Abstract
The gut microbiota, comprising a diverse array of microorganisms in the gastrointestinal tract, has emerged as a key player in human health. Emerging research indicates that this gut microbial composition is influenced by sex. These sex differences are not necessarily static and likely alter across the life course in response to several factors including changing hormone profile. As such, the menopause transition-a pivotal phase in female ageing in which the hormone profile changes dramatically is receiving increasing attention. Declining estrogen which occurs during menopause appears to influence the microbiota, which may in turn contribute to menopause-related conditions such as weight gain, bone health, cancer risk and cognitive health. The modulation of estrogen through the gut’s ‘estrobolome’, a collection of bacterial genes involved in estrogen metabolism, may offer explanation for some of the interindividual differences observed during menopause (e.g. length, symptoms and disease risk). Therapeutic modulation of the gut microbiota therefore represents a potential approach towards managing menopausal symptoms. Indeed, prebiotics and probiotics such as Lactobacillus have been shown to increase bacterial diversity and improve metabolic and overall health in menopausal women. However, evidence remains limited regarding the specific underlying mechanisms, highlighting an urgent need for a research focus in the area. This review summarizes the current understanding of the gut microbiota’s role in menopausal health and the potential of prebiotics and probiotics as therapeutic interventions. Further research into gut microbiota modulation may enable more effective, personalised treatments for menopause-associated health challenges, and supporting women’s health into older ages.
Keywords: Menopause, diet, microbiome, estrogen, fibre
Introduction
The ‘Gut microbiota’ refers to the collection of microorganisms in the gastrointestinal (GI) tract including bacteria, archaea, viruses and fungi. 1 A symbiotic relationship exists between these organisms and the host, which can be beneficial, neutral or pathogenic. The gut microbiota is shaped and influenced by various factors (exogenous and endogenous) encountered throughout the life course including but not limited to birth (e.g. mother’s health and mode of delivery), diet and antibiotic usage. Intriguingly, biological sex has also been identified as an important modulator of gut microbial composition. 1 Sex mediated gut microbiota differentiation starts in early life. On the first day of life, male infants show higher Bifidobacterium levels as compared to female infants. 2 Within the first 30 days, stool sample analysis reveals that male neonates have lower α-diversity, lower abundance of Clostridiales and higher abundance of Enterobacteriales compared to females. 3
Both dynamic changes (during the onset of puberty and menopause) and steady-state (during adulthood) levels of sex hormones play a role in shaping the gut microbiota. 1 In adulthood, gut microbial diversity continues to increase and transitions towards a state of uniqueness (within each person) with advancing age, 4 plateauing typically around the age of 40 years. 5 Sexual dimorphism of the gut microbiota, in adulthood, has been observed in several human studies.6–9 In two large human cohorts, the Belgian Flemish Gut Flora Project (n = 1106) and the Dutch LifeLines-Deep study (n = 1135), sex (10th strongest effect size among 69 factors) correlated significantly with overall microbiome community variation. 9 Another large cohort study of adults from four geographical regions showed that young adult women had higher gut microbial diversity than young adult men, but these differences were less pronounced in middle-aged adults. 5 Differences in gut microbiota between pre- and post-menopausal women have been reported, 10 with postmenopausal women’s gut microbiota observed to be more like age-matched men than premenopausal women. 11 A higher relative abundance of Lachnospira and Roseburia, and a lower relative abundance of Prevotella, Parabacteroides and Bilophila were reported in premenopausal women than in postmenopausal women (who had similar levels to the men). 11
Although research on gut microbiota changes during the menopausal transition is emerging. The available studies are inconsistent, with most of them comparing menopausal women with non-menopausal women, which does not account for the age effect. Thus, the current evidence remains limited, highlighting the need for further investigation. This review aims to summarise the existing knowledge on the interplay between the gut microbiota and sex hormones and their potential role in menopausal symptoms. We also present the current evidence on the impact of prebiotic and probiotic interventions (potent gut microbial modulators) and their role in alleviating menopausal symptoms.
Gut microbiota and sex hormones
Sex steroid hormones (including androgens, estrogens and progestogens) are the main determinants of metabolic and physical differences between females and males. Generally, females and males have the same hormones but they differ in their blood concentrations, production sites, and their interaction with different organs and systems. 12
A bidirectional relationship exists between these sex hormones and the gut microbiota: (1) sex hormones (at specific concentrations) appear to modulate the gut microbial composition, 13 whilst (2) the gut microbiota regulates the levels of circulating sex hormones. 14
2.1) Rodent studies have been particularly useful in establishing the modulatory effects of sex hormones on the microbiota. For example, gut bacterial diversity is observed to be similar across males and females prior to puberty, with differences only becoming clear among post-pubescent animals. 15 Similarly, castrated male littermates possess a microbiota more like that of a female microbiota. 15 This sex-specific difference in microbiota was found to be consistent across different strains of mice. 16
In humans this is more difficult, observations in pregnant women have revealed profound effects on the gut microbiota, especially during the third trimester when the estrogen levels are at their peak. 17 Menopause associated sex hormones changes are also linked with decreased gut microbial diversity in women as the microbiota shifts towards more male-like composition. 14 More research needs to be done to better understand these changes, and their role in menopause-linked health status.
