Abstract
Background
Lung adenocarcinoma (LUAD) is one of the common malignant tumors worldwide, and the 5-year survival rate remains unsatisfactory. Reliable prognostic biomarkers are needed to provide references for personalized treatment of patients. Some studies have shown that disulfidptosis-related genes (DRGs) are closely associated with tumorigenesis and development. This study constructed a prognostic risk model to explore the prognostic value of DRGs in LUAD and provide a reference for formulating personalized treatment plans for LUAD patients.
Methods
RNA-seq data of LUAD tissues and adjacent or normal lung tissues were downloaded from TCGA database and GEO database. A risk scores model was constructed through univariate Cox analysis, Lasso analysis, and multivariate Cox analysis. ROC curves and nomogram models were drawn to evaluate the risk model. External validation was performed using LUAD data, data in the LUAD single-cell dataset, and other data in the GEO database. In addition, the immune microenvironment and drug sensitivity of the high-risk and low-risk groups were analyzed. The key gene PPP1R14B in the model was further experimentally verified by in vitro cell experiments.
Results
In this study, a risk model composed of four genes was constructed, and the overall survival (OS) of the low-risk group was higher than that of the high-risk group (P < 0.001). The area under the curve (AUC) of the ROC curves of the training set risk model at 1-, 3-, and 5-year were 0.767, 0.759, and 0.711, respectively. Drug sensitivity analysis showed that there was a statistical significance between the high-risk and low-risk groups of patients for drugs such as gefitinib, afatinib, lapatinib, and paclitaxel (P < 0.001). The results of in vitro cell experiments showed that the proliferation and migration of knockdown PPP1R14B LUAD cells were significantly inhibited, and the number of apoptosis of LUAD cells was significantly increased (P < 0.05).
Conclusion
The risk model constructed based on four DRGs can predict the prognosis of LUAD patients with relative accuracy. There are differences in the immune microenvironment between the high-risk and low-risk groups. Patients in the high-risk group are more sensitive to drugs such as gefitinib, afatinib, lapatinib, and paclitaxel, providing a reference for personalized treatment of LUAD patients. Knockdown PPP1R14B significantly inhibited the proliferation and migration of LUAD cells and promoted the apoptosis of LUAD cells.
Keywords: Lung adenocarcinoma, Disulfidptosis, Prognostic model, Bioinformatics, In vitro validation
Introduction
Lung cancer is the most common malignant tumor of the respiratory system. According to pathological types, lung cancer can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). LUAD is the main pathological type of NSCLC, accounting for approximately 50% of all diagnosed lung cancer cases [1]. Early LUAD can achieve good curative effects through surgical treatment. However, due to factors such as difficulty in early detection, susceptibility to metastasis, and lack of systematic treatment, the survival rate of LUAD patients remains poor, with a five-year survival rate of only 18% [2, 3]. Therefore, LUAD is still a heavy burden on human health, and reliable prognostic biomarkers need to be discovered to improve the prognosis of LUAD patients.
In 2023, a study proposed a new cell death mode, namely disulfidptosis. It refers to cell death in high SLC7A11-expressing cells caused by glucose starvation, resulting in the collapse of disulfide bonds in actin cytoskeletal proteins and actin. This death mode is different from other cell death modes [4]. The role and clinical significance of disulfidptosis-related genes in LUAD are still in the exploratory stage, which provides new ideas for improving the prognosis of LUAD patients.
In this paper, the LUAD transcriptome and clinical data from The Cancer Genome Atlas (TCGA) database and Gene Expression Omnibus (GEO) database were combined and subjected to bioinformatics analysis. A prognostic model for LUAD patients based on disulfidptosis-related genes (DRGs) was constructed to predict the prognosis, immune microenvironment, and immunotherapy of LUAD patients. The model was validated using two other LUAD datasets in GEO, and single-cell analysis of the genes in the model was performed using the GEO single-cell dataset. The key genes in the model were further validated by in vitro cell experiments. Our study may provide relevant evidence for the prognosis and treatment of LUAD patients.
Methods
Data collection and processing
A total of 36 disulfidptosis genes were obtained through the FerrDB V2 database (http://www.zhounan.org/ferrdb/current/, RRID: SCR_026852), The RNA-seq data and clinical data of LUAD patients were downloaded from the TCGA database (https://portal.gdc.cancer.gov/repository, RRID: SCR_003193), which consists of 59 normal samples and 541 samples from LUAD patients. Data from three LUAD patient cohorts were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo, RRID: SCR_005012), 129 LUAD patient samples were included in GSE50081, 85 LUAD patient samples were included in GSE30219, and 182 LUAD patient samples were included in GSE41271.
Co-expression and differential analysis of DRGs
In R software, the RNA-seq data downloaded from the TCGA database and the GSE50081 database were merged using the “limma” package. The expression levels of 36 disulfidptosis genes were extracted, and the filtering conditions were set as|R| > 0.4 and P < 0.05 to obtain DRGs [5, 6]. Then, the filtering conditions of FDR < 0.05 and|logFC| > 2 were set to identify the differentially expressed genes between the normal group and the LUAD group [7]. The “venn” package was used to draw a Venn diagram by taking the intersection of the differentially expressed genes and DRGs, and the results were visualized by a volcano plot.
Establishment and evaluation of the TCGA-LUAD risk prognostic model
The LUAD patients in the TCGA database were randomly divided into a training set and a validation set at a ratio of 7: 3 using the “caret” R package. Univariate Cox regression analysis was performed on the screened DRGs by the “survival” R package. Subsequently, the “glmnet” R package was applied to conduct Lasso-Cox regression analysis to screen out the DRGs with independent prognostic significance and construct the LUAD risk prognostic model. The risk score formula is as follows: LUAD risk score =  , where Coefi is the corresponding regression coefficient and Expi represents the expression value of each mRNA [8]. The LUAD patients were divided into high-risk and low-risk groups according to the median value of the risk score, and Kaplan-Meier survival analysis was carried out for each group. By calculating the survival rates of the patients at 1-, 3-, and 5-year, the receiver operating characteristic (ROC) curve was drawn to evaluate the accuracy of the risk model in predicting the prognosis of LUAD patients. Moreover, two GEO cohorts (GSE30219, GSE41271) were used as external validation.
, where Coefi is the corresponding regression coefficient and Expi represents the expression value of each mRNA [8]. The LUAD patients were divided into high-risk and low-risk groups according to the median value of the risk score, and Kaplan-Meier survival analysis was carried out for each group. By calculating the survival rates of the patients at 1-, 3-, and 5-year, the receiver operating characteristic (ROC) curve was drawn to evaluate the accuracy of the risk model in predicting the prognosis of LUAD patients. Moreover, two GEO cohorts (GSE30219, GSE41271) were used as external validation.
