Abstract
Objective:
To investigate the involvement of oxidative and apoptotic mechanisms in the possible neuroprotective effect of Kaempferide (KPD) and Norbergenin (NRG) against AlCl3-induced cognitive shutdown in rats.
Introduction:
Aluminium chloride (AlCl3) is widely known as a neurotoxic agent that induces memory and cognitive shutdown via induction of oxidative stress and apoptosis. KPD is an O-methylated flavonol that possesses anti-oxidant, anti-inflammatory, anti-dementia and anti-depression properties, whereas NRG, a demethylated compound derived from bergenin, possesses an anti-oxidant property and has neuroprotective effects. Both alleviate D-galactose-induced neurotoxicity in rats.
Methods:
Eighty-four male Wistar rats were randomly divided into two experimental models: prophylactic (pre-treatment with donepezil, KPD or NRG; n = 42) and curative (post-treatment with donepezil, KPD, or NRG; n = 42). In each of these models, the animals were divided into seven groups (n = 6 per group): group 1 (normal saline), group 2 (200 mg/kg AlCl3), group 3 (donepezil + AlCl3), group 4 (5 mg/kg KPD + AlCl3), group 5 (10 mg/kg KPD + AlCl3), group 6 (5 mg/kg NRG + AlCl3) and group 7 (10 mg/kg NRG + AlCl3)
Results:
Kaempferide and Norbergenin averted the increase in TBARS, NO and AChE, and decrease in the number of crossings, time spent and distance moved in the target quadrant, latency of fall, speed, paw withdrawal threshold (PWT), SOD, CAT, GPx, GR and GSH induced by AlCl3. These agents also averted the upregulation of Aβ1-41, p-Tau, caspase-3, Bax and downregulation of Akt, p-CREB, SOD1 and BCl-2 induced by AlCl3
Conclusion:
The neuroprotective effects of KPD and NRG against AlCl3-induced Aβ accumulation and cognitive shutdown are mediated via suppression of oxidative stress and apoptosis.
Keywords: Aluminium chloride, Amyloid-β protein, Apoptosis, Kaempferide, Neurocognition, Norbergenin, Oxidative stress
Introduction
Aluminium, one of the most abundant metals on earth, may enter the human body through drinking water, cooking utensils, food, cosmetics, pharmacological agents, etc.1,2 Aluminium-treated animal model is considered an excellent experimental approach to studying clinical neurodegenerative diseases [NDDs] 3 because it can cross the blood-brain barrier and accumulate in various brain regions like the hippocampus and the cerebral cortex that are associated with the learning and memory formation processes, 4 leading to inflammation and neuronal loss. 5 Its accumulation in the brain is also known to contribute to NDDs like Parkinson disease and Alzheimer’s disease (AD) by eliciting neuronal oxidative stress. 6 In fact, ‘aluminium hypothesis’ of AD was proposed in the 1960s to underscore aluminium as an environmental contributor to the pathogenesis of AD based on various analytical, neurotoxicological and epidemiological findings. The first report of aluminium poisoning-induced memory disorder in humans was in 1921, 7 and it was later shown in animal models that its intracerebral administration induces epilepsy. 8 More than 20,000 persons exposed to accidental contamination of drinking water by aluminium in 1988 in Camelford (Cornwall, UK) exhibited various symptoms of cerebral impairments like short-term memory and loss of concentration in a 10-year follow-up study. 9 A similar association of aluminium poisoning and dementia has been documented in England and Wales, 10 Norway, 11 and Canada. 12
It is established that the formation of intracellular neurofibrillary tangle due to hyperphosphorylated tau protein and extracellular deposition of amyloid beta (Aβ) are the two hallmarks of AD.13–15 Oxidative stress, which is caused by elevation of free radicals and suppression of anti-oxidants, has been associated with the progression of AD as it promotes Aβ deposit, hyperphosphorylated tau levels, cognitive impairment, 16 and neuronal apoptosis.17,18 Interestingly, the use of traditional medicine and dietary supplements with anti-oxidant properties for the prevention and treatment of dementia and other NDDs has gained scientific attention in recent times.19–21 Kaempferide (KPD) is an O-methylated flavonol that is isolated from extensive natural sources such as fruits (Apple, Grapes), vegetables (Broccoli, tomato, cabbage), herbs (dill, tarragon) and other natural plants. It has been reported to possess anti-oxidant, anti-inflammatory, anti-dementia and anti-depression properties.22,23 Norbergenin (NRG, from Bergenia stracheyi) is also a demethylated form of bergenin with a very strong anti-oxidant property, which has been associated with its neuroprotective effect. 24
D-galactose and aluminium induce neurotoxicity through different mechanisms to the extent that combination of both agents has been proposed as the ideal experimental model of inducing AD in animals 25 For instance, D-galactose is a reducing sugar that forms advance glycation end products (AGEs) by reacting with free amines of amino acids in the peptides and proteins, thereby inducing changes that mimic natural aging processes in animals, including cognitive impairment, 26 oxidative stress, 27 decreased immune response, 28 increased acetylcholinesterase (AChE) level 29 and transcriptional changes. 30 Aluminium-induced neurotoxicity has been linked to its ability to affect fast and slow neuronal transports, inhibit long-term potentiation (LTP), induce inflammatory responses, structural and synaptic abnormalities, expression of beta amyloid precursor protein (APP), and deposition of Aβ plaque on the brain cells. 31
While KPD and NRG have been reported to alleviate D-galactose-induced neurotoxicity in rats. 32 it is not known if these agents could alleviate aluminium-induced neurotoxicity, especially as their mechanisms for cognitive decline are different. It is also not known if these agents would be suitable to prevent cognitive shutdown (prophylaxis) or ameliorate it (curative) amidst aluminium challenge, as this information would provide useful pharmacokinetic insights. This study investigated the involvement of oxidative and apoptotic mechanisms in the neuroprotective effect of Kaempferide and Norbergenin against aluminium chloride-induced cognitive impairment in rats.
Methods
Sample size
The sample size of the animal grouping was done following the procedure detailed in Charan and Kantharia. 33 The doses of the drugs are consistent with previous study. 32
Animals and their treatment
Eighty-four (84) male adult Wistar rats weighing between 120 and 180 g were used in the studies. The animals were obtained from the animal house at the Council of Scientific and Industrial Research (CSIR), Hyderabad, Telangana, India, and kept at the animal house under a standard environment (room temperature 24–27°C and 60% humidity with 12-h light and dark cycles). Laboratory pellets (20% protein, 5% fat and 1% multivitamins) and free access to water were provided to the animals.
Experimental protocols
After a week of acclimation, the animals were randomly divided into two experimental models: prophylactic (n = 42) and curative (n = 42). In the prophylactic model, the animals were divided into seven groups (n = 6 per group). Group 1 (Control group) received normal saline every day throughout the study. Groups 2–7 received a single dose of 200 mg/kg AlCl3 34 once daily for 45 days. Group 3 was pre-treated with donepezil (3 mg/kg) 30 min before the AlCl3 challenge, while groups 4 and 5 were pre-treated with 5 mg/kg and 10 mg/kg KPD (Sigma Aldrich, USA), respectively, for a week prior to the 45-day challenge with AlCl3. Groups 6 and 7 were pre-treated with 5 mg/kg and 10 mg/kg NRG (ChemScene chemicals, NJ, USA) 35 respectively, for a week prior to the 45-day challenge with AlCl3.
Similarly, the animals in the curative model were divided into seven groups (n = 6 per group). Group 1 (Control group) received normal saline every day throughout the study. Groups 2–7 received a single dose of 200 mg/kg AlCl3 once daily for 45 days. Group 3–7 were post-treated with donepezil (3 mg/kg), 5 mg/kg KPD, 10 mg/kg KPD, 5 mg/kg NRG and 10 mg/kg NRG respectively for 15 days after 45-day challenge with AlCl3.
Morris water maze (MWM) test
The MWM test was done as previously described.25,36 The animals were tested with a single spatial probe trial to see if it would use a spatial learning technique to find the platform. Two trails on the same day before the probe test. After the 60 s were up, the platform was taken out of the water tank and the animal was released to swim around the tank at will. As part of the probe trail assessment of rats’ spatial learning and memory abilities, we recorded the number of times the rats crossed the platform, the length of time they spent in the target quadrant and the distance they covered within the target quadrant.
