Abstract
Constipation, a common gastrointestinal disorder, significantly impacts quality of life.Its association with gut microbiota has garnered attention.Dietary factors play a crucial role in the development and management of constipation.The recently introduced dietary index for gut microbiota (DI-GM), a measure of gut microbiota diversity, offers insights into this connection.The association between dietary gut microbiota index and constipation is a critical public health issue.This study investigated the association between DI-GM and constipation prevalence in the American population using data from 11,819 individuals from the National Health and Nutrition Examination Survey (NHANES) between 2005 and 2010.Constipation was defined using Bristol stool form scale types 1 and 2.Dietary recall information was used to determine the DI-GM score, indicating the dietary influence on the gut microbiome. Multivariate weighted logistic regression, adjusted for confounders, was performed to analyze the association between DI-GM scores and constipation prevalence.Further analyses included a subgroup analysis and restricted cubic splines to explore this association [restricted cubic spline(RCS)].An increased DI-GM index, indicating a healthier gut microbiome, was related to a decreased risk of constipation.A similar association was observed with a more favorable score for beneficial gut microbiota.Non-linear associations between DI-GM scores and constipation were identified through RCS analysis.Subgroup and interaction analyses confirmed the consistency of these findings across strata, suggesting no significant heterogeneity.These findings suggest that dietary adjustments may be an important method for preventing constipation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12937-025-01164-y.
Keywords: Dietary index for gut microbiota (DI-GM), Constipation, General population, NHANES
Introduction
Economic development has shifted dietary habits, contributing to a rise in digestive diseases, including constipation [1], which has emerged as a significant global health issue affecting quality of life.Women, particularly older adults, are more susceptible to constipation than men [2, 3].Its prevalence varies widely, ranging from 2 to 27% in Western countries [4], with a global estimate of approximately 14% [5].Constipation is also associated with higher risks of mortality and cardiovascular events [6].The etiology and pathophysiology of constipation remain poorly understood, posing substantial challenges for research and clinical management [7].
In recent years, the critical role of the gut microbiota in maintaining intestinal health has garnered considerable attention [8–10].Diet, a primary determinant of gut microbiota composition and function [11, 12], is quantified using the dietary index for gut microbiota (DI-GM) to evaluate its impact. Examining the DI-GM’s association with constipation is highly relevant to clinical practice and public health.Researchers, including Kase, conducted an extensive review of 106 studies investigating the association between adult dietary patterns and the gut microbiota.They identified 14 dietary components that exert either positive or negative effects on the gut microbiota, subsequently developing the DI-GM to assess diet quality in relation to gut microbiota health [13].DI-GM has been shown to correlate positively with urinary indoxyl sulfate and trimethylamine-N-oxide, which are gut microbiota-derived metabolites reflecting specific microbial metabolic activities.While these metabolites are not direct markers of gut microbiota diversity, this positive correlation suggests that the DI-GM has the potential to be a valuable tool for identifying dietary patterns that may either support or compromise gut microbiota health.This index offers a standardized measure for evaluating diets that promote gut microbiota equilibrium, fostering interdisciplinary collaboration across the fields of nutrition, microbiology, gastroenterology, medicine, and epidemiology.
A growing body of research has also examined the association between micronutrient consumption and constipation [14–17].However, the specific influence of DI-GM on constipation remains unclear.This study utilizes comprehensive data from the National Health and Nutrition Examination Survey (NHANES) conducted between 2005 and 2010 to investigate the association between DI-GM and the prevalence of constipation among adults in the United States.The research aims to address this knowledge gap and provide new perspectives and clinical guidance for treating and preventing constipation.Based on the nutritional patterns observed in this cohort, we hypothesize that individuals with constipation will have lower DI-GM scores.
Materials and methods
Survey description
This observational study utilized data from the NHANES conducted by the Centers forDisease Control and Prevention (between 2005 and 2010) [18].The NHANES is a stratified, multistage probability survey assessing the health and nutritional status of the U.S.non-institutionalized population [19].It compiles demographic and health data viain-home interviews, physical examinations, and laboratory assessments at mobile examination centers (MEC).Our study was carried out in accordance with the guidelines for cross-sectional studies.The survey was approved by the National Center for Health Statistics (NCHS) Ethics Review Board, with all participants providing written informed consent. No additional institutional review board approval was required for secondary analysis of the NHANES data [20].NHANES data are publicly available on the NHANES website (http://www.cdc.gov/nchs/nhanes.html), accessed on September 17, 2024.
Study population
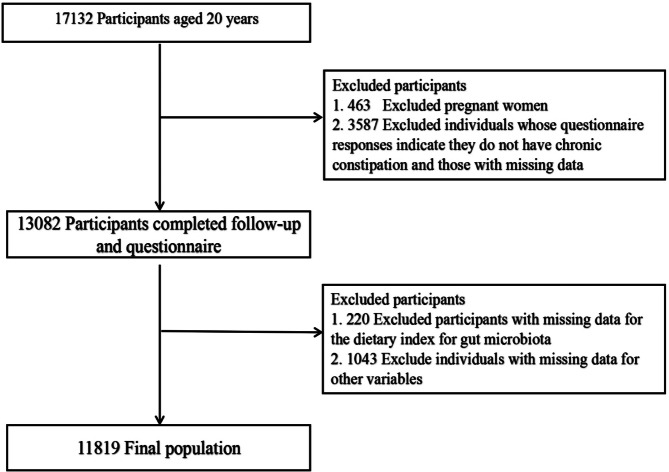
Our study included 17,132 participants aged ≥ 20 years from the 2005 to 2010 NHANES cycles.Exclusion criteria were pregnancy (n = 463),absence of constipation data (n = 3587), missing DI-GM components (n = 220),and incomplete covariate information (n = 1043).The final analysis included 11,819 eligible participants, as outlined in Fig. 1.
