors for rheumatic fever (eg, NZ European or Asian ethnicity), treatment is only recommended if symptoms are severe and a 5-DAY antibiotic course duration is usually sufficient. For patients with risk factors for rheumatic fever (eg, Māori or Pacific aged 3–35 years), current guidance remains 10 days of antibiotics. See 3D HealthPathways “Tonsillitis and Sore Throat” for further information”. No other changes were made to laboratory acceptance criteria, workup, or reporting, and no other AMS initiatives in relation to TSC were undertaken. The HealthPathways referenced in the comment had been updated in April 2021 (during the prechange period; see Supplementary Table 1 for recommended agents) to recommend shorter courses for those without ARF risk factors; however, uptake of these recommendations was minimal until the laboratory comments were updated [16].
Analysis
Creation of the analysis dataset has been described previously [16, 19, 20]; briefly, between 1 October 2020 and 31 October 2024, all community TSC results from our laboratory were matched to a dataset containing all community antibiotic dispensing events from our region, using the National Health Index number, which is a unique identifier given to all patients in NZ. Dispensing data were supplied from the National Pharmaceutical Collection, which records all community dispensing of prescribed antibiotics. This allowed the relationship between sample collection, result reporting, and antibiotic dispensing to be determined at an individual patient level. The prechange period was defined as sample collection before 21 September 2022 and the postchange as after this. The following patients were included in the analysis: (1) all those (regardless of age) who had a TSC reported as growing GAS and (2) were not already on antibiotics at time of laboratory report (ie, no dispensing or ongoing antibiotics from 5 days before TSC collection up until date of report). Repeat episodes in the same patient of TSC with GAS within 30 days of the index sample were excluded and not counted as separate index cases. Patients with risk factors for ARF (Māori or Pacific ethnicity and aged 3–35 years) were also excluded and were used as a comparator group because there was no change in treatment duration between time periods (the altered comment for TSC recommended the status quo 10 days’ treatment for this group) [16]. The antibiotic treatment duration each patient was dispensed following TSC was determined by what was dispensed in the 5 days following TSC report. Antibiotics that are not used for treatment of pharyngitis (eg, nitrofurantoin) were excluded from this determination (Supplementary Table 2 for full list).
Key Outcome Measures
Outcome measures that are relevant to the treatment of GAS pharyngitis were examined: (1) isolation of GAS from a further TSC between completion of the initial antibiotic course and day 30 post completion (microbiological failure); (2) further antibiotic dispensing between completion of the initial antibiotic course and day 30 after completion, with the same nonpharyngitis antibiotics excluded (clinical failure requiring retreatment); (3) any unplanned hospitalization within 30 days of TSC collection (clinical failure with possible suppurative complication); (4) hospitalization with infection relating to the pharynx, head, or neck within 30 days of TSC collection (clinical failure with confirmed suppurative complication); (5) a notified diagnosis of ARF within 90 days of TSC collection (immune sequelae); and (6) isolation of GAS from TSC from another individual with the same street address within 30 days of TSC report (household transmission). The 30-day “at-risk” period for outcome measures (1) and (2) were measured from the end of the index antibiotic course because these outcomes either could not or would be very unlikely to occur while the patient was still taking the index antibiotics. For these measures, where a patient did not receive antibiotics for their index GAS-positive TSC “completion of the initial antibiotic course” was regarded as day of TSC report. The hospitalization outcomes were for the only 2 acute admitting hospitals in our region and included visits to the emergency department without overnight admission.
Two analyses were performed: the first compared the outcome measures between the pre- and postchange periods across the entire cohort; the second compared outcomes according to the specific treatment duration dispensed for those in the postchange period. The chi-squared test was used to compare categorical variables and the Mann–Whitney U test for continuous variables. A logistic regression model was fitted for each analysis and outcome measure, with age, sex, ethnicity, NZ Deprivation Index 2018 (a measure of social deprivation assigned by domicile) [21], and season sample was collected in added as covariates. Analysis was performed in Stata-17 (StataCorp, College Station, TX).