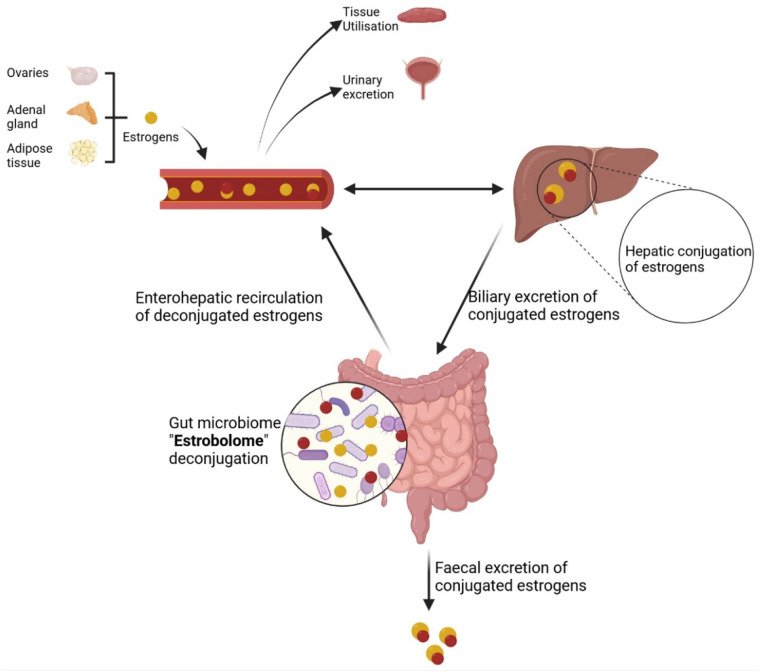
2.2) Endogenous estrogens are metabolised in the liver into glucuronidated or sulphated forms, which allows the conjugated estrogens to reach the intestinal tract via biliary excretion. A number of gut bacterial species across major phyla, and especially Firmicutes and Bacteroidetes, possess enzymes like β-glucuronidases18,19 and sulphatases, 20 which deconjugate the excreted estrogens, thus allowing them to be recycled back into the circulation and carry on their physiological function 21 (Figure 1). An aggregate of these bacterial genes, whose products are capable of metabolising estrogens, is referred to as the ‘estrobolome’. 22 This recycling was further confirmed using radioactively labelled estradiol and estrone when injected in women, and their excretion was recorded in bile and faeces. Approximately, 50% of the injected estrogens are excreted in bile, 23 with a much smaller fraction appearing in the faeces (10–15%) 24 which indicates that a significant portion is reabsorbed in the systemic circulation via bacterial deconjugation. Other sex hormones including progesterone 25 and androgens 26 are also similarly deconjugated and recirculated by the gut microbiota. In a recent human study, the estrogen metabolising activity of the gut microbiota (β-glucuronidase enzyme activity) was found to be inversely associated with estrogen levels in the gut, whereas the systemic levels of estrogens were strongly and directly associated with gut microbial richness (i.e. number of unique bacterial species). 27 The role of gut microbiota in modulating sex hormones is further supported by a rodent study in which the transplantation of gut microbiota from male to female mice resulted in a systemic increase in testosterone levels. 28
Figure 1.
Estrogens are produced by the ovaries, adrenal gland and adipose tissue, and released in the blood stream, and metabolised (in the liver) into biologically inactive conjugated forms which are released via biliary excretion into the small intestine. A specific set of gut bacteria, possessing β-glucuronidase enzymes (referred to as ‘Estrobolome’) deconjugate estrogens into biologically active free-forms. These may be reabsorbed into the blood circulation, used by peripheral tissues, and returned to the liver through enterohepatic recycling for re-conjugation. Some of the conjugated and deconjugated estrogens are excreted via urine and stool.