Fig. 1.
Flow chart of the study
Diagnosis of constipation
In the NHANES database, constipation is determined based on stool consistency.Data on stool textures were recorded in three rounds of the NHANES intestinal health questionnaire from 2005 to 2010.Participants were asked to estimate their stools consistency by referring to cards displaying various colored pictures representing different types of stool.They were then asked to indicate the number corresponding to their typical or most frequently observed stool type according to the Bristol stool form scale (BSFS).Constipation is characterized by BSFS type 1, which refers to hard lumps similar to nuts, or type 2,described as sausage-shaped stools with a lumpy texture.Normal stool consistency is defined as BSFS type 3 (sausage-like but with cracks on the surface), type 4 (sausage or snake-like, smooth, and soft), or type 5(soft mass with clearly defined edges).Chronic diarrhea is characterized by BSFS type 6,which refers to fluffy pieces with ragged or broken edges, or BSFS type 7,which is described as watery stool without solid pieces.Chronic constipation can be classified as either type 1 or type 2 [21, 22].
Assessment of DI-GM
As per the criteria outlined by Kase et al.,the DI-GM is composed of 14 dietary components, including items categorized as beneficial items, such as avocado, broccoli, chickpeas, coffee, cranberries, fermented dairy, fiber, green tea, soybeans, and whole grains, and detrimental items, such as red meat, processed meat, refined grains, and diets high in fat (≥ 40% of total energy). Specific tea types were not detailed in the NHANES, rendering their inclusion in the analysis not applicable [13].The dietary recall index uses data from the National Health and Nutrition Examination Survey (NHANES) 2005–2010 to calculate DI-GM.The components and scoring criteria of DI-GM are shown in Supplementary Table 1. For items beneficial to the gut microbiota, a score of 1 was assigned when the consumption was greater than or equal to the gender-specific median, and a score of 0 otherwise.For items unfavorable to the gut microbiota, a score of 0 was assigned when the consumption was greater than or equal to the gender-specific median or 40% (for high-fat diets), and a score of 1 otherwise. The aggregate of the DI-GM scores resulted in a total ranging from 0 to 13,with scores for positive components scaling from 0 to 9 and those for negative components from 0 to 4.These scores were then categorized into groups of: 0–3,4,5,and 6 or higher.
Assessment of covariates
Based on previous studies [23–25], potential covariates included age, sex, race/ethnicity, education level, family income ratio, body mass index (BMI), smoking status, alcohol consumption, physical activity, carbohydrate intake, cardiovascular disease, hyperlipidemia, diabetes, and hypertension.Self-reported race/ethnicity were categorized into five groups: non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, and other races. Participants were divided into two categories based on their marital status: those living alone (including never-married, separated, divorced, and widowed individuals) and those living with a partner (including married and cohabiting individuals).Education level was divided into three categories based on the number of years of education:<9 years,9–12 years, and > 12 years. Household income was categorized into three levels based on the poverty income ratio (PIR): low income(PIR ≤ 1.3),middle income(PIR = 1.3–3.5),and high income(PIR > 3.5). Smoking status was classified into three categories: never smokers(those who had smoked fewer than 100 cigarettes in their lifetime), former smokers(those who had smoked more than 100 cigarettes but were not currently smoking), and current smokers(those who had smoked more than 100 cigarettes and were currently smoking on some or every day).Participants were categorized into three groups based on alcohol consumption: never drinkers (lifetime consumption of fewer than 12 drinks), former drinkers(at least 12 drinks in a year but none in the last year, or no drinking in the past year but a lifetime total of at least 12 drinks), and current drinkers(at least 12 drinks in any year and drank in the past year).BMI was calculated using standardized techniques based on weight and height.Physical activity (PA) was measured by the weekly duration that individuals spent on activities, such as walking, cycling, chores, work tasks, and leisure, with 0 recorded for those who did not engage in exercise during the week.Diabetes diagnosis was determined by any of the following criteria: being told by a doctor that the individual has diabetes; HbA1c ≥ 6.5%;fasting blood glucose ≥ 7.0mmol/L; random blood glucose ≥ 11.0mmol/L;2-hour oral glucose tolerance test blood glucose ≥ 11.1 mmol/L; or use of diabetes medication or insulin.Hypertension was diagnosed based on meeting any of the following criteria: being previously told to have hypertension; use of antihypertensive medication; average systolic blood pressure ≥ 140mmHg; or average diastolic blood pressure ≥ 90mmHg.A history of cardiovascular disease was diagnosed based on self-reported previous diagnosis of coronary heart disease, angina, stroke, myocardial infarction, or heart failure.Dyslipidemia was defined by meeting any of the following criteria: use of lipid-lowering medication; high triglycerides ≥ 150 mg/dl; or high cholesterol(total cholesterol ≥ 200 mg/dl, or LDL ≥ 130 mg/dl, or HDL < 40 mg/dl).Information on dietary intake collected through NHANES from 2005 to 2010 was assessed by trained professional interviewers.
