Abstract
Veterinarians play a vital role in providing healthcare, detecting zoonotic outbreaks, and safeguarding the livelihood of those relying on animals for subsistence. However, veterinary capacities are unequal between countries, and their geography is seldom documented despite significant implications for healthcare access. Here, we web-scrape 303,745 addresses of veterinary practices from 115 countries and use geospatial models to map their global distribution at 10×10 km2. Animals located more than one hour from veterinarians are overwhelmingly (93.8%) in low- and middle-income countries. Globally, the number of isolated animals could be reduced by 32.9% by increasing the number of veterinarians by 5%, provided that this effort is geographically targeted. Our maps provide a global baseline to allocate resources to improve access to care, enhance veterinary education, and strengthen disease surveillance.
Subject terms: Bacterial infection, Geography
Veterinary care is unevenly distributed globally, limiting access to care. Our maps show that 93.8% of isolated animals (>1h travel time) are in low- and middle-income countries. A 5% targeted increase in veterinarians may reduce isolation by 32.9%.
Introduction
Maps play a critical role in prioritizing interventions for global infectious diseases1. In human medicine, fine-scale maps have helped quantify the burden of diseases such as malaria and dengue, which together affect millions of people worldwide and pose significant public health challenges2. Furthermore, maps facilitated geographically targeted campaigns to target insecticide bed nets in specific regions3. Concomitantly, maps of healthcare facilities4,5 have been used to make international comparisons of access to primary care and guide the deployment of important medicines such as antiretroviral therapy against the human immunodeficiency virus6. In animal health, similar efforts have been conducted to map diseases that threaten the livelihood of those who raise animals for subsistence such as avian influenza7, Rift Valley fever8, or antimicrobial resistance (AMR)9–12.
However, unlike in human medicine, mapping access to veterinarians – those who are the first line of defense against diseases of animal origin13—has thus far lagged behind and was limited to the national-level or, in a few countries, to the regional/province-level (United States, France, Italy, Spain, Switzerland)14–19. The most notable global effort to inventory veterinary capacities—currently led by the World Organisation for Animal Health—is focused on country-level performance assessments20. Despite these efforts, gaps remain in mapping veterinary services at a fine-scale, potentially overshadowing important geographic disparities in access to veterinary care, such as between rural and urban areas, high-income (HICs) and low- and middle-income countries21 (LMICs), or regions with high versus (vs) low food animals’ density. This is particularly true for large LMICs currently transitioning from extensive farming to intensive farming22, a phase associated with an increased risk of emergence of zoonotic pathogens23.
Important insight could be gained from assessing the state of the veterinary workforce at a fine spatial scale. Firstly, by identifying areas akin to medical deserts in human medicine24, i.e., areas with inadequate access to healthcare providers due to a lack of personnel, facilities, or infrastructure. In such areas, long travel times to/for veterinarians are a major obstacle to delivering care. While travel time is only one dimension of the multifaceted challenge of access to care (i.e.,: cost, education, regulatory hurdles), a recent meta-analysis identified this factor as a leading cause for insufficient animal care in LMICs25. Maps could guide capacity building in these regions using travel time as a criterion for resource allocation. In addition, mapping areas where veterinary capacities are currently insufficient could indirectly help strengthen surveillance for potential pandemic pathogens26 in regions associated with a high risk of disease emergence27,28.
Between 2010 and 2020, platforms have appeared in many countries that enable internet users to find veterinary practices in their vicinity using their postcodes/addresses29–32. Although these platforms were not designed for public health purposes, they represent an unprecedented opportunity to investigate the fine-scale geographic distribution of veterinary practices and its determinants. However, to convert this data into actionable insights for capacity-building decisions several challenges must be addressed. First, platforms of veterinary practices addresses must be inventoried. Second, web-scraping tools are needed to extract the large number of veterinary practices addresses listed online, and these addresses must also be curated and geocoded. Third, statistical models that capture variations in veterinary practices density and account for influencing factors must be developed and validated regionally to ensure robust interpolation between regions with extensive available data and those with limited or sparse data. Fourth, model predictions must align with national and international veterinary practices estimates. Finally, models should account for variations in the presence of veterinary practices on online platforms between countries characterized by different levels of economic development and internet penetration.
In this work, we map the global distribution of veterinary practices at 10 × 10 km2 resolution using geospatial models in combination with a global address book of veterinary practices assembled from open-access online platforms. We identify regions where food animals are located over an hour from veterinary practices by motorized transport and highlight areas where veterinary capacity should be increased to improve access to care.
Results
Building a global address book of veterinarians
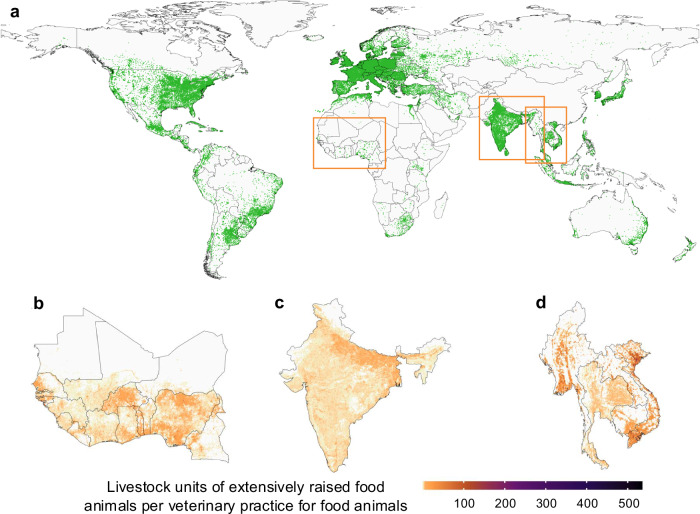
We identified 87 online platforms listing addresses of veterinary practices across 115 countries (Supplementary Fig. 1), including OpenStreetMap and Google Maps, 16 national platforms listing veterinary practices by postcode, 21 websites of veterinary councils, and 48 national phonebooks. From these platforms, web-scraping techniques (Methods) returned 404,635 records of potential addresses of veterinary practices (Supplementary Fig. 2). After a data curation phase to i) remove records that were not veterinary practices (e.g., veterinary pharmacies, that could be listed on national phonebooks when searching for veterinary practices), ii) prevent duplicates across platforms, and iii) remove permanently closed practices, our database consisted of 303,745 addresses of veterinary practices (Fig. 1a and Supplementary Fig. 3). A majority of addresses were retrieved from the Americas (39.7%), Europe (38%), and Asia (18.2%), while Africa and Oceania accounted for 2.1%, and 2% of records, respectively. The specialization of veterinary practices (companion vs food animals) was reported for 9.1% of the practices sampled. Amongst those, 85% cared for companion animals, and 15% cared for food animals.
Fig. 1. Addresses of veterinary practices and livestock units of extensively raised food animals per veterinary practice.
a Green dots represent the geographic locations of veterinary practices, obtained by geocoding the addresses within online platforms. Distribution of livestock units33 of extensively raised food animals per veterinary practice (specialized in food animals) at the 10×10 km2 resolution in West Africa (b), India (c), and Mainland Southeast Asia (d).
Global distribution of veterinarians
A global map of veterinary practices was generated at 10 × 10 km2 resolution using web-scraped addresses of veterinary practices in combination with anthropogenic and environmental covariates in a geospatial model (Methods, Supplementary Table 1, Supplementary Fig. 4a). This map was cross-validated in nine world subregions (Supplementary Fig. 5) and showed that LMICs accounted for 57% of all predicted veterinary practices worldwide. Population density, gross domestic product, and low travel time to cities were positively associated with a high density of veterinary practices and had the highest statistical influence on their geographic distribution (Methods, Supplementary Table 2).
The number of veterinary practices per pixel was re-aggregated nationally and compared with the number of practices reported by veterinary associations, international organizations, and peer-reviewed publications (Supplementary Table 3) in each country. For 70% of countries, re-aggregated predictions were within <40% of national-level reports of the numbers of veterinary practices. For the remaining 30% of countries, re-aggregated predictions were between 40-50% of national-level reports of the numbers of veterinary practices (Supplementary Fig. 6). Furthermore, 92.4% of our predictions were lower than the national numbers of the estimated veterinary graduates (Methods, Supplementary Fig. 6).
For veterinary practices specialized in food animals, the global map of their distribution (vs companion animals, Supplementary Fig. 7) showed that pixels with a majority of veterinary practices specialized in food animals were in Africa (54.6%), Oceania (43.4%), and Latin America (29.8%). In contrast, this proportion decreased to 18.9% in Europe, 13.5% in Asia, and only 6% in North America.
Globally, the number of livestock units (LSUs33, Methods) of food animals raised extensively22 per veterinary practice was, on average, 5.4 across LMICs (Fig. 1b). This number fell to 0.2 in HICs. At the country-level, we identified pixels with more than 5 LSUs of extensively raised food animals per veterinary practice (hereafter referred to as “high animals’ density areas”) in 81.5% of Latin American countries, 92% of African countries, and 63.3% of Asian countries.
Travel time to veterinary services
Global maps of coldspots of veterinary capacity (Fig. 2), i.e., 10 × 10 km2 pixels where food animals were more than 1 h away by motorized transport from the nearest veterinary practice (Methods), showed that 188.8 million LSUs lived more than 1 h away from a veterinary practice. That is equivalent to 1.2 times the biomass of chicken, cattle, and pigs raised for food in the United States.
Fig. 2. Coldspots of veterinary capacity in cattle, chickens, and pigs.
Travel time for cattle (a), chickens (c), and pigs (e) living at least 1 h away by motorized transport from a veterinary practice or school (“coldspots”). Average motorized travel time per cattle (b), chickens (d), and pigs (f) living in coldspots per country. Numbers in the bars represent the percentages of animals per country living in coldspots, relative to the global number of animals in coldspots.
Asia had the highest percentage of animals living in coldspots (44.4%), followed by Latin America (27.9%), and Africa (18.8%). The highest percentages of all cattle in coldspots were in Brazil (22.1%), Sudan (8.1%), China (7.6%), Chad (7.5%), and Australia (4.5%). For chickens, the highest percentages were in China (15.2%), Bolivia (8.6%), Russia (7.4%), Iran (7.6%), and Indonesia (7%), while for pigs were in China (50.2%), Myanmar (7.4%), Papua New Guinea (6%), Russia (5.1%), and Brazil (4.6%). Finally, at the species-level, LMICs accounted for 94% of cattle, 93.4% of chickens, and 99.4% of pigs in coldspots. Countries with the highest average travel time to reach an animal in coldspots were Somalia, China, Guyana, Sudan, Papua New Guinea, and the Central African Republic. These patterns remained consistent when setting maximum travel time thresholds to define coldspots at 2 or 4 h (Supplementary Fig. 8).
