Abstract
We have shown previously that glycogen synthase kinase-3β (GSK-3β), cyclin-dependent kinase 5, and c-Jun NH2-terminal kinase become overactivated and hyperphosphorylate τ in heat-shocked female rats. This hyperphosphorylation of τ is estrogen-independent, prevented by androgens, and similar to Alzheimer's disease. In this study, ovariectomized (OVX) Sprague-Dawley rats (n = 75) received daily injections of 10 μg of 17β-estradiol benzoate (EB), or 250 μg of testosterone propionate (TP), or both EB and TP, or sesame oil (SO) vehicle for 4–6 weeks. In kinase assays of forebrain homogenates, overactivation of GSK-3β at 0–6 h after heat shock toward human recombinant τ, bovine τ, and phosphoglycogen synthase peptide 2 was prevented in OVX + TP and OVX + (EB + TP) but not in sham-OVX + SO, OVX + SO, and OVX + EB. Abs against inactive (pSer9) and activity-enhanced (pTyr216) GSK-3β showed marked increase of pSer9- and decrease of pTyr216-GSK-3β in both OVX + TP and OVX + (EB + TP) but not in sham-OVX + SO, OVX + SO, and OVX + EB. EB enhanced the overactivation of cyclin-dependent kinase 5. The activity of c-Jun NH2-terminal kinase was gonadal hormone-independent. The serum concentrations of testosterone and 17β-estradiol were 2.53 ng/ml and 201 pg/ml in OVX + TP and OVX + EB, respectively. These findings demonstrate that testosterone prevents the hyperphosphorylation of τ by inhibiting the heat shock-induced overactivation of GSK-3β and suggest that androgens given to aging men or, in combination with estrogens, to postmenopausal women could prevent or delay Alzheimer's disease.
About two times more women than men have Alzheimer's disease (AD) (1), partly because women with AD live longer. However, recent studies showed that women carry an innate higher risk for AD (2). The precipitous decline and loss of neuroprotective effects of estrogens in postmenopausal women—in contrast to the gradual decline of androgens in aging men—are offered as an explanation. However, recent studies showed no beneficial effects of estrogens on mild-to-moderate AD (3). On the other hand, a possible advantageous role of androgens in the prevention and/or treatment of AD has not been tried yet despite their neuroprotective effects (4), the century-old suggestion that they may rejuvenate aged men (5), lower serum testosterone concentration in men with AD (6), the increasing evidence that stressful stimuli play a role in the etiopathogenesis of AD (7, 8), and inhibition of stress response by androgens (9).
The cause of AD is not known, but it seems to be a syndrome resulting from an interplay among a genetic predisposition, environmental stress factors, and the aging process. The histologic hallmarks of AD are the senile plaques made of Aβ amyloid, dystrophic neurites, and reactive glial cells, and the neurofibrillary tangles composed of bundles of abnormal filaments, the so-called paired-helical filaments, the major component of which is hyperphosphorylated τ (10–12). However, the earliest manifestation of hyperphosphorylated τ is a granular form in the somatodendritic compartment (11, 12). τ is a group of multiple-phosphorylated and thermostable proteins generated by alternative splicing of the single τ gene. Thus, six human (13), four bovine (14), and three rat (15) τ isoforms are generated. Although many kinases and phosphatases have been shown to act on τ in vitro, the dysregulated processes of dephosphorylation-hyperphosphorylation that lead to paired helical filament τ are not known. However, by studying the morphology, evolution, and distribution of τ immunoreactivity in AD, we suggested that it may represent a defensive response to various stressful stimuli (11). To test this hypothesis, we have shown that heat shock induces hyperphosphorylation of τ similar to paired helical filament-τ in female rats (8, 16), which is prevented by androgens but not estrogens (17, 18). Furthermore, in a recent study, we analyzed six kinases and found that only glycogen synthase kinase-3β (GSK-3β), cyclin-dependent kinase 5 (Cdk5), and c-Jun NH2-terminal kinase (JNK) hyperphosphorylated τ after heat shock (19). We now show that testosterone, but not 17β-estradiol, prevents hyperphosphorylation of τ by inhibiting the overactivation of GSK-3β.
Materials and Methods
Purification of Bovine τ and Expression and Purification of Recombinant Human τ (htau40).
Purification of bovine τ has been described (19). After excision with Ndel and EcoRI and blunting with the Klenow fragment of E. coli polymerase, the τ sequence of htau40 containing the entire coding sequence of the longest adult isoform of cerebral τ was cloned into the Smal cut pQE-32 bacterial expression vector (Qiagen, Chatsworth, CA). Clones in correct orientation were selected through restriction enzyme-site analysis and sequencing. The τ construct was expressed in the E. coli strain BL21 (DE3) after induction with 1 mM isopropyl-β-thiogalactopyranoside. This construct contained an N-terminal polyhistidine tag that allowed for affinity purification on a Ni2+-conjugated agarose column. The recombinant protein was purified further to near homogeneity by Mono-Q ion exchange and Superdex-75 gel filtration chromatography using FPLC.
