Abstract
Sirtuin 1, which is more commonly identified as SIRT1, is a well-recognized NAD+-dependent histone and/or protein deacetylase that operates a wide range of cellular and molecular mechanisms involved in carcinogenic processes. SIRT1 not only regulates the primary mechanisms involved in tumorigenesis but also is responsible for controlling other processes, such as cell migration and metastasis, autophagy, and apoptotic flux, as well as chemotherapeutic resistance. It is well established that SIRT1 works at the upstream of signal transduction pathways, such as p53 signaling, together with FOXO mechanism, as well as the others. Indeed, SIRT1 by its deacetylase activity deacetylates different molecules in those signaling pathways and mostly causes a kind of blockade in the signaling of interest. Nonetheless, many aspects of SIRT1–cancer relationship are ambiguous, and more experiments should be performed for further uncovering of the concept. In the current review, we first highlight the major regulators of SIRT1 in cancer and then underscore key signal transduction pathways regulated by SIRT1. In the following, the role of SIRT1 will be discussed in tumor progression, from tumorigenesis to chemotherapeutic resistance, and at last, the contribution of SIRT1 to human cancers will be exemplified by some studies conducted in the field.
Graphical abstract

Keywords: Sirtuin 1, Neoplasm, Carcinogenesis, Signal transduction, Drug resistance
Introduction
Sirtuins are known as NAD+-dependent deacetylases, involving in a wide spectrum of cellular and molecular processes. In this evolutionary conserved family of deacetylases, SIRT1 has attracted much attention compared to its other counterparts, as it operates several molecular mechanisms and signaling pathways [1, 2]. Mechanistically, SIRT1 acts by deacetylating different histones (e.g., H4K16) and non-histone proteins (e.g., p53), contributing to vital cellular processes such as cell cycle progression/arrest, cell growth and differentiation, metabolism, and apoptosis, coupled with other mechanisms [3, 4].
Other than typical conditions, SIRT1 is also in a strong correlation with malignant circumstances. Nonetheless, despite extensive evaluations conducted in this field, it remains debate as SIRT1 can serve as either a tumor-inducing factor or a tumor suppressor, specifically depending on the cancer species, target signaling mechanism, and cellular context [5]. In detail, as SIRT1 can repress multiple tumor suppressors, such as p53 and p73, it is considered a potent oncogene, provoking tumor growth and expansion, while on the other front, inhibitory effects of SIRT1 on carcinogenic genes, proteins, and signal molecules unveil the tumor-suppressive potentials of this histone/protein deacetylase [5, 6]. By serving as either tumor suppressor or tumor promoter, SIRT1 regulates cancer onset and development from the beginning steps, i.e., tumor cell growth and proliferation, to more advanced processes, including cell migration, invasion, and metastasis [7, 8]. Behind the scenes of these processes, several signaling pathways are working continuously to maintain the relevant conditions. Interestingly, these signal transduction mechanisms are substantially regulated by SIRT1, regarding the observed network between SIRT1 and p53, Wnt, TGF-β, and NF-κB signal molecules [9].
Furthermore, the corresponding sirtuin has intriguingly been found to mediate the response of tumor cells to chemotherapeutic agents, as it functions as a regulator at upstream of chemo-resistance underlying mechanisms, such as drug efflux mediated by ATP-binding cassettes [10, 11]. Together, it is hypothesized that SIRT1 is a crucial cancer modulator, as it links to cancerous conditions in a reciprocal manner. Thus, SIRT1 activation or inhibition provides a therapeutic approach against multiple neoplasms. The current review aims to highlight the most important parts of SIRT1–cancer relationship to elucidate uncovered aspects of this cross talk for further exploration of cancer therapies depending on the regulation of SIRT1.
Key targets modulated by SIRT1 in cancer
SIRT1 and epigenetic alterations in cancer
Under cancerous circumstances, SIRT1 is principally known as an epigenetic modulator once DNA damage is progressed (Fig. 1). In this context, it serves both by deacetylating histones at sites of DNA damage and as a deacetylase of proteins involved in DNA repair and DNA damage response (DDR) [6, 12]. Concurrent with histone deacetylation, the status of chromatin compaction is changed. On the other hand, once non-histone proteins are deacetylated, protein activity will be operated [13]. Both chromatin compaction and protein activity, triggered by SIRT1, provide protection against DNA damage [14]. In connection with environmental impulses, histone and chromatin modifications, which affect temporal control of DNA damage repair as well as its spatial regulation, are considered central mechanisms to epigenetic modulations [15].
Fig. 1.
Key targets modulated by SIRT1 in cancer
Sir proteins in yeasts that are recognized as sirtuin orthologs have the ability of constructing an inactive state of heterochromatin through repressing the transcription process subsequent to polymerization across nucleosomes [16]. Within the context, Sir proteins are transferred from transcriptionally repressed genes to DNA damage sites by MEC1-mediated signaling pathway to derepress the genes, being epigenetically silenced by the Sir2/3/4 complex [6]. Likewise, SIRT1 in eukaryotes was found to be necessary for the repair of double-stranded DNA breaks (DSBs), as well as genome maintenance, especially when a genotoxic stress threats the mammalian stem cell; in this situation, SIRT1 detaches the repressed DNA loci and relocates to DNA break regions to repair the damage by the ataxia–telangiectasia mutated (ATM) signaling mechanism. As a result, chromatin is reorganized following the aforementioned SIRT1 relocalization, leading to transcriptional alterations that are linked to age-correlated modifications [6, 17].
On the gene promoter, SIRT1 localization results in the recruitment of DNA methyltransferases (DNMTs) to enhance the methylation on the promoter for further stimulation of silencing at the damaged regions [18]. Consistently, it was demonstrated that pharmacological knockdown of SIRT1 could mitigate γH2AX and generate decreased DNMTs (esp. DNMT1, DNMT3a, and DNMT3b) in breast cancer cells [19]. Furthermore, H2O2-induced DNA damage in colorectal cancer cells provokes the recruitment of MutS homolog 2 (MSH2)-MSH6 heterodimer for binding to chromatin and further enrollment of DNMT1 and proliferating cell nuclear antigen (PCNA) at the sites of injury [12]. Major epigenetic proteins in this regard, including SIRT1, enhancer of zeste homologue 2 (EZH2), DNMT1, and DNMT3b, are reported to be recruited to DSBs to preserve chromatin compaction around the damaged sites concurrent with the emergence of repressive histone modifications [20, 21]. With a mechanistic view, DNMT1 creates a silencing complex with DNMT3b and SIRT1 and EZH2, as the polycomb repressive complex 4 (PRC4) components to generate DNA damage-induced foci [6, 21].
ADP ribosylation factor (ARF) is another substantial gene to be involved in DNA break repair, as it can be transcriptionally regulated by SIRT1 and the E2F1 transcription factor, which contribute to the PARP1-related signaling pathway [22]. Indeed, ARF is a tumor suppressor in association with p53 modulation, and arf gene is usually inactivated in human neoplasms [23, 24]. E2F1, on the other hand, is SIRT1’s target, which is in relation to the failure to trigger apoptosis-inducing genes [25, 26]. Beyond, SIRT1 is also responsible for the recruitment of key DNA repair proteins, including NBS1, as the Nijmegen breakage syndrome 1, and Rad51 in keratinocytes with episomes of high-risk human papillomaviruses (HPV) species involving in viral activities, like genome maintenance, amplification, and gene transcription [27].
SIRT1-based long noncoding RNAs in cancer
Long noncoding RNAs (lncRNAs), as RNA transcripts with more than 200 nucleotides in length, most of which do not contain the ability of coding functional proteins. LncRNAs are major regulators in many disorders, as they can associate with a wide spectrum of mRNAs and proteins, such as sirtuins [28]. There are several examples of SIRT1-interacting lncRNAs (including GAS5, lincRNA-p21, MCM3AP-AS1, TUG1, UCA1, etc.) that can control SIRT1 expression, especially by sponging miRNAs (Fig. 1). These lncRNAs, along with the others, have been demonstrated to have pathogenic roles in the progression of multiple malignant circumstances. SNHG10 is a well-characterized lncRNA that functions as a molecular sponge for miR-543 in non-small cell lung cancer (NSCLC) [29]. Similarly, SNHG7 has been demonstrated to inhibit NLRP3-mediated pyroptosis by modulating the miR-34a/SIRT1 axis in hepatocellular carcinoma [30]. GAS5 represents another notable example, as it suppresses the malignant progression of colorectal cancer cells by regulating autophagic flux through the miR-34a/mTOR/SIRT1 negative feedback loop [31]. Conversely, certain lncRNAs such as UCA1 have been identified as oncogenic noncoding RNAs; UCA1 promotes cancer cell proliferation and inhibits apoptosis by modulating SIRT1 expression and interacting with specific microRNAs in pediatric acute myeloid leukemia (AML) [32].
Given the intricate regulatory interplay between lncRNAs and SIRT1 deacetylase, several therapeutic agents have been developed to target the lncRNA/SIRT1 axis. Notable compounds include anthocyanins, dexmedetomidine, berberine, sorafenib, 17β-estradiol, phenylpyridinium, cisplatin, resveratrol, and liraglutide, among others. In vivo studies have demonstrated that anthocyanins attenuate inflammatory responses in airway tissues of asthma animal models primarily by suppressing NF-κB signaling via the miR-138-5p/SIRT1 axis [33]. This regulatory pathway is also targeted by dexmedetomidine hydrochloride, which further inhibits apoptosis and inflammatory responses in hepatocytes. Moreover, berberine has been reported to alleviate hepatic insulin resistance through the miR-146b/SIRT1 signaling mechanism [34].
SIRT1 polymorphisms and cancer
Since cancers are complicated maladies with the involvement of both environmental and genetic parameters, different polymorphisms may play a substantial role in the onset or even exacerbation of malignant traits. In this era, SIRT1 gene polymorphisms have been reported to be of great significance (Fig. 1). SIRT1-encoding gene is located on chromosome 10q21.3 and contains 11 exons within its structure [35, 36]. Polymorphic variants of SIRT1 have been found to be correlated with a wide spectrum of disorders from obesity and myocardial infarction (MI) to multiple malignancies [37, 38]. In this regard, rs3758391 T/C and rs369274325 G/T polymorphisms that are located at the SIRT1 promoter region have been realized to be responsible for susceptibility to particular defects [39].
In the case of human neoplasms related to SIRT1 polymorphisms, urinary bladder cancer (UBC) is a well-studied malignancy to be affected by these variations. Relatively, Shafieian et al. conducted a preliminary evaluation and demonstrated that SIRT1 rs3758391 T/C and rs369274325 G/A polymorphisms might confer an elevated risk of UBC in a selected population of Iranian patients [35]. Another research team found SIRT1 polymorphisms to be correlated with breast cancer (BC). They noticed that serum SIRT1 levels were increased in BC patients, relying on the tumor grades. According to their study, SIRT1 rs3758391 and rs12778366 TT genotypes were more common in relation to histopathologic grade and lymph node status. SIRT1 rs12778366 TT genotype was also found to be linked to negative estrogen (ER) and progesterone receptors (PR). The frequency of T allele was realized to be higher for both single-nucleotide polymorphisms (SNPs) in BC patients than in normal participants. Furthermore, in association with ER and PR expression, GG genotype combined with AG genotype of rs3740051 was indicated to be more common than the AA genotype and G allele inheritance was shown to be relative to BC pathology [40]. Unlike UBC and BC, lung cancer pathology was found not to be correlated with SIRT1 polymorphisms. In this regard, patients with non-small cell lung cancer (NSCLC) were comprised and tested for four SNPs of SIRT1 gene, including rs12778366 C/T, rs3758391 C/T, rs2273773 C/T, and rs4746720 C/T. Findings suggested that the evaluated polymorphisms might have no correlation with lung cancer, as there was no significant difference of allele and genotype frequencies among control and cancer groups. Although it was supposed that lung cancer did not depend on SIRT1 SNPs, further analyses would clarify whether these polymorphisms contribute to lung cancer [41].
Future research should prioritize multiethnic cohorts to assess generalizability, functional analyses (e.g., CRISPR editing) to confirm SNP effects, and investigations into how lifestyle factors modulate risks. While SIRT1 polymorphisms show promise as biomarkers for bladder and breast cancer in specific populations, lung cancer links remain unsupported, underscoring the need for rigorous replication studies and mechanistic clarity.