Rota-Rod test
One widely used test for evaluating the extent to which rats with AD can coordinate their movements, balance, learning and performance is the Rota-Rod test. Motor deficits can arise from neurodegenerative processes and may not be fully captured by cognitive tests alone. A 5-station accelerating Rota-Rod was used to assess locomotor coordination in this experiment. The rats were given 2 min of practice on the Rota-Rod at a speed of 4–24 rpm for the training session. After a 20-min rest, the rats underwent the identical training procedure once more. Each rat participated in 10 trials during the testing period; 5 trials were conducted daily, with 30-min breaks between each trial. Each test lasted 10 min, and the speed increased from 4 to 24 rpm in 5 min. The infrared beam was broken during the fall, and the Rota-Rod system captured the data immediately (Med Associates, SOF-ENV-575). 25
Mechanical hyperalgesia (Randall-Selitto paw pressure test)
The brain’s central nociceptive pathways and regions can be affected by structural lesions brought on by dementia. Alzheimer’s disease common symptom is dementia. By measuring mechanical hyperalgesia, we can assess the interaction between cognitive decline and sensory processing, potentially uncovering how pain sensitivity may affect behaviour and quality of life in Neurodegenerative patients. Therefore, the Randall-Selitto Paw Pressure Test is the ideal model to evaluate AD’s influence on nociceptive pathways. The mechanical nociceptive threshold, an indicator of mechano hyperalgesia, was measured. The Randall-Selitto paw pressure instrument was used to measure the nociceptive flexion reflex. The nociceptive threshold was measured by having the subject withdraw their rear paw.25,37
Brain tissue sampling and preparation
Thiopental was used to provide a little sedation in the rats, and they were euthanised via cervical dislocation. Each rat’s brain was frozen in ice and dissected from the olfactory bulb to the cerebellum. After being rinsed in isotonic saline, it was left to dry on filter paper. Each brain was cut in half along its sagittal axis. The first component (the right hemisphere) was frozen at −80°C for later biochemical study. The remaining section (the left hemisphere) was fixed in 10% formalin for immunostaining of tissues. 25
Biochemical parameters
The first step in preparing a 10% brain homogenate was homogenising the first component of the brain with PBS in a quantity 10 times the weight of the tissue, centrifuging the homogenate at 10,000 × g for 15 min at 4°C, and collecting the supernatant. Using the Bradford assay, we calculated the approximate protein concentrations in the samples. Using commercially available kits and following the directions for the appropriate methods described, we measured the level of Bcl-2 and AChE by ELISA and the levels of TBARS and NO by colourimetric assays.
A blue-coloured potassium-biuret complex is formed when the carbonyl group of protein complexes combines potassium and copper reagents to assay the total protein content. The tyrosine and phenolic chemicals in the protein work with this combination to decrease the phospho-molybdate of the folin reagent, increasing the colour of the solution (Lowry method). To precipitate the protein, 100 mg (wet weight) of brain tissue homogenate was mixed in 10% Trichloroacetic acid (TCA). The sample was centrifuged at 3000 rpm for 5 min. The upper layer was removed. In 1N NaOH, the precipitate was dissolved again. This was mixed well with adding 5 ml of reagent C and allowed to sit uninterrupted for 10 min. This was then stirred with 0.5 ml of Folin phenol reagent and set aside for 30 min. 1% BSA and 1N NaOH were used as standard and blank, respectively. The generated blue colour was measured with a UV-visible double beam bio spectrophotometer (Elico), an autoanalyser) at 650 nm, which automatically quantifies the protein content in the sample.
To assay the oxidative stress parameters, the catalase (CAT) concentration was calculated by monitoring the amount of hydrogen peroxide (H2O2) produced and consumed after the enzyme reaction for 120 s at 20-s intervals at 340 nm. The capacity of glutathione reductase (GR) to catalyse the reduction of glutathione in the presence of NADPH was used as a surrogate for GR activity. Using the enzyme’s capacity to oxidise GSH to GSSG (oxidised glutathione), we could infer GPx activity in the brain. In the presence of NADPH, GR recycled the GSSG to its reduced form, with its removal being detected at 340 nm. 25
Histological investigation
The brains of the sacrificial rats were utilised for histopathological analysis after being fixed in 10% buffered saline formalin. Brain tissues were preserved for 24 h in 10% formol saline before examination. After being washed with tap water, the samples were dehydrated using a series of dilutions of methyl, ethyl and absolute ethyl alcohol. To preserve specimens, they were washed with xylene and then immersed in paraffin at 56°C in a hot air oven for 24 h. Tissue blocks of paraffin beeswax were then sectioned using a slide microtome at a thickness of 4 µm. Tissue samples were taken on glass slides, deparaffinised and stained by Congo red stains to examine histology and detect amyloidal protein plaques in brain regions using a light electric microscope.25,38
Western blot analysis
The Lowry technique was used to isolate proteins from brain homogenate and determine their concentration. Proteins were analysed by Western blotting, including amyloid β1-42, phosphorylated Tau (p-Tau), Bax, Caspase-3, Bcl-2, catalase, superoxide dismutase-1 and β-actin. The following primary antibodies purchased from Novus Biologicals Centennial, USA, were used: anti-Aβ1-42 and anti-p-Tau, mouse monoclonal caspase-3 p17 (1:1000; Santa Cruz Biotechnology), anti-Bax (1:400), anti-Bcl-2 (1:400), anti-actin (1:50,000). Goat anti-rabbit secondary antibody and horseradish peroxidase-conjugated anti-mouse antibody (1:20,000) were utilised as appropriate secondary antibodies. The membranes were then rinsed in TBST 3 times for 15 min each time. Western blotting kit (Thermo Fisher Scientific 96 coated well plate) was utilised for protein band visualisation by chemiluminescence. GelQuant.NET, a programme made available by biochemlabsolutions.com, was then used to analyse the protein band density. Before performing statistical analysis, the calculated density was normalised with respect to the density of the housekeeping total actin bands. 25
Inclusion and exclusion criteria
Not applicable
Ethics approval
The PGP Life Sciences approved the study with reference No. PGP/RM-0019/57-21. All animal treatments were carried out in compliance with the CPCSEA guidelines.
Statistical analysis
Graph-pad Prism 8.0 software was employed for the data analysis. Data are expressed as mean ± SEM and analysed with one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test. Statistical significance was accepted at p < 0.05.
Results
Kaempferide and Norbergenin did not change the aluminium chloride-induced increase in escape latency and path length during MWM test
Throughout the assessment periods in the MWM test of both prophylactic and curative models, AlCl3 increased the escape latency period and path length, which were sustained by pre- or post-treatment with Kaempferide and Norbergenin. This was evident as the effects produced by these agents in AlCl3-challenged rats were comparable to those seen in the AlCl3 + vehicle group for both parameters (Figure 1(a)–(d)).
Figure 1.
Prophylactic and curative treatments of rats with Kaempferide and Norbergenin did not change aluminium chloride-induced increase in escape latency and path length during Morris water maze test. A = Escape latency for prophylactic group; B = Escape latency for curative group; C = Path length for prophylactic group; D = Path length for curative group.
*p < 0.05 versus control.