Ovariectomy, Replacement Therapy with Gonadal Hormones, and Heat Shock.
A total of 75, age-matched 2- to 3-month-old Sprague-Dawley rats were anesthetized by i.p. injection of 150–200 μl/200 g of a rat mixture containing ketamine, xylazine, and acepromazine and then ovariectomized (OVX) or sham-OVX. Beginning on the day of OVX, rats were divided into five groups and received daily s.c. injections of 10 μg of 17β-estradiol 3-benzoate (EB; Steraloids, Newport, RI), or 250 μg of testosterone propionate (TP; Steraloids), or 10 μg of EB + 250 μg of TP in 100 μl of sesame oil (SO) vehicle, or 100 μl of SO for 4–6 weeks. EB and TP are slow-release, long-acting derivatives of 17β-estradiol and testosterone, respectively. Rats were heat shocked at 42°C for 15 min as described (16). Control rats were not heat shocked.
Immunocomplex Protein Kinase Assays for GSK-3β, Cdk5, and JNK.
Protein extracts were prepared exactly as described (19). For GSK-3β assay, 100 μg of protein extract was precleared with 20 μl of protein A-agarose beads for 1 h at 4°C. One microgram of Ab against GSK-3β (Transduction Laboratories, Lexington, KY) was added and incubated for 1 h at 4°C, followed by an additional incubation with protein A-agarose beads. The immunocomplexes were washed three times with the extraction buffer containing 1% (vol/vol) Triton X-100 and two times in GSK-3β kinase buffer (250 mM sodium glycerophosphate, pH 7.4/1 M NaCl/100 mM MgCl2/5 mM EGTA/5 mM benzamidine/0.5 mM Na3VO4/5 mM DTT) and incubated with 20 μl of kinase buffer containing 62.5 μM phosphoglycogen synthase peptide 2 (PGSP-2) [contains sites 3β, 3c, and phosphorylated site 4 from glycogen synthase and has the sequence YRRAAVPPSPSLSRHSSPHQ(pS)EDEEE, Upstate Biotechnology, Lake Placid, NY] and [γ-32P] ATP [20 μCi (1 Ci = 37 GBq) per tube, 50 μM final concentration]. After 10 min of incubation at 30°C, the reaction mixture was spotted onto a P-81 filter, and the radioactivity was determined. For Cdk5 and JNK assays, 200 μg of protein extract was precleared with 20 μl of protein G-agarose beads for 1 h at 4°C. An Ab against Cdk5 (sc-173, Santa Cruz Biotechnology) or an Ab against JNK1 (15701A, PharMingen) were added and incubated for 2 h at 4°C, followed by an additional incubation with protein G-agarose beads. The immunocomplexes were washed three times with the extraction buffer containing 1% (vol/vol) Triton X-100 and twice in kinase buffer (20 mM Hepes, pH 7.4/20 mM MgCl2/20 mM β-glycerophosphate/0.1 mM Na3VO4/2 mM DTT). The washed immunocomplexes were incubated with 20 μl of kinase buffer containing 5 μg of histone H1 or 1 μg of the glutathione S-transferase (GST) fusion protein of the truncated human c-Jun (amino acids 1–169; c-Jun-GST; Upstate Biotechnology), respectively, and [γ-32P] ATP (20 μCi per tube, 50 μM final concentration) for 20 min at 30°C. The phosphorylation reactions were terminated by boiling in 20 μl of 2× Laemmli sample buffer (20). Eight microliters (1 μg of histone H1 or 0.2 μg of c-Jun-GST) was resolved on SDS/10% PAGE, followed by autoradiography. Phosphorylated bands were excised and quantified by liquid scintillation counting. For determination of GSK-3β, Cdk5, and JNK activity toward τ, the selective substrates were replaced with 5 μg of htau40 τ or bovine τ. Control reactions were performed in the absence of Abs.
For all kinase assays, the concentrations of τ and selective substrates were above saturation level, as determined in preliminary experiments, so that the initial reaction rate was proportional to enzyme activity alone, linear within the time of assay, and, thus, physiologically relevant.
Immunoblot Analysis of GSK-3β Activity.
We used phosphospecific Abs to probe inactivated pSer9-GSK-3β (Cell Signaling Technology, Beverly, MA) and activity-enhanced pTyr216-GSK-3β (BioSource International, Camarillo, CA) in immunoblots of SDS/10% PAGE from forebrain extracts prepared as described (19). Incubations in primary Abs were done overnight at 4°C and in goat anti-rabbit IgG coupled to horseradish peroxidase for 1 h at room temperature. An activation-independent mAb (G22320, Transduction Laboratories) was used to visualize total GSK-3β. Enhanced chemiluminescence was used as the detection system.
Immunoblot Analysis of the Phosphorylation State of τ in Forebrain Extracts.
Fifty micrograms of protein in extracts, prepared as described (19), from control and heat-shocked rats were mixed with an equal volume of 2× Laemmli buffer, underwent 5–12.5% linear gradient PAGE, and were immunostained as above. We used the following three anti-τ mAbs to correlate the phosphorylation state of τ with the kinase activities: Tau-1 and PHF-1, which require nonphosphorylated Ser (195/198/199/202) and phosphorylated Ser (396/404), respectively, and the phosphate-independent Tau-5. All primary and secondary incubations were for 1 h.