Construction and validation of nomogram
To better evaluate the predictive ability of the model, the “rms” R package was used to construct a nomogram.
Single-cell sequencing analysis
The single-cell dataset of LUAD, GSE189357, was downloaded from the GEO database. This dataset included 9 LUAD samples. The “Seurat” R package was used to process and analyze the single-cell data. The filtering conditions were set as nFeature_RNA > 200 & nFeature_RNA < 5000, percent.mt < = 15 & percent. HB < = 3 for ScRNA data screening [9]. The “SingleR” package was employed to annotate cell types. The results of cell clustering were presented through the UMAP plot, along with the expression of genes in the model in various immune cells.
Immune microenvironment analysis
The “limma” and “estimate” R packages were used to calculate the stromal score, immune score, and tumor purity of LUAD patients based on the ESTIMATE algorithm. Violin plots were drawn to compare whether there were differences in stromal score, immune score, and tumor purity between the high-risk and low-risk groups [10]. In this study, 60 immune checkpoint genes (ICGs) were collected, and box plots were used to show whether there were differences in the expression of ICGs between the high-risk and low-risk groups [11].
Drug sensitivity analysis
The half maximal inhibitory concentration (IC50) of commonly used chemotherapy drugs for LUAD patients was obtained through the “oncoPredict” R package to conduct drug sensitivity analysis, so as to evaluate the different responsive effects of drug sensitivity between patients in the high-risk and low-risk groups [12].
Experimental subjects
Cell lines: the LUAD cell lines A549 (RRID: CVCL_0023) and H1975 (RRID: CVCL_1511) were purchased from the Institute of Cell Biology, Shanghai, China. Specimen sources: five pairs of LUAD tissue specimens and adjacent non-cancerous tissue specimens were obtained from Yantai Shan Hospital, affiliated to Binzhou Medical University. All experiments were approved by the Ethics Committee of Binzhou Medical University.
Cell culture, transfection and qRT-PCR
The A549 and H1975 cell lines were cultured according to standard procedures. siRNA transfection was performed according to the siRNA product instructions (Ribio, China). The siRNA sequences targeting PPP1R14B were as follows: negative control sense strand: 5’-UUCUCCGAACGUGUCACGUTT-3’, antisense strand: 5’-ACGUGACACGUUCGGAGAATT-3’; siRNA-1 sense strand: 5’-CGUCAAGUAUGACCGCAAGTT-3’, antisense strand: 5’-CUUGCGGGUCAUACUUGACGTT-3’; SiRNA-2 sense strand: 5’-AGGACCACGCGUCUACUUUTT-3’, antisense strand: 5’- AAAGUAGACGCGUGGUCCUTT-3’.
Real-time quantitative qRT-PCR
RNA was isolated from cell lines and tissues using the total RNA extraction reagent (R401-01, Vazyme). The RNA was then reverse transcribed into cDNA using HiScriptII.Q RT SuperMix (R223-01, Vazyme). Additionally, qRT-PCR was performed on the StepOnePlus Real-time Systems (ThermoFisher). The primer sequences were as follows: PPP1R14B-QP-FP: 5’-CAAGGGAAGGTCACCGTCAAGTATG-3’; PPP1R14B-QP-RP: 5’-GCTCATCCACGTCAATCTCCAGTTC-3’; GAPDH-QP-FP: 5’-AGAAGGCTGGGGCTCATTTG-3’; GAPDH-QP-RP: 5’-GCAGGAGGCATTGCTGATGAT-3’.
Cell proliferation assay
After transfecting A549 and H1975 cells with NC, siRNA-1, and siRNA-2 for 48 h, the cells were respectively seeded into 96-well plates with 4 replicate wells. After the cells were cultured for 0, 24, 48, and 72 h, 10 μL of cell counting kit 8 (E-CK-A362, Elabscience) was added to each well. After incubation in the cell culture incubator for 2 h, the absorbance at a wavelength of 450 nm was measured using a microplate reader.
After A549 and H1975 cells were transfected with NC, siRNA-1, and siRNA-2 for 48 h, the cells were seeded into 96-well plates with 4 replicate wells respectively. After culturing the cells for 0, 24, 48, and 72 h, 10 µL of cell counting kit 8 (E-CK-A362, Elabscience) was added to each well. After incubating in a cell incubator for 2 h, the absorbance at a wavelength of 450 nm was measured using a microplate reader.
Transwell migration assay
Cell migration was determined using transwell chambers (TCS003024, Biofil). In the migration assay, after cells (2 × 105) were transfected with siRNA for 48 h, they were seeded into the upper chamber with serum-free medium, and the lower chamber was filled with medium containing 30% FBS. Approximately 24 h later, the cells were fixed with 4% paraformaldehyde for 30 min and stained with 1% crystal violet for 2 min. The number of migrated cells was observed under a microscope (DM6000B, Leica).
Wound-healing assay
A549 and H1975 cells were evenly seeded in 6-well plates and then transfected for 48 h. Cells were added to each well approximately, and two horizontal lines were scratched on the cell surface using a 10 µL pipette tip. After that, the cells were cultured in 10% FBS medium, and cell images were collected using an optical microscope (DM6000B, Leica) at 0, 24, 48, and 72 h respectively.
Flow cytometry apoptosis experiment
The A549 cells transfected for 48 h and the untransfected A549 cells were collected separately, centrifuged at 300 rpm for 5 min and then washed. The operation was carried out according to the instructions of the Annexin V-FITC/PI Apoptosis Kit (E-CK-A211, Elabscience), and the analysis was performed using a flow cytometer.
Statistical analysis
The statistical analysis of this study was conducted using R (RRID: SCR_001905, version 4.3.2). Univariate Cox regression, Lasso regression, and multivariate Cox regression analyses were employed to identify prognostic factors. The Wilcoxon rank-sum test was used to compare the clinicopathological characteristics between the two groups. Kaplan-Meier survival analysis was utilized to compare the overall survival and progression-free survival between the two groups. During the statistical analysis, P < 0.05 was considered statistically significant.
Results
Screening of DRGs in LUAD
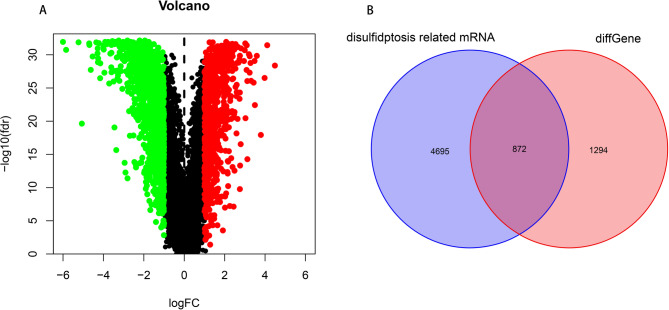
After merging the TCGA and GSE50081 datasets, a co-expression analysis was performed on 36 disulfidptosis genes, resulting in 5,567 DRGs. A variance analysis was conducted on the mRNAs of normal and tumor samples, resulting in 2,166 differentially expressed genes, which were presented in a volcano plot (Fig. 1A). The intersection of the differentially expressed genes and DRGs was taken to obtain 872 differentially expressed DRGs, which were shown in a Venn diagram (Fig. 1B).