Kaempferide and Norbergenin attenuate aluminium chloride-induced decrease in probe tail parameters during MWM test
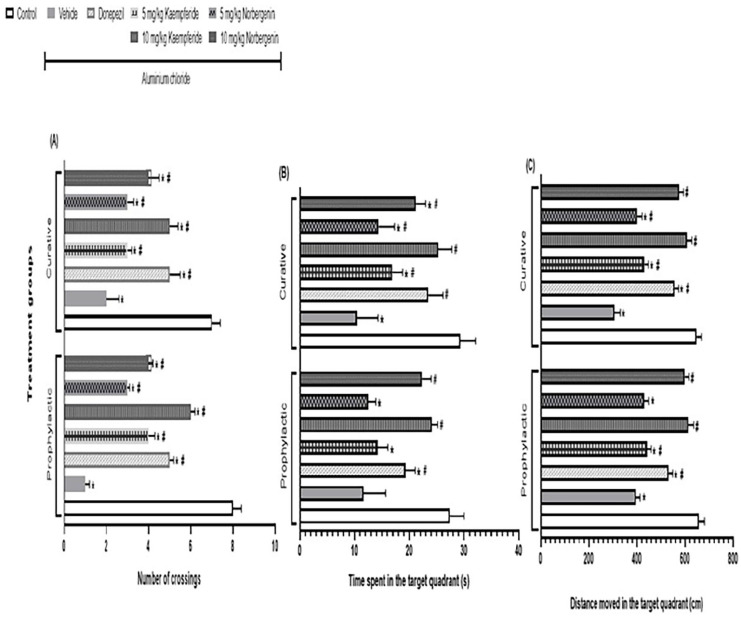
In the prophylactic model, AlCl3 decreased (p < 0.05) the number of crossings (1.0 ± 0.2 vs 8.0 ± 0.4), time spent (11.6 ± 4.1 vs 27.4 ± 2.6 s) and the distance moved (394.5 ± 18.4 vs 657.4 ± 23.6 cm) in the target quadrant. However, pre-treatment of rats with either 5 mg/kg or 10 mg/kg Kaempferide attenuated these AlCl3-induced effects by increasing (p < 0.05) number of crossings (4.0 ± 0.3; 6.0 ± 0.2; Figure 2(a)), time spent (14.2 ± 1.9; 24.1 ± 1.1 s; Figure 2(b)) and distance moved (441.7 ± 17.7; 612.5 ± 23.5 cm; Figure 2(c)) respectively in the target quadrant. Similarly, the attenuations of these AlCl3-induced effects were observed in rats pre-treated with either 5 mg/kg or 10 mg/kg Norbergenin, as they had increased (p < 0.05) number of crossings (3.0 ± 0.1; 4.0 ± 0.2; Figure 2(a)), time spent (12.5 ± 1.4; 22.3 ± 1.7 s; Figure 2(b)) and distance moved (429.8 ± 19.1; 598.6 ± 18.1 cm; Figure 2(c)) respectively in the target quadrant. The attenuations of AlCl3-induced effects by Kaempferide or Norbergenin were comparable (p > 0.05) to the effects of donepezil on number of crossings (5.0 ± 0.2; Figure 2(a)), time spent (19.3 ± 1.8 s; Figure 2(b)) and distance moved (532.1 ± 16.9 cm; Figure 2(c)) in the target quadrant.
Figure 2.
Prophylactic and curative treatments of rats with Kaempferide and Norbergenin attenuate aluminium chloride-induced decrease in probe tail parameters during Morris water maze test. A = Number of crossings; B = Time spent in the target quadrant; C = Distance moved in the target quadrant.
*p < 0.05 versus control; #p < 0.05 versus Aluminium chloride + vehicle group.
In the curative model, the pattern is similar to what was observed in the prophylactic model. For instance, AlCl3 decreased (p < 0.05) the number of crossings (2.0 ± 0.6 vs 7.0 ± 0.4), time spent (10.4 ± 3.9vs 29.3 ± 2.8 s) and the distance moved (305.6 ± 25.2 vs 647.5 ± 21.1 cm) in the target quadrant. However, post-treatment with either 5 mg/kg or 10 mg/kg Kaempferide attenuated these AlCl3-induced effects by increasing (p < 0.05) number of crossings (3.0 ± 0.2; 5.0 ± 0.4; Figure 2(a)), time spent (16.9 ± 1.9 s; 25.3 ± 2.5 s; Figure 2(b)) and distance moved (431.2 ± 16.9 cm; 606.8 ± 21.9 cm; Figure 2(c)) respectively in the target quadrant. Similarly, the attenuations of these AlCl3-induced effects were observed in rats post-treated with either 5 mg/kg or 10 mg/kg Norbergenin, as they had increased (p < 0.05) number of crossings (3.0 ± 0.3; 4.0 ± 0.5; Figure 2(a)), time spent (14.4 ± 2.9 s; 21.2 ± 1.8 s; Figure 2(b)) and distance moved (401.2 ± 20.4 cm; 574.8 ± 18.9 cm; Figure 2(c)) respectively in the target quadrant. The attenuations of AlCl3-induced effects by Kaempferide and Norbergenin were comparable (p > 0.05) to the effects of donepezil on number of crossings (5.0 ± 0.5; Figure 2(a)), time spent (23.5 ± 2.7 s; Figure 2(b)) and distance moved (556.7 ± 17.2 cm; Figure 2(c)) in the target quadrant.
Kaempferide and Norbergenin attenuate aluminium chloride-induced decrease in neurobehaviours during Rotarod and Randall Selitto tests
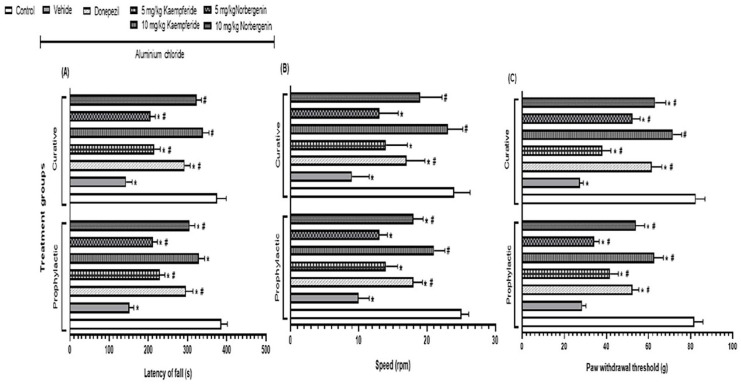
In the prophylactic model, AlCl3 decreased (p < 0.05) the latency of fall (151.4 ± 11.6 vs 386.1 ± 15.4 s), the speed (10.0 ± 1.5 vs 25.0 ± 1.1 rpm) and the paw withdrawal threshold (28.4 ± 1.9 vs 81.7 ± 4.2 g) of rats. However, pre-treatment with either 5 mg/kg or 10 mg/kg Kaempferide attenuated these AlCl3-induced effects by increasing (p < 0.05) the latency of fall (229.4 ± 12.6 s;328.6 ± 14.7 s; Figure 3(a)), speed (14.0 ± 1.7 rpm; 21.0 ± 1.6 rpm; Figure 3(b)) and paw withdrawal threshold (41.8 ± 3.8 g; 62.6 ± 4.5 g; Figure 3(c)). Similarly, the attenuation of AlCl3-induced effects was observed in rats pre-treated with either 5 mg/kg or 10 mg/kg Norbergenin, as they had increased (p < 0.05) latency of fall (211.7 ± 10.9 s; 305.3 ± 12.8 s; Figure 3(a)), speed (13.0 ± 1.2 rpm; 18.0 ± 1.4 rpm; Figure 3(b)) and paw withdrawal threshold (34.1 ± 2.5 g; 53.9 ± 4.2 g; Figure 3(c)) respectively. The attenuations of AlCl3-induced effects by Kaempferide or Norbergenin were comparable (p > 0.05) to the effects of donepezil on the latency of fall (295.2 ± 18.3 s; Figure 3(a)), speed (18.0 ± 1.3 rpm; Figure 3(b)) and paw withdrawal threshold (52.3 ± 3.1 g; Figure 3(c)).
Figure 3.
Prophylactic and curative treatments of rats with Kaempferide and Norbergenin attenuate aluminium chloride-induced decrease neuro-behaviours during Rotarod and Randall selitto tests. A = Latency of fall; B = Speed; C = Paw withdrawal threshold.
*p < 0.05 versus control; #p < 0.05 versus Aluminium chloride + vehicle group.