Statistical analysis
This study represents a secondary analysis of publicly accessible datasets.For the NHANES dataset, we incorporated the complex sampling design and mobile examination center sample weights to ensure that our research represents the overall population of the United States.The sample weight in this study was determined by using the dietary weight variable “WTDRD1” in the three cycles from 2005 to 2010.Categorical variables are presented as unweighted counts (weighted percentages), while continuous variables for normally distributed data are depicted as means (weighted standard deviations, SD), and for non-normally distributed data, continuous variables are depicted as medians (weighted interquartile ranges, IQR).To compare differences between groups, one-way ANOVA (for normally distributed data), the Kruskal-Wallis test (for skewed distribution), and the chi-square test (for categorical variables) were conducted, and the Wilcoxon rank-sum test was used to compare continuous variables between groups.Logistic regression models were used to determine the odds ratio (OR) and 95%confidence interval (95%CI) for the association between DI-GM and constipation.Model 1 was adjusted for sociodemographic characteristics, including age, sex, race/ethnicity, marital status, education level, and household income.Model 2 was additionally adjusted for physical activity, BMI, smoking status, alcohol consumption, and carbohydrate intake, based on Model 1.Model 3 was further adjusted for cardiovascular disease, hyperlipidemia, hypertension, diabetes, and NHANES cycle, based on Model 2.
Furthermore, restricted cubic spline (RCS) regression was applied at the 5th,35th,65th, and 95th percentiles of DI-GM using 4 knots to evaluate linearity and explore the dose-response association between DI-GM and constipation, adjusting for variables in Model 3.
We also examined possible disparities in the association between DI-GM and constipation within various subgroups, including: age (< 60years vs. ≥60years), sex, BMI(< 30 vs. ≥30 kg/m²), hyperlipidemia status (yes vs. no), hypertension status (yes vs. no), diabetes status (yes vs. no), and cardiovascular disease status (yes vs. no).Heterogeneity among the subgroups was assessed using multivariate logistic regression analysis, and the likelihood ratio test was used to evaluate the interactions between the subgroups and DI-GM.The results for each subgroup were stable, and no interactions were detected.
The sample size was determined by the extant data, which precluded a priori statistical power analyses.Statistical analyses were performed using R (version 4.2.3) and Free Software Foundation Statistics Software (version 2.0).Significance was set at a two-tailed p-value of less than 0.05,and the analysis was conducted from September to December 2024.
Results
Characteristics of the participants
Table 1 presents the baseline clinical characteristics of the study participants, categorized by the presence or absence of constipation.Among the 11,819 individuals who met the inclusion criteria and participated in the survey,956 (8.1%) had constipation.The average age of the study participants was 48.1(17.6) years, with 640(71%) being female.Regarding racial demographics, the sample comprised 65% non-Hispanic whites,15% non-Hispanic blacks,15% Mexican Americans,5% other Hispanics, and 5% other ethnic groups.A higher likelihood of constipation was observed among women, those who were married or living with a partner, individuals with medium household income, those with higher education levels, nonsmokers, current drinkers, those with lower physical activity, those with low carbohydrate intake, and those with lower DI-GM scores.
Table 1.
Characteristics of participants in the NHANES 2005–2010 cycles
| Characteristic | Total | Without constipation | constipation | p |
|---|---|---|---|---|
| No. | 11,819 | 10,863 | 956 | |
| Age, Mean ± SD | 49.3(16.6) | 49.5(16.5) | 48.1(17.6) | 0.019 |
| Sex, n (%) | < 0.001 | |||
| Male | 6057 (49) | 5741 (51) | 316 (29) | |
| Female | 5762 (51) | 5122 (49) | 640 (71) | |
| Race, n (%) | < 0.001 | |||
| Non-Hispanic White | 6064 (73) | 5636 (74) | 428 (65) | |
| Non-Hispanic Black | 2351 (11) | 2126 (10) | 225 (15) | |
| Mexican American | 2022 (7) | 1851 (7) | 171 (10) | |
| Other Hispanic | 915 (4) | 815 (4) | 100 (5) | |
| Other Race - Including Multi-Racial | 467 (5) | 435 (5) | 32 (5) | |
| Marry, n (%) | < 0.001 | |||
| Married or living with a partner | 7234 (64) | 6698 (64) | 536 (58) | |
| Living alone | 4585 (36) | 4165 (36) | 420 (42) | |
| Family income ratio, n(%) | < 0.001 | |||
| Low (≤ 1.3) | 3384 (19) | 3041 (18) | 343 (26) | |
| Medium (1.3–3.5) | 4571 (36) | 4184 (35) | 387 (41) | |
| High (> 3.5) | 3864 (45) | 3638 (46) | 226 (33) | |
| Education level (years), n(%) | < 0.001 | |||