Fig. 1.
Screening of DRGs in LUAD (A). Volcano plot of the screened DRGs. (B). Venn diagram
Construction of prognostic model of DRGs in LUAD
Univariate Cox regression was used to screen out 372 DRGs, and Lasso analysis was then performed on them to obtain 6 DRGs (Fig. 2A). Finally, multivariate Cox regression was applied to obtain 4 DRGs that affect the independent prognosis of LUAD for constructing the model (Table 1). Among them, C1QTNF6, RHOV, PLEK2, and PPP1R14B are all risk factors, and their expressions in tumor samples are higher than those in normal samples (Fig. 2B). Based on these 4 genes, the risk score for the prognosis of LUAD patients was obtained as follows: risk score = (1.688 × Exp PPP1R14B) + (0.778 × Exp PLEK2) + (0.513 × Exp RHOV) + (0.649 × Exp C1QTNF6).
Fig. 2.
The prognostic model of LUAD was constructed based on 4 DRGs (A). Lasso regression analysis. (B). Differences of four genes in the model between normal and tumor
Table 1.
Multivariate Cox regression analysis was performed to analyze DRGs affecting the prognosis of LUAD
| Variable | HR | 95%CI | P |
|---|---|---|---|
| PPP1R14B | 3.86 | 1.22–12.24 | 0.022 |
| PLEK2 | 1.86 | 1.04–3.41 | 0.038 |
| RHOV | 1.78 | 1.24–2.56 | 0.002 |
| C1QTNF6 | 2.75 | 1.63–4.65 | < 0.001 |
Evaluation of the prognostic value of the prognostic model
The risk scores of LUAD patients were calculated based on the expression levels and regression coefficients of the genes in the model. The patients were then divided into high-risk and low-risk groups according to the median of the risk scores. Figure 3A-F illustrate the differences in survival risk scores, survival time, and distribution of prognostic gene expressions between the high-risk and low-risk groups in the training set and the validation set. The results of the survival analysis showed that the overall survival (OS) of the low-risk group was higher than that of the high-risk group (P < 0.001 in the training set and P < 0.001 in the validation set, as shown in Fig. 3G, H). ROC curves were drawn for the training set and the validation set respectively to predict the 1-, 3-, and 5-year survival rates of the patients. The areas under the curve (AUC) for the 1-, 3-, and 5-year survival rates in the training set were 0.767, 0.759, and 0.711 respectively (Fig. 3I), while those in the validation set were 0.686, 0.691, and 0.695 respectively (Fig. 3J). To further evaluate the predictive ability of the prognostic model, external validation was conducted using GSE41271 and GSE30219 from the GEO database. ROC curves were drawn, and the AUCs for the 1-, 3-, and 5-year survival rates in the GSE41271 dataset were 0.769, 0.717, and 0.726 respectively, while those in the GSE30219 dataset were 0.625, 0.685, and 0.656 respectively (Fig. 3K, L). By searching relevant literature on previously published prognostic models for LUAD and comparing the ROC values at 1-, 3-, and 5-year of some previously published prognostic models for LUAD related to disulfidptosis, it was found that the prognostic model constructed in this study had a better predictive performance than previous studies [13–19] (Fig. 3M). See Table 2.
Fig. 3.
Evaluation and validation of the LUAD prognostic model (A). Risk scores of high-risk and low-risk groups in the training set. (B). Risk scores of high-risk and low-risk groups in the validation set. (C). Survival time of high-risk and low-risk groups in the training set. (D). Survival time of high-risk and low-risk groups in the validation set. (E). Heat map of gene expression levels of the prognostic model in high-risk and low-risk groups in the training set. (F). Heat map of gene expression levels of the prognostic model in high-risk and low-risk groups in the validation set. (G). OS survival curve in the training set. (H). OS survival curve in the validation set. (I). ROC curve for predicting 1-, 3-, and 5-year survival rates of patients in the training set. (J). ROC curve for predicting 1-, 3-, and 5-year survival rates of patients in the validation set. (K). ROC curve in the GSE41271 dataset. (L). ROC curve in the GSE30219 dataset. (M). Comparison with published literature
Table 2.
The situation of previously published LUAD prognostic models
| Authors | Journal name | Publish date | Signature | PMID |
|---|---|---|---|---|
|
Cui Qi, et al [13]. |
AGING | June 13, 2023 | 7-mRNA signature | 37315289 |
|
Junzhi Liu, et al [14]. |
Current Medicinal Chemistry | April 16, 2024 | 7-mRNA signature | 38685772 |
|
Fangchao Zhao, et al [15]. |
Clinical Epigenetics | February 11, 2024 | 11-mRNA signature | 38342890 |
|
Xiaoxia Pan, et al [16]. |
Frontiers in Medicine | October 23, 2024 | 4-mRNA signature | 39507711 |
|
Jiaqi Huang, et al [17]. |
Computers in Biology and Medicine | August 28, 2023 | 21-mRNA signature | 37657358 |
|
Dabao He, et al [18]. |
Frontiers in Immunology | October 27, 2023 | 9-mRNA signature | 37965333 |
|
Leqi Zhong, et al [19]. |
Cancer Cell International | January 2, 2024 | 5-mRNA signature | 38167017 |
Construction of the nomogram
To determine whether the risk index represents an independent risk factor for the prognosis of LUAD, univariate and multivariate Cox regression analyses were performed (Fig. 4A, B). The results showed that the risk index is a risk factor (HR = 2.093, 95% CI: 1.741–2.517, P < 0.001). A nomogram was constructed by combining the prognostic model with age, gender, risk score, and TNM staging (Fig. 4C). By plotting the C-index curve, it is further verified that the nomogram model has accurate prediction performance (Fig. 4D). The calibration curve indicated that the nomogram could make relatively accurate predictions. Based on the above results, the nomogram can provide a reference for clinical decision-making.
Fig. 4.