Menopausal transition, gastrointestinal health and the gut microbiota
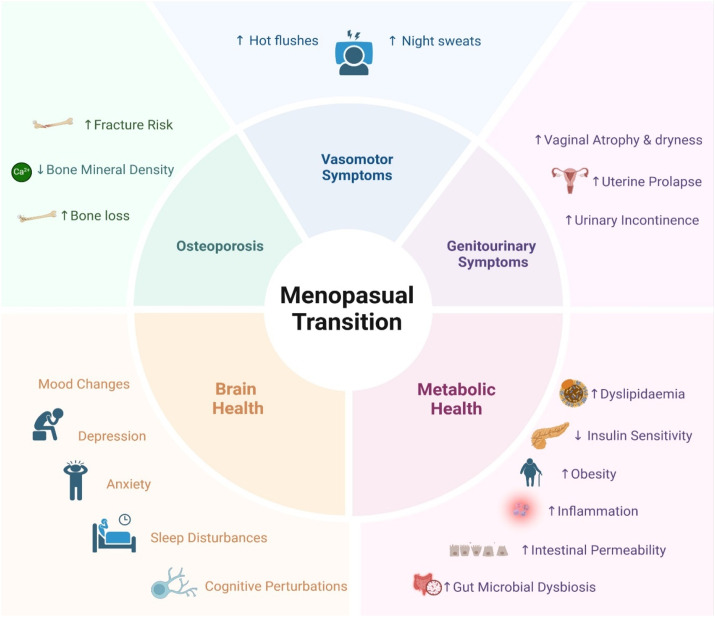
The menopausal transition occurs as a result of natural reproductive ageing when a woman loses primary ovarian follicles and oocytes. It is accompanied by fluctuations in female sex hormones including a decrease in estrogens and progesterone; and an increase in follicle-stimulating hormone (FSH) and luteinising hormone (LH). 29 These hormonal fluctuations are accompanied by various menopausal symptoms (varying in severity among women) including vasomotor symptoms (i.e. hot flushes and night sweats), genitourinary symptoms (i.e. vaginal atrophy and dryness, and urinary incontinence), mood changes (i.e. depression and anxiety), cognitive perturbations (termed brain fog), sleep disturbances and changes in bone mineral density (Figure 2). 30 The menopausal transition usually lasts for an average of 7 years and occurs between the ages of 45 and 55 years. 29 Delayed menopause is associated with higher risk of endometrial and breast cancer, while early menopause increases the risk for osteoporosis and cardiovascular diseases. The age of natural menopause onset depends on various factors including genetic, environmental, reproductive, dietary and lifestyle. 31 The association between diet and menopause onset is not very well researched but there is evidence that dietary factors influence the age of menopause onset via their effect on serum hormone levels.31,32
Figure 2.
Representation of physiological symptoms associated with menopausal transition.
Menopause significantly impacts gastrointestinal health, influencing appetite regulation, weight management and digestion, all of which are linked to hormonal changes during this stage. 33 Postmenopausal women experience changes in bowel function more often compared to premenopausal women, 34 with gastrointestinal conditions such as irritable bowel syndrome presenting more severe symptoms in postmenopausal women than in premenopausal women or men of similar age. 35 Hormonal shifts associated with menopause commonly lead to these gastrointestinal symptoms including abdominal pain, bloating, nausea, constipation and indigestion, 36 which may also be associated with alterations in the gut microbial composition.
As aforementioned, the gut microbiota plays an important role in maintaining circulating levels of sex hormones. Experimental evidence suggests that estrogen and progesterone improve barrier function of intestinal epithelial cells by upregulating the tight junction proteins.37,38 The hormonal fluctuations and decline associated with menopause have been linked to reduced gut barrier integrity and increased microbial translocation. 14 Similarly, in mouse model, ovariectomy has been shown to increase intestinal permeability. 39 Findings from the Study of Women’s Health Across the Nation (SWAN) cohort indicate that gut permeability (as measured by fatty acid binding protein 2 [FABP2]), increases during the menopausal transition and is linked to heightened inflammation. 40 This increased permeability facilitates the movement of microbes and microbial products from the gut into systemic circulation, potentially triggering systemic inflammation and immune activation, which are associated with the pathogenesis of various diseases. 41 Menopause affects the gastrointestinal health as well as gut microbiota composition which potentially play an important role in menopause-associated symptoms.