| < 9 | 3087 (17) | 2775 (16) | 312 (24) | |
| 9– 12 | 2852 (24) | 2589 (24) | 263 (29) | |
| > 12 | 5880 (59) | 5499 (60) | 381 (47) | |
| Smoking status, n(%) | < 0.001 | |||
| Never | 6177 (53) | 5614 (52) | 563 (58) | |
| Former | 3013 (25) | 2815 (25) | 198 (20) | |
| Current | 2629 (23) | 2434 (23) | 195 (22) | |
| Drinking status, n(%) | < 0.001 | |||
| Never | 1466 (10) | 1294 (10) | 172 (15) | |
| Former | 2308 (16) | 2097 (16) | 211 (19) | |
| Current | 8045 (74) | 7472 (74) | 573 (66) | |
| BMI (kg/m2), Mean (SD) | 28.9(6.6) | 29.0(6.6) | 28.2(6.5) | < 0.001 |
| CVD, n (%) | 0.238 | |||
| No | 10,532 (92) | 9691 (92) | 841 (90) | |
| Yes | 1287 (8) | 1172 (8) | 115 (10) | |
| Hyperlipidemia, n (%) | 0.136 | |||
| No | 3400 (29) | 3105 (29) | 295 (30) | |
| Yes | 8419 (71) | 7758 (71) | 661 (70) | |
| Hypertension, n (%) | 0.024 | |||
| No | 6937 (64) | 6343 (63) | 594 (66) | |
| Yes | 4882 (36) | 4520 (37) | 362 (34) | |
| DM, n (%) | 0.547 | |||
| No | 9820 (88) | 9019 (88) | 801 (88) | |
| Yes | 1999 (12) | 1844 (12) | 155 (12) | |
| Beneficial to gut microbiota | 2.2(1.2) | 2.2(1.2) | 1.9(1.2) | < 0.001 |
| Unfavorable to gut microbiota | 2.3(1.0) | 2.3(1.0) | 2.4(1.0) | 0.005 |
| DI-GM | 4.5(1.6) | 4.5(1.6) | 4.3(1.5) | < 0.001 |
| Carbohydrate intake(g/day) | 257.2(127.4) | 258.8(128.5) | 238.5(111.4) | < 0.001 |
| Physical activity, min/week |
169.8 (30.0, 720.0) |
180.0 (30.0, 735.0) |
110.0 (0.0, 513.3) |
< 0.001 |
Abbreviation: NHANES, National Health and Nutrition Examination Survey. Categorical variables data are presented as unweighted counts (weighted percentages), while continuous variables for normally distributed data are depicted as means (weighted standard deviations, SD), and for non-normally distributed data, continuous variables are depicted as medians (weighted interquartile ranges, IQR).The sample size represents the unweighted count of actual observations, while all other results mentioned in the text are adjusted for the complex survey design using weights
Association between DI-GM and constipation
Univariate analysis showed that age, sex, race, marital status, education level, smoking status drinking status, BMI, DI-GM, beneficial components of gut microbiota, and carbohydrate intake were associated with constipation (Table 2).
Table 2.
Associations between variables and constipation in American adults
| Variable | OR_95CI | P_value |
|---|---|---|
| Age | 1.00(0.99,1.00) | 0.22 |
| Sex: Female vs.Male | 2.52 (2.03,3.11) | < 0.001 |
| Race, n (%) | ||
| Non-Hispanic White | 1(Ref) | |
| Non-Hispanic Black | 1.69 (1.33,2.15) | < 0.001 |
| Mexican American | 1.52 (1.12,2.07) | 0.008 |
| Other Hispanic | 1.42 (1.06,1.90) | 0.019 |
| Other Race - Including Multi-Racial | 1.08 (0.66 ~ 1.77) | 0.764 |
| Marital status: ref. = Married or living with a partner | ||
| Living alone | 1.32 (1.08,1.62) | 0.009 |
| Family income ratio ref.≤ 1.3 | ||
| Medium (1.3–3.5) | 0.82 (0.67,1.00) | 0.05 |
| High (> 3.5) | 0.50 (0.41,0.62) | < 0.001 |
| Education level (years): ref. =Less than high schoolr | ||
| High school or equivalent | 0.83(0.66,1.05) | 0.122 |
| Above high school | 0.54 (0.45,0.65) | < 0.001 |
| Smoking status: ref. = Never | ||
| Former | 0.72 (0.59,0.89) | 0.003 |
| Current | 0.89 (0.73,1.09) | 0.263 |
| Drinking status: ref. = Never | ||
| Former | 0.74 (0.53,1.04) | 0.086 |
| Current | 0.55 (0.41,0.71) | < 0.001 |
| PA timeb (minutes) | 1 (1 ~ 1) | 0.22 |
| BMI (kg/m2) | 0.97 (0.96,0.99) | 0.001 |
| CVD, yes vs. no | 1.24(0.97,1.58) | 0.735 |
| Hyperlipidemia, yes vs. no | 0.97(0.80,1.18) | 0.307 |
| Hypertension, yes vs. no | 0.91 (0.77,1.07) | 0.245 |
| Diabetes: yes vs. no | 0.97 (0.76,1.23) | 0.776 |
| DI-GM | 0.90 (0.84 ~ 0.97) | 0.003 |
| Beneficial to gut microbiota | 0.81(0.75,0.88) | < 0.001 |
| Carbohydrate intake (g/day), Median (IQR) | 1.00 (1.00 ~ 1.00) | < 0.001 |
Associations between variables and Constipation.Results are based on weighted data. DI-GM, gut microbiota dietary index, OR odds ratio, CI confidence interval, Ref Refreference
As shown in Table 3, each incremental point of DI-GM was associated with a 10% reduction in the prevalence of constipation (OR = 0.90,95%CI = 0.84,0.97, p = 0.003).This association remained significant in the fully adjusted model (OR = 0.91,95% CI = 0.85,0.98,p = 0.016). When stratified by DI-GM, participants with DI-GM ≥ 6 had a significant negative correlation with the prevalence of constipation (OR = 0.58,95%CI = 0.44,0.78,p < 0.001) in the fully adjusted model.Additionally, an increase in the beneficial components of the gut microbiota was significantly associated with a lower prevalence of constipation (OR = 0.84,95% CI = 0.77,0.93,p < 0.001),whereas no significant difference in the association was found between the harmful components of gut microbiota and constipation.
Table 3.