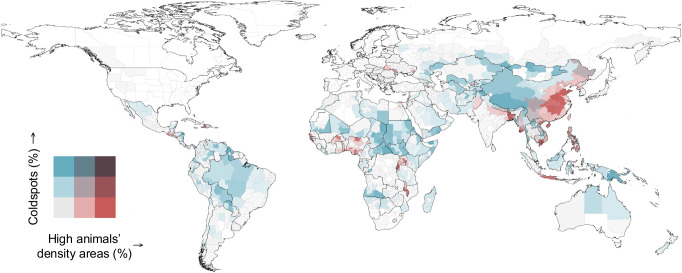
Except for North America and Europe (excluding Russia), regions with high percentages (>50%) of coldspots of veterinary capacity and low animal densities (<5 LSUs/10 × 10 km2) were present in every continent (Fig. 3). Of even greater concerns were regions that combined the presence of coldspots with growing animals’ densities (>5 LSUs/10×10 km2). These were located predominantly in Asia, and to a lesser extent in West Africa, and around the African Great Lakes. In Rwanda, Malawi, Bangladesh, Papua New Guinea, Vietnam, Philippines, and Haiti more than 75% of the aggregated LSUs of cattle, chickens, and pigs were present in coldspots and/or high animals’ density areas.
Fig. 3. Regional veterinary capacities available to food animals.
Proportion of 10×10 km2 pixels per administrative unit where food animals were farther than 1 h away by motorized transport from the nearest veterinary practice or school (“coldspot”), combined with the proportion of pixels classified as high animals’ density areas, i.e., pixels where the number of extensively raised food animals (converted in livestock units33) per veterinary practice was higher than 5. In regions with a white background, the proportion of food animals in coldspots and/or high animals’ density areas was lower than 1%.
A geographically targeted approach to improve access to veterinarians
In countries with coldspots, we identified the locations where a hypothetical 5% scale-up of veterinary practices could take place such as to maximize the number of food animals living within 1 h from a practice. A recursive exhaustive allocation approach (REA) testing every location achieves the maximal possible coverage of food animals, but it is computationally expensive34. Therefore, we compared three approximations to the REA (Methods): i) one based on countries administrative division (administrative approach), ii) one based on random allocations of veterinary practices in space (random approach), and iii) one targeting specific areas of a high density of food animals distant from veterinary practices (“contiguity approach”).
The comparison of these approaches with the REA in nine countries (Kenya, Panama, Ecuador, Liberia, Eritrea, Honduras, Nicaragua, Costa Rica, and Cambodia) showed that, in each country, the contiguity approach was, on average, 18.5 times faster than the REA (Supplementary Fig. 9 and Supplementary Table 4) and was the only one reaching >90% of the REA’s performance for reducing the number of animals in coldspots when scaling up the number of veterinary practices. In contrast, the administrative and the random approach reduced the number of animals in coldspots, on average, only by 33.1% and 42%, respectively, when compared to the REA (average coverage range of the Monte Carlo simulations for the random approach: 22.2–63.2%).
Furthermore, values of kernel density estimation (KDE) maps computed from the veterinary practices allocated by each approach showed that the administrative and the random approach allocated veterinary practices in different geographical locations than the REA. In contrast, the geographical patterns of the veterinary practices allocated through the contiguity approach matched that of the REA (Supplementary Fig. 10). As a result, the average Pearson correlation coefficient (r) computed between the values of each KDE map of the REA and the contiguity approach was 0.96 (95% bootstrapped CIs: [0.94, 0.97]; r2 = 0.92). Therefore, the contiguity approach was applied to all countries, to generate a global map of “supplementary” veterinary practices (Fig. 4).
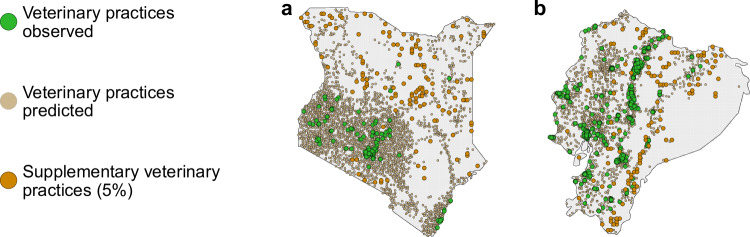
Fig. 4. Veterinary practices allocated through a geographically targeted approach.
Results of the geographically targeted approach used to scale up veterinary practices in two countries with coldspots: (a) Kenya and (b) Ecuador. Coldspots were defined as 10 × 10 km2 pixels where food animals were farther than 1 h of motorized travel time from the nearest veterinary practice. Both panels show the point patterns of veterinary practices observed (green), veterinary practices predicted through the geospatial modeling (light brown, see Methods), and the 5% of supplementary predicted veterinary practices allocated through a geographically targeted approach (orange), i.e., an approach prioritizing the allocation of veterinary practices in areas with a high density of food animals in coldspots and far from predicted veterinary practices (“contiguity approach”, see Methods).
Globally, a 5% increase in the number of veterinary practices could reduce the coldspots area by 6,600,000 km2 (comparable to 85% of the size of Australia, Supplementary Fig. 11). The continents where the contiguity approach removed the highest number of food animals from coldspots were Asia (11%) and Latin America (8.6%). Overall, the contiguity approach reduced the number of animals in coldspots by 27% for cattle, 34.3% for chickens, and 44.8% for pigs (Supplementary Fig. 12). Together, these food animals correspond to 89% of the LSUs of Mexico. The countries that would concentrate the increase (5%) in the global number of veterinary practices if these were targeted geographically were China (39.2%), Brazil (20.2%), Myanmar (5.5%), Venezuela (4%), and Russia (3.8%) (Fig. 5).
Fig. 5. Where to increase veterinary capacities.
Global maps of the regions that concentrate a 5% global increase in veterinary practices to reduce coldspots of veterinary capacity for cattle (a), chickens (c), and pigs (e). Barplots showing countries where veterinary practices could be added to maximize the number of cattle (b), chickens (d), and pigs (f) living within 1 h of veterinary practices, grouped by continent.
At the sub-national level, the regions that would concentrate the increase in the global number of veterinary practices for cattle (Supplementary Fig. 13) were Mato Grosso, Rio Grande do Sul, Pará, and Goiás (Brazil), Xizang, Qinghai, and Yunnan (China), Mahajanga (Madagascar), Central Darfur (Sudan), Zinder (Niger), Kalmyk and Orenburg (Russia), and Queensland and Northern Territory (Australia). For chickens (Supplementary Fig. 14), the regions identified were Mato Grosso (Brazil), Cochabamba and Santa Cruz (Bolivia), Durango (Mexico), Jilin, Yunnan, Nei Mongol, and Guangxi (China), Antsiranana (Madagascar), Northern Cape (South Africa), Zinder (Niger), Stavropol and Leningrad (Russia), New South Wales (Australia) and West Coast (New Zealand). For pigs (Supplementary Fig. 15) the regions were Mato Grosso and Parà (Brazil), Sonora (Mexico), Yunnan, Guizhou, Sichuan, and Guangxi (China), Gaza and Tete (Mozambique), and Omsk and Tatarstan (Russia).
Discussion
In this study, we assembled an address book of >300,000 veterinary practices from 115 countries using web-scraping techniques (Methods), thereby creating a global geospatial database that would have been virtually impossible to build via traditional “manual” data collection. Although this data is irregular in nature, when used in combination with statistical models it showed a good agreement with national estimates of the number of veterinary practices. Furthermore, we showed that it can supplement international efforts that document veterinary capacities by providing insights into the geographic distribution of veterinary practices at unprecedented resolution (10 × 10 km2). The most notable of these efforts is led by the World Organisation for Animal Health (WOAH)35, which established guidelines to evaluate the national performances in veterinary services (PVS)20. To date, more than 140 countries have engaged in the PVS pathway. This consists of a standardized global methodology that every country can use to i) evaluate the status of its national veterinary services, ii) identify its strengths and weaknesses, and iii) plan workforce development. In this context, the methodology underlying the PVS pathway could be supplemented with additional criteria for the evaluation. For instance, the percentage of areas classified as geographic coldspots of veterinary capacity could be integrated into the PVS. In addition, our study introduces a standalone metric for each country and food animal: the average travel time for a veterinarian to reach an animal in coldspots (Methods and Fig. 2). This metric enables cross-country comparisons and offers an immediate overview of veterinary services availability, potentially strengthening the PVS by helping prioritize the allocation and scaling up of resources.
In this study, we also proposed a tentative strategy to reduce coldspots of veterinary services when a limited workforce is available. Our findings suggest that a 5% increase in the global veterinary workforce, if geographically targeted, could bring within 1 h of motorized transport from a veterinary practice 27% of the world’s cattle, 34.3% of chickens, and 44.8% of pigs currently living in coldspots of veterinary care akin to medical deserts. This represents an easily achievable target to improve the livelihood of the 1.3 billion people who rely on food animals for subsistence (of which 600 million are small-scale farmers)36 and to enhance animal welfare37. In addition, it could help strengthen surveillance against emerging pathogens. While numerous studies have explored outbreaks in food animals7–9,38,39, there remains a gap in assessing the capacities of veterinarians, who are among the key actors involved in managing such outbreaks.
The regions that would benefit the most from increased access to veterinary practices are East and Southeast Asia (for chicken and pigs) and Brazil (cattle). However, a lack of access to care for cattle in Brazil could also reflect an overall lack of incentives for health workers to move to rural areas40, rather than a shortage of veterinary practices. On the one hand, rural areas face economic challenges that limit the ability of farmers to afford veterinary services, making practices less viable. On the other hand, veterinarians may prefer urban settings for better work-life balance, as rural jobs often demand long hours and frequent travel. Also, the lack of infrastructure and amenities, along with limited opportunities for collaboration and professional growth, could further discourage veterinarians from working in rural regions41.