Gonadal Hormone Concentrations in Serum.
The serum concentrations of testosterone and 17β-estradiol were determined by liquid RIA at midcourse 2–3 weeks after OVX in blood samples from the tail artery and after heat shock 4–6 weeks after OVX in blood samples obtained by cardiac puncture at the time of terminal perfusion. We used the ultrasensitive estradiol kit DSL-4800 and the testosterone kit DSL-4100 (Diagnostic Systems Laboratories, Webster, TX).
Statistical Analysis.
Statistical analysis was performed with one- and two-way ANOVAs. Significant ANOVA (P < 0.05) was followed by the Tukey test of all pairwise multiple comparisons.
Results
Purification of τ.
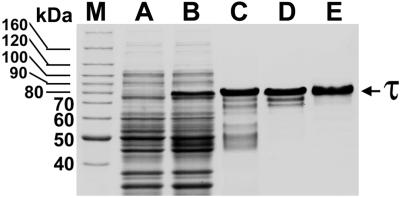
Polyhististine-tagged human recombinant τ was isolated to near homogeneity as illustrated in Fig. 1. Bovine forebrain τ was also purified to near homogeneity as described (19).
Figure 1.
Coomassie blue-stained SDS/PAGE gels show the expression and purification steps of polyhistidine-tagged htau40. Lanes: M, molecular mass standards; A, noninduced extract; B, isopropyl-β thiogalactopyranoside-induced extract; C, after Ni2+ affinity chromatography; D, after Mono-Q column FPLC; and E, purified τ (2 μg) after Superdex-75 column FPLC.
Testosterone Prevents the Heat Shock-Induced Hyperphosphorylation of τ.
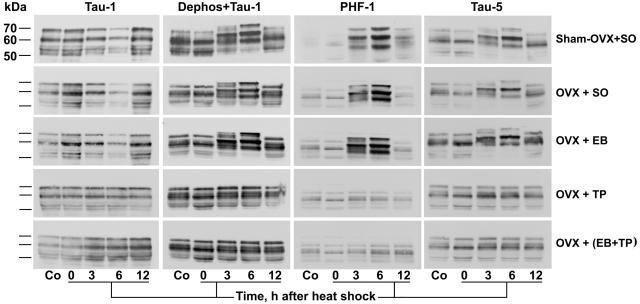
Immunoblot analysis of forebrain extracts showed hyperphosphorylation of τ at 3 and 6 h after heat shock similar to what we have described (8, 16–19), only in sham-OVX + SO, OVX + SO, and OVX + EB, but not in OVX + TP and OVX + (EB + TP) (Fig. 2). Hyperphosphorylation of τ peaked at 6 h after heat shock and was demonstrated by upward gel mobility shift and accentuation of staining intensity with resultant changes in banding patterns; it was recognized by PHF-1 and Tau-5, and by Tau-1 only after prior dephosphorylation of electroblotted proteins. In contrast to OVX + TP and OVX + (EB + TP), hyperphosphorylation of τ was not prevented in OVX + EB.
Figure 2.
Testosterone prevents the heat-shock-induced hyperphosphorylation of τ. Immunoblots of forebrain extracts from control (Co) and heat-shocked rats at 0, 3, 6, or 12 h after heat shock probed with the anti-τ mAbs Tau-1, PHF-1, and Tau-5. Note, at 3 and 6 h: (i) the accentuation and upward mobility shift of τ polypeptides caused by hyperphosphorylation and revealed by Dephos + Tau-1, PHF-1, and Tau-5 in sham-OVX + SO, OVX + SO, and OVX + EB, but not in OVX + TP and OVX + (EB + TP); and (ii) the attenuation of τ polypeptides revealed by Tau-1 in sham-OVX + SO, OVX + SO, and OVX + EB, but not in OVX + TP and OVX + (EB + TP). Dephos, dephosphorylation of electroblotted proteins with alkaline phosphatase before immunostaining.
Initial Rate Kinase Assays.
The results of GSK-3β, Cdk5, and JNK kinase assays toward htau40, bovine τ, and their selective substrates in the five groups of OVX and hormone-treated rats are analyzed below (Figs. 3–7) and summarized in Table 2, which is published as supporting information on the PNAS web site, www.pnas.org.
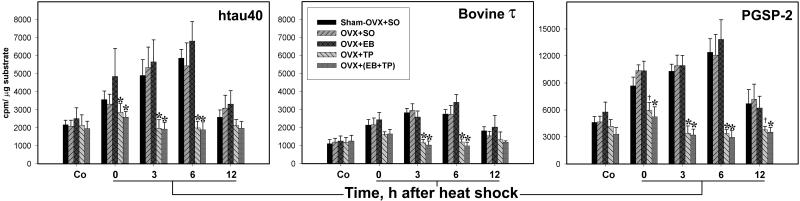
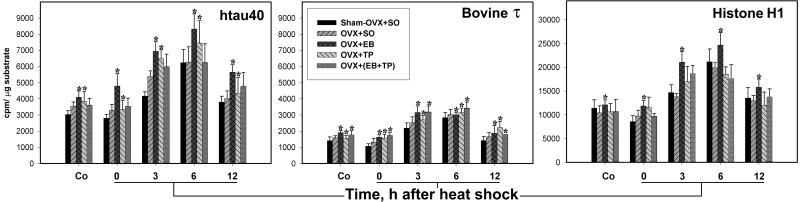
Figure 3.