SIRT1 and gut microbiota in cancer
The homeostasis of intestinal epithelia specifically relies on the existing interactions between epithelial cells, immune cells, and symbiotic gut bacteria. If these complex interconnections become disrupted, the mentioned homeostasis would be disturbed, leading to the progression of intestinal defects such as inflammatory bowel disease (IBD) or even related malignancies in worse conditions. In this context, SIRT1 deacetylase has been found as sensor for environmental stress to change the integrity of intestinal epithelia [42]. In a study conducted by Wellman et al., it was clarified that SIRT1 protein could prevent intestinal inflammation by modulating gut microbiota. Thus, SIRT1 activation might be considered as a therapeutic target for IBD, as this protein mediated the host–microbiome cross talk [42] (Fig. 1).
Colorectal cancer (CRC) is probably the most common malignancy in relation to SIRT1–gut microbiome interactions. FOXQ1 has been found to be highly expressed in CRC tissues, indicating poor prognosis of patients with CRC. Once FOXQ1 is up-regulated, CRC cells become resistant to radiation therapies, while this radio-resistant trait would be reversed concurrent with FOXQ1 suppression. Moreover, FOXQ1 up-modulates SIRT1, transcriptionally, which in turn leads to overexpression and nuclear translocation of β-catenin; in this regard, the reversal of radio-resistance in CRC cells can be explained as the result of FOXQ1 knockdown-mediated inhibition of SIRT1 protein. The inhibition of SIRT1 and β-catenin can also cause a reduction in the number of pathological bacteria in xenograft nude mice, which were increased before [43].
Notwithstanding, there are limited number of evaluations on the possible networks among SIRT1 and gut commensal bacteria in human cancers, and thus, further explorations are still needed to elucidate ambiguous aspects.
Regulation of cancer-related signaling pathways can be mediated by SIRT1
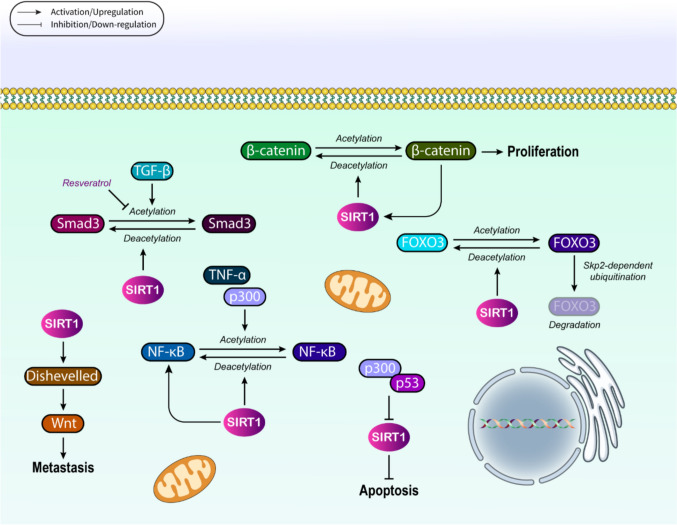
Many signaling pathways have been found to date to be in association with SIRT1 in the process of cancer onset and development (Fig. 2). p53-related signaling, along with FOXO, NF-κB, Wnt, and TGF-β pathways, is the best-known signaling mechanisms, determined to be targeted by SIRT1 during tumor growth and expansion. p53, as a crucial tumor suppressor that represents mutated pattern in a vast array of malignancies, is generally modulated at either transcriptional or posttranscriptional levels [9]. Histone acetylation/deacetylation is one of the most common modifications occurred post-transcriptionally, which is tightly regulated by SIRT1, as it triggers p53 deacetylation for further suppression of the activity of this tumor suppressor at transcriptional level [44, 45]. SIRT1-mediated modulation of p53 relies on its deacetylase activity, as wild-type SIRT1 up-regulation removes the acetylation of p53, while inactive SIRT1 fails to do so [46]. More interestingly, SIRT1 can also regulate p53 through deacetylating the p300, as a p53’s histone acetyltransferase [47]. Once p300 is acetylated, it is triggered to be attached to p53 to suppress SIRT1, thus increasing the p53 activation depending on p300 [47]. We know that the most significant role of activated p53 is in the stimulation of cell cycle stoppage and apoptosis, together with DNA damage repair. Thereby, p53 hyperacetylation triggered by SIRT1 inhibition can result in apoptosis and cell cycle arrest, demonstrating the potential of SIRT1 suppression in cancer therapy [48, 49]. Hypermethylated in cancer 1 (HIC1) as a transcriptional repressor and tumor suppressor has also been reported to interconnect with SIRT1 promoter to suppress its transcription. Once HIC1 reservoir is depleted, SIRT1 is up-modulated, and thus, p53 is deacetylated to be suppressed, leading to apoptosis evasion and tumorigenesis induction [50]. In this situation, SIRT1 suppression can reverse the provoked resistance to apoptosis. SIRT1 deacetylates p53 at lysine residues (e.g., K382), reducing its ability to bind DNA and activate pro-apoptotic targets like BAX and PUMA. Under oxidative stress, SIRT1 redirects cytosolic p53 to mitochondria via deacetylation, enabling transcription-independent apoptosis through cytochrome c release. This dual nuclear/cytosolic regulation explains SIRT1's paradoxical role as both a tumor promoter, by suppressing nuclear p53, and suppressor, by enhancing mitochondrial apoptosis [51].
Fig. 2.
Regulation of cancer-related signaling pathways can be mediated by SIRT1
In the context of FOXO signaling, the first evidence suggested that Sir2 as able to increase the longevity of Caenorhabditis elegans by regulating DAF-16, which is a homolog for human FOXO3a [52, 53]. Afterward, it was revealed that SIRT1 could operate several molecular mechanisms by deacetylating FOXO family members such as FOXO1, FOXO3a, and FOXO4 [2, 54]. FOXOs serve as physiological regulators at the upstream of cell survival and proliferation, as well as other related processes, which their impairment may result in cancer onset. Thus, FOXOs deacetylation mediated by SIRT1 results in tumorigenesis modulation through the mentioned processes [55, 56]. For instance, SIRT1-mediated deacetylation of FOXO3 triggers Skp2-dependent ubiquitination and thus degradation of FOXO3, confirming the regulatory effect of SIRT1 on FOXOs transcriptional activities [57]. Under acute nutrient deprivation, both FOXO3a and SIRT1 are overexpressed, while FOXO3a silencing blocks the expression of SIRT1 induced by starvation conditions [58]. Under such circumstances, p53 connects to FOXO3a and then induces SIRT1 at its transcriptional level. It is worth noting that the presence of p53 is necessary for SIRT1 induction in a starvation-triggered FOXO3a-dependent manner. SIRT1 deacetylates FOXO1/3a via interaction with FHL2 (a scaffolding protein), which recruits SIRT1 to FOXO transcription factors. Deacetylation exposes FOXO's nuclear export signal, sequestering it in the cytoplasm and reducing transcription of cell cycle inhibitors like p27Kip1. Conversely, during nutrient deprivation, SIRT1-FOXO3a-p53 forms a feed-forward loop where FOXO3a activation increases SIRT1 expression, creating metabolic plasticity in tumors [59]. Hence, the SIRT1-FOXO3a-p53 axis, which has been identified to be involved in aging and fasting/fed state-related mechanisms, can substantially regulate the expansion of different tumors, regarding the age-related pattern of tumor onset and development [58].
NF-κB is the other signal molecule contributed to tumorigenesis and malignant phenotypes [60, 61]. Under typical conditions, NF-κB is inhibited by IκB molecules that maintain NF-κB within the cytosol. Concurrent with cytokine induction, IκB molecules are promptly degraded following phosphorylation and ubiquitylation, and thus, NF-κB complex is transferred into the nucleus, triggering target gene transcription, as the result of reversible acetylation [62, 63]. SIRT1 and HDAC3 are principal deacetylases, being responsible for reversing NF-κB acetylation [64]; SIRT1, in this regard, not only blocks p300-mediated acetylation of NF-κB but also inhibits NF-κB acetylation triggered by TNF-α, which in turn up-regulates endothelial CD40 in a manner depending on NF-κB [65]. In addition to acetylation, NF-κB methylation is also modulated by SIRT1. It has been reported that lysine 310 to arginine (K310R) mutation as well as SIRT1 up-regulation can increase p65 methylation. Contrarily, siRNA-mediated depletion of SIRT1 blocks the methylation of RelA/p65 to mitigate its ubiquitination and increases NF-κB transcriptional activity [66]. BCA3, as the breast cancer-associated protein 3, can inhibit gene transcription mediated by NF-κB through binding to NF-κB’s p65 subunit in a process depending on neddylation [67]. In this regard, once wild-type BCA3 is up-regulated together with SENP8, a NEDD8-specific protease begins to block NF-κB transcription, while enhancing the apoptotic flux in a TNF-α-dependent manner principally by recruiting SIRT1. This finding was confirmed by a study in which siRNA-mediated knockdown of BCA3 reversed the repressive effects of this protein on NF-κB transcription [68]. Overall, SIRT1 deacetylates RelA/p65 subunit at K310, which enables RelA methylation by Set9, targeting it for proteasomal degradation, disrupts p300-mediated acetylation required for CD40 transcription in endothelial cells, and synergizes with BCA3-mediated neddylation to block NF-κB survival signals in breast cancer.
SIRT1 has been reported to be able to reverse the acetylation of β-catenin at K345, which this deacetylation disrupts the potential of β-catenin in activating transcription and cell proliferation. In parentheses, β-catenin is a part of Wnt/β-catenin signaling mechanism that is substantially modulated by PTMs, such as acetylation. This signal transduction is responsible for carcinogenesis and tumor expansion of the majority of colorectal cancers, along with breast, ovary, and prostate malignancies [69, 70]. SIRT1 also prolongs β-catenin retention within the cytosol, leading to the blockade of intestinal and colorectal tumorigenesis [71]. Amazingly, the expression of SIRT1 is regulated by β-catenin, as it has been shown that loss of β-catenin significantly lowers both mRNA and protein levels of SIRT1 in sham-treated knockout mice in comparison twith non-treated counterparts [72]. In addition to β-catenin, SIRT1 has inhibitory effects of Wnt signal transduction by regulating its inhibitor, i.e., Dickkopf Wnt signaling pathway inhibitor 1 (DKK1). Experimentally, it was found that being exposed to tobacco smoke condensate (TSC) suppressed the acetylation of H4K16, while induced trimethylation of H3K27 located on the promoter of DKK1, which can be relatively revoked by SIRT1 inhibition mediated by siRNA [73]. Other constituent of the canonical Wnt signaling is Dishevelled (Dvl), which should necessarily be present for LRP6 phosphorylation that is required for subsequent recruitment of Axin, as well as β-catenin stabilization [74, 75]. Evidence suggests that by targeting Dvl, SIRT1 is a key regulator of Wnt signaling mechanism [76, 77]. In the absence of SIRT1, Dvl proteins are significantly down-regulated, leading to a decrease in the expression levels of Wnt target gene. Furthermore, SIRT1 inhibitors can suppress cell migration induced by Wnt, demonstrating the contribution of SIRT1 to Wnt signal transduction [76]. SIRT1 deacetylates both β-catenin (at K345) and its binding partner HINT1 (at K21/K30). This modification disrupts β-catenin's nuclear translocation by enhancing E-cadherin binding at adherens junctions, and also increases HINT1's affinity for β-catenin, preventing TCF/LEF-mediated transcription of c-MYC and CYCLIN D1. Moreover, it stabilizes Dishevelled (Dvl) proteins by deacetylating their PDZ domains, paradoxically promoting Wnt signaling in specific contexts [78].