Patient Consent Statement
Hospital research committee approval was gained. Following screening, NZ Health and Disability Ethics Committees review, and the requirement for individual patient consent, was waived (2024 OOS 21470), as this project formed part of a quality improvement audit cycle for the laboratory. The study was performed and reported according to the STROBE guidelines [22].
RESULTS
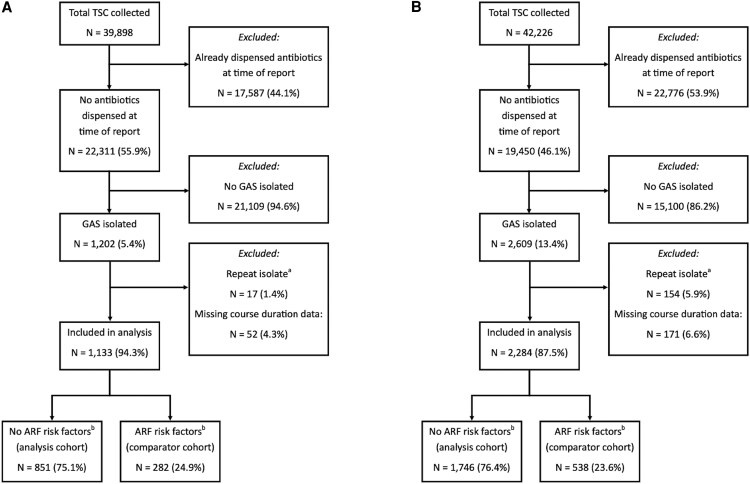
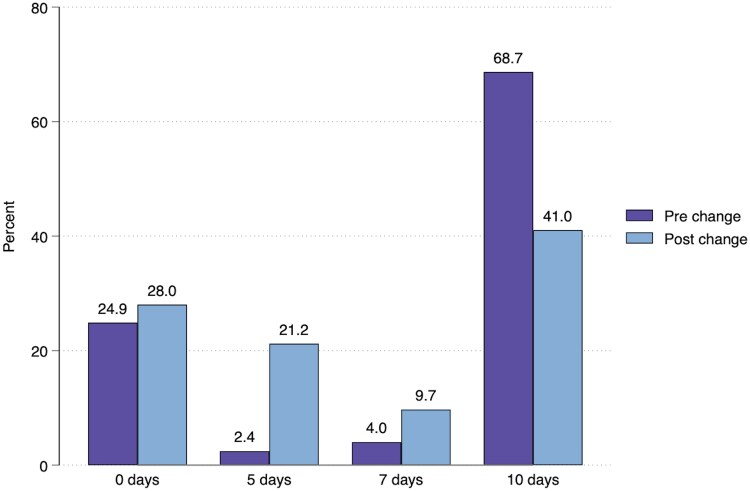
Figure 1 shows the formation of the analysis cohort from all patients having TSC collected in each period. There was a significant increase in TSC positivity for GAS between periods for those not already dispensed antibiotics at time of report (5.4% vs 13.4%, P < .01). A small proportion of patients in each period had missing data for course duration, which was more common for patients dispensed a liquid formulation antibiotic. Those with missing course duration did not differ significantly from those with complete data when stratified by antibiotic formulation (Supplementary Table 3), so data were deemed missing at random and were excluded from the analysis. Demographics of the pre- and postchange cohorts are shown in Table 1. The median age reduced from 20.1 to 18.2 years (P = .02), with a greater reduction observed in the comparator group (17.6 to 11.5 years, P < .01). The age distribution histograms are shown in Supplementary Figures 1a-b. In the postchange period, a higher proportion of patients were from areas of lesser social deprivation (median NZ Deprivation Index 5 prechange vs 4 postchange, P < .01). The antibiotic agents used were similar across periods, although penicillin VK use increased and amoxicillin use decreased. Table 2 shows course durations and outcome measures across periods. There was a reduction in median antibiotic course duration (10 days pre- vs 7 days postchange, P < .01), whereas there was no change in the comparator cohort (median 10 days in both periods, P = .33). Those receiving 10 days of antibiotics reduced from 68.7% prechange to 41.0% postchange (Figure 2), with shorter durations becoming more common, particularly 5 days (2.4% to 21.2% postchange), whereas similar changes were not seen for the comparator cohort (Supplementary Figure 2). There were no significant differences for any outcome measures in the multivariate logistic analyses comparing time periods (Table 2). Hospitalization with infection relating to the pharynx, head, or neck, occurred in only 1 patient in the analysis cohort. Table 3 shows the results of the multivariate logistic analyses comparing outcomes according to the course durations received in the postchange period. There were no significant differences for the adjusted odds ratios (aORs) for any of the measured outcomes, with the exception of higher 30-day repeat antibiotic dispensing in the no antibiotics group compared to ten days (15.6% vs 11.4%; aOR 1.80; 95% confidence interval, 2.26–2.25; P < .01) and