A metagenome-wide association study identified distinct differences in the gut microbiota and associated metabolites between premenopausal and postmenopausal women. 10 Postmenopausal women exhibited an overrepresentation of Bacteroidetes and Tolumonas, while Firmicutes and Roseburia species were depleted. 10 Roseburia, a group of commensal bacteria that produce short-chain fatty acids, are crucial for metabolic health, and their reduction has been linked to various metabolic diseases. 42 Conversely, Tolumonas showed a negative correlation with bone mineral density, 10 potentially due to its role in toluene production, which adversely affects bones by reducing bone density. 43 In addition to differences in bacterial species, significant variations in the metabolic and biological processes of these species were observed between premenopausal and postmenopausal women through GMM analysis. These findings suggest a potential contribution to increased disease risks in postmenopausal women.. 10 Another study comparing gut microbiota between individuals with menopausal syndrome (MPS) and healthy menopausal subjects revealed that gut dysbiosis was present exclusively in those with MPS. MPS is diagnosed by clinical manifestations including (1) menstrual disorders; (2) vasomotor symptoms primarily hot flushes; (3) one or more additional symptoms such as a mental disorder, urogenital atrophy, cardiovascular symptoms, skin and body changes and osteoporosis; along with (4) menopause-related hormonal changes. 44 These individuals exhibited an enrichment of metabolic pathways associated with cardiovascular disease and carbohydrate metabolism, suggesting a higher risk of developing cardiovascular disease, obesity, diabetes, and other related conditions. 44
Notably, menopause-associated gut microbial dysbiosis has been linked to postmenopausal osteoporosis. Changes in bacterial α-diversity (the number and distribution of unique bacterial species) and β-diversity (patterns of microbial shifts) were more pronounced in postmenopausal osteoporosis patients compared to non-osteoporotic postmenopausal women. These microbial alterations were also associated with changes in bone mineral density. 45 In postmenopausal obese women, visceral adiposity was positively associated with gut dysbiosis, and negatively associated with the Firmicutes:Bacteroidetes ratio. It was observed that visceral adiposity in these postmenopausal women was also inversely associated with the abundance of SCFA-producing commensal bacteria. 46 Taken together, literature suggests the association of gut dysbiosis with not only menopausal symptoms but also the severity of these symptoms, although further research is needed to better understand the underlying mechanisms.
Gut-microbiota-brain axis in menopause
Menopause affects brain health altering connectivity, structure and metabolism independent of age. 47 Indeed, brain fog and issues with memory are often reported during the menopause transition. 48 Sex hormones such as estrogen have implications for brain health with estrogen described as neuroprotective, promoting spinogenesis and synaptogenesis, 49 as well as cerebral blood perfusion and overall cerebrovascular function. 48 As such, it is possible that the modulation of estrogens through the ‘estrobolome’ could have neurological implications. Similarly, it could be envisaged that remodelling of the microbiome through various interventions (e.g. dietary interventions) could improve/restore gut brain axis interactions impacted by menopause. To our knowledge a gut-microbiota-brain axis connection in relation to menopause is yet to be reported but should be the focus of future research endeavours given the clear impact of menopause on brain health.
Menopausal transition – prebiotic and probiotic interventions and future directions in microbiota research
Hormone Replacement Therapy (HRT) is often the first line of treatment for improving menopausal symptoms. However, its use is contraindicated for many women, for example, those with a history of breast cancer or blood clotting, with many women also suffering side effects and not wishing to take HRT over extended periods of time. 30 Therefore, alternatives to HRT in menopause are needed.
Modulating the gut microbiota might be a promising strategy to alleviate or relieve menopausal symptoms. 49 The gut microbiota primarily feed on dietary fibres. High quality carbohydrate intake (which means higher intake of fibres, solid carbohydrates and low glycaemic index foods) has been associated with lower somatic and psychological symptoms of menopause. 50 Prebiotic interventions have shown promising potential in managing menopausal symptoms by modulating the gut microbiota and addressing associated physiological and metabolic changes (Table 1). Dietary interventions with prebiotics such as flaxseeds 51 and vegetables 52 have improved intestinal integrity, lipid profiles, and been associated with reductions in body weight and BMI among perimenopausal and menopausal women. Similarly, flavonoid-rich blackcurrants 53 and water-soluble soybean fibres 54 have shown benefits for bone health by enhancing calcium absorption and preserving bone mineral density, thereby mitigating osteoporosis risks in menopausal women. Metabolic health improvements have also been observed with flaxseed mucilage resulting in better insulin sensitivity 55 in obese postmenopausal women. Additionally, rice bran and tea seed oil have reduced both peripheral and neuroinflammation in animal models, 56 while prebiotic-rich yogurt alleviated menopausal symptoms such as anxiety, depression and vasomotor disturbances. 57
Table 1.
Studies on the effect of prebiotics in managing menopausal symptoms.