Nevertheless, physical access is only one of many dimensions of the challenge of access to care42. Costs, for instance, constitute a barrier particularly in LMICs, where smallholder farmers may struggle to afford veterinary services or medications, even from nearby clinics. Training levels and expertise of veterinary personnel are equally critical. An insufficiently trained workforce may limit the quality of care, especially in areas where diseases requiring specialized care, such as foot-and-mouth disease or avian influenza, are prevalent. Furthermore, local disease burden can exacerbate these challenges, as areas with a high prevalence of food animals’ diseases may require more resources and skilled professionals to manage potential outbreaks. Our efforts to build a global database of veterinary practices could be broadened to incorporate information on these additional factors affecting access to care. However, assembling this information must necessarily take place through a multi-stakeholder approach: via international organization, but also potentially by involving individual practitioners in data gathering and validation. In addition, while the “golden hour” has been acknowledged in the context of trauma care as the optimal timeframe for intervention, this threshold is a convenience benchmark that can be subject to variations depending on the nature of the health issue43. The tentative goal of this work is to provide the backbone for an initial evaluation of areas lacking veterinary practices and illustrate the value of high-resolution data for capacity building in animal health.
In HICs, the predictions derived from web-scraped data mirrored past research from the United States, Canada, and France44–46, showing that veterinary practices predominantly cluster in affluent urban areas thereby reflecting the growing trend for specialization in pet care (80–95%) over food animal care (5–20%)47. This translates into a regional imbalance of veterinary practices, especially in North America, Europe, and Asia. In contrast, in LMICs, our predictions suggest that the proportion of veterinary practices specialized in food animals is higher (25–50%) but their number is limited, and their geographic distribution is highly heterogenous leading to >93% of coldspots of access to care being in LMICs. In addition, the number of LSUs per veterinary practice in LMICs is 27 times higher than in HICs, potentially placing an overwhelming burden on veterinary professionals, which can lead to insufficient care and slower disease detection. Causes for limited access to care might include insufficient training and equipment for diagnostic laboratories48, comparatively low salaries21, and low standard of living in rural areas41.
Nevertheless, the data from LMICs that support this assessment is currently limited: of the web-scraped veterinary database, just 33.6% were from LMICs although these make up 79.8% of the global food production. This discrepancy might rely both on gaps in veterinary infrastructure in LMICs but also on limitations in the means to collect addresses in these regions, due to poor internet penetration, less-developed digital infrastructure, and the lack of online platforms listing veterinary services. In this context, our study can provide a baseline for increasing veterinary capacities but also a starting point for better documenting the veterinary workforce at a sub-national scale and target investments to improve it. For example, in China—the largest animal producer in the world—public data on the geographic distribution of veterinary practices could not be identified for this study, and even national estimates of the number of veterinary practices could only be found via press articles49. A unified global database of veterinary practices might serve as a foundation to address this challenge and foster collaboration between the public and private sectors to expand and enhance our database. An increasing number of veterinary practices are being integrated under large private companies, whose business model is to pool resources and create economies of scale50. Partnerships between organizations like the WOAH, the Food and Agriculture Organization (FAO), and such companies could help build on this shared vision, using the database of veterinary practices as a common ground. Such collaboration could help extend the systematic expansion of veterinary coverage and knowledge about the global state of veterinary services.
Our analysis of the access to veterinary practices comes with limitations. First, our study focused on veterinarians—holders of a university degree in veterinary medicine—and did not include “paravets” who are semi-autonomous professionals predominantly present in LMICs whose qualifications vary considerably between countries. Unlike veterinarians, paravets were not systematically inventoried on online platforms and were therefore not included in our study although they may play a crucial role in providing care.
Second, our database of web-scraped veterinary practices is likely incomplete and remains a work in progress. In particular, veterinarians with a stable client base (e.g., those working in rural areas) may not perceive significant benefits in listing their addresses online. Relying on online sources may also introduce a sampling bias—which we attempted to account for—by implicitly overrepresenting areas with high internet penetration where veterinarians are likelier to advertise their services online. This is particularly relevant for some LMICs, where the limited resources to inventory and maintain online infrastructures listing veterinary services can result in an underrepresentation of the national veterinary capacity, including government facilities. In addition, language barriers can pose challenges when scraping non-English sources, leading to potential inaccuracies in data translation or collection, which requires extensive data cleaning and deduplication efforts. Lastly, privacy policies in some countries preclude displaying addresses of veterinary practices online. Although in our study we used spatial covariates to account for the incompleteness of the veterinary database, these limitations underscore the need to complement web-scraping with other methods to improve data accuracy and representativeness, such as encouraging veterinary students and local officers to conduct field surveys and establish bilateral collaborations with local non-governmental organizations and veterinary associations.
Third, information about the capacity (personnel/equipment) of each veterinary practice is currently very challenging to access for animal health. Online platforms typically only report the name of practices or the full name of a veterinarian, without providing details such as the number of personnel, available equipment, or the range of services offered. For this reason, we considered each single address sampled as a standalone veterinary practice. In contrast, human healthcare mapping initiatives, such as “healthsites.io”, enrich geographic data of hospitals collected from OpenStreetMap51 with additional information, including whether they are public or private, the medical specializations offered, and, in some cases, the beds availability5. Similarly, Service Provision Assessments (SPAs) collect data on service availability, quality of care, and facility infrastructure, providing a comprehensive understanding of healthcare capacity within a country. Achieving a comparable level of detail on the “quality” of care at the location of each practice should be a priority for the future. This could include surveys and structured assessments modeled after SPAs, as well as collaborations with local veterinary authorities to facilitate the collection of data on clinic size, personnel qualifications, equipment, diagnostic capabilities, and service types. Additionally, leveraging crowdsourcing platforms or engaging practitioners directly through digital tools could enrich the database with qualitative and quantitative insights. Such advancements would help bridge the gap between veterinary and human healthcare mapping, providing a more nuanced understanding of global veterinary capacity and supporting better-targeted interventions for animal health.
Fourth, the uncertainty associated with spatial interpolations of the veterinary practices maps is reflected in confidence interval maps (Supplementary Fig. 4). These uncertainty levels reflect the spatial cross-validation procedure used to prevent regional overfitting. However, these do not reflect a comparison with independent field surveys since all data sources identified were included in the model training and cross-validation to produce the most accurate maps possible. In this context, covariates such as population density, gross domestic product, and travel time to cities were the most influential in modeling the distribution of veterinary practices. Although they can be used to separate such distribution between HICs and LMICs, they might not fully capture all the barriers associated with access to veterinary care in rural or underdeveloped areas. Similarly, maps of food animals’ density used for this study come with their own uncertainty, and although they have been cross-validated to report the most accurate predictions of food animals at the 10 × 10 km2 resolution52,53, the on-the-ground numbers of such animals can differ from the ones predicted. In addition, other sources of uncertainty concern regions of the world where animals and pastoralist communities are nomadic54. Albeit limited in number globally, the population density of animals raised in these systems may vary over the years and affect the locations of coldspots. However, to the best of our knowledge, global maps of herd movements that would enable us to account for these variations do not exist to this day. Future field campaigns could investigate how the network of predicted veterinary practices should be adapted for optimally serving these communities.
Fifth, considering a 1-h timeframe for coldspots is a benchmark of convenience for its interpretability and its comparability with the “golden hour” used in human medicine55. However, while a 1-h timeframe has been used in the context of trauma care for humans, it is important to note that this is not a universally recognized standard for animal care. The optimal timeframe for intervention can vary significantly based on the specific medical condition43. For example, in the context of access to antivenoms for snakebites, Ochoa and colleagues used travel time intervals to access facilities with antivenoms of 0–30 min, 30–60 min, and >60 min if the neurotoxic effects of snakes’ venom have, respectively, severe, moderate, and mild risk of mortality56. Our choice of a 1-h benchmark is therefore an initial attempt to evaluate the lack of access to veterinary services, which might differ for conditions or be revised for specific applications (i.e., reproductive care, outbreak investigation, etc.).
Sixth, areas of Brazil were identified as coldspot of access to care for cattle. However, veterinary practices that have as sole clients a single large farm—a common business structure in Brazil57—may have little incentive for presence on online registers. Although our models account for variations in the online presence (see “Investigating preferential sampling of addresses”, Methods) Brazil may have been disproportionally affected by low online registration rates, potentially leading to underestimating access to care.
Seventh, 70% of our re-aggregated predictions were within <40% of national-level reports of the numbers of veterinarians while only 30% of our re-aggregated predictions were between 40 and 50% of national-level reports of the numbers of veterinarians. Despite this considerable variation, it is important to note the lack of consensus among national estimates obtained from various sources (Supplementary Fig. 6). This discrepancy may stem from inadequate data sharing among entities responsible for maintaining databases on national veterinary capacity. Similarly, estimates of the number of veterinary graduates were collected in each country for comparison with our maps. While graduates represent an upper plausible limit on the number of veterinary practices active in each country, these estimates did not account for veterinarians working in a country different from the country of graduation.
Finally, our analyses did not incorporate long-distance mobile veterinary practices, present in certain HICs such as Australia, where veterinarians may occasionally travel via airplane to reach remote farms58.
Methods
Collecting national estimates and addresses of veterinary practices
Between March 2020 and June 2021, we conducted an online sampling campaign on a global scale to collect data about veterinary practices. We searched for addresses of veterinary practices to investigate their global distribution and for their national estimates as a reference for the comparison with our country-level predictions. Concretely, in each country, we searched for (A) national estimates of the number of veterinary practices, (B) annual estimates of the number of graduates from veterinary schools, (C) addresses or geographic coordinates of veterinary hospitals, clinics, and private practices, and (D) information about the animals treated in these veterinary practices, i.e., companion animals (cats, dogs, other pets) or food animals.
When searching for national estimates of veterinary practices (A), we consulted websites of international organizations such as the WOAH, the FAO, the World Veterinary Association (WVA), and the World Small Animal Veterinary Association (WSAVA). In Europe, we retrieved data from the website of the European Board of Veterinary Specialization (EBVS) and the 2019 survey of the veterinary profession in Europe59 compiled by the Federation of Veterinarians of Europe (FVE). For the rest of the world, we consulted websites of national associations and universities, governmental reports, and estimates by statistical agencies. We also searched national estimates on scientific reports, peer-reviewed publications, and online newspapers.
In addition, we sent 309 emails to veterinary associations and governmental agencies to request national estimates of veterinary practices (response rate of 16.8%). From this search, we found 117 national-level estimates of the number of veterinary practices across 79 countries. We collected 79 estimates from international organizations and national associations, 13 from governmental agencies, 20 from online newspapers and blogs, and 8 from scientific literature (Supplementary Table 3).