In the curative model, the pattern is similar to what was observed in the prophylactic model. For instance, AlCl3 decreased (p < 0.05) the latency of fall (143.1 ± 15.2 vs 375.2 ± 23.6 s), the speed (9.0 ± 2.5 vs 24.0 ± 2.3 rpm) and the paw withdrawal threshold (27.3 ± 1.8 vs 82.4 ± 4.4 g) of rats. However, both post-treatment with either 5 mg/kg or 10 mg/kg Kaempferide attenuated these AlCl3-induced effects by increasing (p < 0.05) the latency of fall (214.8 ± 15.8 s; 339.5 ± 14.7 s; Figure 3(a)), speed (14.0 ± 3.1 rpm; 23.0 ± 2.2 rpm; Figure 3(b)) and paw withdrawal threshold (38.1 ± 4.1 g; 71.5 ± 4.2 g; Figure 3(c)). Similarly, the attenuation of AlCl3-induced effects were observed in rats pre-treated with either 5 mg/kg or 10 mg/kg Norbergenin, as they had increased (p < 0.05) latency of fall (205.2 ± 12.1 s; 322.6 ± 12.9 s; Figure 3(a)), speed (13.0 ± 2.8 rpm; 19.0 ± 3.2 rpm; Figure 3(b)) and paw withdrawal threshold (52.4 ± 3.5 g; 63.1 ± 5.1 g; Figure 3(c)) respectively. The attenuation of AlCl3-induced effects by Kaempferide or Norbergenin were comparable (p > 0.05) to the effects of donepezil on the latency of fall (291.9 ± 14.7 s; Figure 3(a)), speed (17.0 ± 2.7 rpm; Figure 3(b)) and paw withdrawal threshold (61.6 ± 4.7 g; Figure 3(c)).
Kaempferide and Norbergenin attenuate aluminium chloride-induced oxidative stress in rats
In the prophylactic model, AlCl3 decreased (p < 0.05) total protein (4.41 ± 0.3 vs 14.33 ± 1.5 mg), SOD (519.5 ± 25.6 vs 973.2 ± 21.5 nmol/mg protein), GSH (0.14 ± 0.01 vs 0.35 ± 0.02 nmol/mg protein), CAT (0.21 ± 0.03 vs 0.67 ± 0.02 U/mg protein), GPx (0.89 ± 0.05 vs 1.53 ± 0.12 µmol/mg protein) and GR (2.94 ± 0.3 vs 9.46 ± 0.4 µmol/mg protein) but increased (p < 0.05) TBARS (2.93 ± 0.13 vs 1.28 ± 0.11 nmol/mg protein), NO (2.29 ± 0.08 vs 1.25 ± 0.12 nmol/mg protein) and AChE (8.31 ± 0.5 vs 1.43 ± 0.2 U/g protein) in rats. However, pre-treatment with either 5 mg/kg or 10 mg/kg Kaempferide attenuated these AlCl3-induced effects by increasing (p < 0.05) the total protein (9.66 ± 0.4; 12.54 ± 1.4 mg), SOD (684.5 ± 21.3; 911.9 ± 27.8 nmol/mg protein), GSH (0.22 ± 0.04; 0.31 ± 0.03 nmol/mg protein), CAT (0.35 ± 0.03; 0.59 ± 0.05 U/mg protein), GPx (1.12 ± 0.04; 1.42 ± 0.05 µmol/mg protein) and GR (4.21 ± 0.4 vs 8.57 ± 0.3 µmol/mg protein) but decreasing the TBARS (2.12 ± 0.12; 1.51 ± 0.19 nmol/mg protein), NO (1.93 ± 0.13; 1.48 ± 0.04 nmol/mg protein) and AChE (5.12 ± 0.6; 2.31 ± 0.5 U/g protein) in rats. Similarly, pre-treatment with either 5 mg/kg or 10 mg/kg Norbergenin attenuated these AlCl3-induced effects by increasing (p < 0.05) the total protein (8.25 ± 0.7; 11.04 ± 0.9 mg), SOD (635.2 ± 26.4; 821.8 ± 24.7 nmol/mg protein), GSH (0.18 ± 0.05; 0.26 ± 0.01 nmol/mg protein), CAT (0.31 ± 0.02; 0.52 ± 0.03 U/mg protein), GPx (1.06 ± 0.03; 1.28 ± 0.07 µmol/mg protein) and GR (4.06 ± 0.6; 7.12 ± 0.4 µmol/mg protein) but decreasing the TBARS (2.23 ± 0.21; 1.73 ± 0.23 nmol/mg protein), NO (2.01 ± 0.07; 1.55 ± 0.12 nmol/mg protein) and AChE (5.57 ± 0.4; 2.83 ± 0.2 U/g protein) in rats. The attenuations of AlCl3-induced effects by Kaempferide or Norbergenin were comparable (p > 0.05) to the effects of donepezil on the total protein (10.35 ± 0.6 mg), SOD (878.2 ± 24.8 nmol/mg protein), GSH (0.28 ± 0.03 nmol/mg protein), CAT (0.45 ± 0.04 U/mg protein), GPx (1.31 ± 0.13 µmol/mg protein) and GR (6.87 ± 0.2 µmol/mg protein) but decreasing the TBARS (2.04 ± 0.18 nmol/mg protein), NO (1.76 ± 0.06 nmol/mg protein) and AChE (3.96 ± 0.7 U/g protein) in rats (Figure 4(a)–(i)).
Figure 4.
Prophylactic and curative treatments of rats with Kaempferide and Norbergenin attenuate aluminium chloride-induced oxidative stress. A = total protein level; B = thiobarbituric acid reactive substances level; C = nitric oxide level; D = acetylcholinesterase activity; E = superoxide dismutase activity; F = glutathione level; G = catalase activity; H = glutathione peroxidase reductase; I = glutathione reductase activity.
*p < 0.05 versus control; #p < 0.05 versus Aluminium chloride + vehicle group.
In the curative model, AlCl3 decreased (p < 0.05) total protein (5.14 ± 0.8 vs 14.69 ± 1.5 mg), SOD (524.1 ± 21.2 vs 975.5 ± 25.4 nmol/mg protein), GSH (0.14 ± 0.02 vs 0.33 ± 0.03 nmol/mg protein), CAT (0.24 ± 0.05 vs 0.59 ± 0.03 U/mg protein), GPx (0.82 ± 0.01 vs 1.51 ± 0.17 µmol/mg protein) and GR (2.73 ± 0.5 vs 9.42 ± 0.3 µmol/mg protein) but increased (p < 0.05) TBARS (3.21 ± 0.23 vs 1.33 ± 0.12 nmol/mg protein), NO (2.23 ± 0.05 vs 1.24 ± 0.04 nmol/mg protein) and AChE (8.29 ± 0.4vs 1.42 ± 0.3 U/g protein) in rats. However, pre-treatment with either 5 mg/kg or 10 mg/kg Kaempferide attenuated these AlCl3-induced effects by increasing (p < 0.05) the total protein (9.17 ± 0.8; 13.84 ± 1.1 mg), SOD (677.3 ± 19.4; 891.6 ± 26.7 nmol/mg protein), GSH (0.20 ± 0.01; 0.29 ± 0.02 nmol/mg protein), CAT (0.35 ± 0.06; 0.53 ± 0.02 U/mg protein), GPx (1.08 ± 0.16; 1.43 ± 0.05 µmol/mg protein) and GR (3.86 ± 0.4; 7.83 ± 0.3 µmol/mg protein) but decreasing the TBARS (2.44 ± 0.12;1.59 ± 0.17 nmol/mg protein), NO (1.85 ± 0.03; 1.33 ± 0.07 nmol/mg protein) and AChE (4.72 ± 0.5;2.49 ± 0.6 U/g protein) in rats. Similarly, pre-treatment with either 5 mg/kg or 10 mg/kg Norbergenin attenuated these AlCl3-induced effects by increasing (p < 0.05) the total protein (8.76 ± 0.8;12.75 ± 1.6 mg), SOD (634.5 ± 25.7; 855.1 ± 17.4 nmol/mg protein), GSH (0.17 ± 0.04; 0.23 ± 0.03 nmol/mg protein), CAT (0.31 ± 0.05; 0.48 ± 0.07 U/mg protein), GPx (1.02 ± 0.08;1.34 ± 0.03 µmol/mg protein) and GR (3.79 ± 0.2;7.31 ± 0.4 µmol/mg protein) but decreasing the TBARS (2.65 ± 0.21;1.87 ± 0.12 nmol/mg protein), NO (1.89 ± 0.09;1.55 ± 0.11 nmol/mg protein) and AChE (4.92 ± 0.3; 3.06 ± 0.2 U/g protein) in rats. The attenuations of AlCl3-induced effects by Kaempferide or Norbergenin were comparable (p > 0.05) to the effects of donepezil on the total protein (11.39 ± 0.3 mg), SOD (841.6 ± 23.6 nmol/mg protein), GSH (0.25 ± 0.02 nmol/mg protein), CAT (0.46 ± 0.04 U/mg protein), GPx (1.34 ± 0.02 µmol/mg protein) and GR (6.89 ± 0.6 µmol/mg protein) but decreasing the TBARS (2.06 ± 0.24 nmol/mg protein), NO (1.67 ± 0.07 nmol/mg protein) and AChE (3.11 ± 0.2 U/g protein) in rats (Figure 4(a)–(i)).