Testosterone prevents the heat-shock-induced overactivation of GSK-3β. The bar charts show the initial rate activity of GSK-3β in immunocomplex kinase assays of forebrain extracts from control (Co) and heat shocked rats 0, 3, 6, and 12 h after heat shock. Note the marked inhibition of GSK-3β activity toward htau40, bovine τ, and PGSP-2 at 3 and 6 h after heat shock in OVX + TP and OVX + (EB + TP). Also, note the different ordinate scale for PGSP-2. *, P < 0.05, statistically significant difference in comparison with sham-OVX + SO within each control and time point after heat shock; and †, P = 0.089 (Tukey test) but P < 0.05 by Dunnett's method.
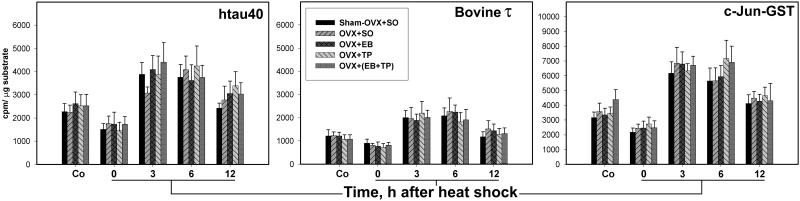
Figure 7.
The kinase activity of JNK is gonadal hormone-independent. The bar charts show the initial rate activity of JNK toward htau40, bovine τ, and c-Jun-GST in immunocomplex kinase assays of forebrain extracts from control (Co) and at 0, 3, 6, and 12 h after heat shock. Note that in both control and heat-shocked rats, there were no statistically significant differences among the five gonadal hormone-treated groups.
Testosterone Prevents the Heat-Shock-Induced Overactivation of GSK-3β.
As in intact rats (19), the activity of GSK-3β toward htau40, bovine τ, and PGSP-2 increased significantly at 0–6 h and peaked at 6 h after heat shock in sham-OVX + SO, OVX + SO, and OVX + EB, but was not significantly different from nonheat-shocked controls in OVX + TP and OVX + (EB + TP) (Fig. 3 and Table 2). The activity of GSK-3β was significantly less in OVX + TP and OVX + (EB + TP) than in the other three groups at 3 and 6 h; it was evidently less at 0 h and became progressively severe at 6 h (Fig. 3). Also, in control rats, the activity of GSK-3β toward PGSP-2 was lowest in OVX + TP and OVX + (EB + TP). The overall GSK-3β activity among the five groups and at all points after heat shock was lowest toward bovine τ, highest toward PGSP-2, and 95% higher toward htau40 than bovine τ (Fig. 3). Furthermore, the GSK-3β activity was not significantly changed in heat-shocked compared with control rats in OVX + TP and OVX + (EB + TP), in contrast to the other three groups (Table 2). Two-way ANOVA showed that the effects of OVX and heat shock and the interaction between them were significant toward all three substrates (P < 0.001). These data are further illustrated in Fig. 4; whereas the concentration of total GSK-3β was similar in all kinase assay mixtures, its increased activity at 0–6 h was increasingly prevented only in OVX + TP and OVX + (EB + TP). Furthermore, immunoblot analysis with activity-dependent phospho-specific Abs showed that the inactivated pSer9-GSK-3β decreased at 0–6 h after heat shock in sham-OVX + SO, OVX + SO, and OVX + EB, and progressively increased in OVX + TP and OVX + (EB + TP); the activity-enhanced pTyr (216)-GSK-3β decreased in groups OVX + TP and OVX + (EB + TP) and increased in OVX + SO and OVX + EB (Fig. 5).
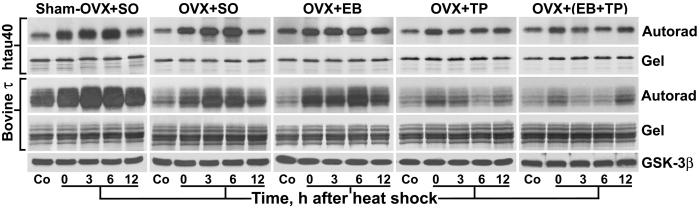
Figure 4.