| Study reference | Study population | Study design | Intervention | Study duration | Results/Observed outcomes |
|---|---|---|---|---|---|
| Rodent studies | |||||
| Lucas et al., 2000 62 | Ovariectomised (OVX) Sprague-Dawley rats (n = 48) | Experimental intervention versus sham-operated and OVX controls | Low-dose (LD) prune diet (5%), High-dose (HD) prune diet (25%) prunes |
45 days | HD prune diet prevented the OVX induced elevation in serum cholesterol |
| Mitmura et al., 2003 54 | OVX Sprague-Dawley rats (n = 40) | Experimental intervention versus sham-operated and OVX controls | Water-soluble soybean fibre (WSSF) (50 g/kg of diet) | 4 weeks | WSSF-fed rats had ↑Ca absorption, ↑femoral Ca content, and prevented the OVX induced elevation in serum cholesterol |
| Chao et al., 2024 56 | OVX C57BL/6 female mice under multiple metabolic stressors (n = 16) | Experimental intervention versus sham-operated young and old mice | Rice bran and tea seed oil (10% w/w of the diet) | 8 weeks | ↑ Clostridia (short chain fatty acid producers) ↓Tannerellaceae (endotoxins producers) ↓hepatic fat accumulation, ↓peripheral inflammation ↓oxidative damage ↓neuroinflammation in the brain |
| Human studies | |||||
| Sari et al., 2020 52 | Menopausal overweight women with hyperlipidaemia (n = 60) | Randomised parallel control study | 400 g/day of vegetables | 21 days | Improvements in plasma lipid profile, body weight, and BMI were observed |
| Sant’Ana et al., 2022 51 | Perimenopausal overweight women (n = 30) | Non-randomised, prospective, parallel clinical trial | Brown and golden flaxseeds (40g) | 12 weeks | Flaxseeds consumption ↓intestinal permeability and improved the blood lipid profile, especially in the golden flaxseeds group |
| Brahe et al., 2015 55 | Obese postmenopausal women (n = 58) | Randomised, blinded, placebo-controlled trial | Flaxseed mucilage (10 g/day) | 6 weeks | ↓serum C-peptide ↓insulin release during an oral glucose tolerance test ↑insulin sensitivity ↓Faecalibacterium species Observed changes in insulin sensitivity were not mediated by bacterial changes |
| Nosal et al., 2024 53 | Peri- and early postmenopausal women aged 45–60 years (n = 40) | Randomised, double-blind, placebo-controlled trial | Blackcurrant (BC) supplementation | 6 months | - Preserved whole-body bone mineral density - Dose-dependent increase in Ruminococcus 2 bacteria, suggestive of the BC’s bone protective effect |
| Kahleova et al., 2023 61 | Postmenopausal women (n = 84) | Randomised controlled trial | Low-fat, vegan diet with cooked soybeans (1/2 cup daily) | 12 weeks | - ↓severe day hot flushes associated with changes in Porphyromonas and Prevotella corporis abundance -↓total severe hot flushes and ↓severe night hot flushes associated with changes in Clostridium asparagiforme abundance |
| Wu et al., 2022 63 | Postmenopausal women with a history of Roux-en-Y gastric bypass (RYGB) (n = 20) | Randomised double-blind, placebo-controlled trial | Soluble corn fibre (SCF) (20 g/day) – a prebiotic | 2 months | ↑fractional calcium absorption following SCF treatment only in those with ↑change in microbial composition |
| Shafie et al., 2022 57 | Menopausal women (n = 60) | Triple-blind, randomised controlled trial | Prebiotic-rich yogurt (100g) | 6 weeks | ↓mean total scores of menopausal symptoms ↓anxiety ↓depression ↓vasomotor symptoms |
Some individual probiotics and probiotic blends also demonstrate beneficial effects in managing menopausal symptoms (Table 2). Lactobacillus strains have shown improvements in managing menopausal symptoms (Table 2). L. acidophilus supplementation improved bone mineral density whilst also enhancing trabecular and cortical bone microarchitecture. 58 L. intestinalis YT2 supplementation helped recover OVX-induced gut dysbiosis and improved other symptoms like pain sensitivity, depressive behaviour, fat deposition and bone loss. 49 L. gasseri CP2305 supplementation improved total, vasomotor, somatic and psychological scores in premenopausal women. 59 A combination of L. rhamnosus GR-1 and L. reuteri RC-14 improved vaginal flora and overall urogenital health in postmenopausal women. 60
Table 2.
Studies on the effect of probiotics supplementation in managing menopausal symptoms.