Kaempferide and Norbergenin altered the expressions of cognition-associated proteins in AlCl3-treated rats
According to behavioural parameter results, the best effective dose for KPD and NRG is 10 mg/kg rather than 5 mg/kg. Therefore, we only select high doses for western blot analysis. AlCl3 increased the expression of Aβ, Tau, caspase-3 and BAX (pro-apoptotic protein) but decreased the expression of Akt, p-CREB, SOD1 and Bcl-2. However, Kaempferide and Norbergenin at 10 mg/kg averted this trend, as they downregulated the expressions of Aβ, Tau, caspase-3 and BAX but upregulated the expressions of Akt, p-CREB, SOD1, Bcl-2 (anti-apoptotic protein) in AlCl3-treated rats (Figures 5 and S1).
Figure 5.

Effect of Kaempferide and Norbergenin on the expression of proteins associated with cognition in rats challenged with aluminium chloride.
AC: aluminium chloride; PC: positive control (donepezil); Kpd: Kaempferide; Nrg: Norbergenin.
Kaempferide and Norbergenin attenuated AlCl3-induced Aβ plaques accumulation in rat brain
AlCl3-induced neurodegenerative models were analysed for Aβ deposition by staining the brain tissue homogenates with Congo red dye in the prophylactic group. AlCl3-treated rats developed significant multifocal Aβ deposits in the form of red plaques in the brain, while pre-treatment with donepezil showed mild dense plaques. KPD and NRG at 10 mg/kg treated groups showed better results than KPD and NRG at 5 mg/kg. Furthermore, Norbergenin moderately reduced Aβ plaque development, while the Kaempferide-treated group showed virtually no Aβ plaque development (Figure 6). Compared to AlCl3-treated animals, tissue sections from animals treated with AlCl3/Donepezil showed a modest to good tissue regeneration effect with mild dense plaques. As observed in the donepezil group, treatment with NRG at 10 mg/kg bw resulted in moderate amyloid beta plaque development reduction. Compared to the AlCl3-treated group, the KPD-treated group showed virtually no amyloid beta plaque development, indicating that KPD had a more significant impact than the donepezil effect demonstrated in the G3 group. According to this study, KPD can reduce the neurotoxicity caused by amyloid beta plaques.
Figure 6.
Effect of Kaempferide and Norbergenin on the expression of proteins associated with cognition in rats challenged with aluminium chloride.
AC: aluminium chloride; PC: positive control (donepezil); Kpd: Kaempferide; Nrg: Norbergenin.
Discussion
In this study, AlCl3 increased the escape latency and path length but decreased the number of crossings, time spent and distance moved in the target quadrant, latency of fall, speed and PWT. This is slightly contrary to some reports that showed no significant effect of AlCl3 on memory and learning abilities (escape latency and time spent in the target quadrant) and object recognition ability (time spent in exploring novel and familiar objects, discrimination index).20,39 However, it agrees with previous studies that reported that aluminium induces learning disorders and memory deficits and inhibits long-term potentiation (LTP).40-42 Thus, the well-established impairment of memory by aluminium is also confirmed in this study. Our study further showed that KPD and NRG avert AlCl3-induced cognitive shutdown, as these agents decreased the escape latency and path length but increased the number of crossings, time spent and distance moved in the target quadrant, latency of fall, speed and PWT. Our observation is consistent with the previous report that demonstrated that KPD reversed Aβ1-42-, 43 AlCl3 42 and D-galactose 38 induced neurocognitive shutdown in rodents.
Aluminium, even at nanomolar concentration, is known to bind negatively charged ligands like DNA and RNA phosphate groups to alter their topology and influence the expression of various genes essential for brain functions. 44 It also alters the expression of neurofilament and tubulin, nerve growth factor and brain-derived neurotrophic factor, 45 pro-inflammatory and pro-apoptotic genes, 46 amyloid precursor protein (APP), anti-oxidant genes, 47 neprilysin (NEP) 48 and β-APP secretases. 49 It also induces non-enzymatic phosphorylation and accumulation of Tau,50,51 accumulation of Aβ protein, 49 lipid peroxidation 50 and neuronal cell death 52 and inhibits de-phosphorylation of Tau. Thus, we investigated if Kaempferide and Norbergenin can inhibit the alteration of expression of some proteins important for brain memory functions.
Sequential secretase cleavage of type-1 transmembrane APP leads to the formation of Aβ, which accumulates in the brain to induce the development of AD. 53 This cleavage is done by β-site amyloid precursor protein-cleaving enzyme 1 (BACE1, a β secretase) and ɣ-secretase.54,55 Advanced glycation end product (RAGE) proteins cause an influx of Aβ from the blood to the brain, while low-density lipoprotein receptor-related protein-1 (LRP1) causes its efflux from the brain to the blood. Also, the degradation of Aβ is elicited by NEP and insulin-degrading enzyme (IDE), zinc-associated metallo-endopeptidase. 56 Therefore, the imbalance between the production-clearance processes can lead to its accumulation in the brain. 57 Studies have shown that AlCl3 increase Aβ by increasing BACE1 and decreasing LRP-1 and NEP expression in the brain cortex and hippocampus. 3 In this study, we observed an increased expression of Aβ1-41 in the rat hippocampus. However, pre-treatment or post-treatment of AlCl3-challenged rats with KPD and NRG reduced the expression of Aβ1-41. Though our study is limited by lack of information on the regulatory proteins influenced by these agents to suppress Aβ1-41 expression, it suggests that KPD and NRG can prevent and reverse the accumulation of Aβ1-41 in the brain, thereby serving as potential agents for the prevention or management of AD. Our finding is consistent with previous reports that demonstrated the ability of KPD to reduce the activity of Aβ secretase and block the conformational transition of Aβ plaques. 42
The accumulation of Aβ in the brain can cause endoplasmic reticulum disruption and stress, which is also associated with AD in human and animal models. 58 In fact, biomarkers of endoplasmic reticulum stress like GRP78 and caspase 12 increase in the cortex and hippocampus of mice models of AD. 59 When prolonged, the stress can induce apoptosis signalling through the regulation of CCAAT enhancer-binding protein homologous protein (CHOP) and caspase-12. 60 Recently, Promyo et al. 20 reported that AlCl3 causes endoplasmic reticulum stress by elevating GRP78, CHOP and caspase-12, which were attenuated by treatment with anti-oxidant melatonin. Bax is known as a pro-apoptotic protein that translocates into the mitochondrial membrane and facilitates cytochrome c release, while Bcl-2 is a part of the pro-survival family that prevents cell death, including neuronal cells. Neurotoxicity induced by Aβ was recently shown to be mediated via upregulation of Bax and downregulation of Bcl-2. 61 This study noted that Kaempferide and Norbergenin averted the increase in caspase-3 and Bax and the decrease in BCl induced by AlCl3. This provides evidence that the neuroprotective effect of KPD and NRG during aluminium toxicity is partly mediated by its anti-apoptotic actions.