Ultimately, TGF-β and its related signaling pathway that involve in the regulation of cell growth, migration, and apoptosis are under control of SIRT1; in detail, TGF-β signaling is typically regulated by a group of inhibitory molecules, called Smads, which can be deacetylated by SIRT1 to operate the state of TGF-β signaling pathway. Smad3, Smad6, and Smad7 are the most common undergoing SIRT1-mediated deacetylation [79]. In the case of Smad7, SIRT1 has been shown to link to the N-terminal region of Smad7 for reversing the acetylation of its K64 and K70. Upon the overexpression of SIRT1, Smad7 is decreased, leading to the suppression of cell apoptosis, whereas SIRT1 inhibition increases the apoptotic flux. According to other assessments conducted in this field, SIRT1-mediated deacetylation of Smad7 induces its ubiquitination and also increases Smad ubiquitination regulatory factor 1 (Smurf1)-dependent degradation of the protein, resulting in the suppression of apoptosis mediated by TGF-β [80]. Smad3 can also be deacetylated by SIRT1; TGF-β-mediated acetylation of Smad3 is reversed by SIRT1, giving rise to the inhibition of collagen IV and fibronectin mRNA levels induced by TGF-β. Once SIRT1 is silenced, the suppressive effects of SIRT1 activators, such as resveratrol, are eliminated on Smad3 acetylation. Resveratrol blocks the acetylation of Smad3 in UUO (unilateral ureteral obstruction) cases at day two of the disease. Thus, studies have suggested that Smad3 acetylation has a substantial role in the pathogenesis of renal fibrosis, and SIRT1-mediated deacetylation of this protein can be considered a promising therapeutic approach for such disorders [81]. Smad7 deacetylation by SIRT1 at K64/K70 results in triggering Smurf1-dependent ubiquitination that reduces Smad7 stability, alleviating its inhibition of TGF-β receptor I and also enhances Smad3/4 complex formation, promoting EMT in metastatic cells. In renal fibrosis models, SIRT1 inhibitors prevent Smad3 deacetylation, blocking collagen IV overproduction [78].
All above-stated mechanisms illustrate how SIRT1's deacetylase activity serves as a molecular switch, with outcomes determined by cellular localization, binding partners (e.g., FHL2 for FOXO, BCA3 for NF-κB), and posttranslational modification cross talk (acetylation ↔ ubiquitination/methylation). Table 1 lists the most common signaling cascades that are modulated by SIRT1 through cancer development.
Table 1.
Effects of SIRT1 on key signaling pathways involved in cancer progression
| Signaling pathway | Contributing signal molecule | SIRT1’s effect | Outcome | Reference(s) |
|---|---|---|---|---|
| p53 | p53 | p53 deacetylation | ↓ p53-related tumor-suppressive activity | [46] |
| p300 | Deacetylation of p300, as a p53’s histone acetyltransferase | ↓ p53-related tumor-suppressive activity | [47] | |
| HIC1 | SIRT1-mediated deaceylation of p53 while HIC1 is depleted | ↓ p53-related tumor-suppressive activity | [50] | |
| FOXO | FOXO1, FOXO3a, and FOXO4 | Deacetylation of FOXOs | ↑ tumorigenesis | [52, 54, 58, 59] |
| NF-κB |
p300 TNF-α |
SIRT1 blocks p300-mediated acetylation of NF-κB, and inhibits NF-κB acetylation triggered by TNF-α | ↑ endothelial CD40 in a NF-κB-dependent manner | [65] |
| p65 | NF-κB methylation through SIRT1-mediated up-regulation of p65 methylation | [66] | ||
| BCA3 | WT BCA3 is up-regulated together with SENP8, a NEDD8-specific protease begins to block NF-κB transcription | ↑ apoptotic flux in a TNF-α-dependent manner | [67] | |
| Wnt/β-catenin | β-catenin | Deacetylation of β-catenin at K345 | Disruption of the potential of β-catenin in activating transcription and cell proliferation | [69] |
| β-catenin | Prolonging the β-catenin retention within the cytosol | Blockade of intestinal tumorigenesis | [71, 72] | |
| DKK1 | SIRT1 inhibits Wnt signal transduction by regulating DKK1 | [73] | ||
| Dvl | In the absence of SIRT1, Dvl proteins are significantly down-regulated | ↓ Wnt target gene expression | [76, 77] | |
| TGF-β | Smad 7 |
Deacetylation of Smad7 By reversing the acetylation of its K64 and K70 |
↓ cell apoptosis | [79] |
| Smurf1 | Deacetylation of Smad7 induces its ubiquitination and also increases Smurf1-dependent degradation of the protein | ↓ cell apoptosis | [80] | |
| Smad3 | Deacetylation of Smad3 | Inhibition of collagen IV and fibronectin mRNA levels | [81] |
Role of SIRT1 in major hallmarks being responsible for cancer onset and progression
SIRT1 and tumorigenicity
Interestingly, SIRT1 can serve as either a tumor-inducing factor or tumor suppressor relative to cancer progression. The evaluations performed on the relationship between SIRT1 and p53, as SIRT1 suppressed p53-mediated functions, were the origin of the hypothesis that SIRT1 could be considered a tumor-promoting factor. Afterward, it was consistently found that SIRT1 overexpression could inhibit cell cycle arrest and apoptosis mediated by p53 in response to DNA damage. In parallel, SIRT1 mutant subtypes carrying point mutations have also identified to be responsible for enhancing cells’ vulnerability to stress responses due to the disruption of SIRT1’s deacetylase activity [5, 82]. On the other hand, once SIRT1 is pharmacologically inhibited, p53 is hyperacetylated, and thus, p53-related transcriptional activity is up-regulated [82]. Furthermore, SIRT1 up-modulation has the ability of suppressing the expression of tumor suppressor genes/proteins, such as FOXOs, RB, p73, and Ku70, which are involved in DNA damage repair. Therefore, siRNA-mediated depletion of SIRT1 can be considered a strategy for triggering growth arrest in various cancers [83].
Otherwise, SIRT1’s expression, or even activity, is being considered to be controlled by a vast array of tumor suppressors, among which HIC1 can interconnect with SIRT1 to generate a complex that further suppresses SIRT1 promoter by directly attachment to it. In parentheses, HIC1 encodes a type of transcriptional repressor, containing zinc finger motifs, which links to p53 in order to block the age-dependent progression of cancer in mouse models of malignancies. Regarding the cross talk between p53 and HIC1, by which p53 transactivates HIC1, it has been supposed that p53, HIC1, and SIRT1 are interconnected by a circular modulatory loop, in which HIC1 first blocks SIRT1 transcription, then SIRT1 deacetylases p53, and p53 triggers HIC1 activity for further regulation of p53 functions in cell cycle development and apoptosis [84, 55, 85]. Deleted in breast cancer 1 (DBC1) is another factor that generates a stable complex with SIRT1 to block SIRT1 activity, resulting in p53 hyperacetylation and increasing p53-related activities. In this context, RNAi-mediated silencing of DBC1 consistently triggers p53 deacetylation mediated by SIRT1 and represses the apoptotic flux mediated by p53 [86]. Together, SIRT1 overexpression may enhance the risk of cancer principally by blocking the p53, as well as other potential tumor suppressors, confirming that SIRT1 can serve as a potent tumor-inducing factor [87].
Contrarily, SIRT1 is literally believed to act as a tumor suppressor, as well. In this era, studies on mice with colon cancer revealed that SIRT1 could deacetylate β-catenin and force nuclear oncogenic form of β-catenin to be transferred into cytoplasm, leading to a decrease in cancer progression. More interestingly, similar human investigations demonstrated that there was an inverse relationship between nuclear SIRT1 and oncogenic type of β-catenin. Earlier in vitro studies had also shown that aberrant up-regulation of SIRT1 could significantly decrease cell proliferation in colon cancer cell lines whose growth was conducted by β-catenin. In the same direction, SIRT1 was also found to deacetylate the NF-κB’s RelA/p65 subunit to make cells sensitive to apoptosis mediated by TNF-α through blocking the transactivation of RelA/p65 protein [88, 89].
It has been indicated that BRCA1-associated breast cancer cases expressed lower levels of SIRT1 in comparison with BRCA1-wild-type breast cancer subjects. Using molecular analyses, they further noticed that BRCA1 could attach to SIRT1 promoter to modulate SIRT-related expression at mRNA level, as well as protein levels, and thus, BRCA1 defects promoted SIRT1 under-expression, which was responsible for neoplastic transformation in BRCA1 mutant cells. In this regard, SIRT1 restoration could suppress the proliferation of BRCA1 mutant cells, as well as tumor formation in nude mice, undergoing BRCA1 mutant cell implantation [90]. All in all, it can be concluded that SIRT1 is a potent tumor suppressor relative to BRCA1 defects, and using SIRT1 activators, like resveratrol, can help oncologists to cure BRCA1-corelated breast cancers. In the context of unveiling the molecular basis of SIRT1 effects on apoptosis induction, scientists noticed that there was a negative correlation between SIRT1 and survivin through deacetylating H3K9 in its promoter; it is worth considering that survivin encodes an antiapoptotic protein [90]. Regarding all the aforementioned findings, by suppressing tumor growth and expansion through inhibiting oncogenes transcription or even oncoproteins activity, SIRT1 is considered not only a potent tumor promoter but also a powerful tumor suppressor.
To address the complex duality of SIRT1 in cancer biology, we present contextual determinants that govern its paradoxical roles as either a tumor promoter or suppressor. This functional dichotomy is shaped by dynamic interactions between the cellular microenvironment, genetic and epigenetic landscapes, and the distinct stages of disease progression.
The functional outcomes of SIRT1 activity are intricately linked to tissue-specific signaling architectures. In breast cancer, SIRT1 exhibits subtype-dependent behavior. In HER2-positive and hormone receptor-positive subtypes, it functions oncogenically by stabilizing estrogen receptor alpha (ERα) signaling and promoting resistance to aromatase inhibitors. Conversely, in triple-negative breast cancer (TNBC), SIRT1 acts as a tumor suppressor through BRCA1-mediated repression of survivin and deacetylation of PARP1 [91, 92].
Regarding prostate cancer, discrepancies in SIRT1 function between species highlight its context dependence. In human models, SIRT1 promotes tumor growth via androgen receptor stabilization, whereas in murine systems, it exhibits protective effects by regulating oxidative stress through the p63 pathway [93].
SIRT1 has also been found to inhibit gastric cancer proliferation and metastasis via STAT3/MMP‐13 signaling. In contrast, in colon cancer, it plays a suppressive role by sequestering β-catenin in the cytoplasm, thus inhibiting Wnt signaling [94].
.
Loss of BRCA1 leads to down-regulation of SIRT1, fostering genomic instability. Interestingly, pharmacological activation of SIRT1 in BRCA1-deficient models has been shown to restore replication fork stability. In breast cancer, disruption of the negative feedback loop between SIRT1 and DBC1 results in their concurrent overexpression, creating a therapeutic vulnerability that can be exploited by SIRT1 inhibitors [92].
SIRT1’s function also evolves throughout different stages of carcinogenesis. Dyring early tumorigenesis, SIRT1 suppresses genomic instability through chromatin silencing mediated by HIC1 and helps maintain stem cell quiescence via deacetylation of the Notch signaling pathway. In metastatic progression, it promotes epithelial-to-mesenchymal transition (EMT) by stabilizing the Smad3/4 transcriptional complex and enhances chemo-resistance in non-small cell lung cancer (NSCLC) by regulating the acetylation status of ABC transporters. In lung cancer, resistance to cisplatin is associated with SIRT1-mediated activation of the nucleotide excision repair (NER) pathway. Similarly, in ER-positive breast cancer, SIRT1 contributes to tamoxifen resistance via the SIRT1-FOXO1 survival axis [92, 95].
.
Together, these context-dependent variables position SIRT1 as a molecular rheostat, an integrative hub that decodes diverse oncogenic cues through its deacetylase activity. Understanding this multifaceted regulatory framework is essential for developing precision oncology strategies that effectively target SIRT1 in a manner tailored to specific tumor contexts.