Next, we estimated the number of graduates who may still be practicing veterinary medicine in the year 2022 (B) as the upper threshold for comparing the number of veterinary practices we predicted at the national level. First, we defined a global list of veterinary schools available from the WOAH questionnaires on the veterinary educational establishments60 and Wikipedia61. In addition, we sent 659 emails to veterinary schools (response rate of 4.7%) and consulted the WOAH questionnaires to collect school-specific data about the range of graduates per year and the average number of years required to obtain a degree in veterinary medicine. Also, we collected the foundation year of each school and the retirement age per country62. For calculating the number of graduates, we made the following assumptions: (1) 10% of students graduate each year from a veterinary school, (2) older schools have a bigger range of graduates per year than newer schools, (3) the first valid year considered to estimate the number of graduates is given by the foundation date of the school plus the years required to obtain the degree, (4) the last valid year considered for graduation is 2022, and (5) veterinarians who are now retired based on a country’s retirement age were excluded from the number of graduates estimated. Then, for each school, we calculated the number of graduates, NG, as Eq. (1):
| 1 |
Where YA is the number of years, net of the retirement age, in which students graduated from the school, and GMAX and GMIN are, respectively, the maximum and minimum number of graduates per year. Through Eq. (1) we summed NG for each national veterinary school, estimating the national-level number of graduates from 680 veterinary schools out of the 719 sampled across 120 countries.
For assembling the global database of addresses of veterinary practices (C-D), we first identified the types of online sources listing their addresses or coordinates. We prioritized the data collection from i) online platforms specifically designed to search veterinary practices by postcode (e.g., https://findavet.rcvs.org.uk/home/), ii) websites of national phonebooks, like countries Yellow Pages, iii) websites of national veterinary associations and governmental agencies as the ministries of agriculture, and iv) open-access web maps like OpenStreetMap and Google Maps.
We retrieved websites of data sources listed in points i) to iii) by querying internet browsers both in English and the main country language. The key search string we used to identify platforms listing veterinary practices addresses combined the country name with terms such as “find a veterinarian”, “veterinarians near me”, “veterinarians by address”, and “veterinarians by postcode”. For finding national phonebooks, we combined the country name with the terms “national phonebook”, “yellow pages”, and “white pages”, and used the list of World’s Yellow Pages websites available from Wikipedia63. Finally, for the websites of veterinary associations and governmental agencies, we combined the country name with the terms “veterinary association”, “veterinary union”, “veterinary council”, “ministry of agriculture”, and “national statistics institute”. In addition, we used generic queries like “list of veterinarians” followed by the country name to find data potentially present in online sources not considered.
Next, we defined the key search terms to use on each online data source to display addresses of veterinary practices. Specifically, for online platforms listing veterinary practices by postcode, we searched the complete list of veterinary practices in the country, without specifying a city or a postcode in the website’s search box. For national phonebooks, we obtained webpages containing lists of addresses through the search terms “veterinarian”, “veterinarians”, “vet”, “veterinary clinic”, “veterinary hospital”, and “veterinary practice”. For the websites of veterinary councils, we collected the online list or the downloadable PDF of the veterinary practices registered. Finally, when sampling addresses through OpenStreetMap, we used the dedicated Application Programming Interface (API) available in the R package osmdata64 to query the OpenStreetMap database one country at a time. As reported by the OpenStreetMap glossary, we built the API query by using the term “amenity” to subset the group of services listed on the database and the term “veterinary” as the key to refine the search. According to the glossary, a query built with these tags gives in return “places where a veterinary surgeon, also known as a veterinarian or vet, practices”65. In contrast, the API to query the Google Maps database, available in the R package googleway66, allowed for only three queries per zone, which are too few to list veterinary practices in a country. Therefore, we defined multiple country-level queries for every city listed on the opendatasoft database67 through strings containing the term “veterinarian” followed by the city and the country name. In addition, we restricted the search only to places offering veterinary care using the tag “veterinary_care” in the function to query Google’s database, as reported in the API’s user manual68.
Each query of Google Maps always returned the names and addresses of veterinary practices complete with geographic coordinates. In contrast, every OpenStreetMap query returned entries with geographic coordinates, but only 37.7% of them were complete with names and addresses of veterinary practices. Every other online source inspected provided only the addresses of veterinary practices, and, where available, their name and specialization, as text strings. Therefore, we used different means to extract such information from PDFs and web pages. For PDFs, we used the R package tabulizer69 to extract the file’s tables containing the addresses of veterinary practices. When addresses were only available on online platforms, we sampled them through web-scraping70. Specifically, we coded web-scraping software (web-scrapers) in the R and Python programming languages through the packages rvest71 and BeautifulSoup72. Before launching the web-scrapers, we used the R package robotstxt73 to ensure that web-scrapers had the right to access the online platforms. This information is contained inside source code of each platform, built through the HyperText Markup Language (HTML). In platforms denying access to web-scrapers (2 out of 87), we collected addresses of veterinary practices manually. In addition, we used the selenium WebDriver package74 to automate web navigation through web-crawlers to visualize and sample data in multiple web pages of the same website. This practice was also used with websites embedding applications displaying data only upon users’ interaction with specific buttons (e.g., buttons that open sub-sections containing the addresses of veterinary practices).
Finally, we used every address sampled as input for Google geocoding API75 to retrieve its geographic coordinates if not already available (detailed point pattern examples in Supplementary Fig. 16).
Data curation of the addresses of veterinary practices
Addresses of veterinary practices from diverse sources led to collecting duplicated addresses or entries not specifically related to the veterinary medical profession. Therefore, assembling a database with unique geographic entries of veterinary practices required screening for (1) duplicated addresses of veterinary practices sampled from different online data sources, (2) entries related to the broader field of veterinary care where the presence of veterinarians is not required (e.g., veterinary pharmacies), and (3) entries wrongly geocoded by Google’s API because of missing information inside the text strings of the addresses.
For step (1), we first combined the database’s strings reporting the name, the address, and the geographic coordinates of each entry. Then, we calculated the pairwise strings’ similarity through the Levenshtein similarity76. For this first screening, we set a threshold of 90% similarity to consider two strings as duplicates and remove one of them from the database. However, we also identified duplicates when two or more online data sources reported a veterinary practice name and address in different ways. For example, several websites reported strings with truncated addresses, buildings’ numbers placed before the street name or vice versa, or missing addresses’ names, as for data collected especially from OpenStreetMap. For this reason, we performed a second screening using geographic coordinates of sampled data and classified an observation as duplicate if falling within an area of ~10 m radius built around another veterinary practice (Supplementary Fig. 17a).
In step (2) we removed entries related to facilities different from veterinary practices. Hence, we first restricted the screening to entries where a facility’s name didn’t imply the presence of a veterinarian working in that facility (e.g., the store “Budget Pet Supplies” in England). Next, we used Google’s language API to translate more than 100 words (e.g., “veterinary pharmacies”, “pet shop”, “pet supplies”, “pet food”, “kennel”, etc.) in every language available in the Google database. Then, we screened the remaining entries for patterns in the facilities’ names matching the translated words and removed the matching entries from the database.
Nevertheless, online data sources often list facilities that are different from veterinary practices but whose names don’t contain information on the type of service they provide (e.g., the dogs’ training center “Pets with Problems”, Supplementary Fig. 17b). For this reason, we combined web-crawling and web-scraping software to perform automatic Google searches using names and addresses of the unscreened veterinary practices to collect information on the service they provide, listed in the webpage of the Google results (Supplementary Fig. 17b). Then, we removed entries not corresponding to veterinary practices. Since Google also lists the status of a facility, with this method we could also identify veterinary practices permanently closed, excluding them from the database.
Step (3) of data curation concerned entries of veterinary practices addresses wrongly geocoded by the Google API. Hence, for each country, we overlayed their shapefiles to spatial points of sampled addresses and retained in the database only the ones falling within the boundaries of the shapefiles.
Predicting the global distribution of veterinary practices
We aggregated the geocoded addresses of veterinary practices in pixels of 0.08333 decimal degrees, or ~10 × 10 km2 at the equator. As a result, we obtained counts of veterinary practices per pixel that we modeled through a geospatial analysis to predict the distribution of veterinary practices at the global level.
First, we selected spatial covariates potentially related to the distribution of veterinary practices. Previous country-level studies showed that veterinary practices distribution depends on the high population density and income levels of a region44,45, and opportunities to share practices for early graduates46. For this reason, we selected spatial covariates that could correctly represent this observed tendency of veterinary practices to aggregate in urban areas. Specifically, we included in our stack maps of population density, Gross Domestic Product (GDP), and travel time to cities with more than 50,000 inhabitants (hereafter referred to as “major cities”). Besides socio-economic indicators, we also selected agricultural covariates potentially useful to represent the separation of veterinary practices between rural and urban areas. Hence, we included the proportion of areas used for croplands and pastures, and the density of cattle, chickens, pigs, and sheep available from the 4th version of the Gridded Livestock of the World database (GLW4). All covariates, except the proportion of areas used for croplands and pastures, were log10-transformed and all of them were resampled at the 10 × 10 km2 resolution. Plots, measure units, and references of these covariates and support maps used in this study are available in Supplementary Fig. 18 and Supplementary Table 1.
Before incorporating covariates in the models, we quantified their correlation through a version of the Variance Inflation Factor (VIF) adapted for spatial objects77. As for the VIF used in linear regression, we used a threshold of 10 to exclude correlated covariates78 (VIF values in Supplementary Table 5). Furthermore, in a similar approach used to create gridded maps of the human population79–81, we used the 10 × 10 km2 resolution map of the world settlement footprint (WSF)82 to outline areas where there are human settlements. We used such areas to identify the pixels for constraining both the modeling analysis and the predictions of veterinary practices.
Second, we modeled the counts of veterinary practices as a Poisson variable using environmental and anthropogenic covariates as fixed effects. In addition, we used a Gaussian Random Field (GRF), discretized to finite elements called mesh through the Stochastic Partial Different Equation method (SPDE)83,84, as a random effect to account for spatial autocorrelation85,86. A statistical model with these characteristics is called Log-Gaussian Poisson Regression model87 (LGPR). Given the global scale of the study, we fitted the LGPR models through the Integrated Nested Laplace Approximation algorithm (INLA) for computational efficiency87–90.
Third, we defined the geographic areas where to train and validate the accuracy of LGPR models. Specifically, we divided the world into subregions according to the division proposed by the United Nations91. However, we divided the “Europe and Northern America” subregion into three different subregions to have a comparable number of subregions consisting of HICs and LMICs as classified by the World Bank21. As a result, from West to East, we defined 9 subregions: North America, Latin America and the Caribbean (abbreviated as “Latin America”), Europe, Northern Africa and Western Asia (abbreviated as “Middle East”), Sub-Saharan Africa, Russian Federation, Central and Southern Asia (abbreviated as “Central Asia”), Eastern and South-Eastern Asia (abbreviated as “Eastern Asia”), and Oceania (Supplementary Fig. 19).