Learning disorders and long-term memory (LTM) impairment are features of cognitive dysfunction. Meanwhile, LTM formation requires activating transcription factor cAMP response element-binding protein (CREB) by various kinases, 62 qualifying CREB as a molecular switch from short-term memory to LTM. Moreover, the LTM requires a transducer of regulated CREB activity 1 (TORC1), a CREB co-activator that shuttles between the nucleus and cytoplasm, transferring intracellular signals into transcription activity of genes. 63 Furthermore, SIRT1 is required for the role of TORC1 on LTM, 64 and its deficiency causes cognitive damage and accumulation of Aβ and Tau in the brain of AD patients. 65 Aluminium has been reported to impair LTM by altering the cAMP-PKA-CREB pathway 66 and inhibiting the nuclear translocation of TORC1 and SIRT1. 41 In this study, we assessed the level of p-CREB expression as one of the markers of proteins required for memory. Consistent with previous observations by Yan et al., 41 we also noted that AlCl3 down-regulated the expressions of p-CREB and Akt. However, treatment with KPD and NRG averts the AlCl3-induced down-regulation of p-CREB, as evidenced by an expression level comparable to the control. This suggests that the cAMP-PKA-CREB pathway is involved in memory potentiation by KPD and NRG. Our confirmation of the effectiveness of KPD in averting AlCl3-induced decrease in p-CREB is also consistent with a previous report that KPD reverses Aβ1-42 induced reduction in p-CREB. It was already established that KPD reduces the ratio of SIRT1/β-actin in mice. 43
Oxidative stress increases the risk of AD by downregulating LRP1 and NEP while upregulating BACE1 and ɣ-secretase, leading to the brain accumulation of Aβ, peroxidation of macromolecules and hyperphosphorylation of Tau.67,68 Accumulation of reactive oxygen species (ROS) in the neuronal cells oxidises polyunsaturated neuronal lipid products to produce active lipid by-products like MDA, F2-isoprostanes, 4-hydroxy-2, 3-non-enal (HNE) or generation of advanced glycation end products (AGEs) from glycated69,70 all of which are neurotoxic and reduce the survival rate of neuronal cells. For example, HNE activates an apoptotic cascade in neurons, 71 while AGEs produce inflammatory mediators like NO, IL-1 and TNF-α. 72 HNE and AGEs can accelerate Aβ plaque production and tau phosphorylation, while AGEs’ interaction with its receptor can activate the NF-kB cascade to produce BACE1. 73 Elevated ROS induces a compensation by PI3K/Akt axis via elevation of Nrf2˗a transcription faction that upregulates anti-oxidant enzymes by recruiting Maf protein to bind to anti-oxidant response elements (ARE) found in the promoter region of anti-oxidant enzymes. 74 Promyo et al. 20 also reported that AlCl3 elicited oxidative stress in mice brains, which was attenuated by anti-oxidant melatonin. In this study, we observed that both pre- and post-treatment with KPD or NRG averted AlCl3-induced oxidative stress, as demonstrated by a reduction in the TBARS, NO and AChE but an increase in total protein, SOD, GSH, CAT, GPx and GR despite AlCl3 challenge. Our observation is consistent with the previous report that demonstrated that KPD and NRG reversed Aβ1-42-and D-galactose-induced oxidative stress in mice’s cerebral cortex and hippocampus 43 and rats. 32 It also agrees with the observation that KPD abolished oxidative stress induced by combined treatment of mice with AlCl3 and D-galactose, as demonstrated by its ability to reduce the content of ROS and MDA while increasing the activities of anti-oxidant enzymes (SOD, GSH-Px) despite in D-galactose-aluminium challenge. 42
The PI3K/Akt pathway is one of the ROS-related signalling pathways associated with AD pathogenesis. 75 This pathway is crucial for regulating cell survival, proliferation, growth, differentiation, motility, intracellular trafficking and complex processes like extension of neurites. 76 This axis also regulates long-term potentiation (LTP), a cellular process that strongly correlates with memory. 77 Activation of Akt phosphorylates glycogen synthase kinase -3β (GSK-3β) to facilitate its degradation,19,78 an event that promotes the survival of neuronal stem cells and their differentiation into neurons, astrocytes, oligodendrocytes. 79 Lack of activation of this pathway induces apoptotic cell death in neurons via activation of GSK-3β, increasing the expression and activity of BAD, caspase-3 and other cell death mediators to propagate apoptosis. 80 Furthermore, accumulation of Aβ prevents the propagation of the PI3K/Akt pathway and eliminates the suppressive effect of this pathway on GSK-3β, whose activation will lead to increased apoptotic signals and diminished cell survival, tau hyper-phosphorylation. 80 In this study, we confirmed that AlCl3 down-regulates the PI3K/Akt pathway via suppression of Akt. We also attempted to know if KPD and NRG can upregulate the pathway despite AlCl3. Interestingly, we found that KPD and NRG increased the expression of Akt in AlCl3-treated rats, which confirms that these agents averted AlCl3-induced downregulation of this pathway. This report aligns withour previous study that showed that KPD and NRG averted D-galactose-induced downregulation of Akt. 32 Our studies add these agents to the long list of herbal and natural compounds that have been reported to have therapeutic value for the management of AD via activation of the PI3K/Akt pathway, 78 irrespective of the animal model of AD.
Structurally, aluminium induces various forms of morphological abnormalities in the hippocampus of rats, including a decrease in cell number, reduced dendrites and disordered cell arrangement. Consequently, abnormal neurons adversely influence spatial navigation. A previous study has reported damaged neuronal ultrastructure in the hippocampus of D-galactose-treated rats, 32 including condensed nuclear density, shrunk nucleus areas, increased heterochromatin and expanded cytoplasmic vacuolar areas. In this study, we observed that KPD and NRG averted the accumulation of Aβ in the brain of rats, demonstrating its ability to prevent AD development.
Limitation
Our finding is limited, being an animal study on dementia. While it has translational potential in humans, we recommend a clinical study of the neuroprotective effects of Kaempferide and Norbergenin in AD patients.
Conclusion
In conclusion, this study demonstrated that Kaempferide and Norbergenin avert Aβ accumulation and neurocognitive shutdown induced by AlCl3 via suppression of oxidative stress and apoptosis.
Supplemental Material
Supplemental material, sj-docx-1-iji-10.1177_03946320251343687 for Kaempferide and Norbergenin avert aluminium chloride-induced amyloid β accumulation and neurocognitive shutdown via oxidative and apoptotic mechanisms by Swathi Nalla, Suhasin Ganta, Sarad Pawar Naik Bukke, Nagaraju bandaru, Hope Onohuean and Abdullateef Isiaka Alagbonsi in International Journal of Immunopathology and Pharmacology
Footnotes
Credit authorship contribution statement: SN, SG, SPNB, NB and HO conceived and designed the study. SN and SG conducted the study. SG, SPNB and NB supervised the study. HO and AIA analysed, Interpreted the results and drafted the manuscript. HO and AIA revised the manuscript. All authors agreed for the final version to be published.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: The study was approved by the PGP Life Sciences with reference No. PGP/RM-0019/57-21.
Animal welfare: The present study followed or in compliance the international CPCSEA guidelines of animal treatments.
ORCID iDs: Suhasin Ganta  https://orcid.org/0000-0002-5099-4280
https://orcid.org/0000-0002-5099-4280
Sarad Pawar Naik Bukke  https://orcid.org/0000-0002-5693-2953
https://orcid.org/0000-0002-5693-2953
Nagaraju bandaru  https://orcid.org/0000-0003-2059-4849
https://orcid.org/0000-0003-2059-4849
Hope Onohuean  https://orcid.org/0000-0002-1890-6324
https://orcid.org/0000-0002-1890-6324
Abdullateef Isiaka Alagbonsi  https://orcid.org/0000-0002-5462-9950
https://orcid.org/0000-0002-5462-9950
Supplemental material: Supplemental material for this article is available online.