SIRT1 and cancer cell proliferation
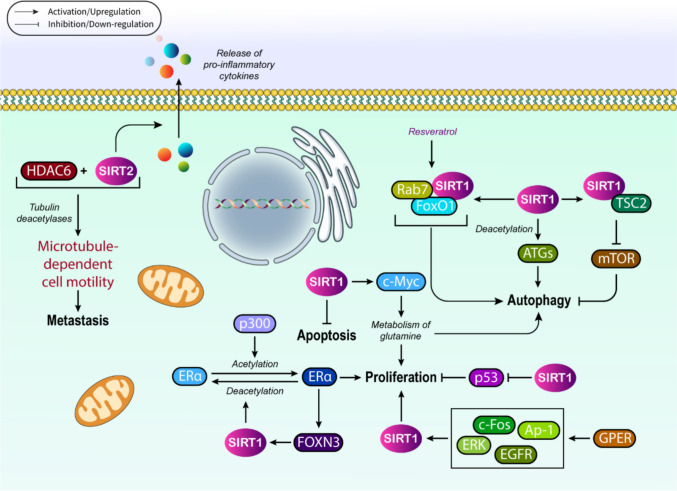
As mentioned before, SIRT1 is responsible for regulating cancer cell proliferation through multiple mechanisms. For instance, in breast cancer, SIRT1 has been found to exert its effects in association with hormone signaling cascades to control malignant cell proliferation. In this framework, estrogen receptor α (ERα) that is acetylated by p300 can be deacetylated by SIRT1 in ER+ breast cancer cells to block the signaling mechanism mediated by estrogen [96]. Moreover, ERα can also recruit the members of FOXO family to become targeted by SIRT1, as ERα links to FOXN3, and then recruits SIRT1 to stimulate deacetylation and suppression of ERα signaling in a SIRT1-dependent manner [97]. This suppression subsequently inhibits the proliferation of breast cancer cells. On the other front, in ER− breast cancer cells, hormone signaling-mediated regulation of SIRT1 is conducted by GPER, which is G protein-coupled ER (GPER) that affects estrogens and antiestrogens in either cancerous or normal cells under malignant conditions. Concurrent with E2 activation, GPER begins the induction of SIRT1 through provoking the EGFR/ERK/c-Fos/Ap-1 signaling cascade, resulting in stimulation of cancer cell survival even after DNA damage, a finding that further supports the dual role of SIRT1 as either oncogene or tumor suppressor. GPERs have also been recognized to mediate fatty acid synthase up-modulation, induced by 17-beta-estradiol, in cancer cells and cancer-associated fibroblasts (CAFs) [5, 98–100].
SIRT1 overexpression has also been realized in colon cancer and melanoma cell lines, as well as chronic leukemias [43, 101, 102]. In colon cancer, this overexpression of SIRT1 can increase c-Myc expression, while in melanoma cells, SIRT1 is being responsible for enhancing the resistance to the adhesion-induced stress through interacting with DNMT3B for further suppression of Mxd1, as the MXD dimerization protein 1, resulting in Myc hyperactivation and subsequent development of melanoma [103]. The up-regulated SIRT1 is also capable of triggering cell cycle arrest through E2F1 activation, leading to the induction of cyclins and pRb, and further inhibition of colon cancer cell proliferation [104]. In transgenic mice models of colon cancer, SIRT1 was also found to deacetylate β-catenin to repress cell proliferation [105]. More interestingly, human evaluations indicated that SIRT1 overexpression could serve as great prognostic factor for colorectal cancer [105].
On the other front, the survival and proliferation of lymphomas are controlled by a SIRT1-dependent axis with the contribution of AMP-activated protein kinase (AMPK). The amplification of SIRT1 expression enhances cell proliferation and release of pro-inflammatory cytokines, while suppresses apoptosis in B lymphocytes derived from mice [106, 107]. In parallel, it has been demonstrated that SIRT1 deletion in intestinal epithelial cells decelerates the progression of intestinal tumors in mouse models of colon cancer [108]. In AML models, interferon regulatory family 9-mediated silencing of SIRT1 provokes p53 activation to decrease cell growth [109]. Moreover, SIRT1 inhibition mediated by shRNA or small molecule inhibitors up-regulates p53 at its transcriptional levels, together with increasing mitochondrial oxidative phosphorylation in the same cells [110].
In addition, c-Myc expression induced by the deletion of one copy of SIRT1 increases the metabolism of glutamine and thus enhances cell proliferation, autophagy, and tumor expansion. On the contrary, once both copies of SIRT1 are deleted, cell apoptosis is induced, while autophagy and tumorigenesis are suppressed. Other than the mentioned malignancies, SIRT1 effects, especially dose-dependent ones, have also been realized in mouse models of skin cancer, in which SIRT1 deletion alters the cancer progression pattern, depending on whether deletion is homozygous or heterozygous [111]. Together, it can be concluded that SIRT1 involves in cancer cell proliferation, as well as tumor cell metabolism as either an oncogene or a tumor suppressor. Considering the significance of SIRT1 in cancer cell proliferation, it is highly suggested that SIRT activators, along with its inhibitors, can be very beneficial to cure different human neoplasms.
SIRT1 and autophagy
There are numerous genes and proteins within the nucleus and/or cytosol to be targeted by SIRT1, among which autophagy-related genes/proteins (Atgs/ATGs), such as ATG5, ATG7, and ATG8, are of great significance [112, 113]. It has been proposed that SIRT1 either directly or indirectly involves in autophagy stimulation in response to specific conditions cellular stress, viz. ER stress and/or oxidative stress, or nutrient deprivation [].
In depth, SIRT1 actively participates in autophagy regulation by deacetylating the ATGs; in this context, it was reported that SIRT1 overexpression, in a transient manner, could induce autophagy even in the absence of nutrient depletion. The autophagic flux was found not to be activated in SIRT−/− mouse embryonic fibroblasts experiencing starvation. In a procedural manner, SIRT1 first generates a complex with autophagy’s major components, i.e., ATG5, ATG7, and ATG8, and then catalyzes their deacetylation in an NAD-dependent mode to trigger autophagy [114]. Accordingly, acetylation/deacetylation can be considered as a crucial posttranslational modification, operating the autophagy flux.
Other than ATGs, H4K16ac, FoxO3, E2F1, p73, PPAR-γ co-activator 1α (PGC1α), S6K, NF-κB, p53, and TSC2 are the other autophagy regulators linked to SIRT1 [112]. In this regard, autophagy has been reported to be induced by resveratrol, in a SIRT1-dependent fashion, to block the proliferation of prostate cancer cells. On the contrary, SIRT1 under-expression causes a decrease in the basal levels of autophagy induced by resveratrol, principally by silencing the phosphorylation of S6K1 and 4E-BP1, as the eukaryotic initiation factor 4E-binding protein 1, which are the substrates of mammalian target of rapamycin complex 1 (mTORC1) [115]. Moreover, this amazing deacetylase has the ability of blocking inflammation through promoting autophagy, which can be exemplified by SIRT1 inactivation in THP-1 cells that stimulated inflammation, depending on NF-kB that blocked autophagy by nutrient-sensing pathways [116].
Autophagy can also be induced by SIRT1 through the FoxO1 deacetylation, especially when starvation and/or glucose deprivation circumstances exist. Evidence in this context indicates that SIRT1 enhances the autophagy activation by involving in a regulatory axis, SIRT1-FoxO1-Rab7, in which Rab7 is a small GTP-binding protein contributing to the process of autophagosome–lysosome fusion [117]. As a pathological example, autophagy is impaired in diabetes mellitus and diabetic cardiomyopathy, which can be modulated in a SIRT1-dependent mode as the results of resveratrol consumption. Once exposure to resveratrol is extended, cardiac oxidative damages are ameliorated by autophagy activation, inducing SIRT1 activity and Rab7 overexpression. Alongside, resveratrol also reversed the oxidative stress in H9C2 cells and increased the FoxO1 DNA binding at the Rab7 promoter, relying on the existence of SIRT1 [112, 118]. Overall, the role of the SIRT1-FoxO1-Rab7 regulatory axis can be highlighted in resveratrol-mediated activation of autophagy.
SIRT1 and cell migration/metastasis in cancer
We know cell migration occurs through the whole process of cancer progression and is especially central to invasion, as the initial step of metastasis. New aspect of SIRT1’s contribution to human neoplasms has been uncovered, especially through cell migration and subsequent invasion. Although several issues are still ambiguous, SIRT1/HDAC6-mediated deacetylation of cortactin, as a class II nucleation-promoting factor (NPF) responsible for binding to F-actin filaments and cofilins to affect their stability, has been accepted to be a crucial event. Cortactin acetylation/deacetylation is also considered to influence other PTMs in relation to cortactin, such as phosphorylation. Mechanistically, both SIRT1 and HDAC6 attach to the residues located in the repeat region of cortactin. In this regard, this is the relative content and activity of the deacetylases that determines which one contributes to the regulation of cortactin deacetylation in a particular cell type. Although SIRT1 and HDAC6 have ubiquitous expression patterns, they represent different degrees of activity in varied types of cancers [119, 120].
Other than SIRT1, it was interestingly determined that SIRT2 could also interact with cortactin deacetylation, as its inhibition had previously been found to be responsible for cortactin acetylation. SIRT2, along with HDAC6, acts as tubulin deacetylases and thus provokes the microtubule-dependent cell motility [121, 122]. Since HDACs have the ability of increasing cell migration and invasion by operating the regulation of actin filaments and microtubule dynamics, their abnormal modulation might result in neoplastic invasion and metastasis [123, 124]. Nonetheless, deacetylation activity of SIRT1 on tubulin is not yet fully elucidated. Regarding the significance of SIRT1-cell migration cross talk, it is worth investigating how much SIRT1-dependent deacetylation could affect the expansion of cell migration and invasion of human malignancies. Now it is an important question whether SIRT1 can be considered an ideal therapeutic target to decelerate and even block the invasion and metastatic traits. In this regard, SIRT1 inhibiting agents, as well as its activators, have been therapeutically analyzed, and their potential clinical efficacy might be an extra parameter to watch out for. Figure 3 comprehensively illustrates key cancer-related hallmarks in association with SIRT1.
Fig. 3.
SIRT1 and cancer proliferation, metastasis, and autophagy
Cancer chemo-resistance can also be controlled in a SIRT1-dependent manner
Chemo-resistance is still a challenging issue in cancer therapy that undesirably leads to failure in therapy response while using different chemotherapeutic agents. In this era, it has been importantly revealed that SIRT1 can induce this chemo-resistant trait and increase carcinogenesis, as well [10, 125]. It is mechanistically accepted that SIRT1, by deacetylating histone proteins, as well as non-histone proteins involved in modulating cancer-associated signaling mechanisms, monitors several cellular pathways from transcription to posttranslational modifications within the cytoplasm or nucleus [126, 127].
Relative to drug resistance, SIRT1 positively controls the resistant phenotype existing against chemotherapeutic drugs, which is principally derived from interconnection with FOXO proteins, together with p53 [128, 129]. The role of FOXO proteins in carcinogenesis and drug resistance depends on the state of malignancy and the contribution of other relevant molecules. FOXO1, in this regard, has been identified as the major modulator of multidrug resistance protein 1 (MDR1), a.k.a. ATP-binding cassette B1 (ABCB1), having significant roles in chemo-resistance [130, 131]. MDR1, p-glycoprotein, ABCG2, and MDR-associated protein 1 are multidrug resistant proteins, being responsible for provoking the stoppage of hydrophobic agents, such as antimetabolites and topoisomerases. Furthermore, Shin et al. noticed that AMPKα stimulates hypoxia-induced drug resistance of lung cancer cells mediated by SIRT to manage cancer cell responses against other therapies [132].
When we take a look at SIRT1 inhibition implications in chemo-resistance, we notice that it is a considerable strategy to improve cancer therapy mostly by attenuating the existing resistance against therapeutic approaches. Regarding that many chemo-drugs act by provoking cell cycle arrest and cell senescence, SIRT1 up-regulation has been found to modulate cell cycle and apoptosis, coupled with autophagy and senescence to affect mechanisms involved in chemo-resistance [133, 134]. A remarkable investigation in this field assessed an array of SIRT1 activators and inhibitors in clinical settings; it was determined that SIRT1 activation mediated by common activators such as resveratrol, SRT1720, and SRT2183 exerted positive effects on either carcinogenesis or drug resistance [135, 136]. On the other front, several SIRT1 inhibitors, including small molecule inhibitors or biological inhibitors, have been identified to be responsible for blocking SIRT1 activities, especially those involved in drug resistance. Tenovins are a well-known family of these inhibitors, acting by targeting the HDAC III. Tenovin 6 is one of these tenovins that inhibits SIRT1, and thus blocks the process of tumor growth in a relation to the p53-dependent pathway. Interestingly, it has been found that co-treatment with MDM2 inhibitors could increase the efficacy of tenovin 6 in a broad spectrum of cell lines. In line with this finding, tenovin 6-mediated suppression of SIRT1-2 has been recognized to activate p53 and provoke the apoptotic flux in ALL cells, as well as relevant clinical trials [137, 138]. Other than MDM2 inhibitors, other chemotherapeutic agents, like docetaxel, can synergize the cytotoxic effects of tenovin 6, when are used in combination with this SIRT1 inhibitor. Moreover, it was determined that co-treatment of NSCLC with tenovin 6 and metformin could efficiently suppress the expression of SIRT1, while enhancing p53 acetylation, as well as caspase 3 activity [139].