In each subregion, we initialized the SPDE model by creating the mesh and assigning priors to the function used to capture the spatial autocorrelation of the outcome (i.e., counts of veterinary practices). First, we used subregions’ shapefiles to define the mesh reticulate where to apply the SPDE algorithm. As suggested by Lindgren et al., we defined a regular mesh inside the whole study area where we sampled the outcome90. Also, we defined a 2.5-decimal degree buffer zone outside the shapefile borders to capture spatial autocorrelation of the outcome present along borders while avoiding issues related to the edge effect92. Then, we defined the mesh resolution of the study area (inner mesh), represented by the subregions, by setting a maximum length of the triangle vertices of 0.15 decimal degrees. In contrast, for the mesh in the buffer zone (outer mesh), we allowed for a maximum length of 10 decimal degrees since this area does not influence predictions’ accuracy93. We set the minimum length of triangle vertices of the inner mesh to 0.01 decimal degrees to cover every part of the study area. Then, we applied a Matérn covariance function to the mesh to capture the spatial autocorrelation of the outcome by specifying its hyperparameters. Since we lacked information about spatial dependence among veterinary practices, we assigned Penalized-Complexity priors94 to the hyperparameters of the covariance function by setting a probability lower than 0.01 both for the range and its standard deviation of being higher, respectively, than 0.2 and 0.05 decimal degrees.
Fourth, we used INLA to fit different LGPR models by including in the formula, besides the SPDE, one spatial covariate at the time to assess the importance of each covariate. For each model, we calculated the Deviance Information Criterion (DIC)95,96, whose lower values suggest better model performances97. If a covariate did not decrease the DIC, we excluded it from the LGPR formula. Then, of all the covariates selected, we retained in the final formula just the ones whose 95% credible intervals of their posterior means did not cross zero98 (Supplementary Fig. 20 and Supplementary Table 6). Once we identified the subregional LGPR model with the lowest DIC (hereafter referred to as “best subregional model”), we reran the models removing one covariate at a time to calculate the change in the DIC and identify the covariates with the highest effect in predicting the distribution of veterinary practices. Second, we compared the DIC of each best subregional LGPR model with and without the SPDE model to understand if adding a random effect to capture spatial autocorrelation to the models’ formula decreased the DIC99 (Supplementary Fig. 21).
Next, we assessed the accuracy of the best subregional models by comparing values of predicted vs observed counts of veterinary practices through the adjusted Coefficient of Determination based on the deviance residuals of the model ()100–102. We validated the predictions’ accuracy of every model by computing the between the predicted and the observed counts of veterinary practices per pixel both in the subregions where we trained the models and in all external subregions (Supplementary Fig. 5). In addition, for each subregion we quantified the uncertainty of predictions by mapping their standard deviation and 95% confidence intervals97 (Supplementary Fig. 4b and 4c).
Fifth, we assembled the global map of veterinary practices distribution. Once assessed the accuracy of the LGPR models, we used the predictions obtained by each subregional model that returned the highest to assemble the global map of the distribution of veterinary practices, regardless of their specialization.
We further verified the goodness of our geospatial analysis by checking the agreement between the national estimates of veterinary practices and their country-level numbers aggregated from the pixel-level predictions. Specifically, for each country, we summed the pixel-level predictions of the number of veterinary practices and compared the result with national estimates of veterinary practices sampled from veterinary associations, governmental agencies, peer-reviewed literature, and online newspapers, and with the estimated number of graduates from veterinary schools.
Furthermore, we investigated the preferential sampling of the addresses of veterinary practices. In our study, we only used online data sources to collect the addresses of veterinary practices. However, if veterinarians register online only in certain areas to compete for a pool of patients, our sampling campaign could be preferential. Although preferential sampling can lead to biased predictions of the response103, works have shown that the inclusion of spatial covariates and a random effect accounting for spatial autocorrelation can typically account for this bias104–106. Hence, we selected five countries (Austria, Belgium, Denmark, Netherlands, and Switzerland) as study areas to inspect for the presence of preferentially sampled addresses of veterinary practices using two methods: (1) a joint model proposed by Pennino et al. based on a Log-Gaussian Cox Process (LGCP) to account for preferential sampling103,104 where we used the to compare the accuracy of the predictions of the LGCP with the accuracy obtained through the LGPR models fitted in the same study areas (Supplementary Fig. 22), and (2) a Monte Carlo test that targets the excess of clustered sampling locations in areas of high or low spatial autocorrelation of data by using the K-Nearest Neighbors algorithm107,108.
Predicting the proportion of veterinary practices specialized in food animals
We used the available information about the specialization of veterinary practices to investigate the separation between veterinary practices specialized in treating food animals and veterinary practices specialized in treating companion animals. The purposes were to (1) model the distribution of the proportion of veterinary practices specialized in treating food animals and predicting it in areas where we didn’t find information about the specialization of veterinary practices and (2) understand the effect of the covariates used to predict the global distribution of veterinary practices on the proportion of food vs companion animals’ veterinary practices.
First, we separated spatial points of veterinary practices specialized in treating food animals from the rest of the veterinary practices sampled. We found this information only through governmental agencies and online platforms listing veterinary practices by postcode in Mexico, Argentina, Chile, Great Britain, Belgium, Switzerland, Italy, South Africa, and Iran. Next, we aggregated these points in pixels with a resolution of 10 × 10 km2 and calculated, for each pixel, the ratio between the count of food animals’ veterinary practices and the other veterinary practices sampled. Since 80.1% of the ratios computed was between 0 and 1, we used this share of values to predict the proportion of veterinary practices specialized in food animals.
Second, we used the beta regression model (BRM)109 to predict the proportion of food animals’ veterinary practices at the 10 × 10 km2 resolution and to investigate its relationship with the spatial covariates selected in this study. We used the best subset selection method110 to compute BRMs with every possible combination of covariates. From the batch of BRMs, we extracted just the ones where every covariate was significantly related to the response according to the z-test on the regression coefficients (p-value < 0.05). We identified the best BRM as the one with the lowest Akaike Information Criterion (AIC) value, performed a 10-fold cross-validation, and analyzed plots of its residuals (Supplementary Fig. 23a and 23b). Then, we mapped the proportion of veterinary practices specialized in food animals. We used the BRM with the lowest AIC value to predict the proportion of food animals’ veterinary practices in every 10 × 10 km2 pixel where we predicted the presence of veterinary practices at the global level.
Third, we compared the distribution of veterinary practices with food animals’ density and AMR. The map of the proportion of food vs companion animals’ veterinary practices was multiplied for the map of the distribution of veterinary practices to obtain the map of the distribution of food animals’ veterinary practices. This map was used to identify pixels where food animals outnumber veterinary practices for food animals. Therefore, we compared the global distributions of veterinary practices for food animals and food animals raised in extensive farm systems. Conversely to intensive farm systems, where food animals are housed in confined spaces that facilitate the regular monitoring of their health, extensive farm systems may involve less frequent contact with veterinarians, who might rely more on visual inspections during routine checks or when specific health concerns arise111. For this task, we expressed the 10 × 10 km2 resolution density maps of food animals raised extensively22 in livestock units (LSUs), which is a means to compare different food animals’ productions according to the Eurostat glossary33; for the conversion we used the coefficients of 0.014 for chickens, and 0.5 for pigs. Then, we summed the LSUs pixels’ values for each species into a single layer, divided it for the global map of the distribution of veterinary practices, and produced a 10 × 10 km2 resolution global map of the geographic distribution of LSUs of extensively raised food animals per veterinary practice.
In addition, we investigated if pixels with high LSUs of extensively raised food animals per veterinary practices could be related to the distribution of AMR in food animals. Therefore, we calculated the Pearson correlation between the distribution of LSUs of extensively raised food animals per food animals’ veterinary practice and the distribution of AMR in cattle, chickens, and pigs available for LMICs9. However, this analysis returned a Pearson’s r of 0.3 (r2 = 0.09).
Identification of coldspots of veterinary capacity in food animals
In the human population, the “golden hour” refers to the evidence that people cared within 1 h after a traumatic event have a high chance of a positive health outcome55. Comparably, we defined a veterinary coldspot as a pixel of 10×10 km2 where cattle, chickens, and pigs, which together represent 84.2% of the biomass of animals farmed worldwide112, are farther than 1 h of motorized travel time (TTM) from the nearest veterinary practice.
For each country, we disaggregated the map of veterinary practices for food animals at a resolution of 1 km2. In addition, we used the Google geocoding API to include in such a map also the locations of the veterinary schools sampled, as they are often equipped with veterinary clinics. Then we intersected this map with the 1 km2 resolution GLW4 maps of cattle, chickens, and pigs’ density. Through this step, we identified pixels with cattle, chickens, and pigs but no veterinary practices. We called these pixels “pixels of isolated animals” (PIA) if they contained, according to the GLW4, at least 1 cattle head, 1 pig, and 10 chickens, since 1 chicken is present almost everywhere in the world52,53. The PIA map of cattle in Kenya is reported in Supplementary Fig. 24a; we will refer to this country throughout the manuscript to show small-scale examples of our results. Next, we defined the starting points of veterinarians that travel to PIA as the centroids of the pixels with veterinary schools and predicted veterinary practices, and we used a friction surface to calculate the cumulative cost, in hours, to travel from these centroids to every pixel of the map. For this study, we used the 2020 version of the global motorized friction surface at 1 km2 resolution produced by The Malaria Atlas Project4,113 (Supplementary Table 1 and Supplementary Fig. 24b). This friction surface is a “walking + motorized friction surface”, i.e., it represents a scenario where people must walk to the closest roads (at an average speed of 5 km/h) where it is possible to use motorized vehicles (e.g., public transport, cars), assuming that such vehicles are available without delay when reaching the roads. Then, through the R package gdistance114,115, we combined veterinary practices coordinates, PIA, and the friction surface to calculate 1 km2 resolution TTM maps. Specifically, we calculated the cumulative TTM required by veterinarians to cross each pixel of the friction surface to reach every other country pixel. This calculation was done using the eight-directional least-cost path algorithm115. Furthermore, no corrections were made for the slopes (isotropic movement), since this is usually applied to pedestrians and cyclists116. From these maps, we extracted only PIA where TTM was higher than 1 h (Supplementary Fig. 25a) to identify coldspots.
We computed coldspots maps separately for each country to exclude potential veterinarians traveling across national borders. Then, we aggregated these country-level maps at 10 × 10 km2 resolution and assembled them to obtain global maps of coldspots of veterinary capacity for cattle, chickens, and pigs. The aggregation of coldspots maps from the 1 km2 resolution (i.e., the resolution of the friction surface) to the 10 × 10 km2 resolution caused a 1% area loss for cattle coldspots, 5% for chickens coldspots, and 7% for pigs coldspots.