Construction of the nomogram (A). Univariate COX analysis of the risk score. (B). Multivariate Cox analysis of the risk score. (C). Nomogram model for LUAD patients. (D). C-index curve for predicting the survival of patients at 1-, 3-, and 5-years
Immune microenvironment analysis
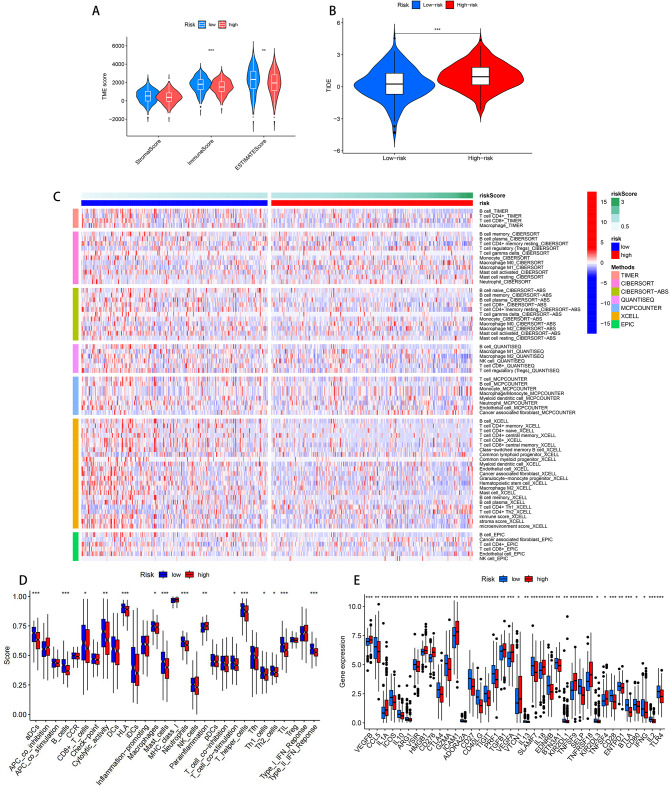
The tumor microenvironment (TME) can affect the clinical outcome of patients and their response to treatment [20]. By calculating the TME score of LUAD patients and the differences in the immune microenvironment between the high-risk and low-risk groups, the results showed that both the immune cell score (P < 0.001) and the tumor purity score (P < 0.01) were significant between the high-risk and low-risk groups (Fig. 5A), indicating that the immune cells and tumor purity in the low-risk group were higher than those in the high-risk group in the tumor microenvironment. The TIDE score was calculated through the online website of Tumor Immune Dysfunction and Exclusion (TIDE) (http://tide.dfci.harvard.edu) to predict the response of LUAD patients in the high-risk and low-risk groups to immunotherapy. The results showed that the score of the high-risk group was higher than that of the low-risk group, indicating that the patients in the low-risk group had a better response to immunotherapy (Fig. 5B). We also compared the immune infiltration levels between the high-risk and low-risk groups, and the results showed statistical significance in cells such as CD8 + T cells, CD4 + memory resting T cells, macrophages, plasma cells, monocytes, etc. (Fig. 5C). Using ssGSEA to explore the differences in immune function between the two groups, we found that there were statistically significant differences in B cells, human leukocyte antigens, neutrophils, type II interferon response, etc. between the high-risk and low-risk groups (Fig. 5D). We also compared the expression of 60 ICGs [11]between the high-risk and low-risk groups, and the results showed that 38 ICGs differed between the high-risk and low-risk groups, with 26 ICGs highly expressed in the low-risk group and 12 ICGs highly expressed in the high-risk group (Fig. 5E).
Fig. 5.
Immune microenvironment analysis (A). TME scores between high-risk and low-risk groups. (B). TIDE scores. (C). Analysis of immune cell infiltration between high-risk and low-risk groups. (D). Analysis of differences in immune function between high-risk and low-risk groups. (E). Analysis of differences in immune checkpoints between highrisk and low-risk groups
Single-cell sequencing analysis of genes in the prognostic model
The expression of 4 genes in the DRGs prognostic model of LUAD in the GSE189357 dataset were analyzed. After unsupervised clustering and annotation, these 4 genes were located on the UMAP map of the GSE189357 dataset. It contains 9 major cell types, namely T cells, natural killer (NK) cells, B cells, monocytes, epithelial cells, macrophages, endothelial cells, dendritic cells, and smooth muscle cells (Fig. 6A). The expression of each gene in each cell population can be observed through Fig. 6B-I. PPP1R14B is mainly expressed in monocytes, macrophages, and endothelial cells, PLEK2 is mainly expressed in monocytes, and RHOV is mainly expressed in endothelial cells.
Fig. 6.
Single-cell sequencing analysis (A). Cell types in the GSE189357 dataset. (B). Expression of PPP1R14B in various cell populations. (C). Expression of PLEK2 in each cell population. (D). Expression of RHOV in each cell population. (E). Expression of C1QTNF6 in each cell population. (F). Expression of PPP1R14B in each cell type. (G). Expression of PLEK2 in each cell type. (H). Expression of RHOV in each cell type. (I). Expression of C1QTNF6 in each cell type
Drug sensitivity analysis
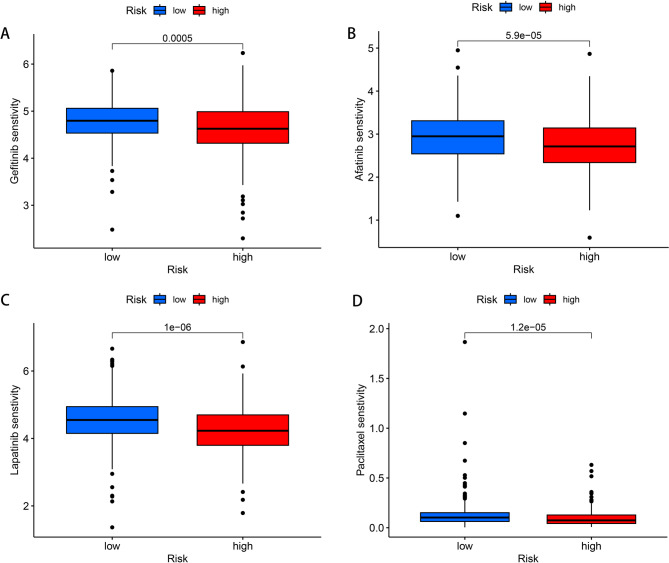
In this study, drug sensitivity analysis was performed on LUAD patients, and the IC50 values of 198 antitumor drugs between the high-risk and low-risk groups were calculated. The results showed that patients in the high-risk group had lower IC50 values, indicating that these patients were more sensitive to drugs such as gefitinib, afatinib, lapatinib, and paclitaxel (P < 0.001), which was more conducive to drug treatment (Fig. 7A-D).
Fig. 7.