References
- 1. Sanusi IO, Olutona GO, Wawata IG, et al. (2023) Occurrence, environmental impact and fate of pharmaceuticals in groundwater and surface water: A critical review. Environmental Science and Pollution Research 30(39): 90595–90614. [DOI] [PubMed] [Google Scholar]
- 2. Stahl T, Falk S, Rohrbeck A, et al. (2017) Migration of aluminum from food contact materials to food—a health risk for consumers? Part I of III: Exposure to aluminum, release of aluminum, tolerable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environmental Sciences Europe 29(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang L, Hu J, Zhao Y, et al. (2014) Effects of aluminium on β-amyloid (1-42) and secretases (APP-cleaving enzymes) in rat brain. Neurochemical Research 39(7): 1338–1345. [DOI] [PubMed] [Google Scholar]
- 4. Baranauskaite J, Sadauskiene I, Liekis A, et al. (2020) Natural compounds rosmarinic acid and carvacrol counteract aluminium-induced oxidative stress. Molecules 25(8): 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadek KM, Lebda MA, Abouzed TK. (2019) The possible neuroprotective effects of melatonin in aluminum chloride-induced neurotoxicity via antioxidant pathway and Nrf2 signaling apart from metal chelation. Environmental Science and Pollution Research 26(9): 9174–9183. [DOI] [PubMed] [Google Scholar]
- 6. Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Frontiers in Cellular Neuroscience 9: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spofforth J. (1921) Case of aluminium poisoning. The Lancet 197(5103): 1301. [Google Scholar]
- 8. Chusid JG, Pacella BL, Kopeloff LM, et al. (1951) Chronic epilepsy in the monkey following multiple intracerebral injections of alumina cream. (18970). Proceedings of the Society for Experimental Biology and Medicine 78(1): 53–54. [DOI] [PubMed] [Google Scholar]
- 9. Altmann P, Cunningham J, Dhanesha U, et al. (1999) Disturbance of cerebral function in people exposed to drinking water contaminated with aluminium sulphate: Retrospective study of the Camelford water incident. BMJ 319(7213): 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martyn CN, Osmond C, Edwardson JA, et al. (1989) Geographical relation between Alzheimer’s disease and aluminium in drinking water. The Lancet 1(8629): 59–62. [PubMed] [Google Scholar]
- 11. Frecker MF. (1991) Dementia in Newfoundland: Identification of a geographical isolate? Journal of Epidemiology and Community Health 45(4): 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neri LC, Hewitt D. (1991) Aluminium, Alzheimer’s disease, and drinking water. The Lancet 338 (8763): 390. [DOI] [PubMed] [Google Scholar]
- 13. Kumar A, Singh A. (2015) A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Frontiers in Pharmacology 6: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Onohuean H, Akiyode AO, Akiyode O, et al. (2022) Epidemiology of neurodegenerative diseases in the East African region: A meta-analysis. Frontiers in Neurology 13: 1024004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Onohuean H, Onohuean FE, Igbinoba SI, et al. (2022) Elucidation of chemical profiles and molecular targets of Mondia whitei leave fractions bioactive as novel therapeutics: An in vitro and in silico assay. Journal, Genetic Engineering & Biotechnology 20(1): 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang SW, Kim SJ, Kim MS. (2017) Oxidative stress with tau hyperphosphorylation in memory impaired 1,2-diacetylbenzene-treated mice. Toxicology Letters 279: 53–59. [DOI] [PubMed] [Google Scholar]
- 17. Onohuean H, Adisa RA, Alagbonsi AI. (2021) Anti-apoptotic effect of Buchholzia coriacea Engl. stem back extracts on AsPC-1 and mechanisms of action. BMC Complementary Medicine and Therapies 21(1): 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sinha K, Das J, Pal PB, et al. (2013) Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Archives of Toxicology 87(7): 1157–1180. [DOI] [PubMed] [Google Scholar]
- 19. Nabirumbi R, Onohuean H, Drago KC, et al. (2024) Fluoxetine attenuates stress-induced depression-like behavior due to decrease in pro-inflammatory cytokines in male rats. Science Progress 107(1): 368504241234786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Promyo K, Iqbal F, Chaidee N, et al. (2020) Aluminum chloride-induced amyloid β accumulation and endoplasmic reticulum stress in rat brain are averted by melatonin. Food and Chemical Toxicology 146: 111829. [DOI] [PubMed] [Google Scholar]
- 21. Esparza JL, Gómez M, Domingo JL. (2019) Role of melatonin in aluminum-related neurodegenerative disorders: A review. Biological Trace Element Research 188(1): 60–67. [DOI] [PubMed] [Google Scholar]
- 22. Rahul, Siddique YH. (2021) Neurodegenerative diseases and flavonoids: Special reference to kaempferol. CNS & Neurological Disorders Drug Targets 20(4): 327–342. [DOI] [PubMed] [Google Scholar]
- 23. Imran M, Rauf A, Shah ZA, et al. (2019) Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytotherapy Research 33(2): 263–275. [DOI] [PubMed] [Google Scholar]
- 24. Haq KU, Rusdipoetra RA, Siswanto I, et al. (2022) Elucidation of reactive oxygen species scavenging pathways of Norbergenin utilizing DFT approaches. Royal Society Open Science 9(12): 221349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiroma SM, Mohd Moklas MA, Mat Taib CN, et al. (2018) D -galactose and aluminium chloride induced rat model with cognitive impairments. Biomedicine & Pharmacotherapy 103: 1602–1608. [DOI] [PubMed] [Google Scholar]
- 26. Wei H, Li L, Song Q, et al. (2005) Behavioural Study of the D -galactose Induced Aging Model in C57BL/6J Mice 157. Behavioural Brain Research 157(2): 245–251. [DOI] [PubMed] [Google Scholar]
- 27. Ho SC, Liu JH, Wu RY. (2003) Establishment of the mimetic aging effect in mice caused by D -galactose. Biogerontology 4: 15–18. [DOI] [PubMed] [Google Scholar]
- 28. Lei H, Wang B, Li WP, et al. (2003) Anti-aging effect of astragalosides and its mechanism of action. Acta Pharmacologica Sinica, 24: 230–234. [PubMed] [Google Scholar]
- 29. Gao J, He H, Jiang W, et al. (2015) Salidroside ameliorates cognitive impairment in a D-galactose-induced rat model of Alzheimer’s disease. Behavioural Brain Research 293: 27–33. [DOI] [PubMed] [Google Scholar]
- 30. Tian J, Ishibashi K, Ishibashi K, et al. (2005) Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: a comprehensive transcriptional response. Proceedings of the National Academy of Sciences 102: 11846–11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Syed Umesalma SA. (2015) Protective effect of Centella asiatica against aluminium-induced neurotoxicity in cerebral cortex, striatum, hypothalamus and hippocampus of rat brain-histopathological, and biochemical approach. Journal of Molecular Biomarkers & Diagnosis 6: 1. [Google Scholar]
- 32. Nalla S, Ganta S. (2023) Defensive impact of Kaempferide against neurodegenerative studies: In vitro and in vivo investigations. Chemistry Africa 6: 2483–2493. [Google Scholar]
- 33. Charan J, Kantharia ND. (2013) How to calculate sample size in animal studies? Journal of Pharmacology and Pharmacotherapeutics 4(4): 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahdi O, Chiroma SM, Hidayat Baharuldin MT, et al. (2021) WIN55,212-2 attenuates cognitive impairments in AlCl3 + D-Galactose-Induced Alzheimer’s disease rats by enhancing neurogenesis and reversing oxidative stress. Biomedicines 9: 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nazir N, Koul S, Kurishi MA, et al.(2007). Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis—A flow cytometric study. Journal of Ethnopharmacology 112: 401–405. [DOI] [PubMed] [Google Scholar]
- 36. Sun D, McGinn MJ, Zhou Z, et al. (2007). Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Experim Neurol 204(1): 264– 272. [DOI] [PubMed] [Google Scholar]
- 37. Anseloni VC, Ennis M, Lidow MS. (2003) Optimization of the mechanical nociceptive threshold testing with the Randall-Selitto assay. Journal of Neuroscience Methods 131: 93–97. [DOI] [PubMed] [Google Scholar]
- 38. Layton C, Suvarna K. (2013) Bancroft’s Theory and Practise of Histological Techniques (7th edition). Elsevier. [Google Scholar]
- 39. Rebai O, Djebli NE. (2008) Chronic exposure to aluminum chloride in mice: Exploratory behaviors and spatial learning. Advances in Biological Research 2(1–2): 26–33. [Google Scholar]
- 40. Ribes D, Colomina MT, Vicens P, et al. (2010) Impaired spatial learning and unaltered neurogenesis in a transgenic model of Alzheimer’s disease after oral aluminum exposure. Current Alzheimer Research 7(5): 401–408. [DOI] [PubMed] [Google Scholar]
- 41. Yan D, Jin C, Cao Y, et al. (2017) Effects of aluminium on long-term memory in rats and on SIRT1 mediating the transcription of CREB-dependent gene in hippocampus. Basic & Clinical Pharmacology & Toxicology 121(4): 342–352. [DOI] [PubMed] [Google Scholar]