Although SIRT1 has been suggested to play substantial roles in molecular mechanisms involved in chemo-resistance, better understanding of this amazing cross talk needs more and more exploration at both preclinical and clinical stages.
Examples of SIRT1’s contribution to cancerous conditions
Leukemias, as the commonest subtypes of hematologic malignancies, have been reported to be pathogenically affected by SIRT deacetylases. Experiments conducted on leukemias have found that SIRT2 and SIRT3 are the major factors that can be considered as prognostic biomarkers for these malignancies. In acute lymphoblastic leukemia (ALL), SIRT2 is responsible for regulating tumor growth, while in chronic lymphocytic leukemia (CLL), SIRT3 is the major sitruin, serving as a modulator of reactive oxygen species (ROS) levels. SIRT3 also provides growth advantages for B-cell malignancies [140, 141]. SIRT2 is an important SIRT in cancer progression, as it participates in cell cycle control, genomic integrity, cell differentiation, metabolic networks, and autophagy [140]. SIRT2 can also control inflammasome pathways in hematopoietic stem cells (HSCs), as well as controlling oxidative stress by the NLRP3-Caspase1, being responsible for cell clearance control [142]. Acute myeloid leukemia (AML) is the other leukemia in association with SIRTs; in silico analyses and the GEPIA database say [110]. Among those SIRTs, SIRT7 has been recognized to serve as a great biomarker for monitoring the response to treatments with inhibitors of AML-related driver mutations. Moreover, SIRT3 has also a strong association with leukemogenesis [141, 143]. Beyond the previously mentioned SIRTs, SIRT1 is the most studied gene involved in a vast array of leukemias. For instance, it is considered a perfect prognostic biomarker in AML, as it has been found to be overexpressed in high-risk patients [102]. .
A 2024 study underscores that SIRT1 can either promote or inhibit leukemia cell growth depending on cellular context, emphasizing its paradoxical functions in DNA repair, metabolic regulation, and chemo-resistance [144]. In AML, SIRT1 overexpression remains a marker of poor prognosis , but 2025 findings reveal that SIRT1 inhibition (e.g., via siRNA or EX-527) induces ferroptosis in cytarabine-resistant AML cells, restoring chemo-sensitivity by elevating ROS and lipid peroxidation [145]. This positions SIRT1 as a therapeutic target to overcome drug resistance in high-risk AML. For CML, earlier work implicates SIRT1 in maintaining leukemic stem cells (LSCs), but recent preclinical models suggest that combining SIRT1 inhibitors (e.g., tenovin 6) with tyrosine kinase inhibitors (TKIs) enhances LSC eradication [146]. In DLBCL, SIRT1 amplification correlates with aggressive subtypes, and the SNP rs3758391 remains a predictor of survival [146]. A 2025 pan-cancer analysis further confirms SIRT1’s variable prognostic roles: High expression is protective in renal and glioma malignancies but risky in gastric cancer, underscoring tissue-specific mechanisms [147].
Other than leukemias, lymphomas have also been reported to be linked to SIRT1 in a p53-dependent mode. In this context, SIRT1 protein overexpression enhances p53 deacetylation, leading to its inactivation, whereas SIRT1 down-regulation that is mostly occurred due to the impact of HDAC inhibitors results in p53 acetylation and its subsequent hyperactivity, which in turn leads to cell apoptosis progression [148]. In the case of other hematologic neoplasms, i.e., myelodysplastic syndromes (MDSs), the involvement of SIRTs is less clarified. Further expression evaluation of SIRT1 in conjugation with the acetylation profile of Tet methylcytosine dioxygenase 2 (TET2) opens new windows to the role of TET2’s non-mutational mechanisms in disease progression [149]. Using the GEPIA database, it was observed that SIRT1-, SIRT3-, SIRT5- and SIRT6-coding genes were also amplified in hematological cancers such as diffuse large B cell lymphoma (DBLC) in comparison with non-cancerous tissues.
SIRT activators have also attracted much attention in blood neoplasms; for instance, cell populations can be enhanced in mice with medullary failure as the consequence of resveratrol (SIRT1 activator) administration [150]. In human multiple myeloma (MM) cell lines, resveratrol can activate the inositol-requiring enzyme 1α (IRE1α) by the XBP1 mRNA splicing and IRE1α phosphorylation. Indeed, resveratrol diminishes the DNA-binding capacity of XBP1 and enhances the enrichment of SIRT1 at the XBP1 promoter. Furthermore, it specifically suppresses the transcriptional activity of XBP1s, whereas inducing the expression levels of IRE1/XBP1 genes, involving in the pathway that control ER stress homeostasis [151]. Nonetheless, further exploitations are still needed to unveil the exact interaction between SIRTs, especially SIRT1, and hematologic malignancies.
On the other hand, and in the case of virus-mediated cancers, human papilloma virus (HPV) is one of the most common viruses leading to cancer progression if still unprevented. Interestingly, SIRT1 has been found to be a target of HPV, which affects p53 by the E6 protein of the virus. This viral protein, along with HPV E7, is up-regulated during the HPV latency-to-malignancy switching process [152]. HPV E7 has the ability of targeting SIRT1, as it stimulates the overexpression of SIRT1 in human cervical cancer (CC) cells, and also is needed to preserve abnormally high levels of SIRT1 in CC cells. Moreover, the E7 protein can stimulate site-specific histone H3 modifications and increase the expression of survivin protein [153]. HPV E7 protein seems to present its survival-promoting functions by enhancing the expression of SIRT1 to decrease p53 pro-apoptotic capabilities, regarding the SIRT1’s ability of deacetylation, and thus, SIRT1 is interconnected with the oncogenic HPVs. Importantly, SIRT1 inhibition by small molecular inhibitors can rescue HPV E7-triggered malignant transformation in human CC cells, which confirms the above-stated findings [154]. Recent work extends this mechanism to HPV + oropharyngeal cancers, where SIRT1 inhibitors (e.g., sirtinol) reverse viral oncoprotein-mediated immune evasion in murine models. For HBV/HCV-associated HCC, HBV X protein (HBx) disrupts SIRT1-β-catenin interactions, driving Wnt signaling. A 2024 study links SIRT1/AMPK axis suppression by HCV core protein to metabolic dysfunction, proposing NAD + boosters (e.g., nicotinamide riboside) as adjuvants to antiviral therapies [147].
SIRT1 also has a strong interaction with virus-mediated hepatocellular carcinomas (HCC). Epidemiological evidence clarifies the relationship between HCC and hepatitis B virus (HBV) infection, as there are approximately 0.5 billion people around the world suffering from chronic HBV infection that are at high risk of developing HCC [155, 156]. HBx, which is the HBV X protein with a negative modulatory domain and a transactivation domain at its N terminus and C terminus, respectively, was experimentally found to transform SV40-immortalized murine liver cells to promote HCC and its development [157, 158]. HBx protein can also trigger hepatocytes toward oncogenicity, i.e., hepatocellular carcinoma, through linking to multiple contributors from p53 to the histone deacetylases , particularly SIRT1 [159, 160]. Relative to SIRT1, HBx has been reported to block β-catenin activity by suppressing the existing interaction between SIRT1 and β-catenin, which in turn results in releasing the inhibitory effects of SIRT1 on this signaling molecule. In parentheses, HBx also interacts with APC (adenomatous polyposis coli) and GSK-3β (glycogen synthase kinase 3β) to protect β-catenin from degradation. Consistently, once SIRT1 is silenced by siRNAs, β-catenin expression is increased, while resveratrol-mediated activation of SIRT1 results in β-catenin down-regulation [161]. SIRT1 has also been suggested to be under-expressed in hepatitis C virus (HCV)-induced liver defects [162]. We know that HCV infection can provoke hepatic steatosis and insulin resistance, both of which exacerbate the development of CHC, as chronic hepatitis C. It was unveiled that HCV core protein exerted its effects on hepatic energy and glucose/lipid metabolism by attenuating the existing interaction between SIRT1 and AMP-activated protein kinase (AMPK). In this context, in vitro evaluations demonstrated that ROS levels were increased in HCV core protein expressing HepG2 cells, whereas SIRT1 and AMPK were reduced at both mRNA and protein expression levels. This finding suggested that HCV core protein could change cellular redox status, affecting SIRT1/AMPK activity, and thus altering the expression pattern of genes involved in glucose and lipid metabolism, which ultimately resulted in hepatic metabolic defects [162, 163]. Although the contribution of SIRT1 to the progression of virus-mediated neoplasms and defects requires further assessments, it is an accepted hypothesis that this protein deacetylase significantly participates in the onset of these cancers, as well as their expansion.
Concluding remarks and future prospects
SIRT1, the most conserved mammalian sirtuin, exerts context-dependent roles in carcinogenesis by targeting tumor suppressors, oncoproteins, and critical pathways governing proliferation, apoptosis, autophagy, metastasis, and chemo-resistance. Its dual functionality, acting as both a tumor promoter and suppressor, highlights its therapeutic potential but also underscores the complexity of targeting SIRT1 in cancer. Recent advances in SIRT1 modulators (activators and inhibitors) have intensified preclinical exploration, though clinical translation remains nascent.
SIRT1 activators (e.g., resveratrol, SRT1720, and newer small molecules) enhance deacetylase activity, potentially reactivating tumor-suppressive functions like DNA repair or metabolic regulation. Preclinical studies show promise in chemoprevention and sensitizing tumors to radiotherapy by reducing oxidative stress. However, paradoxical effects, such as SIRT1-driven metastasis suppression in breast cancer versus pro-survival effects in leukemia, demand tissue-specific dosing strategies.
SIRT1 inhibitors (e.g., EX-527, sirtinol, tenovin) block oncogenic SIRT1 activity, particularly in cancers where SIRT1 stabilizes hypoxia-inducible factor-1α (HIF-1α) or suppresses p53. EX-527, for instance, synergizes with cisplatin in ovarian cancer models by reversing chemo-resistance. Challenges include balancing on-target efficacy with systemic toxicity, as SIRT1 inhibition may impair metabolic homeostasis in normal cells.
In clinical settings, resveratrol has entered early-phase trials for cancer prevention, though poor bioavailability limits efficacy. Next-gen agents (e.g., STAC-3) with improved pharmacokinetics are under evaluation. On the other front, EX-527 is being tested in combination therapies for solid tumors (NCT04887831), while sirtuin pan-inhibitors (e.g., nicotinamide) face specificity challenges.
Dual role dilemma as SIRT1’s opposing effects in different cancers that complicate therapeutic targeting, delivery limitations which many modulators lack tissue specificity or sufficient blood–brain barrier penetration, and biomarker gaps as predictive biomarkers to identify patients likely to benefit from SIRT1 modulation are lacking are the prime challenges in investigating SIRT1’s role in cancer progression. Developing context-specific modulators guided by tumor genetics, such as TP53 status, exploring nanoparticle-based delivery to enhance selectivity, and prioritizing combination regimens such as SIRT1 inhibitors + immunotherapy to mitigate resistance would minimize the existing obstacles in the near future.
Together, SIRT1 represents a high-risk, high-reward target in oncology. While preclinical data underscore its mechanistic versatility, overcoming pharmacological and biological complexities will determine its translational success.
Acknowledgements
Not applicable.
Abbreviations
- ARF
ADP ribosylation factor
- AMPK
AMP-activated protein kinase
- ATM
Ataxia–telangiectasia mutated
- ATP
Adenosine triphosphate
- ABCB1
ATP-binding cassette B1
- DDR
DNA damage response
- DKK1
Dickkopf Wnt signaling pathway inhibitor 1
- Dvl
Dishevelled
- DNMT
DNA methyltransferase
- DSB
Double-stranded DNA break
- EZH2
Enhancer of zeste homologue 2
- FOXO
Forkhead box O
- GPER
G protein-coupled ER
- HIC1
Hypermethylated in cancer 1
- IRE1α
Inositol-requiring enzyme 1α
- LncRNA
Long noncoding RNA
- mTORC1
Mammalian target of rapamycin complex 1
- MDR1
Multidrug resistance protein 1
- MEC1
Mitosis entry checkpoint 1
- MSH2
MutS homolog 2
- NAD
Nicotinamide adenine dinucleotide
- NF-κB
Nuclear factor kappa B
- NSCLC
Non-small cell lung cancer
- NPF
Nucleation-promoting factor
- PRC4
Polycomb repressive complex 4
- PR
Progesterone receptor
- PCNA
Proliferating cell nuclear antigen
- SNP
Single-nucleotide polymorphism
- SIRT1
Sirtuin 1
- TGF-β
Transforming growth factor beta
- TSC
Tobacco smoke condensate
Author contributions
M.A, A.T, N.F, P.R, and M.E were responsible for writing—reviewing and editing, writing—original draft, resources, investigation, supervision, and conceptualization. M.H, N.Z, SZ.GD, B.P, M.A, N.S, and KS.E were involved in writing—original draft, investigation, data curation, visualization, and software. FS.K, S.C, F.A, and F.J took part in investigation and writing—reviewing and editing.