Drug sensitivity analysis (A). Gefitinib. (B). Afatinib. (C). Lapatinib. (D). Paclitaxel
PPP1R14B as a potential therapeutic target for LUAD
In the multifactorial Cox analysis, we found that the PPP1R14B gene had the largest HR value, and there were fewer studies on PPP1R14B in LUAD in previous studies. Therefore, we chose this gene for further study and analysis. First, we investigated the differences in the expression levels of PPP1R14B in normal lung tissues and LUAD in the TCGA and GSE50081 combined dataset, and found that the expression levels were higher in LUAD (Fig. 8A). We conducted qRT-PCR tests on 5 pairs of lung adenocarcinoma tissues and adjacent normal tissues. It was found that the expression of PPP1R14B in cancer tissues was higher than that in adjacent normal tissues, which was consistent with the results in the combined dataset of TCGA and GSE50081 (Fig. 8B). PPP1R14B was knocked down in H1975 and A549 cell lines. Two siRNAs were measured using qRT-PCR to determine their ability to reduce PPP1R14B expression (Fig. 8C, D). CCK-8 assays were performed to show that PPP1R14B knockdown inhibited the proliferative capacity of LUAD cells (Fig. 8E, F). Scratch and Transwell assays showed that PPP1R14B knockdown diminished the migration ability of LUAD cells (Fig. 8G-N). Flow cytometry apoptosis assays showed that PPP1R14B knockdown increased the number of apoptotic cells (Fig. 8O, P). Thus, knockdown of PPP1R14B significantly inhibited the proliferation and migration of LUAD cells and promoted apoptosis of LUAD cells.
Fig. 8.
Experimental verification of PPP1R14B (A). The expression of PPP1R14B in normal and tumor tissues. (B). qRT-PCR was performed to detect the expression of PPP1R14B in cancer tissues as well as in paracancerous tissues (n = 5). (C). qRT-PCR validation confirmed that PPP1R14B was stably knocked down in A549 cell line. (D). qRT-PCR validation confirmed that PPP1R14B was stably knocked down in the H1975 cell line. (E). The CCK8 experiment showed that knocking down PPP1R14B can inhibit the proliferation of A549 cell line. (F). The CCK8 experiment showed that knocking down PPP1R14B can inhibit the proliferation of H1975 cell line. (G-H). scratch assay showed that knocking down PPP1R14B can inhibit the migration of A549 cell line. (I-J). Scratch assay shows that knocking down PPP1R14B can inhibit the migration of H1975 cell line. (K-L). Transwell experiments have shown that knocking down PPP1R14B can inhibit the migration of A549 cell lines. (M-N). Transwell experiments have shown that knocking down PPP1R14B can inhibit the migration of H1975 cell lines. (O-P). Flow cytometry cell apoptosis experiments showed that knocking down PPP1R14B can promote apoptosis in A549 cell lines
Discussion
LUAD is a common subtype of lung cancer. In recent years, with the continuous development in the fields of lung cancer screening as well as molecular targeted therapy and immunotherapy, the prognosis of LUAD patients has been somewhat improved. However, due to the heterogeneity of lung cancer, only a small proportion of patients can benefit from these advancements [21], and its 5-year survival rate remains extremely low [22]. NSCLC is the main histological subtype of lung cancer and is one of the cancers with a relatively poor prognosis. It has been reported that due to factors such as smoking and air pollution, the incidence of lung cancer has been on the rise [23]. LUAD is the most common pathological subtype of NSCLC. Despite the emergence of new therapies including immunotherapy and targeted therapy, its incidence is also increasing continuously. Therefore, it is extremely urgent to improve the poor prognosis situation of patients [24]. Research has shown that programmed cell death plays a crucial role in the elimination of abnormal homeostatic cells and is regarded as a promising cancer treatment strategy [25, 26]. In previous studies, it has been determined that the occurrence and development of LUAD are closely associated with programmed death modalities such as apoptosis, necroptosis, ferroptosis, and cuproptosis [22, 27–33]. Recently, a novel form of cell death has been proposed, which differs from other programmed cell deaths. With the advancement of technology, researchers, based on the currently known cell death mechanisms, have identified numerous therapeutic targets for cancer and developed related targeted drugs, thereby improving the prognosis of cancer patients. However, current targeted-therapy strategies are only effective in some lung adenocarcinoma patients or may cause severe side-effects. Therefore, there is still a need to further explore effective therapeutic targets for lung adenocarcinoma. As a new form of cell death, disulfidptosis is being studied in the hope of providing clues for the discovery of new therapeutic targets [34], thus offering new ideas for the treatment of LUAD. The discovery of disulfidptosis occurred in human clear-cell renal cell adenocarcinoma cells. It was found that high expression of SLC7A11 accelerates the depletion of nicotinamide adenine dinucleotide phosphate (NADPH) in the cytoplasm under glucose starvation. This leads to the accumulation of non-reducible disulfide bonds, triggering disulfide stress, and ultimately resulting in disulfidptosis, i.e., cell death. This form of cell death is different from the known ones and is thus named disulfidptosis [4]. Compared with normal cells, tumor cells show a significantly increased dependence on SLC7A11. Research has revealed that SLC7A11 is highly expressed in various tumor cells, and it is closely related to tumor growth, prognosis, metastasis, and treatment [35, 36]. Tumor cells with high SLC7A11 expression are more likely to trigger disulfidptosis in an environment of glucose deficiency or when exposed to metabolic inhibitors. By targeting and inhibiting SLC7A11 or disrupting the metabolic process of NADPH, tumor cells can be induced to undergo disulfidptosis, thereby achieving the purpose of treatment. In addition, the combined use of endoplasmic reticulum stress inhibitors and glucose transporter inhibitors is expected to further promote the formation of disulfide bonds in cytoskeletal proteins, effectively suppressing tumor growth. As a new form of cell death, disulfidptosis provides new ideas for the treatment of tumors. Consequently, we have studied the prognostic value of DRGs in LUAD, in an attempt to identify potential therapeutic targets and provide references for the prognosis of LUAD patients.
In this study, bioinformatics analysis was conducted by combining the gene expression and survival data from TCGA and GEO databases to construct a prognostic risk model for LUAD. The prognostic model consists of four genes: PPP1R14B, PLEK2, RHOV, and C1QTNF6. Previous studies have found that PPP1R14B can affect the cell cycle and promote cell division through pathways such as E2F, MTORC1, and P53 [37–39]. PLEK2 is also involved in many important processes in tumor development, including tumorigenesis, drug resistance, and immune escape [40, 41]. In addition, PLEK2 plays a multifaceted regulatory role in multiple signaling pathways through interactions. It promotes cell invasion and metastasis through the EGFR/CCL2 pathway. PLEK2 acts as a downstream effector of the JAK2/STAT5 pathway in red blood cells. Therefore, the upregulation of PLEK2 may directly lead to an enhanced invasive ability of cancer cells [42]. C1QTNF6 is a protein-coding gene and is a prognostic indicator of poor survival rates in many cancers, including LUAD [43]. It causes the proliferation of cancer cells in gastric cancer [44] and inhibits apoptosis in oral squamous cell carcinoma [45]. RHOV is a member of the Rho GTPase family. RHOV has been identified to activate p21-activated kinase, which can affect a wide range of physiological and pathological processes [46]. Studies have found that RHOV is closely related to LUAD metastasis. Through activating the JNK/c-Jun signaling pathway and regulating the expression of epithelial-mesenchymal transition markers, the overexpression of RHOV promotes the proliferation, migration, and invasion of LUAD cells [47].