Association between the gut microbiota dietary index and constipation in the NHANES 2005–2010 cycles
| Quartiles | OR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Crude | p-Value | Model 1 | p-Value | Model 2 | p-Value | Model 3 | p-Value | |
|
DI-GM_score (Continuous variable) |
956/11,819 (8.1%) |
0.90 (0.84–0.97) |
0.003 |
0.92 (0.86–0.99) |
0.028 |
0.91 (0.85–0.98) |
0.013 |
0.91 (0.85–0.98) |
0.016 |
| DI-GM_score(classified variable) | |||||||||
| Q1(0–3) |
262/3083 (8.5%) |
0.92 (0.73.1.14) |
0.43 |
0.87 (0.70,1.09) |
0.224 |
0.88 (0.70,1.11) |
0.281 |
0.88 (0.69,1.11) |
0.264 |
| Q2(4) |
281/3029 (9.3%) |
1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | ||||
| Q3(5) |
221/2782 (7.9%) |
0.86 (0.66,1.11) |
0.227 |
0.87 (0.66,1.15) |
0.308 |
0.85 (0.64,1.12) |
0.234 |
0.85 (0.64,1.13) |
0.236 |
| Q5(≥ 6) |
192/2925 (6.6%) |
0.58 (0.45,0.75) |
< 0.001 |
0.60 (0.46,0.79) |
< 0.001 |
0.58 (0.44,0.77) |
< 0.001 |
0.58 (0.44,0.78) |
< 0.001 |
| P for trend test | < 0.001 | 0.001 | < 0.001 | 0.001 | |||||
|
DI-GM_benifi (Continuous variable) |
/ |
0.81 (0.75–0.88) |
< 0.001 |
0.85 (0.78–0.93) |
< 0.001 |
0.84 (0.77–0.92) |
< 0.001 |
0.84 (0.77–0.93) |
< 0.001 |
|
DI-GM_unfav (Continuous variable) |
/ |
1.06 (0.97–1.16) |
0.186 |
1.03 (0.94–1.13) |
0.495 |
1.00 (0.90–1.11) |
0.977 |
1.00 (0.90–1.11) |
0.993 |
Note: Model 1 was adjusted for age, gender, marital status, race/ethnicity, education level and Family income ratio
Model 2 was adjusted for Model 1 + body mass index, smoking status, drinking, physical activities, Carbohydrate intake
Model 3 was adjusted for model 2 + CVD, hypertension, hyperlipidemia, diabetes, NHANES cycle. Abbreviations: CI, confidence interval; OR, odds ratio
Figure 2 shows that both DI-GM (non-linear, p = 0.012) and beneficial components of gut microbiota (non-linear, p < 0.001) exhibited non-linear association with constipation in the RCS analysis, whereas harmful components of gut microbiota (non-linear, p = 0.873) were linearly associated with constipation.
Fig. 2.
Association between DI-GM and Constipation in NHANES 2005–2010 participants by RCS. Association between DI-GM and Constipation in NHANES 2005–2010 participants by RCS Abbreviations: CI, confidence interval; DI-GM, dietary index for gut microbiota; NHANES, National Health and Nutrition Examination Survey; OR, odd ratio; RCS, restricted cubic spline. The model adjusted for age, gender, marital status, race/ethnicity, education level, Family income ratio, body mass index, smoking status, drinking, physical activities, Carbohydrate intake. Model 3 was adjusted for model 2 + CVD, hypertension, hyperlipidemia, diabetes, NHANES cycle
Figure 3 Subgroup analyses
Fig. 3.
Association between the gut microbiota dietary index and Constipation according to general characteristics. The stratifications were adjusted for all variables (age, gender, marital status, race/ethnicity, education level, body mass index, smoking status, drinking, physical activities, Carbohydrate intake, CVD, hypertension, hyperlipidemia, diabetes, NHANES cycle.) except for the stratification factor itself. Circles represent the ORs and horizontal lines represent 95% CIs. BMI, body mass index; CI, confidence interval; OR, odds ratio
Subgroup analyses were conducted to assess potential effect modifications of the association between DI-GM and constipation.Stratification by age, sex, BMI, hyperlipidemia, cardiovascular disease, hypertension patients, and diabetes mellitus revealed no significant interactions in any subgroup (Fig. 3).The interaction p-values for all subgroups were greater than 0.05,suggesting that our findings are stable and consistent across subgroups.
Sensitivity analysis
In the sensitivity analysis, total energy intake was additionally adjusted, DI-GM was associ- ated with constipation (OR = 0.56,95%CI = 0.42–0.75,p < 0.001).The results are shown in Supplementary Table 2.
Discussion
In this large cross-sectional study of the American population, we initially demonstrated that a DI-GM score of 6 or higher, along with beneficial gut microbiota components, had a notably negative correlation with constipation.Even after adjusting for potential confounding factors (including age, sex, marital status, race, education level, PIR, BMI, drinking, smoking, physical activity, carbohydrate intake, cardiovascular disease, hypertension, hyperlipidemia, and diabetes), this negative dose-response association remained significant.Our study found that, based on stool consistency, the overall prevalence of constipation was 8.1%,with women (11.1%) being more likely than men (5.2%) to experience constipation, consistent with previous literature reports [21].However, owing to differences in population and definitions, this prevalence is slightly lower than global prevalence rates, although the risk of developing constipation between sexes remains largely consistent [2].RCS analysis showed that both DI-GM and beneficial gut microbiota had non-linear associations with constipation, whereas harmful gut microbiota exhibited a linear association. Subgroup analysis indicated that the association between DI-GM and constipation remained robust across different subgroups.