- 42. Lin H, Wang X, Zhao J, et al. (2023) Protective effect of kaempferol against cognitive and neurological disturbances induced by D-galactose and aluminum chloride in mice. Journal of Functional Foods 100: 105385. [Google Scholar]
- 43. Yan T, He B, Xu M, et al. (2019) Kaempferide prevents cognitive decline via attenuation of oxidative stress and enhancement of brain-derived neurotrophic factor/tropomyosin receptor kinase B/cAMP response element-binding signaling pathway. Phytotherapy Research 33(4): 1065–1073. [DOI] [PubMed] [Google Scholar]
- 44. Lukiw WJ, LeBlanc HJ, Carver LA, et al. (1998) Run-on gene transcription in human neocortical nuclei: Inhibition by nanomolar aluminum and implications for neurodegenerative disease. Journal of Molecular Neuroscience 11(1): 67–78. [DOI] [PubMed] [Google Scholar]
- 45. Johnson VJ, Sharma RP. (2003) Aluminum disrupts the pro-inflammatory cytokine/neurotrophin balance in primary brain rotation-mediated aggregate cultures: Possible role in neurodegeneration. Neurotoxicology 24(2): 261–268. [DOI] [PubMed] [Google Scholar]
- 46. Lukiw WJ, Percy ME, Kruck TP. (2005) Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. Journal of Inorganic Biochemistry 99(9): 1895–1898. [DOI] [PubMed] [Google Scholar]
- 47. García T, Esparza JL, Giralt M, et al. (2010) Protective role of melatonin on oxidative stress status and RNA expression in cerebral cortex and cerebellum of AβPP transgenic mice after chronic exposure to aluminum. Biological Trace Element Research 135(1–3): 220–232. [DOI] [PubMed] [Google Scholar]
- 48. Luo Y, Niu F, Sun Z, et al. (2009) Altered expression of Aβ metabolism-associated molecules from d-galactose/AlCl3 induced mouse brain. Mechanisms of Ageing and Development 130(4): 248–252. [DOI] [PubMed] [Google Scholar]
- 49. Castorina A, Tiralongo A, Giunta S, et al. (2010) Early effects of aluminum chloride on beta-secretase mRNA expression in a neuronal model of ß-amyloid toxicity. Cell Biology and Toxicology 26(4): 367–377. [DOI] [PubMed] [Google Scholar]
- 50. Abd-Elghaffar SKH, El-Sokkary GH, Sharkawy AA. (2007) Aluminum-induced neurotoxicity and oxidative damage in rabbits: Protective effect of melatonin. Neuro Endocrinology Letters 26(5): 609–616. [PubMed] [Google Scholar]
- 51. Kihira T, Yoshida S, Yase Y, et al. (2002) Chronic low-Ca/Mg high-Al diet induces neuronal loss. Neuropathology 22(3): 171–179. [DOI] [PubMed] [Google Scholar]
- 52. Shaw CA, Petrik MS. (2009) Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. Journal of Inorganic Biochemistry 103(11): 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Strooper B, Vassar R, Golde T. (2010) The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nature Reviews Neurology 6(2): 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Steiner H, Fluhrer R, Haass C. (2008) Intramembrane proteolysis by γ-secretase. Journal of Biological Chemistry 283(44): 29627–29631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vassar R, Kandalepas PC. (2011) The β-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimer’s Research & Therapy 3(3): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yoon SS, Jo SA. (2012) Mechanisms of amyloid-β peptide clearance: Potential therapeutic targets for Alzheimer’s disease. Biomolecules & Therapeutics 20(3): 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bates KA, Verdile G, Li QX, et al. (2009) Clearance mechanisms of Alzheimer’s amyloid-Β peptide: Implications for therapeutic design and diagnostic tests. Molecular Psychiatry 14(5): 469–486. [DOI] [PubMed] [Google Scholar]
- 58. Endres K, Reinhardt S. (2013) ER-stress in Alzheimer’s disease: Turning the scale? American Journal of Neurodegenerative Disease 2(4): 247–265. [PMC free article] [PubMed] [Google Scholar]
- 59. Soejima N, Ohyagi Y, Nakamura N, et al. (2013) Intracellular accumulation of toxic turn amyloid-β is associated with endoplasmic reticulum stress in Alzheimer’s disease. Current Alzheimer Research 10(1) 11–20. [PubMed] [Google Scholar]
- 60. Rao RV, Castro-Obregon S, Frankowski H, et al. (2002) Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. The Journal of Biological Chemistry 277(24): 21836–21842. [DOI] [PubMed] [Google Scholar]
- 61. Kudo W, Lee HP, Smith MA, et al. (2012) Inhibition of Bax protects neuronal cells from oligomeric Aβ neurotoxicity. Cell Death & Disease 3(5): e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alzoubi KH, Alkadhi KA. (2007) A critical role of CREB in the impairment of late-phase LTP by adult onset hypothyroidism. Experimental Neurology 203(1): 63–71. [DOI] [PubMed] [Google Scholar]
- 63. Iourgenko V, Zhang W, Mickanin C, et al. (2003) Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America 100(21): 12147–12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Altarejos JY, Montminy M. (2011) CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nature Reviews Molecular Cell Biology 12(3): 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang F, Wang S, Gan L, et al. (2011) Protective effects and mechanisms of sirtuins in the nervous system. Progress in Neurobiology 95(3): 373–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang L, Jin C, Lu X, et al. (2014) Aluminium chloride impairs long-term memory and downregulates cAMP-PKA-CREB signalling in rats. Toxicology 323: 95–108. [DOI] [PubMed] [Google Scholar]
- 67. Cassidy L, Fernandez F, Johnson JB, et al. (2020) Oxidative stress in Alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complementary Therapies in Medicine 49: 102294. [DOI] [PubMed] [Google Scholar]
- 68. Zhao Y, Zhao B. (2013) Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Medicine and Cellular Longevity 2013: 316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tönnies E, Trushina E. (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. Journal of Alzheimer’s Disease 57(4): 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bradley-Whitman MA, Lovell MA. (2015) Biomarkers of lipid peroxidation in Alzheimer disease (AD): An update. Archives of Toxicology 89(7): 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tamagno E, Robino G, Obbili A, et al. (2003) H2O2 and 4-hydroxynonenal mediate amyloid β-induced neuronal apoptosis by activating JNKs and p38MAPK. Experimental Neurology 180(2): 144–155. [DOI] [PubMed] [Google Scholar]
- 72. Allan Butterfield D, Boyd-Kimball D. (2018) Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. Journal of Alzheimer’s Disease 62(3): 1345–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tamagno E, Guglielmotto M, Monteleone D, et al. (2012) Amyloid-β production: Major link between oxidative stress and BACE1. Neurotoxicity Research 22(3): 208–219. [DOI] [PubMed] [Google Scholar]
- 74. Kim KC, Lee IK, Kang KA, et al. (2013) 7,8-dihydroxyflavone suppresses oxidative stress-induced base modification in DNA via induction of the repair enzyme 8-oxoguanine DNA glycosylase-1. BioMed Research International 2013: 863720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. O’Neill C. (2013) PI3-kinase/Akt/mTOR signaling: Impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Experimental Gerontology 48(7): 647–653. [DOI] [PubMed] [Google Scholar]
- 76. Ye L, Wang X, Cai C, et al. (2019) FGF21 promotes functional recovery after hypoxic-ischemic brain injury in neonatal rats by activating the PI3K/Akt signaling pathway via FGFR1/β-klotho. Experimental Neurology 317: 34–50. [DOI] [PubMed] [Google Scholar]
- 77. Horwood JM, Dufour F, Laroche S, et al. (2006) Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. The European Journal of Neuroscience 23(12): 3375–3384. [DOI] [PubMed] [Google Scholar]
- 78. Su HC, Ma CT, Yu BC, et al. (2012) Glycogen synthase kinase-3β regulates anti-inflammatory property of fluoxetine. International Immunopharmacology 14(2): 150–156. [DOI] [PubMed] [Google Scholar]
- 79. Razani E, Pourbagheri-Sigaroodi A, Safaroghli-Azar A, et al. (2021) The PI3K/Akt signaling axis in Alzheimer’s disease: A valuable target to stimulate or suppress? Cell Stress & Chaperones 26(6): 871–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Koh SH, Lo EH. (2015) The role of the PI3K pathway in the regeneration of the damaged brain by neural stem cells after cerebral infarction. Journal of Clinical Neurology 11(4): 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-iji-10.1177_03946320251343687 for Kaempferide and Norbergenin avert aluminium chloride-induced amyloid β accumulation and neurocognitive shutdown via oxidative and apoptotic mechanisms by Swathi Nalla, Suhasin Ganta, Sarad Pawar Naik Bukke, Nagaraju bandaru, Hope Onohuean and Abdullateef Isiaka Alagbonsi in International Journal of Immunopathology and Pharmacology