Next, we validated the model. The results of the survival analysis showed that in both the training set and the validation set, the OS of the high-risk group was lower than that of the low-risk group, and the PFS results showed the same trend as the OS. The results of the independent prognostic analysis of the model indicated that the risk score was statistically significant. The C-index results of the model also showed that the C-index value of the risk score was higher than that of other clinical characteristic indicators, suggesting that this model can serve as an independent prognostic indicator, independent of other clinical features. In the training set, the AUC values at 1-, 3-, and 5-year were 0.767, 0.759, and 0.711 respectively, while in the internal validation set, the AUC values at 1-, 3-, and 5-year were 0.686, 0.691, and 0.695 respectively. The AUC in the training set was higher than that in the validation set. The model of this study was also compared with previous models proposed by others. In the study by Qi et al. [13], the AUC of the LUAD disulfidptosis prediction model composed of seven genes was 0.694, 0.677, and 0.652; in the study by Pan et al. [16], the AUC of the LUAD disulfidptosis prediction model composed of four genes was 0.68, 0.66, and 0.65; in the study by Liu et al. [14], the AUC of the LUAD disulfidptosis prediction model composed of seven genes was 0.687, 0.604, and 0.588; in the study by Huang et al. [17], the AUC of the LUAD disulfidptosis prediction model composed of twenty-one genes was 0.747, 0.714, and 0.692. The prognostic model in this study was composed of four genes, while the models in previous studies were composed of four or more genes. Therefore, the prediction model of this study is superior to the previous prognostic models for LUAD disulfidptosis proposed by others. The model in this study is more accurate and reliable, and has certain research value. This study also drew a nomogram to predict the survival period of patients and verified it with a calibration curve. We found that the predicted values of the nomogram for the 1-, 3-, and 5-year survival rates of patients were highly consistent with the actual observed values, which demonstrated that the nomogram constructed based on clinical case characteristics had high prediction accuracy. Through the enrichment analysis of differentially expressed genes, we found that the DRGs signature had a wide-ranging impact on LUAD, covering all stages from tumor occurrence to development. In conclusion, the prognostic model we constructed is relatively accurate, and its potential mechanism of action in the field of LUAD is worthy of in-depth research and verification.
Research indicates that the tumor microenvironment is a crucial factor influencing various tumor phenotypes. The infiltration of immune cells within the tumor microenvironment is its prominent immunological characteristic and is of vital importance for the development of immune escape formation by tumor cells [48]. By calculating the TME scores of the high-risk and low-risk groups, it was found that the low-risk group had a higher microenvironment score, including scores for immune cells and tumor purity. The results of the immune cell infiltration analysis revealed that in the low-risk group, CD4 + T cells, CD8 + T cells, CD4 + memory resting T cells, etc., exhibited higher expression levels. The analysis of immune checkpoints discovered that VEGFB, IL1A, HMGB1, CD276, ICAM1, TNFSF9, and TNFSF4 were highly expressed in the high-risk group. TNFSF9 was highly expressed in LUAD tissues and was related to the polarization of M1 macrophages [49]. TNFSF4 is a costimulatory immune checkpoint protein and has been proven to enhance the anti-tumor activity of T cells [50]. Through TIDE analysis, it was found that patients in the low-risk group were more suitable for immunotherapy. These findings suggest that targeting the immunosuppressive microenvironment may enhance the immunotherapy response of LUAD, especially in the context of immune checkpoint inhibitors, which holds greater promise in promoting the anti-LUAD immune response.
This study also evaluated the correlation between commonly used clinical drugs and the risk score. It was found that patients in the low-risk group were more sensitive to drugs such as gefitinib, afatinib, lapatinib, and paclitaxel. This provides a theoretical basis for the selection of clinical anti-tumor drugs.
In addition, we conducted in vitro verification of the PPP1R14B gene in the model through cell scratch assay, Transwell assay, CCK-8 assay, and cell flow apoptosis assay. The results of the above experiments indicate that PPP1R14B is involved in the growth and invasion of LUAD, providing preliminary evidence for the feasibility of using PPP1R14B for targeted therapy of LUAD. This study analyzed the role of disulfidptosis-related genes in the prognosis of LUAD and their correlation with the tumor microenvironment, and constructed a prognostic prediction model. This model has shown good performance in predicting the survival outcomes, tumor immune microenvironment, and immunotherapy response of LUAD patients. Finally, experimental verification has also confirmed these findings.
In this study, a DRGs prognostic model for LUAD was constructed, which was relatively accurate in predicting the prognosis of LUAD patients. However, there are still some limitations. Firstly, the drug screening in this study was conducted based on data from public databases, lacking validation with real-world clinical data. Future studies should incorporate large-scale clinical trials to verify these findings. Secondly, although preliminary bioinformatics analyses and experimental validations have been performed, more in-depth investigations are required to elucidate the molecular regulatory mechanisms of PPP1R14B. Thirdly, the limited variety of machine learning algorithms employed in this research highlights the need to integrate advanced computational models to enhance the reliability of biomarker identification and further advance LUAD research. Finally, future work should prioritize diverse patient populations and validate the robustness, generalizability, and clinical translational potential of the conclusions across multiple independent cohorts.
Author contributions
Yuqing Dong, Ying Zhang and Haoran Liu designed the research, wrote the manuscript, and carried out the experiment; Xintong Jiang analyzed and interpreted the data; Shuyang Xie and Pingyu Wang reviewed the paper and provided comments. All authors contributed to manuscript writing and revisions.All of the authors have read and approved the final manuscript.
Funding
The authors declare financial support was received for the research, authorship, and publication of this article. This work was supported by Nature Foundation Project of Shandong Province (ZR2024MH228), and Yantai Science and Technology Innovation and Development Plan (2024JCYJ058).
Data availability
The datasets presented in this study can be found in online repositories. The names of the repositories and accession number(s) can be found within the article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuqing Dong, Ying Zhang and Haoran Liu contributed equally to this work.