Testosterone progressively prevents the GSK-3β-induced hyperphosphorylation of τ at 0–6 h after heat shock. Immunoprecipitation kinase assays of GSK-3β activity toward htau40 and bovine τ in forebrain extracts from control (Co) and heat-shocked rats. Autorad, autoradiographs of SDS/PAGE gels; Gel, Coomassie blue-stained SDS/PAGE gels; and GSK-3β, immunoblots of the kinase assay mixtures probed with an mAb against total GSK-3β. Note the progressive prevention of hyperphosphorylation of both htau40 and bovine τ at 0–6 h after heat shock in OVX + TP and OVX + (EB + TP). In contrast, in sham-OVX + SO, OVX + SO, and OVX + EB, τ remained hyperphosphorylated at 0–6 h. Equal amounts of GSK-3β were present in all kinase assay mixtures.
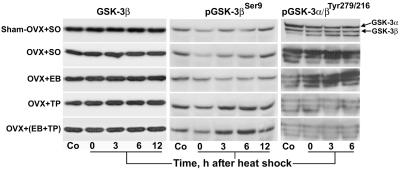
Figure 5.
Activity-dependent Abs confirm the inhibition of GSK-3β by testosterone. Immunoblots of 50 μg of total protein in forebrain extracts from control (Co) and heat-shocked rats were probed with phospho-specific Abs against inactivated (pGSK-3βSer9) and activity-enhanced (pGSK-3α/βTyr279/216) GSK-3β. Note: (i) the progressive accentuation of staining intensity for pGSK-3βSer9 in OVX + TP and OVX + (EB + TP) at 0–6 h after heat shock; (ii) the persistent attenuation of staining intensity for pGSK-3βSer9 in sham-OVX + SO, OVX + SO, and OVX + EB at 0–6 h; (iii) the severe attenuation of staining intensity for pGSK-3α/βTyr279/216 in OVX + TP and OVX + (EB + TP) but not in OVX + SO and OVX + EB; and (iv) the equal amounts of total GSK-3β in all samples. Also, note that because the amino acids surrounding the activity-enhancing pTyr279 in GSK-3α and pTyr216 in GSK-3β are identical (21), both were recognized by the Ab; the identity of the third band is not known.
17β−Estradiol Stimulates Cdk5 but Its Activity Toward τ Is Overridden by the Inhibition of GSK-3β by Testosterone.
The results of Cdk5 kinase activity are shown in Fig. 6 and Table 2. Two-way ANOVA showed that the activity of Cdk5 toward htau40, bovine τ, and histone H1 was significantly higher at 3–6 h after heat shock (P < 0.001) as in intact rats (19) and different among the five OVX and hormone-treated groups (P < 0.001), but there was no significant interaction between the effects of heat shock and hormone treatment. The activity of Cdk5 toward all three substrates was highest in OVX + EB (Fig. 6) with a 48%, 30%, and 23% overall increase toward htau40, bovine τ, and histone H1, respectively, in comparison with sham-OVX + SO. The activity of Cdk5 toward htau40 and histone H1 was significantly higher in OVX + EB than in the other four groups but, toward bovine τ, only in comparison with sham-OVX + SO (Fig. 6). Also, in OVX + TP, the Cdk5 activity was slightly higher toward htau40 and bovine τ but not toward histone H1 in comparison with sham-OVX + SO. However, despite the enhanced overactivation of Cdk5 after heat shock in OVX + EB, the inhibition of GSK-3β by testosterone prevented the hyperphosphorylation of τ, as is demonstrated in OVX + (EB + TP) of Fig. 2.
Figure 6.
17β-Estradiol stimulates the kinase activity of Cdk5. The bar charts show the initial rate activity of Cdk5 toward htau40, bovine τ, and histone H1 in immunoprecipitation kinase assays of forebrain extracts from control (Co) and at 0, 3, 6, and 12 h after heat shock. Note the enhanced overactivation of Cdk5 toward all three substrates and at all time points after heat shock in OVX + EB. Also, the Cdk5 activity toward htau40 in OVX + EB was significantly higher than in the other four groups. *, P < 0.05, a statistically significant difference in comparison with sham-OVX + SO.
The Activity of JNK Is Independent of Gonadal Hormones.
As is shown in Fig. 7 and Table 2, the activity of JNK toward htau40, bovine τ, and c-Jun-GST increased significantly at 3 and 6 h after heat shock in all five OVX and hormone-treated groups and decreased significantly toward htau40 and c-Jun-GST at 0 h. It also remained significantly increased at 12 h toward htau40 and c-Jun-GST. Two-way ANOVA confirmed the significant effects of heat shock on the activity of JNK toward all three substrates (P < 0.001) and its independence from gonadal hormones. Also, there was no significant interaction between the effects of heat shock and hormonal treatment on the JNK activity.
Gonadal Hormone Concentrations in Serum.
As is shown in Table 1, the concentrations of testosterone were within the physiologic range for male rats (22); the concentrations of 17β-estradiol were slightly above the proestrus levels of cycling female rats (23) but within the range used in similar experimental models (24). Although within the normal range of the estrous cycle, the levels of 17β-estradiol in sham-OVX + SO were rather low, but this result was probably caused by the stress of daily injections.
Table 1.