| Study reference | Study population | Study design | Intervention | Study duration | Results/Observed outcomes |
|---|---|---|---|---|---|
| Rodent studies | |||||
| Dar et al., 2018 58 | Ovariectomised (OVX) mouse model (8–10 weeks old) (n = 30) | Experimental intervention versus sham-operated and OVX controls | Lactobacillus acidophilus (109 CFU/ml) – a probiotic | 6 weeks | ↑bone mineral density ↑bone heterogeneity - Enhanced trabecular and cortical bone microarchitecture |
| Lim et al., 2021 49 | OVX rats (n = 60) | Experimental intervention versus sham-operated and OVX controls | Lactobacillus intestinalis YT2 (109 CFU/ml) – a probiotic strain | 18 weeks | - Recovery of OVX induced gut dysbiosis - Improved menopausal symptoms ↓fat mass ↓pain sensitivity ↓depressive behaviour ↓cognitive impairment ↓bone loss |
| Human studies | |||||
| Honda et al., 2024 64 | Healthy peri- and postmenopausal women (n = 111) | Randomised double-blind, placebo-controlled trial | Levilactobacillus brevis KABP052 (a probiotic)- as part of a blend containing ≥1 billion CFU | 12 weeks | ↑estradiol and ↑estrone levels were observed in probiotic group versus placebo |
| Sawada et al., 2022 59 | Premenopausal women aged 40–60 years (n = 80) | Double-blind, placebo-controlled, parallel-group trial | Lactobacillus gasseri CP2305 (1 x 1010 CP2305 bacterial cells) | 6 consecutive menstrual cycles | -Relief in total, vasomotor and psychological score on the Simplified Menopausal Index - Relief in total, somatic, and vasomotor score on Greene Climacteric Scale |
| Szulinska et al., 2018 65 | Obese postmenopausal women (n = 81) | Randomised, placebo-controlled, clinical trial | Ecologic barrier (live multispecies probiotic supplement, 1-2.5 x 1010 CFU per day) | 12 weeks | ↓systolic blood pressure ↓vascular endothelial growth factor ↓serum interleukin-6 ↓serum tumour necrosis factor alpha ↑vascular function |
| Petricevic et al., 2008 60 | Postmenopausal women with Nugent scores between 4 and 6 in initial vaginal swab (n = 72) | Randomised, double-blind, placebo-controlled study | Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 (2.5 x 109 CFU of each strain) | 14 days | -Improved vaginal flora -Improved overall urogenital health |
While some of these studies have examined gut microbial changes as potential mechanistic drivers,53,55,56,61 a significant gap in existing research lies in the inconsistent investigation of these microbial shifts as the underlying mechanism for the observed benefits. Further research into gut microbial changes in response to prebiotic supplementation in menopausal women is essential to deepen our understanding of its role in managing menopausal symptoms. This will not only allow for the development of more targeted and effective therapeutic interventions for menopausal symptom management but could also help identify specific probiotic strains or optimal combinations (e.g. symbiotics) that offer the greatest therapeutic potential.
Conclusion
In conclusion, while emerging evidence points to the gut microbiota’s role in modulating hormonal balance and potentially influencing menopausal symptoms, there remains a substantial need for focused research to clarify these mechanisms and to determine effective gut microbiota-based interventions. Prebiotics and probiotics offer promising, non-hormonal therapeutic avenues to alleviate menopausal symptoms such as hot flushes, inflammation and metabolic symptoms; however, their role in modulating microbial diversity and microbiota-linked relief in menopausal symptoms needs to be further explored. Most existing studies are primarily observational, focussing on associations rather than establishing causality or elucidating underlying mechanisms. Additionally, many studies compare menopausal women to non-menopausal women without adequately accounting for the effects of age. Future research should address these limitations by incorporating designs that distinguish between age-related and menopause-specific changes, while also focussing on demonstrating causative relationships and mechanisms.
Furthermore, studies should aim to identify specific bacterial strains and prebiotic compounds that are most effective in supporting hormonal health, mood, and metabolic function in menopausal women. Such research could lead to innovative, personalised therapies that not only address the symptoms of menopause but also enhance life-long health and well-being in women. As the field advances, these insights may pave the way for gut microbiota-focused approaches as integral components of menopause management.
Footnotes
Contributorship: ML wrote the manuscript, which was reviewed and edited by AMM, DV and MGP. All authors read and agreed on final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: MGP
ORCID iDs
Marrium Liaquat https://orcid.org/0000-0001-7290-2554
Anne Marie Minihane https://orcid.org/0000-0001-9042-4226
David Vauzour https://orcid.org/0000-0001-5952-8756
Matthew G Pontifex https://orcid.org/0000-0003-2174-2313
References
- 1.Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol 2021; 61: 100912. [DOI] [PubMed] [Google Scholar]