References
- 1.Chen P, Quan Z, Song X, Gao Z, Yuan K. MDFI is a novel biomarker for poor prognosis in LUAD. Front Oncol. 2022;12:1005962. 10.3389/fonc.2022.1005962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A, Cancer statistics. 2018. CA Cancer J Clin. 2018;68(1):7–30. 10.3322/caac.21442 [DOI] [PubMed]
- 3.Li Y, Yang Y, Ma Q, Cheng H, Wang H, Ma C, et al. HNRNPK/CLCN3 axis facilitates the progression of LUAD through CAF-tumor interaction. Int J Biol Sci. 2022;18(16):6084–101. 10.7150/ijbs.76083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Nie L, Zhang Y, Yan Y, Wang C, Colic M, et al. Actin cytoskeleton vulnerability to disulfide stress mediates Disulfidptosis. Nat Cell Biol. 2023;25(3):404–14. 10.1038/s41556-023-01091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin Y, Zhang D, Zhang H, Hou L, Wang Z, Yang L, et al. Construction of a ferroptosis-related five-lncRNA signature for predicting prognosis and immune response in thyroid carcinoma. Cancer Cell Int. 2022;22(1):296. 10.1186/s12935-022-02674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Tang L, Huang W, Abisola FH, Zhang Y, Zhang G, et al. Identification of a prognostic cuproptosis-related signature in hepatocellular carcinoma. Biol Direct. 2023;18(1):4. 10.1186/ s13062-023-00358-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng A, He L, Chen T, Xu M. A novel cuproptosis-related LncRNA nomogram to improve the prognosis prediction of gastric cancer. Front Oncol. 2022;12:957966. 10.3389/fonc.2022.957966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao S, Chi H, Ji W, He Q, Lai G, Peng G, et al. A Bioinformatics-Based analysis of an Anoikis-Related gene signature predicts the prognosis of patients with Low-Grade gliomas. Brain Sci. 2022;12(10):1349. 10.3390/brainsci12101349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Cui Y, Zhou G, Zhang Z, Zhang P. Leveraging mitochondrial-programmed cell death dynamics to enhance prognostic accuracy and immunotherapy efficacy in lung adenocarcinoma. J Immunother Cancer. 2024;12(10):e010008. d10.1136/jitc-2024-010008 [DOI] [PMC free article] [PubMed]
- 10.Yu ZL, Zhu ZM. N6-Methyladenosine related long Non-Coding RNAs and immune cell infiltration in the tumor microenvironment of gastric Cancer. Biol Proced Online. 2021;23(1):15. 10.1186/s12575-021-00152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma C, Zhao H, Sun Y, Ding W, Wang H, Li Y, et al. Deciphering disulfidptosis: Uncovering a lncRNA-based signature for prognostic assessment, personalized immunotherapy, and therapeutic agent selection in lung adenocarcinoma patients. Cell Signal. 2024;117:111105. 10.1016/j.cellsig.2024.111105. [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Nie Z, Li Y, Gao Y, Wen X, Cao H, Zhang S. A new Ferroptosis-Related LncRNA signature predicts the prognosis of bladder Cancer patients. Front Cell Dev Biol. 2021;9:699804. 10.3389/fcell.2021.699804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi C, Ma J, Sun J, Wu X, Ding J. The role of molecular subtypes and immune infiltration characteristics based on disulfidptosis-associated genes in lung adenocarcinoma. Aging. 2023;15(11):5075–95. 10.18632/aging.204782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Li H, Zhang N, Dong Q, Liang Z. Multiomics analysis of Disulfidptosis patterns and integrated machine learning to predict immunotherapy response in lung adenocarcinoma. Curr Med Chem. 2024;31(25):4034–55. 10.2174/0109298673313281240425050032. [DOI] [PubMed] [Google Scholar]
- 15.Zhao F, Su L, Wang X, Luan J, Zhang X, Li Y, et al. Molecular map of disulfidptosis-related genes in lung adenocarcinoma: the perspective toward immune microenvironment and prognosis. Clin Epigenetics. 2024;16(1):26. 10.1186/s13148-024-01632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan X, Qian H, Sun Z, Yi Q, Liu Y, Lan G, et al. Investigating the role of Disulfidptosis related genes in radiotherapy resistance of lung adenocarcinoma. Front Med (Lausanne). 2024;11:1473080. 10.3389/fmed.2024.1473080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Zhang J, Zhang F, Lu S, Guo S, Shi R, et al. Identification of a disulfidptosis-related genes signature for prognostic implication in lung adenocarcinoma. Comput Biol Med. 2023;165:107402. 10.1016/j.compbiomed.2023.107402. [DOI] [PubMed] [Google Scholar]
- 18.He D, Tang H, Yang X, Liu X, Zhang Y, Shi J. Elaboration and validation of a prognostic signature associated with disulfidoptosis in lung adenocarcinoma, consolidated with integration of single-cell RNA sequencing and bulk RNA sequencing techniques. Front Immunol. 2023;14:1278496. 10.3389/fimmu.2023.1278496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong L, Chang W, Luo B, Gao W, He H, Fang M, et al. Development and validation of a Disulfidptosis and disulfide metabolism-related risk index for predicting prognosis in lung adenocarcinoma. Cancer Cell Int. 2024;24(1):2. 10.1186/s12935-023-03204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Low V, Li Z, Blenis J. Metabolite activation of tumorigenic signaling pathways in the tumor microenvironment. Sci Signal. 2022;15(759):eabj4220. 10.1126/scisignal.abj4220. [DOI] [PubMed] [Google Scholar]
- 21.Miller M, Hanna N. Advances in systemic therapy for non-small cell lung cancer. BMJ. 2021;375:n2363. 10.1136/bmj.n2363. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y, Li D, Liang Q, Yang M, Pan Y, Li H. Cross-talk between Cuproptosis and ferroptosis regulators defines the tumor microenvironment for the prediction of prognosis and therapies in lung adenocarcinoma. Front Immunol. 2023;13:1029092. 10.3389/fimmu.2022.1029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni L, Yang H, Wu X, Zhou K, Wang S. The expression and prognostic value of Disulfidptosis progress in lung adenocarcinoma. Aging. 2023;15(15):7741–59. 10.18632/aging.204938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Zhang F, Wang J, Wang Y. MicroRNA-mediated regulation in lung adenocarcinoma: signaling pathways and potential therapeutic implications (Review). Oncol Rep. 2023;50(6):211. 10.3892/or.2023.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YC, Jiang Q, Yang KP, Wang L, Sethi G, Ma Z. Extracellular vesicle-mediated ferroptosis, pyroptosis, and necroptosis: potential clinical applications in cancer therapy. Cell Death Discov. 2024;10(1):23. 