Funding
Not applicable.
Data availability
Not applicable.
Declarations
Conflict of interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Afshin Taheriazam, Email: a.taheriazam@iautmu.ac.ir.
Mina Alimohammadi, Email: mina.alimohammadi11@gmail.com.
Payman Rahimzadeh, Email: P.rahimzadeh76@gmail.com.
Najma Farahani, Email: najmafarahani@yahoo.com.
Maliheh Entezari, Email: mentezari@iautmu.ac.ir.
References
- 1.Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J, et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22(10):1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187: 111215. 10.1016/j.mad.2020.111215. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, Jia Y, et al. Regulation of SIRT1 and its roles in inflammation. Front Immunol. 2022;13: 831168. 10.3389/fimmu.2022.831168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JY, Mondaca-Ruff D, Singh S, Wang Y. SIRT1 and Autophagy: Implications in Endocrine Disorders. Front Endocrinol (Lausanne). 2022;13: 930919. 10.3389/fendo.2022.930919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Peterson LM, Li X. Trending topics of SIRT1 in tumorigenicity. Biochim Biophys Acta. 2021;1865(9): 129952. 10.1016/j.bbagen.2021.129952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves-Fernandes DK, Jasiulionis MG. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int J Mol Sci. 2019;20(13): 3153. 10.3390/ijms20133153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Bi Y, Xue L, Wang J, Lu Y, Zhang Z, et al. Multifaceted modulation of SIRT1 in cancer and inflammation. Crit Rev Oncog. 2015;20(1–2):49–64. 10.1615/critrevoncog.2014012374. [DOI] [PubMed] [Google Scholar]
- 8.Simmons GE Jr., Pruitt WM, Pruitt K. Diverse roles of SIRT1 in cancer biology and lipid metabolism. Int J Mol Sci. 2015;16(1):950–65. 10.3390/ijms16010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z, Fang D. The roles of SIRT1 in cancer. Genes Cancer. 2013;4(3–4):97–104. 10.1177/1947601912475079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousafzai NA, Jin H, Ullah M, Wang X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am J Cancer Res. 2021;11(11):5233–48. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Chen W. Emerging roles of SIRT1 in cancer drug resistance. Genes Cancer. 2013;4(3–4):82–90. 10.1177/1947601912473826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding N, Bonham EM, Hannon BE, Amick TR, Baylin SB, O’Hagan HM. Mismatch repair proteins recruit DNA methyltransferase 1 to sites of oxidative DNA damage. J Mol Cell Biol. 2016;8(3):244–54. 10.1093/jmcb/mjv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Hagan HM. Chromatin modifications during repair of environmental exposure-induced DNA damage: a potential mechanism for stable epigenetic alterations. Environ Mol Mutagen. 2014;55(3):278–91. 10.1002/em.21830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apelt K, Lans H, Scharer OD, Luijsterburg MS. Nucleotide excision repair leaves a mark on chromatin: DNA damage detection in nucleosomes. Cell Mol Life Sci. 2021;78(24):7925–42. 10.1007/s00018-021-03984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandita TK, Kumar R, Horikoshi N, Singh M, Gupta A, Misra HS, et al. Chromatin modifications and the DNA damage response to ionizing radiation. Front Oncol. 2013;2:39153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbert PB, Henikoff S. The yin and yang of histone marks in transcription. Annu Rev Genomics Hum Genet. 2021;22:147–70. 10.1146/annurev-genom-120220-085159. [DOI] [PubMed] [Google Scholar]
- 17.Sahu RK, Singh S, Tomar RS. The mechanisms of action of chromatin remodelers and implications in development and disease. Biochem Pharmacol. 2020;180: 114200. 10.1016/j.bcp.2020.114200. [DOI] [PubMed] [Google Scholar]
- 18.Manna S, Mishra J, Baral T, Kirtana R, Nandi P, Roy A, et al. Epigenetic signaling and crosstalk in regulation of gene expression and disease progression. Epigenomics. 2023;15(14):723–40. 10.2217/epi-2023-0235. [DOI] [PubMed] [Google Scholar]
- 19.Kala R, Shah HN, Martin SL, Tollefsbol TO. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent gamma-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer. 2015;15(1):672. 10.1186/s12885-015-1693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouyahya A, Mechchate H, Oumeslakht L, Zeouk I, Aboulaghras S, Balahbib A, et al. The role of epigenetic modifications in human cancers and the use of natural compounds as epidrugs: mechanistic pathways and pharmacodynamic actions. Biomolecules. 2022;12(3):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babar Q, Saeed A, Tabish TA, Pricl S, Townley H, Thorat N. Novel epigenetic therapeutic strategies and targets in cancer. Biochim Biophys Acta. 2022;1868(12): 166552. 10.1016/j.bbadis.2022.166552. [DOI] [PubMed] [Google Scholar]
- 22.Orlando G, Khoronenkova SV, Dianova II, Parsons JL, Dianov GL. ARF induction in response to DNA strand breaks is regulated by PARP1. Nucleic Acids Res. 2014;42(4):2320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontana R, Ranieri M, La Mantia G, Vivo M. Dual role of the alternative reading frame ARF protein in cancer. Biomolecules. 2019;9(3):87. 10.3390/biom9030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo J, Seong D, Lee SR, Oh DB, Song J. Post-translational regulation of ARF: perspective in cancer. Biomolecules. 2020;10(8):1143. 10.3390/biom10081143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin JY, Lu XT, Hou ML, Cao T, Tian Z. Sirtuin1-p53: a potential axis for cancer therapy. Biochem Pharmacol. 2023;212: 115543. 10.1016/j.bcp.2023.115543. [DOI] [PubMed] [Google Scholar]
- 26.Stanelle J, Pützer BM. E2F1-induced apoptosis: turning killers into therapeutics. Trends Mol Med. 2006;12(4):177–85. 10.1016/j.molmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Langsfeld ES, Bodily JM, Laimins LA. The deacetylase sirtuin 1 regulates human papillomavirus replication by modulating histone acetylation and recruitment of DNA damage factors NBS1 and Rad51 to viral genomes. PLoS Pathog. 2015;11(9): e1005181. 10.1371/journal.ppat.1005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabaie H, Salkhordeh Z, Asadi MR, Ghafouri-Fard S, Amirinejad N, Askarinejad Behzadi M, et al. Long non-coding RNA-associated competing endogenous RNA axes in T-cells in multiple sclerosis. Front Immunol. 2021;12: 770679. 10.3389/fimmu.2021.770679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Nong L, Chen ML, Gu XL, Zhao WW, Liu MH, Cheng WW. Long noncoding RNA SNHG10 sponges miR-543 to upregulate tumor suppressive SIRT1 in nonsmall cell lung cancer. Cancer Biother Radiopharm. 2020;35(10):771–5. 10.1089/cbr.2019.3334. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, He M, Chen J, Li C, Zhang Q. Long non-coding RNA SNHG7 inhibits NLRP3-dependent pyroptosis by targeting the miR-34a/SIRT1 axis in liver cancer. Oncol Lett. 2020;20:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang HG, Wang FJ, Wang Y, Zhao ZX, Qiao PF. LncRNA GAS5 inhibits malignant progression by regulating macroautophagy and forms a negative feedback regulatory loop with the miR-34a/mTOR/SIRT1 pathway in colorectal cancer. Oncol Rep. 2021;45(1):202–16. 10.3892/or.2020.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang Y, Li E, Zhang H, Zhang L, Tang Y, Wanyan Y. Silencing of lncRNA UCA1 curbs proliferation and accelerates apoptosis by repressing SIRT1 signals by targeting miR-204 in pediatric AML. J Biochem Mol Toxicol. 2020;34(3): e22435. 10.1002/jbt.22435. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Zhang M, Zhang H, Qian X, Luo L, He Z. Anthocyanins Inhibit Airway Inflammation by Downregulating the NF-κB Pathway via the miR-138-5p/SIRT1 Axis in Asthmatic Mice. Int Arch Allergy Immunol. 2022;183(5):539–51. 10.1159/000520645. [DOI] [PubMed] [Google Scholar]
- 34.Sui M, Jiang X, Sun H, Liu C, Fan Y. Berberine ameliorates hepatic insulin resistance by regulating microRNA-146b/SIRT1 pathway. Diabetes Metab Syndr Obes. 2021. 10.2147/DMSO.S313068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafieian Z, Bahari G, Hashemi M, Nakhaee A. SIRT1 gene polymorphisms are associated with urinary bladder cancer in an iranian population. Rep Biochem Mol Biol. 2019;8(2):194–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Sadeghi, M.B., Nakhaee, A., Saravani, R. et al. SIRT1 functional polymorphisms (rs12778366, rs3758391) as genetic biomarkers of susceptibility to type 2 diabetes mellitus in Iranians: a case-control study and computational analysis. Int J Diabetes Dev Ctries. 2021;41:447–55. 10.1007/s13410-020-00898-1. [Google Scholar]
- 37.Yamac AH, Uysal O, Ismailoglu Z, Erturk M, Celikten M, Bacaksiz A, Kilic U. Premature myocardial infarction: genetic variations in SIRT1 affect disease susceptibility. Cardiol Res Pract. 2019;2019:8921806. 10.1155/2019/8921806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilic U, Gok O, Bacaksiz A, Izmirli M, Elibol-Can B, Uysal O. SIRT1 gene polymorphisms affect the protein expression in cardiovascular diseases. PLoS ONE. 2014;9(2): e90428. 10.1371/journal.pone.0090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohtavinejad N, Nakhaee A, Harati H, Poodineh J, Afzali M. SIRT1 gene is associated with cardiovascular disease in the Iranian population. Egypt J Med Hum Genet. 2015;16(2):117–22. [Google Scholar]
- 40.Rizk SM, Shahin NN, Shaker OG. Association between SIRT1 gene polymorphisms and breast cancer in Egyptians. PLoS ONE. 2016;11(3): e0151901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv Y, Lin S, Peng F. SIRT1 gene polymorphisms and risk of lung cancer. Cancer Manag Res. 2017. 10.2147/CMAR.S142677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wellman AS, Metukuri MR, Kazgan N, Xu X, Xu Q, Ren NSX, et al. Intestinal epithelial Sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology. 2017;153(3):772–86. 10.1053/j.gastro.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang M, Liu Q, Dai M, Peng R, Li X, Zuo W, et al. FOXQ1-mediated SIRT1 upregulation enhances stemness and radio-resistance of colorectal cancer cells and restores intestinal microbiota function by promoting beta-catenin nuclear translocation. J Exp Clin Cancer Res. 2022;41(1):70. 10.1186/s13046-021-02239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahhal R, Seto E. Emerging roles of histone modifications and HDACs in RNA splicing. Nucleic Acids Res. 2019;47(10):4911–26. 10.1093/nar/gkz292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin. 2010;60(6):376–92. 10.3322/caac.20085. [DOI] [PubMed] [Google Scholar]
- 46.Yuan F, Wang J, Li R, Zhao X, Zhang Y, Liu B, et al. A new regulatory mechanism between P53 and YAP crosstalk by SIRT1 mediated deacetylation to regulate cell cycle and apoptosis in A549 cell lines. Cancer Manag Res. 2019. 10.2147/CMAR.S214826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Lee JT, Gu W. SIRT1: Regulator of p53 Deacetylation. Genes Cancer. 2013; 4(3–4):112–7. 10.1177/1947601913484496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu B, Zhang D, Wang X, Lin D, Chen Y, Xu X. Targeting SIRT1 to inhibit the proliferation of multiple myeloma cells. Oncol Lett. 2021;21(4):306. 10.3892/ol.2021.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Feng Y, Guo Q, Guo W, Xu H, Li X, et al. SIRT1 modulates cell cycle progression by regulating CHK2 acetylation-phosphorylation. Cell Death Differ. 2020;27(2):482–96. 10.1038/s41418-019-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005; 123(3):437–48. 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 2010;1804(8):1684–9. 10.1016/j.bbapap.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Y, Yan F, Feng Z, Lazarovici P, Zheng W. Signaling network of Forkhead family of transcription factors (FOXO) in dietary restriction. Cells. 2019;9(1):100. 10.3390/cells9010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–30. 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 54.Dilmac S, Kuscu N, Caner A, Yildirim S, Yoldas B, Farooqi AA, Tanriover G. SIRT1/FOXO signaling pathway in breast cancer progression and metastasis. Int J Mol Sci. 2022;23(18): 10227. 10.3390/ijms231810227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halasa M, Adamczuk K, Adamczuk G, Afshan S, Stepulak A, Cybulski M, Wawruszak A. Deacetylation of transcription factors in carcinogenesis. Int J Mol Sci. 2021;22(21): 11810. 10.3390/ijms222111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li K, Luo J. The role of SIRT1 in tumorigenesis. N Am J Med Sci (Boston). 2011;4(2):104–6. 10.7156/v4i2p104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene. 2012;31(12):1546–57. 10.1038/onc.2011.347. [DOI] [PubMed] [Google Scholar]