- 2.Nagpal R, Kurakawa T, Tsuji H, et al. Evolution of gut Bifidobacterium population in healthy Japanese infants over the first three years of life: a quantitative assessment. Sci Rep 2017; 7(1): 10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cong X, Xu W, Janton S, et al. Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PLoS One 2016; 11(4): e0152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilmanski T, Diener C, Rappaport N, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab 2021; 3(2): 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Cuesta-Zuluaga Jet al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems. 2019; 4(4): 00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature 2014; 509(7500): 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016; 352(6285): 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takagi T, Naito Y, Inoue R, et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol 2019; 54(1): 53–63. [DOI] [PubMed] [Google Scholar]
- 9.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science 2016; 6285: 560–564. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Chen J, Li X, et al. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett 2019; 593(18): 2655–2664. [DOI] [PubMed] [Google Scholar]
- 11.Santos-Marcos JA, Rangel-Zuñiga OA, Jimenez-Lucena R, et al. Influence of gender and menopausal status on gut microbiota. Maturitas 2018; 116: 43–53. [DOI] [PubMed] [Google Scholar]
- 12.Lauretta R, Sansone M, Sansone A, et al. Gender in endocrine diseases: role of sex gonadal hormones. Internet J Endocrinol 2018; 2018: 4847376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos-Marcos JA, Mora-Ortiz M, Tena-Sempere M, et al. Interaction between gut microbiota and sex hormones and their relation to sexual dimorphism in metabolic diseases. Biol Sex Differ 2023; 14(1): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters BA, Santoro N, Kaplan R, et al. Spotlight on the gut microbiome in menopause: current insights. Int J Womens Health 2022; 14: 1059–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yurkovetskiy L, Burrows M, Khan A, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013; 39(2): 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Org E, Mehrabian M, Parks BW, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016; 7(4): 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koren O, Goodrich J, Cullender T, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012; 150(3): 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabek M, McCrae SI, Stevens VJ, et al. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol 2008; 66(3): 487–495. [DOI] [PubMed] [Google Scholar]
- 19.Pollet RM, D'Agostino EH, Walton WG, et al. An atlas of beta-glucuronidases in the human intestinal microbiome. Structure 2017; 25(7): 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ervin SM, Simpson JB, Gibbs ME, et al. Structural insights into endobiotic reactivation by human gut microbiome-encoded sulfatases. Biochemistry 2020; 59(40): 3939–3950. [DOI] [PubMed] [Google Scholar]
- 21.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 2017; 103: 45–53. [DOI] [PubMed] [Google Scholar]
- 22.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe 2011; 10(4): 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandberg AA, Slaunwhite WR, Jr. Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women. J Clin Investig 1957; 36(8): 1266–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adlercrtuez H, Paula J. Assay of estrogens in human feces. J Steroid Biochem 1982; 17(6): 639–645. [DOI] [PubMed] [Google Scholar]
- 25.Martin F, Peltonen J, Laatikainen T, et al. Excretion of progesteone metabolites and estriol in faeces from pregnant women during ampicillin administration. J Steroid Biochem 1975; 6(9): 1339–1346. [DOI] [PubMed] [Google Scholar]
- 26.Collden H, Landin A, Wallenius V, et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiol Endocrinol Metab 2019; 317(6): E1182–E1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flores R, Shi J, Fuhrman B, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med 2021; 10(253): 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markle JGM, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013; 339(6123): 1084–1088. [DOI] [PubMed] [Google Scholar]
- 29.Breeze B, Connell E, Wileman T, et al. Menopause and Alzheimer's disease susceptibility: exploring the potential mechanisms. Brain Res 2024; 1844: 149170. [DOI] [PubMed] [Google Scholar]
- 30.Santoro N, Roeca C, Peters BA, et al. The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab 2021; 106(1): 1–15. [DOI] [PubMed] [Google Scholar]
- 31.Sapre S, Thakur R. Lifestyle and dietary factors determine age at natural menopause. J Midlife Health 2014; 5(1): 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagel G, Altenburg HP, Nieters A, et al. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas 2005; 52(3-4): 337–347. [DOI] [PubMed] [Google Scholar]
- 33.Lurati AR. Effects of menopause on appetite and the gastrointestinal system. Nurs Womens Health 2018; 22(6): 499–505. [DOI] [PubMed] [Google Scholar]
- 34.Triadafilopoulos G, Finlayson MA, Grellet C. Bowel dysfunction in postmenopausal women. Women Health 1998; 27(4): 55–66. [DOI] [PubMed] [Google Scholar]
- 35.Lenhart A, Naliboff B, Shih W, et al. Postmenopausal women with irritable bowel syndrome (IBS) have more severe symptoms than premenopausal women with IBS. Neuro Gastroenterol Motil 2020; 32(10): e13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heitkemper MM, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gend Med 2009; 6: 152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Giessen J, van der Woude CJ, Peppelenbosch MP, et al. A direct effect of sex hormones on epithelial barrier function in inflammatory bowel disease models. Cells 2019; 8(3): 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Bian C, Luo Z, et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci Rep 2019; 9(1): 8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins FL, Rios-Arce ND, Atkinson S, et al. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Phys Rep 2017; 5(9): e13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shieh A, Epeldegui M, Karlamangla ASet al. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight. 2020; 5(2): 134092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol 2012; 30: 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamanai-Shacoori Z, Smida I, Bousarghin L, et al. Roseburia Spp: A marker of health? Future Microbiol 12(2): 157–170. [DOI] [PubMed] [Google Scholar]