10.1038/s41420-024-01799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Song D, Zhou Z, Jiang Z, Zhang N, Zhang H, et al. A comprehensive and visualized analysis of relationship between ferroptosis and tumor using bibliometrics and bioinformatics. Am J Cancer Res. 2023;13(12):6190–209. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Ouyang Y, Feng Z, Jiang Z, Ma J, Zhou X, et al. RASGRP2 is a potential immune-related biomarker and regulates mitochondrial-dependent apoptosis in lung adenocarcinoma. Front Immunol. 2023;14:1100231. 10.3389/fimmu.2023.1100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Ouyang L, Jiang S, Liang L, Chen Y, Mao C, et al. PPP2R1A Silencing suppresses LUAD progression by sensitizing cells to nelfinavir-induced apoptosis and pyroptosis. Cancer Cell Int. 2024;24(1):145. 10.1186/s12935-024-03321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Wang Y, Zhou X, Zhang Z, Ju H, Diao X, et al. Construction of a prognostic and early diagnosis model for LUAD based on necroptosis gene signature and exploration of immunotherapy potential. Cancers (Basel). 2022;14(20):5153. 10.3390/cancers14205153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G, Liu X, Liu H, Zhang X, Shao Y, Jia X. A novel necroptosis related gene signature and regulatory network for overall survival prediction in lung adenocarcinoma. Sci Rep. 2023;13(1):15345. 10.1038/s41598-023-41998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Xu Y, Ma L, Yu K, Niu Y, Xu X, et al. Essential roles of exosome and circrna_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun (Lond). 2022;42(4):287–313. 10.1002/cac2.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Shen N, Wang Z, Yu L, Yang S, Wang Y, et al. TRIM3 facilitates ferroptosis in non-small cell lung cancer through promoting SLC7A11/xCT K11-linked ubiquitination and degradation. Cell Death Differ. 2024;31(1):53–64. 10.1038/s41418-023-01239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Qu H, Ma X, Li L, Wei Y, Wang Y, et al. Identification of Cuproptosis and immune-related gene prognostic signature in lung adenocarcinoma. Front Immunol. 2023;14:1179742. 10.3389/fimmu.2023.1179742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou S, Liu J, Wan A, Zhang Y, Qi X. Epigenetic regulation of diverse cell death modalities in cancer: a focus on pyroptosis, ferroptosis, cuproptosis, and Disulfidptosis. J Hematol Oncol. 2024;17(1):22. 10.1186/s13045-024-01545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y, Dong Y, Liu W, Fan X, Sun Y. Pan-Cancer analyses confirmed the Ferroptosis-Related gene SLC7A11 as a prognostic biomarker for Cancer. Int J Gen Med. 2022;15:2501–13. 10.2147/IJGM.S341502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Lu Z, Sun R, Guo S, Gao F, Cao B, et al. The role of SLC7A11 in cancer: friend or foe?? Cancers (Basel). 2022;14(13):3059. 10.3390/cancers14133059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol Rev. 2021;101(3):1371–426. 10.1152/physrev.00026.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baluapuri A, Wolf E, Eilers M. Target gene-independent functions of MYC oncoproteins. Nat Rev Mol Cell Biol. 2020;21(5):255–67. 10.1038/s41580-020-0215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng YX, Shi S, Jiang XH, Liu KC, Qin ZJ, Wang YY, et al. Comprehensive analysis of protein phosphatase 1 regulatory inhibitor subunit 14B, a molecule related to tumorigenesis, poor prognosis, and immune cell infiltration in lung adenocarcinoma. Am J Transl Res. 2023;15(2):858–77. [PMC free article] [PubMed] [Google Scholar]
- 40.Mao D, Zhou Z, Chen H, Liu X, Li D, Chen X, et al. Pleckstrin-2 promotes tumour immune escape from NK cells by activating the MT1-MMP-MICA signalling axis in gastric cancer. Cancer Lett. 2023;572:216351. 10.1016/j.canlet.2023.216351. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X, Shu D, Sun W, Si S, Ran W, Guo B, et al. PLEK2 promotes cancer stemness and tumorigenesis of head and neck squamous cell carcinoma via the c-Myc-mediated positive feedback loop. Cancer Commun (Lond). 2022;42(10):987–1007. 10.1002/cac2.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Li T, Hu B, Li H. PLEK2 gene upregulation might independently predict shorter Progression-Free survival in lung adenocarcinoma. Technol Cancer Res Treat. 2020;19:1533033820957030. 10.1177/1533033820957030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W, Zhang J, Xie T, Huang X, Wang B, Tian Y, et al. C1QTNF6 is a prognostic biomarker and related to immune infiltration and drug sensitivity: A Pan-Cancer analysis. Front Pharmacol. 2022;13:855485. 10.3389/fphar.2022.855485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qu HX, Cui L, Meng XY, Wang ZJ, Cui YX, Yu YP, et al. C1QTNF6 is overexpressed in gastric carcinoma and contributes to the proliferation and migration of gastric carcinoma cells. Int J Mol Med. 2019;43(1):621–9. 10.3892/ijmm.2018.3978. [DOI] [PubMed] [Google Scholar]
- 45.Song X, Li L, Shi L, Liu X, Qu X, Wei F, et al. C1QTNF6 promotes oral squamous cell carcinoma by enhancing proliferation and inhibiting apoptosis. Cancer Cell Int. 2021;21(1):666. 10.1186/s12935-021-02377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao D, Li C, Rajoka MSR, He Z, Huang J, Wang J, et al. P21-Activated kinase 1: emerging biological functions and potential therapeutic targets in Cancer. Theranostics. 2020;10(21):9741–66. 10.7150/thno.46913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Xia R, Jiang L, Zhou Y, Xu H, Peng W, et al. Overexpression of RhoV promotes the progression and EGFR-TKI resistance of lung adenocarcinoma. Front Oncol. 2021;11:619013. 10.3389/fonc.2021.619013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chi H, Jiang P, Xu K, Zhao Y, Song B, Peng G, et al. A novel anoikis-related gene signature predicts prognosis in patients with head and neck squamous cell carcinoma and reveals immune infiltration. Front Genet. 2022;13:984273. 10.3389/fgene.2022.984273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Wang Y, Jiang Z. Immune induction identified by TMT proteomics analysis in Fusobacterium nucleatum autoinducer-2 treated macrophages. Expert Rev Proteom. 2020;17(2):175–85. 10.1080/14789450.2020.1738223. [DOI] [PubMed] [Google Scholar]
- 50.Yin P, Gui L, Wang C, Yan J, Liu M, Ji L, et al. Targeted delivery of CXCL9 and OX40L by mesenchymal stem cells elicits potent antitumor immunity. Mol Ther. 2020;28(12):2553–63. 10.1016/j.ymthe.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repositories and accession number(s) can be found within the article.