Several dietary factors have been shown to influence the gut microbiota and the development of constipation, and dietary adjustment is considered an effective strategy for alleviating or treating these symptoms.For example, the intake of fermented dairy and dietary fiber, recognized as advantageous for the gut microbiota within the DI-GM, could play a pivotal role. A randomized controlled trial demonstrated that a diet abundant in fermented items progressively enhanced microbial variety and reduced inflammatory markers [26]. Additionally, Bassotti et al. reported that constipation is characterized by low-grade mucosal inflammation [27], whereas Boyer et al. and Krauter et al. noted that it often leads to dysmotility and impairs propulsive forces, affecting defecation [28, 29].Sandler et al. reported that insufficient dietary fiber intake is a significant cause of constipation, and a meta-analysis confirmed the efficacy of fiber supplementation in relieving constipation [30].Fukumoto et al. reported that cellulose can be fermented by the gut microbiota into short-chain fatty acids (SCFAs), which can release 5-HT to promote intestinal motility [31].Kang et al. indicated that caffeine intake was associated with a lower likelihood of constipation, suggesting that daily consumption of caffeinated foods or beverages can help control constipation [32].Furthermore, some studies, including Wichmann et al.,have also confirmed that disruption of the gut microbiota affects SCFA production, further reducing glucagon-like peptide-1 (GLP-1) production and inhibiting intestinal transit function [33].Refined grains, categorized as harmful to the gut microbiota in DI-GM, are common in Western diets.Overconsumption of these grains can lead to elevated blood sugar levels, associated with intestinal and neuroinflammation [34].Similarly, high-fat and high-carbohydrate diets have been shown to reduce gut microbiota diversity [35].
In patients with constipation, the diversity and composition of the gut microbiota often changes.Research indicates a reduction in the number of beneficial bacteria in the intestines of these patients, particularly in species such as Bifidobacterium.Bifidobacterium ferments carbohydrates to produce SCFAs such as acetate, propionate, and butyrate, which are crucial for maintaining intestinal health.These substances not only promote intestinal motility and increase blood supply to the intestinal mucosa but also provide essential energy for intestinal mucosal cells.When the population of Bifidobacterium decreases, the production of SCFAs also diminishes, potentially weakening intestinal motility function and leading to or exacerbating symptoms of constipation [36].Therefore, maintaining a balanced population of beneficial bacteria in the gut is vital for preventing and treating constipation.The stringent data collection protocols and multistage sampling strategy of the NHANES enabled us to analyze this relationship across a broad and varied population of U.S. adults, and subgroup analyses enhanced the robustness and reliability of the study results.
The DI-GM score calculated in this study was based on the 24 - hour dietary recall data from NHANES and has certain limitations.The 24 - hour dietary recall only records dietary intake at a single time point, which may not accurately reflect an individual’s habitual or long-term dietary patterns.Short-term dietary data is key for studying chronic health issues like constipation.This is because long-term dietary patterns can more deeply affect gut microbiota composition and function, and constipation is often associated to long-term changes in gut microbiota.Thus, dietary data from a single time point may not fully capture the association between long-term diet and health.We’re aware that recall bias is a major issue with 24-hour dietary recall data.To minimize this bias, surveyors were rigorously trained to effectively guide respondents in accurately recalling their dietary intake over the past 24 h.Moreover, surveyors used standardized tools like food charts and models to help respondents accurately report food types and amounts.These tools enhance the accuracy of respondents’ memory of food details, thereby reducing biases from memory inaccuracy.Daily dietary intake changes indeed challenge the reliability of using 24-hour dietary recall data to assess long-term dietary patterns.Personal dietary choices can vary significantly due to a multiplicity of factors, including social activities, individual preferences, and food resource availability.Nevertheless, most adults maintain relatively stable dietary habits unless influenced by major health issues or lifestyle changes.This suggests that even though the DI-GM score reflects dietary habits at the time of data collection, it can still reasonably represent the usual dietary patterns of the general population.Despite its limitations, the 24-hour dietary recall is widely used in large- scale surveys like NHANES due to its feasibility and relatively low cost.Previous research has also used similar data to explore the association between DI-GM and chronic diseases [37–40], providing a solid methodological foundation for our study.To further confirm the association between DI-GM and constipation found in this study and explore the potential causal mechanisms of this association, future research will use more comprehensive dietary assessment methods, such as multi-day dietary records or food diaries.This would more accurately capture individuals’ long-term dietary patterns.
However, this study has several limitations.First, the cross-sectional nature of the study precludes the establishment of a causal association between DI-GM and constipation.Further prospective studies and randomized controlled trials are required to establish causality.Second, as is common in many studies, it is difficult to completely exclude the likelihood of confounding effects arising from measurement error residuals because of variables that were not measured or from unknown confounders.Third, although the DI-GM initially encompassed 14 food items, the lack of specific tea consumption records in the NHANES 24-hour dietary recall data meant that these food parameters could not be obtained.Fourth, the study used self-reported 24-hour dietary data to explore the association between DI-GM and constipation, which might have led to recall bias, and some covariates were also self-reported.Finally, although we utilized data from a large sample of the American population, owing to dietary and lifestyle differences between Western and non-Western countries, it is necessary to consider these aspects when generalizing our findings to other populations.
Conclusions
Through an exhaustive analysis of the NHANES data, this study validated the newly proposed DI-GM as an indicator of dietary quality that correlates with gut microbiota diversity.This study found that higher DI-GM scores, especially for beneficial items, were inversely associated with the incidence of constipation.Given the known association between diet, gut microbiota, and constipation, future studies and dietary interventions integrating DI-GM may enhance design of preventative and therapeutic approaches for constipation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to thank all of the participants for their valuable contributions.