In addition, we subset the GLW4 maps through the coldspots maps to quantify the number of food animals living inside coldspots. Hereafter, we will refer to such animals as “isolated animals” (IA). Then, we weighted the TTM of every coldspot based on its IA value. The metric, calculated for cattle, chickens, and pigs coldspots in each country, was defined as Eq. (2):
| 2 |
Where i represents the i-pixel of the coldspots map and NC the number of pixels with coldspots. We used Eq. (2) to compute this metric with three maximum TTM thresholds of 6, 4, and 2 h for each coldspot. The reason was to understand if extremely high TTM values (e.g., TTM > 24 h) could affect the sensitivity of and change the ranking of countries with the highest (Fig. 2).
Geographic allocation of veterinary practices to improve access to care
In each country with coldspots, we tried to minimize IA by developing the fastest spatial approach to allocate an additional 5% of the veterinary practices predicted in a country. For the allocation, we targeted 10 × 10 km2 areas where we didn’t predict veterinary practices and where there are human settlements according to the WSF map82; hereafter, we will refer to these areas as “candidates”. An exhaustive allocation approach that adds veterinary practices in every possible candidate will select only the candidates where additional veterinary practices will bring the highest number of IA within 1 h of motorized travel time from a practice. However, an exhaustive approach can be performed iteratively, i.e., testing one candidate at a time and choosing at each iteration the one minimizing IA (recursive exhaustive approach, REA), or simultaneously, i.e., allocating batches of all the possible combinations of the n-veterinary practices available at the same time (simultaneous exhaustive approach, SEA). In addition, an exhaustive allocation approach is a variation of the maximal coverage location problem (MCLP)117,118, which is computationally expensive to solve the higher the number of candidates and the bigger the size of a country119, and faster approximations of the MCLP have been proposed depending on the nature of the facilities to allocate120,121. Therefore, we performed the allocation analysis through different steps.
In step (1), we compared the results of the REA and the SEA applied to a small testing area symbolizing a country with coldspots. The purpose was to verify that the two exhaustive approaches produced the same results to select the fastest one for our next analysis. Specifically, the REA tests every candidate and allocates a veterinary practice inside the candidate minimizing IA. Then, it repeats this process for the number of veterinary practices to allocate to the remaining candidates. In contrast, the steps of the SEA differ from the ones of the REA for the number of veterinary practices allocated at the same time. Specifically, the SEA adds batches of veterinary practices in each possible combination of the candidates available to find the batch minimizing IA. Hence, depending on the number of veterinary practices to allocate, the SEA re-calculates the coldspots map for as many combinations (C(k,n)) as described by Eq. (3):
| 3 |
Where k is the number of candidates and n is the number of veterinary practices. In contrast, the REA recomputes the coldspots only times.
We compared the results of the REA and SEA by defining a coldspots map of approximately 1000 km2 to reduce the computational time to complete both approaches. Next, we selected a random number of 50 candidates and batches of 2, 3, 4, and 5 veterinary practices and allocated them first recursively and then simultaneously to find the candidates minimizing IA. For each batch, we recorded the indices of pixels selected for the allocation, the IA decrease, and the time to complete both approaches. According to our comparison, the computational time to complete the REA was always the lowest (Supplementary Fig. 26). More specifically, even a slight increase from 4 to 5 veterinary practices to allocate through the SEA resulted in a 10,000-fold increase in the computational time required by the REA. Therefore, we selected the REA as the fastest exhaustive approach to quantify the maximum reachable decrease of IA per country.
In step (2), we selected nine small-sized countries (Kenya, Panama, Ecuador, Liberia, Eritrea, Honduras, Nicaragua, Costa Rica, and Cambodia) to compute the number of IA brought within 1 h of motorized travel time from a veterinary practice through the REA and record the decrease of IA for each veterinary practice allocated. Then, we developed a geographically targeted approach to allocate the same 5% of veterinary practices in less time than the REA. The aim was to reach a comparable number of IA brought within 1 h of motorized travel time from a veterinary practice obtained through an exhaustive approach. Specifically, this approach was defined by sampling properties in the proximity area around each candidate. These properties can vary across countries since they depend on i) the density of IA, ii) the distribution of veterinary practices predicted, and iii) local values of the friction surface, due to the characteristics of the territory and traveling speed limits. These properties were used as a guide in the contiguity approach to choose the candidates where the supplementary 5% of veterinary practices could cover a similar animal population covered by the REA. The steps to perform the contiguity approach are reported below.
In step (2.1) we randomly selected 100 candidates and allocated the 1st veterinary practice in one of them, re-computed the coldspots map, and compared it with the initial one to extract the catchment area122,123, i.e., where TTM decreased to <1 h due to the presence of a new veterinary practice (Supplementary Fig. 27a).
In step (2.2) we calculated the average catchment area radius based on the distance of the allocated veterinary practice, starting from each point of the catchment area perimeter (Supplementary Fig. 27a).
In step (2.3) we repeated step (2.2) independently for all the randomly selected candidates to calculate an average radius of the catchment areas specific to each country.
In step (2.4), for the 1st veterinary practice to permanently allocate in the country, we defined a circle around every candidate available based on the average radius calculated in step (2.3).
In step (2.5), within this circle, we sampled a) IA, calculated intersecting maps of food animals’ density with the coldspot map; we used absolute numbers of food animals since they can better inform intervention priorities to reduce coldspots compared to proportions of food animals, which could be similar among countries (Supplementary Fig. 28), b) NC, c) the minimum distance of the candidate from other veterinary practices already predicted in the country, and d) the average TTM. Property (a) allows prioritizing candidates catchment area where IA is the highest. Property (b) and (c) identify candidates that are still far from other veterinary practices and hence where a new allocation doesn’t create clusters of veterinary practices. Property (d) allows the identification of candidates where the TTM in their catchment areas is the lowest; this permits a newly allocated veterinary practice to travel farther and reach more IF.
In step (2.6), for each candidate, we categorized the values of property (a) to (c) in five different groups by building an ordinal variable based on numerical ranges of equal width.
In step (2.7), we assembled each variable in a database and sorted its rows starting with the groups of property (a), i.e., prioritizing allocation in candidates with a high IA124. Then, the sorting of the rows corresponding to candidates surrounded by the highest IA continued based on the groups of the highest NC values. The group of rows corresponding to candidates with high values of IA and NC was then sorted based on their highest minimum distance from predicted veterinary practices (Supplementary Fig. 27a). Finally, among the sorted rows corresponding to the candidates with the highest IA, NC, and farthest from existing veterinary practices, we selected for the allocation the one whose catchment area presented the minimum average TTM.
In step (2.8), we allocated an additional veterinary practice to this candidate, excluded it from the batch of candidates, and re-computed the coldspots maps. Then, we recorded the updated IA.
In step (2.9), we repeated steps (2.4) to (2.8) for all the remaining veterinary practices to allocate in the country by using each time the updated version of the coldspots map.
Finally, in step (3), we compared the different allocation approaches in the same country selected in step (2). Specifically, we compared the REA and the contiguity approach, which recalculate coldspots iteratively, with two additional approaches that allocate all veterinary practices at once. We based the first approach on the administrative division of a country, prioritizing the allocation in regions with the highest number of IA (“administrative approach”). The second approach assigned veterinary practices randomly within a country (“random approach”). For the random approach, we repeated the allocation for 100 Monte Carlo simulations to avoid selecting the candidates minimizing IA by chance. For each approach, including the 100 Monte Carlo allocations of the random approach, we calculated its performance in terms of isolated animals’ coverage (IAC) as Eq. (4):
| 4 |
Where IAREA is the number of isolated animals living in coldspots after allocating veterinary practices through the REA, while IAApproach is the number of isolated animals living in coldspots after allocating veterinary practices through the contiguity, the administrative, or the random approach. Through Eq. (4), the comparison between IAC calculated for each approach was performed between each of the nine countries selected (Supplementary Fig. 9). Then, we inspected the results of each approach through 10 × 10 km2 resolution KDE maps125 computed from the geographic coordinates of the supplementary veterinarians (Supplementary Fig. 10). Since the contiguity approach was faster than the REA while returning comparable results in each of the nine countries selected (Supplementary Table 4), we applied it at the global scale for coldspots of cattle, chickens, and pigs, allocating an additional 5% of the veterinary practices predicted in each country with coldspots. Furthermore, for each species, all the allocations performed at the national level were merged in a unique database and the coordinates of the top 1,000 veterinary practices that removed the highest number of food animals from coldspots were extracted. Then, we mapped the geographic pattern of these supplementary veterinary practices to identify the regions where to prioritize the increase of veterinary capacities.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank S. Dellicour, D. Pigott, and L. Huber for providing inputs and feedback to improve our study, and C. Zhao, A. Morgan, and R. Mulchandani for research assistance. This work is supported by the Branco Weiss Fellowship and the Eccellenza Fellowship from the Swiss National Science Foundation (Grant n° PCEFP3_181248).
Author contributions
T.P.V.B. conceptualized the research idea and supervised the project. N.G.C. and Y.W. collected the data. N.G.C. and T.P.V.B. defined the methodology to analyze the data and produced the visualization of results. N.G.C. and T.P.V.B. wrote the manuscript. T.P.V.B. procured the research funds.
Peer review
Peer review information
Nature Communications thanks Catrin Moore, Sue M. Neal, Amit Chaudhari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Shapefiles used in this study were downloaded from the Database of Global Administrative Areas (GADM). The global map of the distribution of veterinary practices predicted at the 10 × 10 km2, databases of national estimates of veterinary practices, locations of veterinary schools, the URLs of online platforms listing addresses of veterinary practices, and estimates of the Food and Agriculture global statistical database (FAOSTAT) of food animals per country are publicly available at Zenodo126. The exact locations of each individual veterinary practice are protected due to data privacy laws in place in some of the countries included in the analysis. However, the addresses of these veterinary practices can be retrieved from the listing of veterinary practices provided in our Zenodo repository126 for each country.
Code availability
The code used to produce the results of this study is available at Zenodo126.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-60102-y.
References
- 1.Reich, B. J. & Haran, M. Precision maps for public health. Nature555, 32–33 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni, M. A., Duguay, C. & Ost, K. Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: a scoping review of reviews. Glob. Health18, 1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertozzi-Villa, A. et al. Maps and metrics of insecticide-treated net access, use, and nets-per-capita in Africa from 2000 to 2020. Nat. Commun.12, 1–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss, D. J. et al. Global maps of travel time to healthcare facilities. Nat. Med.26, 1835–1838 (2020). [DOI] [PubMed] [Google Scholar]
- 5.healthsites.io Building an open data commons of health facility data with OpenStreetMap. https://healthsites.io (2023).