Gonadal hormone concentrations in sera
| Treatment | Testosterone*, ng/ml
|
17β-Estradiol†, pg/ml
|
||
|---|---|---|---|---|
| Midcourse | Final | Midcourse | Final | |
| Sham-OVX + SO | — | 0.08 ± 0.02 (n = 10) | — | 11.8 ± 6.4 (n = 8) |
| OVX + SO | < MCD (n = 5) | < MCD (n = 7) | 4.3 ± 1.0 (n = 5) | 9.3 ± 3.5 (n = 9) |
| OVX + EB | 0.06 ± 0.01 (n = 5) | 0.06 ± 0.01 (n = 11) | 129.5 ± 36.7 (n = 5) | 201 ± 100.3 (n = 9) |
| OVX + TP | 4.83 ± 1.30 (n = 10) | 2.53 ± 0.82 (n = 11) | 10.3 ± 2.1 (n = 4) | 10.2 ± 5.2 (n = 11) |
| OVX + (EB + TP) | 4.53 ± 1.18 (n = 10) | 3.20 ± 0.82 (n = 10) | 153.8 ± 36.4 (n = 7) | 138.2 ± 9.3 (n = 7) |
n, number of rats; MCD, minimal concentration detectable.
and
, the ranges of detectable concentrations were 0.05–25.0 ng/ml and 2.2–3,000.0 pg/ml, respectively, but for 17β-estradiol, the standard curve was not linear below 5 pg/ml. Data are means ± SD.
Other Findings.
We performed quantitative immunoblot analysis of the relative total amounts of GSK-3β, Cdk5, and JNK in forebrain extracts from control and heat-shocked rats with primary Abs and 125I-labeled protein A. One-way ANOVA revealed no statistically significant differences among the five gonadal hormone-treated groups (see Table 3, which is published as supporting information on the PNAS web site, www.pnas.org.).
Discussion
The salient finding of our study is the prevention of the heat-shock-induced overactivation of GSK-3β but not of Cdk5 and JNK and the concomitant abolition of τ hyperphosphorylation by testosterone or testosterone combined with 17β-estradiol, but not by 17β-estradiol alone. Because in nonheat-shocked control rats the activity of GSK-3β toward htau40, bovine τ, and PGSP-2 was not significantly different among the five gonadal hormone-treated groups, testosterone seems to prevent the overactivation of GSB-3β under stressful conditions. The lack of GSK-3β inhibition in OVX + EB indicates that testosterone and not its aromatized metabolite 17β-estradiol prevents the overactivation of GSK-3β and concomitant hyperphosphorylation of τ.
GSK-3β is a Ser/Thr multifunctional kinase that phosphorylates Pro and other sites in τ (for a review, see ref. 25). Although Ser/Thr/Pro sites do not require priming phosphorylation, the majority of GSK-3β substrates are formed by prephosphorylation of the second Ser in the target motif Ser-Xaa-Xaa-Xaa-Ser by another kinase. GSK-3β has a high basal activity, phosphorylating its substrates in the default state, whereas stimulation decreases its activity. Many kinases phosphorylate and inactivate GSK-3β, such as the cAMP-dependent protein kinase A (26), protein kinase C (27), and integrin-linked kinase (28), but the Wnt/Wg and insulin-signaling pathways are the two most studied. Both of these signaling pathways lead to inactivation of GSK-3β, but crosstalk between them is prevented and followed by different downstream effects (29). N-terminal Ser9 of GSK-3β phosphorylated by protein kinase B (30) in the insulin pathway behaves as a competitive pseudosubstrate inhibiting phosphorylation of primed substrates (31–33). On the other hand, in the Wnt/Wg pathway, the vertebrate protein GBP/FRAT binds to and disengages GSK-3β from the destruction complex (axin, adenomatous polyposis coli, β-catenin, GSK-3β; refs. 34 and 35), preventing phosphorylation of β-catenin and τ. However, the separation of the two pathways is not perfect, because chronic and prolonged Wnt stimulation leads to phosphorylation of Ser9 by protein kinase B within the axin complex (36).
As for the multiple Pro sites of τ, priming phosphorylation of GSK-3β substrates is not always required (37). Also, FRATtide (a FRAT1 peptide) inhibits the GSK-3β-catalyzed phosphorylation of nonprimed τ but not primed τ (38). In this study, the GSK-3β activity toward htau40, which is devoid of phosphorylation and thus unprimed, was about two times higher than toward native bovine τ, indicating prephosphorylation of GSK-3β sites in bovine τ. As is shown in Fig. 5, not only Ser9 was hyperphosphorylated at 3 and 6 h after heat shock in OVX + TP and OVX + (EB + TP), but also the activity-enhancing Tyr216 was hypophosphorylated. It is believed that phosphorylation of Tyr216 in the activation loop (residues 200–226), which is equivalent to Tyr185 in mitogen-activated protein kinase, enhances (39, 40) but is not essential (31) for GSK-3β activation and can be overridden by phosphorylation of Ser9 (41). Although GSK-3β is constitutively active and phosphorylated on Tyr216, the stimulation conferred by Tyr216 phosphorylation varies in the literature from 2- to 200-fold (39, 42).