lower 30-day household cases in the 5- compared to the 10-day group (4.0% vs 7.0%; aOR 0.52; 95% confidence interval, 0.28–0.97; P = .04). The timings of these events are shown in Supplementary Figures 3a-c. Similar results were seen when the same analysis was performed in the prechange period and when those receiving any antibiotic treatment were compared to those receiving none (Supplementary Tables 4–5). The antibiotic agents used in the different course duration groups were similar, other than the 7-day group, in which amoxicillin was used more frequently (Supplementary Table 6).
Figure 1.
Formation of the analysis and comparator cohorts for aprechange period and bpostchange. aRepeat positive throat swab cultures from the same patient within 30 d were excluded from the analysis. bIn New Zealand, these are Māori or Pacific ethnicity and aged 3–35 y. ARF, acute rheumatic fever; GAS, group A Streptococcus; TSC, throat swab culture.
Table 1.
Demographic and Clinical Characteristics for Patients in the Analysis and Comparator Cohorts
| Prechange | Postchange | ||||
|---|---|---|---|---|---|
| N | % | N | % | P | |
| Analysis cohort (patients without ARF risk factors a ) | … | … | … | … | |
| Total number of samples | 851 | … | 1746 | … | |
| Individual patients | 831 | 97.6% | 1690 | 96.8% | |
| Age (y) median (IQR) | 20.1 | (14.8, 25.0) | 18.2 | (8.5, 35.1) | .02 |
| Female | 539 | 63.3% | 1053 | 60.3% | .09 |
| Ethnic group | … | … | … | … | <.01 |
| European | 690 | 82.8% | 1260 | 75.4% | |
| Māori | 15 | 1.8% | 50 | 3.0% | |
| Pacific | 15 | 1.8% | 38 | 2.3% | |
| Asian | 90 | 10.8% | 258 | 15.4% | |
| Other/unknown | 23 | 2.8% | 65 | 3.9% | |
| NZ Deprivation Index 2018b, median (IQR) | 5 | (2, 7) | 4 | (1, 6) | <.01 |
| Season sample collected in | … | … | … | … | <.01 |
| Spring | 217 | 25.5% | 479 | 27.4% | |
| Summer | 183 | 21.5% | 306 | 17.5% | |
| Autumn | 229 | 26.9% | 395 | 22.6% | |
| Winter | 222 | 26.1% | 566 | 32.4% | |
| Five most common antibiotics dispensed | … | … | … | … | <.01 |
| Phenoxymethylpenicillin (penicillin VK) | 415 | 64.1% | 912 | 71.4% | |
| Amoxicillin | 168 | 26.0% | 238 | 18.6% | |
| Erythromycin | 25 | 3.9% | 36 | 2.8% | |
| Cefalexin | 20 | 3.1% | 68 | 5.3% | |
| Roxithromycin | 9 | 1.4% | 8 | 0.6% | |
| Other | 10 | 1.5% | 16 | 1.3% | |
| Comparator cohort (patients with ARF risk factors a ) | … | … | … | … | |
| Total number of samples | 282 | … | 538 | … | |
| Individual patients | 271 | 96.1% | 524 | 97.4% | |
| Age (y) median (IQR) | 17.6 | (11.0, 22.6) | 11.5 | (8.0, 20.1) | <.01 |
| Female | 172 | 61.0% | 313 | 58.2% | .58 |
| Ethnic group | … | … | … | … | .85 |
| Māori | 170 | 60.3% | 328 | 61.0% | |
| Pacific | 112 | 39.7% | 210 | 39.0% | |
| NZ Deprivation Index 2018b, median (IQR) | 6 | (4, 9) | 7 | (4, 10) | .05 |
| Season sample collected in | … | … | … | … | .21 |
| Spring | 56 | 19.9% | 140 | 26.0% | |
| Summer | 56 | 19.9% | 94 | 17.5% | |
| Autumn | 82 | 29.1% | 136 | 25.3% | |