- 6.Cooke, G. S., Tanser, F. C., Bärnighausen, T. W. & Newell, M. L. Population uptake of antiretroviral treatment through primary care in rural South Africa. BMC Public Health10, 585 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert, M. et al. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat. Commun.5, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements, A. C. A., Pfeiffer, D. U. & Martin, V. Application of knowledge-driven spatial modelling approaches and uncertainty management to a study of Rift Valley fever in Africa. Int. J. Health Geogr.5, 1–12 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Boeckel, T. P. et al. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science365, eaaw1944 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Schar, D. et al. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun.12, 6–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Criscuolo, N. G., Pires, J., Zhao, C. & Van Boeckel, T. P. resistancebank.org, an open-access repository for surveys of antimicrobial resistance in animals. Sci. Data8, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao, C. et al. Geographically targeted surveillance of livestock could help prioritize intervention against antimicrobial resistance in China. Nat. Food2, 596–602 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Bellemain, V. The role of veterinary services in animal health and food safety surveillance, and coordination with other services. Rev. Sci. Technol.32, 371–381 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Neal, S. M. & Greenberg, M. J. Putting access to veterinary care on the map: a veterinary care accessibility index. Front Vet. Sci.9, 857644 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrada, M., Ndiaye, Y., Raboisson, D. & Lhermie, G. Spatial evaluation of animal health care accessibility and veterinary shortage in France. Sci. Rep.12, 13022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministero della Salute. Federazione Nazionale Ordini Veterinari Italiani. Iscritti Ordine Veterinario. https://www.fnovi.it/iscritti-ordine (2023).
- 17.Ordre National des Vétérinaires. Trouver un vétérinaire pour soigner mon animal. https://extranet.veterinaire.fr/annuaires/soigner-mon-animal (2020).
- 18.Istituto Nacional de Estadística. Profesionales sanitarios colegiados 2016—Veterinarios. https://www.ine.es/jaxi/Datos.htm?path=/t15/p416/a2016/&file=s04002.px#!tabs-mapa (2016).
- 19.Swiss Federal Office of Public Health. Health Professions platform. https://www.medregom.admin.ch (2022).
- 20.World Organisation for Animal Health. Performance of Veterinary Service (PVS) Pathway. https://www.woah.org/en/what-we-offer/improving-veterinary-services/pvs-pathway/ #:~:text=The PVS Pathway empowers national,inefficiencies and opportunities for innovation (2022).
- 21.The World Bank - World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (2022).
- 22.Gilbert, M. et al. Income disparities and the global distribution of intensively farmed chicken and pigs. PLoS One10, 1–14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer, J. H., Finucane, M. L., Fox, J. M., Saksena, S. & Sultana, N. Emerging infectious disease, the household built environment characteristics, and urban planning: evidence on avian influenza in Vietnam. Landsc. Urban Plan.193, 103681 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas-Gabrielli, V. & Chevillard, G. ‘Medical deserts’ and accessibility to care: what are we talking about?. Méd./Sci.34, 599–603 (2018). [DOI] [PubMed] [Google Scholar]
- 25.LaVallee, E., Mueller, M. K. & McCobb, E. A systematic review of the literature addressing veterinary care for underserved communities. J. Appl. Anim. Welf. Sci.20, 381–394 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Wikipedia. PREDICT (USAID). https://en.wikipedia.org/wiki/PREDICT_(USAID). (2023).
- 27.Colella, J. P. et al. EcoHealth reframing of disease monitoring Build international biorepository capacity. Science370, 773–775 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Carlson, C. J. et al. The future of zoonotic risk prediction. Philos. Trans. R. Soc. B Biol. Sci.376, 20200358 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Royal College of Veterinary Surgeons. Find a vet. https://findavet.rcvs.org.uk/home/ (2023).
- 30.Ordre National des Vétérinaires. Trouver un vétérinaire pour soigner mon animal. https://extranet.veterinaire.fr/annuaires/soigner-mon-animal (2023).
- 31.Tierarzt-onlineverzeichnis. Tierarzt-Suche nach Bundesländern. https://www.tierarzt-onlineverzeichnis.de/suche (2023).
- 32.Dierenarts kliniek.nl. Alle dierenartsklinieken van Nederland. https://dierenarts-kliniek.nl (2023).
- 33.Eurostat. Livestock Unit. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Livestock_unit_(LSU) (2022).
- 34.Eiselt, H. A. & Marianov, V. Foundations of Location Analysis (Springer, 2011).
- 35.World Organisation for Animal Health. Home. https://www.woah.org/en/home/ (2023).
- 36.WOAH. GBADs—The Global Burden of Animal Diseases. https://gbads.woah.org (2023).
- 37.Radyowijati, A. & Haak, H. Improving antibiotic use in low-income countries: an overview of evidence on determinants. Soc. Sci. Med57, 733–744 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Wint, G. R. W. et al. Mapping bovine tuberculosis in Great Britain using environmental data Europe PMC Funders Group. Trends Microbiol.10, 441–444 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lefrançois, T. & Pineau, T. Public health and livestock: emerging diseases in food animals. Anim. Front.4, 4–6 (2014). [Google Scholar]
- 40.Silva, P. F. A., da, Shimizu, H. E., Sanchez, M. N. & Ramos, M. C. Analysis of the distribution of the health workforce in Brazil. Res. Soc. Dev.11, 1–12 (2022). [Google Scholar]
- 41.Federation of Veterinarians of Europe. Shortage of veterinarians in rural and remote areas. Summary Rep. 1–14 (2020).
- 42.Lem, M. Barriers to Accessible Veterinary Care. https://rucore.libraries (2019). [PMC free article] [PubMed]
- 43.Jang, W. M. et al. Travel time to emergency care not by geographic time, but by optimal time: a nationwide cross-sectional study for establishing optimal hospital access time to emergency medical care in South Korea. PLoS One16, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richards, J. M. An ecological analysis of the geographic distribution of veterinarians in the United States. J. Vocat. Behav.11, 216–231 (1977). [Google Scholar]
- 45.Olfert, M. R., Jelinski, M., Zikos, D. & Campbell, J. Human capital drift up the urban hierarchy: veterinarians in Western Canada. Ann. Reg. Sci.49, 551–570 (2012). [Google Scholar]
- 46.Truchet, S., Mauhe, N. & Herve, M. Veterinarian shortage areas: what determines the location of new graduates?. Rev Agric Food Environ. Stud.98, 255–282 (2017). [Google Scholar]
- 47.Health for Animals. Global state of pet care: stats, facts and trends. https://www.healthforanimals.org/wp-content/uploads/2022/07/Global-State-of-Pet-Care.pdf (2022).
- 48.Lasley, J. N., Appiah, E. O., Kojima, K. & Blacksell, S. D. Global veterinary diagnostic laboratory equipment management and sustainability and implications for pandemic preparedness priorities1. Emerg. Infect. Dis.29, 1–12 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Guardian—China’s new animal health rules alone won’t stop zoonotic outbreaks, experts warn. https://www.theguardian.com/environment/2021/jan/26/chinas-new-animal-health-rules-alone-wont-stop-zoonotic-outbreaks-experts-warn (2021).
- 50.AniCura. Shaping the future of veterinary care, together. https://www.anicuragroup.com (2024).
- 51.OpenStreetMap Foundation. OpenStreetMap. https://www.openstreetmap.org/copyright (2023).
- 52.Gilbert, M. et al. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci Data5, (2018). [DOI] [PMC free article] [PubMed]
- 53.Cinardi, G., Da Re, D., Gilber, M., Robinson, T. P. & Wint, W. G. R. Gridded Livestock of the World—2015 (GLW4). Harvard Dataverse10.7910/DVN/LHBICE (2022).
- 54.Smith, A. B. Origins and spread of African pastoralism. Hist. Compass4, 1–7 (2006). [Google Scholar]
- 55.American College of Surgeon. ATLS—Advanced Trauma Life Support Program for Doctors (American College of Surgeons, 2008).
- 56.Ochoa, C. et al. Vulnerability to snakebite envenoming and access to healthcare in the Terai region of Nepal: a geospatial analysis. Lancet Reg. Health Southeast Asia9, (2023). [DOI] [PMC free article] [PubMed]
- 57.Sen, A. & Chander, M. Privatization of veterinary services in developing countries: a review. Trop. Anim. Health Prod.35, 223–236 (2003). [DOI] [PubMed] [Google Scholar]
- 58.Scott, A. The Flying Vet (Harper Collins Publishers, 2023).
- 59.Federation of Veterinarians of Europe. Survey of the veterinary profession in Europe. https://fve.org/cms/wp-content/uploads/FVE_Survey_2018_WEB.pdf (2019).
- 60.World Organisation for Animal Health. Veterinary Educational Establishments in OiE Member Countries. https://www.woah.org/fileadmin/Home/eng/Support_to_OIE_Members/vee/en_vee_list.php (2013).
- 61.Wikipedia. List of schools of veterinary medicine. https://en.wikipedia.org/wiki/List_of_schools_of_veterinary_medicine (2023).
- 62.Wikipedia. Retirement age. https://en.wikipedia.org/wiki/Retirement_age. (2023).
- 63.Wikipedia. List of yellow pages. https://en.wikipedia.org/wiki/List_of_yellow_pages (2023).
- 64.Padgham, M., Lovelace, R., Salmon, M. & Rudis, B. Osmdata. J. Open Source Softw.2, 305 (2017). [Google Scholar]
- 65.OpenStreetMap Foundation. OpenStreetMap glossary - Tag:amenity=veterinary. https://wiki.openstreetmap.org/wiki/Tag:amenity%3Dveterinary (2023).
- 66.Cooley, D. googleway: Accesses Google Maps APIs to Retrieve Data and Plot Maps. https://cran.r-project.org/package=googleway (2022).
- 67.opnedatasoft. Geonames - All Cities with a population higher than 1000. https://public.opendatasoft.com/explore/dataset/geonames-all-cities-with-a-population-1000/table/?disjunctive.cou_name_en&sort=name. (2023).
- 68.Google Maps Platform. Place Types. https://developers.google.com/maps/documentation/places/web-service/supported_types (2023).
- 69.Jeeper, T. L. tabulizer: Bindings for Tabula PDF Table Extractor Library (2022).