GSK-3β is a proapoptotic kinase (43) and antagonizes the prosurvival effects of heat-shock response (44). Heat-shock proteins inhibit the apoptotic pathway at multiple sites (45), and gonadal hormones have well known anti-apoptotic effects. However, the anti-apoptotic activity of gonadal hormones is nongenotropic, nonsex-specific, involves a Src/Shc/ERK signaling pathway, and is dissociated from the genotropic transcriptional activity, despite the fact that both are mediated through estrogen or androgen receptors (46). Our finding that testosterone but not 17β-estradiol prevented the overactivation of GSK-3β favors a genotropic and sex-specific transcriptional effect mediated through the androgen receptor, which is present in both female and male cerebral cortices (47). How and at what points along the many possible signaling pathways testosterone inhibits GSK-3β remains the subject of future investigations.
Testosterone given together with 17β-estradiol inhibited GSK-3β to a similar degree as testosterone alone. On the other hand, 17β-estradiol given alone had a stimulatory effect on the activity of Cdk5 toward htau40 and histone H1, which was significantly higher than testosterone alone or testosterone combined with 17β-estradiol. In view of the fact that Cdk5 may play a role in AD (48) and, in addition, that estrogen has hypoandrogenic effects (49), the possibility that estrogen given alone may be detrimental rather than protective against AD should be considered. On the contrary, because of the increasing evidence implicating GSK-3β in the pathogenesis of AD (50–52), testosterone given alone to aging men and given combined with 17β-estradiol to postmenopausal women would probably prove beneficial in preventing and/or treating AD. The rat heat-shock model in its transient form and the lack of senile plaques and neurofibrillary tangles is certainly not a model of AD. However, it is simple, highly reproducible, and recapitulates in just 6 h the most important biochemical abnormality of AD that in humans takes a lifetime to develop. In view of the recent findings that neurofibrillary tangles per se may not be necessary for neurotoxicity (53), the rat heat-shock model could be used to test the efficacy of small-molecule inhibitors of GSK-3β (54, 55) in preventing hyperphosphorylation of τ.
Supplementary Material
Acknowledgments
We thank Drs. L. I. Binder and P. Davies for supplying the anti-τ mAbs Tau-1 and PHF-1, respectively, and Dr. M. Goedert for providing the clone htau40. This work was supported by the Alzheimer's Association (S.Ch.P.).
Abbreviations
- AD
Alzheimer's disease
- Cdk5
cyclin-dependent kinase 5
- GSK-3β
glycogen synthase kinase 3β
- JNK
c-Jun NH2-terminal kinase
- OVX
ovariectomized or ovariectomy
- EB
17β-estradiol benzoate
- TP
testosterone propionate
- SO
sesame oil
- PGSP-2
phosphoglycogen synthase peptide 2
- GST
glutathione S-transferase
- c-Jun-GST
truncated human c-Jun
References
- 1.Jorm A F, Korten A E, Henderson A S. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 2.Andersen K, Launer L J, Dewey M E, Letenneur L, Ott A, Copeland J R M, Dartigues J-F, Kragh-Sorensen P, Baldereschi M, Brayne C, et al. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 3.Mulnard R, Cotman C W, Kawas C, van Dyck C H, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, et al. J Am Med Assoc. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 4.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown-Sequard C E. Lancet. 1889;2:105–107. [Google Scholar]
- 6.Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith A D. Neuroendocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- 7.Mori H, Kondo J, Ihara Y. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 8.Papasozomenos S C, Su Y. Proc Natl Acad Sci USA. 1991;88:4543–4547. doi: 10.1073/pnas.88.10.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr J E, Beck S G, Handa R J. Neuroendocrinology. 1996;8:439–447. doi: 10.1046/j.1365-2826.1996.04735.x. [DOI] [PubMed] [Google Scholar]
- 10.Grundke-Iqbal I, Iqbal K, Yung Y-C, Quinlan M, Wisniewski H, Binder L. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papasozomenos S C. Lab Invest. 1989;60:123–137. [PubMed] [Google Scholar]
- 12.Papasozomenos S C. Lab Invest. 1989;60:375–388. [PubMed] [Google Scholar]
- 13.Goedert M, Spillantini M G, Jackes R, Rutherford D, Crowther R A. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 14.Himmler A. Mol Cell Biol. 1989;9:1389–1396. doi: 10.1128/mcb.9.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosik K S, Orecchio L D, Bakalis S, Neve R L. Neuron. 1989;2:1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- 16.Papasozomenos S C. J Neurochem. 1996;66:1140–1149. doi: 10.1046/j.1471-4159.1996.66031140.x. [DOI] [PubMed] [Google Scholar]
- 17.Papasozomenos S C. Proc Natl Acad Sci USA. 1997;94:6612–6617. doi: 10.1073/pnas.94.13.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papasozomenos S C, Papasozomenos T. J Alzheimer's Dis. 1999;1:147–153. doi: 10.3233/jad-1999-1302. [DOI] [PubMed] [Google Scholar]