| Winter | 88 | 31.2% | 168 | 31.2% | |
| Five most common antibiotics dispensed | … | … | … | … | <.01 |
| Phenoxymethylpenicillin (penicillin VK) | 164 | 75.9% | 322 | 80.9% | |
| Amoxicillin | 42 | 19.4% | 44 | 11.1% | |
| Cefalexin | 2 | 0.9% | 15 | 3.8% | |
| Erythromycin | 2 | 0.9% | 12 | 3.0% | |
| Co-amoxiclav | 1 | 0.5% | 5 | 1.3% | |
| Other | 5 | 2.3% | 0 | 0.0% | |
Abbreviations: GAS, group A Streptococcus (Streptococcus pyogenes); IQR, interquartile range; TSC, throat swab culture.
aIn New Zealand these are: Māori or Pacific ethnicity and aged 3–35 y.
bThis is a measure of social deprivation assigned by domicile, with higher numbers indicating increasing levels of social deprivation.
Table 2.
Antibiotic Course Durations and Outcomes for Patients in the Analysis and Comparator Cohorts
| Prechange | Postchange | ||||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | aORa | 95% CI | P | |
| Analysis cohort (patients without ARF risk factors b ) | … | … | … | … | … | … | |
| Total patients | 851 | … | 1746 | … | … | … | |
| Course duration (d), median (IQR) | 10 | (5, 10) | 7 | (0, 10) | - | - | <.01 |
| Course duration (d), mean (SD) | 7.5 | (4.6) | 6.1 | (4.3) | - | - | |
| GAS in TSC within 30 d of index antibiotic course completion | 30 | 3.5% | 48 | 2.7% | 0.78 | (0.48–1.25) | .30 |
| Further antibiotic dispensing within 30 d of index course completion | 109 | 12.8% | 225 | 12.9% | 1.02 | (0.79–1.31) | .88 |
| Any unplanned hospitalization within 30 d of TSC collection | 1 | 0.1% | 9 | 0.5% | 3.98 | (0.50–31.84) | .19 |
| Hospitalization with related infectionc within 30 d of TSC collection | 0 | 0.0% | 1 | 0.1% | - | - | - |
| Household case within 30 d of TSC report | 54 | 6.3% | 113 | 6.5% | 0.96 | (0.69–1.36) | .86 |
| ARF within 90 d of TSC collection | 0 | 0.0% | 0 | 0.0% | - | - | - |
| Comparator cohort (patients with ARF risk factors b ) | … | … | … | … | … | … | |
| Total patients | 282 | … | 538 | … | … | … | |
| Course duration (d), median (IQR) | 10 | (5, 10) | 10 | (0, 10) | - | - | .33 |
| Course duration (d), mean (SD) | 7.4 | (4.3) | 7.1 | (4.5) | - | - | |
| GAS in TSC within 30 d of index antibiotic course completion | 5 | 1.8% | 15 | 2.8% | 1.65 | (0.58–4.65) | .35 |
| Further antibiotic dispensing within 30 d of index course completion | 36 | 12.8% | 81 | 15.1% | 1.22 | (0.79–1.88) | .36 |
| Any unplanned hospitalization within 30 d of TSC collection | 1 | 0.4% | 2 | 0.4% | 1.00 | (0.09–11.52) | 1.00 |
| Hospitalization with related infectionc within 30 d of TSC collection | 0 | 0.0% | 1 | 0.2% | - | - | - |
| Household case within 30 d of TSC report | 8 | 2.8% | 37 | 6.9% | 2.28 | (1.04–5.03) | .04 |
| ARF within 90 d of TSC collection | 1 | 0.4% | 2 | 0.4% | 0.83 | (0.07–9.59) | .88 |
Abbreviations: aOR, adjusted odds ratio; ARF, acute rheumatic fever; CI, confidence interval; GAS, group A Streptococcus (S. pyogenes); IQR, interquartile range; SD, standard deviation; TSC, throat swab culture.