- 70.Khder, M. A. Web scraping or web crawling: State of art, techniques, approaches and application. Int. J. Adv. Soft Comput. Its Appl.13, 144–168 (2021). [Google Scholar]
- 71.Wickham, H. rvest: Easily Harvest (Scrape) Web Pages (2022).
- 72.Richardson, L. Beautiful Soup Documentation. https://beautiful-soup-4.readthedocs.io/en/latest/. (2007).
- 73.Meissner, P. & Ren, K. robotstxt: A ‘robots.txt’ Parser and ‘Webbot’/’Spider’/’Crawler’ Permissions Checker (2020).
- 74.Software Freedom Conservacy. Selenium WebDriver. https://www.selenium.dev/documentation/ (2023).
- 75.Kahle, D. & Wickham, H. ggmap: Spatial visualization with ggplot2. R. J.5, 144–161 (2013). [Google Scholar]
- 76.Levenshtein, V. I. Binary codes capable of correcting deletions, insertions, and reversals. Sov. Phys. - Dokl.10, 707–710 (1966). [Google Scholar]
- 77.Naimi, B., Hamm, N. A. S., Groen, T. A., Skidmore, A. K. & Toxopeus, A. G. Where is positional uncertainty a problem for species distribution modelling?. Ecography37, 191–203 (2014). [Google Scholar]
- 78.Field, A., Miles, J. & Field, Z. Discovering Statistics Using R (SAGE Publications Ltd, 2012).
- 79.WorldPop. WorldPop methods—mapping settlements. https://www.worldpop.org/methods/ (2023).
- 80.Reed, F. J. et al. Gridded population maps informed by different built settlement products. Data3, (2018). [DOI] [PMC free article] [PubMed]
- 81.Stevens, F. R. et al. Comparisons of two global built area land cover datasets in methods to disaggregate human population in eleven countries from the global South. Int J. Digit Earth13, 78–100 (2020). [Google Scholar]
- 82.Marconcini, M. et al. Outlining where humans live, the World Settlement Footprint 2015. Sci. Data7, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lindgren, F., Rue, H. An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic. 423–498 (2011).
- 84.Miller, D. L., Glennie, R. & Seaton, A. E. Understanding the Stochastic Partial Differential Equation Approach to Smoothing. J. Agric Biol. Environ. Stat.25, 1–16 (2020). [Google Scholar]
- 85.Rue, H. & Held, L. Gaussian Markov Random Fields—Theory and Applications (CRC Press,2005).
- 86.Simpson, D., Illian, J. B., Lindgren, F., Sørbye, S. H. & Rue, H. Going off grid: computationally efficient inference for log-Gaussian Cox processes. Biometrika103, 49–70 (2015). [Google Scholar]
- 87.Bachl, F. E., Lindgren, F., Borchers, D. L. & Illian, J. B. inlabru: an R package for Bayesian spatial modelling from ecological survey data. Methods Ecol. Evol.10, 760–766 (2019). [Google Scholar]
- 88.Rue, H., Martino, S. & Chopin, N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Ser. B Stat. Methodol.71, 319–392 (2009). [Google Scholar]
- 89.Bivand, R. S., Gómez-Rubio, V. & Rue, H. Spatial data analysis with R-INLA with some extensions. J. Stat. Softw.63, 1–31 (2015). [Google Scholar]
- 90.Lindgren, F. & Rue, H. Bayesian spatial modelling with R-INLA. J. Stat. Softw.63, 1–25 (2015). [Google Scholar]
- 91.United Nations. World regions in the SDG framework of the United Nations. https://unstats.un.org/sdgs/indicators/regional-groups/ (2021).
- 92.Gao, F., Kihal, W., Meur, N., Souris, M. & Deguen, S. Does the edge effect impact on the measure of spatial accessibility to healthcare providers?. Int J. Health Geogr.16, 1–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Righetto, A. J., Faes, C., Vandendijck, Y. & Ribeiro, P. J. On the choice of the mesh for the analysis of geostatistical data using R-INLA. Commun. Stat. Theory Methods49, 203–220 (2020). [Google Scholar]
- 94.Fuglstad, G. A., Simpson, D., Lindgren, F. & Rue, H. Constructing priors that penalize the complexity of gaussian random fields. J. Am. Stat. Assoc.114, 445–452 (2019). [Google Scholar]
- 95.Berg, A., Meyer, R. & Yu, J. Deviance information criterion for comparing stochastic volatility models. J. Bus. Econ. Stat.22, 107–120 (2004). [Google Scholar]
- 96.Van Der Linde, A. DIC in variable selection. Stat. Neerl.59, 45–56 (2005). [Google Scholar]
- 97.Lezama-Ochoa, N., Pennino, M. G., Hall, M. A., Lopez, J. & Murua, H. Using a Bayesian modelling approach (INLA-SPDE) to predict the occurrence of the Spinetail Devil Ray (Mobular mobular). Sci. Rep.10, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moraga, P. Geospatial Health Data Modeling and Visualization with R-INLA and Shiny. Chapman & Hall/CRC Biostatistics Series10.1201/9780429341823 (2019).
- 99.Gómez-Rubio, V. Bayesian Inference with INLA (Chapman & Hall/CRC Press, 2020).
- 100.Cameron, A. C. & Windmeijer, F. A. G. R-squared measures for count data regression models with applications to health-care utilization. J. Bus. Econ. Stat.14, 209–220 (1996). [Google Scholar]
- 101.Mittlböck, M. Calculating adjusted R2 measures for Poisson regression models. Comput. Methods Prog. Biomed.68, 205–214 (2002). [DOI] [PubMed] [Google Scholar]
- 102.Mohebbi, M., Wolfe, R. & Forbes, A. Disease mapping and regression with count data in the presence of overdispersion and spatial autocorrelation: a Bayesian model averaging approach. Int. J. Environ. Res. Public Health11, 883–902 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pennino, M. G. et al. Accounting for preferential sampling in species distribution models. Ecol. Evol.9, 653–663 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Conn, P. B., Thorson, J. T. & Johnson, D. S. Confronting preferential sampling when analysing population distributions: diagnosis and model-based triage. Methods Ecol. Evol.8, 1535–1546 (2017). [Google Scholar]
- 105.Lee, A., Szpiro, A., Kim, S. Y. & Sheppard, L. Impact of preferential sampling on exposure prediction and health effect inference in the context of air pollution epidemiology. Environmetrics26, 255–267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gelfand, A. E., Sahu, S. K. & Holland, D. M. On the effect of preferential sampling in spatial prediction. Environmetrics23, 565–578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Watson, J. A perceptron for detecting the preferential sampling of locations and times chosen to monitor a spatio-temporal process. Spat. Stat.43, 1–22 (2021).
- 108.Zhao, T. T. et al. Model-based spatial-temporal mapping of opisthorchiasis in endemic countries of Southeast Asia. Elife10, 1–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cribari-Neto, F. & Zeileis, A. Beta regression in R. J. Stat. Softw.34 (2010).
- 110.Hocking, R. R. & Leslie, R. N. Selection of the best subset in regression analysis. Technometrics9, 531–540 (1967). [Google Scholar]
- 111.Temple, D. & Manteca, X. Animal welfare in extensive production systems is still an area of concern. Front Sustain. Food Syst.4, e70789 (2020). [Google Scholar]
- 112.FAOSTAT. Crops and livestock products. https://www.fao.org/faostat/en/#data/QCL (2020).
- 113.The Malaria Atlas Project. Accessibility to healthcare—motorized friction surface. https://malariaatlas.org/project-resources/accessibility-to-healthcare/ (2020).
- 114.van Etten, J. R. Package gdistance: distances and routes on geographical grids. J. Stat. Softw.76, (2017).
- 115.Dijkstra, E. W. A note on two problems in connexion with graphs. Numer. Math.1, 269–271 (1959). [Google Scholar]
- 116.Hierink, F. et al. Differences between gridded population data impact measures of geographic access to healthcare in sub-Saharan Africa. Commun. Med.2, 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Church, R. & Revelle, C. The maximal covering location problem. Pap. Reg. Sci. Assoc.32, 101–118 (1972). [Google Scholar]
- 118.Murray, A. T. Maximal coverage location problem: impacts, significance, and evolution. Int. Reg. Sci. Rev.39, 5–27 (2016). [Google Scholar]
- 119.Vafaeinejad, A., Bolouri, S., Alesheikh, A. A., Panahi, M. & Lee, C. W. The capacitated location-allocation problem using the VAOMP (Vector Assignment Ordered Median Problem) unified approach in GIS. Appl. Sci.10, 1–22 (2020). [Google Scholar]
- 120.Janáček, J. Approximate covering models of location problem. Lect. Notes Manag. Sci.1, 53–61 (2008). [Google Scholar]
- 121.Wei, R. Coverage location models: alternatives, approximation, and uncertainty. Int. Reg. Sci. Rev.39, 48–76 (2016). [Google Scholar]
- 122.Chen, B. Y., Cheng, X. P., Kwan, M. P. & Schwanen, T. Evaluating spatial accessibility to healthcare services under travel time uncertainty: a reliability-based floating catchment area approach. J. Transp. Geogr.87, 102794 (2020). [Google Scholar]
- 123.Macharia, P. M., Ray, N., Gitonga, C. W., Snow, R. W. & Giorgi, E. Combining school-catchment area models with geostatistical models for analysing school survey data from low-resource settings: Inferential benefits and limitations. Spat. Stat.51, 100679 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Falchetta, G., Hammad, A. T. & Shayegh, S. Planning universal accessibility to public health care in sub-Saharan Africa. Proc. Natl. Acad. Sci. USA117, 31760–31769 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duong, T. Statistical visualisation for tidy and geospatial data in R via kernel smoothing methods in the eks package. Comput. Stat. (2024).
- 126.Criscuolo, N. G., Wang, Y. & Van Boeckel, T. P. Data and code for: a global map of travel time to access veterinarians. Available at. Zenodo10.5281/zenodo.14309953 (2024). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Shapefiles used in this study were downloaded from the Database of Global Administrative Areas (GADM). The global map of the distribution of veterinary practices predicted at the 10 × 10 km2, databases of national estimates of veterinary practices, locations of veterinary schools, the URLs of online platforms listing addresses of veterinary practices, and estimates of the Food and Agriculture global statistical database (FAOSTAT) of food animals per country are publicly available at Zenodo126. The exact locations of each individual veterinary practice are protected due to data privacy laws in place in some of the countries included in the analysis. However, the addresses of these veterinary practices can be retrieved from the listing of veterinary practices provided in our Zenodo repository126 for each country.
The code used to produce the results of this study is available at Zenodo126.