- 58.Fasano C, Disciglio V, Bertora S, Lepore Signorile M, Simone C. FOXO3a from the nucleus to the mitochondria: a round trip in cellular stress response. Cells. 2019;8(9):1110. 10.3390/cells8091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24(5):1021–32. 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang T, Ma C, Zhang Z, Zhang H, Hu H. NF-kappaB signaling in inflammation and cancer. MedComm. 2020;2(4):618–53. 10.1002/mco2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. The nuclear factor kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021;8(3):287–97. 10.1016/j.gendis.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-kappaB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5(1):209. 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verzella D, Cornice J, Arboretto P, Vecchiotti D, Di Vito NM, Capece D, et al. The NF-kappaB Pharmacopeia: Novel Strategies to Subdue an Intractable Target. Biomedicines. 2022;10(9):2233. 10.3390/biomedicines10092233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–80. 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Zhang J, Yan C, Zhou J, Lin R, Lin Q, et al. SIRT1 regulates CD40 expression induced by TNF-alpha via NF-kB pathway in endothelial cells. Cell Physiol Biochem. 2012;30(5):1287–98. 10.1159/000343318. [DOI] [PubMed] [Google Scholar]
- 66.Yang X-D, Tajkhorshid E, Chen L-F. Functional interplay between acetylation and methylation of the RelA subunit of NF-κB. Mol Cell Biol. 2010;30(9):2170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao F, Cheng J, Shi T, Yeh ET. Neddylation of a breast cancer-associated protein recruits a class III histone deacetylase that represses NFκB-dependent transcription. Nat Cell Biol. 2006;8(10):1171–7. [DOI] [PubMed] [Google Scholar]
- 68.Zhou L, Jia L. Targeting Protein Neddylation for Cancer Therapy. Adv Exp Med Biol. 2020;1217:297–315. 10.1007/978-981-15-1025-0_18. [DOI] [PubMed] [Google Scholar]
- 69.You H, Li Q, Kong D, Liu X, Kong F, Zheng K, Tang R. The interaction of canonical Wnt/beta-catenin signaling with protein lysine acetylation. Cell Mol Biol Lett. 2022;27(1):7. 10.1186/s11658-021-00305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao C, Xiao G, Hu J. Regulation of Wnt/β-catenin signaling by posttranslational modifications. Cell Biosci. 2014; 4(1):13. 10.1186/2045-3701-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bian J, Dannappel M, Wan C, Firestein R. Transcriptional Regulation of Wnt/beta-Catenin Pathway in Colorectal Cancer. Cells. 2020;9(9):2125. 10.3390/cells9092125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. 10.1038/s41392-021-00762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hussain M, Rao M, Humphries AE, Hong JA, Liu F, Yang M, et al. Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res. 2009;69(8):3570–8. 10.1158/0008-5472.CAN-08-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeong W, Jho EH. Regulation of the low-density lipoprotein receptor-related protein LRP6 and its association with disease: Wnt/beta-catenin signaling and beyond. Front Cell Dev Biol. 2021;9: 714330. 10.3389/fcell.2021.714330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunach M, Del Valle-Perez B, Garcia de Herreros A. p120-catenin in canonical Wnt signaling. Crit Rev Biochem Mol Biol. 2017;52(3):327–39. 10.1080/10409238.2017.1295920. [DOI] [PubMed] [Google Scholar]
- 76.Holloway KR, Calhoun TN, Saxena M, Metoyer CF, Kandler EF, Rivera CA, Pruitt K. SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc Natl Acad Sci U S A. 2010;107(20):9216–21. 10.1073/pnas.0911325107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma M, Castro-Piedras I, Simmons GE Jr., Pruitt K. Dishevelled: a masterful conductor of complex Wnt signals. Cell Signal. 2018;47:52–64. 10.1016/j.cellsig.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jung TY, Jin GR, Koo YB, Jang MM, Kim CW, Lee SY, et al. Deacetylation by SIRT1 promotes the tumor-suppressive activity of HINT1 by enhancing its binding capacity for beta-catenin or MITF in colon cancer and melanoma cells. Exp Mol Med. 2020;52(7):1075–89. 10.1038/s12276-020-0465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chien L-H, Deng J-S, Jiang W-P, Chou Y-N, Lin J-G, Huang G-J. Evaluation of lung protection of Sanghuangporus sanghuang through TLR4/NF-κB/MAPK, keap1/Nrf2/HO-1, CaMKK/AMPK/Sirt1, and TGF-β/SMAD3 signaling pathways mediating apoptosis and autophagy. Biomed Pharmacother. 2023;165: 115080. [DOI] [PubMed] [Google Scholar]
- 80.Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isshiki K, et al. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem. 2007;282(1):151–8. 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- 81.Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177(3):1065–71. 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee JT, Gu W. SIRT1: regulator of p53 deacetylation. Genes Cancer. 2013;4(3–4):112–7. 10.1177/1947601913484496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinha S, Sharma S, Vora J, Shrivastava N. Emerging role of sirtuins in breast cancer metastasis and multidrug resistance: implication for novel therapeutic strategies targeting sirtuins. Pharmacol Res. 2020;158: 104880. 10.1016/j.phrs.2020.104880. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Cooper TK, Zahnow CA, Overholtzer M, Zhao Z, Ladanyi M, et al. Epigenetic and genetic loss of Hic1 function accentuates the role of p53 in tumorigenesis. Cancer Cell. 2004;6(4):387–98. 10.1016/j.ccr.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 85.Tseng RC, Lee CC, Hsu HS, Tzao C, Wang YC. Distinct HIC1-SIRT1-p53 loop deregulation in lung squamous carcinoma and adenocarcinoma patients. Neoplasia. 2009; 11(8):763–70. 10.1593/neo.09470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008; 451(7178):583–6. 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 87.Patil MR, Bihari A. A comprehensive study of p53 protein. J Cell Biochem. 2022;123(12):1891–937. 10.1002/jcb.30331. [DOI] [PubMed] [Google Scholar]
- 88.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004; 23(12):2369–80. 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ashburner BP, Westerheide SD, Baldwin AS Jr. The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001; 21(20):7065–77. 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32(1):11–20. 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carafa V, Altucci L, Nebbioso A. Dual tumor suppressor and tumor promoter action of sirtuins in determining malignant phenotype. Front Pharmacol. 2019;10:38. 10.3389/fphar.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sung JY, Kim R, Kim JE, Lee J. Balance between SIRT1 and DBC1 expression is lost in breast cancer. Cancer Sci. 2010;101(7):1738–44. 10.1111/j.1349-7006.2010.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilking MJ, Ahmad N. The role of SIRT1 in cancer: the saga continues. Am J Pathol. 2015;185(1):26–8. 10.1016/j.ajpath.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S, Yang Y, Huang S, Deng C, Zhou S, Yang J, Cao Y, Xu L, Yuan Y, Yang J, Chen G, Zhou L, Lv Y, Wang L, Zou X. SIRT1 inhibits gastric cancer proliferation and metastasis via STAT3/MMP-13 signaling. J Cell Physiol. 2019; 234(9):15395–406. 10.1002/jcp.28186. [DOI] [PubMed] [Google Scholar]
- 95.Zhang T, Rong N, Chen J, Zou C, Jing H, Zhu X, Zhang W. SIRT1 expression is associated with the chemotherapy response and prognosis of patients with advanced NSCLC. PLoS ONE. 2013;8(11): e79162. 10.1371/journal.pone.0079162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim MY, Woo EM, Chong YTE, Homenko DR, Kraus WL. Acetylation of estrogen receptor α by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20(7):1479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu Z, Yang Y, Li B, Li Y, Xia K, Yang Y, et al. Checkpoint suppressor 1 suppresses transcriptional activity of ERalpha and breast cancer cell proliferation via deacetylase SIRT1. Cell Death Dis. 2018;9(5):559. 10.1038/s41419-018-0629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10(1):47–60. 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 99.Santolla MF, Avino S, Pellegrino M, De Francesco E, De Marco P, Lappano R, et al. SIRT1 is involved in oncogenic signaling mediated by GPER in breast cancer. Cell Death Dis. 2015;6(7):1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santolla MF, Lappano R, De Marco P, Pupo M, Vivacqua A, Sisci D, et al. G protein-coupled estrogen receptor mediates the up-regulation of fatty acid synthase induced by 17beta-estradiol in cancer cells and cancer-associated fibroblasts. J Biol Chem. 2012;287(52):43234–45. 10.1074/jbc.M112.417303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilking MJ, Singh C, Nihal M, Zhong W, Ahmad N. SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation. Arch Biochem Biophys. 2014; 563:94–100. 10.1016/j.abb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fajardo-Orduna GR, Ledesma-Martinez E, Aguiniga-Sanchez I, Weiss-Steider B, Santiago-Osorio E. Role of SIRT1 in chemoresistant leukemia. Int J Mol Sci. 2023;24(19): 14470. 10.3390/ijms241914470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meliso FM, Micali D, Silva CT, Sabedot TS, Coetzee SG, Koch A, et al. SIRT1 regulates Mxd1 during malignant melanoma progression. Oncotarget. 2017;8(70):114540–53. 10.18632/oncotarget.21457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fang Z, Lin M, Li C, Liu H, Gong C. A comprehensive review of the roles of E2F1 in colon cancer. Am J Cancer Res. 2020;10(3):757–68. [PMC free article] [PubMed] [Google Scholar]
- 105.Jia X, Liu H, Ren X, Li P, Song R, Li X, et al. Nucleolar protein NOC4L inhibits tumorigenesis and progression by attenuating SIRT1-mediated p53 deacetylation. Oncogene. 2022;41(39):4474–84. 10.1038/s41388-022-02447-y. [DOI] [PubMed] [Google Scholar]
- 106.He M, Tan B, Vasan K, Yuan H, Cheng F, Ramos da Silva S, et al. SIRT1 and AMPK pathways are essential for the proliferation and survival of primary effusion lymphoma cells. J Pathol. 2017;242(3):309–21. 10.1002/path.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Q, Yan C, Xin M, Han L, Zhang Y, Sun M. Sirtuin 1 (Sirt1) overexpression in BaF3 cells contributes to cell proliferation promotion, apoptosis resistance and pro-inflammatory cytokine production. Med Sci Monit. 2017;23:1477–82. 10.12659/msm.900754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lo Sasso G, Ryu D, Mouchiroud L, Fernando SC, Anderson CL, Katsyuba E, et al. Loss of Sirt1 function improves intestinal anti-bacterial defense and protects from colitis-induced colorectal cancer. PLoS ONE. 2014;9(7): e102495. 10.1371/journal.pone.0102495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian W-L, Guo R, Wang F, Jiang Z-X, Tang P, Huang Y-M, Sun L. The IRF9-SIRT1-P53 axis is involved in the growth of human acute myeloid leukemia. Exp Cell Res. 2018;365(2):185–93. [DOI] [PubMed] [Google Scholar]