aLogistic model compares the odds of each outcome variable according to pre- versus postchange period, with prechange set as the base value and adjusted for age, sex, ethnicity, NZ Deprivation Index 2018, and season sample was collected in.
bIn NZ these are: Māori or Pacific ethnicity and aged 3–35 y.
cRelated infection defined as admission with infection relating to pharynx, head, or neck.
Figure 2.
Percentage of patients in the analysis cohort receiving a given antibiotic course duration following positive throat swab culture for group A Streptococcus in the pre- and postchange periods.
Table 3.
Outcomes According to Antibiotic Course Duration for Patients in the Postchange Period Analysis Cohort
| N | % | aORa | 95% CI | P | |
|---|---|---|---|---|---|
| Total patients b | … | … | … | … | |
| 10 d | 685 | 41.0% | … | … | |
| 7 d | 162 | 9.7% | … | … | |
| 5 d | 354 | 21.2% | … | … | |
| No antibiotics | 468 | 28.0% | … | … | |
| GAS in TSC within 30 d of index antibiotic course completion | … | … | … | … | |
| 10 d | 25 | 3.6% | 1.00 | - | - |
| 7 d | 2 | 1.2% | 0.29 | (0.07–1.26) | .10 |
| 5 d | 9 | 2.5% | 0.66 | (0.30–1.44) | .30 |
| No antibiotics | 10 | 2.1% | 0.59 | (0.27–1.28) | .18 |
| Further antibiotic dispensing within 30 d of index course completion | … | … | … | … | |
| 10 d | 78 | 11.4% | 1.00 | - | - |
| 7 d | 19 | 11.7% | 1.01 | (0.59–1.73) | .98 |
| 5 d | 45 | 12.7% | 1.10 | (0.75–1.64) | .63 |
| No antibiotics | 73 | 15.6% | 1.80 | (2.26–2.55) | <.01 |
| Any unplanned hospitalization within 30 d of TSC collection | … | … | … | … | |
| 10 d | 5 | 0.7% | 1.00 | - | - |
| 7 d | 3 | 1.9% | 2.45 | (0.56–10.64) | .23 |
| 5 d | 0 | 0.0% | - | - | - |
| No antibiotics | 0 | 0.0% | - | - | - |
| Household case within 30 d of TSC report | … | … | … | … | |
| 10 d | 48 | 7.0% | 1.00 | - | - |
| 7 d | 10 | 6.2% | 0.81 | (0.40–1.65) | .57 |
| 5 d | 14 | 4.0% | 0.52 | (0.28–0.97) | .04 |
| No antibiotics | 33 | 7.1% | 1.04 | (0.64–1.68) | .89 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; IQR, interquartile range; GAS, group A Streptococcus (Streptococcus pyogenes); SD, standard deviation; TSC, throat swab culture.
Hospitalization with infection relating to pharynx, head, or neck and acute rheumatic fever have been omitted from this table because there was only 1 case and a logistic model could not be fitted.
aLogistic model compares the odds of each outcome variable according to course duration adjusted for age, sex, ethnicity, NZ Deprivation Index 2018, season sample was collected in, with 10-d antibiotic duration set as the base value.
bTotal (1669) is lower than 1746 from previous table due to a small number of patients being treated with course durations other than 0, 5, 7, or 10 d.