Author contributions
CYS, ZLZ, SXZ, and HCT contributed to study conception and design; CYS, ZLZ, and SXZ contributed to data acquisition and analysis; CYS and ZLZ contributed to the interpretation of data; CYS and HCT had primary responsibility for final content; all authors contributed to critical revision and approved the final manuscript.
Funding
The authors declare that they have no funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The institutional review board approved the NHANES protocol of the Centers for Disease Control and Prevention (CDC), and each participant provided written informed consent.The authors of this study thank the participants of the NHANES and the NHANES staff.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang L, Zhu Q, Qu X, Qin H. Microbial treatment in chronic constipation. Sci China Life Sci. 2018;61(7):744–52. 10.1007/s11427-017-9220-7. (2018). [DOI] [PubMed]
- 2.Barberio B, Judge C, Savarino EV, Ford AC. Global prevalence of functional constipation according to the Rome criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(8):638–648.10.1016/S2468-1253(21)00111-4(2021). [DOI] [PubMed]
- 3.Camilleri M, Ford AC, Mawe GM, Dinning PG, Rao SS, Chey WD, Simrén M, Lembo A, Young-Fadok TM, Chang L. Chronic constipation. Nat Rev Dis Primers. 2017;3:17095.10.1038/nrdp.2017.95(2017). [DOI] [PubMed]
- 4.Sethi S, Mikami S, Leclair J, Park R, Jones M, Wadhwa V, Sethi N, Cheng V, Friedlander E, Bollom A, Lembo A. Inpatient burden of constipation in the united states: an analysis of National trends in the united States from 1997 to 2010. Am J Gastroenterol. 2014;109(2):250–6. 10.1038/ajg.2013.423. (2014). [DOI] [PubMed]
- 5.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(9):1582-91; quiz 1581, 1592.10.1038/ajg.2011.164(2011). [DOI] [PubMed]
- 6.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Yamagata K, Kalantar-Zadeh K, Kovesdy CP. Constipation and risk of death and cardiovascular events. Atherosclerosis. 2019;281:114–20. 10.1016/j.atherosclerosis.2018.12.021(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohkusa T, Koido S, Nishikawa Y, Sato N. Gut microbiota and chronic constipation: A review and update. Front Med (Lausanne). 2019;6:19. 10.3389/fmed.2019.00019. (2019). [DOI] [PMC free article] [PubMed]
- 8.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4):e00036-17.10.1128/MMBR.00036-17(2017). [DOI] [PMC free article] [PubMed]
- 9.Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49.10.1017/S0029665120006916(2021). [DOI] [PubMed]
- 10.Tan H, Nie S. Functional hydrocolloids, gut microbiota and health: picking food additives for personalized nutrition. FEMS Microbiol Rev. 2021;45(4):fuaa065.10.1093/femsre/fuaa065(2021). [DOI] [PubMed]
- 11.Agakidis C, Kotzakioulafi E, Petridis D, Apostolidou K, Karagiozoglou-Lampoudi T. Mediterranean diet adherence is associated with lower prevalence of functional Gastrointestinal disorders in children and adolescents. Nutrients. 2019;11(6):1283. 10.3390/nu11061283. (2019). [DOI] [PMC free article] [PubMed]
- 12.Mitsou EK, Kakali A, Antonopoulou S, Mountzouris KC, Yannakoulia M, Panagiotakos DB, Kyriacou A. Adherence to the mediterranean diet is associated with the gut microbiota pattern and Gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117(12):1645–55. 10.1017/S0007114517001593. (2017). [DOI] [PubMed]
- 13.Kase BE, Liese AD, Zhang J, Murphy EA, Zhao L, Steck SE. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. 2024;16(7):1045.10.3390/nu16071045(2024). [DOI] [PMC free article] [PubMed]
- 14.Du W, Yan C, Wang Y, Li Y, Tian Z, Liu Y, Shen W. Association between dietary copper intake and constipation in US adults. Sci Rep. 2024;14(1):19237. 10.1038/s41598-024-70331-8(2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Zhang L, Li L. Association between selenium intake with chronic constipation and chronic diarrhea in adults: findings from the National health and nutrition examination survey. Biol Trace Elem Res. 2021;199(9):3205–12. 10.1007/s12011-020-02451-x(2021). [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Du Z, Li Z, Yu F, Li L. Association of dietary magnesium intake with chronic constipation among US adults: Evidence from the National Health and Nutrition Examination Survey. Food Sci Nutr. 2021;9(12):6634–6641.10.1002/fsn3.2611(2021). [DOI] [PMC free article] [PubMed]
- 17.Zhao X, Wang L, Quan L. Association between dietary phosphorus intake and chronic constipation in adults: evidence from the National health and nutrition examination survey. BMC Gastroenterol. 2023;23(1):24. 10.1186/s12876-022-02629-8(2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. NHANES Survey Methods and Analytic Guidelines.Accessed September 17, 2024. Available online: https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx
- 19.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and Opera tions, 1999–2010. Vital Health Stat. 2013;1:56:1–37. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. NHANES Data Release and Access Policy. https://www.cdc.gov/nchs/data/nhanes/nhanes_release_policy.pdf