- 43.Atay AA, Kismet E, Turkbay T, et al. Bone mass toxicity associated with inhalation exposure to toluene. Biol Trace Elem Res 2005: 0163–4984. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Zhou Y, Mao T, et al. The relationship between menopausal syndrome and gut microbes. BMC Womens Health 2022; 22(1): 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Liu J, Wu Z, et al. Gut microbiota signatures and fecal metabolites in postmenopausal women with osteoporosis. Gut Pathog 2023; 15(1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaber M, Wilson AS, Millen AE, et al. Visceral adiposity in postmenopausal women is associated with a pro-inflammatory gut microbiome and immunogenic metabolic endotoxemia. Microbiome 2024; 12(1): 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosconi L, Berti V, Dyke J, et al. Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci Rep 2021; 11(1): 10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navarro-Pardo E, Holland CA, Cano A. Sex hormones and healthy psychological aging in women. Front Aging Neurosci 2017; 9: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim EY, Song EJ, Kim J, et al. Lactobacillus intestinalis YT2 restores the gut microbiota and improves menopausal symptoms in ovariectomized rats. Benef Microbes 2021; 12(5): 503–516. [DOI] [PubMed] [Google Scholar]
- 50.Mohsenian S, Shabbidar S, Siassi F, et al. Carbohydrate quality index: its relationship to menopausal symptoms in postmenopausal women. Maturitas 2021; 150: 42–48. [DOI] [PubMed] [Google Scholar]
- 51.Sant’Ana CT, Amorim AD, Gava AP, et al. Brown and golden flaxseed reduce intestinal permeability and endotoxemia, and improve the lipid profile in perimenopausal overweight women. Int J Food Sci Nutr 2022; 73(6): 829–840. [DOI] [PubMed] [Google Scholar]
- 52.Sari IK, Utari DM, Kamoshita Set al. Increasing vegetable intake 400 g/day to control body weight and lipid profile in overweight hyperlipidemia menopausal women. Journal of Public Health Research. 2020; 9(1733): 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nosal BM, Thornton SN, Darooghegi Mofrad M, et al. Blackcurrants shape gut microbiota profile and reduce risk of postmenopausal osteoporosis via the gut-bone axis: evidence from a pilot randomized controlled trial. J Nutr Biochem 2024; 133: 109701. [DOI] [PubMed] [Google Scholar]
- 54.Mitmura R, Hara H, Aoyama Y, et al. Ingestion of water soluble soybean fiber prevents osteopenia and hypercholesterolemia induced by ovariectomy in rats. J Agric Food Chem 2003; 51: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 55.Brahe LK, Le Chatelier E, Prifti E, et al. Dietary modulation of the gut microbiota--a randomised controlled trial in obese postmenopausal women. Br J Nutr 2015; 114(3): 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chao YW, Tung YT, Yang SC, et al. The effects of rice bran on neuroinflammation and gut microbiota in ovariectomized mice fed a drink with fructose. Nutrients 2024; 16(17): 2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shafie M, Homayouni Rad A, Mirghafourvand M. Effects of prebiotic-rich yogurt on menopausal symptoms and metabolic indices in menopausal women: a triple-blind randomised controlled trial. Int J Food Sci Nutr 2022; 73(5): 693–704. [DOI] [PubMed] [Google Scholar]
- 58.Dar HY, Shukla P, Mishra PK, et al. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. BoneKEy Rep 2018; 8: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawada D, Sugawara T, Hirota T, et al. Effects of Lactobacillus gasseri CP2305 on mild menopausal symptoms in middle-aged women. Nutrients 2022; 14(9): 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petricevic L, Unger FM, Viernstein H, et al. Randomized, double-blind, placebo-controlled study of oral lactobacilli to improve the vaginal flora of postmenopausal women. Eur J Obstet Gynecol Reprod Biol 2008; 141(1): 54–57. [DOI] [PubMed] [Google Scholar]
- 61.Kahleova H, Holtz DN, Strom N, et al. A dietary intervention for postmenopausal hot flashes: a potential role of gut microbiome. An exploratory analysis. Compl Ther Med 2023; 79: 103002. [DOI] [PubMed] [Google Scholar]
- 62.Lucas EA, Juma S, Stoecker BJ, et al. Prune suppresses ovariectomy-induced hypercholesterolemia in rats. JNB (J Nutr Biochem) 2000; 11: 255–259. [DOI] [PubMed] [Google Scholar]
- 63.Wu KC, Cao S, Weaver CM, et al. Prebiotic to improve calcium absorption in postmenopausal women after gastric bypass: a randomized controlled trial. J Clin Endocrinol Metab 2022; 107(4): 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Honda S, Tominaga Y, Espadaler-Mazo J, et al. Supplementation with a probiotic formula having beta-glucuronidase activity modulates serum estrogen levels in healthy peri- and postmenopausal women. J Med Food 2024; 27(8): 720–727. [DOI] [PubMed] [Google Scholar]
- 65.Szulinska M, Skrypnik K, Sobieska Met al. Multispecies probiotic supplementation favorably affects vascular function and reduces arterial stiffness in obese postmenopausal women-A 12-week placebo-controlled and randomized clinical study. Nutrients. 2018; 10(11): 1672. [DOI] [PMC free article] [PubMed] [Google Scholar]