DISCUSSION
In this analysis, key outcome variables for patients with GAS pharyngitis were examined across 2 periods, between which there was an abrupt overall reduction in the antibiotic treatment durations used in our region. Because the change in course durations was driven by a laboratory AMS initiative involving a change in laboratory reporting [16] patients receiving shorter antibiotic courses in the postchange period represent individuals who would otherwise likely have been prescribed 10 days of antibiotics. Despite the shorter durations, there was no signal from the data of increased risk of poor patient outcomes, including household transmission, irrespective of duration of short-course antibiotics given, which included patients dispensed no antibiotics. Although the no antibiotics group had more 30-day antibiotic treatment, overall this group still had far less antibiotic exposure than the other groups due to the absence of an index course.
These findings are consistent with prior evidence demonstrating similar treatment efficacy with shorter course antibiotic treatment of GAS pharyngitis. Much of this literature used nonpenicillins [4–9], so this study adds to recent trial evidence showing equivalent clinical outcomes using shorter course penicillin-based treatment [11]. Shorter course treatment has been associated with poorer pharyngeal GAS eradication, with most studies assessing this via programmed TSC at a set time point after treatment [10]. The clinical relevance of ongoing TSC positivity for GAS in patients that have clinically recovered is uncertain, and to our knowledge this study is the first time the effect of short-course therapy on real-world household transmission of GAS has been examined. Here, we found no difference in incident household cases of GAS pharyngitis in those receiving short-course treatment, or indeed no antibiotics. At least part of the explanation for this appears to be that many household cases occurred very shortly after diagnosis of the index case, suggesting transmission likely occurred before any effect of antibiotic treatment (Supplementary Figure 2c).
Strengths of this study include a large sample size, which is important in the context of a study where most outcome measures were found not to differ between groups. Despite the large sample size, matching of laboratory and antibiotic treatment data occurred at the individual patient level, meaning these individual relationships could be precisely defined at scale rather than needing to be aggregated at a population level, as is common with observational studies. The nature of our local health services, with a single laboratory provider and only 2 acute admitting hospitals, permitted near-complete coverage and follow-up for outcome measures. We also examined multiple potential indicators of treatment failure, increasing the sensitivity for detecting suboptimal outcomes due to shorter treatment durations. Finally, a comparator group in which treatment durations did not change was available for comparison, which demonstrated that outcome measures were otherwise stable over time.
Limitations of this study include its observational nature and the potential for residual confounding after adjustment of the logistic models for measurable potential confounders. As such, differences in treatment duration at the individual patient level could have been influenced by unmeasured patient factors that could also influence outcome (eg, clinicians may have felt more comfortable using short-course therapy in patients with milder symptoms, who may also have been less likely to have treatment failure). Outcome measures were also determined via passively collected data, based on re-presentation to healthcare services, rather than systematic follow-up, as would occur in a trial setting. However, this may be both a limitation and a strength, as re-presentation is a demonstration of an outcome that was sufficiently important to the patient to seek further help. This may be a better reflection of the real-world effects of shorter courses. For example, we did not collect TSC on household members of those being treated for GAS pharyngitis to determine the true incidence of household transmission. However, transmission leading to a laboratory diagnosis of GAS pharyngitis in a household member, as in this study, is likely a more relevant outcome. In this study we only examined patients who were not on antibiotics at the time of TSC result reporting, which represented 55.9% and 46.1% of all patients having TSC collected pre- and postchange, respectively (Figure 1). This was because there was a lesser reduction in treatment durations in those prescribed antibiotics prelaboratory report across periods (because the laboratory report itself is what drove the shorter durations) [16]. It is possible that patients included in this study may therefore represent patients with milder symptoms, as clinicians felt comfortable awaiting laboratory results before prescribing. COVID-19–related lockdowns occurred during the prechange period of this study. Effects of this included less seasonal variation of sample collection (Table 1) and lower TSC positivity for GAS (Figure 1). It is possible differing community transmission dynamics or circulating GAS strains may have affected comparisons between periods, for example, the median age in the comparator cohort was significantly lower postchange (17.6 to 11.5 years); however, this would be less likely to affect comparisons limited to the postchange period (Table 3). The effect on the household transmission aspects of this study is uncertain; on one hand, introductions of GAS into households would have been markedly reduced, but households tended to lock down together, so there would still have been ample opportunity for transmission between index cases and household contacts. Our analysis was also based on pharmacy dispensing data, which does not capture a minority of antibiotic dispensing that occurs within practices (eg, intramuscular benzathine penicillin). However, we expect this to have had minimal effect on the analysis cohort, as this is predominantly targeted at those with ARF risk factors [23]. Finally, this study was performed in a single geographical region, so results may not necessarily be generalizable to other populations; however, the population in our region is relatively heterogeneous and likely similar to many populations in other developed countries.