- 110.Abraham A, Qiu S, Chacko BK, Li H, Paterson A, He J, et al. SIRT1 regulates metabolism and leukemogenic potential in CML stem cells. J Clin Invest. 2019;129(7):2685–701. 10.1172/JCI127080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ming M, Soltani K, Shea CR, Li X, He YY. Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene. 2015;34(3):357–63. 10.1038/onc.2013.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiu G, Li X, Che X, Wei C, He S, Lu J, et al. SIRT1 is a regulator of autophagy: implications in gastric cancer progression and treatment. FEBS Lett. 2015;589(16):2034–42. [DOI] [PubMed] [Google Scholar]
- 113.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1(1): e10-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu Y, Wan W. Acetylation in the regulation of autophagy. Autophagy. 2023;19(2):379–87. 10.1080/15548627.2022.2062112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li G, Rivas P, Bedolla R, Thapa D, Reddick RL, Ghosh R, Kumar AP. Dietary resveratrol prevents development of high-grade prostatic intraepithelial neoplastic lesions: involvement of SIRT1/S6K axis. Cancer Prev Res (Phila). 2013;6(1):27–39. 10.1158/1940-6207.CAPR-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D. SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochem Biophys Res Commun. 2012;427(1):191–6. [DOI] [PubMed] [Google Scholar]
- 117.Luo G, Jian Z, Zhu Y, Zhu Y, Chen B, Ma R, et al. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int J Mol Med. 2019;43(5):2033–43. 10.3892/ijmm.2019.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qu X, Chen X, Shi Q, Wang X, Wang D, Yang L. Resveratrol alleviates ischemia/reperfusion injury of diabetic myocardium via inducing autophagy. Exp Ther Med. 2019;18(4):2719–25. 10.3892/etm.2019.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186(4):571–87. 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun J, Tai S, Tang L, Yang H, Chen M, Xiao Y, et al. Acetylation modification during autophagy and vascular aging. Front Physiol. 2021;12: 598267. 10.3389/fphys.2021.598267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim JY, Hwang HG, Lee JY, Kim M, Kim JY. Cortactin deacetylation by HDAC6 and SIRT2 regulates neuronal migration and dendrite morphogenesis during cerebral cortex development. Mol Brain. 2020;13(1): 105. 10.1186/s13041-020-00644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaur S, Rajoria P, Chopra M. HDAC6: a unique HDAC family member as a cancer target. Cell Oncol. 2022;45(5):779–829. [DOI] [PubMed] [Google Scholar]
- 123.Khwaja S, Kumar K, Das R, Negi AS. Microtubule associated proteins as targets for anticancer drug development. Bioorg Chem. 2021;116: 105320. [DOI] [PubMed] [Google Scholar]
- 124.Peng J, Xie F, Qin P, Liu Y, Niu H, Sun J, et al. Recent development of selective inhibitors targeting the HDAC6 as anti-cancer drugs: structure, function and design. Bioorg Chem. 2023. 10.1016/j.bioorg.2023.106622. [DOI] [PubMed] [Google Scholar]
- 125.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins–novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7(10):841–53. 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 126.Hsu YC, Hsieh YH, Liao CC, Chong LW, Lee CY, Yu YL, Chou RH. Targeting post-translational modifications of histones for cancer therapy. Cell Mol Biol (Noisy-le-grand). 2015;61(6):69–84. [PubMed] [Google Scholar]
- 127.Xia C, Tao Y, Li M, Che T, Qu J. Protein acetylation and deacetylation: An important regulatory modification in gene transcription (Review). Exp Ther Med. 2020;20(4):2923–40. 10.3892/etm.2020.9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–5. 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 129.Yaman D, Takmaz T, Yuksel N, Dincer SA, Sahin FI. Evaluation of silent information regulator T (SIRT) 1 and Forkhead Box O (FOXO) transcription factor 1 and 3a genes in glaucoma. Mol Biol Rep. 2020;47(12):9337–44. 10.1007/s11033-020-05994-3. [DOI] [PubMed] [Google Scholar]
- 130.Chakrabarti P, English T, Karki S, Qiang L, Tao R, Kim J, et al. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J Lipid Res. 2011;52(9):1693–701. 10.1194/jlr.M014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ioannilli L, Ciccarone F, Ciriolo MR. Adipose tissue and FoxO1: bridging physiology and mechanisms. Cells. 2020;9(4): 849. 10.3390/cells9040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin DH, Choi YJ, Park JW. SIRT1 and AMPK mediate hypoxia-induced resistance of non-small cell lung cancers to cisplatin and doxorubicin. Cancer Res. 2014;74(1):298–308. 10.1158/0008-5472.CAN-13-2620. [DOI] [PubMed] [Google Scholar]
- 133.Jang J, Huh YJ, Cho HJ, Lee B, Park J, Hwang DY, Kim DW. SIRT1 enhances the survival of human embryonic stem cells by promoting DNA repair. Stem Cell Rep. 2017;9(2):629–41. 10.1016/j.stemcr.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maiese K. Moving to the rhythm with clock (circadian) genes, autophagy, mTOR, and SIRT1 in degenerative disease and cancer. Curr Neurovasc Res. 2017;14(3):299–304. 10.2174/1567202614666170718092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ren Y, Du C, Shi Y, Wei J, Wu H, Cui H. The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int J Mol Med. 2017;39(5):1317–24. 10.3892/ijmm.2017.2931. [DOI] [PubMed] [Google Scholar]
- 136.Huber JL, McBurney MW, Distefano PS, McDonagh T. SIRT1-independent mechanisms of the putative sirtuin enzyme activators SRT1720 and SRT2183. Future Med Chem. 2010;2(12):1751–9. 10.4155/fmc.10.257. [DOI] [PubMed] [Google Scholar]
- 137.Jin Y, Cao Q, Chen C, Du X, Jin B, Pan J. Tenovin-6-mediated inhibition of SIRT1/2 induces apoptosis in acute lymphoblastic leukemia (ALL) cells and eliminates ALL stem/progenitor cells. BMC Cancer. 2015;15(1):226. 10.1186/s12885-015-1282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Levavasseur F, Oussous S, Zubaidan T, Kosmider O, Pendino F, Rombaut D, et al. FOXP1 regulates oxidative stress, SIRT1 expression, and resistance to chemotherapies in acute myeloid leukemia cells. Blood Adv. 2023;7(13):3265–75. 10.1182/bloodadvances.2022008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hirai S, Endo S, Saito R, Hirose M, Ueno T, Suzuki H, et al. Antitumor effects of a sirtuin inhibitor, tenovin-6, against gastric cancer cells via death receptor 5 up-regulation. PLoS ONE. 2014;9(7): e102831. 10.1371/journal.pone.0102831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goes JVC, Carvalho LG, de Oliveira RTG, Melo MML, Novaes LAC, Moreno DA, et al. Role of Sirtuins in the Pathobiology of Onco-Hematological Diseases: A PROSPERO-Registered Study and In Silico Analysis. Cancers (Basel). 2022;14(19):4611. 10.3390/cancers14194611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu W, Denu RA, Krautkramer KA, Grindle KM, Yang DT, Asimakopoulos F, et al. Loss of SIRT3 provides growth advantage for B cell malignancies. J Biol Chem. 2016;291(7):3268–79. 10.1074/jbc.M115.702076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kasbekar M, Mitchell CA, Proven MA, Passegué E. Hematopoietic stem cells through the ages: a lifetime of adaptation to organismal demands. Cell Stem Cell. 2023. 10.1016/j.stem.2023.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma J, Liu B, Yu D, Zuo Y, Cai R, Yang J, Cheng J. SIRT3 deacetylase activity confers chemoresistance in AML via regulation of mitochondrial oxidative phosphorylation. Br J Haematol. 2019;187(1):49–64. 10.1111/bjh.16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu Z, Lyu T, Wu L, Yang H, Li W. The role of SIRT1 in leukemia. Curr Treat Options Oncol. 2024;25(10):1283–8. 10.1007/s11864-024-01265-6. [DOI] [PubMed] [Google Scholar]
- 145.Kong Q, Liang Q, Tan Y, Luo X, Ling Y, Li X, et al. Induction of ferroptosis by SIRT1 knockdown alleviates cytarabine resistance in acute myeloid leukemia by activating the HMGB1/ACSL4 pathway. Int J Oncol. 2025. 10.3892/ijo.2024.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang FT, Sun J, Zhang L, He X, Zhu YH, Dong HJ, et al. Role of SIRT1 in hematologic malignancies. J Zhejiang Univ Sci B. 2019;20(5):391–8. 10.1631/jzus.B1900148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Q, Sun S, Zhou C, Xu H. Comprehensive analysis of the prognostic, immunological, and diagnostic roles of SIRT1 in pan-cancer and its validation in KIRC. Front Immunol. 2024;15:1501867. 10.3389/fimmu.2024.1501867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hassell KN. Histone deacetylases and their inhibitors in cancer epigenetics. Diseases. 2019;7(4): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun J, He X, Zhu Y, Ding Z, Dong H, Feng Y, et al. SIRT1 activation disrupts maintenance of myelodysplastic syndrome stem and progenitor cells by restoring TET2 function. Cell Stem Cell. 2018;23(3):355–69. 10.1016/j.stem.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Song XR, Cheng YQ, Su DF, Liu AJ. The Sirt1 activator resveratrol improved hematopoiesis in pancytopenia mice induced by irradiation. J Pharmacol Sci. 2019;140(1):79–85. 10.1016/j.jphs.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 151.Limonta P, Moretti RM, Marzagalli M, Fontana F, Raimondi M, Montagnani Marelli M. Role of endoplasmic reticulum stress in the anticancer activity of natural compounds. Int J Mol Sci. 2019;20(4): 961. 10.3390/ijms20040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vats A, Trejo-Cerro O, Thomas M, Banks L. Human papillomavirus E6 and E7: What remains? Tumour Virus Res. 2021;11: 200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Wang J, Liu C, Li M. Silent information regulator 1 promotes proliferation, migration, and invasion of cervical cancer cells and is upregulated by human papillomavirus 16 E7 oncoprotein. Gynecol Obstet Invest. 2022;87(1):22–9. [DOI] [PubMed] [Google Scholar]
- 154.Chen J, Chen H, Pan L. SIRT1 and gynecological malignancies (Review). Oncol Rep. 2021;45(4):1–11. 10.3892/or.2021.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gomes C, Wong RJ, Gish RG. Global perspective on hepatitis B virus infections in the era of effective vaccines. Clin Liver Dis. 2019;23(3):383–99. 10.1016/j.cld.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 156.Ponde RAA. Expression and detection of anti-HBs antibodies after hepatitis B virus infection or vaccination in the context of protective immunity. Arch Virol. 2019;164(11):2645–58. 10.1007/s00705-019-04369-9. [DOI] [PubMed] [Google Scholar]
- 157.Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020;182: 104925. 10.1016/j.antiviral.2020.104925. [DOI] [PubMed] [Google Scholar]
- 158.Chaturvedi VK, Singh A, Dubey SK, Hetta HF, John J, Singh MP. Molecular mechanistic insight of hepatitis B virus mediated hepatocellular carcinoma. Microb Pathog. 2019;128:184–94. 10.1016/j.micpath.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 159.Zhao J, Gray SG, Greene CM, Lawless MW. Unmasking the pathological and therapeutic potential of histone deacetylases for liver cancer. Expert Rev Gastroenterol Hepatol. 2019;13(3):247–56. 10.1080/17474124.2019.1568870. [DOI] [PubMed] [Google Scholar]
- 160.Xia H, Huang Z, Wang Z, Liu S, Zhao X, You J, et al. Glucometabolic reprogramming: from trigger to therapeutic target in hepatocellular carcinoma. Front Oncol. 2022;12: 953668. 10.3389/fonc.2022.953668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Q, Cheng ST, Chen J. HBx mediated increase of SIRT1 contributes to HBV-related hepatocellular carcinoma tumorigenesis. Int J Med Sci. 2020;17(12):1783–94. 10.7150/ijms.43491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu JW, Sun LJ, Liu W, Zhao YH, Kang P, Yan BZ. Hepatitis C virus core protein induces hepatic metabolism disorders through down-regulation of the SIRT1-AMPK signaling pathway. Int J Infect Dis. 2013; 17(7):e539-45. 10.1016/j.ijid.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 163.Zhu Y, Liu R, Shen Z, Cai G. Combination of luteolin and lycopene effectively protect against the “two-hit” in NAFLD through Sirt1/AMPK signal pathway. Life Sci. 2020;256: 117990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.