- 19.Shanavas A, Papasozomenos S C. Proc Natl Acad Sci USA. 2000;97:14139–14144. doi: 10.1073/pnas.97.26.14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Woodgett J R. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keating R J, Tcholakian R K. Endocrinology. 1979;104:184–188. doi: 10.1210/endo-104-1-184. [DOI] [PubMed] [Google Scholar]
- 23.Butcher R L, Collins W E, Fugo N W. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 24.Green P S, Simpkins J W. Int J Dev Neurosci. 2000;18:347–358. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- 25.Grimes C A, Jope R S. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 26.Fang X, Yu S X, Lu Y, Bast R C, Jr, Woodgett J R, Mills G B. Proc Natl Acad Sci USA. 2000;97:11960–11961. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsujio I, Tanaka T, Kudo T, Nishikawa T, Shinozaki K, Grundke-Iqbal I, Takeda M. FEBS Lett. 2000;469:111–117. doi: 10.1016/s0014-5793(00)01234-5. [DOI] [PubMed] [Google Scholar]
- 28.Yoganathan T N, Costello P, Chen X, Jabali M, Yan J, Leung D, Shang Z, Yee A, Dedhar S, Sanghera J. Biochem Pharmacol. 2000;60:1115–1119. doi: 10.1016/s0006-2952(00)00444-5. [DOI] [PubMed] [Google Scholar]
- 29.Ding V W, Chen R H, McCormick F. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 30.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 31.Dajani R, Fraser E, Roe S M, Young N, Good V, Dale T C, Pearl L H. Cell. 2001;105:721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 32.Frame S, Cohen P, Biondi R M. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 33.ter Haar E, Coll J T, Austen D A, Hsiao H-M, Swenson L, Jain J. Nat Struct Biol. 2001;8:593–596. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- 34.Yost C, Farr G S, III, Pierce S B, Ferkey D M, Chen M M, Kimelman D. Cell. 1998;93:1031–1041. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Yuan H, Weaver C D, Mao J, Farr G H, III, Sussman D J, Jonkers J, Kimelman D, Wu D. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukumoto S, Hsieh C-M, Maemura K, Layne M D, Yet S-F, Lee K-H, Matsui T, Rosenzweig A, Taylor W G, Rubin J S, et al. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 37.Diehl J A, Cheng M G, Roussel M F, Sherr C J. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas G M, Frame S, Goedert M, Nathke I, Polakis P, Cohen P. FEBS Lett. 1999;458:247–251. doi: 10.1016/s0014-5793(99)01161-8. [DOI] [PubMed] [Google Scholar]
- 39.Kim L, Liu J, Kimmel A R. Cell. 1999;99:399–408. doi: 10.1016/s0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- 40.Hartigan J A, Xiong W-C, Johnson G V W. Biochem Biophys Res Commun. 2001;284:485–489. doi: 10.1006/bbrc.2001.4986. [DOI] [PubMed] [Google Scholar]
- 41.Bhat R V, Shanley J, Correll M P, Fieles W E, Keith R A, Scott C W, Lee C-M. Proc Natl Acad Sci USA. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes K, Nikolakaki E, Plyte S E, Totty N F, Woodgett J R. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hetman M, Cavanaugh J E, Kimelman D, Xia Z. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xavier I J, Mercier P A, McLoughlin C M, Ali A, Woodgett J R, Ovsenek N. J Biol Chem. 2000;275:29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- 45.Beere H M, Green D R. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- 46.Kousteni S, Bellido T, Plotkin L I, O'Brien C A, Bodenner D L, Han K, DiGregorio G B, Katzenellenbogen J A, Katzenellenbogen B S, Roberson P K, et al. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 47.Finley S K, Kritzer M F. J Neurobiol. 1999;40:446–457. [PubMed] [Google Scholar]
- 48.Patrick G N, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai L H. Nature (London) 1999;402:588–589. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 49.Casson P R, Elkind-Hirsch K E, Buster J E, Hornsby P J, Carson S A, Snabes M C. Obstet Gynecol. 1997;90:995–998. doi: 10.1016/s0029-7844(97)00538-3. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi H, Ishiguro K, Uchida T, Taskashima A, Lemere C A, Imahori K. Acta Neuropathol. 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 51.Lucas J J, Hernandez F, Gomez-Ramos P, Moran M A, Hen R, Avila J. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomidokoro Y, Ishiguro K, Harigaya Y, Matsubara E, Ikeda M, Park J-M, Yasutake K, Kawarabayashi T, Okamoto K, Shoji M. Neurosci Lett. 2001;299:169–172. doi: 10.1016/s0304-3940(00)01767-5. [DOI] [PubMed] [Google Scholar]
- 53.Wittmann C W, Wszolek M F, Shulman J M, Salvatera P M, Lewis J, Hutton M, Feany M B. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 54.Coghlan M P, Culbert A A, Cross D A E, Corcoran S L, Yates J W, Pearce N J, Rausch O L, Murphy G J, Carter P S, Cox L R, et al. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 55.Leclerc S, Garnier M, Hoessel R, Marko D, Bibb J A, Snyder G L, Greengard P, Biernat J, Wu Y-Z, Mandelkow E-M, et al. J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.