In this cohort, a widespread reduction in antibiotic treatment duration for GAS pharyngitis does not appear to have had a negative effect across a range of key treatment outcomes. This was demonstrated at a population level between periods and at an individual course duration level. A significant proportion of patients were not dispensed any antibiotic treatment yet had similar overall outcomes to those that were. These results suggest that short-course treatment or management without antibiotics are acceptable approaches for GAS pharyngitis. Although short-course treatment has been associated with lower bacterial eradication rates previously, we could find no evidence in this real-world setting that it results in an increase in subsequent household cases. This is a novel finding as far as we are aware.
Supplementary Material
Contributor Information
Max Bloomfield, Department of Microbiology, Awanui Laboratories Wellington, Wellington, New Zealand; Department of Infection Services, Te Whatu Ora/Health NZ—Capital, Coast and Hutt Valley, Wellington, New Zealand.
Hamish Reed, Resident Medical Officers’ Unit, Te Whatu Ora/Health NZ—Capital, Coast and Hutt Valley, Wellington, New Zealand.
Sue Todd, Ora Toa Cannons Creek General Practice, Porirua, New Zealand.
Koen van der Werff, Department of Microbiology, Awanui Laboratories Wellington, Wellington, New Zealand.
Michelle Balm, Department of Microbiology, Awanui Laboratories Wellington, Wellington, New Zealand; Department of Infection Services, Te Whatu Ora/Health NZ—Capital, Coast and Hutt Valley, Wellington, New Zealand.
Tim Blackmore, Department of Microbiology, Awanui Laboratories Wellington, Wellington, New Zealand; Department of Infection Services, Te Whatu Ora/Health NZ—Capital, Coast and Hutt Valley, Wellington, New Zealand.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Te Whatu Ora—Ministry of Health for help in accessing the National Pharmaceutical Collection data, and Peter Wash and Joe Hart for help in accessing demographic and laboratory data.
Potential conflicts of interest. No conflicts.
Financial support. The study was supported by internal funding.
References
- 1. Gunnarsson RK, Ebell M, Centor R, et al. Best management of patients with an acute sore throat–a critical analysis of current evidence and a consensus of experts from different countries and traditions. Infect Dis 2023; 55:384–95. [DOI] [PubMed] [Google Scholar]
- 2. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012; 55:1279–82. [DOI] [PubMed] [Google Scholar]
- 3. Pelucchi C, Grigoryan L, Galeone C, et al. Guideline for the management of acute sore throat: ESCMID Sore Throat Guideline Troup C. Pelucchi et al. Guideline for management of acute sore throat. Clin Microbiol Infect 2012; 18:1–28. [DOI] [PubMed] [Google Scholar]
- 4. Ioannidis JPA. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for upper respiratory tract infections. J Antimicrob Chemother 2001; 48:677–89. [DOI] [PubMed] [Google Scholar]
- 5. Casey JR, Pichichero ME. Metaanalysis of short course antibiotic treatment for group A streptococcal tonsillopharyngitis. Pediatr Infect Dis J 2005; 24:909–17. [DOI] [PubMed] [Google Scholar]
- 6. Casey JR, Pichichero ME. Higher dosages of azithromycin are more effective in treatment of group A streptococcal tonsillopharyngitis. Clin Infect Dis 2005; 40:1748–55. [DOI] [PubMed] [Google Scholar]
- 7. Pichichero ME, Casey JR. Bacterial eradication rates with shortened courses of 2nd- and 3rd-generation cephalosporins versus 10 days of penicillin for treatment of group A streptococcal tonsillopharyngitis in adults. Diagn Microbiol Infect Dis 2007; 59:127–30. [DOI] [PubMed] [Google Scholar]