- 21.Hong Y, Shen H, Chen X, Li G. Gender differences in the association between dietary protein intake and constipation: findings from NHANES. Front Nutr. 2024;11:1393596. 10.3389/fnut.2024.1393596. (2024). [DOI] [PMC free article] [PubMed]
- 22.Ballou S, Katon J, Singh P, Rangan V, Lee HN, McMahon C, Iturrino J, Lembo A, Nee J. Chronic diarrhea and constipation are more common in depressed individuals. Clin Gastroenterol Hepatol. 2019;17(13):2696–703. 10.1016/j.cgh.2019.03.046(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Yang Q, Huang J, Lin H, Luo N, Tang H. Association of the newly proposed dietary index for gut microbiota and depression: the mediation effect of phenotypic age and body mass index. Eur Arch Psychiatry Clin Neurosci. 2024 Oct. 10.1007/s00406-024-01912-x(2024). [DOI] [PubMed]
- 24.Yang S, Wu XL, Wang SQ, Guo XL, Guo FZ, Sun XF. Association of dietary energy intake with constipation among men and women: results from the national health and nutrition examination survey. Front Nutr. 2022;9:856138.10.3389/fnut.2022.856138(2022). [DOI] [PMC free article] [PubMed]
- 25.Xi Y, Jiao WE, Li F, Li HD, Lu G, Deng YQ, Tao ZZ. Association between 19 allergens and chronic constipation in adults: NHANES 2005–2006. Int Arch Allergy Immunol. 2023;184(3):252–60. 10.1159/000527159(2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, Topf M, Gonzalez CG, Van Treuren W, Han S, Robinson JL, Elias JE, Sonnenburg ED, Gardner CD, Sonnenburg JL. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–53. 10.1016/j.cell.2021.06.019(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassotti G, Villanacci V, Creţoiu D, Creţoiu SM, Becheanu G. Cellular and molecular basis of chronic constipation: taking the functional/idiopathic label out. World J Gastroenterol. 2013;19(26):4099–105. 10.3748/wjg.v19.i26.4099(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyer L, Ghoreishi M, Templeman V, Vallance BA, Buchan AM, Jevon G, Jacobson K. Myenteric plexus injury and apoptosis in experimental colitis. Auton Neurosci. 2005;117(1):41–53. 10.1016/j.autneu.2004.10.006(2005). [DOI] [PubMed] [Google Scholar]
- 29.Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007;19(12):990–1000. 10.1111/j.1365-2982.2007.00986.x(2007). [DOI] [PubMed] [Google Scholar]
- 30.van der Schoot A, Drysdale C, Whelan K, Dimidi E. The effect of Fiber supplementation on chronic constipation in adults: an updated systematic review and Meta-Analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(4):953–69. 10.1093/ajcn/nqac184. (2022). [DOI] [PMC free article] [PubMed]
- 31.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1269. 10.1152/ajpregu.00442.2002(2003). [DOI] [PubMed] [Google Scholar]
- 32.Kang Y, Yan J. Exploring the connection between caffeine intake and constipation: a cross-sectional study using National health and nutrition examination survey data. BMC Public Health. 2024;24(1):3. 10.1186/s12889-023-17502-w(2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wichmann A, Allahyar A, Greiner TU, Plovier H, Lundén GÖ, Larsson T, Drucker DJ, Delzenne NM, Cani PD, Bäckhed F. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14(5):582–90. 10.1016/j.chom.2013.09.012(2013). [DOI] [PubMed] [Google Scholar]
- 34.González Olmo BM, Butler MJ, Barrientos RM. Evolution of the human diet and its impact on gut microbiota, immune responses, and brain health. Nutrients. 2021;13(1):196. 10.3390/nu13010196(2021). [DOI] [PMC free article] [PubMed]
- 35.Takayama K, Takahara C, Tabuchi N, Okamura N. Daiokanzoto (Da-Huang-Gan-Cao-Tang) is an effective laxative in gut microbiota associated with constipation. Sci Rep. 2019;9(1):3833. 10.1038/s41598-019-40278-2(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai H, Li Y, He Y, Chen F, Mi B, Li J, Xie J, Ma G, Yang J, Xu K, Liao X, Yin Y, Liang J, Kong L, Wang X, Li Z, Shen Y, Dang S, Zhang L, Wu Q, Zeng L, Shi L, Zhang X, Tian T, Liu X. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: a double-blinded randomized placebo trial. Gut Microbes. 2023 Jan-Dec;15(1):2197837. 10.1080/19490976.2023.2197837(2023). [DOI] [PMC free article] [PubMed]
- 37.Liu J, Huang S. Dietary index for gut microbiota is associated with stroke among US adults. Food Funct. 2025;16(4):1458–68. 10.1039/d4fo04649h. [DOI] [PubMed]
- 38.Xiao Y, Yang Y, Gao S, Zhang H, Wang J, Lin T, Bai Y. Dietary index for gut microbiota, a novel protective factor for the prevalence of chronic kidney diseases in the adults: insight from NHANES 2007–2018. Front Nutr. 2025;12:1561235. 10.3389/fnut.2025.1561235. [DOI] [PMC free article] [PubMed]
- 39.Huang Y, Liu X, Lin C, Chen X, Li Y, Huang Y, Wang Y, Liu X. Association between the dietary index for gut microbiota and diabetes: the mediating role of phenotypic age and body mass index. Front Nutr. 2025;12:1519346. 10.3389/fnut.2025.1519346. [DOI] [PMC free article] [PubMed]
- 40.Wu Z, Gong C, Wang B. The relationship between dietary index for gut microbiota and diabetes. Sci Rep. 2025;15(1):6234. 10.1038/s41598-025-90854-y. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.