- 8. Altamimi S, Khalil A, Khalaiwi KA, Milner RA, Pusic MV, Al Othman MA. Short-term late-generation antibiotics versus longer term penicillin for acute streptococcal pharyngitis in children. Cochrane Database Syst Rev 2012; 2012:CD004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dawson-Hahn EE, Mickan S, Onakpoya I, et al. Short-course versus long-course oral antibiotic treatment for infections treated in outpatient settings: a review of systematic reviews. Fam Pract 2017; 34:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falagas ME, Vouloumanou EK, Matthaiou DK, Kapaskelis AM, Karageorgopoulos DE. Effectiveness and safety of short-course vs long-course antibiotic therapy for group a beta hemolytic streptococcal tonsillopharyngitis: a meta-analysis of randomized trials. Mayo Clin Proc 2008; 83:880–9. [PubMed] [Google Scholar]
- 11. Skoog Ståhlgren G, Tyrstrup M, Edlund C, et al. Penicillin v four times daily for five days versus three times daily for 10 days in patients with pharyngotonsillitis caused by group A streptococci: randomised controlled, open label, non-inferiority study. BMJ 2019; 367:l5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brundage JF, Gunzenhauser JD, Longfield JN, et al. Epidemiology and control of acute respiratory diseases with emphasis on group A beta-hemolytic streptococcus: a decade of U.S. Army experience. Pediatrics 1996; 97:964–70. [PubMed] [Google Scholar]
- 13. Bennett J, Zhang J, Leung W, et al. Rising ethnic inequalities in acute rheumatic fever and rheumatic heart disease, New Zealand, 2000–2018. Emerg Infect Dis 2021; 27:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Heart Foundation of New Zealand . Group A streptococcal sore throat management guideline. Auckland: National Heart Foundation, 2019. [Google Scholar]
- 15. Community HealthPathways . Tonsillitis and sore throat. 2021. Available at: https://3d.communityhealthpathways.org/17240.htm. Accessed 26 July 2023.
- 16. Bloomfield M, van der Werff K, Todd S, Balm M, Blackmore T. Effect of directive laboratory comments on prescribing response to positive throat swab cultures. J Antimicrob Chemother 2024; 79:334–8. [DOI] [PubMed] [Google Scholar]
- 17. Statistics New Zealand . 2023 Census population counts (by ethnic group, age, and Māori descent) and dwelling counts. 2024. Available at: https://www.stats.govt.nz/information-releases/2023-census-population-counts-by-ethnic-group-age-and-maori-descent-and-dwelling-counts/. Accessed 9 May 2025.
- 18. Health New Zealand . Reducing rheumatic fever. 2024. Available at: https://www.tewhatuora.govt.nz/for-health-professionals/clinical-guidance/diseases-and-conditions/rheumatic-fever-guidance/reducing-rheumatic-fever#:∼:text=In%202023%2F24%2C%20there%20were,23%20(2.7%20per%20100%2C000). Accessed 9 May 2025.
- 19. Bloomfield M, Todd S, van der Werff K, Blackmore T, Balm M. Post-report antibiotic initiation following community non-sterile-site microbiology results: an opportunity for labs to lead stewardship? J Antimicrob Chemother 2023; 78:2715–22. [DOI] [PubMed] [Google Scholar]
- 20. Bloomfield M, van der Werff K, Todd S, et al. An exception-reporting approach for wound swab culture: effect on post-report antibiotic initiation. J Clin Microbiol 2024; 62:e0034224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atkinson J, Salmond C, Crampton P. NZDep2013 Index of Deprivation. 2014. Available at: www.otago.ac.nz/wellington/otago069936.pdf. Accessed 4 November 2022.
- 22. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–7. [DOI] [PubMed] [Google Scholar]
- 23. BPAC NZ . Antibiotics: choices for common infections. 2023; Available at: https://bpac.org.nz/antibiotics/guide.aspx. Accessed 9 May 2025.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.